Page 1

xMAP® Antibody
Product Catalog Number
®
xMAP
Antibody Coupling Kit
Not For Use In Diagnostic Procedures
Coupling Kit
User Manual
40-50016
For Research Use Only
89-00002-00-319
Rev C
December 2011
Page 2

© Luminex Corporation, 2001-2011. All rights reserved. No part of this publication may
be reproduced, transmitted, transcribed, or translated into any language or computer
language, in any form or by any means without prior express, written consent of:
LUMINEX CORPORATION
12212 Technology Boulevard
Austin, Texas 78727-6115
U.S.A.
Voice: (512) 219-8020
Fax: (512) 219-5195
®
Antibody Coupling Kit User Manual
xMAP
PN 89-00002-00-319 Rev. C
December 2011
Luminex Corporation (Luminex) reserves the right to modify its products and services at
any time. This guide is subject to change without notice. Although prepared to ensure
accuracy, Luminex assumes no liability for errors or omissions, or for any damages
resulting from the application or use of this information. This guide may be updated
periodically.
To ensure that you have a current version, access http://www.luminexcorp.com/support
The most recent version of this guide is available for download at that URL.
®
The following are registered trademarks of Luminex: Luminex
®
Luminex
100 IS™, Luminex® 200™, Luminex® SD™, Luminex® XYP™, FLEXMAP
®
3D™, xMAP
xPONENT
, xTAG® Microspheres, MagPlex®, MicroPlex®, MAGPIX™, and
®
.
, Luminex® 100™,
Page 3

Safety.......................................................................................... 1
Introduction ................................................................................. 3
Method........................................................................................ 4
Protein Considerations................................................................... 5
Kit Contents ................................................................................ 5
Storage and Stability................................................................... 6
Necessary Materials and Equipment.......................................... 6
Instructions ................................................................................. 8
General Workflow........................................................................... 8
Coupling Protocol........................................................................... 8
Coupling Assessment ............................................................... 14
Microsphere Enumeration............................................................ 14
Coupling Confirmation.................................................................. 14
Sample Coupling Confirmation Protocol...................................... 14
Troubleshooting........................................................................ 17
Page 4

Page 5

Safety
The following informational notes and cautions are used in this manual.
NOTE: This message is used to provide general helpful information.
IMPORTANT: This message advises users that failure to take action or avoid a
certain action can result in data loss or the reporting of incorrect data.
You may encounter the symbols in the table below, within this manual.These
represent warnings, conditions, identifications, and important information.
Table 1: Symbols
Symbol
Meaning
General Warning,Caution, Risk of Danger
Manufacturer
Decision Point
Page 1 of 20
Page 6
Material Safety Data Sheets (MSDS's) are available upon request. Visit
http://www.luminexcorp.com/support for more information.
CAUTION: xMAP® Antibody Coupling Kit Sulfo-NHS
Solution MAY BE HARMFUL IF ABSORBED THROUGH
SKIN OR IF SWALLOWED. MAY CAUSE EYE AND SKIN
IRRITATION.
CAUTION: xMAP® Antibody Coupling Kit Activation Buffer
MAY BE HARMFUL IF ABSORBED THROUGH SKIN OR
IF SWALLOWED.
CAUTION: EDC causes severe eye irritation. Causes
respiratory tract and skin irritation.
Page 2 of 20
Page 7
Introduction
The xMAP Antibody Coupling (AbC) Kit contains all of the reagents necessary to
covalently couple antibodies to Luminex MagPlex microspheres (beads) in approximately
three hours. This kit can also be used to couple other proteins to Luminex microspheres
but due to the large diversity in protein composition, coupling performance with other
proteins is not guaranteed. For more information on protein coupling please visit:
http://www.luminexcorp.com/support
Coupling is achieved through carbodiimide reactions involving the primary amino groups
on the antibody, or protein of choice, and the carboxyl functional groups on the
microsphere surface.
*
This kit is configured for a one-time use
6
or as many as 50x106 microspheres. The kit can be used to perform as many as
2.5x10
ten individual coupling reactions at scales of 2.5x10
**
; or as many as four reactions at scales of up to 12.5x106 microspheres per
reaction
reaction.
and contains enough reagent to couple as few as
6
to 5x106 microspheres per
Scale
(beads/rxn)
5x106 or less
Up to 12.5x10
IMPORTANT:
**
6
Number of
Reactions
10
4
* Luminex strongly recommends that the EDC reagent be discarded after one use.
** Each coupling reaction requires a minimum of 2.5x106 microspheres.
The coupled microspheres can then be used with a Luminex xMAP instrument to develop
monoplex or multiplex assays.
This kit is ideal for use in two modes:
• Coupling optimization and assay optimization: For users developing a new assay,
the kit will allow them to perform up to 10 small scale coupling reactions to test
multiple concentrations of antibody with multiple bead regions.
• Small-to-medium scale assay manufacturing: For users who have already
developed and optimized an assay, the kit will allow them to couple up to 50 million
beads at once to meet their routine usage needs.
Page 3 of 20
Page 8
With Luminex MagPlex microspheres, if the kit protocol is performed carefully, the percent
recovery of the coupling reaction is typically 90% or greater; enough for more than forty-
five 96-well plates (@ 2,500 beads/well) for every 12.5x10
6
microspheres coupled.
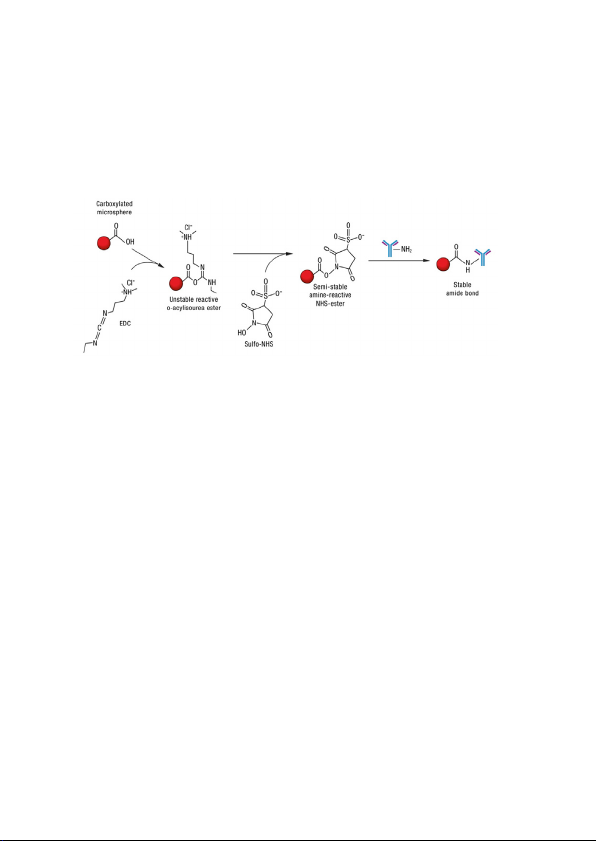
Method
NOTE: The diagram above illustrates the chemical reaction taking place during
coupling and is not intended to be a literal representation of the order in which
reagents are added to the reaction.
The coupling procedure involves a two-step carbodii
on the surface of the polystyrene microspheres must first be activated with a carbodiimide
derivative prior to coupling the antibody. EDC (1-Ethyl-3-[3dimethylaminopropyl]carbodiimide hydrochloride) reacts with the carboxyl groups on the
surface of the microspheres to form an active O-acylisourea intermediate. This
intermediate forms a more stable ester using Sulfo-NHS (N-hydroxysulfosuccinimide).
The ester reacts with the primary amines (NH2 groups) of antibodies to form a covalent
bond (amide linkage). In the protocol described in this manual, the Sulfo-NHS is added to
the reaction prior to the addition of EDC to maximize efficiency of the reaction. The
presence of Sulfo-NHS in the mixture at the time of the EDC addition is critical due to the
limited stability of the EDC microsphere conjugate. The reaction with the carboxylated
microsphere does not begin until EDC is added to the mixture.
mide reaction. The carboxyl groups
Page 4 of 20
Page 9

Protein Considerations
This kit includes a specially formulated Activation Buffer (pH 6.0) which is suitable for
most antibodies.
The protein to be coupled must be free of sodium azide, bovine serum albumin (BSA),
glycine, tris(hydroxymethyl)aminomethane (Tris), glycerol, or amine-containing additives
and should be suspended in phosphate buffered saline (PBS), pH 7.4.
A number of buffers can be used successfully in this coupling reaction. Generally, the pH
at which a coupling reaction occurs should be compatible with the solubility of the protein
of interest. This should be considered when coupling different proteins.
Additionally, coupling efficiency will vary depending on a variety of factors, including type
of antibody, quantity of antibody, quantity of microspheres, etc. As such, it is
recommended that several quantities of antibody input be tested to optimize the coupling
reaction and functionality in the final assay. If coupling for the first time, 2-5μg of antibody
6
microspheres is generally a good starting input.
per 1x10
The amount of antibody to use in the coupling reaction will depend on the quantity of
coupled antibody necessary to promote optimal binding of the desired target molecule.
Kit Contents
The following items are included in this kit:
Component Part Number Volume/Mass Quantity
EDC Reagent 11-40144
(ThermoSci #77149)
Sulfo-NHS 11-25169 250 μL1
Activation Buffer
(green cap)
11-25171 45 mL 1
Wash Buffer 11-25167 30 mL 1
1.5 mL tubes 11-00277 n/a 10
Disposable pipettes 11-00321 n/a 20
10 mg 1
NOTE: The EDC reagent is a Thermo Scientific product and is manufactured for
Luminex for use in this kit. For questions regarding the use of this product with this
kit, please contact Luminex technical support.
Page 5 of 20
Page 10

Storage and Stability
Photosensitive microspheres should be protected from light at all times. All kit
components are to be stored at 2-8ºC. The EDC reagent, in its original packaging, may be
stored at -20°C to ensure the longest shelf-life possible. All other components should
never be frozen.
IMPORTANT: Due to the instability of the EDC reagent in solution, it should
always be stored in its original sealed packaging until needed and discarded after
one use. Reconstituted EDC should never be stored and reused.
All components are guaranteed up to the expiration date found on the label; when stored
in their original packaging and as specified in this manual.
The stability of coupled microspheres is dependent upon several factors; including the
protein stability and composition, aseptic processing conditions, presence of
preservatives, storage buffer, storage temperature conditions etc. However, stability
studies have shown that antibody coupled microspheres, stored in appropriate storage
buffer, are stable over a period of 18 months.
Necessary Materials and Equipment
There are various materials and pieces of equipment that are required for microsphere
coupling which are not included with this kit.
Required Equipment
• Luminex xMAP instrument: MAGPIX, LX100/200, or FLEXMAP 3D
• Luminex xPONENT software
• Luminex MagPlex Microspheres (Cat# MC1XXXX-01, -04, & MC10XXX-ID)
NOTE: Visit http://www.luminexcorp.com/Products/ReagentsMicrospheres/MAGPLEX-MICROSPHERES for a complete list of MagPlex® microspheres and guide-
lines for selecting the right regions for your instrument.
• Luminex Instrument Performance Calibration and Verification Kits
• Luminex Instrument Sheath Fluid (LX100/200 & FLEXMAP 3D) or Drive Fluid
(MAGPIX)
• Magnetic Separator
Other Necessary Equipment
• Tube Rotisserie or Rotator
• Water-bath sonicator
Page 6 of 20
Page 11

• Vortexer
• Pipettor
Optional Equipment
• xMAP Antibody Coupling Kit Quick Guide (89-30000-00-361)
(Available at http://www.luminexcorp.com/downloads)
• Luminex Tube Magnetic Separator (Cat# CN-0288-01)
• Microcentrifuge
• Luminex Plate Magnetic Separator (Cat# CN-0269-01)
Page 7 of 20
Page 12

Instructions
General Workflow
Coupling Protocol
The following is the detailed step-by-step process for coupling antibodies (or similar
proteins) to carboxylated magnetic microspheres. An abridged quick reference guide
(part number 89-30000-00-361) can be found online at http://www.luminexcorp.com/
downloads.
IMPORTANT: Pr
throughout this entire procedure.
1. Remove kit and all reagents from the refrigerator and allow them to equilibrate to
m temperature for 20-30 minutes.
roo
2. While the reagents equilibrate to room temperature, calculate and
volumes for Decision Point steps 4, 8, 11, 13, 19, and 20; for each reaction being
performed.
3. Resuspend the stock microspheres.
otect photosensitive microspheres from light whenever possible
note the required
• If using a 1mL stock microsphere vial, vortex the stock microsphere vial for 10
seconds and then sonicate for 10 seconds to disperse the microspheres.
Alternatively, the microsphere vials can be rotated on a rotator for 15 minutes.
• If using a 4mL stock microsphere vial, rotate the vial for 15 minutes at 15-30
rpm.
Page 8 of 20
Page 13

4. Dispense the desired amount of microspheres from
microcentrifuge tubes (“reaction tube”) provided with the kit.
Example Calculation:
If coupling 5 million microspheres, with m
6
microspheres:
12.5x10
the stock vial into one of the
icrosphere stock concentration of
IMPORTANT: This kit is designed for a maximum of 12.5x106 microspheres per
reaction tube.
5. Wash the microspheres.
A. Place the reaction tube with microspheres i
nto the magnetic separator for 1-2
minutes.
NOTE: If performin
used, in place of a magnetic separator, to pellet the microspheres during wash
steps. Beads can be pelleted by microcentrifugation at ≥ 8000 x g for 1-2 minutes.
B. With the tube still positioned in the mag
with transfer pipette.
NOTE:
included for the removal of supernatant during the various wash steps in this protocol. If using one pipette for the removal
removal of Wash Buffer, the kit should have enough to perform up to ten individual
reactions. (i.e. 2/rxn) Special care should be taken to avoid cross contamination,
when performing multiple reactions simultaneously.
C. Add 500 μL of Ac
D. Vortex the reaction tube for 10 seconds
perse the microspheres.
g multiple reactions simultaneously, a microcentrifuge may be
netic separator, remove the supernatant
For your convenience, twenty disposable transfer pipettes have been
of Activation Buffer and one for the
tivation Buffer into the reaction tube.
and then sonicate for 10 seconds to dis-
6. Repeat Step 5 once for a total of two washes.
Page 9 of 20
Page 14

7. Place the reaction tube with microspheres into the magnetic separator for 1-2
minutes and, with the tube still positioned in the magnetic separator, remove the
supernatant with a transfer pipette.
8. Add Activation Buffer into the reaction tube.
• If coupling more than 5x10
6
microspheres, add 400 μL of Activation Buffer into
the reaction tube.
• If coupling 5x10
6
microspheres or less, add 480 μL of Activation Buffer into the
reaction tube.
9. Vortex the reaction tube for 10 seconds and then sonicate for 10 seconds to
disperse the microspheres.
10. Vortex the provided Sulfo-NHS tube for a minimum of 10 seconds.
11. Add Sulfo-NHS solution to the reaction tube.
• If coupling more than 5x10
6
microspheres, add 50 μL of the Sulfo-NHS solution
into the reaction tube.
• If coupling 5x10
6
microspheres or less, add 10 μL of the Sulfo-NHS solution into
the reaction tube.
12. Add 250 μL of Activation Buffer into the 10 mg vial of EDC. Invert the EDC vial and
then vortex the vial for 10-12 seconds to dissolve the EDC.
IMPORTANT: EDC will begin to degrade once exposed to moisture in the atmosphere and the Activation Buffer. Once opened the EDC solution must be prepared and used quickly. If performing multiple reactions, be sure to prepare all of
them prior to this step so that the EDC solution can be quickly added to all of the
reaction tubes immediately after dissolution. The EDC solution must be made
fresh for each coupling event and the excess should be discarded.
13. Add EDC Solution to reaction tube.
• If coupling more than 5x10
6
microspheres, add 50 μL of the EDC solution into
the reaction tube.
• If coupling 5x10
6
microspheres or less, add 10 μL of the EDC solution into the
reaction tube.
14. Vortex the reaction tube for a minimum of 10 seconds.
15. Protect microspheres from light and rotate on rotator for 20 minutes.
(Rotation speed should be ~15-30 rpm)
Page 10 of 20
Page 15

16. Wash the microspheres.
A. Place the reaction tube with microspheres into the magnetic separator for 1-2
minutes.
B. With the tube still positioned in the mag
with a transfer pipette.
C. Add 500 μL
of Activation Buffer into the reaction tube.
D. Vortex the reaction tube for 10 seconds
disperse the microspheres.
17. Repeat Step 16 twice, for a total of three washes.
18. Place the reaction tube with microspheres into
minutes and, with the tube still positioned in the magnetic separator, remove the
supernatant with a transfer pipette.
19. Calculate volume of antibody
to be used in the reaction.
• Typically, a range of 2-5 μg of antibody per 1x10
netic separator, remove the supernatant
and then sonicate for 10 seconds to
the magnetic separator for 1-2
6
microspheres is a good
starting point, if performing the coupling reaction for the first time.
Example Calculation:
If coupling 5 million microspheres, with an a
and using the suggested starting point of 5 μg of antibody per 1x10
spheres:
ntibody at a concentration of 5 mg/mL,
6
micro-
Page 11 of 20
Page 16

20. Calculate the volume of Activation Buffer needed for the reaction.
• If coupling more than 5x10
6
microspheres, subtract the volume of antibody
calculated in Step 19 from 1000 μL.
• If coupling 5x10
6
microspheres or less, subtract the volume of antibody
calculated in Step 19 from 500 μL.
Example Calculation:
If 5 μL of the stock antibody is needed (as calculated in the example in Step 19) for
the coupling of 5 million microspheres:
Volume of Activation Buffer needed = (1000μL or 500μL) - (Volume of Ab needed)
Volume of Activation Buffer needed = 500μL - 5μL
Volume of Activation Buffer needed = 495μL
21. Add the appropriate volume of Activation Buffer, calculated in Step 20, to the
reaction tube.
22. Add the appropriate volume of antibody, calculated in Step 19, to the reaction tube.
23. Vortex the reaction tube for a minimum of 10 seconds.
24. Protect microspheres from light and rotate on rotator for 2 hours. (Rotation speed
should be ~15-30 rpm)
25. Wash the microspheres.
A. Place the reaction tube with microspheres into the magnetic separator for 1-2
minutes.
B. With the tube still positioned in the magnetic separator, remove the supernatant
with a transfer pipette.
C. Add 500 μL of Wash Buffer into the reaction tube.
D. Vortex the reaction tube for 10 seconds and then sonicate for 10 seconds to
disperse the microspheres.
26. Repeat Step 25 twice, for a total of three washes.
27. Place the reaction tube with microspheres into the magnetic separator for 1-2
minutes and, with the tube still positioned in the magnetic separator, remove the
supernatant with a transfer pipette.
28. Add 1 mL of Wash Buffer into the reaction tube.
NOTE: The Wash Buffer is used as a storage buffer after completing the coupling
reaction.
29. Vortex the reaction tube for 10 seconds and then sonicate for 10 seconds to
disperse the microspheres.
30. Protect from light and store at 2-8 ºC until needed.
Page 12 of 20
Page 17

NOTE: For optimal performance allow the coupled microspheres to block overnight before first use.
Page 13 of 20
Page 18

Coupling Assessment
Once the coupling reaction has been completed, the coupled microspheres can be
enumerated and the efficiency of the coupling reaction assessed.
Microsphere Enumeration
Although, the protocol described in this manual will typically yield over a 90% recovery, it
is recommended that the user count the number of microspheres actually recovered after
each coupling reaction. This can be done with the use of a cell counter or hemacytometer.
Please refer to the cell counter or hemacytometer’s users manual for appropriate
instructions for doing so.
Coupling Confirmation
It is strongly recommended to assess coupling efficiency before proceeding to assay
development. The coupled microspheres can be reacted with a phycoerythrin (PE)labeled target or antibody that binds to the coupled protein. Alternatively, the target or
antibody may be biotinylated, then labeled with PE. This complex can then be analyzed
on a Luminex xMAP instrument. The intensity of the fluorescent signal of this reaction is
directly proportional to the amount of protein on the surface of the microspheres. This
process provides a rapid assessment of the relative amount of protein coupled to the
microspheres; however, this does not necessarily verify the functionality of the protein.
The ultimate test is the functional assay of the coupled protein.
Sample Coupling Confirmation Protocol
1. Select the appropriate antibody-coupled microsphere set or sets.
2. Resuspend the microspheres by vortex and sonication for approximately 20
seconds.
3. Prepare a working microsphere solution by diluting the coupled microsphere stocks
to a final concentration of 50 beads/μL in PBS-1% BSA.
NOTE: Up to 4 different microsphere sets may be prepared in the same mixture (in
multiplex), provided each set is a different microsphere region and the various
antibodies coupled to those regions can be detected with the same detection anti
body.
NOTE: At least 1mL of the microsphere solution is required for each reaction.
NOTE: Either PBS-1% BSA or PBS-BN (PBS, 1% BSA, 0.05% Azide, pH 7.4) may
be used as Assay Buffer.
-
Page 14 of 20
Page 19

4. Prepare a solution of phycoerythrin-labeled anti
μg/mL in PBS-1% BSA. Prepare a 1:2 dilution series of that detection antibody
solution to a concentration of 0.0625 μg/mL as shown in the following table.
-species IgG detection antibody at 4
Dilution Tube
Volume of
PBS-1% BSA
Volume of Detection
Antibody
Concentration
1:1 - - 4 μg/mL
1:2 500 μL 500 μL from Tube 1:1 2 μg/mL
1:4 500 μL 500 μL from Tube 1:2 1 μg/mL
1:8 500 μL 500 μL from Tube 1:4 0.5 μg/mL
1:16 500 μL 500 μL from Tube 1:8 0.25 μg/mL
1:32 500 μL 500 μL from Tube 1:16 0.125 μg/mL
1:64 500 μL 500 μL from Tube 1:32 0.0625 μg/mL
or optimal results use Costar round-bottom 96-well plates and Luminex
NOTE: F
Magnetic Plate Separator with MagPlex microspheres.
5. Aliquot 50 μL of
of wells of the plate (duplicate sets of 8 wells, 16 wells total).
6. Add 50 μL of
the microsphere solution.
7. Add 50 μL of each of the di
the appropriate wells of the plate (as shown in the plate layout below).
1 2 3 4 5 6 7 8 9 10 11 12
Blank Blank
A
1:64 1:64
B
1:32 1:32
C
1:16 1:16
D
1:8 1:8
E
1:4 1:4
F
1:2 1:2
G
1:1 1:1
H
Example plate layout using columns 1 & 2
the microsphere solution prepared in Step 3 into two entire columns
PBS-1% BSA, as a blank sample, into the wells in Row A containing
luted detection antibody solutions prepared in Step 4 into
Page 15 of 20
Page 20

8. Mix the reactions gently by pipetting up an
9. Cover the plate and incubate for 30 minutes at room
10. Clip the plate in place on the Luminex
forcefully invert over a biohazard receptacle to evacuate the liquid from the wells.
NOTE: For information on the MagPlex Manual Wash Method, please visit:
http://www.luminexcorp.com/Products/Re
MICROSPHERES.
11. Wash each well with 100 μL o
several times with a pipettor, and remove the liquid by using the procedure
described in the previous step.
12. Repeat the previous wash step once.
13. Resuspend the microspheres in 100 μL of
down several times with a pipettor.
14. Analyze 50-75 μL o
example of typical results is shown below.
f PBS-1% BSA by gently pipetting up and down
n the Luminex analyzer according to the system manual. An
d down several times with a pipettor.
Magnetic Plate Separator and rapidly and
temperature on a plate shaker.
agentsMicrospheres/MAGPLEX-
PBS-1% BSA by gently pipetting up and
Page 16 of 20
Page 21

Troubleshooting
This troubleshooting guide may be helpful in solving problems that may arise. For more
information, call Technical Support toll free at 1-877-785-BEAD (2323). Outside of U.S.
and Canada, call +1 512-381-4397. Send e-mail inquiries to: support@luminexcorp.com
Problem Possible Cause(s) Recommendations
Low Bead
Count
Uncoupled
microspheres tend to
stick to the inner
surfaces of some
tubes
Microspheres were
lost during washes
Make sure to use tubes supplied in this
kit only.
Use the recommended magnetic
separator to perform all washes. See
Recommended Material list.
Use the transfer pipette to remove all
supernatant during washes. The
microsphere pellets are loose and
using a pipettor could disturb the bead
pellet.
.
Incorrect probe height
adjustment
Incorrect protocol setup on the Luminex
instrument
Adjust probe height according to the
®
Luminex
200™, FLEXMAP 3D® or
®
MAGPIX
user manual.
Make sure correct microsphere
regions are selected based on your
particular custom designed assay.
Page 17 of 20
Page 22

Problem Possible Cause(s) Recommendations
Low/No
signals
The reagents may not
have been stored
correctly
EDC may not have
been stored correctly
or rehydrated long
before use
The stock protein
concentration is
possibly low
The stock protein
suspension possibly
contains foreign
proteins, azide,
glycine, Tris or some
primary amines
The reaction volumes
and/or mixing method
may be incorrect
The anti-species
detection antibody
may not be correct
Store all reagents as recommended.
EDC must remain in the pouch it is
shipped in until ready to use.
EDC must be used immediately in the
coupling procedure after rehydration.
Make sure the protein concentration is
correct and the recommended amount
is coupled to the microspheres.
Dialyze the protein of interest to
remove the competing substances.
The final reaction volume for the 2 hr
incubation is critical for successful
coupling. Refer to the protocol. End-toend mixing during the 2 hr incubation
is very important. Please use an
optical rotator.
Make sure the anti-species detection
antibody is correct, for example if you
are using a mouse monoclonal to
couple to the microspheres, use an
anti-mouse detection antibody.
Make sure the anti-species detection
antibody has the appropriate label on
it, either biotin or phycoerythrin.
Page 18 of 20
Page 23

Page 19 of 20
Page 24

Page 20 of 20
 Loading...
Loading...