Page 1

Q A - I D S
U s e r & S e r v i c e M a n u a l
Q A - I D S I n f u s i o n P u m p T e s t e r
1
Page 2
Copyright 1998 by METRON. All rights reserved.
METRON:
USA _ FRANCE NORWAY
1345 Monroe NW, Suite 255A 30, rue Paul Claudel Travbaneveien 1
Grand Rapids, MI 49505 91000 Evry, France N-7044 Trondheim, Norway
Phone: (+1) 888 863-8766 Phone: (+33) 1 6078 8899 Phone: (+47) 7382 8500
Fax: (+1) 616 454-3350 Fax: (+33) 1 6078 6839 Fax: (+47) 7391 7009
E-mail: metronus@aol.com E-mail: metronfrance@infonie.fr E-mail: support@metron.no
Disclaimer
METRON provides this publication as is without warranty of any kind, either express or implied, including
but not limited to the implied warranties of merchantability or fitness for any particular purpose. Further,
METRON reserves the right to revise this publication and to make changes from time to time to the content
hereof, without obligation to METRON or its local representatives to notify any person of such revision or
changes. Some jurisdictions do not allow disclaimers of expressed or implied warranties in certain transactions;
therefore, this statement may not apply to you.
Limited Warranty
METRON warrants that the QA-IDS Infusion Pump Tester will substantially conform to published specifications and to the documentation, provided that it is used for the purpose for which it was designed. METRON will,
for a period of twelve (12) months from date of purchase, replace or repair any defective analyzer, if the fault is
due to a manufacturing defect. In no event will METRON or its local representatives be liable for direct, indirect, special, incidental, or consequential damages arising out of the use of or inability to use the QA-IDS Infusion Pump Tester, even if advised of the possibility of such damages. METRON or its local representatives are
not responsible for any costs, loss of profits, loss of data, or claims by third parties due to use of, or inability to
use the QA-IDS Infusion Pump Tester. Neither METRON nor its local representatives will accept, nor be bound
by any other form of guarantee concerning the QA-IDS Infusion Pump Tester other than this guarantee. Some jurisdictions do not allow disclaimers of expressed or implied warranties in certain transactions; therefore, this
statement may not apply to you.
Trademarks
IBM is a registered trademark, and PC/XT is a trademark of IBM Corporation. Centronix is a registered trademark of Centronix Corporation. Microsoft is a registered trademark and Windows is a trademark of Microsoft
Corporation.
2
Page 3

Table of Contents
MANUAL REVISION RECORD............................................................................................5
1. INTRODUCTION..................................................................................................................7
1.1 QA-IDS Features .........................................................................................................................7
1.2 Specifications................................................................................................................................7
1.3 General Information......................................................................................................................8
2. INSTALLATION...................................................................................................................9
2.1 Receipt, Inspection and Return.....................................................................................................9
2.2 Setup............................................................................................................................................10
2.3 Power ..........................................................................................................................................10
2.4 PRO-Soft QA-IDS.......................................................................................................................10
3. OPERATING QA-IDS........................................................................................................13
3.1 Control Switches and Connections.............................................................................................13
3.2 Key Pad Functions.....................................................................................................................14
3.3 Menu and Function Keys............................................................................................................14
3.4 Display Menus and Messages ...................................................................................................15
3.5 Printing Test Reports.................................................................................................................20
3.6 Operator Maintenance................................................................................................................22
4. INFUSION PUMP TESTING.............................................................................................23
4.1 Introduction................................................................................................................................23
4.2 Test Preparation..........................................................................................................................23
4.3 Occlusion Pressure/ Bolus Volume Testing ..............................................................................25
4.4 Infusion Flow Rate and Volume Testing....................................................................................26
5. CONTROL AND CALIBRATION....................................................................................29
5.1 Required Test Equipment...........................................................................................................29
5.2 Adjusting the Display Contrast ..................................................................................................29
5.3 Testing Power Supply to IDS.....................................................................................................29
5.4 Testing Pressure Gauge Accuracy..............................................................................................30
5.5 Testing Flow Rate Accuracy.....................................................................................................30
5.6 Checking the Battery Backup.....................................................................................................31
6. COMPONENT FUNCTIONS AND PARTS....................................................................33
6.1 Theory of Operation....................................................................................................................33
6.2 Power Supply..............................................................................................................................33
6.3 Printer Output..............................................................................................................................33
6.4 Serial Port...................................................................................................................................34
6.5 Microprocessor............................................................................................................................34
6.6 Pressure Gauge............................................................................................................................34
6.7 Pump............................................................................................................................................34
6.8 Stepper Motor/Drivers................................................................................................................34
6.9 Control Panel...............................................................................................................................35
6.10 Component Parts......................................................................................................................35
3
Page 4

APPENDIX A: DIAGRAMS......................................................................................39
Processor board Component Location...........................................................................................41
6.10.1 Processor board Schematic Diagram 1 ...............................................................................42
6.10.2 Processor board Schematic Diagram 2 (Digital Part) .......................................................43
6.10.3 Processor board Schematic Diagram 3 (CPU) ..................................................................44
6.10.4 Processor board Schematic Diagram 4 (Memory) ............................................................45
6.10.5 Processor board Schematic Diagram 5 (RS232C and Printer) .........................................46
6.10.6 Processor board Schematic Diagram 6 (Digital Outputs) .................................................47
6.10.7 Processor board Schematic Diagram 7 (Digital Inputs) ....................................................48
6.10.8 Processor board Schematic Diagram 8 (A/D) ...................................................................49
6.10.9 Processor board Schematic Diagram 9 (Stepper Motor and Drivers) ..............................50
6.10.10 Processor board Schematic Diagram 10 (Pressure Sensor and Temperature) ................51
6.10.11 Processor board Schematic Diagram 11 (Main Part) ......................................................58
6.10.12 Processor board Schematic Diagram 12 (Keyboard, Pressure Sensor and Pump Index)
..........................................................................................................................................................59
APPENDIX B: ERROR REPORT FORM, QA-IDS.........................................................62
APPENDIX C: SUGGESTION FORM, QA-IDS...............................................................65
4
Page 5

Manual Revision Record
This record page is for recording revisions to your QA-IDS User and Service Manual that have
been published by METRON or its authorized representatives. We recommend that only the management or facility representative authorized to process changes and revisions to publications:
• make the pen changes or insert the revised pages;
• ensure that obsolete pages are withdrawn and either disposed of immediately, or marked as
superseded and placed in a superseded document file, and;
• enter the information below reflecting that the revisions have been entered.
Rev No Date Entered Reason Signature of Person Entering Change
0 - Initial Release
5
Page 6

This page intentionally left blank.
6
Page 7
1.1 QA-IDS Features
1. Introduction
This chapter describes the Metron’s QA-IDS Infusion Pump Tester’s
features and specifications.
METRON’s QA-IDS Infusion Pump Tester is a precision instrument, designed for use by trained service technicians, for testing all
types of infusion pumps according to International Electrotechnical
Commission (IEC) Draft Standard 62.D (IEC 601.2.24). Tests include:
• volumetric tests
• bolus tests
• occlusion alarm tests
QA-IDS is capable of detecting a minimum volume variation of one
microliter in a range of 0.10 milliliter per hour (ml/hr) to 1000.0
ml/hr. Flow measurements are taken every 30 seconds, so that the
measurements are independent of the infusion pump’s flow rate.
Test results, shown in the QA-IDS's LCD display, can be printed out
directly, or transferred to a PC via the PRO-Soft QA-IDS test automation software. PRO-Soft lets you design test protocols, remotely
control the QA-IDS, and store the test results.
1.2 Specifications
1. Flow Rate
Flow Range 0.10 ml/hr - 1000.0 ml/hr
Min. volume detection 0.72 µl
Display resolution 0.01 ml/hr
2. Time Interval to Achieve ± % Accuracy of Reading
1000 ml/hr 100 ml/hr 10 ml/hr
0.5% 0.6 sec 0.5% 6 sec 0.5% 52 sec
1.0% 0.3 sec 1.0% 3 sec 1.0% 26 sec
1.5% 0.2 sec 1.5% 2 sec 1.5% 18 sec
3. Pressure Generation
Range: - 200 to + 600 mmHg
Accuracy:
- 200 to + 200 mmHg: ± 10 mmHg
+ 201 to + 600 mmHg: ± 20 mmHg
4. Occlusion Alarm Test
Measurement Range: - 400 to +1500 mmHg.
7
Page 8

Accuracy:
- 400 to + 500 mmHg: ± 10 mmHg
+ 501 to + 1500 mmHg: ± 2% of reading
Maximum Input Pressure: 2500 mmHg
5. Bolus Test
Accuracy: ± 20 µl
1.3 General Information
Temperature Requirements
+15°C to +35°C when operating
0°C to +50°C in storage
Display
Type LCD graphic display
Alphanumeric format 4 lines, 40 characters
Display control 7 function keys and a keypad
Data Input/ Output (2) Parallel printer port (1); Bi-directional RS
-232C (1) for Computer control
Power From 110 VAC to 240 VAC, 47 / 63 Hz.
Mechanical Specifications
Housing Metal case
Height 13.5 cm / 5.31 in.
Width 23.5 cm / 9.25 in.
Depth 24.5 cm / 9.65 in.
Weight 4.30 kg / 9.48 lbs.
Printer Port Centronics Interface
Standard Accessories
User and Service Manual QA-IDS (P/N 15035)
Additional Accessories
Carrying case (P/N 15100)
Carrying case, ext. printer (P/N 10500)
PRO-Soft QA-IDS software (P/N 15200)
PRO-Soft QA-IDS DEMO (P/N 15201)
User Manual PRO-Soft QA-IDS (P/N 15205)
Storage
Store in the carrying case in dry surroundings within the temperature range specified. There are no other storage requirements.
Periodic Inspection
The unit should be calibrated every 12 months.
8
Page 9

2.1 Receipt, Inspection
and Return
2. Installation
This chapter explains unpacking, receipt inspection and claims, and
the general procedures for QA-IDS setup.
1. Inspect the outer box for damage.
2. Carefully unpack all items from the box and check to see
that you have the following items:
• QA-IDS Infusion Pump Tester (P/N 15000)
• User and Service Manual QA-IDS (P/N 15035)
3. If you note physical damage, or if the unit fails to function
according to specification, inform the supplier immediately. When METRON AS or the company’s representative, is informed, measures will be taken to either repair the
unit or dispatch a replacement. The customer will not have
to wait for a claim to be investigated by the supplier. The
customer should place a new purchase order to ensure delivery.
4. When returning an instrument to METRON AS, or the
company representative, fill out the address label, describe
what is wrong with the instrument, and provide the model
and serial numbers. If possible, use the original packaging
material for return shipping. Otherwise, repack the unit using :
• a reinforced cardboard box, strong enough to carry the
weight of the unit.
• at least 5 cm of shock-absorbing material around the unit.
• nonabrasive dust-free material for the other parts.
Repack the unit in a manner to ensure that it cannot shift in the
box during shipment.
METRON’s product warranty is on page ii of this manual. The
warranty does not cover freight charges. C.O.D. will not be accepted without authorization from METRON A.S or its representative.
9
Page 10
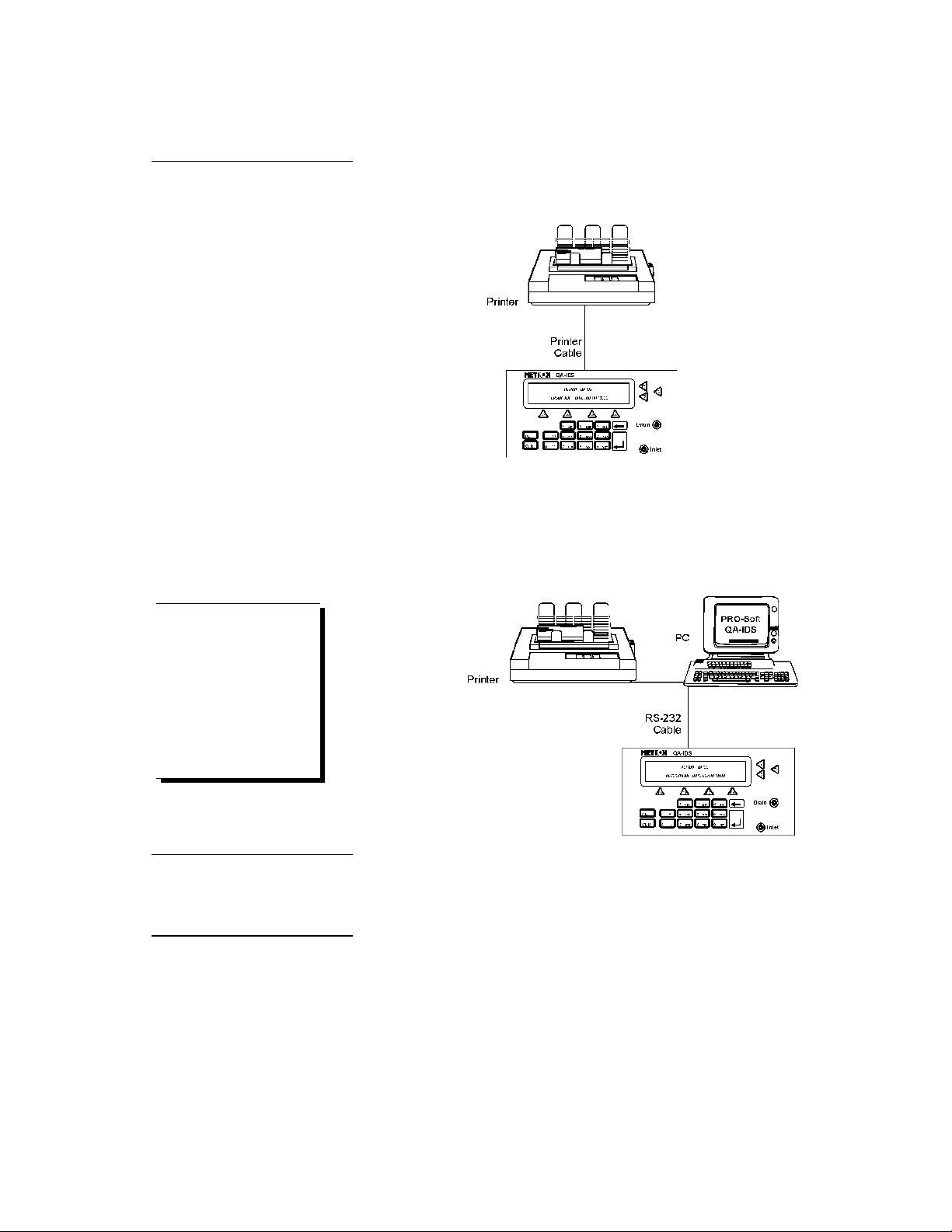
2.2 Setup
1. Equipment connection is as shown in the typical setup below.
2. If PRO-Soft QA-IDS is being used, attach an RS-232 (null
modem/data transfer configured) cable to the 9-pin D-sub
outlet port located at the rear of the QA-IDS. Do not attach
the printer cable to the QA-IDS. See below. However, if
you are not using PRO-Soft QA-IDS, and are sending directly to a printer for printouts, attach the printer cable to
the 25-pin outlet port .
Some RS-232C cables are
NO T E
missing the connection between the seventh and the
eighth wires in the cable.
The cable may still be called
NULL-modem, but it will not
work with the QA-IDS. Refer
to the PRO-Soft QA-IDS
Users Manual for more information.
2.3 Power
2.4 PRO-Soft QA-IDS
Main On/Off Switch. QA-IDS should remain off for at least 5 seconds before switching on again, in order to allow the test circuits to
discharge fully.
PRO-Soft QA-IDS is a front-end test automation and presentation
tool for METRON's QA-IDS Infusion Pump Tester. It allows you to
conduct the same tests, but by remote control via an IBM-compatilbe PC/XT with MS Windows (Version 3.1 or later).
10
Page 11
PRO-Soft QA-IDS has its
NO T E
own user manual which
contains all the information
concerning the program. If
you order a demonstration
version of the program you
also receive the manual.
Each of the QA-IDS tests can be run independently from PRO-Soft
in the “Manual” test mode. Results are shown on the PC screen during testing, and the user is prompted to set the tested equipment accordingly. At the conclusion of tests, the user may print a report,
store the test and results on disk, or both. Combinations of tests can
be created and stored as “Test Sequences.” The program maintains a
library of these sequences. In this way you can store and retrieve sequences that are appropriate for each infusion device being tested at
your facility.
Sequences can then be used independently, or can be attached to a
checklist, written procedure, and equipment data in the form of a
test “Protocol.” The equipment data can be entered manually into
the protocol, or it may be retrieved by PRO-Soft from the QA-MAP
program or other equipment files. Protocols can be created easily for
each infusion pump in your inventory, and stored for use. Test protocols with results can be printed, or stored on disk, and the results
of testing can be sent back to the equipment database to close a
work order and update the service history.
11
Page 12

This page intentionally left blank.
12
Page 13
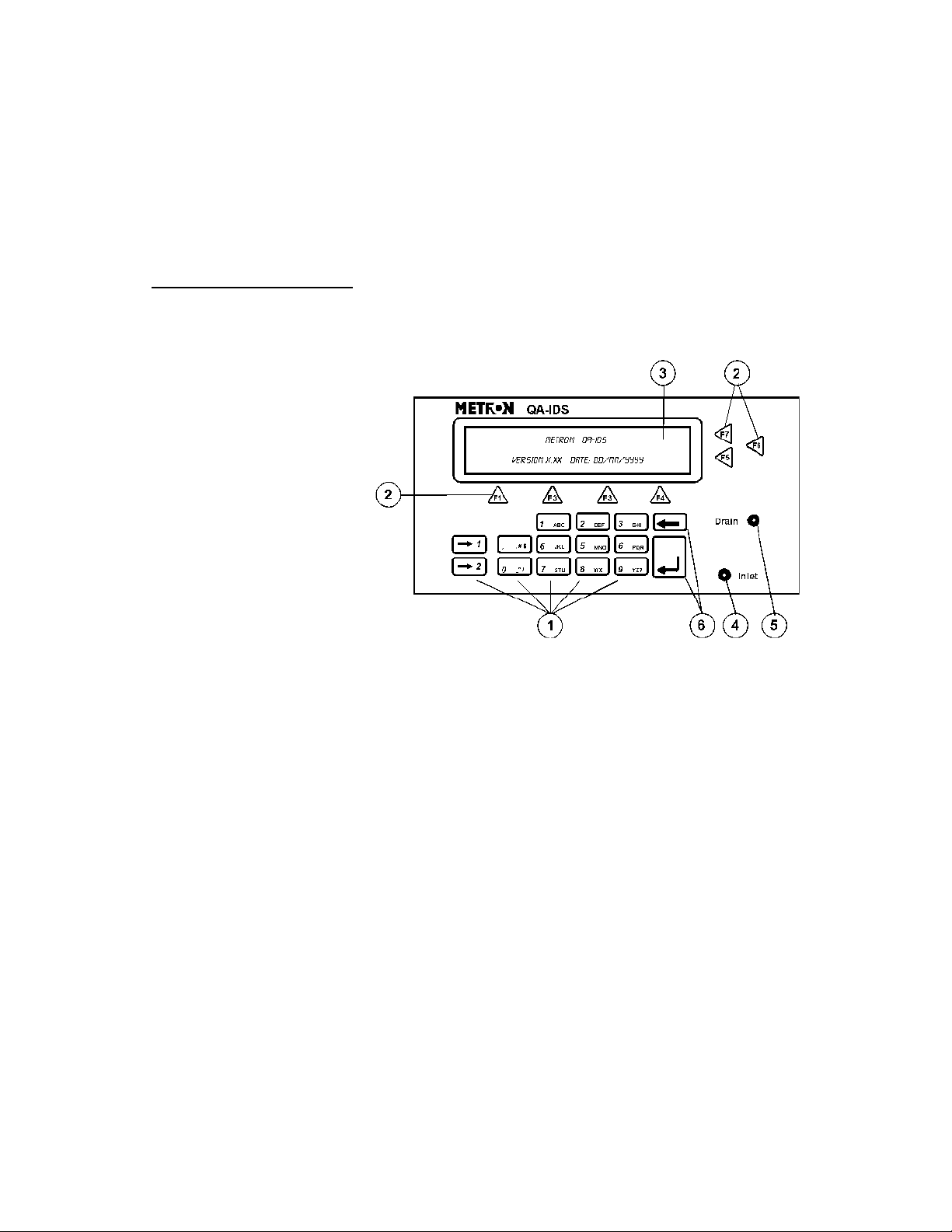
3.1 Control Switches and Connections
Front Panel
3. Operating QA-IDS
This chapter explains the operating controls, switches and menus of
the QA-IDS, details how to use them in testing, and provides instructions for printing reports, and operator maintenance.
1. Key Pad 11 alphanumeric keys, used to enter information.
2. Function Keys F1-F4 are used to select the functions shown in the
3 LCD Display Shows messages, test results and function menus.
4 Inlet Inlet connection for infusion set.
5. Drain Connection for drainage tube.
6. Upgrade Keys Special function keys for firmware upgrade.
menu bar at the bottom of the display, i.e., for selecting the function that is directly above the key.
F5-F7 are used to select the function, or enter information in the message field in the same line.
13
Page 14
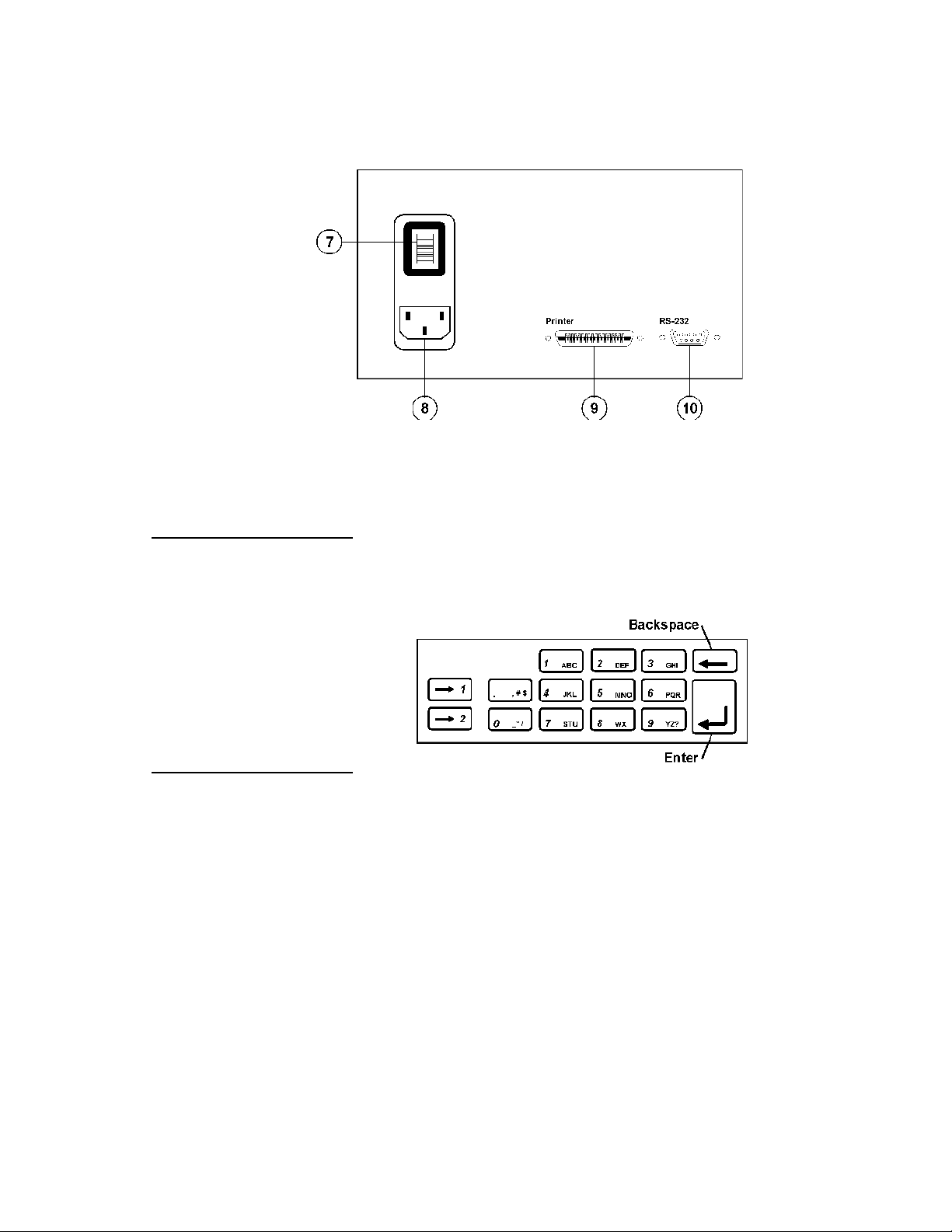
Rear Panel
3.2 Key Pad Func-
tions
7. Power Switch Turns power ON and OFF.
8. Mains QA-IDS Mains connection for test instrument.
9. Printer Outlet Port 25 pin D-sub. Centronic output.
10. RS-232 Serial Port 9-pin D-sub
The alphanumeric keys comprise both numbers and letters. Hold the
key in and it moves automatically from character to character.
3.3 Menu and Function Keys
14
The QA-IDS uses displays, function keys and a keypad to provide
flexibility and control over operations. The top three lines in the display are used for messages, status and results. The menu bar is
shown at the bottom of the display. Function keys are numbered
from F1 to F7.
A function/menu is selected by pressing that key which is directly
below/to the right of the menu unit shown in the display.
Page 15
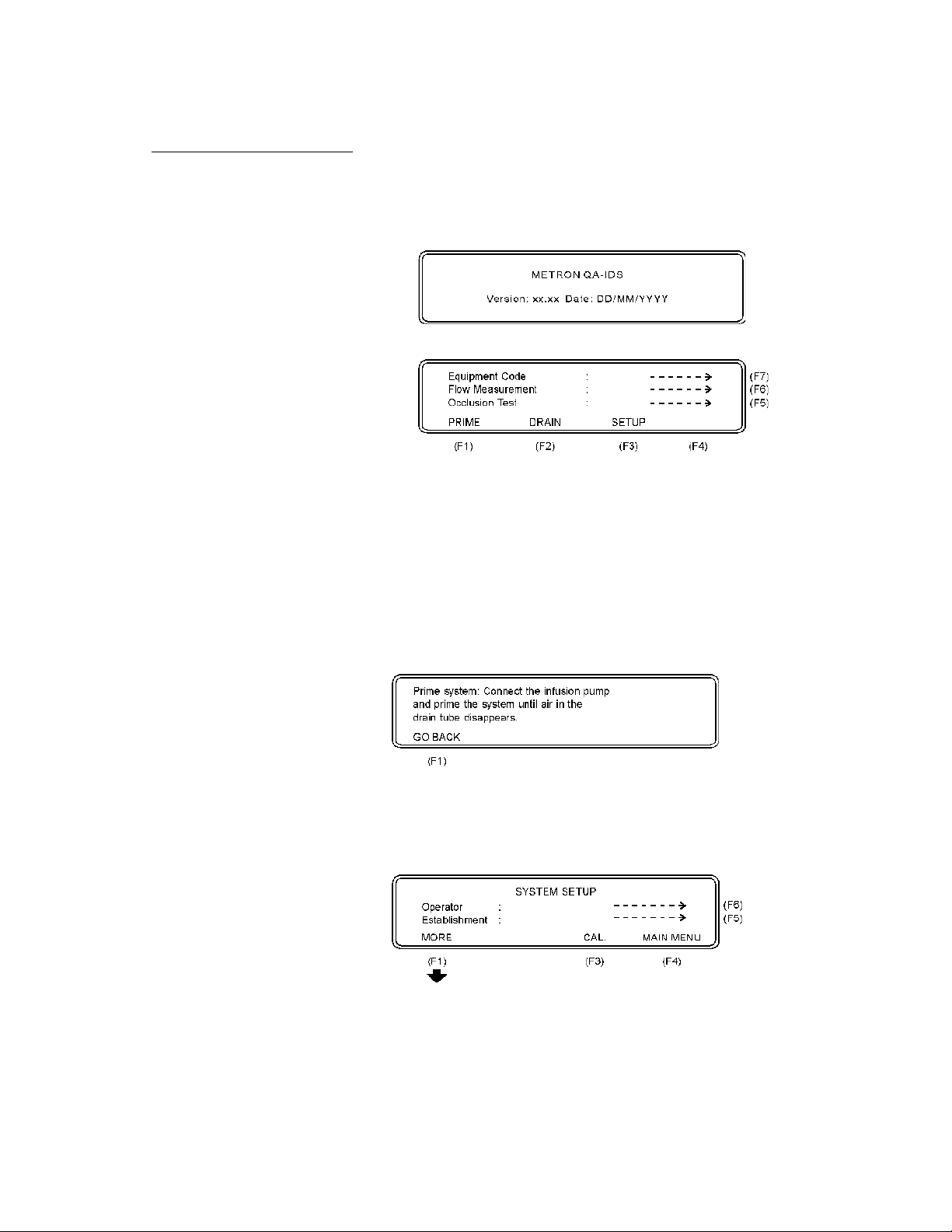
3.4 Display Menus and Messages
1. Startup Screen. The following screens will be displayed
in sequence for the first two seconds after the QA-90 has
been switched ON.
2. Main Menu
This window offers the following functions:
• Press Occlusion Test (F5) to go to the Occlusion Test
Setup Screen. See paragraph 3.4.5 below.
• Press Flow Measurement (F6) to go to the Flow Measure-
ment Test Setup Screen. See paragraph 3.4.6 below.
• Press Equipment Code (F7) to record the code or name of
the device under test. Press Enter (↵ ) to store.
3. PRIME (F1). When PRIME (F1) is pressed, the following display will appear:
• Press GO BACK (F2) to return to the main menu.
4. SETUP (F3)
This function is used for entering general information in connection with the test. Three main displays are shown below.
• Press Operator (F6) to record the operator’s name or ini-
tials.
15
Page 16
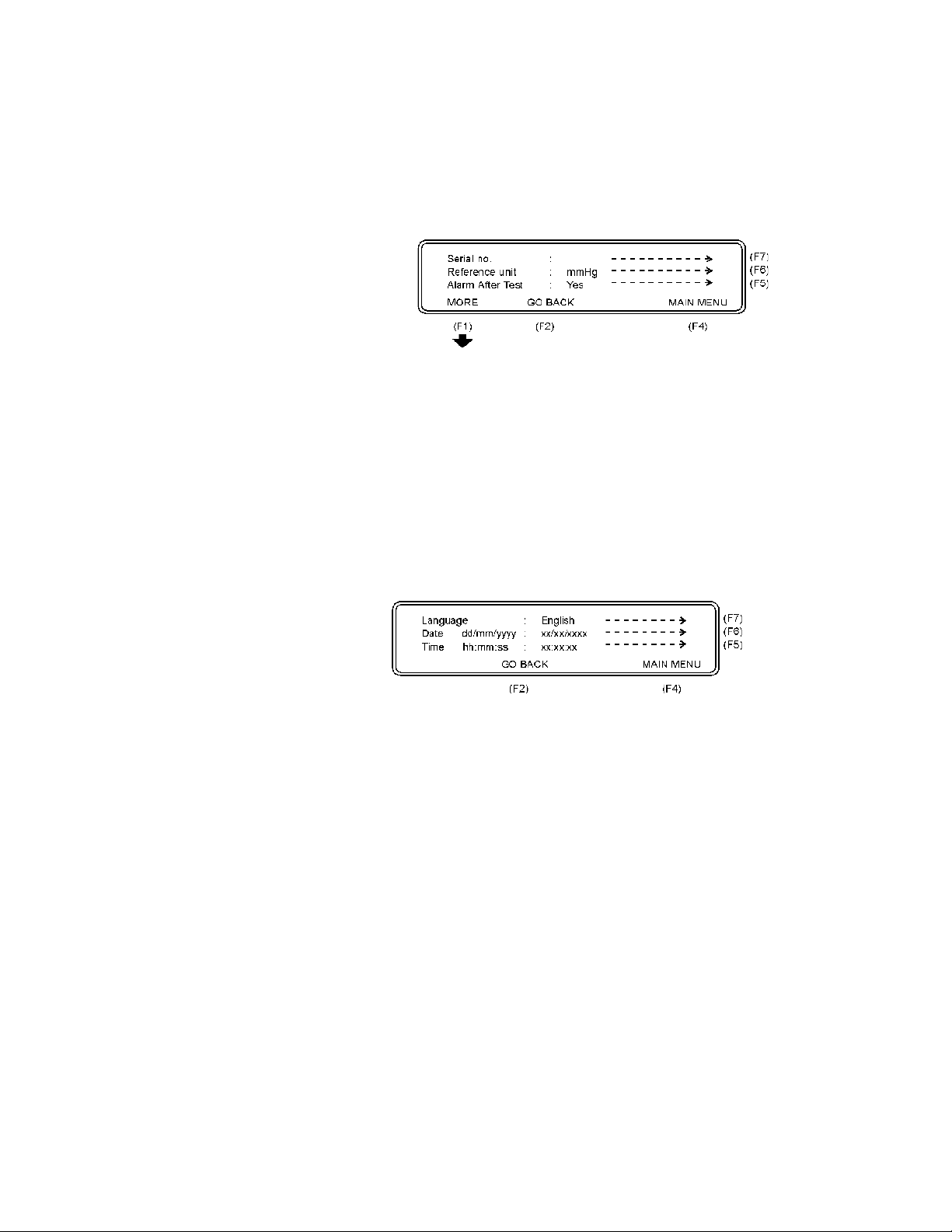
• Press Establishment (F5) to record the establishment.
• Press MAIN MENU (F4) to return to the main menu.
• Press MORE (F1) and the following display will appear:
• Press Serial no (F7) to record the serial number of the QA-
IDS being used.
• Press Reference Unit (F6) to select the reference unit, as
follows:
mmHg mBar kg/cm
cmH2O kPa inHg PSI
2
inH2O
• Press Alarm After Test (F5) and select Yes or No.
• Press GO BACK (F2) to return to the previous display.
• Press MAIN MENU (F4) to return to the main menu.
• Press MORE (F1) and the following display will appear:
• Press Language (F7) to select the language used for the
testing.
• Press Date (F6) to set the day/month/year.
• Press Time (F5) to set the hour/minute/second.
• Press GO BACK (F2) to return to the previous display.
• Press MAIN MENU (F4) to return to the main menu.
5. Occlusion Test (F5)
The Occlusion Alarm Test is used to protect the patient. An Occlusion Pressure Alarm will be activated if the pressure inside
the administration set extends preset levels. The three main test
displays are shown below.
16
Page 17

• Press Flow Set (F7) to set the preset value of the infusion
pump.
• Press Alarm Prsr (F6) to set the preset pump value.
• Press START (F2) to start the Occlusion Test, and the fol-
lowing display will appear:
The following parameters are displayed:
Prsr: The pressure in the connction tube. Measurement results
Time: The time from test start until alarm activation. Measure-
Bolus: The volume expansion within the tube when the infusion
display: max. value > instantaneous measured value >
mean value in mmHg.
ment results display: max. time > elapsed time > mean
time in minutes/seconds.
pump alarm activates. Measurement results display: max.
bolus > last executed calculation after STOP is pressed >
mean bolus in milliliters.
• Press GO BACK (F4) to return to the previous menu.
• Press STOP (F2) to stop the test.
• Press START (F2) to return to the previous menu.
• Press GO BACK (F4) to return to the previous display.
6. Flow Measurement (F6)
This function is used for entering flow information in connection with the test. There are four main displays, shown below:
17
Page 18
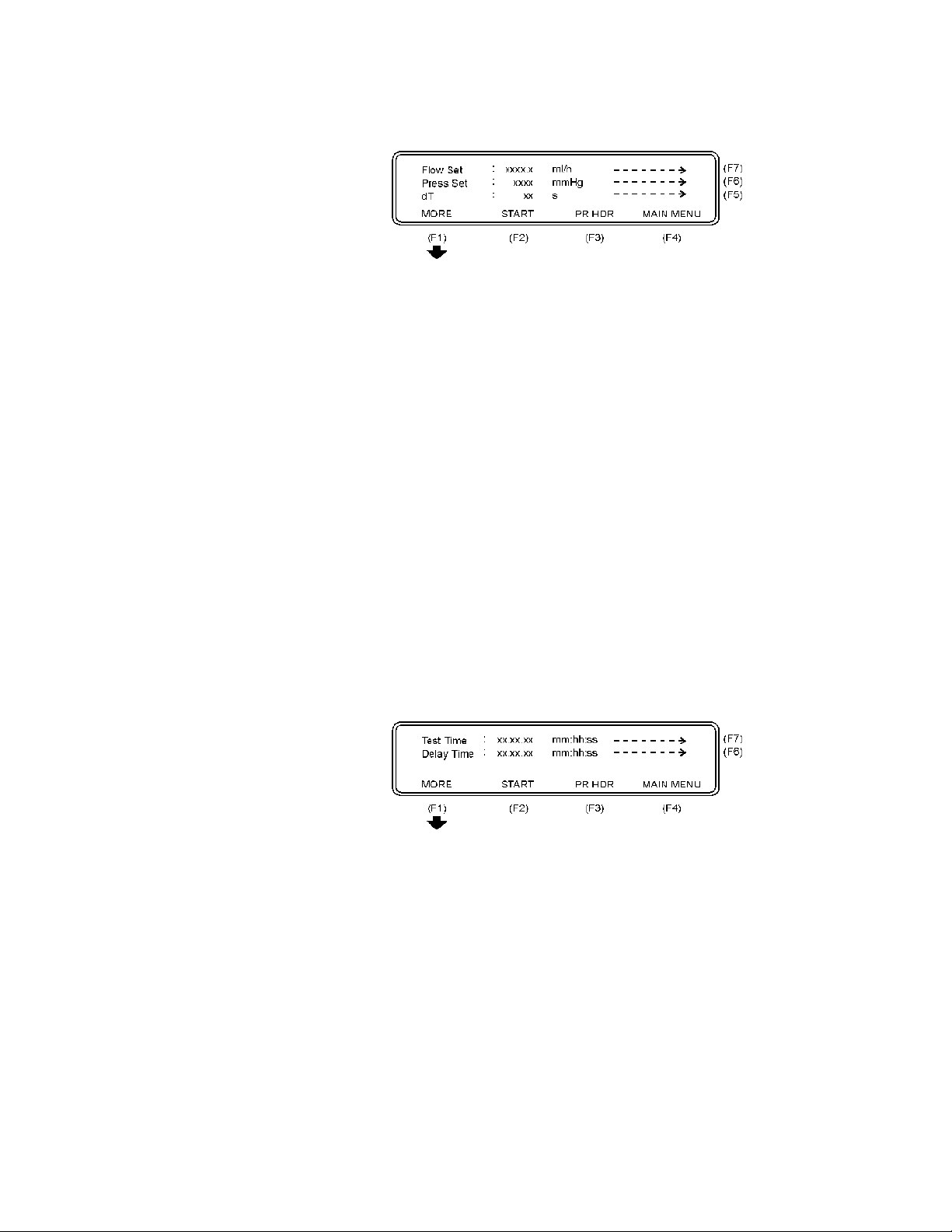
The following parameters are displayed:
Flow Set: This value is the preset value of the infusion pump. All er-
Press Set: This function enables you to enter + (positive) or - (nega-
dT: Interrogation time, or time between two measurements.
ror calculations of the flow measurement are related to
this value. If the Flow Set value is not entered correctly,
this may cause a poor presentation with incorrect overall
errors.
tive) backpressure into the system. Operation range is
from -200 to +600 mmHg.
Average value during the test interval is displayed. Recommended dT from IEC is 30 sec, and this is the minimum value to be selected on the QA-IDS. Maximum dT is
600 sec. or 10 minutes. Default value is 30 sec.
• Press Flow Set (F7) to set the preset value of the infusion
pump.
• Press Press Set (F6) to set the relevant + or - backpressure
into the system.
• Press dT (F5) to set the interrogation time, or time between
two measurements.
• Press PR HDR (F3) to print a header.
• Press MAIN MENU (F4) to return to the Main Menu.
• Press MORE (F1) and the following screen will appear:
18
The following parameters are displayed:
Test Time: This is the preset time for a test. Maximum testing time
is 24 hours. Default value is 1 hour.
Delay Time: This is the delay before the first measuring.
• Press Test Time (F7) to set the actual time of the test.
• Press Delay Time (F6) to set the delay before the first mea-
surement.
• Press MORE (F5) to return to the previous display.
• Press PR HDR (F3) to print a header.
Page 19
• Press MAIN MENU (F4) to return to the Main Menu.
• Press START (F2) to start flow measurement, and the
Flow Measurement 1 display will appear:
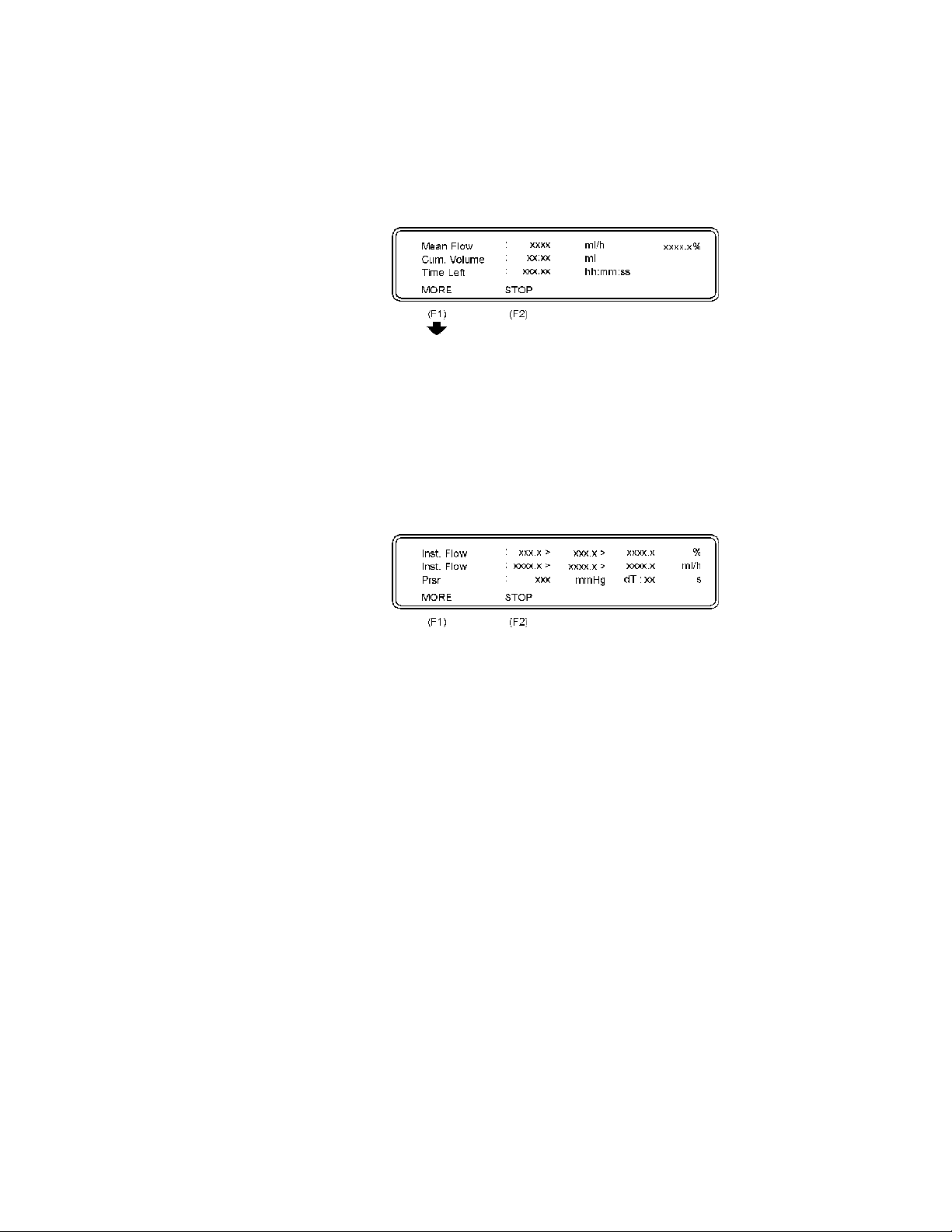
The following parameters are displayed:
Mean Flow: The flow of the liquid volume in infusion pump every
Cum. Volume: The cumulative volume from start of the test.
Time Left: The remaining time before the test is finish.
second displayed in ml/h and %.
• Press STOP (F2) to return to the Flow measurement setup.
• Press MORE (F1) and the Flow Measurement 2 display
will appear.
The following parameters are displayed:
Inst. Flow: Instantaneous Flow shows: max. value > mean value
Inst. Flow: Instantaneous Flow shows: max. value > mean value
Prsr: The pressure in the connection tube.
> minimum value in %.
> minimum value in ml/h.
• Press STOP (F2) to return to the previous display.
• Press MORE (F1) to return to the Flow Measurement
Setup.
19
Page 20

3.5 Printing Test Reports
Hard copy printouts of test results can be made if a printer is connected to the QA-IDS. See paragraph 2.2 for connecting the printer.
To obtain a printout of the test, select PR HDR (F3) at the completion of the test. As can be seen by the following examples, the printouts contain:
• QA-IDS unit, operator and establishment identification.
• Tested equipment identification
• Test setup data, and detailed reselts.
• Test summary
• Comments
1. Example Printout of a Flow Measurement Test.
METRON QA-IDS Infusion pump tester Ver.
1.17
Operator : Establishment :
Equipment Code : ___________________________________________
Serial no. : ___________________________________________
Status : ___________________________________________
Group : ___________________________________________
Manufacturer
:
Type : ___________________________________________
Model : ___________________________________________
Location : ___________________________________________
Flow set : ml/h Test Time : s
Press Set : mmHg Delay Time : s
dT
:
dT
No.
*****************************************************************
*********
Mean Flow : ml/h
Max. Peak Flow : ml/h
Min. Peak Flow : ml/h
Cum. Volume : ml
Measurement Time : s
Comments : ___________________________________________
Date-Time/Signature ______________________________
- - EQUIPMENT INFORMATION - -
___________________________________________
- - SETUP DATA FLOW MEASUREMENT TEST - -
s
Pressure
(mmHg)
Cum. Volume
(ml)
- - TEST SUMMARY - -
___________________________________________
Inst. Flow
(ml/h) (%)
Serial no.:
Mean Flow
(ml/h) (%)
20
Page 21

2. Example Printout of an Occlusion Test.
METRON QA-IDS Infusion pump tester Ver.
1.17
Operator : Establishment :
Equipment Code : ___________________________________________
Serial no. : ___________________________________________
Status : ___________________________________________
Group : ___________________________________________
Manufacturer
:
Type : ___________________________________________
Model : ___________________________________________
Location : ___________________________________________
Flow set : ml/h Pump type :
Alarm pressure : mmHg
Occlusion
Test no.
*****************************************************************
*********
No. of occlusion
tests :
Mean bolus : ml
Mean alarm pressure : mmHg Mean stop pressure :
Comments : ___________________________________________
Date-Time/Signature ______________________________
- - EQUIPMENT INFORMATION - -
___________________________________________
- - SETUP DATA OCCLUSION TEST - -
Alarm time
(mm:ss)
- - TEST SUMMARY - -
Bolus
(ml)
Mean alarm time :
___________________________________________
Serial no.:
Alarm pressure
(mmHg)
Stop pres-
sure
(mmHg)
The tested equipment and comments portions enable the operator
to manually enter appropriate remarks.
3. Presentation of Measuring Data
Instant Flow: Flow rate measured during the last interrogation.
Maximum Flow: Max. flow detected during one interrogation period.
Minimum Flow: Min. flow detected during one interrogation period.
Mean flow: Mean flow from start of test till elapsed time.
Cumulative Volume: Accumulated or delivered volume during the test.
Measured Pressure: Measured pressure in the flowline.
Elapsed Time: Elapsed time from start of test.
Time Left: Time left of a preprogrammed test sequence.
Delay Time: Delay for calculation of measuring values for a test.
4. Scaling Conversion from mmHg to Selected Pressure Unit
mmHg = 1 kg/cm2= 0.0013
21
Page 22

3.6 Operator Maintenance
cmH20 = 1.3 inHg = 0.03937
mBar = 0.013 inH2O = 0.5118
kPa = 0.13 PSI = 0.01885
1. Draining the QA-IDS. To ensure proper functioning, and
reduce the chance of malfunction, the QA-IDS should be
drained:
• Daily after use.
• Prior to storage.
• Prior to being transported.
To drain the QA-IDS:
• Disconnect the infusion set from the Inlet.
• Press DRAIN (F2) in the Main Menu. The following dis-
play will appear:
• Drain the unit until the liquid in the drain tube disappears.
• Press GO BACK (F2) to return to the main menu.
2. Cleaning. The QA-IDS should be cleaned every six
months, in accordance with the following procedure:
• Use any of the following cleaning materials:
- Sodium hypochloride, 4% solution in distilled water.
- Acetic Acid, 4% solution in distilled water.
- Bicarbonate of Soda, 4% solution in distilled water.
• Mix the cleaning fluid in distilled water.
• Prime the QA-IDS with the mixture (See paragraph 3.4.3).
• Allow the mixture to remain in the system for 30 minutes.
• Drain the unit, as above
• Repeat this procedure two to three times.
• Set the QA-IDS on a one-hour flow test, with a flow rate of
100 ml/hr, using distilled water.
22
Page 23

4.1 Introduction
4. Infusion Pump Testing
This chapter details procedures for conducting each of the QA-IDS
tests.
Infusion pump testers calculate flow rates from a measured time period to fill a defined volume. If it takes one hour to fill a defined
volume of 10 milliliters, the flow rate is calculated to be 10 ml/hr.
This flow rate is called the instant flow rate. The minimum volume
detection of this measurement is 10 milliliters, since it needs that
amount to calculate a flow rate. The next instant flow rate calculation may be done when an additional volume of 10 ml is delivered
to the measuring device. When two or more instant flow rates have
been obtained, the calculation of a mean flow rate can be done by
averaging the instant flow rates.
Detecting unexpected flow changes requires detection of tiny infusion volumes, as flow measurements may only be calculated every
time the defined volume is filled up. The ability to detect small infusion volume requires that measurements be made frequently, hence
a high flow-sampling rate. The ability to detect only larger infusion
volumes decreases the sampling rate, as well as the ability to accurately detect flow changes. International standards prescribe flow
sampling to be done every thirty seconds for all flow rates to ensure
a steady rate of infusion from all types of infusion devices. To fulfill
this requirement, minimum volume detection of the measuring device is critical.
Example:
4.2 Test Preparation
To test an infusion pump for unexpected flow changes at a flow rate
of 10 ml/hr, with an accuracy of ± 2%, the following minimum flow
detection is needed:
Expected flow: 10 ml/hr = 166.7 pl pr. minute (divide by 60) = 83.3 µl
pr. 30 sec. (divide by 2).
2% of expected flow: 83.3 µl pr. 30 sec. x 2% = 1.67 µl pr. 30 sec.
A volume detection of 1.67 µl is required to detect a 2% unexpected, sudden flow change from an expected flow rate of 10 ml/hr
within 30 seconds.
The minimum volume detection of QA-IDS is 0.72 µl. That means
that less than 1 µl is needed for QA-IDS to detect any sudden flow
change between 0.10 ml/hr and 1000.0 ml/hr. QA-IDS performs
flow-samplings every second for all flow rates.
23
Page 24

WAR NI N G
Do not start measuring with the QA-IDS without having liquid in the
system, as it may be exposed to wear.
IMP O RT A N T
1. METRON recommends sterile, distilled water in test use, as tap water
may contain too much oxygen, and potentially destructive deposits.
1. Remove all air inside the administration set before connecting it to the
QA-IDS. Air passing through the measurering system will give incorrect
readings.
1. Set up the test components. See below.
• Connect the infusion pump being tested to the QA-IDS In-
let Port and to an infusion container.
• Switch the QA-IDS ON. The following will be displayed in
the LCD display for about two seconds:
• Fill the administration set with liquid (Metron recommends
distilled water).
2. Prime the QA-IDS. Before starting the measurements, the
internal volume of the QA-IDS has to be filled with liquid .
• Connect the administration set to the inlet of the QA-IDS.
• Press PRIME (F1) in the Main menu and the following dis-
play will appear.
24
Page 25

CAUTION
Prime the unit properly.
4.3 Occlusion Pressure/ Bolus Volume Testing
• Start the infusion pump with a high flow rate. Keep the
QA-IDS working until there is no more air in the tubes.
Timeout is max. 10 minutes.
• Press GO BACK (F1) to return to the main menu.
To protect the patient, an infusion device should include an occlusion pressure alarm. This alarm should activate if the pressure inside
the administration set exceeds preset levels. Since an occlusion with
built up pressure also distends the administration line, an excess volume of liquid is stored in the line. This is the Bolus volume, which is
a discrete quantity of liquid delivered in a short time, and is not intended to form a part of the continuous flow output. Such uncontrolled volume may also be a risk to the patient, and must be measured for the infusion pump.
1. Prime the QA-IDS as per paragraph 4.2.2 above.
2. Press Occlusion Test (F5) in the Main Menu and the following display will appear:
3. Press Flow Set (F7) to select an intermediate flow rate on
the infusion device.
4. Press Alarm Prsr (F6) to enter a preset pump value on
pumps having programmable occlusion alarm settings.
5. Press Pump Type (F5) and select between the two options:
Standard or Reversing.
6. Start the infusion, and simultaneously press START (F2) to
start the Occlusion Test, and the following display will appear.
During the test, the pressure (Prsr) in the administration set and
elapsed time will be displayed. When the Occlusion Alarm on
the infusion pump is activated, press STOP (F2) to stop the
test, and the following display will appear.
25
Page 26

4.4 Infusion Flow Rate and Volume Testing
The following parameters will then be displayed:
Prsr: The pressure in the connction tube. Measurement results
display: max. value > instantaneous measured value >
mean value in mmHg. Note: For multiple measurements,
max. measured value > last measured value > mean
value are displayed.
Time: The time from test start until alarm activation. Measure-
ment results display: max. time > elapsed time > mean
time in minutes/seconds.
Bolus: The volume expansion within the tube when the infusion
pump alarm activates. Measurement results display: max.
bolus > last executed calculation after STOP is pressed >
mean bolus in milliliters. Note: Maximum bolus volume to
be measured with a standard QA-IDS is about 1.7
milliliters.
7. A new measurement in a series is initiated by just pressing
START (F2) to start test.
According to the IEC an occlusion test should be run with different
settings of flow rate and, if the pump has a programmable occlusion
pressure alarm, different alarm settings are recommended.
1. Press Flow measurement (F6) in from the Main Menu and the
display will show the Flow Measurement Setup 1 Menu.
26
The following parameters are displayed:
Flow Set: This value is the preset value of the infusion pump. All
error calculations of the flow measurement are related to
this value. If the Flow Set value is not entered correctly,
this may cause a poor presentation with incorrect overall
errors.
Press Set: This function gives the possibility to enter + (positive) or
- (negative) backpressure into the system. Operation
range is from -200 to +600 mmHg.
dT: Interrogation time, or time between two measurements.
Average value during the test interval is displayed. Recommended dT from IEC is 30 sec, and this is the minimum value to be selected on the QA-IDS. Maximum dT
is 600 sec. or 10 minutes. Default value is 30 sec.
Page 27

• Press Flow Set (F7) and enter the preset value of the infu-
sion pump being tested.
• Press Press Set (F6) and enter in the relevant + or - back-
pressure.
• Press dT (F5) if values other than the default 30 seconds is
desired, and enter the new value.
2. Press MORE (F1) in Flow Measurement Setup 1, and the
display will show the Flow Measurement Setup 2.
The following parameters will then be displayed:
Test Time: This is the preset time for a test. Maximum testing
Delay Time: This is the delay before the first measuring.
time is 24 hours. Default value is 1 hour.
• Press Test Time (F7) to select a time for the test other than
the default time of 1 hour.
• Press Delay Time (F6) to set a delay before the first mea-
surement, if desired.
• Press MORE (F1) to return to the Flow Measurement
Setup 1 Menu.
3. Press START (F2) to start flow measurement in either the
Flow Measurement Setup 1 or Flow Measurement Setup
2, and the following display will appear:
The following parameters will then be displayed:
Mean Flow: The flow of the liquid volume in infusion pump every
Cum. Volume: The cumulative volume from start of the test.
Time Left: The remaining time before completion of the test.
second, displayed in milliliters per hour (ml/h) and
percent (%).
• Press STOP (F2) to return to the Flow Measurement 1
display.
27
Page 28

• Press MORE (F1) and the Flow Measurement 2 display
will appear.
The following parameters will then be displayed:
Inst. Flow: Instantaneous Flow shows: max. value > mean value
Inst. Flow: Instantaneous Flow shows: max. value > mean value
Prsr: Shows pressure in the connection tube.
and minimum value in percent (%).
and minimum value in milliliters per hour (ml/h).
• Press STOP (F2) to return to the previous display.
• Press MORE (F1) to return to the Flow Measurement
Setup.
28
Page 29

5.1 Required Test Equipment
5. Control and Calibration
This chapter explains the QA-IDS maintenance procedures, including testing and calibration.
• Digital multimeter, 10 µV resolution, 0.1% accuracy.
• Pressure/vacuum generator, - 400 to + 1700 mmHg pressure.
• Pressure gauge.
• Calibrated infusion pump.
5.2 Adjusting the Display Contrast
5.3 Testing Power Supply to IDS
WARNING!
HIGH VOLTAGES ARE CAPABLE
OF CAUSING DEATH!
USE EXTREME CAUTION WHEN PERFORMING TESTS AND CALIBRATION. USE ONLY INSULATED TOOLS WHEN THE UNIT IS
PLUGGED IN, AND THE METAL CASE HOUSING IS OFF.
1. Remove metal case housing.
1. Set contrast in the display by adjusting potentiometer R160 (located at left of CPU) to desired contrast. See Component Loca-
tion Diagram, page A-2.
1. Disconnect cable to the stepper motor, and connect a power
supply with 24 VDC and current limit set to 400 mA.
2. Measure current drawn from the supply voltage, max. 350
mA.
3. Connect mains unit and adjust the voltage to 24V.
4. Switch ON the mains voltage and measure/adjust the fol-
lowing voltages in the sequence given using the multimeter. There must be 0 mmHg pressure applied to the
QA-IDS:
Test point Level Max. deviation
V24P (J140/1) 24 VDC ± 1 V
Vcc (C1N12/I) + 5 VDC ± 50 mV
V5N (C1N31/2) - 5 VDC ± 200 mV
Uref 4.096 VDC ± 1 mV
Uad 5 VDC ± 2 mV
29
Page 30

5.4 Testing Pressure Gauge Accuracy
1. Connect pressure generator and pressure gauge to the Inlet and
start calibration of the pressure gauge.
2. Set pressure to 0 mmHg and press F3.
3. Set pressure to 1000 mmHg and press F4. The pressure gauge is
now calibrated.
4. Check that the QA-IDS is within specifications at - 400 and
+ 1700 mmHg pressure.
Value Max. deviation
- 400 mmHg ± 10 mmHg
+ 700 mmHg ± 10 mmHg
+ 900 mmHg ± 20 mmHg
+ 1700 mmHg ± 34 mmHg
5. To remove as much air pressure as possible from the pressure
sensor:
• Connect an infusion set with a supply of sterile, distilled
water to the QA-IDS inlet. (Infusion pump or gravity-fed
system can be used.)
• Open the infusion set, or set the infusion pump to a rate of
250 ml/hr.
5.5 Testing Flow Rate Accuracy
• Select PRIME (F1) from the main menu to fill the hose
QA-IDS measuring system with water.
• Once filled, stop the infusion, and set the QA-IDS back-
pressure to - 400 mmHg. The tester will pump to create this
negative pressure in the test circuit.
• Select DRAIN (F2) and allow pump to run until it stops.
• Then, set the back-pressure to 10 mmHg, and open the infu-
sion set, pumping any remaining air out of the QA-IDS
pressure sensor.
1. Set QA-IDS to do a Flow Measurement, with a set flow rate
(Flow Set) of 100 ml/hr, a back pressure setting (Press Set) of 0
mmHg, and a sampling interval (dT) of 30 seconds.
1. Connect infusion set of a calibrated infusion pump to the QAIDS inlet. Note: Metron recommends the use of sterile water
for calibration tests.
2. Prime the QA-IDS:
30
Page 31

5.6 Checking the Battery Backup
• Turn infusion pump ON, and set flow rate at 250 ml/hr.
• Select PRIME (F1) from the QA-IDS menu.
• Select GO BACK (F1) when all air is cleared from the
drain line.
3. Set infusion pump to 100 ml/hr.
4. Press START from the QA-IDS Flow Measurement menu.
5. Run test at each of the below settings for at least 15 minutes and
check that deviations are within tolerance limits.
Flow Rate Max. deviation Volume Max. deviation
- 1 ml/Hr ± 0.05 ml/Hr - 1 ml ± 0.05 ml
+ 10 ml/Hr ± 0.5 ml/Hr + 10 ml ± 0.5 ml
+ 100 ml/Hr ± 5 ml/Hr + 100 ml ± 5 ml
+ 1000 ml/Hr ± 50 ml/Hr + 1000 ml ± 50 ml
1. In the SETUP menu, check the clock for date and time. Correct
if necessary.
2. Turn the QA-IDS OFF, then ON again, and verify that the correct date and time are displayed.
31
Page 32

This page intentionally left blank.
32
Page 33

6.1 Theory of Operation
6. Component Functions and Parts
This chapter provides a description of the functions of the QA-IDS
components, and a parts list for cross reference.
The QA-IDS consists of an independent primary switched power
supply, a keypad board, a processor board, a LCD display and an index transmitter mounted on the pump. The processor board, keypad
board and index transmitter are described in diagrams, contained in
Appendix A. One is a Component Location Diagram. Another,
F190.20.2000.10, is a schematic diagram describing the keyboard,
pressure sensor, and pump index. F190.20.1N00.10 diagrams the
Processor Board Main Part. Set F190.20. 1000.10 through
F190.20.1900.10 are schematic diagrams describing the processor
board by function. The boards are connected by 2 x 16-conductor
flat cables, 1 x 3-conductor and 1 x 2-conductor.
The QA-IDS is an infusion pump tester that runs on 110 - 230 V AC
mains voltage. The unit is based on a Motorola microprocessor.
Controls on the front panel of the unit allow testing of all the functions of an infusion pump.
QA-IDS performs tests according to IEC Draft Standard 62.D and
IEC 601.2.24. Test results, which are shown in the QA-IDS LCD
display, may be printed out on an external printer. A serial port
(RS-232C) enables the unit to be controlled from a PC, simply by
using the PRO-Soft QA-IDS software program.
6.2 Power Supply
6.3 Printer Output
The program in QA-IDS is stored in RAM with battery backup, and
can be updated from a PC via the built-in serial port.
The power supply consists of a primary switched unit, mounted on
the backplane, that supplies 24 VDC, 2A to the processor board
(V24P). The processor board is equipped with a secondary switched
regulator that generates + 5 VDC (Vcc). Via a passive filter, this is
used as the supply voltage to the measurement amplifier and A/D
converter (V5P). A capacitive switched supply generates - 5 VDC to
the measurement amplifier and A/D converter (V5N). A reference
voltage of 4.096 V is generated from V5P, which is used for the A/D
and pressure sensor. The supply voltages are monitored by a watchdog, and the unit is reset if V24P falls below 20 V. The contrast on
the display is regulated using R160. The stepper motor drivers are
supplied directly from V24P.
The QA-IDS has a printer output with a standard 25-pin D-sub contact for Centronics interface. The drivers are built up with TTL in-
33
Page 34

6.4 Serial Port
6.5 Microprocessor
6.6 Pressure Gauge
verters, which are run from a register connected to the data bus. The
inputs are protected against overvoltage.
The serial port has a 9-pin D-sub male (DTE), and is connected to a
PC with a null modem cable. The handshake is carried out in the
software. The control signals RTS, CTS and DTR are connected on
the D-sub contact.
The microprocessor unit consists of a Motorola processor with a
clock frequency of 16 MHz, RAM, A/D converter, watchdog,
real-time clock, parallel and serial I/O. The program is stored in a
128K RAM with battery backup. The setup parameters and calibration data are stored in EEPROM in the CPU. The LCD display and
keypad are connected to the processor's bus via registers. The
built-in A/D converter in the CPU is used to monitor the temperature and battery voltage.
The pressure gauge consists of a differential pressure sensor, a measurement amplifier, the reference voltage and an instrumentation
amplifier.
The pressure sensor, U1B00, is made up of a piezo-resistive element
encapsulated in silicon. It is temperature- and offset-compensated. It
receives its voltage supply from the reference voltage. The output
signal is amplified with an instrumentation amplifier, U1B10, which
is a 4 x operational amplifier. The amplified signal is proportional to
the pressure measured, and is fed to a 12-bit A/D converter, U1700.
To measure negative pressure, an offset of 800 mV is inserted at
zero pressure. The offset is adjusted with R1 B1B. The amplification is adjusted with R1B10 to 4050 mV at 1700 mmHg. The reference voltage is generated from V5P using a variable voltage divider,
R1B26, and an operational amplifier, U1B20. U1B20 is also used
for temperature measurement,
6.7 Pump
6.8 Stepper Motor/Drivers
34
The QA-IDS has a pump from Saphierwerk, driven by a stepper motor. The pump is a rotating piston pump with the pump head and piston in artificial sapphire. It pumps 50 µl per revolution. The pump
has a circuit board with a Hall sensor to provide an index per revolution. The index is used to measure the volume pumped. The stepper
motor is a bipolar type with 200 steps per revolution.
The stepper motors are run by three circuits from Ericsson. The
stepper motor driver U 1810 is connected to the CPU, and generates
the control signal to two driver circuits. The stepper motor can run
in full-and half-step mode. The driver circuits, U1820 and U 1830,
have built-in temperature protection and pulse width modulation to
Page 35

6.9 Control Panel
6.10 Component Parts
COMPONENT PART TYPE/VALUE QTY. DIAGRAM REFERENCE
KEYBOARD, PRESSURE SENSOR, AND PUMP INDEX
Printed cirouit board Keyboard 1
Diode BAS16 22 D2000-D2004, D2010 - D2014, D2020 -
Capacitor X7R 100 nF 63V 1 C2109
Switch 22 S2000-S2004, S2010 - S2014, S2020 -
HALL-switch A3141EU 1 U2100
HD-Connector 16 pin RP 1 J200
MX ENRAD 3pin IMRPL 1 J210
control the current drawn. The motor drivers are supplied with 24V
to achieve rapid current increase and good torque. Each phase of the
motor can be supplied with up to 1.5 A, which is regulated by the
software independently of the step rate. U1840 is used for synchronizing the pulse width modulation to the drivers to prevent high
peak values in the current drawn.
The control panel is made up of a LCD display with 4 x 40 characters and a keypad with 22 push-buttons. The function buttons control
the menu choices shown on the display. The numeric and alphanumeric keys are used to enter parameters and for setups.
D2023, D2030 - D033, D2040 - D2043
S2023, S2030 - S033, S2040 - S2043
CPU
Printed circuit board CPU 1
Op-ampl. 2x LM6142BIM 1 U1B20
Op-ampl. 4x LF;347M 1 U1B10
R Inverter MAX680 1 U1N30
Different. press. MPX200D 1 U1B00
Currentampl. MC34167 1 U1N10
Register HCT574T 7 U1500, U1510, U1520, U1530, U1540,
A/D converter ADC12030CIW 1 U1700
S-RAM 128 x 8 TC55001 BLF-85 1 U1300
Step driver PBD351 1 U1810
Step driver PBL3770AN 2 U1820, U1830
NAND port 4x 2 HCT132T 1 U1840
CPU 16MHz 68HC11F1N4P 1 U1210
Watch dog MAX693CSA 1 U1250
Decoder 4- 8 HCT138T 2 U1240, U1241
High voltage switch MC14066 1 U1260
Realtime watch NJU6355 1 U1270
EEPROM 12C-buss PCF8582AT 1 U1200
Inverterw/OK output LS05 2 U1410, U1411
RS-232 LTC1383CS 1 U1400
Transistor BC846B 1 Q1430
Diode BAS16 8 D1B21, D1430, D1250, D1251, D1420,
Schottkeydiode SS24 2A 40V 5 D1N10, D1820, D1821, D1830, D1831
X-tal 32.768kHz 8 x 3 1 X1270
X-tal 1 X1210
Resistor 10R 0.25W 1 R1252
Resistor 22R 0.25W 7 R150- R154, R1700, R1702
U1600, U1610
D1421, D1840, D1841
35
Page 36

COMPONENT PART TYPE/VALUE QTY. DIAGRAM REFERENCE
Resistor 47R 0.25W 1 R1B1C
Resistor 100R 0.25W 1 R1215
Resistor 330R 0.25W 1 R1B1D
Resistor 470R 0.25W 1 R1B27
Resistor 680R 0.25W 1 R161
Resistor 1k 0.25W 8 R1B21, R1B25, R1214, R1253, R1421,
Resistor 1k3 0.25W 1 R1B11
Resistor 2k0 0.25W 1 R1400
Resistor 3k0 0.25W 1 R1B28
Resistor 3k3 0.25W 2 R1251, R1430
Resistor 4k7 0.25W 11 R1B1A, R1B22, R1270- R1273, R1822,
Resistor 5k6 0.25W 1 R1B20
Resistor 8k8 0.25W 1 R1N11
Resistor 10k 0.25W 33 R1B14- R1B18, R1B23, R1N30, R1210-
Resistor 38k 0.25W 1 R1250
Resistor 47k 0.25W 6 R1431, R1600 - R1604
Resistor 56k 0.25W 5 R1200, R1824, R1830, R1840, R1841
Resistor 68k 0.25W 1 R1N10
Resistor 100k 0.25W 2 R1B12, R1B13
Resistor 510k 0.25W 1 R1B24
Resistor 10M 0.25W 1 R1222
Resistor 000R 0.25W 3 R1820, L1N11, L1430
Resistor 0R47 0.3W 2 R1827, R1837
Resistor 0R68 0.3W 2 R1828, R1838
Potentiometer 200R 1 R1B10
Potentiometer 500R 1 R1826
Potentiometer 1k 2 R1B1B, R160
El.lytt capacitor 1 µF 40V 1 C1N11
El.lytt capacitor 220 µF 40V 4 C1N18, C1N34. C1827, C1837
El.lytt capacitor 470 µF 16V 1 C1N32
Tantal capacitor 2u2 16V 3 C1701, C1707, C1818
Tantal capacitor 4u7 16V 1 C1210
Tantal capacitor 10 µF 16V 1 C1702
Tantal capacitor 33 µF 16V 1 C1N13
Cer. capacitor 820pF 16V 4 C1820, C1821,C1831, C1840
Cer. capacitor 3n3 1 C1200
Cer. capacitor 22 nF 1 C1430
Capacitor NPO 22 pF 63V 2 C1211, C1212
Capacitor NPO 1n5 50V 1 C1830
Capacitor X7R 1nF 63V 1 C1B00
Capacitor X7R 10 nF 63V 2 C1B20, C1B24
Capacitor X7R 100nF 63V 40 C1B09, CIB10,C1B18, C1B19,
Spool 150 uH 1.7A 1 L1N10
Lithium battery 3V 1 B1250
Piezo piper 1 X1430
D-sub 25 pin VS 1 J170
D-sub 9 pin VP 1 J120
HD connector 16 pin RP 2 J100, J160
MX single row 2 pin 1M RPL 1 J150
R1825, R1835, R1844
R1410 - R1412, R1823
R1213, R1216, R1217, R1220, R1221,
R1223, R1420, R1260. R1264, R1811,
R1510- R1514, R1812, R1842, R1843,
R1845, R1848
CIB28,C1B29, C1N10, C1N12, C1N14,
C1N19, C1N30, C1N31, C140, C160, C1250,
CI 279, C1293 - C1296, C1309, C1400 .
C1403, C1409, C1818, C1419, C1595 C1599, C1609, C1619, C1700, C1708,
C1709, C1819, C1828, C1829, C1838,
C1839, C1849
36
Page 37

COMPONENT PART TYPE/VALUE QTY. DIAGRAM REFERENCE
MX single row 3 pin 1M RPL 2 J110, J1210
Connector 5pin 3.9 mm 1 J180
Connector 2 pin 3.9 mm 1 J140
Pin 2 pin 4 S1250- S1252, S1260
37
Page 38

This page intentionally left blank.
38
Page 39

APPENDIX A: DIAGRAMS
Processor Board Component Location.................................................................................................
Processor Board Schematic Diagram 1.................................................................................................
Processor Board Schematic Diagram 2 (Digital Part)..........................................................................
Processor Board Schematic Diagram 3 (CPU).....................................................................................
Processor Board Schematic Diagram 4 (Memory)...............................................................................
Processor Board Schematic Diagram 5 (RS232C and Printer)............................................................
Processor Board Schematic Diagram 6 (Digital Outputs)....................................................................
Processor Board Schematic Diagram 7 (Digital Inputs)......................................................................
Processor Board Schematic Diagram 8 (A/D).....................................................................................
Processor Board Schematic Diagram 9 (Stepper Motor and Drivers)................................................
Processor Board Schematic Diagram 10 (Pressure Sensor and Temperature)....................................
Processor Board Schematic Diagram 11 (Main Part)..........................................................................
Processor Board Schematic Diagram 12 (Keyboard, Pressure Sensor and Pump Index)....................
39
Page 40

This page intentionally left blank.
40
Page 41

Processor board Component Location
41
Page 42

6.10.1 Processor board Schematic Diagram 1
42
Page 43

6.10.2 Processor board Schematic Diagram 2 (Digital Part)
43
Page 44

6.10.3 Processor board Schematic Diagram 3 (CPU)
44
Page 45

6.10.4 Processor board Schematic Diagram 4 (Memory)
45
Page 46

6.10.5 Processor board Schematic Diagram 5 (RS232C and Printer)
46
Page 47

6.10.6 Processor board Schematic Diagram 6 (Digital Outputs)
47
Page 48

6.10.7 Processor board Schematic Diagram 7 (Digital Inputs)
48
Page 49

6.10.8 Processor board Schematic Diagram 8 (A/D)
49
Page 50

6.10.9 Processor board Schematic Diagram 9 (Stepper Motor and Drivers)
50
Page 51

6.10.10 Processor board Schematic Diagram 10 (Pressure Sensor and Temperature)
51
Page 52

52
Page 53

6.10.11 Processor board Schematic Diagram 11 (Main Part)
53
Page 54

54
Page 55

6.10.12 Processor board Schematic Diagram 12 (Keyboard, Pressure Sensor and Pump Index)
55
Page 56

56
Page 57

6.10.13
This page intentionally left blank.
57
Page 58

APPENDIX B: ERROR REPORT FORM, QA-IDS
QA-IDS INFUSION PUMP TESTER ERROR REPORT FORM
USA _ FRANCE NORWAY
1345 Monroe NW, Suite 255A 30, rue Paul Claudel Travbaneveien 1
Grand Rapids, MI 49505 91000 Evry, France N-7044 Trondheim, Norway
Phone: (+1) 888 863-8766 Phone: (+33) 1 6078 8899 Phone: (+47) 7382 8500
Fax: (+1) 616 454-3350 Fax: (+33) 1 6078 6839 Fax: (+47) 7391 7009
E-mail: metronus@aol.com E-mail: metronfrance@infonie.fr E-mail: support@metron.no
From: (name) Phone:
Address: Fax:
Date:
QA-IDS Error Report Product:
Version:
Type
チ Wrong results チ Error messages, without reason
チ Program stops, no reaction チ Wrong responses on commands.
チ Other
Description of the situation prior to the error:
Description of the error:
(METRON use internally)
Received date: Comments:
Correction date:
Ref No.
58
チ Critical
チ Minor
チ Normal
Page 59

This page intentionally left blank.
59
Page 60

APPENDIX C: Suggestion Form, QA-IDS
QA-IDS INFUSION PUMP TESTER SUGGESTION FORM
USA _ FRANCE NORWAY
1345 Monroe NW, Suite 255A 30, rue Paul Claudel Travbaneveien 1
Grand Rapids, MI 49505 91000 Evry, France N-7044 Trondheim, Norway
Phone: (+1) 888 863-8766 Phone: (+33) 1 6078 8899 Phone: (+47) 7382 8500
Fax: (+1) 616 454-3350 Fax: (+33) 1 6078 6839 Fax: (+47) 7391 7009
E-mail: metronus@aol.com E-mail: metronfrance@infonie.fr E-mail: support@metron.no
From: (name) Phone:
Address: Fax:
Date:
QA-IDS Improvement Suggestion Product:
Version:
Type
チ One window チ Presentation
チ Several windows チ Options, configuration possibilities
チ Documentation チ Other
Description of the suggested improvement:
(METRON use internally)
Received date: Comments:
Correction date:
Ref No.
60
Page 61

This page intentionally left blank.
61
Page 62

62
 Loading...
Loading...