Page 1

KODAK 2100 Intraoral
X-ray System
User’s Manual
Page 2

This document is originally written in English.
Manual Name: KODAK 2100 Intraoral X-ray System, User Guide
Document code: SM700
Revision Number: Rev 3
Printed Date: 9/2007
The brand names and logos reproduced in this guide are copyright.
KODAK is a trademark of KODAK used under License.
RVG, Trophy, are registered trademarks of Carestream Health, Inc. All other names
or products referred to in this document are used only for the purpose of
identification and maybe the trademarks or the registered trademarks of their
respective owners.The RVG technology is the subject of and international patent
registered by Carestream Health, Inc.
Page 3

Table of Contents
1 Safety and Regulatory Information
Conventions Used in This Manual............................................................................................................1-1
General Safety Guidelines.........................................................................................................................1-1
Warnings and Safety Instructions .............................................................................................................1-2
Labeling Summary....................................................................................................................................1-4
IEC Symbols Used....................................................................................................................................1-5
Regulatory Information.............................................................................................................................1-5
CE Conformity....................................................................................................................................1-5
U.S. Regulations..................................................................................................................................1-5
2 System Overview
Components...............................................................................................................................................2-1
Ceiling-mounted Unit (Optional)........................................................ ......................................................2-3
Mounted on Mobile Stand (Optional)......................................................................................... ..............2-4
Floor-mounted Unit (Optional).................................................................................................................2-5
Control Timer Unit....................................................................................................................................2-6
3 Using the System
Positioning.................................................................................................................................................3-1
Positioning the patient.........................................................................................................................3-1
Positioning the x-ray generator ...........................................................................................................3-1
Positioning the imaging receptor.........................................................................................................3-2
Exposure....................................................................................................................................................3-2
Exposure parameters ...........................................................................................................................3-2
Procedure.............................................................................................................................................3-3
Processing .................................................................................................................................................3-3
Additional Features...................................................................................................................................3-4
4 User Mode
Entering User Mode..................................................................................................................................4-1
Changing Parameters.................................. ..... .... ...................................................... ................................4-1
Exiting User Mode....................................................................................................................................4-1
5 Care and Maintenance
General Maintenance.................................................................................................................................5-1
Cleaning...............................................................................................................................................5-2
Disinfecting.........................................................................................................................................5-2
Error messages ..........................................................................................................................................5-2
Troubleshooting ........................................................................................................................................5-4
iii
Page 4

Table of Contents
6 Specifications
According to IEC Standard 601-2-7.........................................................................................................6-1
Manufacturer.......................................................................................................................................6-1
X-ray Generator........................................................................................................................................6-3
Equipped X-ray Generator........................................................................................................................6-4
Position of Identification Labels...............................................................................................................6-6
Tables of Exposure Times ........................................................................................................................6-7
Emitted Doses.........................................................................................................................................6-14
iv
Page 5

1
Safety and Regulatory Information
The information contained in this manual is based on the experience and
knowledge relating to the subject matter gained by Carestream Health Inc.
prior to publication. No patent license is granted by this information.
Carestream Health Inc. reserves the right to change this information without
notice, and makes no warranty, express or implied, with respect to this
information. Carestream Health Inc. shall not be liable for any loss or
damage, including consequential or special damages, resulting from any use
of this information, even if loss or damage is caused by Carestream Health
Inc. negligence or other fault.
Conventions Used in This Manual
CAUTION:
Caution points out procedures that you must follow precisely to avoid
damage to the system or any of its components, yourself or others, loss
of data, or corruption of files in software applications.
Note
Notes provide additional information, such
as expanded explanations, hints, or
reminders.
Important
Important highlights critical policy
information that affects how you use this
manual and this product.
General Safety Guidelines
• This product is designed and manufactured to ensure maximum safety
of operation. Operate and maintain it in strict compliance with the safety
precautions and operating instructions contained in this manual.
• This product meets all the safety requirements applicable to medical
equipment. However, anyone attempting to operate the system must be
fully aware of potential safety hazards.
• There are no user serviceable parts in this system. The product must be
installed, maintained, and serviced by qualified service personnel
according to procedures and preventive maintenance schedules in the
product service manual. If your product does not operate as expected,
contact your Service Representative.
• Do not modify this product in whole or in part without prior written
approval from Carestream Health Inc.
• The assembly, extensions, adjustments, modifications, and repairs must
be performed by an authorized Service Representative. Your radiology
system must be installed in premises that comply with applicable
standards.
• Personnel operating and maintaining this system should receive training
and be familiar with all aspects of operation and maintenance.
9/2007 SM700_K2100_03_en 1-1
Page 6

• T o ensure safety, read all user manuals carefully before using the system
and observe all Caution, Important, and Note callouts located
throughout the manual.
• Keep this manual with the equipment.
• Reading this manual does not qualify you to operate, test, or calibrate
this system.
• Unauthorized personnel are not allowed access to the system.
• If the product does not operate properly or fails to respond to the
controls as described in this manual:
– Follow the safety precautions as specified in this manual.
– Stop using the equipment and do not make or authorize any changes
to it.
– Immediately contact your Service Representative, report the problem,
and await further instructions.
• X-ray systems manufactured by Carestream Health Inc. comply with
safety standards throughout the world for optimum protection against
radiation risks.
• Be aware of the product specifications and of system accuracy and
stability limitations. Consider these limitations before making any
decision based on quantitative values. If you have any doubts, consult
your Sales Representative.
CAUTION
X-rays can be dangerous if used incorrectly . Take precautions even when
following the instructions in this manual.
Use conventional commercially available equipment to protect yourself
and your patients against scattered radiation risks.
• If you fail to comply with these instructions, Carestream Health Inc. will
not be responsible for the safety reliability, and characteristics of the
equipment.
:
Warnings and Safety Instructions
CAUTION:
Do not operate the equipment in the presence of explosive liquids,
vapors, or gases. Do not plug in or turn on the system if hazardous
substances are detected in the environment. If these substances are
detected after the system has been turned on, do not attempt to turn off
the unit or unplug it. Evacuate and ventilate the area before turning off
the system.
DANGER: THIS IS AN ELECTRICAL UNIT. DO NOT EXPOSE IT
TO WATER SPRAY. SUCH ACTION MAY CAUSE AN
ELECTRICAL SHOCK OR A MALFUNCTION OF THE
UNIT.
1-2 SM700_K2100_03_en 9/2007
Page 7

WARNING
The user is responsible for the operation and maintenance of this unit.
This unit must only be operated by legally qualified persons.
The cover of the unit must not be opened by the operator.
Inspection and maintenance operations should only be carried out by an
approved technician.
WARNING
This unit must be installed in an x-ray room that complies with current
installation standards. From this location, visual or audio communication
must be maintained with the patient, together with access to the control
interface during exposure.
WARNING
Do not operate the unit if there is the threat of an earthquake.
Following an earthquake, ensure that the unit is operating properly before
using it again.
Failure to observe this precaution may expose patients to hazards.
WARNING
X-ray equipment can be hazardous to patients and the operator if the
exposure safety factors and operating instructions are not observed.
WARNING
Do not place objects within the field of operation of the unit.
WARNING
We recommend that the patient and the operator wear protective lead-lined
aprons, unless other Radiation Protection Protocols apply locally.
Ensure that any parts of the unit that may come into contact with the patient
and the operator have been disinfected after each patient has been exposed to
x-rays.
If the unit develops a fault, turn it off (O) and display a sign that states “Out
of Service.”
WARNING
The operator must ask the patient to refrain from moving during the entire
period of exposure.
In the European Union, this symbol indicates that
when the last user wishes to discard this product,
it must be sent to appropriate facilities for
recovery and recycling.
Contact your local sales representative for
additional information on the collection and
recovery programs available for this product.
9/2007 SM700_K2100_03_en 1-3
Page 8

Labeling Summary
Safety Labels
CHASSIS GROUND STUD
ATTEN TIO N: CONSULT ACCOMPANYING DOCUMENTS
CAUTION: IONIZING RADIATION
1-4 SM700_K2100_03_en 9/2007
Page 9

IEC Symbols Used
The system may have labels with one or more of the following symbols.
These symbols indicate the IEC standards to which the system conforms.
Caution — consult accompanying documents
Protective earth
Power ON
Power OFF
Regulatory Information
The product conforms to the following safety standards: IEC/EN 60 601-1
Medical Electrical Equipment General Requirements for Safety, IEC/EN 60
601-2 Medical Electrical Equipment Electro-Magnetic Compatibility
Requirements and Tests.
CE Conformity
This product conforms to the requirements of EU Council Directive
93/42/EEC. The Kodak intraoral x-ray system is a Class II b medical device,
which bears the following mark of conformity: .
U.S. Regulations
U.S federal law restricts this device to sale by or on the order of a
dentist.
CAUTION:
9/2007 SM700_K2100_03_en 1-5
Page 10

Page 11

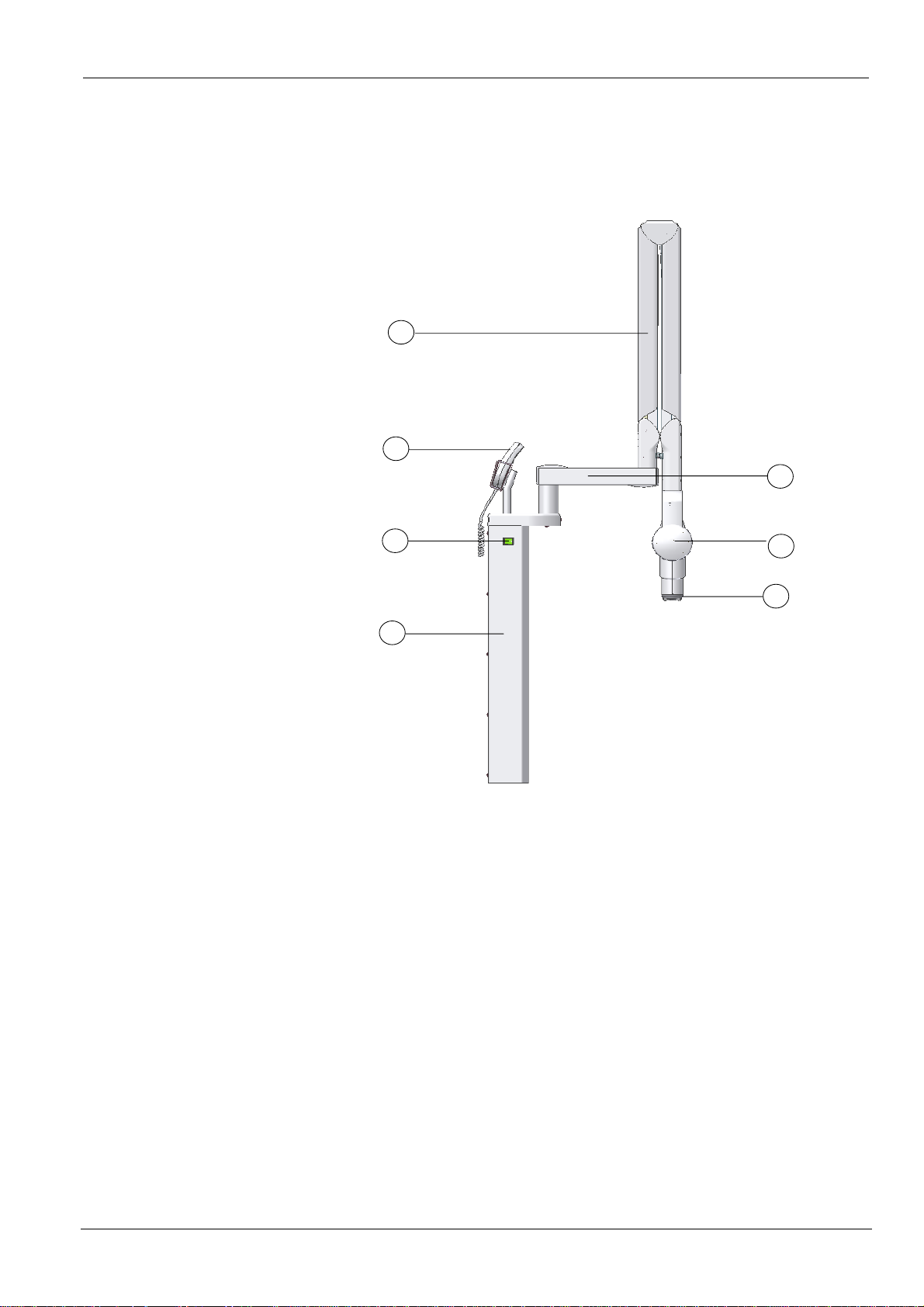
2
System Overview
Components
d
c
e
b
Figure 1. KODAK 2100 Intraoral X-ray System
a. High-frequency x-ray generator
• Transformer and associated electronics, and an oil-bathed x-ray tube
• Beam-limiting device
• Radiation diameter – 6 cm (2 3/8 in.)
•
Distance from x-ray tube focal spot to skin – 20 cm
(7 7/8 in.
• Handle to facilitate positioning
b. Wall framework
• Contains the high-frequency generator’s control electronics designed to
support its mechanical stand
)
a
f
c. Control timer unit
• Selection of exposure times
• Self-test of the microprocessor each time the unit is activated
• Alarm during incorrect operation
• Digital icon that reduces the exposure time range if you are using a
Kodak RVG sensor
9/2007 SM700_K2100_03_en 2-1
Page 12

d. Scissor arm
• Allow you to position the generator precisely and easily
• Wall-mounted with a choice of extensions
Figure 2. Side view of KODAK 2100 Intraoral X-ray System
Table 3. Types of Scissor Arms
Extension R Span A
CG 645 47.0 cm
(18.5 in.)
CG 646 64.8 cm
(25.5 in.)
CG 648 82.5 cm
(32.5 in.)
170.0 cm
(66 15/16 in.)
188.0 cm
(74 in.)
205.0 cm
(80 11/16 in.)
e. On/off switch
• Contains built-in light
f. Rectangular collimator (optional)
• Different sizes adapted to films and RVG sensors
Additional options
• Separate exposure switch (if the control panel is attached to the wall
framework)
• Ceiling-mounted unit
• Floor-mounted unit
• Unit mounted on mobile stand
2-2 SM700_K2100_03_en 9/2007
Page 13

Ceiling-mounted Unit
d
b
c
f
Figure 4. KODAK 2100 Intraoral X-ray System ceiling-mounted unit
a. High-frequency x-ray generator
b. Ceiling-mounted unit containing the high-frequency x-ray
generator’s control electronics
c. Separate timer/control unit for the x-ray generator
d. Scissor arm
e. On/off switch with built-in light
f. Rectangular collimator
e
a
9/2007 SM700_K2100_03_en 2-3
Page 14

Mounted on Mobile Stand (Optional)
d
c
g
e
a
f
b
h
Figure 5. KODAK 2100 Intraoral X-ray System mounted on mobile
stand
a. High-frequency x-ray generator
b. Mobile stand containing the high-frequency x-ray generator’s
control electronics
c. Timer/control unit for the x-ray generator
d. Scissor arm
e. On/off switch with built-in light
f. Rectangular collimator
g. Handle
h. Foot brake
2-4 SM700_K2100_03_en 9/2007
Page 15
Floor-mounted Unit (Optional)
d
c
g
e
b
Figure 6. KODAK 2100 Intraoral X-ray System floor-mounted unit
a
f
a. High-frequency x-ray generator
b. Floor column containing the high-frequency x-ray generator’s
control electronics
c. Timer/control unit for the x-ray generator
d. Scissor arm
e. On/off switch with built-in light
f. Rectangular collimator
g. Extension arm
9/2007 SM700_K2100_03_en 2-5
Page 16

Control Timer Unit
a
b
e
d
i
f
c
g
h
Figure 7. KODAK 2100 Intraoral X-ray System control timer unit
a. Display
b. “Warning, see accompanying documents” sign
c. Exposure time selection
d. Emitting dose calculation
e. Digital mode function
f. Ready mode
g. X-ray emission control light
h. X-ray exposure button
i. Selection knob
• Rotate the knob to select exposure time
• Press knob quickly to display the latest measure dose emitted
• Press and hold knob to switch from film to digital exposure time
frame
2-6 SM700_K2100_03_en 9/2007
Page 17

3
Using the System
Positioning
Positioning the patient
Every dental specialist would like to produce high-quality intraoral
radiographs that reveal maximum detail with the minimum dose to the
patient, show teeth and anatomic structures accurately with a minimum of
distortion or magnification, and have optimal density and contrast to
maximize their use for the detection of dental diseases.
To obtain high-quality intraoral radiography with maximum details, take
extra care in all three steps of the radiography process: positioning the
patient, the x-ray generator, and the imaging system; exposing the film or the
sensor; and processing the film.
Seat the patient with the sagittal plane vertical.
• For radiography of the upper maxillary, the Frankfort plane (nose-ear
plane) must be horizontal
• For radiography of the lower maxillary, the occlusal plane must be
horizontal
Figure 8. Patient positioning
Positioning the x-ray generator
The scissor arm allows you to accurately position the generator for any type
of exposure. The beam-limiting device maintains a distance of at least 20 cm
(8 in.) between the focal spot and the skin, which allows you to use either the
paralleling technique or the bisecting technique.
Paralleling technique
The positioning tool used in the paralleling technique allows you to align the
beam and the receptor. An adapted collimator reduces the dosage by limiting
surface exposure.
Bisecting technique
When using the bisecting technique, do not use a rectangular collimator . This
limits the risk of misaligning the x-ray beam and the image receptor.
9/2007 SM700_K2100_03_en 3-1
Page 18
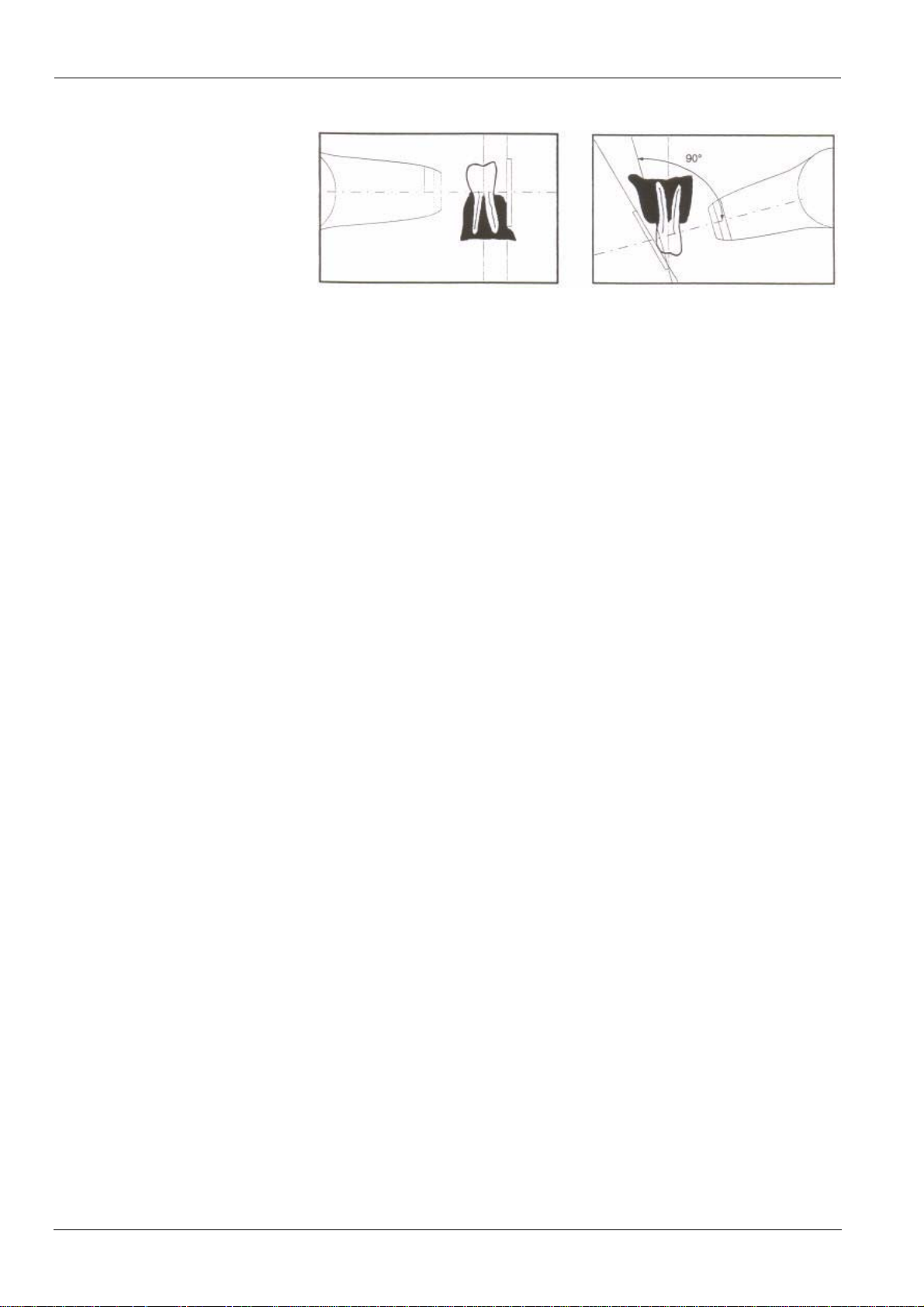
Figure 9. Paralleling technique (left) and Bisecting technique (right)
Positioning the imaging receptor
Using the KODAK 2100 Intraoral X-ray System, you may create an x-ray
image on one of three different types of imaging receptors:
• Conventional silver halide films, such as KODAK INSIGHT or
KODAK ULTRA-SPEED dental films
• Digital sensors, such as KODAK RVG sensor
• Phosphor plate
Properly placing the receptor is critical. Check your dental radiography text
for information about proper placement of the imaging receptor.
Improperly positioning the film or sensor results in errors on the radiograph,
such as distorted teeth and roots, elongation, magnification, and/or
overlapping contacts. The paralleling technique generally reduces the risk of
such errors. However, if you improperly position the system, angulation
errors can occur (angulation of the receptor to the tooth itself).
If the exit pattern of the beam is not aligned with the imaging receptor, then
part of the radiograph will not be exposed to radiation and the final
radiograph will have some clear (unexposed) areas. This defect is called
“cone cuts”.
The imaging receptor is marked to indicate the tube side. If the orientation is
not correct, the resulting radiograph is lighter and may show artifacts, such as
foil pattern or sensor cable.
Exposure
Exposure parameters
Because each receptor (film, digital sensor, or phosphor plate) has its own
sensitivity to x-ray radiation, the choice of receptor affects the exposure
parameters. For instance, sensitivity class for conventional dental films is
characterized with a letter D, E, or F where F is more sensitive than E, and E
more sensitive than D. Consequently, the required dose for the correct
exposure goes down with each increase in sensitivity.
Tables with recommended exposure times are found in Section 6. The
exposure times are based on manufacturers’ recommendations. The table
should be considered as a guideline; adjust to accommodate your conditions.
Adjust the exposure time range based on the type of receptor you use, film or
digital. To change the mode, press and hold the selection knob at least
3 seconds. Then turn the selection knob to set the exposure time.
3-2 SM700_K2100_03_en 9/2007
Page 19

Procedure
1. Turn on the system.
The on/off button and Ready indicator light up.
2. Select the exposure mode (digital or film) by pressing and holding the
selection knob at least 3 seconds until the mode changes.
The digital mode has shortened exposure times to prevent overexposure
of the digital sensor. When you select digital exposure, the digital
indicator lights up.
3. Select the exposure time by turning the selection knob.
Exposure tables are available in Section 6 of this manual. Additional
tables are provided to hang close to your control timer unit.
• For conventional use, the exposure time range goes from 0.05 to
1.25 sec.
• For digital use, the exposure time range goes from 0.010 to 0.063 sec.
4. Acquire the image.
a. Press the x-ray exposure button on the control timer unit.
The x-ray emission indicator lights up and an audible signal is
emitted.
b. Keep pressing until the x-ray emission light goes out and the audible
signal stops.
1
Processing
CAUTION
If you stop pressing the control key before the exposure ends, a
manipulator alarm is activated. It indicates that the x-ray emission was
interrupted prematurely and that there is a risk of underexposure.
5. Read the emitted dose.
Quickly press the selection knob. The “mGy” indicator lights up and the
dose in mGy is displayed. Section 6 provides a table with emitted
dosage based on exposure times.
When using conventional film, process the film according to manufacturer’s
instructions. Develop the film under safelight conditions in an automatic
processor or manually.
If you use an automatic processor, refer to the processor’s manual. Be sure to
maintain the mechanically and keep the solutions replenished.
If you develop film manually, follow precisely the manufacturer’s
recommendations
temperature. Any deviation from the manufacturer’s recommendations (such
as a solution that is too concentrated or diluted, too hot or cold, or if film is
processed for the wrong amount of time), will adversely affect the quality of
the final radiograph.
:
for solution preparation, development time, and solution
1. This function may be disabled, depending on local regulations. See Section 4,
User Mode.
9/2007 SM700_K2100_03_en 3-3
Page 20

Additional Features
• KODAK 2100 Intraoral X-ray System uses a high-frequency technology
that has several advantages:
– Shorter exposure times, reducing the risk of blur due to movement of
the patient or film during exposure
– Reduction in x-ray dose to patients because the KODAK 2100
System emits fewer soft rays absorbed by patients that do not
contribute to the radiological picture
• A thermal safety system prevents the generator from overheating in case
of intensive use. This system can prohibit any exposure as long as the
generator did not cooled down: I01 error message appears on the display
unit and an audible signal is emitted until the cooling period is over.
CAUTION
Do not turn off the system. If you turn off power, the microprocessor
does not calculate the cooling time, and for safety reasons considers that
the system has not gone into the cooling cycle.
• While the exposure is taken, the exposure time counts off on the control
unit display.
If the exposure is interrupted (such as by releasing the key), the audible
and visible manipulator alarm is activated and the remaining exposure
time is displayed. This information makes it easier to decide whether to
develop the film or to start another exposure. (If the remaining time is
short, you may develop the film.)
To stop the manipulator alarm, press the selection knob.
• A self-test automatically activates when you turn on the unit.
The self-test checks the display and alarm lights and all the systems.
If the self-test detects a problem, an error code is displayed.
When the test is completed, a short beep sounds and the display shows
the firmware version and the total number of exposures (divided by 10)
taken by this unit since it was installed.
:
3-4 SM700_K2100_03_en 9/2007
Page 21

4
User Mode
The User mode allows you to choose the length of the cone (which is
necessary to calculate the correct emitted dose) and the type of imaging
receptor (required by local regulatory agencies).
Entering User Mode
1. Turn on the system.
2. Quickly press the selection knob on the control timer to enter the menu.
3. To change from one parameter to another, turn the selection knob one
Changing Parameters
To change parameters:
1. Press and hold the selection knob at least 3 seconds until the display
2. Turn the selection knob to change the parameter value.
The self-test is activated. At the end of the self-test, software
information is displayed (for example, F718 1.00).
You have access to the menu when USER is displayed. The display
intermittently shows the first parameter (P 01) and the setting (for
example, ON).
step in any direction.
shows EDIT and you hear a sound.
The parameter value starts blinking.
• To validate your choice, press and hold the selection knob at least
3 seconds until COPY is displayed and a noise sounds.
• To keep the initial value, press the selection knob briefly. “Abor”
appears on the display.
Exiting User Mode
The system returns to the parameters/programs mode.
To exit the User mode:
• Press the selection knob briefly.
“Quit” is displayed before the system return to operational mode.
N° Parameters
P01 Digital receptor ON / OFF
P02 Long cone ON / OFF
Choice
9/2007 SM700_K2100_03_en 4-1
Page 22

Page 23

5
Care and Maintenance
General Maintenance
To make sure that the system functions correctly, you must have it serviced
annually by an authorized technician. In addition, every three months inspect
the equipment and make sure of the following:
Generator
Mechanical support
Control unit and electrical installation
Functioning
• The certification label is legible.
• There are no oil leaks.
• The wall framework is securely attached to the wall.
• All the labels are legible.
• The scissor arm is stable in all positions.
• The symbols are legible.
• The control unit cable and the power supply cable are in good condition.
• The ground is correctly installed.
• The radiology control key returns to its initial position after use.
• The audible signal is audible and the x-ray emission light is visible when
you make an exposure (for example, 0.1 sec.).
Important
If the result of any of these checks is not
satisfactory, discontinue using the
equipment and contact an authorized
technician.
• The message “E01”, which means Operator Error, is displayed when
you make an exposure (for example, 1.00 sec.) and release the control
button before the exposure time has elapsed.
Timer self-test
• Turn on the system to activate the self-test.
– The test starts with a simultaneous test of the display and alarm
lights.
– The unit proceeds to the systems test. At the end of this test, indicated
by a short beep, the firmware version and the total number of
exposures (divided by 10) made by the machine since installation is
displayed.
– If the test is not successful, an Error code is displayed on the display.
9/2007 SM700_K2100_03_en 5-1
Page 24

Cleaning
Disinfecting
Clean the outside of the system with a damp paper towel or soft cloth using
an alcohol-based, non-corrosive cleaner.
If necessary, wipe off surfaces with disinfectant.
CAUTION
• Do not allow liquids to drip into the system.
• Do not spray cleaner or disinfectant directly onto the machine.
• Protect the system from contamination using barriers available
from dental distributors.
• Follow the manufacturer’s safety recommendations when using the
cleaner or disinfectant.
:
Error messages
Table 10. Error messages
Error message Cause How to cancel
I01 Cooling cycle; this message can appear
during a period of intensive use.
CAUTION
If you turn off power to the system, the microprocessor does not calculate the cooling time, and for
safety reasons considers that the system has not gone into the cooling cycle.
:
Do not turn off the system. The error
message will disappear when the system
returns to a satisfactory temperature.
E01
plus audible alarm
E02 The radiography control was activated
E03–E04 Problems with the exposure time
E10 to E18 kV voltage error.
Release of the radiography control
button before the end of the exposure.
The display shows the remaining
exposure time. (Based on this time,
decide whether to develop the film or
make another exposure.)
while the unit was being powered on.
control.
Press the selector knob to stop the alarm.
Turn off the system and restart. If the
problem persists, call a qualified service
technician and discontinue using the
equipment.
Turn off the system and restart. If the
problem persists, call a qualified service
technician and discontinue using the
equipment.
Turn off the system and restart. If the
problem persists, call a qualified service
technician and discontinue using the
equipment.
5-2 SM700_K2100_03_en 9/2007
Page 25

Table 10. Error messages
Error message Cause How to cancel
E20 to E24 Filament voltage error. Turn off the system and restart. If the
problem persists, call a qualified service
technician and discontinue using the
equipment.
E30 Problem with voltage to main power
supply or to chemical capacitor.
E40 to E46 System error (problems with the
microprocessor on the power board).
E50 to E54 Problems with the I2C bus (the
connection between the control panel
and the power board).
Turn off the system and restart. If the
problem persists, call a qualified service
technician and discontinue using the
equipment.
Turn off the system and restart. If the
problem persists, call a qualified service
technician and discontinue using the
equipment.
Turn off the system and restart. If the
problem persists, call a qualified service
technician and discontinue using the
equipment.
9/2007 SM700_K2100_03_en 5-3
Page 26

Troubleshooting
Table 11. Troubleshooting
Problem Cause Solution
Nothing lights up Unit is disconnected. Connect the unit.
Fuse F1 is burned out or defective. Replace the fuse.
Circuit breaker is off. Turn on the circuit breaker.
Control unit does not
light up
No x-ray emission Generator is cooling. Wait for the I01 message to disappear.
X-ray emission works,
but exposure is too light
or completely white
X-ray emission works,
but exposure is too dark
Control unit is disconnected. Connect the control unit.
Fuse F1 is burned out or defective. Replace the fuse.
Control unit is defective. Call a qualified service technician.
Radiology control key is defective. Call a qualified service technician.
Generator is positioned incorrectly. Adjust the position of the generator.
Exposure time is too short. Increase the exposure time.
Development time is too short. Increase the development time. (Refer to
the development instructions.)
Developer is too cold. Heat the developer.
Developer is too old or diluted. Replace with fresh developer.
RVG mode is incorrectly selected. Verify your exposure settings. (Refer to
the exposure procedure.)
Receptor is facing the wrong way. Reposition the receptor.
Unit was incorrectly installed. Call a qualified service technician.
Exposure time is too long. Decrease the exposure time.
Development time is too long. Decrease the development time. (Refer
to the development instructions.)
Developer is too hot. Cool the developer.
Developer is too concentrated. Adjust the concentration or replace the
developer.
RVG/film mode is incorrectly selected. Verify your exposure settings. (Refer to
the exposure procedure.)
5-4 SM700_K2100_03_en 9/2007
Page 27

6
Specifications
According to IEC Standard 601-2-7
Manufacturer
Trophy
A subsidiary of Carestream Health Inc.
4, rue F. Pelloutier - Croissy-Beaubourg
77435 Marne-la Vallée Cedex 2
France
Models
• Dental X-ray diagnosis devices, class 1, type B, intermittent use
• KODAK 2100-TR: equipped with tube TRX 708 from TROPHY
• KODAK 2100-C: equipped with tube OCX / 65-G from CEI
Electric power supply (during exposure)
• 230–240 V AC (± 10%), 50 Hz, 5 A, apparent resistance 0.5 Ω
• 100–110–130 V AC (± 10%), 50/60 Hz, 12 A, apparent resistance 0.2 Ω
Electric power supply (no exposure)
• 230–240 V AC (± 10%), 50 Hz, 100 mA
• 100–110–130 V AC (± 10%), 50/60 Hz, 100 mA
Rated high voltage and maximum corresponding current
• 60 kV / 7 mA
Current/voltage combinations for a maximum output power of:
• 420 W, 60 kV / 7 mA
Rated power for exposure time of 0.1 sec.
•420 W
Rate of use
• At 60 kV, 7 mA and 0.1 sec. and at the maximum tank temperature:
approximately one exposure every 8 sec.
Minimum value of the current/time product in the range of
conformity
• 0.07 mAs at 7 mA
Fixed parameters
• 60 kV / 7 mA
Area of conformity to IEC standard 60601-2-7 (2002)
• Reproducibility of the emitted radiation: conform
• Linearity of the emitted radiation: conform
• Precision in radiography: conform
9/2007 SM700_K2100_03_en 6-1
Page 28

Measurement conditions
• kV: Indirect measurement using a kV peakmeter
• mAs: Direct measurement in the circuit using a mAs-meter
• Exposure time: Indirect measurement on the kV signal at 75% of the
peak value
Storage and transportation conditions
• Temperature: -10°C to 60°C (14°F to 140°F)
• Relative humidity: 10% to 95%
• Atmospheric pressure: 700 to 1060 hPa
Dimensions and weight
• Control unit:
• Wall framework:
• X-ray emitting unit:
• Scissor arm:
• Mobile stand
(optional):
• Floor column
(optional):
• Ceiling column:
13 x 9 x 4 cm
(5.1 x 3.5 x 1.6 in.)
51.4 x 18.9 x 10.8 cm
(20.2 x 7.4 x 4.3 in.)
43.8 x 22.6 x 12 cm
(17.2 x 8.9 x 4.7 in.)
87.3 x 13.3 x 6.3 cm
(34.4 x 5.2 x 2.5 in.)
90 x 60 x 110 cm
(35.4 x 23.6 x 43.3 in.)
24 x 23 x 90 cm
(9.4 x 9.1 x 35.4 in.)
50 x 50 x 154 cm
(19.7 x 19.7 x 60.6 in.)
0.15 kg
(0.33 lb)
4.3 kg
(9.5 lb)
4.3 kg
(9.5 lb)
9 kg
(19.8 lb)
40 kg
(88.2 lb.)
20 kg
(44.2 lb.)
12,8 kg
(28.2 lb.)
Scissor arm
• Equipped with gas jack specially designed for this application; proven to
function correctly after more than 400,000 cycles
Electromagnetic compatibility
• KODAK 2100 Intraoral System complies with the European Directive
89/336/EEC and the IEC 60601.1.2 (2001) standard.
Classification: Group 1, Class B
6-2 SM700_K2100_03_en 9/2007
Page 29
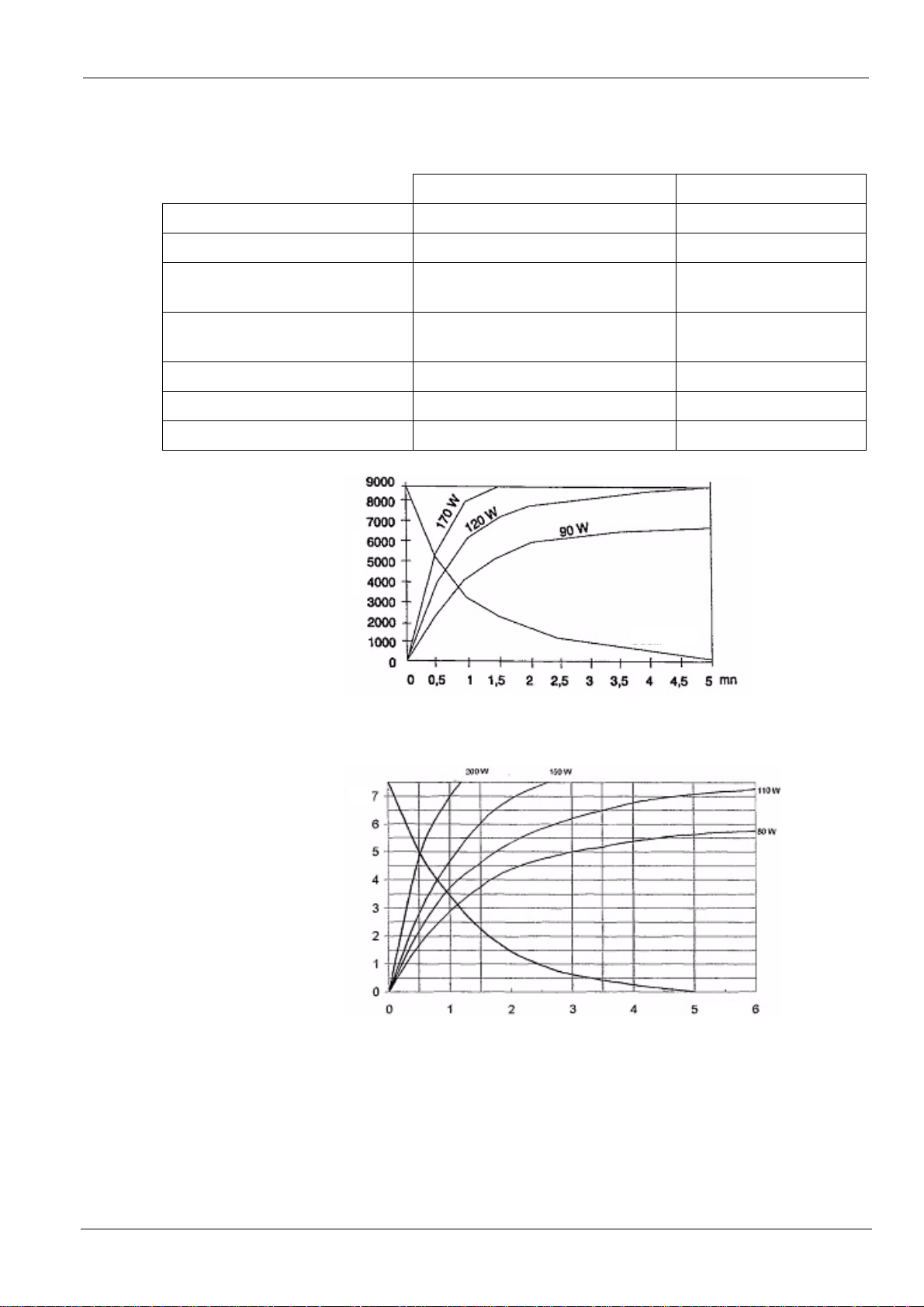
X-ray Generator
Rated high voltage 70 kV 70 kV
Rated anodic power 490 W 490 W
Table 12. Main characteristics of the x-ray ge ne rat o r
TROPHY type TRX 708 CEI type OCX/65-G
Maximum heat accumulated in the
8,700 J 10,000 J
anode
Rated value of focal spot
0.7 mm (0.027 in.) 0.7 mm (0.027 in.)
(IEC 60336/1993)
Target materials Tungsten Tungsten
Target slope 19° 19°
Filtration due to fixed materials 0.6 mm (0.023 in.) eq. Al 0.6 mm (0.023 in.) eq. Al
Joules
Cooling
Figure 13. Heating and cooling curves for TROPHY TRX 708 tube
kJ
min
Figure 14. Heating and cooling curves for CEI OCX/65-G tube
9/2007 SM700_K2100_03_en 6-3
Page 30

Equipped X-ray Generator
IEC standard 60601-2-28 (1993) Conform
Type of protection against electric shocks Class I
Degree of protection against electric shocks Type B
Rated value of inherent filtration 1.5 mm (0.059 in.) eq. Al
Rated value of additional filtration 1.0 mm (0.039 in.) eq. Al
Rated value of total filtration 2.5 mm (0.098 in.) eq. Al
Beam-limiting cone, focal spot/skin distance 20 cm (7 7/8 in.)
Maximum accumulated heat 32,500 J
Maximum continuous thermal dissipation 7 W
Table 15. Equipped x-ray generator
Amount of leaking radiation at maximum rate
during one hour of use
Maximum field of symmetrical radiation 6 cm (2 3/8 in.) diameter
Position and tolerances of the focal point on the
reference axis
< 0.25 mGy
0 mm +/-0.5 mm (0.020 in.)
a
b
c
Figure 16. X-ray generator
a Reference axis
b Target angle
c Focal point
6-4 SM700_K2100_03_en 9/2007
Page 31
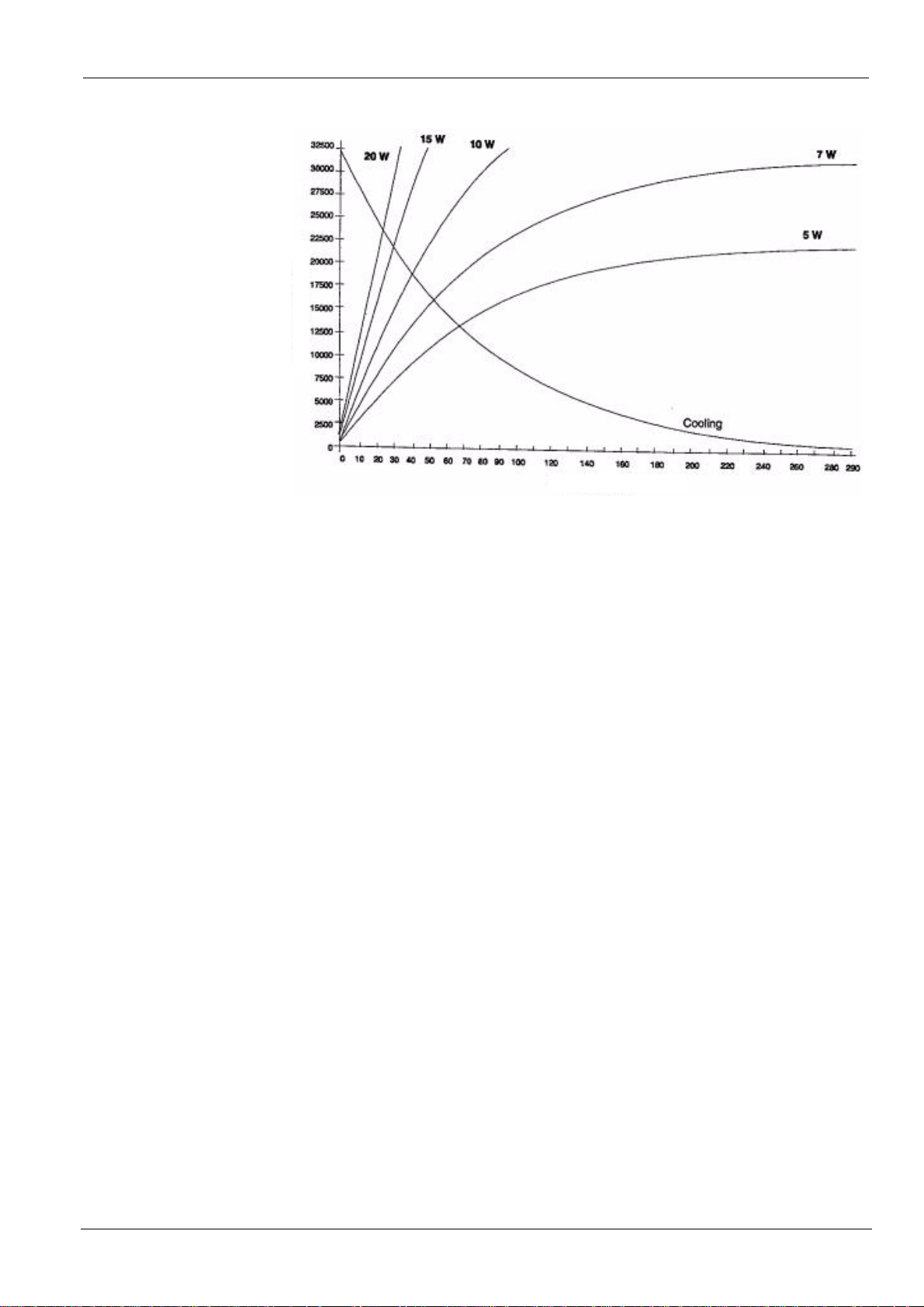
Energy in Joules
Time in minutes
Figure 17. Heating and cooling curves of the KODAK 2100 system
tube head
9/2007 SM700_K2100_03_en 6-5
Page 32

Position of Identification Labels
a b
Figure 18. Position of identification labels
a Machine identification
b X-ray emitting unit identification
6-6 SM700_K2100_03_en 9/2007
Page 33

Tables of Exposure Times
Table 19. Exposure times in seconds for KODAK film
KODAK Ultra-Speed (D) film KODAK Insight (F) film
60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior 0.250 0.400 Anterior 0.100 0.160
Premolar 0.320 0.500 Premolar 0.125 0.200
Molar 0.400 0.630 Molar 0.160 0.250
Mandibular Mandibular
Anterior 0.200 0.320 Anterior 0.080 0.125
Premolar 0.250 0.400 Premolar 0.100 0.160
Molar 0.250 0.400 Molar 0.100 0.160
Bitewing Bitewing
Anterior 0.200 0.320 Anterior 0.080 0.125
Posterior
Occlusal 0.500 0.630 Occlusal 0.200 0.250
KODAK D-Speed (D) film KODAK E-Speed (E) film
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
0.250
Table 20. Exposure times in seconds for KODAK film
60kV - 7mA Cone 20 cm (8 in.)
0.400 Posterior 0.100 0.160
Maxillary Child Adult Maxillary Child Adult
Anterior 0.250 0.400 Anterior 0.125 0.200
Premolar 0.320 0.500 Premolar 0.160 0.250
Molar 0.400 0.630 Molar 0.200 0.250
Mandibular Mandibular
Anterior 0.200 0.320 Anterior 0.100 0.160
Premolar 0.250 0.400 Premolar 0.100 0.160
Molar 0.250 0.400 Molar 0.125 0.200
Bitewing Bitewing
Anterior 0.200 0.320 Anterior 0.100 0.160
Posterior 0.250 0.400 Posterior 0.125 0.200
Occlusal 0.500 0.630 Occlusal 0.200 0.320
9/2007 SM700_K2100_03_en 6-7
Page 34

Table 21. Exposure times in seconds for KODAK CR plates
KODAK CR 7400 plates
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior 0.250 0.400 Anterior
Premolar 0.320 0.500 Premolar
Molar 0.400 0.630 Molar
Mandibular Mandibular
Anterior 0.200 0.320 Anterior
Premolar 0.250 0.400 Premolar
Molar 0.250 0.400 Molar
Bitewing Bitewing
Anterior 0.200 0.320 Anterior
Posterior 0.250 0.400 Posterior
Occlusal 0.500 0.630 Occlusal
Table 22. Exposure times in seconds for KODAK RVG digital sensors
KODAK RVG 6000 KODAK RVG 5000
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior 0.080 0.125 Anterior 0.100 0.160
Premolar 0.100 0.160 Premolar 0.125 0.160
Molar 0.125 0.200 Molar 0.160 0.200
Mandibular Mandibular
Anterior 0.063 0.100 Anterior 0.080 0.125
Premolar 0.080 0.100 Premolar 0.080 0.125
Molar 0.080 0.125 Molar 0.100 0.160
Bitewing Bitewing
Anterior 0.063 0.100 Anterior 0.080 0.125
Posterior 0.080 0.125 Posterior 0.100 0.160
Occlusal 0.125 0.200 Occlusal 0.160 0.250
6-8 SM700_K2100_03_en 9/2007
Page 35

Table 23. Exposure times in seconds for KODAK RVG digital sensors
KODAK RVG 6100 (sizes 1 & 2) KODAK RVG 6100 (size 0)
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior 0.080 0.125 Anterior 0.050 0.080
Premolar 0.100 0.160 Premolar 0.063 0.100
Molar 0.125 0.200 Molar 0.080 0.125
Mandibular Mandibular
Anterior 0.063 0.100 Anterior 0.040 0.063
Premolar 0.080 0.100 Premolar 0.050 0.063
Molar 0.080 0.125 Molar 0.050 0.080
Bitewing Bitewing
Anterior 0.063 0.100 Anterior 0.040 0.063
Posterior 0.080 0.125 Posterior 0.050 0.080
Occlusal 0.125 0.200 Occlusal 0.080 0.125
Table 24. Exposure times in seconds for KODAK RVG digital sensors
KODAK RVG 5100
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior 0.100 0.160 Anterior
Premolar 0.125 0.160 Premolar
Molar 0.160 0.200 Molar
Mandibular Mandibular
Anterior 0.080 0.125 Anterior
Premolar 0.080 0.125 Premolar
Molar 0.100 0.160 Molar
Bitewing Bitewing
Anterior 0.080 0.125 Anterior
Posterior 0.100 0.160 Posterior
Occlusal 0.160 0.250 Occlusal
9/2007 SM700_K2100_03_en 6-9
Page 36

Table 25. Exposure times in seconds for Trophy RVG digital sensors
Trophy RVG Access Trophy RVG Ultimate
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior 0.100 0.160 Anterior 0.080 0.125
Premolar 0.125 0.200 Premolar 0.100 0.160
Molar 0.160 0.200 Molar 0.125 0.200
Mandibular Mandibular
Anterior 0.080 0.125 Anterior 0.063 0.100
Premolar 0.080 0.125 Premolar 0.080 0.100
Molar 0.100 0.160 Molar 0.080 0.125
Bitewing Bitewing
Anterior 0.080 0.125 Anterior 0.063 0.100
Posterior 0.100 0.160 Posterior 0.080 0.125
Occlusal 0.160 0.250 Occlusal 0.125 0.200
Table 26. Exposure times in seconds for Trophy RVG digital sensors
Trophy RVG Reference
High Resolution
Trophy RVG Reference
High Sensitivity
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior 0.080 0.125 Anterior 0.020 0.032
Premolar 0.100 0.160 Premolar 0.025 0.040
Molar 0.125 0.160 Molar 0.032 0.050
Mandibular Mandibular
Anterior 0.063 0.100 Anterior 0.016 0.025
Premolar 0.063 0.100 Premolar 0.020 0.032
Molar 0.080 0.125 Molar 0.020 0.032
Bitewing Bitewing
Anterior 0.063 0.100 Anterior 0.016 0.025
Posterior 0.080 0.125 Posterior 0.020 0.032
Occlusal 0.125 0.200 Occlusal 0.040 0.050
6-10 SM700_K2100_03_en 9/2007
Page 37

Table 27. Exposure times in seconds for Trophy RVG digital sensors
Trophy RVGui High Resolution Trophy RVGui High Sensitivity
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior 0.080 0.125 Anterior 0.020 0.032
Premolar 0.100 0.160 Premolar 0.025 0.040
Molar 0.125 0.160 Molar 0.032 0.050
Mandibular Mandibular
Anterior 0.063 0.100 Anterior 0.016 0.025
Premolar 0.063 0.100 Premolar 0.020 0.032
Molar 0.080 0.125 Molar 0.020 0.032
Bitewing Bitewing
Anterior 0.063 0.100 Anterior 0.160 0.025
Posterior 0.080 0.125 Posterior 0.020 0.032
Occlusal 0.125 0.200 Occlusal 0.040 0.050
Table 28. Exposure times in seconds for Trophy RVG digital sensors
Trophy RVG THD
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior 0.040 0.063 Anterior
Premolar 0.050 0.080 Premolar
Molar 0.063 0.100 Molar
Mandibular Mandibular
Anterior 0.032 0.050 Anterior
Premolar 0.040 0.063 Premolar
Molar 0.040 0.063 Molar
Bitewing Bitewing
Anterior 0.032 0.050 Anterior
Posterior 0.040 0.063 Posterior
Occlusal 0.080 0.100 Occlusal
9/2007 SM700_K2100_03_en 6-11
Page 38

Table 29. Exposure times in seconds for your local con ditions (fill in this chart)
60kV - 7mA Cone 20 cm (8 in.) 60kV - 7mA Cone 20 cm (8 in.)
Maxillary Child Adult Maxillary Child Adult
Anterior Anterior
Premolar Premolar
Molar Molar
Mandibular Mandibular
Anterior Anterior
Premolar Premolar
Molar Molar
Bitewing Bitewing
Anterior Anterior
Posterior Posterior
Occlusal Occlusal
6-12 SM700_K2100_03_en 9/2007
Page 39
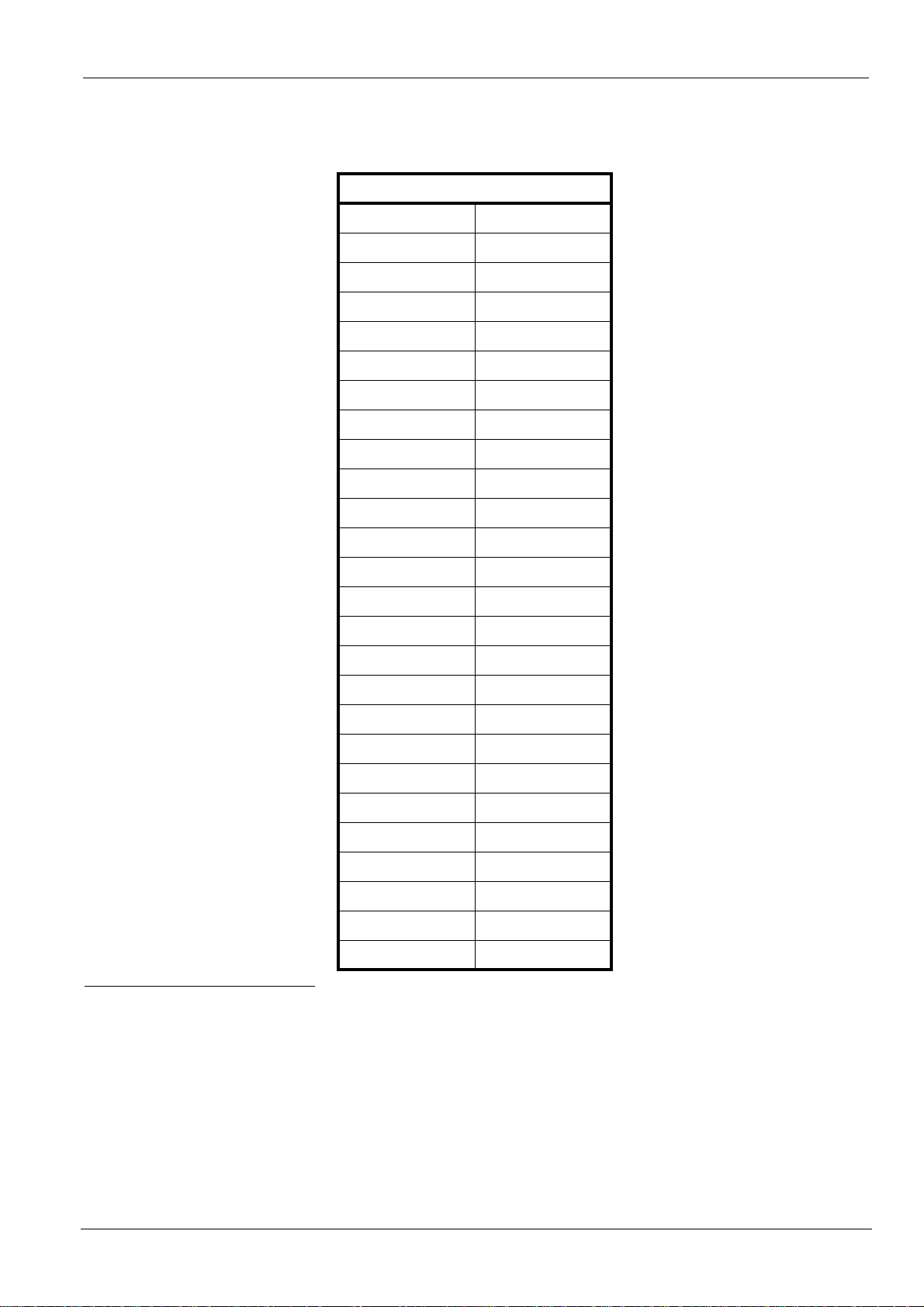
Emitted Doses
Table 30. Dose measured at extremity of cone area
20 cm (8 in.) cone
t (s) D (mGy)
0.010 0.06
0.013 0.08
0.016 0.10
0.020 0.12
0.025 0.15
0.032 0.19
0.040 0.24
0.050 0.30
0.063 0.38
0.080 0.49
0.100 0.61
0.125 0.76
Note
Dose accuracy: +/- 30% (mGray)
To obtain the dose in mGy.cm
values by the exposed surface, which
depends on the collimator that is used.
2
, multiply
0.160 0.97
0.200 1.22
0.250 1.52
0.320 1.95
0.400 2.44
0.500 3.05
0.630 3.84
0.800 4.87
1.000 6.09
1.250 7.61
1.600 9.74
2.000 12.18
2.500 15.23
9/2007 SM700_K2100_03_en 6-13
Page 40

Table 31. Exposure surface versus type of collimator used
Collimator type Format (mm) Used with
digital sensor
A 19 x 24 Size 0 - 4.6
B 23 x 35 Size 1 Size 0 22 x 35 8.3
C 31 x 39 Size 2 Size 1 24 x 40
Standard cone 60 mm
- Size 3 27 x 54
diameter
Used with
film
Size 2 31 x 41
Size 4 57 x 76
Exposure
surface (cm
12.1
28.3
2
)
6-14 SM700_K2100_03_en 9/2007
Page 41

© Carestream Health, Inc., 2007
The Kodak trade mark and trade dress are used under license
from Eastman Kodak Company
SM700-3 – 09/07
Trophy
A subsidiary of Carestream Health, Inc.
4 rue F. Pelloutier
Croissy-Beaubourg
77435 Marne la Vallée Cedex 2 (France)
+33 1 64 80 85 00
 Loading...
Loading...