LaMotte Water Analysis Kit User Manual

STUDIES

WHAT IS WA TER?
Water, a substance which covers approximately 75% of the Earth’s surface,
exists in three forms: solid, liquid and gas. In the solid form, ice, water is
used to cool drinks and other solutions, while the gas form, steam, can be
used to power turbines and engines. As a liquid, water is used in many
ways, from drinking to agriculture to providing homes for many kinds of
aquatic life.
Known as the universal solvent, “pure” water is able to combine with
other substances to form solutions. Even “natural” water found in the
environment contains dissolved gases, such as oxygen and carbon dioxide,
and dissolved minerals, such as iron and calcium. Most of these substances
are not harmful at the levels commonly found, but some substances are
toxic, or poisonous, to living things. Tests are available to determine levels
of many toxic and nontoxic substances in water.
If dangerous or unhealthy substances are discovered, the water can be
treated to remove or destroy them. Treatment is not necessary in many
industries, such as agriculture, but others require water be purified before
use. For instance, many drinking water systems treat the water with
chlorine to disinfect and kill bacteria, making it safe for consumption.
Other industrial processes add dangerous chemicals or bacteria to the
water, which must be removed or rendered harmless before returning it to
the environment.
As the human population increases concern over the availability of usable
water increases as well. Polluted water will purify itself naturally over time,
but as more and more untreated, polluted water is returned to the
environment the system is overloaded and unable to cope. Water
treatment processes, either chemical, biological or filtration, may be used
to supplement the natural purification process.
To determine the efficiency of treatment, and levels of toxic and nontoxic
substances, the water must be frequently tested. When establishing a
testing program it is important to take samples over a period of time
because the character of the water constantly changes. By taking several
samples, determining their composition and keeping accurate records,
scientists are better able to understand water.
2

WHAT IS SOIL?
Soil forms the natural covering of the Earth and supports plant life. It is
formed by weathering, or the breakdown of rocks into smaller particles by
natural forces. Wind, rain, freezing and thawing are all important forms of
weathering. Weathering is a continual process, but it is slow, and the
formation of new soil takes hundreds of years. Therefore, it is important to
conserve existing soil and use it wisely.
Weathered rocks are not the only the components of soil. Soil also
contains nutrients necessary for plant growth and survival. These
nutrients come from weathered rocks, and from dead decomposed plant
and animal material. Plant and animal waste left in soil are decomposed by
bacteria and fungi living in the soil, adding nutrients to the soil. The
practice of plowing or spading plant leaves into the soil, or adding humus
to the soil, will also add nutrients. Humus, composed of decayed organic
material, not only adds nutrients to the soil, it helps improve the texture
and water-holding capacity of the soil.
Since there are many types of rocks, and varying amounts of vegetation,
many different types of soil are formed. Soil types are classified by the size
of the particles; clay particles are the smallest, silt particles are
medium-sized, and sand particles are the largest. Each type of soil has a
unique feel. Sandy soil feels gritty when rubbed between the fingers, silty
soil feels silky or powdery, and clay feels sticky and moist. Many soils are a
combination of these three particles, and are called loam. The percentage
of each type of particle in loam can be determined by measuring settling
rates; sand particles settle fastest, and clay particles settle slowest.
In addition to covering the Earth, soil provides the nutrients necessary for
plants to survive. Plants require many different nutrients. Some nutrients,
such as nitrogen, phosphorus, and potassium are required in large
amounts, while only small amounts of others, such as sulfur, iron,
manganese, and calcium, are necessary. It is important that these nutrients
are not only present in the soil, but that they are in a form available to the
plants. Availability is dependent upon several factors, particularly the pH
of the soil. For instance, if the pH is too high iron will not be in a form
plants can use; it will not be available to the plants.
3
What is pH?
One of the simplest, yet most important, analyses of water and soil is the
pH test. pH is a measurement of the concentration of hydrogen ions in a
substance, or how acidic or basic the substance is. The concentration of
hydrogen ions is inversely proportional to the pH; the higher the
concentration of hydrogen ions the lower the pH.
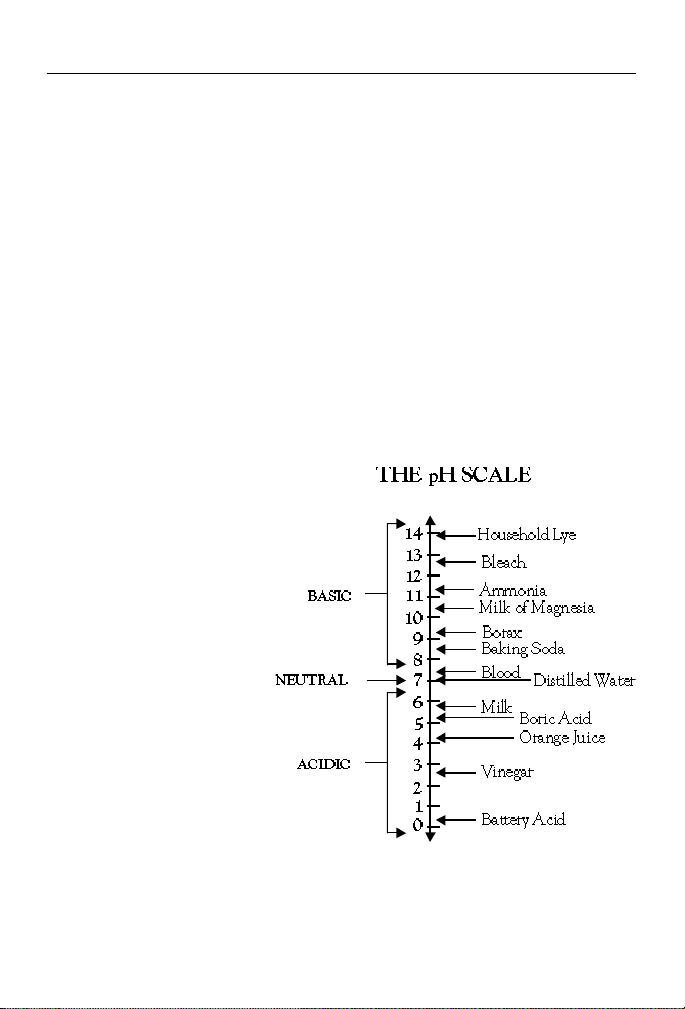
pH is measured on a logarithmic scale which ranges from 0 to 14. A pH of
7.0 is considered neutral; substances with a pH below 7.0 are acidic and
those with a pH above 7.0 are basic, or alkaline. Since the pH scale is
logarithmic, a change of one pH unit reflects a ten fold change in the
acidity. Orange juice, pH 4.0, is ten times more acidic than boric acid, pH
5.0, and 100 times more acidic than milk, pH 6.0. The pH of several
household substances are shown in Fig. 1.
There are many methods which can be used to measure pH. The simplest,
most inexpensive method, is using litmus paper, which, when dipped into
the solution, changes color to indicate whether the solution is acidic,
alkaline or neutral. Litmus paper will only indicate whether a substance is
acidic or alkaline, but not the degree of acidity. pH indicator test papers
are also dipped into the solution, but the resulting color is matched to a
color standard to indicate
the pH of the sample.
Liquid pH indicators can
also be used to determine
pH. When the indicator is
added to a solution, the pH
of the solution causes the
indicator to change color,
which is matched to a
color standard to
determine the pH. The
most sophisticated method
of pH analysis is a pH
meter. When the pH
electrode is immersed in a
sample, the electrode and
meter combine to give a
pH reading which can be
read directly from the
meter.
Fig. 1 The approximate pH values of some
common substances.
4

pH & WA TER
The pH of water is a concern to many people. Many factors contribute to
the pH of water, including the quantity of plant and animal life, the rocks
and other minerals the water is exposed to, and the pH of incoming
substances.
Plant and animal life in the water are constantly undergoing chemical and
biological processes which alter the water’s pH. Two of these processes are
respiration and photosynthesis. When plants and animals respire, or
breathe, carbon dioxide is released. The carbon dioxide reacts with the
water to form carbonic acid, which lowers the pH. Simultaneously, plants
are undergoing photosynthesis, a process which removes carbon dioxide
from the water before it is transformed into carbonic acid, raising the pH
of the water. Since plants need light to photosynthesize, photosynthesis
only occurs during daylight hours, but respiration occurs throughout the
day and night, so the pH of water tends to be higher during the day.
The pH is also dependent on the minerals in the water. As water passes
over and through rocks, minerals from the rocks dissolve into the water.
Some minerals, such as calcium, occur in a form which raises the pH,
while others lower the pH.
Natural waters normally have a pH between 5.0 and 8.0. When the pH is
out of this range, it may be an indication of pollution, and further testing
is necessary. Chemicals and other pollutants intentionally or
unintentionally added to the water can alter the pH.
One pollutant that can cause water to be acidic is acid rain. Acid rain,
defined as rain, snow or other precipitation with a pH of less that 5.6, is
formed from air pollutants, particularly sulfur dioxide and nitrogen oxides,
which are released as gases into the atmosphere. Power plants, smelters,
automobiles, and volcanic activity are all contributors to acid rain. In the
atmosphere, the gases combine with moisture to form sulfuric acid and
nitric acid solutions, which return to the Earth as acid rain.
pH testing is also important in water used for industrial or domestic
purposes. Acidic water can corrode metal pipes, while alkaline water can
leave deposits known as scale, potentially clogging pipes and ruining
equipment.
5
 Loading...
Loading...