Margette Eagle3000 Data manual

Eagle
®
3000 Patient Monitor
Data Manual

NOTE:
Trademarks
Due to continuing product innovation, specifications in this manual are
subject to change without notice.
Trademarked names appear throughout this document. Rather than list the
names and entities that own the trademarks or insert a trademark symbol
with each mention of the trademarked name, the publisher states that it is
using the names only for editorial purposes and to the benefit of the
trademark owner with no intention of improperly using that trademark.
ACCUSKETCH, APEX, AQUA-KNOT, ARCHIVIST, BABY MAC, CASE, CD
TELEMETRY, CENTRA, CHART GUARD, CINE 35, CORO, COROMETRICS,
CRG PLUS, DIGISTORE, Digital DATAQ, E for M, EAGLE, Event-Link,
HELLIGE, IMAGE STORE, LASER SXP, MAC, MAC-LAB, MACTRODE,
MARQUETTE, MARQUETTE UNITY NETWORK, MARS, MAX, MEI, MEI in the
circle logo, MEMOPORT C, MIDAS SYSTEM, MIDASNET, MINISTORE,
MINNOWS, Monarch 8000, MULTI-LINK, MULTISCRIPTOR, MUSE, Neo-Trak,
OXYMONITOR, PRESSURE-SCRIBE, PRES-R-CUFF, QMI, QS, Quantitative
Medicine, Quantitative Sentinel, Qwik Connect Spiral, RAMS, SAM, SEER,
SOLAR, Spectra 400, Spectra-Tel, ST GUARD, TRAM, TRAM-NET, TRAMRAC, TRAMSCOPE, TRIM KNOB, UNITY NETWORK, UNITY twist logo, Vari-X,
Vari-X Cardiomatic, and VAS are trademarks of Marquette Medical Systems,
Inc. registered in the United States Patent and Trademark Office.
12SL, 15SL, AccuVision, ADVANTAGE, AUTOSEQ, BODYTRODE,
CardioMail, CardioServ, CardioSmart, CardioSpeak, CardioSys, CD
TELEMETRY
‚
-LAN, CENTRALSCOPE, Corolation, Corometrics Sensor Tip, CV
Mail, CV-Web, DASH, EDIC, HI-RES, IMAGE VAULT, INTELLIMOTION,
INTER-LEAD, LIFEWATCH, MARQUETTE MEDICAL SYSTEMS,
MARQUETTE
CardioWindow, MUSE CV, MUSEWord, O
‚
RESPONDER, MENTOR, MIDAS Com, MRT, MUSE
SENSOR, OMRS, OnlineABG,
2
Premium, RSVP, SILVERTRACE, SMART-PAC, SMARTLOOK, SOLARVIEW,
Spectra-Overview, Trimline, UNITY, and Universal are trademarks of
Marquette Medical Systems, Inc.
© 1997 Marquette Medical Systems, Inc. All rights reserved.
T-2
Eagle 3000 Patient Monitor
415397-038
Revision A
21 April 1997

Table of Contents
Table of Contents
INTRODUCTION ................................................................1-1
1
Manual Information ................................................................1-2
Revision History ..........................................................1-2
Manual Purpose ..........................................................1-3
Chapter Content ..........................................................1-3
Introduction .....................................................1-3
Upper Level Overview .......................................1-3
Circuit Board Assembly Chapters ..................... 1-3
Related Manuals .........................................................1-4
Tech Memos ................................................................1-4
BBS Tech Memo Service ..............................................1-4
Safety Information ..................................................................1-5
Responsibility of the Manufacturer ..............................1-5
Intended Use ............................................................... 1-5
Equipment Symbols ....................................................1-6
Notes, Cautions, and Warnings ...................................1-7
Service Information .................................................................1-8
Service Requirements ..................................................1-8
Equipment Identification .............................................1-8
Warranty .....................................................................1-8
How to Reach Us… .................................................................1-9
Ordering Supply Items ................................................1-9
Ordering Service Parts .................................................1-9
Service Calls ................................................................1-9
Service Contracts .............................................1-9
Technical Support .....................................................1-10
For All Hardware ............................................1-10
Telemetry .......................................................1-10
Series 7000/7010 ..........................................1-10
48-Hour Turnaround Repair ..........................1-10
Service Address .............................................. 1-10
For Additional Information ........................................1-10
Revision A
2
Abbreviations .......................................................................1-11
UPPER LEVEL OVERVIEW ................................................. 2-1
Upper Level Part Numbers ......................................................2-2
Use Field Service Manual First ....................................2-2
Model Numbers ...........................................................2-2
Circuit Board Part Numbers ...................................................2-3
Exploded View PN 414888-001J/002J/003F/004J/005F ....... 2-4
Parts List PN 414888-001J/002J/003F/004J/005F ..............2-9
Eagle 3000 Patient Monitor
415397-038
i

Table of Contents
Switches and Controls PN 800772-002A ............................... 2-12
PCB Connectors PN 800772-002A ........................................2-13
Parts Location Diagram PN 800772-002A ............................. 2-19
Parts List PN 800772-002A ................................................... 2-21
Schematic Diagram PN SD800772-002A ...............................2-24
POWER SUPPLY ASSEMBLY AND PCB ............................... 3-1
3
4
Introduction ...........................................................................3-2
Exploded View PN 414641-001C .............................................3-3
Parts List PN 414641-001C .....................................................3-5
Switches and Controls PN 800706-001C ................................. 3-6
PCB Connectors PN 800706-001C ..........................................3-7
Parts Location Diagram PN 800706-001C ...............................3-8
Parts List PN 800706-001C ...................................................3-10
Schematic Diagram PN SD800706-001B ............................... 3-14
OPTION ASSEMBLIES AND PCBs .......................................4-1
Introduction ...........................................................................4-2
Exploded View PN 416021-001C/002C ...................................4-3
Parts List PN 416021-001C .....................................................4-5
Parts List PN 416021-002C .....................................................4-6
Exploded View PN 418394-001A/002A ...................................4-7
Parts List PN 418394-001A ..................................................... 4-9
Parts List PN 418394-002A ................................................... 4-10
Switches and Controls PN 800836-002A ............................... 4-11
PCB Connectors PN 800836-002A ........................................4-12
Parts Location Diagram PN 800836-002A ............................. 4-17
Parts List PN 800836-002A ................................................... 4-18
Schematic Diagram PN SD800836-002A ...............................4-20
Switches and Controls PN 801318-001A ............................... 4-29
PCB Connectors PN 801318-001A ........................................4-30
ii
Eagle 3000 Patient Monitor
415397-038
Revision A

Table of Contents
Parts Location Diagram PN 8013138-001A ..........................4-35
Parts List PN 801318-001A ................................................... 4-36
Schematic Diagram SD801318-001A ....................................4-38
Switches and Controls PN 800862-001E ...............................4-47
PCB Connectors PN 800862-001E ........................................4-48
Parts Location Diagram PN 800862-001E .............................4-50
Parts List PN 800862-001E ................................................... 4-53
Schematic Diagram SD800862-001E .................................... 4-56
DATA ACQUISITION ASSEMBLY AND PCB .........................5-1
5
Introduction ...........................................................................5-2
Exploded View PN 414887-001B .............................................5-3
Parts List PN 414887-001B .....................................................5-4
Switches and Controls PN 800776-001B ................................. 5-5
PCB Connectors PN 800776-001B .......................................... 5-6
Parts Location Diagram PN 800776-001B ...............................5-8
Parts List PN 800776-001B ...................................................5-10
Schematic Diagram PN SD800776-001B ............................... 5-14
Revision A
Eagle 3000 Patient Monitor
415397-038
iii

For your notes
Table of Contents
iv
Eagle 3000 Patient Monitor
415397-038
Revision A

1
INTRODUCTION
Manual Information ................................................................ 1-2
Revision History .......................................................... 1-2
Manual Purpose .......................................................... 1-3
Chapter Content .........................................................1-3
Introduction .....................................................1-3
Upper Level Overview ....................................... 1-3
Circuit Board Assembly Chapters ..................... 1-3
Related Manuals .........................................................1-4
Tech Memos ................................................................ 1-4
BBS Tech Memo Service .............................................. 1-4
Safety Information .................................................................. 1-5
Responsibility of the Manufacturer .............................. 1-5
Intended Use ............................................................... 1-5
Equipment Symbols .................................................... 1-6
Notes, Cautions, and Warnings ................................... 1-7
Service Information ................................................................1-8
Service Requirements .................................................. 1-8
Equipment Identification ............................................. 1-8
Warranty .....................................................................1-8
How to Reach Us… ................................................................. 1-9
Ordering Supply Items ................................................1-9
Ordering Service Parts ................................................. 1-9
Service Calls ...............................................................1-9
Service Contracts ............................................. 1-9
Technical Support ..................................................... 1-10
For All Hardware ............................................ 1-10
Telemetry ....................................................... 1-10
Series 7000/7010 .......................................... 1-10
48-Hour Turnaround Repair ..........................1-10
Service Address .............................................. 1-10
For Additional Information ........................................ 1-10
Abbreviations ....................................................................... 1-11
Revision A
Eagle 3000 Patient Monitor
415397-038
1-1

Manual Information
INTRODUCTION: Manual Information
Revision History
Revision Title Manual PN Date Comment
A Eagle 3000 Patient
Monitor Data Manual
Each page of this manual has a revision letter, located at the
bottom of the page, that identifies the manual’s update level. This
may be important if you have different updates to a manual and
don’t know which is the most current.
For the initial release, the manual has the revision letter A. For the
first update of the manual, the manual receives the revision letter
B, and so on. The table below indicates the revision history of this
manual.
Table 1-1. Revision History
415397-038 21 April 1997 Initial release of this
manual.
1-2
Eagle 3000 Patient Monitor
415397-038
Revision A

INTRODUCTION: Manual Information
Manual Purpose
Chapter Content
Introduction
Upper Level Overview
This manual is a supplement to the field service manual, Eagle
3000 Patient Monitor Field Service Manual , PN 415397-003. If you
need equipment overview, maintenance, troubleshooting,
calibration, configuration or upper level assembly information,
order the field service manual.
This manual supplies detailed technical information for service
representatives and technical personnel so they may troubleshoot
the equipment to a circuit board or component level.
Users of this manual are expected to have a strong background in
electronics, including analog and digital circuity with
microprocessor and micro-controller architecture.
This manual is organized into five chapters, summarized as
follows:
Chapter one describes the service manual and chapter contents
and provides general information on safety, service requirements,
equipment symbols, and serial number identification.
Chapter two gives the upper level model part numbers and
presents the upper assembly interconnection diagram and signal
names. Information regarding the main processor PCB,
pn 800772-002, can also be found in chapter two.
Circuit Board Assembly
Chapters
Power Supply
Eagle 3000 Options PCB
Acquisition Processor PCB
Each of the subsequent chapters describe a circuit board or
assembly that may include any or all of the following:
■
General information
■
List of switches and controls
■
List of input and output connector signals
■
Exploded view or parts location diagram
Parts list
■
Schematic diagram
■
■
Updated data describing revisions to the circuit boards and
major assemblies, if applicable
Chapter three provides all information available for the power
supply assembly, pn 414641-001, and for the power supply PCB,
pn 800706-001.
Chapter four provides all information available for the Eagle 3000
option assemblies, pn 416021-001 and -002, as well as
information for the Eagle 3000 option PCBs, pn 800836-002,
800862-001, and 801318-001.
Chapter five provides all information available for the acquisition
processor (DAS) assembly, pn 414887-001, and for the acquisition
processor PCB, pn 800776-001.
Revision A
Eagle 3000 Patient Monitor
415397-038
1-3
INTRODUCTION: Manual Information
Related Manuals
Part Number Name
415397-003 Eagle 3000 Patient Monitor Field Service Manual
405040-088 Centralscope 12 Central Station Service Manual
405040-018 Centralscope 12C Central Station Service Manual
405040-164 Centralscope Central Station Field Service Manual
Part Number Name
415397-042 Eagle 3000 Monitor Operator’s Manual (for software version 3)
415397-043 Eagle 3000 Monitor Instructions (for software version 3)
Check these documents if you need additional information on the
Marquette Monitoring system.
Table 1-2. Service Documents
Table 1-3. Operator Documents
Tech Memos
BBS Tech Memo Service
Marquette Service issues technical memos that aid service
personnel in servicing and maintaining the equipment. Tech
Memos supply important information about changes to the
equipment, typical problems, and how to solve those problems.
Tech Memos are also written to describe hardware and software
upgrades.
Tech Memos are automatically distributed to all Marquette Field
Service personnel, and are available to customers by subscription.
Contact Technical Support for more information or to subscribe.
For the address or telephone number, see the “How to Reach Us...”
pages presented later in this manual.
Designed primarily for biomedical technicians, the BBS Tech
Memo Service provides “instant” on-line access to the latest
Marquette maintenance and repair information. Subscribers with
an IBM or IBM-compatible computer and modem can link-up to
the MEITIS system and download technical publications as well as
send and receive messages from technical support
representatives. Call Service Contracts at 800-552-3248 for
subscription information.
1-4
Eagle 3000 Patient Monitor
415397-038
Revision A

Safety Information
INTRODUCTION: Safety Information
Responsibility of the
Manufacturer
Intended Use
Marquette Medical Systems is responsible for the effects of safety,
reliability, and performance only if:
Assembly operations, extensions, readjustments,
■
modifications, or repairs are carried out by persons authorized
by Marquette.
The electrical installation of the relevant room complies with
■
the requirements of the appropriate regulations.
The equipment is used in accordance with the instructions for
■
use.
This device is intended for use under the direct supervision of a
licensed health care practitioner.
To ensure patient safety, use only parts and accessories
manufactured or recommended by Marquette Medical Systems.
Contact Marquette Medical Systems for information before
connecting any devices to this equipment that are not
recommended in this manual.
Revision A
Eagle 3000 Patient Monitor
415397-038
1-5
CAUTION
INTRODUCTION: Safety Information
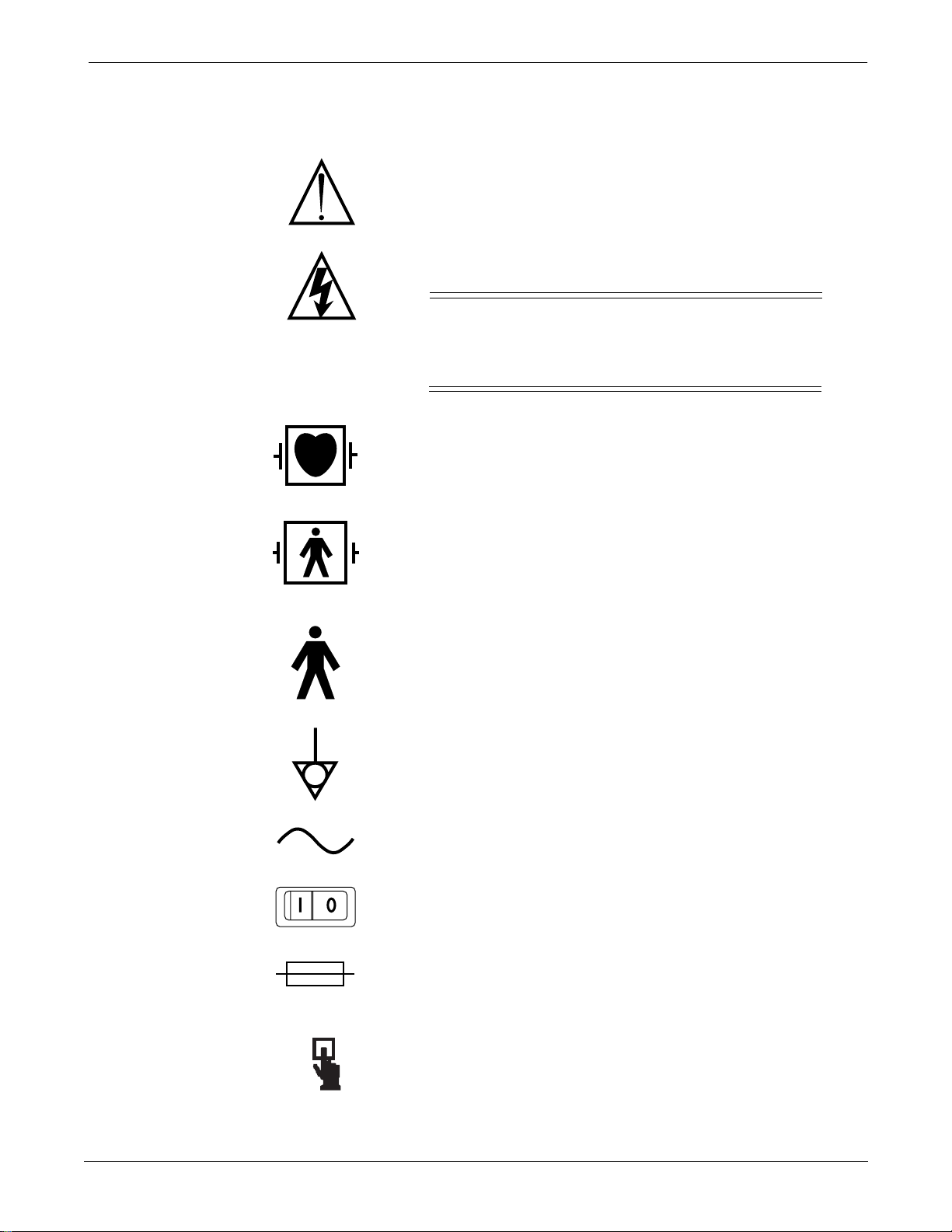
Equipment Symbols
The following symbols appear on the equipment.
NOTE: Some symbols may not appear on all equipment.
ATTENTION: Consult accompanying documents before using
the equipment.
In Europe, this symbol means dangerous or high voltage. In the
United States, this symbol represents the caution notice below:
To reduce the risk of electric shock, do NOT
remove cover (or back). Refer servicing to
qualified personnel.
Defibrillator-proof type CF equipment; type CF equipment is
specifically designed for applications where a conductive
connection directly to the heart is established. The paddles
indicate the equipment is defibrillator proof.
Defibrillator-proof type BF equipment; type BF equipment is
suitable for intentional external and internal application to the
patient, excluding direct cardiac application. Type BF
equipment is type B equipment with an F-type isolated (floating)
part. The paddles indicate the equipment is defibrillator proof.
PRESS
Type B equipment; type B equipment is suitable for intentional
external and internal application to the patient, excluding direct
cardiac application.
Equipotentiality
Alternating current (AC)
Power;
Fuse
Indicates where to press to open the door on the Series 7160
Direct Digital Writer.
II
II
= ON;
OOOO
= OFF
1-6
Eagle 3000 Patient Monitor
415397-038
Revision A

NOTE
CAUTION
WARNING
INTRODUCTION: Safety Information
Notes, Cautions, and
Warnings
Notes, cautions, and warnings are used in this manual to provide
additional information to service personnel.
A note conveys special instructions to service
personnel to highlight an operating procedure,
practice, etc. Notes may precede or follow the
applicable text, depending on the material to be
highlighted.
The purpose of a caution is to inform service
personnel of an operating procedure, practice, etc.,
which if not strictly observed, could result in
possible damage to the equipment. Cautions
always precede the applicable text.
A warning provides instructions to service
personnel that if an operating procedure, practice,
etc., is not followed, personal injury may result.
Warnings always precede the applicable text.
Revision A
Eagle 3000 Patient Monitor
415397-038
1-7
Service Information
INTRODUCTION: Service Information
Service Requirements
Equipment
Identification
Follow the service requirements listed below.
Refer equipment servicing to Marquette’s authorized service
■
personnel only.
Any unauthorized attempt to repair equipment under
■
warranty voids that warranty.
It is the user’s responsibility to report the need for service to
■
Marquette Medical Systems or to one of their authorized
agents.
Failure on the part of the responsible individual, hospital, or
■
institution using this equipment to implement a satisfactory
maintenance schedule may cause undue equipment failure
and possible health hazards.
Regular maintenance, irrespective of usage, is essential to
■
ensure that the equipment will always be functional when
required.
Every Marquette Medical Systems device has a unique serial
number for identification. The serial number appears on the
product label on the base of each unit under the Trim knob
control.
Month
Manufactured
A = January
B = February
C = March
D = April
E = May
F = June
G = July
H = August
J = September
K = October
L = November
M = December
Warranty
D 1 XX 0005 G XX
Year
Manufactured
1 = 1991
2 = 1992
3 = 1993
(and so on)
Product Code
Two-character
product
descriptor
1 year.
Product
Sequence
Number
Manufacturing
number (of
total units
manufactured.)
Division
F = Cardiology
G = Monitoring
Device Characteristics
One or 2 letters that
further describe the unit,
for example:
P = prototype not
conforming to marketing
specification
R = refurbished equipment
S = special product
documented under
Specials part numbers
U = upgraded unit
1-8
Eagle 3000 Patient Monitor
415397-038
Revision A

How to Reach Us…
INTRODUCTION: How to Reach Us…
The following are telephone numbers and addresses for contacting
various Marquette Medical Systems Service and Supplies Division
personnel.
Ordering Supply Items
Ordering Service Parts
Supply items are generally items used during normal operation of
a product. Leadwires, electrode paste, patient cables, and printer
paper are examples of supply items.
Make telephone inquiries about supply items at:
1-800-558-5102 (U.S. only)
1-561-575-5000 (outside the U.S.)
Address orders or inquiries to:
Marquette Medical Systems Service and Supplies
P.O. Box 9100
100 Marquette Drive
Jupiter, FL 33468-9100
Attn: Supplies
Service parts are items that are not expended in the normal
operation of the product. They are generally replacements for
defective or malfunctioning items inside the product. Service
parts include PCB assemblies, electronic components, internal
cables and harnesses, software or firmware, and operator and
service manuals. When ordering additional operator manuals,
remember to notate the software version from the start-up screen.
Service Calls
Service Contracts
A part number for the item to be replaced is necessary for ordering
a service part. If the part number for the desired item is
unobtainable, the following will be necessary to order the item:
■
model and serial number of the equipment,
part number/name of the assembly where the item is used,
■
item name, and
■
where applicable, reference designation (eg, R13, S12, U32).
■
To open a service call with Marquette Medical Systems Service,
contact a Service Dispatcher at:
1-800-558-7044 (U.S. only)
1-561-575-5000 (outside the U.S.)
For any questions about Service Contracts, contact the service
contract operator at:
1-800-552-3248
Revision A
Eagle 3000 Patient Monitor
415397-038
1-9

INTRODUCTION: How to Reach Us…
Technical Support
For All Hardware
Telemetry
Series 7000/7010
48-Hour Turnaround Repair
Technical Support has the most current information about your
equipment and can provide assistance with any technical
questions or problems.
For technical advice concerning any equipment in your Marquette
Medical Systems monitoring system, contact Tech Support—
Monitoring Hardware at:
1-800-558-7822
For technical advice concerning your Telemetry system, contact
Tech Support—Telemetry at:
1-800-552-3243
For technical advice concerning Series 7000/7010 patient
monitoring equipment, contact Tech Support:
1-800-443-0980
Some Marquette products (Input Modules, Tram modules, Series
7700 ECG Telemetry Transmitters, and CD Telemetry
Transmitters) are repaired on a 48-hour turnaround basis.
To inquire about status of 48-hour turnaround repair items, or if
you have questions before shipping an assembly to be repaired,
call:
Service Address
For Additional
Information
1-800-552-3243
Send items for 48-hour repair and all monitoring repair items to:
Marquette Medical Systems Service and Supplies
P.O. Box 9100
100 Marquette Drive
Jupiter, FL 33468-9100
Attn: Monitoring Repair
The main switchboard operator will direct your call to the person
most able to assist you. For any other questions or problems,
contact the main switchboard operator at:
1-800-558-5120
1-10
Eagle 3000 Patient Monitor
415397-038
Revision A
Abbreviations
INTRODUCTION: Abbreviations
A
A ampere
Ampl amplifier
Ave Avenue
B
BDGH binding head
Btu/hr British thermal
units per hour
C
CA California
Cap capacitor
CMOS complimentary
metal-oxide
semiconductor
Comp composition
D
DC, dc direct current
E
eg for example
EPROM electronically
programmable
read only memory
ESD electrostatic
discharge
etc et cetera, and so
forth
F
FL Florida
G
g gram
I
in inch
Inc incorporated
L
lb pound
LED light-emitting
diode
M
mA milliampere
MHz megahertz
mm millimeter
mmHg millimeter of
mercury
MOSFET metal-oxide
semiconductor
field-effect
transistor
MPE metallized
polycarbonate
epitaxial
MPP metallized
polypropylene
N
No number
O
oz ounce
P
PA pulmonary artery
PC printed circuit,
personal
computer
PCB printed circuit
board
pF picofarad
PLCC plastic leaded chip
carrier
PNH pan head
Q
Qty quantity
R
RAM random access
memory
Res resistor
ROM read-only memory
S
SM surface mount
SST stainless steel
SvO
2
Tant tantalum
TTL transistor-
V volt, voltage
W watt, West
w/ with
WI Wisconsin
WW wire wound
➔ continued
< less than
≤ less than or equal
µF microfarad
µH microhenry
Ω ohm
% percent
mixed venous
oxygen saturation
T
transistor logic
V
W
Other
to
K
K kilo, kilohm
Revision A
Eagle 3000 Patient Monitor
415397-038
1-11

For your notes
INTRODUCTION: Abbreviations
1-12 Revision A
Eagle 3000 Patient Monitor
415397-038

2
UPPER LEVEL OVERVIEW
Upper Level Part Numbers ...................................................... 2-2
Use Field Service Manual First .................................... 2-2
Model Numbers ........................................................... 2-2
Circuit Board Part Numbers ................................................... 2-3
Exploded View PN 414888-001J/002J/003F/004J/005F ....... 2-4
Parts List PN 414888-001J/002J/003F/004J/005F ..............2-9
Switches and Controls PN 800772-002A ............................... 2-12
PCB Connectors PN 800772-002A ........................................ 2-13
Parts Location Diagram PN 800772-002A ............................. 2-19
Parts List PN 800772-002A ................................................... 2-21
Schematic Diagram PN SD800772-002A ............................... 2-24
Revision A
Eagle 3000 Patient Monitor
415397-038
2-1

UPPER LEVEL OVERVIEW: Upper Level Part Numbers
Upper Level Part Numbers
Use Field Service
Manual First
Model Numbers
It is recommended that you first familiarize yourself with the
information presented in the Troubleshooting section of the Eagle
3000 Patient Monitor Field Service Manual , pn 415397-003. The
troubleshooting information will help you narrow service problems
down to one of the replaceable assemblies.
After you have determined which assembly requires replacement,
use the input/output connector list, parts location diagram, parts
list, and/or schematic diagram presented in this manual to repair
the assembly.
Also refer to the Eagle 3000 Patient Monitor Field Service Manual ,
pn 415397-003, for preventative maintenance, calibration, and
configuration procedures.
The following model part numbers are discussed in this manual
for the Eagle 3000 patient monitor.
Table 2-1. Model Part Numbers
Model Part Number
Eagle 3000 Patient Monitor 414888-001
414888-002
414888-003
414888-004
414888-005
2-2
Eagle 3000 Patient Monitor
415397-038
Revision A
NOTE
UPPER LEVEL OVERVIEW: Circuit Board Part Numbers
Circuit Board Part Numbers
The various model parameters of the Eagle 3000 patient monitor
are listed in the table below. Refer to your Eagle 3000 model
number for the correct replacement part numbers.
Model PN NBP Writer ETCO2
414888-001 X – – –
414888-002 X – – X
414888-003 X – X X
414888-004 X X – X
414888-005 X X X X
In addition to the main processor PCB, pn 800772-002, the table
below lists the other assemblies and circuit boards described in
this manual.
Table 2-2. List of Options
Defib
Sync/
Remote
Alarm
Table 2-3. Assemblies and Circuit Boards
Item Part Number
Power Supply Assembly 414641-001
Power Supply PCB 800706-001
Option Assemblies 416021-001
416021-002
418394-001
418394-002
Defib Sync/Remote Alarm PCB
1
800836-002
801813-001
ETCO2 PCB 800862-001
Data Acquisition Assembly 414887-001
Data Acquisition PCB 800776-001
1
There are two PCBs for the defib sync/remote alarm assembly. PN
800836-002 is used on older Eagle 3000 monitors that have the
1/4-inch phone jack type connector for remote alarm. PN 801813-001 is
used for new Eagle 3000 monitors, which have a 9-pin subminiature “D”
connector for remote alarm. Refer to chapter 4 for more information
about the option assemblies.
Revision A
The exploded view and parts lists for the upper level
assembly, pn 414888-001J through -005F, on the
following pages show the new, 9-pin “D” connector.
For information about the old, 1/4-inch phone jack
type connector, please refer to chapter 4.
Eagle 3000 Patient Monitor
415397-038
2-3
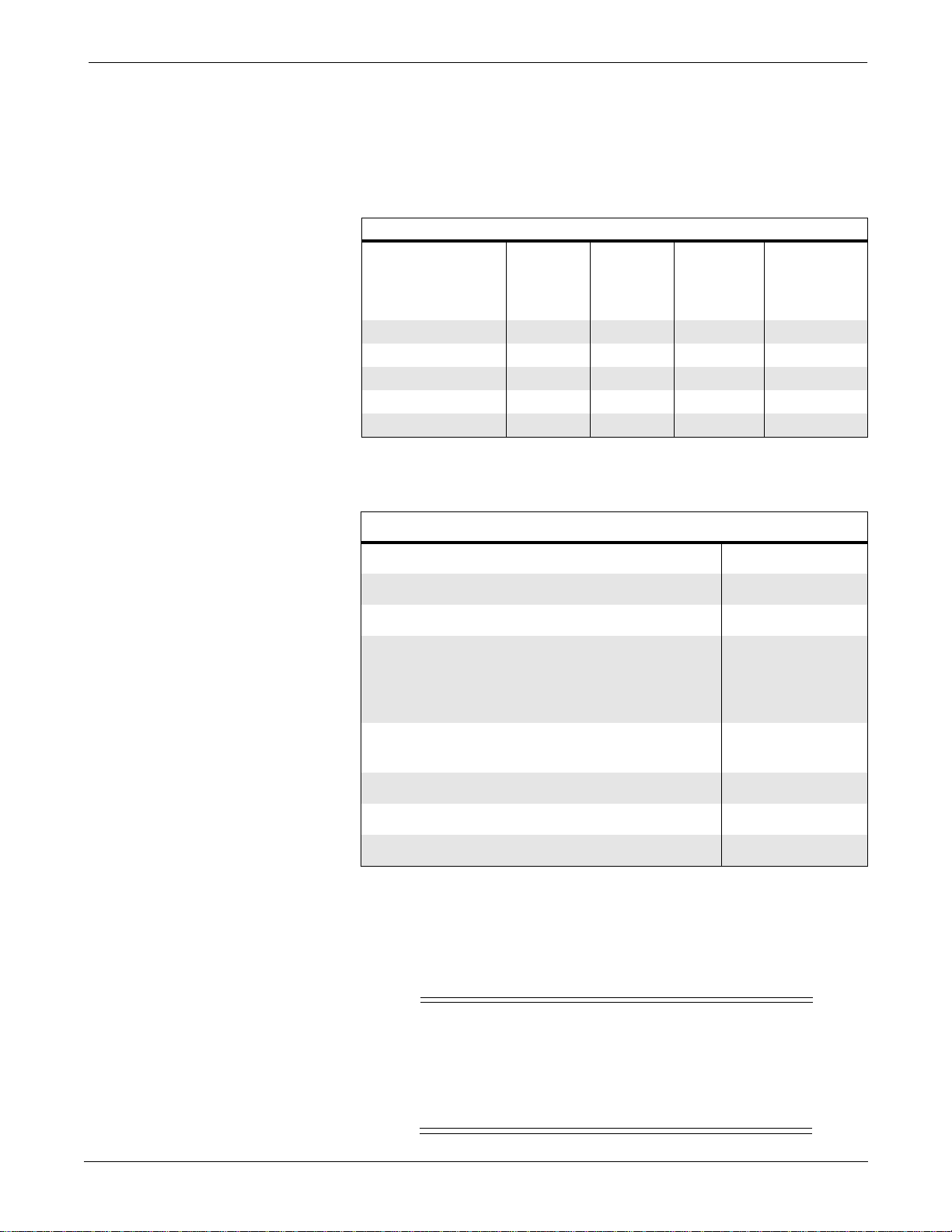
UPPER LEVEL OVERVIEW: Exploded View PN 414888-001J/002J/003F/004J/005F
Exploded View PN 414888-001J/002J/003F/004J/005F
Page 1 of 5
50
58
42
46
A1
2 PLACES
42
INSERT THIS
SCREW SECOND
NOTE:
A2
47
REFER TO ASSEMBLY
DETAIL A, SHEET 3
A4
21
A5
25
4 PLACES
36
4 PLACES
NOTE:
13
2 PLACES
38
INSERT THESE TWO SCREWS,
BUT DO NOT TIGHTEN UNTIL
THE BEZEL AND DAS ASSEMBLIES
HAVE BEEN SECURED.
NOTE:
8
9
16
A3
2 PLACES
36
42
2 PLACES
36
APPLICABLE ONLY
IF ITEM 14 IS NOT
PRESENT
36
4 PLACES
42
42
INSERT THIS
SCREW FIRST
32
51
4 PLACES
36
2 PLACES
39
52
INSTALL PARTS SHOWN,
SCRAP REMAINING PARTS
IN PACKAGE.
NOTE:
19
11
2 PLACES
2 PLACES
41
45
2 PLACES
23
HAND TIGHTEN
26
5
2-4
4 PLACES
36
2 PLACES
36
61
37
E
2 PLACES
W2
4
35
2 PLACES
36
CABLE MUST BE THREADED
THRU THE SLOT, IN THE
WRITER MOUNTING AREA
OF THE FRAME.
NOTE:
Eagle 3000 Patient Monitor
415397-038
W3
OF INLET PORT FITTING.
REMOVE WASHER AND DO NOT USE.
NOTE:
TORQUE NUT TO 10 in-LBS
APPLY ITEM 62 TO THREADS
OF ITEM 34.
NUT AND WASHER ARE PART
10
56
15
A8
40
4 PLACES
SEE NOTE 2
MOUNT LABEL ON VERTICAL
SURFACE, JUST BELOW THE
FLANGE.
NOTE:
34
Revision A
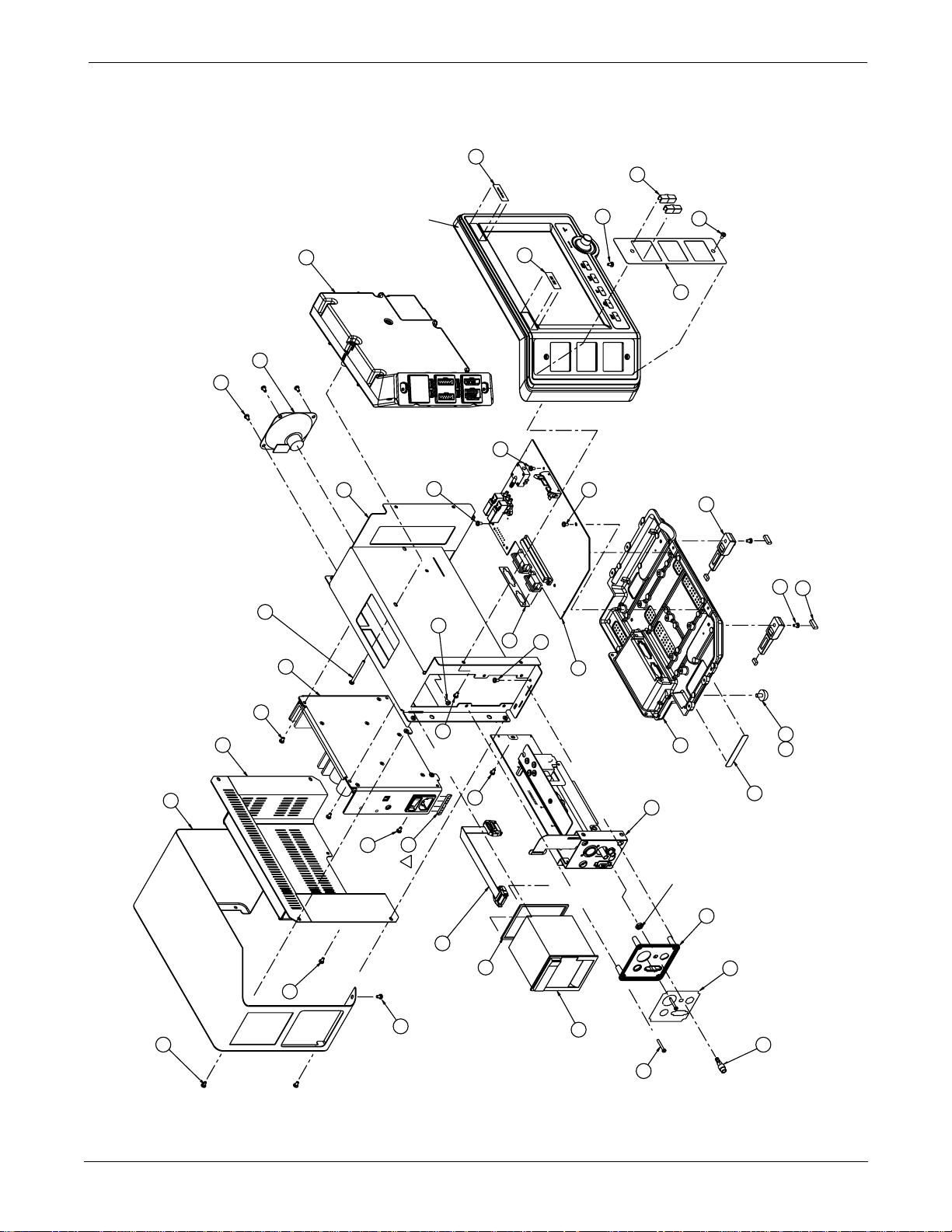
Page 2 of 5
A4
21
UPPER LEVEL OVERVIEW: Exploded View PN 414888-001J/002J/003F/004J/005F
50
58
REFER TO ASSEMBLY
DETAIL A
51
2 PLACES
42
2 PLACES
39
52
4 PLACES
42
42
SCREW FIRST
INSERT THIS
NOTE:
42
SCREW SECOND
INSERT THIS
NOTE:
32
46
A1
SCRAP REMAINING PARTS
IN PACKAGE.
INSTALL PARTS SHOWN,
NOTE:
19
11
REFER TO
ASSEMBLY
DETAIL B
5
2 PLACES
2 PLACES
41
45
2 PLACES
HAND TIGHTEN
26 23
Revision A
Eagle 3000 Patient Monitor
415397-038
SEE NOTE 2
SURFACE, JUST BELOW THE
MOUNT LABEL ON VERTICAL
FLANGE.
NOTE:
2-5
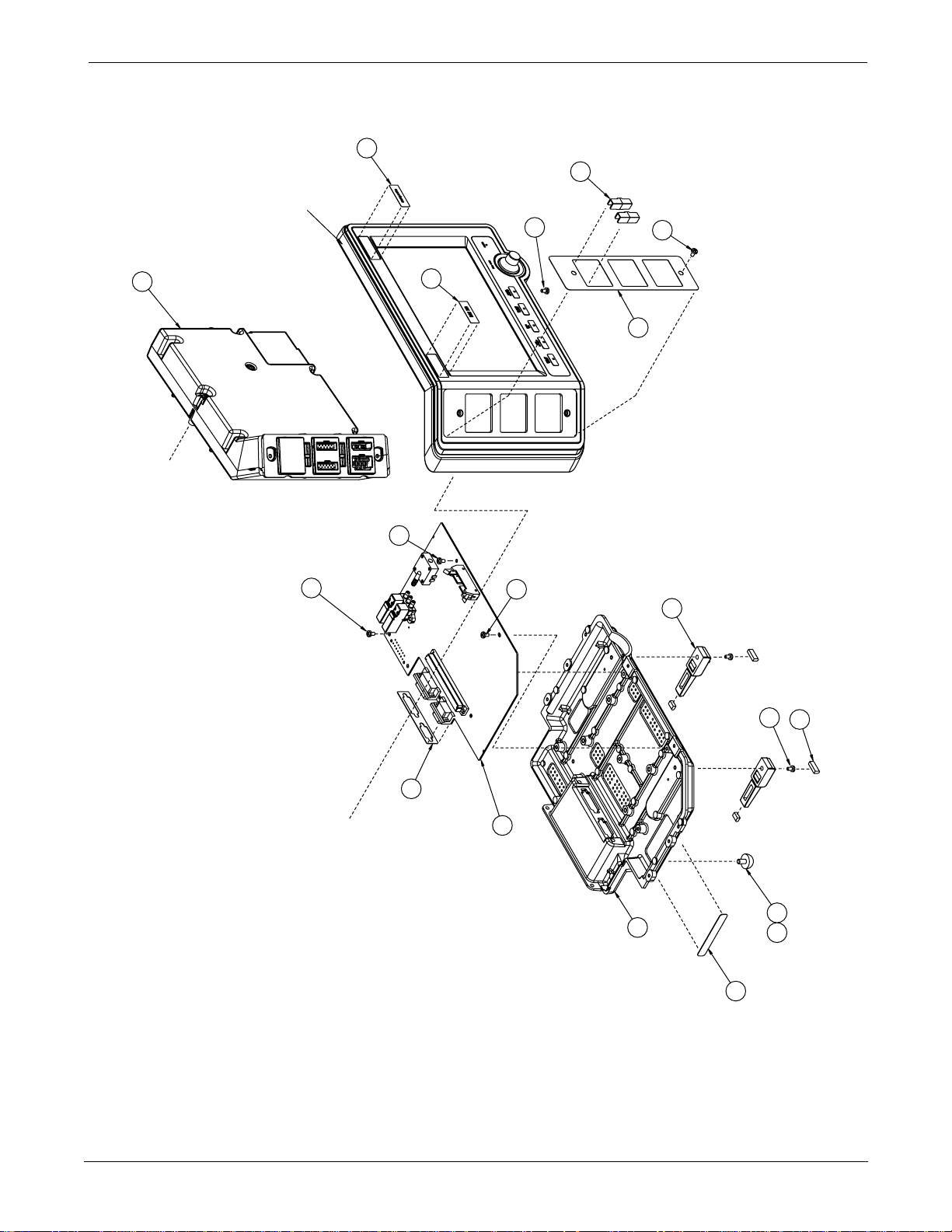
age 3 of 5
UPPER LEVEL OVERVIEW: Exploded View PN 414888-001J/002J/003F/004J/005F
NOTE:
REMOVE ADHESIVE
BACKING 2 PLACES
PLACE UID LABEL
AS SHOWN
15
A9
57
17
NOTE:
HARDWARE ITEMS INCLUDED
WITH SWITCH ASSEMBLY.
TORQUE 10 in/lbs.
REF.
12
17
A10
43
TORQUE 1.5 in/lbs
3 PLACES
A7
18
W1
36
4 PLACES
REMOVE BACKING AND
WRAP PUMP PRIOR TO
MOUNTING CLAMP.
24
6.00 ±10
ASSEMBLY DETAIL A
11
3
1.75 ±.10
3
FLOW
1.00 ±.10
REF.
6
3
28
22
36
2.25 ±.10
3
E
.700 ±.10
7
2-6
ASSEMBLY DETAIL B
Eagle 3000 Patient Monitor
415397-038
Revision A
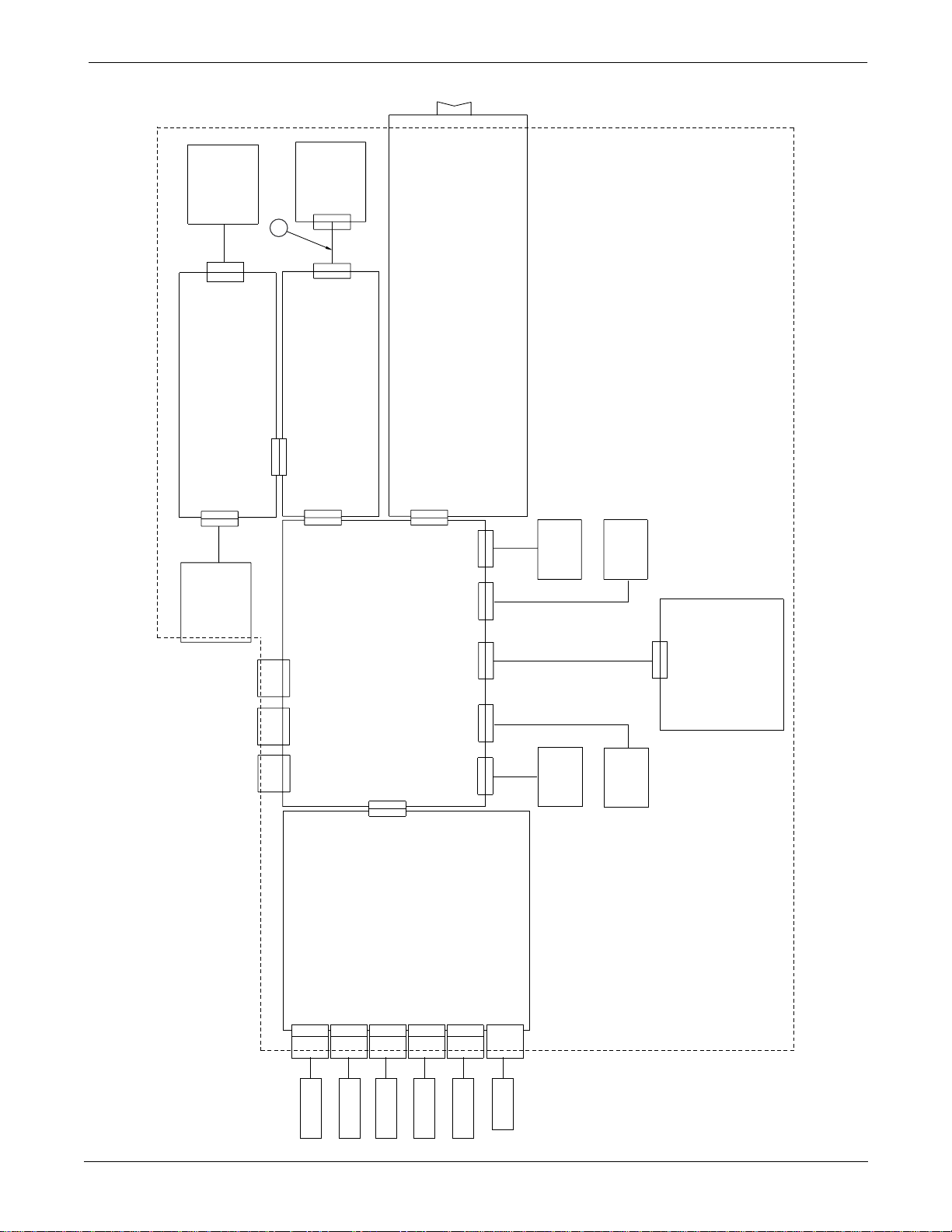
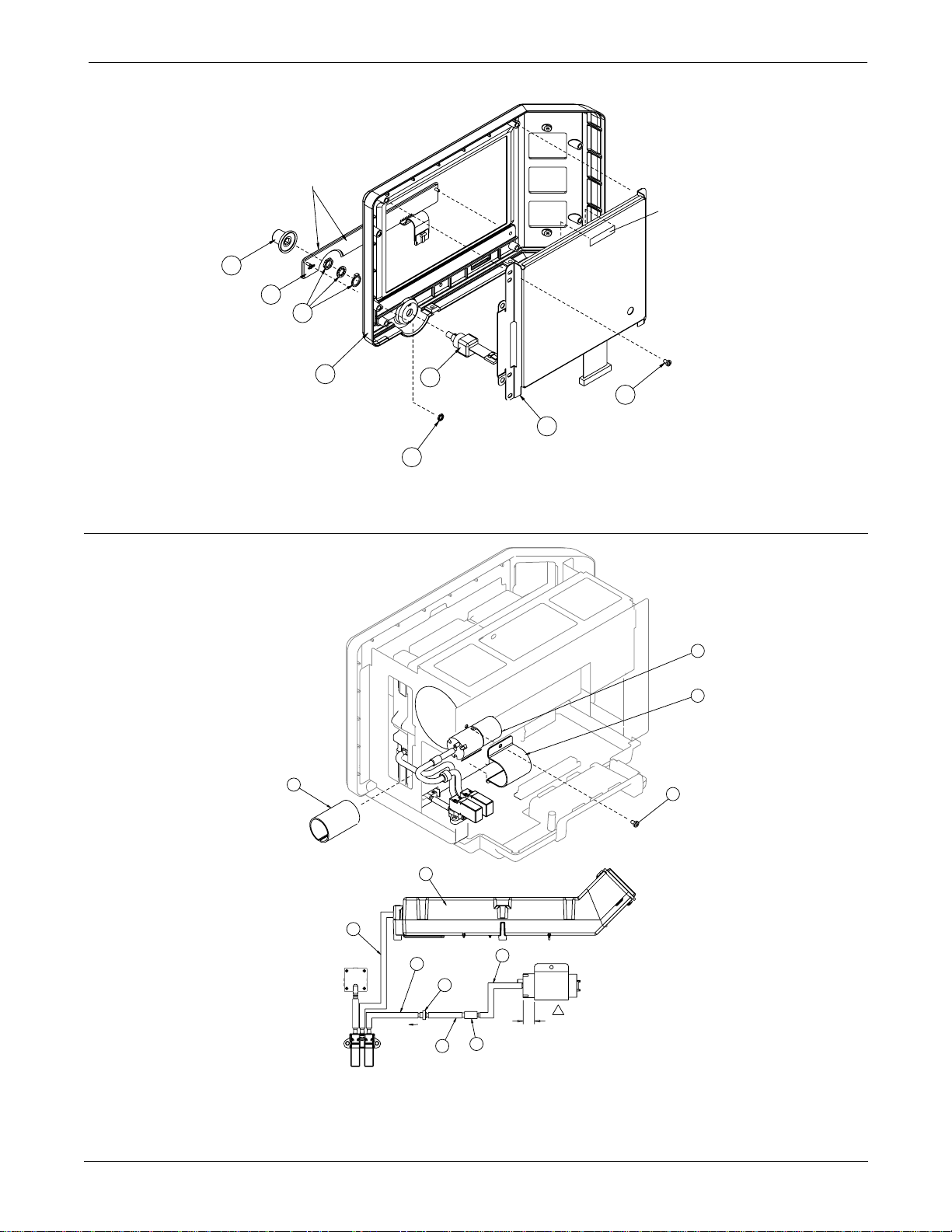
Page 4 of 5
UPPER LEVEL OVERVIEW: Exploded View PN 414888-001J/002J/003F/004J/005F
A12
PUMP
1
P12A
4
J11A
A11
ETCO2 SUBSYSTEM
A
3
J
11
W
1P3
FLEX
W3
REF.
4
A2J14
A11J14
AA6J
2
WRITER
A8
1
J8A
2
P2W
W2
1
P2W
3
J2A
A2
OPTION PCB
6J1
J3A
A
A3
POWER SUPPLY
3
2J1
A5P1
A1J5 A1J4
A6P1
A5
SPEAKER
A6
NBP PUMP
A1J9
A1J10
A1J12
SWITCHA9PANEL
W1
TRIM
KNOB
A10
W1P1
A7J3
A7
DISPLAY
A1
CPU
AA
8J1
8J4
A4
DAS
17J4
A
4
16
J
A15W4A
A1J3
W1P2
A1J25
A10P1
A1J7
A9P1
19AJ4A
18J4
Revision A
ELECTRODES
ECG/RESP
SENSORS
TEMP
PULSE OX
PROBE
TRANSDUCER
PRESSURE
Eagle 3000 Patient Monitor
415397-038
PRESSURE
TRANSDUCER
NBP CUFF
2-7
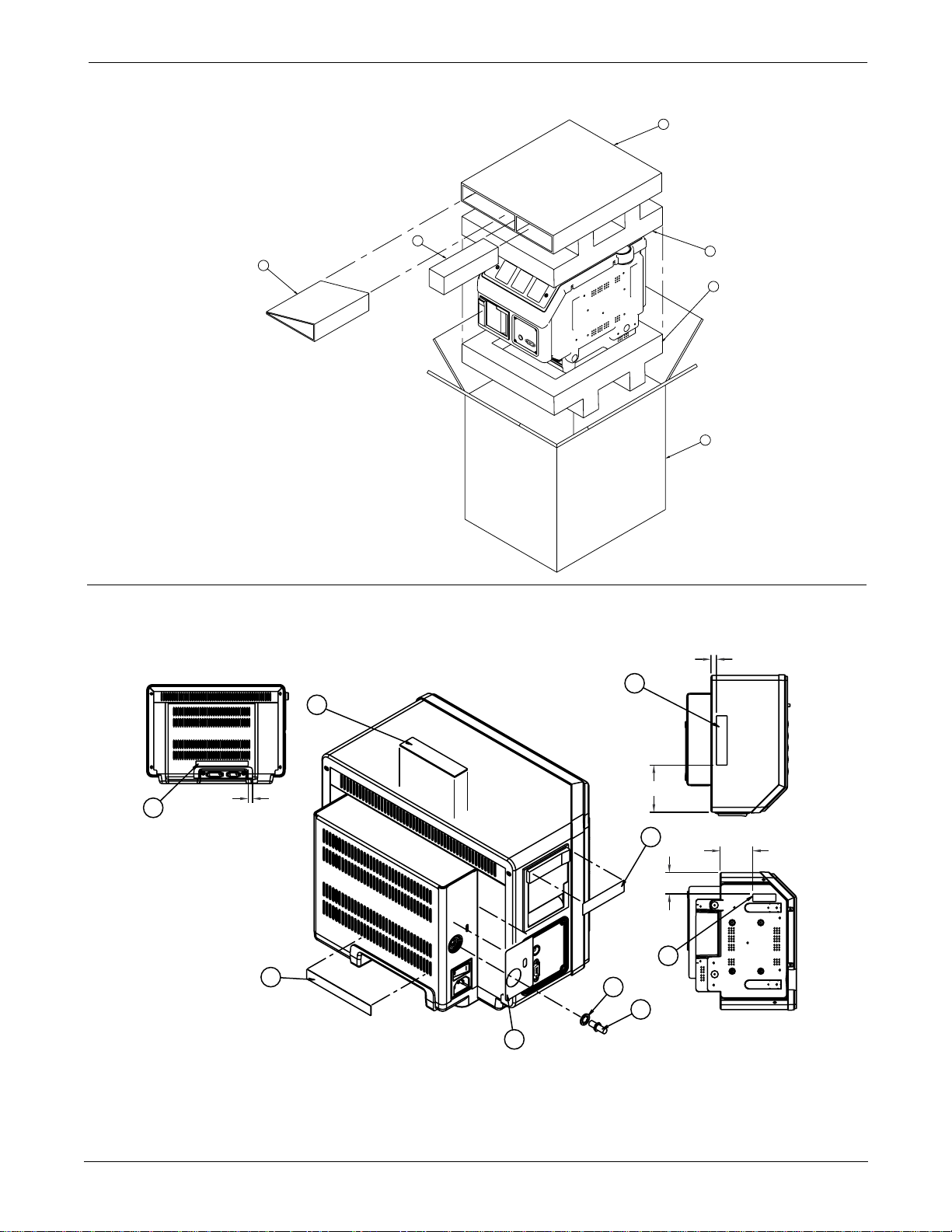
UPPER LEVEL OVERVIEW: Exploded View PN 414888-001J/002J/003F/004J/005F
Page 5 of 5
49
31
NOTES:
1) LOOSE ITEMS MAY BE SHIPPED WITH THE UNIT IF
SPACE PERMITS OR PACKAGED SEPERATELY.
2) MARK LABEL WITH THE FOLLOWING; "MODEL NAME",
SERIAL NUMBER AND APPROPRIATE BARCODE.
33
30
58
29
BACK VIEW
55
54
REF.
NOTE:
LABELS TO BE PLACED APPROX. AS SHOWN.
DIMENSIONS ARE FOR REFERENCE ONLY.
.38 REF.
54
53
NOTE:
CENTER LABEL AROUND
DIMPLE
55
REF.
5.0 REF.
48
2.5 REF
60
2
1
TORQUE 17 in-LBS
.25 REF.
TOP VIEW
2.7 REF
BOTTOM VIEW
2-8
Eagle 3000 Patient Monitor
415397-038
Revision A
UPPER LEVEL
VERVIEW
UPPER LEVEL OVERVIEW: Parts List PN 414888-001J/002J/003F/004J/005F
Parts List PN 414888-001J/002J/003F/004J/005F
Item
1 Plug, MC, Equipotential 400040-001 1
2 Washer, Lock, Serrated, Male/Female, No 6 400041-001 1
3 Tubing, Silastic 401582-001 AR
4 W2 Harness Assembly, Writer Data 402642-007 1
5 Label, Model/Serial Number 404525-006 1
6 Check Valve, 0.125 inch 418437-001 1
7 Filter, 43 Micron 404679-001 1
8 Rear Cover 412847-001 1
9 Top Cover (For 414888-001 Only)
10 Side Connector Cover (For 414888-002 and -004
11 Base, Diecast 412857-002 1
12 Bezel, Monitor 412858-001 1
13 Chassis Frame 412868-003 1
14 A8 Thermal Writer Assembly, 2-inch, STAR (For
15 Knob, Trim Knob, Soft 414622-001 1
16 A3 Power Supply Assembly, 5/12 Volt 414641-001 1
17 A10 Switch Assembly, Rotary 414642-001 1
18 A7 Display Processor PCB Assembly 414704-002 1
19 Foot, Two-Position 414793-001 1
20 Not used in this assembly
21 A4 Data Acquisition PCB Assembly 414887-001 1
22 Clamp, NBP Pump 414992-002 1
23 Foot, Rubber, 0.62 diameter 415054-002 2
24 Foam Pump Mount 415081-001 1
25 A5 Speaker Assembly 415091-001 1
26 Cement, Loctite 4851-003 AR
27 Not used in this assembly
28 A6 NBP Pump Assembly 415321-001 1
29 Carton, Shipping, Eagle 3000 415337-001 1
30 Insert, Foam, Glued, Top Section 415338-001 1
31 Eagle 3000 Field Service Manual 415397-003 AR
32 Gasket, EMI Shield 415478-002 1
33 Insert Spacer, Packaging 415582-001 1
34 Connector, Luer, Female, 1/8 Barb 416229-001 1
35 Gasket, Writer 415650-001 1
36 Screw, PNH, Phillips, 6-32 x 1/4 45000-604 29
Reference
Designation
Parts List Part Number Qty
Top Cover (For 414888-002 and -003 Only)
Top Cover (For 414888-004 and -005 Only)
Only)
Side Connector Cover, ETCO2 (For 414888-003 and
-005 Only)
414888-004 and -005 Only)
412848-003
412848-002
412848-001
412849-002
412849-003
413568-001 1
1
1
1
1
1
Revision A
Eagle 3000 Patient Monitor
415397-038
2-9
UPPER LEVEL OVERVIEW: Parts List PN 414888-001J/002J/003F/004J/005F
Item
Reference
Designation
Parts List Part Number Qty
37 Screw, PNH, Phillips, 6-32 x 3/8 45000-606 2
38 Screw, PNH, Phillips, 6-32 x 1-1/2 45000-817 2
39 Screw, PNH, Phillips, SST, 4-40 x 3/8 4502-412 2
40 Screw, PNH, Phillips, SST, 4-40 x 15/16 4502-430 4
41 Screw, BDGH, Phillips, 6-32 x 3/16 45074-606 2
42 Screw, BDGH, Phillips, 6-32 x 1/4 45074-608 8
43 Nut, Hex, 4-40 4521-104 3
45 Tape, Double-Sided (White) 4813-100 AR
46 A1 Main Processor PCB Assembly 800772-002 1
47 A2
Assembly, Eagle 3000 Options (For 414888-002 and
416021-002
1
-004 Only)
A2
Assembly, Eagle 3000 Options (For 414888-003 and
416021-001
1
-005 Only)
59 Insert, Foam, Glued, Bottom Section 415338-002 1
61 Clip, EMI 416053-002 1
62 Adhesive, Permabond 910FS 4851-074 AR
63 Adhesive, Loctite 4851-070 AR
The following items are determined by customer destination. They are included here for reference.
48 Label, Writer (Press to Open) 415570-001 1
49 Writer Paper, 3-Roll Sample 9402
1
SAMPLE-046
50 Label, Marquette
Label, Corometrics, A Marquette Company
Label, Marquette Hellige
51 Label, Eagle 3000 Patient Monitor
Label, Eagle 3000N Neonatal Monitor
52 Label, Parameter Connectors (English)
Label, Parameter Connectors (German)
Label, Parameter Connectors (French)
Label, Parameter Connectors (Swedish)
Label, Parameter Connectors (Spanish)
Label, Parameter Connectors (Italian)
Label, Parameter Connectors (English International)
413384-001
413383-002
413383-003
413383-001
413383-002
413382-001
413382-002
413382-003
413382-004
413382-005
413382-006
413382-007
-
-
-
-
-
-
-
-
-
-
-
53 Label, Voltage, Equipotential, Ratings (UL/CUL/CE) 415969-001 1
54 Label, Ethernet and ASYNC COMM Connectors 415151-001 1
55 Label, Prescription Device (USA Only) 415043-002 1
2-10
Eagle 3000 Patient Monitor
415397-038
Revision A
UPPER LEVEL OVERVIEW: Parts List PN 414888-001J/002J/003F/004J/005F
Item
56 Label, Defib Sync, CO2, Inlet, Rmt Alm, and
Reference
Designation
Parts List Part Number Qty
415868-001
Exhaust Connectors (English)
Label, Defib Sync, CO2, Inlet, Rmt Alm, and
415858-002
Exhaust Connectors (German)
Label, Defib Sync, CO2, Inlet, Rmt Alm, and
415868-003
Exhaust Connectors (French)
Label, Defib Sync, CO2, Inlet, Rmt Alm, and
415868-004
Exhaust Connectors (Swedish)
Label, Defib Sync, CO2, Inlet, Rmt Alm, and
415868-005
Exhaust Connectors (Spanish)
Label, Defib Sync, CO2, Inlet, Rmt Alm, and
415868-006
Exhaust Connectors (Italian)
Label, Defib Sync, CO2, Inlet, Rmt Alm, and
415868-007
Exhaust Connectors (Dutch)
57 Label, Membrane Switch Panel (English)
Label, Membrane Switch Panel (German)
Label, Membrane Switch Panel (French)
Label, Membrane Switch Panel (Swedish)
Label, Membrane Switch Panel (Spanish)
Label, Membrane Switch Panel (Italian)
Label, Membrane Switch Panel (Dutch)
58 Cover, Patient Connectors (for units w/o invasive
415001-001
415001-002
415001-003
415001-004
415001-005
415001-006
415001-007
408557-002 2
blood pressure)
60 Label, UL 2601 Classification 416338-001 1
-
-
-
-
-
-
-
-
-
-
-
-
-
-
Revision A
Eagle 3000 Patient Monitor
415397-038
2-11
WARNING
UPPER LEVEL OVERVIEW: Switches and Controls PN 800772-002A
Switches and Controls PN 800772-002A
Listed below are all switches, controls, and LEDs found on the
circuit board.
Due to possible high voltage, always use an
insulated screwdriver while making adjustments.
Table 2-1. Controls
Designator Name Function
DS1 Green light-emiting diode Ethernet status LED
DS2 Yellow light-emiting diode Ethernet status LED
DS3 Red light-emiting diode Ethernet status LED
DS4 Green light-emiting diode General purpose diagnostic LED
DS5 Yellow light-emiting diode General purpose diagnostic LED
DS6 Red light-emiting diode General purpose diagnostic LED
DS7 Red light-emiting diode NBP diagnostic LED
DS8 Green light-emiting diode NBP diagnostic LED
Table 2-2. Switches
S1 — 4-Position DIP Switch
Designator Name Function
SW1 Software updates OPEN — Software updates enabled; NBP
calibration enabled
CLOSED — Software updates disabled; NBP
calibration disabled
SW2 Watchdog timer OPEN — Enabled
CLOSED — Disabled
SW3 Reserved for future use
SW4 Reserved for future use Current Usage:
OPEN — Normal operation
CLOSED — Forces boot code menus to be
displayed after reset
S2 — Dual Pressure Sensor Switch
Designator Name Function
S2 Dual pressure sensor switch Detects NBP over pressure condition. One
switch trips when cuff pressure is 150 mmHg
(neonatal mode); the other switch trips when
cuff pressure is 300 mmHg (adult mode).
2-12
Eagle 3000 Patient Monitor
415397-038
Revision A
 Loading...
Loading...