
Dental Film Digitizer
User's Manual
Size 0Size 0
Size 1Size 1
Size 2Size 2
www.microtek.com

Preface
Copyright
© 2013, Microtek International, Inc., all rights reserved. This document may not
be reproduced or copied in any way, stored electronically, or translated into any
language, without the permission of Microtek International, Inc.
Trademarks
Microtek, ScanMaker, ArtixScan, ScanWizard and ColoRescue are trademarks or
registered trademarks of Microtek International, Inc. All other trademarks or
registered trademarks are the property of their respective holders. Specifications,
software and hardware bundles are subject to change without notice. Not
responsible for typographical errors.
Disclaimer
The contents of this manual have been checked carefully for accuracy, and every
effort has been made to ensure that there are no errors. However, Microtek makes
no guarantee as to the accuracy of the contents of this document and reserves the
right to make changes to the contents without prior warning.
I49-004741 B1
September 2013
Microtek International, Inc.
6, Industry East Road 3, Science Based Industrial Park, Hsinchu, Taiwan
Tel: 886-3-5772155, Fax: 886-3-5772598, http://www.microtek.com
2 Medi-2200 Plus User's Manual
Microtek International, Inc.
No. 6, Industry East Road 3,
Science-Based Industrial Park,
Hsinchu, Taiwan
EVESTAR GmbH
Linsellesstraße 127-129
D-47877 Willich, Germany

FCC Compliance Statement
This equipment (Model: MMS-9600TFU2L) has been tested and found to comply
with the limits for a Class B digital device, pursuant to Part 15 of the FCC rules.
These limits are designed to provide reasonable protection against harmful
interference in a residential installation. This equipment generates, uses and can
radiate radio frequency energy and, if not installed and used in accordance with
the instructions, may cause harmful interference to radio communications.
However, there is no guarantee that interference will not occur in a particular
installation. If this equipment does cause harmful interference to radio or
television reception, which can be determined by turning the equipment off and
on, the user is encouraged to try to correct the interference by one or more of the
following measures:
• Reorient or relocate the receiving antenna.
• Increase the separation between the equipment and receiver.
• Connect the equipment into an outlet on a circuit different from that to which
the receiver is connected.
• Consult the dealer or an experienced radio/TV technician for help.
Note: A shielded Hi-Speed USB interface cable with ferrite core installed on the
film digitizer connector end must be used with this equipment.
Caution: Changes or modifications not expressly approved by the manufacturer
responsible for compliance could void the user's authority to operate the
equipment.
This device complies with Part 15 of the FCC Rules. Operation is subject to the
following two conditions: (1) This device may not cause harmful interference, and
(2) this device must accept any interference received, including interference that
may cause undesired operation.
Medi-2200 Plus User's Manual 3

CE 0120 Compliant Device
CE Compliance Statement
This device complies with CE Certification pursuant to
EN 55011:2007+A2:2007
EN 60601-1-2:2007
WEEE Compliance Statement
Microtek International, Inc. operates under full compliance with the WEEE
Directive. Among the requirements of the Directive are the marking of any
applicable equipment placed in the EU market with the WEEE symbol, a crossed
out wheeled bin as shown at left; demonstration of the implementation of a takeback program; and meeting recycling targets.
ISO Compliance Statement
Comply with the requirements of the international standard ISO 9001:2000 and
ISO 13485:2003
4 Medi-2200 Plus User's Manual
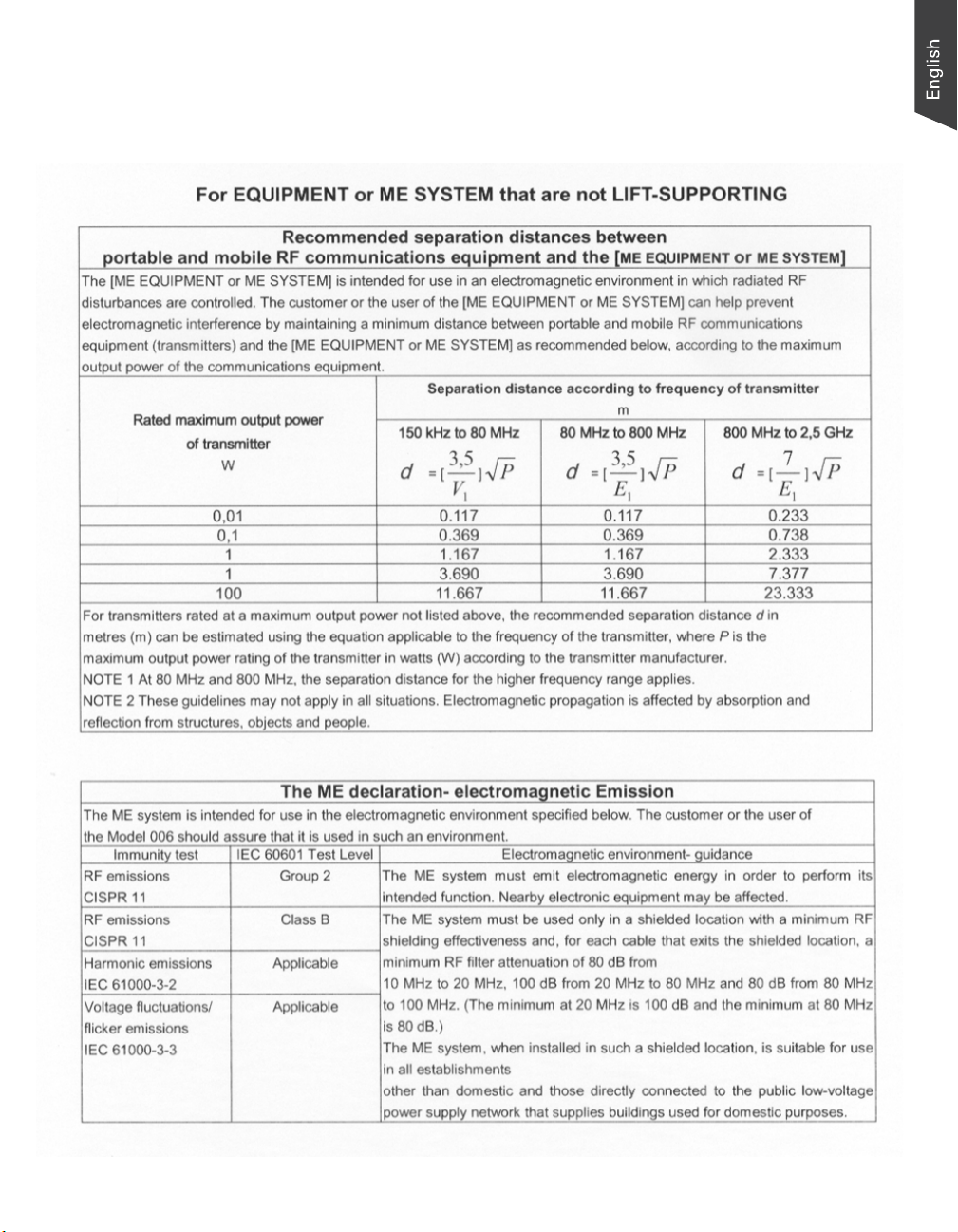
Guidance and Manufacturer's Declaration
for EMC Directive
Medi-2200 Plus User's Manual 5

CE Declaration of Conformity (CE DoC)
6 Medi-2200 Plus User's Manual
Safety Precautions
Before using your digitizer, read the following safety guides carefully, which detail
the proper operation of the digitizer and its accessories to prevent injuries or
damage to users or equipment.
Meanings of Symbol Signs
CAUTION
This indicates hazardous situation which, if not payed
attention, could result injury or damage to users or
equipment.
WARNING
Follow operating instructions or Consult instructions for use
STAND-BY
DIRECT CURRENT
This indicates hazardous situation which, if not payed
attention, could result serious injury or death to users.
Power Supply:
• Only use with adapter MAGIC POWER TECHNOLOGY CO., LTD MPM-X60-
15.
• Grounding reliability can only be achieved when the equipment is connected
to an equivalent receptacle marked "Hospital Only" or "Hospital Grade".
• Do not modify this equipment without authorization of the manufacturer.
• To avoid risk of electric shock, this equipment must only be connected to a
supply mains with protective earth.
• This adapter MAGIC POWER TECHNOLOGY CO., LTD MPM-X60-15 is a
forming part of the medical device.
Medi-2200 Plus User's Manual 7
• Accessory equipment connected to the analog and digital interfaces must be
in compliance with the respective nationally harmonized IEC standards (i.e.
IEC 60950 for data processing equipment, IEC 60065 for video equipment,
IEC 61010-1 for laboratory equipment, and IEC 60601-1 for medical
equipment.) Furthermore all configurations shall comply with the system
standard IEC 60601-1-1. Anyone who connects additional equipment to the
signal input part or signal output part is configuring a medical system, and is
therefore, responsible that the system complies with the requirements of the
system standard IEC 60601-1-1. The unit is for exclusive interconnection
with IEC 60601-1 certified equipment in the patient environment and IEC
60XXX certified equipment out-side of the patient environment. If in doubt,
consult the technical services department or your local representative.
• Users must not allow SIP/SOPs and the patient to come into contact at the
same time.
• Ensure to turn off the power of each device before connecting or
disconnecting the cables.
• Insert the plug completely into the outlet, as a loose connection may cause
arcing and result in fire.
• Ensure to hold the plug or connector to disconnect the cable; otherwise, if
you pull the cable only, it may damage the core wire and result in fire or
electric shock.
• Place and route the power supply cord such that it is not likely to be walked
on or pinched by items placed upon or against them, paying particular
attention to the cord near the power plugs, convenience receptacles, and at
the point where it exits from the outlet.
• When the digitizer is left unattended and unused for long periods of time,
unplug it from the wall outlet.
Moving and Storing the Film Digitizer:
• Always ensure that the digitizer is stored properly before shipping or moving
it. Quick stops, excessive force, and uneven surfaces may cause the product
to overturn when moving.
• Do not hit or drop the digitizer. The digitizer may be damaged if it receives a
strong jolt, which may result in fire or electric shock if the digitizer is used
without being repaired.
• Do not place the digitizer on any slippery, slanted, or unstable surface. The
product may slide or fall, causing serious injury to people as well as serious
damage to the product.
• Do not use the digitizer near water. Never spill liquid of any kinds on the
product, or it may result in electric shock or other hazards.
8 Medi-2200 Plus User's Manual

• This digitizer should be situated away from heat sources such as radiators,
heat registers, stoves, or other products (including amplifiers) that produce
heat.
• Do not store the digitizer in which it may be exposed to direct sunlight.
Using the Digitizer
• Before using the digitizer, make sure the area of the glass surface is clear from
obstacles.
• The digitizer is only designed and used for digitizing mammography films or
reflective materials. Do not attend to use it for other purposes.
Maintenance and Service:
• When the digitizer is going to be cleaned, ensure to turn off the power of each
device and unplug the power cable from the AC outlet.
• When you need a repair service, unplug the digitizer from the power outlet
and consult qualified service personnel.
• When replacement parts are required, use replacement parts that are specified
by the manufacturer or have the same characteristics as the original parts.
• For safety reasons, ensure to inspect the device before using it. In addition,
carrying out a regular inspection at least once a year.
Medi-2200 Plus User's Manual 9
Labels and Markings
On The Device
M
i
c
r
o
N
t
o
e
.
k
6
S
I
,
n
c
I
t
n
i
e
e
d
r
n
u
H
n
c
s
a
s
e
t
t
in
r
I47-0
i
o
y
B
c
n
E
h
a
a
a
u
s
1
l
s
,
e
1084, A
3
t
I
d
0
n
R
0
c
I
o
n
7
a
7
d
d
,
u
T
s
a
t
r
i
w
i
E
a
V
a
l
E
n
S
L
T
i
A
n
s
R
e
D
G
l
l
e
m
4
s
7
b
s
8
H
t
7
r
a
7
ß
W
e
i
1
ll
2
i
c
7
h
1
,
2
G
9
e
r
m
a
n
.
3
,
P
a
r
k
,
y
Microtek International, Inc.
No. 6, Industry East Road 3,
Science-Based Industrial Park,
Hsinchu Taiwan,
I47-0
1
1084,
A
EVES
Linsellesstra
T
AR GmbH
D-47877 Willich, Germany
ß
e 127-129
I47-011084, A
TFDA label
(for Taiwan shipping only)
CE 0120 label
10 Medi-2200 Plus User's Manual
Microtek International, Inc.
No. 6, Industry East Road 3,
Science-Based Industrial Park,
Hsinchu, Taiwan
EVESTAR GmbH
Linsellesstraße 127-129
D-47877 Willich, Germany
TWN
Commodity label
(for Taiwan shipping only)
Power label
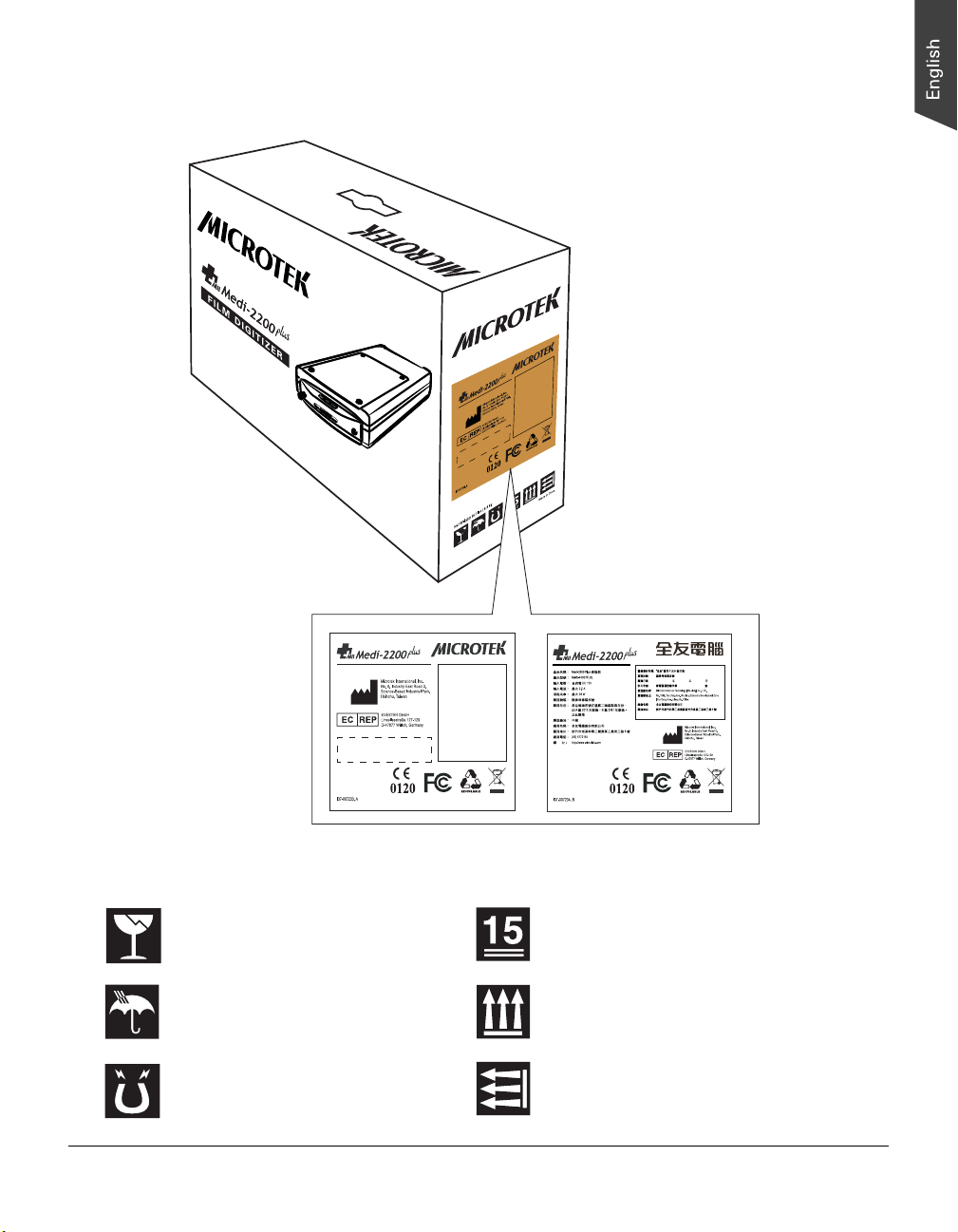
On The Package Carton
Fragile; handle with care
Keep in a dry place
Keep away from magnets
CE 0120 label
(for EU/US/AP shipping)
TFDA label
(for Taiwan shipping)
Store 15 layers of boxes in one pallet
The storage direction of the top of the box
The storage direction of the top of
the machine
Medi-2200 Plus User's Manual 11

12 Medi-2200 Plus User's Manual

Table of Contents
Preface ............................................................................................... 2
Safety Regulations ............................................................................. 3
FCC Compliance Statement ............................................................... 3
Regulations ..................................................................................... 4
Guidance and Manufacturer's Declaration for EMC Directive .................. 5
CE Declaration of Conformity (CE DoC) ............................................... 6
Safety Precautions ........................................................................... 7
Labels and Markings ....................................................................... 10
On The Device ........................................................................ 1 0
On The Package Carton ............................................................ 1 1
Knowing about Your Film Digitizer .................................................. 15
Features of the Medi-2200 Plus ...................................................... 1 5
System Requirements ..................................................................... 16
Unpacking Your Film Digitizer .......................................................... 17
Looking for Damage ....................................................................... 17
Unpacking Package Contents ........................................................... 17
Package Contents ........................................................................... 20
How to Repack Your Film Digitizer for Shipping ................................. 21
Taking a Closer Look ........................................................................ 23
Front/Open View ........................................................................... 23
Rear View ..................................................................................... 24
Installing Your Digitizer .................................................................... 25
Installing the Software .................................................................... 25
Unlocking the Digitizer ................................................................... 2 6
Connecting the Digitizer .................................................................. 27
To the Power Adapter .............................................................. 2 6
To the Hi-Speed USB (USB 2.0) Cable ....................................... 28
Digitizing Dental Films .................................................................... 29
Removing the Black Mat ................................................................. 30
Positioning Dental Films ................................................................. 3 1
Medi-2200 Plus User's Manual 13

A. Using the Panoramic X-Ray Film Holder ................................. 3 1
B. Using the Cephalometric X-Ray Film Holder ............................ 3 2
C. Using the Intraoral X-Ray Film Holder ..................................... 33
Scanning Dental Films .................................................................... 34
Digitizing Dental Films without Film Holder .................................... 37
Using the Film Alignment Ruler ....................................................... 3 7
Scanning Dental Films with Film Alignment Ruler ............................. 3 8
Digitizing Reflective Originals ......................................................... 40
Care and Cleaning ............................................................................ 42
Cleaning the Glass Surfaces ............................................................ 4 2
Maintenance ..................................................................................... 43
Appendix .......................................................................................... 44
Troubleshooting ............................................................................ 4 4
Specifications ................................................................................ 46
14 Medi-2200 Plus User's Manual

Knowing about Your Film Digitizer
The Medi-2200 Plus is a dental film digitizer, which is specifically designed for
the use of the intraoral and extraoral dental films scanning. With the use of
exclusive dental film holders and the Smart-Scan function together, the Medi2200 Plus can automatically perform image auto-cropping and multiple dental
films scanning, easily transferring dental films into digital format in short periods
of time. The digitized images are intended for the use in primary, secondary and
over reading applications; thus, it is specifically designed for the trained medical
professionals or staffs.
The Medi-2200 Plus can capture details in bright and dark areas of dental X-ray
films and provide the medical professionals a convenient method to digitize the
dental X-ray films for the electric data storage. Additionally, the Medi-2200 Plus
features the scanning capabilities of reflectives, such as photographs, documents
or prints as well.
Features of the Medi-2200 Plus
The Medi-2200 Plus comes with several important features, including the
following:
• Energy-saving LED light source — Adopting LEDs as the light source, when
the digitizer is detected by the system, there are no requirements for any
warm-up time before carrying out the scan, which boots your productivity and
reduces energy costs amazingly. With its stable performance, the image
quality will remain consistent even after used for a certain period of time.
• Smart-Scan — The Smart-Scan function provides you an intelligent method
for scanning X-ray films. With this function applied, the digitizer can detect
the film holder with the loaded films, auto-crop the scan frames, perform
scanning in a single pass for multiple films at one time, and save the scanned
images into your selected folder or send them to your selected application. By
using the Smart-Scan button on either the software control panel or the Medi2200 Plus unit, the busy medical professionals or staffs can save time to
convert X-ray films into digital files effortlessly.
• One-pass Scan for Multiple Jobs — This feature allows the digitizer to
perform scanning in a single pass for all the selected scan jobs.
• 8.5"x14" scan bed — The flatbed of the Medi-2200 Plus lets you easily
digitize various sizes of dental X-ray films up to 8"x12" and reflectives up to
8.5" x 13.5", which offers more flexibility for storing old X-ray films or
records.
• Clinically Proven Image Quality — With a high resolution of 4800 dpi, the
16-bit grayscale, and a high dynamic range of 4.0 maximum optical density,
the Medi-2200 Plus is able to capture a wide range of gray tones, providing
excellent details when digitizing dental images.
Medi-2200 Plus User's Manual 15

• Exclusive Dental Film Holders — These specially designed dental film holders
are designed to hold a wide range of dental X-ray films in place. By loading
the dental film holder on the scan bed before scanning films, you can ensure
perfect alignment of your dental images and achieve consistent scans. There
are three kinds of dental film holders come with your digitizer package, which
accommodate five sizes of intraoral and extraoral dental films, such as
intraoral Size 0 through 4, panoramic and cephalometric films. With the use of
exclusive dental film holders, medical professionals can scan multiple intraoral
dental films at a time, making operations more convenient and efficient.
• Microtek ScanWizard Medi software — The friendly user interface of
ScanWizard Medi stores X-ray images in a computer readable format, offering
an easy and quick access and management.
• Easy to operate, clean and maintain — The Medi-2200 Plus requires virtually
no routine maintenance and no daily cleaning, which is ideal for busy
radiology departments.
System Requirements
In order to use your Medi-2200 Plus, your computer must satisfy the following
system requirements:
• CD-ROM drive (for installing software)
• Color display with 24-bit color output capability
• 512 MB RAM or above
• Pentium IV PC or higher with Hi-Speed USB (USB 2.0)
• Microsoft Windows XP, Vista, Windows 7 and 8
16 Medi-2200 Plus User's Manual

Unpacking Your Film Digitizer
Looking for Damage
While unpacking your digitizer, inspect the shipping carton for any signs of
mishandling or damage. The digitizer’s packing carton and padding material have
been carefully chosen to prevent damage to the unit in shipping and can withstand
a reasonable amount of pressure.
After unpacking your digitizer package, refer to the later section to ensure that you
received all of the parts necessary for digitizer setup. If there are any damaged or
missing parts, notify the shipper immediately or contact customer service.
NOTE: Save the carton and all packing materials. If you need to ship the digitizer
later, it is recommended that you should repack it using the original wire ties,
plastic bags, foam supports and cartons, which protects your digitizer from
unnecessary damage. Refer to the later section “How to Repack Your Film Digitizer
for Shipping” for the detail.
Unpacking Package Contents
After unpacking the shipping carton, follow the steps shown below to take out
your digitizer and other accessory components.
1. Ensure that you put your shipping
carton toward the right direction.
Then, open the top cover.
2. Take out the bags containing the
film templates and accessory
components from the sides of the
carton.
Medi-2200 Plus User's Manual 17

3. With both hands, hold and lift up
two sides of the foam supports
firmly. Before putting down the
package on a steady surface, do
not go off either of hands from
holding the foam supports.
4. Turn over the foam supports and
make them stand vertically.
5. Lift up the upper foam support.
18 Medi-2200 Plus User's Manual

6. Then, pull out the digitizer from the
bottom foam support.
Finally, remove the digitizer from the
wrapped plastic bags and place them on a
flat and stable surface where there is
enough space for positioning the
components.
Once the digitizer has been placed in a
suitable location and is ready to be connected
to the computer, you are ready to step
forward to the installation of the necessary
software and hardware for the digitizer.
Medi-2200 Plus User's Manual 19

Package Contents
After you have unpacked your digitizer package, please check for the major
components listed below.
(I41-014879)
Medi-2200 Plus film digitizer
Power adapter
(PN: 545-00-780400)
(I41-015575)
Power cord
(PN/EU: 121-46-260003)
(PN/US: 121-46-260000)
(PN/TWN: 121-46-260001)
Size 4
Size 3
Size 0
Size 1
Size 2
Intraoral
Intraoral
X-Ray Film Holder
X-Ray Film Holder
Intraoral
X-Ray Film Holder
Panoramic
Panoramic
Panoramic
X-Ray Film Holder
X-Ray Film Holder
X-Ray Film Holder
Film Alignment ruler
(PN: 215-00-780108 )
Hi-Speed USB cable
(PN: 121-44-150504)
20 Medi-2200 Plus User's Manual
Software CD
(I41-015794)
Cephalometric
Cephalometric
X-Ray Film Holder
X-Ray Film Holder
Cephalometric
X-Ray Film Holder

How to Repack Your Film Digitizer for
Shipping
To ship back your Medi-2200 Plus for repairing, maintenance services, or other
reasons, in order to not damage your Medi-2200 Plus, it is strongly recommended
that using the original carton and other packing materials. Follow the steps below
to repack your Medi-2200 Plus correctly.
1. Wrap the digitizer with the plastic bag,
and then insert it into one foam support.
2. Cover down the other foam support on the
top of the digitizer.
Medi-2200 Plus User's Manual 21

3. With both hands, hold the two
sides of foam supports firmly and
then insert them into the shipping
carton.
4. Put the bags containing the film
templates and accessory
components into the sides of the
carton.
5. Close the top cover of the shipping
carton and seal it with tape.
22 Medi-2200 Plus User's Manual

Taking a Closer Look
Front/Open View
No. Names of Parts Functions of Parts
1 Digitizer Lid (TMA) Used to scan dental films.
2 Locking/Unlocking switch Used to lock and unlock the TMA.
3 Glass Surface Scan bed for scanning dental films or reflectives.
4 Vertical Ruler Used to measure the length of the images.
5 Power button Presses to turn on or off the digitizer.
6 Smart-Scan buttons Presses to perform the Smart-Scan function. They function
same as the Smart-Scan button on the software control
panel. These buttons are Intraoral, Panoramic and
Cephalometric.
7 Horizontal Ruler Used to measure the width of the images.
8 Black Mat Used when scanning of reflective materials.
Medi-2200 Plus User's Manual 23

Rear View
No. Names of Parts Functions of Parts
1 Hi-Speed USB Port Connects the Hi-Speed USB (USB 2.0) cable to the computer.
2 15-pin Accessory port Connects the TMA to the digitizer.
3 Power Connector Connects the digitizer to the power adapter.
24 Medi-2200 Plus User's Manual

Installing Your Film Digitizer
Installing the Software
Important: Do not remove the yellow stickers from your digitizer until you are
told to do so. You must install all software before connecting your digitizer.
Always close any open programs and turn off Anti-virus utilities before installing
software.
1. Turn on your computer.
2. Place the Medi-2200 Plus CD-
ROM into your CD-ROM drive.
3. Follow the on-screen instructions
to install the driver and software.
NOTE: If the Microtek Software
Installer screen does not come up
automatically, double-click the
following in succession: “My
Computer”; the CD-ROM icon;
then cdsetup.exe to start the
installer program.
4. To install the software on the
Medi-2200 Plus CD, click each
software program in the order that it appears on the screen to install, and
follow the on-screen instructions.
5. Click EXIT on the Microtek Software Installer screen when all the software has
been installed.
6. Restart your computer.
Drivers & Software Upgrades
After you finish the installation of software, if you found that the installed drivers and software
cannot run your product or your computer system properly later, please go to the Microtek
Download Service site at ww7.microtek.com.tw/service.php to download and install any updates
you may require.
For additional information about Microtek products, please visit our website at www.microtek.com.
Medi-2200 Plus User's Manual 25

Unlocking the Digitizer
1. Remove the yellow “Unlock” stickers from your digitizer.
2. With the digitizer power off, tilt the digitizer and locate the locking switch at
the bottom left corner of the digitizer. Do not turn the digitizer upside down
when attempting to unlock, as this may damage the digitizer’s mechanism.
3. Push the locking switch to the position as indicated in the graphic, with the
icon on the lock showing as “unlocked”.
Locked
4. Raise the digitizer lid (TMA), and look for the locking switch at the base of the
TMA.
5. Push the locking switch to the position as indicated in the graphic, with the
icon on the lock showing as “unlocked”.
Locked
Unlocked
Unlocked
NOTE: To lock the digitizer and the TMA (for shipping and other purposes), turn
the locking switch to the “Locked” position. When locking the digitizer, do not
turn the digitizer upside down when locking the digitizer back.
26 Medi-2200 Plus User's Manual

Connecting the Digitizer
Before connecting the Medi-2200 Plus
with your computer, make sure that the
digitizer lid (TMA) has been properly
installed, with its connector securely
connected to the scanner’s 15-pin
accessory port. If not, plug the
connector of the TMA into the digitizer’s
15-pin accessory port.
To the Power adapter
1. Connect the power adapter to the back of the digitizer.
2. Plug one end of the power cord into the power adapter, and plug the other
end of the power cord into a wall outlet or other power source.
Medi-2200 Plus User's Manual 27

To the Hi-Speed USB (USB 2.0) Cable
3. Connect one end of the Hi-Speed USB cable to your computer.
4. Connect the other end of the Hi-Speed cable to the digitizer's USB port.
5. Press the Power button on the front
panel of the digitizer to turn the
digitizer on; the LED of the button
panel will light up .
The system will detect your digitizer
automatically.
28 Medi-2200 Plus User's Manual
Power Button

Digitizing Dental Films
Intraoral
X-Ray Film Holder
Intraoral
X-Ray Film Holder
Size 0
Size 2
Size 1
Size 4
Size 3
To scan dental films, use the film holders included with your digitizer package,
and match them with the correct types of film to be scanned. There are three types
of dental film holders – Panoramic X-Ray Film Holder, Cephalometric X-Ray Film
Holder, and Intraoral X-Ray Film Holder. During scanning, the use of film holders
ensures the precise alignment of film, which yields consistent scans and aids the
correct performance of the automatic cropping. The use of the individual dental
film holder is explained in the succeeding pages of the manual.
Panoramic X-Ray Film Holder Used to hold two standard panoramic dental
Cephalometric X-Ray Film Holder Used to hold cephalometric dental film,
Intraoral X-Ray Film Holder Used to hold five basic intraoral dental film
film, supporting sizes –
6" x 12" (15 cm x 30 cm) and
5" x 12" (12 cm x 30 cm).
supporting size 8" x 10" (20.3 cm x 25.4 cm).
sizes, Size 0, 1, 2, 3, and 4.
Panoramic
Panoramic
X-Ray Film Holder
X-Ray Film Holder
For panoramic films For cephalometric films
Cephalometric
Cephalometric
X-Ray Film Holder
X-Ray Film Holder
For intraoral films
Medi-2200 Plus User's Manual 29

Removing the Black Mat
The Medi-2200 Plus is come with a Black Mat put on the digitizer lid when
shipping. This Black Mat is designed to work with scanning of reflective materials
such as photographs, documents or prints as well. It is not designed to work with
scanning of X-ray films. During scanning X-ray films, the Black Mat should be
removed to reveal the light source in the digitizer for X-ray films.
To remove the Black Mat:
Raise the digitizer lid (TMA) and push the Black Mat to the side (shown in diagram
1) to remove from the digitizer lid (shown in diagram 2).
30 Medi-2200 Plus User's Manual

Positioning Dental Films
A. Using the Panoramic X-Ray Film Holder
To scan a panoramic film, use the Panoramic X-Ray Film Holder, which can hold a
single piece of panoramic film.
1. Make sure that the Black Mat is removed from the digitizer lid.
2. Place the Panoramic X-Ray Film Holder on the digitizer glass surface. Make
sure to orient the film holder with the “Microtek” logo facing up.
U-shaped edge
Important: Align the holder's
top corners (left and right) of
the U-shaped edge firmly
against the top edge of the
glass surface. When placing the
film holder, make sure that the
Calibration window on the
glass surface is kept clear and
free of obstruction at all times.
Note that the red arrow
marks on both the film
holder and digitizer’s top
ruler are pointing to each
other.
Calibration window
Panoramic
Panoramic
X-Ray Film Holder
X-Ray Film Holder
Microtek logo
3. Place the panoramic film to be
scanned inside the frame of the film
holder, with the letter (R or L)
oriented correctly and with the
correct side up. Then, lower down
the digitizer lid.
Panoramic
Panoramic
X-Ray Film Holder
X-Ray Film Holder
Medi-2200 Plus User's Manual 31

B. Using the Cephalometric X-Ray Film Holder
Cephalometric
X-Ray Film Holder
To scan a cephalometric film, use the Cephalometric X-Ray Film Holder, which can
hold a single piece of cephalometric film.
1. Make sure that the Black Mat is removed from the digitizer lid.
2. Place the Cephalometric X-Ray Film Holder on the digitizer glass surface.
Make sure to orient the film holder with the “Microtek” logo facing up.
U-shaped edge
Important: Align the holder's
top corners (left and right) of
the U-shaped edge firmly
against the top edge of the
glass surface. When placing the
film holder, make sure that the
Calibration window on the
glass surface is kept clear and
free of obstruction at all times.
Note that the red arrow
marks on both the film
holder and digitizer’s top
ruler are pointing to each
other.
Calibration window
Microtek logo
3. Place the cephalometric film to be
scanned inside the frame of the film
holder, with the human head facing to
the right of the digitizer. Then, lower
down the digitizer lid.
32 Medi-2200 Plus User's Manual
Cephalometric
X-Ray Film Holder

C. Using the Intraoral X-Ray Film Holder
Size 0
Size 2
Size 1
Size 4
Size 3
Intraoral
X-Ray Film Holder
Intraoral
X-Ray Film Holder
To scan intraoral dental films, use the Intraoral X-Ray Film Holder, which can hold
up to three Size 0, three Size 1, six Size 2, two Size 3 and one Sise 4 films at a
time.
1. Make sure that the Black Mat is removed from the digitizer lid.
2. Place the Intraoral X-Ray Film Holder on the digitizer glass surface. Make sure
to orient the film holder with the “Microtek” logo facing up.
U-shaped edge
Size 4
Important: Align the holder's
top corners (left and right) of
the U-shaped edge firmly
against the top edge of the
glass surface. When placing the
film holder, make sure that the
Calibration window on the
glass surface is kept clear and
free of obstruction at all times.
Size 3
Note that the red arrow
marks on both the film
holder and digitizer’s top
ruler are pointing to each
other.
Calibration window
Microtek logo
3. Place the Size 0, 1, 2, 3 or 4 film to be
scanned inside the individual frame of
the film holder, with the correct side
up. Then, lower down the digitizer lid.
Size 3
Size 4
Medi-2200 Plus User's Manual 33
Size 0
Size 1
Size 2
Intraoral
Intraoral
X-Ray Film Holder
X-Ray Film Holder

Scanning Dental Films
ScanWizard Medi is a scanning software designed by Microtek for X-ray films or
reflectives scanning used for a medical purpose. Its user-friendly interface enables
you to easily start the scanning process and finish all the scan jobs. Refer to the
ScanWizard Medi software manual for more details.
1. Follow the procedures for “Positioning
Dental Films” to place the film holder and
dental films on the digitizer glass surface.
2. Launch ScanWizard Medi (either as a
Size 3
Size 4
Size 0
Size 1
Size 2
der
der
ol
ol
m H
m H
l
l
Fi
Fi
Intraoral
Intraoral
X-Ray
X-Ray
stand-alone by clicking the program icon,
or by using the “File-Import” or “Scan”
command from an application program).
3. Make sure you are in the “X-Ray” scanning
mode. If not, click the “Scan Material” icon
in the Preview window; then, select “XRay” as your scanning mode.
4. Select your image output type in the Type
drop-down menu.
– Select Gray Scale to scan the image
in 8-bit grayscale.
– Select Gray Scale (12-bit) to scan the image in 12-bit grayscale.
– Select Gray Scale (16-bit) to scan the image in 16-bit grayscale.
5. Select your desired image output resolution in the Resolution drop-down
menu. The default setting is 300 dpi for the X-Ray
scanning mode.
6. If necessary, click the Window Expansion button to
reveal the image correction tools to adjust image
quality.
7. Click the Smart-Scan button on the control panel or
the buttons on the Medi-2200 Plus unit to start
scanning.
– Press the
Holder.
– Press the
Holder.
– Press the button on the digitizer if using the Cephalometric X-Ray Film
Holder.
34 Medi-2200 Plus User's Manual
button on the digitizer if using the Intraoral X-Ray Film
button on the digitizer if using the Panoramic X-Ray Film

• If ScanWizard Medi is launched as a stand-alone program, a window
“Scan To: Save As” dialog box will appear. Click the Save button; then,
the scanned image can be saved into your selected folder or sent to your
selected application.
To view the scanned image, double click the “MSmart Images”
icon on your desktop (default folder assigned by the software),
or retrieve it from your assigned folder.
• If ScanWizard Medi is launched as a Plug-In from an image-editing
program, the scanned image is delivered after the scan to your
application, where the image can be saved, viewed, or printed further.
Medi-2200 Plus User's Manual 35

After Scan
Untitled1
Untitled3
36 Medi-2200 Plus User's Manual
Untitled2

Digitizing Dental Films without Film Holder
If the supplied film holder is not going to be used, Medi-2200 Plus also allows
you to scan the dental films without any film holder on the scan bed.
Using the Film Alignment Ruler
To scan non-standard-size film
such as 8" x 10" film, use the
Film Alignment Ruler, which
allows you to scan film up to 8" x
12" in size.
1. Place the Film Alignment Ruler towards the back of the scanner on the
scanner glass surface.
Calibration strip
Important: Align the Film Alignment
Ruler firmly against the top ruler of the
scanner, with the ruler oriented
correctly and with the correct side up.
During placement of the Ruler, make
sure that the calibration strip on the
Ruler is kept clear and free of
obstruction at all times.
2. Place the film (non-standard
size film) to be scanned on the
scanner glass surface, and
center the film along the Film
Alignment Ruler on the
scanner.
Medi-2200 Plus User's Manual 37

Scanning Dental Films with Film Alignment
Ruler
Follow the steps below to perform your scan without using supplied holders.
1. Follow the procedures for "Using the Film Alignment Ruler" to position the
film to be scanned on the digitizer correctly.
2. Follow the procedures (steps 2 through 6) for “Scanning Dental Films” for
Digitizing Dental Films with Film Holder to launch the ScanWizard Medi
software, and to specify your scanning requirements.
3. Click the Smart-Scan button on the control panel or any buttons on the Medi2200 Plus unit to start scanning.
If you do not use a supplied holder,
then start the scanning process,
when the overview is finished,
ScanWizard Medi will prompt you a
window to ask if you want to
continue the scanning.
You can select the Scan Frame tool from the Toolbar in the Preview window,
and directly resize the scan frame (floating dotted line) around the image by
dragging on the edge of corner of the scan frame.
When done, click the Scan button to continue your scanning.
• If ScanWizard Medi is launched as a stand-alone program, a window
“Scan To: Save As” dialog box will appear. Click the Save button; then,
the scanned image can be saved into your selected folder, or sent to your
selected application.
To view the scanned image, double click the “MSmart
Images” icon on your desktop (default folder assigned by the
software), or retrieve it from your assigned folder.
• If ScanWizard Medi is launched as a Plug-In from an image-editing
program, the scanned image is delivered after the scan to your
application, where the image can be saved, viewed, or printed further.
38 Medi-2200 Plus User's Manual

Scan Frame tool
Medi-2200 Plus User's Manual 39

Digitizing Reflective Originals
To scan reflective originals, such as photographs, documents or prints, you can
still use the Medi-2200 Plus with built-in transparency adapter.
1. Raise the digitizer lid, and place the reflective original to be scanned facing
down on the digitizer glass surface.
2. Place the top end of the document towards the back of the digitizer, then
lower the digitizer lid on the glass surface.
Make sure that the Black
Mat is installed on the
digitizer lid (TMA)
3. Launch ScanWizard Medi (either as a stand-alone by clicking the program
icon, or by using the “File-Import” or “Scan” command from an application
program).
4. Make sure you are in the “Reflective” scanning
mode. If not, click the “Scan Material” icon in the
Preview window; then, select “Reflective” as
your scanning mode.
5. Click the Overview button to perform a preliminary scan of the image, which
will appear in the Preview window.
6. In the Preview window, you can select the Scan Frame tool from the Toolbar,
and resize the scan frame (floating dotted line) around the image by dragging
on the edge or corner of the scan frame to determine the final size of the
actual scan.
7. Specify your scanning requirements in the Settings window.
a) Select a desired image type.
b) Select a desired resolution.
c) Adjust the scan frame settings if necessary.
40 Medi-2200 Plus User's Manual

8. Adjust image quality if necessary, using the Advanced Image Correction (AIC)
tools. Clicking the Window Expansion button to reveal the image correction
tools.
9. If the colors in your reflective originals (e.g. photos) are faded and need
restoring, check the “Automatic Color Restoration” box in the Settings
window.
10. Click the Scan to or Scan button to scan the image.
• If ScanWizard Medi is launched as a stand-alone program, the scanned
image can be saved after the scan to a file, opened in an image-editing
program, or sent to a printer.
• If ScanWizard Medi is launched as a plug-In from an image-editing
program, the scanned image is delivered after the scan to your
application, where the image can be saved, printed, or modified further.
Medi-2200 Plus User's Manual 41

Care and Cleaning
To ensure optimal performance for the Medi-2200 Plus, it is important to clean the
glass surface of the digitizer on a regular basis.
Cleaning the Glass Surfaces
1. Lift up the digitizer lid.
2. Use the cleaning cloth come with your package or a soft, non-abrasive and
lint-free cloth to gently wipe the glass surface area on the digitizer's scan bed.
NOTE: Do not use any detergents, synthetic cleaning solutions, cleaning naphtha,
or other solvents to clean the glass surface directly.
42 Medi-2200 Plus User's Manual

Maintenance
After usage for a period of time, the parts inside your digitizer, such as the light
source, may become worn out, and a problem such as insufficient light supply
may occur. In this cases, contact your local dealers or wholesales for the
maintenance services.
NOTE: Your digitizer need a professional to perform the required service or
maintenance. Do not attend to fix or perform the maintenance by yourself, or it
may cause dangers to you or the digitizer.
Medi-2200 Plus User's Manual 43

Troubleshooting
The LED on the button panel indicate the status of the digitizer.
On - Ready to scan
Flashing - Scanning
Off - Digitizer is off or sleeping
If you encounter any problems, check the section below and follow the suggested
solution if your problem is listed. You may also want to go over the installation
instructions described in the “Installing Your Digitizer” chapter of this manual to
make sure that you have followed all procedures properly.
1. No light comes on when the digitizer is turned on.
Make sure your digitizer is connected to your computer and plugged into a
power source.
2. The Add/Remove Hardware Wizard appears on your screen.
Click the “Cancel” button and close the ScanWizard Medi.
Disconnect the Hi-Speed USB cable from the back of your computer, and refer
to the installation instructions in this manual.
3. When trying to scan, an error message appears on your screen that reads,
“Can’t Find Scanners”.
Make sure your digitizer is unlocked (see “Unlocking the Digitizer” section).
Make sure your digitizer is connected to your computer and plugged in to a
power source (see “Connecting the Digitizer” section).
Uninstall and reinstall the ScanWizard Medi software.
4. After clicking scan, a blank screen appears.
Make sure your digitizer is unlocked (see “Unlocking the Digitizer” section).
LED
Uninstall and reinstall the ScanWizard Medi software.
5. Having trouble scanning X-ray films.
Make sure the TMA (Transparency Media Adapter) is properly installed, with
its connector securely connected to the digitizer’s 15-pin accessory port.
Make sure you place the film holder and film properly on the scan bed. Do not
block the calibration window (as indicated in the “Positioning Dental Films”
section).
44 Medi-2200 Plus User's Manual

Make sure you are in the “X-Ray” scanning mode. If not, click the “Scan
Material” icon in the Preview window; then, select “X-Ray” as your scanning
mode.
Uninstall and reinstall the ScanWizard Medi software.
6. Having trouble scanning reflective originals.
Make sure you place the reflective original to be scanned properly on the scan
bed.
Make sure you are in the “Reflective” scanning mode. If not, click the “Scan
Material” icon in the Preview window; then, select “Reflective” as your
scanning mode.
Uninstall and reinstall the ScanWizard Medi software.
Medi-2200 Plus User's Manual 45

Specifications
Type Desktop flatbed film digitizer
Image Sensor CCD (Charge-Coupled Device)
Lamp Source LED (Light Emitting Diode )
Scanning Modes Grayscale in a single scanning pass
Resolution Optical: 4800 dpi (H) x 9600 dpi (V)
Scanning Area Transparent: 8" x 12" (203 mm x 305 mm)
Product Life Cycle 100,000 scans or 5 years
Interfaces Hi-Speed USB (USB 2.0)
Dimensions (L x W x H) 22.6" x 11.7" x 4.6"
Weight 14.1 lbs (6.4 kg)
Voltage AC 100V to 240V, 50-60 Hz (Input)
Power Consumption 38 W (Max)
Power Supply Voltage Manufacturer Model No.
Environment Operating Temperature: 50°F to 95°F
Input:
16-bit grayscale (Approx. 65,536 shades of gray)
8-bit grayscale (Approx. 256 shades of gray)
Output:
16-bit grayscale (Approx. 65,536 shades of gray)
12-bit grayscale (Approx. 4,096 shades of gray)
8-bit grayscale (Approx. 256 shades of gray)
Reflective: 8.5" x 13.5" (216 mm x 343 mm)
(576 mm x 297 mm x 118 mm)
DC 15V, 2.5A (Output)
100V to 240V MAGIC POWER MPM-X60-15
TECHNOLOGY
CO., LTD.
(10°C to 35°C)
Relative Humidity: 30% to 75%
Storage Temperature: -4°F to 140°F
(-20°C to 60°C)
Relative Humidity: 10% to 85%
Altitudes: <= 2,000 m
Atmosphere Pressure: 70-101.3 KPa
Important
Specifications, software bundles, and accessories are subject to change without
notice. Not responsible for typographic errors.
46 Medi-2200 Plus User's Manual
 Loading...
Loading...