Page 1

β
Page 2

Page 3

Dear Owner of GlucoKey Connect
Blood Glucose Plus β-Ketone
Monitoring System:
Thank you for purchasing the GlucoKey Connect Blood
Glucose Plus β-Ketone Monitoring System. This manual
provides important information to help you to use the system
properly. Before using this product, please read and follow
the instructions in this Owner's Manual.
Regular monitoring of your blood glucose levels can help you
and your doctor gain better control of your diabetes. Due to
its compact size and easy operation, you can use the GlucoKey
Connect Blood Glucose Plus
easily monitor your blood glucose levels.
β-Ketone Monitoring System to
Version 1.0 2018/07
311-4183XXX-XXX
Page 4

TABLE OF CONTENTS
Perform a Blood Glucose or β-Ketone Test 21
1 NOITAMROFNI YTEFAS
3 noitamrofnI tnatropmI
5 NOITCUDORTNI
5 esU dednetnI
5 elpicnirP tseT
6 weivrevO tcudorP
7 yalpsiD reteM
8 DETRATS GNITTEG
8 -upteS laitinI
11 GNITSET EROFEB
11 noitarbilaC
11 rebmuN edoC eht gnikcehC
21 gnitseT noituloS lortnoC
61 ESOCULG DOOLB RUOY GNITSET
61 ecnaraeppA pirtS tseT
71 pirtS tseT a gnitresnI
71 eciveD gnicnaL eht gniraperP
81 elpmaS doolB a gniniatbO
Page 5

Disposing Used Test Strip and Lancet 23
42 STLUSER TSET GNIWEIVER
72 ATAD GNIRREFSNART
Data Transmission Via Bluetooth 27
92 ECNANETNIAM
92 yrettaB eht gnignahC
03 eciveD ruoY rof gniraC
03 egarotS eciveD
03 lasopsiD reteM
13 spirtS tseT ruoY rof gniraC
Important Control Solution Information 31
33 EULAV ECNEREFER
53 NOITAMROFNI LOBMYS
63 GNITOOHSELBUORT
Result Readings (for glucose test) 36
Result Readings (for β-Ketone test) 36
73 segasseM rorrE
83 tnemerusaeM esoculG doolB
Page 6

14 SNOITACIFICEPS
WARRANTY TERMS AND CONDITIONS 43
Page 7

SAFETY INFORMATION
Read the following Safety Information thoroughly before
using the device.
Use this device ONLY for the intended use described in
this manual.
DO NOT use accessories which are not specified by the
manufacturer.
DO NOT use the device if it is not working properly or if it
is damaged.
This device DOES NOT serve as a cure for any symptoms
or diseases. The data measured is for reference only.
Always consult your doctor to have the results
interpreted.
The blood glucose test strip can be used for testing of
newborns.
The β-Ketone test strip must NOT be used for testing of
newborns.
Before using this device to test blood glucose or
β-Ketone, read all instructions thoroughly and practice
the test. Carry out all the quality control checks as
directed.
1
Page 8

Keep the device and testing supplies away from young
children. Small items such as the battery cover, batteries,
test strips, lancets and vial caps are choking hazards.
The use of this instrument in a dry environment,
especially if synthetic materials are present (synthetic
clothing, carpets etc.) may cause damaging static
discharges that may cause erroneous results.
DO NOT use this instrument in close proximity to sources
of strong electromagnetic radiation as these may
interfere with the correct operation.
Proper maintenance as well as timely calibration of the
device together with the control solution is essential in
ensuring the longevity of your device. If you are
concerned about the accuracy of the measurement,
please contact customer service for assistance on
1800 451 737.
KEEP THESE INSTRUCTIONS IN A SAFE PLACE
2
Page 9

Important Information
Severe dehydration and excessive water loss may cause
readings which are lower than actual values. If you
believe you are suffering from severe dehydration,
consult a healthcare professional immediately.
If your blood glucose or β-Ketone results are lower or
higher than usual and you do not have symptoms of
illness, first repeat the test. If you have symptoms or
continue to get results which are higher or lower than
usual, follow the treatment advice of your healthcare
professional.
Use only fresh whole blood samples to test your blood
glucose or β-Ketone. Using other substances will lead to
inaccurate results.
If you are experiencing symptoms that are inconsistent
with your blood glucose or β-Ketone test results and you
have followed all the instructi
owner’s manual, contact your healthcare professional.
We do not recommend using this product on severely
hypotensive individuals or patients in shock. Readings
which are lower than actual values may occur for
individuals experiencing a hyperglycemic-hyperosmolar
state, with or without ketosis. Please consult your
healthcare professional before use.
ons described in this
3
Page 10

The measurement unit used for indicating the
concentration of blood glucose is mmol/L.
The measurement unit used for indicating the
concentration of β-Ketone is mmol/L.
4
Page 11

INTRODUCTION
Intended Use
This system is intended for use outside the body (in vitro
diagnostic use) by people with diabetes at home and by
healthcare professionals in clinical settings as an aid to
monitor the effectiveness of diabetes control. It is intended
to be used for the quantitative measurement of glucose
(sugar) with capillary from fingertip, venous, arterial and to
measure β-hydroxybutyrate (Ketone) in fresh whole blood
samples from the finger. It should not be used for the
diagnosis of diabetes or screening for diabetes mellitus.
Professionals may test blood glucose with capillary sampling
from a finger tip or with venous, arterial and neonatal blood
from the heel. Test β-Ketone with a capillary sampling from
the fingertip. Use only heparin for anticoagulation of w
blood.
Home use is limited to capillary blood from the fingertip.
Test Principle
Your system measures the amount of glucose or β-Ketone in
whole blood. The glucose or β-Ketone testing is based on the
measurement of electrical current generated by the reaction
of glucose or β-Ketone with the reagent of the strip. The
meter measures the current, calculates the blood glucose or
β-Ketone level and displays the result. The strength of the
current produced by the reaction depends on the amount of
glucose or β-Ketone in the blood sample.
hole
5
Page 12

Product Overview
1
2
3
4 6 7
1
Test Strip Slot
Strip Indication Light
2
3
Test Strip Ejector
4
Battery Compartment
5
Display Screen
6
Down Button
7
MAIN Button
8
UP Button
6
5
8
Page 13

Meter Display
1
2
3
4
5
6
7
8
9
10
11
11
1 Blood Drop Symbol 9
2 Ketone Warning /
Ketone Symbol
3 Test Result
4 Measurement Mode
5
Memory Symbol
6
Day Average
7 Warning Symbol
8 Test Strip Symbol
Low Battery Symbol
10
Blood Glucose Symbol
11
Measurement Unit
12
Date & Time
7
Page 14

GETTING STARTED
Initial Set-up
Please follow the initial set-up procedure before using the
device for the first time or after you have replaced the battery
When the battery power is extremely low and “E-b & ”
appears on the screen, the meter cannot be turned on.
Step 1: Enter the Setting Mode
1. The meter turns on automatically once a new battery is
inserted.
2. Start with the meter off (no test strip inserted). Press
and at the same time.
Step 2: Configuring the Settings (Date, Time,
Memory Deletion and Reminder Alarm)
Press
setting then press MAIN button to confirm the setting and
switch to another field.
or to adjust the value or enable/disable the
8
Page 15
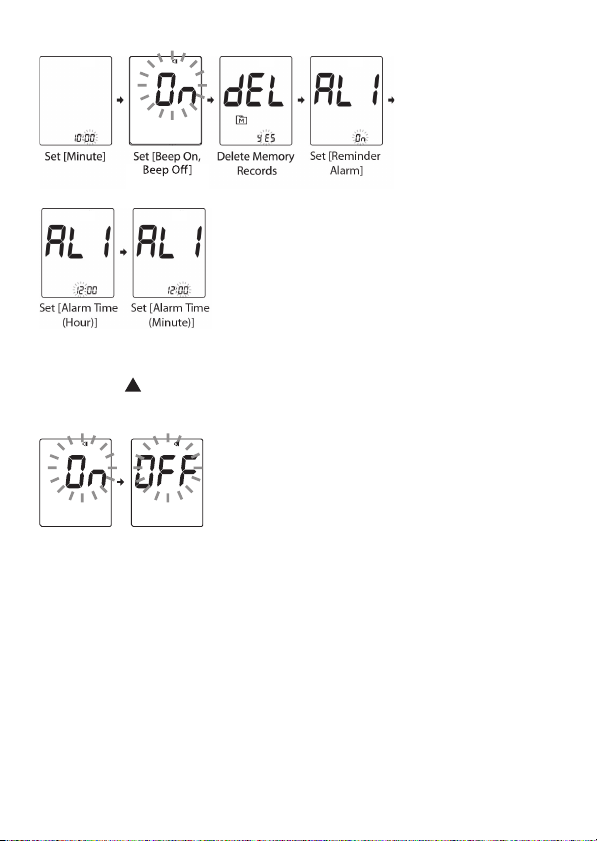
Note:
Press to select Beep On or Beep Off then press
MAIN button to confirm.
When Beep is turned off, the alarm function will remain
effective.
During memory deletion, select “no” to keep all saved
results.
You may set it up to four reminder alarms.
9
Page 16

To turn off the alarm, press or to change On to OFF.
Press MAIN button to confirm.
When the alarm goes off, the device will automatically
turn on. Press
press
or the device will beep for 2 minutes then
or to mute the alarm. If you do not
switch off.
If the device is idle for 3 minutes during the setting mode,
it will turn off automatically.
10
Page 17

BEFORE TESTING
Calibration
You must calibrate the device every time you begin to use a
new box of β-Ketone test strips by setting the meter with the
correct code. To ensure test accuracy, make sure the code
number displayed on the display screen matches the number
printed on the test strip vial or individual foil pack.
Checking the Code Number
1. Insert the β-Ketone strip into the test strip slot of the
device. Wait for the device to display the code number.
Important
Make sure that the code number displayed on the device
matches the number on the test strip vial or individual foil
pack before you proceed.
2. Remove the β-Ketone strip, the display will show “OFF”
indicating the device has finished coding and ready for
β-Ketone or blood glucose testing.
Make sure the code number on display and test strip vial or
individual foil pack are the same.
11
Page 18

If it matches, you can proceed with your test. Otherwise,
g
please stop testing and repeat the calibration procedure. If
the problem persists, contact customer service for further
assistance.
Important
It is important to make sure that the LCD display code is
the same as the code on the test strip vial or individual foil
pack before testing. Failure to do so will cause inaccurate
results.
Control Solution Testin
Our Control Solution contains a known amount of glucose or
β-Ketone that reacts with test strips and is used to ensure
your device and test strips are working together correctly.
Test strips, control solutions or sterile lancets may not be
included in the kit (please check the contents on your product
box). They can be purchased separately. Please make sure you
have those items needed for a blood glucose test beforehand.
12
Page 19

Do a control solution test when:
you first receive the device.
you begin using a new vial of test strips.
you suspect the device or test strips are not working
properly.
your blood glucose or β-Ketone test results are not
consistent with how you feel or if you think the results
are not accurate.
you have dropped or think you may have damaged the
device.
Perform a control solution test:
1. Insert the test strip into the test strip slot of the device.
Wait for the device to display the test strip “ ”and
blood drop “ ”.
2. The meter will detect the difference between control
solution and blood sampl
automatically mark the result as a control solution test
with “QC” displayed.
3. Shake the control solution vial thoroughly before use.
Squeeze out a drop and wipe it off then squeeze another
drop and place it on the tip of the vial cap. Hold the
device to move the absorbent hole of the test strip to
es automatically. It will
13
Page 20

touch the drop of control solution. Once the confirmation
window is filled completely, the device will begin counting
down.
Note:
To avoid contaminating the control solution, do not directly
apply the control solution onto a strip.
4. Read and compare the result. After counting down to 0,
the test result of the control solution will appear on the
display. Compare this result with the range printed on the
test strip vial or individual foil pack and it should fall
within this range. If the test result is out of range, read
the instructions again and repeat the control solution
test.
Note:
Control solution test results are stored in the memory.
The control solution range printed on the test strip vial or
14
Page 21

individual foil pack is for control solution use only. It is not
a recommended range for your blood glucose level.
Refer to the Maintenance section for important
information about your control solutions.
Out of range results:
If you continue to get results that fall outside the range
printed on the test strip vial, it means that the meter and
strips may not be working properly.
15
Page 22

TESTING YOUR BLOOD GLUCOSE
Test Strip Appearance
Absorbent Hole
Apply a drop of blood
here. The blood will be
automatically absorbed.
Test Strip Handle
Hold this part to insert
the test strip into the
meter slot.
1
Blood Glucose Test Strip
2
β-Ketone Test Strip
1
2
Confirmation Window
This is where you confirm
if enough blood has been
applied to the absorbent
hole in the strip.
Contact Bars
Insert this end of the test
strip into the meter. Push
it in firmly until it stops.
16
Page 23

Inserting a Test Stri
p
Insert the test strip into its slot.
Important
The front side of test strip should face up when inserting the
test strip. Test results may be inaccurate if the contact bar is
not fully inserted into the test slot.
To reduce the chance of infection:
Never share a lancet or a lancing device.
Always use a new, sterile lancet. Lancets are for single
use only.
Avoid getting hand cream, oils, dirt or debris in or on the
lancets and the lancing device.
Preparing the Lancing Device
1. Remove the cap.
2. Insert a new lancet firmly into the white lancet holder cup.
3. Remove the protective disk on the lancet. Hold the lancet
firmly in place and twist off the protective disk.
17
Page 24

4. Replace the cap until it snaps or clicks into place.
5. Rotate the dial to set the desired lancing depth.
6. Pull the cocking control out until the orange bar appears
on the release button window.
Obtaining a Blood Sample
Please follow the suggestions below before obtaining a drop
of blood:
Wash and dry your hands before starting.
Select the puncture site either on your fingertips or other
body parts.
Rub the puncture site for about 20 seconds before
penetration.
* Blood from the fingertip
1. Press the lancing device tip firmly against the lower side
of your fingertip.
18
Page 25

2. Press the release button to prick your nger. A click
indicates that the puncture is complete.
* Blood from sites other than the fingertip (For Blood
Glucose Test Only)
Important
AST is not available for β-Ketone test.
Alternative site testing (AST) is when individuals check their
blood glucose levels using other areas of the body other than
the
ngertips. The GlucoKey test strips allow AST to be
performed on sites other than the
your healthcare professional before you begin AST.
ngertips. Please consult
We strongly recommend that you perform AST ONLY at the
following times:
During a pre-meal or fasting state (more than 2 hours
since the last meal).
19
Page 26

Two hours or more after taking insulin.
Two hours or more after exercise.
DO NOT use AST if:
You think your blood glucose is low.
You may not notice if you are hypoglycemic.
Your AST results are inconsistent with the way you feel.
You are testing for hyperglycemia.
Your routine glucose results often fluctuate.
To obtain a blood sample from the alternative sites, please
rub the puncture site for approximately 20 seconds.
1. Replace the lancing device cap with the clear cap.
2. Pull the cocking control out until the orange bar appears
on the release button window.
Important
Choose a different spot each time you test. Repeated
punctures at the same spot may cause soreness and
calluses.
Avoid lancing the areas with obvious veins to avoid
excessive bleeding.
It is recommended to discard the first drop of blood as it
might contain tissue fluid which may affect the test
result.
20
Page 27

Perform a Blood Glucose or β-Ketone Test
1. Insert the test strip into its slot. Wait for the device to
display the test strip “ ”, blood
drop “ ” and
GLU or KETONE.
2. Press ▲ or ▼ to adjust the measuring mode, and
press MAIN button to confirm it.
General Tests (Gen) - any time of day without regard to
time since the last meal.
AC - no food intake for at least 8 hours.
PC - 2 hours after a meal.
QC - testing with control solution.
3. Obtain a blood sample.
Use the preset lancing device to prick your desired site.
After penetration, discard the first drop of blood with a
clean tissue or cotton. Gently squeeze the punctured area
to obtain another drop of blood. Be careful NOT to smear
the blood sample. The volume of blood sample must be
at least 0.5 microliter (μL) for blood
0.8 microliter (μL) for β-Ketone.
glucose or at least
21
Page 28

4. Apply the blood sample.
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
BG BG BG BG BG BG BG BG BG BG BG BG BG
Move your finger to meet the absorbent hole of the test
strip and the drop will be automatically be drawn onto the
test strip. Remove your finger until the confirmation
window is filled. The meter begins to countdown. Do not
remove your finger until you hear a beep sound.
5. Read your result.
The results of your blood glucose test or the result of your
β-Ketone test will appear after the meter counts down to
0. The results will be stored automatically in the meter
memory.
22
Page 29

Disposing Used Test Strip and Lancet
To remove the used test strip, simply push the Test Strip
Ejector button upward to eject the used test strip. The device
will automatically turn off after the test strip is removed.
Remove the used lancet from the lancing device after you
have finished testing. Discard your used strip and lancet
properly in a sharps container.
Important
The used lancet and test strip may be a biohazard. Please
consult your healthcare provider for proper disposal which
complies with your local regulations.
23
Page 30

REVIEWING TEST RESULTS
Your device stores the 1000 most recent test results along
with respective dates and times in its memory. To enter the
device memory, start with the device switched off.
To review all test results, do the following:
1. Press and release MAIN button or▲. The “ ” icon
appears on the screen.
2. Press MAIN to review the test results stored in the device.
Press ▲ or ▼ repeatedly to review other test results
stored in the device.
After the last test result, press MAIN again and the device
will be turned off.
24
Page 31

To review the day-average test results, do the following:
1. Press and release ▼ to enter memory mode for average
results with “ ” and DAY AVG displayed on the screen.
Release MAIN and then your 7-day average result
measured in general mode will appear on the display.
2. Press ▲ to review 14, 21, 28, 60 and 90-day average
results stored in each measuring mode in the order of
Gen, AC, then PC.
25
Page 32

Note:
Press and hold MAIN for 5 seconds to exit the memory
mode or leave it without any action for 3 minutes. The
device will turn off automatically.
If using the device for the first time, the “---” icon will
appear when you recall the test results or review the
average result. This indicates that there is no test result
in the memory.
Control solution results are NOT included in the day
average.
26
Page 33

TRANSFERRING DATA
Data Transmission Via Bluetooth
You can use your device with an iOS (5.0.1 or higher) or
toAndroid (2.3.3 or higher) download data from your
GlucoKey
transmit
your
1. Install the GlucoKey app to your device with an iOS or
2. Every time the GlucoKey Connect is turned off, the
3. Make sure your GlucoKey Connect is already paired with
Connect via Bluetooth. Follow the steps below to
data from your GlucoKey Connect. Please contact
customer service or place of purchase for assistance.
Android system.
Bluetooth will be initiated for data transmission. The
Bluetooth indicator flashes in blue.
your device by following the instructions
below:.
27
Page 34

Note:
This step is recommended when the user needs to pair this
meter to a Bluetooth receiver for the first time or when the
user needs to pair this meter to another Bluetooth receiver.
4. If your device with an iOS or Android is within the receiving
range, the data transmission will start and the Bluetooth
signals in blue. Once it is finished, the GlucoKey Connect
will automatically switch off.
5. If your device with an iOS or Android is not within the
receiving range, the GlucoKey Connect will automatically
switch off in 2 minutes.
Note:
While the meter is in transmission mode, it will be unable
to perform a blood glucose test.
Before transmitting data, ensure the meter is within range
and your iOS or Android device has Bluetooth turned on.
28
Page 35

MAINTENANCE
Changing the Battery
You must change the battery immediately and reset the date
and time when the battery power is extremely low and
“
& E-b” appears on the screen. The meter cannot be
turned on.
To change the battery, do the following:
1. Press the edge of the battery cover and lift it up to
remove the cover.
2. Remove the old battery and replace with one 1.5V AAA
size alkaline battery.
3. Close the battery cover. If the battery is inserted correctly,
you will hear a “beep” afterwards.
CAUTION
RISK OF EXPLOSION IF BATTERY IS REPLACED BY AN
INCORRECT TYPE.
DISPOSE OF USED BATTERIES ACCORDING TO THE
INSTRUCTIONS.
Note:
Replacing the battery does not affect the test results
stored in the memory.
29
Page 36

Keep the battery away from small children. If swallowed,
seek medical assistance immediately.
Battery may leak chemicals if unused for a long time.
Remove the battery if you are not going to use the device
for an extended period.
Properly dispose of the used battery according to your
local environmental regulations.
Caring for Your Device
To clean the exterior of the device, wipe it with a cloth
moistened with tap water or a mild cleaning agent, then
dry the device with a soft dry cloth. DO NOT rinse with
water.
DO NOT use organic solvents to clean the device.
Device Storage
Storage condition: -20°C to 60°C , below 95% relative
humidity.
Always store or transport the device in its original storage
case.
Avoid dropping and heavy impact.
Avoid direct sunlight and high humidity.
Meter Disposal
The used meter should be treated as contaminated and may
carry a risk of infection during measurement. The batteries in
this used meter should be removed and the meter should be
disposed in accordance with local regulations.
30
Page 37

Caring for Your Test Strips
Storage condition: 2°C to 30°C , below 85% relative
humidity. DO NOT freeze.
Store your test strips in their original vial only. Do not
transfer to another container.
Store test strip packages in a cool and dry place. Keep
away from direct sunlight and heat.
After removing a test strip from the vial, immediately
close the vial cap tightly.
Touch the test strip with clean and dry hands.
Use each test strip immediately after removing it from the
vial.
Do not use test strips beyond the expiry date. This may
cause inaccurate results.
Do not bend, cut or alter a test strip in any way.
Keep the strip vial away from children since the cap and
the test strip may be a choking hazard. If swallowed,
promptly see a doctor for assistan
For further information, please refer to the test strip package
insert.
ce.
Important Control Solution Information
Use only our control solutions with your device.
Do not use the control solution beyond the expiry date or
3 months after first opening. Write the opening date on
the control solution vial and discard the remaining
solution after 3 months.
31
Page 38

It is recommended that the control solution test be done
at room temperature 20°C to 25°C. Make sure your
control solution, device and test strips are at this
specified temperature range before testing.
Shake the vial before use, discard the first drop of control
solution and wipe off the dispenser tip to ensure a pure
sample and an accurate result.
Store the control solution tightly closed at temperatures
between 2°C to 30°C. DO NOT freeze.
32
Page 39

REFERENCE VALUE
The device provides you with plasma equivalent blood
glucose results.
Time of day Normal plasma glucose
range for people without
diabetes
Fasting and before meals < 5.6 mmol/L
2 hours after meals < 7.8 mmol/L
Source: American Diabetes Association (2012). Clinical
Practice Recommendations. Diabetes Care, 35 (Supplement
1): S1-100.
The β-Ketone test measures Beta-Hydroxybutyrate
(β-OHB), the most important of the three β-Ketone bodies
in the blood. Normally, levels of β-OHB are expected to be
less than 0.6 mmol/L. β-OHB levels may increase if a
person fasts, exercises vigorously or has diabetes and
becomes ill.
If your β-Ketone result is "Lo", repeat the β-Ketone test
with new test strips. If the same message appears
again or the result does not reflect how you feel,
contact your healthcare professional. Follow your
healthcare professional’s advice before you make any
changes to your diabetes medication program.
33
Page 40

If your -Ketone result is between 0.6 and 1.5 mmol/L, β
this may indicate a development of a problem that may
require medical assistance. Follow your healthcare
professional’s instructions.
If your β-Ketone result is higher than 1.5 mmol/L, contact
your healthcare professional immediately for advice and
assistance. You may be at risk of developing diabetic
ketoacidosis (DKA).
Please consult your doctor to determine a target range
that works best for you.
34
Page 41

SYMBOL INFORMATION
g
SYMBOL
REFERENT
In vitro
diagnostic
medical device
Consult
instructions for
Temperature
limitation
Use by
Batch code
Serial number
Do not reuse
Keep away from
sunlight
SYMBOL
REFERENT
Caution, consult
accompanying
documents
Humidity
Limitation
Collection for
electrical and
CE mark
Manufacturer
Dispose of the
packaging
properly after
use
Sterilized using
irradiation
Keep dry
Do not use if
package is
dama
ed
Manufacturing
date
35
Page 42

TROUBLESHOOTING
If you follow the recommended steps but the problem
persists or error messages other than the ones below
appear, please call customer service on 1800 451 737.
Do not attempt to repair the device yourself and never try to
disassemble the device under any circumstances.
Result Readings (for glucose test)
MESSAGE
< 0.5 mmol/L
≥ 13.3 mmol/L
> 33.3mmol/L
WHAT IT MEANS
Result Readings (for β-Ketone test)
< 0.1 mmol/L
0.1 to 8.0 mmol/L
> 8.0 mmol/L
WHAT IT MEANS
0.6 mmol/L
MESSAGE
36
Page 43

Error Messages
MESSAGE
E-b
E-U
E-E
E-2
E-0
E-A
E-C
E-F
E-t
Cause
The batteries
cannot provide
enough power for
a test.
Strip has been
used.
Problem in
operation.
You may have
removed the strip
after applying blood,
or insufficient blood
volume.
Ambient
temperature is out
of the system’s
operation range.
What To Do
Replace the battery
immediately and reset
date and time on the
meter setting.
Repeat the test with a
new strip.
Review the instructions
and repeat the test with
a new strip. If problem
persists, contact
customer service
for assistance.
Review the instructions
and repeat test with a
new test strip.
System operational range
is 8°C to 45°C. Repeat
the test after the device
and test strip have reached
the above temperature.
37
Page 44

Blood Glucose Measurement
Symptom
Cause
What To Do
The device
does not
display a
message
after
inserting a
test strip.
Batteries
exhausted.
Test strip inserted
upside down or
incompletely.
Defective device or Please contact
test strips. customer service.
Replace the battery
immediately and reset
date and time on the
meter setting.
Fully insert the test strip
with contact bars end
first and facing up.
38
Page 45

Insufficient blood
sample.
Repeat the test using a
new test strip with
larger volume of blood
sample.
The test
does not
start after
applying
the
sample.
The
control
solution
testing
result is
out of
range.
Defective test
strip.
Sample applied
after the device is
automatically
turned off.
Defective device.
Error in performing
the test.
Control solution
vial was poorly
shaken.
Expired or
contaminated
control solutions.
Repeat the test with a
new test strip.
Repeat the test with
anew test strip. Apply
sample only when
flashing “ ” appears on
the display.
Please contact
customer service.
Read instructions
thoroughly and repeat
the test again.
Shake the control
solution vigorously and
repeat the test again.
Check the expiration
date of the control
solution.
39
Page 46

Control solution
that is too warm or
too cold.
Control solution, device
and test strips should
be at room temperature
(20°C to 25°C) before
testing.
Defective test
strip.
Device
malfunction.
Repeat the test with a
new test strip.
Please contact
customer service.
40
Page 47

SPECIFICATIONS
Model No.
Memory
Dimensions
Power Source
Weight
External output
Features
Operating Condition
Storage/Transportation
Condition
Measurement Units Fixed mmol/L
Measurement Range 0.5-33.3 mmol/L for glucose
TD-4183D / GD82a
1000 measurement results with
respective date and time
89.8 (L) x 54.9 (W) x 18 (H) mm
One 1.5V AAA alkaline battery
46.1 g (without battery)
Bluetooth
Auto electrode insertion
detection
Auto sample loading detection
Auto reaction time count-down
Auto switch-off after 3 minutes
without action
Temperature warning
8°C to 45°C, below 85% R.H.
(noncondensing)
-20°C to 60°C, below 95% R.H
test and 0.1~8.0 mmol/L for
β-Ketone test
41
Page 48

Hematocrit range 0~70% for glucose testing and
10~70% for β-Ketone test
Test Result Glucose measurements are
reported as plasma equivalents
This device has been tested to meet the electrical and safety requirements of: IEC/EN
61010-1, IEC/EN 61010-2-101, EN 61326-, IEC/EN 61326-2-6, EN 301 489-17, EN
300 328.
42
Page 49

WARRANTY TERMS AND
CONDITIONS
With respect to disposable products, the manufacturer
warrants to the original purchaser that at time of delivery,
each standard product manufactured by the manufacturer
shall be free from defects in material and workmanship and
when used for the purposes and indications described on the
labeling is fit for the purposes and indications described on
the labeling. All warranties for a product shall expire as of the
product expiration date, or if none, after two (2) years from the
original date of purchase, as long as it has not been modified,
altered or misused. The manufacturer warranty hereunder
shall not apply if:
(i) a product is not used in a
if it is used for a purpose not indicated on the labeling;
(ii) any repairs, alterations or other work has been performed
by the buyer or others on such item, other than work
performed with the manufacturer’s authorization and
according to its approved procedures; or
(iii) the alleged defect is a result of abuse, misuse, improper
maintenance, accident or the negligence of any party other
than the manufacturer. The warranty set forth herein is
ccordance with its instructions or
43
Page 50

conditioned upon proper storage, installation, use and
maintenance in accordance with applicable written
recommendations from the manufacturer. The warranty
furnished hereunder does not extend to damaged items
purchased hereunder resulting in whole or in part from the
use of components, accessories, parts or supplies not
furnished by the manufacturer.
44
Page 51

Page 52

Distributed in Australia by:
NIPRO AUSTRALIA PTY LTD
SUITE 2.02, LEVEL 2,
657 PACIFIC HIGHWAY,
ST. LEONARDS, NSW 2065, AUSTRALIA
For assistance, please call 1800 451 737.
website: www.niproaustralia.com.au
email: info@niproaustralia.com.au
For self-testing
TaiDoc Technology Corporation
B1-7F, No.127, Wugong 2nd Rd.,
Wugu Dist., 24888 New Taipei City,
Taiwan
www.taidoc.com
MedNet GmbH
Borkstraβe 10, 48163
Mϋnster, Germany
 Loading...
Loading...