Page 1
ProAxis
®
Spinal Surgery Table
6988, 6988I
Owner’s Manual
This manual is supplied in the following versions:
English (EN)
Spanish (ES)
French (FR)
German (DE)
Italian (IT)
Portuguese (PT-BR)
Japanese (JA)
Multilingual (ML)
NW0725 Rev K
MIZUHO OSI ©2016
MIZUHO OSI
30031 Ahern Avenue
Union City, CA 94587-1234
Telephone: 510-429-1500
Outside USA: +1-510-429-1500
Toll Free: 800-777-4674
Fax: 510-429-8500
WWW.MIZUHOSI.COM
NEWHIPNEWS.COM
Emergo Europe
Molenstraat 15
2513 BH The Hague
The Netherlands
Telephone: (31) (0) 70 345-8570
Fax: (31) (0) 70 346-7299
Page 2

Table of Contents
1 Important Notices ................................................................................................................ 1
1.1 Trademarks and Patents ......................................................................................................... 5
1.2 Disposal of Electrical Components ........................................................................................ 5
2 Introduction ........................................................................................................................ 6
2.1 General Description ................................................................................................................. 6
2.2 Intended Use ............................................................................................................................. 7
2.3 User Profile ............................................................................................................................... 7
2.4 Training Requirements ............................................................................................................ 7
2.5 Conditions of Use .................................................................................................................... 7
2.6 Product Lifetime ....................................................................................................................... 7
2.7 Specifications ........................................................................................................................... 8
2.8 Shipping and Storage .............................................................................................................. 9
2.9 Glossary of Terms.................................................................................................................. 10
3 Component Identification .................................................................................................12
3.1 Table Orientation .................................................................................................................... 12
3.1.1 IntelliPendant
3.1.2 Cord Wrap ....................................................................................................................................... 14
3.1.3 Emergency Stop ............................................................................................................................. 14
3.1.4 Auxiliary Control Panel ................................................................ .................................................. 15
3.1.5 Advanced Control Pad System™ .................................................................................................. 15
®
................................................................................................................................ 14
3.2 Storage Cart ............................................................................................................................ 16
3.3 Component Part Number ...................................................................................................... 17
3.4 Model Number and Serial Number ....................................................................................... 18
4 Basic Operation .................................................................................................................19
4.1 Control Operation .................................................................................................................. 19
4.2 Emergency Stop ..................................................................................................................... 20
4.3 Auxiliary Control Panel ......................................................................................................... 20
4.4 Floor Locks ............................................................................................................................. 21
4.4.1 IntelliPendant
4.4.2 Manual Floor Lock Override .......................................................................................................... 22
®
Activated Floor Lock Override ............................................................................ 22
4.5 Moving the Table .................................................................................................................... 22
4.6 Torso Trolley
4.7 IntelliPendant
®
......................................................................................................................... 23
®
........................................................................................................................ 23
i
Page 3

4.7.1 Table Functions .............................................................................................................................. 26
4.7.2 Defined Function and Setting Symbols ........................................................................................ 27
4.7.3 Return to Level ............................................................................................................................... 30
4.7.4 Soft Keys Functions and Settings ................................................................................................ 30
4.7.5 Configuration of Table for Transport or Storage ......................................................................... 38
4.7.6 Version Info ..................................................................................................................................... 47
4.8 Independent Monitoring System (IMS) ................................................................................ 47
5 Inspection ..........................................................................................................................49
5.1 Acceptance and Transfer ...................................................................................................... 49
5.2 Pre-Procedure/Post-Procedure ............................................................................................ 49
5.3 Semi-Annual Preventative Maintenance .............................................................................. 49
5.4 Product Lifetime ..................................................................................................................... 49
6 Function Check .................................................................................................................50
7 ProAxis® Spinal Surgery Table Standard Components ..................................................54
7.1 ProAxis
7.2 ProAxis
®
Standard Components .......................................................................................... 54
®
Patient Care Kits .................................................................................................... 56
8 Setup of ProAxis® Spinal Surgery Table for Surgical Procedures .................................57
8.1 ProAxis
8.1.1 Components Used for Prone Patient Positioning ........................................................................ 79
8.2 ProAxis
8.2.1 Prone Positioning with Cervical Management Base Unit ............................................................ 82
8.2.2 Prone Patient Positioning with Cervical Traction Vector Adjustor ............................................ 91
8.2.3 Components for Prone Patient Positioning with Cervical Accessories .................................... 93
8.3 ProAxis
8.3.1 Components Used for Supine Patient Positioning .................................................................... 102
8.3.2 Supine Patient Positioning with Cervical Traction Vector Adjustor ........................................ 103
8.3.3 Components for Supine Patient Positioning with Cervical Accessories ................................. 104
8.4 ProAxis
8.4.1 Components Used for Lateral Patient Positioning .................................................................... 109
®
Prone Patient Positioning ..................................................................................... 59
®
Prone Patient Positioning with Cervical Management ...................................... 82
®
Supine Patient Positioning ................................................................................... 95
®
Lateral Patient Positioning ................................................................................. 105
9 ProAxis® Optional Accessories ...................................................................................... 110
10 Cleaning, Storage, and Maintenance ............................................................................. 112
10.1 Cleaning and Disinfecting ................................................................................................. 112
10.1.1 Table Exterior .............................................................................................................................. 112
10.1.2 ProneView
®
Helmet and Mirror System .................................................................................... 112
ii
Page 4

10.1.3 Mizuho OSI Tempur-Pedic
®
Medical Pads ................................................................................ 113
10.2 Storage ................................................................................................................................ 113
10.3 Maintenance ....................................................................................................................... 113
11 Software Troubleshooting .............................................................................................. 115
11.1 Software Release Notes .................................................................................................... 122
11.1.1 Software Version 1.105 .............................................................................................................. 122
11.1.2 Software Version 1.110 .............................................................................................................. 123
11.1.3 Software Version 1.111 .............................................................................................................. 124
11.2 Manual Floor Lock Override ............................................................................................. 125
12 Electrical System ............................................................................................................ 127
12.1 Description ......................................................................................................................... 127
12.2 Component Identification and Function .......................................................................... 127
12.2.1 Detachable Power Cord ............................................................................................................. 127
12.2.2 Power Switch .............................................................................................................................. 127
12.2.3 Power/Charging Indicator .......................................................................................................... 127
12.2.4 Circuit Breakers .......................................................................................................................... 128
12.2.5 The Emergency Stop Button ..................................................................................................... 128
12.2.6 IntelliPendant
®
............................................................................................................................ 128
12.3 Battery System ................................................................................................................... 128
12.3.1 Charging the Batteries ............................................................................................................... 129
12.3.2 Replacing the Batteries .............................................................................................................. 129
12.3.3 Lithium Battery ........................................................................................................................... 133
13 Technical Support ........................................................................................................... 134
13.1 Contact for Parts and Service ........................................................................................... 134
13.2 Further Technical Information .......................................................................................... 134
13.3 Order Replacement Parts .................................................................................................. 134
13.4 Return Damaged Parts ...................................................................................................... 134
13.5 Send a Part for Repair ....................................................................................................... 135
13.6 Warranty .............................................................................................................................. 135
13.7 European Union EC Representative ................................................................. 135
14 Appendix .......................................................................................................................... 136
14.1 Electromagnetic Emissions .............................................................................................. 136
14.2 Electromagnetic Immunity ................................................................................................ 137
14.3 Recommended Separation Distances.............................................................................. 139
iii
Page 5

ProAxis® Spinal Surgery Table Owner’s Manual
1
Mizuho OSI 2016
NW0725 Rev K
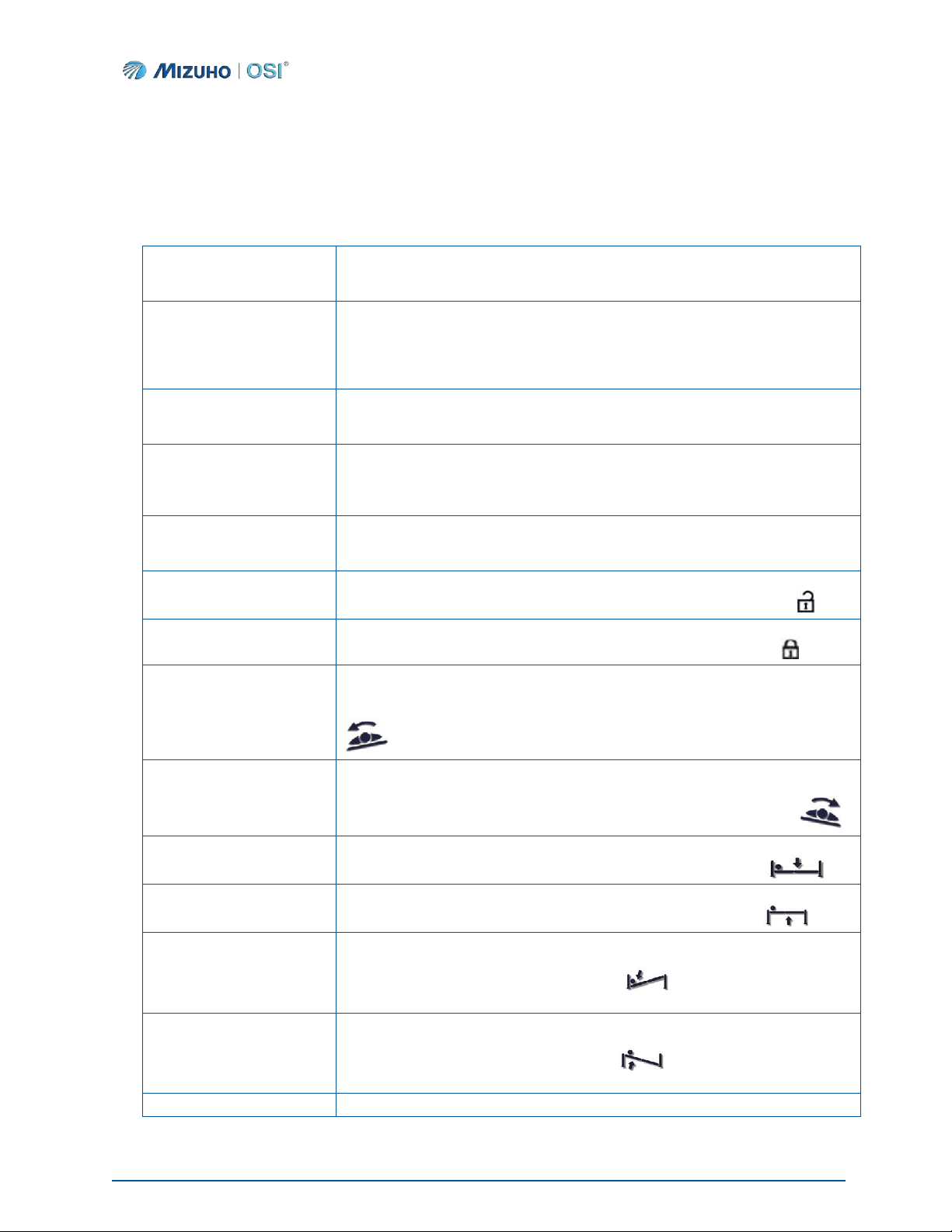
Symbol
Meaning
This symbol indicates an authorized representative in the European Community.
This symbol indicates the Manufacturer of the device.
This symbol indicates the Date of manufacture.
NOTE:
This symbol indicates a comment or instruction of importance.
This symbol is to signify CAUTION. It is intended to alert the user to consult the
documentation for safety-related information such as warnings and precautions that
cannot, for a variety of reasons, be presented on the device itself.
This WARNING symbol is intended to alert the user of important operation,
maintenance, or safety instructions.
This symbol indicates proper disposal instructions.
This symbol indicates a product number.
This symbol indicates a serial number.
This symbol indicates that you must read the owner’s manual before use.
This symbol indicates that you are advised to refer to the owner’s manual before use.
This symbol directs the user to follow operating instructions (related to battery safety).
This symbol indicates an external ground stud that is required for use when the AC
power cable is not connected to a protective earth ground hospital grade AC outlet in
your operating room or facility.
1 Important Notices
CAUTION: To ensure safe operation of the equipment, please READ THESE INSTRUCTIONS
COMPLETELY and keep this manual readily available for future reference.
Carefully observe and comply with all warnings, cautions and instructions placed on the equipment or
described in this manual.
NOTE: This device is intended for use by trained personnel only. To schedule an in-service,
please contact your domestic Mizuho OSI sales representative or call 1-800-777-4674
within the USA or +1-510-429-1500 outside the USA.
NOTE: The application techniques outlined in these instructions are the manufacturer’s
suggested techniques. The final disposition of each patient’s care as related to the use
of this equipment rests with the attending surgeon.
In this manual, the following symbols are used:
Page 6

ProAxis® Spinal Surgery Table Owner’s Manual
2
Mizuho OSI 2016
NW0725 Rev K
This symbol indicates this equipment is an applied part TYPE B in accordance with IEC
60601-1 and is generally suitable for applications involving external or internal contact
with the patient, excluding the heart. The patient circuit is connected to protective earth
and this equipment should be connected only to hospital grade AC outlets with a
protective earth ground.
This symbol represents the visual alarm signal associated with the table being
overloaded.
This symbol represents an Emergency Stop.
This symbol represents the table’s Weight Limit.
This symbol indicates that a Warning exists should the Torso Trolley® Chest Tray not be
locked in place on the Rail Mounts.
This symbol indicates that a Warning exists related to actions to be taken, and the
owner’s manual must be read before use.
This symbol represents a Pinch Hazard.
This symbol indicates Do not touch, housing energized (related to battery safety).
This symbol indicates Warning, dangerous voltage (related to battery safety).
This symbol indicates a medical device that needs to be protected from moisture.
This symbol indicates a medical device that can be broken or damaged if not handled
carefully.
This symbol indicates “This End Up”, and identifies the correct orientation for safe
handling.
This symbol indicates the temperature limits to which the medical device can be exposed
safely.
This symbol indicates the range of humidity to which the medical device can be exposed
safely.
This symbol indicates the range of atmospheric pressure to which the medical device
can be exposed safely.
Page 7

3
Mizuho OSI 2016
NW0725 Rev K
This symbol indicates Do Not Stack.
This symbol identifies POWER OFF.
This symbol identifies POWER ON.
ProAxis® Spinal Surgery Table Owner’s Manual
Page 8

ProAxis® Spinal Surgery Table Owner’s Manual
4
Mizuho OSI 2016
NW0725 Rev K
WARNING: Proper preoperative and intra-operative procedures must be followed to prevent
venous stasis and pooling, pressure sore development, neuropathy, improper electro-surgical
tissue grounding, hypertension/hypotension, and hypothermia.
WARNING: To avoid the risk of electric shock, this equipment must only be connected to a
supply mains with protective earth.
WARNING: The ProAxis® Table is to be used by personnel that receive training from either
Mizuho OSI or from someone qualified by the medical facility to provide this training. Failure to
comply with this requirement may result in damage to the table, possible injury to the patient or
harm to the healthcare workers.
WARNING: This symbol indicates an external ground stud that is required for use
when the AC power cable is not connected to a protective earth ground hospital grade AC outlet
in your operating room or facility. To protect the patient, hospital staff, and the device from
possible electrical hazards, an external ground wire connection is required between the external
ground stud and protective earth ground when the device is in use under battery power or not
connected to a protective earth ground.
WARNING: Medical electrical equipment needs special precautions regarding EMC and
needs to be installed and put into service according to the EMC information provided in this
manual.
WARNING: Use of the ProAxis® Spinal Surgery Table with patients weighing more than
500 lbs (227 kg) could result in damage to the table, possible injury to the patient, or harm to the
healthcare professional.
WARNING: When unlatching the spars from the Head-End of the ProAxis® Spinal Surgery
Table for either storage or cleaning, do not use excessive force to unlatch the spars. If after
unlocking the spar lock, the spar does not unlatch with hand force, seek assistance from qualified
service personnel. Failure to properly unlatch the spars may cause harm to the healthcare
professional and/or the device.
WARNING: Before and after each use, inspect the table, components and accessories for
possible damage, excessive wear, or non-functioning parts. Carefully inspect all accessible areas,
joints, and all moving parts for possible damage or non-function. Damaged or defective parts
should not be used or processed. Contact Mizuho OSI Services for repair or replacement (refer to
Section 13).
WARNING: The ProAxis® Spinal Surgery Table should not be operated in an oxygen-rich
environment nor in the presence of flammable anesthetics, volatile substances, or other explosive
gases, liquids, or atmospheres.
WARNING: No modification of the ProAxis® Spinal Surgery Table or its components is
Page 9
ProAxis® Spinal Surgery Table Owner’s Manual
5
Mizuho OSI 2016
NW0725 Rev K
allowed. Any modification to the equipment may result in damage to the table, possible injury to
the patient or harm to the healthcare professionals.
CAUTION: As outlined in the AORN Recommended Practices for Positioning a Patient in the
Perioperative Setting, following the positioning of the patient, an assessment of the patient’s
alignment, tissue perfusion, and skin integrity should be completed. All contact points of the
patient with the table pads should be monitored during the procedure.
NOTE: If the integrity of the AC power source is in doubt, the equipment shall be operated from
its internal electrical power source (battery).
NOTE: If high-frequency surgical equipment, cardiac defibrillators or cardiac defibrillator monitors
are to be used with the ProAxis® Spinal Surgery Table, refer to the instructions for use
provided by the manufacturer of those devices.
1.1 Trademarks and Patents
ProAxis®, IntelliPendant®, Torso Trolley®, GentleTouch®, ShearGuard®, and Orange Aid® are registered
trademarks of Mizuho OSI.
Advanced Control Pad System™ is a trademark of Mizuho OSI.
DORO® is a registered trademark of pro med instruments GmbH.
Mayfield® is a registered trademark of Schaerer Mayfield USA, Inc.
O-arm® is a registered trademark of Medtronic, Inc.
ProneView® is a registered trademark of Dupaco, Inc.
Tempur-Pedic® is a registered trademark of Tempur-Pedic North America, Inc.
Velcro® is a registered trademark of Velcro Industries.
Product protected by:
US Patent Number: 8,584,281
(Other patents pending)
1.2 Disposal of Electrical Components
In accordance with the European Union Waste Electrical and Electronic Equipment (WEEE)
Directive, all electrical components and batteries must be disposed of in accordance with local regulations
or returned to Mizuho OSI for proper disposal. Please contact Mizuho OSI Services at 1-800-777-4674
within the USA or +1-510-429-1500 outside the USA for further information regarding this requirement.
Page 10

ProAxis® Spinal Surgery Table Owner’s Manual
6
Mizuho OSI 2016
NW0725 Rev K
Foot-End
Head-End
2 Introduction
2.1 General Description
The ProAxis® Spinal Surgery Table (Figure 1) is designed to support and position a patient undergoing
surgical procedures while allowing for the articulation of the patient’s spine intra-operatively through
flexion and extension movements. These intra-operative movements allow the natural movement of the
spine to be replicated during the surgical procedure. The table provides prone, supine, and lateral
positioning capabilities, with enhanced user and patient benefits through key proprietary features. The
table’s radiolucent hinge, spars, and two-piece supine top also allow for excellent intraoperative imaging
using either a C-arm or O-arm®.
The ProAxis® table has electrically powered positioning and operating functions, which are controlled by
means of the IntelliPendant®. The IntelliPendant® provides a visual status display for the Floor Locks,
Hinge Mode, Advanced Control Pad System™ (ACP), Battery Status, Height Status,
Trendelenburg/Reverse Trendelenburg Angle, Hinge Angle, and Lateral Tilt Angle. The table provides a
further feature in providing the user with the option of selecting between two Hinge Mode settings – Fixed
Surgical Site (FSS) or Fixed End (FE) mode.
The table includes Mizuho OSI Tempur-Pedic® medical pad technology. The material used in the
manufacture of the Mizuho OSI pads has viscoelastic properties and is temperature sensitive, becoming
softer where the patient’s body makes the most contact with the surface and remaining firm in the areas
where less body contact is being made. Pressure is distributed evenly over the entire surface area. The
pads are radiolucent and made without natural rubber latex. The benefits of using Mizuho OSI TempurPedic® Medical Pads are improved pressure management, reduced shear forces, and enhanced patient
comfort when used in ambient temperatures. In accordance with AORN recommendations, it is important
to limit skin exposures to lower ambient temperatures, protect the patient by initiating passive warming
interventions (e.g. applied forced-air warming systems, blankets, drapes and reflective composites), and
to maintain an ambient room temperature of 20° to 25°C.
The Advanced Control Pad System™ is utilized when the patient is positioned prone. The pad control
system, which is built into the table, is electronically controlled and pneumatically actuated, providing
massage action and periodic pressure point stimulation through the Contoured ACP Hip Pads.
Figure 1: ProAxis® Spinal Surgery Table – Prone Patient Setup, Fixed Surgical Site Hinge Mode
Page 11

ProAxis® Spinal Surgery Table Owner’s Manual
7
Mizuho OSI 2016
NW0725 Rev K
2.2 Intended Use
The ProAxis® Spinal Surgery Table is a mobile, dual-column, carbon-fiber hinged frame surgery table
designed for temporary support (<24 hours) and positioning of a patient in a prone, supine, or lateral
position. The table is intended for use during surgical procedures, including radiographic imaging during
such procedures. The table is not intended for use in patient transport.
ProAxis® provides a platform designed to support and position adult and pediatric patients with body
weight less than 500 pounds (227 kg) that fall within the height range of 58-81 inches (147-206 cm).
The ProAxis® system, when used with the Torso Trolley® Chest Pad and the Contoured Hip Pads, shall
support patients in a prone position with minimal iliac crest to iliac crest distances of 8.0 inches (20.3 cm).
2.3 User Profile
The ProAxis® Spinal Surgery Table is suitable for use by health care professionals, including but not
limited to surgeons, radiologists, anesthesiologists, circulating nurses, surgical technicians, biomedical
technicians, and radiology technicians.
2.4 Training Requirements
Before using the ProAxis® Spinal Surgery Table, the user must read this ProAxis® Spinal Surgery Table
Owner’s Manual ( NW0725).
It is required that personnel using the ProAxis® Spinal Surgery Table receive training by either Mizuho
OSI or by someone qualified by the medical facility to provide this training.
WARNING: Failure to ensure training prior to use of this device may cause harm to the
patient, healthcare professional, or the device.
2.5 Conditions of Use
The ProAxis® Spinal Surgery Table may be used several times throughout the day and night in medical
facilities; e.g. hospitals, and outpatient surgery/imaging centers. The ProAxis® Spinal Surgery Table will
be used in an operating room or other treatment room, and may be rolled between rooms. It shall not be
used for patient transport.
WARNING: To maximize patient safety, do not move the table with surgical equipment in
vivo that is not free to move with the patient.
2.6 Product Lifetime
The product’s service lifetime is defined as 10 years. At the time of delivery, your product fulfills existing
regulations and standards. However, despite proper use, routine inspection, prescribed service,
maintenance and repairs, the product is subject to aging and wear. Therefore, Mizuho OSI cannot
guarantee the product’s safety after ten (10) years and recommends your product be taken out of service.
For product warranty information, refer to Section 13 of this manual.
Page 12

ProAxis® Spinal Surgery Table Owner’s Manual
8
Mizuho OSI 2016
NW0725 Rev K
2.7 Specifications
The ProAxis® Spinal Surgery Table has the following specifications:
The maximum patient load is 500 pounds (227 kg) in a procedural position at any point within its
physical range.
The patient height range supported by the table is 58-81 inches (147-206 cm).
Table top width:
o The open frame used for prone positioning is 19 inches (48 cm).
o The Supine Tops used for supine and lateral positioning are 21 inches (53 cm).
Table top length is 81 inches (206 cm).
When in use, the overall length of the table is 122-138 inches (310 - 351 cm) dependent on
where the table is in its range of movements.
The table can be configured to a length of 80 inches (204 cm) for storage (Figure 55, page 46).
Defined Home position: Height of 32 inches (81 cm) top of hinged frame to floor with a level table
top (i.e. 0° Hinge, Tilt, and Trendelenburg/Reverse Trendelenburg).
Height Range: 20 inches – 47 inches (51 cm – 119 cm) when table top is Level.
Lateral Tilt (left/right) is 0 – 15 degrees.
Trendelenburg is 0 – 15 degrees from the Home position
Reverse Trendelenburg is 0 – 20 degrees from the Home position
Two Hinge Modes: Fixed Surgical Site (FSS), Fixed End (FE)
Hinge Angle Range: -20 to +35 degrees
o Some height adjustments may be required to achieve the maximum hinge up angle in
FSS only.
o Some height adjustments may be required to achieve the minimum hinge down angle.
The table trapezoidal rail frame and Supine Tops are made of radiolucent carbon-fiber
construction.
Input power requirement is 100/120V, 50/60 Hz ( 6988) or 220/240V, 50/60 Hz ( 6988I) as
indicated on the manufacturer’s label.
As a back-up power source, the table may be operated under battery power. The expected
working life of a fully charged battery is approximately eight (8) hours with two procedures
averaging six (6) minutes of motion time.
The table is IPX4 rated per IEC 60529.
Operating environment: +50 to +86 degrees F (+10 to +30 degrees C), relative humidity 40%-70%.
Class 1 Equipment, Type B per IEC 60601-1.
The table is not suitable for use with flammable anesthetic gas mixtures.
Page 13

ProAxis® Spinal Surgery Table Owner’s Manual
9
Mizuho OSI 2016
NW0725 Rev K
2.8 Shipping and Storage
If required to be transported, the ProAxis® Spinal Surgery Table must be transported using the
appropriate shipping crate. Unpacking instructions are included with the original shipping crate.
When not in use, the ProAxis® Spinal Surgery Table should be stored in a clean, dry environment.
The following conditions are required of the shipping and or storage environment:
Ambient temperature -4°F (-20°C) to 122°F (50°C)
Relative humidity from 10% to 95%, non-condensing
Atmospheric pressure from 75 to 105 kPa
To prepare the table to be shipped or stored in a more compact configuration, utilize the IntelliPendant®
and complete the steps outlined (Section 4.7.5). Transporting or storing the table in the stored
configuration reduces the length of the table from 122 inches (310 cm) to 80 inches (204 cm).
When in storage, the table cover provided serves as a dust cover and should be used. The table cover
can be adjusted with the Velcro® closures so it will fit the ProAxis® in the standard use table position (fully
extended) or stored configuration (reduced length). Also, to ensure that the battery is always fully
charged and ready for use, store the table connected to AC power that matches the ratings on the
manufacturer’s label, located on the Foot-End column (Figure 10, page 18).
Page 14
ProAxis® Spinal Surgery Table Owner’s Manual
10
Mizuho OSI 2016
NW0725 Rev K
Head-End Column
The Head-End Column consists of one set of linkage arms and the base. The
Power Switch, Emergency Stop Button, Auxiliary Control Panel, and port for the
IntelliPendant® are located on the Head-End Column.
Head-End Frame
The Head-End Frame of the ProAxis® is the patient support structure comprised
of the head board, rails and hinge components extending from the Head-End
Column. The Head-End Frame is intended to support the patient’s upper torso,
and therefore, the Head-End Frame Rails are shorter than the Foot-End Frame
Rails.
Foot-End Column
The Foot-End Column consists of one set of linkage arms and the base. The
manufacturer’s label and the port for connecting the Advanced Control Pad
System™ Tubing are located on the Foot-End Column.
Foot-End Frame
The Foot-End Frame of the ProAxis® is the patient support structure comprised of
the rails extending from the hinge to the Foot-End Column. When the patient is
positioned on the table, his/her lower torso and feet are oriented toward the FootEnd Column.
Retractable Center Beam
The Retractable Center Beam connects the Head-End and Foot-End Columns.
The Beam retracts, allowing the table to be transported or stored in smaller
configuration.
Floor Locks Unlocked
Refers to the Floor Locks being fully retracted. The button on the IntelliPendant®
and the Auxiliary Control Panel used to unlock the Floor Locks is labeled .
Floor Locks Locked
Refers to the Floor Locks being fully deployed. The button on the IntelliPendant®
and the Auxiliary Control Panel used to lock the Floor Locks is labeled .
Left Side of the Table
Refers to the left side of the table (as you stand at the Head-End Column and look
to the Foot-End Column). The button on the IntelliPendant® and the Auxiliary
Control Panel used to laterally roll (tilt) the table top to the left is labeled
.
Right Side of the Table
Refers to the right side of the table (as you stand at the Head-End Column and
look to the Foot-End Column). The button on the IntelliPendant® and the Auxiliary
Control Panel used to laterally roll (tilt) the table top to the right is labeled .
Lower the Table
Refers to lowering the Height of the table top. The button on the IntelliPendant®
and the Auxiliary Control Panel used to lower the table top is labeled .
Raise the Table
Refers to raising the Height of the table top. The button on the IntelliPendant®
and the Auxiliary Control Panel used to raise the table top is labeled .
Trendelenburg
Refers to lowering the height of the Head-End of the table top relative to the FootEnd of the table top. The button on the IntelliPendant® and the Auxiliary Control
Panel used to lower the Head-End is labeled . The height of the hinge is
maintained during this motion.
Reverse Trendelenburg
Refers to lowering the height of the Foot-End of the table top relative to the HeadEnd of the table top. The button on the IntelliPendant® and the Auxiliary Control
Panel used to lower the Foot-End is labeled . The height of the hinge is
maintained during this motion.
Hinge Up
Refers to the frame hinging such that the table is flexed upwards. The button on
2.9 Glossary of Terms
This glossary of terms assumes that the patient is positioned prone with his or her head oriented towards
Head-End Column of the table and his or her feet towards the Foot-End Column. The functions of the
IntelliPendant® and Auxiliary Control Panel are oriented for this position.
Page 15

ProAxis® Spinal Surgery Table Owner’s Manual
11
Mizuho OSI 2016
NW0725 Rev K
the IntelliPendant® and the Auxiliary Control Panel used to Hinge Up the frame is
labeled .
Hinge Down
Refers to the frame hinging such that the table is flexed downwards. The button
on the IntelliPendant® and the Auxiliary Control Panel used to hinge down the
table is labeled .
Advanced Control Pad
System™ (ACP)
The Advanced Control Pad System™ provides user-selectable, pressure point
stimulation to the patient’s hips, thighs and chest when positioned prone. The
Contoured Hip Pads have built in cells that alternate between inflating and
deflating with air.
Return to Level
Refers to the motion of the table required to return the table top to the neutral
position. When the Return to Level function is activated, the Lateral Tilt Angle
returns to 0 degrees; Trendelenburg / Reverse Trendelenburg Angle returns to
0 degrees and the Hinge Angle returns to 0 degrees. Following a one (1) second
pause, if the button continues to be depressed, the table top will return to a height
of 32 inches (81 cm) above the floor, which is defined as its Home position.
NOTE: The complete functionality of the Return to Level feature
requires either the Rail Mounts or the Supine Tops to be
installed on the open frame to be operational. If either of these
components is not installed, the Return to Level feature will only
return the Lateral Tilt Angle to 0 degrees and the table top
height to 32 inches (81 cm) above the floor.
The IntelliPendant® button used to return the table to level is labeled .
Home Position
The position of the ProAxis® when the table top is level head-to-foot and side-to
side, the hinge is neutral and the height of the table top is 32 inches (81 cm)
above the floor.
Fixed Surgical Site (FSS)
Hinge Mode
The hinge mode of the Frame that raises and lowers the Head-End and Foot-End
while maintaining the height of the hinge as it is raised or lowered. This motion
approximates “fixing” the surgical site at a constant position.
When FSS Hinge Mode is selected and the Hinge Angle is positive, the
symbol is visible on the display of the IntelliPendant®.
When FSS Hinge Mode is selected and the Hinge Angle is negative, the
symbol is visible on the display of the IntelliPendant®.
Fixed End (FE) Hinge
Mode
The hinge mode of the Frame that maintains the Head-End and Foot-End at a
constant height as the hinge is raised and lowered.
When FE Hinge Mode is selected and the Hinge Angle is positive, the
symbol is visible on the display of the IntelliPendant®.
When FE Hinge Mode is selected and the Hinge Angle is negative, the
symbol is visible on the display of the IntelliPendant®.
Hospital Grade AC Outlet
Refers to specially designated outlets (receptacles) that include additional
grounding reliability, assembly integrity, strength, and durability. A hospital grade
outlet in the United States may be indicated by a green colored dot on the face of
the outlet.
Page 16

ProAxis® Spinal Surgery Table Owner’s Manual
12
Mizuho OSI 2016
NW0725 Rev K
Head-End
Foot-End
Base
Base
3 Component Identification
3.1 Table Orientation
The ProAxis® Spinal Surgery Table is described as having a Head-End and a Foot-End (Figure 2).
When the components are set up and the patient is positioned, the patient’s head is oriented towards the
Head-End of the device and his/her feet are oriented towards the Foot-End of the device.
Figure 2: ProAxis® Spinal Surgery Table
The linkage arms of the columns raise, lower, and rotate when the table is in use, and may come in contact
with items located on or near the table, potentially causing damage to the table or item. The base surfaces
at the Head-End and Foot-End columns are not intended to be used for storage.
CAUTION: Do not place any items on the base of the Head-End or Foot-End columns or below
the table. Storing or placing anything in these areas may result in damage to the device or the item
being stored.
An important safety label regarding Patient Weight Capacity is also located at the Head-End of the table top
(Figure 3).
Figure 3: Patient Weight Capacity Label at Head-End
Page 17

ProAxis® Spinal Surgery Table Owner’s Manual
13
Mizuho OSI 2016
NW0725 Rev K
Power Switch
Reset Button
Circuit Breakers
External Ground Stud
Power Receptacle
AC Power Indicator LED
Battery Indicator LED
WARNING: Use of the ProAxis® Spinal Surgery Table with patients weighing more than
500 pounds (227 kg) could result in damage to the table, possible injury to the patient, or harm to
the healthcare professionals.
The Power Switch, Battery, and AC Power Indicator LEDs, Power Receptacle, and Circuit Breakers are
located on the Head-End table base.
Figure 4: ProAxis® Table Base, Head-End
The Hand Pendant, called the IntelliPendant®, the Auxiliary Control Panel, the Emergency Stop, and Cord
Wrap are located on the Head-End column.
Page 18

ProAxis® Spinal Surgery Table Owner’s Manual
14
Mizuho OSI 2016
NW0725 Rev K
IntelliPendant®
Emergency Stop
Auxiliary Control Panel
Connection Port for
IntelliPendant®
Cord Wrap
Figure 5: ProAxis® Head-End Column
3.1.1 IntelliPendant
®
The ProAxis® Spinal Surgery Table functions are controlled by the single hand pendant referred to as the
IntelliPendant® (Figure 5).
The graphical display of the IntelliPendant® provides real time information about the table’s position: Height,
Trendelenburg / Reverse Trendelenburg angle, Lateral Tilt angle and Hinge angle. The IntelliPendant® also
informs the user of the table’s status for Power, Hinge Motion, ACP Setting, and Floor Locks. The table’s
functions and settings, which include Hinge Mode, Memory Position, Advanced Control Pad System™
Cycles, Floor Lock Override, and Storage Configuration, are managed through the use of the
IntelliPendant®.
When the table is in use, the current Height is identified on the display screen in inches/centimeters. The
angle of Trendelenburg/Reverse Trendelenburg, Lateral Tilt, and the Hinge is displayed in degrees. The
Hinge angle displayed when the hinge is raised or lowered will range from -20 to +35 degrees as the hinge
angle changes.
Detailed instructions for utilizing the IntelliPendant® are provided in Section 4.
3.1.2 Cord Wrap
A Cord Wrap is provided on the inside of the Head-End column for convenient storage of the Power Cord
when not in use (Figure 5).
3.1.3 Emergency Stop
An Emergency Stop is provided on the Head-End column above the connection port for the IntelliPendant®
(Figure 5). Pressing this button will immediately stop any motion of the ProAxis® Spinal Surgery Table.
Detailed instructions for utilizing the Emergency Stop are provided in Section 4.
Page 19

ProAxis® Spinal Surgery Table Owner’s Manual
15
Mizuho OSI 2016
NW0725 Rev K
Connection Port for the Advanced
Control Pad System™ Tubing
Foot-End Base
Not Intended for Storage
3.1.4 Auxiliary Control Panel
The Auxiliary Control Panel (Figure 5) is intended to provide a second option for controlling primary table
motions and Floor Lock function. The Auxiliary Control Panel may be utilized to control table Height,
Trendelenburg/Reverse Trendelenburg, Lateral Tilt, Hinge motion, and Floor Lock operation.
NOTE: Should the IntelliPendant
®
stop functioning, the Auxiliary Control Panel will allow the user to
execute the basic motions of the table and lock/unlock the Floor Locks.
Detailed instructions for utilizing the Auxiliary Control Panel are provided in Section 4, Basic Operation.
3.1.5 Advanced Control Pad System™
The Advanced Control Pad System™ Connection Port is located on the Foot-End column (Figure 6).
Detailed instructions for utilizing the Advanced Control Pad System™ are provided in Section 4.
Figure 6: ProAxis® Foot-End Column
Page 20

ProAxis® Spinal Surgery Table Owner’s Manual
16
Mizuho OSI 2016
NW0725 Rev K
Chest Pad
Cervical Management
Base Unit and Adaptor
5-Way Articulating
Arm Boards (2)
Chest Tray
Cervical Traction
Adjustor Vector
Rail Mounts (2)
2-inch Tempur-Pedic®
Supine Top Pad
3.2 Storage Cart
A Storage Cart is provided for ease of storing the table’s standard components and optional accessories
when not in use. The silhouettes on the Cart provide guidance on where each component or accessory
should be stored. The front side of the Cart is primarily dedicated to the storage of standard components
(Figure 7), while the back side of the Cart is designed for the storage of most optional accessories
(Figure 8).
Figure 7: Front Side of Storage Cart
NOTE: Ensure the Chest Tray is stored as shown within the ledge of the Cart to prevent damage to
the pieces of the component when removing or handling.
Page 21

ProAxis® Spinal Surgery Table Owner’s Manual
17
Mizuho OSI 2016
NW0725 Rev K
ProneView® Mirror
and Helmet
Lateral
Positioners
Lateral Arm Board
Pivoting Arm
Boards (2)
Supine Tops
Cervical Chest Tray
Hip Pads (2)
Cross Arm Support
Safety Straps and Adaptors
3.3 Component Part Number
Each standard component of the ProAxis® Spinal Surgery Table is identified and labeled with its respective
part number.
Figure 8: Back Side of Storage Cart
Figure 9: ProAxis® Component Label
Page 22

18
Mizuho OSI 2016
NW0725 Rev K
3.4 Model Number and Serial Number
Manufacturer’s
Label
ProAxis® Spinal Surgery Table Owner’s Manual
Figure 10: ProAxis® Foot-End Column
In addition to the product number and serial number, the following information is provided on the
manufacturer’s label:
Figure 11: Manufacturer’s Label, Examples
NOTE: Additional product information may also be found on the label.
Page 23
ProAxis® Spinal Surgery Table Owner’s Manual
19
Mizuho OSI 2016
NW0725 Rev K
4 Basic Operation
4.1 Control Operation
For use with AC power:
1. Plug the Power Cord into a properly grounded receptacle. Refer to the manufacturer’s
label at the Foot-End column for input voltage requirements (Figure 10, page 18).
NOTE: Only use the Power Cord supplied by Mizuho OSI with the table or if necessary, one of
equivalent rating.
2. Depress the Power Switch and observe that the switch illuminates with a green ring
indicating that power is applied to the table.
3. If the AC power cable is not connected to an outlet with a protective earth ground, then the
external ground stud should be connected to a protective earth ground (Figure 4, page 13).
WARNING: This symbol indicates an external ground stud that is required for use when
the AC power cable is not connected to a protective earth ground hospital grade AC outlet in your
operating room or facility or when the table is in use under battery power. Failure to ensure ground
may cause harm to the patient, healthcare professionals, or the device.
NOTE: If the integrity of the AC power source is in doubt, the equipment shall be operated from its
internal electrical power source (battery).
Should the table be operated on battery power:
1. Ensure that the battery has been properly charged as outlined in Section 12.3. Depress
the Power Switch and observe that the switch illuminates with a green ring indicating that
power is applied to the table. The battery LED at the base of the Head-End column will not
be illuminated, indicating that the table is being used on battery power (Figure 4, page 13).
2. The battery status symbol on the screen of the IntelliPendant® indicates the level of
charge in the batteries. If the battery status symbol with one green bar appears , the
battery is not charged and the table should only be used with AC power.
WARNING: Failure to ensure the table is properly charged when used on battery power may
cause the table to malfunction and may harm the patient, healthcare professional and the table.
3. To protect the patient, hospital staff, and the table from possible electrical hazards, an
external ground cable connection is required between the external ground stud and
protective earth ground when the table is in use under battery power (Figure 4, page 13).
The expected working life of a fully charged battery is approximately eight (8) hours with two procedures
averaging a total of six (6) minutes of motion time. While in storage, it is recommended that the table be
plugged in to charge the battery. A fully depleted battery will take 24 hours to fully charge. This can be
accomplished when using the table under AC power.
If during use, the battery symbol on the IntelliPendant® appears , the table should be connected
to AC power, and it can continue to be utilized while the battery is charging.
Page 24
ProAxis® Spinal Surgery Table Owner’s Manual
20
Mizuho OSI 2016
NW0725 Rev K
Graphical Display of
Emergency Stop
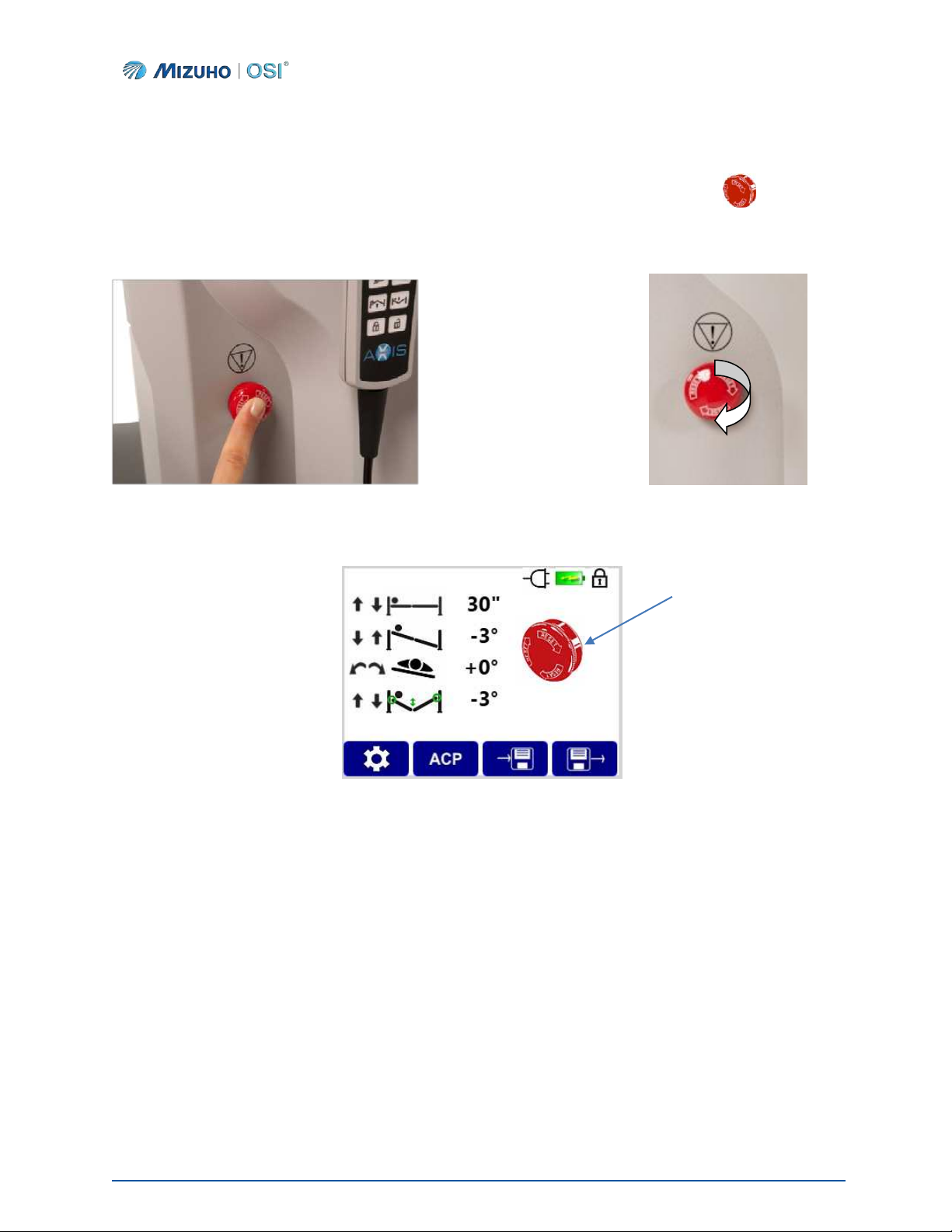
4.2 Emergency Stop
To immediately interrupt the motion of the ProAxis® Spinal Surgery Table, press the Emergency Stop button
located on the side of the Head-End column (Figure 12). The IntelliPendant® will display if the
Emergency Stop is engaged (Figure 14), and the IntelliPendant® will vibrate. No further table functions will
be allowed until the Emergency Stop is reset. To reset the table, turn the Emergency Stop button in the
direction of the arrows until it returns to the original position (Figure 13).
Figure 12: Activating Emergency Stop Figure 13: Reset Emergency Stop
Figure 14: Emergency Stop Display on the IntelliPendant®
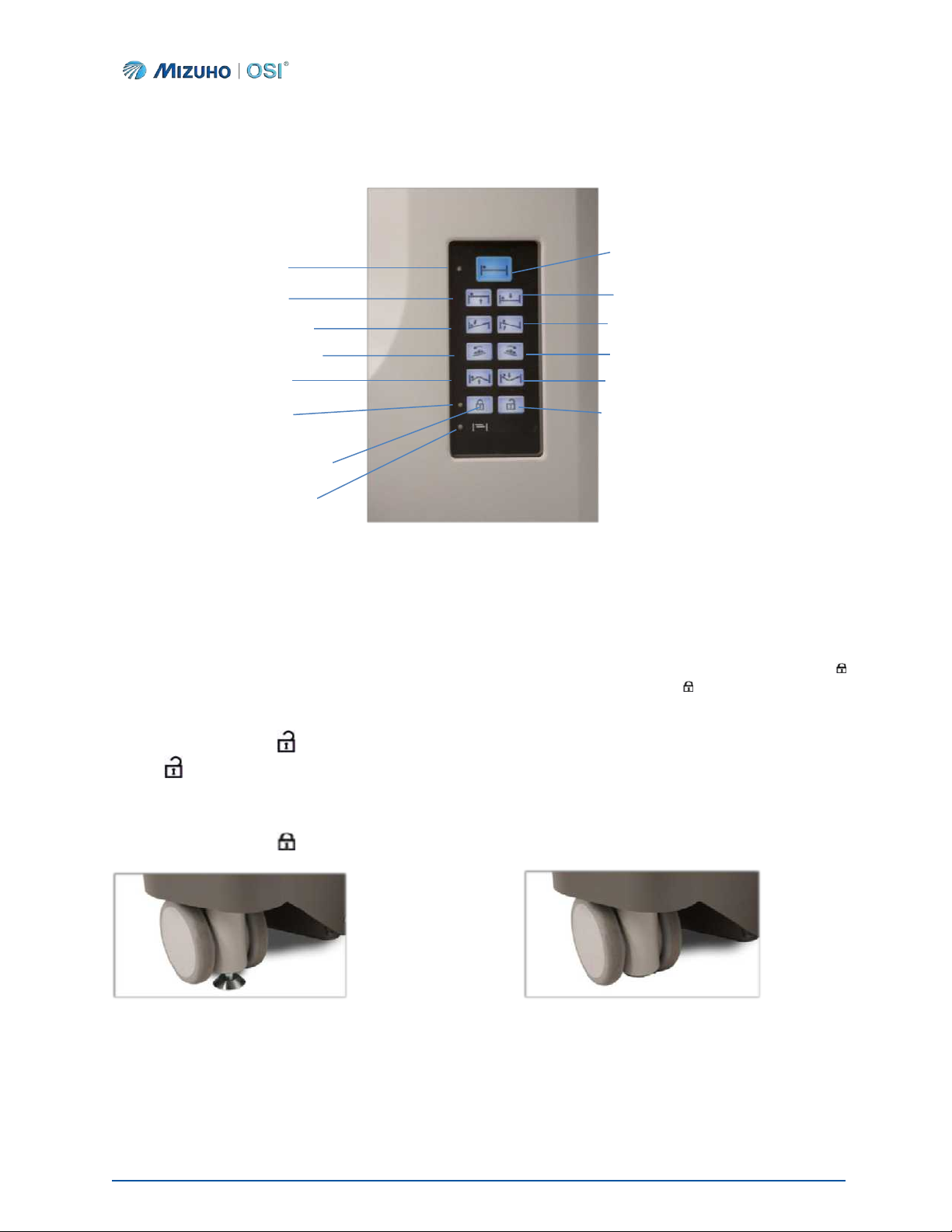
4.3 Auxiliary Control Panel
The Auxiliary Control Panel provides function and motion buttons labeled with symbols identifying what
function or motion can be achieved (Figure 15, page 21). The motion buttons control the Height,
Trendelenburg/Reverse Trendelenburg, Lateral Tilt Right/Left, Hinge Up/Down, and Return to Level. The
two (2) function buttons control the table’s Floor Lock/Unlock capabilities.
A green Light Emitting Diode (LED) illuminates on the face of the Auxiliary Control Panel when the Floor
Locks are fully deployed and locked, when the table frame has returned to a level position, and when the
table is prepared for storage.
In order for the motion buttons on the Auxiliary Control Panel to function, the Floor Locks must be fully
deployed. To lock the Floor Locks, press the Floor Lock button on the Auxiliary Control Panel or the
IntelliPendant®. Confirm the Floor Locks are engaged by observing that the Floor Lock LED illuminates
green and the Floor Lock symbol is visible on the IntelliPendant®.
Page 25
ProAxis® Spinal Surgery Table Owner’s Manual
21
Mizuho OSI 2016
NW0725 Rev K
Return to Level
Height Down
Reverse Trendelenburg
Lateral Tilt, Right
Hinge Down
Unlock Floor Locks
Level LED
Height Up
Trendelenburg
Lateral Tilt, Left
Hinge Up
Floor Locks
Locked LED
Lock Floor Locks
Storage Status
LED
To utilize the Auxiliary Control Panel, press and hold a button until the desired motion is achieved. A
display on the IntelliPendant® will show the symbol of the motion button when it is activated (Figure 23,
page 26).
4.4 Floor Locks
When the ProAxis® Spinal Surgery Table is powered on, the Floor Locks will automatically return to their
previous state of being either fully deployed (locked) or fully retracted (unlocked).
To lock the Floor Locks via the IntelliPendant® or the Auxiliary Control Panel, press the Floor Lock button
once and release to deploy the Locks (Figure 16). The IntelliPendant® will display when the Floor Locks
are fully deployed and locked (Figure 22, page 25). On the Auxiliary Control Panel, the Floor Lock LED will
illuminate (Figure 15). To unlock the Floor Locks via the IntelliPendant® or the Auxiliary Control Panel, press
the Floor Unlock button once to completely retract the Floor Locks (Figure 17). The IntelliPendant® will
display when the Floor Locks are fully retracted and unlocked (Figure 21, page 25). It will take
approximately 10 to 15 seconds for the Floor Locks to change position.
Figure 16: Floor Lock Deployed (Locked) Figure 17: Floor Lock Retracted (Unlocked)
NOTE: The ProAxis
symbol is visible on the IntelliPendant® display.
Figure 15: Auxiliary Control Panel
®
table will not function until the Floor Locks are completely deployed and the
Page 26

ProAxis® Spinal Surgery Table Owner’s Manual
22
Mizuho OSI 2016
NW0725 Rev K
4.4.1 IntelliPendant
®
Activated Floor Lock Override
If the Floor Lock systems fail during table use such that powered table motion is prevented, the
IntelliPendant® can be used to activate the Floor Lock Override function (see Section 4.7.4.4, page 35).
Use of the Floor Lock Override function will allow for 60 seconds of activity during which multiple actions
can be taken.
NOTE: Should a particular motion take longer than 60 seconds, the action will be completed as
long as the function button continues to be depressed.
NOTE: The Floor Lock Override function allows for changes to Height, Trendelenburg/Reverse
Trendelenburg, Lateral Roll, Hinge, and Return to Level functions via the Hand Pendant
and is intended for use in emergency situations only.
4.4.2 Manual Floor Lock Override
In the event the table’s floor locks do not retract properly to allow for table transport, the Floor Locks can be
manually raised or lowered (see Section 0).
NOTE: The manual floor lock override is intended only as a means of transporting the table should
the Floor Lock systems fail. Use of this override will not allow the user to activate any of
the table functions.
4.5 Moving the Table
Before moving the ProAxis® Spinal Surgery Table or preparing it for its storage sequence of movements,
ensure the table is returned to its Home position such that it is neutral and at a maximum height of
32 inches (81 cm).
CAUTION: When moving the ProAxis® Spinal Surgery Table in its normal configuration,
ensure that the table is at a height no greater than its home position of 32 inches (81 cm) and in a
neutral state with the IntelliPendant® reading 0º for Lateral Roll, Trendelenburg/Reverse
Trendelenburg and Hinge to prevent the table from being unstable during relocation.
After all four (4) Floor Locks are unlocked, the table can be rolled for relocation. The ProAxis® Spinal
Surgery Table is heavy, and a minimum of two people is required to move it. Position one person at the
Head-End of the table and one person at the Foot-End. Care should be taken to control the ProAxis® when
rolling it.
CAUTION: If the ProAxis® Spinal Surgery Table is allowed to roll too fast, it may be difficult to
stop or turn. Impact of the table with a stationary object may cause serious damage to the table or
the other object. If an impact does occur the table must be visually inspected for damage, and a
Function Check must be performed (refer to Section 6). If damage is discovered or the table does
not successfully complete the Function Check, call Mizuho OSI Services (refer to Section 13).
NOTE: For easier moving and storage, the ProAxis
to its retracted position of 80 inches (203 cm) (see Section 4.7.5).
®
Spinal Surgery Table may also be configured
Page 27

ProAxis® Spinal Surgery Table Owner’s Manual
23
Mizuho OSI 2016
NW0725 Rev K
Motion Buttons:
Return to Level (Home)
Height Up/Height Down
Trendelenburg /
Reverse Trendelenburg
Lateral Tilt Left / Right
Hinge Up/Hinge Down
Graphical Display
Dynamic Soft Keys
Function Buttons:
Floor Lock
Floor Unlock
Soft Key Function Display
4.6 Torso Trolley
®
The Torso Trolley® is designed to support the patient and simultaneously move the prone patient’s upper
torso in conjunction with the hinge angle being changed, preventing any spinal compression or distraction.
The Torso Trolley® is formed by utilizing the Chest Tray to which the chest pad is mounted. The 5-Way
Adjustable Arm Boards are also mounted to the Chest Tray utilizing the Arm Board Brackets provided.
Refer to Section 8 for detailed instructions on the setup and use of the Torso Trolley®.
4.7 IntelliPendant
®
The ProAxis® Spinal Surgery Table functions are controlled by the IntelliPendant®. When the table is in use,
the display screen provides the user with detailed real time information as to the status of each motion or
function. This includes specific data on the table’s position including Height, Trendelenburg/Reverse
Trendelenburg Angles, Lateral Tilt Angle, and Hinge Angle. To activate a function or motion, a button must
be pressed and held until the function is complete or the desired position is achieved. The IntelliPendant
®
also provides important information about the system’s operational state, including Power status, Hinge
Motion, Advanced Control Pad setting, and Floor Lock status (Figure 18).
Utilizing the Soft Keys allows the user to access and select the following functions and settings: Hinge
Mode, Memory Position, Advanced Control Pad System™ Cycles, and Floor Lock Override (Figure 18).
Figure 18: IntelliPendant®
Page 28

ProAxis® Spinal Surgery Table Owner’s Manual
24
Mizuho OSI 2016
NW0725 Rev K
The IntelliPendant® also provides haptic feedback to the user when a requested action is not possible. This
may occur under the following conditions:
Floor Locks are Unlocked and must be engaged before continuing.
The maximum range of a particular motion has been reached.
A system error has occurred.
A visual indication of the constrained movement will also be displayed on the IntelliPendant® screen
informing the user of the table’s state. For instances where a particular table function has reached its limit,
the image depicted on the screen will show the location of the error or limit through the use of an orange
circle (see Section 4.7.2).
To connect:
1. Align the pins with the port located at the Head-End column of the table (Figure 19). Insert
until the locking collar clicks, securing the pins in the port.
Figure 19: Connecting the IntelliPendant®
NOTE: Only connect the IntelliPendant
®
supplied with the ProAxis® Spinal Surgery Table to the
IntelliPendant® port.
2. The IntelliPendant® should remain connected and be stored on the hand pendant clip on
the Head-End column when not in use (Figure 5, page 14).
Press the Power Switch on the Head-End base once the IntelliPendant® has been connected. A green ring
will illuminate around the switch confirming power is applied to the table (Figure 4, page 13). The Graphical
Display that appears when the ProAxis® table is turned on is identified as the IntelliPendant® screen
(Figure 20).
Page 29
ProAxis® Spinal Surgery Table Owner’s Manual
25
Mizuho OSI 2016
NW0725 Rev K
Floor Lock
Status Icon
Figure 20: IntelliPendant® Screen at Start-Up
The Floor Locks must be fully deployed for the IntelliPendant® or Auxiliary Control Panel motion buttons to
function. To lock the Floor Locks, press the Floor Lock button once on the IntelliPendant® or the Auxiliary
Control Panel and confirm the Floor Lock symbol is visible on the IntelliPendant®, indicating the Floor Locks
have been engaged (Figure 22).
Figure 21: Unlocked Floor Locks Symbol Figure 22: Locked Floor Locks Symbol
Page 30
ProAxis® Spinal Surgery Table Owner’s Manual
26
Mizuho OSI 2016
NW0725 Rev K
Graphical Display
of Function
4.7.1 Table Functions
When in use, the table’s position at any given time is identified on the IntelliPendant® (Figure 18, page 23)
as follows:
Height reported in inches/centimeters
Angle of Trendelenburg/Reverse Trendelenburg reported in degrees
Angle of Lateral Tilt reported in degrees
Angle of the Hinge reported in degrees
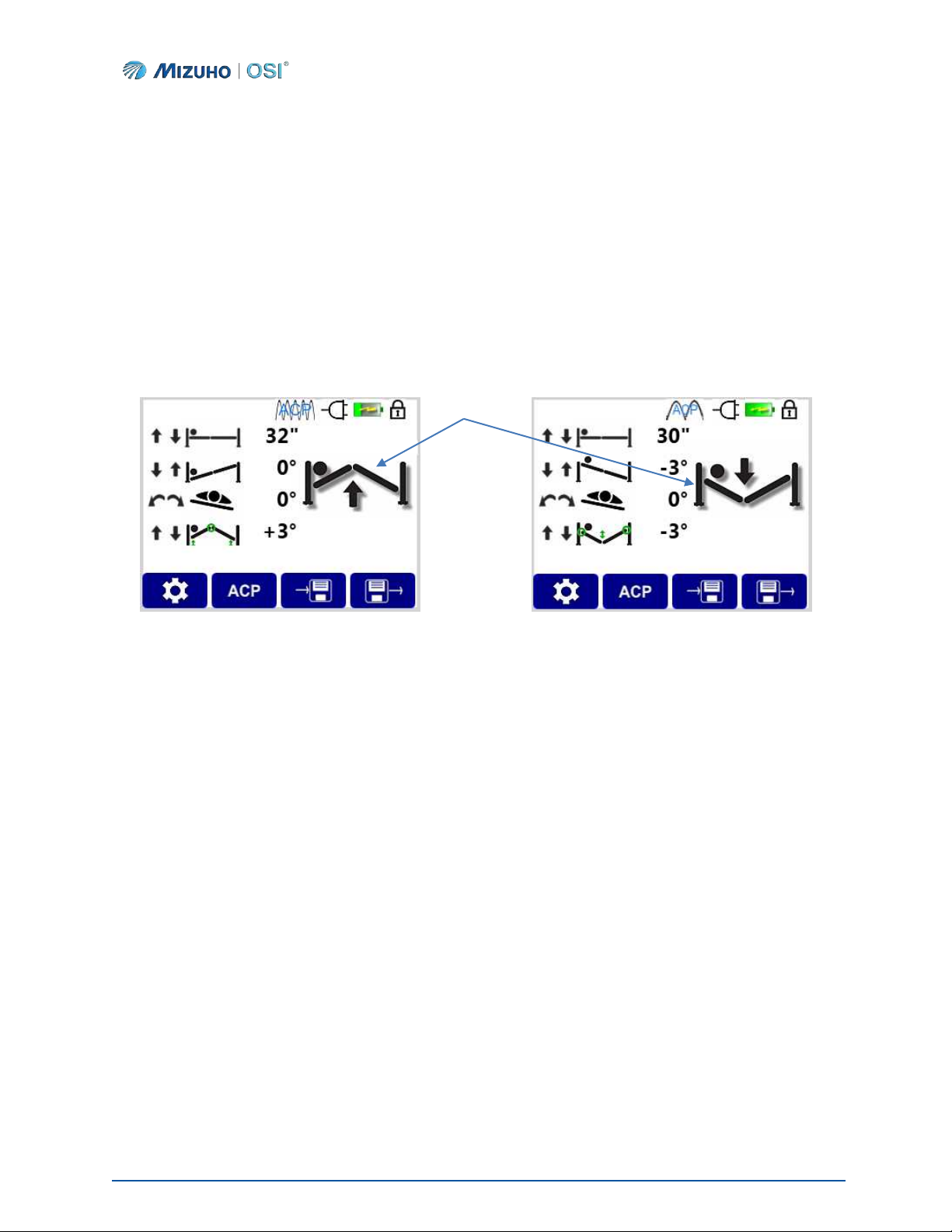
The function being executed is also reflected on the IntelliPendant® screen, informing the user of the action
taking place (Figures 23 and 24).
Figure 23: Hinge Up Action Displayed Figure 24: Hinge Down Action Displayed
Page 31

ProAxis® Spinal Surgery Table Owner’s Manual
27
Mizuho OSI 2016
NW0725 Rev K
Symbol
Meaning
Height Up
Height Down
Trendelenburg
Reverse Trendelenburg
Lateral Tilt, Right
Lateral Tilt, Left
Hinge Up
Hinge Down
Lock (Floor Locks) and Locked Position
Unlock (Floor Locks) and Unlocked Position
Half-lock Position; indicates the transitional stage of the Floor Locks while they are in the
process of being either locked or unlocked
Lock Error; indicates the Floor Locks must be locked before executing any table function
Represents Return to Level on function button, also appears on the screen display to
indicate height position
Menu
ACP ON/OFF Function
Save to Memory Position
Go to Memory Position
Cancel
Accept / Select
4.7.2 Defined Function and Setting Symbols
The following symbols are utilized on the function buttons of the IntelliPendant®, the Auxiliary Control Panel,
or on the display screen itself when in use.
Page 32

ProAxis® Spinal Surgery Table Owner’s Manual
28
Mizuho OSI 2016
NW0725 Rev K
Advanced Control Pad Cycle, Fast
Advanced Control Pad Cycle, Slow
Fixed End Hinge Mode, Hinge Up
Fixed End Hinge Mode, Hinge Down
Fixed Surgical Site Hinge Mode, Hinge Up
Fixed Surgical Site Hinge Mode, Hinge Down
Home (Return to Level) Position Achieved
Floor Lock Override
Indicates the system is running on battery power or AC power, and the battery voltage
meets or exceeds the 100% charged threshold
Indicates the system is running on battery power, and the battery voltage exceeds the
75% threshold but not the 100% threshold
Indicates the system is running on battery power, and the battery voltage exceeds the
50% threshold but not the 75% threshold
Indicates the system is running on battery power, and the battery voltage is below the
50% threshold. Requires Charging and AC Power Connection
Battery Charging
AC Power Connected
Displayed when user has saved the current position as M1, or when the table has arrived
at the stored position M1
Displayed when user has saved the current position as M2, or when the table has arrived
at the stored position M2
Indicates that the requested motion would exceed a boundary for height up, hinge angle
increase, or Trendelenburg angle increase
Indicates that the requested motion would exceed a boundary for height down, hinge
angle decrease, or Trendelenburg angle decrease
Indicates that the requested left tilt motion would exceed a boundary
Indicates that the requested right tilt motion would exceed a boundary
Appears when a “hinge up” button is held, and the maximum hinge angle has been
reached
or
Appears when a “hinge up” button is held, and a boundary has been reached
Page 33

ProAxis® Spinal Surgery Table Owner’s Manual
29
Mizuho OSI 2016
NW0725 Rev K
or
or
Appears when a “hinge down” button is held, and a boundary has been reached
or
Appears when a “height up” button is held, and a boundary has been reached
or
or
Appears when a “height down” or “home” button is held, and a boundary has been
reached
or
Appears when a “Trendelenburg” button is held, and a boundary has been reached
or
Appears when a “Reverse Trendelenburg” button is held, and a boundary has been
reached
Appears when a “Lateral Roll Right” button is held, and a boundary has been reached
Appears when a “Lateral Roll Left” button is held, and a boundary has been reached
Appears when an upward hinge motion is requested, and hinge lockout is in effect (due
to the hinge enable cables on Torso Trolley® or supine top not being plugged in)
Note that hinge and memory buttons can do this.
Appears when a downward hinge motion is requested, and hinge lockout is in effect (due
to the hinge enable cables on Torso Trolley® or supine top not being plugged in)
Note that hinge and memory buttons can do this.
Appears when the Emergency Stop is enabled
Page 34

ProAxis® Spinal Surgery Table Owner’s Manual
30
Mizuho OSI 2016
NW0725 Rev K
Menu Function
ACP On/Off
Save To Memory
Position
Go To
Memory Position
Graphical Display
of Function
4.7.3 Return to Level
The IntelliPendant® is equipped with a Return to Level button that allows the table to be returned to a
neutral position from any current state. When the Return to Level function is activated, the Lateral Tilt
Angle returns to 0 degrees, Trendelenburg/Reverse Trendelenburg Angle returns to 0 degrees, and the
Hinge Angle returns to 0 degrees. Following a one (1) second pause, if the button continues to be
depressed, the table top will return to a height of 32 inches (81 cm) above the floor, which is defined as its
Home position.
NOTE: The complete functionality of the Return to Level feature requires either the Rail Mounts or
the Supine Tops to be installed on the open frame to be operational. If neither of these
components is installed, the Return to Level feature will only return the Lateral Tilt Angle to
0 degrees and the table top height to 32 inches (81 cm) above the floor. This is intended to
prevent unintentional traction via the automatic return of Trendelenburg/Reverse
Trendelenburg to neutral when using Cervical Management. The Hinge capability is not
enabled when neither Rail Mounts nor the Supine Tops are installed.
Figure 25: Display when Return to Level Function is Completed
4.7.4 Soft Keys Functions and Settings
To control system functions and settings, the Soft Keys corresponding to the icons at the bottom of the
screen are utilized (Figure 26).
Figure 26: Soft Key Selections
Page 35

ProAxis® Spinal Surgery Table Owner’s Manual
31
Mizuho OSI 2016
NW0725 Rev K
Pressing the Menu Key provides the user with a list of setting choices for the table, which may be selected
and customized as needed for each procedure (Figure 27).
Figure 27: Menu Key Selections
4.7.4.1 Memory Positioning
The ProAxis® Spinal Surgery Table has the ability to store two specified table positions in memory for
ease of returning to the desired position at a later time. The table positions can be stored to either
Memory Position 1 or Memory Position 2.
Assignment of the table position to memory is completed through the use of the IntelliPendant®.
To assign the table’s current position to a memory position:
1. From the Home Screen, select the Save to Memory Position using the corresponding Soft
Key (Figure 26).
2. Assign the table position to either the Memory Position 1 or Memory Position 2 location
(Figure 28).
Figure 28: Memory Position Selection
3. The table position is now saved.
Page 36

ProAxis® Spinal Surgery Table Owner’s Manual
32
Mizuho OSI 2016
NW0725 Rev K
Up / Down Arrows
Check Mark
Hinge Mode Options
To move the table from its current position to a saved memory position:
1. From the Home Screen, select the Go To Memory Position using the corresponding Soft
Key (Figure 26).
2. Press and hold the Soft Key corresponding to either Memory Position 1 or Memory Position
2 to move to the desired location.
NOTE: If the hinge capability is not enabled (see Section 8 for setup instructions) and the desired
Memory Position requires a change in hinge angle, the selection of Memory Positions will
not provide the desired motion to the stored position requested.
4.7.4.2 Hinge Mode
The ProAxis® Spinal Surgery Table has two operating modes for the hinged frame, Fixed Surgical Site
(FSS) or Fixed End (FE), which can be selected depending on the surgeon’s preference for positioning
the patient.
FSS mode is designed to ensure the height of the top at the hinge remains constant when the hinge is
flexed or extended. This is accomplished through the simultaneous motions of the Head-End and FootEnd of the table. This mode is utilized primarily for prone patient positioning.
FE mode is designed to ensure the height of the Head-End and Foot-End of the table top remains at a
constant height when the hinge is flexed or extended. This mode is utilized primarily for lateral patient
positioning.
NOTE: For the hinge of the frame to be operational, the Rail Mounts or the Supine Top, Head-End
needs to be installed and the cables connected. Refer to Section 8 for setup instructions.
Selection of the hinge mode is completed through the use of the IntelliPendant®.
To activate the desired Hinge Mode:
1. Press the Menu Soft Key on the IntelliPendant® and observe that the Set Hinge Mode
setting is selected (Figure 27).
2. Upon confirming that Set Hinge Mode is highlighted, press the Soft Key that corresponds
to the check mark.
3. Utilize the arrow Soft Keys to highlight the FSS or FE mode, and press the check mark Soft
Key to select the desired hinge mode (Figure 29).
Figure 29: Hinge Mode Selection
Page 37

ProAxis® Spinal Surgery Table Owner’s Manual
33
Mizuho OSI 2016
NW0725 Rev K
FE Mode Icon
FSS
Mode Icon
4. Selection of the desired hinge mode can be visually confirmed through the corresponding
symbol depicted on the IntelliPendant® display (Figures 30 and 31).
Figure 30: FSS Mode, Positive Hinge Angle Figure 31: FE Mode, Negative Hinge Angle
4.7.4.3 Advanced Control Pad System™ (ACP)
The ProAxis® provides the Advanced Control Pad System™ (ACP) for use when the patient is
positioned prone. The ProAxis® Contoured ACP Hip Pads, the Bilateral Winged ACP Chest Pad, and
the Flat ACP Chest Pad are designed specifically with Tempur-Pedic® medical material and air cells
within the pads. The cells inflate and deflate at selectable intervals providing massage action and
periodic pressure point stimulation to the patient’s chest, hips, and thighs when positioned prone. At the
discretion of the surgeon, the pads can also be used for patient support on the ProAxis® without the
massage action being activated.
The ProAxis® Spinal Surgery Table has two speed settings, Fast and Slow, for the ACP System.
The Fast Cycle, alternates inflation and deflation of the air cells every four (4) seconds. The
Fast Cycle is represented with the symbol.
The Slow Cycle, alternates inflation and deflation of the air cells every six (6) seconds. The
Slow Cycle is represented with the symbol.
NOTE: Selection of the ACP System cycle is at the discretion of the surgeon.
Selection of the ACP System cycle is completed through the use of the IntelliPendant®.
To activate the ACP System:
1. Press the Menu Soft Key on the IntelliPendant®.
2. Utilize the Down arrow Soft Key to select Set ACP Mode (Figure 32). Press the Soft Key
that corresponds to the check mark.
Page 38

ProAxis® Spinal Surgery Table Owner’s Manual
34
Mizuho OSI 2016
NW0725 Rev K
Up / Down Arrows
ACP Cycle Options
Check Mark
Figure 32: Set ACP Mode Selection
3. Utilize the Up or Down arrow Soft Keys to highlight either the Fast Cycle or Slow Cycle,
and press the check mark Soft Key to select the desired speed (Figure 33).
4. Return to the Main IntelliPendant® Screen (Figure 26, page 30) and press the ACP Soft
Key to activate.
NOTE: The ACP system will not function if the ACP function is not selected on the main screen of
the IntelliPendant®.
5. Selection of the desired ACP setting can be visually confirmed through the corresponding
symbol depicted on the IntelliPendant® display (Figures 34 and 35).
a. The Fast Cycle is represented with the symbol (1 cycle every four (4) seconds).
b. The Slow Cycle is represented with the symbol (1 cycle every six (6) seconds).
NOTE: If the ACP system has not been selected, no symbol will appear on the screen, indicating
the ACP function is not in use.
Figure 33: ACP Cycle Selection
Page 39

ProAxis® Spinal Surgery Table Owner’s Manual
35
Mizuho OSI 2016
NW0725 Rev K
Slow ACP Icon
Fast ACP Icon
Figure 34: Fast ACP Cycle Selected Figure 35: Slow ACP Cycle Selected
4.7.4.4 Floor Lock Override
To allow for the use of the Floor Lock Override functionality:
1. Press the Menu Soft Key on the IntelliPendant®.
2. Utilize the Down arrow Soft Key to select the Override Floor Locks option (Figure 36).
3. Upon confirming that Override Floor Lock is highlighted, press the Soft Key that
corresponds to the check mark.
Figure 36: Override Floor Locks Selection
4. Utilize the Down arrow Soft Key to select the Override mode (Figure 37).
5. Upon confirming that the Override Floor Lock mode Override is highlighted, press the
check mark Soft Key (Figure 37). The Override functionality will now be enabled.
Page 40

ProAxis® Spinal Surgery Table Owner’s Manual
36
Mizuho OSI 2016
NW0725 Rev K
Figure 37: Floor Lock Override Screen
6. Press the appropriate motion button on the IntelliPendant® to achieve the desired table
motion. The Floor Lock Override capability will allow for 60 seconds of activity during which
multiple actions can be taken. Should a desired motion take longer than 60 seconds, the
action will be completed as long as the motion button continues to be depressed.
7. Should additional time be required beyond the 60 seconds to complete an additional
function or if the motion button has been released once the 60 seconds have elapsed, the
Floor Lock Override capability will have to be activated starting with Step 1 above.
NOTE: The Floor Lock Override function allows for changes to Height, Trendelenburg/Reverse
Trendelenburg, Lateral Roll, Hinge, and Return to Level motions via the Hand Pendant and
is intended for use in emergency situations only.
4.7.4.5 Language Selection
To select the preferred language display for the IntelliPendant®:
1. Press the Menu Soft Key on the IntelliPendant®.
2. Utilize the Down arrow Soft Key to select Set Language (Figure 38). Press the Soft Key
that corresponds to the check mark.
Figure 38: Set Language Selection
Page 41

ProAxis® Spinal Surgery Table Owner’s Manual
37
Mizuho OSI 2016
NW0725 Rev K
3. Utilize the arrow Soft Keys to highlight the desired language, and press the check mark
Soft Key to set the language mode (Figure 39).
Figure 39: Language Selection Menu
NOTE: The language setting is preserved when the table is powered off.
4.7.4.6 Display Units Selection
To select the preferred units display for the IntelliPendant®:
1. Press the Menu Soft Key on the IntelliPendant®.
2. Utilize the Down arrow Soft Key to select Set Units (Figure 40). Press the Soft Key that
corresponds to the check mark.
Figure 40: Set Units Selection
3. Utilize the arrow Soft Keys to highlight the desired units, and press the check mark Soft
Key to set the units mode (Figure 41).
Page 42

ProAxis® Spinal Surgery Table Owner’s Manual
38
Mizuho OSI 2016
NW0725 Rev K
Figure 41: Units Selection Menu
NOTE: The units setting is preserved when the table is powered off.
4.7.5 Configuration of Table for Transport or Storage
The ProAxis® table can be configured to a smaller footprint for transport or storage. To prepare the table to
be shipped or stored in a more compact configuration, utilize the IntelliPendant® and complete the steps
outlined. Transporting or storing the table in the stored configuration reduces the length of the table from
122 inches (310 cm) to 80 inches (204 cm).
NOTE: Do not attempt to disconnect the spars from their connection point without performing the
exact procedure described below.
WARNING: Failure to properly disconnect the spars using the process below may cause
injury to the healthcare professional and/or damage to the table.
To configure the table to its stored position:
1. Press the Menu Soft Key on the IntelliPendant®.
2. Utilize the Down arrow Soft Key to select Store Table (Figure 42). Press the Soft Key that
corresponds to the check mark.
Figure 42: Store Table Screen
Page 43

ProAxis® Spinal Surgery Table Owner’s Manual
39
Mizuho OSI 2016
NW0725 Rev K
NOTE: If the ProAxis
®
Table is under too much load such that it is unsafe to disconnect the spars,
a blue warning screen identifying an “ERROR” will appear on the IntelliPendant® and the
user will not be able to proceed with the storage sequence (Figure 43).
Figure 43: Store Table Screen
WARNING: Attempting to disconnect the spars when this “ERROR” message appears may
cause injury to the healthcare professional and/or damage to the table.
3. Following the instructions depicted on the IntelliPendant®, complete the following steps to
configure the table to its stored position.
a. STEP 1: Remove any components and accessories attached to the table (Figure 44).
If the Supine Top or Chest Tray is detected, the user will be notified via the
IntelliPendant® screen.
Figure 44: ProAxis® with All Components Removed in Preparation for Storage
b. STEP 2: Confirm the next step to allow the table to move to its storage position.
Continue to hold down the Soft Key until the configuration is completed (Figure 45).
Page 44

ProAxis® Spinal Surgery Table Owner’s Manual
40
Mizuho OSI 2016
NW0725 Rev K
Spar Lock
Figure 45: ProAxis® Configured for Release of Spars
c. STEP 3: Confirm selection of storage position using the Soft Key that corresponds to
the check mark. After continuing with this selection, confirm the Storage LED light on
the Control Panel is illuminated green.
NOTE: Upon confirmation, the remaining steps must be completed to store the table.
d. STEP 4: Disconnect the Spars by:
i. Loosening the Spar Lock on either side of the spars by turning the knob counter-
clockwise (Figure 46).
.
Figure 46: Loosening the Spar Lock on Spar
WARNING: Do not use excessive force when disconnecting the Spars. If after loosening the
Spar Lock, the Spar does not disconnect with hand force, seek assistance from qualified service
personnel. Failure to do so may cause harm to the healthcare professional or device.
Page 45

ProAxis® Spinal Surgery Table Owner’s Manual
41
Mizuho OSI 2016
NW0725 Rev K
Black Tab
ii. Simultaneously pressing down on the black tabs on each side of the table to
disconnect the spars from their locked position (Figure 47) and raise them out of
the latches.
Figure 47: Pressing down on the black tab to disconnect the Spar
iii. Remove the spars by lifting up and shifting the pair of spars to either side such that
they can drop down for storage (Figure 48).
Figure 48: Disconnecting the Spars
Page 46

ProAxis® Spinal Surgery Table Owner’s Manual
42
Mizuho OSI 2016
NW0725 Rev K
Cervical Receptacle
Posts
Hinge Pinch Points
iv. Move spars to the side such that one is positioned on the outside of the Head-End
column while the other is positioned between the posts of the cervical receptacle
(Figure 49).
of the spar hinges and healthcare professionals position themselves away from the linkage arms
when moving the table to a storage position. Failure to do so may cause injury to the healthcare
professional or possible damage to the table.
Figure 49: Spars in process of being lowered to their storage position
WARNING: Take note of the pinch points at the spar hinges. Ensure no items are in the path
Page 47

ProAxis® Spinal Surgery Table Owner’s Manual
43
Mizuho OSI 2016
NW0725 Rev K
Figure 50: Spars Positioned Down for Storage
e. STEP 5: Install the protective Spar Cover on each Spar (Figure 51).
Figure 51: Spar Covers Being Installed
Page 48

ProAxis® Spinal Surgery Table Owner’s Manual
44
Mizuho OSI 2016
NW0725 Rev K
Figure 52: Spars in Position for Storage with Spar Covers Applied
f. STEP 6: Loosen the Beam Lock (Figure 53).
Figure 53: Beam Lock Loosened
g. Using the IntelliPendant®, continue to the next step, which allows the table to configure
the Floor Locks such that those at the Head-End are unlocked while those at the FootEnd remain locked.
Page 49

45
Mizuho OSI 2016
NW0725 Rev K
h. STEP 7: Retract the table (Figure 54).
Head-End
Foot-End
PUSH HERE TO
COLLAPSE TABLE
ProAxis® Spinal Surgery Table Owner’s Manual
Figure 54: Collapsing the ProAxis® Table for Storage
i. STEP 8: Secure the Beam Lock.
j. Using the IntelliPendant® or the Auxiliary Panel, unlock the Floor Locks. With the
assistance of two people, the ProAxis® table may now be moved to its storage location.
Page 50

ProAxis® Spinal Surgery Table Owner’s Manual
46
Mizuho OSI 2016
NW0725 Rev K
Figure 55: ProAxis® Configured for Storage or Transport
To expand the table from its retracted position:
1. With the assistance of two people, move the table to the desired location.
2. Press the Power Switch on the Head-End base, and observe that a green ring illuminates
around the switch, confirming power is applied to the table.
3. The IntelliPendant® will now provide step-by-step instructions on how to configure the table
to its full position.
4. Complete the following steps in order as directed on the Hand Pendant:
a. Loosen the Beam Lock.
b. Continue to the next step, which allows the table to configure the Floor Locks such that
those at the Head-End are unlocked while those at the Foot-End remain locked.
c. Expand the table.
d. Tighten the Beam Lock.
e. Remove the protective Spar Cover from each Spar.
f. Attach the Spars by:
i. Raising the spars such that one is positioned on the outside of the Head-End
column while the other is positioned between the posts of the cervical receptacle
(Figure 49, page 42).
ii. Lifting the spars above the latch position.
iii. Lowering the spars into the respective latches.
iv. Tightening the Spar Locks to secure the spars in place.
Page 51

47
Mizuho OSI 2016
NW0725 Rev K
5. Using the IntelliPendant®, lock the Floor Locks. Complete the Function Check (see Section
6).
6. Confirm Storage LED is no longer illuminated on the Auxiliary Control Panel.
NOTE: Upon expanding the table, the table will still be in the hinged state required for storage. To
level the table top, install the Head-End Supine Top or the two rail mounts to activate the
table’s hinge functionality. Then, press either the Hinge Down button or the Return to Level
button until the Hinge Angle reads 0°.
4.7.6 Version Info
To access information on the table’s software version:
1. Press the Menu Soft Key on the IntelliPendant®.
2. Utilize the Down arrow Soft Key to select Version Info (Figure 56). Press the Soft Key that
corresponds to the check mark.
3. Information on the table’s current software version will be displayed.
NOTE: Software version information will be primarily used by a service technician should it need to
be accessed.
ProAxis® Spinal Surgery Table Owner’s Manual
Figure 56: Version Info Menu Selection
4.8 Independent Monitoring System (IMS)
The ProAxis® Spinal Surgery Table includes an Independent Monitoring System that monitors the load on
the table and notifies the user in the event the table is overloaded. The system works by monitoring the
load on the Head-End plate to which the spars are attached. If the load exceeds the allowable amount for
the maximum-weight patient, the system will notify the user through the use of an error message and table
motion will be interrupted.
The table can be overloaded for three (3) reasons, any of which will be detected by the IMS and prevent
motion of the table. No other conditions are monitored that will trigger the alarm system.
1. A patient whose weight is over the maximum weight limit is placed on the table.
2. Extreme external forces.
3. A loss of coordination between the Head-End and Foot-End, which can place additional
load or tension on the table.
Page 52

ProAxis® Spinal Surgery Table Owner’s Manual
48
Mizuho OSI 2016
NW0725 Rev K
Should the table be overloaded due to any of these three conditions, the overload notification of the IMS will
be triggered and the user will be notified via the Overload screen (Figure 57). In this state, the user has the
opportunity to reverse the table’s overloaded state by removing the load.
Figure 57: IntelliPendant® Display when the Overload Notification of the IMS is Triggered
If the load on the table increases beyond the overload notification level, the IMS will trigger an overload
alarm to notify the user and prevent any further motion of the table (Figure 58).
Figure 58: IntelliPendant® Display when the IMS Overload Alarm is Triggered
For the user, there is no detectable delay from the time the alarm system detects the table is overloaded to
when a warning is displayed on the IntelliPendant®.
The specific load on the table that triggers the alarm system is preset at the factory and is not adjustable.
The functionality of the alarm system should be verified as part of the periodic maintenance of the table.
Page 53

ProAxis® Spinal Surgery Table Owner’s Manual
49
Mizuho OSI 2016
NW0725 Rev K
5 Inspection
5.1 Acceptance and Transfer
1. Upon receipt of your ProAxis® Spinal Surgery Table, remove it from the shipping crate.
Remove any protective wrapping or packaging. Visually inspect all surfaces for freight
damage.
NOTE: Any freight damage must be reported to the freight carrier immediately upon delivery. It is
the responsibility of the recipient to make freight damage claims.
2. Read the model number, serial number and confirm the power requirements on the
manufacturer’s label located on the Foot-End column of the table.
3. Perform Function Check (refer to Section 6).
5.2 Pre-Procedure/Post-Procedure
Before and after each use of the ProAxis® Spinal Surgery Table, visually inspect all accessible areas,
electrical cords, the table and all movable parts and components for possible damage that may adversely
affect the proper operation of the table. Verify Beam Lock and Silver Spar Knobs are tightened. Examine
the patient safety straps for any sign of wear or damage. Examine the covers of all pads for tears or other
damage that might cause the pads to trap fluids or other contaminants. Damaged or defective products
should not be used or processed. Contact Mizuho OSI Services for repair or replacement (refer to Section
13).
5.3 Semi-Annual Preventative Maintenance
A Semi-Annual Preventative Maintenance (PM) check (refer to Section 10.3) on your ProAxis® Spinal
Surgery Table is required at least once every six (6) months.
To obtain the PM checklist call Mizuho OSI Services at 1-800-777-4674 within the USA or +1-510-429-1500
outside the USA.
5.4 Product Lifetime
At the time of delivery, your product fulfills existing regulations and standards; however, in spite of proper
use, routine inspection, prescribed service, maintenance and repairs, the product is subject to aging and
wear. Therefore, Mizuho OSI cannot guarantee the product’s safety after ten (10) years and recommends
your product be taken out of service. For product warranty information, refer to Section 13.6 of this manual.
Page 54

50
Mizuho OSI 2016
NW0725 Rev K
6 Function Check
ProAxis® Spinal Surgery Table Owner’s Manual
NOTE: Perform all steps of the Function Check before every use of the ProAxis
For a complete definition of terms used in this procedure, please refer to the Glossary of Terms in
Section 2.9.
1. Confirm the Beam Lock is tightened.
2. Confirm the silver Spar Locks at the Head-End of the table are tightened, securing the
spars in place.
3. Connect the IntelliPendant® cable to the IntelliPendant® port on the Head-End column
(Figure 5, page 14).
4. Plug the Power Cord into a properly grounded receptacle to use with AC power. Refer to
the manufacturer’s label on the Foot-End column for input voltage requirements (Figure 10,
page 18).
5. Press the Power Switch on the Head-End base, and observe that a green ring illuminates
around the switch, confirming power is applied to the table.
6. If the AC power cable is not connected to an outlet with a protective earth ground, then the
external ground stud should be connected to a protective earth ground (Figure 4, page 13).
7. If the table needs to be used under battery power due to AC outage, ensure that the battery
has been properly charged as outlined in Section 12.3. Press the Power Switch on the
Head-End base, and observe that a green ring illuminates around the switch confirming
power is applied to the table. The battery status symbol on the IntelliPendant® should
be visible indicating a charged battery. If the battery status symbol on the IntelliPendant® is
the battery is not adequately charged and the table should only be used with AC
power. To protect the patient, hospital staff, and the table from possible electrical hazards,
an external ground cable connection is required between the external ground stud and
protective earth ground when the table is in use under battery power (Figure 4, page 13).
®
Surgery Table.
8. Lockout mode: The ProAxis® Surgery Table motions are only operational if the Floor Locks
are locked. The green indicator located on the Auxiliary Control Panel is illuminated and the
Floor Lock symbol is visible on the IntelliPendant® display screen when the Floor Locks
are completely engaged. Unlock the Floor Locks and verify that neither the IntelliPendant®
nor the Auxiliary Control Panel functions operate except to activate the Floor Locks.
9. Complete a Floor Lock check using the Auxiliary Control Panel (Figure 15, page 21).
a. Press the Floor Lock button and observe the LED indicator is illuminated on the
Auxiliary Panel and the Floor Lock symbol is visible on the IntelliPendant® display. The
table is locked if it cannot be moved on its wheels.
b. Press the Floor Unlock button and observe the LED is no longer illuminated on the
Auxiliary Panel and the Floor Unlock symbol is visible on the IntelliPendant® display.
The table is unlocked if it can move on its wheels.
Page 55

51
Mizuho OSI 2016
NW0725 Rev K
c. Floor Lock Override Check:
i. With the Floor Locks unlocked, utilize the IntelliPendant® and select the Override
Floor Locks setting.
ii. With the Override function active, utilize the Height Up, Height Down, Lateral Tilt
Right and Left, or Trendelenburg/Reverse Trendelenburg buttons. These buttons
should function without the Floor Locks being locked while the Override function is
utilized.
10. IntelliPendant® Check:
a. Utilizing the IntelliPendant®, press the Floor Lock button. Observe on the Auxiliary
Control Panel that the LED illuminates and that the Floor Lock symbol displays on the
IntelliPendant® display after the floor locks are completely deployed and locked. Look
under the Head-End and Foot-End base, and observe that all four feet have moved
down and are in direct contact with the floor (Figure 16, page 21).
b. Press the Floor Unlock button. Observe that all four locking feet move up. Confirm
the LED is not illuminated on the Auxiliary Control Panel and the Floor Unlock symbol
displays on the IntelliPendant® display after the floor locks are completely retracted and
unlocked. Look under the Head-End and Foot-End base and observe that all four feet
have moved up and are not contacting the floor (Figure 16, page 21).
c. Repeat Step (a) to ensure the Floor Locks are fully engaged such that the rest of the
table’s functionality may be tested.
ProAxis® Spinal Surgery Table Owner’s Manual
d. Press and hold the Height Up button. Observe that the table top raises through its
entire range. Observe the IntelliPendant® display, and confirm that the Height Up
symbol is visible in the action display area of the screen. The number beside the
Height Up symbol in the motion status area of the screen should be changing,
corresponding to where the table top is within the height range.
e. Press and hold the Height Down button. Observe that the table top lowers through its
entire range. Observe the IntelliPendant® display, and confirm that the Height Down
symbol is visible in the action display area of the screen. The number beside the
Height Down symbol in the motion status area of the screen should be changing,
corresponding to where the table top is within the height range.
f. Press and hold the Reverse Trendelenburg button. Observe that the Foot-End of the
table top moves down through its entire range. Observe the IntelliPendant® display,
and confirm the Reverse Trendelenburg symbol is visible in the action display area of
the screen. The number beside the Reverse Trendelenburg symbol in the motion
status area of the screen should be changing, corresponding to where the table top is
within the Reverse Trendelenburg range.
g. Press and hold the Trendelenburg button. Observe that the Head-End of the table top
moves down through its entire range. Observe the IntelliPendant® display, and confirm
that the Trendelenburg symbol is visible in the action display area of the screen. The
number beside the Trendelenburg symbol in the motion status area of the screen
should be changing, corresponding to where the table top is within the Trendelenburg
range.
h. Press and hold the Lateral Tilt, Left button. Observe that the table top tilts to the left
through its entire range. Observe the IntelliPendant® display, and confirm that the
Lateral Tilt, Left symbol is visible in the action display area of the screen. The number
beside the Lateral Tilt, Left symbol in the motion status area of the screen should be
changing, corresponding to where the table top is within the Lateral Tilt range.
Page 56

ProAxis® Spinal Surgery Table Owner’s Manual
52
Mizuho OSI 2016
NW0725 Rev K
i. Press and hold the Lateral Tilt, Right button. Observe that the table top tilts to the
right through its entire range. Observe the IntelliPendant® display, and confirm that the
Lateral Tilt, Right symbol is visible in the action display area of the screen. The number
beside the Lateral Tilt, Right symbol in the motion status area of the screen should be
changing, corresponding to where the table top is within the Lateral Tilt range.
j. Install the Two-Piece Supine Top on the frame (refer to Section 8) and test the table’s
hinge functionality:
1. Select the FSS Hinge Mode Setting using the IntelliPendant®.
2. Press and hold the Hinge Up button. Observe that the table top hinge angle
increases through its entire range. Observe the IntelliPendant® display, and confirm
the FSS Hinge Up symbol is visible on the screen. The number beside the
Hinge Up symbol in the motion status area of the screen should be changing,
corresponding to where the hinge is within the range of travel.
3. Press and hold the Hinge Down button. Observe that the table top hinge angle
decreases through its entire range. Observe the IntelliPendant® display, and
confirm that the FSS Hinge Down symbol is visible on the screen. The
number beside the Hinge Down symbol in the motion status area of the screen
should be changing, corresponding to where the hinge is within the range of travel.
4. Select the FE Hinge Mode Setting using the IntelliPendant®.
5. Press and hold the Hinge Up button. Observe that the table top hinge raises
through its entire range. Observe the IntelliPendant® display, and confirm the FE
Hinge Up symbol is visible on the screen. The number beside the Hinge
Up symbol in the motion status area of the screen should be changing,
corresponding to where the hinge is within the range of travel.
6. Press and hold the Hinge Down button. Observe that the table top hinge lowers
through its entire range. Observe the IntelliPendant® display, and confirm that the
FE Hinge Down symbol is visible on the screen. The number beside the
Hinge Down symbol in the motion status area of the screen should be changing,
corresponding to where the hinge is within the range of travel.
7. Return to the FSS Hinge Mode Setting using the IntelliPendant® if desired.
k. With the Two-Piece Supine Top installed on the frame, re-position the table top by
raising the Height to 36 inches (91 cm); laterally Tilt the table top left or right six (6)
degrees; raise or lower the Hinge 10 degrees; and place the table top in five (5)
degrees of Trendelenburg. Confirm this position by reading the display on the
IntelliPendant® screen. Press and hold the Return to Level button. Observe that the
table top levels side to side and head to foot. Continue to depress the button, and after
a one-second delay, the table top will move down to its Home height. Observe that the
table top is level and at the Home height position of 32 inches (81 cm) off the floor
when movement stops. Observe the IntelliPendant® display, and confirm the Height
display reads 32 inches (81 cm), the Hinge angle reads 0 degrees, the Tilt angle reads
0 degrees, and the Trendelenburg/Reverse Trendelenburg angle reads 0 degrees.
l. Using the Menu key, navigate to the ACP setting and select the Fast setting. Using the
ACP Soft Key on the Main Screen, activate the ACP function. Observe the Fast ACP
symbol is present on the IntelliPendant® display screen. Repeat the process, this time
selecting the Slow setting. Observe that the Slow ACP symbol is now present on the
IntelliPendant® display screen.
Page 57

ProAxis® Spinal Surgery Table Owner’s Manual
53
Mizuho OSI 2016
NW0725 Rev K
11. Battery operation check:
a. Observe the Battery Status on the base of the Head-End column. If the LED is
illuminated green - OK, then the battery is properly charged. Confirm that the Battery
Status icon on the IntelliPendant® is indicating the battery is fully charged and the
table is ready to operate on battery power.
b. If the LED is illuminated red or the Battery Status icon on the IntelliPendant® is , the
battery must be charged prior to using the table. To charge the battery, ensure the
Power Cord is plugged into a functioning receptacle. The AC LED at the base of the
Head-End column will illuminate green indicating that appropriate AC power is applied
to the table. The table must remain plugged in for a minimum of three (3) hours to
ensure sufficient charging of the battery to operate the table.
c. If the LED remains red and the Battery Status icon is still after three (3) hours,
continue to charge the battery for up to 24 hours.
d. If the LED does not illuminate green-OK and the Battery Status icon does not reflect
after 24 hours, refer to Section 12.3 for servicing instructions.
NOTE: The table may be used with AC power even when the Battery Status icon is indicating
the battery needs charging. Confirm the green AC power LED at the base of the Head-End
column is illuminated.
Page 58

ProAxis® Spinal Surgery Table Owner’s Manual
54
Mizuho OSI 2016
NW0725 Rev K
6988-899
Rail Mount, Left
The Rail Mount, Left slides on the left side of the Head-End hinged, open frame
and works in conjunction with the Chest Tray to translate the patient’s upper
torso when the hinge of the frame is raised or lowered.
6988-887
Rail Mount, Right
The Rail Mount, Right slides on the right side of the Head-End hinged, open
frame and works in conjunction with the Chest Tray to translate the patient’s
upper torso when the hinge of the frame is raised or lowered.
6988-950
Chest Tray
The Chest Tray sits on the Rail Mounts and provides the platform to which to
attach one of the three available Chest Pads used to support the patient. The
Chest Tray has a numeric scale to allow for ease in patient positioning.
6988-530
ProAxis®
5-Way Articulating Arm
Board (2)
- includes 6988-526
Arm Board Bracket (2)
The 5-Way Articulating Arm Boards provide support for the patient’s arms when
positioned prone on the ProAxis®. When in place, the Arm Board can be
translated, articulated, raised, lowered, and laterally adjusted depending on the
positioning needs of the patient. The Ball Joint controls the articulating motion of
the Arm Board, which may be required for positioning a patient with decreased
shoulder or arm range of motion. The 1-inch (2.5 cm) pad provided is of Mizuho
OSI Tempur-Pedic® medical construction. The bracket required to attach the
Arm Board on the Chest Tray is provided with the Arm Board assembly.
6988-758
ProAxis® Chest Pad
The ProAxis® Chest Pad is of Mizuho OSI Tempur-Pedic® medical construction.
When properly positioned, the top edge of the pad should rest at the bottom of
the patient’s supra-sternal notch.
D28502CE
Re-usable ProneView®
Adjustable Protective
Helmet
The re-usable ProneView® Adjustable Protective Helmet is part of the
ProneView® system and supports the one-time use disposable cushion insert
provided in the Patient Care Kit. The ProneView® Adjustable Protective Helmet
has four posts with 2.2 inches (5.6 cm) of height adjustment.
D28580CE
Re-usable ProneView®
Mirror Platform
with Post Holes
The re-usable Mirror Platform with Post Holes provides support for the
ProneView® Adjustable Protective Helmet and allows the patient’s eyes to be
viewed from three sides when positioned prone. The ProneView® Adjustable
Protective Helmet posts seat in the post holes of the mirror.
6988-969
Cervical Chest Tray
The Cervical Chest Tray sits directly on the rails of the open frame and the
ProAxis® Chest Pad attaches to the tray to support the patient. The Chest Tray
has Velcro® straps for securing it to the frame.
6988-725
Cervical Traction
Vector Adjustor
The Cervical Traction Vector Adjustor mounts on the Head-End of the table and
maintains alignment of the traction rope when cervical traction is utilized.
®
7 ProAxis
Spinal Surgery Table
Standard Components
7.1 ProAxis® Standard Components
In addition to the IntelliPendant® and the Power Cord, the following standard patient positioning
components are shipped with the ProAxis® Spinal Surgery Table. Pictures of the individual components
and instructions for use are provided in Section 8.
NOTE: Use proper lifting and carrying techniques when moving components and accessories due
to their weight and size.
Page 59

ProAxis® Spinal Surgery Table Owner’s Manual
55
Mizuho OSI 2016
NW0725 Rev K
6988-209
Cervical Management
Mounting Bracket
The Cervical Management Mounting Bracket installs in the Head Board and
allows the Mizuho OSI optional accessory, the Cervical Management Base Unit,
to be utilized with an aluminum or radiolucent DORO® or Mayfield® skull clamp.
6988-387
Contoured ACP Hip Pad,
Left
The Contoured ACP Hip Pad, Left is of Mizuho OSI Tempur-Pedic® medical
construction and provides support for the patient when positioned prone on the
open frame. When positioned correctly, the patient’s left iliac crest should be
positioned in the top third of the pad labeled Left.
6988-388
Contoured ACP Hip Pad,
Right
The Contoured ACP Hip Pad, Right is of Mizuho OSI Tempur-Pedic® medical
construction and provides support for the patient when positioned prone on the
open frame. When positioned correctly, the patient’s right iliac crest should be
positioned in the top third of the pad labeled Right.
6977-739
Buttocks Strap
The Buttocks Strap rests across the patient’s lower gluteal area when positioned
prone to secure the patient in place on the Contoured ACP Hip Pads and open
frame.
6844-148
Leg Board with Pad (2)
The two (2) Leg Boards, when used together, provide support for the patient’s
legs when positioned prone on the open frame. The 1-inch (2.5 cm) pad is
made of Mizuho OSI Tempur-Pedic® medical construction.
5840-450
Leg Sling
The Leg Sling is placed over the Foot-End section of the hinged, open frame
and is secured in place with Velcro® straps. Positioning pillows should be utilized
in the sling to achieve the desired positioning and support of the patient’s legs
when positioned prone.
6988-880
Retractor Adaptor
The Retractor Adaptor provides six (6) inches (15 cm) of side rail and is required
for mounting ancillary equipment onto the carbon fiber open frame.
6946
Tempur-Pedic® Medical
Pillow, Standard Size
A standard size pillow of Tempur-Pedic® medical construction is provided for
patient positioning. A cleanable, zippered cover encases the pillow.
6947
Tempur-Pedic® Medical
Pillow, Queen Size (2)
A queen size pillow of Tempur-Pedic® medical construction is provided for
patient positioning. A cleanable, zippered cover encases the pillow.
6988-623
Supine Top, Head-End
Assembly
The Supine Top, Head-End is of carbon fiber construction and mounts on the
Head-End section of the hinged, open frame. It is secured to the frame with
locking brackets and accommodates supine and lateral patient positioning.
6988-621
Supine Top, Foot-End
Assembly
The Supine Top, Foot-End is of carbon fiber construction and mounts on the
Foot-End section of the hinged, open frame. It is secured to the frame with
locking brackets and accommodates supine and lateral patient positioning.
6988-442
Tempur-Pedic® Medical
2-inch (5 cm) Supine Top
Pad (one piece)
The one piece Tempur-Pedic® Medical 2-inch (5 cm) Pad mounts on the Supine
Tops. The pad is of viscoelastic foam construction and has a built-in translation
slide on the bottom of the pad that interfaces with the surface of the Supine Top,
Foot-End, allowing for support and movement when the hinge is raised or
lowered.
6977-959
Universal Side Rail
Adaptor (4)
Utilized on the Supine Top, the Universal Side Rail Adaptor is designed to
protect the carbon fiber and provide 6-in. (15 cm) of side rail for mounting
ancillary equipment.
5840-43
Safety Strap, 60 inches
(152 cm) (2)
The Safety Straps are provided to secure the patient when positioned prone on
the ProAxis®.
Page 60

ProAxis® Spinal Surgery Table Owner’s Manual
56
Mizuho OSI 2016
NW0725 Rev K
5855-550
Safety Strap for
Supine Top (2)
The Safety Strap slides on the carbon fiber rails of the Supine Top and is
provided to secure the patient when positioned supine or lateral on the ProAxis®.
6988A-PV-1-ACP
ProAxis® Spinal Surgery
Table ProneView® Patient
Care Kit (3)
The Patient Care Kits are single-use pad covers intended to prevent crosscontamination and protect the equipment. Three (3) ProneView® Patient Care
Kits are included with the ProAxis® table.
6988-900
ProAxis®
Component Cart
The ProAxis® Component Cart is provided to safely store all the standard
components of the table. It is also designed to store optional accessories which
may be acquired for use with the ProAxis®. A cover is provided with the cart and
serves as a dust cover when the cart is not in use.
6988-70
ProAxis® Table Cover
The ProAxis® Table Cover is provided to serve as a dust cover when the table is
not in use. The cover fits the table when set up (fully extended) or it can be
adjusted with the Velcro® closures to cover the table when it is configured for
transport or storage.
6988-90
Spar Cover (2)
The Spar Covers are provided to serve as a protective cover for the spars when
the table is not in use and configured for transport or storage.
7.2 ProAxis
®
Patient Care Kits
The ProAxis® Surgery Table ships with three (3) individual ProAxis® ProneView® Patient Care Kits. A case
of six (6) individual kits is provided when ordering 6988A-PV-ACP.
Each individual ProAxis® ProneView® Patient Care Kit includes the following:
ProneView® Cushion Insert
Chest Pad Cover for ProAxis® Chest Pad
Pair of Hip Pad Covers with Aprons
Pair of Foam Arm Cradles
1 Set of Single-Use, Disposable Advanced Control Pad System™ Tubing
WARNING: Patient Care Kits are single use-only. Discard after use. Re-use of any component
in the Patient Care Kit may result in cross contamination.
The components should be used as described in the Patient Care Kit instructions included in each kit.
The ProneView® Patient Care Kit works with the provided ProneView® Adjustable Protective Helmet
System. The system is designed by the manufacturer to support the patient’s head while in a prone
position. Additionally, the position of the patient’s facial alignment in the ProneView® Adjustable Protective
Helmet can be readily viewed while in the prone position.
NOTE: Remove the Patient Care Kit components from the packaging and visually inspect the
components. The ProneView® Cushion Insert should be opened sixty (60) minutes prior to
use and the Arm Cradles should be opened twenty (20) minutes prior to use for complete
expansion of the foam.
NOTE: The Mirror Platform with Post Holes is a rectangular shaped mirror with four holes
patterned to accept the adjustable posts on the helmet. The mirror can be seated on the
Face Plate with the longer side oriented either parallel or perpendicular to the overall length
of the table.
Page 61

ProAxis® Spinal Surgery Table Owner’s Manual
57
Mizuho OSI 2016
NW0725 Rev K
®
8 Setup of ProAxis
Spinal Surgery
Table for Surgical Procedures
NOTE: This device is intended for use by trained personnel. Prior to setup and use of the ProAxis
Spinal Surgery Table, ensure personnel have been trained. To schedule an in-service,
please contact your domestic Mizuho OSI sales representative or call 1-800-777-4674
within the USA or +1-510-429-1500 outside the USA.
To prepare the ProAxis® Spinal Surgery Table for patient positioning, complete the following steps:
1. Connect the IntelliPendant® to the port on the Head-End column (Figure 19, page 24).
2. Press the Power Switch and observe that the switch illuminates with a green ring indicating
that power is applied to the table.
3. Utilizing the IntelliPendant®, press the Floor Unlock button. Observe that the Floor Locks
are completely retracted and the Floor Unlock symbol is visible on the display of the hand
pendant (Figure 21, page 25).
4. With the assistance of a second person, move the table to where it will be used. One
person should be located at the Head-End and the other person at the Foot-End of the
table. Care should be taken to control the table when rolling.
WARNING: If the ProAxis® Spinal Surgery Table is allowed to roll too fast, it may be difficult to
stop or turn. Impact of the table top with a stationary object may cause serious damage to the table
top. If an impact does occur, the table must be visually inspected for damage and a Function Check
must be performed (refer to Section 6). If damage is discovered or the table does not successfully
complete the Function Check, call Mizuho OSI Services (refer to Section 13).
®
5. Orient the Head-End of the table in the room where the anesthesiologist work station is
located.
6. Plug the Power Cord into a properly grounded receptacle. Refer to the manufacturer’s
label on the Foot-End column for input voltage requirements (Figure 10, page 18). If the
AC power cable is not connected to an outlet with a protective earth ground, then the
external ground stud should be connected to a protective earth ground (Figure 10, page
18).
7. Using the IntelliPendant®, press the Floor Lock button. Observe that the Floor Lock symbol
is visible on the hand pendant display (Figure 22, page 25).
8. Complete a Function Check with the IntelliPendant® (refer to Section 6).
NOTE: Perform all steps of the Function Check before every use of the ProAxis
Table.
NOTE: The ProAxis
components prior to patient transfer. Components have been designed to specifically
mount on the carbon-fiber, trapezoidal frame or the Supine Top, while safely supporting the
patient and not damaging the frame.
®
Spinal Surgery Table is now ready for completing the setup of the patient
®
Spinal Surgery
Page 62

ProAxis® Spinal Surgery Table Owner’s Manual
58
Mizuho OSI 2016
NW0725 Rev K
Figure 59: ProAxis® Spinal Surgery Table Open Frame Ready for Setup
Page 63

ProAxis® Spinal Surgery Table Owner’s Manual
59
Mizuho OSI 2016
NW0725 Rev K
Torso Trolley®
Flat ACP Chest
ProneView
®
Helmet System
Hinged Open Frame
Contoured ACP
Hip Pads
Built-in ACP System
Built-in Foot Board
5-Way Articulating
Arm Boards
Adjustable
Leg Sling
IntelliPendant®
8.1 ProAxis
NOTE: Only Mizuho OSI components and accessories have been tested and approved for use
®
Prone Patient Positioning
with the ProAxis® Spinal Surgery Table. Other manufacturers’ products have not been
tested for proper performance when used with the ProAxis® Spinal Surgery Table, and
therefore are not endorsed for use by Mizuho OSI. Use of other manufacturers’ products
may void the warranty.
WARNING: Use of components not manufactured by Mizuho OSI may result in harm to the
patient, the table top, the device, or the healthcare professional.
The following instructions provide the recommended steps for setup. Final determination of patient
positioning and setup is at the discretion of the surgeon and surgical team.
Figure 60: ProAxis® Prone Setup – Fixed Surgical Site Hinge Mode
1. Confirm the ProAxis® Spinal Surgery Table is prepared for setup (refer to Section 8). When
the patient is positioned prone, their head is oriented to the Head-End of the table (Figure
1, page 6).
2. Confirm that the Floor Locks are locked. Ensure the Floor Lock symbol is visible on the
IntelliPendant® display.
3. Open the following: the Patient Care Kit, the ProneView® Cushion Insert or the
GentleTouch® Face Pillow, and the package of Arm Cradles and set aside.
NOTE: The surgeon may decide to use the ProneView
®
Cushion or the GentleTouch® Face Pillow
depending on which option allows for the achievement of proper cervical spine position.
Page 64

ProAxis® Spinal Surgery Table Owner’s Manual
60
Mizuho OSI 2016
NW0725 Rev K
Rail Mount,
Slides into
Bracket
Hinged Frame
Bracket
4. Slide the Rail Mount, Right and Rail Mount, Left onto the corresponding Head-End sections
of the Hinged Frame. The nipple will engage the bracket, locking the Rail Mount in place.
To confirm, pull back on the Rail Mount; it should remain secure in the Hinged Frame
bracket (Figure 61).
Figure 61: Installing the Rail Mount
5. Connect the cable connector extending from the Rail Mount, Right/Left to the port on the
corresponding side of the Head-End Assembly. Align the pins of the cable connector with
the port, insert and turn the locking collar to the right securing the pins in the port
(Figure 62).
NOTE: To remove the Rail Mount, turn the locking collar to the left and pull the cable connector out
of the port, disconnecting it from the Head-End Assembly. With the cable connector
disconnected, depress the Black Tab on the frame while sliding the Rail Mount towards the
hinge.
NOTE: When both rail mounts are connected, the Torso Trolley
®
drive pins may reposition to align
properly.
Page 65

ProAxis® Spinal Surgery Table Owner’s Manual
61
Mizuho OSI 2016
NW0725 Rev K
Depress Black Tab
to Remove Rail
Mount
Port for Cable
Connector of Rail
Mount
Figure 62: Attaching the Cable to the Port
6. The Torso Trolley® consists of a Chest Tray to which the Flat ACP or non-ACP Chest Pad
or optional Bilateral Winged ACP Chest Pad and 5-way Adjustable Arm Boards are
mounted (Figure 69, page 66).
a. Prior to installing the Chest Tray, ensure the Rail Mounts are correctly installed and the
Hinged Frame is in the neutral position.
Figure 63: Rail Mount Installed
Page 66

ProAxis® Spinal Surgery Table Owner’s Manual
62
Mizuho OSI 2016
NW0725 Rev K
Yellow Strip
b. Confirm the display on the IntelliPendant® shows the Hinge, Trendelenburg, and
Lateral Tilt angles are all 0° (Figure 22, page 25). If necessary, utilize the
IntelliPendant® and press the Return to Level button to return the Hinged Frame to the
neutral position.
Figure 64: Left and Right Rail Mounts Attached
7. To install the Chest Tray:
a. Orient the Chest Tray over the posts of the Rail Mounts and lower into place. The
Chest Slide can be placed anywhere on the Rail Mounts that allows the posts to sit
between the teeth of the Chest Slide (Figure 65).
b. Confirm the Chest Tray is not placed on the rails at a point lower than Position 1 on the
Rail Mounts in order to ensure the Chest Tray is properly engaged.
NOTE: When mounting the Chest Tray onto the rails, ensure the yellow strip on each of the Rail
Mounts is not visible. Appearance of the yellow strips indicates that the Chest Tray is not
properly engaged with Rail Mounts.
WARNING: Visibility of the yellow strips on the Rail Mounts indicate that Chest Tray is not
properly engaged with the Rail Mounts, which will prevent it from sliding correctly during hinge
motion. Failure to ensure the Chest Tray is correctly mounted may result in harm to the patient or
the device.
c. The Chest Tray may be moved up or down the length of the Rail Mounts by
simultaneously squeezing the grips of the Chest Tray and moving it to the desired
location. Release both grips to lock the Chest Tray into place.
Page 67

ProAxis® Spinal Surgery Table Owner’s Manual
63
Mizuho OSI 2016
NW0725 Rev K
Chest Slide Grip
Chest Slide Grip
Pad
Indentation
Tab of Flat Chest Pad
Secures Pad to Chest
Tray
Depress Tab to Remove
Pad
Figure 65: Chest Slide Mounted on the Rail Mounts
8. To attach the ProAxis® Chest Pad, orient the opening of the pad bracket so it surrounds the
lower surface of the Chest Tray. Slide onto the Chest Tray until the tab of the bracket is
secure on the tray. The indentation in the pad for the supra-sternal notch should be
oriented towards the Head-End of the table (Figure 66). Confirm the tab of the bracket is
secure on the tray. To remove, press the tab of the bracket and slide the pad towards the
Foot-End of the table.
Figure 66: ProAxis® Chest Pad Mounted on the Chest Tray
Page 68

ProAxis® Spinal Surgery Table Owner’s Manual
64
Mizuho OSI 2016
NW0725 Rev K
NOTE: Also at the discretion of the physician, additional foam or padding can be placed between
the patient and the pad as needed to accommodate varying sizes of patients.
NOTE: With ideal positioning, the Chest Pad should support the patient such that the load is borne
predominantly by the sternum. When the patient is positioned correctly, the top edge of the
pad should rest below the patient’s anatomical landmark, the supra-sternal notch.
WARNING: Positioning the Chest Pad superior to the supra-sternal notch may apply pressure
to the throat and the patient’s airway. Failure to ensure the patient is positioned correctly on the
Chest Pad may result in harm to the patient or the device.
9. Install the 5-Way Adjustable Arm Boards.
a. Slide the Arm Board Mounting Brackets into each opening of the bracket on the
underside of the Chest Slide (Figure 67). Advance the brackets fully and tighten the THandles.
b. Attach the Articulating Arm Board to the non-patient transfer side of the table. Insert the
arm board assembly into the Arm Board Bracket and tighten the T-Handle on the Arm
Board Mounting Bracket.
c. Attach the Articulating Arm Board to the patient transfer side of the table. Insert the arm
board assembly into the Arm Board Bracket and tighten the T-Handle on the Arm
Board Mounting Bracket.
d. Adjust the arm boards as needed in preparation for patient transfer (Figure 68). The
Height, Translation, and Angle of the arm board surface can be adjusted. To adjust the
Height, loosen the Black Knob and raise or lower the assembly, or raise or lower the
assembly on the mounting bracket. Tighten the Black Knob and or the T-Handle when
the desired Height is achieved. To translate the arm board towards the Head-End or
Foot-End of the table, pull the pin on the underside of the arm board, translate and then
re-position and release the pin to lock in place. To articulate the arm board, loosen the
drop handle, pivot in the ball joint to the desired position, and tighten T-Handle.
CAUTION: Use care when positioning the arm board to ensure that it does not come in
contact with the floor or surrounding equipment positioned near the table. Failure to allow for
proper clearance of the arm board with its surroundings may result in damage to the arm board or
other equipment.
e. Pivot the Articulating Arm Board on the patient transfer side out of the way by turning
the Black Knob and moving the arm board. Secure in place by re-tightening the Black
Knob (Figure 68).
Page 69

ProAxis® Spinal Surgery Table Owner’s Manual
65
Mizuho OSI 2016
NW0725 Rev K
Pull knob to Release and
Translate Arm Board
North/South.
Bracket on the bottom of
the Chest Tray includes
T- Handles to secure the
Arm Board Mounting
Bracket.
T-Handle Allows Lateral
Adjustment of Arm Board
in Place
T-Handle Allows Height
Adjustment of Arm Board
Arm Board Mounting Bracket
Height Adjustments
Articulating Ball Joint
Pivot &
Lateral Adjustment
Figure 67: Arm Board Mounting Bracket Installed
Figure 68: 5-Way Adjustable Arm Board Installed
Page 70

ProAxis® Spinal Surgery Table Owner’s Manual
66
Mizuho OSI 2016
NW0725 Rev K
Figure 69: Torso Trolley® with Chest Pad and Arm Board Mounted
10. Confirm the Torso Trolley® slides properly. Simultaneously squeeze the grips of the Chest
Tray (Figure 70). This releases the post in the Rail Mount from the teeth of the Chest Tray
and allows for the Chest Tray with Arm Boards to be moved easily toward the Head-End or
Foot-End of the table. The 1-8 scale on the Chest Tray assists in identifying where the
Chest Tray is in its range of movement.
Figure 70: Manually Moving the Torso Trolley®
Page 71

ProAxis® Spinal Surgery Table Owner’s Manual
67
Mizuho OSI 2016
NW0725 Rev K
Slide on Rail Frame
Pad Stop Here
Locking Bracket, Open
White tab
Lip of black component
of clamp
11. Attach the Contoured ACP Hip Pads. The pads are labeled right and left specific to be
oriented to the patient’s iliac crest when lying prone. Open the Locking Bracket, orient the
Pad with Locking Bracket over the rail of the open frame on the Foot-End section of the
frame, lower into place and slide towards the Hinge until it rests against the Pad Stop
(Figure 71).
Figure 71: Mounting the Contoured ACP Hip Pad on the Frame
12. Press up on the white tab until the black component of the clamp fits into the open notch of
the white tab. Press down on the lip of the black component of the clamp to lock the pad in
place (Figure 72).
Figure 72: Locking the Contoured ACP Hip Pad Bracket
13. When installed correctly, the pad should rest against the Pad Stop and the arrow on the
side of the Bracket should be pointed toward the Foot-End of the table (Figure 73). To
remove the Contoured Hip Pad, lift up on the lip of the Bracket so that it disconnects, and
lift the pad off the rail of the frame.
Page 72

ProAxis® Spinal Surgery Table Owner’s Manual
68
Mizuho OSI 2016
NW0725 Rev K
Loop for Buttocks Strap
Oriented Correctly,
Arrow Points to Foot-End
Positioned at Hinge,
Pad Rests against Stop
Locking Bracket, Locked
ACP Tubing Hangs Freely
NOTE: Depending on desired area of flexion and patient’s anatomy, the hip pads may be moved
towards the Foot-End of the table at the surgeon’s discretion to achieve proper positioning.
Figure 73: Contoured ACP Hip Pad Locked in Place
14. To install the Adjustable Leg Sling, place the sling over the Foot-End section of the open
frame (Figure 74). Feed the four straps through their respective buckles on the opposite
side of the sling and secure. The sling should be adjusted for each patient. The straps on
the sling consist of a double–sided hook and loop closure that allows for infinite adjustment
along the length of the strap. The hook end of the strap may be folded over onto the loop
portion of the strap to allow for greater adjustability. The strap may be folded over itself
again to shorten, thereby raising the sling and decreasing the degree to which the patient’s
hips are flexed. If the double-sided hook and loop closure is worn from repetitive use or
misuse, replacement of the sling is required.
Figure 74: Sling Attached to ProAxis® Spinal Frame
15. Place two (2) or three (3) 6946 or 6947 Tempur-Pedic® Medical Pillows in the
sling approximately where the patient’s lower legs will rest to achieve the desired amount of
knee flexion and support (Figure 75).
Page 73

ProAxis® Spinal Surgery Table Owner’s Manual
69
Mizuho OSI 2016
NW0725 Rev K
Figure 75: Sling with Pillow Placement
16. The Leg Boards may be used in place of the Leg Sling to support the legs. Attach the Leg
Boards with the 1-inch (2.5 cm) Tempur-Pedic® Medical Pad. Release the Velcro® Straps
on the sides of the Leg Board. Place one Leg Board over the rails of the open frame and
slide into place. Secure with the Velcro® Straps. Repeat this process with the second Leg
Board. Ensure that the two Leg Boards abut each other (Figure 76).
Figure 76: Leg Boards Installed on the ProAxis® Spinal Frame
17. If using the ProneView® Mirror and Helmet System, place the Mirror Platform with the
ProneView® Adjustable Helmet on the Chest Tray (Figure 77). Ensure that the adjustable
posts are in their lowest position in preparation for patient transfer. Fit the ProneView®
Cushion Insert into the ProneView® Helmet and within the tabs provided at the chin end of
the ProneView® Helmet. Adjust the Cushion Insert openings to be centered relative to the
ProneView® Helmet openings.
Page 74

ProAxis® Spinal Surgery Table Owner’s Manual
70
Mizuho OSI 2016
NW0725 Rev K
Figure 77: ProneView® Mirror and Helmet System Properly Positioned
18. If using the GentleTouch® Face Pillow, place the Pillow on the Chest Tray (Figure 78).
Figure 78: GentleTouch® Face Pillow Properly Positioned
NOTE: In some cases if use of tongs or a head halter is desired in conjunction with the use of the
Torso Trolley®, an optional short Cervical Traction Vector ( 6988-720) is available.
Page 75

ProAxis® Spinal Surgery Table Owner’s Manual
71
Mizuho OSI 2016
NW0725 Rev K
19. After the desired prone components are in place on the frame, the tubing can be connected
to the Contoured ACP Hip Pads.
a. Secure the ACP tubing with the Velcro® wraps along the frame and by routing it
through the underside of the Leg Sling. Ensure the tubing is secure and out of the way
such that the tubing will be able to move when the Hinged Frame is moved.
b. Ensure the third port that does not connect to the Hip Pads is cinched to prevent air
from escaping the system.
c. Connect the tubing to the port at the Foot-End of the table.
d. Utilize the IntelliPendant® and raise and lower the Hinge to the extent of its travel. Re-
adjust the ACP tubing as may be required.
NOTE: Verify the ACP tubing will not be stretched or pinched during hinge movement, which may
interrupt or obstruct air flow to the pads. Failure to do so may result in ACP malfunction.
20. Place the Patient Kit Covers on the Chest and Hip Pads (Figure 79).
Figure 79: ProAxis® Spinal Surgery Table Prone Setup with Patient Care Kits Applied
21. Trial position the imaging equipment if it will be used. If necessary, re-locate the ProAxis®
Surgery Table in the room. Unlock the Floor Locks by using the IntelliPendant® and with
the assistance of a second person move the ProAxis® Surgery Table. When the table is
located where it will be used, lock the Floor Locks.
CAUTION: The ProAxis® Surgery Table is not for patient transport. The location of the table
relative to any imaging equipment that may be used needs to be confirmed prior to transferring the
patient to the table.
Page 76

ProAxis® Spinal Surgery Table Owner’s Manual
72
Mizuho OSI 2016
NW0725 Rev K
22. Position the patient bed or stretcher so the patient’s anatomical landmarks are aligned with
the support pads on the hinged frame of the ProAxis® Surgery Table. It may be necessary
to re-position the Chest Pad by manually moving the Torso Trolley®. The top edge of the
Chest Pad should align with the patient’s anatomical landmark, the bottom of supra-sternal
notch, and the top third of the Contoured ACP Hip Pad should align with the patient’s
anatomical landmark, the iliac crest.
23. The height of the patient bed or stretcher should be level with the top of the Contoured ACP
Hip Pad. To assist the patient transfer process, you may choose to use the IntelliPendant®
and laterally tilt the table toward the patient bed or stretcher by pressing the appropriate tilt
button.
NOTE: When the patient is correctly positioned prone on the ProAxis
®
, the hinge will flex or extend
the patient’s spine at L3.
24. Lock the patient stretcher or bed. Confirm by observing on the IntelliPendant® display that
the table Floor Locks are locked. The Floor Locks Locked symbol should be visible (Figure
22, page 25).
25. Ensure that the ProneView® Adjustable Protective Helmet legs are set to the lowest
position.
26. Place the ProneView® Adjustable Protective Helmet with Cushion Insert on the patient’s
face. Ensure the patient’s eyes are visible. With the assistance of others, log-roll the
patient onto the ProAxis® Spinal Surgery Table supporting the patient’s head, torso and
legs.
27. After log-rolling the patient to a prone position and onto the Mirror Platform on the Chest
Tray, verify there is adequate clearance between the patient’s nose and the mirror, and
verify the endotracheal tube is not kinked.
WARNING: Failure to confirm the positioning of the patient and patient tubes may cause harm
to the patient.
28. Continue to support the patient on the table until the safety straps are in place. DO NOT
leave the patient unattended on the table.
WARNING: Patient must be restrained at all time by a safety strap(s) when positioned on the
table.
29. Remove the patient bed or stretcher while supporting the patient on the table.
30. Install and secure the Articulating Arm Board on the patient transfer side of the table
(Figure 68, page 65). Position the patient’s arms in the Arm Cradles and on the Arm
Boards. Final positioning of the patient’s arms should be completed after confirming the
patient’s orientation on the Chest Pad and the Contoured ACP Hip Pads.
31. Confirm that the patient’s face is seated in the Cushion Insert; the eyes should be visible in
the mirror; the neck should be in a neutral position (Figure 80). Raise the height of the
ProneView® Adjustable Protective Helmet until a neutral neck position is achieved by
turning the posts of the helmet. There is a 2.3-inch (5.6 cm) height adjustment range
available.
Page 77

ProAxis® Spinal Surgery Table Owner’s Manual
73
Mizuho OSI 2016
NW0725 Rev K
WARNING: Frequently monitor the patient’s neck, head, eyes, nose, and mouth to ensure that
a safe position is maintained and the ET tube has not been kinked or displaced. Failure to do so can
lead to serious adverse consequences including blindness and failure to ventilate.
WARNING: Failure to maintain neutral neck position can result in neck damage and/or chin
abrasions. Some erythema may result from skin contact with the materials in this product or from
compression during prolonged cases.
Figure 80: Patient Positioned Properly in ProneView® Helmet
32. Visually confirm the placement of the Contoured ACP Hip Pads. The pad should be
mounted abutting the Pad Stop (Figure 71, page 68). When positioned correctly, the
patient’s anatomical landmark, the iliac crest, should rest in the top one-third of the pad.
The Patient Kit Hip Pad Cover should be smooth against the patient’s skin. Ensure that the
patient’s skin is smooth and flat against the Patient Kit Hip Pad Cover. To re-position the
patient on the Pad, the patient should be lifted and moved such that the iliac crest contacts
the top third of the pad. Ensure the Hip Pad Cover is smoothed and lower the patient back
onto the Pad. Confirm correct placement of the Pads such that the each of the patient’s
iliac crests rest in the top one-third of each respective Hip Pad (Figure 81).
CAUTION: Failure to ensure that the patient is positioned correctly on the Contoured ACP Hip
Pad, may result in harm to the patient or the device.
Page 78

ProAxis® Spinal Surgery Table Owner’s Manual
74
Mizuho OSI 2016
NW0725 Rev K
Supra-sternal Notch
Figure 81: Proper Lower Body Patient Positioning
33. Visually confirm the placement of the Chest Pad. When positioned correctly, the edge of
the Pad should rest below the patient’s anatomical landmark, the supra-sternal notch
(Figure 82). Breasts should be down and lying flat with the nipples oriented towards the
Foot-End of the table. The Patient Kit Chest Pad Cover should be smooth against the
patient’s skin. Ensure that the patient’s skin is smooth and positioned flat against the
cover. If necessary, re-position the Chest Pad. While lifting and supporting the patient,
move the Torso Trolley® with the attached Chest Pad to the desired location and smooth
the Chest Pad Cover. The patient should then be lowered back onto the Chest Pad
(Figure 83).
NOTE: With ideal positioning, the Chest Pad should contact the patient such that the load is borne
predominantly by the sternum.
WARNING: Positioning the Chest Pad superior to the supra-sternal notch may apply pressure
to the throat and patient’s airway. Failure to ensure the patient is positioned correctly on the Chest
Pad may result in harm to the patient or the device.
Figure 82: Example of Patient’s Supra-sternal Notch
Page 79

ProAxis® Spinal Surgery Table Owner’s Manual
75
Mizuho OSI 2016
NW0725 Rev K
Figure 83: Proper Upper Body Patient Positioning
34. Visually confirm that the Leg Sling or Leg Boards provide support to the patient’s knees,
with legs supported on pillows. If using the Leg Sling, the patient’s knees should be flexed
and legs supported on the pillows. Re-position the legs and add pillows as required under
the patient’s tibias to ensure that the ankles are not hyper-extended and there is no
pressure on the patient’s feet (Figure 81, page 74).
CAUTION: Failure to ensure that the patient is positioned correctly with the Leg Sling or Leg
Boards may result in harm to the patient or the device.
CAUTION: When the table is positioned at a low height, take care in positioning the patient in
the Leg Sling such that the Sling does not come in contact with the center beam. Failure to properly
monitor the position of the Leg Sling with the patient’s legs positioned in it may result in harm to
the patient or the device.
35. Place the Buttocks Strap across the lower gluteal area of the patient. A blanket may be
used under the Buttocks Strap to protect the patient from direct contact with the strap. The
strap can be fed through the buckle on the sides of the Contoured ACP Hip Pads and then
back on itself, securing the Velcro®. Confirm that the Buttocks Strap is positioned low on
the gluteal area, and that it surrounds both Contoured ACP Hip Pads, and is secured
snugly around the buttocks. Ensure that the patient does not directly contact the frame of
the table. Use foam or gel padding as needed to pad the area between the patient and the
rails of the frame.
36. Apply a Safety Strap over the patient’s lower legs. Secure the Safety Strap in place with
use of the buckle.
NOTE: The placement and location of the Straps are at the discretion of the surgeon and vary by
procedure.
Page 80

ProAxis® Spinal Surgery Table Owner’s Manual
76
Mizuho OSI 2016
NW0725 Rev K
Pull to Release and
Translate Arm
Board North/South
Black Knob to
Control Pivoting of
Arm Board
Drop Handle
Releases Ball Joint
so that Arm Board
can be angled
T-Handle Allows
Height Adjustment
on Post
NOTE: Due to the open nature of the ProAxis
®
frame and the long duration of many spinal
procedures, the use of forced air warmers, fluid warmers, and blankets should be
considered to help prevent hypothermia. Blankets may be placed over the feet up to the
gluteal area and across the shoulders and arms out of the surgical field to aid in
maintaining the patient’s body temperature. These devices should be used according to
the manufacturer’s directions and at the discretion of the surgeon.
37. Position the patient’s arms and adjust the Articulating Arm Boards as needed. Support the
arms in the foam Arm Cradles. The V of the cradle should rest against the inside of the
patient’s elbow. Translate, raise, lower, or articulate the Arm Board as needed and then
secure in place (Figure 84). Confirm that all the T-Handles and the Drop Handle are
tightened. Confirm that the patient’s arms are positioned so there is no more than
90 degrees flexion at the shoulder and no more than 90 degrees flexion at the elbow. Use
the Velcro® Arm Board Straps provided to secure the arms.
Figure 84: Articulating Arm Board Functionality
WARNING: Hyperextension of the shoulder may cause compression of the brachial plexus
resulting in a potential nerve or vascular injury. Failure to ensure proper arm positioning may result
in harm to the patient.
Figure 85: Final Neutral Prone Positioning
Page 81

ProAxis® Spinal Surgery Table Owner’s Manual
77
Mizuho OSI 2016
NW0725 Rev K
Retractor Adaptor
WARNING: Proper pre-operative and intra-operative procedures must be followed to prevent
venous stasis and pooling, pressure sore development, neuropathy, improper electrosurgical tissue
grounding, hypertension/hypotension, and hypothermia.
CAUTION: Use care and monitor the patient when changing the position of the ProAxis®
Surgery Table (Height Up/Down, Hinge Up/Down, Trendelenburg/Reverse Trendelenburg, and
Lateral Roll) to ensure that the patient or table does not interfere with other equipment and the
components and accessories have proper clearance in relation to the surrounding areas. Failure to
do so may cause harm to the patient or device.
38. Confirm that the patient is properly positioned. If a Retractor Adaptor is required to mount
auxiliary equipment, mount it on the rail frame. Open the Adaptor by turning the black knob
and releasing the bottom of the Adaptor. Place the Adaptor around the frame, align the
black knob, turn to tighten and secure the Adaptor in place (Figure 86). Ensure that the
patient does not directly contact the Retractor Adaptor. Use foam or gel padding as
needed to pad the area between the patient and the Adaptor.
Figure 86: Retractor Adaptor Mounted on Carbon Fiber Rail
WARNING: Only the metal Retractor Adaptor may be positioned in the area above the hinge
and below the rail mounts. Use of the optional Radiolucent Retractor Adaptor at this location
results in a pinch point when using the hinge capabilities of the table, which may cause harm to the
patient or the device.
NOTE: Use of the Radiolucent Retractor Adaptor at any location below the Hip Pads is allowed
when the hinge functionality of the table is engaged. The Radiolucent Retractor Adaptor
can also be used above the hinge when the table is configured for cervical and the hinge
functionality is deactivated.
39. Refer to Section 4 for instructions on how to utilize the IntelliPendant® and operate the
table.
Page 82

ProAxis® Spinal Surgery Table Owner’s Manual
78
Mizuho OSI 2016
NW0725 Rev K
CAUTION: The linkage arms of the columns raise, lower, and rotate when the table is in use.
Ensure no items are in the path of the moving arms and healthcare professionals position
themselves away from the linkage arms when the table is in motion. Failure to do so may cause
injury to the healthcare professional or possible damage to the table.
Figure 87: Prone Positioning with Fixed Surgical Site Mode, Hinge Up
Page 83

ProAxis® Spinal Surgery Table Owner’s Manual
79
Mizuho OSI 2016
NW0725 Rev K
Rail Mount
Chest Tray
5 Way Articulating Arm Board
6988-899, Left
6988-887, Right
6988-950
6988-530, Arm Board
Assembly with Bracket & Pad (2)
5379-7722 1-inch (2.5)
Tempur-Pedic® Medical Arm Board
Pad
Flat Chest Pad
Contoured ACP
Hip Pad, Right
Contoured ACP
Hip Pad, Left
6988-758 ProAxis®
Chest Pad
6988-388
6988-387
Leg Board
Leg Sling
Buttocks Strap
6844-148 (2)
5840-450
6977-739
Left
Right
8.1.1 Components Used for Prone Patient Positioning
Page 84

ProAxis® Spinal Surgery Table Owner’s Manual
80
Mizuho OSI 2016
NW0725 Rev K
Safety Strap, 60 in (152 cm)
Retractor Adaptor
ProAxis® ProneView®
Patient Care Kit
5840-43 (2)
6988-880
6988A-PV-ACP 6/case
Re-usable ProneView®
Adjustable Protective Helmet
System
Tempur-Pedic® Medical
Positioning Pillows (3)
ProAxis® Component Cart
D28502CE ProneView®
Adjustable Protective Helmet
D28580CE Mirror Platform
with Post Holes
6946 Standard Pillow (1)
6947 Queen Pillow (2)
6988-900
Includes a Cart Cover
ProAxis® Table Cover
Optional Accessory
Optional Accessory
6988-70
6988A-GT-ACP
ProAxis® GentleTouch®
Patient Care Kit
6988-449
Radiolucent Retractor Adaptor
Front - Prone
Component
Storage
Back - Optional
Accessory
Storage
Page 85

ProAxis® Spinal Surgery Table Owner’s Manual
81
Mizuho OSI 2016
NW0725 Rev K
Optional Accessory
6988-720
Cervical Traction Vector, Short
Page 86

ProAxis® Spinal Surgery Table Owner’s Manual
82
Mizuho OSI 2016
NW0725 Rev K
8.2 ProAxis
®
Prone Patient Positioning with Cervical Management
Figure 88: ProAxis® Prone Positioning with Cervical Management Base Unit
The following instructions provide the recommended steps for setup. Final determination of patient
positioning and setup is at the discretion of the surgeon and surgical team.
NOTE: When setting up the ProAxis
®
Table for prone patient positioning with cervical
management, the hinge capability of the table should not be enabled.
8.2.1 Prone Positioning with Cervical Management Base Unit
1. Confirm that the ProAxis® Spinal Surgery Table is prepared for setup (refer to Section 8).
2. Confirm that the Floor Lock symbol is visible on the display of the IntelliPendant® (Figure
22, page 25). Observe the display on the IntelliPendant® and confirm the frame is level and
the hinge neutral. The Hinge, Trendelenburg, and Lateral Tilt angles should read 0°
(Figure 22, page 25). If necessary, install the Right and Left Rail Mounts (refer to Section
8.1) to temporarily enable the hinge functionality. Utilizing the IntelliPendant®, press the
Return to Level button to return the Hinged Frame to a neutral position. Remove the Rail
Mounts and return them to their storage location on the ProAxis® Component Cart.
NOTE: DO NOT install the Left and Right Rail Mounts and the Torso Trolley
absence of these components will allow the hinge capabilities of the table to be disabled
and the cervical components to be properly mounted.
®
Chest Tray. The
3. Install the ProAxis® Cervical Management Mounting Bracket on the Head-End Assembly
(Figure 90).
Page 87

ProAxis® Spinal Surgery Table Owner’s Manual
83
Mizuho OSI 2016
NW0725 Rev K
Posts - Slide into
Head-End Assembly
Horizontal Slide
Locking Lever
Traction Rope Pulley
T-Handle Tightens
Securing the Bracket
in Place
Traction Pulley Faces Up
Open Rail Frame
Horizontal Slide
Locking Lever
Figure 89: ProAxis® Cervical Management Base Unit Mounting Bracket
4. Orient the posts of the Bracket over the Head-End Assembly receptacle. Ensure the
T-Handles of the receptacle are unscrewed. Slide the posts into the receptacle. The
Bracket will rest on the top of the receptacle. Tighten both T-Handles (Figure 90).
Figure 90: ProAxis® Cervical Management Mounting Bracket Installed
5. To use the optional Mizuho OSI Cervical Management Base Unit on the ProAxis® Spinal
Surgery Table:
a. Remove the Cervical Management Base Unit Sub-Assembly from the Table Adaptor
Assembly by:
i. First, loosening the Horizontal Slide Locking Lever on the Table Adaptor
Assembly.
ii. Aligning the Stop Cap of the Horizontal Slide so it can pass through and out of
the Table Adapter Assembly. Pull the Sub-Assembly away from the Table
Adaptor Assembly to remove (Figure 91).
Page 88

ProAxis® Spinal Surgery Table Owner’s Manual
84
Mizuho OSI 2016
NW0725 Rev K
Table Adapter
Assembly
Stop Cap
Horizontal Slide
Locking Lever
Cervical Management
Base Unit SubAssembly
Stop Cap
Horizontal Slide
Locking Lever
Figure 91: Removal of Cervical Management Base Unit Sub-Assembly
b. Attach the Cervical Management Base Unit Sub-Assembly into the ProAxis® Cervical
Management Mounting Bracket. To attach:
i. Ensure the Horizontal Slide Locking Lever on the ProAxis® Cervical
Management Mounting Bracket is loosened and ready to receive the Cervical
Management Base Unit Sub-Assembly.
ii. Align the Stop Cap and insert the posts of the Base Unit into the ProAxis®
Bracket (Figure 92).
iii. Tighten the Horizontal Slide Locking Lever when the desired position of the
Horizontal Slide is achieved.
Figure 92: Inserting Cervical Management Base Unit Sub-Assembly into the
ProAxis® Cervical Management Mounting Bracket
Page 89

ProAxis® Spinal Surgery Table Owner’s Manual
85
Mizuho OSI 2016
NW0725 Rev K
Transitional Arm
Knobs #2
and #3
Horizontal Slide
Locking Lever
#1 Black Knob
Yoke Height
Adjustment
Aluminum Adaptor
6. In preparation for attaching a Skull Clamp, attach the appropriate adaptor to the end of the
Cervical Management Base Unit. The Cervical Management Base Unit includes the
aluminum Swivel Adaptor used to mount an aluminum DORO® or Mayfield® Skull Clamp. A
specific Radiolucent Adaptor is required to mount a radiolucent DORO® or a radiolucent
Mayfield® device. Refer to the Cervical Management Base Unit Owner’s Manual for
ordering information and detailed instructions on the use of the Radiolucent Adaptors.
7. With the Cervical Management Base Unit mounted on the ProAxis®, adjustments can be
made to the position of the Adaptor to which the Skull Clamp is connected (Figure 93).
a. Refer to the Mizuho OSI Owner’s Manual NW0658 provided with the Cervical
Management Base Unit for detailed information on the functionality of the Cervical
Management Base Unit Sub-Assembly and its various adjustment options.
b. Each of the Black Knobs (# 1, 2, and 3 in Figure 93) on the Sub-Assembly allow for
making adjustments to the position of the assembly until the desired position is
achieved.
WARNING: Failure to ensure that all teeth of the starbursts are aligned and the knobs are
properly tightened may cause harm to the patient, healthcare professional or the device.
Figure 93: Cervical Management Components
c. To raise the height of the Yoke and Transitional Arms, turn the Black Knob #1 until the
desired height of the assembly is achieved. Loosen the Black Knobs, #2 and #3,
controlling the position of the Transitional Arms holding the Adaptor. Adjustments can
be made to the position and angle of the Assembly utilizing the Black Knobs. Tighten
the Black Knobs when the desired position is achieved.
8. The components of the Cervical Management Base Unit Sub-Assembly have a starburst
locking mechanism. Ensure that all teeth of the starburst are aligned and the
corresponding knob is properly tightened when assembling or repositioning the device. All
knobs should be tightened by hand; DO NOT use a wrench of any kind.
Page 90

ProAxis® Spinal Surgery Table Owner’s Manual
86
Mizuho OSI 2016
NW0725 Rev K
Chest Tray
9. Attach the Contoured ACP Hip Pads. The pads are labeled right and left specific to be
oriented to the patient’s iliac crest when lying prone. Open the Locking Bracket, orient the
Pad with Locking Bracket over the rail of the open frame on the Foot-End section of the
frame, lower into place and slide towards the Hinge until it rests against the Pad Stop
(Figure 71, page 67). Press up on the white tab until the black component of the clamp fits
into the open notch of the white tab. Press down on the lip of the black component of the
clamp to lock the pad in place. (Figure 72, page 67). When installed correctly, the pad
should rest against the Pad Stop and the arrow on the side of the Bracket should be
pointed toward the Foot-End of the table. To remove the Contoured Hip Pad, lift up on the
lip of the Bracket so that it disconnects and lift the pad off the rail of the frame.
NOTE: Depending on the patient’s anatomy, the hip pads may be moved towards the Foot-End of
the table at the surgeon’s discretion to achieve proper positioning.
10. Attach the Cervical Chest Tray by releasing the Velcro® Straps on both sides and orienting
the Cervical Chest Tray above the rails of the frame in the desired location. Slide the Chest
Tray over the Frame until it sits on the Frame, and secure with the Velcro® Straps
(Figure 94).
Figure 94: Cervical Chest Tray Installed on ProAxis® Frame
11. To attach the Flat ACP or Non-ACP Chest Pad, orient the opening of the pad bracket so it
surrounds the edge of the Cervical Chest Tray. Slide onto the Cervical Chest Tray until the
tab of the bracket is secure on the tray (Figure 95). To remove, press the tab of the bracket
and slide the pad towards the Foot-End of the table.
NOTE: With ideal positioning, the Chest Pad should support the patient such that the load is borne
predominantly by the sternum. When the patient is positioned correctly, the top edge of the
pad should rest below the patient’s anatomical landmark, the supra-sternal notch.
WARNING: Positioning the Chest Pad superior to the supra-sternal notch may apply pressure
to the throat and the patient’s airway. Failure to ensure the patient is positioned correctly on the
Chest Pad may result in harm to the patient or the device.
Page 91

ProAxis® Spinal Surgery Table Owner’s Manual
87
Mizuho OSI 2016
NW0725 Rev K
Tab of Chest
Pad bracket
Chest Pad
Figure 95: Cervical Chest Tray with Chest Pad Installed
12. To install the Adjustable Leg Sling, place the sling over the Foot-End section of the open
frame (Figure 74, page 68). Feed the four straps through their respective buckles on the
opposite side of the sling and secure. The sling should be adjusted for each patient. The
straps on the sling consist of a double–sided hook and loop closure that allows for infinite
adjustment along the length of the strap. The hook end of the strap may be folded over
onto the loop portion of the strap to allow for greater adjustability. The strap may be folded
over itself again to shorten, thereby raising the sling and decreasing the degree to which
the patient’s hips are flexed. Place two (2) or three (3) 6946 or 6947 TempurPedic® Medical Pillows in the sling approximately where the patient’s lower legs will rest to
achieve the desired amount of knee flexion and support (Figure 75, page 69).
13. The Leg Boards may be used in place of the Leg Sling to support the legs. Attach the Leg
Boards with the 1-inch (2.5 cm) Tempur-Pedic® Medical Pad. Release the Velcro® Straps
on the sides of the Leg Board. Place one Leg Board over the rails of the open frame and
slide into place. Secure with the Velcro® Straps. Repeat this process with the second Leg
Board. Ensure that the two Leg Boards abut each other (Figure 76).
14. After the desired Flat Chest Pad and Contoured ACP Hip Pads are in place on the frame,
the tubing can be connected to the pads.
a. Align the tubing with the long end directed to the pad furthest away from the port at the
Foot-End column (Figure 6, page 15).
b. Secure the ACP tubing with the Velcro® wraps along the frame and by routing it
through the underside of the Leg Sling. Ensure the tubing is secure and out of the way.
c. Connect to the tubing to the port at the Foot-End of the table.
d. Re-adjust the ACP tubing as may be required.
15. Place the Patient Kit Covers on the Chest and Hip Pads.
Page 92

ProAxis® Spinal Surgery Table Owner’s Manual
88
Mizuho OSI 2016
NW0725 Rev K
16. Trial position the imaging equipment if it will be used. If necessary, re-locate the ProAxis®
Spinal Surgery Table in the room. Unlock the Floor Locks by using the IntelliPendant® and
with the assistance of a second person move the ProAxis® Spinal Surgery Table. When
the table is located where it will be used, lock the Floor Locks.
CAUTION: The ProAxis® Surgery Table is not for patient transport. The location of the table
relative to any imaging equipment that may be used needs to be confirmed prior to the patient being
transferred to the table.
17. Refer to the specific manufacturer’s instructions for use regarding the Skull Clamp being
attached to the Cervical Management Base Unit. The surgeon determines the best
approach to secure the patient to the Skull Clamp.
18. Confirm the Black Knobs of the Cervical Management Base Unit are loosened so that the
Transitional Arms and Adaptor can be readily positioned when connecting the Skull Clamp.
19. Position the patient bed or stretcher so the patient’s anatomical landmarks are aligned with
the support pads on the hinged frame of the ProAxis® Spinal Surgery Table. It may be
necessary to re-position the Chest Pad. The top edge of the Chest Pad should align with
the patient’s anatomical landmark, the bottom of supra-sternal notch, and the top third of
the Contoured ACP Hip Pad should align with the patient’s anatomical landmark, the iliac
crest.
20. The height of the patient’s bed or stretcher should be level with the top of the Contoured
ACP Hip Pad. To assist the patient transfer process, you may choose to use the
IntelliPendant® and laterally tilt the table toward the patient bed or stretcher.
21. Lock the patient stretcher or bed. Confirm by observing the IntelliPendant® display that the
table Floor Locks are locked. The Floor Locks Locked symbol should be visible (Figure 22,
page 25).
22. The surgeon supports the patient’s head in the Skull Clamp while others assist to log-roll
the patient onto the ProAxis® Spinal Surgery Table. The surgeon continues to support the
patient’s head and Skull Clamp until final positioning is achieved with the Cervical
Management Base Unit.
WARNING: After log-rolling the patient to a prone position, confirm that the endotracheal tube
is not kinked. Failure to confirm may cause harm to the patient.
23. Continue to support the patient on the table until the safety straps are in place. DO NOT
leave the patient unattended on the table.
WARNING: Patient must be restrained at all times by a safety strap(s) when positioned on the
table.
24. Remove the patient bed or stretcher while supporting the patient on the table.
Page 93

ProAxis® Spinal Surgery Table Owner’s Manual
89
Mizuho OSI 2016
NW0725 Rev K
25. Visually confirm the placement of the Contoured ACP Hip Pads. The pad should be
mounted abutting the Pad Stop (Figure 73, page 68). When positioned correctly, the
patient’s anatomical landmark, the iliac crest, should rest in the top one-third of the pad.
The Patient Kit Hip Pad Cover should be smooth against the patient’s skin. Ensure that the
patient’s skin is smooth and flat against the Patient Kit Hip Pad Cover. To re-position the
patient on the Pad, the patient should be lifted and moved such that the iliac crest contacts
the top third of the pad. Ensure the Hip Pad Cover is smoothed and lower the patient back
onto the Pad. Confirm correct placement of the Pads such that each of the patient’s iliac
crests rest in the top one-third of each respective Hip Pad (Figure 81, page 74).
CAUTION: Failure to ensure that the patient is positioned correctly on the Contoured ACP Hip
Pad may result in harm to the patient or the device.
26. Visually confirm the placement of the Chest Pad. When positioned correctly, the edge of
the Pad should rest at the patient’s anatomical landmark, the supra-sternal notch (Figure
82, page 74). Breasts should be down and lying flat, with the nipples oriented towards the
Foot-End of the table. The Patient Kit Chest Pad Cover should be smooth against the
patient’s skin. Ensure that the patient’s skin is smooth and positioned flat against the
cover. If necessary, re-position the Chest Tray. While lifting and supporting the patient,
move the Chest Tray to the desired location and smooth the Chest Pad Cover. The patient
should then be lowered back onto the Chest Pad.
NOTE: With ideal positioning, the Chest Pad should contact the patient such that the load is borne
predominantly by the sternum.
WARNING: Positioning the Chest Pad superior to the supra-sternal notch may apply pressure
to the throat and patient’s airway. Failure to ensure the patient is positioned correctly on the Chest
Pad may result in harm to the patient or the device.
27. Visually confirm that the Leg Sling or Leg Boards provide support to the patient’s knees,
and the legs are supported on the pillows. If the Leg Sling is being used, the patient’s
knees should be flexed and legs supported on the pillows. Re-position the legs and add
pillows as required under the patient’s tibias to ensure that the ankles are not hyper-
extended and there is no pressure on the patient’s feet (Figure 81, page 74).
CAUTION: Failure to ensure that the patient is positioned correctly with the Leg Sling or Leg
Boards may result in harm to the patient or the device.
28. Position the patient’s arms back to his/her sides and secure. The disposable Positioning
Straps may be used to assist in final positioning. The Arm Cradles may be used to provide
padding.
29. Place the Buttocks Strap across the lower gluteal area of the patient. A blanket may be
used under the Buttocks Strap to protect the patient from direct contact with the strap. The
strap can be fed through the buckle on the sides of the Contoured ACP Hip Pads and then
back on itself, securing the Velcro®. Confirm that the Buttocks Strap is positioned low on
the gluteal area, and that it surrounds both Contoured ACP Hip Pads, and is secured
snugly around the buttocks. Ensure that the patient does not directly contact the frame of
the table. Use foam or gel padding as needed to pad the area between the patient and the
rails of the frame.
30. Apply a Safety Strap over the patient’s lower legs. Secure the Safety Strap in place with
use of the buckle.
Page 94

ProAxis® Spinal Surgery Table Owner’s Manual
90
Mizuho OSI 2016
NW0725 Rev K
Figure 96: Final Prone Positioning with Cervical Management Base Unit
NOTE: The placement and location of the Straps are at the discretion of the surgeon and vary by
procedure.
31. Due to the open nature of the ProAxis® frame and the long duration of many spinal
procedures, the use of forced air warmers, fluid warmers, and blankets should be
considered to help prevent hypothermia. Blankets may be placed over the feet up to the
gluteal area and across the shoulders and arms out of the surgical field to aid in
maintaining the patient’s body temperature. These devices should be used according to
the manufacturer’s directions and at the discretion of the surgeon.
WARNING: Proper pre-operative and intra-operative procedures must be followed to prevent
venous stasis and pooling, pressure sore development, neuropathy, improper electrosurgical tissue
grounding, hypertension/hypotension, and hypothermia.
WARNING: Use care and monitor the patient when changing the position of the ProAxis®
Surgery Table (Height Up/Down, Trendelenburg/Reverse Trendelenburg, and Lateral Roll) to ensure
that the patient or table does not interfere with other equipment, and the components and
accessories have proper clearance in relation to the surrounding areas. Failure to do so may cause
harm to the patient or device.
32. Confirm that the patient is in their final position. If a Retractor Adaptor is required to mount
auxiliary equipment, mount it on the rail frame. Open the Adaptor by turning the black knob
and releasing the bottom of the Adaptor. Place the Adaptor around the frame, align the
black knob, turn to tighten and secure the Adaptor in place. Ensure that the patient does
not directly contact the Retractor Adaptor. Use foam or gel padding as needed to pad the
area between the patient and the Adaptor.
33. Refer to Section 4, Basic Operation, for instructions on how to utilize the IntelliPendant®
and operate the table.
CAUTION: The linkage arms of the columns raise, lower, and rotate when the table is in use.
Ensure no items are in the path of the moving arms and healthcare professionals position
themselves away from the linkage arms when the table is in motion. Failure to do so may cause
injury to the healthcare professional or possible damage to the table.
Page 95

ProAxis® Spinal Surgery Table Owner’s Manual
91
Mizuho OSI 2016
NW0725 Rev K
Cervical Traction
Vector Adjustor
8.2.2 Prone Patient Positioning with Cervical Traction Vector Adjustor
The Cervical Traction Vector Adjustor is designed to provide adjustability of the angle of pull of cervical
traction as well as to maintain alignment of the traction rope when skull tongs or a head halter is used.
Figure 97: Cervical Traction Vector Adjustor Properly Installed on ProAxis®
1. Confirm that the ProAxis® Surgery Table is prepared for setup (refer to Section 8).
2. Confirm that the Floor Lock symbol is visible on the display of the IntelliPendant® (Figure
22, page 25). Observe the display on the IntelliPendant® and confirm the frame is level and
the hinge neutral. The Hinge, Trendelenburg, and Lateral Tilt angles should read 0°
(Figure 22, page 25). If necessary, temporarily install the Right and Left Rail Mounts (refer
to Section 8.1) in order to enable the hinge functionality. Utilizing the IntelliPendant®, press
the Return to Level button to return the Hinged Frame to the neutral position. Remove the
Rail Mounts and return them to their storage location on the ProAxis® Component Cart.
3. Install the ProAxis® Cervical Traction Vector Adjustor. Extend the Drop Lock on the post of
the Cervical Traction Vector Adjustor, align the posts with the Head-End Assembly
receptacle, and lower the Vector into place (Figure 98). Position the Vector at the desired
height and tighten both T-Handles on the Head-End Assembly (Figure 99).
Page 96

ProAxis® Spinal Surgery Table Owner’s Manual
92
Mizuho OSI 2016
NW0725 Rev K
Notch to Guide
Traction Rope
Drop Lock
Tighten T-Handle to
Secure Traction Vector
Adjustor at Desired
Height
Figure 98: Installation of Cervical Traction Vector Adjustor
NOTE: DO NOT install the Left and Right Rail Mounts and the Torso Trolley
®
Chest Tray. The
absence of these components will allow the hinge capabilities of the table to be disabled
and the cervical components to be properly mounted.
Figure 99: Cervical Traction Vector Adjustor Properly Installed on ProAxis®
4. A traction rope can be directed over the Traction Vector Adjustor, with the length of rope
adjusted so the attached weight(s) can hang freely. While monitoring the position of the
weights, utilize the IntelliPendant® to raise and lower the table through its full range of
motion. Ensure the weight(s) continues to hang freely in all table positions. Adjust the
length of traction rope if necessary.
5. Install the remaining prone positioning components as instructed in Section 8.2.2.
Page 97

ProAxis® Spinal Surgery Table Owner’s Manual
93
Mizuho OSI 2016
NW0725 Rev K
ProAxis® Cervical Management
Base Unit Mounting Bracket
Cervical Chest Tray
Flat Chest Pad
6988-209
6988-969
6988-758 ProAxis® Chest
Pad
Contoured ACP
Hip Pad, Left
Contoured ACP
Hip Pad, Right
Leg Sling
6988-387
6988-388
5840-450
Leg Board
Buttocks Strap
Safety Strap, 60 in. (152 cm)
6844-148 (2)
6977-739
5840-43 (2)
8.2.3 Components for Prone Patient Positioning with Cervical Accessories
Page 98

ProAxis® Spinal Surgery Table Owner’s Manual
94
Mizuho OSI 2016
NW0725 Rev K
Tempur-Pedic® Medical Pillow
Retractor Adaptor
Cervical Traction Vector Adjustor
6946 Standard Pillow (1)
6947 Queen Pillow (2)
6988-880
6988-725
Optional Accessory
Optional Accessory
Optional Accessory
5979-1 Cervical
Management Base Unit
6910-3034 DORO®
Radiolucent Skull Clamp
(pictured)
6910-1001 DORO® QR3
Aluminum Skull Clamp
6910-3006-2 Titanium, Adult
Skull Pins for DORO®
6910-3006-5 Stainless, Adult
Skull Pins for DORO®
Optional Accessory
Optional Accessory
5979-300 Radiolucent
DORO® Adaptor for Cervical
Management Base Unit (pictured)
5979-200 Radiolucent
Mayfield® Adaptor for Cervical
Management Base Unit
6988-449
Radiolucent Retractor Adaptor
Page 99

ProAxis® Spinal Surgery Table Owner’s Manual
95
Mizuho OSI 2016
NW0725 Rev K
Hinge Angle -20º
Tempur-Pedic® Medical
2-inch Supine Table Pad
with Translation Slide
Carbon Fiber Removable Two-Piece Supine Top
8.3 ProAxis
®
Supine Patient Positioning
Figure 100: ProAxis® Supine Setup, Fixed Surgical Site Mode
The following instructions provide the recommended steps for setup. Final determination of patient
positioning and setup is at the discretion of the surgeon and surgical team.
1. Confirm the ProAxis® Surgery Table is prepared for setup (refer to Section 8).
2. Confirm that the Floor Lock symbol is visible on the display of the IntelliPendant® (Figure
22, page 25). Observe the display on the IntelliPendant® and confirm the frame is level
and the hinge neutral. The Hinge, Trendelenburg, and Lateral Tilt angles should read 0°
(Figure 22, page 25). If necessary, temporarily install the Right and Left Rail Mounts
(refer to Section 8.1) in order to enable the hinge functionality. Utilizing the
IntelliPendant®, press the Return to Level button to return the Hinged Frame to a neutral
position. Remove the Rail Mounts and return them to their storage location on the
ProAxis® Component Cart.
3. Confirm that there are no accessories mounted to the rails before attempting to mount
the Two-Piece Supine Top.
4. The ProAxis® Two-Piece Supine Top is made of carbon fiber construction and mounts on
the open rail frame. Each section (Head-End and Foot-End) has a set of Locking
Brackets that secure the top to the frame. The Head-End section also has a Cable
Connector that plugs into the Head-End port, allowing the hinge to function (Figure 104,
page 97).
a. To install the Head-End section on the frame, open the Locking Brackets, align the
Locking Brackets over the rails, and lower into place (Figure 101). The top should
rest flat on the frame. Slide the Top toward the Head-End Column until it rests
against the Brackets of the rail frame on both sides (Figure 107, page 99).
Page 100

ProAxis® Spinal Surgery Table Owner’s Manual
96
Mizuho OSI 2016
NW0725 Rev K
Locking Bracket
Aligned over Rail
Frame
Figure 101: Supine Top Locking Bracket Placed over Rail in Open Position
b. To engage the Locking Brackets on each side, first press up on the white tab until the
black portion of the clamp fits into the open notch of the white tab. Press down on
the lip of the black component of the clamp to lock it into place (Figure 102).
Figure 102: Latching of Locking Bracket
 Loading...
Loading...