
REF 9515-190-50-ENG Rev C1
Surveyor S4
MOBILE MONITOR
USER MANUAL
Manufactured by Mortara Instrument, Inc., Milwaukee, Wisconsin U.S.A.
CAUTION: Federal law restricts this device to sale by or on the order of a physician.
i
Copyright © 2015
by Mortara Instrument, Inc.
7865 N. 86th Street
Milwaukee, Wisconsin 53224
E-mail: techsupport@mortara.com

This document contains confidential information that belongs to Mortara Instrument, Inc. No part of this document
may be transmitted, reproduced, used, or disclosed outside of the receiving organization without the express written
consent of Mortara Instrument, Inc. V1.1.0 2015-11
Mortara is a registered trademark of Mortara Instrument, Inc. Surveyor™ is a trademark of Mortara Instrument,
Inc. All other trademarks and registered trademarks are the property of their respective owners.
Product License
This product and its accompanying software are subject to Mortara Instrument’s End User License Agreement. By
using this product and its accompanying software, you agree to the terms and conditions specified therein.
Open-Source Software
Mortara Instrument uses a variety of software and components created by the open-source community. The use of
open-source software and components is essential to creating robust, high quality products, as these have been
under constant scrutiny and review by the open-source community. Upon explicit written request, Mortara
Instrument will make available to customers the source code for open-source components used in this product
within three years after the purchase of the product. Mortara Instrument may charge a fee for delivering such
source code. Permission to use, copy, modify, and/or distribute this software for any purpose with or without fee is
hereby granted, provided that the above copyright notice and this permission notice appear in all copies.
Additional Notices
Portions of this product incorporate software and components that are copyrighted by their respective owners
including:
Linux Operating System Kernel
Licensed under the GNU General Public License version 2 (GPLv2).
Copyright (C) 1989, 1991 Free Software Foundation, Inc.
All rights reserved.
BusyBox Utilities
Licensed under the GNU General Public License version 2 (GPLv2).
See http://www.busybox.net/license.html
All rights reserved.
Linux WLAN Driver SD8787
Licensed under the GNU General Public License version 2 (GPLv2).
© Marvel® Technology Group, Ltd.
All rights reserved
wpa_supplicant
Copyright (c) 2003-2013, Jouni Malinen <j@w1.fi> and contributors.
This software may be distributed, used, and modified under the terms of BSD license.
All rights reserved.
Dropbear SSH
Copyright (c) 2002-2013 Matt Johnston
Portions copyright (c) 2004 Mihnea Stoenescu
All rights reserved.
i

QT Framework
© 2013 Digia Plc and/or its subsidiary(ies) and other contributors.
License under GNU Lesser General Public License version 2.1 (LGPLv 2 .1).
SOFTWARE IS PROVIDED BY THE COPYRIGHT HOLDERS AND CONTRIBUTORS "AS IS" AND ANY
EXPRESS OR IMPLIED WARRANTIES, INCLUDING (BUT NOT LIMITED TO) THE IMPLIED WARRANTIES
OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE, ARE DISCLAIMED. IN NO EVENT
SHALL THE COPYRIGHT OWNER OR CONTRIBUTORS BE LIABLE FOR ANY DIRECT, INDIRECT,
INCIDENTAL, SPECIAL, EXEMPLARY, OR CONSEQUENTIAL DAMAGES (INCLUDING, BUT NOT LIMITED
TO, PROCUREMENT OF SUBSTITUTE GOODS OR SERVICES; LOSS OF USE, DATA, OR PROFITS; OR
BUSINESS INTERRUPTION), HOWEVER CAUSED AND ON ANY THEORY OF LIABILITY; WHETHER IN
CONTRACT, STRICT LIABILITY, OR TORT (INCLUDING NEGLIGENCE OR OTHERWISE); ARISING IN ANY
WAY OUT OF THE USE OF THIS SOFTWARE, EVEN IF ADVISED OF THE POSSIBILITY OF SUCH DAMAGE.
ii

TABLE OF CONTENTS
1.
GENERAL STATEMENTS ................................................................................................................ 1
TECHNICALSUPPORTANDSERVICE.....................................................................................................................................1
2. NOTICES ........................................................................................................................................... 2
MANUFACTURER’SRESPONSIBILITY............................................................................................................................... .....2
RESPONSIBILITYOFTHECUSTOMER....................................................................................................................................2
EQUIPMENTIDENTIFICATION.............................................................................................................................................2
COPYRIGHTANDTRADEMARKNOTICES............................................................................................................................... 2
OTHERIMPORTANTINFORMATION.....................................................................................................................................2
3. WARRANTY INFORMATION ............................................................................................................ 3
YOURMORTARAWARRANTY............................................................................................................................................3
4. USER SAFETY INFORMATION ........................................................................................................ 5
SAFETYREGULATIONS......................................................................................................................................................5
WARNINGS....................................................................................................................................................................5
PowerSupplyWarnings.............................................................................................................................................6
Accessories,Cables,andExternalConnectionsWarnings..........................................................................................7
DefibrillationandElectrosurgeryWarnings...............................................................................................................7
ECGWarnings............................................................................................................................................................7
CAUTIONS.....................................................................................................................................................................8
5. EQUIPMENT SYMBOLS AND MARKINGS ................................................................................... 10
SYMBOLDELINEATION............................................................................................................................... ....................10
6. GENERAL CARE ............................................................................................................................. 11
PRECAUTIONSFORS4....................................................................................................................................................11
PRECAUTIONSFORLI‐IONBATTERYCHARGER,ACPOWERPACKANDCORD............................................................................11
INSPECTIONPRIORTOCLINICALUSE.................................................................................................................................11
CLEANINGANDDISINFECTING.........................................................................................................................................12
MAINTENANCE.............................................................................................................................................................13
BATTERYLIFEANDCHARGETIME.....................................................................................................................................15
PRODUCTLIFE..............................................................................................................................................................16
DECOMMISSIONINGANDDISPOSAL..................................................................................................................................16
7. ELECTROMAGNETIC COMPATABILITY (EMC) ........................................................................... 17
GUIDANCEANDMANUFACTURER’SDECLARATION:ELECTROMAGNETICEMISSIONS..................................................................18
GUIDANCEANDMANUFACTURER’SDECLARATION:ELECTROMAGNETICIMMUNITY...................................................................18
GUIDANCEANDMANUFACTURER’SDECLARATION:ELECTROMAGNETICIMMUNITY...................................................................19
RECOMMENDEDSEPARATIONDISTANCESBETWEENPORTABLEANDMOBILERFCOMMUNICATIONSEQUIPMENTANDTHEEQUIPMENT
............................................................................................................................... ..................................................20
USAANDCANADARADIOREGULATIONS..........................................................................................................................21
8. INTRODUCTION .............................................................................................................................. 23
GENERALINFORMATION................................................................................................................................................23
INDICATIONSFORUSE...................................................................................................................................................23
SYSTEMOVERVIEW.......................................................................................................................................................24
SAM10......................................................................................................................................................................24
SAM5........................................................................................................................................................................24
iii

TABLE OF CONTENTS
C
ARRYINGPOUCHES......................................................................................................................................................27
BATTERYCHARGER.......................................................................................................................................................28
9. UNPACKING AND SET UP ............................................................................................................. 29
CHECKINGCONTENTS....................................................................................................................................................29
BATTERYINSTALLATION.................................................................................................................................................30
10. PATIENT PREPARATION FOR QUALITY ECG ............................................................................ 31
QUALITYECGDATAACQUISITION...................................................................................................................................31
SKINPREPARATION.......................................................................................................................................................31
ELECTRODEPLACEMENT............................................................................................................................... ..................32
PACEMAKERPATIENTS...................................................................................................................................................32
ELECTRODELOCATIONSFOR12‐LEADECG.......................................................................................................................32
UsingtheLeadForm12‐LeadCable..........................................................................................................................33
ELECTRODELOCATIONSFOR5‐WIRECABLE.......................................................................................................................34
ELECTRODELOCATIONSFOR3‐WIRECABLE.......................................................................................................................35
PACEMAKERPATIENTS...................................................................................................................................................35
CHECKINGECGELECTRODEANDLEADWIRESIGNALQUALITY..............................................................................................36
11. OPERATION .................................................................................................................................... 37
POWERINGONTHES4..................................................................................................................................................37
SCREENTIME‐OUT&REACTIVATION............................................................................................................................... .37
ONSCREENQUICKSETUPGUIDE.....................................................................................................................................37
POWERINGOFFTHES4.................................................................................................................................................38
MAINSCREEN..............................................................................................................................................................39
Device/Patient..........................................................................................................................................................40
StatusArea...............................................................................................................................................................40
LockIcon...................................................................................................................................................................40
TimeofDay..............................................................................................................................................................41
BatteryLevelIndicator.............................................................................................................................................41
CentralStationSlotName+UnitID&BedID..........................................................................................................41
MonitoringStatus....................................................................................................................................................41
PatientHookupDisplay&LeadQualityIndicators..................................................................................................42
PatientDemographicsScreen..................................................................................................................................43
WaveformReview....................................................................................................................................................43
MONITORINGMENUS...................................................................................................................................................46
HEARTRATEDISPLAY....................................................................................................................................................49
PRINTING............................................................................................................................... .....................................50
TOOLKIT.....................................................................................................................................................................51
WaveformGain(Amplitude)....................................................................................................................................53
WaveformSpeed......................................................................................................................................................53
PowerOff–SuspendMonitoring.............................................................................................................................54
12. CONFIGURING THE S4 .................................................................................................................. 57
HOSTSETTINGS............................................................................................................................................................58
NETWORKSETTINGS......................................................................................................................................................59
LANGUAGESETTINGS....................................................................................................................................................59
WIFIDIAGNOSTICS.......................................................................................................................................................60
RESETPASSCODE............................................................................................................................... ...........................60
HARDWAREDIAGNOSTICS..............................................................................................................................................60
S4VERSION................................................................................................................................................................61
SAMVERSION.............................................................................................................................................................61
iv

TABLE OF CONTENTS
13.
PERFORMING ECG MONITORING ................................................................................................ 63
POWER‐ON.................................................................................................................................................................63
PATIENTPREP..............................................................................................................................................................63
PATIENTCABLEHOOKUP................................................................................................................................................63
CONFIRMGOODSIGNAL................................................................................................................................................64
NEWPATIENTMONITORING...........................................................................................................................................64
SAMEPATIENTMONITORING..........................................................................................................................................65
CONFIRMMONITORINGACTIVE......................................................................................................................................65
ENDINGAMONITORINGSESSION....................................................................................................................................66
14. PRODUCT SPECIFICATIONS ........................................................................................................ 69
GENERALSPECIFICATIONS..............................................................................................................................................69
ENVIRONMENTALCONDITIONS........................................................................................................................................69
POWERREQUIREMENTS&BATTERY.................................................................................................................................69
DISPLAYSPECIFICATIONS................................................................................................................................................70
ECGSPECIFICATIONS....................................................................................................................................................70
WIRELESSNETWORKSPECIFICATIONS............................................................................................................................... 71
15. TROUBLESHOOTING & ERROR CODES ..................................................................................... 73
POWERANDBATTERY............................................................................................................................... .....................73
DISPLAYANDTOUCHSCREEN...........................................................................................................................................73
ECGTRACE............................................................................................................................... ..................................73
NETWORKTRANSMISSION............................................................................................................................... ...............74
ERRORMESSAGES........................................................................................................................................................76
16. REORDERING ACCESSORIES & CONSUMABLES ..................................................................... 77
17. APPLICABLE STANDARDS ........................................................................................................... 79
v

TABLE OF CONTENTS
vi

1. GENERAL STATEMENTS
Technical Support and Service
Headquarters
Mortara Instrument, Inc.
7865 North 86th Street
Milwaukee, WI 53224
U.S.A.
Tel: 414.354.1600
Tel: 800.231.7437
Fax: 414.354.4760
Internet: http://www.mortara.com
European Union
Representative
Mortara Instrument Eu rope s.r.l.
(European Headquarters)
Via Cimarosa 103/105
40033 Casalecchio di Reno (BO)
Italy
Tel: +39.051.298.7811
Fax: +39.051.613.3582
Service/Technical
Support Group
Mortara Instrument, Inc.
7865 North 86th Street
Milwaukee, WI 53224
U.S.A.
Tel: 414.354.1600
Service: 888.MORTARA
(888.667.8272)
Fax: 414.354.4760
E-mail: techsupport@mortara.com
Sales Support/
Supplies & Accessories
Mortara Instrument, Inc.
7865 North 86th Street
Milwaukee, WI 53224
U.S.A.
Tel: 414.354.1600
Fax: 414.354.4760
Hospital Customers: orders.us@mortara.com
Physician Practice: orderspc.us@mortara.com
U.S. Distribution: orderspc.us@mortara.com
Mortara Instrument Germany
Bonifaciusring 15
45309 Essen
Germany
Tel: +49.201.18 55 69 70
Fax: +49.201.18 55 69 77
Mortara Instrument Netherlands
Postbus 324
5680 AH Best
Industrieweg 160b
5683 CG Best
Netherlands
Tel: +31.499.377310
Fax: +31.499.377908
Mortara Instrument Aus tralia
PO Box 7568
Baulkham Hills NSW 2153
Unit 28, 9 Hoyle Avenue
Castle Hill NSW 2154
Australia
Tel: +61 2 8070 9303
Fax: +61 2 9899 9478
Mortara Instrument UK Ltd
Units 11 & 12, Scion House
Stirling University Innovation Park
Stirling FK9 4NF
Scotland
Tel: +44.1786.444980
Fax: +44.1786.446630
1

2. NOTICES
Manufacturer’s Responsibility
Mortara Instrument, Inc. (Mortara) is responsible for the effects on safety and performance of the Surveyor™ S4
mobile monitor, as indicated by the label, only if article 2 of 93/42/EEC directive is applied, in particular:
WARNING: System installation and assembly operations, extensions, readjustments, modifications or
repairs are carried out by personnel authorized by Mortara only.
The mobile monitor is used in accordance with the instructions for use.
The mobile monitor is correctly maintained according to the standards authorized by Mortara using original
spare parts.
The mobile monitor is used with original accessories and supplies that are in compliance with the standard
specifications described in this manual.
The electrical installation of the relevant room complies with the requirements of appropriate regulations.
Responsibility of the Customer
The user of this mobile monitor is responsible for ensuring the implementation of a satisfactory maintenance
schedule. Failure to do so may cause undue failure and possible health hazards. This manual must be kept in a safe
place to prevent its deterioration and/or alteration. The user and Mortara authorized personnel must have access to
this manual at any time. The user of this mobile monitor must periodically check the accessories, their functionality
and integrity.
Equipment Identification
Mortara equipment is identified by a serial and reference number on the back of the mobile monitor. Care should be
taken so that these numbers are not defaced.
Copyright and Trademark Notices
This document contains information that is protected by copyright. All rights are reserved. No part of this
document may be photocopied, reproduced, or translated into another language without prior written consent of
Mortara.
Other Important Information
The information in this document is subject to change without notice.
Mortara makes no warranty of any kind with regard to this material including, but not limited to, implied warranties
of merchantability and fitness for a particular purpose. Mortara assumes no responsibility for any errors or
omissions that may appear in this document. Mortara makes no commitment to update or to keep current the
information contained in this document.
2

3. WARRANTY INFORMATION
Your Mortara Warranty
MORTARA INSTRUMENT, INC. (hereafter referred to as “Mortara”) warrants that components within Mortara
products (hereafter referred to as “Product/s”) will be free from defects in workmanship and materials for the
number of years specified on documentation accompanying the product, or previously agreed to by the purchaser
and Mortara, or if not otherwise noted, for a period of twelve (12) months from the date of shipment.
Consumable, disposable or single use products such are warranted to be free from defects in workmanship and
materials for a period of 90 days from the date of shipment or the date of first use, whichever is sooner.
Reusable product such as, but not limited to, BATTERIES, PATIENT CABLES, LEAD WIRES, MAGNETIC
STORAGE MEDIUMS, CARRY CASES or MOUNTS, are warranted to be free from defects in workmanship and
materials for a period of 90 days. This warranty does not apply to damage to the Product/s caused by any or all of
the following circumstances or conditions:
a) Freight damage;
b) Supplies, accessories and internal parts NOT approved by Mortara;
c) Misapplication, misuse, abuse, and/or failure to follow the Product/s instruction sheets and/or information
guides;
d) Accident;
e) A disaster affecting the Product/s;
f) Alterations and/or modifications to the Product/s not authorized by Mortara;
g) Other events outside of Mortara’s reasonable control or not arising under normal operating conditions.
THE REMEDY UNDER THIS WARRANTY IS LIMITED TO THE REPAIR OR REPLACEMENT WITHOUT
CHARGE FOR LABOR OR MATERIALS, OR ANY PRODUCT/S FOUND UPON EXAMINATION BY
MORTARA TO HAVE BEEN DEFECTIVE. This remedy shall be conditioned upon receipt of notice by Mortara
of any alleged defects promptly after discovery thereof within the warranty period. Mortara’s obligations under the
foregoing warranty will further be conditioned upon the assumption by the purchaser of the Product/s (i) of all
carrier charges with respect to any Product/s returned to Mortara’s principal place or any other place as specifically
designated by Mortara or an authorized distributor or representative of Mortara, and (ii) all risk of loss in transit. It
is expressly agreed that the liability of Mortara is limited and that Mortara does not function as an insurer. A
purchaser of a Product/s, by its acceptance and purchase thereof, acknowledges and agrees that Mortara is not liable
for loss, harm, or damage due directly or indirectly to an occurrence or consequence there from relating to the
Product/s. If Mortara should be found liable to anyone under any theory (except the expressed warranty set forth
herein) for loss, harm, or damage, the liability of Mortara shall be limited to the lesser of the actual loss, harm, or
damage, or the original purchase price of the Product/s when sold.
EXCEPT AS SET FORTH HEREIN WITH RESPECT TO REIMBURSEMENT OF LABOR CHARGES, A
PURCHASER’S SOLE EXCLUSIVE REMEDY AGAINST MORTARA FOR CLAIMS RELATING TO THE
PRODUCT/S FOR ANY AND ALL LOSSES AND DAMAGES RESULTING FROM ANY CAUSE SHALL BE
THE REPAIR OR REPLACEMENT OF DEFECTIVE PRODUCT/S TO THE EXTENT THAT THE DEFECT IS
NOTICED AND MORTARA IS NOTIFIED WITHIN THE WARRANTY PERIOD. IN NO EVENT,
INCLUDING THE CLAIM FOR NEGLIGENCE, SHALL MORTARA BE LIABLE FOR INCIDENTAL,
SPECIAL, OR CONSEQUENTIAL DAMAGES, OR FOR ANY OTHER LOSS, DAMAGE, OR EXPENSE OF
ANY KIND, INCLUDING LOSS OF PROFITS, WHETHER UNDER TORT, NEGLIGENCE OR STRICT
LIABILITY THEORIES OF LAW, OR OTHERWISE. THIS WARRANTY IS EXPRESSLY IN LIEU OF ANY
OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO THE IMPLIED
WARRANTY OF MERCHANTABILITY AND THE WARRANTY OF FITNESS FOR A PARTICULAR
PURPOSE.
3

WARRANTY INFORMATION
4
4. USER SAFETY INFORMATION
WARNING: Means there is the possibility of personal injury to you or others.
CAUTION: Means there is the possibility of damage to the mobile monitor.
NOTE:
NOTE: This manual may contain screen shots and pictures. Any screen shots and pictures are provided
for reference only and are not intended to convey actual operating techniques. Consult the actual screen in
the host language for specific wording.
Provides information to further assist in the use of the mobile monitor.
Safety Regulations
The Surveyor S4 (henceforth referred to as either Surveyor S4 or S4) is a medical mobile monitor.
The S4 and its accessories are labeled, according to applicable standards.
The S4 with all accessories that have a physical or logical connection with it, forms part of a Medical Electrical
System.
The S4 complies with various safety and performance regulations as mentioned in this manual (Applied
Standards).
Warnings
This manual gives important information about the use and safety of this mobile monitor. Deviating from
operating procedures, misuse or misapplication of the mobile monitor, or ignoring specifications and
recommendations could result in increased risk of harm to users, patients and bystanders, or damage to the
mobile monitor.
Users are expected to be licensed clinical professionals knowledgeable about medical procedures and patient
care, and adequately trained in the use of this mobile monitor. The S4 mobile monitor captures and presents
data reflecting a patient’s physiological condition that when reviewed by a trained physician or clinician can be
useful in determining a diagnosis; however, the data should not be used as a sole means for determining a
patient’s diagnosis.
Before attempting to use this device for clinical applications, the operator must read and understand the contents
of the user manual and other accompanying documents. Inadequate knowledge or training could result in
increased risk of harm to users, patients and bystanders, or damage to the mobile monitor. Contact Mortara for
additional training options.
Operation of the equipment beyond its specified ranges, or beyond normal physiological conditions of human
subjects, may cause inaccurate results.
A possible explosion hazard exists. Do not use the device in the presence of a flammable anesthetic mixture.
Do not mount any part of the device closer than 25 cm from outlets of flammable gases, including oxygen.
For proper operation and the safety of users or patients and bystanders, equipment and accessories must be
connected only as described in this manual.
5

USER SAFETY INFORMATION
Repairs and modification must be made by authorized and trained technical personnel. Unauthorized
modifications and repairs will void the S4 warranty and may pose a danger to patients and users.
If additional devices beyond the S4 are connected to the patient, leakage currents could add up and should be
accounted for.
The S4, as all medical equipment or systems, requires special precautions regarding EMC, and should be
installed and put into service according to the EMC information provided in the installation procedure to obtain
a sufficient degree of immunity as well as not to create disturbance to other equipment. Refer to the specific
EMC instructions in this manual.
The quality of the signal produced by the device may be adversely affected by the use of other medical
equipment, including but not limited to defibrillators, electrosurgery equipment and ultrasound machines. Do
not use the S4 system in the presence of imaging equipment such as magnetic resonance imaging (MRI) and
tomography systems. Simultaneous operation may damage the device or lead to erroneous results.
There is no known safety hazard if other equipment, such as pacemakers or other stimulators, is used
simultaneously with the device; however, disturbance to the signal may occur.
Portable and mobile RF communications equipment may affect medical electrical equipment or systems as well
as the S4 and its accessories. Do not operate the S4 near sources of high frequency emissions (e.g.
microwaves). Unauthorized wireless devices, such as personal access points and WiFi hotspots including those
available on personal smartphones, may also interfere with the operation of the system.
A mobile monitor is not intended to replace clinical assessments. It is important that a qualified individual
regularly supervise the patient.
The S4 is restricted to use on one patient at a time.
Power Supply Warnings
Only use the recommended batteries. Use of alternate batteries may damage the device or cause other hazards.
Only charge Mortara Rechargeable Battery (Mortara Re-Order Number 4800-018) in the Mortara Li-Ion
Battery Charger. Attempting to charge unauthorized batteries may result in damage to the unauthorized battery
and/or the Li-Ion Battery Charger.
The S4 is a battery operated device that transmits data reflecting a patient’s physiological condition to a
receiving device. During operation failure, data transmission and LCD information will cease to occur. In the
case of battery depletion, replace the batteries on the device to resume monitoring. In mission critical
conditions, it is advisable to have a backup device available.
Only use the Mortara-provided external battery charger and adapter with the S4. Ensure that the electrical
installation also complies with local safety requirements for the environment where it is used.
Regularly check all cables for damage and proper connection. Do not use equipme
nt with a damaged cable.
The S4 contains an internal battery. The following precautions should be taken regarding the internal battery:
o Do not immerse in water.
o Do not heat or throw in fire.
o Do not leave in conditions over 60 ºC.
o Do not crush or drop.
o Only use the approved batteries.
o Follow the instructions in the disposal section of this manual when taken out of service.
6

USER SAFETY INFORMATION
The S4 rechargeable battery must be initially fully charged prior to use.
When the S4 initially powers on, the screen will illuminate if the batteries are installed properly and charged.
Remove the S4 from service and contact Mortara if the screen does not activate when new or fully charged
batteries are initially installed.
AA batteries are known to leak their contents when stored for an extended period of time in unused equipment.
Always remove the batteries after completing operating the mobile monitor. Always place rechargeable
batteries in the battery charger when not in use. This ensures that the batteries are recharged for the next time
the mobile monitor is operated.
There is a potential pinch hazard when applying the battery compartment cover to the device that could result in
minor injury. Care should be taken to avoid entrapment of fingers when performing this operation.
Accessories, Cables, and External Connections Warnings
The S4 is designed to meet applicable specifications when using Mortara-approved patient cables and
accessories. Use of non-approved cables and accessories may result in reduced performance or electromagnetic
interference, and may pose possible patient and user safety concerns.
Do not use excessive force on any of the connection cables and handle all accessories with care.
Conductive parts of the ECG patient cables, electrodes, and associated connections of type CF applied parts,
including the neutral conductor of the patient cable and electrode should not come into contact with other
conductive parts including earth ground.
To avoid the possibility of serious injury or death during patient defibrillation, do not come into contact with
mobile monitor or patient cables.
Accessories may be provided with separate user manuals. Read these manuals thoroughly and refer to them for
specific functions. It is recommended to keep all manuals together.
To avoid potential for spread of disease or infection, single-use components and accessories (e.g., electrodes,
pouches, etc.) must not be reused. To maintain safety and effectiveness, ECG electrodes and sensors must not
be used beyond their expiration date or useful life.
All accessories including cables, connectors and other patient-applied parts supplied with the S4 do NOT
contain any Latex. If the patient develops an allergic reaction or rash, immediately remove the accessory and
inform Mortara.
Defibrillation and Electrosurgery Warnings
The S4 has not been designed for use together with electrosurgery equipment.
The S4 is defibrillator protected in compliance with IEC 60601-2-25 and/or IEC 60601-2-27 standards if used
with Mortara-approved patient cables. Defibrillation while using non-approved patient cables may damage the
device beyond repair and cause a safety hazard to the patient.
ECG Warnings
Excessive patient movement could interfere with the operation of the system.
Proper clinical procedure must be employed to prep the electrode and sensor sites, and to monitor the patient for
excessive skin irritation, inflammation, or other adverse reactions. Electrodes and other sensors that are
intended for short-term use should be removed from the patient promptly following use.
7

USER SAFETY INFORMATION
12-lead ECGs acquired through the S4 with 10-wire cable will normally use a modified lead system with the
limb electrodes positioned on the torso. Although this is a generally accepted practice (e.g., in stress testing),
the different electrode positions can cause morphology changes on the ECG, thus influencing their
interpretation. Most frequently seen differences are a vertical and rightward axis shift, minor changes of
evidence of old inferior infarction and changes in the T-wave in the limb leads. It is recommended that you
place the electrodes as close as possible to the normal limb positions avoiding the possibility of causing artifact.
The right arm and left arm electrodes should be placed on the clavicles as close as possible to the arms. The left
leg electrode should be placed as close as possible to the left leg without subjecting it to the possibility of
motion artifact.
During periods of lead fail and when a reduced lead set is used for the S4, 12-lead ECG interpretation cannot be
reliably used in determining a diagnosis.
When using the 3-wire or 5-wire ECG cables, it is not possible to acquire a 12-lead ECG with the S4.
Cautions
This device must be installed as part of a system in conjunction with the Surveyor Central Station and in
accordance to guidance and minimum characteristics per requirements provided by Mortara for deployment of
the system on the hospital/clinic’s IT network. Refer to those requirements as well as Manufacturer Disclosure
Statement for Medical Device Security (MDS2) statements provided by Mortara before deploying and using the
system.
The device and patient cable should be cleaned between each use. Cleaning must be performed with the system
turned off and battery removed. Let all parts dry well before turning the power back on.
Prevent liquids from penetrating the system, components, or the monitor. Do not spray the system with liquid
cleaning agents. Do not allow the system, components or accessories to become in contact with unknown
substances which may compromise its mechanical or electrical integrity. If liquids have penetrated the system,
open by authorized personnel for inspection and let dry completely.
Do not attempt to clean the mobile monitor or patient cables by submersing into a liquid, autoclaving, or steam
cleaning as this may damage equipment or reduce its usable life. Wipe the exterior surfaces with a warm water
and mild detergent solution and then dry with a clean cloth. Use of unspecified cleaning/disinfecting agents,
failure to follow recommended procedures, or contact with unspecified materials could result in increased risk
of harm to users, patients and bystanders, or damage to the mobile monitor.
No user-serviceable parts inside. Screw removal by authorized service personnel only. Damaged or suspected
inoperative equipment must be immediately removed from use and must be checked/repaired by authorized
service personnel prior to continued use.
The S4 accommodates single-use or rechargeable internal batteries. If the rechargeable battery appears to
become defective, refer to Mortara Technical Support.
Do not pull or stretch patient cables as this could result in mechanical and/or electrical failures. Patient cables
should be stored off of the floor away from bedrails and wheels to avoid cable damage. Roll the patient cables
into a loose loop prior to storage.
When necessary, dispose of the mobile monitor, its components and accessories (e.g., batteries, cables,
electrodes), and/or packing materials in accordance with local regulations.
Check that all operating and environment conditions such as ambient temperature meet the device’s
specifications. Allow the device to stabilize within its intended operating environment for a minimum of two
hours prior to use.
8

USER SAFETY INFORMATION
Do not exert excessive pressure on the touchscreen LCD or use a sharp or hard object with it. Excessive
pressure may permanently damage the display.
This device is not recommended for use in the presence of imaging equipment such as Magnetic Resonance
Imaging (MRI) and Computed Tomography (CT) devices, etc.
The device is UL listed:
UL-Listed device in the USA and Canada.
Upon request, Mortara can supply a service manual that includes test instructions as well as a list of spare parts
that must be used with the S4.
9
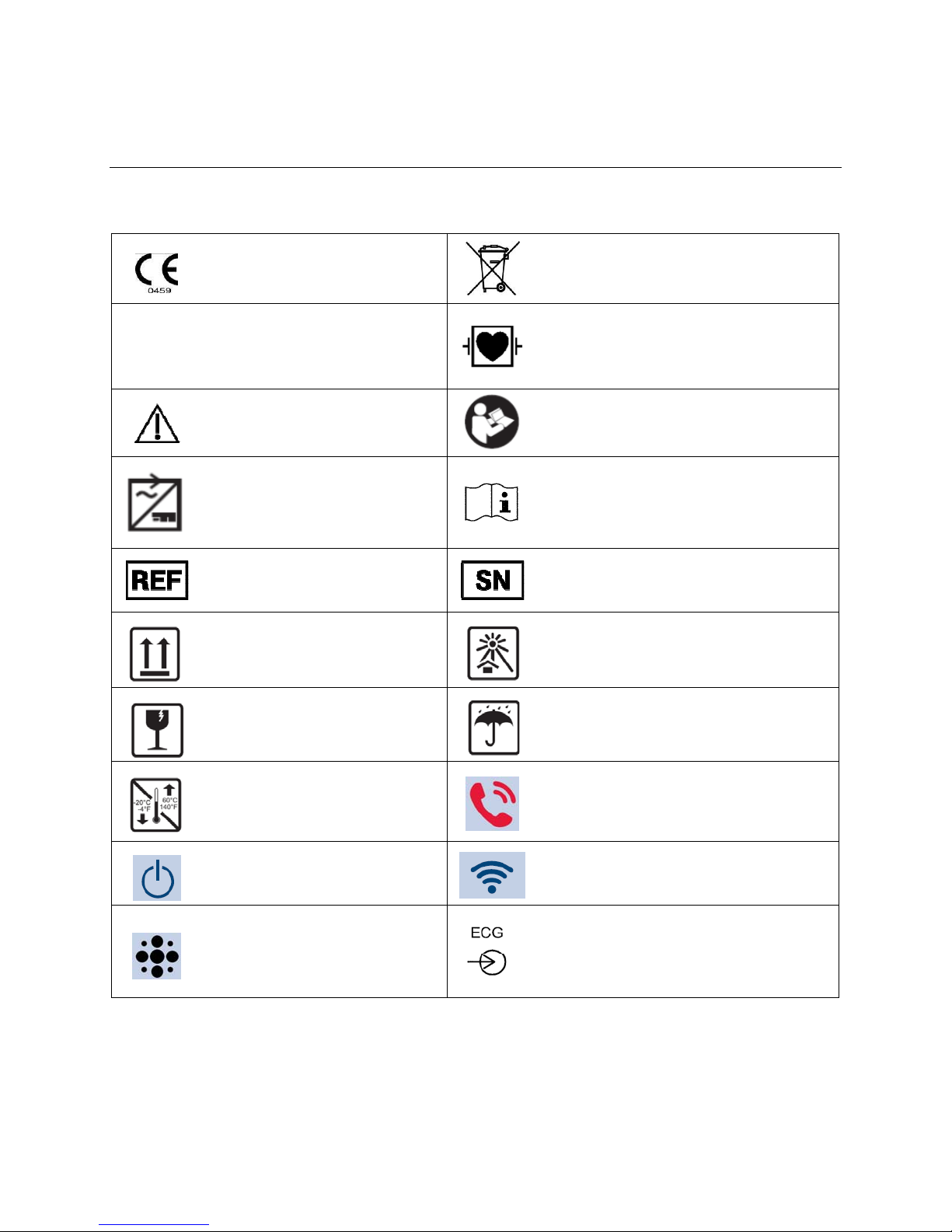
5. EQUIPMENT SYMBOLS AND MARKINGS
Symbol Delineation
Do not dispose as unsorted municipal waste.
Per European Union Directive 2002/96,
requires separate handling for waste disposal
according to national requirements
Defibrillator-proof type CF applied part
IPX4
Indicates compliance to applicable
European Union directives
Indicates the device has been
tested for safety and shall have no
harmful effect from water splashing
against the enclosure from any
direction
Caution Consult accompanying documents
Power input Consult accompanying documents
Catalog number for relevant Mortara
part
This end up Keep away from sunli ght
Serial number
Fragile, handle with care Keep dry
Storage temperature range
The illuminated LED below this icon
indicates the Power On/Off status
Speaker
(RESERVED FOR FUTURE USE)
Nurse Call/Control Button
The illuminated LED below this icon indicates
the status of the WiFi connection
Patient Cable Input
10

6. GENERAL CARE
Precautions for S4
Power off and remove the battery from the S4 before inspecting or cleaning.
Protect the S4 from water and other liquids.
Never immerse the S4 in water or other fluids.
Do not drop the S4 or subject to shock and/or vibration.
Do not use organic solvents, ammonia-based solutions, or abrasive cleaning agents that may damage
equipment surfaces.
Precautions for Li-Ion Battery Charger, AC Power Pack and Cord
Remove the AC Power Pack and AC power cord from the Li-Ion Battery Charger before inspecting or
cleaning.
Protect the AC Power Pack, AC power cord and the Li-Ion Battery Charger from water or other liquids.
Never immerse the AC Power Pack, AC power cord or the Li-Ion Battery Charger in water or other fluids.
Do not subject the AC Power Pack or the Li-Ion Battery Charger to shock and/or vibration.
Do not use organic solvents, ammonia-based solutions, or abrasive cleaning agents that may damage
equipment surfaces.
Inspection Prior to Clinical Use
Inspect your equipment prior to clinical operation. Do not use the equipment and contact an authorized service
representative for servicing if there are concerns about integrity of the system.
Verify that all cables and connectors are securely seated.
Check the case and chassis of the S4, AC Power Pack, AC power cord or the Li-Ion Battery Charger for
any visible damage.
Inspect the S4 touchscreen and controls for proper function and appearance.
Inspect the Li-Ion Battery Charger indicator LEDs for proper operation and indication of battery charging
and AC connection.
Inspect patient accessories for any visual damage.
S4 patient input connector - Verify the pins on the patient input connector are all present and are not bent or
damaged in any way. The recessed area for the patient connector should be free from debris and clean. Use
compressed air to remove any debris that has entered into the connector area.
S4 display – Verify there are no deep scratches or physical damage to the S4 mobile monitor’s display.
Inspect the display bezel to ensure it is firmly adhered to the device housing. Contact Mortara Technical
Support if the display or display bezel require replacement.
S4 battery door – Verify the S4’s battery door for proper opening and closing. Inspect the plastic door
assembly for signs of excessive wear or cracking, including the door seal to prevent fluid ingress. Replace
the battery door assembly if necessary.
S4 Battery Compartment – Inspect the S4’s battery spring contacts and the battery door latching
mechanism for signs of excessive wear. If the battery compartment has been damaged, contact Mortara
Technical Support for assistance.
11

GENERAL CARE
Battery Charger bays – Inspect the Li-Ion Battery Charger’s spring contacts and latching mechanisms for
signs of excessive wear. If a battery bay is damaged, contact Mortara for replacement.
Rechargeable Li-Ion Battery – Follow the instructions and note the cautions labeled on the rechargeable
battery. Contact Mortara for replacement.
AC Power Pack – Inspect the Li-Ion Battery Charger connector on the power pack to ensure adequate
contact to the Li-Ion Battery Charger. Inspect the AC Power Cord connector on the power pack to ensure
adequate contact to the AC Power Cord.
AC Power Cord – With the cord unplugged from AC power, visually and mechanically inspect the AC
Power Cord connectors and along its cable length for cracks in the insulating jacket or other abnormalities
that would inhibit its function. Contact Mortara for replacement.
Device Labeling – Inspect the device labeling for signs of wear and legibility. If the labeling is no longer
clear and legible, contact Mortara Technical Support for assistance.
Cleaning and Disinfecting
The following section provides information on proper cleaning directions for the S4, patient accessories, Li-Ion
battery Charger, AC Power Pack and AC Power Cord. Patient accessories should be cleaned before they are applied
to a new patient. The mobile monitor should be cleaned as per facility standard of care. The Li-Ion battery Charger,
AC Power pack and AC Power Cord should be cleaned in accordance with facility standards pertaining to power
components.
Clean the exterior surface of the device and patient cables with a mild soap and water solution. After cleaning
thoroughly dry off the device with a clean, lint-free, soft cloth.
Disinfect the device after cleaning the exterior surface as required. To disinfect the device wipe the exterior surface
with a damp, soft, lint-free cloth using a solution of 10% household bleach and water (Sodium Hypochlorite solution
consisting of a minimum 1:500 dilution and maximum of 1:10 dilution). Dry off the device with a clean, lint-free,
soft cloth.
Warnings:
Remove the batteries from the device before inspecting or cleaning.
Do not immerse the device in water or other fluids.
Do not drop the device or subject it to shock and/or vibration.
Do not use organic solvents, ammonia-based solutions, or abrasive cleaning agents which may damage
equipment surfaces.
Be careful not to use an excessive amount of disinfecting solution that could lead to fluid entering the
device. Fluid ingress may cause irreparable damage to the internal circuitry.
12
GENERAL CARE
Cautions:
Always disconnect the S4 from power source before cleaning.
Do not use harsh chemicals for cleaning. Do not use disinfectants that contain phenol as they can spot
plastics. Do not steam autoclave, gas sterilize, irradiate, subject to intense vacuum, or immerse in
water or cleaning solution. Be careful to avoid getting cleaning liquids into connectors or the mobile
monitor. If this occurs, allow the mobile monitor to dry in warm air for 2 hours, then check to make
sure all monitoring functions are working properly.
Keep the patient accessories off of the floor. Accessories that fall on the floor should be inspected for
defects, contamination, proper functionality, and cleaned or discarded according to the approved
recommendations.
The user has the responsibility to validate any deviations from the recommended method of cleaning
and disinfection.
Maintenance
The following table shows the recommended maintenance procedures for the S4, patient accessories, Li-Ion Battery
Charger, AC Power Pack and AC Power Cord. The S4 should be serviced once a year by a Mortara authorized
service technician; however, it is good practice to periodically ensure the mobile monitor is in proper working order.
This can be performed by a clinician or biomed at the hospital or healthcare delivery organization familiar with the
S4 mobile monitor, ECG signal acquisition, as well as general maintenance/calibration of biomedical equipment.
To accomplish these steps in their entirety and verify the correct operation of the system, appropriate patient
simulators or other equipment may be required.
Functionality Procedure
Mechanical Integrity Check for cracks, abrasive edges and other signs of damage.
ECG
Li-Ion Battery Charger,
AC Power Pack, AC
Power Cord
Connect ECG leads to Patient Simulator.
Start a new monitoring session.
Verify that waveforms for all leads are properly shown on the LCD.
Check for cracks, abrasive edges and other signs of damage.
Check that all connectors and AC cord length is unbroken and smooth along its
length.
Refer to the service manual for further details.
13

Functionality Procedure
Touchscreen Display
Li-Ion Battery Charger,
AC Power Pack, AC
Power Cord
ECG Cables
AC Power Cord, AC
Power Pack Captive
Cable
Approved Cleaning Agents
Clean the touchscreen with a soft cloth moistened with either a solution of
70% isopropyl alcohol in distilled water or soapy water.
Do not spray cleaner directly onto the touchscreen. Spray the cleaner onto a lint-free
cloth and then wipe the S4 mobile monitor.
To clean the touchscreen display,
1. Ensure that the touchscreen display is off. If on, press the Nurse Call button
once to turn off the display.
2. Clean the display.
Verify proper LED indicators during battery charging.
Approved Cleaning Agents
Enzymatic detergent such as ENZOL (US) or CIDEZYME (outside the US).
Distilled water.
Disinfectant solution (such as CIDEX OPA, or a 10% solution of household
bleach (5.25% sodium hypochlorite) in distilled water).
Soft, lint-free cloths and/or soft-bristled brushes.
Protective gloves and eyewear.
Procedure
1. Disconnect the mobile monito r from its power source.
2. Put on gloves and protective eyewear.
3. Prepare the detergent according to the manufacturer's instructions, and also
4. Apply detergent to product using a soft, lint-free cloth. If material is dried on,
5. Wipe smooth surfaces with the cloth.
6. Use a soft-bristle brush on visibly soiled areas and irregular surfaces.
7. Remove detergent from product using cloth dampened in distilled water.
8. Repeat as necessary.
9. Apply disinfectant solution on affected area using a soft cloth. Allow product to
10. Wipe excess solution and clean product again with cloth dampened in distilled
11. Allow 2 hours for drying.
Approved Cleaning Agents
the disinfectant solution, in separate containers.
allow to sit for 1 minute. Do not immerse cable ends or lead wires in liquid as
it can cause corrosion.
sit for 5 minutes.
water.
Enzymatic detergent such as ENZOL (US) or CIDEZYME (outside the US).
Distilled water.
Disinfectant solution (such as CIDEX OPA, or a 10% solution of household
bleach (5.25% sodium hypochlorite) in distilled water).
Soft, lint-free cloths and/or soft-bristled brushes.
Protective gloves and eyewear.
GENERAL CARE
14
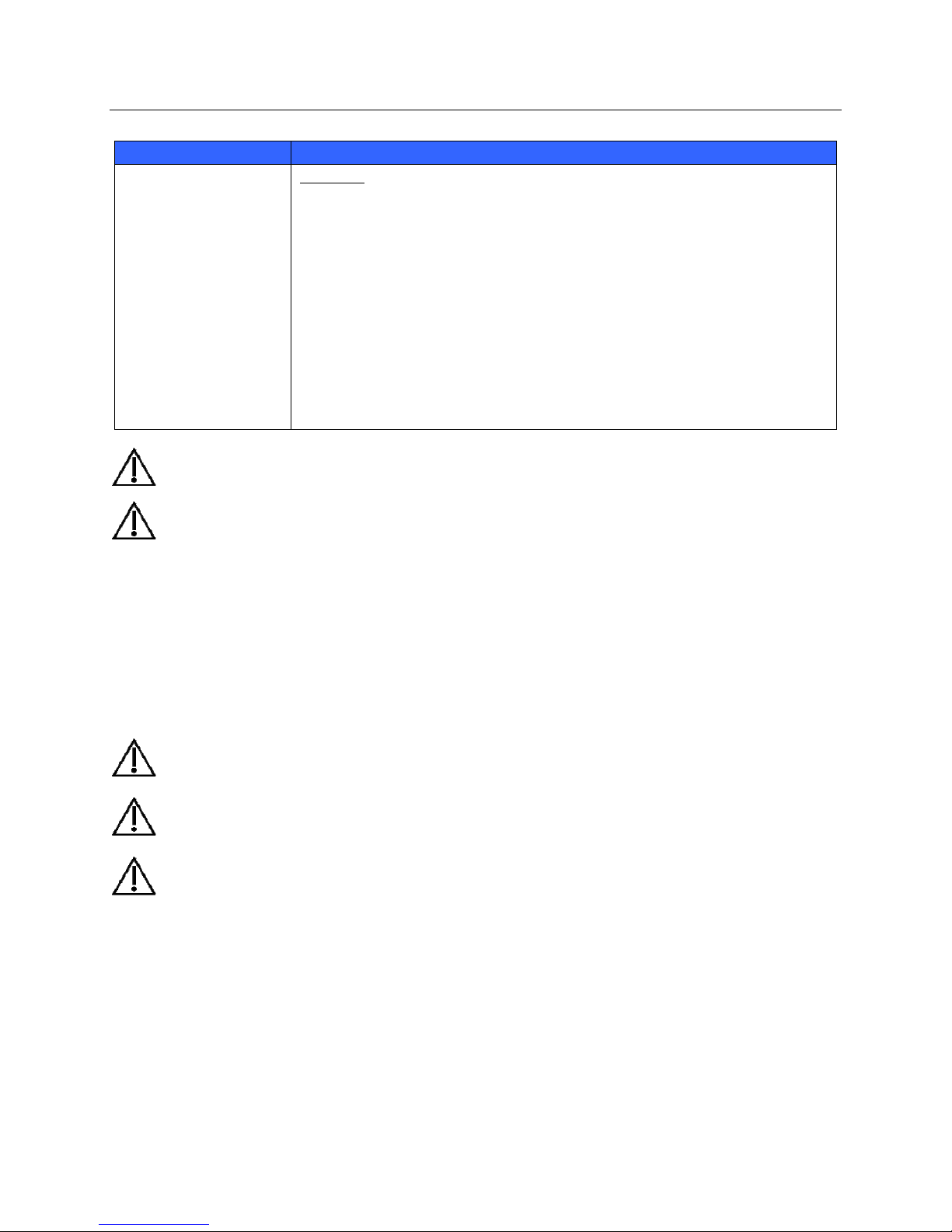
p
Functionality Procedure
Procedure
1. Disconnect the cables from other devices and AC power source.
2. Put on gloves and protective eyewear.
3. Prepare the detergent according to the manufacturer's instructions, and also
4. Apply detergent to product using a soft, lint-free cloth. If material is dried on,
5. Wipe smooth surfaces with the cloth.
6. Use a soft-bristle brush on visibly soiled areas and irregular surfaces.
7. Remove detergent from product using cloth dampened in distilled water.
8. Repeat as necessary.
9. Apply disinfectant solution on affected area using a soft cloth. Allow product
10. Wipe excess solution and clean product again with cloth dampened in distilled
WARNING: Servicing of this device should only be performed by Mortara authorized service personnel.
WARNING: The S4 mobile monitor and/or its accessories and parts should not be used beyond their
roduct life.
GENERAL CARE
the disinfectant solution, in separate containers.
allow to sit for 1 minute. Do not immerse cable ends or lead wires in liquid as
it can cause corrosion.
to sit for 5 minutes.
water. Allow 2 hours for drying.
Battery Life and Charge Time
Battery Life
1. With a new, fully charged rechargeable battery pack installed and under normal operating conditions, the
device should operate for a minimum of 24 hours.
2. When a fresh set (3) of single-use alkaline batteries are installed and under normal operating conditions, the
device should operate for a minimum of 12 hours.
CAUTION: The battery should be removed from the mobile monitor upon completing each clinical use.
The battery should be replaced if it is no longer holding a charge.
WARNING: Use only APPROVED BATTERIES as listed in the Accessories section. Use of unapproved
batteries may cause a hazard and will void the warranty.
CAUTION: Batteries should only be replaced by a trained user.
NOTE: Excessive wireless network traffic and network dropouts may affect the battery life.
15

GENERAL CARE
Product Life
The S4 has a defined product life of 5 years excluding accessories, cables and batteries. As required, product
service, accessories and spare parts are available through Mortara or its authorized partners. Using the mobile
monitor or its accessories and components beyond their defined life may lead to damage to the equipment or a safety
hazard to the user.
Decommissioning and Disposal
Dispose of the mobile monitor, its components and accessories (e.g., batteries, cables, electrodes), and/or packing
materials in accordance with local regulations. Do NOT incinerate the battery.
16

7. ELECTROMAGNETIC COMPATABILITY (EMC)
When using the mobile monitor, assess the electromagnetic environment affected by surrounding devices.
An electronic device may either generate or receive electromagnetic interference. Testing for electromagnetic
compatibility (EMC) has been performed on the mobile monitor according to the applicable international standards.
The mobile monitor should not be used adjacent to or stacked with other equipment. If the mobile monitor is used
in this manner, verify the mobile monitor operates in an acceptable manner in the configuration in which it will be
used.
Fixed, portable, and mobile radio frequency communications equipment may affect the performance of medical
equipment. See the appropriate EMC table for recommended separation distances between the radio equipment and
the mobile monitor.
The use of accessories, transducers, and cables other than those specified by Mortara may result in increased
emissions or decreased immunity of the equipment.
17
ELECTROMAGNETIC COMPATABILITY (EMC)
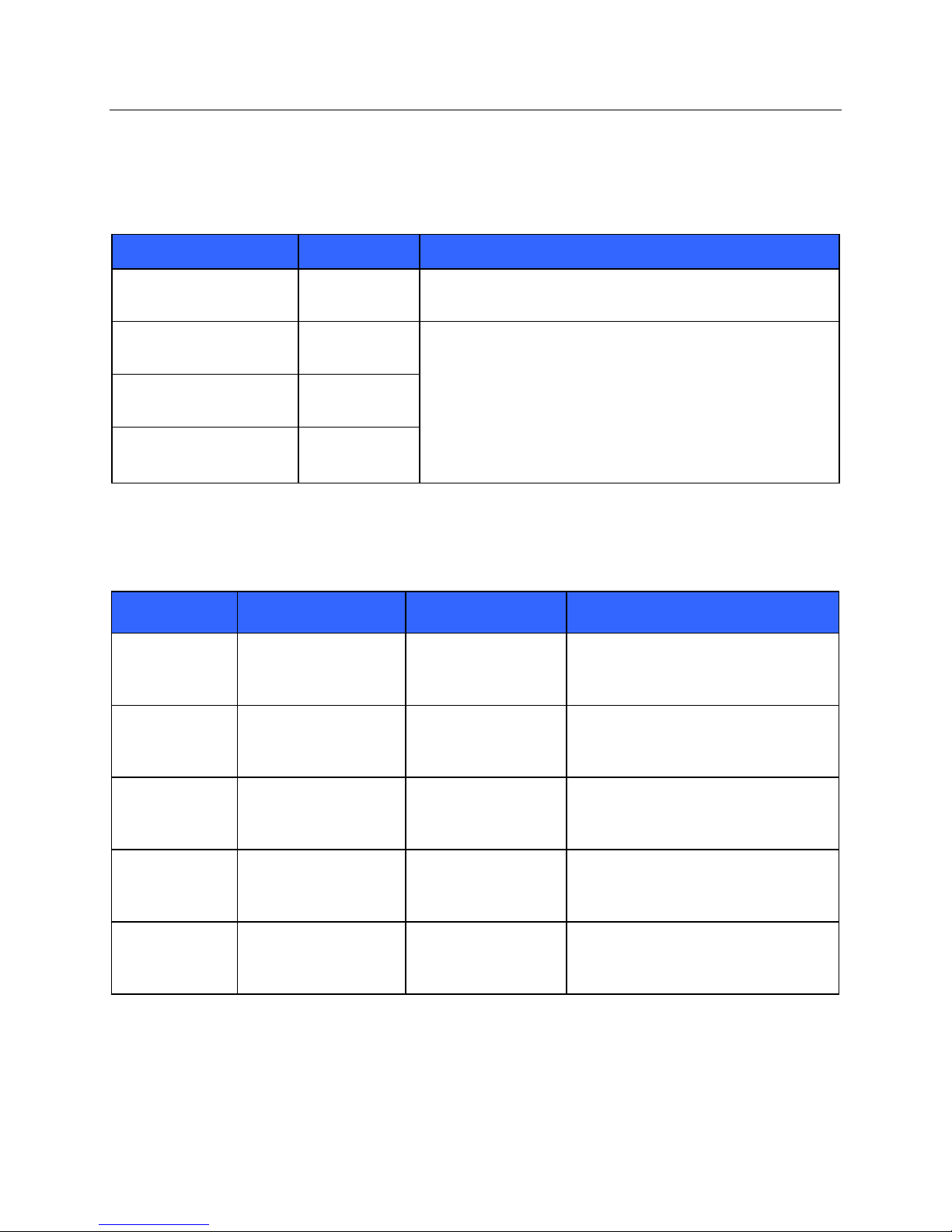
Guidance and Manufacturer’s Declaration: Electromagnetic Emissions
The equipment is intended for use in the electromagnetic environment specified in the table below. The customer or
the user of the equipment should ensure that it is used in such an environment.
Emissions Test Compliance Electromagnetic Environment: Guidance
RF Emissions
CISPR 11
RF Emissions
CISPR 11
Harmonic Emissions
IEC 61000-3-2
Voltage Fluctuations/
Flicker Emissions
IEC 61000-3-3
Group 2 The S4 must emit electromagnetic energy in order to perform its
intended function. Nearby electronic equipment may be affected
Class A The S4 is suitable for use in all establishments other than
domestic and those directly connected to the public low-voltage
power supply network that supplies buildings used for domestic
Not Applicable
Not Applicable
purposes.
Guidance and Manufacturer’s Declaration: Electromagnetic Immunity
The equipment is intended for use in the electromagnetic environment specified in the table below. The customer or
the user of the equipment should ensure that it is used in such an environment.
Immunity Test IEC 60601 Test Level Compliance Level
Electrostatic
discharge (ESD)
EN 61000-4-2
Electrical fast
transient/burst
EN 61000-4-4
Surge
IEC 61000-4-5
Voltage
fluctuations and
Interruptions
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
+/- 6 kV contact
+/- 8 kV air
+/- 2 kV for
power supply lines
+/- 1 kV for
input/output lines
+/- 1 kV differential
mode
+/- 2 kV common mode
<5% UT for 0.5 cycles
40% UT for 5 cycles
70% UT for 25 cycles
<5% UT for 5s
3 A/m 3 A/m Power frequency magnetic fields should
+/- 6 kV contact
+/- 8 kV air
+/- 2 kV for
power supply lines
+/- 1 kV for
input/output lines
+/- 1 kV differential
mode
+/- 2 kV common
mode
<5% UT for 0.5 cycles
40% UT for 5 cycles
70% UT for 25 cycles
<5% UT for 5s
NOTE: UT is the AC Mains voltage prior to application of the test level.
Electromagnetic Environment:
Guidance
Floors should be wood, concrete, or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30%.
Mains power quality should be that of a
typical commercial or hospital
environment.
Mains power quality should be that of a
typical commercial or hospital
environment.
Note that monitoring is interrupted at the
level “< 5% UT for 5s”, but equipment
remains safe (as specified in
EN 60601-1-2).
be at levels characteristic of a typical
location in a typical commercial or
hospital environment.
18
ELECTROMAGNETIC COMPATABILITY (EMC)
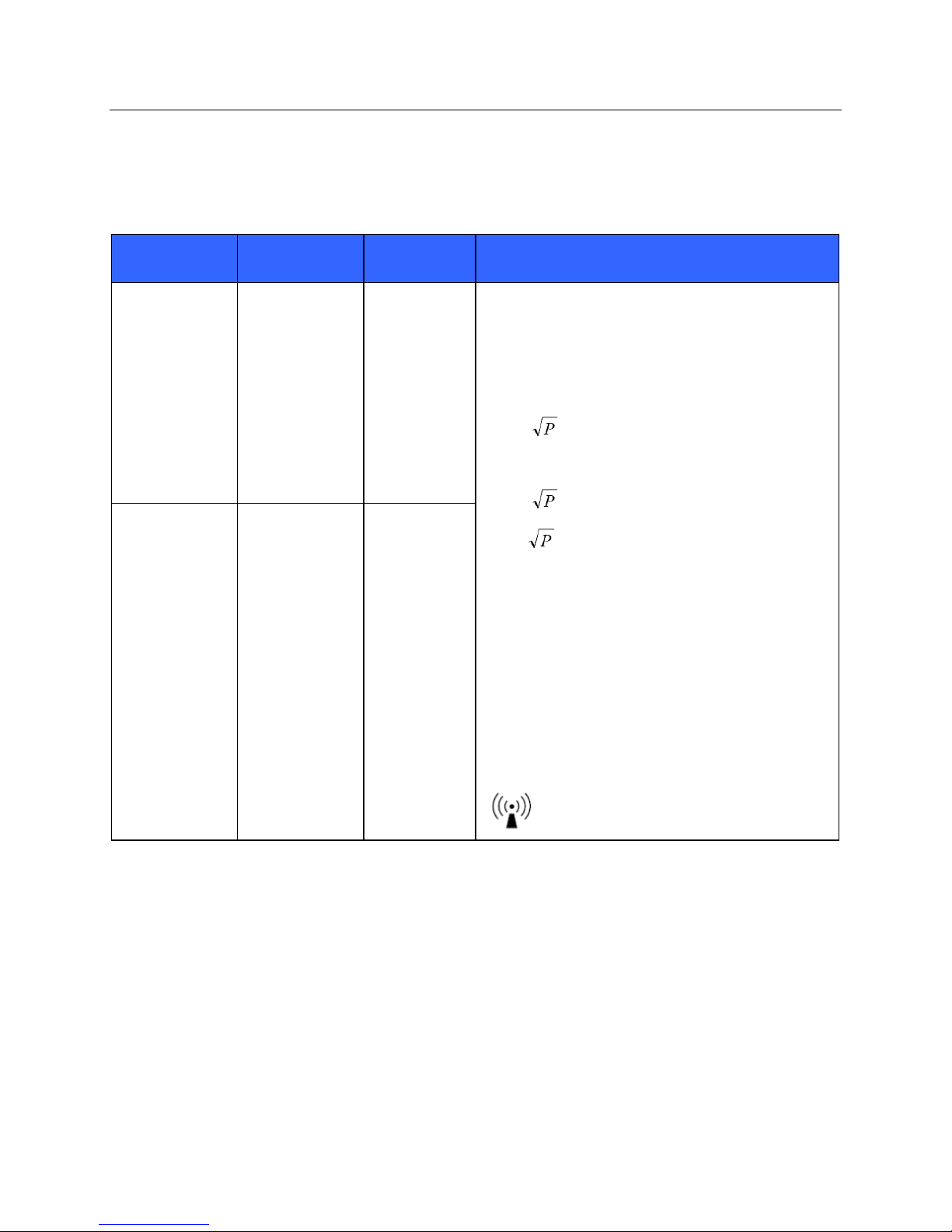
Guidance and Manufacturer’s Declaration: Electromagnetic Immunity
The equipment is intended for use in the electromagnetic environment specified in the table below. The customer or
the user of the equipment should ensure that it is used in such an environment.
Immunity Test
Conducted RF
EN 61000-4-6
Radiated RF
IEC 61000-4-3
IEC 60601 Test
Level
3 Vrms
150 kHz to
80 MHz
3 V/m
80 MHz to
2.5 GHz
Compliance
Level
3 Vrms
150 kHz to
80 MHz
3 V/m
80 MHz to
2.5 GHz
Electromagnetic Environment: Guidance
Portable and mobile RF communications equipment
should be used no closer to any part of the equipment,
including cables, than the recommended separation
distance calculated from the equation applicable to the
frequency of the transmitter.
Recommended separation distance
d = 1.2
d = 1.2 80 MHz to 800 MHz
d = 2.3 800 MHz to 2.5 GHz
Where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter
manufacturer and d is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site surveya, should
be less than the compliance level in each frequency
rangeb.
Interference may occur in the vicinity of equipment
marked with the following symbol:
a. Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land
mobile radios, amateur radios, AM and FM radio broadcast, and TV broadcast cannot be predicted theoretically
with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site
survey should be considered. If the measured field strength in the location in which the equipment is used
exceeds the applicable RF compliance level above, the equipment should be observed to verify normal operation.
If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the
equipment.
b. Over the frequency range 150 kHz to 80 MHz, field strengths should be less than [3] V/m.
19

ELECTROMAGNETIC COMPATABILITY (EMC)
Recommended Separation Distances Between Portable and Mobile RF
Communications Equipment and the Equipment
The equipment is intended for use in the electromagnetic environment in which radiated RF disturbances are
controlled. The customer or the user of the equipment can help to prevent electromagnetic interference by
maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the
equipment as recommended in the table below, according to the maximum output power of the communications
equipment.
Rated Maximum Output
Power of Transmitter (W)
150 KHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
0.01 0.12 m 0.12 m 0.23 m
0.1 0.38 m 0.38 m 0.73 m
1 1.2 m 1.2 m 2.3 m
10 3.8 m 3.8 m 7.3 m
100 12.0 m 12.0 m 23.0 m
Separation Distance According to Frequency of Transmitter (m)
d = 1.2 d = 1.2 d = 2.3
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in
meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the
maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures , o bj ect s, an d pe o pl e.
20

ELECTROMAGNETIC COMPATABILITY (EMC)
USA and Canada Radio Regulations
USA (FCC)
This device is equipped with Transmitter Module with FCC ID:RYYWYSAAVDX7.
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1) This device may not cause harmful interference, and
(2) This device must accept any interference received, including interference that may cause undesired operation.
CAUTION: Changes or modifications not expressly approved by the party responsible for compliance
could void the user’s authority to operate the equipment.
This device with transmitter module has been tested to SAR and complies with FCC exposure requirements for
portable devices. SAR testing has been done at a distance of 10 mm from the face and 0 mm from the body.
Canada (IC)
This device is equipped with Transmitter Module with IC:4389B-WYSAAVDX7.
This device complies with Industry Canada license-exempt RSS standard(s).
Operation is subject to the following two conditions:
(1) This device may not cause interference, and
(2) This device must accept any interference, including interference that may cause undesired operation of the
device.
This device with transmitter module has been tested to SAR and complies with IC exposure requirements for
portable devices. SAR testing has been done at a distance of 10 mm from the face and 0 mm from the body.
Cet appareil est équipé d'un module émetteur avec marque IC:4389B-WYSAAVDX7
L’appareil conforme aux CNR d'Industrie Canada applicables aux appareils rad io exempts de licence.
L'exploitation est autorisée aux deux conditions suivantes:
(1) l'appareil ne doit pas produire de brouillage, et
(2) l'appareil doit accepter tout brouillage radioélectrique subi, même si le brouillage est susceptible d'en
compromettre le fonctionnement.
Cet appareil avec module émetteur a été testé pour les taux d'absorption spécifique (DAS) et est conforme aux
normes d'exposition d'IC pour les appareils portables. Tests de DAS ont été réalisés à une distance de 10 mm du
visage et du corps 0 mm.
French Translation
21

ELECTROMAGNETIC COMPATABILITY (EMC)
22

p
8. INTRODUCTION
General Information
This user manual provides information for users of the Mortara Surveyor S4 mobile monitor, the Li-Ion Battery
charger, and its related components. It is written for clinical professionals expected to have a working knowledge of
medical procedures and terminology as required for monitoring cardiac patients.
The Surveyor S4 mobile monitor is a small, lightweight device designed to acquire an ECG and to transmit this data
to a Surveyor Central Monitoring station.
This user manual explains:
Relevant safety cautions, warnings and notices.
How to configure, use and maintain the S4 mobile monitor as well as its components and accessories.
How to change the device configuration to adapt to the hospital IT network environment.
How to properly acquire and transmit patient ECG signals to a Surveyor Central Station.
Please also refer to the Surveyor Central Station user manual for additional information.
NOTE: This manual may contain renderings of vari o us display screens. Any screen images are provided
for reference only and are not intended to convey actual operating techniques.
Indications For Use
The Surveyor S4 mobile monitor is indicated for use in adult & pediatric patient populations. The Mortara
Surveyor S4 mobile monitor facilitates the monitoring of ECG signals.
The Surveyor S4 mobile monitor is a prescription device intended to be used by knowledgeable healthcare
professionals within a healthcare facility or clinical pharmacology unit.
The Surveyor S4 mobile monitor is indicated for use in a clinical setting by a physician, or by trained personnel
acting on the orders of a licensed physician. It is not intended as a sole means of diagnosis.
The Surveyor S4 mobile monitor is indicated for use to acquire and output electrocardiographic data.
The Surveyor S4 mobile monitor is indicated for use as a radiofrequency physiological signal transceiver,
receiving and delivering real-time acquisition and transmission of simultaneous electrocardiographic data, while
allowing the patient to be ambulatory within the range of the antenna network.
The Mortara Li-ion battery charger is intended for charging only the Mortara rechargeable Li-ion battery pack.
WARNING: The Surveyor S4 mobile monitor by itself is not intended to be used as a vital signs
hysiological monitor.
CAUTION: The Surveyor S4 is has not been designed for direct cardiac applications involving direct
contact with a patient’s heart.
CAUTION: The Surveyor S4 has not been designed to be used together with electrosurgery equipment.
23

INTRODUCTION
CAUTION: The Mortara Li-ion Battery Charger and Battery Pack are not intended for unauthorized
batteries or chargers.
System Overview
The S4 mobile monitor provides a means to acquire and transmit simultaneous ECG data, with diagnostic quality, to
a Surveyor Central Station monitoring system while allowing the patient to be ambulatory within the range of the
antenna network.
The S4 uses three (3) AA alkaline batteries or one (1) rechargeable battery pack. The rechargeable battery may only
be charged by the Surveyor Battery Charger.
The following equipment is necessary to use the S4:
One (1) Rechargeable Li-Ion Battery Pack or three (3) AA batteries with battery tray.
Surveyor Central Station monitoring system.
Applicable patient cable, lead wires and electrodes.
Carrying pouches.
Customer provided 802.11 g/n 2.4 GHz wireless access points and network infrastructure.
Each S4 is factory-configured, pairing a Transmitter module with a Surveyor Acquisition Module (SAM). The
Transmitter module includes the display screen and the main processor of the S4. The SAM provides the clinical
parameter measurement capabilities. The S4 is available with the following SAMs:
SAM10
When configured with the SAM10, the S4 mobile monitor provides cardiac monitoring using one of the following
cable options:
LeadForm 5-wire ECG cable, or
LeadForm 10-wire ECG cable, which provides 12-lead ECG capability.
SAM5
When configured with the SAM5, the S4 mobile monitor provides cardiac monitoring using a shielded 3-wire or
5-wire ECG cable.
Refer to Reordering Accessories and Consumables
for part numbers of ECG cables compatible with the S4.
24

The following shows the S4 mobile monitor along with a 5-wire patient cable.
INTRODUCTION
25

INTRODUCTION
Note the following elements on the S4:
1. WiFi Network Connection Indicator LED
This blue LED will flash once every 5 seconds when the S4 is connected to the Surveyor Central station
and transmitting data.
If there are issues with connecting to the WiFi access point, this LED will flash twice a second.
2. Main Screen Display Area (LCD)
Screen allows for display of icons, menus, waveforms and other device, patient and status information.
The S4 uses a touchscreen user interface for operator interaction which allows control over the device via
selection of menu items and icons as well as entry of relevant configuration and patient information by
pressing on the screen.
The S4 provides haptic feedback by means of slight vibration of the device so as to confirm to the user data
entry via the touchscreen.
The S4 device has an embedded accelerometer which senses the position of the S4 display and will
automatically rotate the display 180 degrees to show the correct orientation depending on how it is held.
3. Nurse Call/Control Button
By pressing this button once, the patient can generate a call message on the Surveyor Central station. This
can be used as a nurse call or event marker function by the patient.
By pressing and holding this button, the operator can activate the LCD and choose to cancel the Patient
Call and subsequently interact with the device’s on-screen menus using the touchscreen.
When the LCD is on and in the main menu, pressing the button once will cause the LCD to be turned off.
Pressing the button when in the configuration menus will NOT turn off the display.
When the S4 is off with batteries installed (typically after a manual power off function), pressing this
button once turns on the device.
4. Speaker
Reserved for future functionality.
5. ECG Cable Connection
6. Power On Indicator LED
This green LED will flash when the S4 is on.
7. Battery Door
26

INTRODUCTION
Carrying Pouches
The S4 may be worn by the patient in a disposable pouch that is either tied to the patient using straps or attached to
the patient’s clothing via adhesive backing. The pouches are designed to fit the contours of the S4 and
accommodate extended wearing of the device while the patient is ambulatory or stationary. The transparent film
allows viewing of the screen and operation of the nurse call button.
CAUTION: Pouches are designed for single patient use, and should NOT be reused.
CAUTION: While the adhesive pouches are made of biocompatible material and contain no latex, they
should NOT be applied directly to the patient’s skin.
Stick-On Pouch
Ref #: 8485-030-51
Tie-On Pouch
Ref #: 8485-029-51
27

INTRODUCTION
Battery Charger
The Li-Ion Battery Charger charges up to five (5) rechargeable Li-Ion Batteries. The five bay Battery Charger is
powered by the AC Power Pack. To prepare the Li-Ion Battery Charger first, plug the AC Power Cord into the AC
Power Pack. Next, plug the AC Power Pack’s captive cable into the Li-Ion Battery Charger. Last, plug the AC
power cord’s AC connector into an active AC power source.
To charge up to five (5) rechargeable Li-Ion batteries, load a battery into an open battery bay. The battery fits
snugly in its charging bay in only one orientation. It snaps in place when slid down onto the charging contacts on
the battery bay.
As shown in the diagram below, the LEDs on the battery charger indicate the status of battery charging: green
indicates the battery is fully charged; orange indicates that the battery is charging; flashing orange indicates that
there is an error in battery charging. If the flashing orange persists, remove the battery, repower the battery charger
and reinsert the battery. If the orange LED continues to flash, check the battery and contact Mortara Technical
Support.
28

9. UNPACKING AND SET UP
Checking Contents
Your S4 is shipped with the following components:
Surveyor S4 Mobile Monitor configured with one of the following SAMs:
o SAM5 ECG
o SAM10 ECG
Li-ion rechargeable battery
AA battery tray (installed in the battery compartment of the S4)
CD-ROM containing the S4 User Manuals
The following optional S4 accessories are available separately:
Pouch Options:
o Disposable Tie-On Pouch
o Disposable Stick-On Pouch
Li-ion Battery Charger Components, including:
o Five (5) Bay Battery Charger
o AC Power Pack and Cord
29

UNPACKING AND SET UP
Battery Installation
NOTE: If using the S4 rechargeable Li-ion battery, ensure it is fully charged prior to first use.
The battery compartment is accessible via the removable battery door.
1. Remove the battery door by firmly pinching the grips located on each side of the door and remove.
2. Load the battery into the battery compartment:
If using the S4 rechargeable Li-ion battery:
a. Ensure the AA battery tray has been
removed from the battery compartment.
b. Align the battery above the metal contacts in
the compartment.
c. Insert the battery into the compartment and
slide to lock it mechanically in place.
Do not apply excessive force. The battery is
designed to lock in only when in the correct
orientation.
3. Replace the battery door. As shown below, position the hinged corners first, then rock the lid down until
the battery door locks snap the grips into place.
OR If using AA alkaline batteries:
a. Use of the AA battery tray is recommended.
Insert the tray into the battery compartment,
and slide to lock it into place.
b. Insert three (3) AA alkaline batteries,
aligning them with the positive (+) and
negative (-) indicators in the battery
compartment.
30

10. PATIENT PREPARATION FOR QUALITY ECG
Quality ECG Data Acquisition
Obtaining quality ECG data is important in continuous ECG monitoring. A quality ECG signal depends largely on
the patient prep and electrode placement. Good contact between the electrodes and the patient's skin and correct
placement of the electrode can help ensure obtaining quality ECG data.
A good quality ECG contains:
Clearly discernible P waves, QRS complexes, and T waves.
Steady, even, crisp baseline.
Absent of respiratory variability, artifact, noise, and other interference.
A good quality ECG may enhance the performance of the arrhythmia algorithm and may lessen false erroneous
alarm notifications.
A poor quality ECG may be caused by many factors:
Poor site preparation.
Poor electrode application or failure to refresh electrodes regularly.
Patient movement.
Interference by other equipment in the room.
Poor quality ECG becomes synonymous with artifact and interference in the ECG waveforms.
A poor quality ECG may manifest in several ways:
Fast baseline artifact.
Erratic baseline.
Sharp “spikes.”
Rolling, wandering waveforms as seen with patient breathing patterns.
Difficult to discern P waves or atrial fib waves from noise.
Inability to discern P waves, QRS complexes, T waves.
Artifact and interference in the ECG waveforms may be caused by using accessories, lead wires, and ECG cables
other than those specified to work with the S4. Always use accessories, lead wires, ECG cables, and other
accessories specified to work with the S4.
Skin Preparation
In continuous ECG monitoring, the goal of skin preparation is to minimize the contact resistance between the
patient’s skin and the ECG electrode. Follow the facility’s standard of care when preparing the patient’s skin for
ECG electrode placement and ECG monitoring.
To prepare the patient’s skin for electrode placement:
1. Explain the procedure to the patient.
2. Maintain patient privacy during skin prep and electrode placement.
3. Locate the correct anatomical landmarks for electrode placement.
4. Clip or shave excess hair in the areas marked for electrode placement.
5. Remove residual skin oils, creams, and lotions by gently abrading the skin with a small gauze pad.
NOTE: With elderly or frail patients take care to not abrade the skin causing discomfort or bruising.
Clinical discretion should alw ays be use d i n pat i e nt preparation.
31

Electrode Placement
To apply electrodes:
1. Use pre-gelled, Ag/AgCl disposable electrodes.
a. Do not use electrodes after their expiration date, or if the gel has dried out.
Store electrodes in an air tight container.
Electrodes dry out if not stored properly leading to loss of adhesion and conductivity.
b. Always use the same electrodes. Do not mix electrode brands or types. Using different types of
electrodes may cause baseline artifact and noise in the ECG tracing.
2. Apply the electrodes in the following manner:
a. Attach the electrode to the ECG lead wires prior to attaching the electrode to the patient’s chest.
b. Place the electrode in the properly prepared, correct location by using a circular motion on the
electrode adhesive area.
c. Gently press the electrode adhesive to the patient’s skin until the entire outer surface of the electrode is
adhered to the patient’s chest.
d. Once the electrode adhesive is attached, gently press on the gel area to ensure proper gel to chest
contact. Avoid dislodging the gel as the displaced gel can increase baseline artifact and noise in the
ECG tracing.
e. Test for firm electrode contact by slightly tugging on the electrode to check for adhesion among the
entire electrode surface. If the electrode moves freely, change the electrode. If the electrode does not
move easily, a good adhesive contact has been obtained.
PATIENT PREPARATION FOR QUALITY ECG
Refer to the Electrode Location section below for further details on correct anatomical landmarks for electrode
placement.
Best Practice Recommendation:
enhance patient skin care and the acquisition of quality ECG data. Clinical discretion should always be used in
patient preparation.
Change electrodes as per hospital standard of care, or at least every 24 hours to
Pacemaker Patients
The S4 contains special circuitry to detect pacemaker spikes up to 2 ms duration. The spike detection points are
transmitted with the ECG samples to the Surveyor Central for processing.
Electrode Locations for 12-Lead ECG
Your S4 should be already configured with the 12-lead, 10-wire Signal Acquisition Module (SAM) and the
12-lead LeadForm ECG cable set.
The LeadForm patient cable consists of lead wires and connector block that connects to the 12-lead SAM
module attached to the S4. Each lead wire terminates in a snap connector.
Before attaching electrodes, review this chapter carefully regarding skin preparation and electrode
placement. Attach electrodes per these recommendations.
Insert the ECG connector into the input connector on the 12-lead SAM of the S4.
32

PATIENT PREPARATION FOR QUALITY ECG
Using the LeadForm 12-Lead Cable
The S4 may be used for continuous simultaneous monitoring of 12 vectors of ECG. The S4 sends the 12-lead ECG
data to the Surveyor Central Station where Arrhythmia and ST analysis is performed. Refer to the Arrhythmia and
ST sections in the Surveyor Central user manual for further details.
The following diagram describes the recommended electrode placement for using the S4 in a continuous monitoring
mode.
IEC AHA Lead Placement
R (red) RA (white) Just below the right clavicle.
L (yellow) LA (black) Just below the left clavicle.
N (black) RL (green) On the sternum
F (green)* LL (red)* Lower left side of body, as close to hip as possible,
on the iliac crest (original Mason-Likar position) or
lowest rib on the left side of chest (modified Mason-
Likar position)
C1 (white) V1 (brown) 4th intercostal space, right sternal border.
C2 (yellow) V2 (yellow) 4th intercostal space, left sternal border.
C3 (green) V3 (green) Midway between C2/V2 and C4/V4.
C4 (brown) V4 (blue) 5th intercostal space, mid-clavicular line.
C5 (black) V5 (orange) Left anterior axillary line at C4/V4 level.
C6 (purple) V6 (purple) Mid-axillary line at C4/V4 and C5/V5 levels.
Electrode Locations: Continuous 12-Lead Monitoring
For accurate V-lead placement and monitoring, it is important to locate the 4
space is determined by first locating the 1
st
intercostal space.
th
intercostal space. The 4th intercostal
33

PATIENT PREPARATION FOR QUALITY ECG
Because patients vary with respect to body shape, it may be difficult to palpate the 1
accuracy. Thus, locate the 2
nd
intercostal space by first palpating the little bony prominence called the Angle of
Lewis, where the body of the sternum joins the manubrium.
This rise in the sternum identifies where the second rib is attached, and the space just below it is the 2
space. Palpate and count down the chest until the 4
th
intercostal space is located.
NOTE AND CAUTION: Placement of the Left Leg (LL)* electrode in the original Mason-Likar position
increases the similarity of the acquired ECG with a standard 12-lead ECG and is therefore recommended;
however, clothing may interfere with this position and increase the amount of artifact. The modified
position may decrease the sensitivity of inferior ECG leads and cause axis shift with respect to the standard
12-lead ECG. Accurate skin preparation and suitable clothing are the most important factors in excessive
artifact prevention.
NOTE: The Right Leg (RL) electrode may be positioned in any location least subject to motion artifact
according to clinician preference and specific patient requirements.
Electrode Locations for 5-Wire Cable
st
intercostal space with
nd
intercostal
5-Wire Lead Placement (AHA) 5-Wire Lead Placement (IEC)
Place the RA (white) electrode under the patient’s
right clavicle, at the midclavicular line within the rib
cage frame.
Place the LA (black) electrode under the patient’s
left clavicle, at the midclavicular line within the rib
cage frame.
Place the LL (red) electrode on the patient’s lower
left abdomen within the rib cage frame.
Place the RL (green) electrode on the patient’s lower
right abdomen within the rib cage frame.
Place the V (brown) electrode in one of the V-lead
positions (V1 – V6) depicted in the following
section.
Place the R (red) electrode under the patient’s right
clavicle, at the midclavicular line within the rib
cage frame.
Place the L (yellow) electrode under the patient’s
left clavicle, at the midclavicular line within the rib
cage frame.
Place the F (green) electrode on the patient’s lower
left abdomen within the rib cage frame.
Place the N (black) electrode on the patient’s lower
right abdomen within the rib cage frame.
Place the C (white) electrode in one of the C-lead
(C1 – C6) positions depicted in the following
section.
34

Electrode Locations for 3-Wire Cable
3-Wire Lead Placement (AHA) 3-Wire Lead Placement (IEC)
Place the RA (white) electrode under the patient’s
right clavicle, at the midclavicular line within the rib
cage frame.
Place the LA (black) electrode under the patient’s left
clavicle, at the midclavicular line within the rib cage
frame.
Place the LL (red) electrode on the patient’s lower
left abdomen within the rib cage frame.
Pacemaker Patients
PATIENT PREPARATION FOR QUALITY ECG
Place the R (red) electrode under the patient’s right
clavicle, at the midclavicular line within the rib
cage frame.
Place the L (yellow) electrode under the patient’s
left clavicle, at the midclavicular line within the rib
cage frame.
Place the F (green) electrode on the patient’s lower
left abdomen within the rib cage frame.
Pacemaker patients may require a modified electrode placement based on the physical location of the patient’s
pacemaker generator device. Do not place an ECG electrode directly over the pacemaker generator as this may lead
to artifact and noise on the ECG tracings.
Best Practice Recommendation
: Place the electrode patches 3 – 5 inches away from the pacemaker generator area.
For example, if the pacemaker generator is located in the left subclavian area, relocate the Left Arm electrode closer
in towards the center of the chest.
Use the following lead placement for monitoring a pacemaker patient using 5-wire cable:
5-Wire Lead Placement for Pacemaker Patients (AHA) 5-Wire Lead Placement for Pacemaker Patients (IEC)
35

PATIENT PREPARATION FOR QUALITY ECG
Checking ECG Electrode and Lead Wire Signal Quality
Once the patient has been properly prepared, the electrodes have been attached in the correct anatomical location,
and the patient ECG cable is connected to the S4, the S4’s ECG screen displays the patient’s ECG tracings.
Check to ensure the ECG tracing is free of artifact and noise with a clean ECG baseline as patient condition permits.
Refer to the Operation chapter of this user manual to check the quality and waveforms for each lead. If the ECG
contains artifact or noise, review the steps for proper electrode site preparation and placement and repeat these steps
to obtain proper ECG signals.
36

11. OPERATION
Powering On the S4
To power ON the S4, place batteries in the unit as described in Unpacking and Set Up.
The monitor will power up automatically.
The power and WiFi LEDs will illuminate and then flash.
The display will show the Mortara logo momentarily and then display the main screen.
Screen Time-Out & Reactivation
To preserve power and to protect against accidental activation of menus, the display will dim after
30 seconds of inactivity and will go blank after another 30 seconds.
To reactivate the display, press and hold the control button until the screen is activated. Press the on-screen
button to cancel the Nurse Call function and activate the main screen.
On Screen Quick Setup Guide
When the S4 first powers up, it may display an on screen, quick setup guide to assist in performing a
monitoring session.
The quick setup screen will only be displayed if the S4 detects an “All Leads Fail” condition implying that
no patient is connected.
To exit the quick setup guide, press the exit button:
37

E
OPERATION
Powering Off the S4
To power OFF the S4, you can either remove the batteries or access the shutdown menu from the settings menu*.
In the latter case, the shutdown menu also stops any active monitoring session on the Surveyor Central Station.
NOTE: Removing the battery when a monitoring session is active will generate an alarm at the Centra l
Station.
*NOTE: The shutdown menu is a protected function and requires the Administrative passcode.
WARNING: Powering off the monitor will terminate the monitoring of the patient and cease all alarms.
nsure that the patient is properly discharged or otherwise cared for prior to stopping the monitoring
session.
38

S
C
Main Screen
The following diagram shows the general layout of the S4’s main functional screens:
Device/Patient
tatus Area
ommand
Buttons
OPERATION
The top portion of the Main Screen is the Device/Patient Status Area. The remainder of the Main Screen shows one
of the following functional displays:
Patient Hookup display, which is used to guide the positioning of sensor on the patient, and provides the
operator with quick indication sensor connection quality,
Waveform display, showing traces from of the physiological parameters,
Parameter Numerics display, showing numeric values calculated from the physiological parameters,
Patient Demographics display, showing information about the currently associated patient, as well as
various command and option selection screens.
Command buttons appear along the bottom of the screen, as appropriate to each display screen.
39

p
M
OPERATION
Device/Patient Status Area
The top portion of the LCD screen shows status information regarding the current state of the device and the patient.
It automatically cycles between Device Status (1), and Patient Status displays (2a, 2b, or 2c) described below:
(1) Device Status
The following information is shown:
Lock Icon, showing whether configuration functions are accessible
WiFi signal strength indicator
battery status indicator
slot name, unit ID, and bed ID assigned to this S4
current monitoring status, and
current time of day
(2a) Patient Status – Patient Monitoring Active
The Status Area shows the current time of day and the patient’s name or
ID, depending on settings.
(2b) Patient Status – Blind Session Active
When patient demographics are not available for a monitoring session
the Status Area displays “[Blind Session]” in place of the patient’s
name/ID.
(2c) Patient Status – Unable to Connect
A red background in the Status Area with the message “Trying to
connect to Central…” indicates that the S4 is not connected to the
Central Station and is attempting to establish a connection.
WARNING: The phrase “EMULATION ACTIVE” in the Status Area indicates that the S4 is not acquiring
hysiological signals from the patient but is generating sample data from an internal source. Contact your
ortara representative to have your S4 reconfigured for use with patients.
Lock Icon
Certain configuration functions of the S4 are not directly accessible to the operator, except when a passcode has
been entered. The lock icon indicates whether the unit is locked and requires the correct passcode to access these
locked configuration functions. Once the passcode has been entered, this icon changes to the unlocked icon that
depicts the passcode has been correctly entered and the configuration functions are accessible. The device will
relock when the LCD turns off either automatically or manually.
Locked – Configuration functions are not currently accessible. A passcode is required to access
them.
Unlocked – Configuration functions are currently accessible. Once unlocked, the configuration
functions are accessible only until the LCD display is turned off, either manually by command, or
automatically by timeout.
The S4 is factory shipped with passcode 7865, but may be changed as described in Configuring the S4
40
.

OPERATION
Time of Day
The current time of day is displayed on the top of the status area. The current date and time is synchronized between
the S4 and the Central Station. The time information is maintained by the S4 even if the connection between the
Central Station is disrupted or the battery removed. The time information is resynchronized on the next connection
to the Central Station.
Battery Level Indicator
The battery level indicator in the Device/Patient Status Area displays one of the following symbols depending on the
status of the S4’s battery:
Central Station Slot Name + Unit ID & Bed ID
The slot name is provided by the Central Station once the S4 device has established communication. The Unit ID
and Bed ID are configuration settings which define the monitoring slot on the Central Station.
Monitoring Status
Monitoring statuses are:
SUSPENDED – The S4 is currently not monitoring a patient, but the monitoring slot is still associated with
a patient; the patient name or ID is alternated with the status.
MONITORING – The S4 is currently monitoring a patient and data is transmitting to the Central Station;
the patient name or ID is alternated with the status.
DISCHARGED – The previously monitored patient is discharged and the S4 is now available to start a new
monitoring session for a new patient.
The battery is fully charged.
The battery is partially charged.
Low Battery – There are 30 minutes or less of battery power available. A low battery status is
also communicated to the Central Station.
Critically Low Battery – There is 1 minute or less of battery power available, after which the
monitoring will be suspended, and the S4 will power off.
41

OPERATION
Patient Hookup Display & Lead Quality Indicators
The displayed picture of the patient torso is an aid in determining the exact positioning of the ECG leads.
The hookup display also allows the operator to ensure quality electrode connection.
The connection quality between the ECG electrode and patient skin is noted by the color of the small circle
positioned on the torso shown on the screen. Green indicates a good quality connection; red indicates bad quality or
no connection. Note that the number of electrode locations will change depending on the type of cable defined or
detected.
All leads OK All leads disconnected
V6 lead in failure
Tapping on any location of the picture of the torso will bring up the patient demographics window.
42

OPERATION
Patient Demographics Screen
The Patient Demographics Screen displays information identifying the current patient, including name, ID, date of
birth (DOB), age, and gender. The information is obtained from the Surveyor Central Station, and is not modifiable
on the S4.
Selecting the “Patient ID” button will cause the patient ID to be displayed in the status area. Selecting “Patient
Patient Demographics Display
Waveform Review
Pressing the waveforms review icon in the main menu displays all available waveforms.
AllWaveformDisplay
All leads, including V-leads are displayed as bipolar leads in order to facilitate the culprit electrode generating
artifact. If the SAME artifact is shown on all waveforms, then the culprit electrode is the Right Arm electrode. If the
artifact is showing up in one lead, for instance LL-RA, the culprit is the lead-specific electrode, in this case LL.
NOTE: Waveform display on the S4 is meant for lead quality evaluation only, not for diagnostic purposes.
43

OPERATION
Pressing anywhere on the waveforms displays the first set of waveforms. If 10-wire, 12-lead monitoring cables are
being used, additional presses will display the remaining sets of waveforms. For 10-wire.
First Set of Waveforms Display Second Set of Waveforms Display
Third Set of Waveform Displays
As depicted below, one or more waveforms are shown as a square wave if the patient connection is not properly in
place. When all leads are in failure mode, the “ALL FAIL” message is shown. Otherwise, the name of the leads in
failure are specifically shown.
44

OPERATION
All Leads in Failure V6 in Failure
45

OPERATION
Monitoring Menus
When initially powered on, the S4 will attempt to connect to the Central Station. Once a connection has been
established, ECG monitoring can begin.
To start monitoring, press the monitoring start icon (1) as shown below.
Main Screen with Monitoring Start Icon
In the monitoring screen, there are a number of choices:
1) Return to the main screen.
2) Start monitoring the same patient as before.
3) Monitor a new patient with a new profile.
NOTE: If the monitoring session is started from the Surveyor Central while this screen is open, the S4 may
still display these options until this screen is closed by pressing any of the ico ns or pr essing the control
button. In such cases, the status area will correctly display that the S4 is actively monitoring a patient.
Monitoring Screen Options
46

Select same patient to begin monitoring the previously
monitored patient. In this case, the patient name and
demographics are displayed as shown below and
confirmation requested before monitoring begins.
OPERATION
Monitor Same Patient
When the device is restarted without previously
discharging a patient (e.g., perhaps due to changing of
batteries), the S4 will automatically reconnect to the
Central Station and resume monitoring the previous
patient.
When monitoring a new patient, patient demographics
for that patient are undefined until and unless patient
information is defined on the Central Station by creating
or importing a patient for the currently monitored slot.
Sessions not associated with a particular patient are
referred to as “[Blind Session]” and shown as such on
the S4’s status area.
Main Screen When Monitoring
47

To start monitoring a new patient, you first have to
select a monitoring profile. Profiles are defined at the
Central Station and include alarm limits and other
settings appropriate for a type of patient. The “_factory”
profile is the default profile shipped with the Central
Station. Other profiles can be defined by the Central
Station administrator. Profiles are communicated from
the Central Station to the S4 and are available as a list of
drop-down menu items at the new patient monitoring
screen.
OPERATION
Monitor New Patient
Once monitoring of a new patient begins, patient demographics for that patient are undefined until and unless patient
information is defined on the Central Station by creating or importing a patient for the currently monitored slot.
Sessions not associated with a particular patient are referred to as “[Blind Session]” and shown as such on the S4’s
status area.
NOTE: It is not possible to define or update patient demographics on the S4. Patient demographics must
be defined and updated on the Central Stati on.
48

OPERATION
Heart Rate Display
When the S4 is in monitoring mode, it is possible to display the patient’s heart rate by pressing the numeric icon (1)
as shown below.
Heart Rate Numerics Icon
This will show the patient’s current heart rate as reported by the Central Station.
NOTE: By design, no other parameters or alarms are shown on the S4’s display. Refer to the Central
Station user manual for relevant parameters, alarm s an d other information available there.
To return to the main screen, press the main screen icon with the patient hookup icon (1) shown below.
Main Screen Patient Hookup Icon
49

OPERATION
Printing
During a monitoring session, it is possible to print a report directly on the Central Station from the S4. To do this,
select the waveforms display icon (1) and then the print icon (2) as shown below. When using specified LeadForm
cables with the SAM10, the printed report is a 12-lead ECG. When using the shielded 3-wire or 5-wire cable with
the SAM5, the report is a rhythm report.
Select the waveforms icon Select the print icon
The “Print in progress…” message is displayed and the
print icon is unavailable until the printing process is
complete.
Print in Process Screen
50

Toolkit
To access special functions and configuration of the S4, tap on the toolkit icon (1) shown below.
Toolkit Icon
OPERATION
In this screen, it is possible to:
1) Adjust the waveform gain.
2) Adjust the waveform speed.
3) Return to the main menu.
4) Set or change configuration.
5) Suspend monitoring and power off.
Toolkit Screen
51

Additionally, in the setup screen it is also possible to
define:
If 3-wire cable has been detected, define the
lead to be monitored, whether Lead I, Lead II
or Lead II.
If SAM10 module has been detected, allow the
user to select 5-wire or 10-wire monitoring
depending on the type of LeadForm cable being
used.
OPERATION
52

Waveform Gain (Amplitude)
OPERATION
The waveform gain can be set to 1/2x, 1X or 2X.
Waveform Speed
The waveform speed can be set to slow, normal or fast.
Waveform Gain Screen
Waveform Speed Screen
NOTE: Waveform gain and speed are not in a particular scale such as mm/mV and only representative.
Waveforms displayed on the S4 LCD display are for lead quality check purposes and not intended for
diagnostic measurements. Refer to the Central Station and its user manual for scaled display and
measurement of ECG waveforms.
53

Power Off – Suspend Monitoring
Suspend monitoring and turn off the S4 by pressing the suspend monitoring icon (1) as shown below.
Power Off Icon
OPERATION
54

Suspending an active monitoring session is a passwordprotected function which requires the entry of the
administrative passcode. The S4 is factory shipped with
passcode 7865. Refer to your facility’s administrator for
additional information.
If the wrong passcode is entered, the “Unlock failed”
message is displayed and the correct passcode must be
entered for access to this screen. Alternatively, this
operation may be cancelled.
OPERATION
Passcode Entry Screen
55

It is highly recommended to suspend monitoring in this
way instead of removing the battery, to avoid a “No
patient monitoring” nuisance alarm on the Central
Station. The Central Station will stop displaying data for
this patient, but the patient slot will still remain
associated with the patient. Monitoring can be resumed
with the same patient later, if needed. The S4 will turn
off after having completed the “suspend monitoring”
action. This action requires confirmation as shown.
OPERATION
Power Off Confirmation Screen
CAUTION: Powering off the S4 during an active monitoring session that is a “blind session” will end the
session and will not save
the monitored data on the Central Station.
56

12. CONFIGURING THE S4
This chapter describes how to configure the S4 including changing the settings during the operation as well as
general configuration for setup of the S4.
Toolkit Screen
Select the configuration icon on the toolkit screen to
access the configuration settings of the S4.
NOTE: This particular screen is a passwordprotected function and only available through a
passcode. The S4 is factory shipped with passcode
7865. Refer to your facility’s administrator for
additional information.
Upon entry of the passcode, the menus for this screen
will be available. If the wrong passcode is entered, the
“Unlock failed” message is displayed and the correct
passcode must be entered to enter this screen.
Alternatively, this operation may be cancelled.
Passcode Entry Screen
57

Upon successful entry of the passcode, the S4 is
unlocked and the configuration settings screen is
displayed as shown.
NOTE: The device will remain in this unlocked
state (as shown by the unlock icon in the main
screen’s status area) until the monitor’s display
turns off again.
NOTE: The configuration settings screen is
displayed on the S4 only in landscape mode.
NOTE: Configuration changes cannot be saved
while an active monitoring session is in progress.
CONFIGURING THE S4
Configuration Settings Screen
Host Settings
The settings for the Central Station including its IP
address, and the unit and bed ID for this particular S4, as
well as base IP port and IP port number are defined here.
If you are uncertain about these settings, please consult
Mortara or your IT Network Administrator.
Host Settings Screen
58

CONFIGURING THE S4
Network Settings
The network settings of the S4 are confgured as shown below.
Network Settings Screen
Available settings include:
IP Address Method: Whether dynamically assigned by the network through a DHCP service or manually
assigned here to a static address. If “Manual” is selected, the IP address, subnet mask and gateway address
must be defined.
WiFi Network Security: The S4 supports any of WPA-PSK-AES, WPA-PSK-TKIP, WPA2-PSK-AES, or
WPA2-PSK-TKIP authentication and encryption methods.
SSID & Passkey: The SSID and Passkey of the WiFi network that will be used for telemetry transmission.
The passkey may be entered as text or hexadecimal digits.
If you are uncertain about these settings, please consult Mortara or your IT Network Administrator.
Language Settings
The S4’s user interface supports various languages
which can be set from this screen.
Language Settings Screen
59

WiFi Diagnostics
CONFIGURING THE S4
The WiFi Diagnostics screen is for assessing the
network interface including confirming network settings,
determining signal strength and using a PING tool to test
the connection to the Central Station. The IP address of
the Central Station defined in the host settings is
automatically populated. Another address may be
manually entered to test alternative IP address as
necessary.
In case of persistent network problems, please see the Troubleshooting chapter and if necessary, contact Mortara or
your IT Network Administrator.
WiFi Diagnostics Screen
Reset Passcode
Use this menu to change the passcode used for
administrative access to this S4. The entered passcode
must be 2 to 5 numeric characters.
Passcode Reset Screen
NOTE: Changing the administrative passcode on a S4 affects only that particular mobile monitor. Other
S4 in your location need to be updated individually.
NOTE: Ensure you safekeep the administrative passcode for the S4. If this information is lost or
forgotten, you may not be able to access the configurat i o n se t t i ngs of the S 4 and w o ul d be required to
return the device to Mortara for authorized service.
Hardware Diagnostics
This hardware diagnostics screen provides an easy
method for service personnel to check the basic
functionality of the device including the built-in
accelerometer, touch screen, general-purpose
input/output as well as analog-to-digial converters plus
battery voltage level and charging status.
HW Diagnostics Screen
60

S4 Version
CONFIGURING THE S4
This screen displays the current version of the S4
software as well as the device serial number. In case of
problems or issues, it may be necessary to share this
information with Mortara.
SAM Version
This screen displays the current version of the Signal
Acquisition Module (SAM) including module number
and part number, as well as hardware and software
version plus serial number. In case of problems or
issues, it may be necessary to share this information with
Mortara.
S4 Version Screen
SAM Version Screen
61

CONFIGURING THE S4
62

13. PERFORMING ECG MONITORING
To perform ECG monitoring on a patient using the S4 and Surveyor Central Station:
Power-On
1. Insert freshly charged rechargeable or brand new disposable batteries
in the device so it powers on.
Patient Prep
2. Prep the patient and place electrodes per clinical guidelines and
provided instructions.
Patient Cable Hookup
3. Attach the patient cable to the electrodes on the one end, and to the
S4 on the other end. The example here demonstrates the 10-wire
LeadForm cable; use the hook-up appropriate to your available
patient cable.
63

Confirm Good Signal
4. Confirm that all leads are OK (i.e., green dots in the electrode
placements as shown on the LCD). Select the waveforms icon to view
and confirm good signal on all leads.
PERFORMING ECG MONITORING
New Patient Monitoring
5. Select the start monitoring icon.
To start monitoring a new patient, select the new patient icon, confirm a profile and select OK. Confirm that the
status is indicated as “Monitoring” and the session labeled as a “[Blind Session]”.
To define patient demographics, create patient demographics or import patient demographics for the slot
associated with this telemetry session.
64

PERFORMING ECG MONITORING
Same Patient Monitoring
6. To resume monitoring the previous patient, select same patient and confirm the patient name and press OK.
Confirm that the status is indicated as “Monitoring” and the session labeled with the correct patient
demographics.
Confirm Monitoring Active
7. Confirm that the monitoring session is active on the Central Station or by confirming the heart rate shown on the
S4 display.
65

PERFORMING ECG MONITORING
Ending a Monitoring Session
NOTE: Monitoring sessions are typically managed at the Central Station and ended there though it can be
done on the S4 by removing the battery or by following the Ending a Monitoring Session procedure.
8. To end a monitoring session, press and hold the patient call button until the LCD activates. Press the
“Abort Call and resume” icon on the display to cancel the nurse call and activate the screen.
Select the settings icon.
Press the power down icon.
66

Enter the administrative passcode.
Press OK to confirm.
The S4 will turn off (including the
LCD and indicator LED’s) and stop
monitoring the patient. This will also
dismiss the patient on the Central
Station.
PERFORMING ECG MONITORING
67

PERFORMING ECG MONITORING
68

14. PRODUCT SPECIFICATIONS
General Specifications
Instrument Type ECG digital mobile monitor
Input Channels
ECG Leads Transmitted
Device Classification Type CF, battery operated
Frequency Range
Special Functions
Defibrillator Protection Complies with IEC 60601-2-25 (with 10-wire cable) and/or IEC 60601-2-27
Function Keys Control button; touchscreen menu navigation
Continuous 12-lead signal acquisition and transmission with 10-wire LeadForm cable
Continuous 7-lead signal acquisition and transmission with 5-wire cables
10-wire: I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5 and V6
5-wire: I, II, III, aVR, aVL, aVF, V
3-wire: I, II or III
2400.96 MHz to 2482.56 MHz
Lead impedance check, ECG display, lead fail, battery notification, multi-purpose
call; 10-wire
Environmental Conditions
Temperature
Humidity
Operating temperature: +10° to +42° C (+50° to +108° F)
Storage temperature: -40° to +70° C (-40° to +158° F)
Operating humidity: 10% to 95% RH, non-condensing
Storage humidity: 10% to 95% RH, non-condensing
Altitude Operating & Storage: 54 kPa to 106 kPa
Cooling Convection (no fan)
Weight 300g with rechargeable battery pack
Dimensions 5.5” x 3.1” x 1.3” (139 x 79 x 33 mm)
Power Requirements & Battery
Disposable Battery Type Three (3) AA alkaline batteries
Disposable Battery Run-Time 12 hours
Rechargeable Battery Type Rechargeable Lithium-ion battery pack
Rechargeable Battery Run-Time 24 hours
Battery Charging Time 8 hours
69

Display Specifications
PRODUCT SPECIFICATIONS
Type
Size & Resolution 4 inches (10 cm) diagonal; active area; 480 x 800 pixels
High definition, antiglare Color TFT-LCD with LED backlight and resistive touch
panel controls
ECG Specifications
ECG
Simultaneous Leads Available
Acquisition Rate: 40,000 samples/s. for pacemaker detection, 500 samples/s. for analysis
Resolution: .117µV originally, reduced to 2.5 µV for processing and transmission
Dynamic Range ± 350mV
ECG Gain ½x, 1x, 2x
Trace Speed slow, normal, fast
CMRR: According to applicable standards (IEC 60601-2-25 and/or IEC 60601-2-27)
Pacemaker Spikes Detect and report pacemaker spikes up to 2 ms duration with 1 samples precision
Max. Auxiliary Patient
Current:
12-lead ECG with specific Signal Acquisition Module (SAM10)
7-lead ECG with specific Signal Acquisition Module (SAM 5)
10-wire: I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, V6
5-wire: I, II, III, aVR, aVL, aVF, V
3-wire: I, II or III
< 10 µA
Frequency Response: 0.05 to 150 Hz
Input Impedance: >2.5 MΩ at 10 Hz
Electrodes: Compatible with Ag/AgCl disposable electrodes
HR Report from the Central
Station
Minimum QRS Amplitude: 300 µV
Recovery from Defibrillation
Discharge
Less than one second. Heart rate is only available when the S4 is actively
communicating with the Central Station.
Less than 5 seconds, compliant with IEC 60601-2-25 and/or IEC 60601-2-27.
70

PRODUCT SPECIFICATIONS
Wireless Network Specifications
Wireless protocol 802.11 g/n 2.4GHz
Disable 802.11b support on WLAN infrastructure.
If 802.11b support is required, do not specify any 802.11b data rate as mandatory.
802.11g supported rates 6, 9, 12, 18, 24, 36, 48, 54 Mbps
802.11n supported rates MCS0 through MCS7
6.5, 13, 19.5, 26, 39, 52, 58.5, 65 Mbps
Transmit Power 15 dBm
Configure WLAN APs maximum transmitting power to 15 dBm
Channels 1 to 13 supported.
Need to configure used channels on devices prior to installation (default is 1, 6, 11)
If dynamic channel selection on WLAN infrastructure is enabled, channels change
will cause short data gaps. Reduce the number of channel changes per day.
Wireless security protocols WPA2-PSK-AES
WPA2-PSK-TKIP (supported but not recommended)
WPA-PSK-AES(supported but not recommended)
WPA-PSK-TKIP (supported but not recommended)
Passphrase or HEX psk
WMM / QoS Yes
Device uses class AC_VI (Video)
SSID Requirements Dedicated SSID is required. Hidden SSID not supported.
SSID can be up to 32 ASCII characters long, per 802.11.
SSID prioritization and minimum bandwidth configuration on WLAN infrastructure is
recommended.
Period Session Timeout or similar policies should be disabled or excluded in the SSID
dedicated to the S4: these policies might cause data gaps of a few seconds whenever
the AP terminates the session.
Dedicated Monitoring VLAN is recommended.
IP address assignment Static or DHCP
IP protocols and ports UDP: one port per device, according to the system configuration (i.e. 20100, 20101,
20102, etc…). Actual ports will be communicated upon definition of the system
configuration;
SSH
ICMP and mDNS for management and service
Maximum devices per Access
16
Point
Data Rate (payload)
Outbound: ~50 - 150 Kbps (depending on cable and network load); Inbound: <
16 Kbps
Minimum RSSI / SNR -75 dBm in the coverage area
Minimum SNR 20 dB in the coverage area
71

PRODUCT SPECIFICATIONS
72

15. TROUBLESHOOTING & ERROR CODES
The following table provides guidance for investigating issues that may occur during operation of the S4. Contact
Mortara Technical Support and Service by calling 1-888-MORTARA (US & Canada) or +1-414-354-1600
(Worldwide) or via techsupport@mortara.com for further assistance.
Power and Battery
Symptom Possible Causes Suggested Resolution
Power cycle the S4 with fresh batteries and try
The S4 is not working
and display does not
light up.
Internal system failure.
Battery will not hold charge.
Display and Touchscreen
Symptom Possible Causes Suggested Resolution
again. If problems persist, stop using the S4 and
contact Mortara Technical Support.
Replace the battery according to instructions in
the General Care & Maintenance chapter.
The touchscreen is not
working properly.
The display is not
working properly.
Touchscreen failure.
Display failure.
Power cycle the S4 and try again. If problems
persist, stop using the S4 and contact Mortara
Technical Support.
Power cycle the S4 and try again. If problems
persist, stop using the S4 and contact Mortara
Technical Support.
ECG Trace
Symptom Possible Causes Suggested Resolution
ECG signal is noisy.
Excessive patient movement.
Electrical noise from auxiliary
equipment.
Bad electrode contact.
Confirm electrode site preparation; confirm correct
ECG positioning, move electrodes if needed.
Isolate the patient from auxiliary equipment, if
possible.
Check to make sure electrodes are still securely
attached to the patient, and reattach if necessary.
Remember the importance of good skin
preparation techniques.
73

Network Transmission
Symptom Possible Causes Suggested Resolution
1. "No ECG Monitoring"
alarm.
2. “Trying to connect to
Central" error
message displayed.
3. X symbol on WiFi
signal indicator.
4. Fast blinking blue
LED.
5. "AP MAC: Not-
Associated" in the
WiFi diagnostic
screen.
6. Invalid IP address if
configured for DHCP.
7. Inability to ping in
WiFi diagnostic
screen.
1. "No ECG
Monitoring" alarm
and no traces on the
Central Station.
2. “Trying to connect to
Central" error
message displayed.
3. Normal symbol on
WiFi signal indicator.
4. Slow blinking blue
LED.
5. Network info
correctly populated
in WiFi diagnostic
screen.
6. Invalid IP address if
configured for
DHCP.
7. Inability to ping in
WiFi diagnostic
screen.
Incorrect WLAN configuration
(e.g., SSID, Security protocol,
Passkey, Passkey type).
WLAN infrastructure issues
(e.g. device placed on black
list).
Incorrect LAN configuration
(Method, IP, Subnet,
Gateway).
LAN infrastructure issues (e.g.
DHCP server is not providing
an IP address).
Check WiFi network settings; contact your IT
Network Administrator to correct issues.
Check WiFi network settings; contact your IT
Network Administrator to correct issues.
Check LAN network settings; contact your IT
Network Administrator to correct issues.
Check LAN IPv4 network settings; contact your IT
Network Administrator to correct issues.
TROUBLESHOOTING & ERROR CODES
74

Symptom Possible Causes Suggested Resolution
TROUBLESHOOTING & ERROR CODES
1. "No ECG Monitoring"
alarm and no traces
on the Central
Station.
2. “Trying to connect
to Central" error
message displayed.
3. Normal symbol on
WiFi signal indicator.
4. Slow blinking blue
LED.
5. Network info correctly
populated in WiFi
diagnostic screen.
6. Valid IP address if
configured for DHCP.
7. Ping successful for
some IP addresses in
WiFi diagnostic
screen.
Any of the following:
1. Intermittent traces on
Central Station,
intermittent "No ECG
Monitoring" alarm.
2. Intermittent "Trying to
connect to Central"
error in top bar.
3. X or low signal symbol
on WiFi signal
indicator.
4. Intermittent fast
blinking blue LED.
5. Intermittent "AP MAC:
Not-Associated" in
WiFi diagnostic
screen, low signal,
high noise, low quality
indexes.
6. Intermittent Valid IP
address if configured
for DHCP.
7. Inconsistent Ping
results in WiFi
diagnostic screen.
Incorrect Host/Central
stations configuration.
Mismatch between Unit/Bed
ID and Base Port
configuration on S4 and
Central stations.
Surveyor Central Station is
not running.
Security router not correctly
configured.
LAN infrastructure issues (i.e.
ports not routed to Security
router, disconnected cables).
Poor WLAN network
coverage.
Check S4 Host settings and/or Central Station
configuration.
Check S4 Host settings and/or Central Station
configuration.
Check Central Station configuration.
Check Security router configuration and status.
Check LAN IPv4 network settings; contact your IT
Network Administrator to correct issues.
Check IT Network Administrator to correct issues.
75

Error Messages
Message Possible Causes Suggested Reso lution
TROUBLESHOOTING & ERROR CODES
Trying to connect to
Central...
The configured slot is
already used by
another device.
Unlock failed. Incorrect passcode used. Enter the correct passcode.
Enter New Passcode. Mismatch of passcodes entered.
ECG Frontend
calibration failed,
restart the device. If
the problem persists
contact service.
Unable to open ECG
Frontend, restart the
device. If the problem
persists contact
service.
Not able to connect to the
Surveyor Central Station.
Selected slot for the Surveyor
Central Station is currently in use.
Calibration of the ECG frontend
failed.
Interface to the ECG frontend
failed.
Check network settings and access of WiFi
network. Also refer to the Network Transmission
items above.
Select a different slot for this monitor.
Ensure that the configuration passcode entered as
well as the confirmation passcode values match.
Power the system on and off. Contact Mortara if
the problem persists.
Power the system on and off. Contact Mortara if
the problem persists.
76

16. REORDERING ACCESSORIES & CONSUMABLES
Use the following Mortara part numbers to obtain spare parts or to reorder accessories:
Description Part Numbers
LI-ION BATTERY PACK 4800-018
TIE-ON DISPOSABLE POUCH - BOX 100** 8485-029-51
STICK-ON DISPOSABLE POUCH - BOX 100**
PAT CBL T12x 10 WIRE AHA SNAPS
PAT CBL T12x 10 WIRE IEC SNAPS
PAT CBL T12x 10 WIRE LARGE AHA SNAPS
PAT CBL T12x 10 WIRE LARGE IEC SNAPS
ECG LEAD SET 5 WIRE CLIP ENDS AHA GRAY
ECG LEAD SET 5 WIRE CLIP ENDS IEC GRAY
ECG LEAD SET 5 WIRE SNAP ENDS AHA GRAY
ECG LEAD SET 5 WIRE SNAP ENDS IEC GRAY
ECG LEAD SET 3 WIRE CLIP ENDS AHA GRAY
ECG LEAD SET 3 WIRE CLIP ENDS IEC GRAY
ECG LEAD SET 3 WIRE SNAP ENDS AHA GRAY
ECG LEAD SET 3 WIRE SNAP ENDS IEC GRAY
HOOKUP KIT MONITORING 10E SINGLE** 9294-009-50
S4 USER MANUAL 9515-190-50-CD
KIT, CHARGER, LI-ION-5, US/CAN 41000-035-50
KIT, CHARGER, LI-ION-5, INTN'L 41000-035-51
8485-030-51
9293-044-50
9293-044-51
9293-044-60
9293-044-61
9293-059-60
9293-059-61
9293-059-62
9293-059-63
9293-059-50
9293-059-51
9293-029-52
9293-059-53
KIT, CHARGER, LI-ION-5, AUSTRALIA 41000-035-52
KIT, CHARGER, LI-ION-5, UK 41000-035-53
AC POWER SUPPLY, FOR CHARGER KIT 4101-012
CONVENIENCE TRAY, FOR AA ALKALINE BATTERIES 8364-005-50
ELECTRODES, BOX OF 10 ** 9300-032-52
ELECTRODES, CASE OF 300 ** 9300-032-50
* Technical Service Installation Required.
** This item is intended for single patient use. Items warranted to be free of defects in workmanship and materials
for a period of 90 days or first use, whichever comes first.
To order additional supplies, contact your Mortara Customer Service Representative.
77

REORDERING ACCESSORIES & CONSUMABLES
78

17. APPLICABLE STANDARDS
IEC 60601-1:2005 Ed:3 Medical electrical equipment Part 1: General requirements for basic safety and essential
performance.
European Union: CENELEC EN60601-1:2006 Medical Electrical Equipment Part 1: General Requirements for
Basic Safety and Essential Performance.
USA: AAMI ES60601-1:2005 Medical electrical equipment Part 1: General Requirements for Basic Safety and
Essential Performance.
Canada: CSA C22.2#60601-1:2008 Ed:3 Medical Electrical Equipment - Part 1: General requirements for basic
Safety and essential performance.
IEC 60601-1-2:2007 Medical Electrical Equipment – Part 1-2: General requirements for safety – Collateral Standard:
Electromagnetic Compatibility.
EN 60601-1-2:2007 Medical Electrical Equipment – Part 1-2: General requirements for safety – Collateral
Standard: Electromagnetic Compatibility – European Union National Differences.
IEC 60601-1-6:2010-01 Edition 3.0, Medical Electrical Equipment – Part 1-6: General Requirements for Safety and
Essential Performance – Collateral Standard: Usability.
IEC 60601-2-25:2011-10 Edition 2.0, Medical Electrical Equipment. Part 2-25: Particular requirements for the basic
safety and essential requirements of electrocardiographs.
(Notes: Applies to 10-wire acquisition module only; when used in conjunction with a Central Station.)
IEC 60601-2-27:2011-08 Edition 3.0, Medical Electrical Equipment. Part 2-27: Particular requirements for the basic
safety and essential performance of electrocardiographic monitoring equipment.
(Notes: Applies to 5-wire and 10-wire acquisition modules; when used in conjunction with a Central Station.)
IEC 60529:2001-02, Degrees of protection provided by enclosures (IP Code), Edition 2.1.
IEC 62366:2007-10 Edition 1.0, Medical devices – Application of usability engineering to medical devices.
ETSI EN 301 489 –1:2011-09 v1.9.2, Electromagnetic compatibility and Radio Spectrum Matters, Part1: Common
technical requirements.
ETSI EN 301 489 –17:2012-09 v2.2.1, Electromagnetic compatibility and Radio Spectrum Matters, Part17: Specific
conditions for Broadband Data Transmissions Systems.
ETSI EN 300 328:2006-10 v1.7.1, Electromagnetic compatibility and Radio Spectrum Matters, Wideband
transmission systems; Data transmission equipment operating in the 2.4 GHz ISM band and using wide band
modulation techniques.
ISO10993-1:1997-12-15, 2nd Edition, ISO10993-5: 1995-05-15, 2nd Edition, ISO10993-10: 2002-09-01, 2nd
Edition + AM1: Biological evaluation of medical devices, Part 1, Part 5, and Part 10 for in vitro cytotoxicity,
irritation, and delayed-type hypersensitivity.
Title 21 CFR Part 820, US FDA Quality System Regulation (QSR).
Title 47 CFR Part 15, US Federal Communications Commission Part 15 Rules for Intentional Radiators.
93/42/EEC: Council Directive of 14 June 1993 concerning medical devices. (Medical Device Directive)
99/5/EC: Council Directive on radio equipment and telecommunications terminal equipment and the mutual
recognition of their conformity. (R & TTE Directive)
2002/96/EC:2003, Waste Electrical and Electronic Equipment (WEEE).
2002/95/EC:2003, Restriction of the use of Certain Hazardous Substances in Electrical and Electronic Equipment
(RoHS).
Canadian Medical Device Regulation (MDR).
79

APPLICABLE STANDARDS
ISO 14971:2007 Application of Risk Management to Medical Devices.
Section 510(k) of the USA Food, Drug and Cosmetic Act (Premarket Notification - also called PMN or 510(k)).
REFERENCE ONLY
EN 62304:2006+AC:2008 - Medical device software - Software life-cycle processes.
80
 Loading...
Loading...