Page 1

Page 2

Page 3

Pool Manager
Wa ter Quality Handbook
for aquatic specialists
Copyright 2002, LaMotte Company,
PO Box 329, Chestertown, Maryland 21620
First printing December, 1989
1505 • 6/07
Page 4

Page 5

T able of Contents
Water Analysis ................................................5
Why? ............................................................
How?............................................................7
Water Sampling ..............................................9
Water Balance.................................................10
pH................................................................
Alkalinity ........................................................15
Calcium Hardness...........................................17
Temperature ...................................................19
Total Dissolved Solids (TDS) ...............................20
Water Sanitizers ..............................................21
Chlorine ........................................................
“Shocking” the Pool.........................................25
Cyanuric Acid.................................................
Bromine.........................................................28
Ozone...........................................................30
Alternative Systems..........................................32
Metal Systems.................................................32
Biguinide Systems ............................................32
Salt Chlorinators..............................................33
Hot Water/Spas...............................................33
Pool Problem Solver.........................................34
Algae (Nitrate and Phosphate) ...........................
Cloudy Water & Filtration ..................................36
Colored Water ................................................38
Stains ............................................................40
Scale.............................................................42
Eye & Skin Irritations.........................................44
Water Treatment Tables ....................................45
Lowering pH...................................................
Raising pH .....................................................47
Raising Chlorine..............................................47
Lowering Alkalinity ...........................................48
Raising Alkalinity..............................................50
Raising Hardness.............................................51
Raising Cyanuric Acid.......................................52
5
11
21
26
34
46
Page 6

Page 7

Water Analysis
Why?
The pool manager’s foremost responsibility is to
maintain a safe recreational environment for the
swimmer. To assure safety, the pool area must be
evaluated regularly for possible sources of injury. To the
inexperienced, a missing “No Diving” sign or an
insufficient level of chlorine in the water may seem
insignificant, but a properly trained pool manager
recognizes that these are serious safety violations which
require immediate attention.
Maintaining proper water quality is an extremely
important part of an overall pool safety program. A
water analysis serves three vital purposes: protecting the
swimmer, protecting the pool, and protecting against
wasteful chemical expenses. In addition. the pool
manager balances the water so it is cosmetically clear
and clean.
Protecting the swimmer
Every public pool is visited regularly by local health
officials who conduct a water analysis while evaluating
the overall safety of the pool area. The health official
and the pool manager share the responsibility of
protecting the swimmer. Cooperating with health
officials to ensure that the swimming facility complies
with state and local regulations is very important. The
pool manager should have a copy of these regulations or
codes and be familiar with them.
To prevent exposure to harmful bacteria, the most
important water quality test is for adequate levels of
sanitizer. Insufficient levels can cause swimmer
irritations which may later lead to severe health
problems. The most common sanitizers used in pool
water are chlorine and bromine. These are used to
prevent bacteria and algae within the pool and will be
discussed later in detail in Chapter 3. The tests for pH
5
Page 8

and alkalinity are also very important to the overall safe
operation of the pool. Cloudy water, skin and eye
irritation all result from improper pH and alkalinity
levels in swimming pool water. The pH must also be
properly maintained to maximize the sanitizing
effectiveness of chlorine. The remaining tests which are
performed on pool water are more important to the
overall protection of the pool and its mechanical parts.
Protecting the pool
Maintaining a proper pH level is the first step toward
protecting the pool. Corrosive conditions, which result
from a low pH, can severely damage pool surfaces,
plumbing, and cause bad staining problems.
Scale can result from a high pH and can clog waterlines,
filters, and leave residues on pool surfaces and parts. By
using daily water tests to make proper chemical
adjustments, the pool and its parts are better protected,
and should last much longer.
Protecting against chemical expense
The most common mistake made in treating pool and
spa water is using improper amounts of chemicals.
However, there are numerous occasions when chemicals
are added in excess of the water’s actual need. This
contributes to an imbalance in the pool chemistry,
which can be harmful to both the swimmer and the
pool, and as a result, more chemicals have to be added
in order to bring the pool back into balance. In extreme
cases swimmers may be restricted from using the pool.
Accurate water analyses should be used to determine
which chemicals need to be added. Chemical treatment
charts have been provided in the back of this handbook.
While there is no substitute for formal instruction in
operating a swimming pool, a retail pool professional
should be consulted for advice on how to add chemicals.
6
Page 9

How?
There are a variety of water test kits available to the
pool manager. Each has its own unique set of directions
which should be read very carefully. Though reagents
may look similar from one kit to the next, the color
standards in the color comparator or viewer can vary
from one manufacturer to another. Therefore, it is
important to realize that reagents or procedures are not
interchangeable from test kit to test kit.
One of the most ignored, yet vital, directions in every
test kit instruction is always to rinse and clean the test
tubes and sampling equipment thoroughly. This should
be done before and after each test. Unclean tubes can
result in test container staining, and may inadvertently
cause false readings if reagent or water remains in a
container from a previous test.
When using a color comparator always read test results
against a white background. If your comparator does
not have a diffusion screen or a transparent white screen
behind the color standards, it may be necessary to hold a
piece of white plastic or paper behind the comparator
when reading results. This procedure will neutralize
background interferences which can significantly affect
test results.
If you ever have trouble determining test results, take
your kit, along with a water sample, to a local pool
retailer or service professional for advice. Several “do’s”
and “don’ts” are listed on the following page.
7
Page 10
Do’s
1. Always hold reagent dropper bottles
vertically and squeeze gently to
obtain a uniform drop size. Never
hold dropper bottles on an angle.
2. Always fill test tubes so the bottom
of the water line is precisely on the
indicated “fill-to” line.
3. Keep reagent bottles tightly sealed
and avoid excessive heat or freezing
temperatures.
4. Keep DPD liquids away from heat.
Don’ts
1. Never leave the test kit where
children can find it or reach the
components within the kit. Remember that safety is
top priority.
2. Do not handle reagent tablets and avoid contact
with test reagents.
3. Do not store your test kit in direct sunlight or next
to water treatment chemicals. These may destroy
instructions and slowly deteriorate components
within the test kit.
8
Page 11
Water Sampling
For best results the analyst should take samples from three
to four areas around the pool each day. This can be
especially beneficial in larger pools. Keep in mind that
samples obtained on the surface should always be avoided
since this may not be representative of the actual water
chemistry. Several “do’s” and “don’ts” include:
Do’s
1. Rinse the sampling container several times with the
water to be tested.
2. Holding the sampling container sides, immerse to
elbow depth, approximately 15 inches or more
below the surface, keeping the container six inches
away from the side wall.
3. Always test the water sample promptly after
collecting it.
Don’ts
1. Never collect a sample
near a make-up water
inlet, return area, or next
to chemical feeders.
2. Nevertestawatersample
immediately following a
shock treatment.
3. Never use a sampling container that is in any way
dirty or has a rusty lid—use plastic whenever
possible. Glass should not be allowed in the pool
area.
9
Page 12

Water Balance
Corrosive
Balanced
Scale Forming
Water balance is defined as a condition where the water
is neither corrosive nor scale forming. The factors in
determining water balance include pH, total alkalinity,
calcium hardness, temperature, and total dissolved solids
(TDS).
Since water in motion tends to be corrosive, water
balance is very important within the pool. To avoid
corrosive conditions which can etch pool surfaces and
mechanical parts, maintain all water quality factors
within the proper range. Proper levels allow the water to
become saturated, or non-corrosive, a key goal for
prolonged trouble-free pool operation. When pool water
becomes oversaturated with dissolved substances,
especially calcium salts (primarily calcium carbonate),
these substances fall out of solution and can make the
water cloudy or deposit on
pool surfaces. This is called
scale and is a menace to
pool surfaces and plumbing
fixtures, clogging water
lines and filters.
As water balance is so
important in maintaining
the pool surfaces and parts,
it constantly needs to be
monitored with test
equipment.
Pipe diagram
10
Page 13
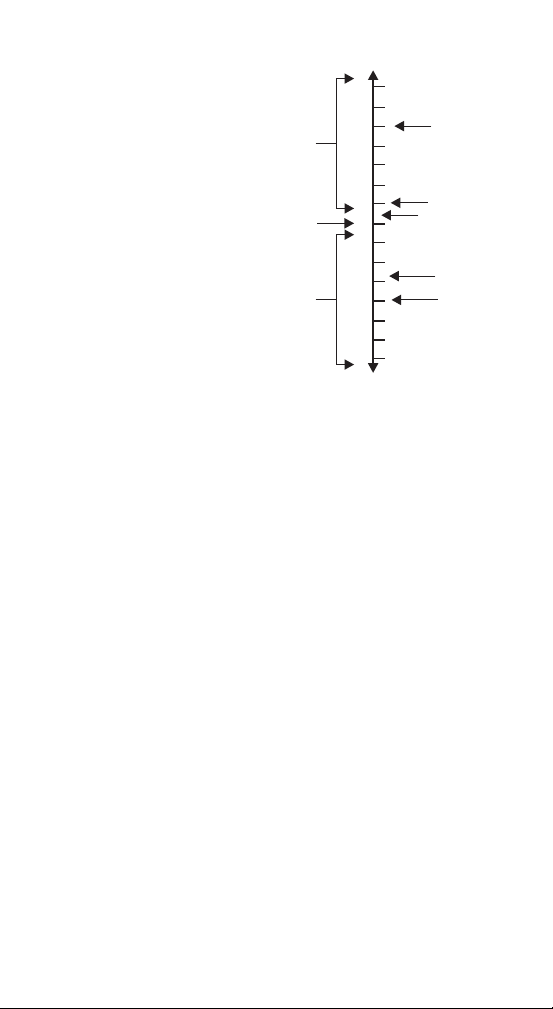
pH
14
13
12
11
10
9
8
7
6
5
4
3
2
1
0
Basic
Neutral
Acidic
Bleach
Ocean Water
Pool Water
Orange Juice
Trichlor
(liquid Cl )
2
What is pH?
The term pH refers to
the concentration of
hydrogen ions in water.
The pH test determines
if a substance is acidic,
neutral or basic. A
substance with a pH of
7.0 is neutral, neither
acidic nor basic. Those
with a pH of less than
7.0 are acidic (orange
juice has an acidic pH of
4.2). A pH above 7.0 is
basic (ocean water is basic with a pH of about 8.0). The
ideal pH for pool water is slightly basic, between pH 7.2
and 7.6. This range is most comfortable for the
swimmer, protects the pool equipment, and allows
sanitizers to work efficiently.
Why Do We Test pH?
The pH test is critical to protecting the pool. Though
low or high pH levels can irritate swimmers’ eyes or skin,
pH is normally considered the best indicator of overall
“pool health.”
Controlling pH is critical for protecting the pool and its
equipment from costly damages due to corrosive/acidic
water or scale-forming/basic water.
For an indication of just how the pH affects the overall
condition of the pool and its parts, other tests must be
performed to obtain a clear answer. These tests are
discussed later.
11
Page 14
The pool water pH should be tested several times a day
pH
LOW
If pH is too low (Acidic)
•
•
•
•
corrosion of pool equipment/staining
swimming eye irritation
etching plaster pool surfaces
chlorine dissipates quickly
pH
HIGH
If pH is too high (Basic)
•
•
•
•
scale accumulates on pool equipment
cloudy, turbid water
chlorine sanitizing power is weakened
swimmer eye irritation
in a public pool with moderate-to-heavy swimmer usage.
The pool’s pH level is constantly being changed by
chlorine or other treatment chemical additions.
Swimmer usage, additions of fresh make-up water,
leaves, and debris can also alter the pH slightly.
The obvious reason to test pH is not only to identify a
problem but to accurately decide how to remedy the
problem. If a water test reveals a highly basic pH, such as
8.2, then the water supply needs to have an acidic
substance added to bring the water back down to a pH
of about 7.4. This need is called an “acid demand” and
can be corrected by adding a liquid acid (muriatic acid)
or a dry acid (sodium bisulfate).
If a water test shows an acidic pH level, such as 6.8, then
a basic substance should be added to bring the water
back up to a pH of about 7.6. This need is called a “base
demand” and can be corrected by adding soda ash
(sodium carbonate) to the water.
High pH
Low pH
®
®
Acid Demand
Base Demand
See pages 44-45 for pH treatment charts.
12
Page 15

How do we test pH?
pH is one of the most vital tests
performed daily on pool water,
anditisalsooneofthemost
simple to perform. A single
liquid or tablet indicator is used
to provide a distinct pH color
reaction which varies from
yellow to deep red. This
indicator (called Phenol Red)
measures pH from 6.8 to 8.4.
Most state public health codes require that pH color
comparator be suitable for reading to 0.1 pH unit.
Check local regulations to confirm that the on-site test
equipment is in compliance.
Testing pH is simple: the test tube or sample cell is
rinsed and filled to a specified line with sample water.
Phenol red liquid or tablet is added to the sample as
specified in the instruction. The sample is capped and
mixed. The resulting colored solution is compared to
color standards to determine the precise pH level.
Alternative methods of testing pH include two types of
instrumentation. One is an electronic colorimeter
which analyzes the color development with the phenol
red pH test by passing a light beam through the sample.
The amount of light that is absorbed by the reacted test
sample determines the result, which is indicated by a
meter display.
The pH meter relies upon a sensor called an electrode,
which is immersed in a water sample. The electrode
measures the electrical activity within the solution and
displays the pH value. Though the pH electrode needs
no phenol red reagent to provide a test result, it does
need constant calibration with specific pH solutions
(known as buffers) to assure accurate readings.
13
Page 16

Paper test strips are gaining acceptance at public pools.
Although they are utilized in smaller private pools and
hot tubs, managers should check the local health
regulations before using test papers as their only testing
device.
Ideal Range for pH
7.2 - 7.6
14
Page 17
Alkalinity
Ideal Alkalinity
Low Alkalinity
Allows to bouncepH
Allows proper pH control
High Alkalinity
Usually means high pH
and is hard to adjust
pH reading
pH reading
pH reading
What is T otal Alkalinity?
Total alkalinity is a measure of the acid-neutralizing
capacity of the water supply which enables it to resist
abrupt changes in pH. Total alkalinity is commonly
known as a pH stabilizer because, at proper levels, a
consistent pH level can be maintained while treatment
chemicals or fresh make-up water is added.
Commercially available chlorine treatment compounds
can range in pH from 3.0 to 13.0, and make-up water
can range in pH from 5.0 to 8.0. Therefore maintaining
total alkalinity at recommended ranges is extremely
important.
Why do we test Alkalinity?
The purpose of testing alkalinity is to determine how
susceptible the pool water is to rapid pH changes. A low
level of total alkalinity allows the pH to fluctuate or
“bounce” when materials are added, even in small
amounts. A high level of total alkalinity can have the
reverse effect, limiting the ability to change pH levels
which may be too high. Total alkalinity is a measure of
acid-neutralizing materials in the water, therefore it is
normal to see high
alkalinity when the
pH is also too high.
A moderate or
ideal alkalinity
allows the pH to be
maintained
without requiring
constant chemical
adjustments.
Alkalinity tests
should be
conducted once a
week.
15
Page 18

How do we test Alkalinity?
The level of alkalinity is usually determined using a
titration method: an indicator is added to a water
sample to produce a distinct color. A weak acid (titrant)
is then added slowly until the original color changes.
The amount of titrant added to produce the color
change determines the result, either by counting the
number of drops added or by measuring the volume of
titrant. Read the test instruction carefully to determine
the proper color change and procedure required. If there
is ever doubt in the test result, repeat the test and
carefully stop the titration procedure when the endpoint
color is reached.
Ideal Ranges for Alkalinity
80 - 100 ppm
sodium hypochlorite
100 - 120 ppm
or bromine
Note: Cyanuric acid contributes to the alkalinity test. The
alkalinity reading should be adjusted to compensate for this. Do
this by multiplying the cyanuric acid reading by 1/3 and
subtracting this from the alkalinity reading.
CYA = 60 ppm
TA = 100 ppm
100 - (60 x 1/3) = 100 - 20 = 80
• In pools using calcium hypochlorite or
• In pools using dichlor, trichlor, chlorine gas
Thus 80 ppm is the true carbonate alkalinity value that should
be used in water balance calculations.
16
Page 19

Calcium Hardness
Hard Water
stains dishes and clothes
Soft water
leaves items clean and clear
What is Calcium Hardness?
Calcium hardness refers to the level of calcium dissolved
in water. If the water has an abundance of calcium, the
water is described as “hard”; water with a low content is
described as “soft.” The most common source of calcium
in pools is fill water, especially if the source or aquifer
contains high calcium. When pool water is low in
calcium content, calcium may dissolve from plaster
surfaces. The term “total hardness” refers to both the
calcium and the magnesium content of the water. Do
not confuse total hardness test results with those for
calcium hardness.
Why do we test Calcium Hardness?
Testing calcium hardness evaluates
the pool water’s aggressive, or
saturated nature. Water is naturally
aggressive and is known as “the
universal solvent.”
In pool water it is important to
evaluate the level of calcium
hardness since water has a natural
tendency to dissolve certain
minerals that are a component of
the pool surfaces. If the water
contains too many minerals it
becomes saturated and the calcium
will begin to fall out of the water,
settle around the pool, and leave
noticeable crusty, white deposits.
These deposits are called scale and
can be seen on pool parts and walls.
Scale can clog filters, heaters, and
plumbing fixtures—leading to poor
water circulation and costly repairs.
17
Page 20

Inadequate levels of calcium
hardness also need to be avoided. In
plaster pools, aggressive low
hardness water can result in etching
or pitting in the plaster. Pools with
inadequate hardness levels are
susceptible to corrosion of metal
parts in the pool or heat exchange
systems.
Scale can clog pipes
Fortunately water does have a saturation point where it
is no longer aggressive and will not deposit scale on pool
surfaces, providing maximum protection to the pool
surface and its parts. This condition is called “water
balance,” and can only be obtained when the pH, total
alkalinity, calcium hardness, and temperature factors are
all at recommended levels.
How do we test Calcium Hardness?
To test calcium hardness a titration procedure is utilized.
An indicator is added to the test sample to produce a
color indicating the presence of calcium. The sample is
titrated with another reagent until a color change occurs
and the titration is complete. Next, the result is
calculated from the volume of titrant used or is read
from a calibrated dispenser or test vial. Always read the
test instruction carefully to determine the proper color
change and procedure, watching closely for the
complete color change from the original color.
Ideal Ranges for Calcium Hardness
Spas: 175 - 300 ppm
Pools: 200 - 400 ppm
18
Page 21

Tempera t u re
0
10
20
30
40
50
60
70
80
90
100
OK
ºF
Water temperature is an important
comfort factor for swimmers, and for the
health of the pool as well. Pool water
should be maintained between
75°-85°F. Every pool should have an
accurate thermometer for measuring
water temperature.
As water warms, the substances within
the water become more reactive and
aggressive. Higher water temperatures
provide an environment where scale
and cloudy water are more likely to
occur, and evaporation increases.
At cooler temperatures, a pool is prone to corrosion if
the water is not properly saturated with minerals.
Water temperature is actually another key element in
calculating the pool’s saturation index, but plays a
smaller role than pH, total alkalinity and calcium
hardness. In spas, where water temperatures reach 104°F,
it is very important to maintain proper saturation index
levels since heaters and other fixtures can be quickly
damaged.
Ideal Range for T emperature
Pools: 75 - 85°F
19
Page 22

T otal Dissolved Solids (TDS)
What is TDS?
Total dissolved solids refer to the amount of dissolved
substances or minerals (actually charged ions) within
the pool. These substances enter the pool either through
the original water supply or by the addition of treatment
chemicals. As water evaporates total dissolved solids
remain behind and increase over time. Distilled water is
a solution that contains no dissolved solids. Sea water,
on the other hand, contains a vast amount of total
dissolved solids.
Due to evaporation and intense chemical treatments,
dissolved solids should be closely monitored in spas.
Why do we test TDS?
Water with high TDS readings may be cloudy and cause
corrosion. Total dissolved solids should be kept under
2000 parts per million. If TDS exceeds this amount, part
of the pool water should be drained and replaced with
fresh (low TDS) water.
How do we test TDS?
Most public pools do not have on-site equipment for
analyzing total dissolved solids, and a water sample must
be taken to a local pool supply retailer who has a TDS
meter. A TDS meter measures the electrical
conductivity of a water sample. An electrical current is
passed between a two-part electrode and the meter then
displays the total dissolved solids level. The dissolved
minerals in water conduct electricity and a total
dissolved solids probe or instrument can be effectively
used to measure TDS in parts per million.
Ideal Range for TDS
Less than 2000 ppm. As a general rule TDS should not
increase 1500 ppm above the initial start-up amount.
Note: salt chlorine pools will run higher .
20
Page 23

Water Sanitizers
Chlorine
What Is Chlorine?
The chlorine used in sanitizing pool water is
commercially available in liquid, dry, or gas forms.
The dry form of chlorine is sold as granular, tablet, or
stick products. Any form of chlorine, when added to
water produces hypochlorous acid (HOCl).
Hypochlorous acid kills bacteria, algae, and
disease-causing organisms and is commonly referred to
as Free Available Chlorine or Free Active Chlorine
(F.A.C.). It is the killing power of free chlorine which is
important in protecting the swimmer.
The amount of free chlorine available in pool water is
significantly affected by pH, sunlight, and impurities.
A high pH level reduces the overall sanitizing power of
free chlorine, while sunlight destroys chlorine within
water. Nitrogen-containing wastes reduce free chlorine
levels by forming less active combined chlorine or
chloramines. Combined chlorine has a pungent odor
and can become irritating to the swimmer. When the
combined chlorine measures 0.2 ppm or more in a pool
it is necessary to superchlorinate or shock treat the pool
to oxidize the combined chlorine.
Several types of chlorine are used in public pools such as
gas or liquid chlorine which are added by automated
control-and-release systems.
Chlorine tablets or sticks are also used in public pools by
adding them to feeders (never add different types of
chemicals to a feeder at the same time). Listed on the
next page are the types of chlorine currently available in
the United States. Each has a different strength which is
represented by % available chlorine.
21
Page 24

The pH of each chlorine is given to show how chemical
additions can affect your ideal pH of 7.2-7.6.
Ideal Ranges for Chlorine
Pools: 2.0 - 4.0 ppm
Spas: 4.0 - 6.0 ppm
Chlorine Treatment Compounds
Trade Name
(Proper Name)
Gas (Gas Chlorine) 100% approx. 2.0
Liquid Chlorine
(Sodium Hypochlorite)
Litho (Lithium Hypochlorite) 35% 10.7
Cal Hypo (Calcium Hypochlorite 65% - 75% 11.8
Dichlor (Sodium
Dichloro-s-triazinetrione Dihydrate)
Dichlor (Sodium
Dichloro-s-triazinetrione Anhydrous)
Trichlor (Trichloro-s-triazinetrione) 90% 3.0
% Available
Chlorine
5% -15% 13.0
56% 6.0
62% 6.0
pH
22
Page 25

Why do we test Chlorine?
Sanitized water in a public pool is top priority for public
health inspectors. Testing and maintaining an adequate
level of Free Available Chlorine is a daily goal of the
pool manager. Most states require free chlorine levels of
at least 1.0 ppm. Tests should be taken several times a
day, two to three times under normal bather loads, and
more frequently when heavier bather loads are
experienced. Local health departments usually have
requirements for testing frequency.
Testing for chlorine is not limited to measuring Free
Available Chlorine. Regular analyses for the amount of
combined chlorine should be made as well. By testing
combined chlorine once a day or several times a week,
the need for superchlorination can be determined.
Combined chlorine can cause chlorine odor and severe
eye irritation. A combined chlorine test will indicate
that superchlorination is required rather than a simple
pH adjustment. Combined chlorine levels should not
exceed 0.2 ppm.
How do we test Chlorine?
Prior to the introduction of DPD (diethyl-p-phenylene
diamine) in 1969, most chlorinated pools in the United
States were tested with OTO (orthotolidine). OTO is a
colorless liquid that produces a yellow color when it
reacts with chlorine, and as with most colorimetric tests,
the color is proportional to the concentration. Since
the test is not suitable for or measuring the Free
Available Chlorine, its use is not permitted at public
pools in almost every state in the nation. Most state
health codes specify that a test kit must be on site for
measuring Free Available Chlorine, and some even
specify that the kit must use the DPD test.
23
Page 26

The DPD test method for free
chlorine uses either a single foil
stripped tablet, identified as
DPD #1, or a dual liquid
reagent system. In the tablet
system one Chlorine DPD #1
tablet is added to a measured
water sample in a tube and
mixed to disintegrate the tablet.
Color develops from a faint
pink to a vivid red, depending
on the concentration of free chlorine, and is matched to
standards with known values in a comparator.
The dual liquid reagent system produces virtually the
same color reaction after drops of each solution are
added to the test sample.
To obtain a reading for Combined Chlorine the DPD #3
tablet or DPD #3 liquid is added to the original free
chlorine test sample. The result is read as Total Chlorine
and the increase in color represents combined chlorine.
Example:
Total Chlorine Free Chlorine Combined Chlorine
2.0 — 1.5 = 0.5
If the combined chlorine is above 0.2 ppm,
superchlorination or a shock treatment should be
considered to achieve “breakpoint chlorination”
†
.A
chlorine-based treatment should be used if free chlorine
is too low. If additional free chlorine is not needed, an
oxygen-based shock treatment can be utilized to
eliminate combined chlorine.
†
Breakpoint is achieved by multiplying the combined chlorine
level times 10. Dose the resulting amount in ppm.
24
Page 27

“Shocking” the Pool
Pool water that contains combined chlorine
(chloramines) and other organic contaminants such as
deodorants and lotions must be treated to keep the
water clear and sanitary. There are two primary
treatment processes for “shocking” pool and spa water:
1. Use a liquid or granular chlorine compound in a
concentrated or excessive amount to increase the
free chlorine to a level high enough to break down
the undesirable chloramines and organics. In most
cases, the chlorine level is raised to 10 ppm (about 5
times the normal level). Following a high chlorine
shock treatment, the pool is often closed for 6-12
hours to allow chlorine levels to return to normal. 4
ppm is considered a safe concentration for re-entry
into the pool.
2. “Non-chlorine” shock is a chlorine-free
persulfate-based compound which is designed to
eliminate undesirable combined chlorine. This type
of treatment has the unique advantage of allowing
bathers to return to the pool shortly after use.
Fecal, vomit or blood accidents
If fecal matter, vomit or blood enters the water,
immediately evacuate the pool and remove as much of
the material as possible. The pool should then be closed
and treated with at least 10 ppm chlorine. Pool
managers should train all personnel on the guidelines for
treating such accidents. These may be obtained from
local health officials or from the Center for Disease
Control website (www.cdc.gov/healthy swimming).
A note about superchlorination
Some organic chlorine cannot be oxidized by shock or
superchlorination. These are sometimes referred to as
nuisance compounds. Usually the concentration of
these is very minimal, but they do contribute to the
combined chlorine reading.
25
Page 28

Cyanuric Acid
What is Cyanuric Acid?
Since chlorine in water is rapidly destroyed by direct
sunlight, cyanuric acid is added to increase the overall
time chlorine will remain in the pool. Cyanuric acid is a
chemical that bonds to free available chlorine, enabling
it to sanitize the water while being protected from the
sun.
Cyanuric acid is available in granular or powdered forms
and is usually added through the skimmer. However,
there are “stabilized chlorine” compounds (dichlor and
trichlor) that already include cyanuric acid. These allow
the pool operator to avoid making periodic additions of
cyanuric acid. A considerable majority of home pool
owners now utilize stabilized forms of chlorine in
outdoor pools.
Why do we test Cyanuric Acid?
Like any chemical additive, cyanuric acid must be
periodically checked to determine if the pool contains
sufficient levels. Insufficient levels of cyanuric acid will
not protect the free available chlorine from sunlight.
Most state public health standards dictate that 100 ppm
is the maximum level of cyanuric acid permitted in
swimming pools. While ideal levels are 30-50 ppm, an
upper limit of 100 ppm was established because cyanuric
acid can only be reduced by pool water removal,
replacement, or by “splash-out.” Sometimes very large
amounts of cyanuric acid can build up if stabilized
chlorine compounds are used regularly over long periods
of time.
Public pools can be closed by local health agencies if
they are not maintained below the maximum level.
26
Page 29

How do we test Cyanuric Acid?
Test results are determined by the degree of cloudiness or
turbidity that develops when a test reagent is reacted
with cyanuric acid. The amount of turbidity indicates
the level of cyanuric acid, with low readings being more
transparent and high readings being very cloudy.
The cyanuric acid test is usually made by viewing the
reacted test sample in a special reading tube containing
a black dot on the bottom. The reacted sample is
dispensed into the tube to a point where the black dot
disappears from sight. At this point the depth of the
solution indicates the cyanuric acid concentration. In
most test kits the values for concentration in ppm are
displayed on the side of the tube.
The analyst should always keep in mind that cold or hot
water samples will interfere with the test results. It is best
to test samples between 70°-85°F.
Ideal Range for Cyanuric Acid
30 - 50 ppm (maximum level 100 ppm)
Note: Cyanuric acid contributes to the alkalinity test. The
alkalinity reading should be adjusted to compensate for this.
Do this by multiplying the cyanuric acid reading by 1/3 and
subtracting this from the alkalinity reading.
CYA = 60 ppm
TA = 100 ppm
100 - (60 x 1/3) = 100 - 20 = 80
Thus 80 ppm is the true carbonate alkalinity value that
should be used in water balance calculations..
27
Page 30

Bromine
What is Bromine?
Bromine is a sanitizer sometimes used in place of
chlorine, particularly in hot water systems. In the same
way that chlorine forms hypochlorous acid, bromine in
water forms hypobromous acid. It is introduced into
water using one of three systems:
1. A two-step system uses a bromide salt added to the
water as sodium bromide, which is oxidized to
hypobromous acid by monopersulfate compounds,
chlorine or ozone.
2. Electrolytic generators can convert bromide salt to
bromine.
3. The third system uses a stick or briquette that
contains chlorine and bromine. This is called
BCDMH
†
. When bromine reacts, it forms a bromide
ion. The chlorine in BCDMH reacts with the
bromide to regenerate bromine. In 1999 a similar
product was introduced, dibromodimethylhydatoin
(DBDMH), which does not contain chlorine. Both
of these bromine products are usually erosion-fed in
an automatic feeder.
Bromine, like chlorine, may be degraded by sunlight.
Bromine destroys bacteria, algae, and water-borne
diseases in much the same way that chlorine sanitizes
water. Like chlorine, bromine forms free available
bromine and combined bromine or bromamines.
Combined bromine is a very active sanitizer, and unlike
combined chlorine, does not have the pungent odor of
combined chlorine.
†
1-bromo-3-chloro-5,5-dimethyhydantoin
28
Page 31

Why do we test Bromine?
Since the test for adequate sanitizer is always the most
important for protecting swimmer health, the bromine
test is vital. When used, a minimum bromine residual of
3 ppm should be maintained within the pool which will
assure proper levels of sanitization within the water.
Always monitor bromine frequently during periods of
heavy bather use.
How do we test Bromine?
Operators should use DPD to test bromine. This is read
against color standards in a visual comparator. Since
Free Bromine and Combined Bromine are similar in
sanitizing strength, they both react with a DPD #1 tablet
or 1A and 1B liquids. This reading represents the level
of Total Bromine.
If a bromine comparator is not available, operators can
use a chlorine comparator and multiply the reading by
2.25 to calculate ppm bromine. But for best results, a
bromine comparator should be used.
Ideal Ranges for Bromine
Pools and Spas: 3 - 6 ppm
29
Page 32

Ozone
O
2
O
3
What is Ozone?
Most people think of
ozone as a layer of gas up
above the earth which
diffuses and protects us
from harmful ultraviolet
radiation. That ozone
layer is actually made by
sunlight striking oxygen
molecules (O
)and
2
adding another atom of
oxygen to produce ozone
(O
). Ozone can also be produced when an intense
3
electrical discharge splits the oxygen molecules, such as
lightning and photocopy processing. Both produce
relatively low levels of ozone which are quickly diffused
by the atmosphere.
There are two types of ozone generators for pools being
manufactured today: the ultraviolet (UV) method, and
the electrical discharge method (commonly known as
corona discharge). The UV method uses special UV
light bulbs which produce a specific bright light to break
up oxygen molecules in the air blown past the bulbs and
into the water. The corona discharge method utilizes
high voltage electrical charges in a confined space where
compressed air is forced through the chamber and into
the water.
Ozone is an effective oxidizer but it dissipates quite
rapidly. Because of this, ozone must be used in
combination with chlorine or bromine to meet public
pool health requirements.
Ozone is very effective against organics and can help to
eliminate organic combined chlorine compounds.
Why do we test Ozone?
Ozone is generated and added to pool water in a
bubbling fashion. If a UV lamp or discharge system
30
Page 33

should malfunction, the air may still be flowing into the
view
water but without the required ozone residuals. As long
as some ozone is going into the water, most experts agree
that the oxidation process is being properly
accomplished. As little as 0.001 parts per million (ppm)
has been determined to be effective.
How do we test Ozone?
Because ozone is required at
such low levels and it
dissipates in minutes after
being produced, it can be
quite difficult to determine
the ozone content of water.
Most current testing is done
with the DPD test method
which produces a pink color in proportion to the
amount of ozone in the water sample. However, DPD
will also react with any chlorine or bromine in the
water. Often, testing with DPD is sufficient to identify
the total level of the safe combined oxidizers.
Another test method is the indigo-trisulfonate method.
This method is best suited for use with electronic
colorimeters since visual color distinction is extremely
difficult for measuring at the low levels of 0.01 ppm.
Ideal Range for Ozone
0.01 - 0.1 ppm
31
Page 34

Alternative Systems
Some health jurisdictions have approved the systems
below for use in pools and/or spas. Check with the local
health department for compliance and recommended
concentrations.
Metal Systems
There are systems that introduce metals or
combinations of metals such as copper, silver or zinc into
water to inhibit bacteria and algae. There are 3 ways
these can be added to the water.
1) Some systems are copper and silver electrodes that
use an electrical current to release copper and silver into
the water.
2) Some systems are mineral beds that contain metals. a
Water passes through the bed, the metals dissolve and
areaddedtothewater.
3) One can add metals to water by adding them directly
as a liquid. Copper sulfate and silver oxide are examples
of these.
Chlorine or bromine must be used with all metal
systems. Zinc and silver are usually not tested in these
systems. Copper concentrations can vary from 0.3 to
over 1.0 ppm. Color comparators or test strips are used
to determine copper concentrations. Follow the
manufacturer’s recommendations.
Biguinide Systems
Polyhexamethylene biguinide (PHMB) is a bactericide
used in a 3 part system. The EPA has approved this
system for use as a recreational water sanitizer. The other
2 parts of the system are an algicide (quaternary
ammonium compound (QAC) or a polyquat) and
hydrogen peroxide, which serves as an oxidizer.
Chlorine, bromine, metals or monopersulfate should not
be used with this system.
The recommended concentration of PHMB is 30-50 ppm. T est
strips and color comparators may be used to test PHMB.
32
Page 35

Regular maintenance doses of the algicide and oxidizer are
recommended. Check the manufacturer’s recommendations for
dosages.
Salt Chlorinators
Chlorine can be produced by applying a low voltage
direct current to salt (sodium chloride). If sodium
bromide salt is used, bromine will be produced.
The salt can be in a separate brine tank or in the pool
water. There are minimum concentrations of salt
required, usually 2,000-6,000 ppm – follow the
manufacturer’s recommendations. It is also important to
size the units properly so that enough halogen is
produced to meet the demand.
To test the salt concentration, one can use a field kit or a
test strip.
Hot Water
The chemistry of pools and spas is similar, but there are
some things to keep in mind when working with hot
water systems. Since there is a much smaller volume of
water,thebatherloadismuchhigher.Thisleadstoan
increase in bacteria and waste products. Chemical
reactions are faster at higher temperatures. Calcium
carbonate is less soluble, so the tendency for scaling and
cloudy water is greater. Particular consideration must be
given to the heater element, where the water
temperature is higher than the rest of the spa. Scale will
form on this before it forms elsewhere in the system.
The higher temperatures also provide a better
environment for some bacteria. Pseudomonas is a type
of bacteria that frequently causes skin rashes in hot
water. Because of this, hot water systems need higher
sanitizer concentrations and should be shocked
regularly.
Jetted spas and tubs can force carbon dioxide from the
water. This causes the pH to increase. Because of
evaporation and chemical addition, the TDS will
increase faster than in a pool. TDS is a good way to
33
Page 36

determine when a spa or hot tub should be drained. If
the TDS exceeds 1,500 ppm from the TDS at start-up, it
is a good idea to drain and refill.
Hot water systems should be kept below 104 degrees.
Small children and expectant mothers should limit their
time in hot water environments. As always, check the
local health department regulations
Pool Problems
Algae
Algae is probably the most annoying water problem in
outdoor pools since it is so unsightly and difficult to
destroy. Daily brushing and several treatments may be
required to successfully eliminate an algae problem.
Algae multiplies rapidly, so by the time the human eye
can notice it there are billions of algae cells in the pool.
The two most frequent complaints received about
public pools are related to algae and cloudy water (the
three most common colors of algae are green, black and
yellow/mustard). Green algae can make a pool especially
cloudy. Algae can clog filter systems and make pool
surfaces slippery. The best way to avoid an algae problem
is to keep at least 2.0 ppm of free available chlorine
circulating throughout the pool water at all times. For
persistent algae problems, an algicide may be used.
Nitrate and Phosphate
Nitrate and Phosphate are the two building blocks for
algae. Nitrates may enter the water from leaves or debris
but other sources of nitrates include well water supplies
and localized spraying of lawn or crop fertilizers.
Because nitrates can only be removed by draining the
water, some manufacturers have focused on removing
the other algae nutrient, phosphate. Phosphate can
occur naturally, come from fertilizers or from the
breakdown of Phosphate-based sequestering agents. A
variety of phosphate removal systems have been
introduced to eliminate the potential for algae.
34
Page 37

Ideal Ranges
Pools and spas: <10 ppm Nitrate
Pools and spas: <100 ppb Phosphate
Algae
Color
Green Algae Black Mustard Algae
Pea green color,
sometimes colors
entire body of water.
Also attaches to
pool surfaces.
Cause
Insufficient or inactive levels of sanitizer. Inadequate water
circulation.
Treatment
• Check pH and
adjust if necessary.
• Shock treat pool
water.
• Brush surfaces if
necessary.
• Retest pH and
repeat treatments
if necessary.
Note: There are a number of specialized algae treatments on
the market. If the problem is persistent, ask a pool professional
about these.
Better known as
“black spots” on
pool walls and
surfaces.
• Brush affected
areas
thoroughly.
• Spot treat affected
areas with
sanitizer.
• Shock treat pool
water to 30 ppm
chlorine and later
add algicide.
• Brush and
vacuum
as necessary.
A yellow film, usually
found on steps or
walls.
• Brush affected
areas thoroughly.
• Spot treat
affected areas
with sanitizer.
• Shock treat pool
water and later
add algicide.
• Retest pH and
repeat treatments
if necessary.
35
Page 38

Cloudy Water
Cloudy pool water is a common problem in swimming
pools. The usual causes are improper filtration, and/or
improperly balanced water. An algae condition or severe
chloramine condition can also cloud pool water.
If a cloudy water condition should occur, check the filter
system for clogs and/or damage and determine if
adequate flow rates exist. In moderate to heavily used
pools a minimum six hour turnover rate is usually
suggested. This means that every six hours the entire
body of pool water has been recirculated through the
filter system. Clogged filters can often be cleared by
backwashing.
After a thorough evaluation of the filter system, the
water balance should be checked. Look for signs of high
calcium hardness, high pH and alkalinity levels.
Filtration
Sand Filters: Check the sand for gaps or hard spots
and/or replace the sand. Generally sand should be
replaced every 3-5 years.
DE Filters: Soak the “fingers” in a filter cleaner. If the
filter is a grid-type filter, hose the grids off and inspect
them for damage.
Cartridge Filter: Replace dirty cartridges with clean ones
and clean the dirty ones for the next replacement.
Consider contacting your service pro for advice before
investigating a suspected filter problem.
36
Page 39

Cloudy Water
Cause
Poor Filtration Algae Growth Unbalanced Water
Confirmation
Slow filter turnover
rates
Treatment
• Backwash and
clean filter
• Determine if
filter media
needs
replacement
• Run filter for
24 hours
Note: Consult a pool professional if a cloudy condition persists.
Repeated treatments or the use of a clarifier may be
recommended.
Hazy pool
water with
slightly green
appearance
• Super-
chlorinate to
30 ppm
chlorine and
brush pool
surfaces
HIGH
•Calcium
hardness
• TDS
•Cyanuric acid
• Replace a
portion of
the pool
water with
fresh water
of low
hardness
and TDS
LOW
• pH
•Alkalinity
• Add dry
acid or
liquid acid
to reduce
pH to
7.2-7.6
and
alkalinity
to 80-120
37
Page 40

Colored Water
Black?
Green?
Red?
Brown?
Clear, colorless pool water is the goal, but sometimes it is
difficult to achieve. Colored water is a nuisance caused
by oxidized metals and algae, and can result in stained
pool surfaces. A turbid green pool water condition is
usually attributed to algae. To gain a better
understanding of algae treatment see the section on
algae earlier in this chapter.
Water color resulting from oxidized metals is translucent
in its early stages. Green, red, brown, and black are some
of the more common colors produced by dissolved
metals. Green , red or brown colors are usually produced
by iron. Blue or blue-green water is due to copper.
Brown or black color is usually due to manganese, but
iron can also cause these colors. These metals can come
from the water source or result from corrosion of pipes or
fixtures.
Often these colored water conditions
appear after a pool is first filled or after
a shock treatment. If the fill water
contains metals it should be treated
with a sequestering agent and filtered
prior to chlorine additions. A shock
treatment can cause metals to oxidize
which allows them to fall out of
solution and become more visually apparent.
38
Page 41

Colored Water
Color
Green/Red/Brown Blue/Green Brown/Black
Iron Copper Manganese
Treatment
• Brush
• Shock
treat and
brush
• Vacuum
Note: T ake a pool sample to a pool professional for dissolved
metals testing immediately after treatment and at least once a
month.
• Adjust pH & alkalinity to recommended ranges
• Add sequestering agent & run filter
• After 12 hours, shock treat the pool
• Retest pH & alkalinity. Test hardness levels, and,
if necessary, raise to 200 ppm
39
Page 42

Stains
When stains appear on swimming pool surfaces
immediate action should be taken to avoid costly and
annoying repairs. Brushing can often remove fresh
stains. Neglected stains in plaster pools may ultimately
require draining the pool and applying an acid wash to
the surfaces. Like colored water, stains are the result of
metal ions in pool water and they indicate that either
the source water contains metals (such as copper, iron
and manganese) or that a corrosive pool water condition
is dissolving metal pool components.
After noticing a pool stain, determine what caused it by
testing the water for metals. If the test indicates a metal
problem, the pH should be adjusted to be within the
proper range of pH 7.2-7.6. If the problem persists, add a
sequestering agent to chemically bind the metals to
keep them from causing staining problems.
40
Page 43

Stains
Color
Green/Red/Brown Blue/Green Brown/Black
Cause
Iron Copper Manganese
Treatment
• Adjust pH & alkalinity to recommended ranges
• Vigorously brush the stained areas
• Add sequestering agent and run filter
• After 12 hours, shock treat the pool
• Retest pH and alkalinity. Test hardness levels, and, if necessary,
raise to 200 ppm
Note: T ake a pool sample to a pool professional for dissolved
metals testing immediately after treatment and at least once a
month.
41
Page 44

Scale
Crusty white deposits on pool surfaces indicate a
severely high level of one or more water balance factors.
Scale deposits not only make pool surfaces rough, but
also reduce water circulation by building up within the
filter and plumbing system.
If scale deposits are readily noticeable on pool surfaces,
pH, calcium hardness, and total alkalinity must be tested
and adjusted immediately. One, if not all three, is much
too high and needs to be reduced. Reduce the pH and
alkalinity levels first, because reducing the calcium
hardness level is difficult.
If high hardness or total dissolved solids is causing the
scale, it is best to drain a portion of the pool water and
replace it with fresh make-up water low in hardness and
total dissolved solids.
Scale formation on pool walls
42
Page 45

Scale
Confirmation
Crusty deposits on pool surfaces
Cause
• High calcium hardness
• High pH and alkalinity
• High TDS
Treatment
• Adjust pH and alkalinity to ideal ranges (7.2-7.6 and 80-120
respectively)
• Replace a volume of pool water with water low in hardness
and dissolved solids. Consult a pool professional to determine
the replacement amount
• Use a sequestering agent to prevent scale build-up if high
hardness levels are a continuing problem
43
Page 46

Eye & Skin Irritations
Eye and skin irritations are another common problem
for swimming pool bathers. Nasal irritations can also be
noticed in indoor pool areas with poor ventilation and
excessive levels of combined chlorine.
There are two basic causes of eye
and skin irritations: an improper
pH, and a chloramine problem.
The human eye is most
comfortable in water with a pH of
about 7.5. Therefore a pH below
7.2, or above 8.0 can become irritating. Low and high
pH levels irritate both eyes and skin.
A chloramine problem is caused when combined
chlorine levels exceed 0.2 ppm as determined by a DPD
test. Though many people incorrectly blame high
chlorine for stinging eyes, it is actually the presence of
chloramines which causes this.
Cause
High or low pH Combined Chlorine
Treatment
Adjust pH to recommended
range & test.
Perform “Breakpoint Chlorination”
as noted on page 24.
44
Page 47

Water T reatment Tables
Dry chemicals should first be mixed into a small amount
of water in increments of about two pounds, and then
this predissolved mixture can be distributed evenly
around the pool unless directed otherwise.
Precautions:
Never add water to acid; always add acid to water.
·
Never add calcium chloride or other corrosive chemicals
·
to skimmers. These can damage pump and meter ele
ments.
Always follow manufacturer’s recommendations and
·
warnings on product labeling.
Conversions
1 ounce (dry) = 28.35 grams
1 ounce (liquid) = 29.57 milliliters
1 pint = 0.4732 liter
1 gallon - 3.785 liters
1 pound = 453.6 grams
1 foot = 0.3048 meter
-
45
Page 48

Lowering pH with Muriatic Acid*
1,000
gallons
pH pt oz pts oz pt oz pt oz pt oz
7.6-7.8 0 1. 3 0 6.4 0 12.8 1 9.6 4 0
7.8-8.0 0 1.9 0 9.6 1 3.2 2 6.4 6 0
8.0-8.4 0 2.6 0 12.8 1 9.6 3 3.2 8 0
>8.4 03.210 2040100
5,000
gallons
10,000
gallons
20,000
gallons
50,000
gallons
*Treatment recommendations are affected by total alkalinity. At
low alkalinity levels less acid may be required and at higher
alkalinity levels more acid may be required.
Lowering pH with Dry Acid* (sodium bisulfate)
1,000
gallons
pH lb oz lb oz lb oz lb oz lb oz
7.6-7.8 0 1.6 0 8.0 0 16.0 1 12.0 5 0
7.8-8.0 0 2.4 0 12.0 1 4.0 3 8.0 8 0
8.0-8.4 0 3.2 0 16.0 1 12.0 4 4.0 10 0
>8.4 04.0103050 130
5,000
gallons
10,000
gallons
20,000
gallons
50,000
gallons
*Treatment recommendations are affected by total alkalinity. At
low alkalinity levels less acid may be required and at higher
alkalinity levels more acid may be required.
46
Page 49

Raising pH with Soda Ash*
1,000
gallons
lb oz lb oz lb oz lb oz lb oz
pH
7.2-7.4 0 .6 0 3.2 0 6.4 0 12.8 2 0
7.0-7.2 0 1.0 0 4.8 0 9.6 1 3.2 3 0
6.8-7.0 0 1.3 0 6.4 0 12.8 1 9.6 4 0
£6.7 0 1.6 1 8.0 1 0 2 0 5 0
5,000
gallons
10,000
gallons
20,000
gallons
50,000
gallons
*Treatments in low alkalinity waters require less soda ash while
treatments in high alkalinity waters may require more soda ash.
Raising Chlorine 1 ppm
Chlorine Type
(% active)
Sodhypo* (5%)
Sodhypo* (10%)
Lithium (35%)
Calhypo (65%)
Dichlor (56%)
Dichlor (62%)
Trichlor (90%)
1,000
gallons
3oz 13oz 1.5pt 3pt 1gal
1 oz 7 oz 13 oz 1.5 pt 2 qt
0.4 oz 2 oz 4 oz 8 oz 19 oz
0.2 oz 1 oz 2 oz 4 oz 10 oz
0.2 oz 1 oz 2 oz 5 oz 12 oz
0.2 oz 1 oz 2 oz 4 oz 11 oz
0.1oz 1oz 1.5oz 3oz 7oz
5,000
gallons
10,000
gallons
20,000
gallons
50,000
gallons
*This is a liquid and the calculation assumes:
1 liq. oz. = 1 dry oz., 16 oz. = 1 pint, 32 oz. = 1 quart,
128 oz. = 1 gallon
Routine shock treatments may require a 10 ppm dose while
algae problems may require even higher doses. Consult
product label before adding any chemical products.
47
Page 50

Lowering Alkalinity with Dry Acid
1,000
gallons
ppm lb oz lb oz lb oz lb oz lb oz
10 0 3 102 0 4 0 100
20 0 6 204 0 8 0 200
30 0 10306 0120 300
40 0 13408 0160 400
50 1 0 50100200 500
60 1 3 60120240 600
70 1 6 70140280 700
80 1 1080160 320 800
90 1 1390180 360 900
100 2 0 10 0 20 0 40 0 100 0
5,000
gallons
10,000
gallons
20,000
gallons
50,000
gallons
Be sure to note chemical precautions on page 45.
Always follow manufacturer’s recommendations.
48
Page 51

Lowering Alkalinity with Muriatic Acid
1,000
gallons
ppm pt oz pt oz pt oz pt oz pt oz
10 0 2.5 0 13.0 1 10.0 3 4.0 8 2.5
20 0 5.0 1 10.0 3 4.0 6 8.5 16 0.0
30 0 8.0 2 7.0 4 14.0 9 12.5 24 0.0
40 0 10.5 3 4.0 6 8.5 13 0.5 32 0.0
50 0 13.0 4 1.0 8 2.5 16 0.0 40 0.0
60 0 15.5 4 14.0 9 12.5 19 0.0 48 0.0
70 1 2.0 5 11.0 11 6.5 22 0.0 57 0.0
80 1 5.0 6 8.5 13 0.5 26 0.0 65 0.0
90 1 7.5 7 5.5 14 10.5 29 0.0 73 0.0
100 1 10.0 8 2.5 16 4.5 32 0.0 81 0.0
5,000
gallons
10,000
gallons
20,000
gallons
50,000
gallons
Be sure to note chemical precautions on page 45.
Always follow manufacturer’s recommendations.
49
Page 52

Raising Alkalinity with Sodium Bicarbonate
1,000
gallons
ppm lb oz lb oz lb oz lb oz lb oz
10 020111721371
20 0 5 1 7 2 13 5 10 14 1
30 07224487212
40 0 9 2 13 5 10 11 4 28 2
50 0113871141353
60 01444871614423
70 0164159141911494
80 1 2510114228564
90 1 4 6 5 12 11 25 5 63 5
100 1 7 7 1 14 1 28 2 70 5
5,000
gallons
10,000
gallons
20,000
gallons
50,000
gallons
Be sure to note chemical precautions on page 45.
Always follow manufacturer’s recommendations.
50
Page 53

Raising Hardness with Calcium Chloride
1,000
gallons
ppm lb oz lb oz lb oz lb oz lb oz
10 020 1014 2 7 6 2
20 0 4 1 4 2 7 4 15 12 4
30 061 133117 6187
40 082 74159 13249
50 0 10 3 1 6 2 12 4 30 11
60 0 12 3 11 7 6 14 12 36 13
70 0 14 4 5 8 10 17 3 42 16
80 0164 159131910492
90 125 8111 222554
100 146 2124 249616
5,000
gallons
10,000
gallons
20,000
gallons
50,000
gallons
A significant amount of heat can be generated when mixing
calcium chloride in water. Follow manufacturer’s
recommendations carefully.
51
Page 54

Establishing or raising Cyanuric Acid level
1,000
gallons
ppm lb oz lb oz lb oz lb oz lb oz
10 01070 1311143
20 030131 113 586
30 04142 85 0128
40 0 5 1 11 3 5 6 11 16 11
50 0 7 2 1 4 3 8 6 20 14
5,000
gallons
10,000
gallons
20,000
gallons
50,000
gallons
Be sure to note chemical precautions on page 45.
Always follow manufacturer’s recommendations.
52
Page 55

Page 56

LaMotte Company
Helping People Solve Analytical Challenges
PO Box 329 • Chestertown • Maryland • 21620 • USA
800-344-3100 • 410-778-3100 (Outside U.S.A.)
Visit us on the web at www.lamotte.com
®
1505 • 7/07
 Loading...
Loading...