LaMotte Pool Manager Water Quality User Manual



TABLE OF CONTENTS
Chapter 1:
WhyYouShouldTestTheWater........................2
Chapter 2:
Usingthe5-WayTestStrip............................4
Chapter 3:
TheRoleofEachPrimaryTestFactor......................6
Chlorine......................................6
Bromine......................................8
pH.........................................9
TotalAlkalinity.................................10
TotalHardness..................................10
Chapter 4:
TheImportanceofOtherWaterTestFactors.................12
CyanuricAcid..................................12
Temperature...................................13
TotalDissolvedSolids..............................13
Metals (Copper, Iron, & Manganese) .....................13
NitrateandPhosphate.............................14
Chapter 5:
Trouble Shooting Water Problems .......................15
Algae.......................................15
Cloudy Water ..................................17
ColoredWater..................................18
Stains.......................................19
ScaleFormations ................................20
Eye & Skin Irritations..............................21
Filtration.....................................21
Chapter 6:
WaterTreatmentTables ............................22
CalculatingPoolorSpaVolume........................22
AdjustingpH ..................................24
AdjustingChlorine...............................27
AdjustingAlkalinity ..............................29
AdjustingHardness...............................33
AdjustingCyanuricAcid............................35
RecordingYourTestResults................inside back cover

WHY YOU SHOULD TEST THE WA TER
The two most important reasons to test the water:
#1 To protect the bather
The goal of every pool and spa owner is to have sparkling, clean water.
Unfortunately, a variety of undesirable substances will often enter a pool
or spa. Such items can make the water unhealthy. There are bacteria
on every person’s body that can get into water; some forms can cause
infections or rashes. Bather perspiration and urine break down to
undesirable nitrogen compounds and “Mother Nature” can also
contribute: rain, algae spores, leaves and other organic materials. All of
these necessitate the use of a sanitizer, such as chlorine. The Insta-Test 5
color chart shows the ideal range for free chlorine and total chlorine in
swimming pools. Spa owners should keep in mind that the ideal free
chlorine range in spas (hot tubs) is between 2 and 4 ppm.
#2 To protect the pool or spa
Everything that the water contacts is affected by the chemistry of the
water itself. The surfaces of the pool or spa, the heater, the filter, the steps,
and the pump can be damaged if the water is either corrosive or scale
forming. The goal of the water analyst is to keep the water “in balance”
so it does not damage the pool and its equipment. By maintaining each
test factor of pH, alkalinity, and hardness within the “Ideal Range” [shown
on the test strip color chart] the water will not harm the pool
or spa surfaces and components.
2

Additional reasons to routinely test your water:
In addition to the obvious goal of determining what treatment chemicals
are needed to protect the bather and the pool there are many other good
reasons to test the water.
How much treatment chemical to add - By testing the water
and using the treatment tables found in the back of this book (or on the
chemical product label), the analyst can closely predict how much
treatment chemicals are needed. This testing avoids a costly overdose of
chemicals that can create larger problems than the original one.
Total Alkalinity - To avoid undesirable changes in the pH level the
alkalinity must be maintained in the ideal range (near 100 ppm). Total
alkalinity helps to stabilize the pH of the water so that chemical
treatments or environmental conditions will not rapidly raise or lower the
pH to a damaging level. Such changes can also occur after adding large
doses of chlorine, since one form of chlorine may have a very low pH
while another a very high pH. If the pH of your source water is high or
low, consult with a pool professional to select a type
of chlorine or sanitizer that makes sense for
your water and the environmental conditions.
Total Chlori ne - If the total chlorine level
ever exceeds the free chlorine level,
a superchlorination or shock treatment
is needed. High total chlorine levels
indicate that undesirable ammonia
or nitrogen compounds have
“combined” with the free chlorine to
make the sanitizer much less active and
create odor and swimmer irritation.
Total Hardness - When a hardness
level is too low, it can cause corrosion.
If it is too high, it can settle or “drop out”
of solution as chalky white or tan colored
deposits on component surfaces.
These deposits, often referred to as
“scale,” could eventually lead to clogged
pipes, or damaged motors, heaters,
and filters.
3
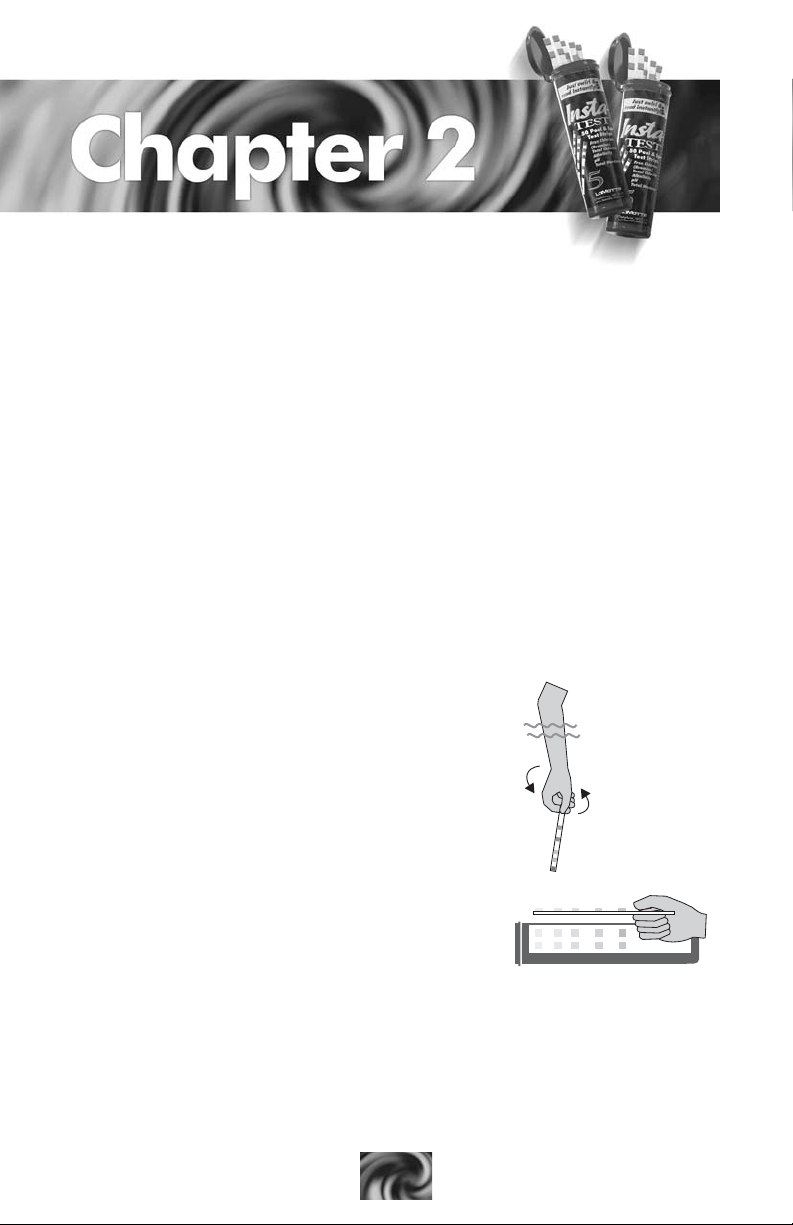
USING THE 5-WAY TEST STRIP
0
1
0
(
0
)
1
(
2
)
80
0
6.8
7
.2
50
100
It is important to carefully read the instructions on the test strip bottle
for proper use. In order to consistently obtain accurate results, there are
several tips that help the user. For example, the reason the instruction says
to read the strip starting with chlorine (Cl) at the top is because the pH
and hardness pads need a few extra seconds to fully develop (about 10 to
15). By the time the strip is removed from the water and the initial three
test factors are carefully read, the colors for pH and hardness are ready for
evaluation.
Note: a color that appears on the strip may be between two color chart
values. If this happens, record the result as a number in between the values
shown.
Note: the magenta (hot pink) values represent the bromine test results,
and are found just below the free chlorine values printed in black. Below
are explanations as to why some of the instructions and tips are important
to follow.
Instruction:
1. Immerse strip. SWIRL 3 TIMES or DIP
according to directions on bottle.
Why? Exposes each pad to the correct amount
of pool/spa water.
2. Remove with pads face up.
Why? Hold strip level to avoid reactant
from one pad running into another pad.
3. DO NOT SHAKE OFF EXCESS WA TER.
Read immediately Cl->TCl-> etc.
Why? This could shake off the colored reaction. Read the pads reactions
in sequence to allow the proper reaction time for each pad.
4
Tips
Keep wet fingers out of the bottle. Strip pads will react if they get wet so shake
Close vial tightly after removing strip. To keep moisture out of bottle press down
Immerse strip to a depth of 12"-18". In pools, this assures a representative sample
Read in natural daylight. Sodium vapor light bulbs can make color
Store in a cool, dry place. Indoor is best, since extreme heat
out a strip and pick it up with dry fingers.
the center of the cap to seal it quickly after
removing a strip.
of the pool water and not just the surface
where evaporation takes place. In spas, swirl
with the jets not running.
matching difficult.
and moisture could reduce the shelf-life
of the strips.
The PopTop Vial
The unique PopTop vial comes with a molded desiccant sleeve insert. This
eliminates the need for a desiccant pillow and provides more protection
for strips from moisture intrusion. Do not remove the sleeve. A properly
closed vial is 100% leak proof and airtight.
Simple Do’s and Don’ts
In addition to the important tips on the bottle here are some key DO’s and
DON’Ts to remember.
DO’s
1. Use the test strip “on-site” and swirl in a one-foot circle.
2. Always read the result promptly after swirling and lifting it level
from the water.
3. Always remove sunglasses since they can make color matching
more difficult.
Don’ts
1. Never dip the strip next to a make-up water inlet, return line
or a chemical feeder.
2. Never test the water prior to one complete filter cycle after a large
amount of chemical has been added.
3. Never swirl a strip in spa water with the air jets running.
5
THEROLEOFEACHPRIMARY
TEST FA CTOR
Sanitizers - Protecting the Bather
A sanitizer must work quickly and efficiently to keep the water
environment just as healthy for 2 bathers as for 200 bathers. There are
many sanitizing systems available. The most common pool sanitizer is
chlorine and the most common spa sanitizer is bromine. Both sanitizers
are excellent oxidizers, which means they destroy or “burn out”
contaminants in the water. There are “alternative” sanitizers available
which use small amounts of chlorine or bromine to support their system
and some that
do not use chlorine or bromine at all. For the pool professionals that
recommend small amounts of chlorine or bromine be present, just
maintain a test result on the free chlorine pad with a very faint pink color.
If the pad shows a light yellow color, the result is zero and more sanitizer is
needed.
CHLORINE
When chlorine enters the water, it is in a form that is an active sanitizer
and an oxidizer called “free chlorine”. It will react with any number
of contaminants in the water. When it reacts with ammonia compounds
in the water, which come from bathers’ perspiration and urine, it becomes
“combined chlorine”. In this form, chlorine is a much slower sanitizer.
This form also causes chlorine odor and eye irritation. When using the
5-way strip, the difference between the free chlorine reading (pad 1) and
the total chlorine reading (pad 2) is the combined chlorine reading.
When the total chlorine reading is higher than the free chlorine reading,
it is time to oxidize or destroy the combined chlorine. The simplest way
is to increase the chlorine level in the pool to 10 ppm. This higher level
of chlorine will oxidize or eliminate the combined chlorine and is called
superchlorination or shock treating. Use the chlorine treatment table
found in the back of this book to determine how much chlorine should
be added based on the volume of water in your pool or spa.
6
There are also non-chlorine shocks available, such as potassium
IDEAL
RANGE!
monopersulfate and sodium dipersulfate. These will eliminate combined
chlorine. Keep in mind that these non-chlorine shocks are oxidizers only,
not sanitizers. Chlorine or bromine must be added to maintain an
adequate level of sanitizer.
Stabilizing your Free Chlorine
In an outdoor swimming pool, the use of a chlorine stabilizer (cyanuric
acid) is usually recommended to reduce the degradation of free chlorine by
sunlight. Cyanuric acid acts like a shield for chlorine from ultraviolet
light. It can be added by itself, usually at an initial dose of 30-40 ppm,
or cyanuric acid can be added as part of a chlorine compound. Two forms
of chlorine that contain cyanuric acid are known as Sodium
Dichloro-s-triazinetrione Dihydrate (dichlor) and
Trichloro-s-triazinetrione (trichlor). When these are added to water, they
form free chlorine and cyanuric acid. Since trichlor has more active
stabilizer and chlorine it usually costs more.
There are three common unstabilized forms of chlorine. They are sodium
hypochlorite (liquid bleach), calcium hypochlorite(cal-hypo) and lithium
hypochlorite. These are normally used for indoor pools, superchlorination
treatments or when cyanuric acid levels are too high.
Chlorine Demand
The National Spa and Pool Institute defines chlorine demand as “the
amount of chlorine that will be consumed by readily oxidizable impurities
in water”. In simpler terms, if a 3 ppm dosage of chlorine is added and is
promptly tested to find only 2 ppm is on hand, the water had a 1 ppm
demand. Many things contribute to chlorine demand such as bacteria,
organics (like dirt and leaves), fertilizers (including nitrate and
phosphate), and bather wastes. When opening a pool in the spring or after
a heavy storm, expect higher than usual chlorine demands. Once the
demand is met, the remaining free chlorine residual is there to take care of
the additional demand.
The ideal free chlorine range for pools is 1-3 ppm
and for spas is 2-4 ppm.
7
Chlorine Treatment Compounds
IDEAL
RANGE!
% Available
Trade Name(Proper Name)
Liquid Chlorine (Sodium Hypochlorite) 12% 13.0
Litho (Lithium Hypochlorite) 35% 10.7
Cal Hypo (Calcium Hypochlorite) 65% 11.8
Dichlor (Sodium Dichloro-s-triazinetrione Dihydrate) 56% 6.0
Dichlor (Sodium Dichloro-s-triazinetrione Anhydrous) 62% 6.0
Trichlor (Trichloro-s-triazinetrione) 90% 3.0
Chlorine pH
BROMINE
There are two types of bromine systems. One type is a solid tablet form
that is added to a skimmer and feeds sanitizer into the passing water as it
slowly dissolves. It contains both bromine and chlorine. The function
ofthechlorineistomakemorebrominewhennoneremains.Thesecond
type uses a bromide salt which requires the addition of a separate oxidizer
such as chlorine, ozone or non-chlorine shock.
When bromine is added to water, it forms free bromine. Like chlorine, free
bromine can also combine with ammonia compounds, but the combined
bromine reacts as quickly as free bromine. Thus, there is no need to
distinguish between free and combined bromine. A bromine system
should be shocked with 10 ppm of chlorine periodically. Spa owners may
need to do this frequently. Check with your local chemical supplier for
advice.
Determine bromine readings by using pad 1 on the test strip. Note: the
magenta (hot pink) values represent the bromine test results, and are
found just below the free chlorine values printed in black.
The ideal range for bromine in pools
and spas is 3-6 ppm.
8

pH
Water Balance - Protecting the Pool or Spa
pH is the measure of the acidity of the water. The pH scale extends from
0 to 14 with 7 being neutral. As the pH moves lower than 7.0, the water
becomes more acidic and tends to be corrosive; as pH moves up higher
than 7.0, the water becomes less acidic (or more basic) and could lead to a
scale forming condition.
Since most water has the tendency to either corrode or leave small, crusty
“scale” deposits, it is important to properly balance the factors of pH, total
alkalinity and hardness. Depending on where you live, the water can
contain a variety of minerals. These minerals directly affect whether the
water will corrode, scale or be in balance. In addition to pH, total
alkalinity and calcium hardness, temperature plays a role in water balance
and must be considered when determining ideal levels in pools or spas.
The reason is because in warmer water a substance called calcium
carbonate tends to fall out of solution more rapidly, thus leaving behind
scale deposits. For example, in 60°F (16°C) pool water, a good practice
would be to keep the pH level closer to 7.6 if the alkalinity and hardness
are in the ideal range. In 80°F (27°C) pool water, a pH of 7.3 would be
best. A professional pool and spa retailer can provide a “saturation index”
calculation to target your ideal pH level.
Note: a high pH level (above 7.8), will inhibit the ability of free chlorine
to sanitize water efficiently. If your water source has a high pH, the form of
chlorine selected can actually help to lower the pH. (For example: If your
water source is a pH of 8.2 you may wish to use Trichlor since it has a pH
of 3.0). For several reasons, including bather comfort, the ideal pH of pool
or spa water is 7.2 - 7.6.
When using the 5-way strip, read pad 4 to determine pH. Keep your results
in the orange, ideal range, colors. If the color turns yellow, add pH
increaser as shown in the charts at the end of this book. If the pH is not up
to the ideal range within 24 hours, add more pH increaser or consult
a local pool professional (the pH could be far below pH 6.8 and in need of
serious attention). If the pad turns red, add pH decreaser to bring the pH
down into the ideal range. The same process should be followed if the pH
remains high after you have added pH decreaser , continue to add more or
consult a professional. Note: if the pH test pad ever turns purple it means
the chlorine or bromine level is well above 10 ppm. Bathers should not
enter the water until the chlorine or bromine level is below 10 ppm.
9
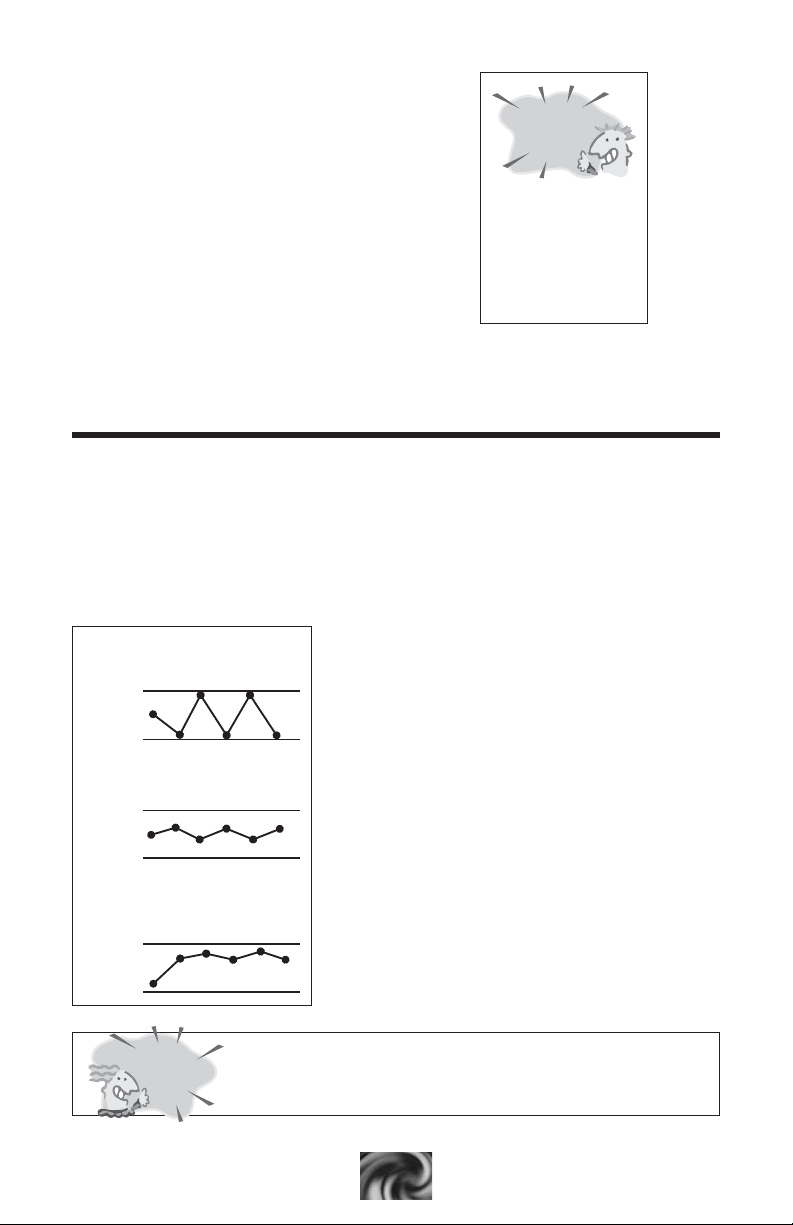
IDEAL
RANGE!
LOW
ALKALINITY
IDEAL
ALKALINITY
HIGH
ALKALINITY
pH
Value
pH
Value
pH
Value
Allows pH to bounce
Allows proper pH control
Usually means a high pH
and is hard to adjust
IDEAL
RANGE!
If pH Is Too LOW (Acidic)
corrosion of pool equipment/staining
n
swimmer eye irritation
n
etching plaster pool surfaces
n
chlorine dissipates quickly
n
If pH Is Too HIGH (Basic)
scale accumulates on pool equipment
n
cloudy, turbid water
n
sanitizing power of chlorine is weakened
n
swimmer eye irritation
n
The ideal pH
range for pools
and spas is
7.2 - 7.6.
TOTAL ALKALINITY
Total alkalinity refers to the buffering capacity of the water or how well
the water can resist changes in pH. If the alkalinity is too low , the pH
could potentially change daily. This is known as “pH bounce” and leaves
the pool and spa water vulnerable to pH problems from chlorine
treatments, environmental conditions and even from fresh make-up water.
Low alkalinity water will tend to be corrosive, thus eroding pool surfaces
and equipment.
If the alkalinity level is too high, the pH may
also drift to a very high level. Then, it is very
difficult to reduce the pH and the water may
be cloudy and prone to scaling.
When using the 5-way strip, use pad 3
to determine the total alkalinity reading.
Try to keep results in the blue-green color
range and treat the water promptly if it falls
outside the ideal range. Note: when raising or
The ideal alkalinity range for pools and spas is
80 -120 ppm (100-150 if using cyanuric acid).
lowering the alkalinity level, the pH of the
water will also be affected respectively.
Be sure to check the pH level carefully
within 24 hours after any significant
alkalinity treatment.
10
 Loading...
Loading...