Page 1

Instructions for use
KaVo In eXam 3510
Always be on the safe side.
Page 2

Sales:
KaVo Dental GmbH
Bismarckring 39
D-88400 Biberach
Tel. +49 7351 56-0
Fax +49 7351 56-1488
Manufacturer:
Kaltenbach & Voigt GmbH
Bismarckring 39
D-88400 Biberach
www.kavo.com
Page 3

Instructions for use KaVo In eXam 3510
Contents
Contents
Contents ............................................................................................................................................................1
1 User notes ......................................................................................................................................................3
1.1 User guidelines .........................................................................................................................................3
1.1.1 Standard documentation ..................................................................................................................3
1.1.2 Abbreviations ....................................................................................................................................3
1.1.3 Symbols used ...................................................................................................................................3
1.1.4 Definitions .........................................................................................................................................5
1.2 Target audience .......................................................................................................................................6
1.3 Service .....................................................................................................................................................7
1.3.1 For North America ............................................................................................................................7
1.4 Implied warranty .......................................................................................................................................8
1.5 Restrictions on the guarantee ..................................................................................................................9
1.6 Transport and storage ............................................................................................................................10
1.6.1 Transportation damage ..................................................................................................................10
1.6.2 Storage ...........................................................................................................................................10
2 Safety ...........................................................................................................................................................12
2.1 Explanation of safety symbols ................................................................................................................12
2.1.1 Warning symbol ..............................................................................................................................12
2.1.2 Structure .........................................................................................................................................12
2.1.3 Description of danger levels ...........................................................................................................12
2.2 Intended purpose ...................................................................................................................................13
2.2.1 General ...........................................................................................................................................13
2.2.2 Product-specific ..............................................................................................................................14
2.3 Safety instructions ..................................................................................................................................15
2.3.1 Attaching to KaVo Primus 1058 ......................................................................................................16
2.4 Protective devices ..................................................................................................................................17
2.4.1 Closing the casing ..........................................................................................................................17
2.5 Requirements for correct operation ........................................................................................................18
2.5.1 Standard conformity .......................................................................................................................18
3 Product description ......................................................................................................................................19
3.1 Entire system ..........................................................................................................................................19
3.2 Operating unit .........................................................................................................................................20
3.2.1 Assignment of button/area to be X-rayed .......................................................................................20
3.3 Focal spot determination ........................................................................................................................22
3.4 Where to affix nameplates, power rating plates and caution labels .......................................................23
3.5 Nameplates, power rating plates and caution labels ..............................................................................24
3.6 Technical data ........................................................................................................................................28
3.6.1 Information on electromagnetic compatibility .................................................................................32
3.6.2 Standard conformity .......................................................................................................................35
3.7 Determination of the patient's X-ray exposure .......................................................................................36
3.7.1 Exposure diagram ..........................................................................................................................36
4 Operation .....................................................................................................................................................37
4.1 Switch on machine .................................................................................................................................37
4.2 Specifying the imaging parameters ........................................................................................................38
4.3 Laser highlighting of central beam .........................................................................................................39
4.4 Positioning patients ................................................................................................................................40
4.5 Position X-ray source .............................................................................................................................41
4.6 Positioning the image receiver ...............................................................................................................42
4.7 Take X-ray ..............................................................................................................................................45
4.8 Finishing off activities .............................................................................................................................46
1/64
Page 4

Instructions for use KaVo In eXam 3510
Contents
5 Setting default doses, film sensitivity and sensor sensitivity ........................................................................47
5.1 Correction of the exposure time / dose default setting ...........................................................................47
5.2 Selection of the suitable basic dose .......................................................................................................48
5.2.1 With film ..........................................................................................................................................48
5.2.2 With digital image receiver .............................................................................................................48
5.3 Exposure time table ................................................................................................................................49
5.3.1 Default dose setting for X-rays taken using film .............................................................................49
5.3.2 Default dose for X-rays taken with digital image receivers .............................................................52
6 Preparation methods DIN EN ISO 17664 ....................................................................................................56
6.1 Cleaning and disinfection .......................................................................................................................56
6.1.1 Cleaning .........................................................................................................................................56
6.1.2 Disinfection .....................................................................................................................................56
7 Safety checks ...............................................................................................................................................58
8 Troubleshooting ...........................................................................................................................................59
9 Accessories and compatibility ......................................................................................................................62
9.1 Accessories and kits ...............................................................................................................................62
9.1.1 Accessories ....................................................................................................................................62
9.1.2 Kits ..................................................................................................................................................63
9.2 Compatibility ...........................................................................................................................................64
2/64
Page 5
Instructions for use KaVo In eXam 3510
1 User notes | 1.1 User guidelines
1 User notes
1.1 User guidelines
Requirement
You should read these instructions prior to installing the product, in order to avoid
maloperations and damage.
This document has been translated from the German original.
1.1.1 Standard documentation
The product-related documentation forKaVo In eXam consists of the following com‐
ponents. You can order more of these from KaVo, if necessary:
▪ User instructions
▪ Assembly instructions
▪ Customer documents
1.1.2 Abbreviations
Short
form
GA Instructions for use
PA Care instructions
MA Assembly instructions
TA Technician's instructions
STK Safety check
IEC International Electrotechnical Commission
RA Repair instructions
EMC Electromagnetic compatibility
Explanation
1.1.3 Symbols used
Symbols in this document
See the section Safety/Warning Symbol
Important information for users and technicians
Caution: ionising radiation!
Laser beam
3/64
Page 6
Instructions for use KaVo In eXam 3510
1 User notes | 1.1 User guidelines
CE mark (Communauté Européenne). A product with this mark meets the
requirements of the relevant EC directives, i.e. the applicable standards in
Europe.
Action request
Symbols on the operating unit and X-ray head
kV button
"Consult user instructions!" symbol
Take X-ray
Bitewing function area "Molars"
Bitewing function area "Canines/Incisors"
X-ray emission pilot lamp
Dig eXam button
Dosage preselection film (7mA), digital image receivers (4mA).
Adult/child selection button
Occlusal function selection
Selection button for default dose setting
"Ionising radiation" symbol
Symbols on identification labels
Type B application part [IEC 878-02-02]
Focus size [IEC 417-5326-a]
CE marking
X-ray filtration [IEC 417-5381]
X-ray tube CE marking [IEC 417-5381]
4/64
Page 7
Instructions for use KaVo In eXam 3510
1 User notes | 1.1 User guidelines
CSA label
Consult user instructions
For information about proper disposal, see intended purpose
1.1.4 Definitions
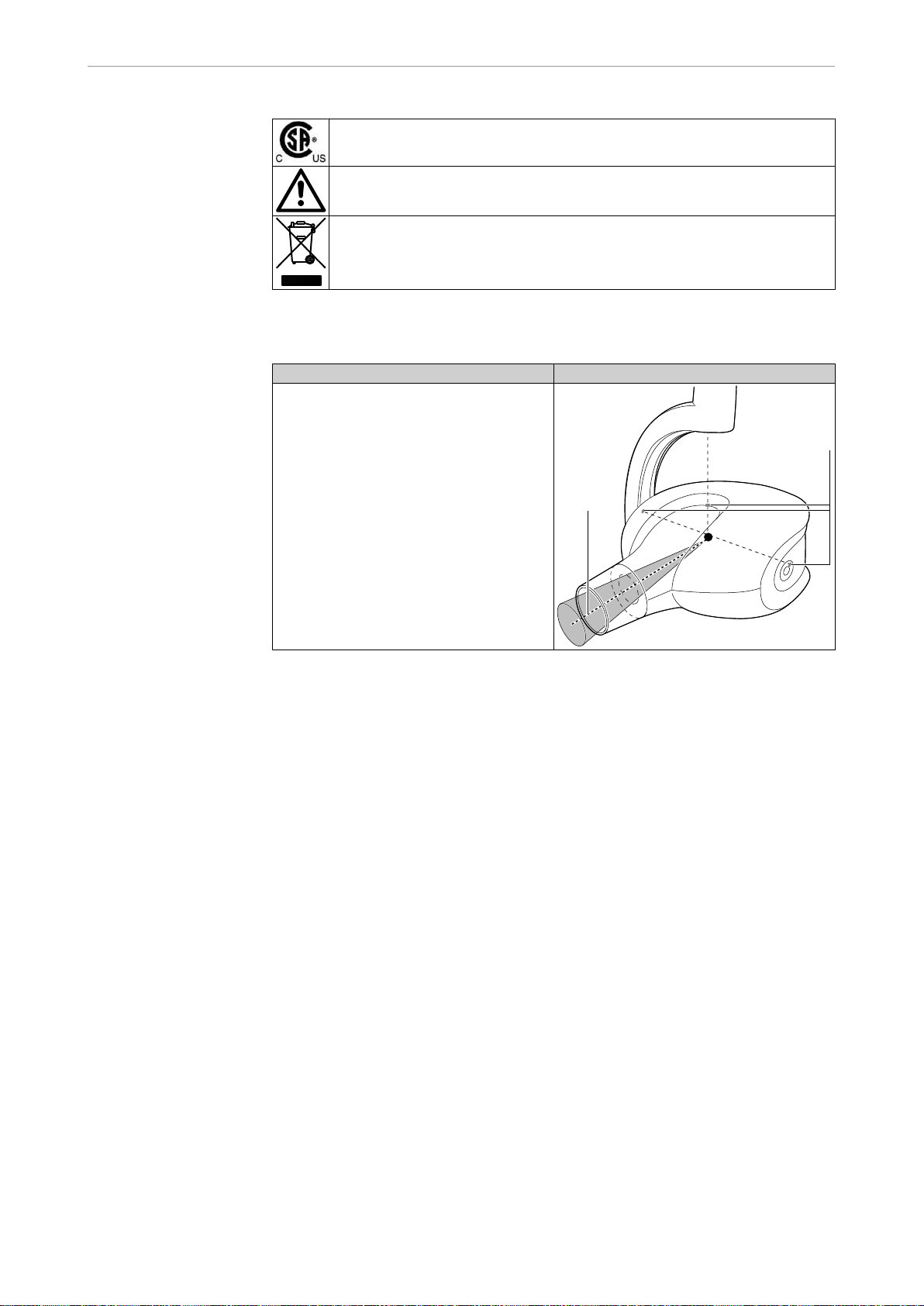
Definition Meaning
Focus-skin distance (FSD) ① refers to
the distance between the focal spot and
the patient's skin. The focal spot is loca‐
ted at the point where both axes inter‐
cept, identified by the focal spot markers
② .
2
1
5/64
Page 8

Instructions for use KaVo In eXam 3510
1 User notes | 1.2 Target audience
1.2 Target audience
This document is intended for use by dentists and other practice employees as well
as for use by clinic staff, who possess the appropriate 'expert knowledge' and who
have undergone instruction on the use of X-ray machines in accordance with the
stipulations of the country in which they are practising.
6/64
Page 9
Instructions for use KaVo In eXam 3510
1 User notes | 1.3 Service
1.3 Service
Service hotline:
++ 49 (0) 7351 56-2900
Service.Roentgen@kavo.com
Please indicate the product serial number in all requests.
Additional information can be obtained at: www.kavo.com
1.3.1 For North America
GENDEX Dental Systems
019 West Oakton Street
Des Plaines, IL 60018-1884
USA
+1 (847) 640-48 00
7/64
Page 10

Instructions for use KaVo In eXam 3510
1 User notes | 1.4 Implied warranty
1.4 Implied warranty
The KaVo end-user customer guarantee for the product named in the completion
certificate guarantees that the product functions correctly and that there are no faults
in the material or workmanship for a duration of 12 months following the purchase
date, according to the following conditions:
Following a reasonable complaint relating to defects or short delivery, KaVo will
provide a replacement or perform repairs. KaVo reserves the right to perform re‐
pairs.
Claims of any other nature, damages in particular, are excluded. In case of default
and gross negligence or intent, the latter only applies if there are no compelling legal
provisions opposing it.
KaVo shall not be liable for defects and their consequences, which occur as a result
of normal normal wear and tear, or of improper cleaning and maintenance, nonobservance of the operating, maintenance or connection regulations; calcination or
corrosion; a contaminated air or water supply; or chemical or electrical effects, which
are non-standard or not permitted according to company regulations.
As a general rule, this guarantee does not apply to lamps, glassware, rubber parts
or the colour durability of synthetic materials.
KaVo shall not be liable for defects or their consequences if they are likely to be a
direct result of actions or modifications by a customer or third party.
Any claims arising from this guarantee can only be lodged if the completion certifi‐
cate (carbon copy) has been sent in to KaVo and the operator/user is able to produce
the original.
8/64
Page 11

Instructions for use KaVo In eXam 3510
1 User notes | 1.5 Restrictions on the guarantee
1.5 Restrictions on the guarantee
KaVo shall assume responsibility for the safety, reliability and performance of all
KaVo system components provided that:
▪ Assembly, upgrades, new settings, modifications or repairs toKaVo In eXam
exclusively carried out be KaVo or third parties authoirsed by KaVo
forKaVo In eXam .
▪ The system has been installed and operated in accordance with the User in‐
structions and Assembly instructions.
▪ All servicing is performed in full compliance withDIN VDE-0751-1 must be com‐
plied with to its full extent.
▪ In Germany, the location in whichKaVo In eXam is installed is compliant with
standardsDIN VDE 0100-710: 2002-11 andVDE 0100-560:1995 in its design. In
other countries, the corresponding national regulations must be respected.
9/64
Page 12
Instructions for use KaVo In eXam 3510
1 User notes | 1.6 Transport and storage
1.6 Transport and storage
1.6.1 Transportation damage
Outside of Germany
Note
KaVo is not liable for damage arising from transportation.
Immediately inspect the delivery after receipt!
If external damage to the packaging is visible upon delivery, follow the procedure
below:
1. The recipient must record the loss or damage in the notice of delivery. The re‐
cipient and employee of the transportation firm must sign the notice of delivery.
The recipient can only assert damages against the transportation company ba‐
sed on these records.
2. Leave the product and packaging unchanged.
3. Do not use the product.
If the product is damaged and there is no discernable damage to the packaging
upon delivery, proceed as follows:
1. Report the damage immediately or at least 7 days after the delivery to the deli‐
very company .
2. Leave the product and packaging unchanged.
3. Do not use a damaged product.
Note
If the recipient does not follow one of the above instructions, the damage will be
held to have occurred after the delivery (according to . CMR law , section 5, Art.
30).
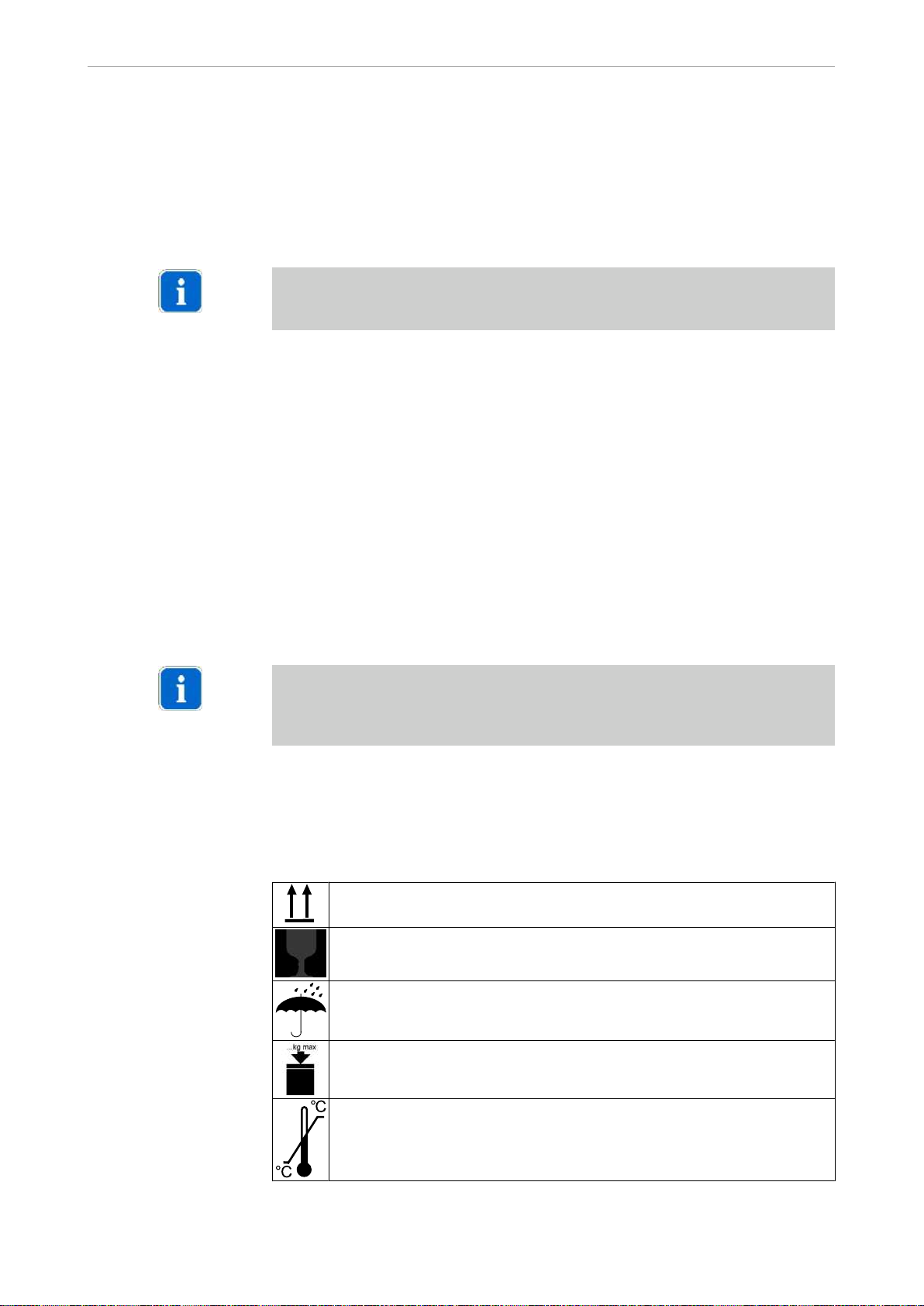
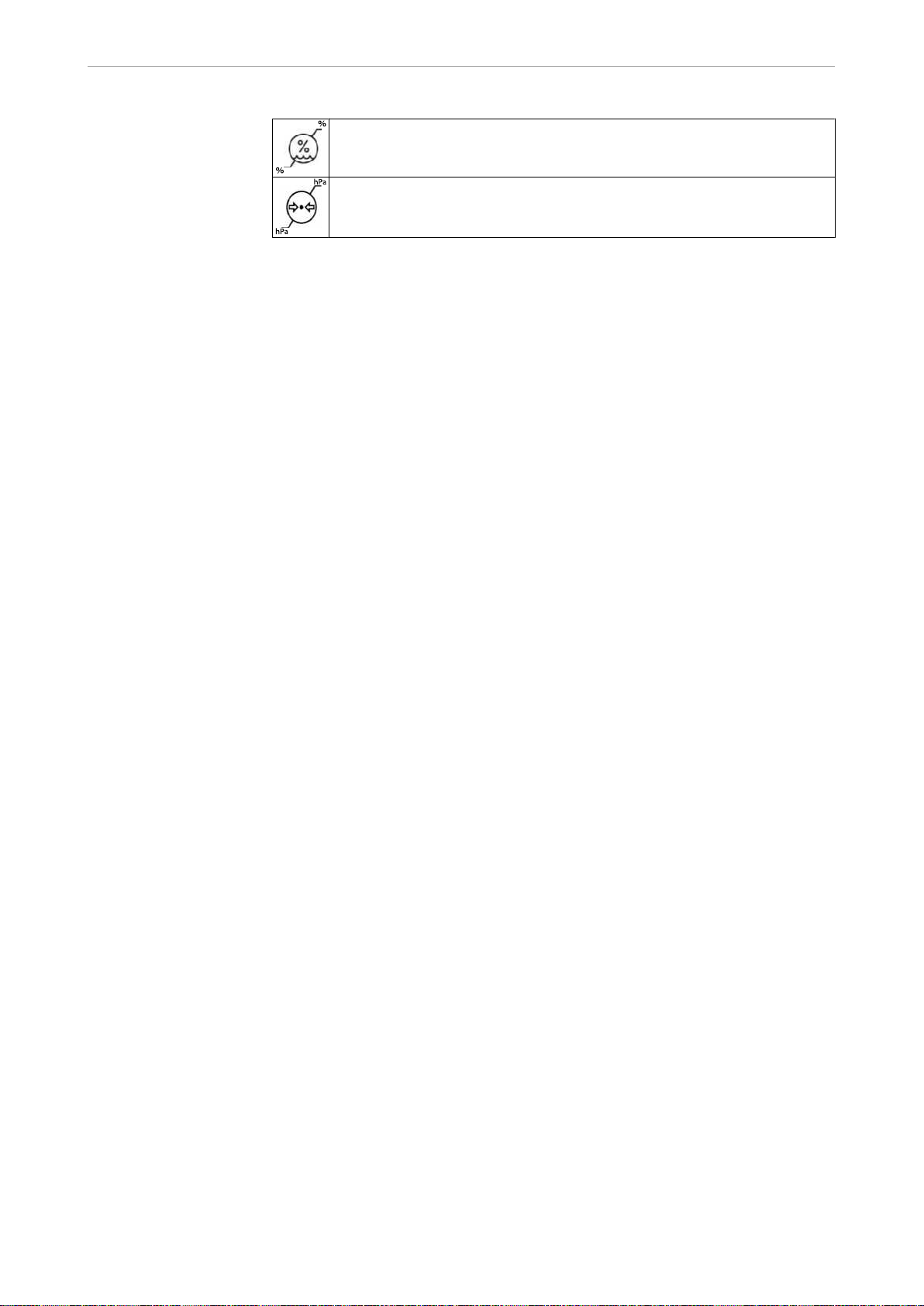
1.6.2 Storage
The symbols printed on the outside are for transportation and storage, and have the
following meaning:
Transport upright with the arrows pointing upwards
Fragile - protect against knocks
Keep dry
Maximum permitted stacking load
Temperature range
10/64
Page 13
Instructions for use KaVo In eXam 3510
1 User notes | 1.6 Transport and storage
Humidity
Air pressure
11/64
Page 14
Instructions for use KaVo In eXam 3510
2 Safety | 2.1 Explanation of safety symbols
2 Safety
2.1 Explanation of safety symbols
2.1.1 Warning symbol
Warning symbol
2.1.2 Structure
The introduction describes the type and source of the hazard.
This section describes the potential consequences of non-observance.
DANGER
▶ The optional step contains necessary measures for avoiding hazards.
2.1.3 Description of danger levels
CAUTION
WARNING
DANGER
Safety instructions with three hazard levels are used in this document for avoiding
personal and property damage.
CAUTION
indicates a hazardous situation that can lead to property damage or minor to mo‐
derate injury.
WARNING
indicates a hazardous situation that can lead to serious injury or death.
DANGER
indicates a maximum hazardous situation that can directly cause serious injury or
death.
12/64
Page 15

Instructions for use KaVo In eXam 3510
2 Safety | 2.2 Intended purpose
2.2 Intended purpose
2.2.1 General
This KaVo product is intended only for use in the field of dentistry. It is impermissible
to use the product for a purpose for which it was not intended.
"Proper use" includes following all the instructions for use and ensuring that all in‐
spections and service tasks are performed.
Apply and meet the overarching guidelines and/or national laws, national regulati‐
ons and the rules of technology for medical devices applicable for startup and use
of the KaVo product for the intended purpose.
The user must ensure that that the device works properly and is in a satisfactory
condition before each use.
During use, national legal regulations must be observed, in particular:
▪ the applicable health and safety regulations.
▪ the applicable accident prevention regulations.
The user must observe the following:
▪ - only use properly operating equipment.
▪ protect himself or herself and third parties from danger.
▪ avoid contamination from the product.
To guarantee constant readiness for use and maintenance of value of the KaVo
product, the recommended annual servicing must be done.
Yearly safety inspections are required.
Note
The product must be cleaned and serviced according to instructions if it is not to
be used for a long period.
13/64
Page 16
Instructions for use KaVo In eXam 3510
2 Safety | 2.2 Intended purpose
Observe the corresponding country-specific regulations when finally shutting down
a KaVo product. Please direct all questions regarding the proper disposal of KaVo
products to the nearest KaVo branch.
Note
The waste that arises must be recycled or disposed of in a manner safe for humans
and the environment. Observe the applicable national regulations.
Please direct all questions regarding the proper disposal of KaVo products to the
nearest KaVo branch.
Note
According to the EC Directive 2002/96 concerning electrical and electronic used
devices, this product is subject to the cited directive and must be disposed accor‐
dingly within Europe.
Before disassembling and disposing of the product, it must be completely proces‐
sed (disinfected, sterilised) according to the section "Preparation methods".
Additional information can be obtained from KaVo (www.kavo.com) or your dental
supplier.
2.2.2 Product-specific
KaVo In eXam is an intraoral X-ray machine.
KaVo In eXam complies with all regulations that are in effect for X-ray and radiation
protection.
During use, diagnostic X-ray radiation is generated that could harm patients or third
parties if incorrectly used.
Compliance with the national quality assurance regulations that are in place, parti‐
cularly those relating to the operator/handler minimising exposure to high doses, is
mandatory.
KaVo In eXam has a class 2 laser for making the X-ray's central beam visible. Cor‐
respondingly, the pertinentIEC 825-1 andIEC 60825-1 regulations have to be con‐
sulted.
X-ray machine disposal
The x-ray tube head contains materials whose disposal must be clarified after the
device stops being used. Follow national regulations (consult your dental supplier
if necessary).
This concerns the systems that generate radiation (tubes, lead sheath), and all
electronic components.
14/64
Page 17

Instructions for use KaVo In eXam 3510
2 Safety | 2.3 Safety instructions
2.3 Safety instructions
Radiation damage due to improper installation
Unnecessary overexposure to X-ray radiation.
CAUTION
CAUTION
CAUTION
▶ Only individuals who have completed a relevant KaVo In eXam training course
are permitted to undertake assembly, upgrade, tuning or repair activities.
▶ Consult the country-specific regulations, guidelines and laws that are in place
to regulate the installation and operation of X-ray equipment.
Ionising radiation
Overexposure to X-ray radiation.
▶ The handler can and must take measures, in accordance with country-specific
legal stipulations, to minimise their personal dose of radiation!
Malfunctions from electromagnetic fields.
The product meets the applicable requirements regarding electromagnetic fields.
Given the complex interactions between equipment and cell phones, the product
may be influenced by a cell phone that is in use.
▶ Do not use cell phones in medical offices, hospitals, or laboratories.
▶ Turn off electronic devices such as computer storage media, hearing aids, etc.
during operation .
CAUTION
WARNING
CAUTION
CAUTION
Risks from electromagnetic fields.
The functions of implanted systems (such as pacemakers) can be influenced by
electromagnetic fields.
▶ Ask patients before treatment.
Injury or damage from damaged functional parts.
When functional parts are damaged, it can cause additional damage or personal
injury.
▶ When operating parts are damaged: Stop working and eliminatethe damage,
or notify a service technician.
▶ Check the electrode lines and accessories for damage to the insulation.
Laser beam (class 2)
Looking directly into the laser beam can cause irritation or permanent changes.
▶ Do not look directly into the laser beam.
▶ Instruct patients not to do this either.
▶ Proceed with caution when positioning the X-ray tube head, switching the laser
off if necessary!
Ignition of ignitable mixtures due to electric equipment in operation.
Deflagration
▶ The In eXam machine must not be operated when using ignitable mixtures.
15/64
Page 18

Instructions for use KaVo In eXam 3510
2 Safety | 2.3 Safety instructions
2.3.1 Attaching to KaVo Primus 1058
Risk of injury from falling or broken parts
Safety and compliance with standards for the installation of In eXam in KaVo Pri‐
CAUTION
mus 1058 can be impaired if the parts supplied as standard in the kit were not
installed in line with the assembly instructions.
▶ Make sure you follow the procedure described in the separate assembly in‐
structions for the kit!
16/64
Page 19
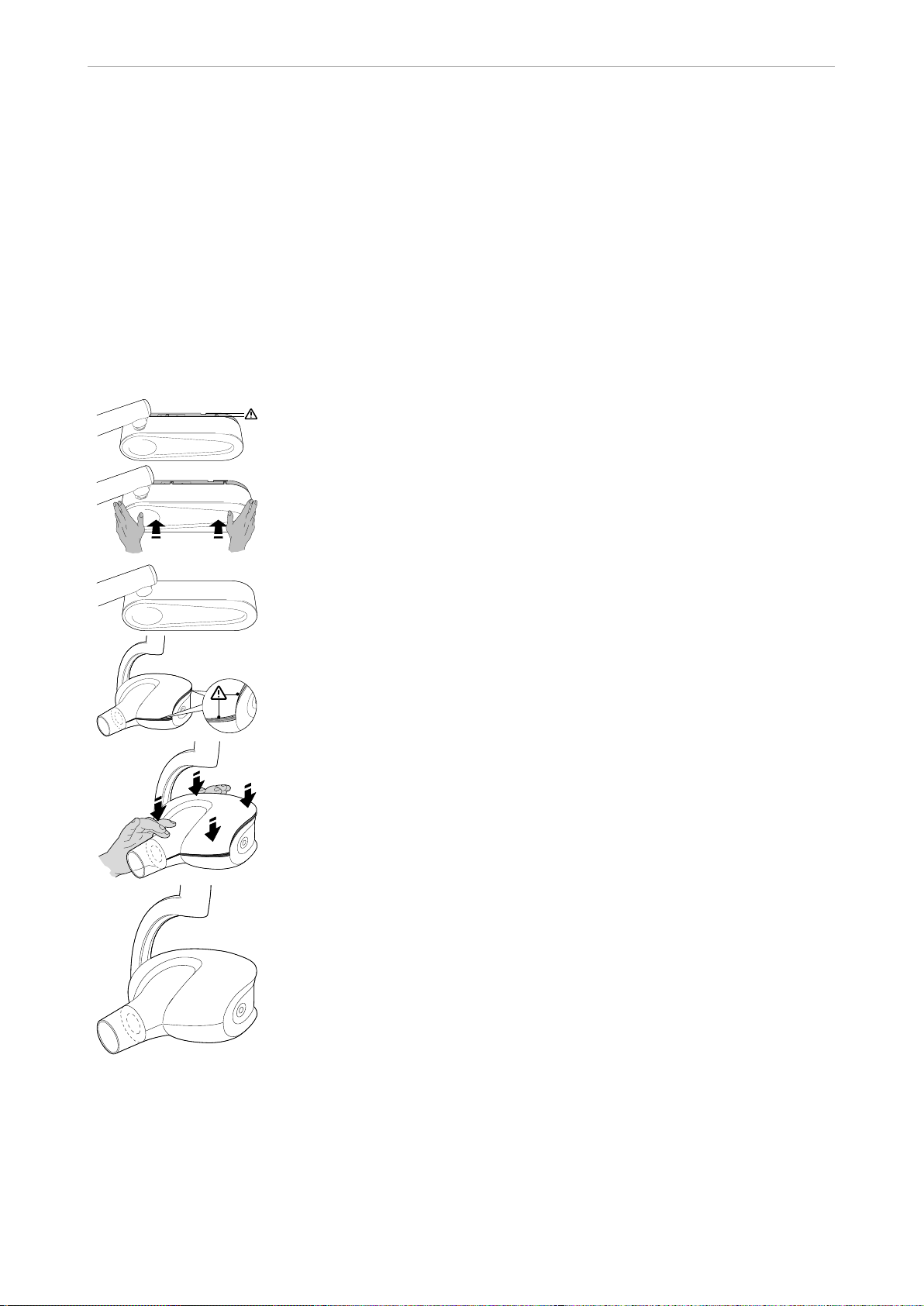
Instructions for use KaVo In eXam 3510
2 Safety | 2.4 Protective devices
2.4 Protective devices
The casing components of KaVo In eXam 3510 are securely fastened with concea‐
led, mounted nuts to safely prevent anybody from accidentally coming into contact
with electrically charged components.
These nuts can only be unscrewed by a qualified Service Technician.
2.4.1 Closing the casing
By pulling on the housing the wall cover or X-ray head casing can be detached from
the snap-fit fastenings, so that a 5mm-wide opening can be seen.
To close the casing, push the relevant part to fix it back into place.
Fix wall panel cover back on
▶ Using both hands, carefully push the wall panel cover to lock it back in place.
The cover is locked securely back in place.
Fix X-ray head cover back on
▶ Using both hands, carefully push the X-ray head cover to lock it back in place.
The cover is locked securely back in place.
17/64
Page 20

Instructions for use KaVo In eXam 3510
2 Safety | 2.5 Requirements for correct operation
2.5 Requirements for correct operation
The X-ray machine has to be installed in such a way as to make it impossible for
anyone other than authorised individuals to take an X-ray.
Responsibility is assumed for the safety, reliability and performance of the unit when:
▪ Installation, expansions, adjustments, changes or repairs is done by technicians
trained by KaVo or third parties authorised by KaVo for the Dig eXam, or by the
personnel of authorised distributors.
▪ The unit is operated according to the instructions for use.
▪ When setting up the unit, follow all the requirements of VDE 0751-1, "Repeated
tests and test before startup of electronic medical devices and systems - general
guidelines".
▪ In Germany, the room in which the In eXam is installed must be designed ac‐
cording to the specifications DIN VDE 0100-710:2002-11 ad VDE
0100-560:1995. In other countries, follow the corresponding national specifica‐
tions.
▪ The KaVo In eXam is an electronic medical device, and is subject to the special
precautions necessary for EMC. The KaVo In eXam may only be operated when
it has been installed according to the EMC instructions in the instructions for
installation.
CAUTION
Unauthorised accessories
Only original replacement parts and accessories should be used, to ensure that
there is no increase in the emissions and/or a reduction in the stability in terms of
the electromagnetic compatibility.
▶ Only use authorised or recommended spare parts and accessories.
See also:
3.6 Technical data, Page 28
KaVo In eXam user instructions.
2.5.1 Standard conformity
In eXam conforms to the following standards:
▪ IEC 60601-1:1988 +A1:1991 + A2:1995 [for US: UL 60601-1:2003. For Canada:
CAN/CSA 22.2 N0.: 601-1]
▪ IEC 60601-1-2:2001
▪ IEC 60601-1-3:1994
▪ IEC 60601-2-7:1998
▪ IEC 60601-2-28:1993
▪ IEC 60601-2-32:1994
▪ IEC 60825-1:1993 +A1:1997 + A2:2001
18/64
Page 21
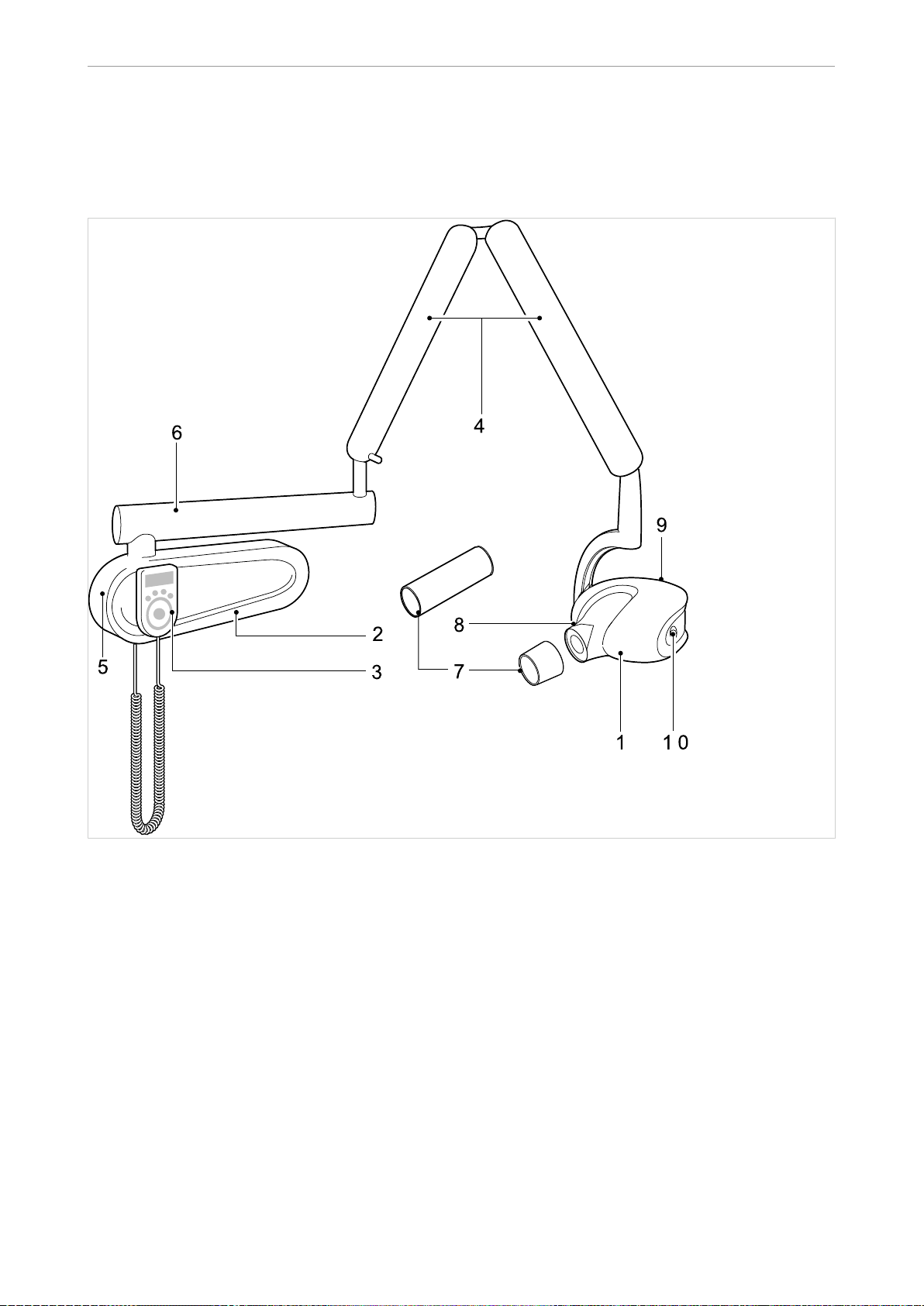
Instructions for use KaVo In eXam 3510
3 Product description | 3.1 Entire system
3 Product description
3.1 Entire system
① The KaVo In eXam high-frequency X-
ray emitter
② Wall panel with high-frequency gene‐
rator electronics
③ Operating unit ⑧ Tilt scale
④ Scissor arm ⑨ Sensor socket
⑤ On/off button ⑩ Laser on/off button
⑥ Extension arm
⑦ Cone
19/64
Page 22

Instructions for use KaVo In eXam 3510
3 Product description | 3.2 Operating unit
3.2 Operating unit
Operating unit control panel
① "Consult user instructions!" symbol ⑧ Default dose setting selection
② Lower jaw select buttons ⑨ Select button: Occlusal function
③ Upper jaw select buttons ⑩ Select button: Bitewing function for
molars
④ Adult/child selection ⑪ Select button: Bitewing function for
anterior teeth and cuspids
⑤ Dig eXam button ⑫ X-ray emission pilot lamp
⑥ kV selection (60/70 kV) ⑬ Take X-ray
⑦ Display (LCD) ⑭ "Ionising radiation" symbol
3.2.1 Assignment of button/area to be X-rayed
The exposure parameters of the KaVo In eXam can be adjusted by the buttons
(②,③,⑨,⑩,⑪) that are assigned to the anatomical regions of the patient.
20/64
Page 23

Instructions for use KaVo In eXam 3510
3 Product description | 3.2 Operating unit
Upper jaw
17 18 15 16 13 14 11 12 21 22 23 24 25 26 27 28
Lower jaw
47 48 45 46 43 44 41 42 31 32 33 34 35 36 37 38
Full mouth X-ray
Occlusal function Bitewing function area "la‐
teral teeth"
Bitewing function area "ca‐
nines/incisors"
21/64
Page 24

Instructions for use KaVo In eXam 3510
3 Product description | 3.3 Focal spot determination
3.3 Focal spot determination
Focus/skin distance (FHA) identifies the distance between the focus and the patient's skin. The focus
lies at the intersection between the two axes that are identified by the focus marks.
Note
The centre of the "laser" positioning button is used to mark the focal point.
22/64
Page 25

Instructions for use KaVo In eXam 3510
1
237
6
A
8
9
10
3 Product description | 3.4 Where to affix nameplates, power rating plates and caution labels
3.4 Where to affix nameplates, power rating plates and caution labels
Locations for affixing the rating plate, nameplate and warning signs
① Rating plate for the overall device ⑦ Laser warning plate
② X-ray tube label ⑧ X-ray warning plate (for USA)
③ Plate for radiation field for 200 mm
focus/skin distance
⑥ Plate for operating unit ⑩ Plate for extension to 300 mm focus/
⑨ Rectangular radiation field limitation
plate
skin distance
(A) Warning symbols on the operating unit
See also: 3.2 Operating unit, Page 20
23/64
Page 26

Instructions for use KaVo In eXam 3510
56468513
Kaltenbach & Voigt GmbH
TRX 708, manufactored for KaVo SN:
0.7 [IEC 60336:1993]
2.5 mm Al 70kV Total filtration
60/70 kV 4/ 7 mA DC
Tube / HV Housing Assembly
TYPE: 3510 [230V] SN: YYYY-
This product complies with FDA regulation 21 CFR
Subchapter J at the date of manufacture
21651
55555555
Kaltenbach & Voigt GmbH
60/70 kV 4/ 7 mA DC
TRX 708, manufactored for KaVo SN:
0.7 [IEC 60336:1993]
2.5 mm Al 70kV Total filtration
Tube / HV Housing Assembly
TYPE: 3510 [110V] SN: YYYY-
This product complies with FDA regulation 21 CFR
Subchapter J at the date of manufacture
88888
88888888
Kaltenbach & Voigt
GmbH
SN:
This product complies
with FDA regulation 21
CFR Subchapter J at the
date of manufacture
Radiation Area:
max. Ø 60mm
@ 200mm Focal distance
Beam Limiting 200mm
TYPE:
3510
YYYY-
3 Product description | 3.5 Nameplates, power rating plates and caution labels
3.5 Nameplates, power rating plates and caution labels
Position number with plate name Plate
①Rating plate for entire machine, 230 V
①Rating plate for entire machine, 110 V
②X-ray tube head plate 230 V
②X-ray tube head plate 110 V
③Shielding system for 200 mm focus/skin distance
24/64
Page 27

Instructions for use KaVo In eXam 3510
88888888
Kaltenbach & Voigt GmbH
REF:
SN:
Timer Unit
TYPE:
1.002.8217
3510
YYYY-
3 Product description | 3.5 Nameplates, power rating plates and caution labels
Position number with plate name Plate
⑥ Plate for operating unit
⑦ Laser warning plate
⑧ X-ray caution label USA
25/64
Page 28

Instructions for use KaVo In eXam 3510
3 Product description | 3.5 Nameplates, power rating plates and caution labels
⑨Rectangular aperture
Size 35 * 45 mm² (shown here as example), black
Size 27 * 37 mm² (not shown), blue
⑩Tube extension to 300 mm focus/skin distance
Symbols on identification labels
Type B application part [IEC 878-02-02]
Focus size [IEC 417-5326-a]
CE marking
X-ray filtration [IEC 417-5381]
26/64
Page 29

Instructions for use KaVo In eXam 3510
3 Product description | 3.5 Nameplates, power rating plates and caution labels
X-ray tube CE marking [IEC 417-5381]
CSA label
Consult user instructions
For information about proper disposal, see intended purpose
27/64
Page 30

Instructions for use KaVo In eXam 3510
3 Product description | 3.6 Technical data
3.6 Technical data
Ambient conditions
Permissible operating temperature min. 10°C, max. 40°C
Humidity No condensate may form on the device;
Storage min. 5°C, max. 70°C
Transport and storage conditions in the original packaging
Temperature min. -20°C, max. +70°C
Air humidity min: 5 % max: 95 %
Air pressure 700 – 1060 hPa
relative humidity of 30% - 75%.
Equipment classification
CE directive 93/42/EEC Class IIb product
IEC 601-1:Type of protection from elec‐
tric shock
IEC 601-1: Degree of protection from
electric shock
IEC 601-1: Continuous operation with
high radiation exposure
Suitable and not suitable:
▪ Not protected from the penetration of water.
▪ Not suitable for sterilisation.
▪ Suitable for wipe or surface disinfection using the cleansers described in the
instructions for use.
▪ Not suitable for operation in environments with combustible mixtures.
Device belongs to protection class I
Type B application part
Intermittent operation
Electricity supply variant with a rated voltage of 230V
Alternating voltage 230 V; ± 10% (207 V – 253 V)
Nominal frequency 50 Hz
Max. current 5A
Permissible impedance 0.5 Ohms
Cross-section of the power line Up to a max. 16 m long: 1.5 mm² (or
AWG14); the diameter must be greater if
the line is any longer.
Design of the mains power input Nominal voltage 250 V, corresponding to
applicable national regulations
28/64
Page 31

Instructions for use KaVo In eXam 3510
3 Product description | 3.6 Technical data
Electricity supply variant with a rated voltage of 110V
Alternating voltage 110 V; ± 10% (99 V – 121 V)
Nominal frequency 50/60 Hz
Max. current 10A
Permissible impedance 0.2 Ohms
Cross-section of the power line Up to a max. 8 m long: 1.5 mm² (or
Design of the mains power input Nominal voltage 110 V, corresponding to
Power consumption of both power supply voltage variants
AWG14); the diameter must be greater if
the line is any longer.
applicable national regulations [ANSI/
NFPA + UL for USA]
Power consumption when X-ray is being
taken
Nominal long-term output < 25 W
490 W [in film mode] 280 W [in sensor
mode]
Nominal values of X-ray radiation
High voltage generation DC (high frequency), 300kHz
Anode voltage 60 / 70 kV± 10%
Anode current 7mA ± 15% [in film mode] 4mA ± 15% [in
sensor mode]
Exposure time accuracy ± 10%
Minimum current-time-product 0.14 mAs [in film mode] 0.08 mAs [in
sensor mode]
Maximum current-time-product 13.2 mAs [in film mode] 3.5 mAs [in sen‐
sor mode
Nominal value of focal spot 0.7 mm [IEC 336:1982]
Anode material Tungsten
Total filtering > 2.5 mm Al / 70 kV [IEC 60 522:1999]
Leakage radiation < 0.25 mGy/h [IEC407:1973]
Pulse:break ratio for 70 kV and 7mA [electronically moni‐
tored]: One X-ray at 0.1s every 14s for
the first hour, followed by one X-ray at
0.1s every 60s.
29/64
Page 32

Instructions for use KaVo In eXam 3510
3 Product description | 3.6 Technical data
Thermal characteristic of the x-ray tube.
Thermal characteristic of the radiation generating system.
30/64
Page 33

Instructions for use KaVo In eXam 3510
3 Product description | 3.6 Technical data
Dimensions, mass
The focus lies at the intersection between the two axes that are identified by the focus marks.
Focus-skin distance [FSD] 200 mm or 300 mm [optional with
Exit X-ray radiation field <60mm; < 35 * 45 mm² with rectangular
Size of packaging ca. 420 x 420 x 1250 mm
Weight, packaged with additional equip‐
ment
"Reach" of the X-ray arm 1,518 mm [optional], 1,800 mm, 2,100
1.003.1576] For the proper and correct
operation of the device according to its
intended purpose, use of the transparent
cone is mandatory!
radiation field limitation, material no.
1.003.0341, < 27 * 37 mm² with radiation
field limitation, material no. 10037200
< 35 kg
mm [optional]
Nominal values of laser used
Laser type Laser diode
Classification according to IEC 825-1 Laser class 2 / < 1mW
Wavelength 650 nm
31/64
Page 34

Instructions for use KaVo In eXam 3510
3 Product description | 3.6 Technical data
3.6.1 Information on electromagnetic compatibility
Guidelines and manufacturer's declaration - electromagnetic emissi‐
ons
In eXam is intended for use in an environment as stipulated below. The customer
or user of In eXam should ensure that it is operated in an environment of this type.
Emissions measurements Conformity Electromagnetic environment - gui‐
de
HF emissions in accordance with
CISPR 11
HF emissions in accordance with
CISPR 11
Emissions of harmonic oscillations
in accordance with IEC61000-3-2
Emissions of voltage fluctuations /
flicker in accordance with
IEC61000-3-3
Group 1 In eXam uses HF energy solely for
its internal functions. Its HF radiati‐
on is therefore very low, and it is
unlikely that nearby devices will be
subject to interference.
Class B In eXam is suitable for use in all de‐
vices, including those used in do‐
mestic environments and similar
which are connected directly to a
public supply grid which also sup‐
plies buildings which are used for
residence purposes.
Class A In eXam is suitable for use in all de‐
vices, including those used in do‐
mestic environments and similar
which are connected directly to a
public supply grid which also sup‐
plies buildings which are used for
residence purposes.
Compliant In eXam is suitable for use in all de‐
vices, including those used in do‐
mestic environments and similar
which are connected directly to a
public supply grid which also sup‐
plies buildings which are used for
residence purposes.
32/64
Page 35

Instructions for use KaVo In eXam 3510
3 Product description | 3.6 Technical data
Guidelines and manufacturer's declaration - electromagnetic stability
In eXam is intended for use in the electromagnetic environment stipulated below.
The customer or user of In eXam should ensure that it is operated in an environment
of this type.
Stability tests IEC 60601 - Test level Compliance level Electromagnetic environment - gui‐
de
Discharge of static
electricity in accor‐
dance with IEC
61000-4-2
Rapid transient electri‐
cal disturbances / burst
in accordance with IEC
61000-4-4
Surges in accordance
with IEC 61000-4-5
Voltage drops, shortli‐
ved interruptions und
fluctuations in the sup‐
ply voltage in accor‐
dance with IEC
61000-4-11
Magnetic field at the
supply frequency
(50/60 Hz) in accor‐
dance with IEC
61000-4-8
+/- 6 kV Contact
discharge
+/- 8 kV Air discharge
+/- 2 kV for mains ca‐
bles +/- 1 kV for input
and output cables
+/- 1 kV Normal mode
voltage
+/- 2 kV Common mode
voltage
< 5 % UT (> 95 % drop
in UT) for 1/2 period)
40 % UT (60 % drop in
UT) for 5 periods)
70 % UT (30 % drop in
UT) for 25 periods)
< 5 % UT (> 95 % drop
in UT) for 1/2 periods
3 A/m 3 A/m Magnetic fields at the mains fre‐
+/- 6 kV Contact
discharge
+/- 8 kV Air discharge
+/- 2 kV for mains ca‐
bles
+/- 1 kV for input and
output cables
+/- 1 kV Normal mode
voltage
+/- 2 kV Common mode
voltage
< 5 % UT (> 95 % drop
in UT) for 1/2 period)
40 % UT (60 % drop in
UT) for 5 periods)
70 % UT (30 % drop in
UT) for 25 periods)
< 5 % UT (> 95 % drop
in UT) for 1/2 period)
Floors should be made of wood or
concrete or be covered with cera‐
mic tiles. If the floor is covered with
a synthetic material versehen ist,
the relative air humidity must be at
least 30 %.
The quality of the supply voltage
should be equivalent to that of a ty‐
pical business or hospital environ‐
ment.
The quality of the supply voltage
should be equivalent to that of a ty‐
pical business or hospital environ‐
ment.
The quality of the supply voltage
should be equivalent to that of a ty‐
pical business or hospital environ‐
ment. When the user of In eXam
demands continuing function of the
device even when the power supply
is interrupted, it is recommended
that In eXam be powered via an un‐
interruptible power supply or a bat‐
tery.
quency should correspond to the
typical values found in business
and hospital environments.
33/64
Page 36

Instructions for use KaVo In eXam 3510
3 Product description | 3.6 Technical data
Guidelines and manufacturer's declaration - electromagnetic stability
In eXam is intended for use in the electromagnetic environment stipulated below.
The customer or user of In eXam should ensure that it is operated in an environment
of this type.
Stability tests IEC 60601 - Test level Compliance level Electromagnetic environment - gui‐
de
Directed HF disturban‐
ces in accordance with
IEC 61000-4-6
Directed HF disturban‐
ces in accordance with
IEC 61000-4-3
3 V
eff
150 kHz to 80 MHz
3 V/m
80 MHz to 2.5 GHz
3 V
3 V/m
Portable and mobile radio equip‐
ment should not be used near to In
eXam or its cables, as the recom‐
mended safe distance has been
calculated in accordance with the
equation applicable for the trans‐
mitting frequency.
Recommended safe distance:
d = 1.2 *
P
to 800 MHz
d = 2.3 * P for 800 MHz to 2.5 GHz
where P is the power rating of the
transmitter in watts (W) according
to information from the manufactur‐
er of the transmitter, and d is the
recommended safe distance in me‐
tres (m).
The field intensity of stationary ra‐
dio transmitters should, at all fre‐
quencies according to local investi‐
gations,a be lower than the
compliance levelb.
Near to devices bearing the symbol
, interference is possible.
Note:
The higher frequency range is applicable at 80 MHz und 800 MHz.
a
The field intensity of stationary transmitters such as the base stations of radio
telephones and mobile land radio devices, amateur radio stations, AM and FM
broadcasting services or television transmitters cannot, in theory, be determined
precisely in advance. To determine the electromagnetic environment in respect of
stationary transmitters, a study of the location should be considered.
If the measured field intensity at the location in which In eXam is being used exceeds
the compliance level above, care should be taken with In eXam to ensure that it is
functioning in accordance with regulations. If unusual performance features are no‐
ted, additional measures may be required, such as changing the alignment or
moving In eXam somewhere else.
b
The field intensity should be less than 3 V/m over the frequency range 150 kHz to
80 MHz.
34/64
Page 37

Instructions for use KaVo In eXam 3510
3 Product description | 3.6 Technical data
Recommended safe distances between portable and mobile telecom‐
munications equipment and In eXam
In eXam is intended for use in an electromagnetic environment in which HF distur‐
bances are checked. The customer or user of In eXam can help to prevent electro‐
magnetic disturbances by complying with the minimum distance between portable
and mobile HF telecommunications equipment (transmitters) and In eXam, depen‐
ding on the output power of the communication device, as stipulated below.
The table shows the necessary safe distance, as a function of the transmission
frequency, in m:
Power rating of the trans‐
mitter in W
150 kHz to 80 MHz
D=1.2 *
P
80 MHz to 800 MHz
D=1.2 *
P
800 MHz to 2.5 GHz
D= 2.3 *
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters for which the rated power is not shown in the table above, the di‐
stance can be determined using the equation belonging to the relevant column,
where P is the rated power of the transmitter in watts (W) according to information
from the manufacturer of the transmitter.
Note
these guidelines may not be applicable in all situations. The propagation of elec‐
tromagnetic waves is affected by absorption and reflection due to buildings, objects
and people.
3.6.2 Standard conformity
See also: 2.5.1 Standard conformity, Page 18
P
35/64
Page 38

Instructions for use KaVo In eXam 3510
3 Product description | 3.7 Determination of the patient's X-ray exposure
3.7 Determination of the patient's X-ray exposure
3.7.1 Exposure diagram
The adjacent diagram directly shows what is known as the Kerma in mGy for the
focus-skin distance (FSD) of 20 cm for the corresponding exposure time, which is
displayed after exposure.
Note
Exposure is measured in units of Gray. For dental applications, the conversion
factor of the technical unit Gray to a dose in Sievert is 1.
Note
If the focus-skin distance changes during exposure, the read amount needs to be
corrected by the distance law.
Note
Exposure by the read-off value relates to the area irradiated by the X-ray, and so
it is necessary to record whether the normal radiation field of the cone of 60 mm
diameter or the reduced radiation field of radiation field limitation
facilityMat. no. 1.003.0341 orMat. no. 1.003.7200 was used.
36/64
Page 39

Instructions for use KaVo In eXam 3510
4 Operation | 4.1 Switch on machine
4 Operation
Note
The description of how to operate the KaVo In eXam applies to both sensors and
films.
Note
A visible laser beam can be switched on to highlight the X-ray's central beam.
Avoidable exposure to radiation.
An individual could fall ill as a result of receiving an excessive dose of X-ray.
CAUTION
▶ Provide patient protection measures (such as lead-lined collars)
▶ Take operator protection measures, such as applying the distance law, or using
screens during X-ray exposure
▶ Monitor the patient before and during X-ray exposure; if necessary, abort the
X-ray procedure and start again
CAUTION
Danger due to deviation from the defined focus-skin distance
Not maintaining the specified focus-skin distance (i.e. skin surface too close to
focus) can lead to unnecessary overexposure to radiation.
▶ Before using the machine, attach the transparent cone (which is detachable for
disinfection purposes) to make sure that the correct focus-skin distance is
maintained during the X-ray prodedure.
4.1 Switch on machine
Requirement
The unit's basic dose setting corresponds to the sensitivity class of the films or
sensors being used.
▶ Switch on KaVo In eXam .
▶
If necessary, use the
See also: 5 Setting default doses, film sensitivity and sensor sensitivity, Page
47
▶
If necessary, use the
operation.
The operating unit's display shows the X-ray image parameters (kV value, mA value,
basic dose setting) and exposure time based on the selected area to be X-rayed.
A green, light-emitting diode highlights the chosen area to be X-rayed.
button to adjust the basic dose setting.
button to specify the basic setting for film or sensor
37/64
Page 40

Instructions for use KaVo In eXam 3510
4 Operation | 4.2 Specifying the imaging parameters
4.2 Specifying the imaging parameters
▶ Choose the required kV.
Use the
want to take.
▶ Choose patient type.
Use the
▶ Choose area to be X-rayed.
Use the buttons assigned to the individual anatomical regions to choose the area
to be X-rayed.
See also:
3.2.1 Assignment of button/area to be X-rayed, Page 20
The specified X-ray time for the chosen X-ray image parameters is shown on the
dispay.
button to select the 60kV or 70kV value according to the X-ray you
button to select the patient type (adult or child).
38/64
Page 41

Instructions for use KaVo In eXam 3510
4 Operation | 4.3 Laser highlighting of central beam
4.3 Laser highlighting of central beam
A laser beam can be used to simulate the X-ray's central beam axis.
Laser beam (class 2)
Looking directly into the laser beam can cause irritation or permanent changes.
CAUTION
▶ Do not look directly into the laser beam.
▶ Instruct patients not to do this either.
▶ Proceed with caution when positioning the X-ray tube head, switching the laser
off if necessary!
▶
Press the laser switch
central beam indicator (aiming laser).
(at the side of the X-ray tube head) to switch on the
39/64
Page 42

Instructions for use KaVo In eXam 3510
4 Operation | 4.4 Positioning patients
4.4 Positioning patients
Note
The following positioning examples are to be understood as principles. Appropriate
positioning is described in the relevant literature.
The patient should preferably sit in
a sagittal-vertical position.
For X-rays of the upper jaw, the no‐
se must be horizontally in line with
the ear.
For X-rays of the lower jaw, the oc‐
clusion level must be horizontal.
40/64
Page 43

Instructions for use KaVo In eXam 3510
4 Operation | 4.5 Position X-ray source
4.5 Position X-ray source
The scissor arm makes possible correct positioning of the generator for all types of
X-rays. The cone assures that there is a distance of at least 20 cm between the focal
spot and the skin.
Parallel technique/right-angle technique
When using the right-angle technique, KaVo recommends working with a handle
and using the radiation field limitiation facility provided as
standard:Mat. no. 1.003.0341
Dissecting angle technique
41/64
Page 44

Instructions for use KaVo In eXam 3510
MAXILLA
MAXILLA
MANDIBLE
MANDIBLE
INCISOR CUSPID PRAEMOLAR MOLAR
+40°
-15° -20° -10° -5°
+45° +30° +20°
4 Operation | 4.6 Positioning the image receiver
4.6 Positioning the image receiver
Dissecting angle technique
▶ Direct the image receivers and generators as shown here (with the beam per‐
pendicular to the bisecting line of the tooth/image receiver angle.)
42/64
Page 45

Instructions for use KaVo In eXam 3510
MAXILLA
MAXILLA
INCISOR CUSPID PRAEMOLAR MOLAR
MANDIBLE
MANDIBLE
4 Operation | 4.6 Positioning the image receiver
Parallel technique / right-angle technique
▶ Direct the image receiver and generator as shown here (with beam perpendi‐
cular to the film). Use a holding system here, if possible.
43/64
Page 46

Instructions for use KaVo In eXam 3510
INCISOR MAXILLA
OKKLUSAL
MANDIBLE
MOLAR
60 ¡
65 ¡
90 ¡ 90 ¡
4 Operation | 4.6 Positioning the image receiver
Occlusion X-rays
Bitewing X-rays
44/64
Page 47

Instructions for use KaVo In eXam 3510
4 Operation | 4.7 Take X-ray
4.7 Take X-ray
▶
Press the X-ray start button
indicator lamp goes out and the audio signal stops.
Note
If the trigger button is released before the exposure ends, an operating error alarm
sounds. It indicates that the transmission of the x-rays was prematurely terminated
and the radiograph may be underexposed. The digital display alternately shows
OP. ERROR and the time that was selected and not maintained. The alarm can be
stopped by selecting another tooth on the control unit.
and keep depressed until the X-ray emission
45/64
Page 48

Instructions for use KaVo In eXam 3510
4 Operation | 4.8 Finishing off activities
4.8 Finishing off activities
▶ Switch off the central beam indicator (pilot beam) with the button.
▶ Disinfect the cone that has come into contact with the patient.
46/64
Page 49

Instructions for use KaVo In eXam 3510
5 Setting default doses, film sensitivity and sensor sensitivity | 5.1 Correction of the exposure time / dose
default setting
5 Setting default doses, film sensitivity and sensor sensitivity
Ionising radiation
Possible consequence of receiving an excessive dose of X-ray.
CAUTION
▶ Depending on the indication and any country-specific regulations in force, use
the film type with the greatest possible sensitivity.
Requirement
To obtain high-quality radiographs, great care is required when developing the film.
Note
The film must be developed in accordance with the film manufacturer guidelines
and developer equipment instructions.
5.1 Correction of the exposure time / dose default setting
The specified default dose for the machine refers to option "6" in the table below.
If needed, adapt the basic dose setting with key
.
ITEM CORRECTION COEFFICIENT
0 - 73 % 0.27
1 - 67 % 0.33
2 - 59 % 0.41
3 - 49 % 0.51
4 - 36 % 0.64
5 - 20 % 0.80
6 0 1
7 + 25 % 1,25
8 + 56 % 1,56
9 + 95 % 1,95
47/64
Page 50

Instructions for use KaVo In eXam 3510
5 Setting default doses, film sensitivity and sensor sensitivity | 5.2 Selection of the suitable basic dose
5.2 Selection of the suitable basic dose
5.2.1 With film
Requirement
The
button is not active, meaning that the LED is not lit up and the display reads
"7mA".
The In eXam is set to the respective film type by using the keys to change
the default dose to the value indicated in the following table.
Supplier Trade name Dose pre-selection with In
eXam
AGFA Dentus M2 5
AGFA Dentus M4 3
AGFA Normal 6
DENTAL UNION Bleu Star 3
DUPONT Lightning fast 6
GEVAERT Dentus Ultra Rapid 6
KODAK Insight 2
KODAK Ekta Speed 3
KODAK Ekta Speed Plus 3
KODAK Ultra Speed 6
MINIMAX Intermediate 8
RINN Extra Fast 9
RINN Super Fast 6
5.2.2 With digital image receiver
Requirement
Keep the
"4mA".
▶ Select adjustment of the preselected exposure according to the values indicated
in the corresponding instructions on operation of the digital image receiver
button is active, meaning that the LED is lit up and the display reads
48/64
Page 51

Instructions for use KaVo In eXam 3510
5 Setting default doses, film sensitivity and sensor sensitivity | 5.3 Exposure time table
5.3 Exposure time table
The default doses used by the device are presented in the following sections.
The specifications are based on a reference setting of level "6" and can be corre‐
spondingly adjusted using the default dose button
as mentioned above.
5.3.1 Default dose setting for X-rays taken using film
Requirement
The
button is not active, meaning that the LED is not lit up and the display reads
"7mA".
Adult setting
Requirement
The adult LED shines.
Program Tooth Bracket Expos‐
ure time
in se‐
conds for
film type
6 and 7
mA 70
kV
Mandible
Adult
41 42 31 32 -15 0.150 0.273
43 44 33 34 -20 0.150 0.273
45 46 35 36 -10 0.150 0.273
47 48 37 38 -5 0.178 0.323
Expos‐
ure time
in se‐
conds for
film type
6 and 7
mA 60
kV
49/64
Page 52

Instructions for use KaVo In eXam 3510
5 Setting default doses, film sensitivity and sensor sensitivity | 5.3 Exposure time table
Program Tooth Bracket Expos‐
ure time
in se‐
conds for
film type
6 and 7
mA 70
kV
Maxilla
Adult
12 11 21 22 +40 0.178 0.323
13 14 23 24 +45 0.178 0.323
15 16 25 26 +30 0.232 0.423
17 18 27 28 +20 0.260 0.472
Expos‐
ure time
in se‐
conds for
film type
6 and 7
mA 60
kV
BITE‐
WING
OCCLU‐
SAL
Canines/
incisors
Molars - 0.178 0.323
Mandible
or maxil‐
la
- 0.150 0.273
- 0.530 0.964
50/64
Page 53

Instructions for use KaVo In eXam 3510
5 Setting default doses, film sensitivity and sensor sensitivity | 5.3 Exposure time table
Child setting
Requirement
The child LED shines.
Program Tooth Bracket Expos‐
ure time
in se‐
conds for
film type
6 and 7
mA 70
kV
Mandible
Child
81 82 71 72 -15 0.075 0.137
83 73 -20 0.075 0.137
84 74 -20 0.075 0.137
85 75 -10 0.089 0.162
Expos‐
ure time
in se‐
conds for
film type
6 and 7
mA 60
kV
Maxilla
Child
51 52 61 62 +40 0.089 0.162
53 63 +45 0.089 0.162
54 64 +45 0.116 0.211
55 65 +30 0.130 0.236
51/64
Page 54

Instructions for use KaVo In eXam 3510
5 Setting default doses, film sensitivity and sensor sensitivity | 5.3 Exposure time table
Program Tooth Bracket Expos‐
ure time
in se‐
conds for
film type
6 and 7
mA 70
kV
BITE‐
WING
OCCLU‐
SAL
Canines/
incisors
Molars - 0.089 0.162
Mandible
or maxil‐
la
- 0.075 0.137
- 0.265 0.482
Expos‐
ure time
in se‐
conds for
film type
6 and 7
mA 60
kV
5.3.2 Default dose for X-rays taken with digital image receivers
Requirement
Keep the
"4mA".
button is active, meaning that the LED is lit up and the display reads
Adult setting
Requirement
The adult LED shines.
52/64
Page 55

Instructions for use KaVo In eXam 3510
5 Setting default doses, film sensitivity and sensor sensitivity | 5.3 Exposure time table
Program Tooth Bracket Expos‐
ure time
in se‐
conds for
film type
6 and 4
mA 70
kV
Mandible
Adult
41 42 31 32 -15 0.088 0.159
43 44 33 34 -20 0.088 0.159
45 46 35 36 -10 0.088 0.159
47 48 37 38 -5 0.104 0.188
Expos‐
ure time
in se‐
conds for
film type
6 and 4
mA 60
kV
Maxilla
Adult
BITE‐
WING
OCCLU‐
SAL
12 11 21 22 +40 0.104 0.188
13 14 23 24 +45 0.104 0.188
15 16 25 26 +30 0.136 0.247
17 18 27 28 +20 0.151 0.275
Canines/
incisors
Molars - 0.104 0.188
Mandible
or maxil‐
la
- 0.088 0.159
- 0.245 0.445
53/64
Page 56

Instructions for use KaVo In eXam 3510
5 Setting default doses, film sensitivity and sensor sensitivity | 5.3 Exposure time table
Child setting
Requirement
The child LED shines.
Program Tooth Bracket Expos‐
ure time
in se‐
conds for
film type
6 and 4
mA 70
kV
Mandible
Child
81 82 71 72 -15 0.044 0.080
83 73 -20 0.044 0.080
84 74 -20 0.044 0.080
85 75 -10 0.052 0.094
Expos‐
ure time
in se‐
conds for
film type
6 and 4
mA 60
kV
Maxilla
Child
51 52 61 62 +40 0.052 0.094
53 63 +45 0.052 0.094
54 64 +45 0.068 0.123
55 65 +30 0.076 0.138
54/64
Page 57

Instructions for use KaVo In eXam 3510
5 Setting default doses, film sensitivity and sensor sensitivity | 5.3 Exposure time table
Program Tooth Bracket Expos‐
ure time
in se‐
conds for
film type
6 and 4
mA 70
kV
BITE‐
WING
OCCLU‐
SAL
Canines/
incisors
Molars - 0.052 0.094
Mandible
or maxil‐
la
- 0.044 0.080
- 0.122 0.222
Expos‐
ure time
in se‐
conds for
film type
6 and 4
mA 60
kV
55/64
Page 58

Instructions for use KaVo In eXam 3510
6 Preparation methods DIN EN ISO 17664 | 6.1 Cleaning and disinfection
6 Preparation methods DIN EN ISO 17664
6.1 Cleaning and disinfection
Damage from improper cleaning and disinfection
The use of unsuitable cleansers and disinfectants can impair the function or da‐
CAUTION
CAUTION
mage the device.
▶ Only clean the outer surfaces!
▶ Only use a soft cloth and mild cleaning solution!
▶ Do not use any solvents or aggressive chemicals!
Damage caused by liquid on the inside of the equipment
If cleaning and disinfection agents are improperly used, liquid can access the inside
of the equipment and result in functional impairment or even destruction.
▶ Exercise care that no cleaning or disinfiection fluid enters the inside of the
equipment.
The unit's transparent cone can be removed for easier cleaning and disinfection. All
other surfaces of the unit can be wiped or disinfected with a damp cloth.
Note
The In eXam unit does not include any parts requiring sterilisation as part of their
intended use.
6.1.1 Cleaning
All external surfaces of the KaVo In eXam can be cleaned with a soft cloth and a
mild cleaning fluid.
Clean the mirror(s)
Note
The mirror is part of the internal filtering of the unit and must not be removed or
altered. If the mirror is damaged, stop using the unit until it has been inpsected and
repaired.
See also: 7 Safety checks, Page 58
Dust, fibres, etc. can be removed from the mirrors for projection of the laser of the
can be cleaned--with the cone removed--using a soft cloth.
6.1.2 Disinfection
Requirement
The unit must not be switched on.
56/64
Page 59

Instructions for use KaVo In eXam 3510
6 Preparation methods DIN EN ISO 17664 | 6.1 Cleaning and disinfection
▶ After each use of any machine part that has come into contact with the patient,
wipe it clean using a disinfectant
Wipe disinfection with disinfecting agents containing alcohol
All surfaces of the KaVo In eXam can be wipe disinfected using a suitable disinfec‐
ting agent containing alcohol.
The following disinfectants are permitted:
▪ Elastoclean spray 2
Manufacturer: KaVo Dental GmbH
▪ MIKROZID pump spray
Manufacturer: Schülke & Mayr
▪ Dürr System Hygiene FD 322 disinfectant spray
Manufacturer: DÜRR Dental GmbH
▪ Incidin liquid
Manufacturer: Ecolab
▪ USA: Aerosol spray
Manufacturer: Ecolab
Sterilisation using heat
When circumstances dictate, the removable transparent cone may be sterilised in
an autoclave at 135° C (up to a maximum of 50 times) in accordance with the
manufacturer's instructions.
57/64
Page 60

Instructions for use KaVo In eXam 3510
7 Safety checks
7 Safety checks
Note
The safety checks (SFC) must be performed as the final part of the installation
process.
Subsequent to this, safety checks have to be performed once a year as part of the
standard maintenance activities by KaVo Service Technicians who have received
KaVo product training.
Note
If a defect is detected during these checks, it must be repaired by KaVo Service
Technicians who have received KaVo product training. Use of the unit is not per‐
mitted until the defect is repaired.
See also: Setup instructionsKaVo In eXam
58/64
Page 61

Instructions for use KaVo In eXam 3510
8 Troubleshooting
8 Troubleshooting
Malfunction Cause Remedy
No display lights up. Machine not connected. ▶ Connect machine.
Main switch OFF ▶ Set to ON.
▶ Replace damaged fuses.
No indicator lamps light up on con‐
trol panel.
X-rays not emitted. Generator is cooling down. ▶ Wait for the "COOLING" messa‐
Starting OK, but X-ray image too
pale or white.
Starting OK; but X-ray image too
dark.
The message "OP.ERROR“ ap‐
pears in the display
The message "POWER ERROR“
appears in the display
COOLING message appears on the
display.
Operating unit not connected. ▶ Connect operating unit.
Fuse F1 or F2 damaged. ▶ Replace damaged fuses.
Operating unit out of order. ▶ Replace operating unit.
ge to disappear.
X-ray start button damaged. ▶ Replace operating unit.
The dose was set too low in the eX‐
am.
Generator incorrectly directed. ▶ Check positioning.
Exposure time not long enough. ▶ Check times chosen.
Development time not long enough. See also: Film manufacturer guide‐
Developer too cold. ▶ Warm.
Developer too old.
Dig eXam button incorrectly selec‐
ted.
Film wrong way round. ▶ Insert film correctly.
Incorrect installation. ▶ Call a qualified Service Techni‐
Dig eXam button incorrectly selec‐
ted.
Development time too long. See also:
Start button was released before
exposure time had come to an end.
The microprocessor has detected a
problem.
The KaVo In eXam is only turned on
for one exposure and turned off di‐
rectly after exposure.
The KaVo In eXam hence calcula‐
tes and stores the theoretical hea‐
ting value for the X-ray emitter while
the X-ray is being taken. Because
no voltage is subsequently applied,
it cannot be decremeted.
As a result, these values are incre‐
mented as each X-ray is taken, and
See also: 5 Setting default doses,
film sensitivity and sensor sensitivi‐
ty, Page 47
lines and developer equipment in‐
structions.
▶ Adjust in line with the material
used.
cian.
▶ Adjust in line with the material
used.
5 Setting default doses, film sensiti‐
vity and sensor sensitivity, Page
47
▶ Choose tooth to stop alarm. The
display shows the exposure time
remaining.
Decide whether to develop the
current image or take a new X-
ray.
▶ Switch off machine, then switch
back on.
If the problem does not go away,
call a qualified Service Techni‐
cian.
▶ Leave the KaVo In eXam turned
on until the error message dis‐
appears.
59/64
Page 62

Instructions for use KaVo In eXam 3510
8 Troubleshooting
Malfunction Cause Remedy
after a certain exposure time (num‐
ber), the display shows the messa‐
ge COOLING irrespective of the
actual temperature since excessive
heating theoretically exists.
The X-ray head cover or wall panel
cover has a 5mm-wide opening.
Arc formation after relatively long in‐
tervals without use.
The message "KV Error“ appears in
the display
The casing was "pulled" out from its
locked closure position; the screws
of the casing are accessible.
If the X-ray emitter has not been
used for more tha three weeks, an
arc may form when maximum
power is used immediately due to
the dispersal of gas molecules from
the anode.
The microprocessor has identified a
problem.
Too much gas in the X-ray tube (e.g.
after a relatively long time without
use) interrupted the exposure as a
result of a high voltage discharge in
the X-ray head.
See also:
2.4.1 Closing the casing, Page 17
▶ Trigger 4 exposures at 60 KV / 4
mA with the maximum exposure
time.
▶ Wait ten minutes.
▶ Trigger 4 exposures at 60 KV / 4
mA with the maximum exposure
time.
▶ Wait ten minutes.
▶ Trigger 4 exposures at 60 KV / 7
mA with the maximum exposure
time.
▶ Wait ten minutes.
▶ Trigger 2 exposures at 70 KV / 7
mA with the maximum exposure
time.
▶ Wait two minutes.
▶ Trigger 2 exposures at 70 KV / 7
mA with the maximum exposure
time.
The X-ray emitter can again be used
as normal.
If the error message occurs again
within a few days, call the service
technician.
▶ Turn off device and turn it back
on after a short interruption.
If the error occurs repeatedly, in‐
itialise as described above.
▶ Wait 15 minutes and then pro‐
ceed as follows:
▶ Trigger 4 exposures at 60 KV / 4
mA with the maximum exposure
time.
▶ Wait ten minutes.
▶ Trigger 4 exposures at 60 KV / 4
mA with the maximum exposure
time.
▶ Wait ten minutes.
▶ Trigger 4 exposures at 60 KV / 7
mA with the maximum exposure
time.
▶ Wait ten minutes.
▶ Trigger 2 exposures at 70 KV / 7
mA with the maximum exposure
time.
▶ Wait two minutes.
60/64
Page 63

Instructions for use KaVo In eXam 3510
8 Troubleshooting
Malfunction Cause Remedy
▶ Trigger 2 exposures at 70 KV / 7
mA with the maximum exposure
time.
The X-ray emitter can again be used
as normal.
If the error message occurs again
within a few days, call the service
technician.
61/64
Page 64

Instructions for use KaVo In eXam 3510
9 Accessories and compatibility | 9.1 Accessories and kits
9 Accessories and compatibility
As a rule, KaVo recommends additional equipment from the eXam range.
See also:
5 Setting default doses, film sensitivity and sensor sensitivity, Page 47
DENTAL X-RAY FILMS
9.1 Accessories and kits
9.1.1 Accessories
Presentation Material summary Mat. no.
Rectangular radiation field limi‐
tation, max. radiation field 35 *
45 mm²
1.003.0341
Rectangular radiation field limi‐
tation, max. radiation field 35 *
45 mm²
Cone extension 30 cm 1.003.1576
Test object for constancy
check, digital
1.003.7200
1.003.0252
62/64
Page 65

Instructions for use KaVo In eXam 3510
9 Accessories and compatibility | 9.1 Accessories and kits
Presentation Material summary Mat. no.
Test object for constancy
check, film
Wall-mounted remote control 1.003.1574
1.003.0250
9.1.2 Kits
The In eXam has the following adaptation options:
▪ Adapt to the KaVo Primus 1058.
The installation requirements are found in the corresponding installation instruc‐
tions.
▪ Adapt to the Centro 1540
63/64
Page 66

Instructions for use KaVo In eXam 3510
9 Accessories and compatibility | 9.2 Compatibility
9.2 Compatibility
The third-party accessory parts listed below are compatible with In eXam, as long
as the said requirements relating to corresponding accessory parts are met.
HOLDING SYSTEMS FOR RIGHT-ANGLE TECHNIQUE
▪ These are matched to the configured image receivers.
▪ These are matched to a centrical cone diameter of 60mm.
DIGITAL SENSORS FROM THIRD-PARTY SUPPLIERS
▪ These bear a CE mark
▪ These are designed by the manufacturer for operation at 60/70 kV in within an
exposure range of 0.08 to 3.5 mAs.
▪ The default dose of the In eXam corresponds to the film type cited as a reference
by the manufacturer.
PHOSPHOR STORAGE PLATES FROM THIRD-PARTY SUPPLIERS
▪ These are designed by the manufacturer for operation at 60/70 kV in within an
exposure range of 0.14 to 13.2 mAs.
▪ The default dose of the In eXam corresponds to the film type cited as a reference
by the manufacturer.
64/64
Page 67

Page 68

1.003.1763 · KF · 20071024 - 04 · en
 Loading...
Loading...