Orphee Mythic 22-CT User manual

mythic 22 CT
User’s Manual
Orphée SA
19, chemin du Champ des Filles - 1228 CH-Plan Les Ouates / SWITZERLAND
Tel : +41.(0)22.884.90.90 Fax : +41.(0)22.884.90.99 - Web Site : www.orphee-medical.com
Cod. M22CT/UM-EN/003
|
|
|
|
|
REF : M22CT/UM/EN/003 |
|
|
|
REVISIONS |
|
|
|
|
|
|
|
|
Revision Nb |
Date |
Author |
Software |
|
Comments |
|
|
|
|
|
|
|
|
|
|
|
|
01 |
23/10/06 |
HC |
> V1.0 |
|
Creation |
02 |
11/01/08 |
HC |
> V2.0 |
|
English correction, Section 4.2 modified |
03 |
25/05/08 |
CM/HC |
> V2.2.0 |
|
Update of the prompts |
|
|
|
|
|
|
|
|
|
|
|
|
CONTACT ADDRESS
MANUFACTURER
M
Manufactured in France for
ORPHEE SA
19, chemin du champ des filles CH-1228 Geneva / Plan-les-Ouates SWITZERLAND
Tel : +41 (0) 22 706 1840 Fax : +41 (0) 22 794 4391
http://www.orphee-medical.com
LOCAL AGENT
Page 2/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |
REF : M22CT/UM/EN/003
READ THIS BEFORE USING THE EQUIPMENT
|
|
|
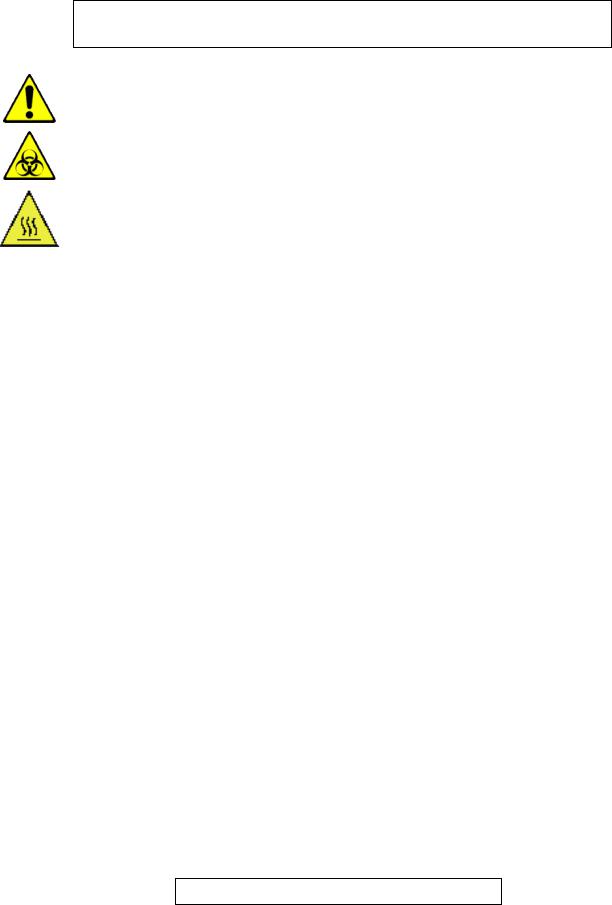
WARNING ! RISK OF DANGER ! Indicates a procedure to be strictly respected in |
|
|
|
order to avoid any risks for the operator (user) or damages on the instrument or on |
|
|
|
the quality of results. |
|
|
|
Indicates that wearing gloves is mandatory before performing the described |
|
|
|
operation due to risk of contact with materials that may be infectious. |
|
|
|
|
|
|
|
Indicates hot temperatures surfaces and risk of burns |
|
|
|
|
|
|
|
|
|
NOTA |
|
Indicates important additional information |
|
|
|
|
|
|
|
|
|
|
|
DANGER |
|
|
|
Misuse of electrical equipment may cause electrocution, burns, fire and others hazards. |
Check that the voltage setting matches the supply voltage.
Protective grounding is required, plug the MYTHIC 22 CT into a supply outlet which has an earth connection.
Preserve a good access to the supply outlet to be able to unplug the MYTHIC 22 CT in emergency case.
Do not place the power supply adapter in liquid, nor put it where it could fall into liquid. If the power supply adapter becomes wet, unplug it before touching it.
Do not use the MYTHIC 22 CT if it is not working properly, or if it has suffered any damage ( damage to the supply cord or its plug; damage caused by dropping the power supply adapter).
Do not let the power supply adapter or its flexible cord coming in contact with surfaces which are too hot to touch.
Do not place anything on top of the MYTHIC 22 CT
Do not use the MYTHIC 22 CT where aerosol sprays are being used, or where oxygen is being administered.
Do not use the MYTHIC 22 CT outside.
Always switch off the MYTHIC 22 CT and disconnect the power adaptor before dismantling any part.
The MYTHIC 22 CT is an automated hematology analyzer for in vitro diagnostic to be used in clinical laboratories by an authorized people.
-Only human blood or artificial control blood should be run.
-Only the reagents mentioned in this manual are permitted to be used.
-The optimum performances can be only achieved if the cleaning and maintenance procedures are carefully
followed.
Due to the use of this equipment, all parts and surfaces of the MYTHIC 22 CT are potentially infective. Wearing rubber gloves is highly recommended and after completion of work, wash hands with disinfectant.
Always replace or use parts of the equipment supplied by ORPHEE’s representative.
Basic safety precautions should always be taken. If the equipment is not used according to the manufacturer’s instructions, the protective by the equipment may be impaired.
The treatment of waste and the elimination of a part or the complete instrument must be done in compliance with the local legislation.
Any output or input connections (except the printer and the barcode reader supplied by ORPHEE) cannot be done without the ORPHEE representative authorization.
Do not open the door located on the right side of the instrument (see section 1.1.3) when a fluidic cycle is in progress for it would lead to an immediate stop. To re-start, shut the door and run a Control cycle (see section 9.3.1)
KEEP THESE INSTRUCTIONS
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 3/102 |

REF : M22CT/UM/EN/003
This equipment needs special precautions regarding general requirements for safety.
Guidance and manufacturer’s declaration – Electromagnetic emissions
The MYTHIC 22 CT is intended for use in the electromagnetic environment specified below. The customer or the user of the MYTHIC 22 CT should assure that it is used in such an environment.
Emissions test |
Compliance level |
Electromagnetic environment - guidance |
Harmonic emissions |
Class A |
The MYTHIC 22 CT is suitable for use in all establishments, including |
|
|
domestic establishments and those directly connected to the public |
IEC 61000-3-2 |
|
low-voltage power supply network that supplies buildings used for |
Voltage fluctuations/flicker |
Complies |
domestic purposes. |
emissions |
|
|
IEC 61000-3-3 |
|
|
Guidance and manufacturer’s declaration – Electromagnetic immunity
The MYTHIC 22 CT is intended for use in the electromagnetic environment specified below. The customer or the user of the MYTHIC 22 CT should assure that it is used in such an environment.
Immunity test |
IEC 60601 test level |
Electrostatic |
±6 kV contact |
discharge (ESD) |
±8 kV air |
IEC 61000-4-2 |
|
Electrical fast |
±2 kV for power supply lines |
transient/burst |
±1 kV for input/output lines |
IEC 61000-4-4 |
|
Surge |
±1 kV differential mode |
IEC 61000-4-5 |
±2 kV common mode |
Voltage dips, short |
<5 % UT |
interruptions and |
(>95 % dip in UT) for 0,5 cycle |
voltage variations |
40 % UT |
on power supply |
(60 % dip in UT) for 5 cycles |
input lines |
70 % UT |
IEC 61000-4-11 |
(30 % dip in UT) for 25 cycles |
|
<5 % UT |
|
(>95 % dip in UT) for 5 sec |
Power frequency |
3 A/m |
(50/60 Hz) |
|
magnetic field |
|
IEC 61000-4-8 |
|
|
|
Compliance
level
Complies
Complies
Complies
Complies
Complies
Electromagnetic environment - guidance
Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, the relative humidity should be at least 30 %.
Mains power quality should be that of a typical commercial or hospital environment.
Mains power quality should be that of a typical commercial or hospital environment.
Mains power quality should be that of a typical commercial or hospital environment. If the user of the MYTHIC 22 CT requires continued operation during power
mains interruptions, it is recommended that the MYTHIC 22 CT be powered from an uninterruptible power
supply or a battery.
Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment.
NOTE UT is the a.c. mains voltage prior to application of the test level.
Page 4/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |

REF : M22CT/UM/EN/003
Guidance and manufacturer’s declaration – Electromagnetic immunity
The MYTHIC 22 CT is intended for use in the electromagnetic environment specified below. The customer or the user of the MYTHIC 22 CT should assure that it is used in such an environment.
Immunity test |
IEC 60601 test level |
Compliance |
Electromagnetic environment - guidance |
|
|
level |
|
|
|
|
Portable and mobile RF communications equipment should |
|
|
|
be used no closer to any part of the MYTHIC 22 CT, |
|
|
|
including cables, than the recommended separation |
|
|
|
distance calculated from the equation applicable to the |
|
|
|
frequency of the transmitter. |
|
|
|
Recommended separation distance |
Conducted RF |
3 Vrms |
3 Vrms |
d = 1,2√P |
IEC 61000-4-6 |
150Khz to 80Mhz |
|
|
Radiated RF |
3 Vrms |
3 Vrms |
d = 1,2√P 80MHz to 800MHz |
IEC 61000-4-3 |
80Mhz to 2,5Ghz |
|
|
|
|
|
d = 2,3√P 800MHz to 2,5GHz |
|
|
|
Where P is the maximum output power rating of the |
|
|
|
transmitter in watts (W) according to the transmitter |
|
|
|
manufacturer and d is the recommended separation |
|
|
|
distance in meters (m). |
|
|
|
Field strengths from fixed RF transmitters, as |
|
|
|
determined by an electromagnetic site survey,a should be |
|
|
|
less than the compliance level in each frequency range |
|
|
|
Interference may occur in the vicinity of equipment |
|
|
|
marked with the following symbol: |
|
|
|
|
NOTE 1 At 80Mhz and 800MHz, the higher frequency range applies.
NOTE 2 Theses guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
aField strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM an FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should considered. If the measured field strength in the location in which the MYTHIC 22 CT is used exceeds the applicable RF compliance level above, the MYTHIC 22 CT should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the MYTHIC 22 CT.
bOver the frequency range 150KHz to 80MHz, field strengths should be less than 3V/m.
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 5/102 |

REF : M22CT/UM/EN/003
NOTE: This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential installation. This equipment generates, uses and can radiate radio frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to radio communications. However, there is no guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try to correct the interference by one or more of the following measures:
-Reorient or relocate the receiving antenna.
-Increase the separation between the equipment and receiver.
-Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
-Consult the dealer or an experienced radio/TV technician for help.
The user may find the following booklet, prepared by the Federal Communications Commission, helpful:
How to identify and Resolve Radio/TV Interference Problems. This booklet is available from the U.S. Government Printing Office, Washington, D.C. 20402, Stock No. 004-000-00345-4.
Pursuant to Part 15.21 of the FCC Rules, any changes or modifications to this equipment not expressly approved by C2
The symbol  on the product indicates that this product may not be treated as household waste. Instead it shall be handed over the applicable collection point for the recycling of electrical and electronic equipment. By ensuring this product is disposed of correctly, you will help prevent potential negative consequences for the environment and human health, which could otherwise be caused by inappropriate waste handling of this product. For more detailed information about recycling of this product, please contact your local city office or your distributor of this product.
on the product indicates that this product may not be treated as household waste. Instead it shall be handed over the applicable collection point for the recycling of electrical and electronic equipment. By ensuring this product is disposed of correctly, you will help prevent potential negative consequences for the environment and human health, which could otherwise be caused by inappropriate waste handling of this product. For more detailed information about recycling of this product, please contact your local city office or your distributor of this product.
Page 6/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |

REF : M22CT/UM/EN/003
KONFORMITÄTSERKLÄRUNG /
DECLARATION DE CONFORMITE
DECLARATION OF CONFORMITY /
DICHIARAZIONE DI CONFORMITA
Name und Adresse der Firma
Nom et adresse de l’entreprise
Nome e indirizzo della ditta
Name and address of the firm
Wir erklären in alleiniger Verantwortung, dass
Nous déclarons sous notre propre responsabilité que Dichiariamo sotto nostra responsabilità che
We declare under our sole responsibility that
das Medizinprodukt für die In-vitro-Diagnostik le dispositif médical de diagnostic in vitro
il dispositivo medico-diagnostico in vitro the in vitro diagnostic medical device
Orphée S.A.
19 Chemin du Champ des Filles
1228 Plan Les Ouates
Mythic 22 CT
Ref. M22CT
mit folgender Klassifizierung nach der Richtlinie über In-vitro-Diagnostika 98/79/EG
avec la classification selon la directive relative aux dispositifs médicaux de diagnostic in vitro 98/79/CE con la classificazione secondo la direttiva relativa ai dispositivi medico-diagnostici in vitro 98/79/CE classified as follows according to the directive on in vitro diagnostic medical devices 98/79/EC

 Produkt der Liste A, Anhang II / Dispositif de la liste A, annexe II /
Produkt der Liste A, Anhang II / Dispositif de la liste A, annexe II /
Dispositivo dell’elenco A, allegato II / Device of List A, Annex II

 Produkt der Liste B, Anhang II / Dispositif de la liste B, annexe II /
Produkt der Liste B, Anhang II / Dispositif de la liste B, annexe II /
Dispositivo dell’elenco B, allegato II / Device of List B, Annex II

 Produkt zur Eigenanwendung, das nicht in Anhang II genannt ist /
Produkt zur Eigenanwendung, das nicht in Anhang II genannt ist /
Dispositif destiné à l’autodiagnostic non listé dans l’annexe II /
Dispositivo per test autodiagnostico non elencato nell’allegato II /
Device for self-testing not listed in Annex II

 Sonstiges Produkt / Autre dispositif / Altro dispostivo / Other device
Sonstiges Produkt / Autre dispositif / Altro dispostivo / Other device
allen Anforderungen der Richtlinie über In-vitro-Diagnostika 98/79/EG entspricht, die anwendbar sind.
remplit toutes les exigences de la directive relative aux dispositifs médicaux de diagnostic in vitro 98/79/CE qui le concernent.
soddisfa tutte le disposizioni della direttiva relativa ai dispositivi medico-diagnostici in vitro 98/79/CE che lo riguardano.
meets all the provisions of the directive on in vitro diagnostic medical devices 98/79/EC which apply to it.
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 7/102 |

REF : M22CT/UM/EN/003
Angewandte Gemeinsame Technische Spezifikationen, harmonisierte Normen, nationale Normen oder andere normative Dokumente
Spécifications techniques communes, normes harmonisées, normes nationales et autres documents normatifs appliqués
Specifiche tecniche comuni, norme armonizzate o nazionali applicate, altri documenti normativi applicati
Applied common technical specifications, harmonised standards, national standards or other normative documents
Konformitätsbewertungsverfahren
Procédure d’évaluation de la conformité
Procedimentodi valutazionedellaconformità Conformity assessment procedure
Konformitätsbewertungsstelle (falls beigezogen) Organe respons. de l'évaluat.de la conformité(si consulté)
Organo incaric. della valutaz. della conform. (se consultato)
Notified Body (if consulted)
Ort, Datum / Lieu, date /
Luogo, data / Place, date
Genève le 19 Juin 2008
Annex III
N/A
Name und Funktion / Nom et fonction /Nome e funzione / Name and function
Philippe Daire
RA & QA
Page 8/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |

REF : M22CT/UM/EN/003
TABLE OF CONTENTS
READ THIS BEFORE USING THE EQUIPMENT.......................................................................... |
3 |
|
1. INSTALLATION ....................................................................................................... |
|
12 |
1.1 UNPACKING................................................................................................................................................................................................ |
|
12 |
1.1.1 Introduction ............................................................................................................................................................................................ |
|
12 |
1.1.2 Unpacking Procedure |
............................................................................................................................................................................. |
12 |
1.1.3 Visual checking........................................................................................................................................................................................ |
|
13 |
1.2 INSTALLATION CONSTRAINTS............................................................................................................................................................... |
13 |
|
1.2.1 Installation place.................................................................................................................................................................................... |
|
13 |
1.2.2 Installation environment ...................................................................................................................................................................... |
13 |
|
1.3 ELECTRICAL CONNECTIONS..................................................................................................................................................................... |
|
14 |
1.3.1 Power supply block ................................................................................................................................................................................. |
|
14 |
1.4 PRINTER CONNECTION............................................................................................................................................................................. |
|
14 |
1.5 CONNECTION, CHANGE AND PRIMING REAGENTS ................................................................................................................................. |
15 |
|
1.5.1 Connection ............................................................................................................................................................................................... |
|
15 |
1.5.2 Priming ..................................................................................................................................................................................................... |
|
16 |
1.6 TRANSPORTATION AND STORAGE .......................................................................................................................................................... |
18 |
|
2. GENERAL OVERVIEW ................................................................................................. |
|
19 |
2.1 GENERALITIES.......................................................................................................................................................................................... |
|
19 |
2.2 OVERVIEW............................................................................................................................................................................................... |
|
20 |
2.3 MAIN PART DESCRIPTION...................................................................................................................................................................... |
|
20 |
2.3.1 Display / Keyboard ................................................................................................................................................................................ |
|
20 |
2.3.2 Dilution fluidic part .............................................................................................................................................................................. |
|
22 |
2.3.3 Mono electronic board ......................................................................................................................................................................... |
23 |
|
2.3.4 Power Supply Block ............................................................................................................................................................................... |
|
23 |
2.3.5 Reagent tray .......................................................................................................................................................................................... |
|
24 |
3. INSTRUMENT SET UP................................................................................................ |
|
25 |
3.1 USER’S IDENTIFICATION ...................................................................................................................................................................... |
|
25 |
3.1.1 Start Up................................................................................................................................................................................................... |
|
25 |
3.1.2 In process ............................................................................................................................................................................................... |
|
25 |
3.2 SYSTEM STATUS..................................................................................................................................................................................... |
|
26 |
3.3 SET UP ..................................................................................................................................................................................................... |
|
27 |
3.4 ADVANCED SET-UP.................................................................................................................................................................................. |
|
28 |
3.4.1 Printer set up :....................................................................................................................................................................................... |
|
28 |
3.4.2 Communication: ...................................................................................................................................................................................... |
|
28 |
3.4.3 Analysis options:.................................................................................................................................................................................... |
|
29 |
3.4.4 Lab. parameters: ................................................................................................................................................................................... |
|
29 |
3.4.5 Calibration factor: ................................................................................................................................................................................ |
|
33 |
3.4.6 Other Setting:....................................................................................................................................................................................... |
|
33 |
3.4.7 Storage options :................................................................................................................................................................................... |
|
33 |
3.4.8 Version release : ................................................................................................................................................................................... |
|
34 |
4. SPECIFICATIONS ..................................................................................................... |
|
35 |
4.1 ANALYTICAL SPECIFICATIONS.............................................................................................................................................................. |
35 |
|
4.2 PHYSICAL SPECIFICATIONS |
.................................................................................................................................................................. |
37 |
4.3 REAGENTS SPECIFICATIONS ................................................................................................................................................................ |
39 |
|
4.3.1 Diluent...................................................................................................................................................................................................... |
|
39 |
4.3.2 Lytic reagent “OnlyOne”...................................................................................................................................................................... |
40 |
|
4.3.3 Cleaning solution.................................................................................................................................................................................... |
|
41 |
4.4 ANALYTICAL LIMITATIONS................................................................................................................................................................... |
|
42 |
4.3.1 Recommendations .................................................................................................................................................................................. |
|
42 |
4.3.1 Interferences ........................................................................................................................................................................................ |
|
42 |
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 9/102 |

|
|
REF : M22CT/UM/EN/003 |
5. SAMPLE ANALYSIS ................................................................................................... |
|
47 |
5.1 VERIFICATIONS BEFORE STARTING ...................................................................................................................................................... |
47 |
|
5.2 START UP ................................................................................................................................................................................................. |
|
47 |
5.3 REAGENT REPLACEMENT ......................................................................................................................................................................... |
|
48 |
5.4 START UP RINSING................................................................................................................................................................................. |
|
49 |
5.5 PREPARATIONS BEFORE ANALYSIS ....................................................................................................................................................... |
49 |
|
5.5.1 Anticoagulant and mixing ..................................................................................................................................................................... |
49 |
|
5.5.2 Blood sample collection tube .............................................................................................................................................................. |
49 |
|
5.6 ANALYSIS ............................................................................................................................................................................................... |
|
50 |
5.6.1 Introduction ........................................................................................................................................................................................... |
|
50 |
5.6.2 Sample Identification.......................................................................................................................................................................... |
51 |
|
5.6.3 Close tube sample run .......................................................................................................................................................................... |
52 |
|
5.6.4 Other vials sample run......................................................................................................................................................................... |
53 |
|
5.7 RESULTS .................................................................................................................................................................................................. |
|
54 |
5.8 PRINTING ................................................................................................................................................................................................ |
|
56 |
5.8.1 Model report (A4) – external printer ............................................................................................................................................... |
56 |
|
5.8.2 Model report – Thermal printer ........................................................................................................................................................ |
57 |
|
5.9 LOGS ........................................................................................................................................................................................................ |
|
58 |
5.10 ARCHIVE ................................................................................................................................................................................................ |
|
58 |
5.10.1 Results ................................................................................................................................................................................................... |
|
59 |
5.10.2 View........................................................................................................................................................................................................ |
|
60 |
5.11 STAND BY AND SHUT DOWN.................................................................................................................................................................. |
61 |
|
6. QUALITY CONTROL .................................................................................................. |
|
62 |
6.1 INTRODUCTION....................................................................................................................................................................................... |
|
62 |
6.2 QC ........................................................................................................................................................................................................... |
|
62 |
6.2.1 Change...................................................................................................................................................................................................... |
|
63 |
6.2.2 Run control blood .................................................................................................................................................................................. |
|
64 |
6.2.3 Levey-Jennings graph .......................................................................................................................................................................... |
65 |
|
6.2.4 Restore.................................................................................................................................................................................................... |
|
65 |
6.3 REPEATABILITY....................................................................................................................................................................................... |
|
66 |
7. CALIBRATION ......................................................................................................... |
|
68 |
7.1 RUN CALIBRATOR ............................................................................................................................................................................. |
|
69 |
7.1.1 Calibration blood analysis ..................................................................................................................................................................... |
69 |
|
7.1.2 Calibration............................................................................................................................................................................................... |
|
70 |
7.2 TARGET VALUE MODIFICATIONS ........................................................................................................................................................... |
70 |
|
8. TECHNOLOGY.......................................................................................................... |
|
72 |
8.1 DETECTION PRINCIPLE............................................................................................................................................................................ |
|
72 |
8.1.1 WBC, RBC, PLT Counting....................................................................................................................................................................... |
72 |
|
8.1.2 Five part diff measurement ................................................................................................................................................................ |
73 |
|
8.1.3 Hemoglobin measurement .................................................................................................................................................................... |
74 |
|
8.2 LEUCOCYTE ANALYSIS............................................................................................................................................................................ |
|
74 |
8.3 ERYTHROCYTE ANALYSIS ....................................................................................................................................................................... |
|
75 |
8.4 ANALYSIS OF PLATELETS....................................................................................................................................................................... |
|
76 |
8.5 FLAGS ...................................................................................................................................................................................................... |
|
76 |
8.5.1 General Flags .......................................................................................................................................................................................... |
|
76 |
8.5.2 Instrument Flags .................................................................................................................................................................................. |
|
77 |
8.5.3 Leucocytes Flags ................................................................................................................................................................................... |
|
77 |
8.5.4 Erythrocyte and HGB Flags ................................................................................................................................................................ |
78 |
|
8.5.4 Platelet Flags ......................................................................................................................................................................................... |
|
78 |
8.5.5 QC Flags.................................................................................................................................................................................................. |
|
78 |
8.5.6 STARTUP Flags ..................................................................................................................................................................................... |
|
78 |
8.6 HYDRAULIC DESCRIPTION...................................................................................................................................................................... |
|
79 |
8.6.1 Sampling module..................................................................................................................................................................................... |
|
79 |
8.6.2 Counting bath module........................................................................................................................................................................... |
79 |
|
8.6.3 Syringes module .................................................................................................................................................................................... |
|
79 |
8.6.4 Optical manifold.................................................................................................................................................................................... |
|
79 |
8.6.5 Optical bench......................................................................................................................................................................................... |
|
80 |
8.8 SOFTWARE .............................................................................................................................................................................................. |
|
80 |
8.8.1 Windows .................................................................................................................................................................................................. |
|
80 |
8.8.2 Menu tree ............................................................................................................................................................................................... |
|
80 |
9. SERVICE ............................................................................................................... |
|
82 |
Page 10/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |
REF : M22CT/UM/EN/003 |
|
9.1 MAINTENANCE ........................................................................................................................................................................................ |
82 |
9.1.1 Maintenance table.................................................................................................................................................................................. |
82 |
9.1.2 Concentrate cleaning ............................................................................................................................................................................ |
83 |
9.1.3 Piston greasing ....................................................................................................................................................................................... |
84 |
9.1.4 In line filter cleaning ............................................................................................................................................................................ |
85 |
9.2 HYDRAULIC CYCLES................................................................................................................................................................................. |
86 |
9.3 MECHANICS............................................................................................................................................................................................. |
87 |
9.4 REPAIRING............................................................................................................................................................................................... |
88 |
9.4.1 Emergency stop...................................................................................................................................................................................... |
88 |
9.4.2 Needle or o-ring replacement ............................................................................................................................................................ |
88 |
9.4.3 Baths dismantling.................................................................................................................................................................................. |
91 |
9.4.4 Baths o-ring replacement.................................................................................................................................................................... |
94 |
9.4.5 Aperture block replacement............................................................................................................................................................... |
94 |
9.5 TROUBLESHOOTING ............................................................................................................................................................................... |
95 |
9.5.1 Analytical problems............................................................................................................................................................................... |
95 |
9.5.2 Other problems ..................................................................................................................................................................................... |
96 |
9.6 TROUBLESHOOTING MESSAGE .............................................................................................................................................................. |
97 |
9.7 LOGS ERRORS ......................................................................................................................................................................................... |
100 |
9.8 HYDRAULIC DIAGRAM ............................................................................................................................................................................ |
101 |
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 11/102 |

1. INSTALLATION
REF : M22CT/UM/EN/003
1.INSTALLATION
1.1UNPACKING
1.1.1 Introduction
The MYTHIC 22 CT is an automated hematology analyzer for in vitro diagnostic use in clinical laboratories by an authorized people.
-Only human blood or artificial control blood should be run.
-Only the reagents mentioned in this manual are permitted to be used.
-The optimum performances can be only achieved if the cleaning and maintenance procedures are carefully followed.
If the MYTHIC 22 CT has been stored at a temperature lower than 10°C it must be let at room temperature during 24 hours.
1.1.2 Unpacking Procedure
Before unpacking the instrument, we recommend to check the box of the instrument and notify any damage to the carrier.
-Open the box on the top, remove the starter kit.
-Remove the MYTHIC 22 CT from the box.
Starter kit contents :
|
INSTALLATION KIT |
|
QTY |
|
Designation |
1 |
Tubing 23 - DILUENT |
|
1 |
Tubing 24 - WASTE |
|
1 |
150W switching adapter |
|
1 |
European Power line cord |
|
1 |
MYTHIC 22 |
CT User manual |
1 |
MYTHIC 22 |
CT Installation Report form |
1 |
MYTHIC 22 |
CT Quality Control Certificate |
1 |
Screwdriver Slot 1/4" |
|
MAINTENANCE KIT
QTY Designation
1 Tygon tubing L=500mm 1.02mm
1 Tygon tubing L=500mm 1.3mm
1 Tygon tubing L=1000mm 1.52x3.2mm
1 Tygon tubing L=500mm 2.06x4mm
1 Tubing 50
4Bottle cap filter 1 In line filter
5Tie wraps
2 Rinsing Head O-ring
1 Silicon grease (3gr)
1 Short Arm TORX T10 Tool
1 Short Arm TORX T20 Tool
Page 12/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |

REF : M22CT/UM/EN/003
1. INSTALLATION
1.1.3 Visual checking
 3
3  2
2
 1
1
Open the door on the right side with the key provided in the kit.
To be checked :
1- Counting chambers perfectly locked in their manifold locations.
2- Needle’s dismountable system located in the rocker. 3- Rocker in front position at the maximum course.
HAZARDOUS MOVING PARTS, BEWARE TO STAY AWAY FROM THESE PARTS WHEN THE MACHINE IS SWITCH ON.
1.2 INSTALLATION CONSTRAINTS 1.2.1 Installation place
To ensure that the MYTHIC 22 CT fulfills its function, place the instrument on a table which supports the weight of the instrument, printer and reagents (around 40 Kg). Leave a space of 10 cm in the rear of the instrument to ensure a well-ventilated place. Avoid a place that can be exposed to direct sunlight.
1.2.2Installation environment
a)Indoor use;
b)Altitude up to 2 000 m;
c)Temperature 18 °C to 34 °C;
d)Maximum relative humidity 80 % for temperatures up to 31 °C decreasing linearly to 50 % relative humidity at 40 °C;
e)MAINS supply voltage fluctuations up to ±10 % of the nominal voltage;
f)Transient over voltages typically present on the MAINS supply.
g)Rated pollution degree II.
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 13/102 |

1. INSTALLATION
REF : M22CT/UM/EN/003
Please contact Orphée’s representative if you want to use the instrument in special conditions (altitude higher than 2000 m or special power supply conditions).
1.3 ELECTRICAL CONNECTIONS
All the connectors are in the rear of the MYTHIC 22 CT |
|
CONNECTION : |
SYMBOL : |
- 2 USB ports : |
|
- Ethernet connection (TCP/IP) : |
|
- External barcode reader (RS232C) : |
|
- Host connection (RS232C) : |
|
- Printer connection (Centronics) : |
|
- Power supply cord connection : |
|
- Equipotentiality : |
|
Any output or input connections (except the printer and the bar code reader supplied by ORPHEE) cannot be done without the ORPHEE representative authorization.
1.3.1 Power supply block
MYTHIC 22 CT must be connected to the power with the power supply block provided with the starter kit. Choose a well-ventilated place for the block and be sure to connect this power supply in a socket-outlet with a correct earth connection.
The power supply block must be placed at the rear of the MYTHIC 22 CT and, if possible in an upper position to avoid the contact with any liquid.
To disconnect electrically the MYTHIC 22 CT, remove the power supply plug from the main circuit.
In case of replacement of the main power wire, supplied with the MYTHIC 22 CT, the new one must comply with the local regulation (31.5mm cable and 250V 10A plug).
The MYTHIC 22 CT has been certified with the power supply provided with the machine. If another power supply is used with the instrument, Orphée or its representative will not apply any warranty on this power supply and on the instrument. Please contact Orphée or its local representative before to use such material.
1.4 PRINTER CONNECTION
Connect the printer cable in conformity with the printer user’s manual.
Use the parallel rear plug of the MYTHIC 22 ( ) or the USB plug (
) or the USB plug ( ) to connect the printer cable. Select the printer driver (section 3.3).
) to connect the printer cable. Select the printer driver (section 3.3).
Page 14/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |

REF : M22CT/UM/EN/003
1. INSTALLATION
1.5 CONNECTION, CHANGE AND PRIMING REAGENTS
MYTHIC 22 CT works exclusively with the reagents described in section 4.3. Orphée or its local representative will not be responsible of the quality of the results and of the maintenance of the instrument if other commercial reagents are used.
1.5.1 Connection
Lytic reagent and cleaning solution :
Before handling the reagents, read carefully their specifications described in section 4.3.
-Remove the door on the left side of the instrument.
-Put the reagent bottles in the dedicated location.
-Remove the caps of the bottles.
-Tighten the red caps on the OnlyOne bottle (red sticker) and the blue one on the cleaning solution bottle (blue sticker).
Diluent and waste :
Waste
Diluent
-Connect the diluent tube (male connector) on the outlet on the bottom and tighten the cap on the diluent container.
-To use a 20 liter diluent container add the tubing straw adaptor supplied with the installation kit.
-Connect the waste tube (female connector) on the outlet on the top and tighten the cap on an empty container.
Do not modify the type and the length of the diluent and waste tubes.
The diluent must be placed on the same level as the MYTHIC 22 CT.
It is mandatory to collect the waste in a container and to treat it in compliance with your local regulation.
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 15/102 |
1. INSTALLATION
REF : M22CT/UM/EN/003
1.5.2 Priming
To use the MYTHIC 22 CT for the first time, it is necessary to perform a complete prime of the fluidic circuit. This operation should be done by a Field Service Engineer.
Before starting, be sure that all the reagent and waste tubes are properly connected. The reagents must be stored 24 hours minimum at room temperature before use.
Priming procedure :
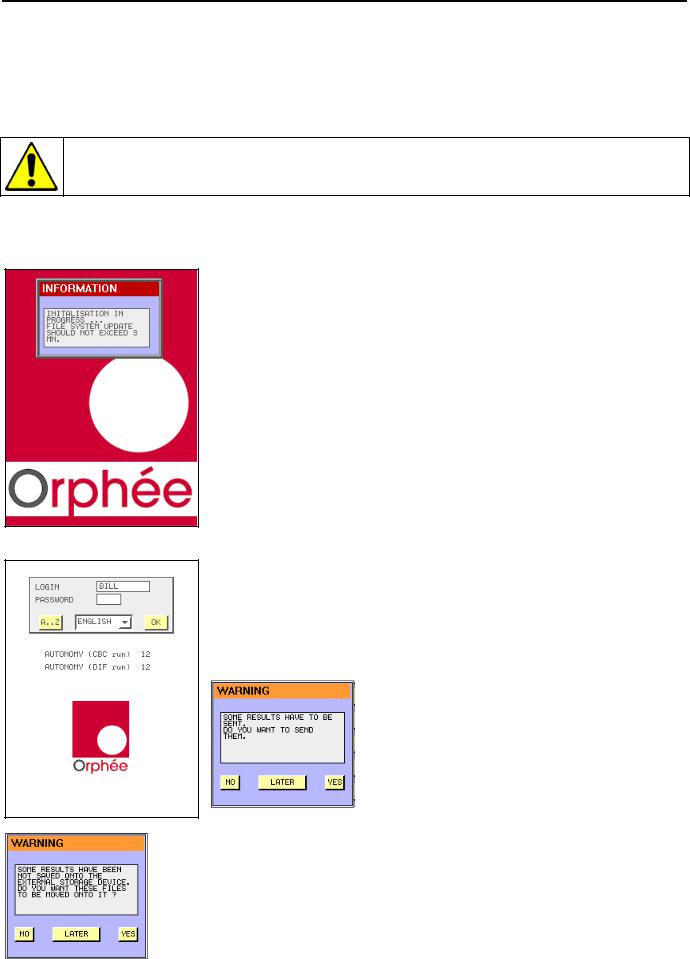
Switch on :
-Connect the power supply block. Switch on the power supply (see section 2.3.4). The power supply can stay switch on all the time.
-Press the ON/OFF button. 
-The cycle LED  turns red. No cycle can be performed before it turns green.
turns red. No cycle can be performed before it turns green.
-The information window could stay up to 3 min to enable the update of all the files.
Login :
-The operator’s identification display appears.
-Enter the user’s identification, the password (see section 3.1) and press
 to validate.
to validate.
- AUTONOMY (run) indicates the number of samples (runs) you can perform (calculated with the smaller volume of reagents).
- If this window appears, it means that several results in memory have not been sent before the MYTHIC 22 was switched off.
- Press YES to send them immediately, or press LATER to postpone them or NO if you do not want to send them.
- No USB key is available, connect an USB key then press YES or see section 3.4.7 to change the archive mode.
Page 16/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |

REF : M22CT/UM/EN/003
1. INSTALLATION
System priming :
-The main menu is displayed.
-Press on  .
.
NOTA To do an emergency stop in case of problem push shortly on the on/off button 
-Press  : The MYTHIC 22 CT performs a complete priming cycle.
: The MYTHIC 22 CT performs a complete priming cycle.
-The cycle LED  turns red. No cycle can be performed before it turns green.
turns red. No cycle can be performed before it turns green.
-AUTONOMY (run) indicates the number of samples (runs).
-To prime or to know the quantity of reagent press the dedicated button.
-Press  to display the cycle counters.
to display the cycle counters.
-To reset the counter with the button  , please contact your Orphée’s representative.
, please contact your Orphée’s representative.
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 17/102 |
1. INSTALLATION
REF : M22CT/UM/EN/003
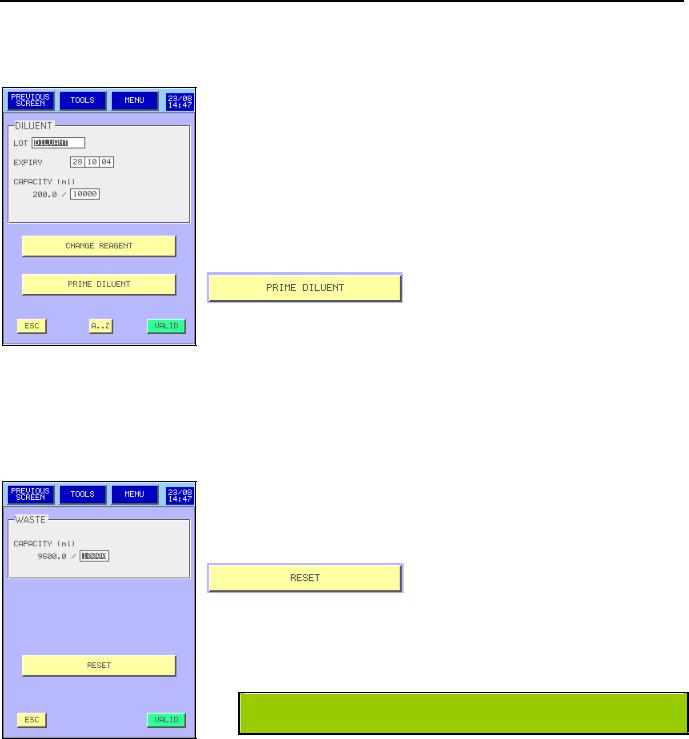
DILUENT PRIME:
- From the MAIN MENU press  then
then
 to have access to this screen.
to have access to this screen.
-Enter lot number, the expiry date and the container capacity.
-Press  to validate the new entry or after changing a new container with the same information.
to validate the new entry or after changing a new container with the same information.
-If needed enter the container volume in milliliter.
-After the replacement of a new container or to prime the diluent, press
-A new entry is automatically done in the logs (see section 5.9)
LYSE AND CLEANER PRIME:
Proceed as described above for the diluent.
WASTE :
-Enter only the capacity of the container.
-After replacement of the waste container press
to initialize the waste calculation.
MYTHIC 22 CT IS NOW READY TO WORK.
1.6 TRANSPORTATION AND STORAGE
Storage temperature: -10°C to +50°C.
If the MYTHIC 22 CT has been stored at a temperature lower than 10°C it must be left at room temperature during 24 hours.
Before transportation outside the laboratory, perform a complete cleaning with a disinfectant in compliance with the local regulation.
Page 18/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |

REF : M22CT/UM/EN/003
2. GENERAL OVERVIEW
2. GENERAL OVERVIEW
2.1 GENERALITIES
MYTHIC 22 CT is a fully automated analyzer performing hematological analysis on whole blood collected on EDTA K2 or K3 tubes .
-Sampling volume : 18,2 µl
-Two sampling mode : closed tube or other vial
-Throughput: close tube mode: > 40 samples/hour
other vial mode: > 45 samples/hour
-22 analysis parameters in DIF mode and 12 parameters in CBC mode :
Leukocyte parameters :
WBC |
White Blood Cells |
LYM |
Lymphocytes in % & # (DIF mode only) |
MON |
Monocytes in % & # (DIF mode only) |
NEU |
Neutrophils in % & # (DIF mode only) |
EOS |
Eosinophils in % & # (DIF mode only) |
BAS |
Basophiles in % & # (DIF mode only) |
Erythrocyte parameters |
|
RBC |
Red Blood Cells |
HGB |
Hemoglobin |
HCT |
Hematocrit |
MCV |
Mean Corpuscular Volume |
MCH |
Mean Corpuscular Hemoglobin |
MCHC |
Mean Corpuscular Hemoglobin Concentration |
RDW |
Red Blood cells Distribution Width |
Thrombocyte parameters |
|
PLT |
Platelet |
MPV |
Mean Platelet Volume |
PDW* |
Platelet Distribution Width |
PCT* |
Thrombocrit |
* For Investigation Use only in the United States of America
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 19/102 |

2. GENERAL OVERVIEW
REF : M22CT/UM/EN/003
2.2 OVERVIEW
MYTHIC 22 CT consists of 8 main parts:
1.Display / Keyboard.
2.Dilution hydraulic part.
3.Mono electronic board.
4.Reagent tray.
5.Connection.
6.External power supply block.
7.Printer.
8.Barcode reader (option).
2.3MAIN PART DESCRIPTION
2.3.1 Display / Keyboard |
Stand By and |
|
|
|
|
ON/OFF button |
|
|
|
|
|
|
|
|
|
|
|
Numeric keyboard |
|
|
|
|
|
|
|
|
|
|
|
Delete (DEL) |
|
|
|
|
|
|
|
|
|
|
|
Exit (ESCAPE) |
|
|
|
|
|
|
|
|
|
|
|
Cursors |
|
|
|
|
|
|
|
|
|
|
|
Enter (ENTER) |
|
|
|
|
|
|
|
|
|
|
|
Cycle in process |
|
|
|
Led |
|
|
|
|
|
|
|
|
|
|
|
Touch screen LCD |
|
|
|
display 380*240 |
|
|
|
|
|
|
|
|
|
|
|
|
|
Page 20/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |
|
REF : M22CT/UM/EN/003
2. GENERAL OVERVIEW
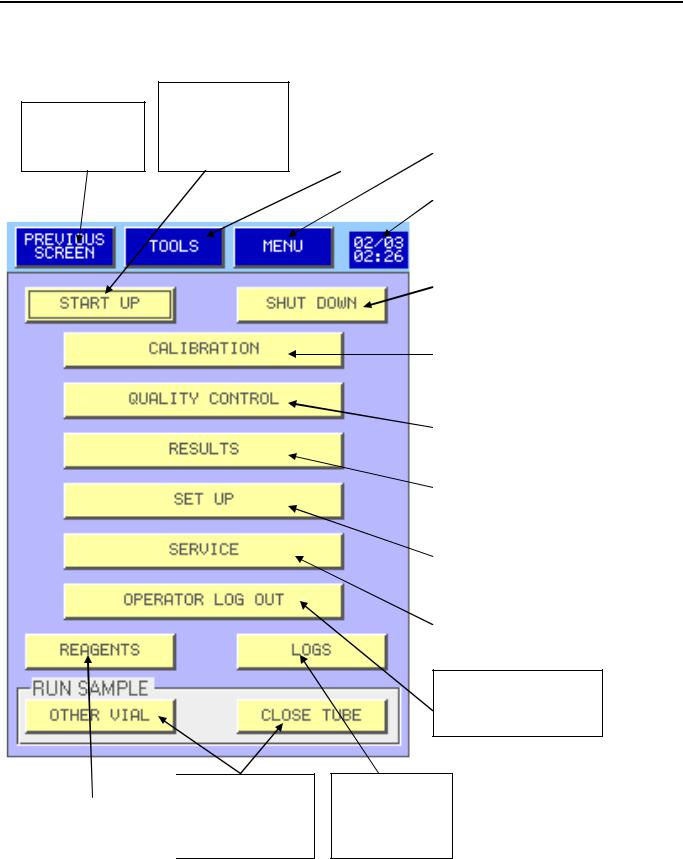
Main Menu description
Start Up rinsing Back to previous and Blank control screen
Reagent |
|
Analysis |
replacement |
|
performing |
(section 5.3). |
|
(section 5.5). |
|
|
|
Print, |
|
|
|
|
|
|
|
Send, |
|
Direct access to |
|
|
|
|
|
Select |
|
|
|
|
|
||
|
main menu |
|
|
|
|
||
Options |
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Date and Time. |
|
|
|
|
|
|
|
|||||
|
|
|
System status |
|
|
|
|
|
|
|
(section 3.2). |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Cleaning and stand by |
|
|
||
|
|
|
mode |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Calibration |
|
|
||
|
|
|
(section 7). |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Quality Control |
|
|
||
|
|
|
(section 6). |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Patient Archive |
|
|
||
|
|
|
(section 5.10). |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Mythic Parameters |
|
|
||
|
|
|
(section 3). |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Maintenance and |
|
|
||
|
|
|
Service Menu |
|
|
||
|
|
|
|
|
|
|
|
Log In and Log Out (section 3.1).
Mythic Events Logs
(section 5.9).
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 21/102 |
2. GENERAL OVERVIEW
REF : M22CT/UM/EN/003
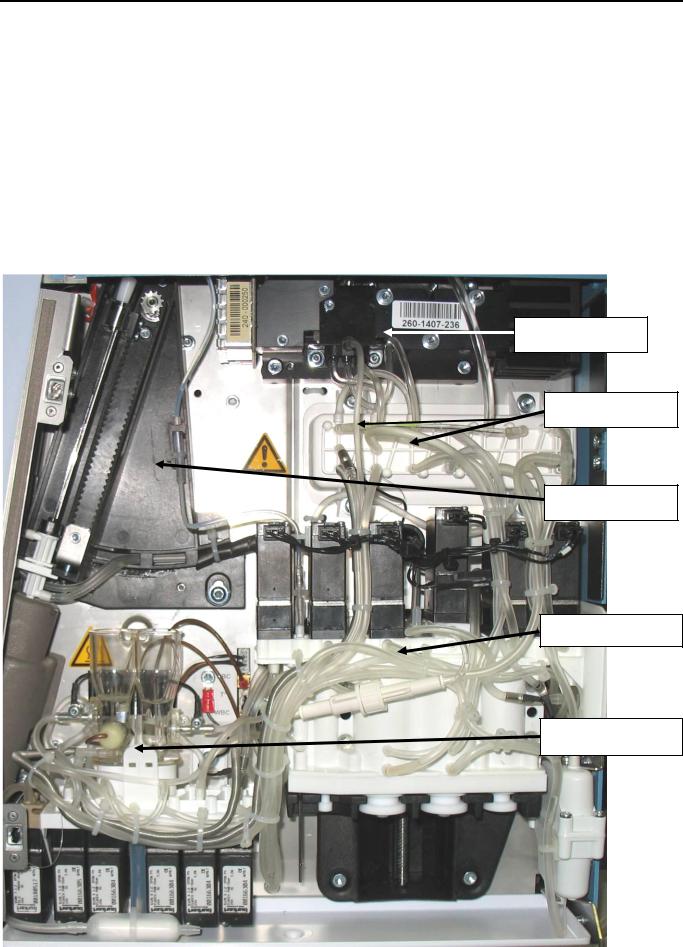
2.3.2 Dilution fluidic part
All the fluidic part is on the right side of the instrument and consist of only three modules:
-Sampling module :
oRocker (patented) : Manages the rise and descent of the needle.
-Syringe module (patented) consists of one block :
oReagent syringes (Diluent, lysis), sampling and air syringes.
oLiquid valve manifold assembly and tubing.
-Counting chambers :
oWBC and RBC counting chambers and hemoglobin measurement.
oLiquid valve manifold assembly and tubing.
Optical bench
Optical manifold
Sampling module
Syringe module
Counting module
Page 22/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |
REF : M22CT/UM/EN/003
2. GENERAL OVERVIEW
2.3.3 Mono electronic board
The mono electronic board is located between the hydraulic part and the reagent tray. The board, driven by a 32-bit processor, manages the following parts :
-Sample needle, rocker, syringe block motors.
-Display and keyboard.
-Connexion mode (RS232, Ethernet, …).
-Printer.
-Measurement (Counting, hemoglobin measurement).
-Data processing.
-External barcode reader.
To avoid all deterioration risks, only the field service engineer can touch this electronic board.
2.3.4 Power Supply Block
Switch on/off
MYTHIC 22 CT is supplied with an external power supply block.
-In the case of replacement of the main power wire supplied with the MYTHIC 22 CT the new one must be in compliance with the local regulation.
-The MYTHIC 22 CT has been certified with the power supply box provided with the machine. - If another power supply is used with the instrument, Orphée or its representative will not apply any warranty on this power supply and on the instrument. Please contact Orphée or its local representative before to use such material.
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 23/102 |

2. GENERAL OVERVIEW
REF : M22CT/UM/EN/003
2.3.5 Reagent tray
The reagent tray is dedicated to the OnlyOne lysing reagent and cleaning solution bottles.
Page 24/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |

REF : M22CT/UM/EN/003
3. INSTRUMENT SET UP
3.INSTRUMENT SET UP
3.1USER’S IDENTIFICATION
3.1.1Start Up
-After the instrument’s initialization, the identification window is displayed.
-In the window  , the last operator’s identification appears.
, the last operator’s identification appears.
-Either the identification is yours, press  and enter your
and enter your
password or the identification is not, press  to enter your login.
to enter your login.
-The window  enables to change the language. Press
enables to change the language. Press  to validate it.
to validate it.
-AUTONOMY (run) indicates the number of samples (runs) you can perform (calculated with the smaller quantity of reagents).
-Enter your identification name with the alphabetic keyboard.
-Place the cursor in the Password window.
-Enter your password for identification.
-For the first login, MYTHIC 22 CT proposes 3 access levels :
o User : No password
o Biologist : Password by default 1- 2- 3
oService people
-Biologist Password can be modified in section 3.3.6.
3.1.2 In process
-To change operator during the process, press  to return to the main menu, and then press on
to return to the main menu, and then press on 
-To change identification, proceed as described above (section 3.1.1).
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 25/102 |

3. INSTRUMENT SET UP
REF : M22CT/UM/EN/003
-If this window appears, it means that several results in memory have not been sent before the MYTHIC 22 was switched off.
-Press YES to send them immediately, or press LATER to wait NO if you do not want to send them.
-No USB key is available, connect an USB key then press YES or see section 3.4.7 to change the archive mode.
3.2SYSTEM STATUS
-Press on the date and hour  to have access to the system status window.
to have access to the system status window.
-Different system status information are displayed.
-To change the screen luminosity press L , N or H
-To return to the MAIN MENU press 
Page 26/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |
REF : M22CT/UM/EN/003
3. INSTRUMENT SET UP
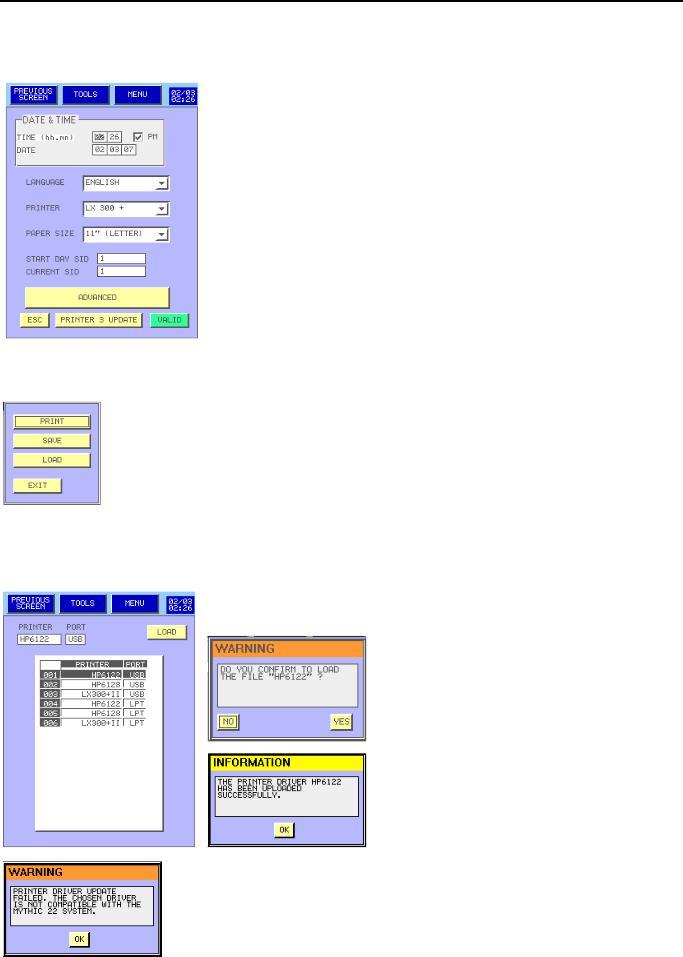
3.3 SET UP
-From the MAIN MENU press on 
-This menu is available for all users.
-The DATE & TIME window enables to modify the time and the date
-To select the language of the Mythic menu, choose the right one in the
Language combo box. 
-  : Select the printer or no printing.
: Select the printer or no printing.
-  : Select the paper size per result.
: Select the paper size per result.
-  : Two SID are available; Start day SID enables to select the first SID for each new day.
: Two SID are available; Start day SID enables to select the first SID for each new day.
-  : If you want to select a new SID number
: If you want to select a new SID number
-  : Biologist reserved for complete settings. (See section 3.4).
: Biologist reserved for complete settings. (See section 3.4).
-Once modifications are done, press either  to valid or
to valid or  to exit keeping the previous setting.
to exit keeping the previous setting.
-Press  to print, save or load from an USB key all the set up.
to print, save or load from an USB key all the set up.
-To load new printers drivers plug the USB key then press on  in the previous screen.
in the previous screen.
-Select the printer and his connection mode.
-Then press 
-Press  then, the driver is loaded in the MYTHIC 22CT
then, the driver is loaded in the MYTHIC 22CT
This prompt appears to confirm the Driver update.
- This prompt appears if the release is failed, check the USB connection or change the USB key or call your Orphée’s representative.
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 27/102 |
3. INSTRUMENT SET UP
REF : M22CT/UM/EN/003
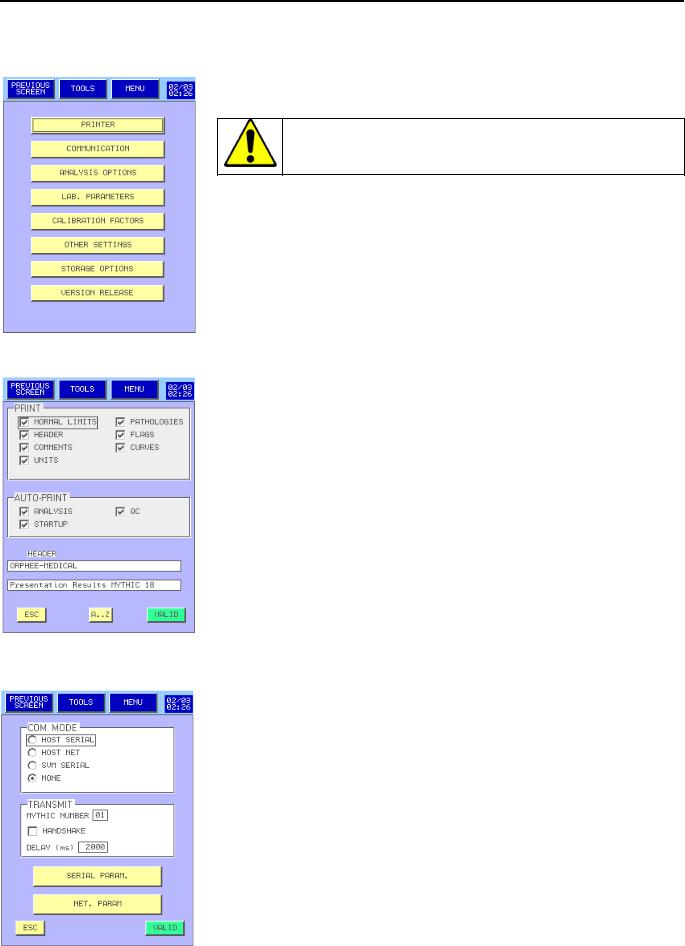
3.4ADVANCED SET-UP
-This menu is reserved to biologist (see section 3.1).
Any modifications can affect the quality of the results. We recommend modifying these values only after an Orphée’s training.
-Please refer below for the description of each key.
3.4.1Printer set up :
-Printer set up menu is intended to present the printing report
-To select an option on the report, press on the corresponding case.
-To enter a header, press  key.
key.
-To exit the menu, either press  to keep the last setting, or
to keep the last setting, or  to save the last modifications.
to save the last modifications.
3.4.2Communication:
-Reserved for Field Service Engineer.
-To set up the connection between MYTHIC and Host.
Page 28/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |
REF : M22CT/UM/EN/003
3. INSTRUMENT SET UP
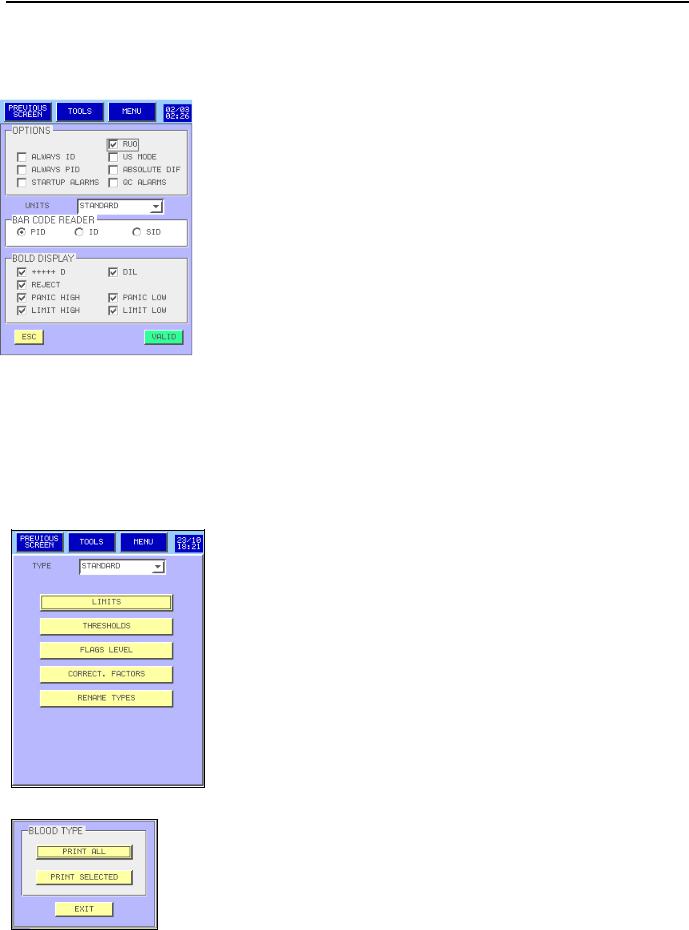
3.4.3Analysis options:
-OPTIONS box:
o ALWAYS ID and ALWAYS PID: To run a sample, user mandatory needs to enter a ID and/or a PID.
oRUO: With tag the PCT and PDW parameters are displayed, printed and send.
oUS MODE: The Research Use Only message is printing below the printing report.
oABSOLUTE DIFF: With tag absolute values for sub-populations of leucocytes are displayed. In the other case, percentages are
displayed.
oQC ALARMS: The message “QC failed” appear below the printing report when the QC result is out of tolerance or expired; The message “QC not done” appear below the printing report when it is not run.
-  : Gives a choice of three unit systems: Standard, International System, and mmol.
: Gives a choice of three unit systems: Standard, International System, and mmol.
-CDB box: The bar code reader is dedicated to the PID or ID or SID.
-BOLD DISPLAY box: display and print in bold-faced type the different choices in this box.
Once modifications are done, press either  to validate your choices or
to validate your choices or  to exit keeping the previous setting.
to exit keeping the previous setting.
3.4.4Lab. parameters:
-Select the blood type in the combo box  then press:
then press:
 to adjust the normal and panic limits (see section 3.4.4.1).
to adjust the normal and panic limits (see section 3.4.4.1).
 to adjust the parameters thresholds (see section 3.4.4.2).
to adjust the parameters thresholds (see section 3.4.4.2).
 to adjust the flags level (see section 3.4.4.3).
to adjust the flags level (see section 3.4.4.3).
 to adjust the corrections factors (see section 3.4.4.4).NOTA: No correction factors with type STANDARD.
to adjust the corrections factors (see section 3.4.4.4).NOTA: No correction factors with type STANDARD.
- To enter a new blood type, press  NOTA: The name of the first type STANDARD cannot be change.
NOTA: The name of the first type STANDARD cannot be change.
- Press  to print the blood type set up.
to print the blood type set up.
-  allow to print all the blood type set up (about 20 pages are printed)
allow to print all the blood type set up (about 20 pages are printed)
-  only the blood type in the combo box is printed.
only the blood type in the combo box is printed.
MYTHIC 22 CT |
Copyright© Orphée SA. All Rights Reserved. |
Page 29/102 |

3. INSTRUMENT SET UP
REF : M22CT/UM/EN/003
3.4.4.1 Limits :
To rename a blood type press  then select it then press
then select it then press  to accede at the alphabetic keyboard then press
to accede at the alphabetic keyboard then press  to validate or
to validate or  to exit without any modification.
to exit without any modification.
-This display enables to enter normal and panic limits for every 22 parameters given by the MYTHIC 22 CT (see section 8).
-To validate the new values, press VALID key in the next page (see below).
-Once modifications are done, press  to validate or
to validate or  to exit without any modification.
to exit without any modification.
-Press  to return to the parameter setting of the standard type.
to return to the parameter setting of the standard type.
Page 30/102 |
Copyright© Orphée SA. All Rights Reserved. |
MYTHIC 22 CT |
 Loading...
Loading...