Neoprobe 2000, 2100 User manual
neo2000®Gamma Detection System
6
Service Manual
Models 2000 & 2100
Manufacturer:
Neoprobe Corporation
425 Metro Place North, Suite 300
Dublin, Ohio 43017-1367 USA
Tel: +1 (614) 793 7500
Fax: + 1 (614) 793 7531
008
Medical Electrical Equipment
With respect to electrical shock,
fire, and mechanical hazards.
only.
00-0059 Rev B © 2001 Neoprobe Corporation
Authorized representative:
AR-MED, Ltd.
Runnymede Malthouse
Egham TW20 9BD, UK
Tel: +44 (0) 1748 497851
Fax: +44 (0) 1748 497801
“neo2000” is a registered trademark of Neoprobe Corporation.
“BlueTip” is a trademark of Neoprobe Corporation.

Table of Contents
1. Introduction 1-1
1.1 System Warnings, Cautions and Notes 1.1-1
2. Specifications 2-1
3. Technical Overview 3-1
4. Diagnostic Troubleshooting 4-1
5. Disassembly, Service Part Installation and Re-assembly 5-1
6. Performance Verification 6-1
7. Disclaimers 7-1
8. Service Policy 8-1
i
Table of Contents (continued)
9. Warranty 9-1
10. Notices 10-1
Appendix A
A.1 neo2000
A.2 neo2000 Grounding & Isolation Diagram A.2-1
A.2 Front Housing Assembly A.3-1
A.3 Rear Housing Assembly A.4-1
Appendix B Neoprobe Authorized Service Parts B-1
Appendix C Service Checklist C-1
®
Functional Schematic A.1-1
ii

Introduction
1
Purpose
This manual provides the necessary
information for an experienced biomedical
instrumentation technician to perform
routine service operations on the Neoprobe
neo2000® Gamma Detection System
(neo2000) in Model 2000 and 2100
configurations.
Scope
This manual gives instructions for the
servicing of the neo2000, including
diagnostic sequences to troubleshoot and
isolate failures, assembly sequences
required to remove and replace suspect
service parts, and performance verification
sequences to ensure that the performance
of the repaired unit meets Neoprobe
specifications. This manual does not
include any servicing capability for the
Neoprobe gamma detection probes, cables
or accessories. These items are not
serviceable in the field. This manual does
not provide detailed repair procedures and
technical documentation to isolate failures
to the lowest possible level. It
also does not discuss how to
perform surgery and procedures
for nuclear medicine.
The intent of this manual is not
to support the remanufacture
and resale of the neo2000 by
third parties.
Servicing Personnel
This manual is intended for use
by biomedical instrumentation
technicians and other
individuals who are familiar
with the routine servicing and maintenance
of medical electronic instrumentation. (For
additional information see Chapter 7.)
Those individuals should also be
thoroughly familiar with the neo2000
Operation Manual.
Customers are not authorized to perform
service during the initial one (1) year
period.
Training
This manual in conjunction with the
neo2000 Operation Manual provides all
the information that should be necessary
for a competent biomedical
instrumentation technician to perform the
authorized servicing operations. Neoprobe
does not provide any supplemental training
in the servicing of the neo2000.
1-1
Introduction (continued)1
Customer Feedback
Neoprobe understands the importance of
feedback to continual quality
improvement, and encourages users and
servicing personnel to provide any
suggestions for improving the products
and this manual. Please forward any
suggestions to:
Neoprobe Corporation
425 Metro Place North, Suite 300
Dublin, OH 43017-1367
Attn: Technical Support
or telephone:
(800) 793 0079 (USA only) or
+1 (614) 793 7500
or email:
service@neoprobe.com.
Note: In the unlikely event of a malfunction or failure,
Neoprobe recommends that owners of neo2000
instruments contact Neoprobe for service.
®
1-2

1.1
Warnings, Cautions and Notes
Overview
Read this section before servicing
the neo2000®. Included below are
warnings, cautions and notes for
safe service of the neo2000.
Definitions
Service Part: Component used in
the neo2000 that is available as a
replacement part for order from
Neoprobe by registered owners of
Neoprobe neo2000 products.
Warning: Specific information
provided to the service technician to
advise of improper servicing of the
device that could present potential
harm to the service technician, user
or patient and/or could result in
irreparable damage to the device or
property.
Caution: Specific information
provided to the service technician to
prevent the incorrect servicing of the
device which may affect performance
or safety of the device.
Note: General information provided
to the service technician to further
explain or clarify the proper servicing
of the device.
Warnings
• Only biomedical instrumentation
technicians or other individuals who
are familiar with the routine servicing and maintenance of medical
grade electronic instrumentation
should service the neo2000.
• Neoprobe shall have no liability for
any incident resulting from improper
servicing of the neo2000, or from the
use of any service parts that are not
obtained by authorized Neoprobe
sources of supply. (see chapter 9)
• Before servicing, complete the
necessary cleaning and inspection
process. (see chapter 4.2 of the
neo2000 Operation Manual)
• Disconnect the power cord from the
power outlet before opening.
• Do not open in the presence of
fluids, flammable anesthetics or
other explosive gases.
• The internal electronics of the
neo2000 are sensitive to damage
from electrostatic discharge (ESD).
Use appropriate precaution when
opening and servicing the unit.
• Neoprobe Corporation does not
support the remanufacture and
resale of the neo2000 by third
parties.
• Returned products must be properly
decontaminated in accordance with
all applicable regulations for
biohazardous material.
• Proper configuration of grounding
required for safety.
Cautions
• Replace wire ties and reroute wires
as they were initially found.
• Do not leave loose hardware inside
the console.
• Conduct normal in-house product
safety testing for the neo2000 after
servicing and before returning the
unit for use on patients.
1.1-1
Warnings, Cautions and Notes (continued)1.1
Notes
• The volume knob may be damaged
by mechanical shock.
• In the unlikely event of a malfunc-
tion or failure, Neoprobe recommends that owners of neo2000
instruments contact Neoprobe for
service.
• Calibration & Preventive Mainte-
nance: The neo2000 is shipped
set to factory specifications.
Preventive maintenance is limited
to external cleaning of the
neo2000, fuse replacements and
functional diagnostics.
®
• Replacement of certain items will
require reprogramming of the unit.
Contact Neoprobe to arrange for
reprogramming of the unit.
• Regarding Year 2000 compliance,
the neo2000 Gamma Detection
System does not use a Real Time
Clock, and subsequently is not
adversely affected by Year 2000.
• “Error 1” or “Error No Probe” is a
normal operating message when
no probe is present. Check probe
and cable connections.
1.1-2
Specifications2
Product Specifications
The neo2000® complies with
the following specifications.
Service replacement parts
ordered through Neoprobe also
comply with the appropriate
specifications listed. Personnel
performing service on the
neo2000 must understand
these specifications and must
ensure that all servicing
activities performed on the
neo2000 do not compromise
any of these specifications.
Product Classification Standard or Regulation
System: Class I, Exempt USA, 21 CFR § 892.1320
Class IIa Europe, Medical Device Directive
Class II Canada, Therapeutic Device Regulation
Class II Australia, Therapeutic Device Regulation
Model 2000 Type B EN 60601-1/2
Model 2100 Type BF EN 60601-1/2
(1)(2)
(2)
Console: Explosion Equipment not suitable for use in the
presence of Flammable ANASTHETIC
MIXTURE WITH AIR or OXYGEN or
NITROUS OXIDE
Fluid Ingress Ordinary Equipment
Mode of Operation Continuous Operation
Degree of Mobility Stationary, non-mobile
Protection against Fused and removable power cable
electrical shock
BlueTip
TM
Fluid Ingress IPX4
Probes
(1)
The degree of protection from electrical shock is based on the system configured with
reusable metal encased probes (Model 1017). When used with the BlueTipTM Probes (Model
2001, 2002, & 2003), the system is Type BF due to the mechanical isolation barrier the
plastic material provides.
2)
Product safety testing of the initial design is certified through laboratory testing conducted
by a certified third party testing laboratory. Ongoing production testing assures that each
device continues to comply with these criteria.
2-1
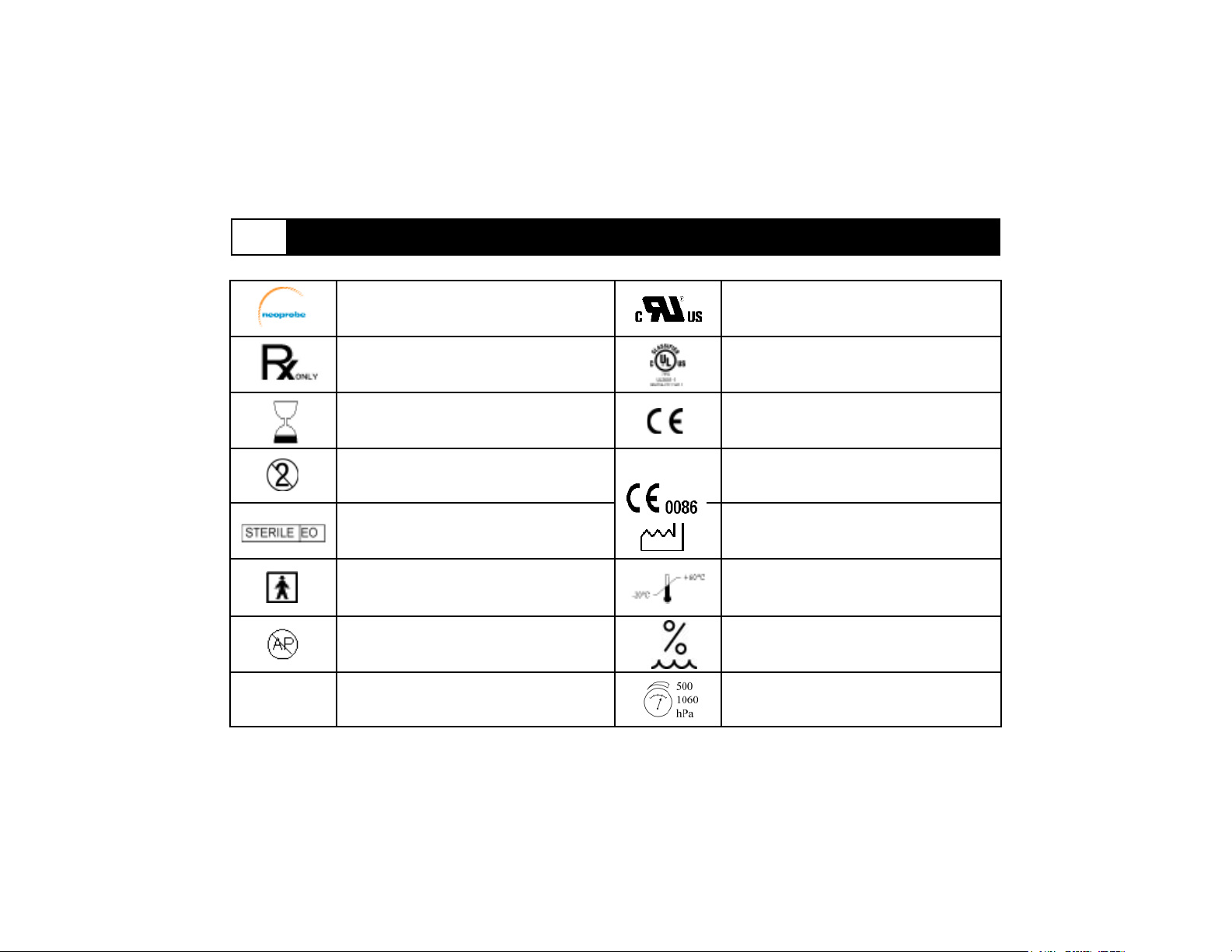
2 Specifications (continued)
IPX4
Neoprobe Corporation logo
In the U.S.A., Caution: Federal Law
restricts this device to sale by or on the
order of a physician
Use by
Do not reuse
Sterile using Ethylene Oxide
Type BF
Not for use in the presence of flammable
anesthetics
Splash-proof equipment
Underwriters Laboratory (UL) recognized
symbol component
UL Classified device
CE Mark, Class I
CE Mark, Class II, Class I Sterile
Date of manufacture
Storage and Transit Temperature
Storage and Transit Pressure
Storage and Transit Humidity
2-2
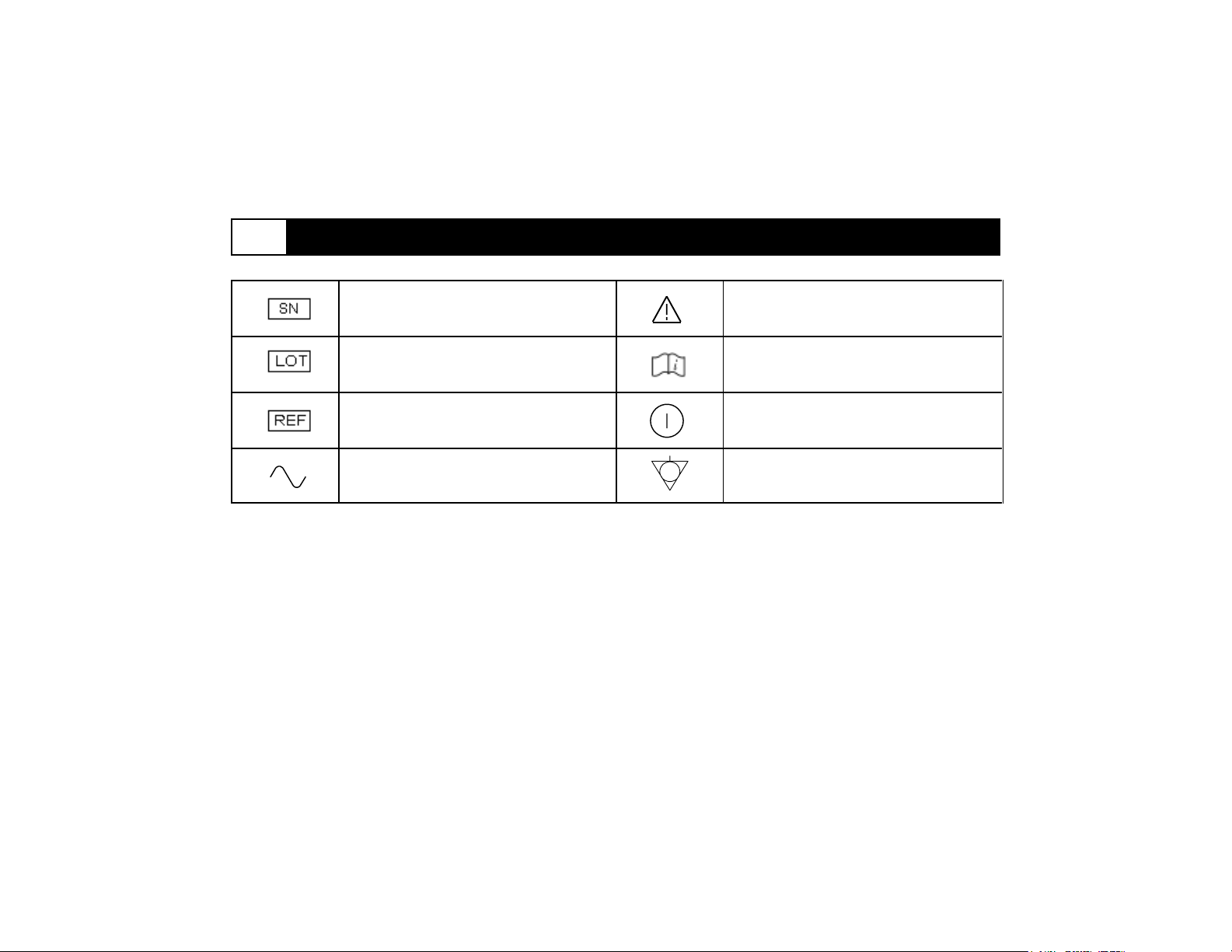
2 Specifications (continued)
Serial number
Batch code
Catalog number
Alternating current
Caution: consult accompanying
documents
Consult instructions for use
Standby (push-push) (power on:
connections to mains; power off:
disconnection from mains)
Equipotentiality
2-3
Technical Overview
3
Overview
This section describes the basic technical
features of the neo2000® console. Refer
to Appendix A.
Processing Architecture
The neo2000® contains two processors
with distinct functions: the central
processing unit (CPU) and the digital signal
processor (DSP). The CPU controls the
operator interface and some higher level
processing functions. The DSP performs a
majority of the processing of the probe
input signals including many of the
formatting and statistical processes
associated with counting input pulses. The
processors are located on separate circuit
boards and use a standard bus structure
with the CPU as the master of the bus.
Operator Inputs
Operator control over the console is
accomplished through the use of momentary pushbuttons. Most buttons have only
one function within a single operating mode.
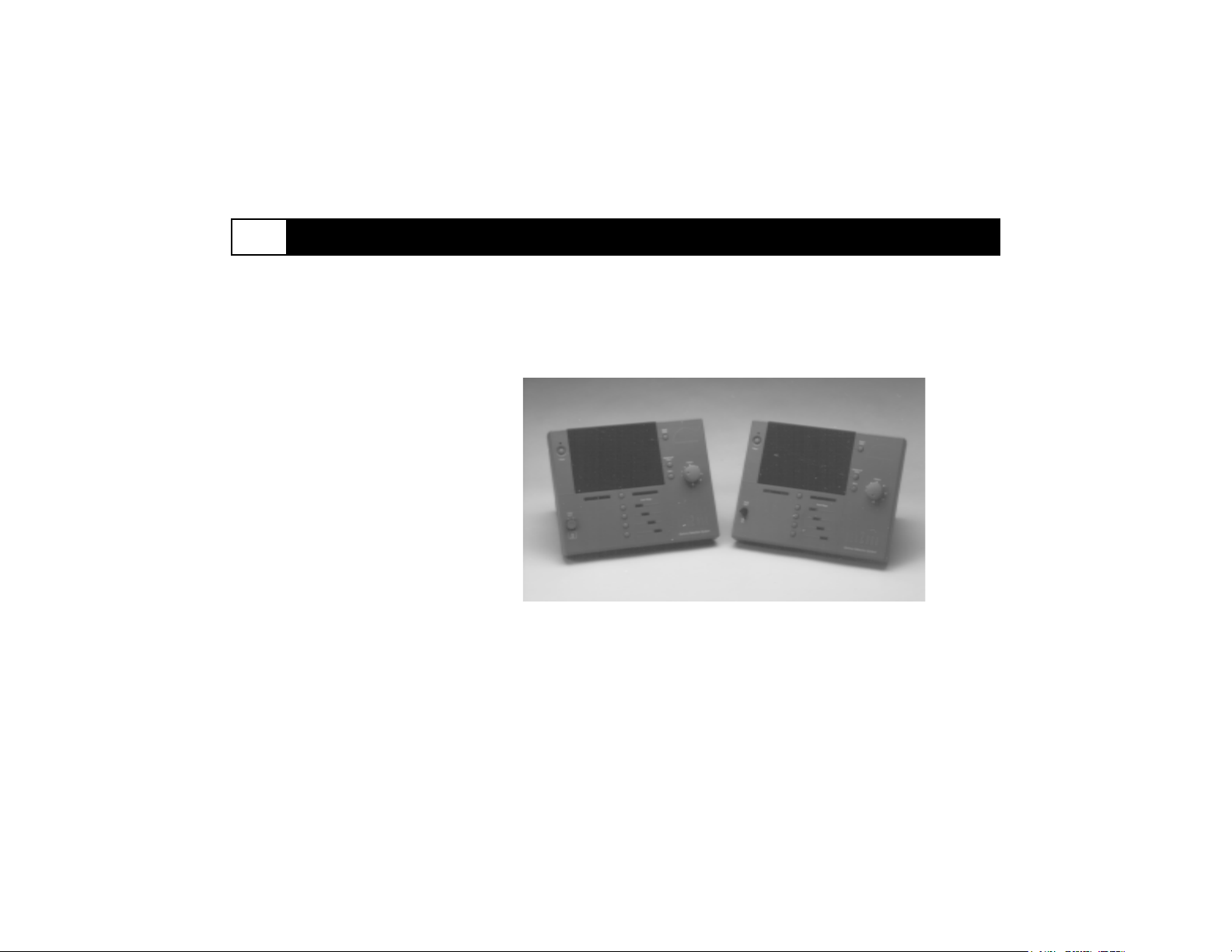
Figure 3-1. neo2000 Console (Left: Model 2100; Right: Model 2000)
3-1

Technical Overview (continued)
3
Volume control is accomplished
through use of a rotary input. To
maximize flexibility, this rotary input
is readable by the software and does
not directly control the audible
output level. Audible feedback of
button presses is provided electronically rather than mechanically.
Operator Outputs
Data from the console is presented to the
operator using both visual and audible
means. Operational state indicators are
provided to clearly indicate the operating
mode of the system to the user. Numeric
displays present quantitative data for
evaluation by the operator. A graphics
display and an audio speaker present
information in a qualitative manner.
Audible data is presented in order to allow
the surgeon to keep visually focused on
the patient. The audible output produces
data and status information simultaneously.
Electronic Input/Output (I/O) Ports
There are two serial I/O ports on the rear
of the console. These are used at the
factory to program the unit and for future
expansion.
Enclosure
The
enclosure
design
complies
with the
following
regulatory
requirements: 1)
EN 60601-11 drop test; 2)
EN 60601-1-2
electromagnetic
susceptibility and
emissions tests. To meet these
design requirements, a two piece
clamshell enclosure is used.
The enclosure has interlocking ribs
combined with a tongue and groove joint
to provide strength to pass the drop test.
Rigidity of the housing is obtained by
screwing the front and rear housings
together.
The walls of the housings are
angled to allow fluids to flow off
of the console. The tongue and
groove allows the placement of
a gasket to prevent liquid
ingress at the location of the
seam. The buttons are
designed to prevent liquid
entry without sacrificing
tactile feedback. External
components are resistant to
most common disinfectants used
to clean devices outside of the sterile
field.
Connectors and Cabling
The console contains both externally
accessible connectors as well as internal
cabling. Connectors and cables meet
applicable regulatory requirements and
environmental conditions.
3-2

Technical Overview (continued)
3
A
E
B
Figure 3-2. A. DSP Board B. PDM Board C. I/O Board D. CPU Board
E. PIM Board(Model 2100 only)
D
C
3-3
Technical Overview (continued)
3
Technical Overview (continued)
neo2000® Components
CPU Board (P101)
The main CPU board is a 100/133 MHz
486DX based PC/104 board with onboard
programming of Flash memory, floppy/IDE
interface, serial ports, parallel port, and
serial boot loader capability.
I/O Board (P105)
The I/O board provides digital expansion
channels to meet the total I/O requirements for the neo2000.
DSP Board (P102)
The DSP is a Starburst DSP board
containing a DSP running at 32 MHz. This
processor is capable of both 32-bit
floating point and 32-bit fixed point
operations and has two timers. This board
also provides the necessary analog and
discrete I/O channels to control and
interface with the probe input circuitry.
PDM Board (P103* or P104**)
The pulse descriminator module (PDM)
board contains the interface circuitry for
the external probe. The major components
of the PDM board are the signal conditioning circuitry, the power circuitry and the
Pulse Discriminator.
PIM Board (P107**)
The probe isolation module (PIM) board
provides isolation circuits for the interface
to the external probe.
Main Board (P100)
The Main board serves as a mounting point
for most of the displays and switches in
the console and also serves as the
motherboard for the console.
Auxiliary Board (P106)
The auxiliary board serves as a junction
box for the rear enclosure half and acts as
a mounting point for the I/O connectors
and the radionuclide switch.
Power Supplies (P140, P141)
The +5V and +12V power supplies are
medical grade universal input supplies
which accept single phase power between
85 and 264 VAC and 47 to 63 Hz.
Cabling
There are a total of eleven cables and four
ground wires in the console. Refer to A.3.
The cables consist of:
Serial port cables (P120, P121)
I/O cables (2) (P122)
Auxiliary board cable (P132)
Line Filter cable (P124)
Power converter cable (P126)
Probe input cable (P128* or P129**)
PIM to PDM cable (P123**)
Speaker cable (P131)
Fan cable assembly (P150)
Line filter ground cable (P125)
Power converter ground cable (2 xP127)
Probe input ground cable (P130*)
* Specific to Model 2000
** Specific to Model 2100
3-4
 Loading...
Loading...