KCI InfoVAC User manual

InfoV.A.C.® Therapy Unit
Owner Service Manual
Important Document
File in your maintenance records.
1
Table Of Contents |
|
WARNING.......................................................................................................................................................................................................... |
3 |
Important Safety Information Accompanies This Device............................................................................................................. |
3 |
DISCLAIMER OF WARRANTY AND LIMITATION OF LIABILITY......................................................................................................... |
3 |
Important Information For Users............................................................................................................................................................. |
4 |
InfoV.A.C.® Therapy Unit Feature Identification.................................................................................................................................. |
5 |
Introduction / About This Manual........................................................................................................................................................... |
6 |
Preparation For Use....................................................................................................................................................................................... |
6 |
Unpack the Unit........................................................................................................................................................................................... |
6 |
Initial Inspection.......................................................................................................................................................................................... |
6 |
Serial Number Location............................................................................................................................................................................. |
7 |
Cleaning and Disinfection.......................................................................................................................................................................... |
7 |
Infection Control.......................................................................................................................................................................................... |
7 |
Supplies and Equipment Needed.......................................................................................................................................................... |
7 |
General Cleaning Recommendations.................................................................................................................................................. |
7 |
Therapy Unit.................................................................................................................................................................................................. |
8 |
Power Supply................................................................................................................................................................................................ |
9 |
Service Procedures...................................................................................................................................................................................... |
10 |
Inspect Unit For Damage........................................................................................................................................................................ |
10 |
•• Hanger Arm and Rubber Anti-slip Pad Inspection................................................................................................................. |
12 |
Battery Check / Change........................................................................................................................................................................... |
13 |
Charge Battery............................................................................................................................................................................................ |
15 |
Power On / Screen Inspection............................................................................................................................................................... |
16 |
Data Transfer............................................................................................................................................................................................... |
16 |
Canister Bellows Check............................................................................................................................................................................ |
17 |
Canister Fit................................................................................................................................................................................................... |
18 |
Verify Time and Date................................................................................................................................................................................ |
19 |
Testing Procedures...................................................................................................................................................................................... |
20 |
Pressure Transducer Check..................................................................................................................................................................... |
20 |
•• Test Canister Preparation................................................................................................................................................................ |
20 |
•• Unit Preparation................................................................................................................................................................................. |
21 |
•• Engineering Screens Access........................................................................................................................................................... |
21 |
•• Sensor Accuracy Test........................................................................................................................................................................ |
22 |
•• Zero Pressures..................................................................................................................................................................................... |
22 |
Pressure Checks.......................................................................................................................................................................................... |
23 |
•• Test Canister Preparation................................................................................................................................................................ |
23 |
•• Unit Preparation................................................................................................................................................................................. |
23 |
•• Test Procedure..................................................................................................................................................................................... |
23 |
Alarm Tests................................................................................................................................................................................................... |
25 |
•• Canister Preparation......................................................................................................................................................................... |
25 |
•• Unit Setup............................................................................................................................................................................................. |
26 |
•• Leak Alarm............................................................................................................................................................................................ |
26 |
•• Blockage Alert..................................................................................................................................................................................... |
26 |
•• Canister Not Engaged...................................................................................................................................................................... |
27 |
•• Canister Full Therapy Interrupted................................................................................................................................................ |
27 |
Testing Complete....................................................................................................................................................................................... |
27 |
Final Settings............................................................................................................................................................................................... |
28 |
Recharge Battery....................................................................................................................................................................................... |
28 |
2
Preparation for Transport and Patient Use......................................................................................................................................... |
28 |
Hanger Arm Rubber Block Replacement............................................................................................................................................ |
29 |
Rubber V-Groove Anti-slip Pad Repair.................................................................................................................................................. |
31 |
Specifications................................................................................................................................................................................................ |
32 |
Spare Parts ..................................................................................................................................................................................................... |
33 |
Symbols Used................................................................................................................................................................................................ |
34 |
Service Manual........................................................................................................................................................................................... |
34 |
Therapy Unit................................................................................................................................................................................................ |
34 |
Customer Contact Information............................................................................................................................................................... |
34 |
Technical Report........................................................................................................................................................................................... |
35 |
InfoV.A.C.® Therapy System Required Service Record.................................................................................................................... |
36 |
3
WARNING
Important Safety Information Accompanies This Device
Indications, Contraindications, Warnings, Precautions and other Safety Information are contained in the V.A.C.® Therapy System Safety Information Sheet. This information sheet is included with the therapy unit and also included in
V.A.C.® Dressing cartons. Please consult the V.A.C.® Therapy System’s User Manual and the Safety Information Sheet before applying V.A.C.® Therapy. If there are questions, or if this information sheet is missing, immediately contact your
local KCI representative.
Additional product information can be found at www.kci1.com (US) or www.kci-medical.com (outside the US).
As with all prescription medical devices, failure to follow product instructions or adjusting settings and performing therapy applications without the express direction and / or supervision of your trained clinical caregiver may lead to improper product performance and the potential for serious or fatal injury. For medical questions, please consult a physician. In case of medical emergency, immediately contact your local emergency services provider.
CAUTION: Federal law (US) restricts this device to sale or rental by or on the order of a physician.
DISCLAIMER OF WARRANTY AND LIMITATION OF LIABILITY
KCI HEREBY DISCLAIMS ALL EXPRESS OR IMPLIED WARRANTIES, INCLUDING WITHOUT LIMITATION ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE, ON THE KCI PRODUCT(S) DESCRIBED IN THIS PUBLICATION. ANY WRITTEN WARRANTY OFFERED BY KCI SHALL BE EXPRESSLY SET FORTH IN THIS PUBLICATION OR INCLUDED WITH
THE PRODUCT. UNDER NO CIRCUMSTANCES SHALL KCI BE LIABLE FOR ANY INDIRECT, INCIDENTAL, OR CONSEQUENTIAL DAMAGES AND EXPENSES, INCLUDING DAMAGES OR INJURY TO PERSON OR PROPERTY, DUE IN WHOLE OR IN PART TO THE USE OR REPAIR OF THE PRODUCT OTHER THAN THOSE FOR WHICH DISCLAIMER OF WARRANTY OR LIMITATION OF LIABILITY IS EXPRESSLY PROHIBITED BY SPECIFIC, APPLICABLE LAW. NO PERSON HAS THE AUTHORITY TO BIND KCI TO ANY REPRESENTATION OR WARRANTY EXCEPT AS SPECIFICALLY SET FORTH IN
THIS PARAGRAPH.
Descriptions or specifications in KCI printed matter, including this publication, are meant solely to generally describe the product at the time of manufacture and do not constitute any express warranties except as set forth in the written limited warranty included with this product. Information in this publication may be subject to change at any time.
Contact KCI for updates.
4
Important Information For Users
In order for KCI products to perform properly, KCI recommends the following conditions. Failure to comply with these conditions will void any applicable warranties.
•• Use this product only in accordance with this manual, the device user manual and applicable product labeling.
•• Assembly, operations, extensions, re-adjustments, modifications, technical maintenance or repairs must be performed by qualified personnel authorized by KCI. Certain repairs will require KCI trained technicians. Please contact KCI at (in the
US) 1-800-275-4524 for repairs not provided for in this service manual.
•• Ensure the electrical installation of the room complies with the appropriate national electrical wiring standards.
•• Do not operate this product if it has a damaged power cord, power supply or plug. If these components are worn or damaged, contact KCI.
•• Do not drop or insert any object into any opening or tubing of this product.
•• Do not connect this product or its components to devices not recommended by KCI.
•• Use only V.A.C.® Dressings and KCI approved parts with this product.
•• Keep this product away from heated surfaces.
•• Although this product conforms to the intent of the standard IEC 60601-1-2 in relation to Electromagnetic Compatibility, electrical equipment may produce interference. If interference is suspected, separate the equipment and contact KCI.
•• Avoid spilling fluids on any part of this product.
Fluids remaining on the electronic controls can cause corrosion that may cause the electronic components to fail. Component failures may cause the unit to operate erratically, possibly producing potential hazards to patient and staff. If spills do occur, unplug the unit immediately and clean with an absorbent cloth. Ensure there is no moisture in or near the power connection and power supply components before reconnecting power. If the product does not work properly, contact KCI.
•• Do not use this product while bathing / showering or where it can fall or be pulled into a tub, shower or sink.
•• Do not reach for a product that has fallen into water. Unplug the unit immediately if plugged into electrical source. Disconnect the unit from dressing and contact KCI.
•• Refer to the Cleaning and Disinfection chapter of this manual for information on infection control.
Notice
This product has been configured from the manufacturer to meet specific voltage requirements. Refer to the product information label for specific voltage.
5
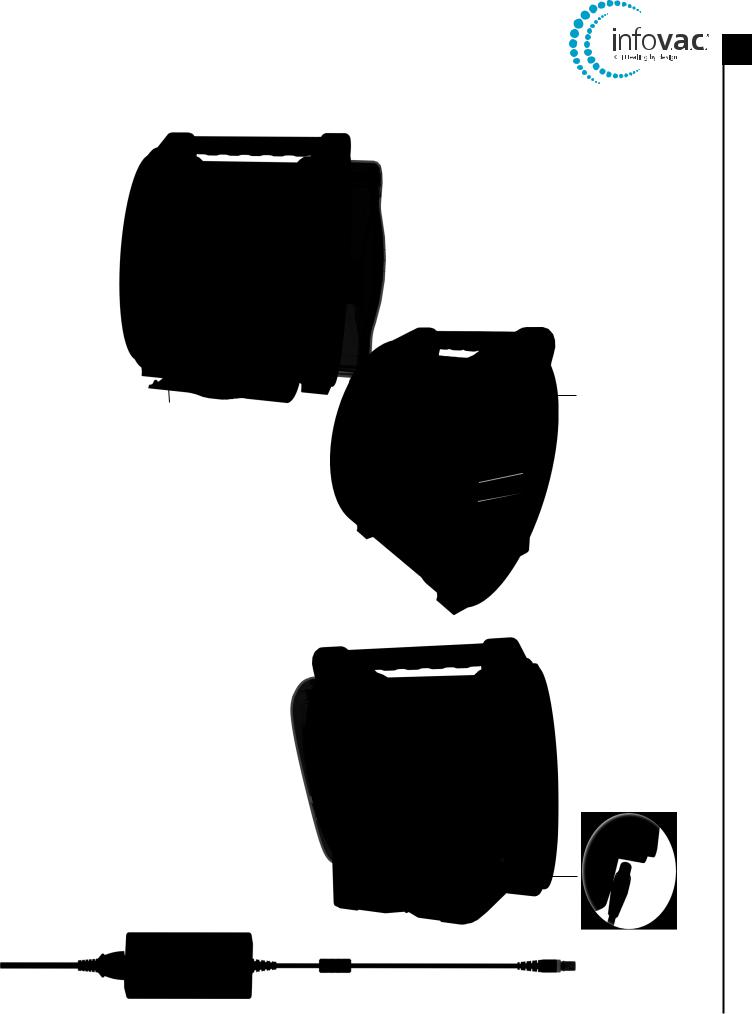
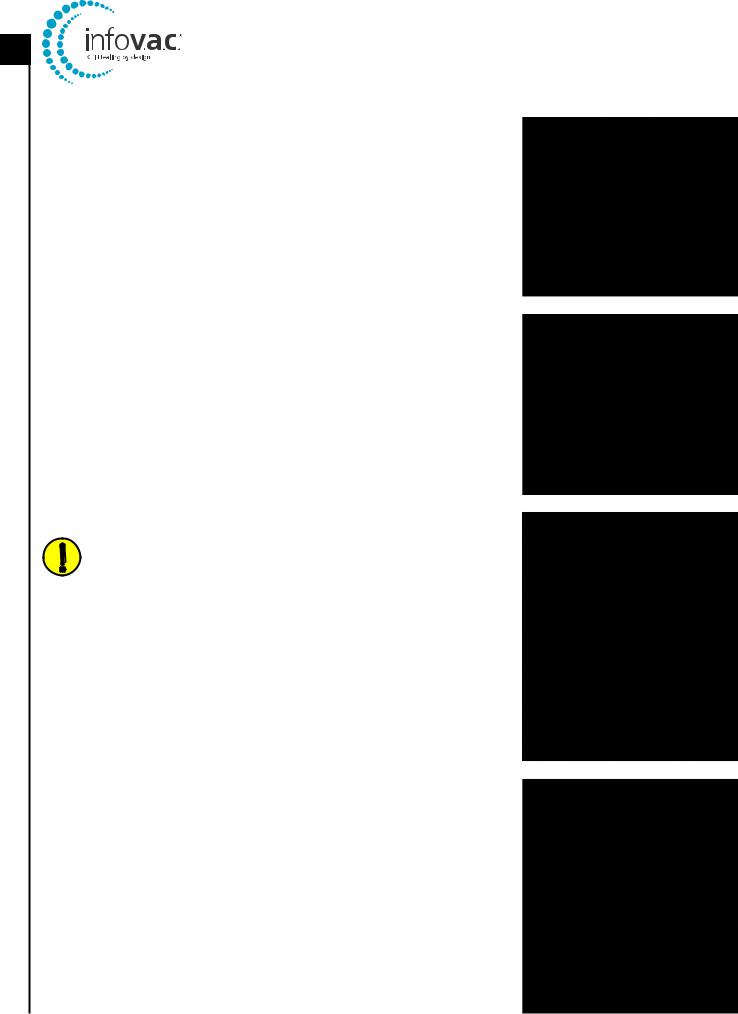
InfoV.A.C.® Therapy Unit Feature Identification
Power On / Off |
|
|
|
Canister Release Button |
|||
Button |
|
|
|||||
|
|
||||||
Touch Screen |
|
|
|
|
|
|
InfoV.A.C.® 500 mL Canister |
|
|
|
|
||||
|
|
|
|
|
|
|
|
Stylus |
|
|
|
|
|
|
|
|
|
Canister Bellows |
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|||
UDI Door |
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
Memory Card Slot |
|
Infrared Data Port |
|
Patient Information |
||||||
|
||||||||||
|
|
|
|
|
USB Data Port |
|
Label |
|||
|
|
|
|
|
|
|
|
|
|
|














 Serial Number
Serial Number
Label
Carry Handle |
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
||||
Over Pressure Warning / Caution Label |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Port Cover |
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||
Hanger Arm Instruction Label |
|
|
|
|
|
|
|
|
|
|
|
|
|
Battery Charging |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Hanger Lock Knob |
|
|
|
|
|
|
|
|
|
|
|
Indicator LED |
|||
|
|
|
|
|
|
||||||||||
Hanger Arm |
|
|
|
|
|
|
|
|
|
|
|
Power Connection |
|||
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
||||
Manufacturer Identification Label |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Regulatory Label |
|
|
|
|
|
|
|
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
InfoV.A.C.® Power Supply
6
Introduction / About This Manual
This manual is designed to assist with routine service procedures for the InfoV.A.C.® Therapy Unit sold by KCI. Following these procedures ensures that the InfoV.A.C.® Therapy Unit is properly cleaned, fully functional and ready for patient use. These procedures include:
•• unpacking and initial unit inspection
•• cleaning and disinfection
•• inspection for damaged and / or missing parts
•• verifying unit function
•• ensuring battery is fully charged
•• verifying default settings are correct
All steps in these procedures must be followed in the order presented to provide proper functionality and reliability of the InfoV.A.C.® Therapy Unit. Opening the therapy unit to gain access to the internal components (other than the battery) may void the warranty.
Each InfoV.A.C.® Therapy Unit must be cleaned, disinfected, inspected and charged between each patient use. For further questions about the required frequency of service procedures, contact KCI (see page 34).
It is recommended that all sections of this manual be reviewed before beginning any routine service procedures on the InfoV.A.C.® Therapy Unit. Please follow all applicable warnings and cautions and use universal precautions where necessary.
Preparation For Use
Preparing the InfoV.A.C.® Therapy Unit for use includes unpacking, inspection for any damaged or missing parts and an initial round of service procedures to ensure the battery is fully charged and the unit is ready for patient use. The following procedures should be performed when the unit is first received from KCI.
Unpack the Unit
1.Inspect the cardboard shipping box for visible signs of damage.
2.Unpack the unit from the shipping box. Use caution when opening to ensure box contents are not damaged.
3.Inspect the contents of the shipping box for damaged items. If damage is noted, contact the shipping company for reporting / return procedures.
4.Inventory all items in shipping box against the packing slip. Contact KCI (see page 34). if there are any items missing.
Initial Inspection
The purpose of this inspection is to ensure the unit has arrived without any internal damage and that the battery is fully charged prior to the initial patient placement.
1.Using a copy machine, reproduce the InfoV.A.C.® Therapy System Required Service Record form from the back of this manual. Fill in the required information for the unit in the spaces provided.
2.Ensure the date of the last Pressure Transducer Check is recorded on the form.
3.Perform the required service procedures as listed on the form. Start with Inspect Unit For Damage on page 10 of this manual.
4.After initial inspection and service are complete, retain the completed form for each unit as a permanent record.

7
Serial Number Location
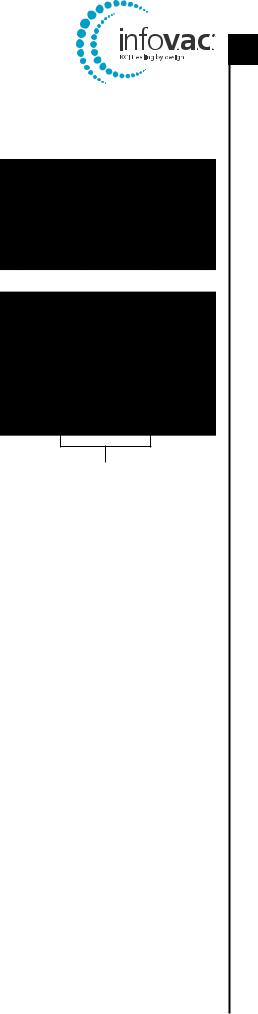
The InfoV.A.C.® has a serial number label, illustrated at right, that is located on the side of the unit in the canister recess. This serial number will be recorded on the InfoV.A.C.® Therapy System Required Service Record form.
Serial Number
Cleaning and Disinfection
Cleaning and disinfection of the InfoV.A.C.® Therapy Unit includes wipedown of all hard surface components. The InfoV.A.C.® Therapy Unit must be cleaned and disinfected:
1.if it becomes soiled during patient use, or
2.between each patient use.
Infection Control
Institutional policies regarding infection control may vary; however, KCI recommends the following regarding infection control when processing KCI V.A.C.® Therapy devices:
•• Designate contaminated and clean areas for separating and storing equipment before and after transport, cleaning and disinfection. Follow protocols to ensure no cross-contamination occurs between unclean and clean units.
•• Use personal protective equipment (PPE) and hand hygiene protocols in accordance with the following standards:
•• 29 CFR 1910.1030, OSHA Bloodborne Pathogens Standard
•• MMWR October 2002;51 (No. RR-16), Guidelines for Hand Hygiene in Healthcare Settings
•• Clean all organic material from the therapy unit prior to disinfection.
•• Use hospital-grade cleaners and disinfectants according to the CDC 2008 Guideline for Disinfection and Sterilization in Healthcare Facilities.
•• Do not immerse or saturate the therapy unit with fluids to avoid damage to the electronics in the device. Follow institutional procedures used for the cleaning and disinfection of other hard surface durable electronic medical equipment.
Ensure that the InfoV.A.C.® Therapy Unit and its power supply are not connected to AC power when using cleaning fluids of any nature.
Supplies and Equipment Needed
•• antiseptic wipes such as PDI Sani-Cloth® Plus wipes (or equivalent)
•• clear plastic bags (as appropriate)
•• cotton tipped applicators
General Cleaning Recommendations
•• Use PPE as appropriate.
•• For items that are wiped down, ensure item’s entire surface is completely covered with the cleaning fluid and remains wet for a minimum of 60 seconds.
8
Therapy Unit
1.Ensure therapy unit is unplugged from power supply.
2.Remove canister bellows from unit. Wipe / clean bellows with an antiseptic wipe. Ensure the bellows cavities and adjacent surfaces are clean of any foreign material. Use a cotton tipped applicator if necessary. Replace canister bellows when finished.
3. Wipe down the unit using an antiseptic wipe.
Do not allow excess fluid to pool in the areas surrounding the touch screen or touch screen gasket.
4.Once the unit is thoroughly cleaned, allow to air dry.
5.Place the clean unit in a clear plastic bag and move it to the service area.
Power Supply
1.Ensure the power supply is unplugged from the therapy unit and / or any power source.
2.Wipe the power supply and cords with an antiseptic wipe. Allow to air dry.
3.Inspect the power supply brick and cords for damage and cracked or exposed wiring. Contact KCI (see page 34) if replacement is necessary.
4.Inspect the caution labels attached to the power cords for legibility. Replace as necessary.
5.Loosely loop the power cords.
6.Place the clean power supply in a clear plastic bag and move it to the service area.
9
Power Cord Labels
10
Service Procedures
The following service procedures are used to verify that the InfoV.A.C.® Therapy Unit is functioning properly and that the battery is fully charged. Once these procedures are complete, the unit will be ready for patient use. These procedures should be performed as listed and in order:
1.When the unit is first received from KCI, as part of the initial inspection (starting at Inspect Unit for Damage).
2.Between each patient use.
All service should occur in a clean area that is protected from contamination from unclean units or other potential contamination sources. Use PPE as appropriate or as specified in local protocols.
Inspect Unit For Damage
Tools and Supplies:
None.
The following replacement parts may be required:
As needed and identified during inspection.
The purpose of this procedure is to inspect the InfoV.A.C.® unit for damage using the following criteria:
1.Examine the unit top, bottom and sides for:
•• Cracks of any size.
•• Holes of any size that expose internal components.
2.Examine the canister recess area for:
•• Cracks of any size.
•• Damage to the canister bellows.
3.Ensure the battery tray (bottom of unit) rubber non-slip strips are present and secure. KCI recommends replacing the battery tray if necessary.
4.Open the User Data Interface (UDI) door and ensure the stylus is present, as shown. Replace the stylus if necessary.
 Loading...
Loading...