Medical Research Laboratories MRL Lite, MRL Pic Monitor, MRL Pic System Service Manual
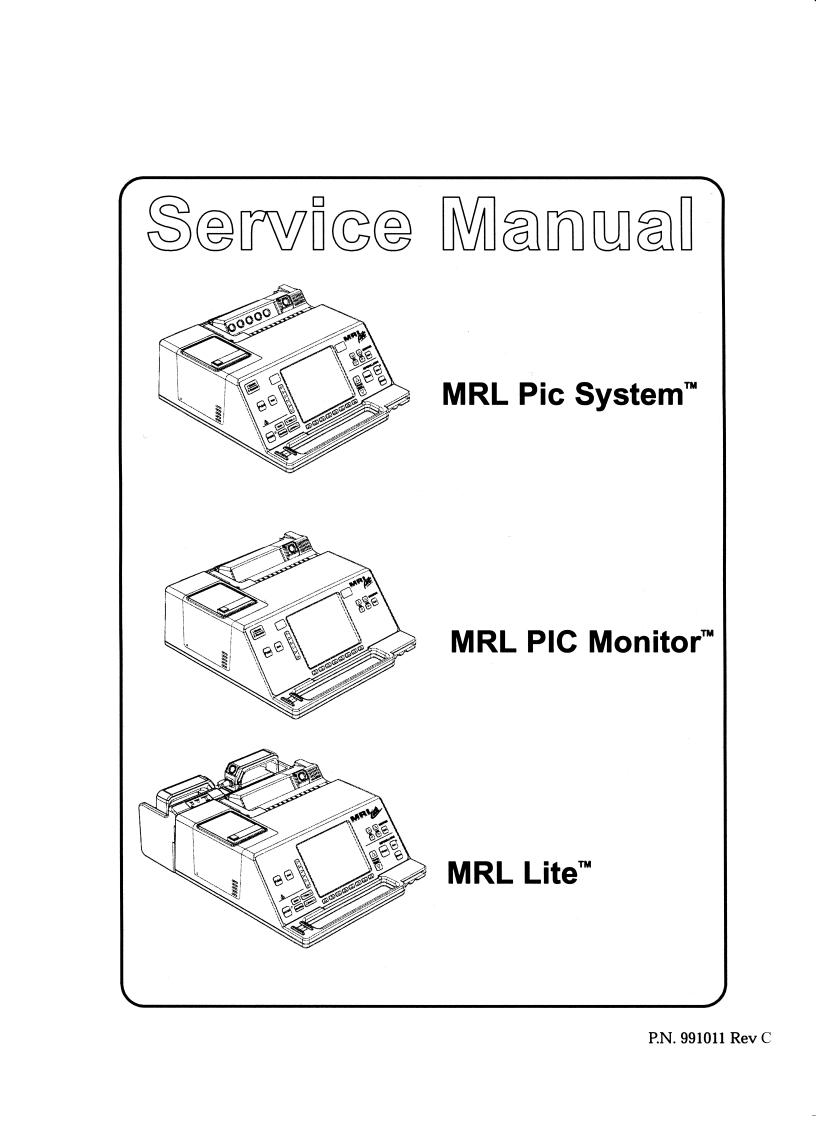

Portable Intensive Care System
SERVICE INSTRUCTION MANUAL
Models: PIC, PIC 2, PIC 2H PIC Monitor
MRL Lite, MRL Lite 2, MRL Lite 2H
Medical Research Laboratories, Inc.
1000 Asbury Drive, Buffalo Grove, Illinois 60089
847/520-0300 (Telephone) 800/462-0777 (Toll-Free) 847/520-0303 (Fax) www.mrlinc.com (Internet) info@mrlinc.com (E-mail)
©1998, 1999, 2000, 2001 Medical Research Laboratories, Inc. All rights reserved. Printed in the U.S.A.
MRL Part Number 991011
MRL PORTABLE INTENSIVE CARE SERVICE MANUAL |
I |

Foreword
|
This manual is intended to provide information for the proper |
|
servicing of the MRL PIC. |
|
DO NOT ATTEMPT TO USE THIS EQUIPMENT WITHOUT |
|
THOROUGHLY READING AND UNDERSTANDING THESE |
|
INSTRUCTIONS. |
User's |
The user is required to be trained in basic monitoring, vital signs |
Responsibility |
assessment and emergency cardiac care. The user should be |
|
completely knowledgeable of the information in the User Instruction |
|
Manual. As with all other electronic patient care monitors, good |
|
clinical judgment should be used when operating the MRL PIC. |
|
User must save all shipping containers and packaging materials. |
|
When shipping the PIC System and accessories for calibration, |
|
service, or upgrades, the original shipping containers and packaging |
|
materials must be used. |
Manufacturer's Medical Research Laboratories, Inc. (MRL), is responsible for the Responsibility safety, reliability and performance of the MRL Portable Intensive
Care System, only if the following three conditions are met:
•Assembly operations, extensions, readjustments, modifications or repairs are carried out by persons authorized by MRL.
•The electrical installation of the relevant room complies with the appropriate requirements.
•The PIC equipment is used in accordance with the instructions for use.
To ensure patient safety and proper operation, use only MRL - authorized parts and accessories.
II |
MRL PORTABLE INTENSIVE CARE SERVICE MANUAL |

FDA Medical Device Registration
The FDA Safe Medical Device Act stipulates that each end-user is required under penalty of law, to register with the manufacturer all information pertinent to each medical device.
Please fill out the attached FDA Medical Device Registration postcard and return it promptly to MRL. This card must be filled in and returned within 30 days of product delivery.
If the medical device is transferred from your possession, you must notify MRL of the new registration information.
Please contact MRL (800/462-0777) if you have any questions regarding this notice.
MRL PORTABLE INTENSIVE CARE SERVICE MANUAL |
III |

Declaration of Conformity
Manufacturer: Medical Research Laboratories, Inc. |
Response Medical Equipment, Ltd. |
||||||||||
|
|
1000 Asbury Drive |
|
Bracken House - Battlebrook |
|||||||
|
|
Buffalo Grove, IL 60089 |
|
Chipping Campden GL55 6JX |
|||||||
|
|
USA |
|
|
|
|
Gloucestershire, United Kingdom |
||||
|
|
Phone |
(847) 520-0300 |
Phone |
11-44-1-386-841926 |
||||||
|
|
Fax |
(847) 520-0303 |
Fax |
11-44-1-386-841230 |
||||||
declares that the CE-marked product |
|
|
|
|
|
|
|||||
Product Name: |
PIC (Portable Intensive Care) |
|
|
|
|
|
|||||
|
Base Units |
|
|
|
|
|
|
Options |
|
||
971009 |
5 lead, EL display |
|
|
971021 |
SpO2 |
|
|
971023 |
Data recording |
||
|
|
|
|
|
|
|
|
|
|
|
|
971013 |
5 lead, Color display |
|
|
971074 |
|
|
971024 |
Data playback |
|||
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
||
971026 |
12 lead, EL display |
|
|
971016 |
CO2 |
|
|
971073 |
RS-232 |
||
971027 |
12 lead, color display |
|
|
971001 |
NIBP |
|
|
971019 |
FAX |
||
|
|
|
|
|
|
|
|
|
|
||
971042 |
5 lead, LCD display |
|
|
971005 |
Voice Memo |
|
|
971104 |
Battery charger |
||
|
|
|
|
|
|
|
|
|
|
||
971044 |
5 lead, without defib |
|
|
971008 |
SAED |
|
|
971029 |
Integral mains supply |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
971017 |
IBP |
|
|
|
|
|
|
Device Type: Defibrillator / External Transcutaneous Pacemaker / Multifunction Monitor
complies with Council Directive 93/42/EEC (Medical Device Directive) of 14 June 1993 class IIb Annex II
|
Standards |
General: IS0 9001 |
|
Safety: |
EN 46001 |
IEC 601-1 / EN 60601-1 Class I, Continuous operation |
|
|
Type BF (with external paddles) or CF (with internal paddles) |
|
IEC 601-1-4 / EN 60601-1-4 |
|
IEC 601-2-4 / EN 60601-2-4 |
|
IEC 601-2-25 / EN60601-2-25 |
|
IEC 601-2-27 / EN 60601-2-27 |
|
IEC 601-2-30 / EN 60601-2-30 |
|
IEC 601-2-34 / EN 60601-2-34 |
|
IEC 1441 / EN1441 |
|
EN 865 |
EMC: |
EN 475 |
IEC 601-1-2 / EN 60601-1-2 |
|
Date January 23, 2003
Joel Orlinsky
Director of Q. A. and Regulatory Affairs

Table of Contents
Title Page .......................................................................... |
i |
Forward........................................................................... |
ii |
FDA Medical Device Registration ................................ |
iii |
Declaration of Conformity.............................................. |
iv |
Table of Contents ............................................................. |
v |
Safety Information ......................................................... |
1.1 |
Symbols and Icons ........................................................ |
1.2 |
General Precautions ...................................................... |
1.5 |
Monitoring Precautions ................................................ |
1.7 |
Defibrillator Precautions............................................... |
1.8 |
External Pacing Precautions ....................................... |
1.10 |
Pulse Oximeter Precautions ........................................ |
1.12 |
Non-Invasive Blood Pressure Precautions.................. |
1.13 |
Battery Precautions ..................................................... |
1.14 |
Charger Precautions .................................................... |
1.15 |
SAED Precautions ...................................................... |
1.16 |
IBP Precautions .......................................................... |
1.16 |
CO2 Precautions ......................................................... |
1.17 |
General Information ...................................................... |
2.1 |
Product Overview ......................................................... |
2.2 |
General Description ...................................................... |
2.2 |
PIC System Interfaces................................................... |
2.3 |
PIC System Controls and Indicators............................. |
2.5 |
PIC System Modes ....................................................... |
2.7 |
Initial Installation Evaluation ....................................... |
2.9 |
Equipment Setup......................................................... |
2.11 |
Summary of Operations .............................................. |
2.18 |
Part Numbering System .............................................. |
2.21 |
Options and Accessories............................................. |
2.22 |
Technical Specifications ............................................. |
2.24 |
Menus ............................................................................. |
3.1 |
User Menu Overview.................................................... |
3.2 |
Supervisor Menu Overview .......................................... |
3.3 |
Quick Access Buttons and Icons .................................. |
3.5 |
Quick Access Buttons and Pop-up Menus.................... |
3.6 |
User Menus ................................................................... |
3.7 |
User Menus – Display .................................................. |
3.8 |
User Menus – SPO2 .................................................... |
3.11 |
User Menus – Non-Invasive Blood Pressure.............. |
3.12 |
User Menus – Respiration (ECG).............................. |
3.13 |
User Menus – Respiration (CO2)................................ |
3.15 |
User Menus – Respiration (Trend) ............................. |
3.17 |
VI |
MRL PORTABLE INTENSIVE CARE SERVICE MANUAL |

|
|
|
..............................................User Menus – Recorder |
3.18 |
|
User Menus – Setup.................................................... |
3.20 |
|
Supervisor Menus ...................................................... |
3.22 |
|
Supervisor Menus – Defibrillator ............................... |
3.23 |
|
Supervisor Menus – Pacer .......................................... |
3.25 |
|
Supervisor Menus – SAED......................................... |
3.26 |
|
Supervisor Menus – 12-Lead...................................... |
3.28 |
|
Supervisor Menus – Setup .......................................... |
3.30 |
|
Supervisor Menus – Calibration ................................. |
3.35 |
|
MRL LITE Program Menu Setup .................................. |
4.1 |
|
MRL Lite Menu Structure ............................................ |
4.1 |
|
Basic Menu Structure ................................................... |
4.3 |
|
MRL Lite Menus .......................................................... |
4.4 |
|
Setup Menus ................................................................. |
4.8 |
|
ECG Configuration Menu .......................................... |
4.10 |
|
Supervisor Menus ....................................................... |
4.12 |
|
Supervisor – Defibrillator Menu................................. |
4.13 |
|
Supervisor – Pacer Menu (optional) ........................... |
4.15 |
|
Supervisor – Diag Menu............................................. |
4.16 |
|
Supervisor – Setup Menu ........................................... |
4.17 |
|
Supervisor – Upgrade Menu....................................... |
4.19 |
|
Performance Test Procedures ..................................... |
5.1 |
|
Inspection Procedures................................................... |
5.2 |
|
Recommended Test Equipment .................................... |
5.4 |
|
Safety Testing ............................................................... |
5.6 |
|
ECG Monitor ................................................................ |
5.7 |
|
ECG Amplitude Calibration ......................................... |
5.9 |
|
Heart Rate Display Accuracy ..................................... |
5.10 |
|
Defibrillator ................................................................ |
5.11 |
|
Synchronized Discharge ............................................. |
5.13 |
|
Transthoracic Pacemaker (if equipped) ...................... |
5.14 |
|
Advisory Option (if equipped) ................................... |
5.15 |
|
Battery Charger Test Procedure.................................. |
5.16 |
|
3-Volt Lithium Battery Check .................................... |
5.17 |
|
Battery Capacity Test and Reconditioning |
|
|
Procedure .................................................................... |
5.18 |
|
Guidelines for Maintaining Peak Battery |
|
|
Performance................................................................ |
5.20 |
|
12-Lead ECG Data Acquisition and |
|
|
Fax Modem Test ......................................................... |
5.21 |
|
Non-Invasive Blood Pressure Performance Test ........ |
5.22 |
|
Pulse Oximeter Performance Test .............................. |
5.24 |
|
IBP Performance Test................................................. |
5.25 |
|
CO2 Performance Test ................................................ |
5.26 |
|
MRL PORTABLE INTENSIVE CARE SERVICE MANUAL |
VII |

|
|
|
|
......................................................Theory of Operation |
6.1 |
|
Overview....................................................................... |
6.1 |
|
Power Distribution........................................................ |
6.2 |
|
Motherboard ................................................................. |
6.3 |
|
5-Lead ECG Monitor .................................................... |
6.4 |
|
Displays ........................................................................ |
6.5 |
|
Chart Recorder.............................................................. |
6.6 |
|
Front Switch Panel........................................................ |
6.6 |
|
Voice Memo and Voice Prompts................................... |
6.7 |
|
1-Volt Output ................................................................ |
6.7 |
|
PCMCIA Interface........................................................ |
6.7 |
|
Blood Pressure .............................................................. |
6.8 |
|
Oximeters...................................................................... |
6.8 |
|
Defibrillator/External Pacemaker ................................. |
6.9 |
|
Options........................................................................ |
6.10 |
|
Interconnect Diagram ................................................. |
6.13 |
Troubleshooting Guide ................................................. |
7.1 |
Malfunctions ................................................................. |
7.1 |
Error Messages ............................................................. |
7.9 |
Power Supply Outputs ................................................ |
7.14 |
Removal and Replacement Instructions ..................... |
8.1 |
Required Tools .............................................................. |
8.1 |
List of Items .................................................................. |
8.2 |
Removal and Replacement Instructions ....................... |
8.4 |
Item 15 – Main Memory Card Cable............................ |
8.4 |
Item 17 – AC Supply/Paddle Tray Module .................. |
8.5 |
Item 18 – CO2 Cable .................................................... |
8.6 |
Item 19 – Defib Main Cable ......................................... |
8.7 |
Item 20 – Chart Recorder Cover................................... |
8.9 |
Item 21 – Bottom Enclosure Assembly ...................... |
8.10 |
Item 22 – Top Enclosure Assembly............................ |
8.12 |
Item 23 – Defibrillator Module................................... |
8.14 |
Item 24 – Input Panel.................................................. |
8.16 |
Item 26 – Memory Card Board................................... |
8.17 |
Item 27 – 5-Lead Paddle Preamp Board..................... |
8.18 |
Item 28 – Barrier for 5-Lead Paddle |
|
Preamp Board ............................................. |
8.19 |
Item 29 – 5-Lead Paddle Preamp Shield .................... |
8.20 |
Item 30 – Pacer Panel Cable....................................... |
8.21 |
Item 31 – Front Panel Cable ....................................... |
8.23 |
Item 32 – 5-Lead Preamp-Protection Cable ............... |
8.25 |
Item 34 – Mono LCD Inverter Cable ......................... |
8.26 |
Item 35 – Mono LCD Cable ....................................... |
8.27 |
Item 36 – Chart Recorder ........................................... |
8.29 |
Item 38 – Battery Board Cover................................... |
8.30 |
VIII |
MRL PORTABLE INTENSIVE CARE SERVICE MANUAL |

|
|
|
...................Item 39 – Patient Input Bracket (5-Lead) |
8.31 |
|
Item 40 – Patient Input Connector |
|
|
Board (5-Lead) ........................................................... |
8.32 |
|
Item 43 – Backlight Inverter....................................... |
8.34 |
|
Item 44 – Power Supply Board................................... |
8.36 |
|
Item 45 – Card Cage Plate.......................................... |
8.38 |
|
Item 46 – Motherboard ............................................... |
8.40 |
|
Item 47 – Card Cage Bracket ..................................... |
8.41 |
|
Item 48 – Card Cage Pre-Assembly ........................... |
8.44 |
|
Item 49 – LCD Display Adapter................................. |
8.48 |
|
Item 50 – Flat Panel Display Cable ............................ |
8.50 |
|
Item 51 – ECG Preamp Board (5-Lead)..................... |
8.52 |
|
Item 52 – Preamp-Motherboard Cable (5-Lead) ........ |
8.54 |
|
Item 53 – Chart Recorder Cable ................................. |
8.55 |
|
Item 54 – Battery Board ............................................. |
8.56 |
|
Item 55 – Speaker Assembly ...................................... |
8.58 |
|
Item 56 – 3V Coin-Cell Battery ................................. |
8.60 |
|
Item 57 – Power Switch Cable ................................... |
8.61 |
|
Item 58 – Power Switch.............................................. |
8.63 |
|
Item 68 & 69 – Blood Pressure Pump/Valve |
|
|
Assembly & Foam Block ........................... |
8.64 |
|
Item 71 – EL Display Cable ....................................... |
8.66 |
|
Item 73 – Blood Pressure Coupling............................ |
8.67 |
|
Item 74 – Blood Pressure Tube .................................. |
8.68 |
|
Item 81 – 12-Lead Preamp-Protection Cable ............. |
8.69 |
|
Item 82 – 12-Lead Motherboard Cable ...................... |
8.70 |
|
Item 83 – 12-Lead Paddle Preamp Board................... |
8.72 |
|
Item 84 – 12-Lead Preamp Board............................... |
8.74 |
|
Item 85 – Color Display Adapter Board..................... |
8.76 |
|
Item 89 – EL Display Adapter Board......................... |
8.78 |
|
Item 90 – Oximeter Board, without CO2.................... |
8.80 |
|
Item 91 – Main Oximeter Cable ................................. |
8.82 |
|
Item 92 – Blood Pressure Board................................. |
8.83 |
|
Item 93 – Main Blood Pressure Cable ........................ |
8.85 |
|
Item 94 – Speech Board.............................................. |
8.86 |
|
Item 96 – Dual Backlight Inverter .............................. |
8.87 |
|
Item 97 – Paddle Pickup Cable................................... |
8.89 |
|
Item 100 – Color Display Shield and EL |
|
|
Display Shield........................................... |
8.90 |
|
Item 103 – Oximeter Board, with CO2....................... |
8.92 |
|
Item 105 – Modem Board........................................... |
8.94 |
|
Item 106 – 12-Lead Paddle Barrier............................ |
8.96 |
|
Item 109 – Color Inverter Cable................................. |
8.97 |
|
MRL PORTABLE INTENSIVE CARE SERVICE MANUAL |
IX |

|
|
|
|
......................................................Assembly Drawings |
9.1 |
|
Table of Item Numbers .......................................... |
Sheet 1 |
|
Top Assembly ........................................................ |
Sheet 2 |
|
5-Lead Unit ............................................................ |
Sheet 3 |
|
12-Lead Unit .......................................................... |
Sheet 4 |
|
Monitor-No Defib .................................................. |
Sheet 5 |
|
MRL Oximeter Option and Power Wiring ............ |
Sheet 6 |
|
MRL LITE ............................................................. |
Sheet 7 |
|
EL, Color TFT, and Mono Display Options .......... |
Sheet 8 |
|
Card Cage Assembly ............................................. |
Sheet 9 |
Service Part Numbers ................................................. |
10.1 |
|
Item 21 – Bottom Assembly ....................................... |
10.2 |
|
Item 22 – Top Enclosure Assembly............................ |
10.2 |
|
Item 23 – Defibrillator Module................................... |
10.3 |
|
Item 24 |
– Input Panel.................................................. |
10.4 |
Item 46 |
– Motherboard ............................................... |
10.4 |
Item 65 |
– Label Kit..................................................... |
10.5 |
Item 105 – Fax Modem Board.................................... |
10.5 |
|
X |
MRL PORTABLE INTENSIVE CARE SERVICE MANUAL |

CHAPTER 1: SAFETY INFORMATION
This chapter provides informaton on the safe operation of the Portable Intensive Care
(PIC) System.
Chapter Overview: |
• |
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Symbols and Icons |
1.2 |
|
• |
General Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1.5 |
|
• |
Monitoring Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1.7 |
|
• |
Defibrillator Precautions. . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1.8 |
|
• |
External Pacing Precautions . . . . . . . . . . . . . . . . . . . . . . . . |
1.10 |
|
• |
Pulse Oximeter Precautions . . . . . . . . . . . . . . . . . . . . . . . . |
1.12 |
|
• |
Non-Invasive Blood Pressure Precautions . . . . . . . . . . . . . |
1.13 |
|
• |
Battery Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1.14 |
|
• |
Charger Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1.15 |
|
• SAED Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1.16 |
|
|
• |
IBP Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1.16 |
|
• CO2 Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
1.17 |
|
|
|
|
|
PORTABLE INTENSIVE CARE SERVICE MANUAL |
1.1 |

|
|
S AFETY INFO RM ATIO N |
|
|
|
Symbols and Icons |
|
|
Symbols |
Graphical symbols, letter symbols and signs listed below may be |
|
|
found on the PIC System and accessories distributed by MRL. Please |
|
|
note the use of these symbols for safe and proper use of the |
|
|
equipment. |
|
|
Alternating current |
For indoor use only |
|
|
(on battery charger |
|
|
only) |
|
Attention, consult |
Negative input |
|
accompanying |
terminal |
|
documents |
|
|
Auxiliary power |
Positive input |
|
operation |
terminal |
|
Caution, high |
Power off |
|
voltage |
|
|
Dangerous voltage |
Power on |
|
Defibrillator |
Recycle battery |
|
protected, type BF |
|
|
patient connection |
|
|
Defibrillator |
Protective earth |
|
protected, type CF |
(ground) |
|
patient connection |
|
|
Earth (ground) |
Defibrillator |
|
|
discharge button |
Release
The symbols listed below may by found throughout this manual.
Warning: Hazards or unsafe practices that could result in severe personal injury or death.
Caution: Hazards or unsafe practices that could result in minor personal injury or product damage.
Note: Points of particular interest for more efficient or convenient operation.
1.2 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |

SAFETY INFO RM ATIO N
Icons
Graphical and text icons listed below may be found on the display of the PIC System during operation.
|
Check chart |
Alarm off |
recorder |
|
Auto heart rate |
Alarm on |
undetermined |
Alarm lower limit |
Auto heart rate set |
set |
at 60 BPM |
Alarm upper limit |
|
set |
Modulated outputv |
Automatic HR |
|
Alarm set |
Mute |
Alarm - push to |
|
disable |
One volt output |
Animated |
|
recording icon |
QRS beeper off |
Battery full |
QRS beeper on |
Battery low |
|
warning |
Volume level |
Battery (partially |
Supervisor menu |
depleted) |
locked |
|
Supervisor menu |
Auxiliary power |
unlock |
Blood pressure |
|
pump 1 |
Notch filter On |
Blood pressure |
|
pump 2 |
Analyze |
Calibration Pulse |
Internal log |
|
Card Review card |
12-Lead |
usage |
PORTABLE INTENSIVE CARE SERVICE MANUAL |
1.3 |

|
|
S AFETY INFO RM ATIO N |
|
|
|
|
Invasive Blood |
|
|
Pressure |
Carbon Dioxide On |
|
Analyze 12-lead |
Carbon Dioxide Off |
|
Card Review/12- |
Resets to patient |
|
lead Next Page |
001 in card review |
|
12-lead save |
|
|
function |
SAED CPR timer |
|
|
Latching |
|
Fax/modem |
connection |
|
Card Review/12- |
|
|
lead printer |
Do Not Sterilize |
|
Card Review card |
Press here to |
|
usage and location |
unlatch |
1.4 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |

SAFETY INFO RM ATIO N
General Precautions
|
The MRL PIC is intended for use by trained, authorized medical |
|
personnel who are familiar with basic monitoring, vital signs |
|
assessment, and emergency cardiac care. The MRL PIC is also |
|
intended for use by physicians at the scene of an emergency or in a |
|
hospital emergency room. |
|
Federal (USA) law restricts this device to sale by or on the order of a |
|
physician. |
|
Any authorized person using the MRL PIC should be completely |
|
knowledgeable of the information in the User Instruction Manual. |
Accessories |
Use only authorized MRL accessories listed in the Introduction |
|
chapter of this manual. Use of unauthorized accessories may cause |
|
the device to operate improperly and provide false measurements. |
Sterilization |
Do Not attempt to sterilize any accessory or equipment part except |
|
internal defibrillator electrodes and the internal paddle cable. Refer to |
|
chapter 16 of the PIC User Instruction Manual for more information. |
Battery Care |
Proper care and maintenance of the MRL batteries is important to |
|
insure continuous operation during patient care. If the batteries are |
|
not maintained properly, loss of power during patient care could |
|
result, affecting patient care. Always have a fully charged battery |
|
pack available as a back-up. |
Dropped or |
If this device has been dropped or damaged in any way, refer the |
Damaged |
device to qualified service personnel for verification of performance |
|
and/or servicing. |
Ingress of Liquids |
To achieve the specified level of protection against spilled or splashed |
|
liquids, thoroughly dry all exposed surfaces of this device prior to |
|
operation or connections to mains power. |
Electrical Shock |
Hazard: Do not use the MRL PIC if it has been immersed in a liquid |
|
or if liquid has spilled on it. Do not clean the MRL PIC with alcohol, |
|
ketone, or any flammable agent. Do not autoclave the MRL PIC. |
|
Conductive parts of electrodes and connectors, for applied parts, |
|
should not contact other conductive parts including earth. |
Electrical Shock |
Hazard: This device does not contain any user-serviceable parts. Do |
(Internal) |
not remove instrument covers or attempt to repair the MRL PIC |
|
System. Refer servicing to qualified personnel. |
PORTABLE INTENSIVE CARE SERVICE MANUAL |
1.5 |

S AFETY INFO RM ATIO N
|
|
Electrodes |
When obtaining a new supply of disposable electrodes for |
(Disposable) |
monitoring, defibrillation or pacing, verify that they will properly |
|
connect to the existing MRL cables prior to putting in service. Do not |
|
use if gel is dry. |
Energy Discharge |
Hazard: The MRL PIC can deliver 360 joules of electrical energy. If |
|
this electrical energy is not discharged properly, as described in the |
|
User Instruction Manual, the electrical energy could cause personal |
|
injury or death to the operator or bystander. |
Expiration Date |
Always verify expiration dates on dated items such as disposable |
|
defibrillation or pacing pads, monitoring electrodes and battery packs. |
|
If the expiration date has passed, replace the disposable items |
|
immediately. |
Ferromagnetic
Equipment
Labels
Operating Near
Oxygen
Patient Physical
Harm
Performance
Treatment Summary
Log
Biomedical equipment and accessories, such as ECG electrodes, cables, and oximeter probes contain ferromagnetic materials. Ferromagnetic equipment must not be used in the presence of high magnetic fields created by magnetic resonance imaging (MRI) equipment.
The large magnetic fields generated by an MRI device can attract ferromagnetic equipment with an extremely violent force, which could cause serious personal injury or death to persons between the equipment and the MRI device.
Observe all PRECAUTION and WARNING labels on the MRL PIC System and Quick Charger/Conditioner.
Hazard: Care should be exercised when operating the MRL PIC and MRL Quick Charger/Conditioner in the presence of oxygen sources (such as near bag-valve-mask devices or ventilators), flammable gases or anesthetics. These environments can produce fire or explosion hazards.
Place the PIC System, accessories and cables in a position where they cannot harm the patient should they fall. Keep all cables and hoses away from patient’s neck.
The MRL PIC System may not meet performance specifications if stored, transported, or used outside the specified storage or operating environmental range limits.
To prevent incorrect trending data from being printed, clear the Treatment Summary Log from the Recorder-Log menu prior to use on a new patient.
1.6 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |

SAFETY INFO RM ATIO N
Monitoring Precautions
•WARNING: PACEMAKER PATIENTS. The MRL PIC includes a pacemaker rejection circuit. The following warning is in accordance with the disclosure requirement of AAMI Standard EC13-3.1.2.1 (8): The rate meter may continue to count the pacemaker rate during some occurrences of cardiac arrest or some arrhythmias. Do not rely upon the heart rate meter alarms to assess the patient’s condition. Keep pacemaker patients under close surveillance. Pacemaker pulses of the type specified in AAMI EC13-1992, section 3.1.4, are detected at amplitudes greater than ± 20mV and rejected by the heart rate display. However, pacemaker pulses that are superimposed on the ECG at very low amplitudes may be counted by the heart rate display.
Note: This warning is an AAMI requirement that applies to all ECG monitors, regardless of make or model.
•Use only MRL patient cables. Other cables can produce excessive artifact, causing an inability to interpret the ECG.
•Use only ECG electrodes that meet the AAMI standard for electrode performance (AAMI EC-12). Use of electrodes not meeting this AAMI standard could cause the ECG trace recovery after defibrillation to be significantly delayed.
•The type of surface electrode and the technique used in applying the electrodes are major factors in determining the quality of the signal obtained. Use high-quality, silver-silver chloride electrodes. These electrodes are designed to provide excellent baseline stability, provide rapid recovery from defibrillation, and minimize artifacts from patient movement.
•When attempting to interpret subtle ECG changes (ST segments, etc.), use only the diagnostic frequency response mode. Other frequency response settings may cause misinterpretation of the patient’s ECG. See Frequency Response in chapter 13 of the PIC User Instruction Manual for more information.
•Excessive artifact can result due to improper skin preparation of the electrode sites. Follow skin preparation instructions in chapter 4 of the PIC User Instruction Manual.
•Do not operate the PIC System in conjunction with electrocautery or diathermy equipment. Such equipment, as well as equipment that emits strong radio frequency signals, can cause electrical interference and distort the ECG signal displayed by the monitor, thereby preventing accurate rhythm analysis.
PORTABLE INTENSIVE CARE SERVICE MANUAL |
1.7 |

S AFETY INFO RM ATIO N
•Do not operate the PIC in close proximity to any other monitor with respiration measurements. The two devices could affect the respiration accuracy.
•Any external connection to the 1V or MOD outputs must comply with clause 19 of IEC 601-1 for leakage current and must not exceed 450 mA.
•Shock Hazard: Use of accessories, other than those specified in the operating instructions, may adversely affect patient leakage currents.
•Certain line-isolation monitors may cause interference on the ECG display and may inhibit heart rate alarms.
Defibrillator Precautions
•The MRL PIC can deliver 360 joules of electrical energy. If this electrical energy is not discharged properly, as described in the User Instruction Manual, the electrical energy could cause personal injury or death to the operator or bystander.
•The operator and all other people must stand clear of the patient, the bed and all conductive surfaces (that are in contact with the patient) during defibrillation. The electrical energy delivered to the patient could also be delivered to any other person who is in contact with the patient or the conductive surface.
•Do not use the defibrillator in the presence of oxygen sources (such as near bag-valve-mask devices or ventilators), flammable gases or anesthetics. These environments can produce fire or explosion hazards.
•WARNING: Never position defibrillator paddles very close to or over ECG electrodes or jewlery. Severe burns may result from improper contact of defibrillator paddles. Before using defibirllator, consult operating instructions for proper procedures.
•After a synchronized cardioversion, the SYNC mode may be cleared after each shock or disarm. The user may have to reselect (press) the SYNC switch after each synchronized cardioversion shock performed on a patient. The PIC can be configured in the Supervisor-Defibrillation Set-up menu to remain in the SYNC mode after each synchronized cardioversion.
1.8 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |

SAFETY INFO RM ATIO N
•Synchronized cardioversion can be performed in the paddle monitoring mode. However, it is possible that artifact can be produced by the moving paddles, which could cause the defibrillator to trigger on the artifact. It is recommended that monitoring in leads I, II or III be used during synchronized cardioversion. Paddle monitoring should not be used for elective cardioversions procedures.
•To avoid stress to the defibrillator or the tester, never attempt to repeatedly charge and discharge the defibrillator in rapid succession. If a need for repetitive testing arises, allow a waiting period of at least 2 minutes for every third discharge.
•Monitoring ECG through the paddles may result in inaccurate heart rate display due to artifact.
•In the SYNC mode the defibrillator will not discharge without a command
•(R-Wave) signal indicated by a SYNC marker, flashing SYNC indicator and an audible beep if the R-wave beeper is enabled.
•Do not use the defibrillator if excessive condensation is visible on the device. Wipe only the outside with a damp cloth.
•Use only MRL-approved disposable defibrillation and pacing pads and cables.
•Defibrillator paddles should be kept clean and dry when not in use. When preparing electrodes and during defibrillation procedures, extreme care should be exercised to prevent gel or any conductive material from forming a contact between the operator and the paddles. Do not allow gel or any other conductive material to form an electrical bridge between the defibrillator electrodes or to the monitoring electrodes. Electrical arcing and/or patient burns could occur during defibrillation. Arcing and patient burns could prevent sufficient energy delivery to the patient.
•WARNING: If conductive gel forms a continuous path between the defibrillator electrodes, delivered energy may be dramatically reduced to zero. In this case, reposition the electrodes to eliminate the shunting path before attempting additional shocks.
•Improper defibrillation technique can cause skin burns. To limit possible skin burns, use only MRL defibrillation gel on paddles, insure the gel covers the entire paddle surface and press firmly against patient’s chest.
PORTABLE INTENSIVE CARE SERVICE MANUAL |
1.9 |

S AFETY INFO RM ATIO N
•Disposable defibrillation electrodes must be used in accordance with the manufacturer’s instructions. Do not use expired, dry electrodes or reuse disposable electrodes, as improper patient contact may result in patient burns and inability of the device to function properly.
•The device contains an automatic disarm of the capacitor bank. If the operator has not delivered the charge to a patient or test load, an internal timer will disarm the capacitor bank 1 minute in manual mode and 30 seconds in SAED Basic or SAED Basic+ mode after the ready charge signal. The ready charge signal is indicated by a continuous audible tone and the energy availability graph displayed on the monitor.
•If a new energy level is selected after the charge button is pushed and while the defibrillator is charging, defibrillator will automatically charge to the new energy selection. The CHARGE button need not be pressed again to select the new energy level.
•Disconnect from the patient any medical electronic device that is not labeled “defibrillation protected.”
•Before charging the defibrillator, verify that the energy selected on the display is the desired output.
•Some erythema of the skin and/or minor burns may occur during defibrillation. Use proper defibrillation techniques, as outlined in the User Instruction Manual, to minimize erythema/burns.
External Pacing Precautions
•Defibrillation will take priority over external pacing. Should the defibrillator be charged during the administration of external pacing, the pacer will automatically be turned off and the defibrillator will charge to the selected energy.
•Transcutaneous pacing should not be used to treat V FIB (ventricular fibrillation). In cases of V FIB, immediate defibrillation is advised.
•Transcutaneous pacing may cause discomfort ranging from mild to severe, depending on the patient’s tolerance level, muscle contractions and electrode placement. In certain cases, discomfort may be decreased by slightly relocating the pacing pads.
1.10 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |

SAFETY INFO RM ATIO N
•It is important to monitor the patient closely to verify that both mechanical and electrical capture are occurring. Electrical capture can be verified by observing the presence of a large ectopic beat after the pacing pulse is delivered. The size and morphology of the beat are dependent on the patient. In some instances the beat may appear as a relatively normal looking QRS pulse. Mechanical capture can be verified by checking for signs of increased blood flow i.e., reddening of the skin, palpable pulses, increased blood pressure, etc. (See chapter 8 of the PIC User Information Manual). Continuously observe the patient during pacing administration, to insure capture retention. Do not leave the patient unattended when administering external pacing therapy.
•Some erythema of the skin and/or minor burns may occur under the pacing electrodes in some patients. For prolonged periods of pacing (>4 hours), periodically inspecting the skin beneath the electrodes (when patient’s condition allows) is recommended. Discontinue external pacing if the skin is affected and if another form of pacing is available.
•Disposable defibrillation/pacing electrodes must be used in accordance with the manufacturer’s instructions. Do not use expired, dry electrodes or reuse disposable electrodes, as improper patient contact may result in patient burns and inability of the device to function properly.
•The pacing rate determination can be adversely affected by artifact. If the patient’s pulse and the heart rate display are significantly different, external pacing pulses may not be delivered when required.
•WARNING: PACEMAKER PATIENTS. The MRL PIC includes a pacemaker rejection circuit. The following warning is in accordance with the disclosure requirement of AAMI Standard EC-13-3.1.2.1 (8): The rate meter may continue to count the pacemaker rate during some occurrences of cardiac arrest or some arrhythmias. Do not rely upon the heart rate meter alarms to assess the patient’s condition. Keep pacemaker patients under close surveillance. Note: This warning is an AAMI requirement that applies to all ECG monitors, regardless of make or model.
•Artifact and ECG noise can make R-wave detection unreliable, affecting the HR meter and the demand mode pacing rate. Always observe the patient closely during pacing operations. Consider using asynchronous pacing mode if a reliable ECG trace is unobtainable.
PORTABLE INTENSIVE CARE SERVICE MANUAL |
1.11 |

S AFETY INFO RM ATIO N
Pulse Oximeter Precautions
•Keep the MRL finger probe clean and dry.
•SpO2 measurements may be affected by certain patient conditions: severe right heart failure, tricuspid regurgitation or obstructed venous return.
•SpO2 measurements may be affected when using intravascular dyes, in extreme vasoconstriction or hypovolemia or under conditions where there is no pulsating arterial vascular bed.
•SpO2 measurements may be affected in the presence of strong EMI fields, electrosurgical devices, IR lamps, bright lights, improperly applied sensors; the use of non-MRL sensors, or damaged sensors; in patients with smoke inhalation, or carbon monoxide poisoning, or with patient movement.
•Tissue damage can result if sensors are applied incorrectly, or left in the same location for an extended period of time. Move sensor every 4 hours to reduce possibility of tissue damage.
•Do not use any oximetry sensors during MRI scanning. MRI procedures can cause conducted current to flow through the sensors, causing patient burns.
•Do not apply SpO2 sensor to the same limb that has an NIBP cuff. The SpO2 alarm may sound when the arterial circulation is cut off during NIBP measurements, and may affect SpO2 measurements.
•WARNING: In some instances, such as obstructed airway, the patient's breathing attempts may not produce any air exchange. These breathing attempts can still produce chest size changes, creating impedance changes, which can be detected by the respiration detector. It is best to use the pulse oximeter whenever monitoring the respirations to accurately depict the patient's respiratory condition.
1.12 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |

SAFETY INFO RM ATIO N
Non-Invasive Blood Pressure Precautions
•Only a physician can interpret pressure measurements.
•Blood pressure measurement results may be affected by the position of the patient, his or her physiological condition and other factors.
•Substitution of a component different from that supplied by MRL (e.g., cuff, hoses, etc...) may result in measurement error. Use only MRL cuffs and hoses.
•Do not use a blood pressure cuff on the limb being used for IV infusion or for SpO2 monitoring.
•Accurate pressure readings may not be achieved on a person experiencing arrhythmias, shaking, convulsions or seizures. Medication may also affect pressure readings. The correctsize cuff is essential for accurate blood pressure readings.
•Blood pressure hoses must be free of obstructions and crimps.
•If the patient’s cuff is not at heart level, an error in measurement may result.
•When monitoring blood pressure at frequent intervals, observe the cuffed extremity of the patient for signs of impeded blood flow.
•WARNING: THIS DEVICE IS NOT APPROVED FOR
USE ON NEO-NATALPATIENTS.
•Do not monitor one patient’s NIBP while monitoring another patient’s ECG.
•Blood pressure measurement may be inaccurate if taken while accelerating or decelerating in a moving vehicle.
•If an NIBP measurement result is questionable or “motion” indication is displayed, repeat the measurement. If the repeated measurement result is still questionable, use another blood pressure measurement method.
•Do not use the NIBP on cardiopulmonary bypass patients.
PORTABLE INTENSIVE CARE SERVICE MANUAL |
1.13 |

S AFETY INFO RM ATIO N
Battery Precautions
•Use only MRL SmartPak or MRL SuperPac batteries in the MRL PIC. Use of any other battery can damage the MRL PIC and not provide sufficient power, inhibiting patient care.
•If the Low Battery indication occurs at any time during operation, immediately replace the battery pack with a battery pack known to be fully charged. Always have a fully charged battery pack available as a back-up.
•Due to the critical nature of all batteries, replacement of the MRL batteries is recommended at 24-month intervals.
•Proper care and maintenance of the MRL batteries is important to ensure continuous operation during patient care. If the batteries are not maintained properly, loss of power during patient care could result, affecting patient care.
•The battery packs contain materials such as stainless steel, cadmium and nickel, which can be recycled. They must be disposed of properly. Consult local authorities for proper disposal.
1.14 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |

SAFETY INFO RM ATIO N
Charger Precautions
•Charge only MRL SmartPak or MRL SuperPac batteries in the MRL Quick Charger/ Conditioner. Charging any other battery can cause damage to the MRL Quick Charger.
•Do not insert objects into or block the charger’s ventilation ports.
•When testing the defibrillator on the charger’s defibrillation output tester, ensure that the paddle surface is positioned properly in the paddle test well. Do not use gel during this test, and ensure that the paddle surface is not contacting the metal charger frame. When discharging the paddles into the tester, press the paddles firmly into the test well to prevent pitting the paddle surfaces.
•Only test MRL defibrillators on the charger’s defibrillation output tester. Testing other brands of defibrillators will damage the charger’s defibrillation output tester.
•Do not take charger or paddle holder apart or attempt to repair it yourself.
•The MRL charger should not be used in the presence of flammable anesthetics or materials.
•If the charger has been dropped or shows visible signs of abuse, refer device to qualified service personnel for verification of proper operation.
•Do not immerse the charger or expose it to water or other liquids.
•Wipe only the outside with a damp cloth.
•Tighten clamp onto power cord to prevent its accidental removal.
•Unplug the charger prior to changing the fuse.
•Use only the MRL Quick Charger to power the MRL PIC System from an auxiliary power source.
•Do not use the MRL Quick Charger to power any non-MRL devices.
•A depleted battery could increase defibrillator charge times.
•It is recommended that a fully charged battery be inserted in the PIC System even when operating on auxiliary power.
•Note: The MRL PIC System will operate from an auxiliary power source without a battery inserted or if the inserted battery is depleted. However, under these circumstances, defibrillator charge time will be slightly longer (10 seconds typical, 15 sec. maximum).
PORTABLE INTENSIVE CARE SERVICE MANUAL |
1.15 |

S AFETY INFO RM ATIO N
SAED Precautions
•WARNING: Cardiac Pacemakers. The presence of an internal cardiac pacemaker may adversely affect analysis results. If it is known, or suspected, that the patient is fitted with a cardiac pacemaker, follow your own locallyestablished procedure for dealing with defibrillation of such patients.
•The PIC, in SAED mode, should only be applied to victims of cardiac arrest who exhibit unconsciousness, absence of breathing, and absence of pulse.
•Excessive motion may affect analysis results. ECG analysis should not be performed when the patient is being moved. Stop all patient movement and do not touch patient when the ECG analysis is in process. Take precautions to eliminate sources of motion or artifact before monitoring in SAED mode.
•SAED mode automatically selects 200, 300, 360J for defibrillation energy. Use of SAED mode on patients weighing less than 80 lbs. may increase the risk of myocardial tissue damage.
IBP Precautions
•To insure compatability and electrical safety, accessory pressure sensors should comply with ANSI/AAMI BP-22 and IEC 601-2-34 for IBP or ANSI/AAMI NS28 for ICP
•Follow instructions supplied with any accessory pressure sensor regarding calibration and removal of trapped air.
•Avoid touching metal parts of any transducer while it is in contact with the patient.
•Do not reuse any components that are labeled for single use only.
•Transducers should be rated to withstand an accidental drop of at least a meter onto a hard surface.
•Transducers that are subject to immersion in liquids should be rated as watertight.
1.16 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |

SAFETY INFO RM ATIO N
CO2 Precautions
•Do not use CO2 sensor during MRI scanning. MRI procedures can permanently damage the CO2 sensor.
•CO2/ETCO2 measurements may be affected by the presence of interfering gases or vapors. Do not use on a patient being administered oxygen or nitrous oxide.
•Use only MRL CO2 sensors and adapters.
•Do not reuse airway adapters that are labeled for single patient use.
•Prior to using airway adapter check for lodged obstructions. After attaching, check the sensor for proper placement of the sensor.
•If using the CO2 monitor for extended critical care, replace the airway adapter every 24 hours or when it becomes occlued.
•Do not use with patients with a low tidal volume, such as patients younger than 3 years of age or weighing less than 22 pounds, or patients with a respiration rate greater than or equal to 60 breaths per minute.
•Accuracy is based upon 1 atmospheric pressure and no residual CO2 gas left in the sensor from previous expiration. The CO2 trace will be displayed as if that is the case.
PORTABLE INTENSIVE CARE SERVICE MANUAL |
1.17 |
S AFETY INFO RM ATIO N
|
|
|
|
1.18 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |

CHAPTER 2: GENERAL INFORMATION
This chapter introduces the MRL Portable Intensive Care (PIC) System. It contains a product overview, a general description and equipment setup, summary of operation procedures for servicing the unit, an explanation of the part numbering system, and a list of available options and accessories system. The chapter concludes with the technical specifications.
Chapter Overview: |
• |
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .Product Overview |
2.2 |
|
• |
General Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2.2 |
|
• |
PIC System Interfaces . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2.3 |
|
• |
PIC System Controls and Indicators . . . . . . . . . . . . . . . . . . |
2.5 |
|
• PIC System Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2.7 |
|
|
• |
Initial Installation Evaluation. . . . . . . . . . . . . . . . . . . . . . . . . |
2.9 |
|
• |
Equipment Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2.11 |
|
• Summary of Operations . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2.18 |
|
|
• Part Numbering System . . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2.21 |
|
|
• |
Options and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2.22 |
|
• |
Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . . . |
2.24 |
|
|
|
|
CAUTION! Federal law restricts this device to use by or on the order of a physician.
NOTE: See the PIC User Instruction Manual for more detailed operation procedures, when using this system on a patient).
NOTE: The disassembly, performance verification, and adjustment
procedures described in this manual are intended to be
performed by qualified service technicians using the
recommended tools and equipment.
PORTABLE INTENSIVE CARE SERVICE MANUAL |
2.1 |

G ENERAL INFO RM ATIO N
Product Overview
The MRL PIC System is an extremely flexible device that incorporates an ECG monitor, defibrillator (manual or semiautomated), external pacer, pulse oximeter, and a non-invasive blood pressure/respiration monitor. The PIC System's small and lightweight design makes it ideal for transport situations or for use in and out of the hospital.
General Description
|
Refer to the following User’s Manuals for instructions on the |
|
operation of the corresponding MRL product: PIC System, PIC |
|
Monitor (991010), and Lite (991022). All MRL PIC products include |
|
a 6.4” VGA display and an annotating chart recorder. An integral |
|
paddle tray/AC supply, shown on the MRL Lite, is optional on all |
|
units. |
MRL PIC System™ |
The MRL PIC System is a multi-parameter Monitor/ |
|
Defibrillator/Pacer. Standard functions for the PIC are |
|
ECG, DEFIB, PACER, and RESP. Upgradeable options |
|
include 12-Lead, NIBP, SpO2, TEMP, AED, CO2, IBP, |
|
Voice Memo, Fax, and Data Record/Review. Display |
|
options include EL, Color TFT, and Mono LCD. Acceptable |
|
batteries include the SmartPak PlusTM and SuperPacTM. |
MRL PIC Monitor™ |
The MRL PIC Monitor standard functions are ECG and |
|
RESP. Upgradeable options include 12-Lead, NIBP, SpO2, |
|
TEMP, CO2, and IBP. The PIC Monitor uses a monochrome |
|
LCD display. Acceptable batteries include the SmartPakTM, |
|
SmartPak PlusTM+, and SuperPacTM. |
MRL Lite™ |
The MRL Lite is a Monitor/Defibrillator Pacer. Standard |
|
functions for the Lite are ECG and DEFIB. Upgradeable |
|
options include Pacing and Advisory. The Lite has a |
|
monochrome LCD display. Acceptable batteries include the |
|
SmartPakTM, SmartPak PlusTM, and SuperPacTM. A |
|
PCMCIA data card is not available for the Lite. |
2.2 |
PORTABLE INTENSIVE CARE SERVICE MANUAL |
 Loading...
Loading...