Page 1

INSTRUCTIONS
DISPOSABLE BALLOON CATHETER
B5-2C/2Q/2LA
B7-2C/2Q/2LA
USA: CAUTION:
• This Product Contains Natural Rubber Latex
Which May Cause Allergic Reactions.
• Federal law restricts this device to sale by or on the
order of a physician.
Page 2

Page 3

Contents
Contents
Symbols.................................................................. 1
Important Information — Please Read Before Use 2
Intended Use ..................................................................... 2
Instruction Manual ............................................................. 2
User Qualifications............................................................. 3
Instrument Compatibility .................................................... 3
Storage .............................................................................. 3
Repair and Modification ..................................................... 3
Signal Words ..................................................................... 4
Natural Rubber Latex Medical Alert................................... 4
Chapter 1 Checking the Package Contents..... 5
1.1 Checking the Package Contents .............................. 5
Chapter 2 Instrument Nomenclature and
Specifications................................... 6
2.1 Nomenclature and Functions.................................... 6
2.2 Specifications............................................................ 9
Chapter 3 Storage .............................................. 14
3.1 Inspection Before Storage........................................ 14
3.2 Storage ..................................................................... 14
Chapter 4 Preparation, Inspection and
Operation .......................................... 15
4.1 Preparation ............................................................... 16
4.2 Inspection ................................................................. 16
4.3 Operation .................................................................. 21
DISPOSABLE BALLOON CATHETER
i
Page 4

Contents
ii
DISPOSABLE BALLOON CATHETER
Page 5
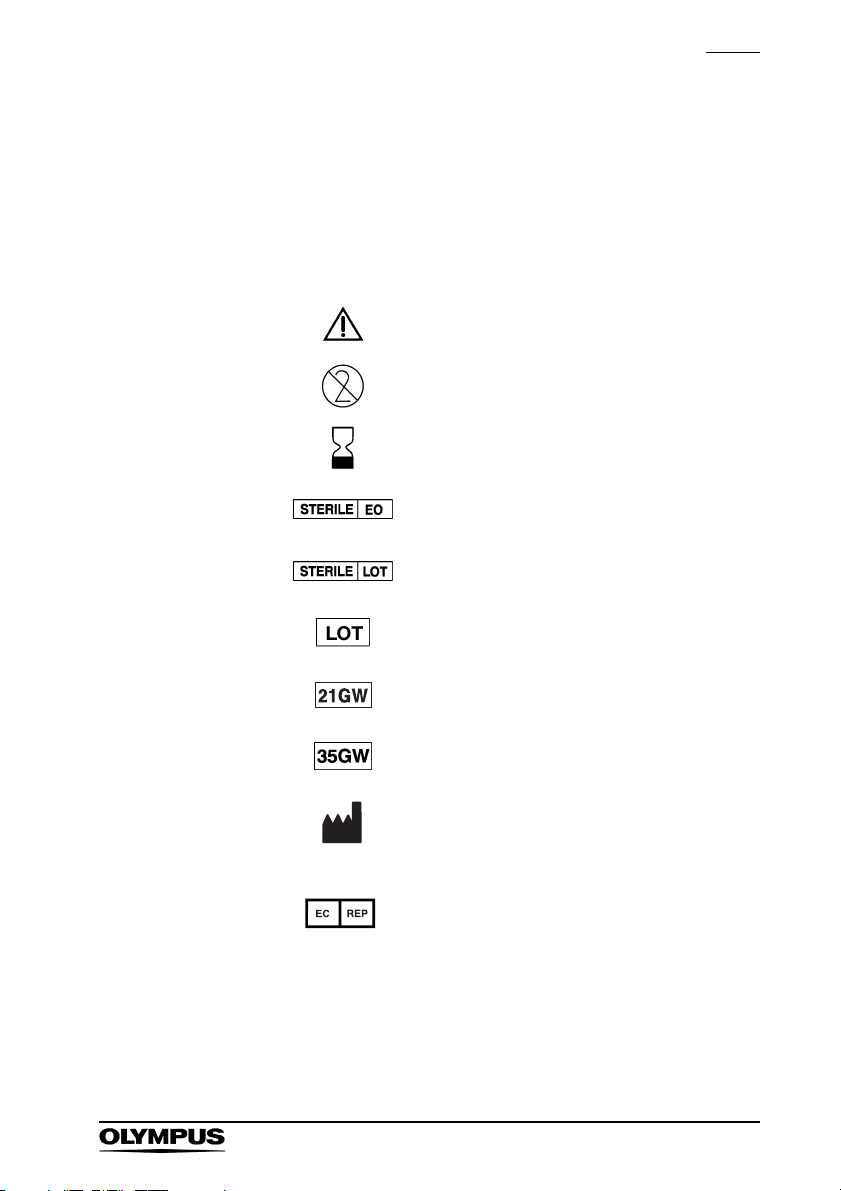
Symbols
Symbols
The meaning(s) of the symbol(s) shown on the package,
the back cover of this instruction manual and/or this
instrument are as follows:
Refer to instructions.
Single use only
Use by (expiration date)
Sterilized using ethylene oxide
Sterilization lot number
Lot number
Compatible with a ø0.53 mm
(0.021 inch) guidewire.
Compatible with a ø0.89 mm
(0.035 inch) guidewire.
Manufacturer
Authorised representative in the
European Community
DISPOSABLE BALLOON CATHETER
1
Page 6

Important Information — Please Read Before Use
Important Information — Please
Read Before Use
Intended Use
B5-2C, B7-2C
These instruments have been designed to be used with
Olympus endoscopes to inject contrast medium or other
medical fluid into the bile duct, pancreatic duct, and
respiratory organs. They can also be used for irrigation,
hemostasis within the respiratory organs, and retrieval of
foreign bodies within the bile duct, pancreatic duct and
respiratory organs. Do not use these instruments for any
purpose other than their intended use.
B5-2Q/2LA, B7-2Q/2LA
These instruments have been designed to be used with
Olympus endoscopes to inject contrast media into the
biliary or pancreatic tract. They can also be used for
retrieval of biliary or pancreatic stones. Do not use these
instruments for any purpose other than their intended
use.
Instruction Manual
This instruction manual contains essential information on
using this instrument safely and effectively. Before use,
thoroughly review this manual and the manuals of all
equipment which will be used during the procedure and
use the instruments as instructed.
Keep this and all related instruction manuals in a safe,
accessible location.
If you have any questions or comments about any
information in this manual, please contact Olympus.
2
DISPOSABLE BALLOON CATHETER
Page 7

Important Information — Please Read Before Use
User Qualifications
The operator of this instrument must be a physician or
medical personnel under the supervision of a physician
and must have received sufficient training in clinical
endoscopic technique. This manual, therefore, does not
explain or discuss clinical endoscopic procedures.
Instrument Compatibility
Refer to the Tables in Section 2.2, “Specifications” to
confirm that this instrument is compatible with the
ancillary equipment being used. Using incompatible
equipment can result in patient injury or equipment
damage.
Storage
This instrument was shipped in a sterile condition. Store
it following the instructions in Chapter 3, “Storage”.
Improper storage can present an infection control risk,
cause equipment damage or reduce performance.
This instrument is a single-use, disposable item that is
not to be reprocessed. Do not reuse or attempt to
sterilize.
Repair and Modification
This instrument contains no user-serviceable parts. Do
not disassemble, modify or attempt to repair; patient or
user injury and equipment damage can result.
DISPOSABLE BALLOON CATHETER
3
Page 8

Important Information — Please Read Before Use
Signal Words
The following signal words are used throughout this
manual:
Indicates a potentially hazardous
situation which, if not avoided, could
result in death or serious injury.
Indicates a potentially hazardous
situation which, if not avoided, may result
in minor or moderate injury. It may also
be used to alert against unsafe practices
or potential equipment damage.
Indicates additional helpful information.
Natural Rubber Latex Medical Alert
This product contains natural rubber latex which may
cause allergic reactions. The balloon at the distal end of
the insertion portion is made of natural rubber latex. Do
not use this product on a latex–sensitive patient.
4
DISPOSABLE BALLOON CATHETER
Page 9
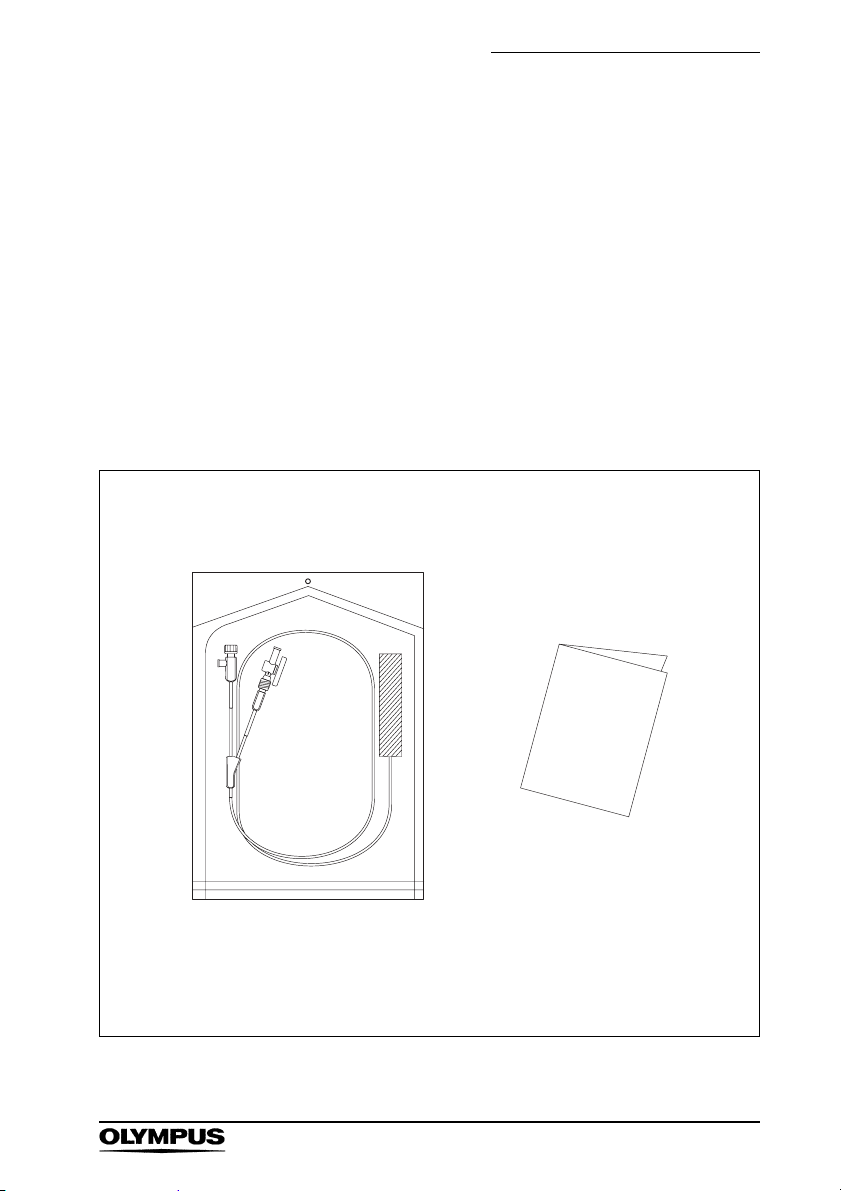
Chapter 1 Checking the Package Contents
Chapter 1 Checking the Package
Contents
1.1 Checking the Package Contents
Match all items in the package with the components
shown below. Inspect each item for damage. If the
instrument is damaged, a component is missing, or you
have any questions, do not use the instrument;
immediately contact Olympus.
Balloon Catheter
(Sterile, Single use only)
DISPOSABLE BALLOON CATHETER
Instruction Manual
5
Page 10
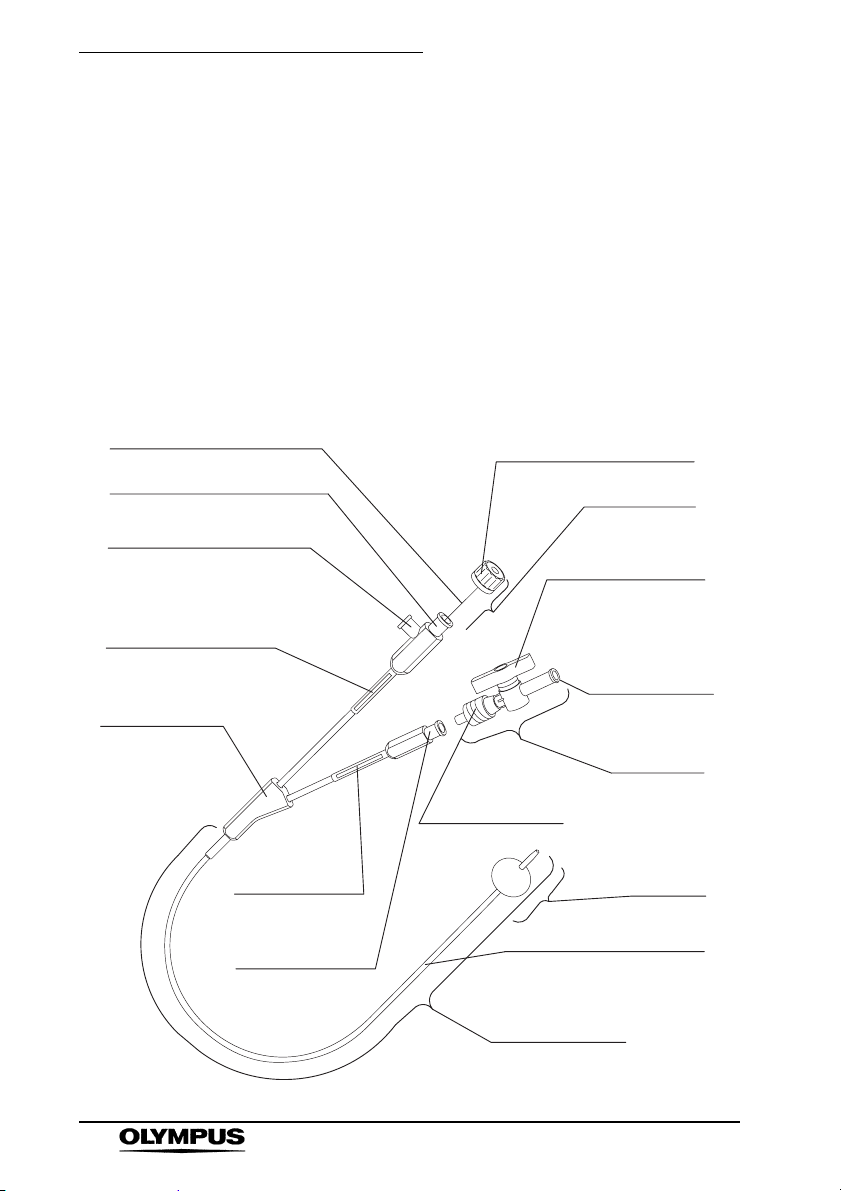
Chapter 2 Instrument Nomenclature and Specifications
Chapter 2 Instrument
Nomenclature and
Specifications
2.1 Nomenclature and Functions
The sterile instrument is sealed in a package.
1. Wire
3. Luer-lock Connector-1
5. Injection Port
7. Model Reference
Label
9. Branch
11. Air Volume
Label
12. Luer-lock
Connector-2
2. Grip
4. Stylet
6. Knob
8. Air Feeding
Port
Stopcock
10. Stopcock
Connector
Distal End
13. Tube
Insertion Portion
/Working Length
6
DISPOSABLE BALLOON CATHETER
Page 11
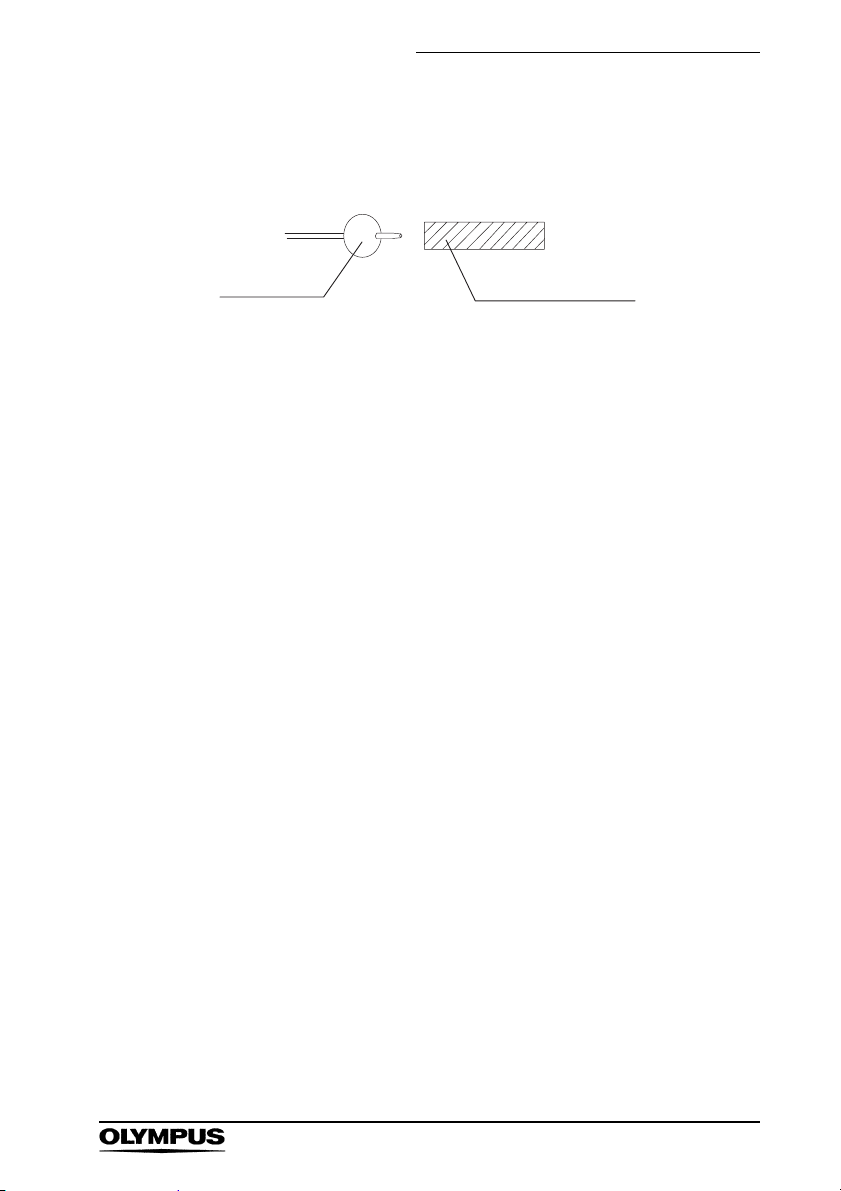
Chapter 2 Instrument Nomenclature and Specifications
Distal End
14. Balloon
1. Wire
The wire is available with the B7-2C/2Q/2LA only.
Inserted in the tube to prevent the tube from kinking.
2. Grip
The grip is attached to Luer-lock connector-1.
3. Luer-lock Connector-1
The Luer-lock is attached to the grip.
4. Stylet
The stylet is available with the B7-2C/2Q/2LA only
and incorporates the grip and wire.
5. Injection Port
Attach a syringe the injection port to inject contrast
medium, medical fluid or saline.
6. Knob
The knob switches a valve to control the airflow.
When the valve is opened, the balloon can be
inflated. After inflation, the knob is rotated and the
valve is shut, maintaining inflation.
7. Model Reference Label
The model reference label indicates the product
number.
15. Lightproof Cap
DISPOSABLE BALLOON CATHETER
7
Page 12

Chapter 2 Instrument Nomenclature and Specifications
8. Air Feeding Port
A syringe is mounted on the air feeding port, and air
is injected into the balloon to inflate it.
9. Branch
The color of the branch indicates the minimum
instrument channel diameter required for the
endoscope to be compatible.
10. Stopcock Connector
The stopcock connector is attached to Luer-lock
connector-2 to secure the stopcock.
11. Air Volume Label
Indicates the air volume required for maximum
balloon inflation.
12. Luer-lock Connector-2
The Luer-lock connector is attached to the stopcock
connector.
13. Tube
The tube works as the channel through which air is
fed into the balloon from the air feeding port and as
the channel through which contrast medium,
medical fluid or saline is injected.
14. Balloon
The balloon can be inflated and deflated using the
syringe attached to the air feeding port.
15. Lightproof Cap
The lightproof cap prevents the latex rubber balloon
from deteriorating when transported or stored.
8
DISPOSABLE BALLOON CATHETER
Page 13

2.2 Specifications
The compatible Olympus endoscopes are listed in the
table on the following page. New endoscopes released
after the introduction of this instrument may also be
compatible for use in combination with this instrument.
For further details, contact Olympus.
Operating Environment
Chapter 2 Instrument Nomenclature and Specifications
Use this instrument only in combination
with products recommended by
Olympus. If combined with products not
recommended by Olympus, patient or
operator injury, malfunction or equipment
damage may result.
Ambient Temperature
Relative Humidity 30 to 85%
Air Pressure 700 to 1060 hPa
10 to 40
(0.71 to 1.08 kgf/cm
(10.1 to 15.4 psia)
DISPOSABLE BALLOON CATHETER
°C (50 to 104°F)
2
)
9
Page 14

Chapter 2 Instrument Nomenclature and Specifications
Specifications
The volume of the air in the balloon
should not exceed the parameters
specified in the following tables.
Otherwise, the balloon may burst or may
not deflate. When the balloon does not
deflate, do not operate the balloon
catheter with excessive force. Otherwise,
the distal end of the instrument may
break off and could remain inside the
patient.
Model B5-2C B7-2C
Shape of the
Balloon
10
Maximum
Insertion
Portion
Diameter (mm)
Working Length
(mm)
Diameter after
inflation (mm)
Maximum Air
Vol ume
(ml (cc))
Compatible
Guide Wire
(mm (inch))
ø 1.95 ø 2.55
ø 11.0 ø 13.0
ø 0.53
(0.021)
DISPOSABLE BALLOON CATHETER
1050
1.5 1.7
ø 0.89
(0.035)
Page 15

Chapter 2 Instrument Nomenclature and Specifications
Compatible
Olympus
Endoscopes
(All of these
parameters
should be met.)
Length and
Model
Channel
Inner
Diameter
(mm)
(Color Code)
Working length less than
600 mm;
CHF, BF
ø 2, ø 2.2
(Blue);
ø2.6
(Green);
ø 2.8, ø 3.2
(Yellow)
ø 2.8, ø 3.2
(Yellow)
The G35-2LB and G35-2LD guidewires
are available from Olympus for use in
combination with the B7-2C.
Medical
Device
Directive
Model B5-2Q B7-2Q
Shape of the
Balloon
This device complies with the
requirements of Directive
93/42/EEC concerning medical
devices. Classification: Class II a
Maximum
Insertion
Portion
Diameter (mm)
Working Length
(mm)
Diameter after
inflation (mm)
DISPOSABLE BALLOON CATHETER
ø1.95 ø2.55
1950
ø11.0 ø13.0
11
Page 16

Chapter 2 Instrument Nomenclature and Specifications
Maximum Air
Vol ume
(ml (cc))
Compatible
Guide Wire
(mm (inch))
Compatible
Olympus
Endoscopes
(All of these
parameters
should be met.)
The G35-2LB and G35-2LD guidewires
are available from Olympus for use in
combination with the B7-2Q.
Length and
Model
Channel
Inner
Diameter
(mm)
(Color Code)
1.6 1.8
ø 0.53
(0.021)
Working length less than
1400 mm;
ø2.2
(Blue);
ø 2.8, ø 3.2
(Yellow);
ø 3.7, ø 4.2
(Orange);
ø5.5
(Pink)
ø 0.89
(0.035)
JF, TJF
ø 2.8, ø 3.2
(Yellow);
ø 3.7, ø 4.2
(Orange);
ø5.5
(Pink)
12
Model B5-2LA B7-2LA
Shape of the
Balloon
Maximum
Insertion
Portion
Diameter (mm)
Working Length
(mm)
Diameter after
inflation (mm)
ø 1.95 ø 2.55
3500
ø 11.0 ø 13.0
DISPOSABLE BALLOON CATHETER
Page 17

Maximum Air
Vol ume
(ml (cc))
Compatible
Guide Wire
(mm (inch))
Compatible
Olympus
Endoscopes
(All of these
parameters
should be met.)
Chapter 2 Instrument Nomenclature and Specifications
1.8 2.0
Length and
Model
Channel
Inner
Diameter
(mm)
(Color Code)
ø 0.53
(0.021)
Working length less than
1400 mm;
ø2.2
(Blue);
ø 2.8, ø 3.2
(Yellow);
ø 3.7, ø 4.2
(Orange);
ø5.5
(Pink)
ø 0.89
(0.035)
JF, TJF
ø 2.8, ø 3.2
(Yellow);
ø 3.7, ø 4.2
(Orange);
ø5.5
(Pink)
The G35-2LB and G35-2LD guidewires
are available from Olympus for use in
combination with the B7-2LA.
Medical
Device
Directive
This device complies with the
requirements of Directive
93/42/EEC concerning medical
devices. Classification: Class I
DISPOSABLE BALLOON CATHETER
13
Page 18

Chapter 3 Storage
Chapter 3 Storage
• Do not store the instrument in a sterile
package that is damaged, wet or
improperly sealed. Otherwise, the
sterility of the instrument may be
compromised and pose an infection
control risk or cause tissue irritation.
• Do not store the sterile package
containing the instrument in place where
it will be damaged, wet or improperly
sealed. Otherwise, the sterility of the
instrument may be compromised and
pose an infection control risk or cause
tissue irritation.
3.1 Inspection Before Storage
Prior to storage, inspect the sterile package as follows:
Confirm that the sterile package containing the
instrument is free from tears, inadequate sealing or water
damage. If tears, inadequate sealing, or water damage is
detected, do not use the instrument; contact Olympus.
3.2 Storage
Store the instrument in the sterile package at room
temperature in a clean and dry environment. Do not store
it in direct sunlight. Ensure that the package is not
crushed by surrounding objects during storage.
14
DISPOSABLE BALLOON CATHETER
Page 19

Chapter 4 Preparation, Inspection and Operation
Chapter 4 Preparation,
Inspection and
Operation
The instrument was shipped in a sterile condition.
• This product contains natural rubber
latex which may cause allergic reactions.
The balloon at the distal end of the
insertion section is made of natural
rubber latex. Do not use this product on a
latex–sensitive patient.
• Do not use an instrument after the
expiration date displayed on the sterile
package. Doing so may pose an infection
control risk or cause tissue irritation.
• Before each case, prepare and inspect
the instrument as instructed below.
Inspect other equipment to be used with
the instrument as instructed in their
respective instruction manuals. Should
the slightest irregularity be suspected, do
not use the instrument; contact Olympus.
Damage or irregularity may compromise
patient or user safety, such as an
infection control risk, tissue irritation,
punctures, hemorrhages or mucous
membrane damage and may result in
more-severe equipment damage.
Do not coil the insertion portion with a
diameter of less than 15 cm. This could
damage the insertion portion.
DISPOSABLE BALLOON CATHETER
15
Page 20

Chapter 4 Preparation, Inspection and Operation
4.1 Preparation
Equipment and Personal Protective Equipment
Prepare all equipment and personal protective
equipment that will be used with the instrument in
accordance with their respective instruction manuals.
Appropriate personal protective equipment may include:
eye wear, a face mask, moisture-resistant clothing and
chemical-resistant gloves that fit properly and are long
enough so that your skin is not exposed.
Spare Instrument
Always have a spare instrument available.
Sterile Syringe, Contrast Medium, Medical Fluid ,
or Saline for Inspection
Prepare a sterile syringe and contrast medium, and
medical fluid or saline solution for inspection.
4.2 Inspection
Wear the personal protective equipment as specified
above.
Before each case, always inspect the instrument
according to the following procedures.
If an abnormality in the instrument is detected, use a
spare instrument, inspecting it thoroughly before use.
16
DISPOSABLE BALLOON CATHETER
Page 21

Chapter 4 Preparation, Inspection and Operation
Inspection of the Sterile Package
Inspect the sterile package for tears, inadequate sealing,
or water damage. If the sterile package shows any
irregularities, the sterile condition of the instrument may
have been compromised. Use a spare instead.
Appearance Inspection
If any of following steps reveals irregularities, do not use
the instrument; use a spare instead.
1. Remove the lightproof cap from the distal end of the
instrument.
2. Gently run your fingertips over the entire length of
the insertion portion to check for any crushed areas,
excessive bends, broken areas or other damages.
3. Confirm that the distal end of the instrument appears
exactly as shown in the tables in Section 2.2,
“Specifications” and is not damaged.
Making and Inspecting the Connections
If any of following steps reveals irregularities, do not use
the instrument; use a spare instead.
1. When using the B5-2C/2Q/2LA, push the grip into
Luer-lock connector-1. Confirm that the grip is
securely attached to Luer-lock connector-1.
2. When using the B7-2C/2Q/2LA, insert the wire of the
stylet through Luer-lock connector-1 into the tube.
Mount the grip onto Luer-lock connector-1. Make
sure the stylet is securely attached.
3. Screw the stopcock connector into Luer-lock
connector-2. Make sure the stopcock is securely
attached.
DISPOSABLE BALLOON CATHETER
17
Page 22

Chapter 4 Preparation, Inspection and Operation
4. Confirm that the branch and Luer-lock connectors
are free from disconnection or looseness.
Inspection of Operation
• The volume of air in the balloon should
• Do not inflate the balloon rapidly.
• Inflate the balloon with air only. Inflation
If any of following steps reveals irregularities, do not use
the instrument; use a spare instead.
not exceed the parameters specified in
the tables in Section 2.2,
“Specifications”. Otherwise, the balloon
may burst.
Otherwise, the balloon may burst.
with anything other than air may hinder
expansion and contraction of the balloon.
18
1. Confirm that the stopcock knob is positioned as
shown in Figure 4.1.
Knob position
when the stopcock
is open.
Stopcock
Figure 4.1
Knob
Air
Feeding
Port
Syringe
2. Connect the sterile syringe onto the air feeding port
(see Figure 4.1).
DISPOSABLE BALLOON CATHETER
Page 23

Chapter 4 Preparation, Inspection and Operation
3. While referring to the tables in Section 2.2,
“Specifications”, slowly inflate the balloon to the
desired size. Confirm that the balloon inflates.
4. With the balloon inflated, turn the knob on the
stopcock 90° to close the stopcock.
5. Confirm that the balloon does not contract.
6. Turn the knob on the stopcock back 90° to open the
stopcock.
7. Pull the sterile syringe’s plunger to deflate the
balloon.
8. Remove the grip from Luer-lock connector-1.
9. When using the B7-2C/2Q/2LA, withdraw the stylet
from the tube.
10. Insert the guidewire into the opening at the distal
end of the tube. Confirm that the guidewire pokes
smoothly and sufficiently out of Luer-lock
connector-1.
Inspecting Irrigation
Do not use the instrument if the contrast medium,
medical fluid or saline solution cannot be injected or if it
leaks from any area other than the distal end. In this
case, use a spare instead.
Use a contrast medium, medical fluid or
saline solution intended for patient use
when inspecting irrigation. Other fluids
may remain inside the channel and could
pose an infection control risk or cause
tissue irritation.
DISPOSABLE BALLOON CATHETER
19
Page 24

Chapter 4 Preparation, Inspection and Operation
1. When using the B5-2C/2Q/2LA, push the grip into
Luer-lock connector-1. Confirm that the grip is
securely attached to Luer-lock connector-1.
2. When using the B7-2C/2Q/2LA, insert the stylet wire
into the tube through Luer-lock connector-1. Then
mount the grip onto Luer-lock connector-1 and
confirm that the stylet is securely attached.
3. Inject contrast medium, medical fluid or saline into
the instrument’s injection port using a sterile syringe.
Confirm that the fluid comes out of the distal end
(see Figure 4.2).
Injection Port
Syringe
20
Figure 4.2
4. Make sure that the contrast medium, medical fluid or
saline does not leak from any area other than the
distal end of the instrument.
5. Connect another syringe containing air to the
instrument’s injection port. Inject air into the insertion
portion to discharge the contrast medium, medical
fluid or saline solution.
DISPOSABLE BALLOON CATHETER
Page 25

4.3 Operation
The operator of the instrument must be a physician or
medical personnel under the supervision of a physician
and must have received sufficient training in clinical
endoscopic technique.
This manual, therefore, does not explain or discuss
clinical endoscopic procedures. It only describes basic
operation and precautions related to the operation of this
instrument.
Chapter 4 Preparation, Inspection and Operation
• When using the instrument, always wear
appropriate personal protective
equipment. Otherwise, blood, mucous
and other potentially infectious material
from the patient could pose an infection
control risk. Appropriate personal
protective equipment may include: Eye
wear, a face mask, moisture-resistant
clothing and chemical-resistant gloves
that fit properly and are long enough so
that your skin is not exposed.
• Do not use the balloon catheter to
retrieve a calculus which is larger than
the fistula or lumen size. Doing so could
cause patient injury such as
hemorrhages or mucous membrane
damage. It could also burst the balloon or
cause the balloon to become stuck in the
fistula or lumen, resulting that the distal
end of the instrument breaks off and
remains inside the fistula or lumen.
DISPOSABLE BALLOON CATHETER
21
Page 26

Chapter 4 Preparation, Inspection and Operation
• Do not insert the instrument into the
• Do not angulate the bending section of
• Do not force the distal end of the
endoscope unless you have a clear
endoscopic field of view. If you cannot
see the distal end of the insertion portion
in the endoscopic field of view or in the
X-ray images, do not use it. This could
cause patient injury, such as punctures,
hemorrhages or mucous membrane
damage. It may also damage the
endoscope and/or instrument.
the endoscope (or operate the forceps
elevator if applicable) abruptly while the
distal end of the insertion portion is
extended from the distal end of the
endoscope. This could cause patient
injury, such as punctures, hemorrhages
or mucous membrane damage.
insertion portion against body cavity
tissue. This could cause patient injury,
such as punctures, hemorrhages or
mucous membrane damage.
22
• The volume of the air in the balloon
should not exceed the parameters
specified in the tables in Section 2.2,
“Specifications”. Otherwise, the balloon
may burst or may not deflate properly.
When the balloon does not deflate, do
not operate the balloon catheter with
excessive force. Otherwise, the distal
end of the instrument may break off and
could remain inside the patient.
• Do not inflate the balloon rapidly. The
balloon may burst and mucous
membrane damage can result.
DISPOSABLE BALLOON CATHETER
Page 27

Chapter 4 Preparation, Inspection and Operation
• Inflate the balloon with air only. Inflation
with anything other than air may hinder
contraction of the balloon and make
impossible to withdraw the instrument
from the body cavity.
• When using the B7-2C/2Q/2LA, do not
withdraw the stylet from the tube quickly.
Infectious substances attached to the
stylet such as the patient’s blood and
mucous may scatter, posing an infection
control risk.
• When using the B7-2C/2Q/2LA, be
careful when handling the distal end of
the removed stylet. The stylet has a very
sharp tip and accidental punctures may
pose an infection control risk or cause
tissue irritation.
Inserting Into the Endoscope
• Confirm that the balloon is completely
deflated when inserting the instrument
into the endoscope. If the balloon is
inflated, the distal end of the instrument
may extend from the distal end of the
endoscope abruptly. This could cause
patient injury, such as punctures,
hemorrhages or mucous membrane
damage. It may also damage the
endoscope and/or instrument.
• When using a guidewire, hold the
guidewire when inserting the instrument.
Otherwise, it will move with the
instrument. This could cause patient
injury, such as punctures, hemorrhages
or mucous membrane damage.
DISPOSABLE BALLOON CATHETER
23
Page 28

Chapter 4 Preparation, Inspection and Operation
• Do not force the instrument if resistance
• Do not advance or extend the instrument
• When inserting the instrument into the
• Insert the instrument slowly. Abrupt
to insertion is encountered. Reduce the
angulation (or lower the forceps elevator
if applicable) until the instrument passes
smoothly. This could cause patient injury,
such as punctures, hemorrhages or
mucous membrane damage. It may also
damage the endoscope and/or
instrument.
abruptly. This could cause patient injury,
such as punctures, hemorrhages or
mucous membrane damage. It could
also damage the endoscope and/or
instrument.
endoscope, hold it close to the biopsy
valve and keep it as straight as possible
relative to the biopsy valve. Otherwise,
the insertion portion could be damaged.
insertion could damage the endoscope
and/or instrument.
24
DISPOSABLE BALLOON CATHETER
Page 29

Chapter 4 Preparation, Inspection and Operation
Using the B5-2C, B7-2C
1. Carefully insert the instrument into the biopsy valve
or T-plug (see Figure 4.3).
Keep as straight as
possible.
Hold the
insertion portion
close to the
biopsy valve.
Biopsy Valve
Figure 4.3
2. Advance the instrument until the distal end of the
insertion portion appears within the endoscopic field
of view.
Using the B5-2Q/2LA, B7-2Q/2LA
When using the B5-2Q/2LA or
B7-2Q/2LA, raise the forceps elevator to
its maximum height. If the forceps
elevator is down, you will not be able to
see the distal end of the insertion portion
in the endoscopic field of view. This could
cause patient injury, such as punctures,
hemorrhages or mucous membrane
damage.
1. Raise the forceps elevator to its maximum height.
2. Carefully insert the instrument into the biopsy valve
(see Figure 4.3).
DISPOSABLE BALLOON CATHETER
25
Page 30

Chapter 4 Preparation, Inspection and Operation
3. When using a guidewire, hold the guidewire in
position and insert the instrument into the
endoscope along the guidewire.
4. When the distal end of the insertion portion contacts
the forceps elevator, lower the forceps elevator.
5. Advance the instrument another 20 mm and raise
the forceps elevator. The distal end of the instrument
will be visible in the endoscopic field of view.
Applications
Radiography, Injecting medical fluid,
and Irrigation
1. When using the B5-2C/2Q/2LA, push the grip into
Luer-lock connector-1. Confirm that the grip is
securely attached to Luer-lock connector-1.
2. When using the B7-2C/2Q/2LA, insert the stylet wire
into the tube through Luer-lock connector-1. Then
mount the grip onto Luer-lock connector-1 and
confirm that the stylet is securely attached.
26
3. Connect a sterile syringe filled with a contrast
medium, medical fluid or saline solution to the
injection port. Inject the fluid until the air inside the
Tube is forced out.
4. Insert the distal end of the instrument into the target
site.
5. Confirm that the stopcock is open (see Figure 4.1).
6. Mount the sterile syringe onto the air feeding port.
Inject the specified amount of air into the instrument
(see the tables in Section 2.2, “Specifications” to
inflate the balloon).
7. Turn the knob on the stopcock 90° to close the
stopcock.
DISPOSABLE BALLOON CATHETER
Page 31

Chapter 4 Preparation, Inspection and Operation
8. Depress the sterile syringe’s plunger to inject the
fluid.
Retrieval
• When retrieving a foreign object, do not
operate the instrument abruptly or with
excessive force. Doing so could cause
patient injury, such as hemorrhages or
mucous membrane damage. It could
also burst the balloon or impede its
deflation, which could cause the balloon
catheter to become stuck inside the
patient, or cause the distal end of the
instrument to break off and remain inside
the patient.
• If the balloon becomes stuck, slowly
withdraw it together with the endoscope
and confirm that there is no bleeding.
Forcibly withdrawing the instrument
could cause its distal end to break off and
remain inside the patient.
1. Insert the distal end of the instrument into the target
site.
2. Confirm that the stopcock is open (see Figure 4.1).
3. Mount the sterile syringe onto the air feeding port.
Inject the specified amount of air into the instrument
(see the tables in Section 2.2, “Specifications” to
inflate the balloon).
4. Turn the knob on the stopcock 90° to close the
stopcock.
5. Pull the instrument to clear the foreign object.
DISPOSABLE BALLOON CATHETER
27
Page 32

Chapter 4 Preparation, Inspection and Operation
Hemostasis
1. Insert the distal end of the instrument into the target
site.
2. Confirm that the stopcock is open (see Figure 4.1).
3. Mount the sterile syringe onto the air feeding port.
Inject the specified amount of air into the instrument
(see the tables in Section 2.2, “Specifications” to
inflate the balloon).
4. Turn the knob on the stopcock 90° to close the
stopcock.
Changing the Patient’s Position (B5-2LA
and B7-2LA Only)
• Do not remove the endoscope from the
body cavity quickly. Infectious
substances attached to the endoscope
such as blood or mucous may scatter,
posing an infection control risk.
28
• Hold the instrument when reinserting the
endoscope. Otherwise, the instrument
will move with the endoscope. This could
cause patient injury, such as punctures,
hemorrhages or mucous membrane
damage.
• Do not withdraw the endoscope past the
instrument’s Branch. If the endoscope is
withdrawn further, the instrument will
move with the endoscope. This may
cause mucous membrane damage.
1. Leave the instrument inside the patient and
completely withdraw the endoscope from the body
cavity.
DISPOSABLE BALLOON CATHETER
Page 33

Chapter 4 Preparation, Inspection and Operation
2. Change the patient’s position.
3. Insert the endoscope into the body cavity again.
Withdrawing the Instrument From the Endoscope
Do not withdraw the instrument from the
endoscope quickly. This could scatter
blood, mucous, or other patient debris
and pose an infection control risk.
• Do not withdraw the instrument from the
endoscope while the balloon is inflated.
This could damage the endoscope
and/or instrument.
• Do not withdraw the instrument from the
endoscope if the forceps elevator is up.
This could damage the instrument.
1. Turn the knob on the stopcock back 90° to open the
stopcock.
2. Pull the sterile syringe’s plunger to deflate the
balloon.
3. If the endoscope is equipped with a forceps elevator,
lower the forceps elevator.
4. Withdraw the instrument from the endoscope.
Disposal
• After use, dispose of the instrument in an
appropriate manner. If it is not properly
disposed of, it could pose an infection
control risk.
DISPOSABLE BALLOON CATHETER
29
Page 34

Chapter 4 Preparation, Inspection and Operation
• The instrument is a single-use,
After using the instrument, dispose of it in an appropriate
manner.
disposable item. Do not reuse or attempt
to sterilize. Reusing the instrument could
pose an infection control risk, cause
tissue irritation or malfunction.
30
DISPOSABLE BALLOON CATHETER
Page 35

©1997 OLYMPUS MEDICAL SYSTEMS CORP. All rights reserved.
No part of this publication may be reproduced or distributed without the
express written permission of OLYMPUS MEDICAL SYSTEMS CORP.
OLYMPUS is a registered trademark of OLYMPUS CORPORATION.
Page 36

Manufacutured by
2951 Ishikawa-cho, Hachioji-shi, Tokyo 192-8507, Japan
Fax: (042)646-2429 Telephone: (042)642-2111
Distributed by
3500 Corporate Parkway, P.O. Box 610 Center Valley, PA
Fax: (484)896-7128 Telephone: (484)896-5000
One Corporate Drive, Orangeburg, N.Y. 10962, U.S.A.
Fax: (845)398-9444 Telephone: (845)398-9400
5301 Blue Lagoon Drive, Suite 290 Miami, FL 33126-2097, U.S.A.
Fax: (305)261-4421 Telephone: (305)266-2332
(Premises/Goods delivery) Wendenstrasse 14-18, 20097 Hamburg, Germany
(Letters) Postfach 10 49 08, 20034 Hamburg, Germany Telephone: (040)237730
KeyMed House, Stock Road, Southend-on-Sea, Essex SS2 5QH, United Kingdom
8F, Hyundai Marines Bldg., 646-1, Yeoksam-Dong, Kangnam-Gu, Seoul 135-080 Korea
491B, River Valley Road #12-01/04, Valley Point Office Tower, Singapore 248373
Fax: (01702)465677 Telephone: (01702)616333
117071, Moscow, Malaya Kaluzhskaya 19, bld. 1, fl.2, Russia
Fax: (095)958-2277 Telephone: (095)958-2245
Room 1202, NCI Tower, A21 Jianguomenwai Avenue Chaoyang
Fax: (10)6569-3545 Telephone: (10)6569-3535
Fax: (02)6255-3499 Telephone: (02)1544-3200
31 Gilby Road, Mount Waverley, VIC., 3149, Australia
Fax: (03)9543-1350 Telephone: (03)9265-5400
18034-0610, U.S.A.
District Beijing 100022 PRC
Fax: 6834-2438 Telephone: 6834-0010
GW8712 14
Printed in Japan 20050223 *0000
 Loading...
Loading...