Page 1
•
SMART
••••••••••••••••••••••••••••••••••••••••••••••
OPERATOR’S
MANUAL
2
Colorimeter
V. 2 . 4 • Printed 08/10
1919-MN
Page 2

Page 3

T ABLE OF CONTENTS
GENERAL INFORMATION
Packaging & Delivery ······································································5
General Precautions ········································································5
Safety Precautions ···········································································5
Limits of Liability ·············································································5
Limited Warranty··············································································6
Limitations························································································6
Specifications ···················································································6
Statistical and Technical Definitions
Related to Product Specifications··················································· 7-8
Contents and Accessories································································· 8
EPA Compliance ··············································································8
CE Compliance·················································································9
CHEMICAL TESTING
Water Sampling for Chemical Analysis ··········································· 10-11
Filtration ··························································································11
An Introduction to Colorimetric Analysis ······································ 12
Reagent Blank ·················································································13
Colorimeter Tubes ···········································································13
Meter Care························································································13
Selecting an Appropriate Wavelength ············································· 13-14
Calibration························································································14-16
Calibration Curves ···········································································14-16
Standard Additions ··········································································16-17
Sample Dilution Techniques & Volumetric Measurements ············ 17
Interferences ····················································································18
Stray Light Interference ···································································18
OPERATION OF THE SMART 2 COLORIMETER
Overview ·························································································19
Power Source ····················································································19
Components ····················································································20
Quick Start ······················································································21-22
GENERAL OPERATING PROCEDURES
The Keypad ······················································································23
Sample Holders·················································································23
The Display & the Menus ······························································· 24-25
Looping Menus ·················································································26
TESTING
Testing Menu ··················································································· 27
Sequences of Tests ···········································································28
General Testing Procedures ····························································· 29
Testing With the Pre-Programmed Tests ········································· 29-30
SMART2 COLORIMETER 1.07 3
Page 4

T ABLE OF CONTENTS (cont.)
Calibrating LaMotte Pre-Progammed Tests······································ 31-32
Measuring in the %T/ABS Mode ····················································· 33-34
EDITING MENU
Edit a Sequence ···············································································35-36
Adding or Deleting Tests ·································································36-38
Edit User Tests ·················································································39
Naming the Test ··············································································40-41
Selecting the Vial and Wavelength ·················································· 42
Entering a New Calibration ····························································· 43-44
Selecting the Numerical Format of the Result ································ 45
Selecting Units of Concentration····················································· 46
Setting the Clock··············································································47
Turning the Data Logger On and Off ··············································· 48
Factory Setup····················································································49
Setting the Power Saver Function ···················································· 49
PC LINK
Output ·····························································································50
Computer Connection······································································50
BATTERY OPERATION
Replacing the Battery ······································································· 50
MAINTENANCE
Cleaning ···························································································51
Meter Care························································································51
Meter Disposal··················································································51
TROUBLESHOOTING GUIDE
Error Messages·················································································· 52
Helpful Hints ····················································································53
SMART2 COLORIMETER TEST INSTRUCTIONS
APPENDIX
4 SMART2 COLORIMETER 1.07
Page 5

GENERAL INFORMATION
PACKAGING & DELIVERY
n
Experienced packaging personnel at LaMotte Company assure adequate
protection against normal hazards encountered in transportation of shipments.
After the product leaves the manufacturer, all responsibility for its safe delivery
is assured by the transportation company. Damage claims must be filed
immediately with the transportation company to receive compensation for
damaged goods.
Should it be necessary to return the instrument for repair or servicing, pack
instrument carefully in suitable container with adequate packing material. A
return authorization number must be obtained from LaMotte Company by
calling 1-800-344-3100. Attach a letter with the authorization number to the
shipping carton which describes the kind of trouble experienced. This valuable
information will enable the service department to make the required repairs
more efficiently.
GENERAL PRECAUTIONS
n
Before attempting to set up or operate this instrument it is important to read
the instruction manual. Failure to do so could result in personal injury or
damage to the equipment.
The SMART2 Colorimeter should not be stored or used in a wet or corrosive
environment. Care should be taken to prevent water or reagent chemicals from
wet colorimeter tubes from entering the colorimeter chamber.
NEVER PUT WET TUBES IN COLORIMETER.
SAFETY PRECAUTIONS
n
Read the labels on all LaMotte reagent containers prior to use. Some
containers include precautionary notices and first aid information. Certain
reagents are considered hazardous substances and are designated with a * in the
instruction manual. Material Safety Data Sheets (MSDS) are supplied for these
reagents. Read the accompanying MSDS before using these reagents.
Additional emergency information for all LaMotte reagents is available 24
hours a day from the Poison Control Center listed in the front of the phone
book. Be prepared to supply the name and four-digit LaMotte code number
found on the container label or at the top of the MSDS. LaMotte reagents are
registered with a computerized poison control information system available to
all local poison control centers.
Keep equipment and reagent chemicals out of the reach of young children.
Protect Yourself and Equipment: Use Proper Analytical Techniques
LIMITS OF LIABILITY
n
Under no circumstances shall LaMotte Company be liable for loss of life,
property, profits, or other damages incurred through the use or misuse of its
products.
SMART2 COLORIMETER 1.07 5
Page 6

LIMITED WARRANTY
n
This instrument is guaranteed to be free from defects in material and workmanship
for a period of two (2) years from original purchase date. In the event that a defect
is found during the warranty time frame, LaMotte Company agrees that it will be
repaired or replaced without charge except for the transporation costs. This
guarantee does not cover batteries.
This product can not be returned without a return authorization number from
Lamotte Company. For warranty support or a Return Authorization Number,
contact LaMotte Company at 1-800-344-3100 or tech @ lamotte.com.
n
LIMITATIONS
This guarantee is void under the following circumstances:
• Damage due to operator negligence, misuse, accident or improper application.
• Damage or alterations from attempted repairs by an unauthorized
(non-LaMotte) service.
• Damage due to improper power source, AC adapter or battery.
• Damage caused by acts of God or natural disaster.
• Damage occurred while in transit with a shipping carrier.
LaMotte Company will service and repair out-of warranty products at a nominal
charge.
SPECIFICATIONS
n
n
INSTRUMENT TYPE: Colorimeter
Readout Graphical 4 line, 16 character per line LCD
Wavelengths 430nm, 520 nm, 570 nm, 620 nm
Wavelength Accuracy ±2
Readable Resolution Determined by reagent system
Wavelength Bandwidth 10 typical
Photometric Range –2 to + 2AU
Photometric Precision ± 0.001AU at 1.0AU
Photometric Accuracy ± 0.005AU at 1.0AU
Sample Chamber Accepts 25 mm diameter flat-bottomed test tubes, 10 mm square cuvettes, 16 mm COD test tubes
Light Sources 4LEDs
Detectors 4 silicon photodiodes with integrated interference filters
Modes Absorbance, pre-programmed tests
Pre-Programmed Tests YES, with automatic wavelength selection
User Defined Tests Up to 10 user tests can be input
RS232 Port 8 pin mini-DIN, 9600b, 8, 1, n
Power Requirements Battery Operation: 9 volt alkaline, Line Operation: 110/ AC; 50/60 Hz with adapter, 6V 500 mA DC
Dimensions (LxWxH) 8.5 x 16.2 x 6.7 cm, 3.4 x 6
Weight 312 g, 11 oz (meter only)
Data Logger 350 test results stored for download to a PC
6 SMART2 COLORIMETER 10.07
.4 x 2.6 inches
Page 7

STATISTICAL AND TECHNICAL DEFINITIONS RELATED
n
TO PRODUCT SPECIFICATIONS
Method Detection Limit (MDL): “The method detection limit (MDL) is
defined as the minimum concentration of a substance that can be measured
and reported with 99% confidence that the analyte concentration is greater
than zero and is determined from analysis of a sample in a given matrix
containing the analyte.”
almost all cases when dealing with a limit of detection or limit of
determination, the primary purpose of determining that limit is to stay away
from it.’”
2
1. CFR 40, part 136, appendix B
2. Statistics in Analytical Chemistry: Part 7 – A Review, D. Coleman and
L Vanatta, American Laboratory, Sept 2003, P. 31.
Precision: Precision is the numerical agreement between two or more
measurements.
3
The precision can be reported as a range for a measurement
(difference between the min and max). It can also be reported as the standard
deviation or the relative standard deviation. It is a measure of how close
together the measurements are, not how close they are to the correct or true
value. The precision can be very good and the accuracy very bad. This is a useful
measure of the performance of a test method.
3. Skoog, D.A., West, D. M., Fundamental of Analytical Chemistry, 2
Holt Rinehart and Winston, Inc, 1969, p. 26.
Accuracy: Accuracy is the nearness of a measurement to the accepted or true
4
value.
The accuracy can be expressed as a range, about the true value, in
which a measurement occurs (i.e. ±0.5 ppm). It can also be expressed as the %
recovery of a know amount of analyte in a determination of the analyte (i.e.
103.5 %). This is a useful measure and what most customers are interested in when
they want to know about the performance of a test method.
4. Skoog D.A., West D. M., Fundamental of Analytical Chemistry, 2
Holt Rinehart and Winston, Inc, 1969, p. 26.
Resolution: Resolution is the smallest discernible difference between any two
measurements that can be made.
places are displayed. (i.e. 0.01). For titrations and various comparators it is the
smallest interval the device is calibrated or marked to (i.e. 1 drop = 10 ppm,
0.2 ppm for a DRT, or ±half a unit difference for an octaslide or color chart).
Note that the resolution many change with concentration or range. In some
cases the resolution may be less than the smallest interval, if it is possible to
make a reading that falls between calibration marks. This is often done with
various comparators. One caveat is, that resolution has very little relationship to
accuracy or precision. The resolution will always be less than the accuracy or
precision but it is not a statistical measure of how well a method of analysis works.
The resolution can be very very good and the accuracy and precision can be very, very
bad! This is not a useful measure of the performance of a test method.
5. Statistics in Analytical Chemistry: Part 7 – A Review, D. Coleman and
L Vanatta, American Laboratory, Sept 2003, P. 34.
1
Note that, “As Dr. William Horwitz once stated, ‘In
nd
ed.,
nd
ed.,
5
For meters this is usually how many decimal
SMART2 COLORIMETER 1.07 7
Page 8

Sensitivity: Sensitivity is the resolution based on how this term is used in
LaMotte catalogs. This term is not listed in any of the references. Sometimes it
is used for detection limit. It is a confusing term and should be avoided.
Repeatability: Repeatability is the within-run precision.
6
A run is a single
data set, from set up to clean up. Generally, one run occurs on one day.
However, for meter calibrations, a single calibration is considered a single run
or data set, even though it may take 2 or 3 days.
6. Jeffery G. H., Basset J., Mendham J., Denney R. C., Vogel’s Textbook of
Quantitative Chemical Analysis, 5
th
ed., Longman Scientific & Technical,
1989, p. 130.
Reproducibility: Reproducibility is the between-run precision.
7. Jeffery G. H., Basset J., Mendham J., Denney R. C., Vogel’s Textbook of
Quantitative Chemical Analysis, 5
th
ed., Longman Scientific & Technical,
7
1989, p. 130.
CONTENTS AND ACCESSORIES
n
n
CONTENTS
SMART2 Colorimeter
Test Tubes, with Caps
Power Supply, 110/220V
SMART2 Colorimeter Quick Start Guide
SMART2 Colorimeter Manual
n
ACCESSORIES
COD Adapter Code 5-0087
UDV Adapter Code 5-0086
Small Field Carrying Case Code 1919-GCS150
Large Field Carrying Case Code 1919-BCS440
SMARTLink2 Program & Interface Cable (3.5 disk) Code 1912-3
SMARTLink2 Program & Interface Cable (CD) Code 1912-CD
EPA COMPLIANCE
n
The SMART2 Colorimeter is an EPA-Accepted instrument. EPA-Accepted
means that the instrument meets the requirements for instrumentation as
found in test procedures that are approved for the National Primary Drinking
Water Regulations (NPDWR) or National Pollutant Discharge Elimination
System (NPDES) compliance monitoring programs. EPA-Accepted
instruments may be used with approved test procedures without additional
approval.
8 SMART2 COLORIMETER 1.07
Page 9

CE COMPLIANCE
n
The SMART2 Colorimeter has earned the European CE Mark of
Compliance for electromagnetic compatibility and safety.
DECLARA TION OF CONFORMITY
Standards to which
Conformity Declared:
Manufacturer's Name:
Manufacturer's Address:
Type of Equipment:
Model Name:
Year of Manufacture:
Testing Performed By:
Chestertown, Maryland
Place
EN61326:1998, IEC61326:1997,
IEC61000-4-2:1995, IEC61000-4-3:1995
IEC61000-4-4:1995, IEC61000-4-5:1995
IEC61000-4-6:1996, IEC61000-4-11:1994,
EN61000-3-2:1995, EN61000-3-3:1994-12,
EN55011/CISPR11, FCCCFR47 Part 15,
EN61558
LaMotte Company
802 Washington Avenue
PO Box 329
Chestertown, MD 21620
Colorimeter
SMART 2
2001
Windermere
2000 Windermere Court
Annapolis, MD 21401
I, the undersigned, hereby declare that the equipment specified above
conforms to the above Directive and Standards.
Signature
1/15/02
Date
SMART2 COLORIMETER 1.07 9
Scott H. Steffen
Name
VP New Products & Quality
Position
Page 10

CHEMICAL TESTING
WA TER SAMPLING FOR CHEMICAL ANALYSIS
n
n
Taking Representative Samples
The underlying factor to be considered for any type of water sampling is
whether or not the sample is truly representative of the source. To properly
collect a representative sample:
l
Sample as frequently as possible.
l
Collect a large sample or at least enough to conduct whatever tests are
necessary.
l
Make a composite sample for the same sampling area.
l
Handle the sample in such a way as to prevent deterioration or
contamination before the analysis is performed.
l
Perform analysis for dissolved gases such as dissolved oxygen, carbon
dioxide, and hydrogen sulfide immediately at the site of sampling. These
factors, as well as samples for pH, cannot be stored for later examination.
l
Make a list of conditions or observations which may affect the sample.
Other considerations for taking representative samples are dependent
upon the source of the sample. Taking samples from surface waters
involves different considerations than taking samples from impounded and
sub-surface waters.
n
Sampling of Open Water Systems
Surface waters, such as those found in streams and rivers, are usually well
mixed. The sample should be taken downstream from any tributary, industrial
or sewage pollution source. For comparison purposes samples may be taken
upstream and at the source of the pollution before mixing.
In ponds, lakes, and reservoirs with restricted flow, it is necessary to collect a
number of samples in a cross section of the body of water, and where possible
composite samples should be made to ensure representative samples.
To collect samples from surface waters, select a suitable plastic container with a
tight fitting screw cap. Rinse the container several times with the sample to be
tested, then immerse the container below the surface until it is filled to
overflowing and replace the cap. If the sample is not to be tested immediately,
pour a small part of the sample out and reseal. This will allow for any
expansion. Any condition which might affect the sample should be listed.
Sub-surface sampling is required to obtain a vertical profile of streams, lakes,
ponds, and reservoirs at specific depths. This type of sampling requires more
sophisticated sampling equipment.
For dissolved oxygen studies, or for tests requiring small sample sizes, a Water
Sampler (LaMotte Code 1060) will serve as a subsurface or in-depth sampler.
10 SMART2 COLORIMETER 1.07
Page 11

This weighted device is lowered to the sampling depth and allowed to rest at
this depth for a few minutes. The water percolates into the sample chamber
displacing the air which bubbles to the surface. When the bubbles cease to rise,
the device has flushed itself approximately five times and it may be raised to
the surface for examination. The inner chamber of the sampling device is lifted
out and portions of the water sample are carefully dispensed for subsequent
chemical analysis.
A Snap-Plunger Water Sampler (LaMotte Code 1077) is another “in-depth”
sampling device which is designed to collect large samples which can be used
for a multitude of tests. Basically , this collection apparatus is a hollow cylinder
with a spring loaded plunger attached to each end. The device is cocked above
the surface of the water and lowered to the desired depth. A weighted
messenger is sent down the calibrated line to trip the closing mechanism and
the plungers seal the sample from mixing with intermediate layers as it is
brought to the surface. A special drain outlet is provided to draw off samples for
chemical analysis.
n
Sampling of Closed System
To obtain representative samples from confined water systems, such as pipe
lines, tanks, vats, filters, water softeners, evaporators and condensers, different
considerations are required because of chemical changes which occur between
the inlet and outlet water. One must have a basic understanding of the type of
chemical changes which occur for the type of equipment used. Also,
consideration should be given to the rate of passage and retaining time for the
process water.
Temperature changes play an important part in deciding exactly what test
should be performed. Process water should be allowed to come to room
temperature, 20–25°C, before conducting any tests.
When drawing off samples from an outlet pipe such as a tap, allow sample to
run for several minutes, rinsing the container several times before taking the
final sample. Avoid splashing and introduction of any contaminating material.
FILTRATION
n
When testing natural waters that contain significant turbidity due to suspended
solids and algae, filtration is an option. Reagent systems, whether EPA,
Standard Methods, LaMotte or any others, will generally only determine
dissolved constituents. Both EPA and Standard Methods suggest filtration
through a 0.45 micron filter membrane, to remove turbidity, for the
determination of dissolved constituents.** To test for total constituents,
organically bound and suspended or colloidal materials, a rigorous high
temperature acid digestion is necessary.
**LaMotte offers a filtering apparatus: syringe assembly (Code 1050) and membrane
filters, 0.45 micron, (Code 1103).
SMART2 COLORIMETER 1.07 11
Page 12

AN INTRODUCTION TO COLORIMETRIC ANALYSIS
n
Most test substances in water are colorless and undetectable to the human eye.
To test for their presence we must find a way to “see” them. The SMART2
Colorimeter can be used to measure any test substance that is itself colored or
can be reacted to produce a color. In fact a simple definition of colorimetry is
“the measurement of color” and a colorimetric method is “any technique used
to evaluate an unknown color in reference to known colors”. In a colorimetric
chemical test the intensity of the color from the reaction must be proportional
to the concentration of the substance being tested. Some reactions have
limitations or variances inherent to them that may give misleading results.
Many such interferences are discussed with each particular test instruction. In
the most basic colorimetric method the reacted test sample is visually
compared to a known color standard. However, accurate and reproducible
results are limited by the eyesight of the analyst, inconsistencies in the light
sources, and the fading of color standards.
To avoid these sources of error, a colorimeter can be used to photoelectrically
measure the amount of colored light absorbed by a colored sample in reference
to a colorless sample (blank).
White light is made up of many different colors or wavelengths of light. A
colored sample typically absorbs only one color or one band of wavelengths
from the white light. Only a small difference would be measured between white
light before it passes through a colored sample versus after it passes through a
colored sample. The reason for this is that the one color absorbed by the
sample is only a small portion of the total amount of light passing through the
sample. However, if we could select only that one color or band of wavelengths
of light to which the test sample is most sensitive, we would see a large
difference between the light before it passes through the sample and after it
passes through the sample.
The SMART2 Colorimeter passes one of four colored light beams through one
of four optical filters which transmits only one particular color or band of
wavelengths of light to the photodectector where it is measured. The difference
in the amount of colored light transmitted by a colored sample is a
measurement of the amount of colored light absorbed by the sample. In most
colorimetric tests the amount of colored light absorbed is directly proportional
to the concentration of the test factor producing the color and the path length
through the sample. However, for some tests the amount of colored light
absorbed is inversely proportional to the concentration.
The choice of the correct wavelength for testing is important. It is interesting
to note that the wavelength that gives the most sensitivity (lower detection
limit) for a test factor is the complementary color of the test sample. For
example the Nitrate-Nitrogen test produces a pink color proportional to the
nitrate concentration in the sample (the greater the nitrate concentration, the
darker the pink color). A wavelength in the green region should be selected to
analyze this sample since a pinkish-red solution absorbs mostly green light.
12 SMART2 COLORIMETER 1.07
Page 13

REAGENT BLANK
n
Some tests will provide greater accuracy if a reagent blank is determined to
compensate for any color or turbidity resulting from the reagents
themselves. A reagent blank is performed by running the test procedure on
10 mL of demineralized water . Use sample water to SCAN BLANK.Insert
the reagent blank in the colorimeter chamber and select SCAN SAMPLE.
Note result of reagent blank. Perform the tests on the sample water as
described. Subtract results of reagent blank from all subsequent test results.
NOTE: Some tests require a reagent blank to be used to SCAN BLANK.
COLORIMETER TUBES
n
Colorimeter tubes which have been scratched through excessive use should
be discarded and replaced with new ones. Dirty tubes should be cleaned on
both the inside and outside. Fingerprints on the exterior of the tubes can
cause excessive light scattering and result in errors. Handle the tubes
carefully, making sure the bottom half of the tube is not handled.
LaMotte Company makes every effort to provide high quality colorimeter
tubes. However, wall thicknesses and diameter of tubes may still vary
slightly. This may lead to slight variations in results (e.g. if a tube is turned
while in the sample chamber, the reading will likely change slightly). To
eliminate this error put the tubes into the sample chamber with the same
orientation every time.
The tubes that are included with the colorimeter have an index mark to
facilitate this. If possible, use the same tube to SCAN BLANK and SCAN
SAMPLE.
METER CARE
n
The optical system of the SMART2 must be kept clean and dry for optimal
performance. Dry the colorimeter tubes before placing them in the
chamber to avoid introducing moisture. For best results store the
instrument in a area that is dry and free from aggressive chemical vapors.
SELECTING AN APPROPRIATE WAVELENG TH
n
The most appropriate wavelength to use when creating a calibration curve
is usually the one which gives the greatest change from the lowest reacted
standard concentration to the highest reacted standard concentration.
However, the absorbance of the highest reacted standard concentration
should never be greater than 2.0 absorbance units. Scan the lowest and
highest reacted standards at different wavelengths using the absorbance
mode to find the wavelength which gives the greatest change in
absorbance without exceeding 2.0 absorbance units. Use this wavelength to
create a calibration curve.
SMART2 COLORIMETER 1.07 13
Page 14

Below is a list of suggested wavelengths for the color of the reacted samples.
Use these as a starting point.
CALIBRATION
n
Sample
Color
Yellow 430
Pink 520
Red 570
Green and Blue 620
Wavelength
Range
As with all pre-calibrated meters, it is highly recommended, even if not
required by regulations, that the user periodically verify the performance of the
meter by running standards with a predetermined concentration. Results
outside of specification are an indication that the meter needs to be adjusted.
This can be done following the user calibration described on page 31. If the
user calibration fails to properly adjust the meter then the meter should be
returned to LaMotte Company for recalibration. (See page 5).
CALIBRATION CURVES
n
The Smart2 Colorimeter contains tests for the LaMotte reagent systems (see
Page 49). The first step in using a non-LaMotte reagent system with your
Smart2 Colorimeter is to create a calibration curve for the reagent system. To
create a calibration curve, prepare standard solutions of the test factor and use
the reagent system to test the standard solutions with the Smart2 Colorimeter .
Select a wavelength for the test as described above.
Plot the results (in ABS or %Transmittance) versus concentration to create a
calibration curve. The calibration curve may then be used to identify the
concentration of an unknown sample by testing the unknown, reading
Absorbance or %T, and finding the corresponding concentration from the
curve. The linear range of the reagent system can be determined and this
information can be used to input a User Test into the Smart2 Colorimeter (see
EDIT USER TESTS, page 36).
n
PROCEDURE
1. Prepare 5 or 6 standard solutions of the factor being tested. The
concentration of these standards should be evenly distributed throughout
the range of the reagent system, and should include a 0 ppm standard
(distilled water). For instance, the solutions could measure 0, 10%, 30%,
50%, 70%, and 90% of the system’s maximum range.
2. T urn on the Smart2 Colorimeter. Select the appropriate wavelength from
the absorbance mode. Be sure to select the appropriate wavelength for the
color produced by the reagent system.
14 SMART2 COLORIMETER 1.07
Page 15
3. Use the unreacted 0 ppm standard to standardize the colorimeter by using
it to scan blank.
4. Following the individual reagent system instructions, react each standard
solution beginning with 0 ppm. Continue with standards in increasing
concentration. Record the reading and the standard solution
concentration on a chart. Readings can be recorded as percent
transmittance (%T) or absorbance (A).
5. Plot results on graph paper or computer using any available plotting
program. If results are as %T versus concentration, semilog graph paper
must be used. Plot the standard solution concentrations on the horizontal,
linear axis, and the %T on the vertical, logarithmic axis. If results are as
absorbance versus standard solution concentration, simple linear graph
paper can be used. Plot the standard solution concentration on the
horizontal axis, and the absorbance on the vertical axis.
6. After plotting the results, draw a line, or curve, of best fit through the
plotted points. The best fit may not connect the points. There should be
approximately an equal number of points above the curve as below the
curve. Some reagent systems will produce a straight line, while others
produce a curve. Many computer spreadsheet programs can produce the
curve of best fit by regression analysis of the standard solution data.
NOTE: Only reagent systems which produce a straight line can be used for a
User Test.
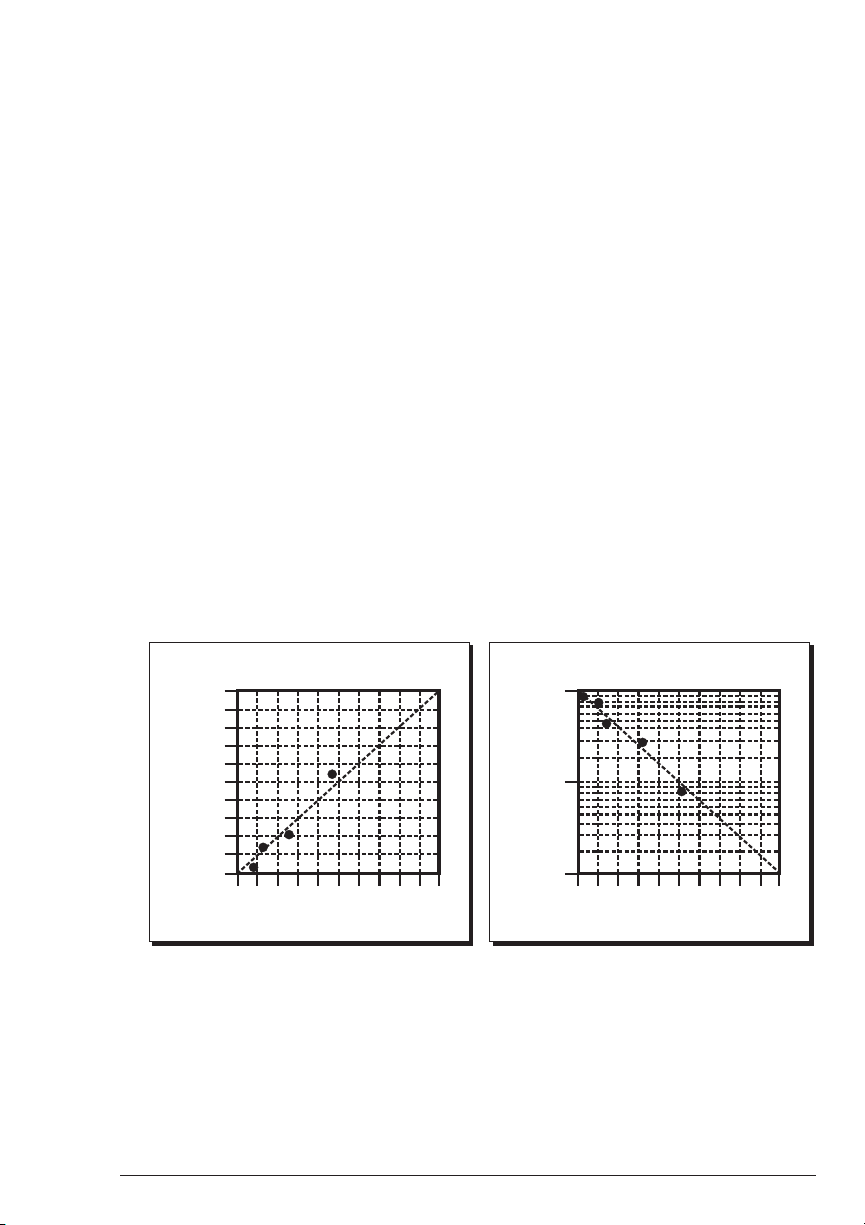
A sample of each type of graph appears below:
CALIBRATION CURVE
Absorbance vs. Concentration
2.0
1.8
1.6
1.4
1.2
1.0
0.8
0.6
Absorbance
0.4
0.2
0.0
123456
Concentration in ppm
SMART2 COLORIMETER 1.07 15
89107
%T Transmission
CALIBRATION CURVE
%T vs. Concentration
100
10
1
0
123456
Concentration in ppm
891070
Page 16
n
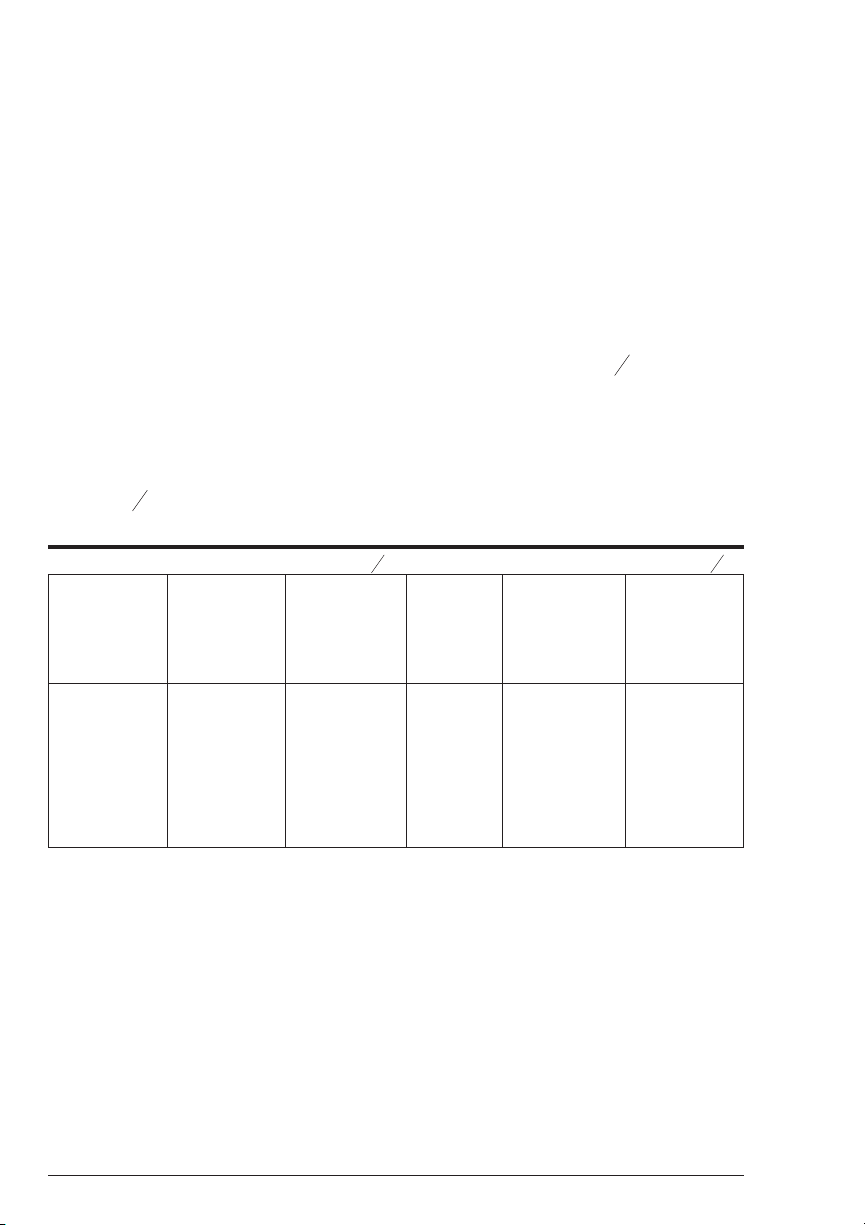
PREPARING DILUTE STANDARD SOLUTIONS
Standard solutions should be prepared to create a calibration curve. Standard
solutions can be prepared by diluting a known concentrated standard by
specified amounts. A chart or computer spreadsheet can be created to
determine the proper dilutions. Use volumetric flasks and volumetric pipets for
all dilutions.
1. In Column A – Record the maximum concentration of test as determined
by the range and path length.
2. In Column B – Record the percent of the maximum concentration the
standard solution will be.
3. In Column C – Calculate the final concentration of the diluted standard
solutions by multiplying the maximum concentration (In Column A) by
B
the % of maximum concentration divided by 100. (C = A x
100
).
4. In Column D – Record the final volume of the diluted sample (i.e. volume
of volumetric flask).
5. In Column E – Record the concentration of the original standard.
6. In Column F – Calculate the milliliters of original standard required
D
(C x
=F).
E
A sample chart appears below:
Final
B
I 00
DEF=Cx
Concentration
Volume of
Standard
of Original
Standard
Standard
Required
ABC=Ax
Maximum
concentration
of test
10.0 ppm 90 9.0 ppm 100 mL 1000 ppm 0.90 mL
10.0 ppm 70 7.0 ppm 100 mL 1000 ppm 0.70 mL
10.0 ppm 50 5.0 ppm 100 mL 1000 ppm 0.50 mL
10.0 ppm 30 3.0 ppm 100 mL 1000 ppm 0.30 mL
10.0 ppm 10 1.0 ppm 100 mL 1000 ppm 0.10 mL
10.0 ppm 0 0 ppm 100 mL 1000 ppm 0 mL
%of
Maximum
concentration
concentration
of Diluted
Standard
mL of
Original
D
E
STAND ARD ADDITIONS
n
A common method to check the accuracy and precision of a test is by standard
additions. In this method a sample is tested to determine the concentration of
the test substance. A second sample is then “spiked” by the addition of a
known quantity of the test substance. The second sample is then tested. The
determined concentration of the spiked sample should equal the concentration
of the first plus the amount added with the spike. The procedure can be
repeated with larger and larger “spikes.” If the determined concentrations do
not equal the concentration of the sample plus that added with the “spike”,
then an interference may exist.
For example, a 10.0 mL water sample was determined to contain 0.3 ppm iron.
To a second 10.0 mL sample, 0.1 mL of 50 ppm iron standard was added. The
16 SMART2 COLORIMETER 1.07
Page 17

concentration of iron due to the “spike” was (0.10 mL x 50 ppm)/10.0 mL =
0.50 ppm. The concentration of iron determined in the spiked sample should
be 0.3 + 0.5 = 0.8 ppm iron. (Note: any error due to the increased volume from
the “spike” is negligible).
LaMotte offers a line of calibration standards which can be used to generate
calibration curves and perform standard additions.
SAMPLE DILUTION TECHNIQUES
n
& VOLUMETRIC MEASUREMENTS
If a test result using the Smart2 Colorimeter gives an over range message then
the the sample must be diluted. The test should be repeated on the diluted
sample to obtain a reading which is in the concentration range for the test.
(Note: This is not true for colorimetric determination of pH.)
Example:
Measure 5 mL of the water sample into a graduated cylinder. Add
demineralized water until the cylinder is filled to the 10 mL line. The sample
has been diluted by one-half, and the dilution factor is therefore 2. Perform the
test procedure, then multiply the resulting concentration by 2 to obtain the
test result.
The following table gives quick reference guidelines on dilutions of various
proportions. All dilutions are based on a 10 mL volume, so several dilutions
will require small volumes of the water sample. Graduated pipets should be
used for all dilutions.
Size of Sample
10 mL 0 mL 1
5mL 5mL 2
2.5 mL 7.5 mL 4
1mL 9mL 10
0.5 mL 9.5 mL 20
Deionized W ater to Bring
Volume to 10 mL Multiplication Factor
If the above glassware is not available, dilutions can be made with the
colorimeter tube. Fill the tube to the 10 mL line with the sample then transfer
it to another container. Add 10 mL volumes of demineralized water to the
container and mix. Transfer back 10 mL of the diluted sample to the tube and
follow the test procedure. Continue diluting and testing until a reading, which
is in the concentration range for the test, is obtained. Be sure to multiply the
concentration found by the dilution factor (the number of total 10 mL volumes
used).
Example:
10 mL of sample is diluted with three 10 mL volumes of demineralized water;
the dilution factor is four.
SMART2 COLORIMETER 1.07 17
Page 18

INTERFERENCES
n
LaMotte reagent systems are designed to minimize most common interferences.
Each individual test instruction discusses interferences unique to that test. Be
aware of possible interferences in the water being tested.
The reagent systems also contain buffers to adjust the water sample to the ideal
pH for the reaction. It is possible that the buffer capacity of the water sample
may exceed the buffer capacity of the reagent system and the ideal pH will not
be obtained. If this is suspected, measure the pH of a reacted distilled water
reagent blank using a pH meter. This is the ideal pH for the test. Measure the
pH of a reacted water sample using the pH meter. If the pH is significantly
different from the ideal value, the pH of the sample should be adjusted before
testing.
Interferences due to high concentration of the substance being tested, can be
overcome by sample dilution (see page 16)
STRAY LIGHT INTERFERENCE
n
When scanning samples in 16 mm tubes, such as COD, the sample chamber lid
can not be closed. The COD adapter minimizes stray light. To further reduce
stray light interference, do not scan sample in direct sunlight.
18 SMART2 COLORIMETER 1.07
Page 19

OPERA TION OF THE
SMART2 COLORIMETER
OVERVIEW
n
The SMART2 Colorimeter is a portable, microprocessor controlled, direct
reading colorimeter. It has a graphical 4 line, 16 character liquid crystal display
for graphical, alphabetical and numerical messages. The operation is controlled
with the keypad through menu driven software in response to selections shown
on the display.
The test library consists of 100 LaMotte tests (not all 100 may be available at
present) and 10 “User Tests”. The LaMotte tests are precalibrated for LaMotte
reagent systems. The colorimeter displays the results of these tests directly in
units of concentration. The 10 “User Tests” may be used to enter additional
calibrations. All of these tests may be arranged in any of 3 sequences. These
sequences can be modified a limitless number of times to meet changing testing
needs.
The optics feature 4 different colored LEDs. Each LED has a corresponding
silicon photodiode with an integrated interference filter. The interference
filters select a narrow band of light from the corresponding LED for the
colorimetric measurements. The microprocessor automatically selects the
correct LED/photodiode combination for a test.`
A RS-232 serial port on the back of the colorimeter, and optional software,
allows the SMAR T2 to be interfaced with an IBM compatible personal
computer for real time data acquisition and data storage. This port also allows
an interface with a RS-232 serial printer.
Due to its portability, alternate power sources, and rugged construction, the
SMART2 Colorimeter is ideal for lab and field use.
POWER SOURCE
n
The SMART2 Colorimeter uses a 500 mA AC adapter. Please refer to the
Parts List for the code number for the correct adapter.
USE OF ANY AC ADAPTER OTHER THAN THE ONE SPECIFIED
FOR USE WITH THE Smart2 COLORIMETER MA Y DAMAGE THE
METER AND WILL VOID THE WARRANTY . Do not use the adapter
sold with the original SMART Colorimeter.
To use the adapter , slide the connector pin from the AC adapter into the small
hole on the left side of the meter. Plug the AC adapter into an appropriate wall
socket or power source.
SMART2 COLORIMETER 1.07 19
Page 20
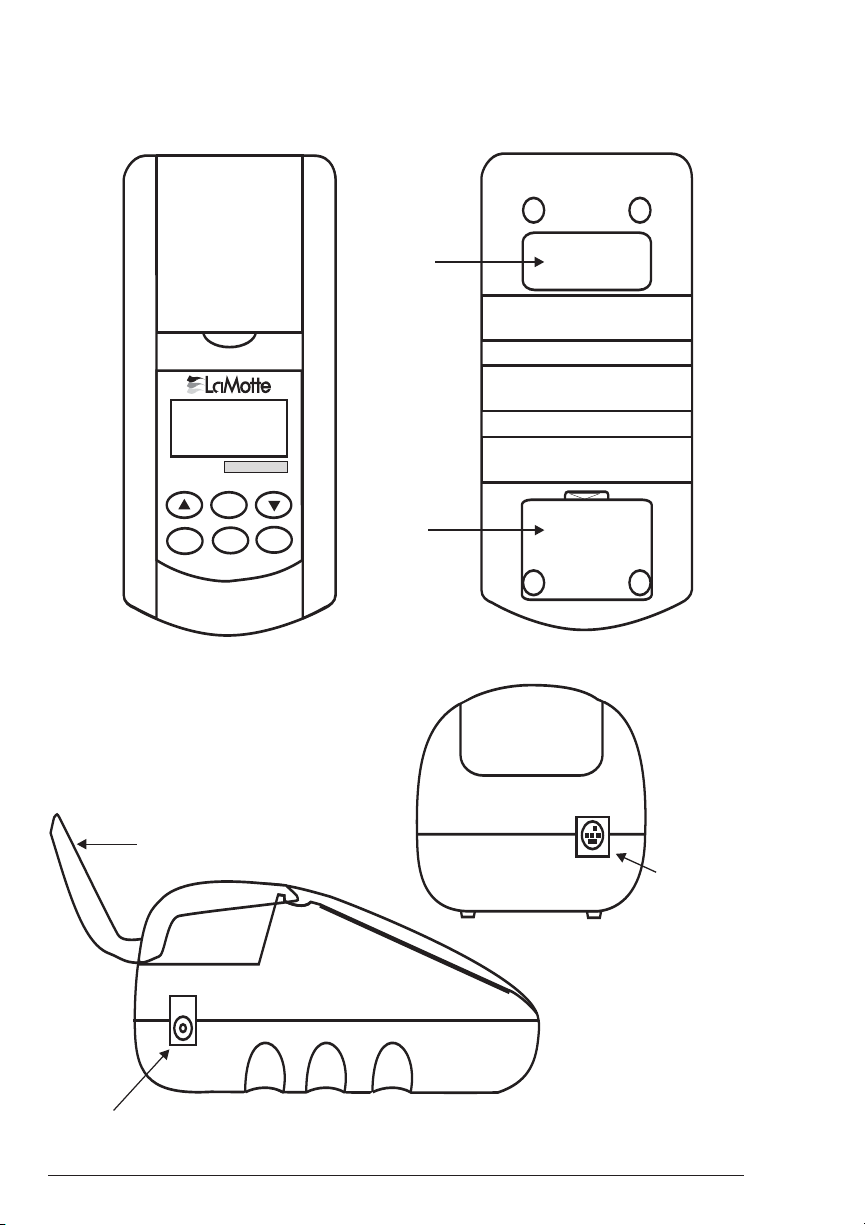
COMPONENTS
p
n
Figure 1 shows a diagram of the Smart2 Colorimeter and its components.
Top View
SMART2
••••••••••••••••••
*
ENTER
OFF EXIT
ON
Bottom View
Serial
Number
Battery
Compartment
Side Views
Lid
AC
ter Socket
Ada
20 SMART2 COLORIMETER 1.07
Figure 1
RS232
Serial Port
Page 21
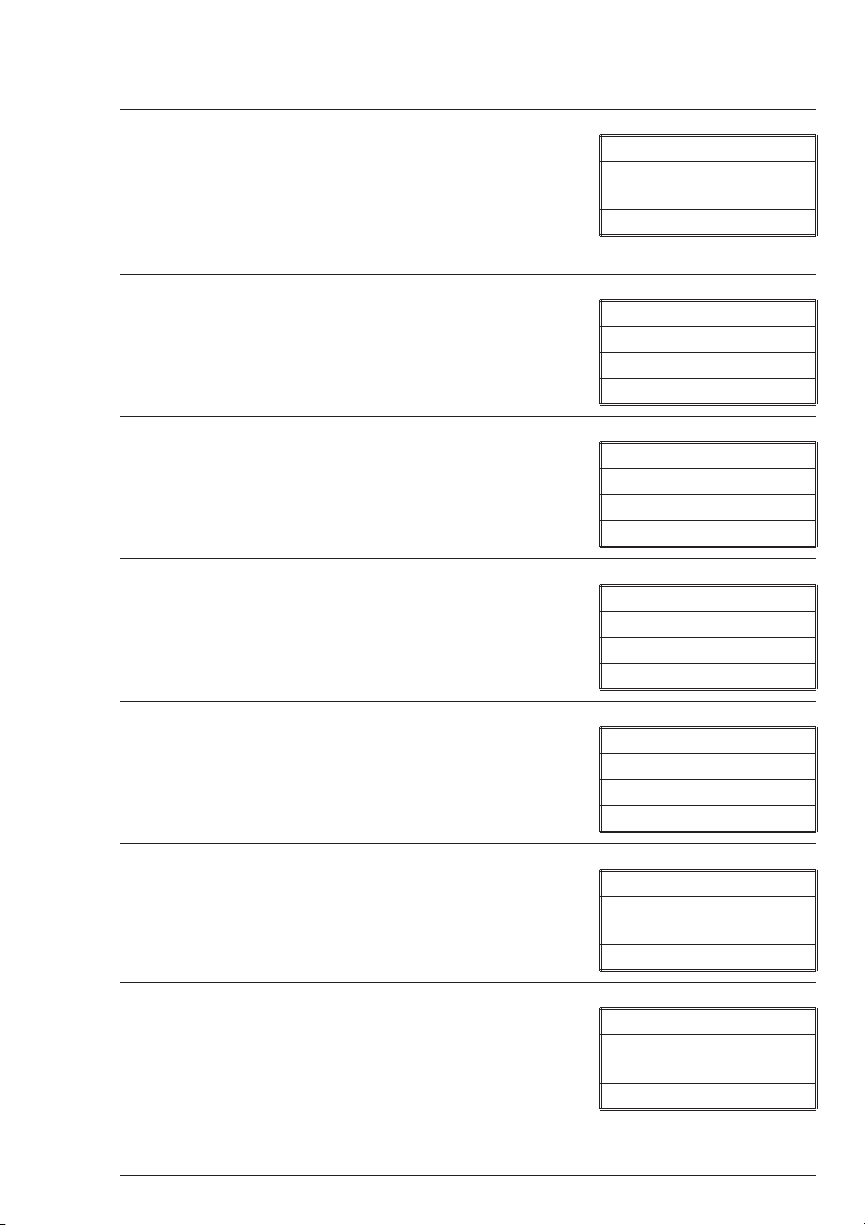
QUICK START
n
Some quick instructions to get into testing.
1. Press ON to turn on the SMART2. The
LaMotte logo screen will appear for about 2
seconds and then the Start screen appears. Press
Q/ENTER to start testing.
2. The Main Menu will appear. Press
Q/ENTER to select TESTING MENU.
3. Press Q/ENTER to select All Tests.
4. Press t or s to move the * to the desired
test.
VER 1.0
Smart2
* Start
MAIN MENU
* Testing Menu
Editing Menu
PC Link
TESTING MENU
* All Tests
Sequence 1
Sequence 2
ALL TESTS
* 001 Alk - UDV
002 Aluminum
003 Ammonia - NLF
5. Press Q/ENTER to select test.
6. Insert blank, press Q/ENTER to scan blank.
7. The screen will display Blank Done for
about 1 second.
SMART2 COLORIMETER 1.07 21
ALL TESTS
* 015 Chlorine
016 Cl F-UDV
017 Cl Liq-DPD
015 Chlorine
* Scan Blank
015 Chlorine
Blank Done
* Scan Blank
Page 22
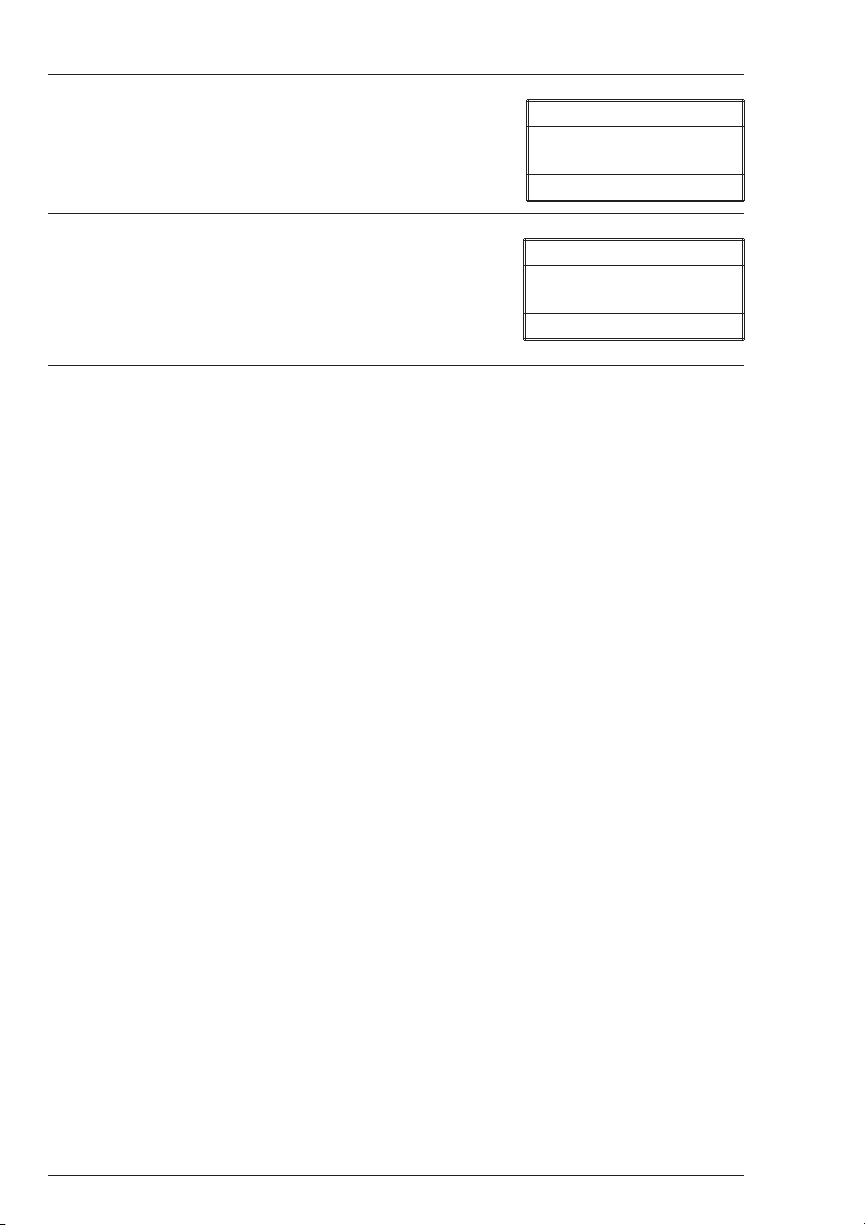
8. Insert the reacted sample. Press Q/ENTER to
scan sample. The SMART2 will scan the sample
and display the concentration.
015 Chlorine
* Scan Sample
9. After recording test result, scroll with t or s
and make another selection with Q/ENTER.
Press EXIT to escape to previous menus.
015 Chlorine
1.28 ppm
* Scan Sample
22 SMART2 COLORIMETER 1.07
Page 23

GENERAL OPERATING PROCEDURES
The operation of the SMART2 Colorimeter is controlled by a microprocessor.
The microprocessor is programmed with menu driven software. A menu is a list
of choices. This allows a selection of various tasks for the colorimeter to
perform, such as, scan blank, scan sample, and edit test sequences. The keypad
is used to make menu selections which are viewed in the display. There are
three selections accessible from the MAIN MENU: Testing Menu, Editing
Menu and PC Link.
THE KEYP AD
n
The keypad has 6 buttons which are used to perform specific tasks.
ON
t This button will cause the display to scroll down through a list of
s This button will cause the display to scroll up in a list of menu
ENTER
Q
EXIT
OFF
SAMPLE HOLDERS
n
The sample chamber is designed for 25 mm round tubes. Additional sample
holders for 16 mm COD tubes and for 1 cm square UDV cuvettes are available
for the SMART2 Colorimeter.
Position the COD adapter in the SMART2 chamber so that the grooves in the
adapter are aligned with the ridges located at the rear of the chamber. The
adapter should be inserted with the small hole, containing the ball plunger, at
the top. The ball plunger can be adjusted with a small screwdriver to control
the tightness of the fit of the tube in the adapter.
This button is used to turn the colorimeter on.
menu choices. It will move through a list viewed in the display. It
will auto scroll when held down.
choices. It will move through a list viewed in the display. It will
auto scroll when held down.
This button is used to select the menu choice adjacent to the “*”in
amenuviewedinthedisplay.
This button is an exit or escape button. When pressed, the display
will exit from the current menu and go to the previous menu.
This button turns the colorimeter off.
SMART2 COLORIMETER 1.07 23
Page 24
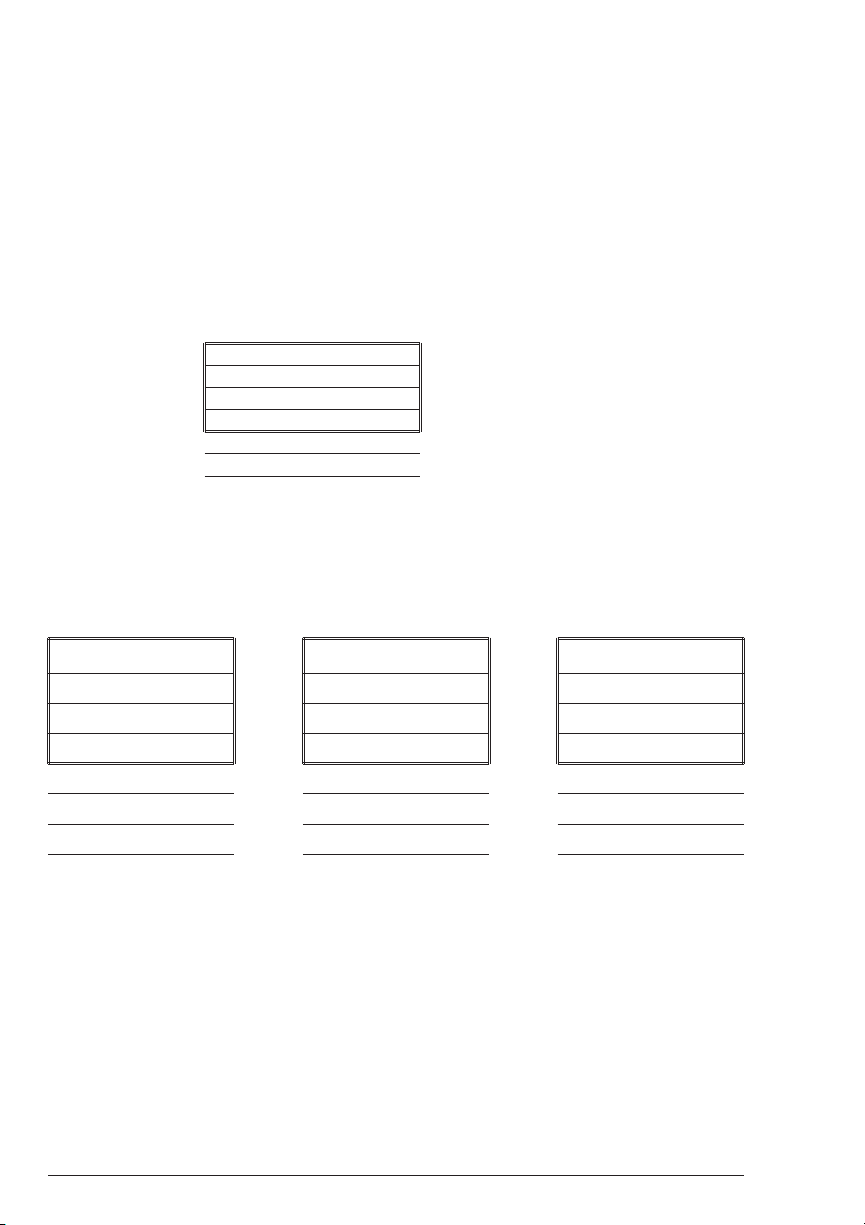
THE DISPLAY & THE MENUS
n
The display allows menu selections to be viewed and chosen. These choices
instruct the colorimeter to perform specific tasks. The menus are viewed in the
display using two general formats which are followed from one menu to the
next. Each menu is a list of choices or selections.
There are four lines in the display. The top line in each menu is a title or
pertinent instruction. The top line does not change unless a new menu is
selected. The second and third lines are used in two ways. One way is to display
menu choices. The second way takes advantage of the graphical capabilities of
the display. Both lines are used to display important messages, such as test
results, in a large, easy to read format. The fourth line is used for menu choices.
DISPLAY
TESTING MENU
* FIRST CHOICE
SECOND CHOICE
ANOTHER
AND ANOTHER
AND SO ON
TITLE or INSTRUCTION
MENU CHOICE WINDOW
Think of the menu choices as a vertical list in the display which moves up or
down each time an arrow button is pressed. This list or menu is viewed through
a window, the menu choice window, in the display. The menu choice window
is the lower 2 or 3 lines of the display. Pushing the arrow buttons brings
another portion of the menu into menu choice window. This is referred to as
scrolling through the menu.
TESTING MENU
* FIRST CHOICE SECOND CHOICE ANOTHER
SECOND CHOICE * ANOTHER AND ANOTHER
ANOTHER AND ANOTHER * AND SO ON
AND ANOTHER AND SO ON LAST CHOICE
AND SO ON LAST CHOICE
LAST CHOICE
t
TESTING MENU
t
TESTING MENU
An asterisk, “*”, will start in the far left position of the top line in the menu
choice window. As the menu is scrolled through, different choices appear next
to the “*”. The “*” in the display corresponds with the Q/ENTER button.
Pushing the Q/ENTER button selects the menu choice which is adjacent to
the “*” in the menu choice window.
24 SMART2 COLORIMETER 1.07
Page 25

The second general format of the display takes advantage of the graphics
capabilities of the display. The top line of the display is still a title line. The
middle two lines of the display are used to display important messages, results
or graphics in a large, easy to read format. The menus work in the same way as
described previously but only one line of the menu is visible at the bottom of
the display.
TESTING MENU
TESTING MENU
t
TESTING MENU
t
Result or Message Result or Message Result or Message
* ANOTHER * AND ANOTHER * AND SO ON
AND ANOTHER AND SO ON LAST CHOICE
AND SO ON LAST CHOICE
LAST CHOICE
As described previously, the EXIT button allows an exit or escape from the
current menu and a return to the previous menu. This allows a rapid exit from
an inner menu to the main menu by repeatedly pushing the
Pushing
OFF at any time will turn the colorimeter off.
EXIT button.
SMART2 COLORIMETER 1.07 25
Page 26
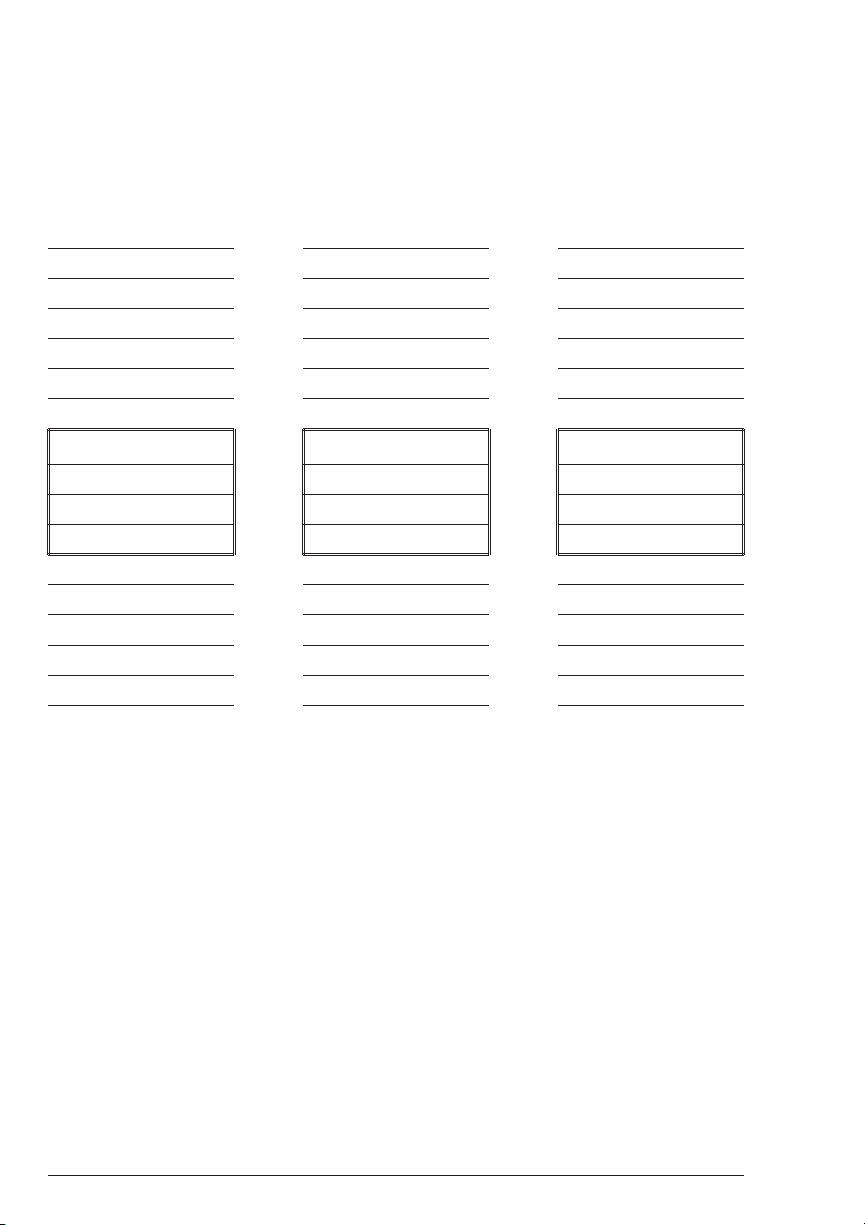
LOOPING MENUS
n
Long menus, such as All Tests, incorporate a looping feature which allow
the user to quickly reach the last choice in the menu from the first choice. In a
looping menu the last choices in the menu are above the first choice and
scrolling upward moves through the menu in reverse order. Scrolling downward
moves through the menu from first choice to last but the menu starts over
following the last choice. So all menu choices can be reached by scrolling in
either direction. The diagrams below demonstrate a looping menu.
AND SO ON AND ANOTHER ANOTHER
: : : AND SO ON AND ANOTHER
: : : : : : AND SON ON
THIRD TO LAST : : : : : :
SECOND TO LAST THIRD TO LAST : : :
LAST CHOICE SECOND TO LAST THIRD TO LAST
TESTING MENU
* FIRST CHOICE * LAST CHOICE * SECOND TO LAST
SECOND CHOICE FIRST CHOICE LAST CHOICE
ANOTHER SECOND CHOICE FIRST CHOICE
AND ANOTHER ANOTHER SECOND CHOICE
AND SO ON AND ANOTHER ANOTHER
: : : AND SO ON AND ANOTHER
: : : : : : AND SO ON
LAST CHOICE : : : : : :
TESTING MENU
s
TESTING MENU
s
26 SMART2 COLORIMETER 1.07
Page 27
TESTING
TESTING MENU
n
The Testing Menu is used to run all LaMotte pre-programmed tests, USER
TESTS and Absorbance tests at one of four wavelengths. Testing from any of
three sequences can also be done.
1. Press the ON button to turn on the
SMART2 Colorimeter. The LaMotte logo
will appear for about 2 seconds and the the
Start screen appears. Press the Q/ENTER
button to begin testing.
2. The MAIN MENU will appear. Press the
Q/ENTER button to select Testing Menu.
3. Scroll with the t or s buttons and make
a selection with the Q/ENTER button.
All Tests has all the available tests. The
three sequences have selected tests and
Absorbance has %T/ABS tests.
VER 1.0
Smart2
* Start
MAIN MENU
* Testing Menu
Editing Menu
PC Link
TESTING MENU
* All Tests
Sequence 1
Sequence 2
Sequence 3
Absorbance
SMART2 COLORIMETER 1.07 27
Page 28

SEQUENCES OF TESTS
n
SEQUENCE 1, SEQUENCE 2,andSEQUENCE 3 are alterable sequences.
They may be edited using the Editing Menu. Any of the LaMotte
pre-programmed tests or User Tests may be placed in these sequences in
whatever testing order that is preferred. Some examples of typical sequences
are given below.
SEQUENCE 1 SEQUENCE 2 SEQUENCE 3
* 015 Chlorine * 002 Aluminum * 003 Ammonia-N LF
079 Phosphate H 035 Cyanide 032 Cu-DDC
009 Bromine-LR 041 Fluoride 064 Nitrate-N L
076 pH TB 053 Iron Phen 067 Nitrite-N L
061 Moly-HR 055 Manganese L 074 pH CPR
086 Silica Hi 064 Nitrate-N L 078 Phosphate L
045 Hydrazine 067 Nitrite-N L 085 Silica Lo
032 Cu-DDC 077 Phenol
051 Iron Bipyr 078 Phosphate L
090 Sulfide-LR
These alterable sequences allow a series of tests to be setup that are run
frequently. The order of the individual tests in the sequence is determined by
the user. After running a test, use the * button to select the next test in the
sequence. Continue this pattern until the entire sequence has been completed.
All Tests is a fixed sequence containing the LaMotte pre-programmed tests,
User Tests, and Absorbance tests.
Modification of the alterable sequences is accomplished through the Editing
Menu. This menu is explained in greater detail in EDITING MENU (p. 32).
Pressing the EXIT button while in a sequence menu will escape back to the
Testing Menu.
Pressing the OFF button at any time will turn the colorimeter off.
28 SMART2 COLORIMETER 1.07
Page 29
GENERAL TESTING PROCEDURES
n
The following are some step by step examples of how to run tests from the
Testing Menu. These test procedures are designed to be used with LaMotte
SMART Reagent Systems.
LaMotte Company continuously updates the list of pre-programmed tests as the
calibrations become available. Pre-programmed calibrations can be added to
the SMART2 Colorimeter in the field. A Windows-based computer running a
Windows Operating System and an 8 pin mini-DIN/9 pin F D-submin serial
cable (order Code 1771) are required.
Call LaMotte Technical Services at 1-800-344-3100 (410-778-3100 outside
the USA) or email at tech@lamotte.com for a current list of available
calibrations and downloading instructions.
TESTING WITH THE LaMOTTE
n
PRE-PROGRAMMED TESTS
Press ON to turn on the Smart2 Colorimeter.
The LaMotte logo will appear for about 2 seconds
and then the Start screen appears. Press the
Q/ENTER button to start testing.
The MAIN MENU will appear. Press the
Q/ENTER button to select Testing Menu.
Press the Q/ENTER button to select All
Tests.
Press the t button to move to the 002
Aluminum to *.
VER 1.0
Smart2
* Start
MAIN MENU
* Testing Menu
Editing Menu
PC Link
TESTING MENU
* All Tests
Sequence 1
Sequence 2
ALL TESTS
* 001 Alk - UDV
002 Aluminum
003 Ammonia - NLF
SMART2 COLORIMETER 1.07 29
Page 30
Press the Q/ENTER button to select
002 Aluminum.
ALL TESTS
* 002 Aluminum
003 Ammonia - NLF
004 Ammonia - NLS
The SMART2 Colorimeter is ready to scan at the
correct wavelength. Place the blank in the sample
chamber, close the lid and press the Q/ENTER
button to scan blank.
NOTE: Do not keep the button depressed.
The screen will display Blank Done for about
1 second. Scan Sample will be positioned next
to *.
Place the reacted sample in the chamber, close
the lid and press the Q/ENTER button to scan
sample. The colorimeter will scan the sample and
the results screen will appear.
Record test result. To repeat the test, press the
Q/ENTER button to scan the sample again. The
last blank scanned is used to zero the colorimeter
for repeated scans. A different blank can be used
by pressing the s buttontoscrollbackto Scan
Blank and then scanning another blank. Scroll
with the t or s buttons and make another
selection with the Q/ENTER button. The %T or
Absorbance of the last test can be viewed by
choosing %T/Abs. Press the EXIT button to
escape to previous menus.
NOTE: The menus loop in this screen so either
the s or t buttons will lead to the menu
selection needed.
002 Aluminum
* Scan Blank
002 Aluminum
Blank Done
* Scan Blank
002 Aluminum
* Scan Sample
002 Aluminum
0.09 ppm
* Scan Sample
Next Test
Previous Test
%/Abs
Calibrate
Scan Blank
30 SMART2 COLORIMETER 1.07
Page 31

CALIBRATING LaMOTTE PRE-PROGRAMMED TESTS
n
The LaMotte Pre-Programmed Tests have been pre-calibrated. Recalibration of
the pre-programmed tests by the user is not possible. However, a procedure to
standardize the calibration can be performed to obtain the most accurate
readings or to meet regulatory requirements.
The LaMotte Pre-Programmed tests are standardized with one standard
solution. To standardize over the full range of the test, the concentration of the
standard should be chosen from the high end of the range. Alternatively, if
samples do not cover the full range of the test, a standard should be chosen
that is close to the concentration of the samples.
The standardization procedure should be followed as often as required by
regulations and laws for compliance monitoring.
In the example below the Aluminum calibration will be standardized.
Prepare a standard solution to be tested. Use 0.10 ppm aluminum.
Use the s or t button to scroll to
002 Aluminum. Follow instructions in the
SMART2 Manual for testing the aluminum
standard. Scan the blank.
The screen will display Blank Done for about
1 second. Scan Sample will be positioned next
to *.
Place the reacted sample in the chamber, close
the lid and press Q/ENTER to scan sample. The
result will be displayed.
The displayed result can now be standardized.
Use the s or t buttons to scroll to
Calibrate. Press Q/ENTER to select.
002 Aluminum
* Scan Blank
002 Aluminum
Blank Done
* Scan Sample
002 Aluminum
* Scan Sample
002 Aluminum
0.09 ppm
* Scan Sample
Next Test
Previous Test
%T/Abs
Calibrate
Scan Blank
SMART2 COLORIMETER 1.07 31
Page 32

A reverse font (dark background with light
characters) will appear to indicate that the
reading can be adjusted. Use s or t to scroll to
the concentration of the sample, 0.10 ppm in this
example.
002 Alumninum
0.09
* Calibrate
Set the calibration by pressing Q/ENTER to
select Calibrate.
Two menu choices will be offered, Set
Calibration and Factory Setting.Set
the calibration by pressing Q/ENTER to select
Set Calibration;oruses or t to scroll to
and select Factory Setting t o revert to the
factory calibration.
The meter will display the message “Storing”
and return to 002 Aluminum test.
The calibration for 002 Aluminum has now
been standardized and can be used for testing.
The standardization can be removed by repeating
the calibration and selecting Factory
Setting.
002 Aluminum
0.10
* Calibrate
002 Aluminum
0.10
* Set Calibration
Factory Setting
Storing
002 Aluminum
* Scan Sample
Next Test
Previous Test
%/Abs
Calibrate
Scan Blank
32 SMART2 COLORIMETER 1.07
Page 33

MEASURING IN THE ABSORBANCE MODE
n
Press ON to turn on the SMART2 Colorimeter.
The LaMotte logo will appear for about 2 seconds
and then the Start screen appears. Press the
Q/ENTER button to start testing.
The MAIN MENU will appear. Press the
Q/ENTER button to select Testing Menu.
Press the t button to scroll to Absorbance.
Press the Q/ENTER button to select
Absorbance.
VER 1.0
Smart2
* Start
MAIN MENU
* Testing Menu
Editing Menu
PC Link
TESTING MENU
All Tests
Sequence 1
Sequence 2
Sequence 3
* Absorbance
TESTING MENU
* Absorbance
Press the tor s buttons to move to the desired
test.
Press the Q/ENTER button to select test.
SMART2 COLORIMETER 1.07 33
Absorbance
* 101 Abs 430
102 Abs 520
103 Abs 570
104 Abs 620
Absorbance
* 102 Abs 520
103 Abs 570
104 Abs 620
Page 34

Insert blank, press the Q/ENTER button to scan
blank.
102 Abs 520
* Scan Blank
The screen will display Blank Done for about
1 second.
Insert the reacted sample. Press the Q/ENTER
button to scan the sample.
Record test result. To repeat the test, press the
Q/ENTER button to scan the sample again. The
last blank scanned is used to zero the colorimeter
for repeated scans. A different blank can be used
by pressing the s buttontoscrollbackto Scan
Blank and then scanning another blank. Scroll
with t or s and make another selection with
Q/ENTER. The %T or Absorbance of the last
test can be viewed by choosing %T/Abs. Press
EXIT to escape to previous menus.
NOTE: The menus loop in this screen so either
t or s will lead to the menu selection needed.
NOTE: The Calibrate function does not work in
the Absorbance mode.
102 Abs 520
Blank Done
* Scan Blank
102 Abs 520
* Scan Sample
102 Abs 520
0.95
* Scan Sample
Next Test
Previous Test
%T/Abs
Calibrate
Scan Blank
34 SMART2 COLORIMETER 1.07
Page 35

EDITING MENU
The EDITING MENU allows the user to edit sequences, edit user tests, set the
clock, edit the logging function, and set the power saving function.
EDIT A SEQUENCE
n
The EDIT SEQUENCE menu allows three alterable test sequences (SEQUENCE
1, SEQUENCE 2,andSEQUENCE 3) to be edited.
Press ON to turn on the SMART2 Colorimeter.
The LaMotte logo will appear for about 2 seconds
and then the Start screen appears. Press the
Q/ENTER button to start testing.
The Main Menu will appear. Press the t
button to scroll to Editing Menu.
Press the Q/ENTER button to select Editing
Menu.
The Editing Menu appears. Press the
Q/ENTER button to select
Editing Sequence.
VER 1.0
Smart2
*START
MAIN MENU
* Testing Menu
Editing Menu
PC Link
MAIN MENU
*Editing Menu
PC Link
EDITING MENU
* Edit Sequence
Edit User Test
Set Clock
The Edit Sequence menu appears. Press the
Q/ENTER button to scroll to select Edit
Sequence 1.
SMART2 COLORIMETER 1.07 35
EDIT SEQUENCE
*Edit Sequence 1
Edit Sequence 2
Edit Sequence 3
Page 36

Sequence 1 appears.
ADDING OR DELETING TESTS
n
EDIT SEQUENCE 1
*015 Chlorine
079 Phosphate H
009 Bromine-LR
There are three ways to alter a sequence: Insert Before, Insert After,
and Delete. Insert Before adds a new test to the sequence before the
selected test. Insert After adds a new test to the sequence after the selected
test. Delete is used to remove an existing test from a sequence.
Below is a step by step example of how to add a test to SEQUENCE 1 starting
from the EDIT SEQUENCE 1 menu.
Press the t button to scroll to 009
Bromine-LR.
Press the Q/ENTER button to select 009
Bromine-LR.
Press the Q/ENTER button to select Insert
Before.
The ALL TESTS menu appears. Press the t
button to move the 002 Aluminum to *.
EDIT SEQUENCE 1
015 Chlorine
079 Phosphate H
* 009 Bromine-LR
EDIT SEQUENCE 1
* 009 Bromine-LR
076 pH TB
060 Moly-LR
EDIT SEQUENCE 1
* Insert Before
Insert After
Delete
ALL TESTS
* 002 Aluminum
003 Ammonia-N LF
004 Ammonia-N LS
Continued...
36 SMART2 COLORIMETER 1.07
Page 37

Press the Q/ENTER button to select
002 Aluminum.
ALL TESTS
* 002 Aluminum
003 Ammonia-N LF
004 Ammonia-N LS
Sequence 1 appears in EDIT SEQUENCE 1
menu and 002 Aluminum is now before
Bromine-LR in the sequence. All changes to
Sequence 1 are automatically saved. Press the
EXIT button to exit the EDIT SEQUENCE 1
menu and return to the EDIT SEQUENCE menu
or continue editing.
The EDIT SEQUENCE menu appears. Select
another sequence to edit or press the EXIT
button to return to the EDITING MENU. Press
the EXIT button again to return the the MAIN
EDIT SEQUENCE 1
* 015 Chlorine
079 Phosphate H
002 Aluminum
009 Bromine-LR
076 pH TB
060 Moly-LR
EDIT SEQUENCE 1
* Edit Sequence 1
Edit Sequence 2
Edit Sequence 3
MENU.
Below is a step by step example of how to delete a test from SEQUENCE
1 starting from the EDIT SEQUENCE 1 menu. The test 002
Aluminum, added in the previous example, will be deleted.
Press the t button to scroll to 002 Aluminum.
EDIT SEQUENCE 1
* 015 Chlorine
079 Phosphate H
002 Aluminum
009 Bromine-LR
076 pH TB
060 Moly-LR
Press the Q/ENTER button to select
002 Aluminum.
SMART2 COLORIMETER 1.07 37
EDIT SEQUENCE 1
* 002 Aluminum
009 Bromine-LR
076 pH TB
Page 38

Press the t button to scroll to Delete.
EDIT SEQUENCE 1
* Insert Before
Insert After
Delete
Press the Q/ENTER button to select Delete.
Sequence 1 appears in the EDIT SEQUENCE 1
menu and 002 Aluminum has been deleted.
All changes to SEQUENCE 1 are automatically
saved.
Press the EXIT button to exit the EDIT
SEQUENCE 1 menu and return to the EDIT
SEQUENCE menu or continue editing.
The EDIT SEQUENCE menu appears. Select
another sequence to edit or press the EXIT
button to return to the EDITING MENU. Press
the EXIT button again to return the MAIN
MENU.
EDIT SEQUENCE 1
* Delete
EDIT SEQUENCE 1
* 015 Chlorine
079 Phosphate H
009 Bromine-LR
076 pH TB
060 Moly-LR
EDIT SEQUENCE 1
* Edit Sequence 1
Edit Sequence 2
Edit Sequence 3
38 SMART2 COLORIMETER 1.07
Page 39

EDIT USER TESTS
n
If a test other than the LaMotte programmed tests is performed regularly, a
calibration for it may be entered in one of the 10 User Tests. These tests are
originally named “User Test1-10". It will be possible to rename the test,
select a wavelength, enter a new calibration, select the number of decimal
places used to display the results, and select the units. A User Test may be
added for a reagent system for which no precalibrated test exists. A calibration
of a LaMotte reagent system may also be entered. The calibration of a User
Test can be changed at any time.
The User Tests have the ability to handle 2 data points. The colorimeter will
determine the absorbance of the standards and calculate a response that will be
stored to determine the concentration of future samples of unknown
concentration. These standards should cover all the concentrations for the
range of the test being performed and be scanned beginning with the low
concentration and finishing with the high concentration (for more
information about this, see CALIBRATION CURVES, page 13). Prepare these
solutions prior to entering a new calibration.
NOTE: A calibration procedure must be performed before using any of the
User Tests.
The User Tests can be placed in any of the alterable sequences using EDIT
SEQUENCES.
To edit a User Test, start at the EDITING
MENU. Scroll down to Edit User Test.
Press the Q/ENTER button to select the Edit
User Test.
From the EDIT USER TEST menu, select the
User Test to be entered or changed. In this
example, choose 105 User Test 01. Use the
t and s buttons to scroll to other User Tests if
desired. Select the User Test by pressing the
Q/ENTER button.
EDITING MENU
* Edit Sequences
Edit User Test
Set Clock
EDITING MENU
* Edit User Test
Set Clock
Edit Logging
EDIT USER TEST
* 105 User Test01
106 User Test02
107 User Test03
108 User Test04
:::
114 User Test10
SMART2 COLORIMETER 1.07 39
Page 40

NAMING THE TEST
n
A User Test can be up to 11 characters long. The menu choices for each
character are 26 upper case letters A to Z, 26 lower case letters a to z, ten
numerals 0 to 9, a space (SP), a dash (-) and a decimal point (.). The existing
name is displayed on the bottom line of the display. A cursor will be over the
character which is to be edited and that character is also displayed in the
center of the display. The character can be changed by using the t and s
buttons to scroll to other characters. Use the Q/ENTER button to select a
character. The edited name is saved at any time by pressing EXIT or by
pressing the Q/ENTER button after selecting the eleventh character.
From the Edit User Test01 menu press the
Q/ENTER button to select Name The Test
and change the name of UserTest01.
The cursor is over the letter “U”in 105 User
Test01 and the letter “U” is displayed in the
large font in the center of the display.
Change the name to H2O. Use the t and s
buttons to scroll to the letter “H” into the center
of the display . Press the Q/ENTER button to
select the letter “H”.
The letter “H” has been entered in the first
position of the name and the cursor has moved to
the second letter “s”.
EDIT USER TEST01
* Name The Test
Select Vial/WL
New Calibration
Decimal Places
Select Units
NAME THE TEST
U
105 User Test01
NAME THE TEST
H
105 User Test01
NAME THE TEST
s
105 User Test01
Use the t and s buttons to scroll to the number
“2” into the center of the display. Press the
Q/ENTER button to select the number “2”.
NAME THE TEST
2
105 Hser Test01
Continued...
40 SMART2 COLORIMETER 1.07
Page 41

The number “2” has been entered in the second
position of the name and the cursor has moved to
the third letter “e”.
NAME THE TEST
e
105 H2er Test01
Use the t and s buttons to scroll to the letter
“O” into the center of the display. Press the
Q/ENTER button to select the letter “O”.
The letter “O” has been entered in the third
position of the name and the cursor has moved to
the fourth letter “r”. Press the EXIT button to
save the name entered up to this point.
The meter will display the message “Storing”
and return to the EDIT USER TEST01 menu.
NAME THE TEST
O
105 H2Or Test01
NAME THE TEST
r
* 105 H2Or Test01
Storing
EDIT USER TEST01
* Name The Test
Select The Vial/WL
New Calibration
Decimal Places
Select Units
SMART2 COLORIMETER 1.07 41
Page 42

SELECTING THE VIAL AND WAVELENGTH
n
The Smart2 Colorimeter has three different vials (the 25 mm 0290 tube,
UDVs and COD tubes) and 4 different wavelengths (430, 520, 570, and
620 nm). The colorimeter uses different settings for each of the twelve
combinations of vial and wavelength. These twelve settings are called
channels. Choose the channel with the correct wavelength and vial for the
test.
Use the t button to scroll to Select
Vial/WL and press Q/ENTER button to select.
Use the t and s buttons to scroll to the
appropriate channel and press Q/ENTER button
to select.
NOTE: This is a looping menu.
The meter will display the message “Storing”
and return to the EDIT USER TEST01 menu.
EDIT USER TEST01
* Name The Test
Select Vial/WL
New Calibration
Decimal Places
Select Units
:::
Ch11 620nm COD
Ch12 570nm COD
SELECT CHANNEL
* Ch1 520nm 25mm
Ch2 430nm 25mm
Ch3 620nm 25mm
Ch4 570nm 25mm
Ch5 520nm UDV
Ch6 430nm UDV
:::
Storing
EDIT USER TEST01
* Select The Vial/WL
New Calibration
Decimal Places
Select Units
42 SMART2 COLORIMETER 1.07
Page 43

ENTERING A NEW CALIBRATION
n
To enter a new calibration two reacted standards solutions of known
concentration are required: a “low standard” and a “high standard”. These
shouldbereadytouse.
Use the t button to scroll to New
Calibration and press Q/ENTER button to
select.
Input the concentration of the LOW STANDARD
by using the t and s buttons to scroll the first
digit of the concentration into the first position
on the display . Press Q/ENTER button to select
that digit (1 for this example).
The number “0” is always the starting point for
the next digit. Continue selecting digits or a
decimal point to enter the concentration (up to
seven characters).
“1.5” has been entered in this example. Press
Q/ENTER button four times to input “0” as the
last four digits. Pressing Q/ENTER after selecting
the last digit saves the concentration.
EDIT USER TEST01
* Select Vial/WL
New Calibration
Decimal Places
Select Units
LOW STANDARD
0______
* Continue
LOW STANDARD
10_____
* Continue
LOW STANDARD
1.50___
* Continue
Input the concentration of the HIGH STANDARD
by using the same method as for the low standard.
SMART2 COLORIMETER 1.07 43
HIGH STANDARD
0______
* Continue
Page 44

Place a clear blank in the sample chamber. Press
the Q/ENTER button to scan the blank.
The screen will display Blank Done for about
1 second.
Insert Blank
* Continue
Blank Done
* Scan Blank
Place the reacted low standard in the sample
chamber. Press Q/ENTER to scan the low
standard.
Place the reacted high standard in the sample
chamber. Press Q/ENTER to scan the high
standard.
The meter will display the message “Storing”
and return to the EDIT USER TEST01 menu.
Insert Lo Standard
* Continue
Insert Hi Standard
* Continue
Storing
EDIT USER TEST01
* New Calibration
Decimal Places
Select Units
44 SMART2 COLORIMETER 1.07
Page 45

SELECTING THE NUMERICAL FORMA T OF THE RESULT
n
To input tests with very different ranges, the number of decimal places
displayed for a result can be selected. A test which ranges from 20 to 1000 ppm
should not be displayed with three decimal places. A test with a range from
0.010 to 0.500 needs three decimal places (the microprocessor will always
calculate the concentration to many more significant figures than will be
displayed). Menu choices of 0, 1, 2, or 3 decimal places will be given for the
display.
Use the t button to scroll to Decimal
Places and press Q/ENTER button to select.
Use the t button to scroll to the number of
decimal places to be shown and press Q/ENTER
to select.
The meter will display the message “Storing”
and return to the EDIT USER TEST01 menu.
EDIT USER TEST01
* New Calibration
Decimal Places
Select Units
DECIMAL PLACES?
* None 0
One 0.0
Two 0.00
Three 0.000
Storing
EDIT USER TEST01
* Decimal Places
Select Units
SMART2 COLORIMETER 1.07 45
Page 46

SELECTING THE UNITS OF CONCENTRATION
n
The SMART2 Colorimeter has seven options for units of concentration. They
are No Units, ppm, pH, FTU, ppb, ppt and mgL.
Use the t button to scroll to Select Units
and press Q/ENTER to select.
Use the t button to scroll to the appropriate unit
and press Q/ENTER to select.
The meter will display the message “Storing”
and return to the EDIT USER TEST01 menu.
EDIT USER TEST01
* Decimal Places
Select Units
SELECT UNITS
* No Units
ppm
pH
FTU
ppb
ppt
mgL
Storing
EDIT USER TEST01
* Select Units
46 SMART2 COLORIMETER 1.07
Page 47

SETTING THE CLOCK
n
Setting the clock allows the correct time and date stamp to be stored with each
reading in the data logger and with each reading sent out the serial port.
From the EDITING MENU use the t button to
scroll to Set Clock. Press Q/ENTER to select.
The current date and time are displayed as
month - day - year on the first line and as hours :
minutes : seconds on the second line. A
two-digit number is displayed for each setting.
Use the t and s buttons to scroll to the
appropriate number and press Q/ENTER to
select. The cursor will move to the next digit. Set
all subsequent numbers in the same manner.
Selecting the final digit in the seconds field stores
the date and time and returns to the
EDITING MENU.
NOTE: These are looping menus.
EDITING MENU
* Edit Sequences
Edit User Test
Set Clock
Editing Logging
Factory Setup
Set PWR Save
SET TIME
MM-DD-YY
HH:MM:SS
EDITING MENU
* Set Clock
Editing Logging
Factory Setup
Set PWR Save
SMART2 COLORIMETER 1.07 47
Page 48

TURNING THE DATA LOGGER ON AND OFF
n
The default setting for the datalogger is “Enabled” or turned off. If there is no
need for data logging, this setting is suggested. If data logging is needed, the
data logger can be “Enabled” or turned on.
From the EDITING MENU use the t button
to scroll to Edit Logging.Press
Q/ENTER
to select.
The current setting is always displayed next to
the *. To change the setting, use the t or s
buttons to scroll to the other setting. Press
Q/ENTER to select.
The meter will display the message “Storing”
and return to the EDITING MENU.
EDITING MENU
* Edit Sequences
Edit User Test
Set Clock
Edit Logging
Factory Setup
Set PWR Save
EDIT LOGGING
* Enabled
Disabled
Storing
EDITING MENU
*Edit Logging
Factory Setup
Set PWR Save
48 SMART2 COLORIMETER 1.07
Page 49

FACTORY SETUP
n
The Factory Setup menu is used in the manufacturing of the SMAR T2
Colorimeter. This menu is not for use by the operator in the field.
SETTING THE POWER SAVING FUNCTION
n
The SMART2 Colorimeter has a power saving function that turns the meter
off after an interval of inactivity. If no buttons have been pressed during that
interval the meter will turn itself off. This interval can be disabled or set for 5,
15, 30 or 60 minutes. The default setting is 5 minutes.
From the EDITING MENU use the t button to
scroll to Set PWR Save. Press Q/ENTER to
select.
The current setting is always displayed next to
the *. To change the setting, use the t or s
buttons to scroll to the appropriate setting. Press
Q/ENTER to select.
The meter will display the message “Storing”
and return to the EDITING MENU.
EDITING MENU
* Edit Sequences
Edit User Test
Set Clock
Edit Logging
Factory Setup
Set PWR Save
Disabled
AUTO SHUTOFF
* 5 Minutes
15 Minutes
30 Minutes
60 Minutes
Storing
EDITING MENU
* Set PWR Save
SMART2 COLORIMETER 1.07 49
Page 50

PC LINK
The SMART2 Colorimeter may be interfaced with any Windows-based
computer by using the LaMotte SMARTLink2 Program and Interface Cable
(Order Code 1912-3 [3.5 disk] or 1912-CD [compact disk]). The program
stores customer information and test data in a database. It can be used to
download data stored in the Smart2 datalogger for each test site.
The colorimeter may also be interfaced with an RS-232 serial printer, using an
interface cable (Order Code 1772) and setting the printer configuration to the
Output as described below.
Choose PC Link from the Main Menu. The user can download the entire
datalogging buffer. Downloading does not delete or empty the datalogger.
OUTPUT
n
RS-232 compatible, asynchronous serial, 9600 baud, no parity, 8 data bits, 1
stop bit.
COMPUTER CONNECTION
n
RS-232 interface connection, 8 pin mini-DIN/9 pin F D-submin. (Order Code
1772).
BA TTERY OPERATION
The colorimeter may be run on battery power or AC using the AC adapter. If
using the meter as a benchtop unit, keep it plugged in if possible. If used on
only battery power, always have a spare battery on hand.
If the battery power is low, the SMART2 will
display “LOW BATT” and turn off.
REPLACING THE BATTERY
n
The SMART2 Colorimeter uses a standard 9-volt alkaline battery that is
available worldwide. The battery compartment is located on the bottom of the
the case.
To replace the battery:
1. Open the battery compartment lid.
2. Remove the battery and disconnect the battery from the polarized plug.
3. Carefully connect the new battery to the polarized plug and insert it into
the compartment.
4. Close the battery compartment lid.
50 SMART2 COLORIMETER 1.07
LOW BATT
Page 51

MAINTENANCE
CLEANING
n
Clean with a damp, lint-free cloth.
DO NOT ALLOW WATER TO ENTER THE COLORIMETER
CHAMBER OR ANY OTHER PAR TS OF THE METER.
METER CARE
n
The optical system of the SMART2 must be kept clean and dry for optimal
performance. Dry the colorimeter tubes before placing them in the chamber to
avoid introducing moisture. For best results store the instrument in an area that
is dry and free from aggressive chemical vapors.
METER DISPOSAL
n
Waste Electrical and Electronic Equipment (WEEE)
Natural resources were used in the production of this equipment. This
equipment may contain materials that are hazardous to health and the
environment. To avoid harm to the environment and natural resources, the use
of appropriate take-back systems is recommended. The crossed out wheeled bin
symbol on the meter encourages you to use these systems when disposing of
this equipment.
Take-back systems will allow the materials to be reused or recycled in a way
that will not harm the environment. For more information on approved
collection, reuse, and recycling systems contact your local or regional waste
administration or recycling service.
SMART2 COLORIMETER 1.07 51
Page 52

TROUBLESHOOTING GUIDE
ERROR MESSAGES
n
n
OVER RANGE
If the message OVERRANGE is displayed when scanning a sample, the sample
may be over range or under range. If the sample is over range the sample should
be diluted and tested again (see Sample Dilution Techniques and Volumetric
Measurements, page 16).
If OVERRANGE is displayed, press the Q/ENTER
button to continue testing on diluted samples.
n
BLANK
If the message Blank? is displayed when scanning a sample, the sample had a
lower reading than the blank. Review test procedure to determine whether a
reagent blank is required.Visually check for color development in reacted
sample. Repeat test if necessary.
If Blank? is displayed, press the Q/ENTER
button to continue. Check to see if the meter
was blanked properly.
CALIBRATION
n
As with all pre-calibrated meters, it is highly recommended, even if not
required by regulations, that the user periodically verify the performance of the
meter by running standards with a predetermined concentration. Results
outside of specification are an indication that the meter needs to be adjusted.
This can be done following the user calibration described on page 31. If the
user calibration fails to properly adjust the meter then the meter should be
returned to LaMotte Company for recalibration. (See page 5).
015 Chlorine
OVERRANGE
* Continue
002 Aluminum
Blank?
* Continue
52 SMART2 COLORIMETER 1.07
Page 53

HELPFUL HINTS
n
n
STRAY LIGHT
The SMART2 Colorimeter should have no problems with stray light. Make
sure that the sample compartment lid is always fully closed, except when
testing COD with the adapter.
SMART2 COLORIMETER 1.07 53
Page 54

54 SMART2 COLORIMETER 1.07
Page 55

•
SMART
••••••••••••••••••••••••••••••••••••••••••••••
REAGENT SYSTEMS
INSTRUCTIONS
2
Colorimeter
TEST
v.2.4 • Printed 08.10
1919-test
Page 56

Page 57

SMART2 COLORIMETER
REAGENT SYSTEMS
SMART2 REAGENT SYSTEMS LIST
LaMotte Company continuously updates the list of pre-programmed tests as the
calibrations become available. Pre-programmed calibrations can be added to
the Smart2 Colorimeter in the field. A Windows-based computer running a
Windows Operating System and an 8 pin mini-DIN/9 pin F D-submin serial
cable (order Code 1771) are required.
Call LaMotte Technical Services at 1-800-344-3100 (410-778-3100 outside
the USA) or email at tech@lamotte.com for a current list of available
calibrations and downloading instructions.
Test Factor (Test #)
Alkalinity-UDV (1) 0–200 Unit Dose Vials (1) 50
Aluminum (2) 0.00–0.30 Eriochrome Cyanine R (4) 50
Ammonia NitrogenLow Range, Fresh Water (3)
Ammonia NitrogenLow Range, Salt Water (4)
Ammonia NitrogenHigh Range (5)
Benzotrizole (10) 0.0–30.0 UV Photolysis (3) 50
Biguanide (7) 0–70 Colorimetric 50
Boron (8) 0.00–0.80 Azomethine-H (2) 25
Bromine-Low Range (9)
See Chlorine-Bromine-Iodine
Bromine-UDV (11) 0.0–22.0 DPD (1) 50
Cadmium (12) 0.00–1.00 PAN (4) 50
Ca & Mg Hardness-UDV (13) 0–400 Unit Dose Vials (1) 50
Carbohydrazide (14)
See Oxygen Scavengers
Chloride-TesTab (21) 0.0–30.0 Argentometric (1) 50
Chlorine (15) 0.00–4.00 DPD (3) 100
Chlorine-Free-UDV (16) 0.00–10.00 DPD (1) 50
Chlorine-Liquid DPD (17) 0.00–4.00 DPD (3) 144
Chlorine-Total-UDV (18) 0.00–10.00 DPD (1) 50
Chlorine Dioxide (20) 0.00–8.00 DPD (2) 100
Chromium (22) 0.00–1.00 Diphenylcarbohydrazide (1) or (5) 100
Chromium-TesTab (23) 0.00–1.00 Diphenylcarbohydrazide (1) 50
Range
(ppm) Test Method (# of Reagents)
0.00–1.00 Salicylate (3) 25
0.00–1.00 Salicylate (3) 25
0.00–4.00 Nesslerization (2) 50
0.00–9.00 DPD (3) 100
0.000–0.900 Iron Reduction (3) 100
#of
Tests
Page 58

Test Factor (Test #)
Range
(ppm) Test Method (# of Reagents)
#of
Tests
Cobalt (24) 0.00–2.00 PAN (3) 50
COD-Low Range (25) 5–150 Digestion (1) 25
COD-Standard Range (26) 0-1500 Digestion (1) 25
COD-High Range (27) 0–15000 Digestion (1) 25
Color (28) 0–1000 Platinum Cobalt (0) ¥
Copper-BCA-Low Range (29) 0.00–3.50 Bicinchoninic Acid (1) 50
Copper-Cuprizone (31) 0.00–2.00 Cuprizone (2) 50
Copper-DDC (32) 0.00–6.00 Diethyldithiocarbamate (1) 50
Copper-UDV (33) 0.0–4.0 Bicinchoninic Acid (1) 50
Cyanide (35) 0.00–0.50 Pyridine-Barbituric Acid (5) 50
Cyanuric Acid (36) 5–200 Melamine (1) 50
Cyanuric Acid-UDV (37) 5–150 Melamine (1) 50
DEHA (38)
0.000–0.700 Iron Reduction (3) 100
See Oxygen Scavengers
Dissolved Oxygen (39) 0.0–11.0 Winkler Colorimetric (3) 100
Erythorbic Acid (40)
0.00–3.00 Iron Reduction (3) 100
See Oxygen Scavengers
Fluoride (41) 0.00–2.00 SPADNS (2) 50
Hardness (Total) UDV (13) 15-400 U nit dose Vial (1) 50
Hydrazine (45) 0.00–1.00 P-dimethylaminobenzaldehyde (2) 50
Hydrogen Peroxide-
0.00–1.50 DPD (2) 100
Low Range (46)
Hydrogen Peroxide-
0–60 DPD (2) 50
High Range (47)
Hydrogen Peroxide-Shock (48) 0–225 DPD (2) 100
Hydroquinone (49)
0.00–2.00 Iron Reduction (3) 100
See Oxygen Scavengers
Iodine (50)
0.00–14.00 DPD (2) 100
See Chlorine-Bromine-Iodine
Iron-Bipyridyl (51) 0.00–6.00 Bipyridyl (2) 50
Iron-UDV (52) 0.00–10.00 Bipyridyl (1) 50
Iron-Phenanthroline (53) 0.00–5.00 1,10 Phenanthroline (2) 50
Lead (54) 0.00–5.00 PAR (5) 50
Manganese-Low Range (55) 0.00–0.70 PAN (3) 50
Manganese-High Range (56) 0.0–15.0 Periodate (2) 50
Mercury (57) 0.00–1.50 TMK (3) 50
Page 59

Test Factor (Test #)
Methylethylketoxime (58)
See Oxygen Scavengers
Molybdenum-High Range (61) 0.0–50.0 Thioglycolate (3) 50
Nickel (63) 0.00–8.00 Dimethylglyoxime (6) 50
Nitrate Nitrogen-Low Range (64) 0.00–3.00 Cadmium Reduction (2) 20
Nitrate-TesTab (66) 0.0–60.0 Zinc Reduction (1) 50
Nitrite Nitrogen-Low Range (67) 0.00–0.80 Diazotization (2) 20
Nitrite-TesTab (69) 0.00–1.25 Diazotization (1) 50
Nitrogen, Total (70) 0–25 mg/L Chromotropic Acid/Digestion (6) 25
Oxygen Scanvengers various DEHA (3) 50
Ozone-DPD (73) 0.00–3.00 DPD (3) 144
Ozone-Low Range (71) 0.00–0.40 Indigo Trisulfonate (3) 100
Ozone-High Range (72) 0.00–2.50 Indigo Trisulfonate (3) 20
pH-Chlorophenol Red (74) 5.0–6.8 Chlorophenol Red (1) 10 0
pH-Phenol Red (75) 6.6–8.4 Phenol Red (1) 100
pH-Thymol Blue (76) 8.0–9.6 Thymol Blue (1) 100
Phenol (77) 0.00–6.00 Aminoantipyrine (3) 50
Phosphate-Low Range (78) 0.00–3.00 Ascorbic Acid Reduction (2) 50
Phosphate-High Range (79) 0.0–70.0 Vanodomolybdphosphoric Acid (1) 50
Phosphorus, Total Low-Range (82) 0.00–3.50 mg/L Ascorbic Acid/Digestion (5) 25
Phosphorus, Total High-Range (83) 0.0–100.0 mg/L Molybdovanadate/Digestion (5) 25
Potassium (81) 0.0–10.0 Tetraphenylboron (2) 100
Silica-Low Range (85) 0.0–4.0 Heteropoly Blue (4) 50
Silica-High Range (86) 0–75 Silicomolybdate (3) 50
Sulfate-High Range (89) 5–100 Barium Chloride (1) 50
Sulfide-Low Range (90) 0.00–1.50 Methylene Blue (3) 50
Surfactants (94) 0.0–8.0 Bromphenol Blue (3) 50
Tannin (96) 0.0–10.0 Tungsto-molybdophosphoric Acid (2) 50
Tolytriazole (97)
See Benzotriazole
Turbidity (98) 0–400 FTU Absorption (0) ¥
Zinc-Low Range (99) 0.00–3.00 Zincon (6) 50
Range
(ppm) Test Method (# of Reagents)
0.00–3.00 Iron Reduction (3) 100
0–30 UV Photolysis (3) 50
#of
Tests
On the meter display, “NA” following the test number indicates that a
calibrationforthattestnumberisnotavailable.
Page 60

Page 61

ALKALINITY–UDV
UNIT DOSE VIALS • CODE 4318-H
QUANTITY CONTENTS CODE
1 Alkalinity Unit Dose Vials, 10 pouches 4318-H
Equipment needed but not supplied:
STANDARD ACCESSORY PACKAGE · CODE 1961
1 Package of 3 Vials (empty) 0156
1 Syringe, 3 mL, plastic 1184
1 Foil Storage Bag 9467
Or:
ADVANCED ACCESSORY PACKAGE · CODE 1962
1 Pipettor, 3mL 30528
1 Pipet Tip (0-5 mL) 30695
1 Cuvette Rack 31695
1 Package of 3 Vials (empty) 0156
1 Foil Storage Bag 9467
APPLICATION: Drinking and surface waters; swimming pool water.
RANGE: 0–200 ppm as CaCO
METHOD: The sample is added to a buffered indicator reagent. The
3
color that develops, ranging from yellow to blue, will
indicate the amount of alkalinity in the sample.
SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCES: Quats and poly quats at high concentrations will interfere.
Smart2 TEST PROCEDURES 2.04 Alkalinity–UDV 1/2
Samples should be analyzed as soon as possible after
collection. Sample may be refrigerated for 24 hours.
Page 62

PROCEDURE
Use 10 mm square cell adapter
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing
1 ALKALINTIY-UDV)from TESTING MENU.
5. Scroll to and select 1 ALKALINITY-UDV from menu.
6. Rinse a clean vial (0156) with sample water.
7. Use the syringe (1184) to add 3 mL of sample to the vial.
8. Insert the vial into chamber, close lid and select SCAN BLANK.
9. Remove vial from the colorimeter.
10. Use the syringe (1184) to add 3 mL of sample to an Alkalinity-UDV vial
(4318).
11. Wait 2 minutes.
12. Invert vial 3 times to mix.
þ NOTE: If powder residue remains in the bottom of the vial after inverting,
invert once more and tap bottom of vial sharply once or twice to dislodge
powder. Mix.
13. Insert tube into chamber, close lid and select SCAN SAMPLE. Record
result.
14. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ NOTES: For best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
It is necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
UDVs from opened pouches should be used promptly. Store unused vials from
opened pouches in the Foil Storage Bag (9467) to extend the shelf life of the
reagent. Generally, UDVs stored in the bag should be used within 10 days if
the humidity is less than 50% and within 5 days if humidity is greater than
50%. The Foil Storage Bag contains a desiccant pack with indicator. When
the indicator in the window turns from blue to pink, the bag should be
replaced.
Alkalinity–UDV 2/2 Smart2 TEST PROCEDURES 2.04
Page 63

ALUMINUM
ERIOCHROME CYANINE R METHOD • CODE 364I-SC
QUANTITY CONTENTS CODE
5 g *Aluminum Inhibitor Reagent *7865-C
2 x 120 mL *Aluminum Buffer Reagent *7866-J
120 mL Aluminum Indicator Reagent 7867-J
15 mL Aluminum Complexing Reagent 7868-E
1 Spoon, 0.05 g, plastic 0696
2 Pipets, 1.0 mL, plastic 0354
1 Test Tube, glass, 5 mL w/cap 0230
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
Aluminum is the third most common element in the earth’s crust, which
accounts for its wide appearance in many water supplies. Aluminum exists in
water as soluble salts, colloidal compounds, and insoluble compounds. In
wastewater that has been treated by alum coagulation it will appear in one or
more of the above forms. Properly treated drinking water should have an
aluminum concentration below 0.05 mg/L.
APPLICATION: Drinking, surface, and saline waters; domestic and industrial
wastewater.
RANGE: 0.00–0.30 ppm Aluminum
METHOD: Aluminum ions buffered to a pH of 6.0 react with
Eriochrome Cyanine R dye to produce a pink to red complex
in proportion to the concentration.
SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCES: Fluoride and polyphosphate will interfere. Interference from
Collect sample in acid washed glass or plastic bottle. Analyze
as soon as possible.
iron and manganese is eliminated by the addition of an
inhibitor.
Smart2 TEST PROCEDURES 2.04 Aluminum 1/2
Page 64

PROCEDURE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing 2 Aluminum).
5. Scroll to and select 2 Aluminum from menu.
6. Rinse a clean colorimeter tube (0290) with sample water. Fill to the 10
mL line with sample.
7. Insert tube into colorimeter chamber and select SCAN BLANK.
8. Rinse a clean test tube (0230) with sample water. Fill to the 5 mL line
with sample.
9. Remove tube from colorimeter. Empty sample from tube (0290).
10. Add 5 mL sample from test tube (0230) to empty tube (0290).
11. Use the 0.05 g spoon (0696) to add one measure of *Aluminum Inhibitor
Reagent (7865). Cap and mix to dissolve powder.
12. Use a 1.0 mL pipet (0354) to add 2 mL of *Aluminum Buffer Reagent
(7866). Cap and mix.
13. Use a second 1.0 mL pipet (0354) to add 1 mL of Aluminum Indicator
Reagent (7867). Cap and mix contents. Wait 5 minutes for maximum
color development.
14. At end of 5 minute waiting period, mix, insert tube into chamber, close lid
and select SCAN SAMPLE. Record result.
15. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ NOTE: For the best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Add 5 drops of Aluminum Complexing Reagent
(7868). Then follow the above procedure to perform the test on a distilled or
deionized water sample. This test result is the reagent blank. Subtract the
reagent blank from all subsequent test results of unknown samples. It is
necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
Aluminum 2/2 Smart2 TEST PROCEDURES 2.04
Page 65

AMMONIA-NITROGEN - LOW RANGE
SALICYLATE METHOD • CODE 3659-01-SC
QUANTITY CONTENTS CODE
60 mL *Salicylate Ammonia #1 *3978-H
10 g *Salicylate #2 *7457-D
2x5g *Salicylate#3Reagent Powder *7458-C
1 Spoon, 0.1 g, plastic 0699
1 Spoon, 0.15 g, plastic 0727
1 Pipet, 1.0 mL, plastic 0354
*WARNING: Reagents marked with an * are considered to be potential
health hazards. To view or print a Material Safety Data Sheet (MSDS) for
these reagents go to www.lamotte.com. To obtain a printed copy, contact
LaMotte by e-mail, phone or fax.
Ammonia nitrogen is present in various concentrations in many surface and
ground water supplies. Any sudden change in the concentration of ammonia
nitrogen in a water supply is cause for suspicion. A product of microbiological
activity, ammonia nitrogen is sometimes accepted as chemical evidence of
pollution when encountered in natural waters.
Ammonia is rapidly oxidized in natural water systems by special bacterial
groups that produce nitrite and nitrate. This oxidation requires that dissolved
oxygen be available in the water. Ammonia is an additional source of nitrogen
as a nutrient which may contribute to the expanded growth of undesirable
algae and other forms of plant growth that overload the natural system and
cause pollution.
APPLICATION: Low concentrations of ammonia in fresh, brackish and salt
water; fresh and salt water aquariums.
RANGE: 0.00 - 1.00 ppm Ammonia-Nitrogen
METHOD: Salicylate and ammonia react at high pH in the presence of
a chlorine donor and an iron catalyst to form a blue
indophenol dye, the concentration of which is proportional
to the ammonia concentration in the sample.
SAMPLE
HANDLE &
PRESERVATION:
INTERFERENCES: There are few interferences in most natural waters. High
Ammonia solutions tend to be unstable and should be
analyzed immediately. Samples may be stored for 24 hours at
4°C or 28 days at –20°C.
concentrations of reducing agents, such as hydrazine, react
with the chlorine donor and can result in negative
interferences. Color and turbidity can also interfere.
Smart2 TEST PROCEDURES 2.04 Ammonia-Nitrogen - LR 1/3
Page 66

PROCEDURE - FRESH WATER
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing
3 Ammonia-NLF)fromTESTING MENU.
5. Scroll to and select 3 Ammonia-NLF from menu.
6. Rinse a clean tube (0290) with sample water. Fill to the 10 mL line with
sample.
7. Insert tube into chamber, close lid and select SCAN BLANK. (See Note.)
8. Remove tube from colorimeter. Use the 1.0 mL plastic pipet (0354) to add
2.0 mL of *Salicylate Ammonia #1 (3978). Cap and mix.
9. Use the 0.15 g spoon (0727) to add two measures of *Salicylate #2
Reagent (7457). Cap and mix until dissolved. Wait 1 minute.
10. At end of 1 minute waiting period use 0.1 g spoon (0699) to add two
measures of *Salicylate #3 Reagent Powder (7458). Cap and shake
vigorously for at least 30 seconds and all solid has dissolved. W ait 12
minutes for maximum color development.
11. At the end of the 12 minute waiting period, immediately mix and insert
tube into chamber , close lid and select SCAN SAMPLE. Record result.
12. Press OFF button to turn colorimeter off or press EXIT button to exit to a
previous menu or make another menu selection.
CALCULATIONS:
To express results as Unionized Ammonia (NH
ppm Unionized Ammonia (NH3)=
ppm Ammonia-Nitrogen (NH
To express results as Ionized Ammonia (NH4):
ppm Ionized Ammonia (NH
ppm Ammonia-Nitrogen (NH
To determine the percentages of Unionized and Ionized Ammonia-Nitrogen,
consult the Appendix.
NOTE:
For the best possible results, a reagent blank should be determined to account
for any contribution to the test result by the reagent system. To determine the
reagent blank, follow the above test procedure to scan a distilled or deionized
water blank. Then follow the above procedure to perform the test on a distilled
or deionized water sample. This test result is the reagent blank. Subtract the
reagent blank from all subsequent test results of unknown samples. It is
necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
):
3
–N) x 1.2
3
4
–N) x 1.3
3
+
)=
Ammonia-Nitrogen - LR 2/3 Smart2 TEST PROCEDURES 2.04
Page 67

PROCEDURE - SALT WATER
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing
4 Ammonia-NLS)fromTESTING MENU.
5. Scroll to and select 4 Ammonia-NLS from menu.
6. Rinse a clean tube (0290) with sample water. Fill to the 10 mL line with
sample.
7. Insert tube into chamber, close lid and select SCAN BLANK. (See Note.)
8. Remove tube from colorimeter. Use the 1.0 mL plastic pipet (0354) to add
2.0 mL of *Salicylate Ammonia #1 (3978). Cap and mix.
9. Use the 0.15 g spoon (0727) to add two measures of *Salicylate #2
Reagent (7457). Cap and mix until dissolved. Wait 1 minute.
10. At end of 1 minute waiting period use 0.1 g spoon (0699) to add two
measures of *Salicylate #3 Reagent Powder (7458). Cap and shake
vigorously for at least 30 seconds and all solid has dissolved. W ait 20
minutes for maximum color development.
11. At the end of the 20 minute waiting period, immediately mix and insert
tube into chamber , close lid and select SCAN SAMPLE. Record result.
12. Press OFF button to turn colorimeter off or press EXIT button to exit to a
previous menu or make another menu selection.
CALCULATIONS:
To express results as Unionized Ammonia (NH3):
ppm Unionized Ammonia (NH3)=
ppm Ammonia-Nitrogen (NH
To express results as Ionized Ammonia (NH4):
ppm Ionized Ammonia (NH
ppm Ammonia-Nitrogen (NH
To determine the percentages of Unionized and Ionized Ammonia-Nitrogen,
consult the Appendix.
NOTE:
For the best possible results, a reagent blank should be determined to account
for any contribution to the test result by the reagent system. To determine the
reagent blank, follow the above test procedure to scan a distilled or deionized
water blank. Then follow the above procedure to perform the test on a distilled
or deionized water sample. This test result is the reagent blank. Subtract the
reagent blank from all subsequent test results of unknown samples. It is
necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
–N) x 1.2
3
+
)=
4
–N) x 1.3
3
Smart2 TEST PROCEDURES 2.04 Ammonia-Nitrogen - LR 3/3
Page 68

Smart2 TEST PROCEDURES 2.04
Page 69

AMMONIA-NITROGEN HIGH RANGE
NESSLERIZATION METHOD • CODE 3642-SC
QUANTITY CONTENTS CODE
30 mL Ammonia Nitrogen Reagent #1 V-4797-G
2 x 30 mL *Ammonia Nitrogen Reagent #2 *V-4798-G
1 Pipet, 1 mL, plastic 0354
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
Ammonia nitrogen is present in various concentrations in many surface and
ground water supplies. Any sudden change in the concentration of ammonia
nitrogen in a water supply is cause for suspicion. A product of microbiological
activity, ammonia nitrogen is sometimes accepted as chemical evidence of
pollution when encountered in natural waters.
Ammonia is rapidly oxidized in natural water systems by special bacterial
groups that produce nitrite and nitrate. This oxidation requires that dissolved
oxygen be available in the water. Ammonia is an additional source of nitrogen
as a nutrient which may contribute to the expanded growth of undesirable
algae and other forms of plant growth that overload the natural system and
cause pollution.
APPLICATION: Drinking, surface, and saline waters; domestic and industrial
wastes.
RANGE: 0.00–4.00 Ammonia Nitrogen
METHOD: Ammonia forms a colored complex with Nessler’s Reagent in
proportion to the amount of ammonia present in the sample.
Rochelle salt is added to prevent precipitation of calcium or
magnesium in undistilled samples.
SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCES: Sample turbidity and color may interfere. Turbidity may be
Ammonia solutions tend to be unstable and should be
analyzed immediately. Sample may be stored for 24 hours at
4°C or 28 days at –20°C.
removed by a filtration procedure. Color interference may be
eliminated by blanking the instrument with a sample blank.
Smart2 TEST PROCEDURES 2.04 Ammonia-Nitrogen–HR 1/2
Page 70

PROCEDURE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Scroll to and select ALL TESTS (or another sequence containing
5 Ammonia-N H)from TESTING MENU.
5. Scroll to and select 5 Ammonia-N H from menu.
6. Rinse a clean tube (0290) with sample water. Fill to the 10 mL line with
sample.
7. Insert tube into chamber, close lid and select SCAN BLANK.(SeeNote)
8. Remove tube from colorimeter. Add 8 drops of Ammonia Nitrogen
Reagent #1 (V-4797). Cap and mix. Wait 1 minute.
9. Use the 1.0 mL pipet (0354) to add 1.0 mL of *Ammonia Nitrogen
Reagent #2 (V-4798). Cap and mix. Allow 5 minutes for maximum color
development.
10. At end of the 5 minute waiting period, immediately mix, insert tube into
chamber, close lid and select SCAN SAMPLE. Record result.
11. Press OFF button to turn the colorimeter off or press the EXIT button exit
to a previous menu or make another menu selection.
CALCULATIONS:
To express results as Unionized Ammonia (NH
ppm Unionized Ammonia (NH3)=
ppm Ammonia-Nitrogen (NH
To express results as Ionized Ammonia (NH4):
ppm Ionized Ammonia (NH
ppm Ammonia-Nitrogen (NH
To determine the percentages of Unionized and Ionized Ammonia-Nitrogen,
consult the Appendix.
):
3
–N) x 1.2
3
+
4
–N) x 1.3
3
)=
þ
NOTE: For the best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
It is necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
Ammonia-Nitrogen–HR 2/2 Smart2 TEST PROCEDURES 2.04
Page 71

BENZOTRIAZOLE/TOLYLTRIAZOLE
UV Photolysis Method • CODE 4047
QUANTITY CONTENTS CODE
15 g *Benzotriazole Reagent *3818-E
25 mL Potassium Sodium Tartrate Solution 7841WT-G
25 mL *Sulfuric Acid *6139WT-G
1 pH Test Papers, 1–11 2956
1 Spoon, 0.25 g, plastic 0695
1 Erlenmeyer Flask, 25 mL, glass 2-2109
1 Graduated Cylinder, 25 mL, glass 0417
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
Equipment needed but not supplied:
QUANTITY CONTENTS CODE
1 UV Shielding Goggles 31041
1 Pen-Ray UV Lamp 31041-1
1 Pen-Ray Lamp Power Source 31041-2
Proper safety precautions must be followed when using the Pen-Ray UV lamp
and power source (31041-1 and 31041-2) to prevent eye and skin damage.
Always wear the UV Shielding Goggles (31041) while the lamp is turned on.
Never handle the lamp itself; always hold it by the socket. Wipe the lamp dry
with a clean, soft tissue after each test. Do not operate the lamp outside the
Erlenmeyer Flask filled with water .
Benzotriazole and tolyltriazole form strong complexes with metals. They are
used in antifreeze for cars, lubricating oil, and photographic anti-fogging
agents. In cooling water systems benzotriazole and tolyltriazole are used as
corrosion and rust inhibitors together with many kinds of scale inhibitors,
bactericides and algaecides.
APPLICATION: Corrosion and rust inhibitors in cooling water systems
RANGE: 0.0 – 30.0 ppm Benzotriazole
0.0 – 30.0 ppm Tolyltriazole
METHOD: Benzotriazole and tolyltriazole are UV-photolyzed in a
buffered solution with a pH between 4 and 6. A yellow color
develops in proportion to the concentration of triazole
present.
Smart2 TEST PROCEDURES 4.08 Benzotriazole/Tolyltriazole 1/4
Page 72

SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCES: Tolyltriazole with interfere in the benzotriazole test.
Samples should be analyzed as soon as possible after
collection.
Benzotriazole will interfere in the tolyltriazole test. Strong
reducing or oxidizing agents will interfere.
Benzotriazole/Tolyltriazole 2/4 Smart2 TEST PROCEDURES 4.08
Page 73

BENZOTRIAZOLE PROCEDURE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select Testing Menu.
4. Select ALL TESTS (or another sequence containing 10 B triazole
from TESTING MENU.
5. Scroll to and select 10 B triazole from menu.
6. Rinse a tube (0290) with sample water. Fill to 10 mL with sample.
7. Insert the tube into chamber, close lid and select SCAN BLANK.
8. Remove the tube from colorimeter. Discard the sample.
9. Adjust the sample water temperature to between 20 and 25°C if necessary.
10. Fill the graduated cylinder (0417) to the 25 mL line with sample water.
Transfer to the Erlenmeyer Flask (2-2109).
11. Use the pH Test Paper (2956) to check the pH of the sample. If the pH is
not between 4 and 6, add one drop of *Sulfuric Acid, 1.0N (6139). Swirl
to mix. Continue adding *Sulfuric Acid, 1.0N (6139) one drop at a time,
swirling to mix and checking the pH after each drop, until the pH is
between 4 and 6.
12. Add 10 drops of Potassium Sodium Tartrate (7841WT).
13. Use the 0.25 g spoon (0695) to add one measure of *Benzotriazole
Reagent (3818). Swirl to mix until the powder has dissolved.
14. Replace the flask in the slot in the case. Insert the Pen-Ray Lamp
(31041-1) into the flask. Plug in the Pen-Ray Power Source (31041-2)
and turn the lamp on for exactly 5 minutes. Remove the lamp from the
flask. Rinse and wipe the lamp dry.
15. Fill a test tube (0290) to the 10 mL line with the digested sample. Cap
tube.
16. Insert tube into chamber, close lid and select SCAN SAMPLE. Record
result in ppm Benzotriazole.
17. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ
NOTE: For best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
It is necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
Smart2 TEST PROCEDURES 7.06 Benzotriazole/Tolyltriazole 3/4
Page 74

TOLY LTRIAZOLE PROCEDURE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select Testing Menu.
4. Select ALL TESTS (or another sequence containing 97 T triazole
from TESTING MENU.
5. Scroll to and select 97 T triazole from menu.
6. Rinse a tube (0290) with sample water. Fill to 10 mL with sample.
7. Insert the tube into chamber, close lid and select SCAN BLANK.
8. Remove the tube from colorimeter. Discard the sample.
9. Adjust the sample water temperature to between 20 and 25°C if necessary.
10. Fill the graduated cylinder (0417) to the 25 mL line with sample water.
Transfer to the Erlenmeyer Flask (2-2109).
11. Use the pH Test Paper (2956) to check the pH of the sample. If the pH is
not between 4 and 6, add one drop of *Sulfuric Acid, 1.0N (6139). Swirl
to mix. Continue adding *Sulfuric Acid, 1.0N (6139) one drop at a time,
swirling to mix and checking the pH after each drop, until the pH is
between 4 and 6.
12. Add 10 drops of Potassium Sodium Tartrate (7841WT).
13. Use the 0.25 g spoon (0695) to add one measure of *Benzotriazole
Reagent (3818). Swirl to mix until the powder has dissolved.
14. Replace the flask in the slot in the case. Insert the Pen-Ray Lamp
(31041-1) into the flask. Plug in the Pen-Ray Power Source (31041-2)
and turn the lamp on for exactly 5 minutes. Remove the lamp from the
flask. Rinse and wipe the lamp dry.
15. Fill a test tube (0290) to the 10 mL line with the digested sample. Cap
tube.
16. Insert tube into chamber, close lid and select SCAN SAMPLE. Record
result in ppm Tolyltriazole.
17. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ
NOTE: For best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
It is necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
Benzotriazole/Tolyltriazole 4/4 Smart2 TEST PROCEDURES 7.06
Page 75

BIGUANIDE
COLORIMETRIC METHOD • CODE 4044
QUANTITY CONTENTS CODE
2 X 60 mL Biguanide Indicator 3994-H
1 Pipet, plastic, 1.0 mL 0354
Biguanide is a non-chlorine, non-bromine chemical sanitizer. It is more stable
than chlorine or bromine and has little chemical odor. Biquanide is an
effective bacteriacide but, unlike chlorine and bromine, it does not destroy
organic contaminants. Therefore, hydrogen peroxide is added to biguanide
pools on a regular basis to eliminate organic contaminants. The optimum
recommended level of biguanide is 30 to 50 ppm.
APPLICATION: Swimming pools
RANGE: 0–70 ppm
METHOD: Biguanide complexes with the proprietary indicator to
produce a colored solution. The color ranges from yellow
through green to blue depending on the biguanide
concentration.
SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCES: The only interfering substances that are likely to be
Samples should be analyzed as soon as possible.
encountered in pool water are oxidized manganese and
oxidizing agents, such as chlorine, bromine and ozone.
Smart2 TEST PROCEDURES 2.04 Biguanide 1/2
Page 76

PROCEDURE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select Testing Menu.
4. Select ALL TESTS (or another sequence containing 7 Biguanide
from TESTING MENU.
5. Scroll to and select 7 Biguanide from menu.
6. Rinse a tube (0290) with sample water. Fill to 10 mL with sample.
7. Insert the tube into chamber, close lid and select SCAN BLANK.
8. Remove the tube from colorimeter.
9. Use the 1.0 mL pipet (0354) to add 2.0 mL of Biguanide Indicator (3994).
Cap and invert three times to mix.
10. Wait 1 minute.
11. Insert the tube into chamber. Close lid.
12. Select SCAN SAMPLE. Record result in ppm Biguanide
13. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ NOTE: For best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
It is necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
Biguanide 2/2 Smart2 TEST PROCEDURES 2.04
Page 77

BORON
AZOMETHINE-H METHOD · CODE 4868
QUANTITY CONTENTS CODE
120 mL *Boron Buffer *4869-J
10 g *Boron Indicator Powder *4870-D
1 Pipet, plastic, 1.0 mL 0354
1 Spoon, 0.15 g 0727
1 Dark storage chamber, brown 0108
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax..
Small amounts of boron are necessary for plant growth but large amounts can
be toxic. In humans, boron aids in the uptake of calcium and the production of
strong bones. An excess of boron can affect the central nervous system
resulting in a syndrome known as borism. Some natural waters may contain
small amounts of boron. Large concentrations may be due to industrial effluent
entering waterways. Boron compounds are used in cleaning compounds, paper
and paints, fertilizers, glass and ceramics, fire retardants and the production of
alloys. In the atomic energy field, boron is a component of neutron shields and
nuclear reactors. Some swimming pools use boron buffering systems.
APPLICATION: Surface and saline waters, hydroponic solutions, industrial
waste, swimming pools.
RANGE: 0.00–0.80 ppm Boron
METHOD: Azomethine-H and borate form a yellow complex at pH 6 in
proportion to the concentration of boron present.
SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCES: Interferences in drinking water are unlikely. Manganese,
Store samples in polyethylene bottles. Do not use borate
detergents or glassware.
zirconium, chromium, titanium, copper, vanadium,
aluminum, beryllium and iron may cause high results.
Smart2 TEST PROCEDURES 2.04 Boron 1/2
Page 78

PROCEDURE
1. This test requires a Reagent Blank. Rinse a tube (0290) with clear,
colorless, boron free water. Fill to 10 mL line with clear, colorless, boron
free water.
2. Use the 1.0 mL pipet (0354) to add 2 mL of *Boron Buffer (4869). Cap
and mix.
3. Use the 0.15 g spoon (0727) to add one level measure of *Boron Indicator
Powder (4870). Press full spoon against side of jar to compress powder .
Scrape off excess powder on inside neck of bottle. Tap excess off spoon
handle.
4. Cap and shake vigorously for 30 seconds.
5. Insert the tube into meter chamber. Close lid.
6. Start a timer set for 30 minutes. Do not open the lid during the waiting
time. The reaction is photosensitive.
7. Rinse a clean tube (0290) with Sample Water. Fill to the 10 mL line with
sample water. Repeat steps 2–4.
8. Insert the tube into the Dark Storage Chamber (0108). Close top.
9. Start a second timer set for 30 minutes. Do not open the chamber during
the waiting time. The reaction is photosensitive.
10. When 2 minutes remain on the first timer (Reagent Blank), press and
hold ON button until colorimeter turns on.
11. Press ENTER to start.
12. Press ENTER to select Testing Menu.
13. Select ALL TESTS (or another sequence containing 8 Boron)from
TESTING MENU.
14. Scroll to and select 8 Boron from menu.
15. At the end of the Reagent Blank 30 minute waiting period, remove
Reagent Blank tube from meter chamber. Invert several times to mix.
16. Insert the tube into meter chamber, close lid and select SCAN BLANK.
17. Remove the tube from colorimeter.
18. At the end of the Sample Water 30 minute waiting period, remove
Sample Water tube from Dark Storage Chamber. Invert several times to
mix.
19. Insert tube into meter chamber, close lid and select SCAN SAMPLE.
Record result in ppm boron.
20. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
Boron 2/2 Smart2 TEST PROCEDURES 2.04
Page 79

BROMINE - UDV
DPD METHOD–UNIT DOSE VIALS · CODE 4311-H
QUANTITY CONTENTS CODE
1 *Free Chlorine Unit Dose Vials, 10 pouches *4311-H
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
Equipment needed but not supplied:
STANDARD ACCESSORY PACKAGE · CODE 1961
1 Package of 3 Vials (empty) 0156
1 Syringe, 3 mL, plastic 1184
1 Foil Storage Bag 9467
Or:
ADVANCED ACCESSORY PACKAGE · CODE 1962
1 Pipettor, 3mL 30528
1 Pipet Tip (0-5 mL) 30695
1 Cuvette Rack 31695
1 Package of 3 Vials (empty) 0156
1 Foil Storage Bag 9467
Like chlorine, bromine is an effective germicidal agent employed in drinking
water treatment, pool and spa water sanitization, food service sanitation, and
other public health applications.
APPLICATION: Drinking, surface, and saline waters; swimming pool water;
domestic and industrial waters and wastes.
RANGE: 0.0–22.0 ppm Bromine
METHOD: In buffered sample bromine reacts with diethyl-p-phenylene
diamine (DPD) to produce a pink-red color in proportion to
the concentration of bromine present.
SAMPLE
HANDLING &
PRESERVATION:
Bromine in aqueous solutions is not stable, and the bromine
content of samples or solutions, particularly weak solutions,
will rapidly decrease. Exposure to sunlight or agitation will
accelerate the reduction of bromine present in such
solutions. For best results start analysis immediately after
sampling. Samples to be analyzed for bromine cannot be
preserved or stored.
Smart2 TEST PROCEDURES 2.04 Bromine - UDV 1/3
Page 80

INTERFERENCE: The only interfering substance likely to be encountered in
water is oxidized manganese. The extent of this interference
can be determined by treating a sample with sodium arsenite
to destroy the bromine present so that the degree of
interference can be estimated.
Iodine and chlorine can also interfere, but these are not
normally present unless they have been added as sanitizers.
Bromine - UDV 2/3 Smart2 TEST PROCEDURES 2.04
Page 81

PROCEDURE
Use 10 mm square cell adapter.
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select Testing Menu.
4. Select ALL TESTS (or another sequence containing 11 Bromine-UDV)
from TESTING MENU.
5. Scroll to and select 11 Bromine-UDV from menu.
6. Rinse a clean vial (0156) with sample water.
7. Use the syringe (1184) to add 3mL of sample to the vial.
8. Insert the vial into chamber, close the lid and select SCAN BLANK.
9. Remove the vial from the colorimeter.
10. Use the syringe (1184) to add 3mL of sample to a *Free Chlorine UDV
vial (4311).
11. Shake vigorously until powder dissolves completely.
NOTE: If powder residue remains in the bottom of the vial after inverting
þ
or air bubbles form, invert once more and tap bottom of vial sharply once
or twice to dislodge powder and bubbles. Mix.
12. Immediately insert tube into chamber, close lid and select SCAN SAMPLE.
Record result in ppm bromine.
13. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ NOTES: For best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
It is necessary to determine the reagent blank only when a new lot number of
reagents are obtained.
UDVs from opened pouches should be used promptly. Store unused vials from
opened pouches in the Foil Storage Bag (9467) to extend the shelf life of the
reagent. Generally, UDVs stored in the bag should be used within 10 days if
the humidity is less than 50% and within 5 days if humidity is greater than
50%. The Foil Storage Bag contains a desiccant pack with indicator. When
the indicator in the window turns from blue to pink, the bag should be
replaced.
Smart2 TEST PROCEDURES 2.04 Bromine - UDV 3/3
Page 82

Smart2 TEST PROCEDURES 2.04
Page 83

CADMIUM
PAN METHOD • CODE 4017
QUANTITY CONTENTS CODE
60 mL *Buffered Ammonia Reagent *4020-H
15 mL Sodium Citrate, 10% 6253-E
30 mL *PAN Indicator *4021-G
30 mL Stabilizing Reagent 4022-G
1 Pipet, 1.0 mL, plastic 0354
2 Pipet, 0.5 mL, plastic 0369
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
Cadmium is used in batteries, paint pigments, electroplating processes, and
with other metals in the preparation of alloys. The solubility of cadmium in
natural water is proportional to the hardness or alkalinity of the water.
Cadmium is not an essential nutrient for plants and animals. It is extremely
toxic and can accumulate in the kidneys and liver.
APPLICATION: Drinking and surface waters; domestic and industrial
wastewater.
RANGE: 0.00–1.00 Cadmium
METHOD: PAN (1-[2-Pyridylazo]-2-Naphthol) forms a red complex
with Cadmium (Cd
SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCES: Ag
Analyze sample as soon as possible. If sample must be stored,
acidify with nitric acid to a pH below 2.
+2
,Co+2,Cu+2,Mn+2,Ni+2,Zn+2,Y+3,In
+2
) at a pH of 10.
+3
Smart2 TEST PROCEDURES 2.04 Cadmium 1/2
Page 84

PROCEDURE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing 12 Cadmium)
from TESTING MENU.
5. Scroll to and select 12 Cadmium from menu.
6. Rinse a tube (0290) with sample water. Fill to the 10 mL line with sample.
7. Insert the tube into chamber, close lid and select SCAN BLANK.
8. Remove tube from colorimeter. Use the 1.0 mL pipet (0354) to add 1.0
mL of *Buffered Ammonia Reagent (4020). Swirl to mix.
9. Add two drops of Sodium Citrate, 10% (6253). Swirl to mix.
10. Use a 0.5 mL pipet (0369) to add 0.5 mL of PAN Indicator (4021). Swirl
to mix.
11. Use a 0.5 mL pipet (0369) to add 0.5 mL Stabilizing Reagent (4022). Cap
and mix.
12. Immediately insert tube into chamber, close lid and select SCAN SAMPLE.
Record result.
13. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ NOTE: For best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
It is necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
Cadmium 2/2 Smart2 TEST PROCEDURES 2.04
Page 85

CALCIUM & MAGNESIUM (TOTAL)
HARDNESS–UDV
UNIT DOSE VIALS • CODE 4309-H
QUANTITY CONTENTS
1 Calcium Hardness Unit Dose Vials, 10 pouches 4309-H
CODE
Equipment needed but not supplied:
STANDARD ACCESSORY PACKAGE · CODE 1961
1 Package of 3 Vials (empty) 0156
1 Syringe, 3 mL, plastic 1184
1 Foil Storage Bag 9467
Or:
ADVANCED ACCESSORY PACKAGE · CODE 1962
1 Pipettor, 3 mL 30528
1 Pipet Tips (0-5 mL) 30695
1 Cuvette Rack 31695
1 Package of 3 Vials (empty) 0156
1 Foil Storage Bag 9467
APPLICATION: Drinking and surface waters; swimming pool water.
RANGE: 0–400 ppm as CaCO
METHOD: Calcium and magnesium react in a strongly buffered medium
Total Hardness
3
with an indicator to develop a pale purple color in
proportion to the concentration.
SAMPLE
HANDLING &
PRESERVATION:
Samples should be analyzed as soon as possible after
collection. If storage is necessary, add 0.5 mL of 20 %
hydrochloric acid per 100 mL of sample. However, the added
acid will have to be neutralized with NaOH before testing.
INTERFERENCES: Heavy metals will interfere.
Smart2 TEST PROCEDURES 2.04 Calcium & Magnesium (Total) Hardness–UDV 1/2
Page 86

PROCEDURE
Use 10 mm square cell adapter.
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing 13 Ca&Mg H-UDV)
from TESTING MENU.
5. Scroll to and selec t 13 Ca&Mg Hard-UDV from menu.
6. Rinse a clean vial (0156) with sample water.
7. Use the syringe (1184) to add 3 mL of sample to the vial.
8. Insert the vial into chamber, close lid and select SCAN BLANK.
9. Remove vial from the colorimeter.
10. Use the syringe (1184) to add 3 mL of sample to a Calcium Hardness
UDV vial (4309).
11. Shake vigorously for 10 seconds.
12. Wait one minute.
13. Invert vial 3 times to mix.
þ NOTE: Firmly tap side of vial 5-10 times to remove all air bubbles.
14. Insert tube into chamber, close lid and select SCAN SAMPLE. Record
result.
15. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ NOTES: For best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
It is necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
UDVs from opened pouches should be used promptly. Store unused vials from
opened pouches in the Foil Storage Bag (9467) to extend the shelf life of the
reagent. Generally, UDVs stored in the bag should be used within 10 days if
the humidity is less than 50% and within 5 days if humidity is greater than
50%. The Foil Storage Bag contains a desiccant pack with indicator. When
the indicator in the window turns from blue to pink, the bag should be
replaced.
Calcium & Magnesium (Total) Hardness–UDV 2/2 Smart2 TEST PROCEDURES 2.04
Page 87

CHLORIDE
ARGENTOMETRIC METHOD · CODE 3693-SC
QUANTITY CONTENTS CODE
50 *Chloride Spectrophotometric Grade Tablets *3885A-H
1 Tablet Crusher 0175
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
Chloride is one of the major anions found in water and sewage. The presence
of chlorides in large amounts may be due to the natural process of water passing
through salt formations in the earth, or it may be evidence of the intrusion of
seawater or pollution from industrial processes or domestic wastes. The salt
content of water affects the distribution of plant and animal life in an aquatic
system, based on the amount of salt they can tolerate.
APPLICATION: Drinking, surface, and saline waters; domestic and industrial
wastewaters.
RANGE: 0.0–30.0 ppm Chloride
METHOD: Silver nitrate reacts with chloride to form turbid silver
chloride in proportion to the amount of chloride in the
sample.
SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCES: Substances in amounts normally found in drinking water
Collect samples in clean, chemically resistant glass or plastic
containers. No preservative is needed if sample is to be
stored.
will not interfere. Bromide, iodide, cyanide, sulfide,
thiosulfate, sulfide and orthophosphate will interfere.
Smart2 TEST PROCEDURES 1.07 Chloride 1/2
Page 88

PROCEDURE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing 21
Chloride-TT)from TESTING MENU.
5. Scroll to and select 21 Chloride-TT from menu.
6. Rinse a tube (0290) with sample water. Fill to 10 mL line with sample.
7. Insert the tube into chamber, close lid and select SCAN BLANK.
8. Remove the tube from colorimeter.
9. Add one *Chloride Spectrophotometric Grade Tablet (3885A).
10. Use Tablet Crusher (0175) to crush tablet.
11. Cap tube.
12. Invert 2 times.
13. Wait 3 minutes. Do NOT mix.
14. Insert tube into chamber, close lid and select SCAN SAMPLE. Record
result in ppm chloride.
15. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ NOTE: For best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
The reagent system is temperature sensitive. The calibration is for 25ºCIf
sample is at 30ºC, multiply resulting ppm by 1.1. If the sample is at 20º,
multiply ppm by 0.9.
Chloride 2/2 Smart2 TEST PROCEDURES 1.07
Page 89

CHLORINE
LIQUID DPD METHOD · CODE 4859
QUANTITY CONTENTS CODE
30 mL DPD 1A Free Chlorine Reagent P-6740-G
30 mL *DPD 1B Free Chlorine Reagent *P-6741-G
30 mL *DPD 3 Total Chlorine Reagent *P-6743-G
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
All water for cities and communities must be sanitized; even waters that come
from clean sources, protected watersheds, reservoirs, and deep wells, are
commonly sanitized to assure safety. Chlorine is the most commonly used
sanitizer for several reasons: it is effective against a wide range of
microorganisms, the cost is low, and the methods of applying it have been well
developed. If an adequate concentration of chlorine is present in the water for
a few minutes, disease producing bacteria will be destroyed. A number of
conditions affect the sanitizing action of chlorine. In municipal systems these
can be controlled so that if chlorine is detectable, it can be assumed that
bacteria have been killed. The factors that influence the rate of sanitization are
temperature, pH, presence of other materials that react with chlorine, time,
and the concentrations of the various chlorine combinations that are formed in
the water with ammonia and other substances that react with chlorine.
The fact that chlorine can be easily detected and measured makes chlorine a
favorite water sanitizer of those concerned with the public safety of water
supplies. Chlorine concentrations in the range of 0.1 to 0.4 parts per million
are usually maintained in municipal supplies.
Chlorine can be added in the form of chlorine gas, liquid sodium hypochlorite
(bleach), granular calcium hypochlorite or as organic chlorine compounds.
Chlorine is not present in natural water supplies; if it is present it is the result
of chlorination of a water supply or of chlorinated compounds being discharged
as waste from industrial operations. The presence of chlorine in concentrations
above 0.5 parts per million should be considered evidence of pollution from
chlorine treated effluents or from a process in which high concentrations of
chlorine are used.
APPLICATION: Drinking, surface, and saline waters; swimming pool water;
domestic and industrial wastes.
RANGE: 0.00–4.00 ppm Chlorine
METHOD: In the absence of iodide, free available chlorine reacts
instantly with DPD to produce a red color. Subsequent
addition of potassium iodide evokes a rapid color response
from the combined forms of chlorine (chloramines).
Smart2 TEST PROCEDURES 2.04 Chlorine- Liquid DPD 1/3
Page 90

SAMPLE
HANDLING &
PRESERVATION:
Chlorine in aqueous solutions is not stable, and the chlorine
content of samples or solutions, particularly weak solutions,
will rapidly decrease. Exposure to sunlight or agitation will
accelerate the reduction of chlorine present in such
solutions. For best results, start analysis immediately after
sampling. Samples to be analyzed for chlorine cannot be
preserved or stored.
INTERFERENCE: The only interfering substance likely to be encountered in
water is oxidized manganese. The extent of this interference
can be determined by treating a sample with sodium arsenite
to destroy the chlorine present so that the degree of
interference can be measured.
Iodine and bromine can give a positive interference, but
these are not normally present unless they have been added
as sanitizers.
Chlorine- Liquid DPD 2/3 Smart2 TEST PROCEDURES 2.04
Page 91

PROCEDURE–FREE CHLORINE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select Testing Menu.
4. Select ALL TESTS (or another sequence containing 17 Cl DPD-Liq)
from TESTING MENU.
5. Scroll to and select 17 Cl DPD-Liq from menu.
6. Rinse a tube (0290) with sample water. Fill to 10 mL with sample.
7. Insert the tube into chamber, close lid and select SCAN BLANK.
8. Remove the tube from colorimeter.
9. Add 5 drops of DPD 1A Free Chlorine Reagent (P-6740).
10. Add 5 drops of *DPD 1B Free Chlorine Reagent (P-6741). Cap and mix.
11. Insert tube into chamber, close lid and select SCAN SAMPLE. Record
result as ppm free chlorine.
PROCEDURE–TOTAL CHLORINE
12. Add 5 drops of *DPD 3 Total Chlorine Reagent (P-6743). Cap and mix.
þ NOTE: For wastewater samples, Standard Methods for the Examination of
Water and Wastewater recommends waiting 2 minutes for full color
development.
13. Insert tube into chamber, close lid and select SCAN SAMPLE. Record
result as ppm total chlorine.
14. SubtracttheFreeChlorinereadingfromtheTotalChlorinereadingto
determine ppm combined chlorine.
15. Press OFF button to turn the colorimeter off or press EXIT button to exit
to a previous menu or make another menu selection.
þ
NOTE: For best possible results, a reagent blank should be determined to
account for any contribution to the test result by the reagent system. To
determine the reagent blank, follow the above test procedure to scan a distilled
or deionized water blank. Then follow the above procedure to perform the test
on a distilled or deionized water sample. This test result is the reagent blank.
Subtract the reagent blank from all subsequent test results of unknown samples.
It is necessary to determine the reagent blank only when a new lot number of
reagents is obtained.
Smart2 TEST PROCEDURES 2.04 Chlorine- Liquid DPD 3/3
Page 92

Smart2 TEST PROCEDURES 2.04
Page 93

CHLORINE–BROMINE–IODINE
DPD METHOD • CODE 3643-SC
QUANTITY CONTENTS CODE
100 *DPD #1 Instrument Grade Tablets *6903A-J
100 *DPD #3 Instrument Grade Tablets *6197A-J
15 mL Glycine Solution 6811-E
1 Tablet Crusher 0175
*WARNING: Reagents marked with an * are considered to be potential health
hazards. To view or print a Material Safety Data Sheet (MSDS) for these reagents go to
www.lamotte.com. To obtain a printed copy, contact LaMotte by e-mail, phone or fax.
All water for cities and communities must be sanitized; even waters that come
from clean sources, protected watersheds, reservoirs, and deep wells, are
commonly sanitized to assure safety. Chlorine is the most commonly used
sanitizer for several reasons: it is effective against a wide range of
microorganisms, the cost is low, and the methods of applying it have been well
developed. If an adequate concentration of chlorine is present in the water for
a few minutes, disease producing bacteria will be destroyed. A number of
conditions affect the sanitizing action of chlorine. In municipal systems these
can be controlled so that if chlorine is detectable, it can be assumed that
bacteria have been killed. The factors that influence the rate of sanitization are
temperature, pH, presence of other materials that react with chlorine, time,
and the concentrations of the various chlorine combinations that are formed in
the water with ammonia and other substances that react with chlorine.
The fact that chlorine can be easily detected and measured makes chlorine a
favorite water sanitizer of those concerned with the public safety of water
supplies. Chlorine concentrations in the range of 0.1 to 0.4 parts per million
are usually maintained in municipal supplies.
Chlorine can be added in the form of chlorine gas, liquid sodium hypochlorite
(bleach), granular calcium hypochlorite or as organic chlorine compounds.
Chlorine is not present in natural water supplies; if it is present it is the result
of chlorination of a water supply or of chlorinated compounds being discharged
as waste from industrial operations. The presence of chlorine in concentrations
above 0.5 parts per million should be considered evidence of pollution from
chlorine treated effluents or from a process in which high concentrations of
chlorine are used.
APPLICATION: Drinking, surface, and saline waters; swimming pool water;
domestic and industrial wastes.
RANGE: 0.00–4.00 Chlorine
METHOD: In the absence of iodide, free available chlorine reacts
instantly with DPD to produce a red color. Subsequent
addition of potassium iodide evokes a rapid color response
from the combined forms of chlorine (chloramines).
Smart2 TEST PROCEDURES 4.08 Chlorine–Bromine–Iodine 1/8
Page 94

SAMPLE
HANDLING &
PRESERVATION:
Chlorine in aqueous solutions is not stable, and the chlorine
content of samples or solutions, particularly weak solutions,
will rapidly decrease. Exposure to sunlight or agitation will
accelerate the reduction of chlorine present in such
solutions. For best results, start analysis immediately after
sampling. Samples to be analyzed for chlorine cannot be
preserved or stored.
INTERFERENCE: The only interfering substance likely to be encountered in
water is oxidized manganese. The extent of this interference
can be determined by treating a sample with sodium arsenite
to destroy the chlorine present so that the degree of
interference can be measured.
Iodine and bromine can give a positive interference, but
these are not normally present unless they have been added
as sanitizers.
Chlorine-Bromine-Iodine 2/8 Smart2 TEST PROCEDURES 2.04
Page 95

PROCEDURE–FREE CHLORINE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing 15 Chlorine)
from TESTING MENU.
5. Scroll to and select 15 Chlorine from menu.
6. Rinse a clean tube (0290) with sample water. Fill to the 10 mL line with
sample.
7. Insert tube into chamber, close lid and select SCAN BLANK.
8. Remove tube from colorimeter and pour off all but a sufficient amount of
sample water to cover a tablet. Add one *Chlorine DPD #1 Instrument
Grade Tablet (6903A). Crush tablet with a tablet crusher (0175), then
add sample water until tube is filled to 10 mL line. Cap tube and shake
until tablet has dissolved. Solution will turn pink if free chlorine is
present. Wait 15 seconds, but no longer than 30 seconds. Mix.
9. Insert tube into chamber, close lid and select SCAN SAMPLE.
PROCEDURE–COMBINED CHLORINE
10. Add one *Chlorine DPD #3 Instrument Grade Tablet (6197A) to sample
from Step 8 above. Crush tablet with tablet crusher (0175). Cap tube and
shake until tablet dissolves. An increase in color represents combined
chlorine.
þ NOTE: For wastewater samples, Standard Methods for the Examination of
Water and Wastewater recommends waiting 2 minutes for full color
development.
11. Insert sample into chamber, close lid and select SCAN SAMPLE. Record
result as Total Chlorine.
12. Subtract free chlorine reading from total chlorine reading to obtain
concentration of combined chlorine.
13. Press the OFF button to turn off the colorimeter or press the EXIT button
to exit to a previous menu or make another menu selection.
Smart2 TEST PROCEDURES 4.08 Chlorine–Bromine–Iodine 3/8
Page 96

BROMINE
Like chlorine, bromine is an effective germicidal agent employed in drinking
water treatment, pool and spa water sanitization, food service sanitation, and
other public health applications.
APPLICATION: Drinking, surface, and saline waters; swimming pool water;
domestic and industrial waters and wastes.
RANGE: 0.00–9.00 Bromine
METHOD: In buffered sample bromine reacts with diethyl-p-phenylene
diamine (DPD) to produce a pink-red color in proportion to
the concentration of bromine present.
SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCE: The only interfering substance likely to be encountered in
Bromine in aqueous solutions is not stable, and the bromine
content of samples or solutions, particularly weak solutions,
will rapidly decrease. Exposure to sunlight or agitation will
accelerate the reduction of bromine present in such
solutions. For best results start analysis immediately after
sampling. Samples to be analyzed for bromine cannot be
preserved or stored.
water is oxidized manganese. The extent of this interference
can be determined by treating a sample with sodium arsenite
to destroy the bromine present so that the degree of
interference can be estimated.
Iodine and chlorine can also interfere, but these are not
normally present unless they have been added as sanitizers.
Chlorine-Bromine-Iodine 4/8 Smart2 TEST PROCEDURES 4.08
Page 97

PROCEDURE A: BROMINE (NO CHLORINE)
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing 9 Bromine-LR)
from TESTING MENU.
5. Scroll to and select 9 Bromine-LR from menu.
6. Rinse a clean tube (0290) with sample water. Fill to the 10 mL line with
sample.
7. Insert tube into chamber, close lid and select SCAN BLANK.
8. Remove tube from colorimeter. Pour out all but a sufficient amount of
sample water to cover a tablet. Add one *DPD #1 Instrument Grade
Tablet (6903A). Crush tablet with tablet crusher (0175), then add sample
water until tube is filled to 10 mL line. Cap tube and shake until tablet is
dissolved. Solution will turn pink if bromine is present. Wait 15 seconds.
Mix.
9. Insert tube into chamber, close lid and select SCAN SAMPLE.
10. Press OFF button to turn colorimeter off or press the EXIT button to exit
to a previous menu or make another menu selection.
Smart2 TEST PROCEDURES 4.08 Chlorine–Bromine–Iodine 5/8
Page 98

PROCEDURE B:
BROMINE IN THE PRESENCE OF CHLORINE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing 9 Bromine-LR)
from TESTING MENU.
5. Scroll to and select 9 Bromine-LR from menu.
6. Rinse a clean tube (0290) with sample water. Fill to the 10 mL line with
sample.
7. Insert tube into chamber close lid and select SCAN BLANK.
8. Rinse a second clean tube (0290) with sample water. Fill to the 10 mL line
with sample. Add 5 drops of Glycine Solution (6811). Cap and mix.
9. Remove blank from colorimeter. Pour out all of the sample water. To this
tube add just enough of Glycine treated sample (Step 8) to cover a tablet.
Add one *DPD#1 Instrument Grade Tablet (6903). Crush tablet with a
tablet crusher (0175). Add all remaining Glycine-treated sample. Cap
tube and shake until tablet dissolves. Solution will turn pink if bromine is
present. Wait 15 seconds. Mix.
10. Insert tube into chamber, close lid and select SCAN SAMPLE.
11. Press OFF button to exit to previous menu or make another menu
selection.
PROCEDURE C: FREE AVAILABLE, TOTAL AVAILABLE &
COMBINED CHLORINE IN THE PRESENCE OF BROMINE
1. Perform the test for free and combined chlorine as previously described.
2. Perform the test for bromine in the presence of chlorine.
3. Calculations:
Residual Bromine (ppm) = Reading BR
Free Chlorine in the Presence of Bromine =
Free Chlorine - 0.45 (Reading BR)
Total Chlorine in the Presence of Bromine =
Tot a l C h l o ri ne - 0.45 (Reading BR)
Combined Chlorine in the Presence of Bromine =
Tot a l C h l o ri ne - Free Chlorine
þ
NOTE: Combined chlorine is not affected by the presence of bromine, so the
calculationisthesameaswhenonlychlorineispresent.
Chlorine-Bromine-Iodine 6/8 Smart2 TEST PROCEDURES 4.08
Page 99

IODINE
Like chlorine and bromine, iodine is an effective germicidal agent employed in
drinking water treatment, pool and spa water sanitization, food service
sanitation, and other public health applications.
APPLICATION: Drinking, surface, and saline waters; swimming pool water;
domestic and industrial wastes.
RANGE: 0.00–14.00 ppm Iodine
METHOD: In a buffered sample iodine reacts with
diethyl-p-phenylene-diamine (DPD) to produce a pink-red
color in proportion to the concentration of iodine present.
SAMPLE
HANDLING &
PRESERVATION:
INTERFERENCE: The only interfering substance likely to be encountered in
Iodine in aqueous solutions is not stable, and the iodine
content of samples or solutions, particularly weak solutions,
will rapidly decrease. Exposure to sunlight or agitation will
accelerate the reduction of iodine present in such solutions.
For best results start analysis immediately after sampling.
Samples to be analyzed for iodine cannot be preserved or
stored.
water is oxidized manganese. The extent of this interference
can be determined by treating a sample with sodium arsenite
to destroy the iodine present so that the degree of
interference can be measured.
Chlorine and bromine can give a positive interference, but
these are not normally present unless they have been added
as sanitizers.
Smart2 TEST PROCEDURES 4.08 Chlorine–Bromine–Iodine 7/8
Page 100

PROCEDURE
1. Press and hold ON button until colorimeter turns on.
2. Press ENTER to start.
3. Press ENTER to select TESTING MENU.
4. Select ALL TESTS (or another sequence containing 50 Iodine)from
TESTING MENU.
5. Scroll to and select 50 Iodine from menu.
6. Rinse a clean tube (0290) with sample water. Fill tube to the 10 mL line
with sample.
7. Insert tube into chamber, close lid and select SCAN BLANK.
8. Remove tube from colorimeter. Pour off all but a sufficient amount of
sample water to cover a tablet. Add one *DPD #1 Tablet Instrument
Grade (6903A). Crush tablet with tablet crusher (0175). Add sample
water until tube is filled to 10 mL line. Cap and shake until tablet
dissolves. Solution will turn pink if iodine is present. Wait 15 seconds.
Mix.
9. Insert tube into chamber, close lid and select SCAN SAMPLE. Record
result.
10. Press OFF button to turn colorimeter off or press EXIT button to exit to a
previous menu or make another menu selection.
Chlorine-Bromine-Iodine 8/8 Smart2 TEST PROCEDURES 4.08
 Loading...
Loading...