
REF: 9502-057-50 A1
ELI 100
Operator’s
Manual
________________________________________________________________
CAUTION: Federal law restricts this device for sale to and use by or on the order of a physician.

®
Copyright© 2002
by Mortara Instrument, Inc.
7865 N. 86th Street
Milwaukee, Wisconsin 53224
This document contains confidential information that belongs to Mortara Instrument, Inc. No part of this document
may be transmitted, reproduced, used, or disclosed outside of the receiving organization without the express written
consent of Mortara Instrument, Inc. Mortara is a registered trademark of Mortara Instrument, Inc. ELI 100 is a
trademark of Mortara Instrument, Inc.
9500-090-01 A1

Technical Support and Service
Following are telephone numbers and addresses for contacting various technical support and service personnel.
Mortara Instrument, Inc.
7865 N. 86th St.
Milwaukee, WI 53224
Telephone Number: 414-354-1600
Toll-free Telephone Number: 800-231-7437
Toll-free Service Number: 888-MORTARA
Fax: 414-354-4760
E-mail address: sales@mortara.com
24 hour technical support
Over 120 trained field service technicians
Same day shipment of replacement parts
Biomedical training classes
Extended warranties/service contracts
Sales Support/Supplies & Accessories
Mortara Instrument, Inc.
7865 N. 86th St.
Milwaukee, WI 53224
Phone: 414-354-1600
Fax: 414-354-4760
Internet: http://www.mortara.com
European Economic Community Representative
Mortara Rangoni
Via Oradour, 7 40016
San Giorgio di Piano
Bologna, Italy
Phone: 39-051-6645-360
Fax: 39-051-6651-012
Mortara Instrument, Inc., GMBH (Germany)
Henricistr. 124
45136 Essen
Telephone number: 49-201-268311
Fax: 49-201-268313
Mortara Instrument, Inc., B.V.
(The Netherlands).
H. Dunantplein 6
3731 CL De Bilt
Postbus 131
3720 AC Bilthoven
Telephone number: 31-30-2205050
Fax: 31-30-2201531
i

Notices
Manufacturer’s Responsibility
Mortara Instrument, Inc., is responsible for the effects on safety, and performance only if
• Assembly operations, extensions, re-adjustments, modifications or repairs are carried out by persons
authorized by Mortara Instrument,
• The electrical installation of the relevant room complies with the requirements of appropriate regulations, and
• The ELI 100 is used in accordance with the instructions for use.
• Upon request, Mortara will make available a service manual containing technical information to assist an
appropriately qualified individual with potential service related issues, though it is highly recommended that if
there are concerns regarding the devices performance, Mortara Service is contacted at 1-800-877-8942.
Responsibility of the Customer
The user of this product is responsible for ensuring the implementation of a satisfactory maintenance schedule.
Failure to do so may cause undue failure and possible health hazards.
Equipment Identification
Mortara Instrument equipment is identified by serial numbers on the back or bottom of the device. Care should be
taken so that these numbers are not defaced.
Information pertinent to tracking and manufacturing is found on the bottom of the product and may be called upon
if service of the device is required.
Copyright and Trademark Notices
This document contains information that is protected by copyright. All rights are reserved. No part of this
document may be photocopied, reproduced or translated to another language without prior written consent of
Mortara Instrument, Inc. The information contained in this document is subject to change without notice.
Other Important Information
The information in this document is subject to change without notice.
Mortara Instrument, Inc., makes no warranty of any kind with regard to this material, including, but not limited to
implied warranties of merchant ability and fitness for a particular purpose. Mortara Instrument, Inc., assumes no
responsibility for any errors of omissions that may appears in this document. Mortara Instrument makes no
commitment to update nor to keep current the information contained in this document.
ii

Warranty Information
Your Mortara Warranty
MORTARA INSTRUMENT, INC. (hereinafter referred to as “Mortara”) hereby warrants that Mortara products
(hereinafter referred to as “Products”) shall be free from defects in material and workmanship under normal use,
service and maintenance for the warranty period of such Product from Mortara or an authorized distributor or
representative of Mortara. Normal use, service and maintenance means operation and maintenance in accordance
with appropriate instructions and/or information guides. This Warranty does not apply to damage to the Products
caused by any or all of the following circumstances or conditions:
a) Freight damage;
b) Parts and/or accessories of the Products not obtained from or approved by Mortara;
c) Misapplication, misuse, abuse and failure to follow the Product instruction sheets and/or information
guides;
d) Accident, a disaster affecting the Products;
e) Alterations or modifications to the Products not authorized by Mortara;
f) Other events outside of Mortara’s reasonable control or not arising under normal operating
conditions.
THE REMEDY UNDER THIS WARRANTY IS LIMITED TO THE REPAIR OR REPLACEMENT WITHOUT
CHARGE FOR LABOR OR MATERIALS, OR ANY PRODUCTS FOUND UPON EXAMINATION BY
MORTARA TO HAVE BEEN DEFECTIVE. This remedy shall be conditioned upon receipt of notice by Mortara
of any alleged defects promptly after discovery thereof within the warranty period. Mortara’s obligations under the
foregoing warranty will further be conditioned upon the assumption by the purchaser of the Products (i) of all
carrier charges with respect to any Products returned to Mortara’s principal place or any other place as specifically
designated by Mortara or an authorized distributor or representative of Mortara, and (ii) all risk of loss in transit. It
is expressly agreed that the liability of Mortara is limited and that Mortara does not function as an insurer. A
purchaser of a Product, by its acceptance and purchase thereof, acknowledges and agrees that Mortara is not liable
for loss, harm or damage due directly or indirectly to an occurrence or consequence therefrom relating to the
Products. If Mortara should be found liable to anyone under any theory (except the expressed warranty set forth
herein) for loss, harm or damage, the liability of Mortara shall be limited to the lesser of the actual loss, harm or
damage, or the original purchase price of the Product when sold.
EXCLUDED FROM THE LIMITED WARRANTY SET FORTH ABOVE ARE CONSUMABLE ITEMS SUCH
AS PAPER, BATTERIES, ELECTRODES, PATIENT CABLES, LEAD WIRES AND MAGNETIC STORAGE
MEDIUMS.
EXCEPT AS SET FORTH HEREIN WITH RESPECT TO REIMBURSEMENT OF LABOR CHARGES, A
PURCHASER’S SOLE EXCLUSIVE REMEDY AGAINST MORTARA FOR CLAIMS RELATING TO THE
PRODUCTS FOR ANY AND ALL LOSSES AND DAMAGES RESULTING FROM ANY CAUSE SHALL BE
THE REPAIR OR REPLACEMENT OF DEFECTIVE PRODUCTS TO THE EXTENT THAT THE DEFECT IS
NOTICED AND MORTARA IS NOTIFIED WITHIN THE WARRANTY PERIOD. IN NO EVENT,
INCLUDING THE CLAIM FOR NEGLIGENCE, SHALL MORTARA BE LIABLE FOR INCIDENTAL,
SPECIAL OR CONSEQUENTIAL DAMAGES, OR FOR ANY OTHER LOSS, DAMAGE OR EXPENSE OF
ANY KIND, INCLUDING LOSS OF PROFITS, WHETHER UNDER TORT, NEGLIGENCE OR STRICT
LIABILITY THEORIES OF LAW, OR OTHERWISE. THIS WARRANTY IS EXPRESSLY IN LIEU OF ANY
OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO THE IMPLIED
WARRANTY OF MERCHANT ABILITY AND THE WARRANTY OF FITNESS FOR A PARTICULAR
PURPOSE.
iii
User Safety Information
Means there is the possibility of personal
Warning:
Caution:
Note:
Federal law restricts this device to sale by or on the order of a physician.
Warning(s)
• Device (electrocardiograph, Class 1) captures and presents data reflecting a patient’s physiological condition
that when reviewed by a trained physician or clinician can be useful in determining a diagnosis. However, the
data should not
• To ensure that electrical safety is maintained during operation from AC (~) power, the device must be plugged
into a Hospital Grade outlet.
• To maintain designed operator and patient safety, peripheral equipment and accessories used that can come in
direct patient contact, must be in compliance with UL 2601-1, IEC 601-1 and IEC 601-2-25.
• To maintain designed operator and patient safety, only use parts and accessories supplied with the device and
available through Mortara Instrument, Inc.
• To avoid the possibility of serious injury or death during patient defibrillation, do not come into contact with
device or patient cables. Additionally, proper placement of defibrillator paddles in relation to the electrodes is
required to minimize harm to the patient.
• To ensure the safety of both the patient and the device, 1.5 meters (5 feet) of open area should surround the
patient.
• A possible explosion hazard exists, do not use the device in the presence of flammable anesthetics.
• ECG electrodes could cause skin irritation and should be examined for signs of irritation or inflammation.
• Before attempting to use the device for clinical applications the operator must read and understand the contents
of the manual and any documents accompanying the device.
• Where the integrity of external PROTECTIVE EARTH CONDUCTOR arrangement is in doubt,
EQUIPMENT shall be operated from its internal ELECTRICAL POWER SOURCE.
• To maintain operator and patient safety, when connecting the ELI 100 to peripheral equipment not in
compliance with UL2601-1, IEC 601-1 or IEC 601-2-25, consideration should be given to the requirements of
IEC 601-1-1.
• The ELI 100 has not been designed for use with high-frequency (HF) surgical equipment and does not provide
a protective means against hazards to the patient.
be used as a sole means for determining a patient’s diagnosis.
injury to you or others.
Means there is the possibility of damage to
the equipment.
Provides information to further assist in the
use of the device.
iv

• When connected to an external modem, a protective earth conductor must be attached to the ELI 100. The
connection may be through the mains power supply cord, or by a separate protective earth conductor attached
to the metal chassis of the ELI 100. The external modem and its power supply must comply with the
requirements of IEC 60950, safety of information technology equipment, including electrical business
equipment.
• Leakage currents can increase if additional devices are connected to the patient.
• All signal input and output (I/O) connectors are intended only for devices complying with EN 60601-1,
excluding the network connectors(s), that must only be used for connections to supporting Mortara Instrument
systems when cabling is supplied or approved by our Mortara Instrument Representative.
• Connecting/disconnecting cable at the back of the device must be done with the device OFF (main power).
Caution(s)
• To prevent possible damage to the keypad, do not use sharp or hard objects to depress keys, only use
fingertips.
• Do not attempt to clean the device or patient cables by submersing into a liquid, autoclaving, or steam
cleaning.
• Wipe the exterior surface of the device and patient cables with a sterilizing disinfectant, then dry with a clean
cloth.
• Conductive parts of the patient cable, electrodes and associated Type CF connections, including the neutral
conductor of the patient cable and electrode, should not come into contact with other conductive parts,
including earth ground.
• The rechargeable internal battery is a sealed lead acid type and it is totally maintenance free. If the battery
appears to become defective, refer to Mortara Instrument Service Department.
• Do not pull or stretch patient cables as this could result in mechanical and/or electrical failures. Patient cables
should be stored after forming them into a loose loop.
• The quality of the signal produced by the electrocardiograph may be adversely affected by the use of other
medical equipment, including but not limited to defibrillators and ultrasound machines.
v

Notes
• Excessive patient movement could interfere with the operation of the device.
• Proper patient preparation is important to proper application of ECG electrodes and operation of the device.
• Patient cables should be checked for cracks or breakage in its exterior properties prior to use.
• There is no known safety hazard if other equipment, such as pacemakers or other stimulators, are used
simultaneously with the ELI 100; however, disturbance to the signal may occur.
• If the ECG input becomes inoperable due to excessive saturation or overload, the display will indicate a lead
fail for the lead(s) that this condition is present and if the signal is being printed the respective lead(s) will print
out as a square wave.
• As defined by IEC 601-1 and IEC 601-2-25, the device is classified as follows:
- Class I equipment
- Type CF applied parts
- Ordinary equipment
- Not suitable for use in the presence of flammable anesthetics
- Continuous operation
• The ELI 100 will automatically turn off (blank screen) if the batteries have been severely discharged.
• After operating the ELI 100 using battery power, always reconnect the power cord and depress the I on the I/O
power switch. This ensures that the batteries will be recharged for the next time you use the ELI 100. The
word CHARGING or AC Power will appear on the LCD screen.
vi
Equipment Symbols
Symbol Delineation
Attention, consult accompanying documents
Alternating current
Protective earth (ground)
Earth (ground)
Fuse
Telephone line (modem)
Defibrillator-proof type CF input
Thermal paper
Equipotentiality
Output/Transmit
vii
Input
ON (power)
OFF (power)
"ON" only for part of Equipment
"OFF" only for part of Equipment
Stop (of action)
Shift key (to enter upper case text)
viii
⌫
Space key
Enter key (accept data/return)
Backspace/Delete key
Initiate printing of 12-Lead ECG
Initiate printing of continuous rhythm strip
High-speed serial data
Eject
Indicates compliance to applicable EEC directives
ix


Table of Contents
Introduction
1
Manual Purpose.............................................................................................................................................. 1-1
Audience ......................................................................................................................................................... 1-1
Conventions .................................................................................................................................................... 1-1
System Description ......................................................................................................................................... 1-1
ELI 100, Illustration ......................................................................................................................................... 1-2
ELI 100 Specifications .................................................................................................................................... 1-3
Setup and Installation ..................................................................................................................................... 1-4
ELI 100, Two Views: Left Side and Rear ........................................................................................................ 1-4
Rear (Fuse Installation)................................................................................................................................... 1-5
Configuration Settings..................................................................................................................................... 1-5
Initial Configuration ......................................................................................................................................... 1-7
Protected Configuration .................................................................................................................................. 1-7
Protected Configuration Screen Summary ..................................................................................................... 1-8
Protected Configuration Screen A .................................................................................................................. 1-8
AC Filter .......................................................................................................................................................... 1-8
Storage Format ............................................................................................................................................... 1-8
Protected Configuration Screen B .................................................................................................................. 1-9
Storage Sensitivity .......................................................................................................................................... 1-9
Protected Configuration Screen C .................................................................................................................. 1-9
Plot Format...................................................................................................................................................... 1-9
Interpretation Format ...................................................................................................................................... 1-9
Protected Configuration Screen D .................................................................................................................. 1-10
Plot Channels.................................................................................................................................................. 1-10
Protected Configuration Screen E .................................................................................................................. 1-11
Units ................................................................................................................................................................ 1-11
Date Format .................................................................................................................................................... 1-11
Protected Configuration Screen F................................................................................................................... 1-11
Baud Rate ....................................................................................................................................................... 1-11
Auto Delete ..................................................................................................................................................... 1-12
Protected Configuration Screen G.................................................................................................................. 1-12
Serial Port ....................................................................................................................................................... 1-12
CAPS Lock...................................................................................................................................................... 1-12
Protected Configuration Screen H .................................................................................................................. 1-12
Pre-append site number to patient ID............................................................................................................. 1-13
Protected Configuration Screen I.................................................................................................................... 1-13
Phone Number ................................................................................................................................................ 1-13
Protected Configuration Screen J................................................................................................................... 1-14
Internal Modem Baud Rate ............................................................................................................................. 1-14
Protected Configuration Screen K .................................................................................................................. 1-14
Auto Save........................................................................................................................................................ 1-14
Cueing............................................................................................................................................................. 1-14
Protected Configuration Screen L................................................................................................................... 1-15
Site Number .................................................................................................................................................... 1-15
Cart Number.................................................................................................................................................... 1-15
Copies ............................................................................................................................................................. 1-15
Retrieve Serials............................................................................................................................................... 1-15
Protected Configuration Screen M.................................................................................................................. 1-15
ID Format ........................................................................................................................................................ 1-16
Protected Configuration Screen N .................................................................................................................. 1-16
Rhythm Lead Selection................................................................................................................................... 1-17
x

q
p
Protected Configuration Screen O...................................................................................................................1-17
Site Name ........................................................................................................................................................1-17
Protected Configuration Screen P...................................................................................................................1-17
Key Click Volume Setting ................................................................................................................................1-18
Persistent Configuration ..................................................................................................................................1-19
Load Thermal Paper.......................................................................................................................................1-20
Apply Power....................................................................................................................................................1-20
ELI 100 Supply List..........................................................................................................................................1-21
100
2
Operation
Starting the System .........................................................................................................................................2-1
Operation .........................................................................................................................................................2-1
AC Power Operation........................................................................................................................................2-1
Battery Operation.............................................................................................................................................2-1
Patient Preparation..........................................................................................................................................2-2
Patient Hookup ................................................................................................................................................2-2
ID Menu Formatting.........................................................................................................................................2-2
Selecting the ID Menu .....................................................................................................................................2-3
ID Menu Prompts.............................................................................................................................................2-3
ID Menu Sequence (Long Format)..................................................................................................................2-5
Acquiring and Printing a 12-Lead ECG
3
ECG Selection Screen Description..................................................................................................................3-1
ECG Analysis...................................................................................................................................................3-2
ECG Activity Screen Description .....................................................................................................................3-2
Taking a Second ECG.....................................................................................................................................3-3
Selecting an E-SCRIBE Patient File................................................................................................................3-4
Request Selection Screen ...............................................................................................................................3-4
4
Ac
uiring and Printing a Rhythm Stri
Rhythm Selection Screen Description.............................................................................................................4-1
Acquiring Routine Rhythm Strips.....................................................................................................................4-1
Rhythm Activity Screen Description ................................................................................................................4-1
Using the Special Functions
5
Special Function Screen Description...............................................................................................................5-1
Directory Description .......................................................................................................................................5-1
Printing a Directory ..........................................................................................................................................5-2
Directory Screen Sequence.............................................................................................................................5-2
Patient Selection Screen Description ..............................................................................................................5-4
Selecting a Patient Name ................................................................................................................................5-4
Erasing a Patient's ECGs ................................................................................................................................5-4
Listing a Patient's ECGs..................................................................................................................................5-5
xi

Selecting a Specific ECG................................................................................................................................ 5-5
Patient ID Screen Description......................................................................................................................... 5-5
Erasing a Specific ECG .................................................................................................................................. 5-6
Maintenance on a Specific ECG ..................................................................................................................... 5-6
Directory Maintenance Screen Description .................................................................................................... 5-6
Updating or Adding Patient ID Information ..................................................................................................... 5-7
Transmitting ECGs.......................................................................................................................................... 5-7
Direct Connection Hookup .............................................................................................................................. 5-7
Telephone Connection Hookup ...................................................................................................................... 5-7
Sending ECGs ................................................................................................................................................ 5-8
Receiving ECGs.............................................................................................................................................. 5-8
Batch Plotting .................................................................................................................................................. 5-9
Downloading a Request List ........................................................................................................................... 5-9
Request Activity Screen Description............................................................................................................... 5-9
Printing the Request List................................................................................................................................5-10
Maintenance and Troubleshooting
6
Troubleshooting Chart .................................................................................................................................... 6-1
Self-Test Printout Description ......................................................................................................................... 6-2
Self-Test Printout Sample, Pg. 1 of 2.............................................................................................................. 6-3
Self-Test Printout Sample, Pg. 2 of 2.............................................................................................................. 6-4
Cleaning and Inspection ................................................................................................................................. 6-5
Inspecting the ELI 100 .................................................................................................................................... 6-5
Cleaning the ELI 100 ...................................................................................................................................... 6-5
Cleaning the Patient Cable ............................................................................................................................. 6-5
Cleaning the Writer Printhead......................................................................................................................... 6-5
System Information LogA
Appendix
System Information Log .................................................................................................................................. A-1
Appendix
Rhythm Strip (RHY) Description ..................................................................................................................... B-1
3-Channel ECG Format Description ............................................................................................................... B-1
4-Channel ECG Format Description ............................................................................................................... B-1
6-Channel ECG Format Description ............................................................................................................... B-1
3+3 ECG Format Description ......................................................................................................................... B-2
Measurement Matrix (MSR) Description......................................................................................................... B-2
Measurement Matrix Acronym List ................................................................................................................. B-2
Standard Rhythm Strip Sample (default, reduced) ......................................................................................... B-4
6-Channel Frontal Rhythm Sample (reduced) ................................................................................................ B-5
6-Channel Precordial Rhythm Sample (reduced) ........................................................................................... B-6
12-Channel Rhythm Sample (reduced) .......................................................................................................... B-7
3-Channel ECG Sample (reduced) ................................................................................................................. B-8
4-Channel ECG Sample (reduced) ................................................................................................................. B-9
6-Channel ECG Sample (reduced) ................................................................................................................B-10
3+3 ECG Sample (reduced)...........................................................................................................................B-11
Measurement Matrix Sample (reduced) ........................................................................................................B-12
B
Sample Print Formats
xii

Glossary ............................................................................................................................................. G-1
Index........................................................................................................................................................ I-1
Figures
Figure 1-1 ELI 100......................................................................................................................................... 1-2
Figure 2-1 ELI 100 Left and Rear.................................................................................................................. 1-4
Figure 3-1 ELI 100 Rear (Fuse Installation) .................................................................................................. 1-5
xiii

____________________________________________________________________________Section 1
1
Introduction
Manual Purpose
The ELI 100 Operator's Manual explains how to operate the ELI 100 interpretive and non-interpretive
electrocardiographs. You can use this manual as a learning tool as well as a source of reference information. It
explains how to:
• Set up the electrocardiograph.
• Use and understand the keyboard, the viewing screen, and the menu sequences.
• Enter, modify, and delete information.
• Acquire a 12-lead electrocardiogram
• Print an ECG rhythm strip
Audience
This manual is written for clinical research professionals. They are expected to have working knowledge of medical
procedures and terminology as required for monitoring cardiac patients.
Conventions
Text that the user must type, such as a:install, appears in bold, Times New Roman font.
Keys, such as ENTER, appear in bold, upper-case, Arial font.
Text that appears on the screen, such as C:\Portrait, appears in normal, Arial font.
System Description
The ELI 100 is a diagnostic electrocardiograph capable of acquiring and printing ECG data in a hospital and/or
clinical environment. If the ELI 100 is equipped with the computerized interpretation option installed and is
enabled, which is at the user’s discretion, the acquired electrocardiogram may assist in allowing the physician to
more quickly determine what care options may be available for the patient.
The printout offers a variety of formats: three, four or six channels in automatic mode and three, six, or twelve
channels during rhythm recording. The ELI 100 can operate on batteries or line power.
1-1
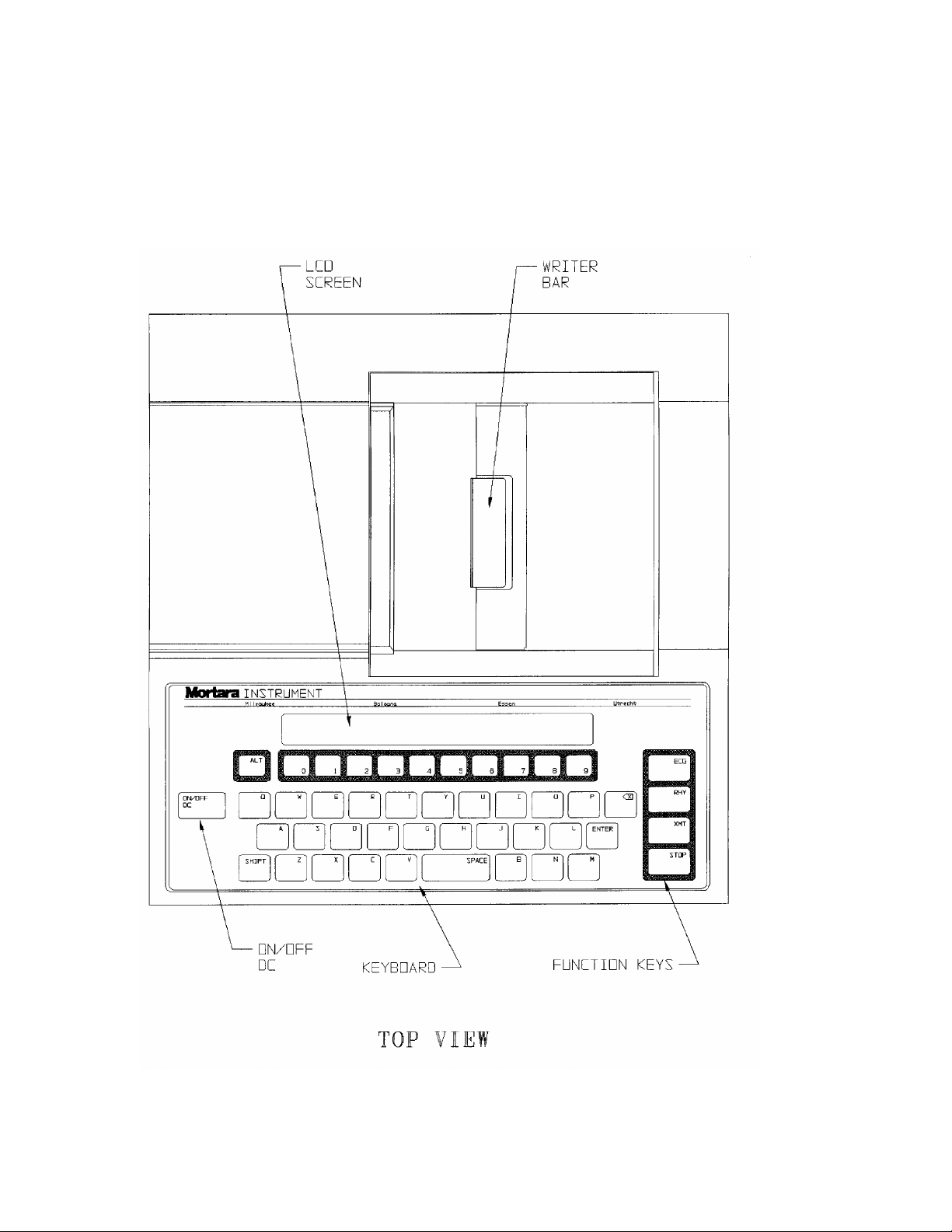
ELI 100_____________________________________________________________________________
The ELI 100 consists of:
• Electrocardiograph
• ELI 100 Operator’s Manual and short form instruction card
• Accessory Kit
Figure 1-1, ELI 100, Illustration
1-2

____________________________________________________________________________Section 1
ELI 100 Specifications
Leads available:
ECG input:
Input impedance:
Input dynamic range:
Electrode offset tolerance:
Common mode rejection:
Patient leakage rejection:
Chassis leakage current:
Frequency response:
Digital sampling rate:
Special computer
functions:
Power:
Weight:
Dimensions:
Recording technique:
Paper type:
Recording speeds:
Recorder time resolution:
Sensitivity:
I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, V6
Simultaneous input from all 12 leads
Meets the requirements of ANSI/AAMI EC11
Meets the requirements of ANSI/AAMI EC11
Meets the requirements of ANSI/AAMI EC11
Meets the requirements of ANSI/AAMI EC11
Meets the requirements of ANSI/AAMI ES1
Meets the requirements of ANSI/AAMI ES1
Meets the requirements of ANSI/AAMI EC11
10,000 samples/sec/channel (used for pacemaker
artifact detection); 500 samples/sec/channel (used for
recording and analysis)
Arm lead reversal detection; lead-off and/or artifact
detection; drift reduction; AC interference rejection
100-240VAC at 50 or 60Hz, or internal batteries
11 pounds (4.9kg)
11 1/4” x 11 1/2” x 3 3/4” (28.1 x 28.7 x 9.3 cm)
Computer controlled thermal dot array (200 dots/inch)
Thermal sensitive, full grid, 108mm width, roll
5, 10, 25, 50mm/sec, computer controlled
1msec
5, 10, and 20mm/mV
1-3
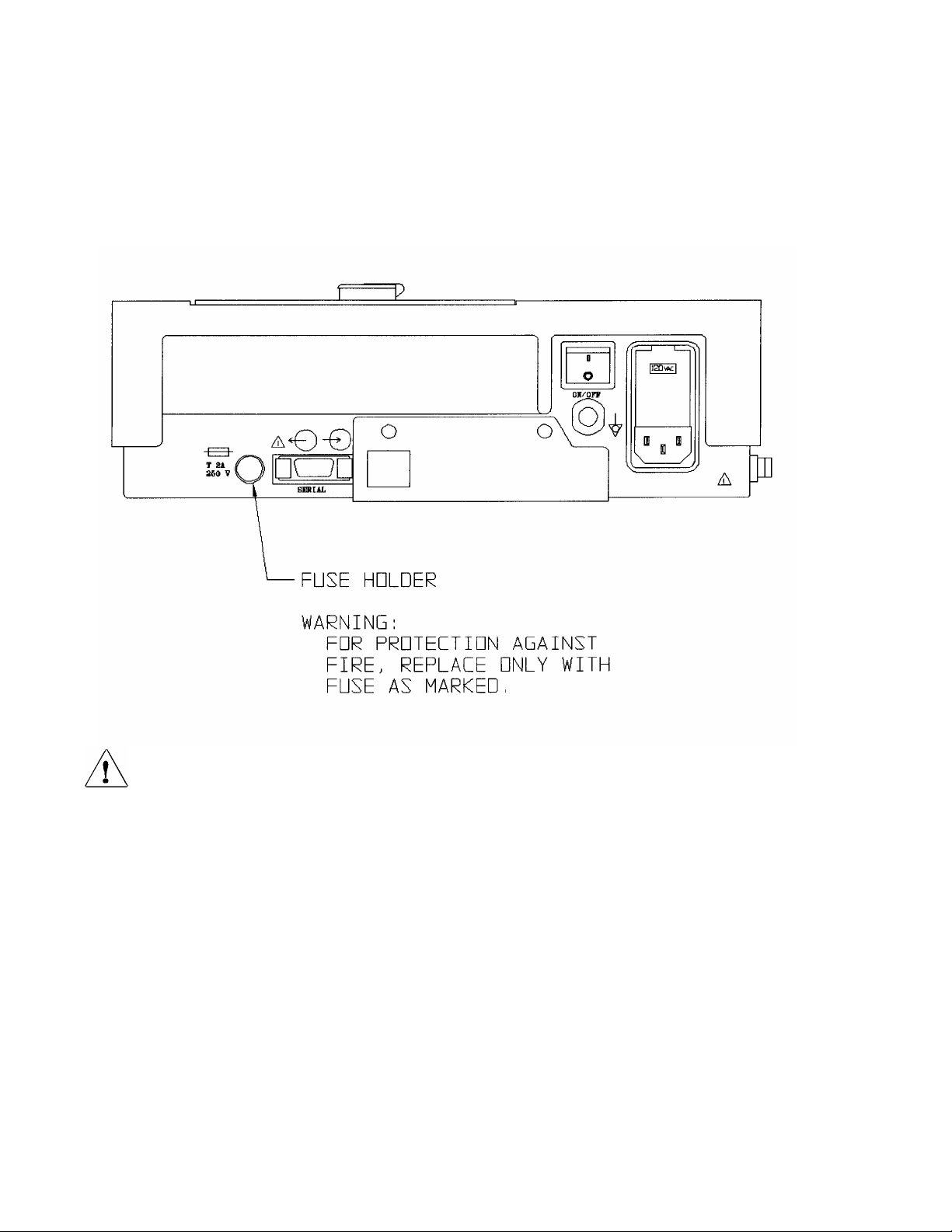
ELI 100_____________________________________________________________________________
Setup and Installation
Figure 2-1, ELI 100
Caution: When the fuse is installed, battery will gradually discharge over time. To
guarantee battery life, always connect the power cord and depress the I on the I/O power
switch to ensure the batteries will be recharged for your next use. The word CHARGING or AC
Power will appear on the LCD screen. If fuse is removed, stored data and configuration
settings will be lost.
100The battery fuse is installed in the back of the unit, as pictured above. The fuse is installed so the ELI 100 can
store data, operate on the internal battery, and save the configuration settings.
One end of the fuse is covered with a plastic tip; the other end has a silver cap. If a new fuse needs to be installed,
follow the steps below:
n Hold the fuse by the plastic tip and insert the silver-capped end into the round socket, which is located near
the fuse “Warning” instructions on the back of the ELI 100.
o Rotate the fuse in the socket so that approximately 1/4" of the plastic cap sticks out.
p In a clockwise motion, press the plastic cap into the socket using your thumb or a screwdriver. The cap
should be almost flush with the unit.
1-4

____________________________________________________________________________Section 1
NOTE: If you remove the battery fuse after the ELI 100 is in operation, any ECGs stored in the unit will be lost
and the initial and protected configuration settings will change to their default settings. The persistent
site and cart number configuration settings will be preserved.
NOTE: If the main switch is ON and the LCD (Liquid Crystal Display) does not display “charging” or “AC
Power,” have a qualified service technician verify that the line fuse(s) are installed.
Configuration Settings
Three configuration programs define all ELI 100 operational conditions:
1. Initial Configuration
a. Download Custom ID
b. Clock and Date settings
2. Protected Configuration
a. AC Filter, Storage Format
b. Storage Sensitivity
c. Plot Format, Interpretation Format
d. Plot Frequency and Plot Channels
e. Units, Date Format
f. Baud Rate, Auto Delete
g. Serial Port, Caps Lock
h. Include Site # in ID
i. Phone Number
j. Auto Save, Cueing
k. Copies, Retrieve Serials
l. ID format
m. Rhythm Lead Selection
n. Site Name
o. Key Click
3. Persistent Configuration
a. Site Number
b. Cart Number
1-5
ELI 100_____________________________________________________________________________
A
A
Initial Configuration
The initial configuration menu allows the user to download a custom ID and change the date and time settings.
To access the initial configuration menu, press the ALT key and the SPF (0) key simultaneously from the main
menu. The following initial configuration menu appears:
DWNLD
ID
0
LT
Custom ID Format Exit to
CLOCK
Date and Time
2
Screen
EX
3
4
5 6 7 8 91
Main Menu
NOTE: To access the Protected Configuration and Persistent Site Number Configuration menus, you must begin
from the initial configuration menu as pictured above. Details to access the protected and persistent
configuration menus follow.
The custom ID format, which is designed in the E-SCRIBE system, can be downloaded to the ELI 100. When you
select (0) DWNLD ID, the following screen appears:
NOTE: The site number must be configured in the cardiograph and recognized as an established, valid site
number at the E-Scribe before downloading the custom ID.
LT
Download custom format?
0
2
YES
3
4
NO
5 6 7 8 91
Back to Initial Configuration
Menu
Select (4) YES to proceed with the download – the screen will display the following message: “connection in
progress”. When the download completes, the new CUSTOM format becomes the ID menu format for all future
ECGs until you reconfigure the ID format. If you reset the format option to SHORT, STD, or LONG in the
protected configuration menu, and then wish to change back to an original or a new CUSTOM format, you must
repeat the download process from the initial configuration menu.
NOTE: CONNECTION FAILURE will be displayed if there was an error in downloading the custom ID.
Press the CLOCK key (2) to access the Clock/Date Selection screen shown below.
Current date and time: 00/00/00 00:00
New date and time: 00/00/00 00:00
n The top line of the LCD screen displays the preprogrammed current date and time set for the ELI 100.
o To reset the clock, type in the correct date and time (using a 24-hour clock) in the same format as
displayed.
US EXAMPLE: 12/31/89, 17:55 for December 31, 1989, 5:55 PM
NOTE: the date format (US or EUROPE) can be switched in the protected configuration settings.
1-6
____________________________________________________________________________Section 1
Protected Configuration
The protected configuration menu allows the user to define the ELI 100 operational conditions that do not change on
a daily or patient-to-patient basis. Once you set these default conditions, you will rarely need to use the protected
configuration program again. To access the protected configuration menu, press the ALT key and the C key
simultaneously from the initial configuration menu.
The chart below summarizes the protected configuration menu screens and the available options for each parameter.
Detailed descriptions of the parameters for each menu option(s) follow the summary chart..
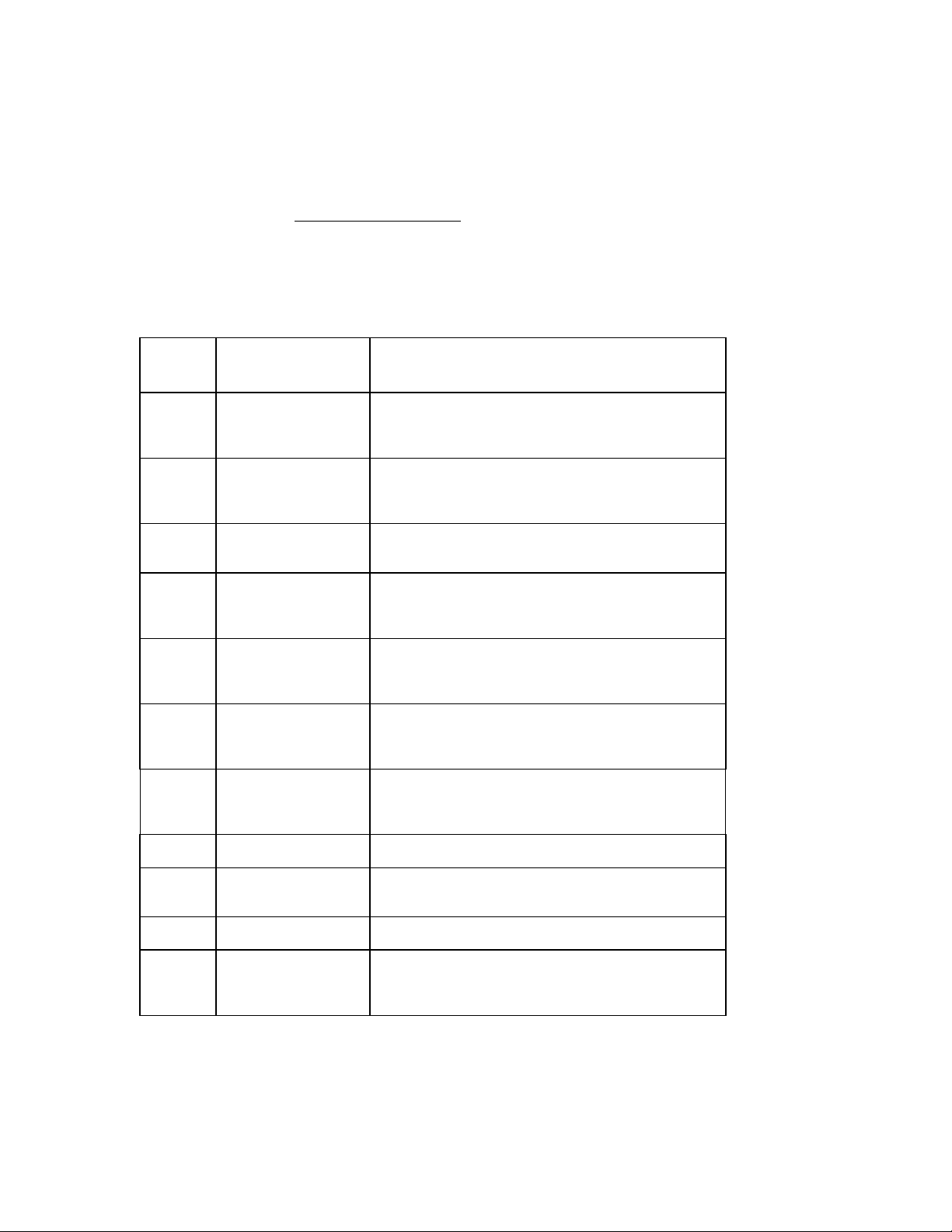
Protected Configuration Screen Summary
Screen Parameter Option
A AC Filter
Storage Format
B
C
D
E Units
F Baud Rate
G
H
I
J Modem Baud Rate 1001100 or 2400
K Auto Save
Storage Sensitivity
Plot Format
Interpretation Format
Plot Frequency
Plot Channels
Date Format
Auto Delete
Serial Port
Caps Lock
Include Site # ID
Phone Number
Cueing
60Hz or 50Hz
2.5 seconds or 10 seconds
2.5 micro-volts or 10 micro-volts.
Interpretation or No Interpretation
Reason Statements or No Reason Statements100
40Hz or 150Hz
3+3 (2pg), 3, 4
LB/IN or kg/cm
US or European
38400
XMT, XMT/PLT, IMMED, OFF, PLT
Direct or Modem
On or Off
Yes or No
User-defined (up to 40 characters including special
characters)
ON or OFF
ON or OFF
1-7
ELI 100_____________________________________________________________________________
A
A
L Site Number
Cart Number
Copies
Retrieve Serials
M ID Format LONG, SHORT, CUSTOM, STD
N Rhythm Lead
Selection
O Site Name User-defined (up to 32 alphanumeric characters)
P Key Click Soft, Medium, Loud
To advance from one configuration screen to the next, press the ENTER key. Select (9) EX from any protected
configuration screen to return to the initial configuration screen.
Display only
Display only
0 = Original copy
1 = Original plus one copy
x = Original plus up to nine copies
0 = Most recent ECG (value should always be
set to 0).
Channel 1, Channel 2, Channel 3: I, II, III, aVR,
aVL, aVF, V1-V6
Protected Configuration Screen A
C
Filter:
60Hz
Storage
Format:
2.5s EX
2
LT
1
0
3 4 5 6 7 8 9
Exit to
Main Menu
AC Filter
The ELI 100 software removes 60Hz or 50Hz interference. Which setting you select depends on the line frequency
in your country. Always use the 60Hz setting in the United States. If the AC interference is present, check to see
that the proper AC filter is selected.
Storage Format
There are two available storage format options in the ELI 100. One format stores 2.5 seconds of each of the twelve
leads. The other storage format option, which is the default setting, stores the full 10 seconds of all twelve leads.
Selecting this storage format option will reduce the amount of storage. For optimum storage capacity, select 2.5
seconds.
Most users select the 2.5 second storage format since it provides the standard 12-lead record. If the default Plot
Channels option is set to 4 channels (see Configuration Screen C), the 2.5 second format is modified so that 10
seconds of Lead II is stored along with 2.5 second segments of the remaining leads.
1-8
____________________________________________________________________________Section 1
A
A
Protected Configuration Screen B
Storage
Sensitivity:
2.5uV EX
LT
2345678910
Exit to
Main Menu
Storage Sensitivity
The default storage sensitivity is 2.5 uV. For optimum storage capacity, select 10 uV.
Protected Configuration Screen C
Plot
Format: INT
LT
2345678910
Plot Format
The ELI 100 automatically analyzes ECGs and prints the interpretation on the ECG printout. The Plot Format
option allows you to select or suppress the “interpretive” text on the ECG report.
NOTE: A qualified physician should review the computer generated ECG interpretation before the treatment of
any patient.
Interpretation Format
The reason statements indicate why a particular interpretive statement was printed. Reason statements print in
[square brackets] within the interpretive text (if the interpretation is turned on). Turning the reason statement
function on or off does not affect the measurements performed or the interpretive statements selected by the analysis
program. See Appendix B for examples of the reason statements on the sample printouts.
Interp
Format: REA EX
Exit to
Main Menu
1-9
ELI 100_____________________________________________________________________________
A
(
)
(
)
grap
(
p
;
p
)
p
Protected Configuration Screen D
Plot
Freq:
150Hz
Plot
Channels:
3
EX
LT
0
2
1
3 4 5 6 7 8 9
Exit to
Main Menu
The ECG plot frequency default setting is 150Hz, or select 40Hz. The recommended setting is 150Hz.
Plot Channels
This option defines the default for the available plot channels. The plot options are:
Format Option ECG Data
STD
Standard
4CH
2.5-second graph of 12 leads in
a 3-channel format
Condensed
in a 4-channel format
contain a labeled median com
4th channel is a 10-second rhythm
of Lead II
stri
h of 12 leads
3 channels
lex
2PG
Two Page
Standard format PLUS a 3-channel
10-second stri
of each Leads V1, II, V5
STD Format Option
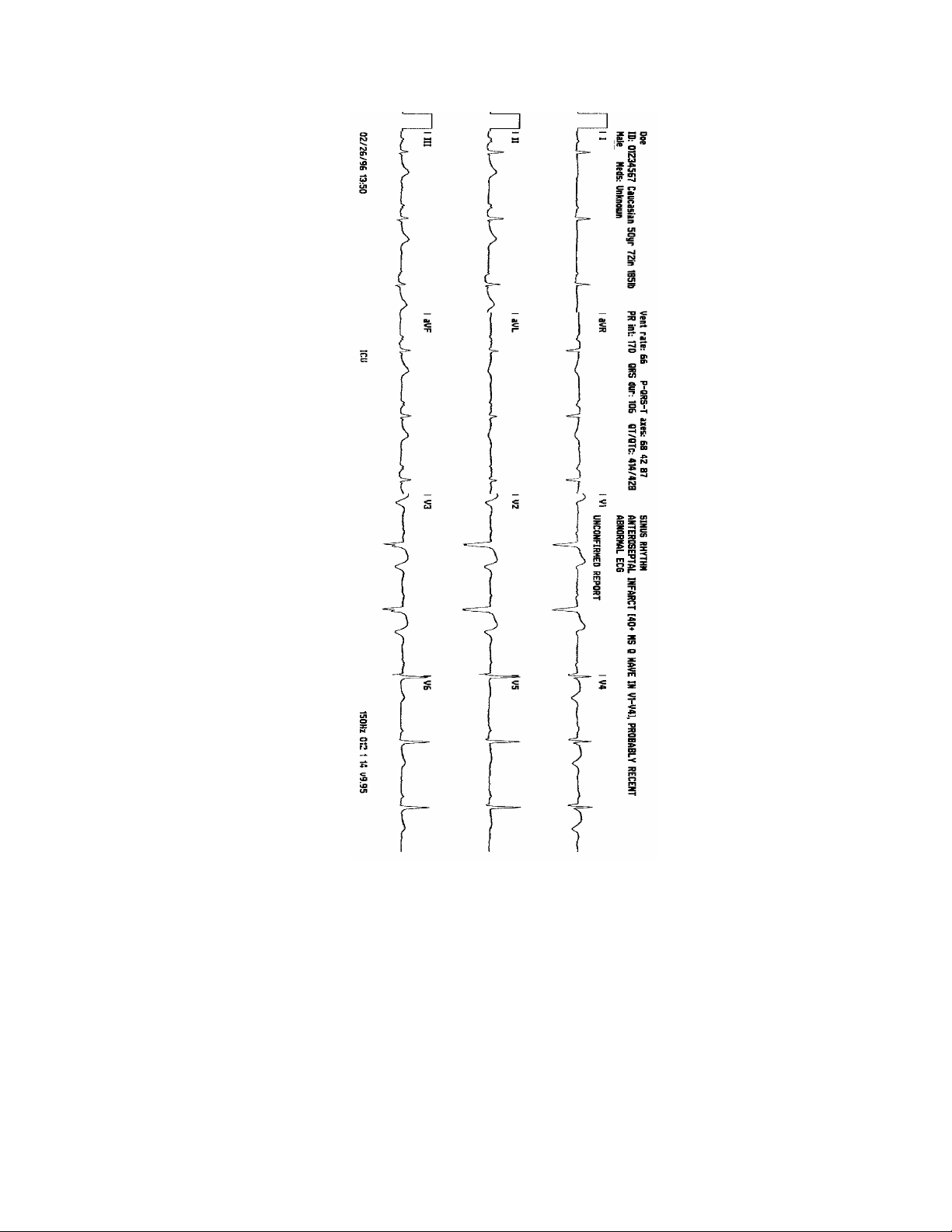
The STD (standard) format is a 4" by 11" strip containing a 2.5-second graph of 12 leads in a 3-channel format.
The following print is an example of the STD format:
1-10
____________________________________________________________________________Section 1
The patient's name, ID number, race, age, height, weight, gender and medications are printed across the top left
section of the printout. Measurements are printed in the middle to include ventricular rate, P-QRS-T axes, PR
interval, QRS duration and QT/QTc. On the top right of the printout, the ECG interpretation and reason statements
are printed.
The date and time of acquisition, location, plot frequency, and software version are printed across the bottom of the
printout.
Two and one-half seconds of leads I, II, III are printed after the calibration pulse, followed by two and one-half
seconds each of the lead sets aVR, aVL, aVF, V1, V2, V3, V4, V5, V6.
1-11

ELI 100_____________________________________________________________________________
STD Format at 50 mm/sec
1-12

____________________________________________________________________________Section 1
STD Format with Long ID
1-13

ELI 100_____________________________________________________________________________
STD Format with Short ID
1-14

____________________________________________________________________________Section 1
4CH Format Option
The 4CH (short) format is a 4" by 11" strip containing a condensed graph of the 12 leads in a 4-channel format.
Three channels contain a labeled median complex for each of the 12 leads, and the fourth channel contains a 10second rhythm strip of lead II.
The following print is an example of the 4CH format:
The patient's name, ID number, race, age, height, weight, gender and medications are printed to the right of the 12
median complexes. Measurements to include ventricular rate, P-QRS-T axes, PR interval, QRS duration and
QT/QTc are printed to the right of the patient ID information. The ECG interpretation and reason statements are
printed below the patient ID information.
1-15

ELI 100_____________________________________________________________________________
The date and time of acquisition, location, writer speed for the median complexes and rhythm strip, plot frequency
and software version are printed across the bottom of the printout.
The median complexes are arranged in columns or leads I, II, III; aVR, aVL, aVF; V1, V2, V3; and V4, V5, V6.
Ten seconds of lead II are printed below the median complexes.
1-16

____________________________________________________________________________Section 1
2PG Format Option
The 2PG (two-page) format is a 4" by 22" strip containing one page in the STD format (refer above) followed by
one page containing 3 channels of rhythm leads II, V1, and V5.
The following print is an example of the 2PG format:
The patient ID information, measurements, ECG interpretation, acquisition time, location, writer speed and software
version number are printed on the rhythm strip in the same format as the STD format.
1-17

ELI 100_____________________________________________________________________________
MSR Format Option
The MSR format option is available when selecting a copy (CPY) of an ECG from the ECG Print Option screen or
ECG Maintenance screen, Section 5. This option is not available when selecting a plot channel format in
Configuration screen D.
The MSR (measurement matrix) option is a table listing the amplitude and interval measurements. The amplitude
measurements are printed in microvolts [µV], and the interval measurements are printed in milliseconds [ms].
The following print is an example of the MSR format:
1-18
PA
PPA
QA
QD
EQD
I II III aVR aVL aVF V1 V2 V3 V4 V5 V6
P wave amplitude
P' amplitude (second phase of a biphasic P) [µV]
Q wave amplitude [µV]
Q wave duration [ms]
Equivalent Q duration [ms]
[µV]

____________________________________________________________________________Section 1
RA
RD
SA
SD
RPA
RPD
SPA
SPD
STJ
STM
R wave amplitude [µV]
R wave duration [ms]
S wave amplitude [µV]
S wave duration [ms]
R' wave amplitude [µV]
R' wave duration [ms]
S' wave amplitude [µV]
S' wave duration [ms]
ST segment displacement at J point [µV]
ST segment displacement at the mid-point between STJ and
STE [µV]
STE
TA
TPA
MTA
QRSA
FLAG
The leads are printed across the top of the printout, and the appropriate acronyms for the measurements are printed
in a column at the left of the page.
ST segment displacement at the end (defined as 1/8 the
average R-R interval from J point) [µV]
T wave amplitude [µV]
T' amplitude [µV]
Modified T amplitude [µV]
Net QRS area
Measurement Matrix Flags
1 = T inflection
2 = Delta waves
3 = 1 and 2
1-19

ELI 100_____________________________________________________________________________
A
A
A
Protected Configuration Screen E
Date
Format: EXUnits: LB/IN
US
LT
2345678910
Exit to
Main Menu
Units
This option defines the units of weight and height to either pounds/inches (LB/IN) or kilograms/centimeters
(kg/cm).
Date Format
This option defines the format for displaying the date and time in either the U.S. format (12/31/98; 17:50) or the
European format (31.12.98; 17:50).
NOTE: To change the date (and time) select (2) CLOCK from the initial configuration menu as explained earlier
in this chapter.
Protected Configuration Screen F
Baud
Rate: 38400
uto
Delete: OFF EX
LT
2345678910
Exit to
Main Menu
Baud Rate
The baud rate determines the serial port’s data transmission rate in bits per second (bps). The baud rate is set to
38400 bps for direct data transmission between the ELI 100 and another unit; and for a direct connection to the ESCRIBE system; and for use with an external modem.
1-20

____________________________________________________________________________Section 1
A
A
Auto Delete
This option defines the ECG auto delete status in the directory. Deleted ECGs will be removed as the directory
becomes full. The selections are:
OFF = ECG is never automatically deleted from the directory.
PLT = ECG is automatically deleted from the directory after plotting.
XMT = ECG is automatically deleted from the directory after transmission.
XMT/PLT = ECG is automatically deleted from the directory after transmission and plotting.
IMMED = ECG is automatically deleted.
NOTE: The initial ECG printout does not activate the procedures described above for the plot option.
Protected Configuration Screen G
Serial
Port:
MODEM
CAPS
Lock: ON EX
LT
2
3
4 5 6 7 8 910
Exit to
Main Menu
Serial Port
This option specifies the serial port mode by either direct connection or external modem. If an internal modem is
present, the cardiograph will check for a dial tone and use it by default.
CAPS Lock
When the CAPS lock is on, all characters shall be translated to upper case, and the shift key shall cause the
characters to be lower case. The default setting is on.
Protected Configuration Screen H
Include
LT
Site # in ID:
NO
2
3
4 5 6 7 8 910
EX
Exit to
Main Menu
1-21

ELI 100_____________________________________________________________________________
A
Pre-append site number to patient ID
When yes is selected for this configuration setting, the site ID, followed by a dash, will be displayed when the user
enters the patient ID. The user will not be able to erase the site ID and dash before entering the patient ID. The
patient ID with the site ID pre-appended shall become the true patient ID and be displayed that way whenever the
patient ID is displayed and printed.
Protected Configuration Screen I
Phone number:
LT
2345678910
Phone Number
This option specifies the phone number for the ECG transmission destination (another unit or an E-SCRIBE
system). Enter up to 40 characters. Special characters may be added using the ALT - key combinations, listed
below. Use the BACKSPACE key to erase an entry error.
ALT-Key
Character ELI Display
1 !
2 @
3 #
4 $
5 %
6 ^
7 &
8 *
9 (
0 )
A ~ right arrow
B \ Japanese Yen
C ,
D E =
I {
K [
L ]
M <
N >
O }
P +
Q ?
R "
S /
T '
U ;
V `
W |
X .
Y :
1-22

____________________________________________________________________________Section 1
A
A
A
To insert a pause for an additional dial tone, use the letter W. For example, you may need to dial a 9 to get an
outside line.
EXAMPLE: 9W14145554321
To change tone dialing to pulse dialing, use the letter P.
EXAMPLE: P14145554321
(If necessary, you can use both the letter W and the letter P in the same phone number.)
Protected Configuration Screen J
ModemBaud
Rate:
LT
2400
2345678910
Internal Modem Baud Rate
The default setting for this option is 2400 bps for telephone transmission. It is recommended that the 2400 default
setting be used at all times. If you find that you are having frequent transmit (XMT) failures due to poor telephone
line quality, press the 2 softkey to change the baud rate to 1100.
Protected Configuration Screen K
UTO
SAVE ON Cueing: ON
LT
2345678910
Exit to
Main Menu
Auto Save
This option defines whether or not the ELI 100 is to save all newly acquired ECGs in the directory. If the selected
configuration option is set to OFF, the ELI 100 asks if you wish to save each new ECG after it prints. A “YES”
answer saves the ECG in the directory.
Cueing
The ELI 100 contains an optical sensor in the writer that aligns a black rectangle on the back of the thermal paper
with a predetermined starting point for printing. If the Cueing option is ON, every 12-lead ECG printout stays
within the perforation lines, which are placed every 11 inches. If the Cueing option is OFF, each printout begins
directly after the preceding printout regardless of the location of the perforation lines.
EX
1-23

ELI 100_____________________________________________________________________________
A
A
Protected Configuration Screen L
LT
Site#:
Cart#:
2345678910
Copies: 0
Retrieve Serials: 0
Site Number
Read only Display
institution for ECG records stored in a Mortara Instrument, Inc., E-SCRIBE system and must be defined for
transmitting. You can use up to four digits for the site number; numbers from 0 to 9999 are supported. See
Persistent Configuration for details to edit site number.
Cart Number
Read only Display
to four digits for the cart number; numbers from 0 to 9999 are supported. See Persistent Configuration for details to
edit cart number.
Copies
This option defines the number of printed copies when an ECG is taken. A zero (0) setting prints the original only;
one (1) prints the original plus 1 copy; two (2) prints the original plus 2 or more copies (up to 9 copies).
You can request copies from the ECG Activity screen, which appears after ECG acquisition. You also can request
copies while in the directory from the Directory Maintenance screen.
Retrieve Serials
This value should always be set to 0.
. This option identifies the site of your ELI 100. Site numbers designate the hospital, clinic, or
. Cart numbers indicate which electrocardiograph transmitted a particular ECG. You can use up
Protected Configuration Screen M
ID
Format: LONG EX
LT
2345678910
Exit to
Main Menu
1-24

____________________________________________________________________________Section 1
A
A
ID Format
This option defines the format for the patient ID information prompts. There are four available formats: SHORT,
STD, LONG, or CUSTOM.
The SHORT format includes the patient's last name, patient ID number, age, and sex prompts.
The STANDARD format includes the patient's last name, patient ID number, age, height, weight, sex, race,
medication 1 selection, medication 2 selection, and the Location field.
The LONG format is identical to the STD format except that it includes the patient's first name, room and comment
fields.
The CUSTOM format, which is designed in the E-SCRIBE system, can be downloaded to the ELI 100. When you
select the CUSTOM format and press the ENTER key, the following screen appears.
Download custom format?
YES NO EX
LT
2345678910
Exit to
Main Menu
Before you download the CUSTOM format, set up the defined configuration for ECG transmission (either a direct
hookup or a telephone connection). Press
YES to receive the customized ID format, and connect the
electrocardiograph accordingly.
When the download completes, the CUSTOM format becomes the ID menu format for all future ECGs until you
reconfigure the ID format. If you reset the format option to SHORT, STD, or LONG, and then wish to change back
to an original or a new CUSTOM format, you must repeat the download process.
NOTE: Also see Initial Configuration section to download custom ID.
Protected Configuration Screen N
Rhythm lead
selection:
CH1
CH2 CH3
II V1 V5
EX
LT
2345678910
Exit to
Main Menu
1-25

ELI 100_____________________________________________________________________________
A
A
Rhythm Lead Selection
This option defines the programmable rhythm strip selections for 3-channel rhythm strips. Using the 4, 5, and 6 soft
keys, you can toggle through each of the 12 leads to select the entries for the three channels.
The factory-set default values are:
Channel 1: Lead II
Channel 2: V1
Channel 3: V5
You can also print the standard lead groups (I-II-III, aVR-aVL-aVF, V1-V2-V3, V4-V5-V6), and the Frontal group
in a 6-channel format (I-II-III-aVR-aVL-aVF), and a Precordial group in a 6-channel format (V1-V2-V3-V4-V5-
V6) by toggling the GRP key on the Rhythm Activity screen while the rhythm strips are printing.
NOTE: You can print a 12-channel format by pressing RHY in the special functions menu. (See Section 5.)
Protected Configuration Screen O
Site Name:
LT
2345678910
Site Name
This option defines your clinic, hospital, or office name. You can enter up to 32 alphanumeric characters. The site
name prints just left of center at the bottom of the ECG printout.
Protected Configuration Screen P
Key
Click: Soft
LT
2345678910
1-26

____________________________________________________________________________Section 1
O
Key Click Volume Setting
This option defines the data entry click loudness. Press the soft key (2) to toggle through and select soft, medium or
loud.
EXTERNAL MODEM
The external modem initialization string is accessed by selecting ALT + SPF from the main menu and then ALT +
I. The modem command sequence can be entered and shall allow the user to enter special characters required by
some modem commands. These special characters can be entered using the ALT key in combination with the
following keys:
ALT-Key
Character ELI Display
1 !
2 @
3 #
4 $
5 %
6 ^
7 &
8 *
9 (
0 )
A ~ right arrow
B \ Japanese Yen
C ,
D E =
I {
K [
L ]
M <
N >
}
P +
Q ?
R "
S /
T '
U ;
V `
W |
X .
Y :
Before using your modem, you must configure it for country-specific operation. This is also true if you move the
modem to a different country after it has been configured for country-specific operation. The following list of
countries require "%T19,0,XX" to be added to the modem initialization string. Where “XX” is replaced by the
number in the following table. The "%T19,0,XX" should be removed for all other countries.
1-27

ELI 100_____________________________________________________________________________
A
A
%T19, 0, XX
Country XX
Australia 01
Czech Republic 25
Hong Kong 30
Hungary 30
India 30
Indonesia 30
Japan 10
Korea 30
New Zealand 09
Philippines 30
Poland 30
Singapore 30
South Africa 35
Slovenia 30
Persistent Configuration
The persistent configuration menu allows the user to define the site number and the cart number.
Begin from the Initial Configuration menu (ALT+SPF from the main screen).
Select ALT + D, to display the current site number and cart number.
Site#:
Cart#:
LT
0
2
3
4
5 6 7 8 91
Select ALT + P, to program the new site number and/or cart number.
Site#:
Cart#:
LT
0
2
3
4
5 6 7 8 91
You may enter up to four digits for the site and cart numbers; numbers from 0 to 9999 are supported.
NOTE: When site # and cart # are left blank and the ecg is printed from memory, the value for site # and cart #
will be 0 on the printout.
1-28

____________________________________________________________________________Section 1
A
A
After the new numbers are entered, the user will have the option of saving them to FLASH memory.
Saving new #’s will erase directory.
Save new #’s?: YES
NO
LT
0
2
3
4 5 6 7 8 91
If you choose to save the newly programmed site and cart numbers, the following message appears:
LT
Program FLASH with new Site/Cart #’s?
YES
0
2
3
4 5 6 7 8 91
NO
1-29

ELI 100_____________________________________________________________________________
Load Thermal Paper
Paper Installation Procedure
To open the writer, push the writer bar to the right. This releases a latch, which makes an audible click. Once
released, pull up and back on the bar to open the writer cover.
To install paper:
n
Insert a four-inch roll of thermal paper into the writer trough, as pictorially represented on the inside of
the writer.
NOTE: Be sure to install the paper grid side up.
o
Grasp the leading edge of the paper roll and pull out the paper approximately four inches beyond the closure
point of the writer.
To close, gently lower the writer cover until it stops. Then press down on the writer bar to latch the cover.
1-30

____________________________________________________________________________Section 1
To cut paper, grasp the lower edge of the paper and pull up along the printhead. The printhead acts as the cutting
edge.
NOTE: Only latch the writer cover by pressing down on the writer bar. Pressure on any other parts of the writer
may damage the writer.
NOTE: Use of paper other than approved by Mortara Instrument, Inc. may damage the writer mechanism or
produce printouts of undesirable quality.
Apply Power
To apply power to the electrocardiograph unit:
n Plug the power cord into an AC wall outlet and into the back of the ELI 100.
o Flip up the I/0 power switch, to the I position (flat to the side of the unit). The I/0 power switch can and
should remain in the
When you turn on the power switch, the word AC POWER (or CHARGING if the batteries are low) appears on the
LCD (liquid crystal display) screen. After eight hours of charging without operation, the internal battery will be
fully charged.
I position at all times.
1-31

ELI 100_____________________________________________________________________________
ELI 100 Supply List
Standard ELI 100 orders include the following accessories:
• P/N 9293-021-50/51 Resting Patient Cable (Domestic/International)
• P/N 3181-008 / -002 Hospital Grade Power Cord (Domestic/International)
• P/N 9100-004-01 ELI 100 Paper - 4 Rolls
• P/N 9300-032-50 Monitoring Electrodes
• P/N 9281-002-50 Snap Adaptors
• P/N 9502-057-50 ELI 100 Operator’s Manual
• P/N 9503-038-01 ELI 100 Short Form Instruction Card
To order additional supplies, contact a Mortara Instrument customer service representative at:
Mortara Instrument, Inc.
7865 N. 86th Street
Milwaukee, WI 53224
Phone: 888-MORTARA
(414) 354-1600
Fax: (414) 354-4760
Internet: http://www.mortara.com
1-32

____________________________________________________________________________Section 2
A
Operation
2
Starting the System
Operation
The ELI 100 operates on AC power or an internal, rechargeable battery.
AC Power Operation
To operate using AC power:
n Plug the AC power cord (use hospital grade) into the left rear receptacle of the ELI 100.
o Depress the I on the I/0 power switch located on the left rear of the ELI 100.
p The words AC POWER appear on the LCD screen.
ELI 100
If the batteries need charging, the word CHARGING is displayed on the LCD screen.
ELI 100
C POWER
CHARGING
The internal batteries are fully charged after eight hours of charging without use of the ELI 100.
Battery Operation
When fully charged, the ELI 100 can operate on batteries for approximately 4 hours.
To operate using battery power:
n Flip up
o Press the ON/OFF/DC key located on the left side of the keyboard.
The words SELF TEST appear briefly on the LCD screen followed by the Main Menu screen.
If the batteries are not charged adequately, the message BATTERY LOW appears on the LCD screen.
If the battery fuse is not installed correctly or is not functioning, a blank screen is displayed on the ELI 100. See
Section 6, “Troubleshooting.”
the I/0 power switch, located on the left side of the unit, to the I position (flat to the side of the unit).
The I/0 power switch can and should remain in the I position at all times.
2-1

ELI 100_____________________________________________________________________________
WARNING: The ELI 100 will automatically turn off (blank screen) if the batteries have been severely
discharged.
NOTE: If no keys are pressed for about four minutes, the ELI 100 turns itself off to conserve battery power. The
only exception to this operation is when the user prints a continuous rhythm strip or if the AC power is
on. (If no keys are pressed for about one minute from the main menu, the Eli 100 will turn off).
WARNING: After operating the ELI 100 using battery power, always reconnect the power cord and depress the I
on the I/O power switch. This ensures that the batteries will be recharged for the next time you use
the ELI 100. The word CHARGING or AC Power will appear on the LCD screen.
Patient Preparation
Correct electrode placement is important for acquiring a successful ECG (see Patient Hookup below). Consider
performing some patient preparation procedures to remove oils, lotions, and hair from the skin, particularly on obese
individuals.
Patient Hookup
ID Menu Formatting
The ELI 100 provides four possible formats (LONG, SHORT, STANDARD, or CUSTOM) for entering patient
identification information.
2-2

____________________________________________________________________________Section 2
A
Selecting the ID Menu
You should enter patient identification information for each patient at the beginning of a session.
To access the ID data entry menu:
• Press the ID key (9) on the Main Menu.
OR
• Press either the ECG (electrocardiogram) or RHY (rhythm strip) function key and answer YES to the
“New Patient?” question that follows.
New Patient? STATYES REQ
LT
2345678910
NOTE: If the patient is connected to the electrocardiograph and you press the ECG or RHY key, the ELI 100
automatically begins the 10-second ECG acquisition while you are entering the patient's identification
data. Therefore, you should instruct the patient to relax in a recumbent position to ensure that the ECG
is free from noise and artifact due to patient activity.
If you press the ID key from the Main Menu to enter new patient information, the entered information is stored in
memory. After completing the ID menu sequence and returning to the Main Menu, you must press the ECG or
RHY function key to run an ECG or rhythm strip.
2-3

ELI 100_____________________________________________________________________________
A
ID Menu Prompts
Depending on the ID format configured at setup, a series of LCD prompts tells you what patient information to
enter. After you type in the requested data and press the ENTER key, the blinking cursor moves sequentially to the
next data entry field. Regardless of the ID format selected in the protected configuration, the patient ID field can be
entered with upper case alphanumeric characters.
NOTE: Site number may be pre-appended to the patient ID depending on the protected configuration setting.
You should always complete the Name and Patient ID Number fields. For units with interpretive software, it is
necessary to enter the patient’s age and sex for an accurate reading. To skip other data entry fields, do not type in
any information in the field and press the ENTER key. Skipped fields appear as a blank field on the ECG report.
NOTE: If date of birth field is used in a custom ID format, the entry of the date of birth will automatically
calculate patient’s age. The date format can be configured for US or European format.
Sample ID Screen Prompt
Last name:
LT
2345678910
Blinking CursorPrompt
2-4

____________________________________________________________________________Section 2
ID Menu Sequence, Long Format
Screen Prompts Data Entry Instructions
Last name:
First name:
Patient ID:
Age:
Ht:
Wt:
Sex:
Race:
Type the patient's last name (up to 20 characters).
Press ENTER.
Type the patient's first name (up to 10 characters).
Press ENTER.
Type the patient's identification number (up to 12 digits)
using the numeric keys. Press ENTER.
Type patient's age (up to 3 digits). Press ENTER.
Type patient's height using inches or centimeters (up to 3
digits). Press ENTER.
Type patient's weight in pounds or kilograms (up to 3
digits). Press ENTER.
Type the letter “M” for male or "F" for female OR press the
forward and backward arrows (soft keys 8 and 9) to enter
male or female. If you do not make a selection, the entry
defaults to male. Press ENTER.
Type the first letter of the patient's race:
C = Caucasian
B = Black
H = Hispanic
O = Oriental
OR press the forward or backward arrows (soft keys 8 and 9) to
scroll the list for a selection. A "blank" selection is provided for
unknown race. Stop at the race you want to select and press
ENTER.
2-5

ELI 100_____________________________________________________________________________
ID Menu Sequence, Long Format, Continued
Medication 1:
Medication 2:
Location:
Room:
Comment: Use this field to further identify a room number, list patient
Press the forward or backward arrows (soft keys 8 and 9) to
scroll the list of 10 groups for a selection:
Blank (no entry)
Digitalis
Beta Blocker
Quinidine/Norpace
Diuretic
Calcium Antagonist
Proc/Lido/Tocainide
Other Antiarrhythmic
Psychotropic
Unknown
Stop at the medication you want to select and press ENTER.
Repeat instructions for Medication 1.
Press ENTER.
You can use this field to enter the patient's room number or any
other user-defined information (up to 5 alphanumeric
characters).
Press ENTER.
Use this field to enter the patient's room number (up to 5
alphanumeric characters).
Press ENTER.
symptoms, or add any other user-defined information (up to 20
character spaces).
Press ENTER.
2-6

______________________________________________________________________Section 3
A
A
3
Acquiring and Printing a 12-Lead ECG
ECG Selection Screen Description
STATNew Patient?
Emergency
ECG
Request
E-SCRIBE Files
LT
YES REQ
2345678910
Enter New Patient
Information
The ECG Selection screen appears if you press the ECG function key before entering patient identification
information on the ID menu.
The YES key (4) allows you to access the ID data entry menu to add new patient information.
The STAT key (7) allows you to acquire an emergency ECG. You can add the patient's identification
information later by editing the ID fields from the ECG directory. See Section 5 for instructions on using
the ECG directory.
The REQ key (9) enables you to select a patient from the E-SCRIBE request list. The list may contain all
the patient's ID information, which you can add to an ECG.
You should begin routine ECGs by connecting the patient to the ELI 100 and entering the patient
identification information as described in Section 2. After you complete the last data entry field on the ID
menu and press the ENTER key, the LCD screen displays the following status condition:
10:21:15
Rate: 60
cquiring ECG
If the patient has been connected correctly for 10 seconds, the “Acquiring ECG” message is displayed only
momentarily.
If there is excessive noise or lead(s) failure during the acquisition of the ECG, the following screen
appears:
10:25:21
WAITING FOR GOOD DATA
LA/V2
Rate: 60
The top line of the LCD screen shows the lead or leads that are not properly attached or that are not sending
clear data. The screen display sample "LA/V2" identifies a lead fail of the left arm (LA) and V2 chest lead.
When the problem is corrected, the ELI 100 waits for 10 seconds of good data before analyzing
the ECG. If you cannot correct the problem, you can override the message by pressing the ECG key a
second time.
3-1

ELI 100_______________________________________________________________________
A
A
ECG Analysis
The ELI 100 analyzes the ECG in approximately three seconds. The LCD screen briefly displays the
following status condition:
NALYZING ECG
The writer then prints an 8 1/2" by 11" strip that graphs the 12-lead ECG data in the format selected at
setup. See Appendix B for descriptions and illustrations of the ECG print formats.
SAVE ECG? YES NO
If the Auto Save screen is set to OFF, the system displays this screen after printing the ECG. Press YES
(4) to save the ECG and store it in the system’s memory. If you press NO (6), the ECG will not be saved
and stored.
ECG Activity Screen Description
Patient Name Patient ID
LT
Name: Robertson
4CH 25mm/s 10mm/mV 100Hz
CPY EX
2345678910
ID: 34567809
INT
Writer Speed Gain Plot FrequencyCopy Exit
Format
Selection Key
Interpretation or
NO Interpretation
The ECG Activity screen appears after the writer prints the ECG data. On this screen you can change the
writer speed, gain, plot frequency, and the interpretation option. You also can print copies of the ECG
report in any of the available plot formats or print the Measurement Matrix. To select the printing format
parameters, toggle the Format Selection (1) key.
3-2

______________________________________________________________________Section 3
(
)
(
)
grap
(
p
;
p
)
p
A
Format Option ECG Data
STD
Standard
4CH
2PG
Two Page
2.5-second graph of 12 leads in
a 3-channel format
Condensed
in a 4-channel format
contain a labeled median com
4th channel is a 10-second rhythm
stri
of Lead II
Standard format PLUS a 3-channel
10-second stri
h of 12 leads
3 channels
lex
of each Leads V1, II, V5
The CPY key (0) allows you to make an additional copy of the ECG report. Select the printing format
instructions for each copy before you press the CPY key.
The Format Selection key (1) allows you to select the copy format.
The INT/NO INT key (8) allows you to copy an ECG with the interpretation (INT) printout or without the
interpretation printout (NO INT).
The EX key (9) returns you to the Main Menu and deletes the ECG if it was not saved.
Taking a Second ECG
If you need to take a second electrocardiogram on the same patient after the first one has printed, press the
EX (exit) key to access the Main Menu. Then press the ECG function key. The LCD displays the
following screen:
Name:Robertson ID:3456709
YES NO RE Q
LT
2 3 4 5 6 7 8 910
STATNew Patient?
Since the patient's ID information is stored in memory, you can acquire another ECG by answering NO
(soft key 5) to the “New Patient?” question. The other keys, STAT, REQ, and YES perform the same
functions as described for the main ECG Selection screen.
3-3

ELI 100_______________________________________________________________________
A
Selecting an E-SCRIBE Patient File
The Request Selection screen (on the next page) appears when you select the
REQ function. The top line of the LCD displays the name and ID of each patient on file
• To scroll through the list of patients, use the << (6) (previous) and >> (7) (next) keys or access the
patient data with the keyboard by entering the first letter of the last name. If there is more than one
patient with the same initial, continue to enter the first letter until the appropriate patient name and ID
appear
• Stop at the patient ID you want to select.
• To add the patient information contained on the request list to an ECG, connect the patient to the ELI
100 and press the ECG function key on the keyboard.
• To continue selecting patient IDs from the request list, you must access the ECG Selection screen from
the Main Menu for each new patient.
Request Selection Screen
Patient Name
Patient ID
LT
Name: Robertson ID: 3456709
<< >> EX
2345678910
Return to ECG
Selection Screen
Scroll List
3-4

____________________________________________________________________________Section 4
q
p
A
A
4
Ac
uiring and Printing a Rhythm Stri
Rhythm Selection Screen Description
YES
LT
The Rhythm Selection screen appears if you press the RHY function key before entering patient identification
information on the ID menu.
The YES key (4) allows you to access the ID data entry menu to add new patient information.
The STAT key (7) allows you to print a rhythm strip immediately and bypass the entry of patient ID
2345678910
Enter New Patient
Information
STATNew Patient?
Emergency
Rhythm Strip
Acquiring Routine Rhythm Strips
You should begin routine rhythm strips by connecting the patient to the ELI 100 and entering the patient
identification information as described in Section 2. After you complete the last data entry field on the ID menu and
press the ENTER key, the rhythm strip begins printing.
See Appendix B for a description and illustration of the rhythm print format.
Rhythm Activity Screen Description
II-V1-V5
GRP
LT
Lead Group
Selection Key
The Rhythm Activity screen appears as soon as the writer begins printing the 3-channel rhythm strip. You can
change the lead groups and printing parameters during printing by pressing the corresponding soft keys.
25mm/s 10mm/mV 100Hz
2345678910
Writer Speed Gain Plot
Frequency
Rate: 60
STBY
Standby/
Continue
Key
EX
Return to
ECG
Selection
Screen
4-1

ELI 100_____________________________________________________________________________
The GRP key (0) selects a new lead group. The available groups are:
)
LEAD GROUP DESCRIPTION
I, II, III 3-channel format
aVR, aVL, aVF 3-channel format
V1, V2, V3 3-channel format
V4, V5, V6 3-channel format
Frontal 6-channel format: I, II, III, aVR, aVL, AVF
Precordial 6-channel format: V1, V2, V3, V4, V5, V6
The Writer Speed key (2) changes paper speed options (25 mm/s, 50 mm/s, 5 mm/s, 10 mm/s).
The Gain key (4) changes complex size options (10 mm/mV, 20 mm/mV, 5 mm/mV).
The Plot Frequency key (6) toggles between 100 Hz and 40 Hz.
You can place the writer in a STBY (standby) mode by pressing key 8. You can also change lead groups, writer
speen, gain and plot frequency while in this mode. To continue printing, press key 8 (CONT) again.
To stop the writer and return to the Main Menu, press the STOP key.
4-2

____________________________________________________________________________Section 5
A
5
Using the Special Functions
The special functions are additional ELI 100 programs that enable you to locate and print ECGs stored in memory,
send and receive ECGs to and from other locations, batch plot ECGs, and perform directory maintenance. To access
the Special Function screen, press the SPF key on the Main Menu.
Special Function Screen Description
DIR
LT
0
LIST
DIR
1
2 3 4 5 6 7 8
BATCH
PLT RCV REQ EX
RHY
9
Directory
Print
Directory
Batch Plot Receive
12-Lead Rhythm
ECGs
ECG
Request
Exit
The DIR key (0) allows you to access the patient directory.
The LIST DIR key (2) prints a list of all ECGs contained in the directory.
The BATCH PLT key (4) allows you to batch plot all unplotted ECGs from the directory.
The RHY key (5) allows printing of a 12-channel format rhythm strip.
The RCV key (7) allows you to receive ECGs from another electrocardiograph.
The REQ key (8) enables you to download requests from E-SCRIBE.
The EX key (9) returns you to the Main Menu.
Directory Description
The directory contains all the saved ECGs, and it can store approximately 75 ECGs depending on the storage space
required for individual records. (Storage capacity increases to approximately 300 ECGs with the serial comparison
and memory upgrade option.) The heart rate, format, and signal quality determine how much memory it takes to
store each record.
An ECG may be listed in the directory but have a “deleted” status. The directory keeps deleted ECGs in the event
that you may want to recover an ECG at some later time. However, the ELI 100 will write over a deleted ECG if the
directory is full.
If the directory is full and there are no deleted ECGs, the following message appears on the LCD:
UNABLE TO STORE ECG
You cannot save a new ECG until you either delete or erase a non-deleted ECG.
5-1

ELI 100_____________________________________________________________________________
A
Printing a Directory
To print a hard copy of a directory, press the LIST DIR key on the Special Function screen. The writer produces a
printout similar to the illustration below.
Patient Directory
dams
Davis
deConstanza
Green
Schmidt
Tolstoy
Wallinski
Patient ID Date TimeName
05/12/90
05/10/90
465238
465238
465238
774021
774021
224313
334212
334212
998742
001742
001742
001742
574391
574391
05/09/90
05/10/90
05/10/90
05/12/90
05/11/90
05/11/90
05/12/90
05/11/90
05/10/90
05/13/90
05/13/90
05/12/90
05/12/90
05/12/90
09:03:53
10:29:13
19:33:01
10:48:44
10:49:25
09:01:30
12:48:12
12:03:05
16:05:37
11:35:35
12:30:56
09:20:19
09:16:41
16:48:00
15:56:29
11:03:47
PLT XMT RTV DEL
X
X
X
The directory lists stored ECGs in alphabetical order using the patients' last names. If multiple ECGs are stored for
one patient, the last name is printed for only the first ECG. ECGs with identical patient ID numbers are grouped
together. ECGs acquired using the STAT key are placed at the top of the directory, and a blank last name field
indicates that the patient's last name was not entered.
The printout also marks ECGs (with an “X” in the appropriate column) to indicate if they have been plotted (PLT),
transmitted (XMT), retrieved (RTV), or deleted (DEL). An ECG is marked as plotted only if it was plotted after
being recalled from the directory or if it was batch plotted. An ECG that is acquired and printed in the normal
process is not marked as being plotted. Only confirmed ECGs retrieved from an E-SCRIBE system will be marked
with an "x" in the retrieved (RTV) column.
NOTE: A question mark appearing in front of a listed ECG indicates corrupt data. A printout of this ECG may
show different wave forms than the original ECG, or it may cause a reset of the electrocardiograph
configuration and directory. If a reset occurs, the ELI 100 displays an error message indicating that you
must reconfigure the setup parameters. If this occurs, contact your Mortara Service Representative for
assistance.
Directory Screen Sequence
You locate ECGs in the directory by entering the first letter of the patient's last name. To access the first level of the
directory, press the DIR (directory) key on the Special Function screen. The LCD screen then displays the Patient
Selection screen. From this screen you can identify a specific patient and erase or list all of that patient's ECGs.
Additionally, you can identify one specific ECG for the selected patient, press the SELECT key, and advance to the
Directory Maintenance screen where you can edit, erase, transmit, delete, or copy that specific ECG.
5-2

____________________________________________________________________________Section 5
Screen Sequence Directory Options
Special Function Screen
Patient Selection Screen Select a patient: Type in the first letter of the patient's last
Patient ID Screen
Directory Maintenance
Screen
The first patient name to appear on the Patient Selection screen depends on the most recent action that took place in
the ELI 100. You will see a patient name for one of the following:
• The most recently stored patient's ECG in the directory.
• The patient's ECG which has been deleted or received since the most recently-stored ECG.
• The patient's ECG which has been transmitted to the ELI 100 from another unit.
DIR key: Advances you to the Patient Selection screen.
LIST DIR key: Prints a list of all ECGs in the directory.
name.
ERASE key: Permanently removes all ECGs for the selected
patient.
LIST key: Prints a list of all the ECGs for the selected patient.
SELECT key: Advances you to the Patient ID screen where you
can select a specific ECG for the selected patient.
EX key: Returns you to the Special Function screen.
<< and >> keys: Allow you to scroll backward and forward
through the ECGs to select a specific ECG.
ERASE key: Permanently removes the ECG identified on the
LCD screen.
SELECT key: Advances you to the Directory Maintenance
screen.
EX key: Returns you to the Patient ID screen.
XMT key: Transmits the selected ECG to another unit or to an
E-SCRIBE system.
DEL key: Deletes the selected ECG record.
CPY key: Takes you to the ECG Activity screen from which you
can print a copy of the selected ECG.
RCR key: Allows you to recover a deleted ECG.
ID key: Allows you to view and edit the patient ID for the
selected ECG.
EX key: Returns you to the Patient ID screen.
5-3

ELI 100_____________________________________________________________________________
A
Patient Selection Screen Description
Selected
Patient Name
Name: Jones ID: 123456789
Patient ID
Numb er
LT
Erase ALL ECG s
for Selected
Patient
ERASE
LIST
2345678910
Display All
ECGs for Selected
Patient
SELECT
Patient ID
Screen
EX
Exit to
The Main
menu
The name displayed in the upper left-hand corner identifies the current patient name selected.
The ERASE key (1) allows you to permanently remove all ECGs for the displayed patient name.
The LIST key (3) lists all the ECGs for the displayed patient name in the same format as the directory printout
(LIST DIR).
The SELECT key (5) allows you to select a specific ECG record (on the Patient ID screen) for the displayed patient
name.
The EX key (9) returns you to the Special Function screen.
Selecting a Patient Name
To select a new patient name, use the keyboard to enter the first letter of the last name. If there are ECGs stored for
several names beginning with the same first letter, repeat pressing the (first letter) character key to cycle through the
names in alphabetical order.
To select an ECG with a blank last name field, press the SPACE bar.
Erasing a Patient's ECGs
To erase a patient's ECGs, select the patient's name and press the ERASE key. The LCD screen displays a message
asking you to confirm your instruction to erase all the ECGs.
ERASE ALL – ARE YOU SURE?
Press the YES key (4) to erase all the selected patient's ECGs or press the NO key (6) to return to the Patient
Selection screen.
NOTE: Erasing ECGs permanently removes the records from the ELI 100. There is no way to recover an erased
ECG.
5-4

____________________________________________________________________________Section 5
A
A
Listing a Patient's ECGs
To list all the ECGs for a specific patient, select the patient's name and press the LIST key. The writer produces a
printout similar to the illustration below.
Patient Directory
Name
dams
Patient ID Date
465238
465238
465238
05/09/90
05/10/90
05/10/90
Time
19:33:02
10:48:05
10:49:24
PLT XMT RTV
X
DEL
X
Selecting a Specific ECG
To access a specific ECG record, select the patient's name and press the SELECT key. Pressing the SELECT key
displays the Patient ID screen where you can select a specific ECG.
Patient ID Screen Description
LT
ERASE
1
Patient Name
Name: Jones 0123456789
0
Patient ID
Number
2 3 4 5 6 7 8
SELECT
Date and Time
03/12/93 09:15:13
Selected ECG
<< >>
EX
9
Erase Selected
ECGs
Directory Maintence
Screen
Scroll
Back
Scroll
Forward
Exit to
the Main
Menu
The ERASE key (1) allows you to permanently remove a specific ECG.
The SELECT key (5) allows you to edit the patient's ID information, transmit, delete, or copy the selected ECG (on
the Directory Maintenance screen).
The << key (7) lets you scroll back through the patient's ECGs. When you have reached the oldest record, a circular
wrap returns you to the most recent record.
The >> key (8) lets you scroll forward through the patient's ECGs. When you have reached the most recent record,
a circular wrap returns you to the oldest record.
5-5

ELI 100_____________________________________________________________________________
A
Erasing a Specific ECG
To erase a specific ECG record for the selected patient's name, use the << or >> keys to select the ECG that you
want to erase. The LCD screen displays a message asking you to confirm your instruction to erase the ECG.
ERASE ECG – ARE YOU SURE?
Press the YES key (4) to erase the selected ECG or press the NO key (6) to return to the Patient Selection screen.
Maintenance on a Specific ECG
Use the scroll keys to select the specific ECG that you wish to edit, transmit, delete, or copy before you press the
SELECT key.
Directory Maintenance Screen Description
Patient Name
Patient ID
Number
Selected ECG
Date and Time
Jones 0123456789
XMT
LT
Transmit ECG
0
DEL
2
Delete
3
CPY
4
Copy
5 6 7 8 91
Patient ID
Information
ID
03/12/90
09:15:13
EX
Exit to the
Main Menu
The XMT key (0) allows you to transmit the selected ECG to another unit or to an
E-SCRIBE system by direct communication or by telephone.
The DEL key (2) deletes the selected ECG record. The DEL key becomes the RCR key when an ECG is deleted.
Press the RCR key to recover the previously deleted ECG.
The CPY key (4) returns you to the ECG Activity screen where you can print a copy of the selected ECG. See
Section 3.
The ID key (6) allows you to view and edit the patient ID information for the selected ECG.
The EX key (9) returns you to the Patient ID screen.
NOTE: The Auto Repeat function is activated when a key is pressed for longer than one second. Since it can
take up to two seconds for the delete function to process, pressing the DEL key for longer than one
second will cause multiple deletes.
5-6

____________________________________________________________________________Section 5
Updating or Adding Patient ID Information
You can edit patient identification information already entered on an ECG or add new patient information to an
ECG that was acquired using the STAT key (emergency key). Follow the steps below:
• Press the ID key (on the Directory Maintenance screen) to enter new patient information or edit the existing
fields for the selected ECG. See Section 2 for a description of the ID menu.
• After you complete the ID menu and press the ENTER key, the Directory Maintenance screen re-appears.
• If you want a printout of the updated ECG, press the CPY key (4) to access the ECG Activity screen. On the
ECG Activity screen, press the CPY key (0)
Transmitting ECGs
You can transmit ECGs between the ELI 100 and another unit or an E-SCRIBE system using a direct connection or
the telephone. Before transmitting ECGs to an E-SCRIBE, the site number must be defined as well as the telephone
number in the configuration program. The baud rate is set automatically.
NOTE: Telephone transmission is available with modem option only.
Direct Connection Hookup
• Connect the ELI 100 to another Mortara Instrument, Inc., electrocardiograph with a unit-to-unit cable (REF
25000-024). Plug each end of the cable into the serial ports located on the left side of the units.
• Select a baud rate of 38400 bps for both units in the configuration program. Use 38400 bps for a direct
connection to E-SCRIBE.
• Turn on the respective units’ appropriate transmitting and receiving options. For the receiving unit, press the
RCV key on the Special Function screen. When the unit is ready to receive a transmission, the LCD message
RECEIVE READY shows on the LCD. For the transmitting unit, select the Main Menu for a batch transmission
or the Directory Maintenance screen for individual ECG transmissions.
Telephone Connection Hookup
To do the following, the modem option must be installed.
• Connect the ELI 100 to a standard telephone jack with the provided phone line cable. Plug the cable into the
telephone jack located on the left side of the ELI 100 and the other end into a telephone wall jack. However, if
you are using an external modem, connect the provided phone line cable to the back of the modem and connect
to a standard telephone jack.
• Turn on the appropriate transmitting and receiving option for the respective units. For the receiving unit, press
the RCV key on the Special Function screen. When the unit is ready to receive a transmission, the message
RECEIVE READY shows on the LCD. For the transmitting unit, select the Main Menu for a batch transmission
or the Directory Maintenance screen for an individual ECG transmission.
If the error message CONNECTION FAILURE appears on the screen, you may have entered an incorrect (or
undefined) telephone number. Check the number and try again.
5-7

ELI 100_____________________________________________________________________________
Sending ECGs
To batch transmit all ECGs from the ELI 100 directory, select the Main Menu and press the XMT function key on
the right side of the keyboard.
NOTE: You cannot batch transmit deleted ECGs.
Once you have used the batch mode of transmission, you cannot batch transmit the same ECGs again. However,
you can transmit the ECGs individually from the Directory Maintenance screen.
To transmit a specific ECG, identify the ECG on the Directory Maintenance screen and press the XMT key (0).
After you submit the transmission, the LCD screen displays the message:
CONNECTION IN PROGRESS
followed by
TRANSMIT IN PROGRESS
ID: (the number of the ECG being transmitted)
followed by
TRANSMIT COMPLETE or BATCH TRANSMIT COMPLETE
After the transmission of a specific ECG, the ELI 100 returns to the Directory Maintenance screen. After a batch
transmission, the ELI 100 returns to the Main Menu.
Receiving ECGs
To receive ECGs from another electrocardiograph (using either the telephone or a direct connection), select the
Special Function screen and press the RCV key. The LCD screen displays the following message:
RECEIVE READY
followed by
RECEIVING ECG (while transmission takes place)
followed by
RECEIVE READY (when reception is complete)
To terminate the receiving mode, press the STOP key. (The RCV mode terminates automatically when the
directory is full.) You can access the received ECGs from the ELI 100 directory.
5-8

____________________________________________________________________________Section 5
A
Batch Plotting
You can batch plot (print) ELI 100 unplotted ECGs. Press the BATCH PLT key on the Special Function screen.
The unit automatically prints all the directory’s received or retrieved unplotted ECGs.
Downloading a Request List
The ELI 100 can download and process a patient request list that identifies the ECGs needed from the E-SCRIBE
system. As with the other forms of ECG data transmission, you can use either the telephone or a direct connection.
Follow the hookup instructions under Transmitting ECGs in this section.
• To enter the request mode, select the Special Function screen and press the REQ (request) key.
• On the Request Activity screen that appears next, press the RCV (receive) key. The LCD screen displays the
following prompt for an identification code:
Request Identification Code:
• Type in the code defined by the E-SCRIBE system and press the ENTER key.
After you submit the code, LCD screen displays the message:
CONNECTION IN PROGRESS
followed by
REQUEST DOWNLOAD IN PROGRESS
followed by
REQUEST DOWNLOAD COMPLETE
When the download completes, the system displays the Request Activity screen.
Request Activity Screen Description
Patient Name
Patient ID
Name: Robertson ID: 34567809
RCV ERASE ID LIST << >> EX
LT
Receive
2345678910
Delete from
List
Edit Patient
ID Informati on
Print
Request List
Scroll List
Return to Special
Function Screen
5-9

ELI 100_____________________________________________________________________________
A
The RCV key (0) brings up the identification code prompt.
The ERASE key (2) allows you to erase a patient from the request list.
The ID key (3) allows you to view and/or edit the patient identification information for the name and ID displayed
on the LCD screen.
The LIST key (4) prints a directory of the request list.
The << and >> keys (6 and 7) allow you to scroll through the request list.
The EX key (9) returns you to the Special Function screen, or you can use the STOP key to return to the Main
Menu.
The top line of the LCD screen shows the name and ID of each patient on the request list. To scroll through the list
of patients, use the << (6) (previous) and >>(7) (next) keys. You can also access the patient name by pressing the
first letter of the patient's last name. If there is more than one patient with the same initial, continue to enter the first
letter until the appropriate patient name and ID appear. You can perform the following functions on the displayed
name and ID:
• Erase the patient from the request list by pressing the ERASE key.
• Edit or update patient identification information by pressing the ID key. The LCD screen displays the ID menu.
See Section 2 for instructions on how to enter patient ID information.
NOTE: You cannot directly enter requests on the ELI 100. To do so, you must first download requests from
an E-SCRIBE.
Printing the Request List printing a request list
To print a hard copy of the request list, press the LIST key. You can use the printout to locate specific patient IDs.
The writer produces a printout similar to the illustration below:
Request Directory
Smith
Johnson
dams
Brown
White
Press the STOP key to return to the Main Menu.
5-10
Patient IDName
198448367
55643010
789665431
224786533
786553420
TimeDate

____________________________________________________________________________Section 6
g
6
Maintenance and Troubleshootin
Troubleshooting Chart
LCD Message Problem Correction
BATTERY LOW Unable to acquire
BATTERY LOW
CANNOT USE PRINTER
(No message; screen is
blank.)
CONNECTION
FAILURE
CUSTOM ID DOWNLOAD
FAILURE
DIRECTORY ERROR
DIRECTORY RESET
CONFIGURATION
ERROR
MUSCLE TREMOR Noise. Check on patient electrode
NO ANSWER Unable to transmit ECG. Check for correct phone
NOTHING TO PLOT Unable to batch plot
RA, LA, LL, V1, V2, V3,
V4, V5, V6
ECG.
Unable to print. Charge the battery with AC
Blank screen. Replace the battery fuse.
Unable to transmit or
receive ECGs.
Unable to download
custom ID format from
E-SCRIBE.
Bad configuration upon
powerup.
Loss of battery power.
ECGs.
Lead fail. Indication of
Charge the battery with AC
power.
power.
Check for correct baud rate,
phone number, and cable
connections or site number.
Check for correct baud rate,
phone number, and cable
connections. Insure site
number and E-SCRIBE custom
ID are valid (if applicable).
Reconfigure parameters.
Check battery fuse.
preparation and/or patient
motion.
number. Insure modem and
E-SCRIBE are online.
None. Unit will batch plot only
unplotted ECGs.
RL/RA/LA/LL/V1/V2/V3/V4/V5/
V6. Check limb leads.
Correct faulty lead(s).
(Continued next page)
6-1

ELI 100_____________________________________________________________________________
Troubleshooting Chart, Continued
REQUEST DOWNLOAD
FAILURE
TRANSMIT FAILURE Unable to transmit ECG. Check phone line. Insure site
UNABLE TO STORE ECG No available memory.
WAITING FOR GOOD
DATA
WRITER COVER OPEN Unable to print. Close writer cover and press
WRITER OUT OF PAPER Unable to print. Insert paper and press ENTER.
Unable to download
from E-SCRIBE.
ECG data too noisy to
store.
Lead fail or noisy ECG
data.
Check for correct baud rate,
phone number, and cable
connections. Insure site
number or E-SCRIBE custom
ID are valid (if applicable).
number is valid. Try again.
Transmit or delete ECGs from
the directory. Correct noise
and try again.
Correct faulty lead or noise, or
press ECG key to override
message.
ENTER.
Self-Test Printout Description
The ELI 100 produces a test pattern that charts the following printing functions:
• Writer speed
• Print density
• Writer calibration – Speed test (100mm line)
• Printhead dots
• Performing a self-test:
n Access the Setup screen by pressing the ALT key and the SPF key simultaneously from the Main Menu.
o On the Setup screen, press the ALT key and the letter “T” simultaneously. The LCD screen displays the
SELF TEST MODE
The self-test performs a “write/read” test on each memory address. After the self-test, the hexadecimal number
(base 16) on the far right identifies the ending or last memory address code for the unit's random access memory
(RAM). If there is an error in the memory's write or read process, the address location of the error is displayed in
brackets [ ] on the screen. If this occurs, contact your Mortara Service Representative.
following message:
p To exit the Self-Test mode, turn the ELI 100 OFF and then back ON.
6-2

____________________________________________________________________________Section 6
Self-Test Printout Sample, Pg. 1 of 2
6-3

ELI 100_____________________________________________________________________________
Self-Test Printout Sample, Pg. 2 of 2
6-4

____________________________________________________________________________Section 6
Cleaning and Inspection
If the hospital or institution fails to implement a satisfactory cleaning and inspection schedule for this equipment, it
may result in equipment failure and health hazards.
NOTE: Only qualified service personnel should repair or replace ELI 100 parts.
Inspecting the ELI 100:
Check for the following conditions on a regular basis:
• No fraying or other damage to power cords, patient cables, or any other cables attached to the machine.
• No bent prongs or pins on the plugs and connectors.
• All cords and connectors are securely seated in their corresponding connections.
• The thermal writer paper is installed grid side up.
CAUTION: Do not attempt to clean the ELI 100 or patient cable(s) by submerging into a liquid, autoclaving, or
steam cleaning.
Cleaning the ELI 100:
Disconnect the power source. Clean the exterior surface of the unit with a damp cloth using a solution of mild
dishwashing detergent diluted in water. After washing, thoroughly dry off the unit with a clean, soft cloth or paper
towel.
NOTE: Do not let soap or water come into contact with the writer, plugs, jacks or vents.
Cleaning the Patient Cable:
After each use, wipe off the patient cable with a damp cloth that has been immersed in a solution of light detergent,
alcohol, or bleach. Make sure the cloth has been thoroughly rung out. Wipe dry immediately.
Cleaning the Writer Printhead:
Clean the printhead once a month. Open the writer lid by pressing the black button on the top of the writer to
release the cover latch. Pull up on the cover from the left to open the writer cover. The printer head is the brown
stripe between the two green stripes on the underside of the writer lid. Gently brush the brown stripe once or twice
with an alcohol wipe. Let the printhead dry completely.
Consumable Materials:
NOTE: There are no known risks associated with the disposal of materials provided with the ELI 100.
PAPER: Paper may be disposed of as normal waste. It is not believed to be recyclable as normal paper due to its
thermal characteristics. Check with your local recycling center or local authorities.
ELECTRODES: Dispose of electrodes in accordance with local regulations.
6-5

ELI 100_____________________________________________________________________________
6-6

__________________________________________________________________________Appendix A
Appendix
A
System Information Log
The following system information log is provided for your convenience. You need this information to set up your
system if it needs servicing. Be sure to update the information log when you add options or your system has been
serviced.
Record the model and serial number of all components, dates of removal, and/or replacement of components, and
the name of the vendor from whom the component was purchased and/or installed.
In addition to having records of this information, the system information provides a warranty record of when your
system was placed in service.
System Information Log
Manufacturer:
Mortara Instrument, Inc.
7865 N. 86th St.
Milwaukee, WI 53224
Telephone Numbers:
Domestic: 800-231-7437
European: +39-51-6650-701
Sales Department: 800-231-7437
Service Department: 888-MORTARA
Product Information:
Name of Unit/Product: ELI 100
Date of Purchase: ___/___/_____
Purchased Unit From: ____________________
Serial Number: __________________________
Software Version: __________
A-1

ELI 100_____________________________________________________________________________
For questions or service information when calling in, have the serial number and the reference number available.
The Serial Number and Part Number (REF) are found in the following places:
Product Information
Serial Number and Ref. Number (REF)
Figure A-a Serial Number – Ref. Number label located on right side of chassis as shown.
A-2

_____________________________________________________________________________Glossary
Glossary
TERM DEFINITION
Arrhythmia Irregularity in the rhythm.
Augmented lead (aVL, aVR, aVF) The difference between
one site and the average of the potential
of two other sites; unipolar extremity
leads.
Baseline drift The QRS complexes are present, but
the baseline wanders due to poor
skin/electrode contact and/or patient
movement.
Bradycardia A slow heart rate.
Calibration pulse Standardization pulse. A base to
compare the relationship of QRS
complexes to one another.
Gain An increase in amount, magnitude or
degree.
J point The end of the QRS complex; the point
where the QRS ends and the ST
segment begins (QRS offset).
Limb lead Bipolar lead that represents the
differences of electrical potential between
two selected sites (leads I, II, III).
Muscle noise Grossly uneven baseline caused by
patient body tremor or other muscle
movement. The artifact may be so large
that it overtakes the complex.
Precordial leads (V1-V6) Unipolar chest leads.
Premature atrial complex (PAC) An arrhythmia that has its origin in the
atrium.
Premature Ventricular Complex (PVC) An arrhythmia that has its origin in the
ventricle.
ST depression The amount of ST deviation below the
baseline.
ST elevation The amount of ST deviation above the
baseline.
ST level The numerical value of the measured ST
segment in microvolts.
G-1

ELI 100_____________________________________________________________________________
ST segment Ventricular repolarization. The end of the
S wave (J point) to the beginning of the T
wave.
Tachycardia A rapid heart rate.
G-2

_______________________________________________________________________________Index
Erasing a Patient's ECGs 5-4
Index
A
AC Filter 1-8
AC Power Operation 2-1
Acquiring and Printing a 12-lead ECG 3-1
Acquiring and Printing a Rhythm Strip 4-1
Acquiring Routine Rhythm Strips 4-1
Apply Power 1-20
Audience 1-1
Auto Delete 1-12
Auto Save 1-14
B
Batch Plotting 5-9
Battery Operation 2-1
Baud Rate 1-11
C
CAPS Lock 1-12
Cart Number 1-15
Cleaning and Inspection 6-5
Cleaning the ELI 100 6-5
Cleaning the Patient Cable 6-5
Cleaning the Writer Printhead 6-5
Configuration Screen Summary 1-6
Configuration Screens 1-7
Conventions 1-1
Copies 1-15
Cueing 1-14
D
Date and Time 1-18
Date Format 1-11
Direct Connection Hookup 5-7
Directory Description 5-1
Directory Maintenance Screen Description 5-6
Directory Screen Sequence 5-2
Downloading a Request List 5-9
E
ECG Activity Screen Description 3-2
ECG Analysis 3-2
ECG Selection Screen Description 3-1
ELI 100 Specifications 1-3
ELI 100 Supply List 1-21
100ELI 100, Illustration 1-2
ELI 100, Two Views: Left Side and Rear 1-4
Erasing a Specific ECG 5-6
F
Figure 1-1 ELI 100 1-2
Figure 2-1 ELI 100 Left and Rear 1-4
Figure 3-1 ELI 100 Rear (Fuse Installation) 1-5
Fuse Installation 1-5
H
Hookup, Patient, 2-2
I
ID Format 1-16
ID Menu Formatting 2-2
ID Menu Prompts 2-3
ID Menu Sequence (Long Format) 2-45
Illustration ELI 100 1-2
Initial Configuration 1-7
Input Mode Selection Screen (WPC) C-2
Inspecting the ELI 100 6-5
Internal Modem Baud Rate 1-14
Interpretation Format 1-9
Introduction 1-1
K
Key Click Volume Setting 1-18
L
Listing a Patient's ECGs 5-5
Load Thermal Paper 1-20
M
Maintenance 6-1
Maintenance on a Specific ECG 5-6
Manual Purpose 1-1
Measurement Matrix (MSR) Description B-2
Measurement Matrix Acronym List B-2
Measurement Matrix Sample (reduced) B-12
O
Operation 2-1
P
Parameters, Operational 1-6
Patient Files, E-SCRIBE 3-4
Patient Hookup 2-2
Patient ID Screen Description 5-5
I-1

ELI 100_____________________________________________________________________________
Patient Preparation 2-2
Patient Selection Screen Description 5-4
Persistent Configuration1-19
Phone Number1-13
Plot Channels1-10
Plot Format1-9
Possible LCD Messages (WPC) C-5
Pre-append site number to patient ID1-13
Printing a 12-lead ECG 3-1
Printing a Directory 5-2
Printing a Rhythm Strip 4-1
Printing the Request List 5-10
Protected Configuration1-7
Protected Configuration Screen A 1-8
Protected Configuration Screen B 1-9
Protected Configuration Screen C 1-9
Protected Configuration Screen D 1-10
Protected Configuration Screen E 1-11
Protected Configuration Screen F 1-11
Protected Configuration Screen G 1-12
Protected Configuration Screen H 1-12
Protected Configuration Screen I 1-13
Protected Configuration Screen J 1-14
Protected Configuration Screen K 1-14
Protected Configuration Screen L 1-15
Protected Configuration Screen M 1-15
Protected Configuration Screen N 1-16
Protected Configuration Screen O 1-17
Protected Configuration Screen P 1-17
Protected Configuration Screen Summary 1-8
R
Rear (Fuse Installation) 1-5
Receiving ECGs 5-8
Request Activity Screen Description 5-9
Request List Downloading 5-9
Request Selection Screen 3-4
Retrieve Serials1-15
Retrieving ECGs from E-SCRIBE 5-9
Rhythm Activity Screen Description 4-1
Rhythm Lead Selection1-17
Rhythm Selection Screen Description 4-1
Rhythm Strip (RHY) Description B-1
S
Scan Functions (WPC) C-3
Select New Channel (WPC) C-3
Setup (WPC) C-2
Sample Print Formats B-1
Selecting a Patient Name 5-4
Selecting a Specific ECG 5-5
Selecting an E-SCRIBE Patient File 3-4
Selecting the ID Menu 2-3
Self-Test Printout Description 6-2
Self-Test Printout Sample, Pg. 1 of 2 6-3
Self-Test Printout Sample, Pg. 2 of 2 6-4
Sending ECGs 5-7
Serial Port1-12
Setting the Date and Time 1-17
Setting the Operational Parameters 1-5
Setup and Installation 1-4
Site Name1-17
Site Number1-15
Special Function Screen Description 5-1
Specifications 1-3
Standard Rhythm Strip Sample (default,
reduced) B-4
Starting the System 2-1
Storage Format1-8
Storage Sensitivity1-9
Supplies, ELI 100 1-18
System Description 1-1
System Information Log A-1
System Startup 2-1
T
Taking a Second ECG 3-3
Telephone Connection Hookup 5-7
Time and Date, Setting 1-18
Transmitting ECGs 5-7
U
Units1-11
Updating or Adding Patient ID Information 5-7
I-2

ATTENTION!
TO ACHIEVE MAXIMUM BATTERY LIFE
EXPECTANCY, PLUG THE
ELECTROCARDIOGRAPH INTO AC CURRENT
WHEN NOT USED. THE BATTERIES MUST BE
CHARGED 12-24 HOURS BEFORE THE
ELECTROCARDIOGRAPH IS USED.
MO92 DR: 2


ATTENTION!
INSTALL ENCLOSED FUSE
BEFORE OPERATING UNIT.
ACHTUNG!
BEIGEFÜGTE SICHERUNG VOR
INBETRIEBNAHME
INSTALLIEREN.
ATTENZIONE!
INSTALLARE IL FUSIBILE
FORNITOPRIMA DI
ACCENDERE L’APPARECCHIO.
ATENCION!
COLOCAR EL FUSIBLE ANTES
DE ENCENDER EL APARATO.
ATTENTION!
INSTALLER LE FUSIBLE CIJOINT AVANT D’UTILSER
L’APPAREIL.
OBS!
MONTERA MEDFÕLJANDE
SÃKRING FÕRE UPPSTART.
M063 Rev: 2


CUIDADO!
Papel Registrador De Malla
Thermal Instrucciones De
Almacentamiento
ATTENTION!
Instructions Sur Le Stockage Du
Papier Quadrille
D’engegistrement Thermique
producirá un color amarillento
en el papel y descoloramiento
de la rejilla.
seco y oscuro, de preferencia a
una temperatura de 26.5 C y a
una Humedad Relative de un
65%.
1. La exposición a la luz del sol
lumière du jour, il jaunira et le
1. Si le papier reste exposé àla
2. Manténgase en un lugar fresco
quadrillage se decolorera.
frais, sec et sombre ayant de
préférence une température de
moins de 26.5° C. et une
2. Conserver dans un endroi
3. Sobres y separadores de
humidité relative de moins de
65%.
3. Les enveloppes ou séparations
páginas fabricados de cloruro
de polivinil (CPV) y de
materiales que contienen
plásticos, producen pérdida de
sensibilidad del papel no
revelado e impresión de
imágines descoloridas. El
polietileno, polipropileno y el
poliéster son recipientes
apropiados para
faites en chlorure de
polyvinyle ou autre substance
contenant un plastifiant
semblable pourraient causer
une perte de sensibilisation du
papier non developpé et la
décolorationdes papiers déjà
imprimés. Les enveloppes en
polyethylène polypropylène et
polyester peuvent être
almacenamiento.
solventes tales como alcohol,
éster y acetona producen el
revelado de la capa de color y
no deben ser empleados.
4. Adhesivos que contienen
employées.
à base de dissolvants tels que
de l’alcool, ed l’ester et de
4. Eviter d’employer tout adhésif
La mayoría de las cintas adhesivas
l’acétone car il pourrait causer
le développement de la couche
colorée du papier.
transparentes producen tambien
imágines descoloridas.
Pour la même raison, la plupart des
papiers collants transparents
cousent la décoloration des images.
VORSICHT!
Anmerkungen Zur
ATTENZIONE!
Istuzioni Per La Conservazione
bewirkt Vergilben des Papiers
und Verblassen des Rasters.
trocken und dunkel sein,
vorzugsweise mit einer
Aufbewahrung Von
Warmegitter-
Aufzeichnungspapier
1. Aussezung an Sonnenlicht
Della Carta Termica Millimetrata
Da Registrazione
1. Se esposta alla luce solare, la
2. Aufbewahrungsort soll kuehl,
carta termica ingiallisce e la
griglia millimetrata scolorisce.
fresco e asciutto. La
temperatura deve essere
2. Conservare al buio in luogo
Temperatur von unter 26.5
Celsius und 65% relativer
Feuchtigkeit.
Blattscheider, die aus
Polyvinylchlori (PVC) und
aehnlichen Plastifikatoren
enthaltenden Materialien
hergestellt sind, resultieren in
3. Umschlaege und
preferibilmente inferiore a 26°
C e l’umidità relativa del
65%.
composti di cloruro di
polivinile (PVC) e simili
materiali contenenti
plastificante causano
scolorimento delle immagini e
3. Buste e fogli di separazione
Empfindlichkeitsverlusten des
nicht entwickelten Papieres
und Verblassen der
gedruckten Bilder.
Polyaethylen, Polypropylen
und Polyester sind
ausreichendals
Aufbewahrungsbehaelter.
Loesungsmittel enthalten, wie
4. Klebestoffe, die
perdita di sensibilità della
carta non avvolta in buste
protettive. Si possono invece
impiegare polietilene,
polipropilene e poliestere
come materiali per contenitori
protettivi.
contenenti solventi come alcool,
estere e chetone che favoriscono
4. Evitare l’uso di adesivi
zum Beispiel Alkohol, Ester
und Keton, verursachen die
Entwicklung der Farbschicht
und sollten vermieden werden.
Auch bewurken die meisten
transparenten, selbsthaftenden
Klebstreifen ein Verblassen der
Bilder.
lo sviluppo della pellicola
termica con conseguente
inscurimento della carta. Allo
stesso modo, la maggior parte
degli adesivi trasparenti causano
lo scolorimento delle immagini.
CAUTION!
Thermal Grid Recording Paper
cause yellowing of the paper
and fading of the grid.
place, preferably below 80° F
and 65% relative humidity.
Storage Notes
1. Exposure to sunlight will
2. Store in a cool, dry, dark
3. Envelopes and sheet
separators made of polyvinyl
chloride (PVC), and similar
plasticizer-containing
material, cause loss of
sensitivity of undeveloped
paper and fading of printed
images. Polyethylene,
polypropylene, and polyester
are satisfactory as storage
containers.
solvents, such as alcohol, ester
and ketone, cause
developments of the colored
coating and should be
avoided.
4. Adhesives containing
Likewise, most clear self-adhesive
tapes cause image fading.
REF.: 9504-001C1

 Loading...
Loading...