KaVo EXPERTsurg LUX 1.008.3500 Instructions For Use Manual
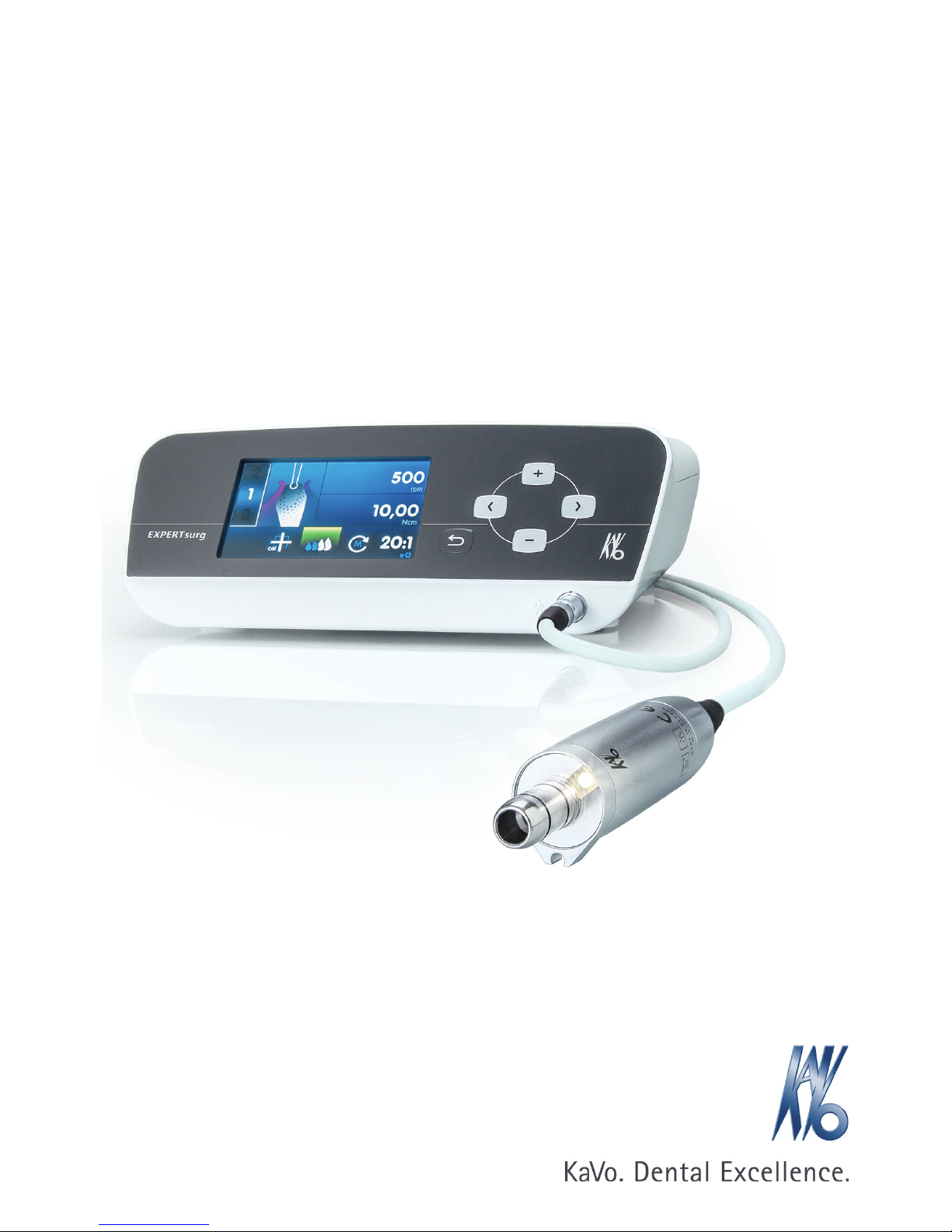
Instructions for use
EXPERTsurg LUX
REF 1.008.3500

Distributed by:
KaVo Dental Corporation
11729 Fruehauf Drive
Charlotte, NC 28273 USA
Phone: 847 550 6800
Fax: 847 550 6825
Manufacturer:
Kaltenbach & Voigt GmbH
Bismarckring 39
D-88400 Biberach
www.kavo.com

Table of contents
1 User instructions............................................................................................................................................. 6
1.1 User guide ............................................................................................................................................. 6
1.1.1 Symbols .................................................................................................................................... 6
1.2 Target group .......................................................................................................................................... 6
1.3 Service................................................................................................................................................... 6
1.3.1 Repair Service .......................................................................................................................... 6
1.4 Terms and conditions of warranty.......................................................................................................... 7
1.5 Transportation and storage.................................................................................................................... 7
1.5.1 Currently valid packaging regulations ....................................................................................... 7
1.5.2 Damage in transit...................................................................................................................... 7
1.5.3 Information on the packaging: Storage and transportation ....................................................... 8
2 Safety ............................................................................................................................................................. 10
2.1 Description of safety instructions ........................................................................................................... 10
2.1.1 Warning symbol ........................................................................................................................ 10
2.1.2 Structure ................................................................................................................................... 10
2.1.3 Description of hazard levels...................................................................................................... 10
2.2 Information about electromagnetic compatibility.................................................................................... 10
2.3 Disposal of electronic and electrical devices ......................................................................................... 11
2.4 Safety instructions ................................................................................................................................. 11
3 Product description......................................................................................................................................... 13
3.1 Intended use .......................................................................................................................................... 13
3.2 EXPERTsurg LUX ................................................................................................................................. 15
3.3 Controls ................................................................................................................................................. 17
3.4 Foot control ........................................................................................................................................... 18
3.5 Rating plates of EXPERTsurg LUX and foot control.............................................................................. 18
3.6 Technical Specifications of the EXPERTsurg LUX................................................................................ 19
3.7 Scope of delivery ................................................................................................................................... 20
4 First use.......................................................................................................................................................... 21
4.1 Unpacking.............................................................................................................................................. 21
4.2 Installing the bottle holder...................................................................................................................... 21
4.3 Plugging in the foot control .................................................................................................................... 21
4.4 Connecting the surgical motor ............................................................................................................... 22
4.5 Connecting the coolant container and hose set..................................................................................... 23
4.6 Electrical connection.............................................................................................................................. 27
5 Operation........................................................................................................................................................ 29
5.1 Switching the device on ......................................................................................................................... 29
5.2 Device settings ...................................................................................................................................... 29
5.2.1 Setting the language ................................................................................................................. 30
5.2.2 Setting the LUX brightness ....................................................................................................... 30
5.2.3 Setting the LUX afterglow time ................................................................................................. 31
5.2.4 Setting the operating mode of the foot control .......................................................................... 31
5.2.5 Setting the pump key operating mode of the foot control ......................................................... 32
5.2.6 Setting the clock time................................................................................................................ 32
5.2.7 Setting the date......................................................................................................................... 33
5.2.8 Setting the LCD brightness ....................................................................................................... 33
Instructions for use EXPERTsurg LUX REF 1.008.3500
Table of contents
3 / 72

5.2.9 Setting the volume .................................................................................................................... 34
5.2.10 Setting the key sound volume ................................................................................................... 34
5.2.11 Exporting settings ..................................................................................................................... 34
5.2.12 Importing settings...................................................................................................................... 35
5.2.13 Factory settings......................................................................................................................... 35
5.2.14 Version ...................................................................................................................................... 36
5.3 Surgical Motor INTRA LUX S600 LED .................................................................................................. 36
5.3.1 Attaching the straight or contra-angle handpiece ..................................................................... 36
5.3.2 Removing the straight or contra-angle handpiece .................................................................... 37
5.4 Setting and executing program steps .................................................................................................... 37
5.4.1 Factory settings......................................................................................................................... 39
5.4.2 Examples of program set sequences........................................................................................ 40
5.4.3 Selecting the program steps ..................................................................................................... 41
5.4.4 Selecting activities .................................................................................................................... 42
5.4.5 Limiting the program steps........................................................................................................ 42
5.5 Changing default values ........................................................................................................................ 43
5.5.1 Setting the maximum speed ..................................................................................................... 43
5.5.2 Setting the torque limit .............................................................................................................. 44
5.5.3 Setting the coolant flow............................................................................................................. 44
5.5.4 Changing the direction of motor rotation................................................................................... 45
5.5.5 Setting the transmission ratio.................................................................................................... 46
5.6 Rinsing function ..................................................................................................................................... 47
5.6.1 Manual rinsing function ............................................................................................................. 47
5.6.2 Program step Rinsing function.................................................................................................. 48
5.7 Activating the one-touch calibration....................................................................................................... 48
5.8 Foot control ........................................................................................................................................... 49
5.8.1 Changing the speed, coolant flow, and direction of motor rotation ........................................... 49
5.8.2 Selecting the program steps ..................................................................................................... 50
5.9 Changing the coolant container ............................................................................................................. 51
6 Decommissioning ........................................................................................................................................... 52
6.1 Disconnecting the electrical connection................................................................................................. 52
6.2 Disposal of the coolant hose.................................................................................................................. 52
6.3 Disconnecting the surgical motor........................................................................................................... 53
6.4 Disconnecting the foot control ............................................................................................................... 53
6.5 Dismantling the bottle holder ................................................................................................................. 53
7 Reprocessing steps in accordance with DIN EN ISO 17664.......................................................................... 55
7.1 Cleaning................................................................................................................................................. 55
7.1.1 Manual cleaning........................................................................................................................ 55
7.1.2 Machine cleaning ...................................................................................................................... 57
7.2 Disinfection ............................................................................................................................................ 57
7.2.1 Manual disinfection ................................................................................................................... 57
7.2.2 Automated disinfection.............................................................................................................. 57
7.2.3 Drying........................................................................................................................................ 58
7.2.4 Service, inspection and testing after preparation...................................................................... 58
7.3 Packaging .............................................................................................................................................. 58
7.4 Sterilisation ............................................................................................................................................ 59
7.4.1 Storage ..................................................................................................................................... 60
Instructions for use EXPERTsurg LUX REF 1.008.3500
Table of contents
4 / 72

8 Troubleshooting.............................................................................................................................................. 61
9 Run a software update ................................................................................................................................... 64
10 Safety checks ("STK") .................................................................................................................................... 65
11 Accessories .................................................................................................................................................... 66
12 Details on electromagnetic compatibility ........................................................................................................ 67
12.1 Guidelines and manufacturer's declaration - electromagnetic transmission.......................................... 67
12.2 Guidelines and manufacturer's declaration - electromagnetic resistance to jamming ........................... 67
12.3 Guidelines and manufacturer's declaration - electromagnetic resistance to jamming ........................... 68
12.4 Recommended safe distance between portable and mobile HF telecommunications equipment and
the EXPERTsurg LUX ........................................................................................................................... 70
Instructions for use EXPERTsurg LUX REF 1.008.3500
Table of contents
5 / 72
Instructions for use EXPERTsurg LUX REF 1.008.3500
1 User instructions | 1.1 User guide
6 / 72
1 User instructions
1.1 User guide
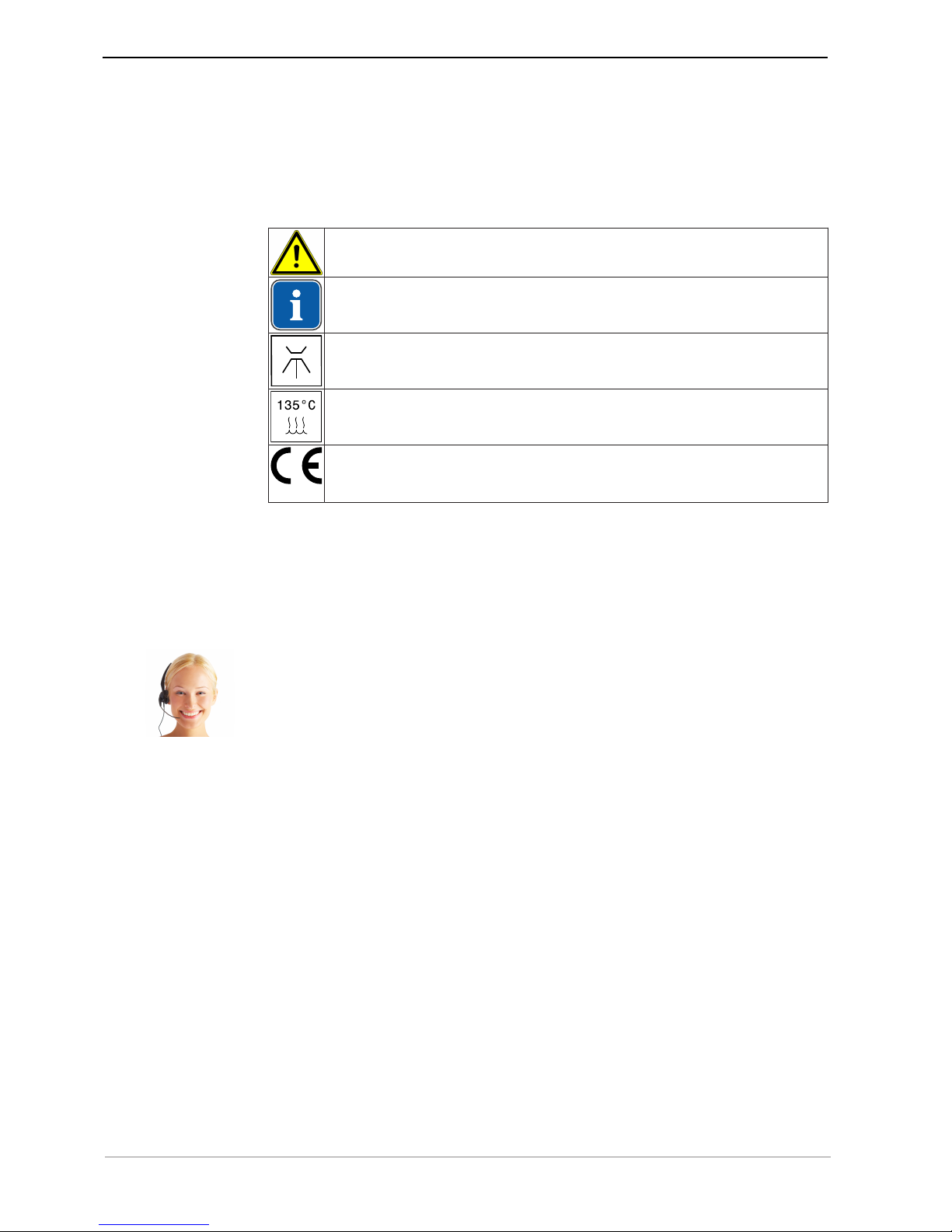
1.1.1 Symbols
Refer to the chapter on Safety/Warning symbol
Important information for users and service technicians
Thermodisinfectable
Sterilisable up to 135 oC
CE mark (European Community). A product bearing this mark meets the requirements of the pertinent EC directives, i.e. the standards applicable in
Europe.
1.2 Target group
This document is for dentists and dental office staff.
1.3 Service
Please direct all questions regarding the product, service and maintenance to the
KaVo Technical Service:
Toll-free: 1-888-ASK-KAVO (888-275-5286)
Email: customerservice@kavo.com
Please refer to the serial number of the product in all inquiries!
1.3.1 Repair Service
KaVo offers a fixed-price service check for the original factory maintenance. You can
use a loaner device for the time of the service check.
For repairs, please contact KaVo Repair Service.
For scheduling or if you have any questions, please contact:
KaVo Repair Service
KaVo Dental Corporation
11729 Fruehauf Drive
Charlotte, NC 28273 USA
Toll-free Direct Customer Service: 1-888-ASK-KAVO (888-275-5286)
Email: techservice@kavo.com
www.kavousa.com

Instructions for use EXPERTsurg LUX REF 1.008.3500
1 User instructions | 1.4 Terms and conditions of warranty
1.4 Terms and conditions of warranty
KaVo provides the final customer with a warranty that the product cited in the handover certificate will function properly and guarantees zero defects in the material or
processing for a period of 12 months from data of purchase, subject to the following
conditions:
Upon justified complaints of flaws or a short delivery, KaVo will make good its warranty by replacing the product free of cost or repairing it according to the customer's
wishes. Other claims of any nature whatsoever, in particular with respect to compensation, are excluded. In the event of default and gross negligence or intent, this shall
only apply in the absence of mandatory legal regulations to the contrary.
KaVo cannot be held liable for defects and their consequences due to natural wear,
improper cleaning or servicing, non-compliance with operating, servicing or connection
instructions, calcification or corrosion, contaminated air or water supplies or chemical
or electrical factors deemed abnormal or impermissible in accordance with factory
specifications.
The warranty does not usually cover bulbs, glassware, rubber parts and the colourfastness of plastics.
Defects or their consequences that can be attributed to interventions on or changes
made to the product by the customer or a third party are excluded from the warranty.
Defects or their consequences that can be attributed to interventions on or changes
made to the product by the customer or a third party are excluded from the warranty.
1.5 Transportation and storage
1.5.1 Currently valid packaging regulations
Note
Only valid for the Federal Republic of Germany.
Dispose of and recycle the sales packaging appropriately in accordance with current
packaging regulations, employing waste management or recycling companies. Comply with the comprehensive return system. KaVo has had its sales packaging licensed
for this purpose. Please comply with the regional public waste-disposal system.
1.5.2 Damage in transit
In Germany
If the packaging is visibly damaged on delivery, please proceed as follows:
1. The recipient of the package must record the loss or damage on the delivery receipt. The recipient and the representative of the shipping company must sign this
delivery receipt.
2. Leave the product and packaging in the condition in which you received it.
3. Do not use the product.
4. Report the damage to the shipping company.
5. Report the damage to KaVo.
6. Consult with KaVo first, before returning a damaged product.
7. Send the signed delivery receipt to KaVo.
7 / 72
Instructions for use EXPERTsurg LUX REF 1.008.3500
1 User instructions | 1.5 Transportation and storage
8 / 72
If the product is damaged but there was no discernable damage to the packaging on
delivery, proceed as follows:
1. Report the damage to the shipping company immediately and no later than 7 days
after delivery.
2. Report the damage to KaVo.
3. Leave the product and packaging in the condition in which you received it.
4. Do not use a damaged product.
Note
Failure on the part of the recipient to comply with any of the above-mentioned obligations will mean that the damage will be considered to have arisen following delivery (in accordance with the General German Freight Forwarders´ Terms and Conditions, Art. 28).
Outside Germany
Note
KaVo shall not be held liable for damage arising from transportation.
The shipment must be checked on arrival.
If the packaging is visibly damaged on delivery, please proceed as follows:
1. The recipient of the package must record the loss or damage on the delivery receipt. The recipient and the representative of the shipping company must sign this
delivery receipt.
Without this evidence, the recipient will not be able to assert a claim for damages
against the shipping company.
2. Leave the product and packaging in the condition in which you received it.
3. Do not use the product.
If the product is damaged but there was no discernable damage to the packaging on
delivery, proceed as follows:
1. Report any damage to the shipping company either immediately or no later than 7
days after delivery.
2. Leave the product and packaging in the condition in which you received it.
3. Do not use a damaged product.
Note
If the recipient fails to comply with any of the above-mentioned obligations, the
damage will be considered to have arisen following delivery
(in accordance with CMR law, Chapter 5, Art. 30).
1.5.3 Information on the packaging: Storage and transportation
Note
Please keep the packaging in case you need to return the product for servicing or
repair.
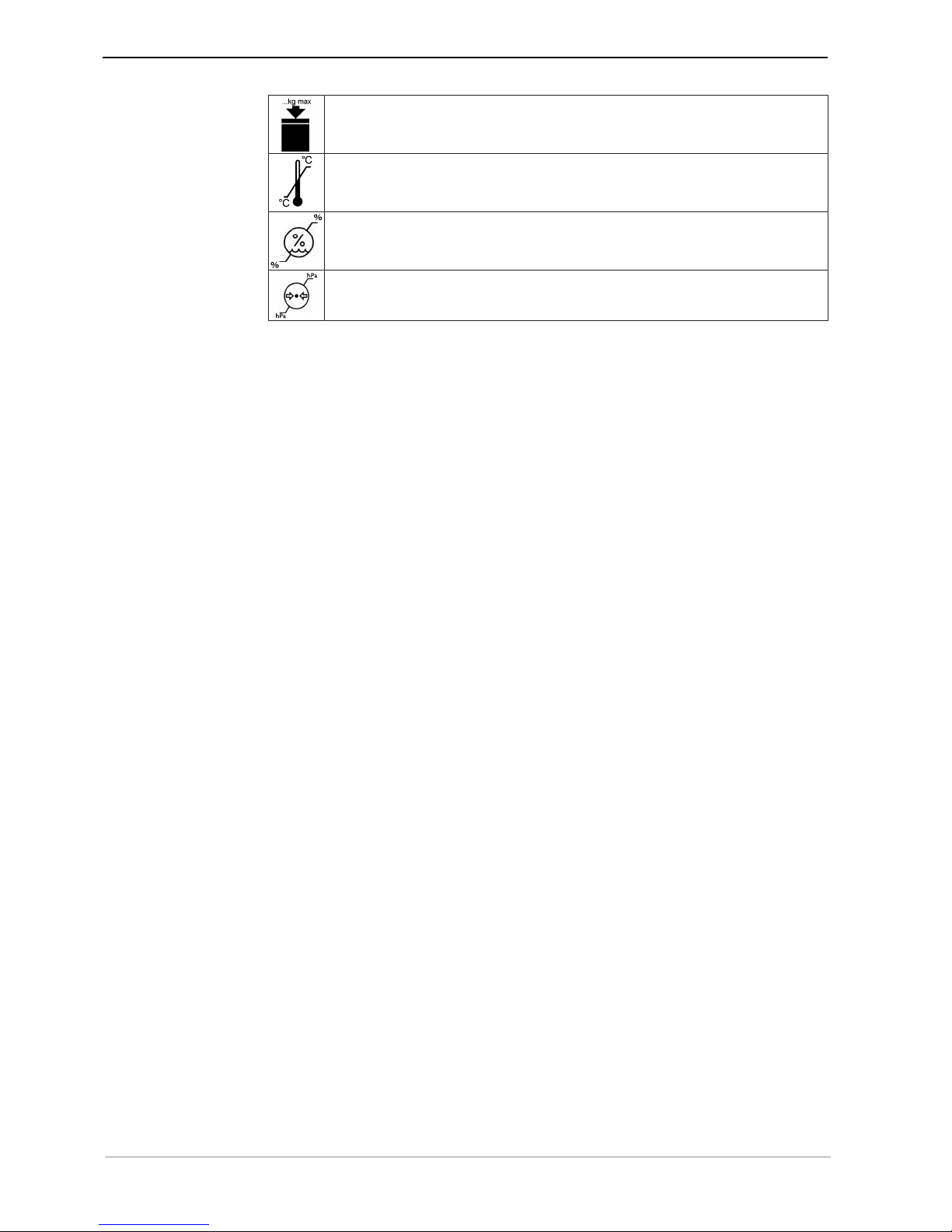
The symbols printed on the outside are for transportation and storage, and have the
following meaning:
Transport upright with the arrows pointing upwards!
Fragile - protect against impact!
Protect from moisture!
Instructions for use EXPERTsurg LUX REF 1.008.3500
1 User instructions | 1.5 Transportation and storage
Permissible stacking load
Temperature range
Humidity
Air pressure
9 / 72
Instructions for use EXPERTsurg LUX REF 1.008.3500
2 Safety | 2.1 Description of safety instructions
10 / 72
2 Safety
2.1 Description of safety instructions
2.1.1 Warning symbol
Warning symbol
2.1.2 Structure
DANGER
The introduction describes the type and source of the hazard.
This section describes potential consequences of non-compliance.
▶ The optional step includes necessary measures for hazard prevention.
2.1.3 Description of hazard levels
Safety instructions distinguishing between three hazard levels are used in this document to prevent personal and property damage.
CAUTION
CAUTION
indicates a hazardous situation that can cause damage to property or mild to moder-
ate injuries.
WARNING
WARNING
indicates a hazardous situation that can lead to serious or fatal injury.
DANGER
DANGER
indicates a maximal hazard due to a situation that can directly cause death or fatal in-
jury.
2.2 Information about electromagnetic compatibility
Note
Based on IEC 60601-1-2 (DIN EN 60601-1-2) concerning the electromagnetic compatibility of electrical medical devices, we must draw your attention to the following
points:
• Medical electrical devices are subject to special precautions concerning the electromagnetic compatibility and must be installed and operated in accordance with the
KaVo assembly instructions.
• High-frequency communications devices may interfere with electrical medical
devices.
See also:
2 12 Information about electromagnetic compatibility, Page 67
Instructions for use EXPERTsurg LUX REF 1.008.3500
2 Safety | 2.3 Disposal of electronic and electrical devices
Note
KaVo cannot guarantee the compliance of accessories, cables, and other components not supplied by KaVo with the EMC requirements of IEC 60601-1-2 (DIN EN
60601-1-2).
2.3 Disposal of electronic and electrical devices
Note
According to EC directive 2012/19 concerning used electrical and electronic
devices, this product is subject to the cited directive and must be disposed of accordingly within Europe.
For more information, please visit www.kavo.com or contact your specialised dental
supplier.
For final disposal:
In Germany
To return an electrical device, you need to proceed as follows:
1. On the homepage www.enretec.de of enretec GmbH, you can download a form for
a disposal order under the menu item eom. Download the disposal order or complete it as an online order.
2. Enter the corresponding information to complete the order, and submit it as an online order or by fax +49 (0)3304 3919 590 to enretec GmbH.
The following contact options are also available for questions and for initiating a
disposal order:
Phone: +49 (0) 3304 3919-500
Email: eom@enretec.de and
Postal address: enretec GmbH, Geschäftsbereich eomRECYCLING®
Kanalstraße 17
D-16727 Velten
3. A unit that is not permanently installed will be picked up at the office.
A permanently installed unit will be picked up at the curb at your address on the
agreed date.
The owner or user of the device will have to bear the cost of disassembly, transportation and packaging.
International
For country-specific information on disposal, contact your dental supplier.
2.4 Safety instructions
WARNING
Application of un-authorised accessories or un-authorised modifications of the
product.
Accessories that have not been approved and/or inadmissible modifications of the
product could lead to hazards and/or personal injury or property damage.
▶ Only use accessories that have been approved for combination with the product
by the manufacturer or are equipped with standardised interfaces (e. g. MULTIflex
couplings, INTRAmatic).
▶ Do not make any modifications to the device unless these have been approved by
the manufacturer of the product.
11 / 72
Instructions for use EXPERTsurg LUX REF 1.008.3500
2 Safety | 2.4 Safety instructions
12 / 72
CAUTION
Electrical sparks in the product.
Explosion and/or fire.
▶ Do not use product in areas subject to an explosion hazard.
▶ Do not operate the product in an oxygen-enriched atmosphere.
CAUTION
Damaged mains cable / missing protective conductor.
Electrical shock.
▶ Check the mains cable before use. The socket outlet must have a protective con-
tact and meet the respective national guidelines.
CAUTION
Damage by liquids.
Faults on electrical components.
▶ Protect openings of the product from any ingress of liquids.
CAUTION
Inadvertent penetration of liquids.
Electrical shock.
▶ Do not place the product in a tub-like container.
▶ Check the coolant containers and lines for absence of leakage. If any liquid is de-
tected on the device, do not touch the device and disconnect the device from the
mains supply without delay. Make sure that the surface of the device is completely dry before plugging the main plug back in the socket.
CAUTION
Rotating parts while the pump is operating
Injuries
▶ Do not stick anything in the pump. Turn off the device when the pump is open.
CAUTION
Risks from electromagnetic fields.
Electromagnetic fields might interfere with the functions of implanted systems (such
as pacemakers).
▶ Ask patients if they have a cardiac pacemaker or other system implanted before
you start the treatment!
CAUTION
Impact of power failure.
Failure of the voltage supply or other errors can cause the surgical motor to come to a
standstill.
▶ Make sure that the power supply is working.
Instructions for use EXPERTsurg LUX REF 1.008.3500
3 Product description | 3.1 Intended use
3 Product description
The EXPERTsurg LUX is a surgical controller according to 21 CFR § 872.4200 (dental
handpiece and accessories). The device consists of a surgical control unit, a foot controller, a surgical motor (separate IFU) + motor cable, an instruments tray, a holder
and a tube set. As a functional principle the software-based surgical control unit controls the speed and torque of a dental micro motor. The unit is equipped with a pump
for the use with external irrigation tubing allowing irrigation of the working area. The
surgical control unit is operated through the buttons on the tabletop console or via foot
control. The device is intended to be used with the INTRA LUX S600 LED (separate
IFU) motor. Straight or contra-angle handpieces with a handpiece connection according to ISO 3964 can be equipped. The instrument tray allows the dentist to deposit the
handpieces in a safe position. The holder is intended to be used for general storage of
the bottle. The tube set is needed to deliver the external irrigation from the bottle to the
different handpieces. A power cord provides electric power to the unit. The EXPERTsurg LUX will be delivered with software on the surgical control unit.
3.1 Intended use
Indications for use
This KaVo product is intended for surgery to expose and dissect oral tissue structures
or endodontic treatments (e.g. periodontal gap, gingiva, bone, jaw, extractions and implantations).
CAUTION
US Federal law restricts this device to sale by or on the order of a health care professional / dentist.
For dental use only.
Proper Use
Note
The EXPERTsurg LUX is approved for use in surgical theatres.
The overarching guidelines and/or national laws, national regulations and the rules of
technology applicable to medical devices for start-up and use of the KaVo product for
the intended purpose must be applied and followed.
The user must ensure that the unit works properly and is in satisfactory condition before each use.
The applicable national legal regulations must be observed during the use of the
device, in particular the following:
▪ Applicable regulations governing the connection and start-up of medical devices.
▪ Current occupational safety regulations.
▪ Current accident prevention regulations.
It is a responsibility of the user:
▪ to only use equipment that is operating correctly,
▪ to protect him or herself, the patient and third parties from hazards, and
13 / 72
Instructions for use EXPERTsurg LUX REF 1.008.3500
3 Product description | 3.1 Intended use
14 / 72
▪ to prevent contamination from the product
To guarantee the consistent readiness for use and to preserve the value of the KaVo
product, the recommended maintenance services must be carried out in 2 year intervals.
The following persons are authorised to repair and service the KaVo product:
▪ Technicians of KaVo branch offices after appropriate product training.
▪ Specifically KaVo-trained technicians of KaVo franchised dealers.
Note
The permitted work is described in the Technician's Instructions available to the
trained service staff.
In Germany, operators, equipment managers and users are obliged to operate their
equipment in accordance with the MPG regulations.
The services encompass all the test tasks required in accordance with § 6 of the medical devices operator ordinance (Medizinprodukte-Betreiberverordnung, MPBetreibV).
After servicing, interventions, and repairs of the device, the device must be tested according to IEC 62353 (according to the state of the art) before re-use.
Note
The product must be cleaned and serviced according to instructions if it is not to be
used for an extended period of time.
Note
Any waste which is generated must be recycled or disposed of in strict compliance
with all applicable national regulations in a manner which is safe both for people
and the environment.
If you have any questions regarding proper disposal of the KaVo product, please
contact the KaVo branch.
Note
A recycling pass can be downloaded from www.kavo.com.
Instructions for use EXPERTsurg LUX REF 1.008.3500
3 Product description | 3.2 EXPERTsurg LUX
3.2 EXPERTsurg LUX
1
2
7
8
3
6
5
4
9
10
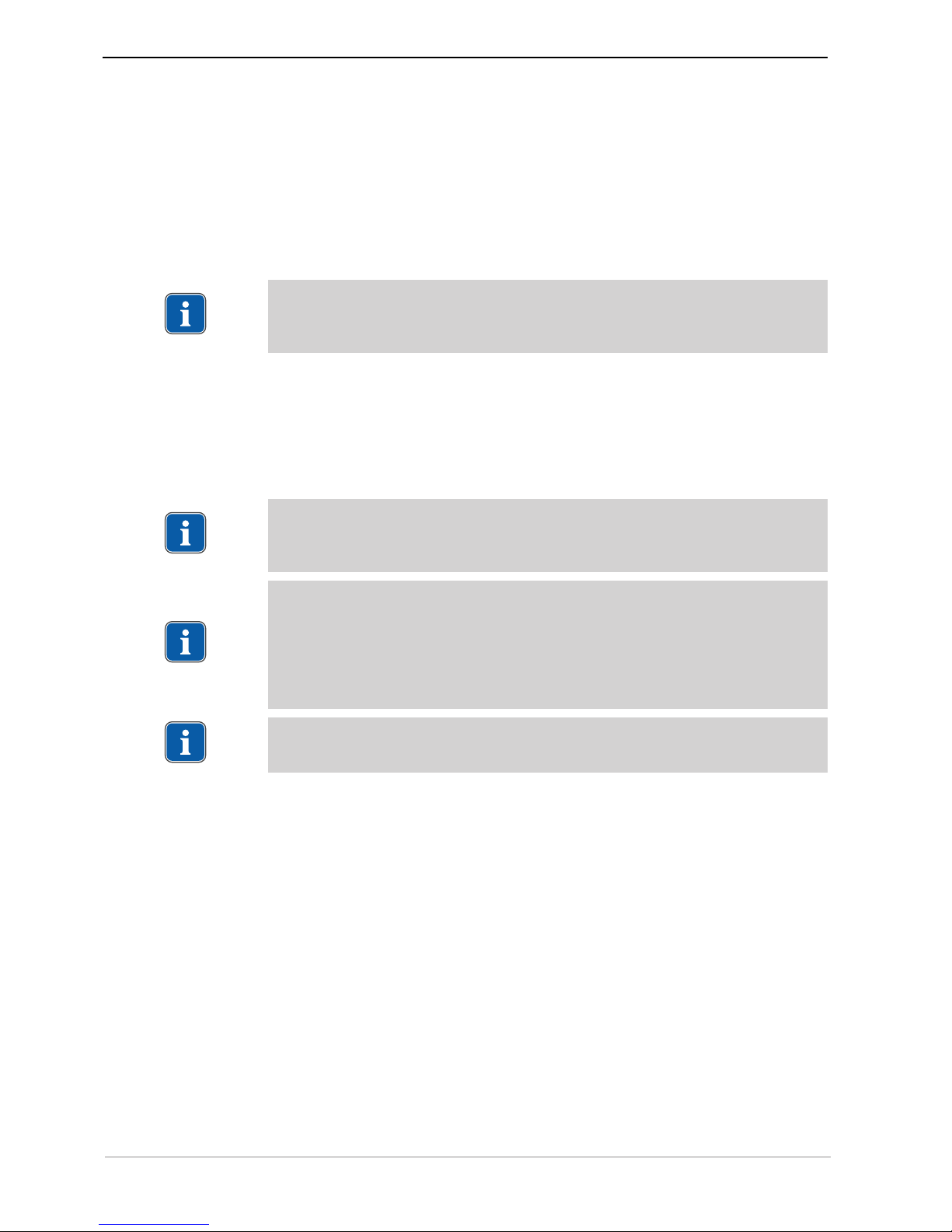
① Hand-held control panel ② Bottle holder
③ Hose pump ④ Hose fixation
⑤ Foot control ⑥ Surgical motor
⑦ Coolant hose ⑧ Handpiece tray
⑨ Motor cable ⑩ Symbol of type B applied part
15 / 72
Instructions for use EXPERTsurg LUX REF 1.008.3500
3 Product description | 3.2 EXPERTsurg LUX
16 / 72
Rear
3
7
2
1
54
6
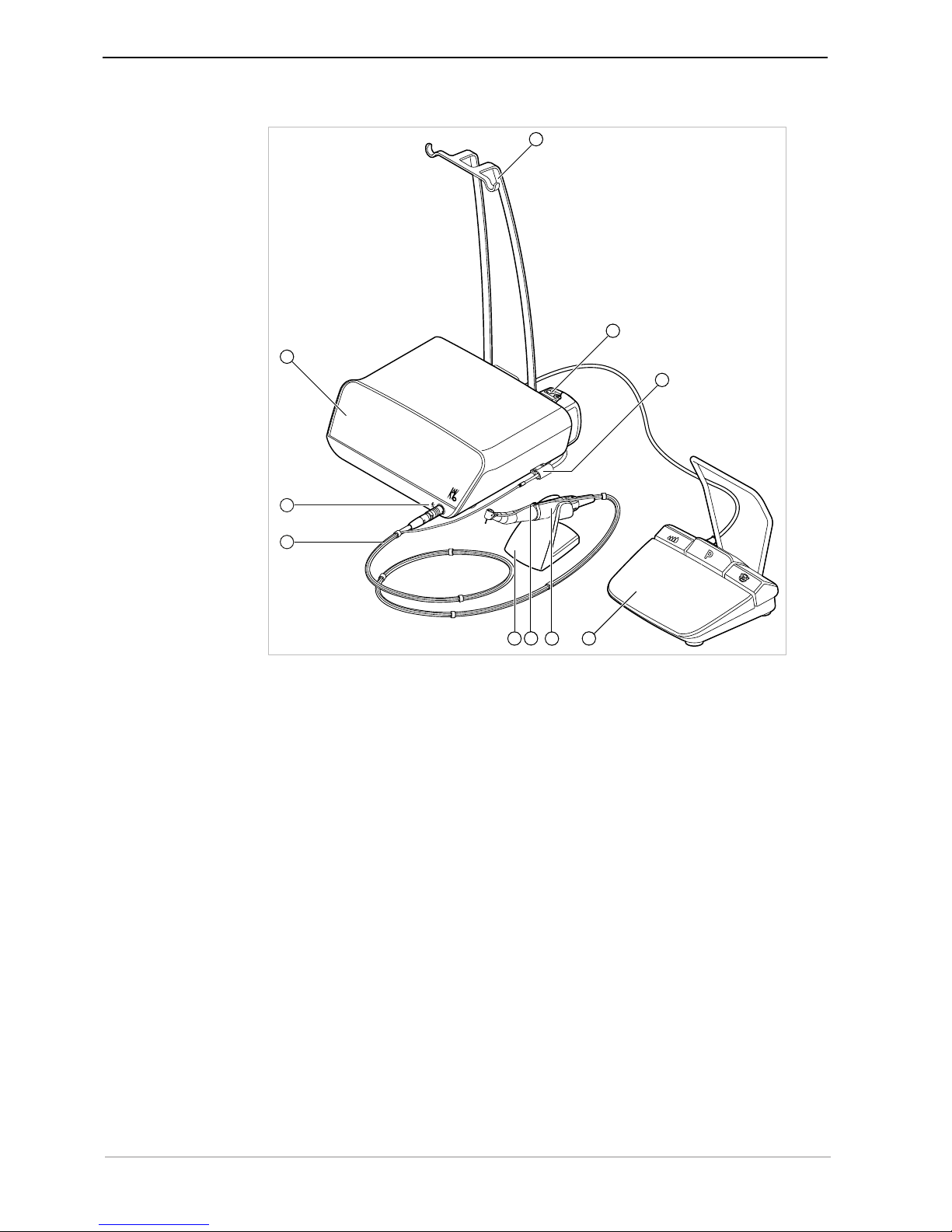
① Hose pump locking mechanism ② On-button
③ Mains plug ④ Please note the instructions for use
⑤ Follow the instructions for use ⑥ SD card slot
⑦ Foot control electrical outlet

Instructions for use EXPERTsurg LUX REF 1.008.3500
3 Product description | 3.3 Controls
3.3 Controls
3
2
6
7
8
1
4 5
1011 913 121415
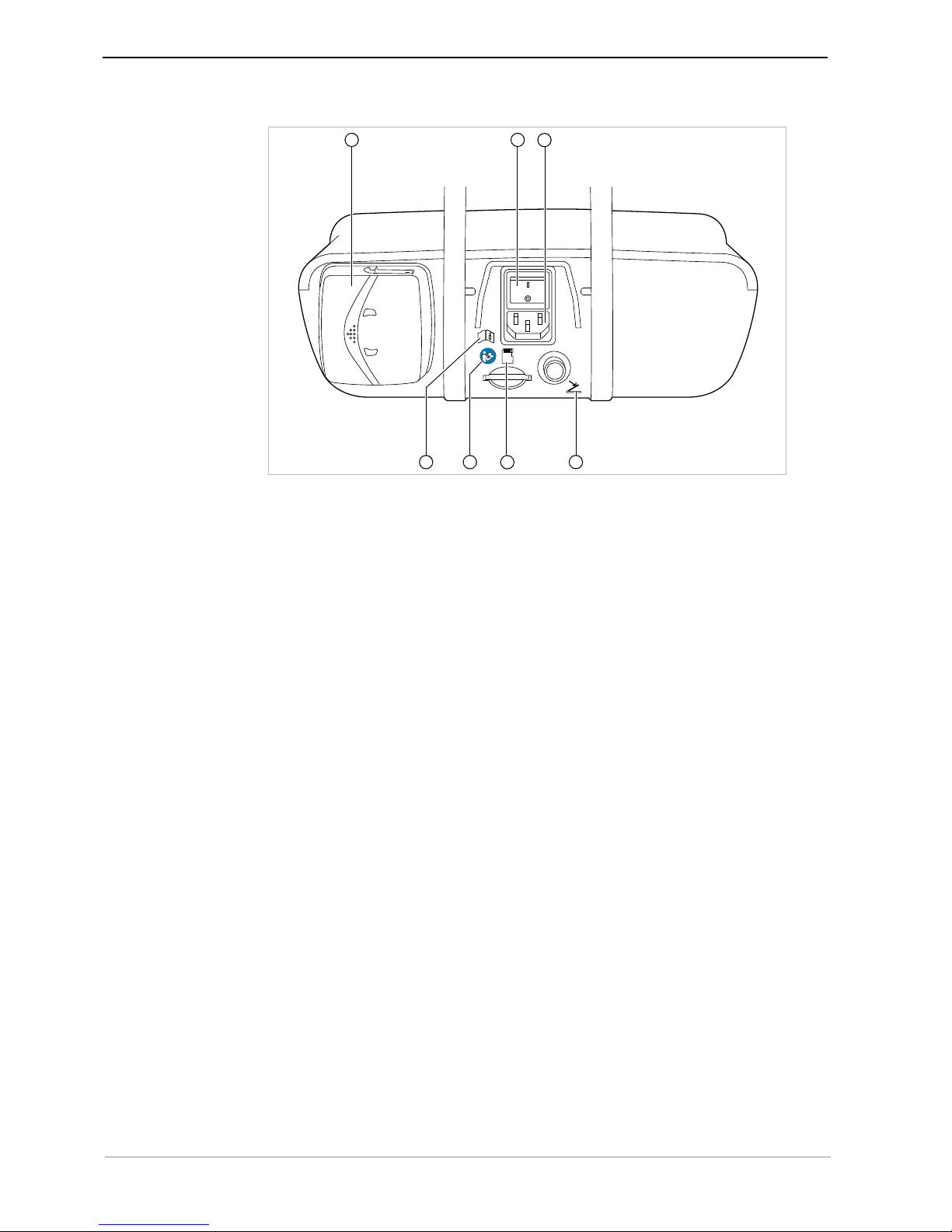
① Program step ② Display of the activity
③ Maximal torque reached ④ Torque limit
⑤ Speed ⑥ Left arrow key
⑦ Plus key, increase value ⑧ Right arrow key
⑨ Minus key, decrease value ⑩ Back key
⑪ Transmission ratio ⑫ Direction of motor rotation
⑬ Coolant pump settings ⑭ Activation of one-touch calibration
⑮ Foot control status indicator / Service
check request
The back key has two functions. Pressing the back key briefly opens the selection of
program steps. Pressing the back key long opens the device settings.
Parameters can be selected using the arrow keys.
Use the plus key to increase the value.
Use the minus key to decrease the value.
17 / 72
Instructions for use EXPERTsurg LUX REF 1.008.3500
3 Product description | 3.4 Foot control
18 / 72
3.4 Foot control
1
3
2
4
① Speed key (grey) ② Pump key (blue)
③ Programme key (grey) ④ Direction of motor rotation key (yellow)
3.5 Rating plates of EXPERTsurg LUX and foot control
The rating plates of EXPERTsurg LUX and foot control are affixed on the underside of
the housing and include the following symbols:
CE mark
VDE mark
CSA mark
Classification, type B
Please note the instructions for use
Please note the electronic instructions for use
Follow the instructions for use

Instructions for use EXPERTsurg LUX REF 1.008.3500
3 Product description | 3.6 Technical Specifications of the EXPERTsurg LUX
Operating mode: continuous operation with intermittent load
Alternating current (AC)
Supply voltage
Protection class II
Manufacturer
YYYY = Year manufactured
XXXXXXX = Serial number
Material number
Type: Device type
For disposal information, see Intended use
GOST R certification
HIBC Code
3.6 Technical Specifications of the EXPERTsurg LUX
Width 265mm
Depth 255mm
Height 100mm
Weight approx. 1.9 kg
Weight of foot control approx. 1.1 kg
Weight of motor approx. 125 g
Input voltage 100 - 240 V ~
Input frequency 50/60 Hz
Rated power max. 150 W
Speed 300 – 40,000 rpm
Max. torque on the motor 5.5 Ncm
Pump delivery rate 30 - 110 ml/min
Foot control: Class of protection IPX8
Foot control: cable length 2.5 m
Length of motor cable 6.5 ft (2 m)
Operating mode
Continuous operation with intermittent
load
30 sec. of operation/ 9 min. pause
19 / 72
Instructions for use EXPERTsurg LUX REF 1.008.3500
3 Product description | 3.7 Scope of delivery
20 / 72
Note
The maximal motor load is 30 seconds operating time / 9 minutes pause (full load at
maximal speed).
Transportation and storage conditions
Ambient temperature -20 ℃ - +50 ℃
Relative humidity 5% - 95%
Air pressure 700 hPa - 1,060 hPa
Operating environment
WARNING
Inappropriate operating conditions.
Impairment of the electrical safety of the device.
▶ It is essential to comply with the operating conditions specified in the "Technical
Specifications" chapter.
Ambient temperature +10 ℃ - +35 ℃
Relative humidity 15% - 80%
Air pressure 700 hPa - 1,060 hPa
Max. elevation for operation up to 3,000 m
3.7 Scope of delivery
The scope of delivery of the EXPERTsurg LUX includes:
▪ EXPERTsurg LUX unit
▪ Foot control
▪ Surgical motor INTRA LUX S600 LED
▪ Motor cable S600
▪ Handpiece tray
▪ Hose set sterile S600 (5 units)
▪ Bottle holder
▪ Instructions for use for EXPERTsurg LUX
▪ Short instructions for the EXPERTsurg LUX
▪ Instructions for use INTRA LUX S600 LED
Instructions for use EXPERTsurg LUX REF 1.008.3500
4 First use | 4.1 Unpacking
4 First use
4.1 Unpacking
Note
You need to keep the cardboard box and all packaging materials to be able to
safely ship the unit in the future.
▶ Open the cardboard box.
▶ Remove the hose boxes.
▶ Take out the foot control and additional equipment.
▶ To take out the unit, pull it vertically upward and place it on a level surface.
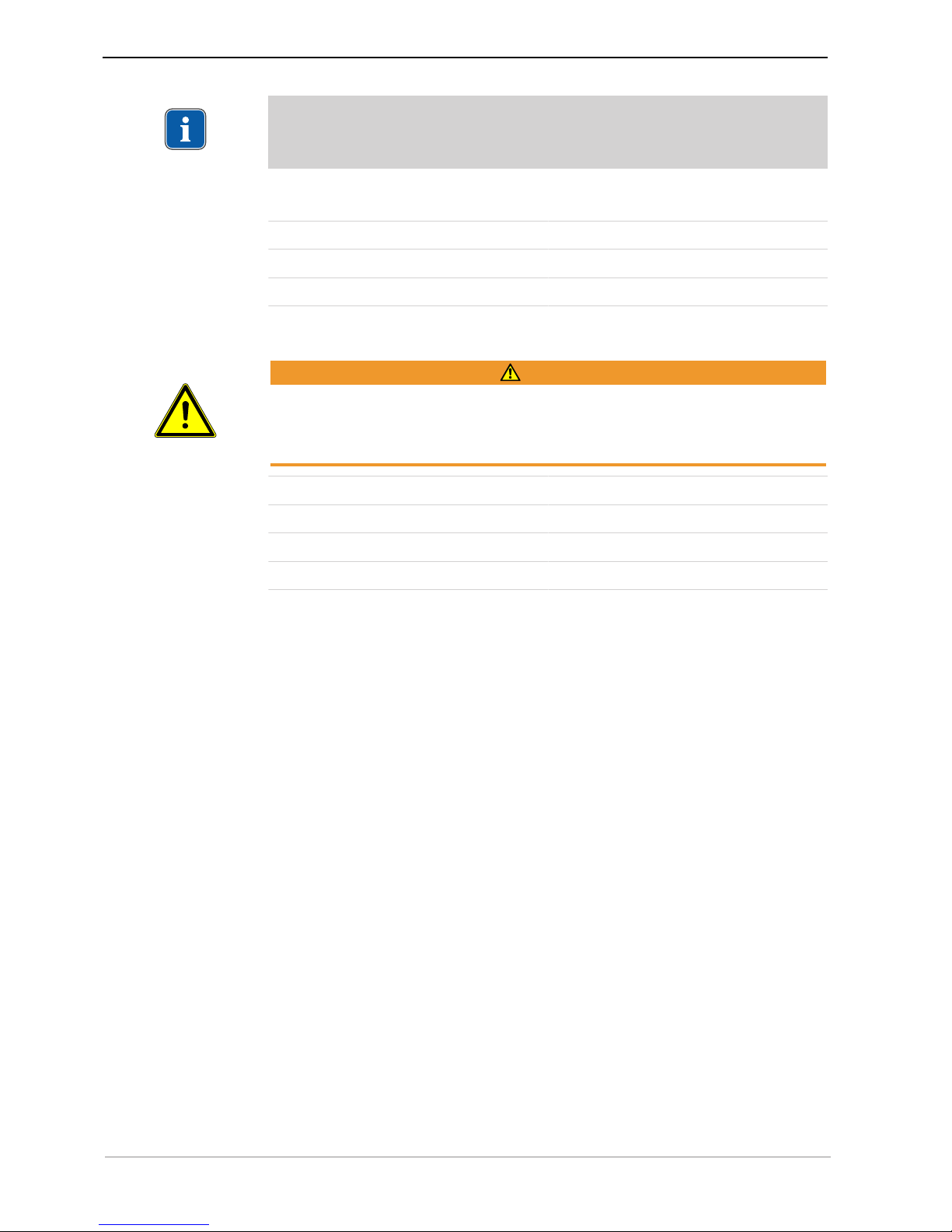
4.2 Installing the bottle holder
▶ Slide the bottle holder ① in the guide on the underside of the unit.
ð The bottle holder ① snaps into place audibly and is then affixed.
4.3 Plugging in the foot control
21 / 72

Instructions for use EXPERTsurg LUX REF 1.008.3500
4 First use | 4.4 Connecting the surgical motor
22 / 72
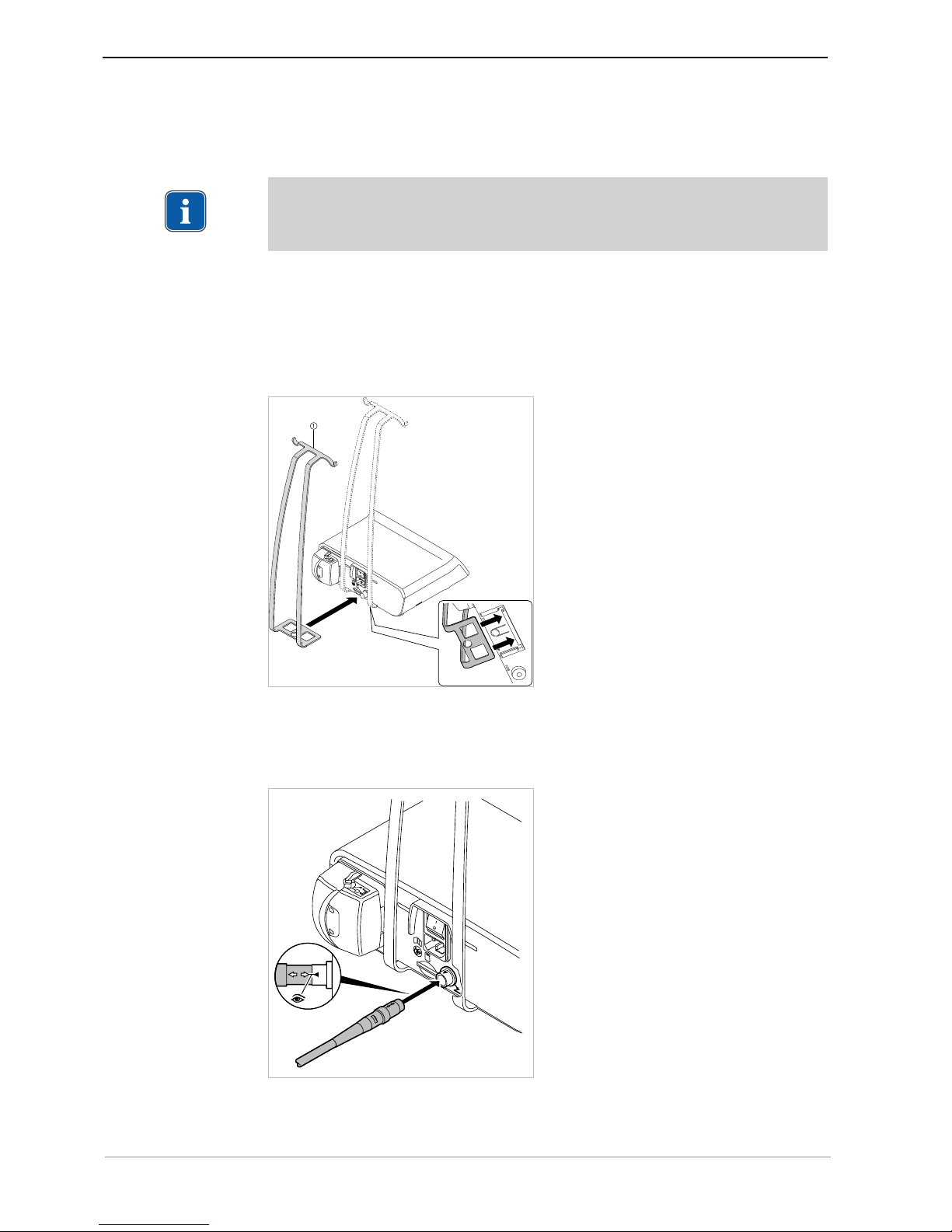
▶ Insert the plug of the foot control in the electrical outlet for the foot control. Make
sure that the marker arrows on the plug and the socket are aligned towards each
other.
▶ Slide the bracket into the designated recesses.
4.4 Connecting the surgical motor
Note
The delivered parts are not sterile (except for the coolant hose). Before the first
treatment of a patient, the surgical motor, motor cable, and the handpiece tray need
to be reprocessed.
See also:
2 Reprocessing steps in accordance with DIN EN ISO 17664
 Loading...
Loading...