
M9 / M9D / M11
Self -Contained Steam Sterilizer
English
Español
Français
For Models:
M9 (-040 / -041 / -042)
M9D (-042)
M11 (-040 / -041 / -042)
User Guide
TP202 20-42-FO-00014 Rev A1 C2169
TP202 20-42-FO-00014 Rev A1 C2169 TP202 20-42-FO-00014 Rev A1 C2169
Style U
003-2915-99 Rev AA8 (12/30/19)
Product Information
Attention Canadian Users - Action Required!
Thank you for purchasing an M9/M11 Steam Sterilizer.
To comply with changes in Canadian regulatory requirements you must now provide documentation, a
Manufacturer’s Data Report (MDR), to your appropriate provincial regulatory agency when purchasing and
using a steam sterilizer.
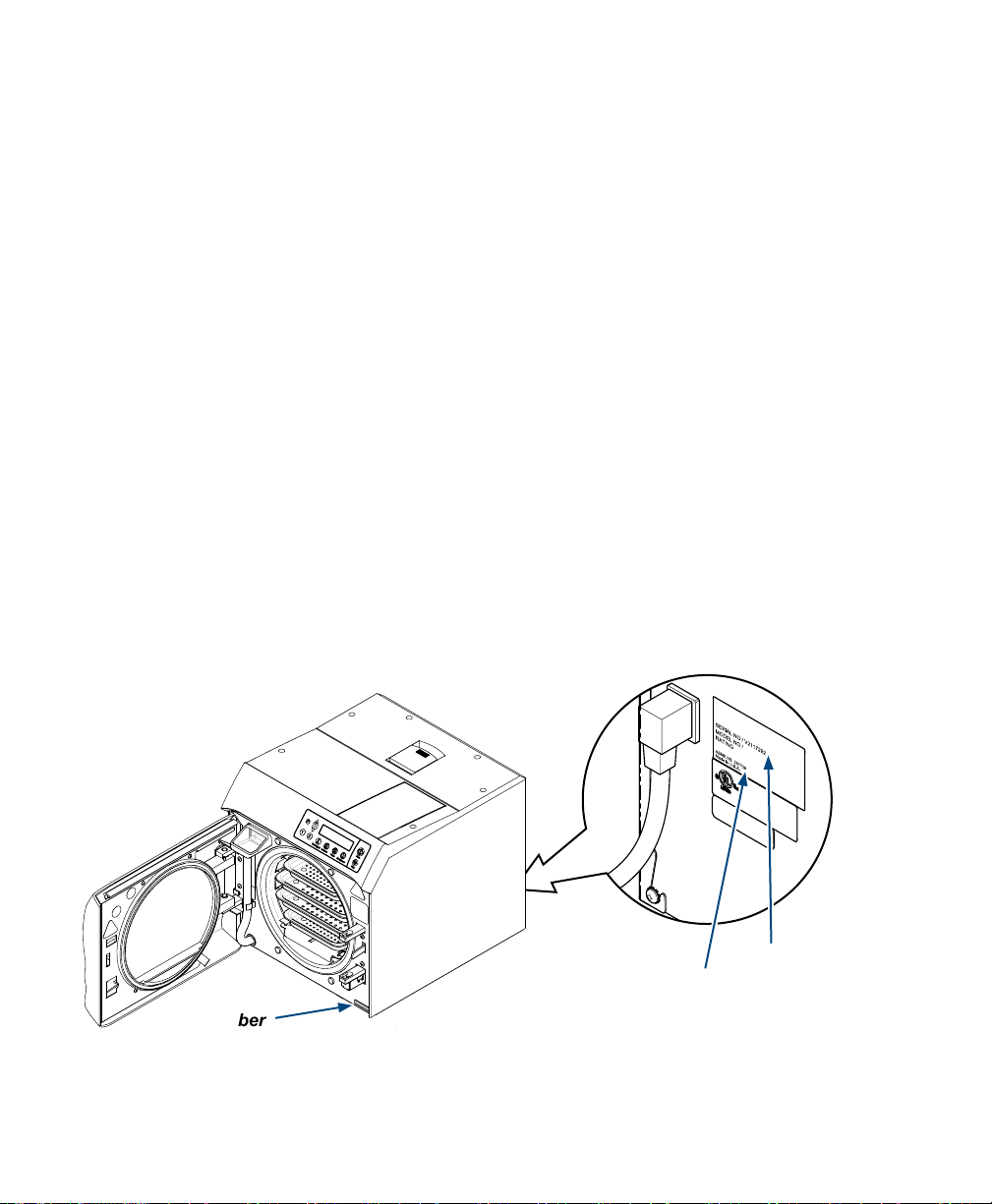
To obtain the MDR from Midmark, record the sterilizer Serial Number and the ASME National Board Number
of your sterilizer. See the illustration below for the location of these numbers. Then, contact Midmark
customer service at 1.800.MIDMARK and provide the recorded numbers.
Midmark will then complete the MDR and send it to you. Once you have the MDR, you must submit it to the
appropriate provincial regulatory agency.
We apologize for any inconvenience and appreciate your assistance in helping maintain compliance
to Canadian regulations for sterilizers. If you have any questions or concerns, please contact 1.800.
MIDMARK.
Serial Number
003-2915-99
ASME National Board
Number Location
English - 2
Product Serial
Number Location
© Midmark Corporation 2018

Table of Contents
Important Information
Safety Instructions ...........................................4
Intended Use....................................................4
Sterilization Technology ...................................4
Electromagnetic Interference ...........................4
Safety Symbols ................................................4
Shipping Symbols ............................................5
Transportation / Storage Conditions ................5
Accessories, Tools and Service Parts. .............5
Included with Sterilizer .....................................6
Component Location ........................................7
Controls and Indicators ....................................8
Sterilization Monitoring Guidelines.................10
Installation
Operating Environment .................................. 11
Location Requirements .................................. 11
Electrical Requirements .................................12
Connecting the Power Cord ...........................13
User Settings .................................................14
Operation
Before Operating the Sterilizer.......................15
Filling the Reservoir .......................................15
Qualication Testing .......................................16
Guidelines for Loading ...................................16
Standard Cycle Parameters ...........................24
Cycle Operation .............................................25
Post-Sterilization Processing .........................28
Programmable Cycle Buttons ........................29
Thermal Printer (Optional)
Operating the Printer......................................38
Printer Tape Description ................................38
Example of Typical Printout
of a Program Cycle ........................................39
Paper Roll Removal / Installation ...................40
Tray and Cassette Tool (optional)
Using the Optional Tray / Cassette Tool.........41
Troubleshooting
Troubleshooting Chart ....................................42
Informational Messages .................................43
Accessing the last 5 Error Codes ..................43
Error Messages..............................................43
Calling for Service ..........................................45
Specications / Compliance
Specications Chart:
M9 / M9D .................................................46
M11 .........................................................47
Water Purity Specications ............................48
Warranty Information
Scope of Warranty .........................................49
Maintenance
Maintenance Messages .................................31
Daily Maintenance .........................................32
Weekly Maintenance ......................................33
Monthly Maintenance .....................................35
Extended Use Maintenance ...........................37
003-2915-99
English - 3
© Midmark Corporation 2018
Important Information
Safety Instructions
The primary concern of Midmark is that this equipment is operated and maintained with the safety of the
patient and sta in mind. To assure safe and reliable operation:
• Read and understand this manual before attempting to install or operate the sterilizer.
• Assure that the appropriate personnel are informed on the contents of this manual.
(This is the responsibility of the purchaser).
• Assure that this manual is located near the sterilizer, or if possible, permanently axed
to the sterilizer.
Intended Use
The Midmark and Ritter M9, M9D and M11 Steam Sterilizers can be used in medical and dental oces,
hospitals, clinics, nursing homes, laboratories, and other facilities to sterilize heat and moisture stable
reusable items (including dental handpieces) that are compatible with steam sterilization. Refer to Guidelines
for loading and Standard Cycle Parameters in this manual for detailed information.
Sterilization Technology
The Midmark M9 / M9D and M11 utilize a dynamic air removal system called Steam Flush Pressure Pulse to
remove air from the chamber.
Electromagnetic Interference
This sterilizer is designed and built to minimize electromagnetic interference with other devices. However, if
interference is noticed between another device and this product:
• Remove interfering device from room
• Plug sterilizer into isolated circuit
• Increase separation between sterilizer and interfering device
• Contact Midmark if interference persists
Safety Symbols
WARNING
Indicates a potentially hazardous situation which could result in serious injury.
Caution
Indicates a potentially hazardous situation which may result in minor or moderate injury.
It may also be used to alert against unsafe practices
Equipment Alert
Indicates a potentially hazardous situation which could result in equipment damage.
003-2915-99
English - 4
© Midmark Corporation 2018
Shipping Symbols
Caution Shipping Damage
Keep dry
Proper shipping orientation
Max. stacking height
Fragile
(Refer to “n” number on package)
Transportation / Storage Conditions
Equipment Alert
Water must be drained from the unit’s reservoir before transporting / storing below +32°F (0°C).
Ambient Temperature Range: -22°F to 140°F (-30°C to +60°C)
Relative Humidity: 10% to 90% (non-condensing)
Atmospheric Pressure: 7.2 psia to 15.4 psia (49.6 kPa to 106.4 kPa)
Accessories, Tools and Service Parts
Unless noted, items can be used on the M9 / M9D and M11.
Accessories Order Number
Speed-Clean, 1 (16oz. [.47 liter]) bottle 002-0396-00
Speed-Clean, 1 case (12 - 16oz. [.47 liter]) bottles 002-0396-05
Thermal Printer 9A599001
Cassette Rack (Horizontal) 9A215001 (M11 only)
Cassette Rack (Vertical) 9A215002 (M11 only)
Printer Thermal Paper Roll (Rell) 060-0016-00
Printer Stick-able Thermal Paper (Rell) 060-0016-01
Door and Dam Gasket Kit (M11 only) 002-0504-00
Door and Dam Gasket Kit (M9 / M9D only) 002-0361-01
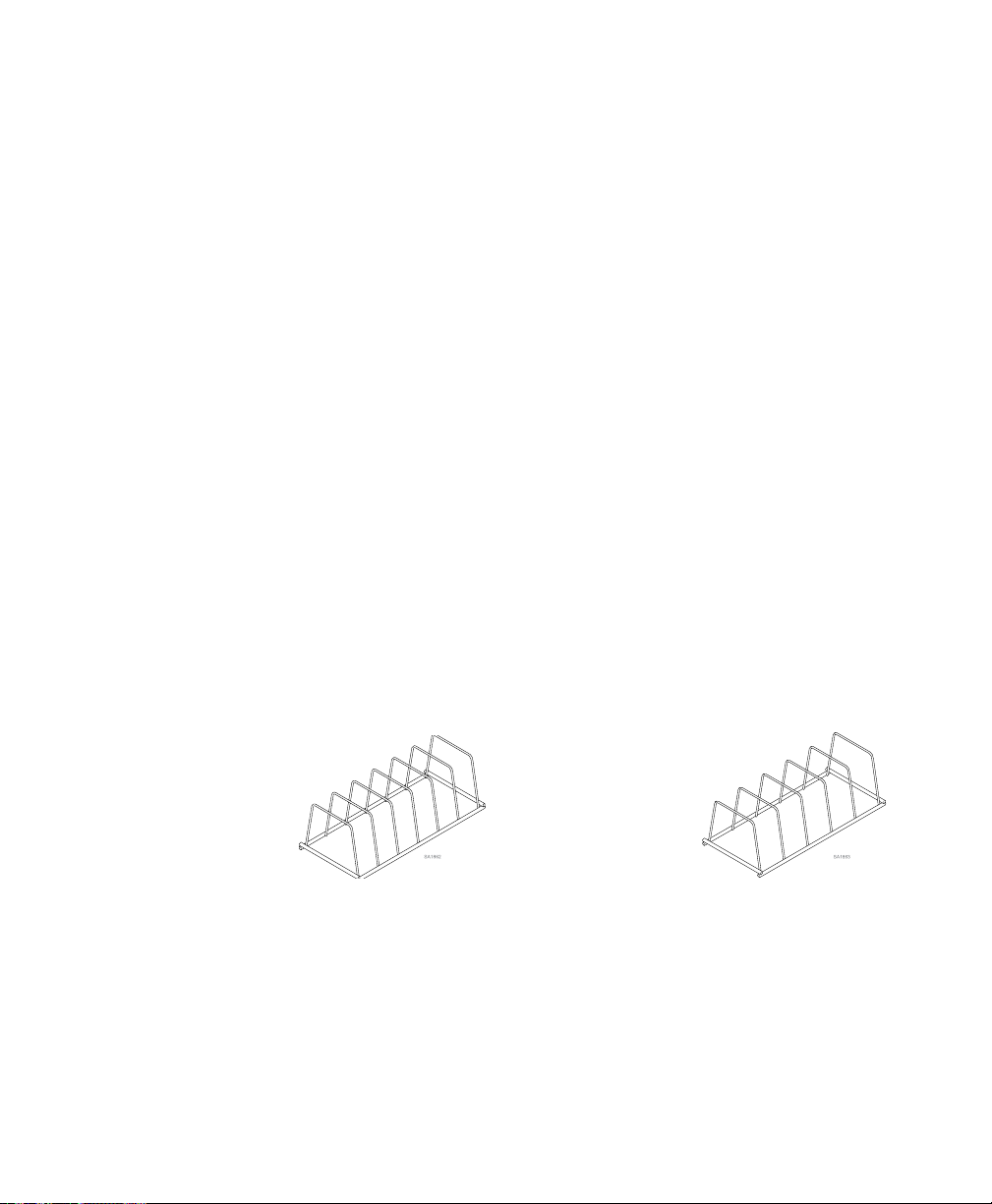
Pouch Rack Kit (M11 only) 002-2108-00
Pouch Rack Kit (M9 / M9D only) 002-2108-01
Tray/Cassette Tool 9A307001
VistaCool ™ direct-to-drain thermal reduction system 9A586002 (Dual)
003-2915-99
Common Service Parts / Tools Order Number
English - 5
© Midmark Corporation 2018

Included with Sterilizer
Speed-Clean
Power Cord
Speed-Clean SDS SheetCare / Operation Card
2 Small Trays
Pouch Rack
(M11 Rack Shown)
003-2915-99
English - 6
2 Large Trays
© Midmark Corporation 2018
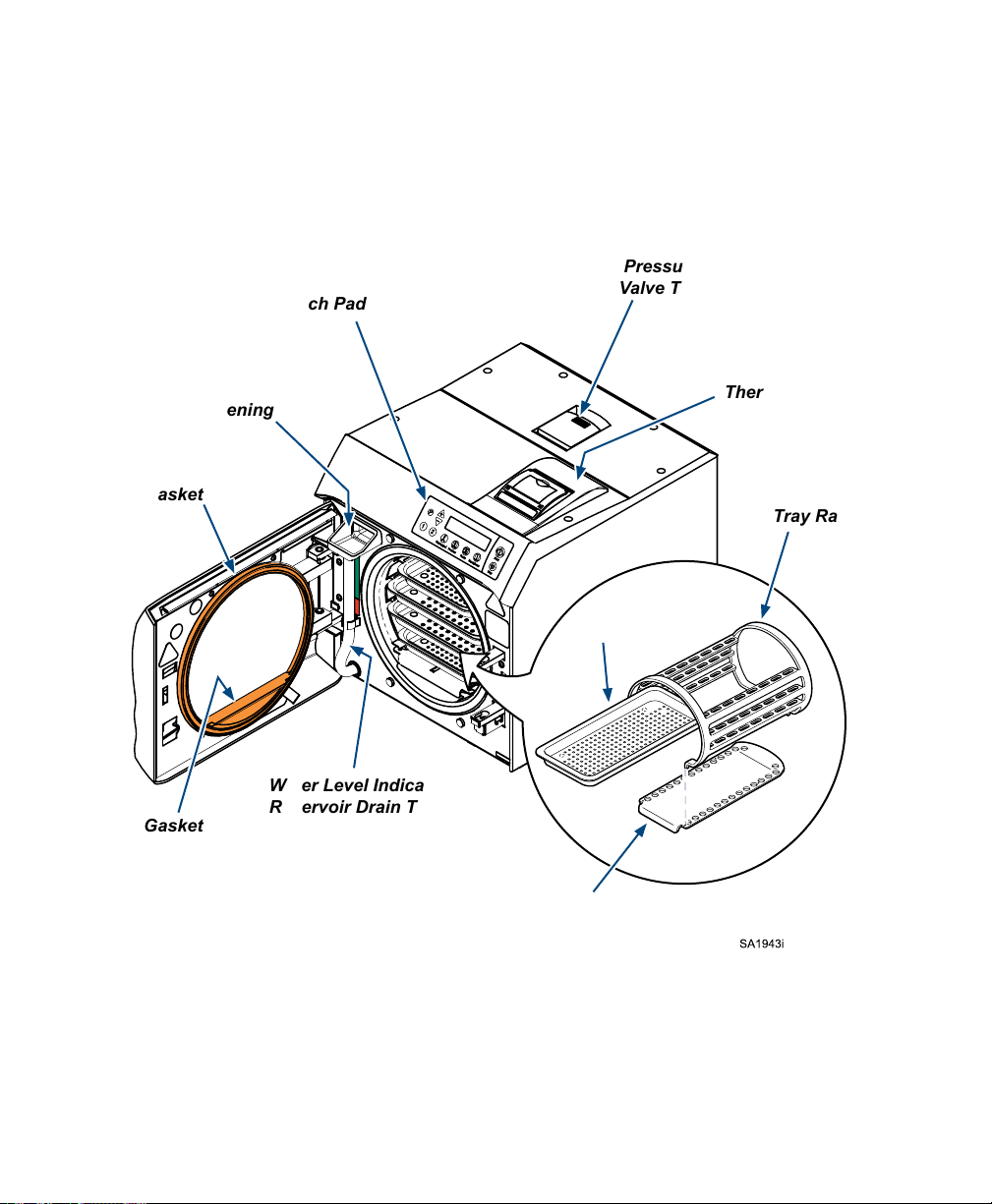
Component Location
Display / Touch Pad
Pressure Relief
Valve Test Lever
Fill Opening
Door Gasket
Dam Gasket
Thermal Printer
(optional)
Tray Rack
Tray(s)
Water Level Indicator /
Reservoir Drain Tube
Tray Plate
003-2915-99
English - 7
© Midmark Corporation 2018
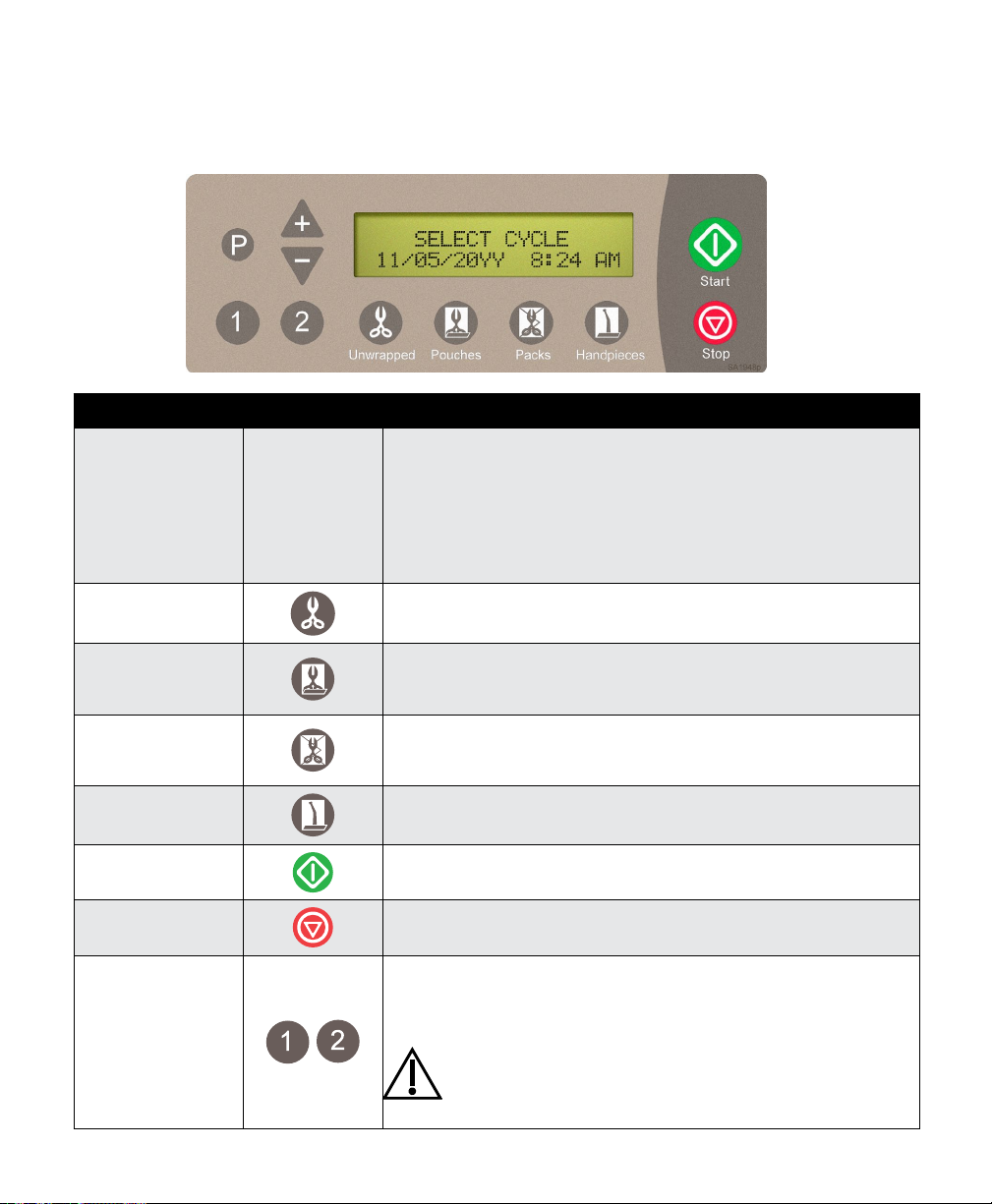
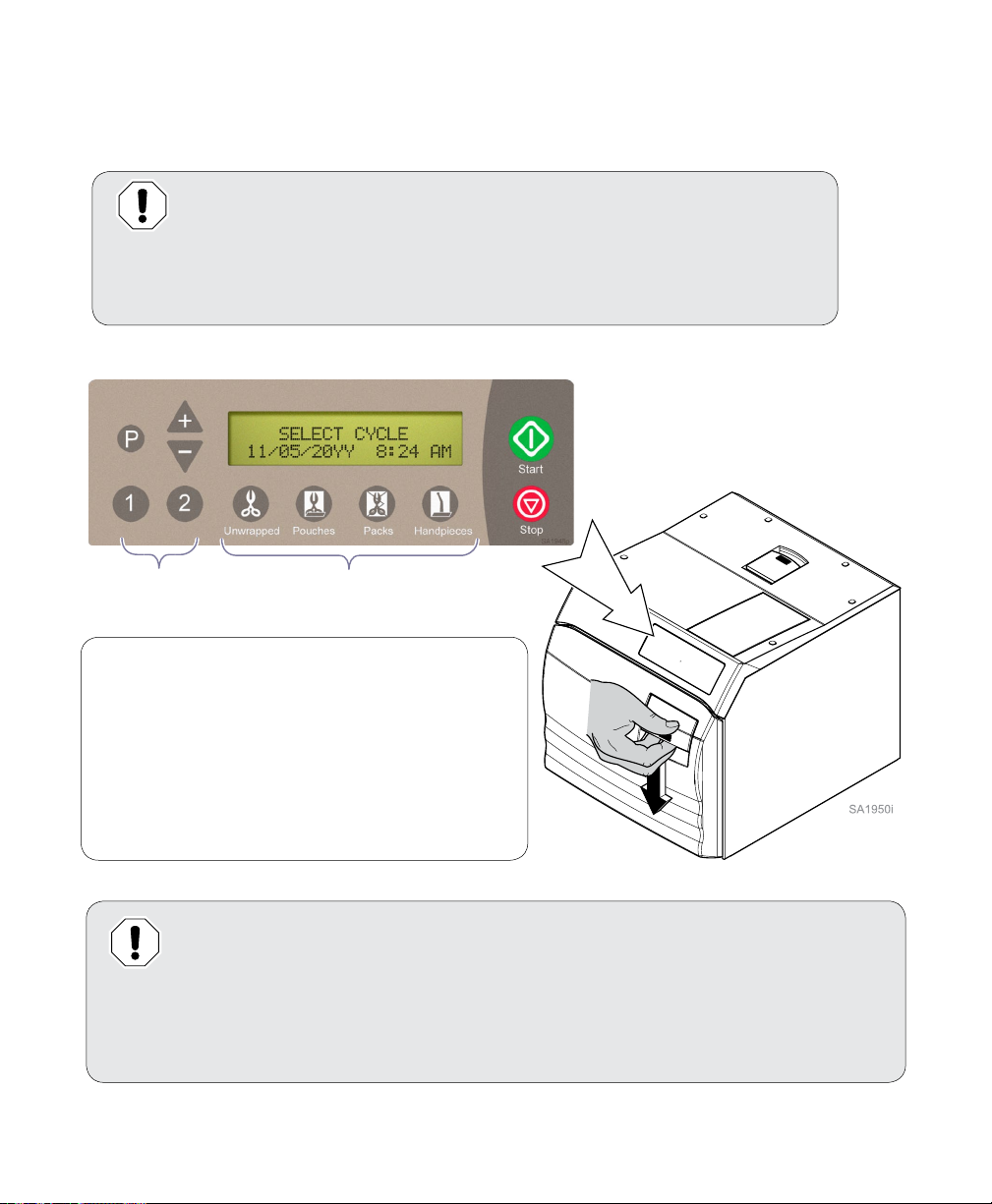
Controls and Indicators
The sterilizer controls and indicators are shown in the illustrations on this, and the following page.
The accompanying tables describe the function of each control / indicator.
Control Function
Display See illustration Indicates cycle selected, cycle temperature and exposure time
for the selected cycle. During the cycle, display shows messages
describing status of cycle. When cycle enters sterilization mode,
remaining cycle time is displayed as well as temperature and
pressure. Display also shows error message if a malfunction
occurs. Refer to the Troubleshooting section of this manual for a
detailed explanation of Informational / Error Messages.
Unwrapped button
A program cycle designed to process unwrapped instruments at:
270°F (132°C) for 3:00 minutes / 30 minute drying cycle.
Pouches button
Packs button
Handpieces
button
Start button
Stop button
1 or 2 buttons
NOTE: All material
run in these cycles
must be validated
for sterilization by
the user.
003-2915-99
A program cycle designed to process instruments in combination
paper / plastic sterilization pouches or wrapped instruments
at: 270°F (132°C) for 4 minutes / 30 minute drying cycle.
A Program cycle designed to process packs of instruments or
textiles at: 250°F (121°C) for 30 minutes / 30 minute drying
cycle.
A program cycle for dental handpieces which runs at:
270°F (132°C) for 4 minutes / 30 minute drying cycle.
Initiates selected program or, when SELECT CYCLE is displayed,
pressing Start will activate heater for 10 minutes.
Terminates selected program or function.
Programmable cycle buttons that allow an operator to create two
dierent programmed cycles for special applications. Sterilization
time and temperature, along with drying time and venting procedure can be adjusted or changed.
Caution
These cycles are not FDA cleared and validation of sterility
of items processed using them is the responsibility of the
user.
English - 8
© Midmark Corporation 2018
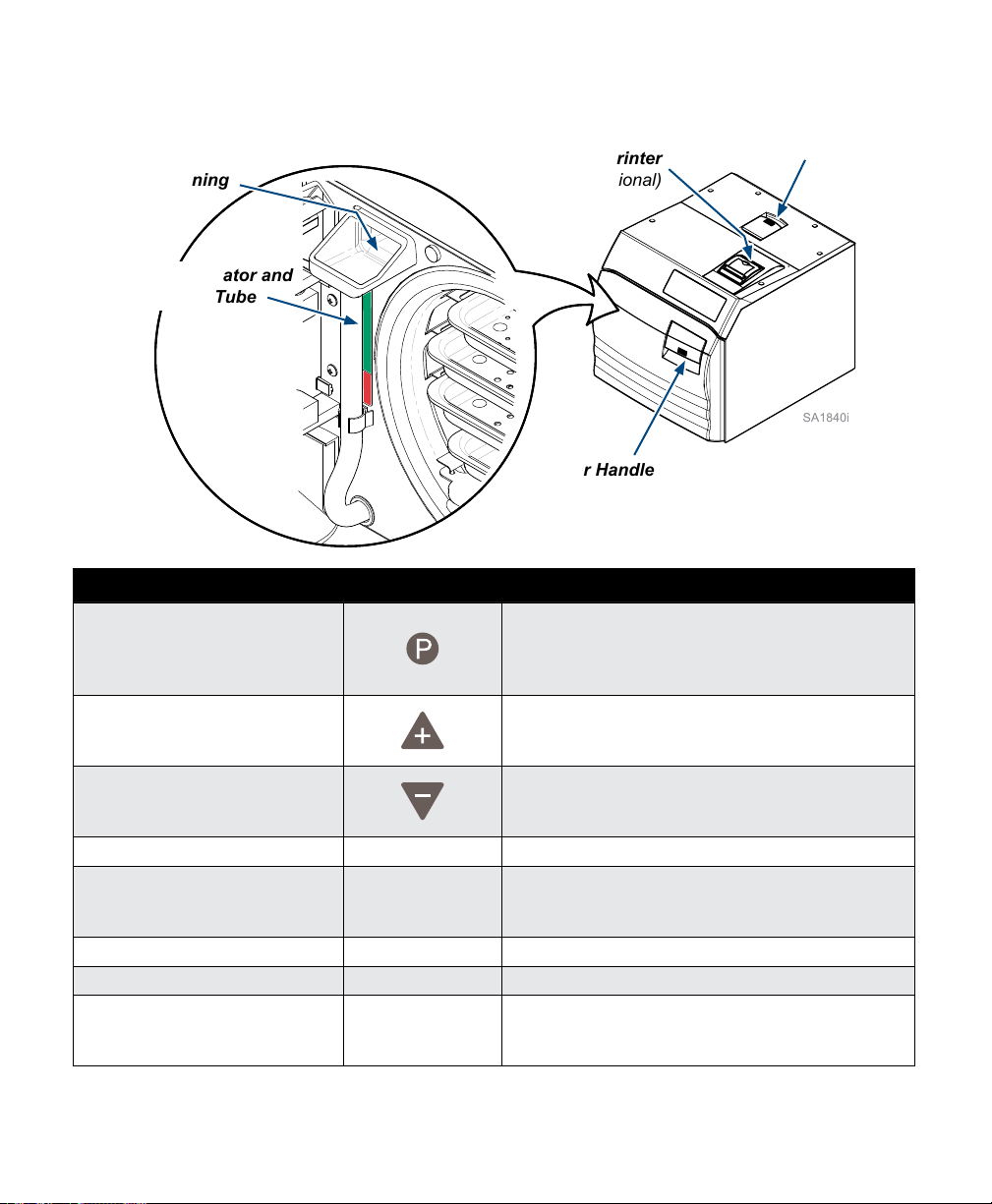
Controls and Indicators - continued...
Pressure Relief
Thermal Printer
Fill Opening
Water Level Indicator and
Reservoir Drain Tube
(optional)
Door Handle
Control Function
P button Programming mode button that allows operator to
change temperature, time, dry time and/or venting
procedure. Used in conjunction with buttons 1 or 2.
(Refer to Programming Mode).
+ (plus) button
- (minus) button
Door Handle refer to illustration For latching / opening door.
Water Level Indicator /
Reservoir Drain Tube refer to illustration
Fill Opening refer to illustration Access for lling reservoir with water.
Pressure Relief Valve Test Lever refer to illustration Allows operator to check pressure relief valve.
Thermal Printer (Optional) refer to illustration The printer (optional equipment) can be used to
Allows temperature, time, vent mode or dry time to
be increased or changed when in location 1 or 2
and the P (programming) mode is activated.
Allows temperature, time, vent mode or dry time to
be decreased or changed when in location 1 or 2
and the P (programming) mode is activated.
Shows amount of water in reservoir. Tube also
used for drainage of reservoir into suitable
container.
provide a permanent record of time, temperature,
and pressure during a cycle.
Valve Test Lever
003-2915-99
English - 9
© Midmark Corporation 2018
Sterilization Monitoring Guidelines
Note
This information below is provided for reference only. Contact appropriate state/local agencies for
specific sterilization guidelines for your office. Additional information on infection control is available
from the Centers for Disease Control and Prevention (CDC), Organization for Safety and Asepsis
Procedures (OSAP) ), Association for the Advancement of Medical Instrumentation (AAMI), and
Association for Professionals in Infection Control and Epidemiology (APIC).
Physical Monitors
Temperature and pressure measuring devices can help detect sterilizer malfunctions. The sterilizer’s control
system aborts the cycle and displays a message if physical conditions go outside established limits. The
optional thermal printer can be used to create a record of each load’s actual cycle time, temperature, and
pressure.
Note
Use only FDA cleared chemical and biological indicators designed for steam sterilization that are
compatible with the particular sterilization cycle temperature and exposure time being monitored.
Use sterility monitors with each sterilization load. If a sterilizing cycle is terminated prematurely,
reprocess instruments to ensure sterility of the load. Follow manufacturer’s instructions for proper
disposal of used indicators.
Chemical Indicators
Chemical indicators are designed to verify that conditions in the sterilizer chamber were adequate to achieve
sterilization. They do not validate that a processed item is sterile. If a chemical indicator shows a failure,
items in that load are considered non-sterile. Potential causes for sterilization failure include: improper
cleaning, packing, loading, or a sterilizer malfunction. Determine the cause of any sterilization failure, and
remedy the situation before running the next cycle. Only FDA cleared chemical indicators labeled for use
with the steam sterilization cycle parameters. e.g. temperature and exposure time, of the M9 / M9D / M11
Sterilizers should be used for monitoring the cycles. Follow the chemical indicator’s instructions for proper
storage, use, interpretation, and disposal.
Biological Indicators
Biological indicators are microbiological devices designed to accompany items being sterilized to monitor
adequacy of the sterilization process. If a biological indicator shows a failure, items in that load are
considered non-sterile. Potential causes for sterilization failure include: improper cleaning, packing, loading,
or a sterilizer malfunction. Determine the cause of any sterilization failure, and remedy the situation before
running the next cycle. Only FDA cleared biological indicators labeled for use with the steam sterilization
cycle parameters. e.g. temperature and exposure time, of the M9 / M9D / M11 Sterilizers should be used for
monitoring the cycles. Follow the biological indicators instructions for proper storage, use, interpretation, and
disposal.
003-2915-99
English - 10
© Midmark Corporation 2018
Installation
Operating Environment
Ambient Temperature Range: 68°F to 104°F (+20°C to +40°C)
Relative Humidity: < 80% (non-condensing)
(Pollution Degree 2, in accordance to IEC664)
Normal Operating Altitude: < 9842 ft. (3000 m) above sea level
Device approved for INDOOR USE ONLY.
Device to be operated in a relatively dust-free environment.
(Pollution Degree 2, in accordance to IEC664)
Device should be connected to a power source with over-voltage limits less than 1500 watts from
mains to ground. (Installation Category II in accordance to IEC664)
The M9 / M9D and M11 will emit 5000 BTU / HR during operation.
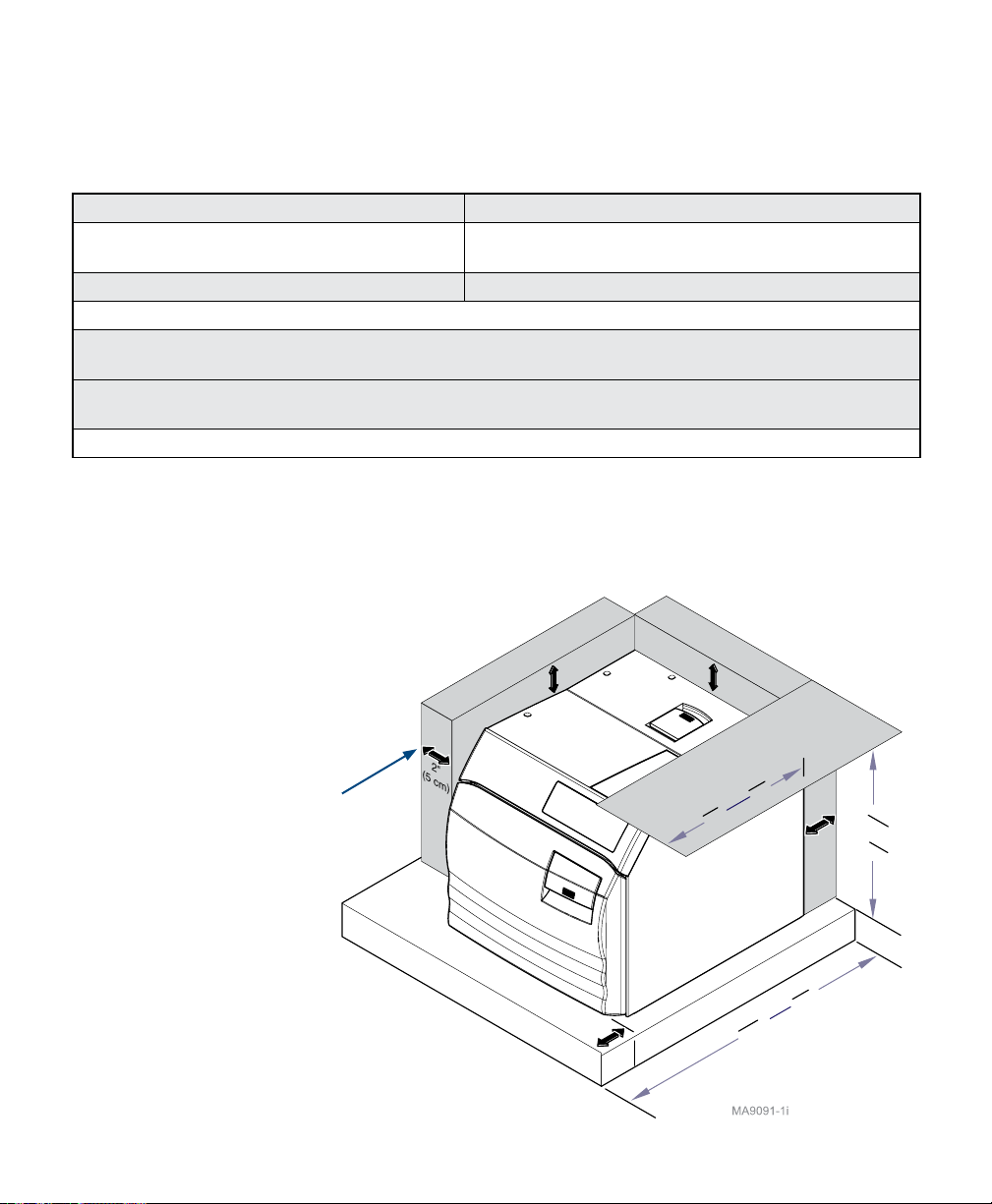
Location Requirements
Allow Clearance
on Both Sides
003-2915-99
Clearance
5"
(13 cm)
2"
(5 cm)
Support Surface
English - 11
1"
(3 cm)
Requirements
5"
(13 cm)
Overhang/Shelf
M9/M9D
15"
(38 cm)
M11
18"
(46 cm)
M9/M9D
M11
21"
(53 cm)
© Midmark Corporation 2018
2"
(5 cm)
18"
(46 cm)
M11
23" (58 cm)
M9/M9D
22" (56)
Location Requirements - continued...
Support Surface
• Material should be water-resistant material. (Ex. laminate, stainless steel, stone, etc.)
• Surface must be level to ensure chamber lls with correct water level.
Improper water level in the chamber could cause a sterilizer malfunction.
• Surface should meet minimum dimensions listed below:
Dimensions
Depth (front to back) M11 - 21” (53 cm) M9 / M9D - 18” (46 cm)
Clearance Requirements
To ensure proper air circulation, and to allow access to the reservoir ll port and drain coupling,
adhere to the minimum clearance requirements listed below. If the sterilizer will be operated
in continuous cycles, locate sterilizer where steam will not damage materials or equipment in
the surrounding area.
Back of Unit - Back Wall ................................. 2” (5 cm)
Front Support Surface - Front Sterilizer ........ 1” (3 cm)
Sides of Unit - Side Wall ................................ 2” (5 cm)
Distance above Unit for Printer Access .......... 5” (13 cm)
Maximum Upper Cabinet Shelf Overhang ..... M11 - 18” (46 cm) M9 / M9D - 15” (38 cm)
Under Cabinet or Shelf................................... M11 - 23” (58 cm) M9 / M9D - 22” (56 cm)
Relocation Requirements for Sterilizer
Disconnect power cord from electrical outlet and allow sterilizer to cool.
Drain water from reservoir or do not tip sterilizer, allowing water to spill.
Electrical Requirements
WARNING
For 115 VAC models: Use 104 - 127 VAC, 50/60 Hz alternating current only.
For 230 VAC models: Use 207 - 253 VAC, 50/60 Hz alternating current only.
Failure to do so may result in electric shock to personnel and / or damage to sterilizer.
Note
For safety, the unit must be connected to a properly polarized and grounded receptacle. Always use a
power cord with grounding connections that match the receptacles in your location.
115 VAC Unit: 115 VAC, 50/60 Hz, 12 amp
Dedicated Supply Circuit*: 120 VAC, 50/60 Hz, 15 amp
Max. Power Consumption: 1425 Watts
230 VAC Unit: 230 VAC, 50/60 Hz, 6.4 amp
Dedicated Supply Circuit*: 230 VAC, 50/60 Hz, 10 amp
Max. Power Consumption: 1500 Watts
* Power source must have over-voltage limits less than 1500 watts from mains to ground.
(Installation Category II in accordance to IEC664)
003-2915-99
English - 12
© Midmark Corporation 2018
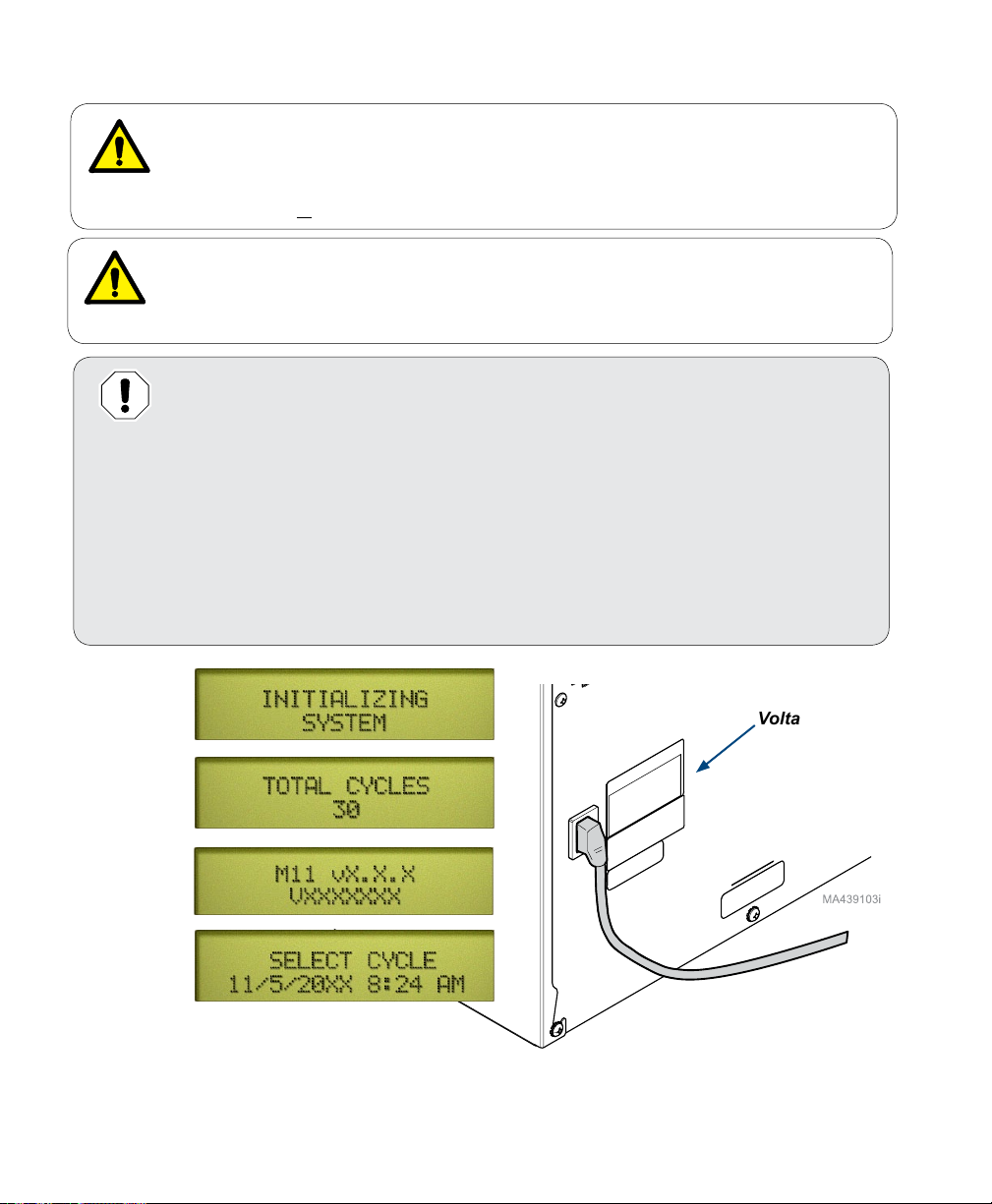
Connecting the Power Cord
WARNING
Equipment is not suitable for use in the presence of a flammable anesthetic mixture
with oxygen, air, or nitrous oxide.
Clarification: Equipment is suitable for use in the presence of oxygen, air, or nitrous oxide.
WARNING
Check the serial number label on back panel of sterilizer to verify voltage rating for
the unit. Failure to connect sterilizer to an appropriate power supply could result in
damage to the unit, and electrical shock to personnel.
Equipment Alert
For optimal performance, allow sterilizer to reach room temperature before operating.
To connect the power cord...
A) Plug power cord into receptacle on back of sterilizer.
B) Plug power cord into a properly polarized and grounded receptacle rated for a
minimum of 15 amps. A dedicated circuit only used for the sterilizer is recommended.
C) M9 / M9D and M11 are not equipped with an on/off switch, the display operates off
very low power. (example: microwave oven display)
Note: When power is connected, the messages shown below will appear on the display.
Display:
Voltage Rating
*
*
* These screens will display the total number of cycles run on the unit, the model number
(M9 / M9D or M11), the software version number, serial number date, and time.
003-2915-99
English - 13
© Midmark Corporation 2018
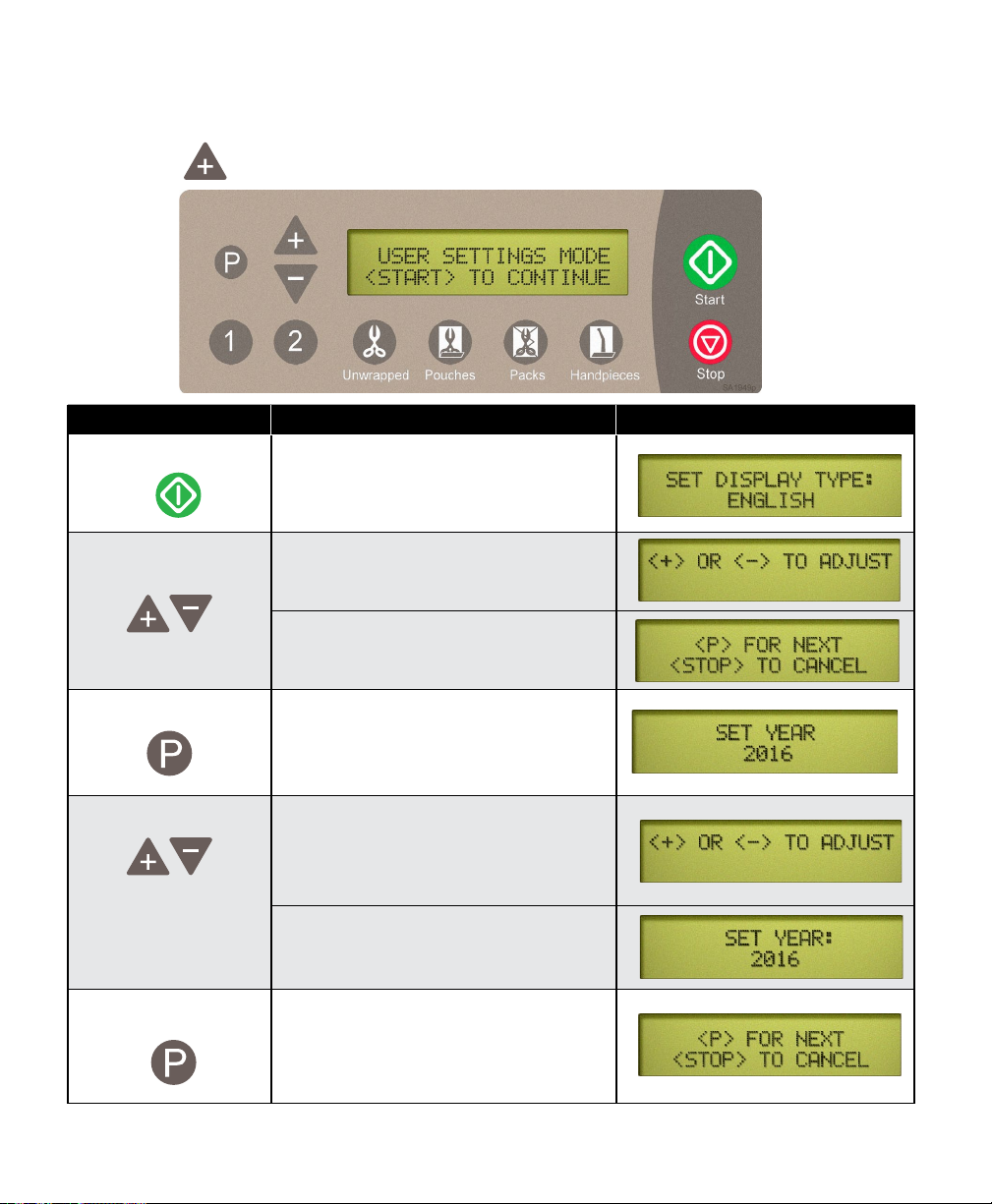
User Settings
User setting mode will enable the user to set the desired unit of measure and adjust the time
clock. To enter the user settings mode, unplug and replug the power cord while pressing and
holding the button until the User Settings screen below appears.
Action Description Display
Press Press start to initialize the User Settings
menus.
Adjust Units of
Measure
Press
Adjust Time Clock The “+“ and “-” buttons will adjust the
Press To store setting and bring up the next
NOTE: User Settings for Date and Clock must be updated manually for day light savings.
003-2915-99
The “+” and “-” buttons alternate the
setting between English or Metric
When the desired Units of Measure
appear on the display...
To store the desired Units of Measure
This brings up the Time Clock programing
display
values of the following settings
- Year / Month / Day / Hour / AM or PM /
Minute.
When the desired values appear on the
display...
setting in the list. Repeat as necessary
for all settings. When all settings are
completed pressing the “P” will initiate
normal power up.
English - 14
© Midmark Corporation 2018
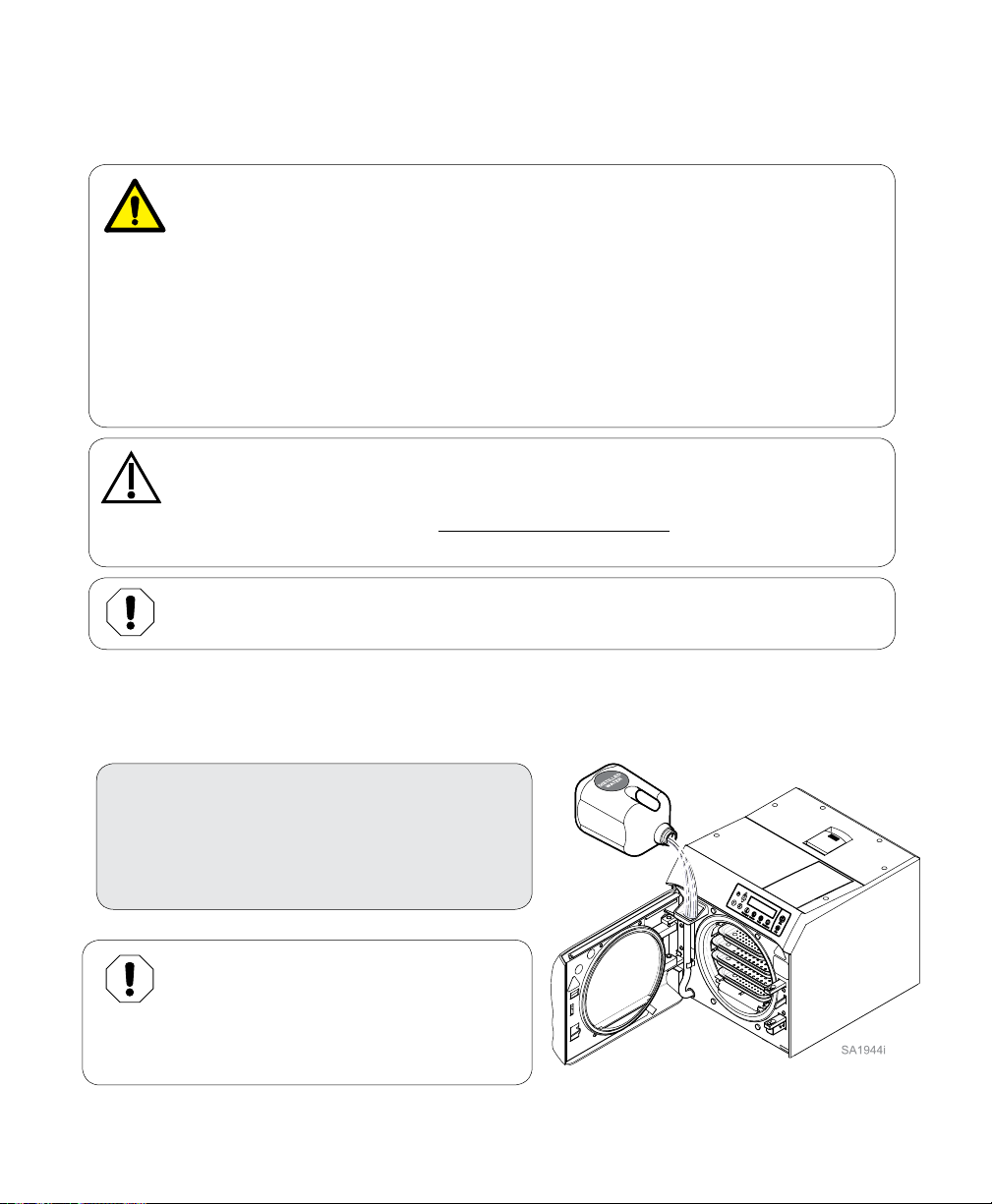
Operation
Before Operating the Sterilizer
WARNING
Do not use this sterilizer for sterilizing volatile substances or for any purpose other
than its intended design. Burns and toxic or explosive conditions could result.
Do not force door handle at any time. Chamber pressure may cause door to open with
extreme force. If door handle does not move freely, allow unit to cool and depressurize for 40
minutes before opening door. Failure to adhere could result in serious personal injury.
Do not run the sterilizer without the tray plate in place. If the sterilizer malfunctions,
immediately unplug sterilizer, and call for service; do not attempt to repair the sterilizer
yourself. Doing so could result in serious injury.
Caution
Programmable cycles 1 and 2 are provided for those applications requiring sterilization
parameters different than the preset cycles. These cycles are not FDA cleared for medical
use. All material processed in these cycles must be validated by the user to ensure sterility of the
processed load.
For optimal performance, allow the sterilizer to reach room temperature before operating.
Equipment Alert
Filling the Reservoir
To fill reservoir...
A) Open door to unit.
B) Pour distilled water into fill opening until
water level reaches the top of the fill level
label on the water level indicator tube.
Equipment Alert
Use distilled water or water that meets the
referenced water purity specifications.
Failure to comply may result in sterilizer
malfunction and/or premature failure due
to excessive corrosion.
003-2915-99
English - 15
© Midmark Corporation 2018
Qualication Testing
Your sterilizer should be tested after sterilizer installation, malfunctions, relocation, major repairs, and
after sterilization process failure. Qualication testing should be performed prior to placing the sterilizer in
service. If multiple cycle types are used, e.g. “Pouches” and “Packs” each cycle type should be qualied.
Qualication testing should include at least one Biological Indicator (BI) (sometimes referred to as Spore
Tests) and one Chemical Indicator (CI). The test pack should be placed on the bottom tray near the chamber
door and performed with items routinely processed and considered to be the most dicult to sterilize.
Additional items should be placed in the chamber along with the Biological Indicator and Chemical Indicator
so that chamber is fully loaded (don’t exceed the maximum capacities listed in the tables under “Guidelines
for Loading” in this manual). Three consecutive test runs, for each cycle type tested, with negative results
from the BIs, and the appropriate readings from all physical monitors and chemical indicators demonstrating
complete sterilization, provide verication that the sterilizer has been properly installed (or reinstalled after
relocation) or repaired to the manufacturer’s specications and that it will function eectively in the facility in
which it is installed. All items processed during qualication testing should be quarantined until the results of
the biological testing for all three test runs are available.
Guidelines for Loading
All items must be processed in accordance with Centers for Disease Control and Prevention (CDC),
“Guidelines for Infection Control in Dental Healthcare Settings” – 2003, MMWR; 52 (no.RR-17), and
“Guidelines for Disinfection and Sterilization in Healthcare Facilities” – 2008, which states:
“Items to be sterilized should be arranged to permit free circulation of the sterilizing agent (e.g.,
steam, chemical vapor, or dry heat); manufacturer’s instructions for loading the sterilizer should
be followed.”
Equipment Alert
Loads must be placed on trays at all times – unless the optional cassette racks are
used – otherwise, serious instrument or equipment damage may occur.
Types of Items that can be processed in the M9 / M9D and M11
Before placing any instrument in the M9 / M9D or M11, check with the instrument manufacturer to be sure
the
materials are compatible with steam sterilization, and to verify the acceptability of sterilization parameters.
The M9 / M9D and M11 are designed to sterilize the following:
• Metal instruments
• Rubber / plastic devices (ex. suction cannulas, impression trays, etc.)
• Wrapping / bundling materials (ex. CSR wrap, instrument pouches, etc.)
• Cassettes (which t in the sterilizer trays or the cassette rack accessories)
• High / low speed handpieces
• Surgical instruments (ex. opthalmologic instruments)
003-2915-99
English - 16
© Midmark Corporation 2018
Types of Items that can be processed in the M9 / M9D and M11 - Continued...
Equipment Alert
Do not sterilize items composed of any of the following materials in the M9 / M9D or M11.
• Corrosion sensitive metal (ex. carbon steel, iron, etc.)
• Fragile items susceptible to breaking under pressure / high temperature
• Biomedical waste
• Plastics that may break down or produce residue when exposed to steam / high
temperatures.
Examples
Polyethylene, Styrene, Cellulosics, ABS, PVC, Acrylic (Plexiglass™), PPO (Noryl™),
Latex, and Neoprene
Preparing Items for Sterilization
WARNING
Clean and dry instruments thoroughly before placing them into tray. Improper cleaning may
result in non-sterile instruments or damage to the unit. Follow instrument manufacturer’s
guidelines and CDC recommendations for handling and cleaning instruments prior to sterilization.
Instruments must be thoroughly cleaned to remove all residual matter, such as debris, disinfectant
residuals, blood, organic tissue, etc. General cleaning guidelines are listed below but the device
manufacturer’s instructions for proper cleaning and preparation of the device for sterilization should always
be followed:
• Clean instruments immediately after use to avoid drying of residual matter.
• The use of automated cleaning equipment (e.g. ultrasonic cleaner or washer-disinfector) is
recommended over manual cleaning for clinician safety and cleaning eectiveness.
• After cleaning, thoroughly rinse instruments with tap water to remove any loosened debris or
residual cleaning uid. The purity of tap water varies signicantly thus, it’s recommended the nal
rinse be done with water of adequate quality to avoid instrument staining. After rinsing
instruments should be inspected for damage, debris, detergent residue and then dried before
packaging.
• If the instrument manufacturer’s instructions require lubrication of the instruments after cleaning,
wipe o excess lubricant before packing for sterilization or loading into the sterilizer.
Immediate Use Sterilization
The M9 / M9D and M11 are capable of Immediate Use sterilization - sterilizing unwrapped instruments for
immediate use. Place a surgical cotton towel, paper tray liner or CSR wrap folded to t on the tray bottom
before putting unwrapped items in the tray. Arrange unwrapped items on the towel so they do not touch one
another (See Photo 1).
003-2915-99
English - 17
© Midmark Corporation 2018

Immediate Use Sterilization - Continued...
Photo 1
Please consider the following when choosing whether or not to sterilize your instruments unwrapped:
• The sterility of unwrapped instruments is compromised upon exposure to a non-sterile
environment. Follow CDC guidelines for using unwrapped, sterilized instruments.
• Due to the sensitive nature of some types of surgery (including, but not limited to
ophthalmological), instruments used in such procedures must be wrapped or pouched in order
to reduce their exposure to sterilization process residues. The water reservoir should also be
drained and relled with fresh distilled water on a daily basis when processing instruments for
these procedures on a routine basis
WARNING
Do not overload the chamber! Adequate space is required around items in trays for
steam circulation and drying. Failure to allow adequate space will compromise
sterilization and drying. Items and packaging should be completely dry when removed from
the sterilizer to minimize the potential for recontamination.
General Guidelines
• Use only M9 / M9D and M11 trays in their appropriate sterilizer. Using other trays could restrict air
/steam ow to items resulting in inadequate sterilization and drying.
• All items must t within the tray and not extend over the lip of the sterilizer tray. Instruments
must not scrape the chamber walls when sliding the tray into the chamber.
• Jointed items must be sterilized in an open position so all surfaces are exposed to the steam
(See Photo 2).
003-2915-99
English - 18
© Midmark Corporation 2018

General Guidelines - Continued...
Photo 2
• Handpieces and instruments must be arranged in a single layer on the trays (not piled or
stacked), to permit proper steam ow and drying.
• Glassware or utensils capable of holding water, e.g. bottles, basins, beakers, etc., should be
positioned on the tray with the open side down so any water condensate drains from the
container (See Photo 3). When sterilizing glassware check with the manufacturer to make sure
it is compatible with steam sterilization.
Photo 3
• Rinse tubing with distilled water or water that meets the referenced water purity specications
and do not dry prior to sterilizing. Arrange tubing on the tray so there are no sharp bends and
the tubing ends are open and unobstructed (See Photo 4).
Photo 4
003-2915-99
English - 19
© Midmark Corporation 2018
General Guidelines - Continued...
• Follow the device manufacturer’s instructions for disassembly of multi-part instruments prior to
packaging / sterilization to assure all parts are adequately exposed to the steam.
• If items are being sterilized and stored for later use they must be packaged, e.g. pouched,
wrapped, etc., and completely dry when removed from the sterilizer chamber to avoid potential
recontamination.
• Variations in load conguration, size, wrapping materials, and the environment may require the
operator to increase the default drying time to assure all packaging and instruments are
completely dry. See “Cycle Operation” section of this manual for instructions on adjusting drying
time.
• When sterilizing a load that contains one or more handpieces, utilize the hand piece cycle, not
Pouches or Unwrapped Cycle.
Pouching and Wrapping Items
The M9 / M9D and M11 are capable of sterilizing pouched or wrapped items to preserve sterility
after processing.
• When pouching or wrapping items, use only sterilization pouches and wraps that have been
cleared by the FDA and labeled for use with the steam sterilization cycle being used. Follow the
manufacturer’s instructions for use.
• Instruments made from dierent materials (stainless steel, carbon steel, plastic, etc.) should not
be mixed in the same pouch or wrapped pack to avoid potential instrument damage.
• Pouches, wraps and the included instruments should not touch the chamber wall to allow proper
steam circulation and avoid potential instrument damage.
• For proper steam circulation and drying the preferred orientation of pouches is resting on their
edge, best accomplished using the Midmark Pouch Rack supplied with the M9 / M9D / M11
sterilizer. If additional pouch racks are needed order kit P.N. 002-2108-00 (M11- 6 slot) or
002-2108-01 (M9 / M9D - 5 slot). If more than two (2) pouch racks are used in a single load
additional dry time may be required.
M11 Pouch Rack
002-2108-00
• The use of the Pouch Rack requires the removal of some of the sterilizer trays. When using the
Pouch Rack load a single pouch per rack slot. When using paper / plastic sterilization pouches
the pouches should be oriented in the rack so the plastic side of one pouch faces the paper side
of the adjacent pouch.
• Pouches loaded directly in the trays will dry best if loaded with plastic side up.
• Do not layer pouches in the trays. Pouches should be loosely packed with handpieces and
instruments single height loaded (not piled or stacked), to permit proper steam ow and
penetration to the items (See Photo 2).
003-2915-99
English - 20
M9 / M9D Pouch Rack
002-2108-01
© Midmark Corporation 2018

Pouching and Wrapping Items - Continued...
• If pouches or wrapped packs are labeled using a marking pen the ink should be nontoxic. On
paper-plastic pouches only mark on the plastic side of the pouch. On wrapped packs mark the
labeling information on the indicator tape or apply a separate label.
Shown below are some recommended pouch load congurations for the M9 / M9D (Photo 5 and 6)
and M11 (Photo 7 and 8):
M9 / M9D Pouches with Pouch Rack
Photo 5
M11 Pouches with Pouch Rack
Photo 7
M9 / M9D Pouches Mixed
Photo 6
M11 Pouches Mixed
Photo 8
• When using cassettes in the M9 / M9D / M11, follow the cassette manufacturer’s instructions for
use.
• Do not wrap items too tightly. Sterilization can be compromised if an item is excessively
wrapped and the wrap is more likely to tear if wrapped too tightly.
• Cassettes can be loaded on the trays but they must t within the boundaries of the tray and they
should not touch each other, the tray above, or the chamber wall to allow proper steam
circulation. The total instrument load, including the cassettes, should not exceed the limits listed
in maximum capacities tables.
• When using the optional Horizontal and Vertical cassette racks (9A215001 and 9A215002) in
the M11 the maximum load including the cassettes can be increased to 12 lbs. (5.44 kg).
Additional dry time may be required.
003-2915-99
English - 21
© Midmark Corporation 2018

Pouching and Wrapping Items - Continued...
Shown below are some recommended cassette load congurations for the M9 / M9D (Photos 9 and10) and
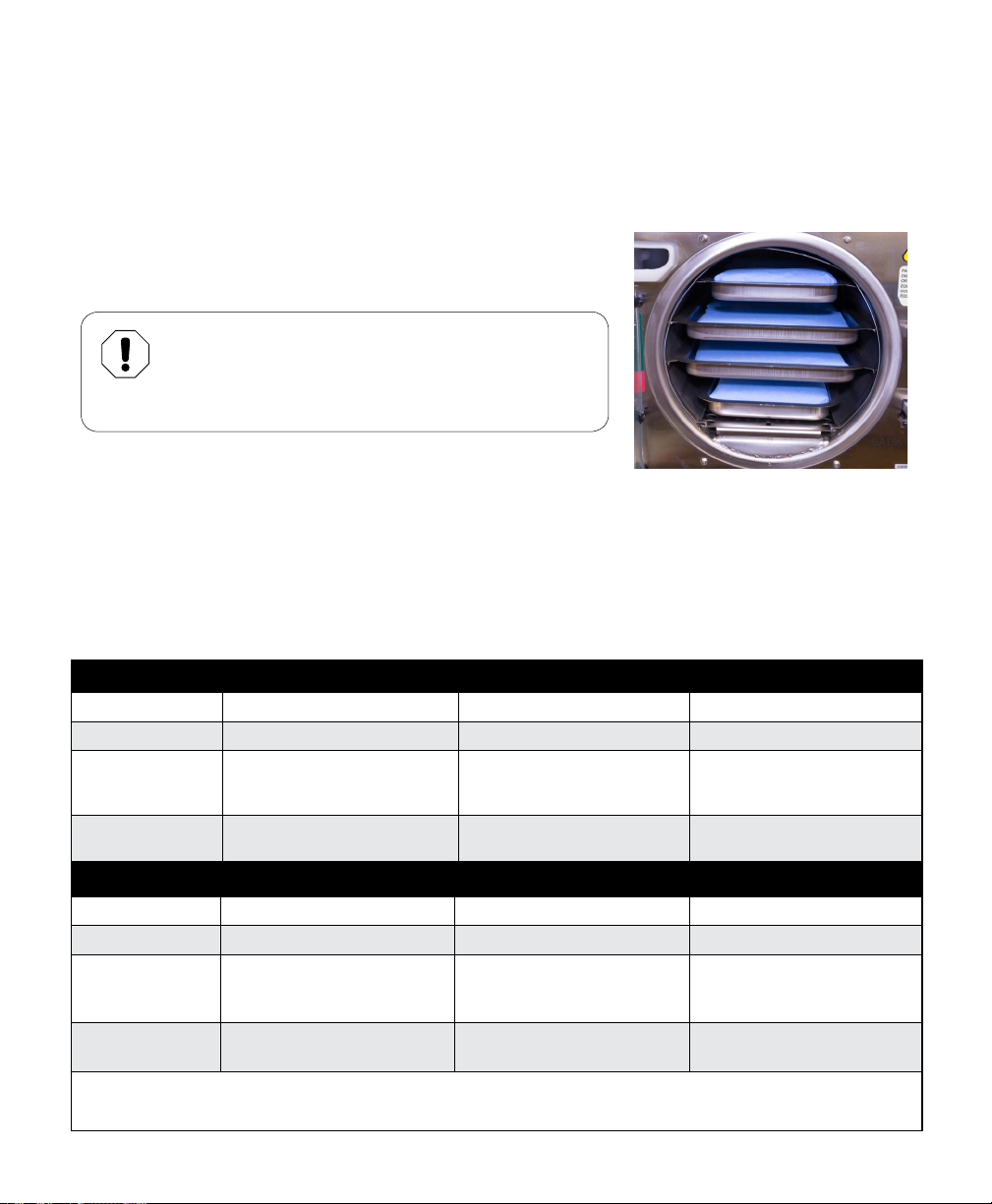
M11 (Photos 11, 12, 13, and 14):
M9 / M9D Large Cassette
Photo 9
M11 Mixed Load with Cassette and Pouches
Photo 11
M9 / M9D Mixed Load with Small Cassette and Pouches
Photo 10
M11 with Vertical Cassette Rack
Photo 12
003-2915-99
M11 with Horizontal Cassette Rack
Photo 13
English - 22
M11 with Horizontal Cassette Rack and Top Tray
Photo 14
© Midmark Corporation 2018
Textile Loads
• Clean textiles recommended for steam sterilization can be processed in the Packs cycle. Verify the
acceptability of the Packs cycle sterilization parameters with the textile manufacturer to assure the
textiles are compatible.
• All textile packs must not exceed the thickness limits specied in the maximum capacity tables below
and must t within the boundaries of the sterilizer trays.
• If multiple packs are placed on a single tray maintain a minimum of 1/4” (6.4 mm) between packs for
proper steam circulation and drying. (Photo 15)
Equipment Alert
Do not use towels or packaging containing chlorine bleach
residue. Failure to comply may cause rusting or discoloration
of the chamber / trays, and significantly shorten the life of the
sterilizer.
Textile load
Photo 15
Load Size
Successful sterilization is dependent on correct loading of the sterilizer. Do not overload the sterilizer
chamber! Adequate space must be maintained around all items placed in the chamber to assure proper
steam circulation and adequate drying. The charts below are provided as a reference regarding the
maximum loads that can be processed in the sterilizers but maintaining proper spacing between all items
processed to assure good steam circulation and drying should be the guiding factor in determining the
maximum load that can be processed.
M9 / M9D Maximum Capacities **
Load Type Large Tray Small Tray Total
Solid Instruments 2.4 lbs. (1089 grams) or 1.6 lbs. (726 grams) or 8.0 lbs. (3629 grams) or
Handpieces 2 handpieces with other
Packs* 90 in
instruments
2.4 lbs. (1089 grams) or
3
≤ 1.25 in. thick
(1475 cm3 ≤ 3.2 cm thick)
2 handpieces with other
instruments
1.6 lbs. (726 grams) or
55 in3 ≤ .75 in. thick
(901 cm3 ≤ 1.9 cm thick)
8 handpieces with other
instruments
8.0 lbs. (3629 grams) or
3
290 in
(4752 cm3)
M11 Maximum Capacities **
Load Type Large Tray Small Tray Total
Solid Instruments 2.7 lbs., (1225 grams) or 1.8 lbs., (816 grams) or 9.0 lbs., (4082 grams) or
Handpieces 2 handpieces with other
Packs* 145 in
* Allow a minimum of 1/4” (6.4 mm) space between each pack, and from the chamber wall.
** The default dry time may need increased due to variations in load conguration, wrapping materials, and the environment to
completely dry the chamber contents at these capacities.
003-2915-99
instruments
2.7 lbs. (1225 grams) or
3
≤ 1.5 in. thick
(2376 cm3 ≤ 3.8 cm thick)
English - 23
2 handpieces with other
instruments
1.8 lbs. (816 grams) or
108 in3 ≤ 1.5 in. thick
(1770 cm3 ≤ 3.8 cm thick)
8 handpieces with other
instruments
9.0 lbs. (4082 grams) or
3
505 in
(8275 cm3)
© Midmark Corporation 2018
Standard Cycle Parameters
Cycle Parameters
Drying
(Ref.)
1
Time
30 min. • Instruments loose on a tray.
30 min. • Pouched or loosely wrapped instruments.
30 min. • Textiles and surgical packs wrapped for
30 min. • Dental handpieces (wrapped or unwrapped).
Cycle Type
Temp.
Time Press.
(Min.)
270°F
(132°C)
Unwrapped
Pouches
Packs
Handpieces
1. The pressure shown in this table is for reference only. It’s the ideal pressure of saturated steam
at the sterilization temperature. The pressure shown on the sterilizer display may be higher.
2. Dry time can be changed from 0 to 60 minutes. Refer to Cycle Operation.
270°F
(132°C)
250°F
(121°C)
270°F
(132°C)
3 min. 27.1 psi
(186 kPa)
4 min. 27.1 psi
(186 kPa)
30 min. 15 psi
(104 kPa)
4 min. 27.1 psi
(186 kPa)
(Always consult the item manufacturer’s
2
• Open glass or metal canisters.
• Tubing not used in surgical procedures.
(Max. length - 40” and Min. inside diameter - .187”)
• Loose items manufacturers recommend for
exposure at 270°F (132°C) for 3 minutes.
Note: The sterility of unwrapped items is compromised on exposure to a non-sterile environment.
• Multiple layers of instruments separated by
fabric.
• Wrapped trays of loose instruments.
• Wrapped cassettes.
• Wrapped items manufactures recommend for
exposure at 270°F (132°C) for 4 minutes.
sterilization.
• Items, except liquids, manufacturers
recommend for exposure at 250°F (121°C) for
30 minutes.
Note: Verify acceptability of sterilization
parameters with handpiece manufacturer.
Items to be Sterilized
recommendations for sterilization.)
003-2915-99
English - 24
© Midmark Corporation 2018
Cycle Operation
Refer to the following steps for a detailed description of cycle operation:
Equipment Alert
The sterilizer will not operate unless the door is closed and latched properly.
Step 1: Close and latch the door.
A) Lift the door handle, then push the door closed.
B) While pushing in on the door, slide the door handle down to engage the latch.
Cycle Buttons
(Programmable)
Cycle Buttons
(Pre-set)
Note
Pressing the Start button when ‘SELECT CYCLE’ is
displayed, at beginning or end of a cycle, activates
the heater for 10 minutes. The display flashes
‘ADDITIONAL HEAT’. This allows the operator to
preheat the chamber before starting a cycle or to
add additional time to the Dry mode at the end of a
cycle. Pressing the Stop button will end the
‘ADDITIONAL HEAT’ time.
Equipment Alert
Using an incorrect sterilization program could result in non-sterile goods and may
damage instruments. Consult instrument manufacturer for specific sterilization instructions.
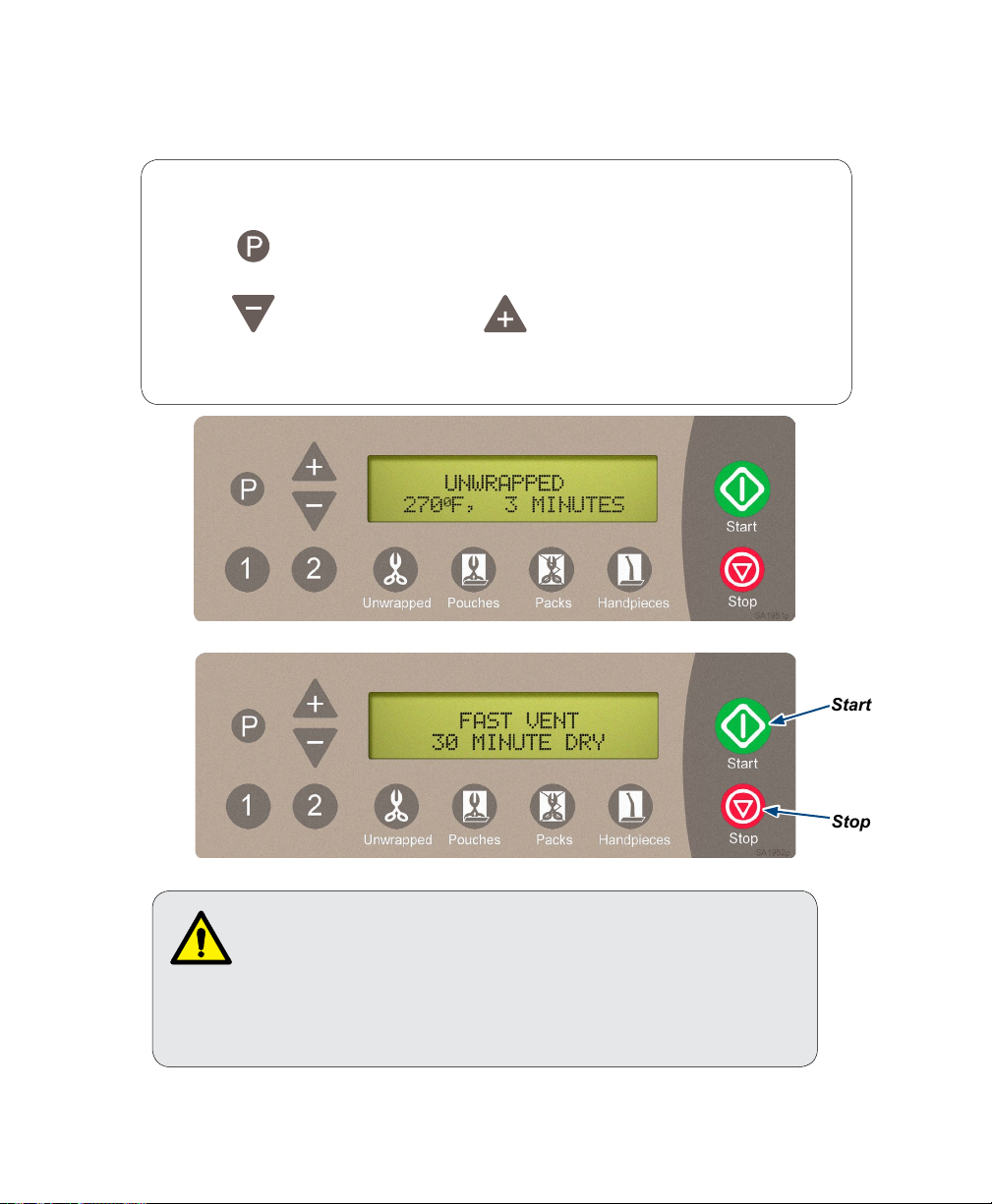
Step 2: Select the desired cycle.
A) Press the appropriate cycle button on the display panel.
(Refer to “Cycle Parameters” chart for time / temperature specifications).
003-2915-99
English - 25
© Midmark Corporation 2018
Cycle Operation - continued...
After the cycle button is pressed, the parameters for that cycle will appear on the display.
Note
On units using the metric display,°F will display as°C and PSI will display as KPA.
Pressing enables operator to change DRY time from 0 to 60 minutes in 1 minute
increments on a pre-set cycle.
Pressing decreases time. Pressing increases time.
If the new Drying Time is desired on a regular basis pressing “P” after adjusting the dry time will
store the new time as the default dry time for the selected cycle.
003-2915-99
WARNING
STOP button may be pressed at any time to stop or interrupt
a cycle. Goods must not be considered sterile if this occurs
before the Dry Cycle. Sterilizer will return to SELECT CYCLE mode.
Step 3: Press ‘Start’ button to initiate cycle.
You will hear a “beep” for two seconds, indicating the cycle has started.
English - 26
© Midmark Corporation 2018
Start
Stop
Cycle Operation - continued...
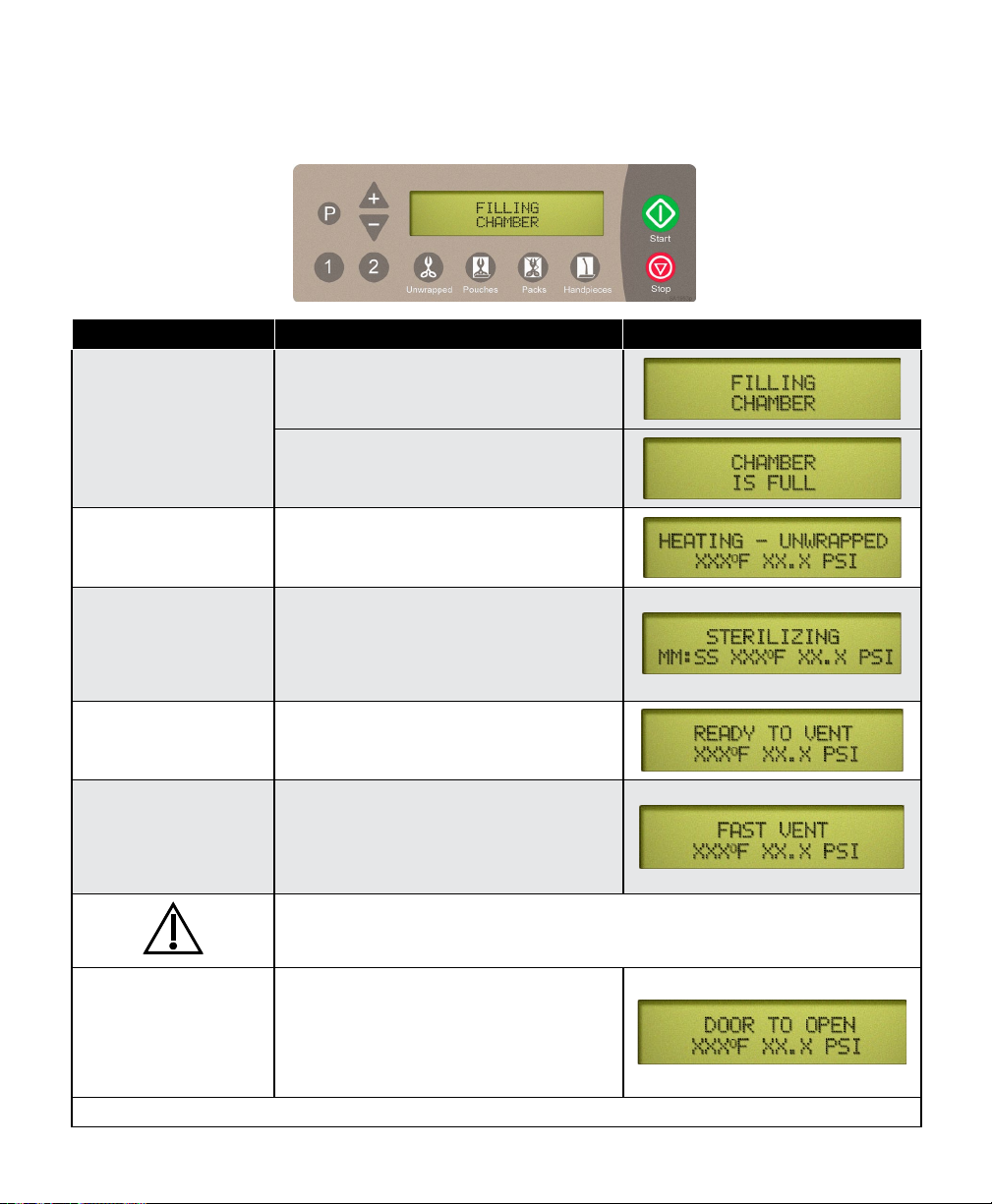
After the ‘Start’ button is pressed, the stage / status of the current cycle will appear on the display.
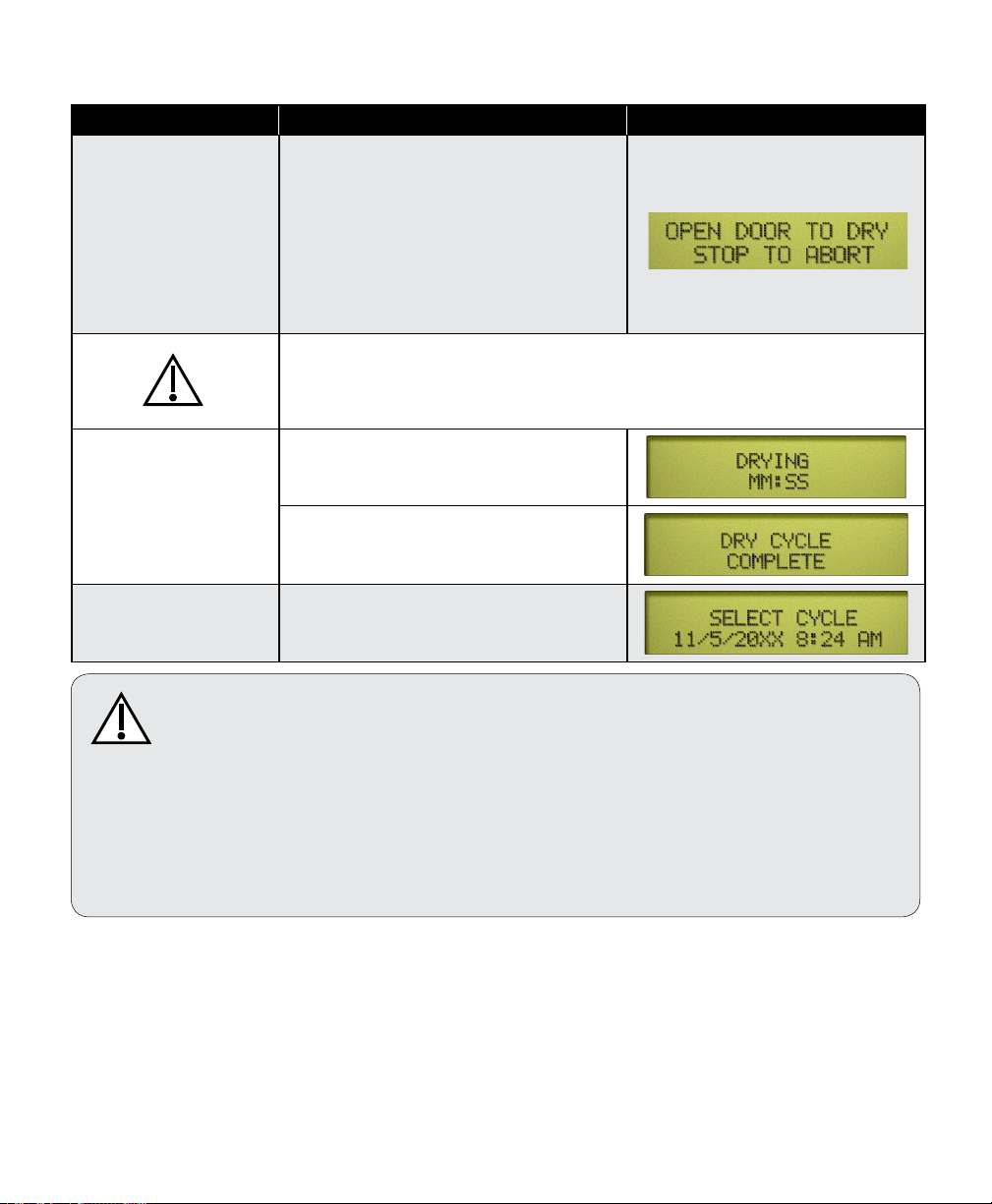
The chart below illustrates the display messages that will appear during each stage of the cycle.
Stage of Cycle Description Display
Filling Chamber begins lling with water.
When water reaches the proper level...
Heating Display changes as temperature and
pressure in chamber changes.
(Metric units may be displayed if desired).
Sterilizing Sterilizing begins when correct
temperature / pressure is reached.
Time remaining in cycle counts down
while current temperature / pressure in
chamber is continuously updated.
Ready to Vent ‘READY TO VENT’ is displayed when
10 seconds remain in sterilization cycle.
Fast Vent When time runs out in sterilizing mode,
Door To Open
(automatic)
Pertains only to
M9/M11 Steam Sterilizers
003-2915-99
the vent valve opens. Steam / water are
released back into the reservoir.
The display changes as temperature and
pressure in chamber changes.
Caution
Keep clear when M9 / M11 door is ready to open!
Failure to do so could result in severe burns from steam being released.
An audible signal is emitted to indicate
that the door is about to open.
When pressure in chamber reaches zero,
the door actuates to partially open (drying
mode) position.
This chart continues on the following page...
English - 27
© Midmark Corporation 2018
Cycle Operation - continued...
Stage of Cycle Description Display
Manual Door Open
Pertains only to
M9D Steam Sterilizer
Drying Time of Dry Cycle is counted down. If
Cycle Complete An audible signal is emitted for 10
An Audible signal is emitted when
pressure inside chambers reaches zero
to indicate Operator to open door. The
door should be opened to the rst stop
(drying mode) position. The audible signal
will continue to repeat every minute until
either the door is opened to the DRY
(partially opened) position, or by pressing
the STOP button, aborting the DRY cycle.
Caution
The processed loads may still be wet if the Dry Cycle is aborted
prior to completion. Always allow processed loads to dry in the
sterilizer before they are handled to avoid re-contamination.
desired, the Dry Cycle can be aborted by
pressing the STOP button.
When Drying time reaches 0:00...
seconds.
Caution
• The processed load and inner surfaces will be hot. Avoid contact with hot surfaces. Failure
to do so could result in serious burns.
• Do not apply downward pressure to the open sterilizer door while unloading the chamber.
Doing so could cause the sterilizer to tip resulting in serious burns or injury from the trays or
instruments sliding out of the chamber.
Step 4: Remove processed load from the chamber.
A) Refer to “Using the Optional Tray/Cassette Tool” later in this manual.
B) Sterilizer is now ready for another cycle.
Post-Sterilization Processing
After sterilization is complete, all items must be handled in accordance with accepted and documented
standards, such as the Centers for Disease Control and Prevention (CDC) documents, “Guidelines for
Infection Control in Dental Healthcare Settings” - 2003, MMWR; 52 (no. RR-17), and “Guidelines for
Disinfection and Sterilization in Healthcare Facilities” - 2008, as well as any local requirements that may
apply.
Qualied personnel responsible for infection control should prepare a protocol for handling sterilized items.
This protocol should be followed by all personnel responsible for handling sterilized items.
003-2915-99
English - 28
© Midmark Corporation 2018

Programmable Cycle Buttons
Cycle buttons can be used for custom applications that are not covered by standard cycle
programs. These cycles have not been FDA cleared for medical use. The programmable cycles allow the
user to adjust cycle parameters in order to sterilize items that cannot be sterilized in any of the standard
pre-programmed cycles. Once a custom program has been stored, it can be used just by pushing the
or button. Use the instructions in the chart below to set time / temperature parameters for these
buttons. (If you wish to change the settings, these buttons may be reprogrammed at any time).
Note:
The programmable cycles can
be programmed for the monthly
cleaning cycles with no dry time
to expedite monthly maintenance.
NOTE:
Pressing the STOP button during this procedure will abort the changes, and revert to the original settings.
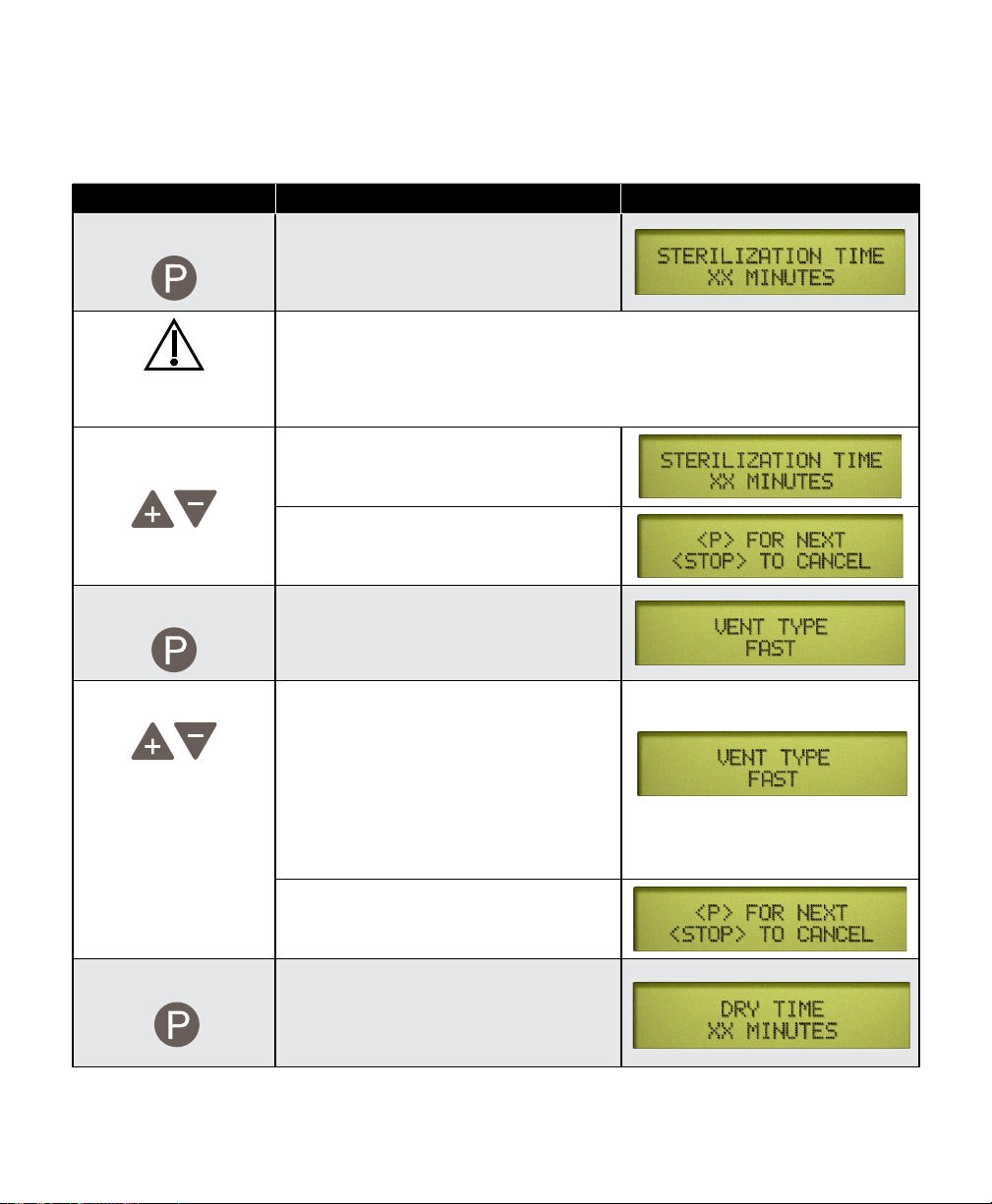
Action Description Display
Press desired
button
This selects the button that will be
programmed.
Press This brings up the sterilization
Adjust Sterilization
Temperature
003-2915-99
temperature programing display.
Caution
Sterilization temperature can be adjusted from a minimum of 250°F (121°C) to
a maximum of 275°F (135°C). At high altitudes sterilization temperatures above
270° F (132° C) may cause the pressure relief valve to leak.
The “+” and “-” buttons adjust the
temperature by 1°increments.
When the desired temperature appears
on the display...
This continues on the following page...
English - 29
© Midmark Corporation 2018
Programmable Cycle Buttons - continued...
NOTE:
Pressing the STOP button during this procedure will abort the changes, and revert to the original settings.
Action Description Display
Press To store the temperature. This brings up
the sterilization time programing display
showing the current value.
Caution
Sterilization time can be adjusted from a minimum of 3 minutes to a maximum
of 45 minutes. Because it is important to properly coordinate the cycle time with
the sterilization temperature the minimum selectable time will be determined by
the temperature setting.
Adjust Sterilization
Time
Press To store the sterilization time. This brings
The “+” and “-” buttons adjust the
time by 1 minute increments.
When the desired time appears on the
display...
up the vent speed programing display and
the current value.
Adjust Vent Speed In FAST vent, the valve fully opens and
vents the chamber.
In SLOW vent, the valve opens for a
fraction of a second (once per minute)
to slowly vent the chamber.
Pressing “+” sets it to: FAST
Pressing “-” sets it to: SLOW
When the desired setting appears on the
display...
Press To store the setting and bring up the dry
time programing display and the current
value.
This chart continues on the following page...
003-2915-99
English - 30
© Midmark Corporation 2018

Programmable Cycle Buttons - continued...
NOTE:
Pressing the STOP button during this procedure will abort the changes, and revert to the original settings.
Action Description Display
Adjust Dry Time Dry time can be adjusted from 0 to 60
minutes.
The “+” and “-” buttons adjust the
time by 1 minute increments.
When the desired time appears on the
display...
Press To store the setting and complete the
programming process. The display will
show the new cycle parameters.
Note
The programmed settings are retained under Program # button <1> or <2>.
Even if power is interrupted, or the unit is unplugged the setting will be retained.
Caution
All materials processed using these cycles must be validated by the user.
Failure to do so could result in incomplete sterilization.
Maintenance
Maintenance Messages
To assure proper operation and maximum sterilizer life, carefully follow all recommendations for periodic
maintenance. Recommended maintenance is easy to do and takes very little time.
One of the MOST important steps you can take to prevent problems with your sterilizer, is to ensure that
ONLY distilled water or water that meets the referenced water purity specifications - NOT TAP WATER is used in the sterilizer. Since the sterilizer operates with high water temperatures, any minerals dissolved
in the water will form mineral deposits. This can prevent valves from opening or closing properly and can
also lead to corrosion in the chamber and tubing.
Maintenance reminders will be displayed on the screen at the appropriate intervals to assist the operator.
These reminders are removed from the display screen once a cycle is started.
The user is responsible for establishing a periodic maintenance procedure to assure correct operation of
equipment and reliable sterilization of loads. Contact your local Midmark distributor or service representative
to develop a program for planned maintenance.
003-2915-99
English - 31
© Midmark Corporation 2018

Daily Maintenance
Equipment Alert
If the sterilizer is used frequently to process dental handpieces that have been
lubricated or dipped in dental milks, drain the water from the reservoir daily.
Refill the reservoir with distilled water or water that meets the referenced water
purity specifications.
Clean External Surfaces
A) Wash the exterior of the sterilizer each day according to your facility’s procedure for clinical contact
surfaces, noting the following: (Use only quaternary disinfectants to disinfect unit. Staining, pitting,
discoloration, or softening could occur if phenolic, iodophor, or glutaraldehyde-based disinfectant is
used on plastic surfaces of the unit. Also, use of alcohol or aerosol spray cleaner / disinfectant
containing substantial amounts of alcohol in the formula can damage the faceplate).
B) Wring excess solution from the cloth.
C) Using soft cloth, wipe all external surfaces.
D) Follow the instructions provided with the cleaner / disinfectant used regarding rinsing and drying
of the external surfaces.
Clean sterilizer door / dam gaskets
Caution
To prevent burns, allow unit to cool before
cleaning gaskets and mating surfaces.
A) Examine gaskets for possible damage.
B) Clean gaskets and mating surfaces with a damp cloth.
003-2915-99
Door Gasket
Dam Gasket
English - 32
© Midmark Corporation 2018

Weekly Maintenance
Equipment Alert
Failure to change water may result in sterilizer malfunction. Do not use bleaching
agents or any abrasive materials / substances in chamber (i.e. bleach, steel wool, wire
brush, scouring powder, etc.). Failure to comply may result in damage to the chamber and/or
other components.
Note
Every seven days, the autoclave will automatically display the PERFORM WEEKLY MAINTENANCE
message. If power is disconnected, the cycle of weekly messages will be reset.
Caution
To prevent burns, allow unit to cool before
draining reservoir.
Clean Chamber / Trays (including Rack and Plate)
A) Disconnect the upper portion of the reservoir drain tube from the panel clips, bend it downward,
and drain the reservoir water into a suitable container, e.g. a bucket, and dispose of the water.
B) Remove the trays, tray rack, and tray plate from the sterilizer.
(Refer to the following page for instructions on removing / installing the tray rack and tray plate).
C) Wash trays, rack, plate, and inside of chamber with mild soap or Speed-Clean and distilled water or
water that meets the referenced water purity specifications.
D) Refill reservoir with distilled water or water that meets the referenced water purity specifications.
003-2915-99
Trays
English - 33
Tray Rack
Tray Plate
© Midmark Corporation 2018

Weekly Maintenance - continued...
WARNING
Allow unit to cool before removing or installing tray rack and plate.
Handle metal tray rack carefully to avoid injury. Do not run sterilizer without
tray plate in place.
To remove tray rack / plate...
A) Remove trays.
B) Using a screwdriver, pry plate up while pulling tray rack / plate out of chamber.
Tray Rack
003-2915-99
Tray Plate
(Angled end must face back of chamber)
Equipment Alert
Install tray rack / plate with angled end of plate
toward the back of the chamber.
Do not allow plate to contact the water level sensor.
To install tray rack / plate...
A) Insert the tray rack into the tray plate.
B) Place back of tray plate in chamber.
C) Press down on tray rack, while sliding into chamber.
English - 34
© Midmark Corporation 2018

Monthly Maintenance
WARNING
Do not process instruments while flushing system.
Equipment Alert
Use only Speed-Clean to flush system. Failure to flush system with Speed-Clean may result in the
premature failure of sterilizer components.
Note
Every 28 days, the sterilizer will automatically display the PERFORM MONTHLY MAINTENANCE
message. If power is disconnected, the cycle of monthly messages will be reset.
Caution
Clean Chamber / Plumbing
A) With a cooled chamber, drain the sterilizer’s reservoir and refill with clean distilled water or water that
meets the referenced water purity specifications. Add one ounce of Speed-Clean sterilizer cleaner
directly to the bottom of chamber.
B) Run one Pouches cycle.
C) Press Stop button when Dry Cycle begins. (Dry Cycle is not needed during maintenance.)
D) Drain reservoir and refill a second time with clean distilled water or water that meets the referenced
water purity specifications. .
E) Rinse by running one Unwrapped cycle
begins.
F) Drain and refill reservoir with clean distilled water or water that meets the referenced water purity
specifications, then allow sterilizer to cool.
G) Remove tray plate, rack, and trays. Wipe off with a damp cloth.
To prevent burns, allow unit to cool before
draining reservoir.
. Push the “Stop” button when the drying cycle
Sterilizer Cleaner
003-2915-99
Air Filter Screen
Fill / Vent
Filter Screen
Speed-Clean
English - 35
MA8195-1i
Steam Temperature Probe
Level Sensor Probe
Heating Element
© Midmark Corporation 2018

Clean Chamber / Plumbing - continued...
Equipment Alert
Use care when wiping the inside of the chamber. Failure to comply may result in
damage to the heating element, steam temperature probe, and/or level sensor probe.
Equipment Alert
Do not operate sterilizer without filters in place.
H) Remove and clean filters. The filters are intended to prevent debris from causing valve failures.
Between regular monthly cleanings if the fill or vent times become too long or items will not dry the
filters should be cleaned. (Refer to the illustration for location of filter screens.)
I) Grasp filter and gently pull away from chamber wall while twisting slightly.
(If necessary, pliers may be used to remove filters)
J) Clean filters with Speed-Clean and distilled water. A small stiff bristled brush or ultrasonic cleaner
may be helpful. Rinse filters with distilled water. Replace filter(s) if debris cannot be removed by
cleaning.
K) Wipe out the inside of chamber.
L) Install filters. (Press inward, toward chamber wall while twisting slightly).
M) Install tray plate, rack, and trays.
Remove / Clean Door and Dam Gaskets
A) Remove door and dam gaskets from chamber door,
then remove the gasket ring from the door gasket.
B) Clean gaskets and ring with Speed-Clean,
distilled water, and a soft brush.
C) Inspect gaskets for damage / shrinking / swelling.
Replace gaskets if damage is apparent.
D) Press gasket ring into the channel in the
door gasket and reinstall the gasket in the door.
E) Install dam gasket.
003-2915-99
English - 36
© Midmark Corporation 2018

Check Pressure Relief Valve
(must be checked each month to assure it functions properly)
A) Press Unwrapped button
B) Press Start button
During the pressure relief valve check, steam will be vented from under the sterilizer. To keep
from being burned, place a steam barrier (a rolled up towel) around the bottom of the sterilizer.
C) Wait until pressure in chamber
reaches 20 PSI (138 kPa).
D) Pull upward firmly on the pressure relief lever for approximately 3 seconds, then release.
(Steam should discharge freely from beneath rear of unit when lever is pulled.
If the valve does not close completely when lever is released,
pull lever again and release quickly so that it snaps closed.
Repeat this until valve seats properly).
E) Press Stop button
(This aborts the cycle to prevent overheating).
Caution
Pressure Relief
Lever
Equipment Alert
If excessive force is required to open the
pressure relief valve, or if the valve will
not reseat properly, the valve must be replaced.
(Refer to “Calling for Service” in this manual).
MA601301i
Extended Use Maintenance
The M9 / M9D and M11 are designed and tested to provide exceptional reliability throughout their
service life. However, like all electro-mechanical devices they are subject to wear and degradation with use.
To ensure the integrity, performance, and safety of all major components it is the responsibility of the user to
have the sterilizer performance / operation veried by a Midmark Authorized Service Provider at least every
10 years or 10,000 cycles, which ever comes rst. After 10 years or 10,000 cycles of use, an annual
inspection by a Midmark Authorized Service Provider is recommended.
003-2915-99
English - 37
© Midmark Corporation 2018

Thermal Printer (Optional)
Operating the Printer
After the optional printer is installed and the sterilizer is plugged in, the printer is automatically powered up
and initialized. No user intervention or setup is required.
Printer Tape Description
The printer will print the following information for each sterilizer cycle:
• Model Number: indicates sterilizer model and software version.
• Cycle Number: reects actual cycle count of sterilizer.
• Sterilizer ID: Serial Number of sterilizer.
• Operator: a line is printed so the operator’s signature can be recorded on the printer tape.
• Date and Time: of start of each cycle
• “BEGIN selected CYCLE”: to indicate the beginning of the cycle selected by the operator.
• Summary of selected cycle set points.
Once the cycle starts, the printer will print the words “FILLING CHAMBER” to show that the sterilizer is ling
with water.
Once the sterilizer begins the Heating Phase of the sterilization cycle, the word “HEATING” is printed and
the printer will print the chamber temperature, pressure, and elapsed time in 2 minute increments until the
heating phase is completed.
When the sterilizer enters the sterilization phase, the word “STERILIZING” is printed and the printer will print
the chamber temperature, pressure, and elapsed time in 1 minute increments until the sterilization phase is
completed.
When the sterilizer has completed the sterilization phase of the program cycle, the printer will print the words
“VENTING CHAMBER” to show that the steam pressure is being exhausted from the chamber.
When the sterilizer enters the drying phase, the word “DRYING” is printed and the printer will print the
words “DRYING START” and show temperature, pressure, and elapsed time in 5 minute increments starting
with 5:00. The printer continues to print the elapsed time in 5 minute increments until the drying phase
is completed. The nal record for the drying phase will include the words “DRYING COMPLETE”. In the
event the drying time is programmed to a time that isn’t divisible by 5, the nal printed record for the drying
phase will reect the actual programmed drying time in 1 minute increments, e.g. a programmed dry time of
12 minutes will have 5,10,and 12 minutes printed on the printer tape.
When the sterilizer has completed the drying phase of the sterilization cycle, the printer will print a summary
of the sterilization cycle with the duration of each phase of the cycle and the Total Cycle Time. Following the
summary the printer will print “CYCLE COMPLETE”.
[NOTE: If drying cycle is aborted, “DRYING COMPLETE” and “CYCLE COMPLETE” will not print].
003-2915-99
English - 38
© Midmark Corporation 2018

Example of Typical Printout of a Program Cycle
Sterilizer Identication
(Serial Number)
Date and Time
of cycle start
Sterilization temperature,
time, and dry time settings
During Heat-Up phase, the
printer records chamber
temperature and pressure
in 2 minute increments
During Sterilization phase,
the printer records chamber
temperature and pressure in
1 minute increments
Indicates Venting
phase initiated
During Drying phase,
the printer records time in
5-minute increments
These lines will show the
total dry time completed
STERILIZING: 3:00
TOTAL CYCLE 00:51:44
Midmark M9 - v0.1.1
Total Cycles: 10
Sterilizer ID: V0000000
--------------------------------------------
Operator
1/27/2016 8:08 AM
BEGIN UNWRAPPED CYCLE
Temp: 270 Degrees F
Time: 3 Minutes
Dry: 30 Minutes
FILLING CHAMBER
HEATING
mm:ss Degrees PSI
0:00 77.2F 0.0
2:00 82.7F 0.7
4:00 152.2F 5.4
STERILIZING
mm:ss Degrees PSI
0:00 272.1 F 28.0
1:00 273.0 F 28.7
2:00 272.2 F 27.9
3:00 272.4 F 28.7
Min 272.0 F 27.9
Max 273.1 F 29.0
VENTING CHAMBER
DRYING
Drying Start
mm:ss Degrees PSI
5:00 192.8 F 0.0
10:00 192.7 F 0.0
30:00 192.8 F 0.0
Drying Complete
FILLING: 0:36
HEATING: 15:40
VENTING: 2:28
DRYING: 30:00
CYCLE COMPLETE
Summary of
Sterilization phase
Cycle summary
Model # and
Software Version
Total # of cycles
run on unit
Line provided for
operator signature
Selected Cycle
Indicates Filling
phase initiated
These lines will not print
if Dry Cycle is aborted
before it completes
003-2915-99
English - 39
© Midmark Corporation 2018

Paper Roll Removal / Installation
Equipment Alert
Use only thermal paper rolls with a maximum diameter of 1.89” (48 mm) and
width of 2.28” (58mm).
To remove paper...
A) Insert finger into groove at the back of the small cover and lift upward until
lid is released from locked position. To avoid damage do not use excessive
force.
B) The printer lid will hinge up towards the back of the printer.
C) Remove any remaining paper.
To install the paper roll...
A) Unroll 3” to 4” (7.6 to 10.2 cm) of paper.
B) Place the paper roll into cradle, unrolled side down.
C) Hold unrolled edge of paper out the front of the printer and shut the lid by
applying equal amounts of pressure on each side of lid until it latches.
D) Press the paper feed button for additional paper.
Note
A pink stripe that gets progressively darker on the paper indicates when paper is low.
Printer Paper Rell Order Number
Printer Thermal Paper Roll 060-0016-00
Printer Stick-able Thermal Paper 060-0016-01
Paper Feed Button
Paper Slot
003-2915-99
English - 40
Status Light
SA1841i
Printer Housing
© Midmark Corporation 2018

Using the Optional Tray / Cassette Tool
Equipment Alert
Only use the 9A307001 Tray / Cassette Tool with Midmark manufactured
trays. Slide the proper plate into the handle as shown.
Note: There are three plates included. Each plate is designed for a specific purpose.
(M9 / M9D trays, M11 trays, cassettes)
To remove M9 / M9D / M11 trays and cassettes...
A) Hook the top, saw-toothed tab of tool to top center of tray lip / cassette.
B) Rotate tool downward until the tool plate is completely under tray / cassette.
C) Check to ensure tray / cassette is being held securely and then remove from chamber.
Note: Cassette tool can handle cassettes up to 1 1/2” (3.8 cm) thick.
Caution
Trays / cassettes may be HOT - use care when removing or transporting. Hold the tray level
and slightly elevated to prevent it from shifting and becoming dislodged. Failure to comply
may result in personal injury due to burns.
003-2915-99
English - 41
© Midmark Corporation 2018

Troubleshooting
Troubleshooting Chart
Use the following chart to assist in correcting minor problems with the sterilizer.
Problem Possible Cause Solution
Sterilizer does not
operate (no display).
Steam escaping from
pressure relief valve.
Sterilization
failure evidence from
process monitor
(chemical indicator,
biological indicator,
etc.).
Door gasket leaks. Door gasket is damaged or dirty. Clean or replace door gasket.
Items are not dry at
end of Dry Cycle.
Printer not printing. Printer cables are not connected
Display goes blank /
black / is non legible
003-2915-99
Sterilizer power cord came loose from
supply outlet or back of sterilizer.
No power at Sterilizer supply outlet. Check circuit breaker for supply outlet. If problem
Fuse open on main P.C. Board. Unplug unit power cord and contact an authorized
Pressure relief valve not properly
seated.
Sterilization conditions were not
present at location of the indicator.
Indicator is out-of-date, is inappropriate
for sterilization cycle, or has otherwise
malfunctioned.
Sterilizer is improperly loaded. Reload sterilizer in accordance with guidelines for
Filter screen(s) clogged. Clean or replace lter screen(s).
properly.
Printer malfunction. (status light ashes
multiple times per second)
Printer is out of paper. (status light
ashes once per second)
Wrong paper Assure printer paper is thermal paper
Electromagnetic Interference Press any key except Stop on the keypad to reset
Assure Sterilizer power cord is plugged into outlet
and sterilizer.
recurs, unplug unit power cord and contact an
authorized service technician.
(see “Calling for Service”)
service technician. (see “Calling for Service”)
Reseat pressure relief valve.
(see “Monthly Maintenance”)
Reload sterilizer in accordance with guidelines for
loading the M9 / M9D or M11. If problem recurs,
unplug unit power cord and contact an authorized
service technician. (see “Calling for Service”)
Use an indicator, appropriate for the load and cycle
selected, that has been stored properly. Contact the
indicator manufacturer for additional information on
proper selection, use, storage, and potential misapplication or malfunction.
(see “Weekly Maintenance”)
loading the M9 / M9D or M11.
If the particular load conguration being used is
consistently not dry at the end of the dry cycle
increase the cycle drying time (see note on page 26)
or reduce the load.
(see “Monthly Maintenance”)
Ensure that printer cables are connected to printer.
Unplug sterilizer power cord, wait 15 seconds, and
then plug sterilizer power cord back in.
Insert a new paper roll. (see paper roll removal and
installation section)
the display without aborting the cycle. Pressing stop
will also reset the display but will also abort the cycle
in process.
English - 42
© Midmark Corporation 2018

Informational Messages
The chart below lists the informational messages that may appear during operation.
Message Possible Cause Solution
INITIALIZING SYSTEM
TOTAL CYCLES XXX
M9, VX.X.X
MM/DD/YYYY 8:07 Am
VXXXXXXX
PERFORM WEEKLY
MAINTENANCE
PERFORM MONTHLY
MAINTENANCE
Unit power cord was just plugged in standard informational
message.
Unit power cord was just plugged
in - standard informational message.
(Displays # of cycles, model number
(M9 / M9D or M11) and software
version)
Unit power cord was just plugged
in - standard informational message.
(Displays date, time, and serial number.)
This message is displayed every 7, 14,
and 21 days after the unit is plugged
into a power source to prompt the
operator to perform weekly maintenance
described in this manual.
This message is displayed every 28
days to prompt operator to perform
monthly maintenance described in this
manual.
Normal operation will occur after a
4 second pause.
Normal operation will occur after this
message.
Normal operation will occur after this
message.
Perform weekly maintenance.
The message will automatically clear
after the next cycle is completed.
Perform monthly maintenance.
The message will automatically clear
after the next cycle is completed.
Accessing the last 5 Error Codes
Action Description Display
Press and hold
during power up
Press the “stop” button
This menu shows the last ve error
codes displayed on the unit.
NOTE: “1” is the most recent
error code; “5” is the oldest.
To exit the error code menu press
the stop button.
Error Messages
The chart below and on the following pages, lists the error messages that may appear during operation.
Caution
If an error occurs more than once, stop using the sterilizer. Record the message or error
code, unplug unit, then call an authorized service representative. (see “Calling for Service”)
If an error message includes: “Items Not Sterile”, the items in sterilizer shall not be considered sterile;
they must be run through a successful sterilization cycle.
003-2915-99
English - 43
© Midmark Corporation 2018

Error Messages - continued...
Message Possible Cause Solution
C010: POWER UP MODE
SYSTEM PWR LOSS
ITEMS NOT STERILE
PUSH STOP TO RESTART
CO60: POWER UP MODE
SYSTEM HARDWARE
C102: FILL MODE
STOP PRESSED
C103: THROUGH C105
HEATUP, STERILIZE OR
VENT MODE
STOP PRESSED
C106: DOOR MODE
STOP PRESSED
C232: FILL MODE
WATER LOW
C326: DOOR MODE DOOR
CLOSED
C382: FILL
DOOR OPEN
C383: HEATUP MODE
DOOR OPEN
C384: STERILIZE MODE
DOOR OPEN
C533 through C633:
STEAM TEMP LOW
or
STEAM TEMP HARDWARE
or
PRESSURE LOW
Unit had loss of power
during cycle.
Power was interrupted
briey or an internal
glitch.
STOP button was pressed
during cycle.
STOP button was pressed
during cycle.
STOP button was pressed
during cycle.
Water level in
reservoir is too low.
Fill / Vent lter (in bottom
of chamber) is clogged.
Door latch safety switch is
still making contact after
door motor operated.
Sterilizer detects that door
safety switch contacts
opened.
Sterilizer detects that door
safety switch contacts
opened.
Sterilizer detects that door
safety switch contacts
opened.
Sterilizer detects that
temperature and / or
pressure is outside the
limits for normal
operation.
This chart continues on the following page...
Press STOP button to restart.
Unplug unit power cord for 1 minute and then plug
back in. If problem persists, contact an authorized
service representative. (see “Calling For Service”)
Press STOP button to restart.
Wait briey (up to one minute) while chamber pressure
/ temperature dissipates. Press STOP button to return
to select cycle mode where a new cycle may be
initiated.
Press STOP button to restart.
Rell water reservoir with distilled water, or water
that meets the referenced water purity specications.
Wait briey (up to one minute). Press STOP button to
return to select cycle mode where a new cycle may be
initiated.
Clean Fill / Vent lter.
(see “Monthly Maintenance”)
Open door.
Close the sterilizer door.
(Cycle will continue where left o.)
Wait briey (1 minute) while chamber pressure / tem-
perature dissipates. Press STOP button to return to
select cycle mode. Initiate a new cycle.
Unplug unit power cord for 1 minute and then plug
back in. If problem persists, contact an authorized
service representative. (see “Calling For Service”)
Unplug unit power cord for 1 minute and then plug
back in. If problem persists, contact an authorized
service representative. (see “Calling For Service”)
003-2915-99
English - 44
© Midmark Corporation 2018

Error Messages - continued...
Message Possible Cause Solution
C642 THROUGH C647
PRESSURE HIGH
C660 THROUGH C677
PRESSURE HARDWARE
or
PRESSURE OVERLIMIT
C980 THROUGH C987
HI-LIMIT OPEN
Chamber pressure is
outside the limits for
normal operation.
Chamber pressure is
outside the limits for
normal operation.
High limit switch has
opened for at least 1/4
second during specic
operational mode.
Wait briey (up to one minute) while chamber pressure
/ temperature dissipates. Press STOP button to return
to select cycle mode. Initiate a new cycle.
Unplug unit power cord for 1 minute and then plug
back in. If problem persists, contact an authorized
service representative. (see “Calling For Service”)
Unplug unit power cord for 30 minute and then plug
back in. If problem persists, contact an authorized
service representative. (see “Calling For Service”)
Calling for Service
Note
Please mark down any displayed code(s) and be sure to relay this information to the service technician.
Contact your Midmark Authorized Dealer, or log on to www.midmark.com/technical-library.
Model and serial number information will be required when calling for service.
To contact Midmark directly:
1-800-Midmark (1-800-643-6275) or 937-526-3662
8:00 am to 5:00 pm EST (Monday thru Friday)
[excluding standard U.S. holidays]
003-2915-99
English - 45
© Midmark Corporation 2018

Specications Chart: M9 / M9D
Physical Dimensions:
Overall Length with Plug 20.38 in. (51.8 cm)
Overall Width 15.3 in. (38.9 cm)
Overall Height with Printer 16.1 in. (40.9 cm)
Counter Area 15.3 in. x 18 in. (38.9 cm x 45.7 cm)
Chamber 9 in. dia. x 15 deep (22.9 cm x 38 cm)
Standard Tray, Large 7 5/16 in. x 12 in. x 7/8 in.
(18.6 cm x 30.5 cm x 2.2 cm)
Standard Tray, Small 5 5/8 in. x 12 in. x 7/8 in.
(14.3 cm x 30.5 cm x 2.2 cm)
Weights:
Weight with Empty Reservoir 73 lbs. (33.1 kg)
Weight with Shipping Carton 81 lbs (36.7 kg)
Water Reservoir Capacity 1.1 Gallons (4.1 liters) to Full Mark
usable volume is 0.5 gallons (1.9 liters)
Electrical Rating:
Note: A separate (dedicated) circuit is required for this sterilizer. Sterilizer should not be connected into an
electrical circuit with other appliances or equipment unless circuit is rated for the additional load.
115 V models 115 VAC (+/- 10%), 12 Amp, 50/60 Hz
230 V models 230 VAC (+/- 10%), 6.4 Amp, 50/60 Hz
Fuse Ratings:
115 VAC F1........0.25 Amp, 250 V, Slo-blo, 1/4” x 1 1/4”
F2........15 Amp, 250 V, Fast Acting, 1/4” x 1 1/4”
230 VAC F1........0.125 Amp, 250 V, Slo-blo, 5 x 20 mm
F2........8 Amp, 250 V, Fast Acting, 5 x 20 mm
Safety Valve Setting 40 psi (275.8 kPa)
Heat Emission 5000 BTU / hr during operation
Certications
ASME Boiler and Pressure Vessel Code,
Section VIII, Division 1.
Canadian Registration Number available
Midmark is an ISO 13485 and ISO 9001 Certied
Company
UL61010-1, 2nd Edition
IEC 61010-2-040:2005, 1st Edition
CAN/CSA C22.2, No. 61010-1, 2nd Edition
CSA C22.2, No. 61010-2-040-07 Part 2-040, 1st Edition
IEC 60601-1-2 Edition 4.0 2014-02
CISPR II:2009, A1:2010 Class A, Group 1
IEC 61000-3-2:2014, Part 3-2
IEC 61000-3-3:2014, Part 3-3
ANSI/AAMI ST55:2010(R)2014
ANSI/AAMI/IEC62304
003-2915-99
English - 46
© Midmark Corporation 2018

Specications Chart: M11
Physical Dimensions:
Overall Length with Plug 23.8 in. (60.5 cm)
Overall Width 17.8 in. (45.2 cm)
Overall Height with Printer 18.1 in. (50.0 cm)
Counter Area 17.8. x 21 in. (45.2 cm x 53.3 cm)
Chamber 11 in. dia. x 18 in. deep (28 cm x 46 cm)
Standard Tray, Large 9 in. x 15 in. x 1 1/8 in.
(22.9 cm x 38 cm x 2.9 cm)
Standard Tray, Small 6 5/8 in. x 15 in. x 1 1/8 in.
(16.8 cm x 38 cm x 2.9 cm)
Weights:
Weight with Empty Reservoir 99 lbs. (44.9 kg)
Weight with Shipping Carton 131 lbs (59.4 kg)
Water Reservoir Capacity 1.4 Gallons (5.3 liters) to Full Mark
usable volume is 1.0 gallons (3.8 liters)
Electrical Rating:
Note: A separate (dedicated) circuit is required for this sterilizer. Sterilizer should not be connected into an
electrical circuit with other appliances or equipment unless circuit is rated for the additional load.
115 V models 115 VAC (+/- 10%), 12 Amp, 50/60 Hz
230 V models 230 VAC (+/- 10%), 6.4 Amp, 50/60 Hz
Fuse Ratings:
115 VAC F1........0.25 Amp, 250 V, Slo-blo, 1/4” x 1 1/4”
F2........15 Amp, 250 V, Fast Acting, 1/4” x 1 1/4”
230 VAC F1........0.125 Amp, 250 V, Slo-blo, 5 x 20 mm
F2........8 Amp, 250 V, Fast Acting, 5 x 20 mm
Safety Valve Setting 40 psi (275.8 kPa)
Heat Emission 5000 BTU / hr during operation
Certications
ASME Boiler and Pressure Vessel Code,
Section VIII, Division 1.
Canadian Registration Number available
Midmark is an ISO 13485 and ISO 9001 Certied
Company
UL61010-1, 2nd Edition
IEC 61010-2-040:2005, 1st Edition
CAN/CSA C22.2, No. 61010-1, 2nd Edition
CSA C22.2, No. 61010-2-040-07 Part 2-040, 1st Edition
IEC 60601-1-2 Edition4.0 2014-02
CISPR II:2009, A1:2010 Class A, Group 1
IEC 61000-3-2:2014, Part 3-2
IEC 61000-3-3:2014, Part 3-3
ANSI/AAMI ST55:2010(R)2014
ANSI/AAMI/IEC62304
003-2915-99
English - 47
© Midmark Corporation 2018

Water Purity Specications
Water Purity Specication Chart
Evaporate Residue ≤ 15 mg/l ≤ 15 ppm
Silica ≤ 2 mg/l ≤ 2 ppm
Iron ≤ 0.2 mg/l ≤ 0.2 ppm
Cadmium ≤ 0.005 mg/l ≤ 0.005 ppm
Lead ≤ 0.05 mg/l ≤ 0.05 ppm
Rest of heavy metals, excluding
Iron, Cadmium, and Lead
Chloride ≤ 3 mg/l ≤ 3 ppm
Phosphate ≤ 0.5 mg/l ≤ 0.5 ppm
Conductivity (at 20°C)
pH 6.5 to 8
Appearance Colorless, clean, without sediment
Hardness ≤ 0.1 mmol/l (.585 gr/gal) ≤ 10 ppm
1
2
AAMI ST-46 (ref.)
≤ 0.1 mg/l ≤ 0.1 ppm
≤ 50 μs/cm TDS ≤ 25 ppm
003-2915-99
English - 48
© Midmark Corporation 2018

Midmark Sterilizer Warranty
1. SCOPE OF WARRANTY
Midmark Corporation (“Midmark”) warrants to the original retail purchaser that it will at Midmark’s option repair or
replace components of the sterilizer products manufactured by Midmark (except for components not warranted under
“Exclusions”) that are defective in material or workmanship under normal use and service. Midmark’s obligation under
this limited warranty is limited to the repair or replacement, at Midmark’s option of the applicable components. This limited
warranty shall only apply to defects that are reported to Midmark within the applicable warranty period and which, upon
examination by Midmark, prove to be defective. This warranty extends only to the rst retail purchaser of a product and
is not transferable or assignable. Replacement components or products may be used and/or refurbished components or
products, provided they are of like quality and specications as new components or products.
2. APPLICABLE WARRANTY PERIOD
The applicable warranty period, measured from the date of invoice to the original user, shall be as follows:
(1) STERILIZER PRODUCTS are warranted for a period of one (1) year.
(2) MIDMARK Replacement Parts and Accessories carry a ninety (90) day warranty.
3. OBTAINING WARRANTY SERVICE
Warranty service must be obtained through either Midmark or an authorized dealer in the Midmark product line for which
warranty service is requested. Midmark may be contacted for warranty service inquiries or issues via our website at www.
midmark.com, by mail to Midmark Corporation, 60 Vista Drive, Versailles, Ohio 45380, or by phone at: 1-800-MIDMARK.
It is the retail purchaser’s obligation to arrange for delivery of a product to Midmark or one of its authorized dealers for
warranty service, which delivery shall be at retail purchaser’s expense. It is also the retail purchaser’s obligation to comply
with the warranty service instructions provided either by Midmark or its authorized dealer. The retail purchaser must
provide Midmark with completed warranty registration information within thirty (30) days after purchase in order to obtain
the benets of this limited warranty.
4. EXCLUSIONS
This limited warranty does not cover and Midmark shall not be liable for the following;
(1) defects, damage or other conditions caused, in whole or in part, by misuse, abuse, negligence, alteration,
accident, freight damage, negligent storage, tampering or failure to seek and obtain repair or replacement in a
timely manner;
(2) products which are not installed, used, and properly cleaned and maintained as required or recommended in
the Midmark “Installation” and/or “Installation/Operation Manual” for the applicable product, including the speci-
ed structural and operational environment conditions and electrical power requirements;
(3) products considered to be of a consumable or sterile nature;
(4) accessories or parts not manufactured by Midmark;
(5) charges by anyone for adjustments, repairs, replacement parts, installation or other work performed upon or
in connection with such products which are not expressly authorized in writing in advance by Midmark;
(6) costs and expenses of routine maintenance and cleaning;
(7) representations and warranties made by any person or entity other than Midmark;
003-2915-99
English - 49
© Midmark Corporation 2018

Warranty Information (continued)
(8) matching of color, grain or texture except to commercially acceptable standards;
(9) changes in color caused by natural or articial light;
(10) custom manufactured products;
(11) alterations or modications to the product by any person or entity other than Midmark;
(12) Products that would otherwise by covered under Sections 1 and 2 of this limited warranty, but are ac quired: (i) from a person or entity that is not Midmark or one of its authorized dealers; or (ii) from a Midmark
dealer that is not authorized to sell the product at issue in the geographic territory where the purchaser is
located, or is not authorized to sell the product at issue outside of their market.
5. EXCLUSIVE REMEDY; CONSEQUENTIAL DAMAGES DISCLAIMER
MIDMARK’S ONLY OBLIGATION UNDER THIS LIMITED WARRANTY IS THE REPAIR OR REPLACEMENT OF
DEFECTIVE PARTS. MIDMARK SHALL NOT BE LIABLE FOR AND HEREBY DISCLAIMS ANY DIRECT, SPECIAL,
INDIRECT, INCIDENTAL, EXEMPLARY OR CONSEQUENTIAL DAMAGES OR DELAYS, INCLUDING, BUT NOT
LIMITED TO, DAMAGES FOR LOSS OF PROFITS OR INCOME, LOSS OF DATA, LOSS OF USE, DOWNTIME,
COVER AND EMPLOYEE OR INDEPENDENT CONTRACTOR WAGES, PAYMENTS AND BENEFITS. THIS
DISCLAIMER SHALL SURVIVE ANY FAILURE OR ASSERTED FAILURE OF THE ESSENTIAL PURPOSE OF THIS
LIMITED WARRANTY OR ITS REMEDIES SPECIFIED HEREIN.
6. WARRANTY DISCLAIMER
THIS LIMITED WARRANTY IS MIDMARK’S ONLY WARRANTY AND IS IN LIEU OF ALL OTHER WARRANTIES,
EXPRESS OR IMPLIED. MIDMARK MAKES NO IMPLIED WARRANTIES OF ANY KIND INCLUDING ANY IMPLIED
WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. THIS WARRANTY IS
LIMITED TO THE REPAIR OR REPLACEMENT OF DEFECTIVE PARTS.
7. STATUE OF LIMITATIONS
No action may be brought against Midmark for breach of this limited warranty, an implied warranty, if any, or for any
other claim arising out of or relating to the products, more than ninety (90) days following expiration of the limited
warranty period. In the event multiple warranty periods exist with respect to a product, the ninety (90) day period
provided for herein shall begin to run from expiration of the warranty period for the component to which the claim relates.
8. NO AUTHORIZATION
No person or rm is authorized to create or approve for Midmark any other obligation or liability in connection with the
products.
003-2915-99
English - 50
© Midmark Corporation 2018

NOTES:
003-2915-99
English - 51
© Midmark Corporation 2018

NOTES:
003-2915-99
English - 52
© Midmark Corporation 2018

M9 / M9D / M11
Esterilizador de vapor autónomo
Inglés
Español
Francés
Para los modelos:
M9 (-040 / -041 / -042)
M9D (-042)
M11 (-040 / -041 / -042)
Guía del usuario
003-2915-99
Español - 1
© Midmark Corporation 2018

Información del producto
Número de serie
Ubicación del número de
la Junta Nacional ASME
Etiqueta de modelo/
número de serie
003-2915-99
Español - 2
© Midmark Corporation 2018

Índice
Información importante ........................4
Instrucciones de seguridad ..............................4
Uso previsto .....................................................4
Tecnología de esterilización .............................4
Interferencia electromagnética.........................4
Símbolos de seguridad ....................................4
Símbolos del transporte ...................................5
Condiciones de transporte/almacenamiento....5
Accesorios, herramientas
y piezas de servicio.......................................5
Incluido con el esterilizador..............................6
Ubicación de componentes..............................7
Controles e indicadores ...................................8
Directrices para supervisar la esterilización...10
Instalación .................................................. 11
Entorno de funcionamiento ............................ 11
Requisitos para el emplazamiento ................. 11
Requisitos eléctricos ......................................12
Conexión del cable de alimentación ..............13
Conguración del usuario ..............................14
Funcionamiento ........................................15
Antes de utilizar el esterilizador .....................15
Llenado del depósito ......................................15
Pruebas de calicación ..................................16
Instrucciones de carga ...................................16
Parámetros de ciclo estándar ........................24
Ejecución del ciclo .........................................25
Procesamiento posterior a la esterilización ..28
Botones de ciclo programables......................29
Impresora térmica (opcional) .............. 38
Uso de la impresora .......................................38
Descripción de la cinta de impresión .............38
Ejemplo de impresión de
un ciclo de programa .................................39
Instalación/Retirada del rollo de papel ...........40
Herramienta de bandejas y cajas
(opcional)
Uso de la herramienta para bandejas/cajas
opcional..........................................................41
Resolución de problemas ...................42
Tabla de resolución de problemas .................42
Mensajes informativos ...................................43
Acceso a los últimos 5 códigos de error .......43
Mensajes de error ..........................................43
Contactar con el servicio técnico ...................46
Especicaciones/Conformidad
Tabla de especicaciones:
M9 / M9D ..................................................47
M11 ..........................................................48
Especicaciones de pureza del agua ............49
Información sobre la garantía
Garantía del esterilizador de Midmark ...........50
Mantenimiento ...........................................31
Mensajes de mantenimiento ..........................31
Mantenimiento diario......................................32
Mantenimiento semanal .................................33
Mantenimiento mensual .................................35
Mantenimiento a largo plazo ..........................37
003-2915-99
Español - 3
© Midmark Corporation 2018

Información importante
Instrucciones de seguridad
El aspecto más importante para Midmark es que este equipo se maneje y mantenga pensando en la
seguridad del paciente y del personal. Para garantizar el funcionamiento seguro y able:
• Lea y comprenda este manual antes de instalar o poner en funcionamiento el esterilizador.
• Asegúrese de que se informa al personal interesado sobre el contenido de este manual.
(Esto es responsabilidad del comprador).
• Asegúrese de que este manual se encuentra cerca del esterilizador o, si es posible, jado de
manera permanente a éste.
Uso previsto
Los esterilizadores por vapor M9, M9D y M11 de Midmark y Ritter pueden utilizarse en hospitales, clínicas,
centros dentales, geriátricos y otras instalaciones para esterilizar instrumental reutilizable que sea estable al calor
y la humedad (incluidas piezas manuales odontológicas), y compatible con la esterilización por vapor. Consulte
La guía de carga y los Parámetros de ciclo estándar, que encontrará más adelante en el presente manual.
Tecnología de esterilización
Midmark M9 / M9D y M11 utilizan un sistema dinámico de eliminación de aire llamado Steam Flush
Pressure Pulse para eliminar el aire de la cámara.
Interferencia electromagnética
Este esterilizador se ha diseñado y construido para reducir al mínimo la interferencia electromagnética con
otros dispositivos. Sin embargo, si detecta interferencias entre este producto y otros dispositivos:
• Retire el dispositivo que cause interferencias de la sala
• Enchufe el esterilizador en un circuito aislado
• Aumente la separación entre el esterilizador y el dispositivo que cause interferencias.
• Póngase en contacto con Midmark si las interferencias persisten
Símbolos de seguridad
ADVERTENCIA
Indica una situación potencialmente peligrosa que podría ocasionar lesiones graves.
Precaución
Indica una situación potencialmente peligrosa que puede ocasionar lesiones leves o
moderadas. También puede usarse para alertar contra prácticas peligrosas.
Advertencia sobre el equipo
Indica una situación potencialmente peligrosa que podría provocar daños en el equipo.
003-2915-99
Español - 4
© Midmark Corporation 2018

Símbolos del transporte
Precaución por daños
de transporte
Orientación correcta
para el transporte
Frágil
Mantener seco
Altura máx. apilamiento
(consultar número "n" en embalaje)
Condiciones de transporte/almacenamiento
Advertencia sobre el equipo
Debe drenarse el agua de los depósitos de la unidad antes de transportarla o almacenarla a
0 °C (+32°F).
Rango de temperatura ambiente: De -30°C a +60°C (de -22°F a 140°F)
Humedad relativa: De 10% a 90% (sin condensación)
Presión atmosférica: De 7,2 psia a 15,4 psia (de 49,6 kPa a 106,4 kPa)
Accesorios, herramientas y piezas de servicio
A menos que se indique lo contrario, los elementos se pueden usar en el M9 / M9D y M11.
Accesorios Número de pedido
Speed-Clean, 1 (0,47 litros [16 oz]) botella 002-0396-00
Speedclean, 1 caja (12 - 0,47 litros [16 oz]) botellas 002-0396-05
Impresora térmica 9A599001
Separador para caja (horizontal) 9A215001 (solo M11)
Bastidor de cartuchos (Vertical) 9A215002 (solo M11)
Piezas de servicio/herramientas comunes Número de pedido
Rollo de papel térmico de la impresora (recarga) 060-0016-00
Papel térmico adherente de la impresora (recarga) 060-0016-01
Juego de junta de puerta y dique (solo M11) 002-0504-00
Juego de junta de puerta y dique (solo M9 / M9D) 002-0361-01
Juego de separador de bolsas (solo M11) 002-2108-00
Juego de separador de bolsas (solo M9 / M9D) 002-2108-01
Herramienta para bandejas/cajas 9A307001
VistaCool ™ sistema de reducción térmica directo al drenaje 9A586002 (Dual)
003-2915-99
Español - 5
© Midmark Corporation 2018

Incluido con el esterilizador
Speed-Clean
Cable de alimentación
Instrucciones de cuidado y
funcionamiento
Hoja SDS de Speed-Clean
2 bandejas
pequeñas
Separador de
(Se muestra
el separador de M11)
003-2915-99
bolsas
Español - 6
2 bandejas
grandes
© Midmark Corporation 2018

Ubicación de componentes
Pantalla / panel táctil
Palanca de
comprobación
de la válvula de
despresurización
Apertura de llenado
Junta de la puerta
Junta de contención
Impresora térmica
(opcional)
Bastidor de
bandejas
Bandeja(s)
Indicador de nivel de
agua / tubo de drenaje
del depósito
Portabandejas
003-2915-99
Español - 7
© Midmark Corporation 2018

Controles e indicadores
Los controles y los indicadores del esterilizador se muestran en la presente página y en la siguiente. En las
tablas se describe la función de cada control e indicador.
Control Función
Pantalla Vea la
ilustración
Botón Unwrapped
(Sin envolver)
Botón Pouches
(Bolsas)
Botón Packs
(Paquetes)
Piezas manuales
botón
Botón Start (Inicio)
Botón Stop
(Detener)
Indica el ciclo seleccionado, la temperatura y el tiempo de exposición
del ciclo seleccionado. Durante el ciclo, la pantalla muestra mensajes
que describen el estado del ciclo. Cuando el ciclo entra en el modo
de esterilización, se muestra el tiempo de ciclo restante así como la
temperatura y la presión. La pantalla también muestra un mensaje de
error en caso de mal funcionamiento. Consulte la sección Resolución de
problemas de este manual para ver una explicación detallada sobre los
Mensajes informativos/de error.
Un ciclo de programas diseñado para procesar instrumentos sin
envolver a: 132°C (270°F) durante 3:00 minutos / ciclo de secado de
30 minutos.
Un ciclo de programas diseñado para procesar los instrumentos en
bolsas de esterilización papel/plástico o instrumentos envueltos a:
132°C (270°F) durante 4 minutos / ciclo de secado de 30 minutos.
Un ciclo de programas diseñado para procesar paquetes de
instrumentos o textiles a: 121°C (250°F) durante 30 minutos / ciclo de
secado de 30 minutos.
Un ciclo de programas para piezas manuales odontológicas que se
ejecuta a: 132°C (270°F) durante 4 minutos / ciclo de secado de
30 minutos.
Inicia el programa seleccionado. Cuando se muestra SELECT CYCLE
(SELECCIONAR CICLO), si se pulsa Start (Inicio), se activa el
calentador durante 10 minutos.
Termina el programa o la función seleccionados.
1 ó 2 botones
NOTA: el usuario
debe asegurarse de
que los materiales
son aptos para
cada ciclo de
esterilización.
003-2915-99
Botones de ciclos programables que permiten al operador crear dos
ciclos programables diferentes para aplicaciones especiales. El tiempo
y la temperatura de esterilización pueden ajustarse o modicarse junto
con el tiempo de secado y el procedimiento de ventilación.
Precaución
Estos ciclos no están aprobados por la FDA y la validación
de la esterilización de los elementos procesados que los
usan es responsabilidad del usuario.
Español - 8
© Midmark Corporation 2018

Controles e indicadores (continuación)
Apertura de llenado
Indicador de nivel
de agua y tubo de
drenaje del depósito
Impresora térmica
Manija de la puerta
(opcional)
Palanca de
comprobación
de la válvula de
despresurización
Control Función
Botón P Botón de modo de programación que permite
regular la temperatura, el horario, el tiempo de
secado o el procedimiento de ventilación. Se usa
junto con los botones 1 o 2.
(Consulte Modo de programación).
Botón + (más)
Botón - (menos)
Manija de la puerta ver ilustración Cerrar y abrir la puerta.
Indicador de nivel de agua /
Tubo de drenaje del depósito
Apertura de llenado ver ilustración Acceso para llenar el depósito de agua.
Palanca de comprobación de la
válvula de despresurización
Impresora térmica (opcional) ver ilustración La impresora (equipo opcional) proporciona un
003-2915-99
ver ilustración
ver ilustración Permite comprobar la válvula de despresurización.
Español - 9
Permite incrementar o modicar la temperatura,
el tiempo, el modo de ventilación o el tiempo de
secado cuando se activa en 1 o 2 y el modo P
(programación) está activado.
Permite disminuir o modicar la temperatura, el
tiempo, el modo de ventilación o el tiempo de
secado cuando se activa en 1 o 2 y el modo P
(programación) está activado.
Muestra el nivel de agua del depósito. El tubo
también se utiliza para el drenaje del depósito
hacia un recipiente apropiado.
registro permanente del tiempo, la temperatura y la
presión durante un ciclo.
© Midmark Corporation 2018

Directrices para supervisar la esterilización
Nota
La siguiente información se proporciona solo a modo de referencia. Póngase en contacto con los
organismos estatales o locales adecuados si necesita unas directrices de esterilización específicas
para su oficina. Hay información adicional sobre el control de las infecciones en los Centros para el
Control y la Prevención de Enfermedades (CDC), la Organización de Seguridad y Procedimientos
de Asepsia (OSAP), la Asociación para el Avance del Instrumental Médico (AAMI) y la Asociación
de Profesionales en Control de Infecciones y Epidemiología (APIC).
Monitores físicos
Los dispositivos de medición de temperatura y presión pueden ayudar a detectar problemas del
esterilizador. El sistema de control del esterilizador aborta el ciclo y muestra un mensaje si las condiciones
físicas se salen de los límites establecidos. La impresora térmica opcional puede utilizarse para crear un
registro del tiempo, la temperatura y la presión del ciclo real de cada carga.
Nota
Utilice solo indicadores químicos y biológicos aprobados por la FDA y diseñados para la
esterilización con vapor que sean compatibles con la temperatura particular del ciclo de
esterilización y el tiempo de exposición que se monitoriza. Use los monitores de esterilización con
cada carga de esterilización. Si un ciclo de esterilización termina de manera prematura, vuelva
a procesar los instrumentos para asegurar la esterilidad de la carga. Siga las instrucciones del
fabricante para desechar de forma adecuada los indicadores usados.
Indicadores químicos
Los indicadores químicos están diseñados para vericar que las condiciones de la cámara de esterilización
eran adecuadas para lograr la esterilización. No validan que un artículo procesado es estéril. Si un indicador
químico indica un fallo, los artículos de esa carga se consideran no estériles. Los fallos de esterilización
pueden deberse a las siguientes causas: limpieza, embalaje o carga incorrectos, o fallo en el esterilizador.
Determine la causa del fallo de la esterilización y soluciónelo antes de ejecutar el siguiente ciclo. Solo
deben utilizarse indicadores químicos aprobados por la FDA y etiquetados para el uso con parámetros del
ciclo de esterilización con vapor (por ejemplo, temperatura y tiempo de exposición) de los esterilizadores
M9 / M9D / M11 para monitorizar los ciclos. Siga las instrucciones del indicador químico para almacenarlo,
utilizarlo, interpretarlo y desecharlo de forma adecuada.
Indicadores biológicos
Los indicadores biológicos son dispositivos microbiológicos, diseñados para acompañar a los artículos que
se esterilizan y controlar que el proceso de esterilización es adecuado. Si un indicador biológico indica un
fallo, los artículos de esa carga se consideran no estériles. Los fallos de esterilización pueden deberse a las
siguientes causas: limpieza, embalaje o carga incorrectos, o fallo en el esterilizador. Determine la causa del
fallo de la esterilización y soluciónelo antes de ejecutar el siguiente ciclo. Solo deben utilizarse indicadores
biológico aprobados por la FDA y etiquetados para el uso con parámetros de ciclo de esterilización con
vapor (por ejemplo, temperatura y tiempo de exposición) de los esterilizadores M9 / M9D / M11 para
monitorizar los ciclos. Siga las instrucciones de los indicadores biológicos para almacenarlos, utilizarlos,
interpretarlos y desecharlos de forma adecuada.
003-2915-99
Español - 10
© Midmark Corporation 2018

Instalación
Entorno de funcionamiento
Rango de temperatura ambiente: +20°C a +40°C (68°F a 104ºF)
Humedad relativa: < de 80 % (sin condensación)
(grado de polución 2, según IEC664)
Altitud normal de funcionamiento: < 3000 m (9842 ft) sobre el nivel del mar
Dispositivo apto SOLO PARA USO EN INTERIORES.
El entorno en que se utilice el dispositivo debe estar relativamente limpio de polvo.
(Grado de polución 2, conforme a la IEC664)
El dispositivo debe conectarse a una fuente alimentación con límites de sobretensión inferiores a
1500 W de la red eléctrica a tierra. (Categoría de instalación II conforme a IEC664)
El M9 / M9D y el M11 emiten 1,47 kW (5000 BTU/HR) durante su funcionamiento.
Requisitos para el emplazamiento
Requirements
003-2915-99
Deje espacio libre
a ambos lados
Clearance
5"
(13 cm)
2"
(5 cm)
Support Surface
Español - 11
1"
(3 cm)
5"
(13 cm)
Overhang/Shelf
M9/M9D
15"
(38 cm)
M11
18"
(46 cm)
(5 cm)
M9/M9D
18"
M11
(46 cm)
21"
(53 cm)
© Midmark Corporation 2018
2"
M11
23" (58 cm)
M9/M9D
22" (56)

Requisitos del emplazamiento -(continuación)
Supercie de soporte
• El material debe ser resistente al agua. (P. ej. laminados, acero inoxidable, piedra, etc).
• La supercie debe estar nivelada para garantizar que la cámara se llena con el nivel de
agua correcto.
Un nivel de agua inadecuado en la cámara podría causar un error de funcionamiento en el
esterilizador.
• La supercie debe tener las dimensiones mínimas que se indican a continuación:
Dimensiones
Profundidad (de adelante hacia atrás) M 11 - 53 cm (21”) M9 / M9D - 46 cm (18”)
Requisitos de distancia
Para garantizar una circulación de aire adecuada y permitir el acceso al puerto de llenado del de-
pósito y al acoplamiento de drenaje, cumpla con los requisitos de espacio mínimo que se detallan
a continuación.Si el esterilizador se va hacer funcionar en ciclos continuos, sitúe el esterilizador
donde el vapor no dañe los materiales o equipos del área que lo rodea.
Parte posterior de la unidad - Pared posterior ................ 5 cm (2”)
Supercie de soporte delantera - Esterilizador frontal ... 3 cm (1”)
Laterales de la unidad - Pared lateral ............................. 5 cm (2”)
Distancia sobre la Unidad para acceso a la impresora .. 13 cm (5”)
Proyección máxima del estante superior del gabinete ... M11 - 46 cm (18”) M9 / M9D - 38 cm (15”)
Debajo del gabinete o estante ........................................ M11 - 58 cm (23”) M9 / M9D - 56 cm (22”)
Requisitos de reubicación para el esterilizador
Desconecte el cable de alimentación de la toma eléctrica y permita que el esterilizador se enfríe.
Drene el agua del depósito o no incline el esterilizador para permitir que el agua se expulse.
Requisitos eléctricos
ADVERTENCIA
Para modelos de 115 V AC: use solo corriente alterna 104 - 127 V AC, 50/60 Hz.
Para modelos de 230 V AC: use solo corriente alterna 207 - 127 V AC, 50/60 Hz.
De lo contrario, el personal puede recibir sacudidas eléctricas y el esterilizador puede sufrir daños.
Nota
Por razones de seguridad, la unidad debe estar conectada a una toma con la polaridad correcta y con
conexión a tierra. Use siempre un cable de alimentación con conexiones a tierra que coincida con las tomas
de sus instalaciones.
Unidad de 115 V AC: 115 V AC, 50/60 Hz, 12 A
Circuito de alimentación exclusivo*: 120 V AC, 50/60 Hz, 15 A
Consumo de energía máx.: 1425 W
Unidad de 230 V AC: 230 VAC, 50/60 Hz, 6,4 amp
Circuito de alimentación exclusivo*: 230 V AC, 50/60 Hz, 10 A
Consumo de energía máx.: 1500 W
* La fuente de alimentación debe tener límites de sobretensión inferiores a 1500 w de la red eléctrica a tierra.
(Categoría de instalación II conforme a IEC664)
003-2915-99
Español - 12
© Midmark Corporation 2018

Conexión del cable de alimentación
ADVERTENCIA
El equipo no debe utilizarse en presencia de mezclas anestésicas inflamables con
oxígeno, aire u óxido nitroso.
Aclaración: El equipo puede utilizarse en presencia de oxígeno, aire u óxido nitroso.
ADVERTENCIA
Compruebe el número de serie en el panel trasero del esterilizador para verificar la
capacidad de voltaje de la unidad. Si no conecta el esterilizador a una fuente de alimentación
adecuada, podría provocar daños a la unidad y sacudidas eléctricas al personal.
Advertencia sobre el equipo
Para garantizar un rendimiento óptimo, deje que el esterilizador alcance la temperatura ambiente
antes de ponerlo en marcha.
Para conectar el cable de alimentación...
A) Enchufe el cable de alimentación a la toma en la parte trasera del esterilizador.
B) Enchufe el cable de alimentación a una toma con la polaridad correcta y con conexión a
tierra para una asignación mínima de 15 A. Se recomienda el uso de un circuito dedicado
exclusivamente al esterilizador.
C) M9 / M9D y M11 no disponen de un interruptor de encendido/apagado; la pantalla funciona a
muy baja potencia. (ejemplo: pantalla del horno microondas)
Nota: Cuando la energía está desconectada, los mensajes que se muestran a continuación aparecerán en la pantalla.
Pantalla:
Capacidad de
voltaje
*
*
* Estas pantallas mostrará el número total de ciclos que se ejecutaron en la unidad, el número de modelo
(M9 / M9D o M11), el número de la versión del software y la fecha y la hora del número de serie.
003-2915-99
Español - 13
© Midmark Corporation 2018

Conguración del usuario
El modo de configuración del usuario permitirá al usuario establecer la unidad de medida deseada
y ajustar el reloj de tiempo. Para ingresar al modo de configuración del usuario, desenchufe
y vuelva a conectar el cable de alimentación mientras mantiene presionado el botón hasta
que aparezca la pantalla Configuración de usuario a continuación..
Acción Descripción Pantalla
Pulse Presione iniciar para inicializar los menús
de Conguración de usuario.
Ajuste las Unidades
de Medida
Pulse
Ajuste reloj de
tiempo
Pulse
NOTA: La conguración del usuario para la fecha y la hora debe actualizarse manualmente para el ahorro
003-2915-99
Los botones "+" y "-" alternan la
conguración entre el idioma o el sistema
de medida
Cuando las unidades de medida deseadas
aparecen en la pantalla...
Almacena las unidades de medida
deseadas Esto hace aparecer la pantalla de
programación del reloj
Los botones "+" y "-" ajustarán los valores
de las siguientes conguraciones
- Año / Mes / Día / Hora / AM o PM / Minuto.
Cuando los valores deseados aparecen en
la pantalla...
Almacena la conguración y muestra la
siguiente conguración en la lista. Repita
según sea necesario para todas las
conguraciones. Cuando se completen
todas las conguraciones, presionar la "P"
iniciará el encendido normal.
de luz diurna.
Español - 14
© Midmark Corporation 2018

Funcionamiento
Antes de utilizar el esterilizador
ADVERTENCIA
No utilice este esterilizador para esterilizar sustancias volátiles ni para otro propósito
que no sea aquel para el cual fue diseñado. Pueden producirse quemaduras y
condiciones tóxicas o explosivas.
Nunca fuerce la manija de la puerta. La presión de la cámara puede hacer que la puerta se abra
con una fuerza extrema. Si la manija de la puerta no se mueve libremente, deje que la unidad
se refresque y se despresurice durante 40 minutos antes de abrir la puerta. Si no lo hace puede
provocar lesiones personales graves.
No ejecute el esterilizador sin el portabandejas en su lugar. En caso de mal funcionamiento del
esterilizador, desenchúfelo inmediatamente y llame al servicio técnico; no intente reparar usted
mismo el esterilizador. Si lo hace, puede provocar lesiones graves.
Precaución
Los ciclos programables 1 y 2 se proporcionan para las aplicaciones que requieren parámetros
de esterilización diferentes a los de los ciclos predefinidos. Estos ciclos no han sido aprobados
por la FDA para su uso médico. El usuario debe asegurarse de que los materiales procesados son aptos
para estos ciclos y confirmar así la esterilidad de la carga procesada.
Advertencia sobre el equipo
Para un óptimo rendimiento, deje que el esterilizador alcance la temperatura ambiente antes de
ponerlo en marcha.
Llenado del depósito
Para llenar el depósito...
A) Abra la puerta de la unidad.
B) Vierta el agua destilada dentro de la apertura
de llenado hasta que su nivel llegue a la parte
superior de la etiqueta de nivel de llenado en
el indicador del nivel de agua.
Advertencia sobre el equipo
Utilice agua destilada o agua que cumpla
con las especificaciones de pureza de agua
indicadas. Si no respeta las indicaciones,
el esterilizador podría no funcionar
correctamente o podría fallar de forma
prematura por exceso de corrosión.
003-2915-99
Español - 15
© Midmark Corporation 2018

Pruebas de calicación
Debe someter a prueba su esterilizador cada vez que se instale, sufra una avería, cambie de ubicación o se
someta a una reparación importante, así como después de un proceso de esterilización fallido. Las pruebas
de calicación deben realizarse antes de poner el esterilizador en funcionamiento. Si se utilizan múltiples
tipos de ciclo, por ejemplo, "Pouches" (Bolsas) y "Packs" (Paquetes), cada tipo de ciclo debe calicarse.
Las pruebas de calicación deben incluir como mínimo un indicador biológico (BI) (también conocido como
prueba de esporas) y un indicador químico (CI). El paquete de prueba se debe colocar en la bandeja
inferior cerca de la puerta de la cámara y se debe realizar con elementos procesados rutinariamente que se
consideren los más difíciles de esterilizar. En la cámara, deben colocarse más artículos junto al indicador
biológico y el químico para que esta se encuentre cargada por completo (no exceda las capacidades
máximas que se enumeran en las tablas en "Indicaciones para la carga" del presente manual). El
esterilizador se ha instalado correctamente (o reinstalado después de una reubicación), o ha sido reparado
según las especicaciones de los fabricantes, y funcionará de forma efectiva cuando, después de tres
pruebas consecutivas, para cada tipo de ciclo probado se obtienen resultados negativos de los BI y lecturas
adecuadas de todos los monitores físicos y CI, que demuestran la esterilización completa. Todos los
artículos procesados durante las pruebas de calicación deben mantenerse en cuarentena hasta que estén
disponibles los resultados de las tres pruebas biológicas.
Instrucciones de carga
Todos los artículos deben procesarse según las pautas "Guidelines for Infection Control in Dental
Healthcare Settings" (Directrices para el control de infecciones en los centros sanitarios dentales) - 2003,
MMWR; 52 (n.º RR-17), y "Guidelines for Disinfection and Sterilization in Healthcare Facilities" (Directrices
para la desinfección y la esterilización en centros sanitarios) - 2008, de los Centros para el Control y la
Prevención de Enfermedades (CDC), que dicen lo siguiente:
"Los artículos que se van a esterilizar deben colocarse de manera que permitan la libre circulación
del agente esterilizante (por ejemplo, vapor, vapor químico o calor seco); se deben respetar las
instrucciones del fabricante al cargar el esterilizador".
Advertencia sobre el equipo
Las bandejas deben contener en todo momento una carga (a menos que
se utilicen los separadores para cajas opcionales); de lo contrario, podrían
ocasionarse daños graves en el instrumento o en el equipo.
Tipos de artículos que se pueden procesar en el M9 / M9D y el M11
Antes de colocar cualquier instrumento en el M9 / M9D o el M11, compruebe las especicaciones del
fabricante para asegurarse de que los materiales son compatibles con la esterilización por vapor y para
vericar la aceptabilidad de los parámetros de esterilización. El M9 / M9D y M11 están diseñados para
esterilizar los siguientes artículos:
• Instrumentos de metal
• Dispositivos de goma / plástico (por ejemplo, cánulas de succión, bandejas de impresión, etc.)
• Materiales de envoltura y empaquetado (p. ej. Envoltura CSR, bolsas de instrumentos, etc.)
• Cajas (que caben en las bandejas del esterilizador o los accesorios de la bandeja de la caja)
• Piezas manuales de alta/baja velocidad
• Instrumentos quirúrgicos (p. ej., instrumentos oftalmológicos)
003-2915-99
Español - 16
© Midmark Corporation 2018

Tipos de artículos que se pueden procesar en el M9 / M9D y el M11- (continuación)
Advertencia sobre el equipo
No esterilice artículos formados por cualquiera de los siguientes materiales en el M9 /M9D o M11.
• Metales sensibles a la corrosión (p.ej., acero al carbono, hierro, etc.)
• Artículos frágiles que puedan romperse si se exponen a altas temperaturas o alta presión
• Desechos biomédicos
• Plásticos que puedan romperse o generar residuos cuando se exponen al vapor o altas
temperaturas.
Ejemplos
Polietileno, estireno, celulosa, ABS, PVC, acrílicos (Plexiglass™), PPO (Noryl™), látex y neopre-
no
Preparación de los artículos para la esterilización
ADVERTENCIA
Limpie y seque los instrumentos a fondo antes de colocarlos en la bandeja. La limpieza
inadecuada puede no esterilizar los instrumentos o dañar la unidad. Siga las indicaciones del
fabricante del instrumento y las recomendaciones de CDC para manipular y limpiar instrumentos antes
de la esterilización.
Los instrumentos se deberán lavar a fondo para eliminar todas las partículas residuales como restos, residuos
generados por los desinfectantes, sangre, tejidos orgánicos, etc. A continuación, se enumeran las directrices
generales sobre limpieza, pero deberán respetarse en todo momento las instrucciones del fabricante del
dispositivo para realizar una limpieza adecuada y preparar el dispositivo para su esterilización.
• Limpie los instrumentos inmediatamente después de haberlos utilizado para evitar que se sequen
• Se recomienda utilizar un equipo de limpieza automático (p. ej., limpiadores por ultrasonidos o
• Después de haber limpiado los instrumentos, aclárelos cuidadosamente con agua corriente
• En caso de que en las instrucciones del fabricante del instrumento se especique que es
las partículas residuales.
lavadoras desinfectadoras) en lugar de realizar una limpieza manual para mayor seguridad del
facultativo y para que la limpieza resulte más ecaz.
para eliminar todos los restos que se hayan desprendido o los líquidos de limpieza residuales.
La pureza del agua corriente puede variar considerablemente de un sitio a otro, por lo que se
recomienda realizar un aclarado nal con agua que posea una calidad adecuada para que no
se manche el instrumento. Después de haber aclarado los instrumentos, deberá comprobarse
si estos presentan algún daño, restos o residuos de detergentes y, a continuación, se deberán
secar antes de empaquetarlos.
necesario lubricar los instrumentos después de haberlos limpiado, retire el exceso de lubricante
antes de envolverlos para su esterilización o de cargarlos en el esterilizador.
Esterilización para el uso inmediato
El M9 /M9D y el M11 pueden esterilizar para el uso inmediato, es decir, esterilizan instrumentos sin envolver
para utilizarlos inmediatamente. Antes de introducir artículos sin envolver en la bandeja, coloque un paño de
campo de algodón, un revestimiento de papel o una envoltura CSR doblada según las dimensiones del fondo
de la bandeja. Coloque los artículos sin envolver en el paño de forma que no se toquen entre sí (véase la
Imagen 1).
003-2915-99
Español - 17
© Midmark Corporation 2018

Esterilización para un uso inmediato (continuación)
Imagen 1
Tenga en cuenta los siguientes puntos a la hora de elegir si esterilizar o no sus instrumentos sin envolver:
• La esterilidad de los instrumentos no envueltos se ve comprometida al exponerlos a un ambiente
no estéril. Siga las indicaciones del CDC para usar instrumentos sin envolver y esterilizados.
• Dada la naturaleza sensible de algunos tipos de cirugía (que incluye, entre otras, la oftalmológica),
los instrumentos utilizados en esos procedimientos deben estar envueltos o embolsados a n
de reducir su exposición a los residuos del proceso de esterilización. Cuando se procesan los
instrumentos para estos procedimientos de forma rutinaria, el depósito de agua también debe
vaciarse y volverse a llenar a diario con agua fresca destilada.
ADVERTENCIA
No sobrecargue la cámara. Es necesario dejar el espacio adecuado alrededor de
los artículos de las bandejas para la circulación del vapor y el secado. Si no se deja
el espacio adecuado, la esterilización y el secado se verán comprometidos. Los artículos y
sus paquetes deben encontrarse totalmente secos cuando se saquen del esterilizador para
reducir al mínimo las posibilidades de que se vuelvan a contaminar.
Directrices generales
• Utilice solo bandejas M9 / M9D y M11 en su esterilizador apropiado. Si se usan otras bandejas,
se podría limitar el ujo de aire / vapor que se dirige a los artículos y provocar una esterilización y
un secado incorrectos.
• Todos los artículos deben caber dentro de la bandeja y no sobrepasar el borde de la bandeja del
esterilizador. Los instrumentos no deben raspar las paredes de la cámara al introducir la bandeja
en el interior de la cámara.
• Los artículos plegables deberán esterilizarse en una posición abierta para que todas las
003-2915-99
supercies queden expuestas al vapor (véase la Imagen 2).
Español - 18
© Midmark Corporation 2018

Directrices generales - (continuación)
Imagen 2
• Las piezas manuales y los instrumentos se deberán colocar en las bandejas formando una sola
capa (ni apilados ni amontonados) a n de lograr una circulación del vapor y un secado adecuados.
• Los materiales de vidrio o los utensilios que puedan albergar agua como las botellas, los
cuencos, los vasos de precipitados, etc., deberán colocarse en la bandeja con el lado abierto
hacia abajo para que el condensado de agua caiga del recipiente (véase la Imagen 3). Al
esterilizar materiales de vidrio, compruebe las especicaciones del fabricante para asegurarse
de que estos son compatibles con la esterilización por vapor.
Imagen 3
• Aclare los tubos con agua destilada o con agua que cumpla con las especicaciones de pureza
mencionadas y no los seque antes de esterilizarlos. Coloque los tubos sobre la bandeja de forma
que no presenten grandes dobleces y que sus extremos estén abiertos y sin obstrucciones (véase
la Imagen 4).
003-2915-99
Imagen 4
Español - 19
© Midmark Corporation 2018

Directrices generales - (continuación)
• Siga las instrucciones del fabricante del dispositivo para desmontar los instrumentos que estén
compuestos por múltiples piezas antes de envasarlos o de esterilizarlos para asegurarse de que
todas las piezas queden expuestas correctamente al vapor.
• En caso de que los artículos se vayan a esterilizar y almacenar para utilizarlos más adelante,
deberán envasarse (p. ej., embolsados, envueltos, etc.) y secarse por completo cuando se
saquen de la cámara del esterilizador para evitar la posibilidad de que se vuelvan a contaminar.
• El operador puede tener que aumentar el tiempo de secado predeterminado en función de las
variaciones en la conguración de la carga, el tamaño, los materiales del envoltorio y el entorno
para garantizar que todos los envoltorios e instrumentos se sequen por completo. Para obtener
más instrucciones sobre cómo ajustar el tiempo de secado, véase el apartado "Funcionamiento
del ciclo" del presente manual.
• Cuando se esterilice una carga que contenga una o más piezas manuales, utilice el ciclo
Handpieces (Piezas manuales) y no los ciclos Pouches (Bolsas) o Unwrapped (Sin envolver).
Embolsado y envoltura de artículos
El M9 / M9D y el M11 pueden esterilizar artículos envueltos o en bolsas que preservan su esterilidad tras el
procesamiento.
• Para embolsar o envolver artículos, utilice únicamente bolsas y envoltorios de esterilización que
hayan sido aprobados por la FDA y que estén indicados para utilizar con el ciclo de esterilización
por vapor que se utilice. Siga las instrucciones de uso del fabricante.
• No se deben mezclar en la misma bolsa ni envolverse en el mismo paquete instrumentos
fabricados a partir de materiales diferentes (acero inoxidable, acero al carbono, plástico, etc.)
para evitar la posibilidad de que se dañen los instrumentos.
• Las bolsas, los envoltorios y los instrumentos incluidos no deberán tocar la pared de la cámara
para que el vapor pueda circular de forma adecuada y evitar posibles daños en el instrumento.
• Para lograr una circulación del vapor y un secado apropiados, la mejor posición de colocación de
las bolsas es apoyadas sobre su borde, para lo cual se utiliza el separador de bolsas opcional
de Midmark que se suministra con el esterilizador M9 / M9D / M11. Si se necesitan bastidores de
bolsas adicionales, solicite el juego P.N. 002-2108-00 (M11- 6 ranuras) o 002-2108-01 (M9 / M9D
- 5 ranuras) Si se usan más de dos (2) bastidores de bolsa en una sola carga, se puede necesitar
un tiempo de secado adicional.
Bastidor de
bolsas de M11
002-2108-00
Bastidor de bolsas
de M9 / M9D
002-2108-01
• Para utilizar el separador de bolsas, es necesario quitar algunas de las bandejas del esterilizador.
Cuando se use el separador de bolsas, cargue una sola bolsa en cada hueco del separador. Cuando
se utilicen bolsas de esterilización de papel y plástico, no deberán colocarse en el separador de
forma que la cara de plástico de una bolsa quede en frente a la de papel de la bolsa contigua.
• Las bolsas cargadas directamente en las bandejas se secarán mejor si se cargan con la cara de
plástico hacia arriba.
• No apile las bolsas en las bandejas. Las bolsas deben estar envueltas sin tensión con las piezas
manuales y los instrumentos cargados en una sola altura (ni apilados ni amontonados) para
003-2915-99
permitir que el vapor uya y penetre en los artículos correctamente (véase la Imagen 2).
Español - 20
© Midmark Corporation 2018

Embolsado y envoltura de artículos (continuación)
• Si se utiliza un rotulador para etiquetar las bolsas o los paquetes envueltos, su tinta no debe
ser tóxica. En las bolsas de papel y plástico, escriba únicamente por la cara de plástico de la bolsa. En
los paquetes envueltos, escriba la información de etiquetado en la cinta indicadora o añada una etiqueta
diferente.
A continuación, se muestran algunas de las conguraciones recomendadas para cargar las bolsas
en el M9 / M9D (imágenes 5 y 6) y el M11 (imágenes 7 y 8).
M9 / M9D: Bolsas con separador de bolsas
Imagen 5
M11: Bolsas con separador de bolsas
Imagen 7
M9 / M9D: Bolsas mezcladas
Imagen 6
M11: Bolsas mezcladas
Imagen 8
• Cuando use cajas en el M9 / M9D / M11, siga las instrucciones de uso del fabricante.
• No apriete demasiado los artículos al envolverlos. La esterilización podría verse afectada si
un artículo está excesivamente envuelto, y es más probable que la envoltura se rompa si se
aprieta demasiado.
• Se pueden cargar cajas en las bandejas, pero deben caber dentro de los límites de estas y no
se deben tocar entre sí, ni a la bandeja superior ni a la pared de la cámara a n de que el vapor
pueda circular correctamente. La carga total del instrumento, incluidas las cajas, no deberá
sobrepasar los límites mencionados en las tablas de capacidad máxima.
• Al usar los bastidores de cajas horizontales y verticales opcionales (9A215001 y 9A215002) en
el M11, la carga máxima, incluidas las cajas, puede aumentarse a 5,44 kg (12 lb). Se puede
necesitar más tiempo de secado.
003-2915-99
Español - 21
© Midmark Corporation 2018

Embolsado y envoltura de artículos (continuación)
A continuación, se muestran algunas de las conguraciones recomendadas para cargar las cajas en el
M9 / M9D (imágenes 9 y 10) y el M11 (imágenes 11, 12, 13 y 14).
M9 / M9D: Caja grande
Imagen 9
M11: Carga mixta con caja pequeña y bolsas
Imagen 11
M9 / M9D: Carga mixta con caja pequeña y bolsas
Imagen 10
M11: Con separador de cajas vertical
Imagen 12
003-2915-99
M11: Con separador de cajas horizontal
Imagen 13
Español - 22
M11 con separador de cajas horizontal y bandeja superior
Imagen 14
© Midmark Corporation 2018

Cargas de productos textiles
• En el ciclo Packs (Paquetes) se pueden procesar los productos textiles limpios que se recomienda
esterilizar con vapor. Compruebe si los parámetros de esterilización del ciclo Packs (Paquetes) son
aceptables para las especicaciones del fabricante del producto textil a n de garantizar su compatibilidad.
• Los paquetes textiles no deben poseer un grosor superior al de los límites especicados en las tablas
de capacidades máximas que se recogen a continuación y deberán caber dentro de los bordes de las
bandejas del esterilizador.
• Si se colocan varios paquetes en una sola bandeja, deberá mantenerse una separación mínima de
1/4” (6,4 mm) entre ellos para lograr una circulación del vapor y un secado apropiados. (Imagen 15)
Advertencia sobre el equipo
No use toallas o embalajes que puedan contener residuos de
lejía.No respetar estas indicaciones puede causar oxidación
o decoloración en la cámara o las bandejas, y reducir
significativamente la vida útil del esterilizador.
Tamaño de la carga
Carga de un producto textil
Imagen 15
Una esterilización correcta depende de que se realice una carga adecuada de los artículos en el
esterilizador. No sobrecargue la cámara del esterilizador. Deberá mantenerse una separación adecuada
alrededor de todos los artículos que se coloquen en la cámara para garantizar una circulación del vapor
correcta y un secado adecuado. Las siguientes tablas sobre las cargas máximas que se pueden procesar
en los esterilizadores se incluyen como referencia; sin embargo, a la hora de determinar la carga máxima
que se puede procesar, debemos tener en cuenta que es necesario mantener una separación adecuada
entre todos los artículos procesados para garantizar una buena circulación del vapor y el secado.
Capacidad máxima del M9 / M9D **
Tipo de carga Bandeja grande Bandeja pequeña Total
Instrumentos sólidos 1089 gramos (2,4 lb) o 726 gramos (1,6 lb) o 3629 gramos (8,0 lb) o
Piezas manuales 2 piezas manuales con otros
Paquetes* 1475 cm
instrumentos
1089 gramos (2,4 lb) o
3
(90 in3 ≤ 1,25 in de grosor)
≤ 3,2 cm de grosor
2 piezas manuales con otros
instrumentos
726 gramos (1,6 lb) o
901 cm3 ≤ 1,9 cm de grosor
(55 in3 ≤ 0,75 in de grosor)
8 piezas manuales con otros
instrumentos
3629 gramos (8,0 lb) o
4752 cm
(290 in3)
3
Capacidad máxima del M11 **
Tipo de carga Bandeja grande Bandeja pequeña Total
Instrumentos sólidos 1225 gramos (2,7 lb) o 816 gramos (1,8 lb) o 4082 gramos (9,0 lb) o
Piezas de mano 2 piezas manuales con otros
Paquetes* 145 in
* Deje un mínimo de 6,4 mm (1/4") de espacio entre cada paquete y con respecto a la pared de la cámara.
** Es posible que sea necesario aumentar el tiempo de secado predeterminado debido a las variaciones en la conguración de la carga,
los materiales de envoltura y el entorno para secar completamente el contenido de la cámara a estas capacidades.
003-2915-99
instrumentos
1225 gramos (2,7 lb) o
3
≤ 1,5 in de grosor
(2376 cm3 ≤ 3,8 cm de grosor)
Español - 23
2 piezas manuales con otros
instrumentos
816 gramos (1,8 lb) o
108 in3 ≤ 1,5 in de grosor
(1770 cm3 ≤ 3,8 cm de grosor)
8 piezas manuales con otros
instrumentos
4082 gramos (9,0 lb) o
3
505 in
(8275 cm3)
© Midmark Corporation 2018

Parámetros de ciclo estándar
Tipo de
ciclo
Sin envoltura
Bolsas
Paquetes
Parámetros del ciclo
Temperatura
(Mín.)
132°C
(270°F)
132°C
(270°F)
121°C
(250°F)
132°C
(270°F)
Tiempo Presione
(Ref.)
3 min 186 kPa
(27,1 psi)
4 min 186 kPa
(27,1 psi)
30 min 104 kPa
(15 psi)
4 min 186 kPa
(27,1 psi)
Artículos para esterilizar
Secado
1
Tiempo
2
(Consulte siempre las
recomendaciones de esterilización
del fabricante del artículo).
30 min • Instrumentos sueltos en una
bandeja.
• Frascos de cristal o metal
abiertos.
• Tubos que no se utilicen en
intervenciones quirúrgicas.
(Longitud máx. - 101,5 cm (40”) y
diámetro interior mín. - 475 cm [0,187”])
• Artículos sueltos cuyos
fabricantes recomienden exponer
a 132°C (270°F) durante 3 minutos.
Nota: la esterilidad de artículos no
envueltos se verá comprometida al
exponerlos a un ambiente no estéril.
30 min • Instrumentos en bolsas o
envueltos de manera individual.
• Varias capas de instrumentos
separadas por tela.
• Bandejas con instrumentos
sueltos envueltas.
• Cajas envueltas.
• Artículos envueltos cuyos
fabricantes recomienden exponer
a 132°C (270°F) durante 4 minutos.
30 min • Paquetes textiles y quirúrgicos
envueltos para la esterilización.
• Los fabricantes de artículos
recomiendan exponer a 121°C
(250°F) durante 30 minutos,
excepto los líquidos.
30 min • Piezas manuales odontológicas
(envueltas o no envueltas)
Piezas
manuales
1. La presión que se muestra en la tabla sirve solo a modo de referencia. Esta es la presión ideal que tiene el
vapor saturado cuando alcanza la temperatura de esterilización. La presión que aparece en la pantalla del
esterilizador puede ser más elevada.
2. El tiempo de secado puede cambiarse de 0 a 60 minutos. Consulte Ejecución del ciclo.
003-2915-99
Español - 24
Nota: verique que los parámetros de
esterilización son aptos contrastando
las indicaciones del fabricante de la
pieza de mano.
© Midmark Corporation 2018

Ejecución del ciclo
Los siguientes pasos son una descripción detallada de la ejecución del ciclo:
Advertencia sobre el equipo
El esterilizador no funcionará a menos que la puerta esté cerrada y bloqueada correctamente.
Paso 1: Cierre y asegure la puerta.
A) Eleve la manilla de la puerta, luego cierre la puerta.
B) Al empujar la puerta, deslice la manilla de la puerta hacia abajo para bloquearla.
Botones de ciclo
(programables)
Botones de ciclo
(preprogramados)
Nota
Al comenzar o finalizar un ciclo, si pulsa el botón
Start (Inicio) cuando se muestra "SELECT CYCLE"
(SELECCIONAR CICLO), se activa el calentador
durante 10 minutos. En la pantalla parpadea
"ADDITIONAL HEAT" (CALOR ADICIONAL). Esto
permite precalentar la cámara antes de iniciar un
ciclo o añadir tiempo al modo de secado cuando un
ciclo finaliza. Si pulsa Stop (Detener), el tiempo de
calor adicional (ADDITIONAL HEAT) finaliza.
Advertencia sobre el equipo
El uso de un programa de esterilización incorrecto puede dar lugar a la no esterilización de
los artículos y dañar los instrumentos. Obtenga las instrucciones de esterilización específicas
del instrumento contactando a su fabricante.
Paso 2: Seleccione el ciclo deseado.
A) Pulse el botón del ciclo que desee en el panel indicador.
(Consulte la tabla de especificaciones de temperatura y tiempo en "Parámetros del ciclo").
003-2915-99
Español - 25
© Midmark Corporation 2018

Ejecución del ciclo (continuación)
Después de presionar el botón del ciclo, en la pantalla se muestran los parámetros de ese ciclo.
Nota
En unidades que utilizan el sistema métrico,°F se mostrará como ºC, y PSI se mostrará como kPa.
Al pulsar, permite modificar el tiempo DRY (secado) de 0 a 60 minutos en incrementos de 1
minuto en un ciclo preprogramado.
Al pulsar, se reduce el tiempo. Al pulsar, se incrementa el tiempo.
Si quiere que el nuevo Tiempo de secado se repita regularmente, presione "P" después de ajustar el tiempo de
secado. Esto almacenará la nueva hora como el tiempo de secado predeterminado para el ciclo seleccionado.
003-2915-99
ADVERTENCIA
El botón STOP (DETENER) puede pulsarse en cualquier momento
para detener o interrumpir un ciclo. Los objetos no se han esterilizado
correctamente si esto se produce antes del ciclo de secado. El esterilizador
volverá al modo SELECT CICLE (SELECCIONAR CICLO).
Paso 3: Presione el botón "Start" (Inicio) para iniciar el ciclo.
Oirá un "bip" durante dos segundos, esto indica que ha comenzado el ciclo.
Español - 26
© Midmark Corporation 2018
Start (Inicio)
Detener

Ejecución del ciclo (continuación)
Después de pulsar el botón "Start" (Inicio), en la pantalla se muestra la etapa/estado del ciclo. La siguiente
tabla ilustra los mensajes que se emiten en cada etapa del ciclo.
Etapa del ciclo Descripción Pantalla
Filling (Llenado) La cámara comienza a llenarse con agua.
Cuando el agua alcanza el nivel adecuado...
Heating
(Calentamiento)
Sterilizing
(Esterilización)
Ready to Vent
(Listo para
ventilación)
Fast Vent
(Ventilación rápida)
Door To Open
(Apertura de puerta)
(automático)
Solo corresponde
a los esterilizadores por
vapor M9/M11
003-2915-99
La pantalla cambia según cambian la
temperatura y la presión de la cámara.
(Las unidades métricas se pueden mostrar si
así se desea).
La esterilización comienza cuando se
alcanza la temperatura y presión correctas.
El tiempo restante del ciclo se cuenta
regresivamente mientras la temperatura y la
presión de la cámara se actualizan de forma
continua.
Cuando quedan 10 segundos para nalizar
el ciclo de esterilización, se muestra "READY
TO VENT" (LISTO PARA VENTILACIÓN).
Cuando se acaba el tiempo del modo de
esterilización, se abre la válvula de ventilación.
El vapor y el agua vuelven al depósito.
La pantalla cambia según cambian la
temperatura y la presión de la cámara.
Precaución
Manténgase alejado cuando vaya a abrir la puerta del M9/M11.
De lo contrario, pueden sufrirse quemaduras graves por el vapor liberado.
Se emite una señal audible que indica que la
puerta va a abrirse.
Cuando la presión en la cámara llega a cero,
la puerta se acciona para pasar a la posición
de apertura parcial (modo de secado).
La tabla continúa en la página siguiente...
Español - 27
© Midmark Corporation 2018

Ejecución del ciclo (continuación)
Etapa del ciclo Descripción Pantalla
Manual Door Open
(Apertura manual
de puerta)
Solo corresponde
al esterilizador por
vapor M9D
Drying (Secado) El tiempo del ciclo de secado se cuenta
Se emitirá una señal sonora cuando la
presión de las cámaras llegue a cero para
indicarle al operador que abra la puerta. La
puerta debe abrirse en la primera posición
de parada (modo de secado). La señal
sonora continuará repitiéndose cada minuto
hasta que la puerta se abra en la posición
DRY (SECADO) (parcialmente abierta), o
se pulse el botón STOP (DETENER) y se
cancele el ciclo de secado.
Precaución
La carga procesada puede seguir mojada si se cancela el ciclo de secado antes
de finalizar. Espere siempre el tiempo necesario a que la carga se seque en el
esterilizador para evitar que se contaminen.
regresivamente. Si lo desea, puede cancelar
el ciclo de secado pulsando el botón STOP
(DETENER).
Cuando el tiempo de secado llega a 0:00...
Cycle Complete
(Ciclo completo)
Se emite una señal audible durante
10 segundos.
Precaución
• La carga procesada y las superficies internas estarán calientes. Evite el contacto con las
superficies calientes. De lo contrario, pueden sufrirse quemaduras graves.
• No aplique presión hacia abajo a la puerta abierta del esterilizador mientras descarga la
cámara. De lo contrario, el esterilizador podría volcarse y provocar quemaduras graves o
lesiones en las bandejas o los instrumentos al deslizarse fuera de la cámara.
Paso 4: Retire la carga procesada de la cámara.
A) Consulte el "Uso de la bandeja opcional/herramienta de caja" más adelante en este manual.
B) Ahora, el esterilizador está listo para iniciar otro ciclo.
Procesamiento posterior a la esterilización
Después de nalizar la esterilización, los artículos deben procesarse según los estándares aceptados
y documentados, como son las pautas "Guidelines for Infection Control in Dental Healthcare Settings"
(Directrices para el control de infecciones en los centros sanitarios dentales) - 2003, MMWR;
52 (N.º RR -17), y "Guidelines for Disinfection and Sterilization in Healthcare Facilities" (Directrices para la
desinfección y la esterilización en centros sanitarios) - 2008, de los Centros para el Control y la Prevención
de Enfermedades (CDC), al igual que todos los requisitos locales relevantes.
El personal cualicado responsable del control de infecciones debe preparar un protocolo de manejo de
artículos esterilizados. Todo el personal encargado de la manipulación de instrumental estéril debe seguir
este protocolo.
003-2915-99
Español - 28
© Midmark Corporation 2018

Botones de ciclo programables
Los botones de ciclo sirven para aplicaciones personalizadas que no cubren los programas de
ciclo estándar.Estos ciclos no han sido aprobados por la FDA para su uso médico.Los ciclos programables
permiten al usuario ajustar los parámetros del ciclo para esterilizar los elementos que no se pueden
esterilizar en ninguno de los ciclos preprogramados normales. Después de guardar un programa
personalizado, se utiliza pulsando los botones . Utilice las instrucciones de la siguiente tabla para
ajustar los parámetros de tiempo y de temperatura para estos botones. (Si desea modificar los ajustes,
estos botones pueden volverse a programar cuando quiera).
NOTA:
Si pulsa el botón STOP (DETENER) durante este procedimiento, se cancelarán los cambios y se volverá
a los ajustes originales.
Acción Descripción Pantalla
Pulse el
botón deseado
Selección del botón que va a
programarse.
Nota:
Los ciclos programables se
pueden programar para los
ciclos mensuales de limpieza sin
tiempo de secado para agilizar el
mantenimiento mensual.
Pulse Esto muestra la pantalla de programación
Ajuste Sterilization
Temperature
(Temperatura de
esterilización)
003-2915-99
de la temperatura de esterilización.
Precaución
La temperatura de esterilización puede ajustarse entre un mínimo de 121°C (250°F)
y un máximo de 135°C (275°F). A grandes altitudes, las temperaturas de esterilización
superiores a 270 ºF (132 ºC) pueden provocar fugas en la válvula de descompresión.
Los botones "+" y "-" ajustan la
temperatura en incrementos de 1°.
Cuando la temperatura deseada aparece
en la pantalla...
Esto continúa en la página siguiente...
Español - 29
© Midmark Corporation 2018

Botones de ciclo programables (continuación)
NOTA:
Si pulsa el botón STOP (DETENER) durante este procedimiento, se cancelarán los cambios y se volverá
a los ajustes originales.
Acción Descripción Pantalla
Pulse Para guardar la temperatura. Esto muestra
la pantalla de programación del tiempo de
esterilización que muestra el valor actual.
Precaución
El tiempo de esterilización puede ajustarse entre un mínimo de 3 minutos y un
máximo de 45 minutos. Puesto que es importante coordinar adecuadamente
el tiempo de ciclo con la temperatura de esterilización, el tiempo mínimo
seleccionable vendrá determinado por la configuración de temperatura.
Tiempo de
esterilización
Pulse Para guardar el tiempo de esterilización.
Ajuste la velocidad de
ventilación
Los botones "+" y "-" ajustan el tiempo en
incrementos de 1 minuto.
Cuando el tiempo deseado se muestre en
la pantalla...
Esto muestra la pantalla de programación
de la velocidad de ventilación y el valor
actual.
En ventilación FAST (RÁPIDA), la válvula
se abre por completo y ventila la cámara.
En ventilación SLOW (LENTA), la válvula
se abre durante una fracción de segundo
(una vez por minuto) para ventilar la
cámara lentamente.
Si pulsa "+", se ajusta: FAST (RÁPIDA)
Si pulsa "-" se ajusta: SLOW (LENTO)
Cuando la conguración deseada
aparece en la pantalla...
003-2915-99
Pulse Para almacenar la conguración y
mostrar la pantalla de programación de
Dry Time (Tiempo de secado) y el valor
actual.
La tabla continúa en la página siguiente...
Español - 30
© Midmark Corporation 2018

Botones de ciclo programables (continuación)
NOTA:
Si pulsa el botón STOP (DETENER) durante este procedimiento, se cancelarán los cambios y se volverá
a los ajustes originales.
Acción Descripción Pantalla
Tiempo de secado Dry Time (Tiempo de secado) puede
ajustarse de 0 a 60 minutos.
Los botones "+" y "-" ajustan el tiempo en
incrementos de 1 minuto.
Cuando el tiempo deseado se muestre en
la pantalla...
Pulse Para almacenar la conguración y
completar el proceso de programación.
La pantalla mostrará los nuevos
parámetros de ciclo.
Nota
La configuración programada se retiene bajo el botón de número de programa
<1> o <2>. Aunque se corte la alimentación o se desenchufe la unidad, se
mantendrá la configuración.
Precaución
El usuario debe asegurarse de que los materiales procesados son aptos para
estos ciclos.
De lo contrario, la esterilización puede no realizarse correctamente.
Mantenimiento
Mensajes de mantenimiento
Para asegurar el funcionamiento adecuado y la máxima vida útil de esterilizador, siga minuciosamente
todas las recomendaciones para el mantenimiento periódico. El mantenimiento recomendado es fácil de
hacer y toma muy poco tiempo.
Una de las medidas MÁS importantes para prevenir problemas en el esterilizador es garantizar que SOLO
se usa agua destilada o agua que cumple con las especificaciones de pureza de agua indicadas (NO AGUA
DEL GRIFO). Dado que el esterilizador funciona con agua a altas temperaturas, cualquier mineral disuelto
en el agua formará depósitos minerales. Esto puede hacer que las válvulas no se abran o cierren de la
forma adecuada y también puede producir corrosión en la cámara y los tubos.
La pantalla mostrará recordatorios de mantenimiento a intervalos adecuados para asistir al operador. Estos
recordatorios desaparecen de la pantalla cuando se inicia un ciclo.
El usuario es responsable de establecer un procedimiento de mantenimiento periódico para asegurar el
correcto funcionamiento del equipo y la esterilización able de las cargas. Contacte con su distribuidor o
representante de servicios Midmark para planicar un programa de mantenimiento.
003-2915-99
Español - 31
© Midmark Corporation 2018

Mantenimiento diario
Advertencia sobre el equipo
Si el esterilizador suele utilizarse para procesar piezas manuales dentales que han sido
lubricadas o sumergidas en soluciones dentales, drene el agua del depósito a diario.
Rellene el depósito con agua destilada o con agua que cumpla con las especificaciones
de pureza de agua indicadas.
Lavado de supercies externas
A) Lave el exterior del esterilizador todos los días según el procedimiento de sus instalaciones
para las supercies de contacto clínico teniendo en cuenta lo siguiente: (Desinfecte la unidad
solo con desinfectantes cuaternarios.Las superficies plásticas de la unidad pueden mancharse,
picarse, decolorarse o ablandarse si se limpian con desinfectantes a base de fenol, iodofor o
glutaraldehído.Además, el alcohol o los limpiadores y desinfectantes de aerosol con alto contenido
en alcohol pueden dañar la tapa frontal).
B) Escurra el exceso de líquido del paño.
C) Limpie las superficies externas con un paño suave.
D) Siga las instrucciones indicadas en el limpiador/desinfectante sobre aclarado y secado de
superficies exteriores.
Limpie las juntas de la puerta y de contención del esterilizador.
Precaución
Para evitar quemaduras, espere a que se
enfríe la unidad antes de realizar la limpieza.
A) Examine las juntas para detectar posibles daños.
B) Limpie las juntas y las supercies de contacto
con un paño húmedo.
003-2915-99
Junta de la puerta
Junta de contención
Español - 32
© Midmark Corporation 2018

Mantenimiento semanal
Advertencia sobre el equipo
Si no cambia el agua, el esterilizador puede no funcionar correctamente. No utilice
agentes blanqueadores ni materiales abrasivos en la cámara (p. ej., lejía, estropajo de
acero, cepillos de alambre, detergente en polvo, etc.). Si no se respetan estos puntos, la
cámara u otros componentes pueden sufrir daños.
Nota
Cada siete días, el autoclave mostrará de forma automática el mensaje de PERFORM WEEKLY
MAINTENANCE (REALIZAR MANTENIMIENTO SEMANAL). Si se desconecta de la fuente de
energía, el ciclo de mensajes semanales se reinicia.
Precaución
Para evitar quemaduras, espere a que se
enfríe la unidad antes de drenar el depósito.
Limpie la cámara y las bandejas (incluido el separador y el portabandejas)
A) Desconecte la parte superior del tubo de drenaje del depósito de las pestañas del panel, dóblelo
hacia abajo y drene el agua del depósito en un recipiente apropiado, por ejemplo, un cubo, y luego
deseche el agua.
B) Retire las bandejas, el bastidor de bandejas y el portabandejas del esterilizador. (Consulte la
página siguiente para ver instrucciones sobre la extracción/instalación del bastidor de bandejas y el
portabandejas).
C) Lave las bandejas, la rejilla, la placa y el interior de la cámara con jabón suave o Speed-Clean y
agua destilada o un agua que cumpla las especificaciones de pureza del agua mencionadas.
D) Llene el depósito con agua destilada o con agua que cumpla con las especicaciones de pureza de
agua indicadas.
003-2915-99
Bandejas
Español - 33
Bastidor de bandejas
Portabandejas
© Midmark Corporation 2018

Mantenimiento semanal (continuación)
ADVERTENCIA
Deje enfriar la unidad antes de retirar o colocar el bastidor de bandejas y el
portabandejas. Maneje el bastidor de bandejas metálico con cuidado para evitar
lesiones. No ejecute el esterilizador sin el portabandejas en su lugar.
Para retirar el bastidor de bandejas y el portabandejas...
A) Retire las bandejas.
B) Con un destornillador empuje el portabandejas hacia arriba y extraiga el bastidor de
bandejas y el portabandejas de la cámara.
Bastidor de bandejas
003-2915-99
Portabandejas
(El extremo angular debe mirar la parte
trasera de la cámara)
Advertencia sobre el equipo
Instale el bastidor de bandejas y el portabandejas con el extremo
angular del portabandejas mirando la parte trasera de la cámara.
No deje que el portabandejas haga contacto con el sensor de nivel de agua.
Para instalar el bastidor de bandejas y el portabandejas...
A) Introduzca el bastidor de bandejas en el portabandejas.
B) Coloque la parte trasera del portabandejas en la cámara.
C) Presione hacia abajo el bastidor de bandejas mientras lo
introduce en la cámara.
Español - 34
© Midmark Corporation 2018

Mantenimiento mensual
ADVERTENCIA
No procese los instrumentos mientras se lava el sistema.
Advertencia sobre el equipo
Utilice únicamente Speed-Clean para lavar el sistema. No lavar el sistema con Speed-Clean puede
Nota
Cada 28 días, el esterilizador mostrará de forma automática el mensaje de PERFORM MONTHLY
MAINTENANCE (REALIZAR MANTENIMIENTO MENSUAL). Si se desconecta de la fuente de energía,
el ciclo de mensajes mensuales se reinicia.
provocar el fallo prematuro de los componentes del esterilizador.
Limpie la cámara y las tuberías
A) Con la cámara fría, drene el depósito del esterilizador y rellénelo con agua destilada limpia, o agua
que cumpla con las especificaciones de pureza de agua indicadas.Añada 29 g (1 onza) del limpiador
para esterilizadores Speed-Clean directamente en la parte inferior de la cámara.
B) Ejecute un ciclo Pouches (Bolsas).
C) Pulse el botón de Stop (Detener) cuando comience el ciclo Dry (Secado).No se requiere Dry
Cycle durante el mantenimiento.
D) Vacíe el depósito y rellénelo por segunda vez con agua destilada o con agua que cumpla con las
especificaciones de pureza de agua indicadas. .
E) Enjuague ejecutando un ciclo Unwrapped (Sin envoltura)
cuando se inicie el ciclo de secado.
F) Vacíe y rellene el depósito con agua destilada limpia o agua que cumpla con las especificaciones de
pureza de agua indicadas, y deje que se enfríe el esterilizador.
G) Coloque el portabandejas, el bastidor y las bandejas. Limpie con un paño húmedo.
Filtro de aire
Filtro de llenado
y ventilación
Para evitar quemaduras, espere a que se enfríe
la unidad antes de drenar el depósito.
. Pulse el botón Stop (Detener)
Sonda de temperatura del vapor
Sonda del sensor de nivel
Precaución
003-2915-99
Speed-Clean
Limpiador
esterilizante
Español - 35
MA8195-1i
Elemento de
calentamiento
© Midmark Corporation 2018

Limpie la cámara y las tuberías (continuación)
Advertencia sobre el equipo
Limpie el interior de la cámara con precaución. No respetar estas indicaciones puede
generar un daño al elemento de calentamiento, la sonda de temperatura del vapor y/o
la sonda del sensor de nivel.
Advertencia sobre el equipo
No ponga en marcha el esterilizador sin que los filtros estén colocados en su sitio.
H) Retire y limpie los filtros. Los filtros tienen evitan que la suciedad provoque fallos en la válvula. Entre
cada limpieza mensual regular, los filtros deben limpiarse si están obstruidos, si los tiempos de
ventilación son muy prolongados o si los artículos no se secan. (Vea la ilustración para saber dónde
se encuentran las pantallas del filtro.)
I) Sujete el filtro y sepárelo con suavidad de la pared de la cámara mientras lo gira ligeramente.
(Si es necesario, utilice alicates para retirar los filtros)
J) Limpie los filtros con SpeedClean y agua destilada. Un pequeño cepillo de cerdas duras o un
limpiador ultrasónico pueden serle de utilidad. Enjuague los filtros con agua destilada. Sustituya los
filtros si no puede eliminar la suciedad.
K) Limpie el interior de la cámara.
L) Instale los filtros.(Presione hacia dentro, hacia la pared de la cámara, mientras lo gira ligeramente).
M) Coloque el portabandejas, el bastidor y las bandejas.
Retirada/limpieza de junta de puerta y dique
A) Extraiga la junta de la puerta y la de contención de la
puerta, luego extraiga el anillo de la junta de la puerta.
B) Limpie las juntas y el anillo con SpeedClean, agua
destilada y un cepillo suave.
C) Compruebe si las juntas están dañadas, han encogido
o aumentado de tamaño. Sustituya las juntas si observa
daños.
D) Introduzca el anillo en el canal de la junta de la puerta y
vuelva a colocarla en la puerta.
E) Coloque la junta de contención.
003-2915-99
Español - 36
© Midmark Corporation 2018

Compruebe la válvula de despresurización
(debe revisarse cada mes para garantizar que funciona correctamente)
A) Presione el botón Unwrapped (Sin envoltura)
B) Pulse el botón Start (Inicio)
Durante la comprobación de la válvula de despresurización, el vapor se ventila por la parte
C) Espere hasta que la presión en
Precaución
inferior del esterilizador. Para evitar quemarse, coloque una barrera contra el vapor (o una
toalla enrollada) alrededor de la parte inferior del esterilizador.
la cámara alcance los 138 kPa (20 PSI).
D) Tire firmemente de la palanca de despresurización hacia arriba durante aproximadamente
E) Pulse el botón Stop (Parada)
(El ciclo se cancela para evitar el sobrecalentamiento).
3 segundos, y suéltela. (El vapor debe salir por debajo de la parte trasera de la unidad cuando tira
de la palanca.
Si la válvula no se cierra por completo cuando se suelte la palanca, tire otra vez de la palanca y
libérela rápidamente para que vuelva a encajar en su posición. Repita esto hasta que la válvula se
asiente adecuadamente).
Palanca de
despresurización
Advertencia sobre el equipo
Si se necesita demasiada fuerza para
abrir la válvula de despresurización, o si
la válvula no asienta correctamente, debe sustituir
la válvula. (Consulte "Solicitud de servicio técnico"
en este manual).
MA601301i
Mantenimiento a largo plazo
El M9 / M9D y el M11 están diseñados y probados para ofrecer una abilidad excepcional durante su vida
útil. Sin embargo, como todos los dispositivos electromecánicos, sufren desgaste y degradación por el uso.
Para garantizar la integridad, el rendimiento y la seguridad de los principales componentes, el usuario debe
realizar una inspección en un proveedor de servicios autorizado Midmark cada 10 años o 10 000 ciclos de
uso, según lo primero que tenga lugar. Después de 10 años o 10.000 ciclos de uso, se recomienda una
inspección anual por un proveedor de servicios autorizado por Midmark.
003-2915-99
Español - 37
© Midmark Corporation 2018

Impresora térmica (opcional)
Uso de la impresora
Cuando la impresora opcional está instalada y el esterilizador está enchufado, la impresora se enciende
automáticamente y se inicia. No se necesita intervención ni conguración por parte del usuario.
Descripción de la cinta de impresión
La impresora imprimirá la siguiente información para cada ciclo del esterilizador:
• Número de modelo: indica el modelo del esterilizador y la versión del software.
• Número de ciclo: reeja el recuento de ciclo real del esterilizador.
• Sterilizer ID (ID del esterilizador) Números de serie del esterilizador.
• Operador: se imprime una línea para que el operador pueda rmar en la cinta de impresión.
• Fecha y hora: del inicio de cada ciclo
• Seleccione “BEGIN selected CYCLE” (COMIENCE CICLO seleccionado): para indicar el comienzo
del ciclo seleccionado por el operador.
• Resumen de los puntos de ajuste del ciclo seleccionados.
Una vez que comienza el ciclo, la impresora imprime "FILLING CHAMBER" (LLENADO DE LA CÁMARA)
para mostrar que el esterilizador se está llenando con agua.
Una vez que el esterilizador comienza la fase de calentamiento del ciclo de esterilización, se imprime
"HEATING" (CALENTAMIENTO), y la impresora imprime la temperatura de la cámara, la presión y el tiempo
transcurrido en incrementos de 2 minutos hasta que se completa esta fase.
Cuando el esterilizador comienza la fase de esterilización, se imprime la palabra "STERILIZING"
(ESTERILIZACIÓN) y la impresora imprime la temperatura de la cámara, la presión y el tiempo transcurrido
en incrementos de 1 minuto hasta que se completa esta fase.
Cuando el esterilizador completa la fase de esterilización del ciclo de programa, la impresora imprime
"VENTING CHAMBER" (VENTILACIÓN DE LA CÁMARA) para indicar que se está reduciendo la presión de
vapor de la cámara.
Cuando el esterilizador comienza la fase de secado, se imprime la palabra "DRYING (SECADO)" y la
impresora imprime las palabras "DRYING START" (INICIO DEL SECADO) y mostrará la temperatura, la
presión y el tiempo transcurrido en incrementos de 5 minutos, comenzando a partir de 5:00. La impresora
continua imprimiendo el tiempo transcurrido en incrementos de 5 minutos hasta que se complete la fase
de secado. El registro nal para la fase de secado incluye las palabras "DRYING COMPLETE" (SECADO
FINALIZADO). Si el tiempo de secado se programa con un tiempo no divisible entre 5, el registro nal
impreso de la fase de secado reejará el tiempo de secado programado real en incrementos de 1 minuto,
por ejemplo, un tiempo de secado de 12 minutos tendrá 5, 10 y 12 minutos impreso en la cinta de
impresión.
Cuando el esterilizador completa la fase de secado del ciclo de esterilización, la impresora imprime un
resumen del ciclo de esterilización con la duración de cada fase del ciclo y el tiempo total del ciclo. Después
del resumen, la impresora imprime "CYCLE COMPLETE" (CICLO COMPLETO).
[NOTA: Si se cancela el ciclo de secado, no se imprimirán "DRYING COMPLETE" (SECADO COMPLETO)
ni "CYCLE COMPLETE" (CICLO COMPLETO)].
003-2915-99
Español - 38
© Midmark Corporation 2018

Ejemplo de impresión de un ciclo de programa
Midmark M9 - v0.1.1
Identicación del esterilizador
(Número de serie)
Fecha y hora
del inicio del ciclo
Conguración de la temperatura,
el tiempo de esterilización y el
tiempo de secado
Durante la fase de calentamiento,
la impresora registra la temperatura
y la presión de la cámara en
incrementos de 2 minutos.
Durante la fase de calentamiento,
la impresora registra la
temperatura y la presión
Incrementos de 1 minuto
Indica que la fase de
ventilación se ha iniciado
Durante la fase de
secado, la impresora
registra el tiempo en
incrementos de 5 minutos
Estas líneas muestran
el tiempo total de
secado completo
BEGIN UNWRAPPED CYCLE (INICIO CICLO SIN ENVOLVER)
VENTING CHAMBER (VENTILACIÓN CÁMARA)
FILLING (LLENADO): 0:36
HEATING (CALENTAMIENTO): 15:40
STERILIZING (ESTERILIZACIÓN): 3:00
VENTING (VENTILACIÓN): 2:28
DRYING (SECADO): 30:00
TOTAL CYCLE (CICLO TOTAL) 0:51:44
Total Cycles (Ciclos totales): 10
Sterilizer ID (ID del esterilizador) V0000000
--------------------------------------------
Operator (Operario)
1/27/2016 8:08 AM
Temp.: 270 Degrees F (Grados F)
Time (Tiempo): 3 Minutes (Minutos)
Dry (Secado): 30 Minutes (Minutos)
FILLING CHAMBER (LLENADO DE CÁMARA)
HEATING (CALENTAMIENTO)
mm:ss Degrees (Grados) PSI (kPA)
0:00 77,2 F 0,0
2:00 82,7 F 0,7
4:00 152,2 F 5,4
STERILIZING (ESTERILIZACIÓN)
mm:ss Degrees (Grados) PSI (kPA)
0:00 272,1 F (112,1 C) 28,0
1:00 273,0 F (112,1 C) 28,7
2:00 272,2 F (112,1 C) 27,9
3:00 272,4 F (112,1 C) 28,7
Min (Mín.) 272.0 F (133,2 C) 27,9
Max (Máx.) 273,1 F (112,1 C) 29,0
DRYING (SECADO)
Drying Start (Inicio de secado)
mm:ss Degrees (Grados) PSI (kPA)
5:00 192,8 F (112,1 C) 0,0
10:00 192,7 F (112,1 C) 0,0
30:00 192,8 F (112,1 C) 0,0
Drying Complete (Secado completo)
CYCLE COMPLETE (CICLO COMPLETO)
Núm. de modelo y
Versión de software
Núm. total de ciclos
tiempo en la unidad
Línea provista para la
rma del operador
Ciclo seleccionado
Indica que la fase de
llenado se ha iniciado
Resumen de
Fase de esterilización
Estas líneas no se
imprimirán si el ciclo
de secado se cancela
antes de nalizar
Resumen del ciclo
003-2915-99
Español - 39
© Midmark Corporation 2018

Instalación/Retirada del rollo de papel
Advertencia sobre el equipo
Use solamente rollos de papel térmico con un diámetro máximo de 48 mm (1,89") y de
58 mm (2,28”) de ancho.
Para retirar el papel...
A) Inserte el dedo en la ranura en la parte posterior de la cubierta pequeña y levántela
hacia arriba hasta que la tapa se libere de la posición de bloqueo. Para evitar daños,
no use una fuerza excesiva.
B) La tapa de la impresora se fijará hacia la parte posterior de la impresora.
C) Retire el papel que quede.
Para instalar el rollo de papel...
A) Desenrolle entre 7,6 y 10,2 cm (entre 3 y 4”) de papel.
B) Coloque el rollo de papel en el receptáculo, desenrollado hacia abajo.
C) Mantenga el borde del papel desenrollado en la parte delantera de la impresora y cierre
la tapa aplicando la misma presión en ambos lados de la tapa hasta que se cierre.
D) Presione el botón de alimentación de papel para obtener papel adicional.
Nota
Una franja rosa que se hace progresivamente más oscura en el papel indica que este se está terminando.
Recarga de papel de la impresora Número de pedido
Rollo de papel térmico de la impresora 060-0016-00
Papel térmico adherente de la impresora 060-0016-01
Botón de alimentación
de papel
Ranura de
papel
003-2915-99
Español - 40
Luz de
estado
SA1841i
Carcasa de la
impresora
© Midmark Corporation 2018

Uso de la herramienta para bandejas/cajas opcional
Advertencia sobre el equipo
Utilice la herramienta para bandejas y cajas 9A307001 solo con bandejas fabricadas por
Nota: Se incluyen tres placas. Cada una cumple una función específica.(bandejas M9 / M9D , bandejas M11,
Para retirar bandejas y cajas M9 / M9D / M11...
A) Enganche la pestaña dentada superior de la herramienta a la parte central superior del borde de
B) Gire la herramienta hacia abajo hasta que la placa de la herramienta se haya introducido por
C) Asegúrese de que la caja/bandeja esté bien sujeta, y extráigala de la cámara.
Nota: La herramienta para casetes puede manipular casetes de hasta 3,8 cm (1 1/2") de grosor.
Las bandejas y las cajas pueden estar CALIENTES. Tenga precaución cuando las retire
Midmark. Deslice la placa adecuada en la manilla tal y como se muestra.
cajas).
la caja o la bandeja.
completo por debajo de la bandeja.
Precaución
o las transporte. Mantenga las bandejas niveladas y ligeramente elevadas para que no se
inclinen y se desordene su contenido. Si no lo hace, puede sufrir lesiones por quemaduras.
003-2915-99
Español - 41
© Midmark Corporation 2018

Resolución de problemas
Tabla de resolución de problemas
Utilice la siguiente tabla para corregir problemas menores del esterilizador.
Problema Posible causa Solución
El esterilizador no
funciona (no se
visualiza nada en la
pantalla).
Se escapa vapor
por la válvula de
despresurización.
Evidencia de
fallo de esterilización
en el monitor de
proceso (indicador
químico, indicador
biológico, etc.).
Escapes por la junta
de la puerta.
Los artículos no están
secos al nal del ciclo
de secado.
La impresora no
imprime.
La pantalla aparece
en blanco/negro/no es
legible
003-2915-99
El cable de alimentación del esterilizador se
ha desenchufado de la toma de corriente o
de la parte trasera del esterilizador.
No llega la alimentación a la toma de la
fuente del esterilizador.
Fusible abierto en el circuito impreso
principal.
La válvula de despresurización no está
correctamente asentada.
No existen las condiciones necesarias para
la esterilización en el emplazamiento del
indicador.
El indicador es obsoleto, es inadecuado
para el ciclo de esterilización o ha tenido un
mal funcionamiento.
La junta de puerta está dañada o sucia. Limpie o sustituya la junta de la puerta.
El esterilizador no se ha cargado
correctamente.
Filtros obstruidos. Limpie o sustituya los ltros.
Los cables de la impresora no están
correctamente conectados.
Mal funcionamiento de la impresora. (la
luz de estado parpadea varias veces por
segundo)
La impresora no tiene papel. (la luz de
estado parpadea cada segundo)
Papel incorrecto Asegúrese de que el papel de la impresora sea papel
Interferencia electromagnética Presione cualquier tecla excepto "Detener" en el
Español - 42
Asegúrese de que el cable de alimentación
esté enchufado en la toma de corriente o en el
esterilizador.
Compruebe el disyuntor para la toma de la fuente.
Si el problema persiste, desenchufe el cable de
alimentación de la unidad y llame a un técnico
autorizado. (Consulte "Solicitud de servicio técnico")
Desenchufe el cable de alimentación de la unidad y
llame a un técnico autorizado. (Consulte "Solicitud de
servicio técnico")
Coloque la válvula de despresurización
correctamente.(Consulte "Mantenimiento mensual").
Recargue el esterilizador conforme a las
"Instrucciones de carga" del M9 / M9D o M11.
Si el problema persiste, desenchufe el cable de
alimentación de la unidad y llame a un técnico
autorizado. (Consulte "Solicitud de servicio técnico")
Utilice un indicador adecuado para la carga y
el ciclo seleccionado que se haya almacenado
correctamente. Contacte con el fabricante del
indicador para obtener información adicional sobre su
correcta selección, uso y almacenamiento, así como
su posible mal uso o mal funcionamiento.
(Consulte "Mantenimiento semanal").
Recargue el esterilizador conforme a las
"Instrucciones de carga" del M9 / M9D o M11.
Si la conguración de carga particular que se utiliza
no está seca al nal del ciclo de secado, aumente el
tiempo de secado del ciclo (vea la nota en la página
26) o reduzca la carga.
(Consulte "Mantenimiento mensual").
Asegúrese de que los cables estén correctamente
conectados a la impresora.
Desenchufe el cable de alimentación del esterilizador,
espere 15 segundos, y vuelva a enchufarlo.
Introduzca un rollo de papel nuevo. (Consulte la
sección sobre retirada e instalación del rollo de papel).
térmico
teclado para reiniciar la pantalla sin interrumpir el
ciclo. Al presionar "Detener", se reiniciará la pantalla,
pero también se cancelará el ciclo en proceso.
© Midmark Corporation 2018

Mensajes informativos
La siguiente tabla incluye los mensajes informativos que pueden aparecer durante el funcionamiento.
Mensaje Posible causa Solución
INITIALIZING SYSTEM
(INICIO DEL SISTEMA)
CICLOS TOTALES XXX
M9, VX.X.X
DD/MM/AAAA 8:07 Am
VXXXXXXX
PERFORM WEEKLY
MAINTENANCE (REALIZAR
MANTENIMIENTO
SEMANAL)
PERFORM MONTHLY
MAINTENANCE (REALIZAR
MANTENIMIENTO
MENSUAL)
El cable de alimentación acaba de
enchufarse - mensaje informativo estándar.
El cable de alimentación acaba de
enchufarse - mensaje informativo estándar.
(Muestra el núm. de ciclos, número de
modelo (M9 / M9D o M11) y versión de
software)
El cable de alimentación acaba de
enchufarse - mensaje informativo estándar.
(Muestra la fecha, hora y número de serie).
Este mensaje se muestra a los 7, 14 y
21 días después que la unidad se ha
enchufado a una fuente de alimentación,
para indicarle al operador que realice el
mantenimiento semanal descrito en este
manual.
Este mensaje se muestra cada 28 días
para indicarle que realice el mantenimiento
mensual descrito en este manual.
El equipo funcionará normalmente
después de 4 segundos.
El equipo sigue funcionando con
normalidad después de este mensaje.
El equipo sigue funcionando con
normalidad después de este mensaje.
Realice el mantenimiento
semanal. El mensaje desaparecerá
automáticamente después de nalizar el
siguiente ciclo.
Realice el mantenimiento
mensual. El mensaje desaparecerá
automáticamente después de nalizar el
siguiente ciclo.
Acceso a los últimos 5 códigos de error
Acción Descripción Pantalla
MANTENER PULSADO
Este menú muestra los últimos cinco
códigos de error que se muestran en
la unidad.
durante el encendido
Pulse el botón Stop
(Detener)
NOTA: "1" es el código de error más
reciente; "5" es el más antiguo.
Para salir del menú del código de
error, pulse el botón de parada.
Mensajes de error
La siguiente tabla y las siguientes páginas incluyen los mensajes de error que pueden aparecer durante el
funcionamiento.
Precaución
Si se producen errores repetidamente, deje de usar el esterilizador. Anote el mensaje o
el código de error, desconecte la unidad y póngase en contacto con un representante de
servicio técnico autorizado. (Consulte "Solicitud de servicio técnico")
Si aparece un mensaje de error: "Items Not Sterile" (Artículos no estériles), los artículos en el
esterilizador no se han esterilizado; deben someterse a un proceso de esterilización.
003-2915-99
Español - 43
© Midmark Corporation 2018

Mensajes de error (continuación)
Mensaje Posible causa Solución
C010: POWER UP MODE
SYSTEM PWR LOSS
(PÉRDIDA DE CORRIENTE
DEL SISTEMA EN MODO DE
ENCENDIDO)
ITEMS NOT STERILE
(ARTÍCULOS NO ESTÉRILES)
PUSH STOP TO RESTART
(PULSE DETENER PARA
REINICIAR)
CO60: POWER UP MODE
SYSTEM HARDWARE
(HARDWARE DEL SISTEMA
EN MODO DE ENCENDIDO)
C102: FILL MODE
STOP PRESSED (SE HA
PULSADO STOP EN MODO DE
LLENADO)
C103: HASTA C105
HEATUP, STERILIZE OR VENT
MODE
STOP PRESSED (SE HA
PULSADO DETENER
EN MODO CALENTAR,
ESTERILIZAR O VENTILAR)
C106: DOOR MODE
STOP PRESSED (SE HA
PULSADO STOP EN MODO DE
PUERTA)
C232: FILL MODE
WATER LOW (BAJO NIVEL
DE AGUA EN MODO DE
LLENADO)
Se ha cortado la corriente
de la unidad durante el
ciclo.
Ha ocurrido un breve corte
de corriente o un fallo
interno imprevisto.
El botón STOP (DETENER)
se ha pulsado durante el
ciclo.
El botón STOP (DETENER)
se ha pulsado durante el
ciclo.
El botón STOP (DETENER)
se ha pulsado durante el
ciclo.
El nivel de agua del
depósito es demasiado
bajo.
Filtro de llenado/ventilación
(en la parte inferior de la
cámara) obstruido.
La tabla continúa en la página siguiente...
Pulse el botón STOP (DETENER) para reiniciar.
Desenchufe el cable de alimentación de la unidad
durante 1 minuto y vuelva a enchufarlo. Si el
problema persiste llame a un representante de
servicio técnico autorizado. (Consulte "Solicitud
de servicio técnico")
Pulse el botón STOP (DETENER) para reiniciar.
Espere unos instantes (hasta un minuto) mientras
baja la presión / temperatura de la cámara. Pulse
el botón STOP (DETENER) para volver al modo
Select Cycle (Seleccionar ciclo) y empezar un
nuevo ciclo.
Pulse el botón STOP (DETENER) para reiniciar.
Rellene el depósito con agua destilada o con agua
que cumpla con las especicaciones de pureza de
agua indicadas. Espere unos instantes (hasta un
minuto). Pulse el botón STOP (DETENER) para
volver al modo Select Cycle (Seleccionar ciclo) y
empezar un nuevo ciclo.
Limpie el ltro de llenado/ventilación. (Consulte
"Mantenimiento mensual").
003-2915-99
Español - 44
© Midmark Corporation 2018

Mensajes de error (continuación)
Mensaje Posible causa Solución
C326: DOOR MODE DOOR
CLOSED (PUERTA CERRADA
EN MODO DE PUERTA)
C382: FILL
DOOR OPEN
(PUERTA ABIERTA
EN LLENADO)
C383: HEATUP MODE
DOOR OPEN (PUERTA
ABIERTA EN MODO DE
CALENTAMIENTO)
C384: STERILIZE MODE
DOOR OPEN (PUERTA
ABIERTA EN MODO DE
ESTERILIZACIÓN)
C533 a C633:
STEAM TEMP LOW
(TEMP DE VAPOR BAJA)
o
STEAM TEMP HARDWARE
(HARDWARE TEMP DE
VAPOR)
o
PRESSURE LOW
(PRESIÓN BAJA)
El interruptor de seguridad
de la puerta todavía está
haciendo contacto tras
ponerse en marcha el
motor de la puerta.
El esterilizador ha
detectado que los
contactos del interruptor
de seguridad de la puerta
están abiertos.
El esterilizador ha
detectado que los
contactos del interruptor
de seguridad de la puerta
están abiertos.
El esterilizador ha
detectado que los
contactos del interruptor
de seguridad de la puerta
están abiertos.
El esterilizador ha
detectado que la
temperatura o la presión
han superado los límites
del funcionamiento normal.
Abra la puerta.
Cierre la puerta del esterilizador. (El ciclo se
reanudará donde se interrumpió)
Espere un poco (1 minuto) mientras baja la
presión / temperatura de la cámara. Presione
el botón STOP (DETENER) para seleccionar el
modo del ciclo. Inicie un nuevo ciclo.
Desenchufe el cable de alimentación de la unidad
durante 1 minuto y vuelva a enchufarlo. Si el
problema persiste llame a un representante de
servicio técnico autorizado. (Consulte "Solicitud
de servicio técnico")
Desenchufe el cable de alimentación de la unidad
durante 1 minuto y vuelva a enchufarlo. Si el
problema persiste llame a un representante de
servicio técnico autorizado. (Consulte "Solicitud
de servicio técnico")
C642 A C647
PRESSURE HIGH
(PRESIÓN ALTA)
C660 A C677
PRESSURE HARDWARE
(HARDWARE DE PRESIÓN)
o
PRESSURE OVERLIMIT
(PRESIÓN SOBRE EL LÍMITE)
003-2915-99
La presión de la cámara
está fuera de los límites
para un funcionamiento
normal.
La presión de la cámara
está fuera de los límites
para un funcionamiento
normal.
Espere unos instantes (hasta un minuto) mientras
baja la presión / temperatura de la cámara.
Presione el botón STOP (DETENER) para
seleccionar el modo del ciclo. Inicie un nuevo
ciclo.
Desenchufe el cable de alimentación de la unidad
durante 1 minuto y vuelva a enchufarlo. Si el
problema persiste llame a un representante de
servicio técnico autorizado. (Consulte "Solicitud
de servicio técnico")
La tabla continúa en la página siguiente...
Español - 45
© Midmark Corporation 2018

Mensajes de error (continuación)
Mensaje Posible causa Solución
C980 A C987
HI-LIMIT OPEN (LÍMITE
SUP. ABIERTO)
El interruptor de
límite superior se ha
abierto al menos 1/4
de segundo durante el
modo de funcionamiento
especíco.
Desenchufe el cable de alimentación de la unidad durante 30 minuto y vuelva a enchufarlo. Si el problema
persiste llame a un representante de servicio autorizado. (Consulte "Solicitud de servicio técnico")
Contactar con el servicio técnico
Nota
Anote cualquier código que se muestre y asegúrese de enviar esta información al técnico de servicio.
Póngase en contacto con su distribuidor Midmark autorizado o inicie sesión en www.midmark.com/
technical-library.
Cuando se ponga en contacto con el servicio técnico se le solicitará información sobre el modelo y el
número de serie.
Para ponerse en contacto directamente con Midmark:
+1-937-526-3662
8:00 am a 5:00 pm EST (Lunes a viernes)
[excepto festivos en EE. UU.]
003-2915-99
Español - 46
© Midmark Corporation 2018

Tabla de especicaciones: M9 / M9D
Dimensiones físicas:
Longitud total con enchufe 51,8 cm (20,38'')
Ancho total 38,9 cm (15,3'')
Altura total con impresora 40,9 cm (16,1'')
Área del contador 38,9 cm x 45,7 cm (15,3" x 18")
Cámara 22,9 cm diá. x 38 cm prof. (9" x 15 in")
Bandeja estándar, grande 18,6 cm x 30,5 cm x 2,2 cm (7 5/16" x 12" x 7/8")
Bandeja estándar, pequeña 14,3 cm x 30,5 cm x 2,2 cm (5 5/8" x 12" x 7/8")
Pesos:
Peso con depósito vacío 33,1 kg (73 lb)
Peso con embalaje 36,7 kg (81 lb)
Capacidad del depósito de agua 4,1 litros (1,1 galones) hasta la marca de llenado el
volumen utilizable es de 1,9 litros (0,5 galones)
Clasicación eléctrica:
Nota: se necesita un circuito independiente (exclusivo) para este esterilizador. El esterilizador no debe
conectarse a un circuito eléctrico con otros dispositivos o equipos a menos que el circuito soporte
cargas adicionales.
Modelos de 115 V 115 V CA (+/- 10%), 12 amps, 50/60 Hz
Modelos de 230 V 230 V CA (+/- 10%), 6,4 amps, 50/60 Hz
Clasicación de fusibles:
115 V AC F1........0,25 A, 250 V, fusible de acción lenta, 1/4" x 1 1/4"
F2........15 A, 250 V, fusible de acción rápida, 1/4” x 1 1/4”
230 V AC F1........0,125 A, 250 V, fusible de acción lenta, 5 x 20 mm
F2........8 A, 250 V, fusible de acción rápida, 5 x 20 mm
Conguración de la válvula de seguridad 275,8 kPa (40 psi)
Emisión de calor 1,47 kW (5000 BTU/HR) durante el funcionamiento
Certicaciones
ASME Boiler and Pressure Vessel Code,
Section VIII, Division 1.
Número de registro canadiense disponible
Midmark es una compañía certicada por las
normas ISO 13485 e ISO 9001.
UL61010-1, 2da Edición
IEC 61010-2-040:2005, 1ra edición
CAN/CSA C22.2, No. 61010-1, 2da Edición
CSA C22.2, No. 61010-2-040-07 Part 2-040, - 1ra edición
IEC 60601-1-2 Edition 4.0 2014-02
CISPR II:2009, A1:2010 clase A, grupo 1
IEC 61000-3-2:2014, Parte 3-2
IEC 61000-3-3:2014, Parte 3-3
ANSI/AAMI ST55:2010(R)2014
ANSI/AAMI/IEC62304
003-2915-99
Español - 47
© Midmark Corporation 2018

Tabla de especicaciones: M11
Dimensiones físicas:
Longitud total con enchufe 60,5 cm (23,8'')
Ancho total 45,2 cm (17,8'')
Altura total con impresora 50,0 cm (18,1'')
Área del contador 45,2 cm x 53,3 cm (17,8" x 21")
Cámara 28 cm diá. x 46 cm prof. (11" x 18")
Bandeja estándar, grande 22,9 cm x 38 cm x 2,9 cm (9" x 15" x 1 1/8")
Bandeja estándar, pequeña 16,8 cm x 38 cm x 2,9 cm (6 5/8" x 15" x 1 1/8")
Pesos:
Peso con depósito vacío 44,9 kg (99 lb)
Peso con embalaje 59,4 kg (131 lb)
Capacidad del depósito de agua 5,3 litros (1,4 galones) hasta la marca de llenado el
volumen utilizable es de 3,8 litros (1 galón)
Clasicación eléctrica:
Nota: se necesita un circuito independiente (exclusivo) para este esterilizador. El esterilizador no debe
conectarse a un circuito eléctrico con otros dispositivos o equipos a menos que el circuito soporte
cargas adicionales.
Modelos de 115 V 115 V CA (+/- 10%), 12 amps, 50/60 Hz
Modelos de 230 V 230 V CA (+/- 10%), 6,4 amps, 50/60 Hz
Clasicación de fusibles:
115 V AC F1........0,25 A, 250 V, fusible de acción lenta, 1/4” x 1 1/4”
F2........15 A, 250 V, fusible de acción rápida, 1/4” x 1 1/4”
230 V AC F1........0,125 A, 250 V, fusible de acción lenta, 5 x 20 mm
F2........8 A, 250 V, fusible de acción rápida, 5 x 20 mm
Conguración de la válvula de seguridad 275,8 kPa (40 psi)
Emisión de calor 1,47 kW (5000 BTU/HR) durante el funcionamiento
Certicaciones
ASME Boiler and Pressure Vessel Code,
Section VIII, Division 1.
Número de registro canadiense disponible
Midmark es una compañía certicada por las
normas ISO 13485 e ISO 9001.
UL61010-1,2da Edición
IEC 61010-2-040:2005, 1ra edición
CAN/CSA C22.2, No. 61010-1, 2da Edición
CSA C22.2, No. 61010-2-040-07 Part 2-040, - 1ra edición
IEC 60601-1-2 Edition4.0 2014-02
CISPR II:2009, A1:2010 clase A, grupo 1
IEC 61000-3-2:2014, Parte 3-2
IEC 61000-3-3:2014, Parte 3-3
ANSI/AAMI ST55:2010(R)2014
ANSI/AAMI/IEC62304
003-2915-99
Español - 48
© Midmark Corporation 2018
 Loading...
Loading...