Page 1

TOTAL, CALCIUM & MAGNESIUM
HARDNESS KIT
DIRECT READING TITRATOR, FRESH & SALT WATER
CODE 4824-DR-LT-01
QUANTITY CONTENTS CODE
15 mL *Sodium Hydroxide Reagent with Metal Inhibitors *4259-E
50 Calcium Hardness Indicator Tablets 5250A-H
15 mL *Hardness Reagent #5 *4483-E
50 Hardness Reagent #6 Tablets 4484A-H
60 mL Hardness Reagent #7 4487DR-H
1 Test Tube, 5-10-12.9-15-20-25 mL, glass, w/cap 0608
1 Direct Reading Titrator, 0-200 Range 0382
1 Pipet, 0.5 mL, plastic 0353
*WARNING: Reagents marked with an * are considered to be potential health hazards.
To view or print a Safety Data Sheet (SDS) for these reagents go to www.lamotte.com.
Search for the four digit reagent code number listed on the reagent label, in the contents
list or in the test procedures. Omit any letter that follows or precedes the four digit
code number. For example, if the code is 4450WT-H, search 4450. To obtain a printed
copy, contact LaMotte by e-mail, phone or fax.
Emergency information for all LaMotte reagents is available from Chem-Tel:
(US, 1-800-255-3924) (International, call collect, 813-248-0585)
To order individual reagents or test kit components, use the specifi ed code number.
NOTE: Read Direct Reading Titrator Manual before proceeding. The Titrator is
calibrated in terms of hardness expressed as parts per million (ppm) Calcium Carbonate
as CaCO3. Each minor division on the Titrator scale equals 4 ppm CaCO3.
Warning! This set contains chemicals
that may be harmful if misused. Read
cautions on individual containers
carefully. Not to be used by children
except under adult supervision.
Page 2
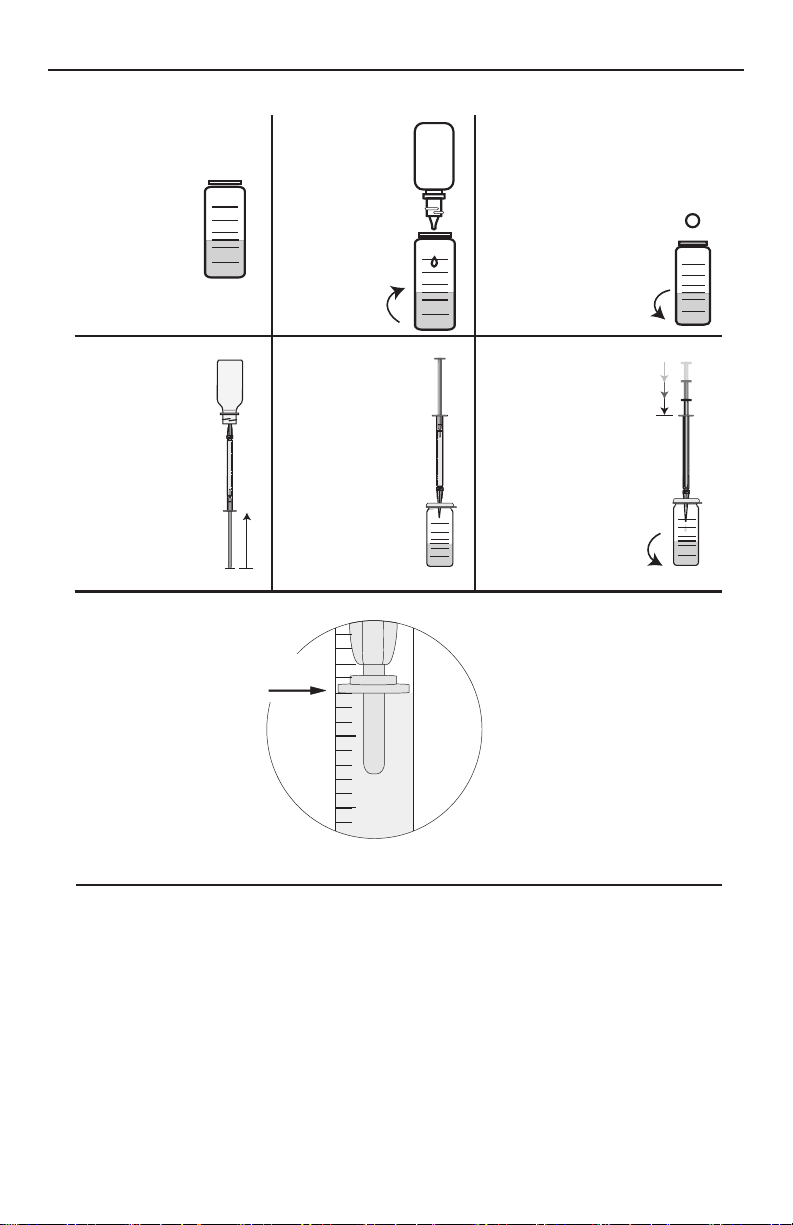
TOTAL HARDNESS TEST PROCEDURE
1
Fill a test
tube (0608)
to the 12.9
mL line with
the sample
water.
4
Fill Direct
Reading
Titrator (0382)
with Hardness
Reagent #7
(4487DR).
7
Read the test
result directly
from the scale
where the large
ring on the
Titrator meets the
Titrator barrel.
Record as ppm
Total Hardness as
(CaCO3).
Add one Hardness
2
Add 5 drops
of *Hardness
Reagent #5
(4483). Swirl
to mix.
3
Reagent #6 Tablet
(4484A). Cap and swirl until
tablet disintegrates. Solution
will turn red if hardness
is present. If solution
is blue, there is no
measurable amount
of hardness.
56
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
Result:
88 ppm
Insert the
Titrator into
the center
hole of the
test tube cap.
80
100
120
While gently
swirling the tube,
0
0.1
0.2
slowly press the
0.3
0.4
0.5
0.6
plunger to titrate
0.7
0.8
0.9
1.0
until the red color
changes to clear
blue.
EXAMPLE:
Plunger tip is 2 minor
divisions below line 80.
Test result is:
80 + (2 divisions x 4) = 88 ppm.
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
:
NOTE:
Parts per million CaCO3 may be converted to grains per gallon (gpg) CaCO3:
gpg CaCO3 = ppm CaCO3 x 0.058
If the plunger reaches the bottom line on the scale (200 ppm)
before the endpoint color change occurs, refi ll the Titrator and
continue the titration. When recording the test result, be sure to
include the value of the original amount of reagent dispensed
(200 ppm).
Page 3

CALCIUM HARDNESS TEST PROCEDURE
1
Fill a test
tube (0608)
to the 12.9
mL line with
the sample
water.
4
Fill Direct
Reading
Titrator (0382)
with Hardness
Reagent #7
(4487DR).
7
Read the test
result directly
from the scale
where the large ring
on the
Titrator meets
the
Titrator barrel.
Record as ppm
Calcium Hardness as
(CaCO3).
Add one Calcium
2
3
Hardness Indicator
Add 6 drops
of *Sodium
Hydroxide with
Metal
Inhibitor
(4259). Cap
and swirl
to
mix.
Tablet (5250A). Cap and
swirl until tablet disintegrates.
Solution will turn red
if hardness is present.
If solution is blue,
there is no measurable
amount of hardness.
56
1.0
0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
0
Result:
88 ppm
Immediately
insert the
Titrator into
the center
hole of the
test tube cap.
80
100
120
While gently
swirling the tube,
0
0.1
0.2
0.3
slowly press the
0.4
0.5
0.6
0.7
plunger to titrate
0.8
0.9
1.0
until the red color
changes to clear
blue.
EXAMPLE:
Plunger tip is 2 minor
divisions below line 80.
Test result is:
80 + (2 divisions x 4) = 88 ppm.
0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
NOTE:
If the plunger reaches the bottom line on the scale (200 ppm) before
the endpoint color change occurs, refi ll the Titrator and continue the
titration. When recording the test result, be sure to include the value
of the original amount of reagent dispensed (200 ppm).
MAGNESIUM HARDNESS TEST PROCEDURE
Subtract Calcium Hardness from Total Hardness. Record as ppm
Magnesium Hardness as CaCO3.
Magnesium Hardness (ppm CaCO3) = Total Hardness - Calcium Hardness
Page 4

ANALYSIS OF HARDNESS IN SALT WATER
When sea and estuarine waters containing very high levels of mineral salts are to be
tested, the sample must be diluted to a feasible concentration before titration. This test
is supplied with a calibrated pipet for performing the kit dilutions described below.
TOTAL HARDNESS DILUTION (1 TO 25.8)
1. Use the 0.5 mL pipet (0353) to transfer 0.5 mL of the salt water to be tested to the
test tube (0608).
2. Dilute to the 12.9 mL line with distilled water.
3. Follow Steps 2 through 7 under the Total Hardness Test Procedure. Multiply
Titrator reading by 25.8. Record as ppm Total Hardness as CaCO3.
CALCIUM HARDNESS DILUTION (1 TO 12.9)
1. Use the 0.5 mL pipet (0353) to transfer 1.0 mL (two measures) of the salt water to
be tested to the test tube (0608).
2. Dilute to the 12.9 mL line with distilled water.
3. Follow Steps 2 through 7 under Calcium Hardness test procedure. Multiply
Titrator reading by 12.9. Record as ppm Calcium Hardness as CaCO3.
4. To convert Calcium Carbonate to Calcium Chloride, multiply by 1.11. Record as
ppm CaCl2 = ppm CaCO3 x 1.11
5. To convert Calcium Carbonate to Calcium, multiply by 0.4. Record as ppm
Calcium.
ppm Ca = ppm CaCO3 x 0.4
MAGNESIUM HARDNESS OF SALT WATER
Subtract Calcium Hardness from Total Hardness. Record as ppm Magnesium Hardness
as CaCO3.
Magnesium Hardness (ppm CaCO3) = Total Hardness - Calcium Hardness
To convert Magnesium Hardness as CaCO3 to Magnesium Chloride, multiply by 0.95.
Record as ppm Magnesium Chloride.
ppm MgCl2 = ppm CaCO3 x 0.95
To convert Magnesium Hardness to Magnesium, multiply by 0.24. Record as ppm
Magnesium.
ppm Mg = ppm CaCO3 x 0.24
LaMOTTE COMPANY
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.) • Fax 410-778-6394
Visit us on the web at www.lamotte.com
64824-DR-LT-01 11/19
 Loading...
Loading...