Instrumentarium Dental OP-300 Service manual
ENGLISH
ORTHOPANTOMOGRAPH® OP300
3D Dental X-Ray System
Service Manual
210986 rev. 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

ORTHOPANTOMOGRAPH® OP300
Copyright Code: 210986 rev 2 Date: June 12, 2014
Copyright © 6/12/14 by Instrumentarium Dental, PaloDEx
Group Oy.
All rights reserved.
ORTHOPANTOMOGRAPH®/ INSTRUMENTARIUM
DENTAL™/ CLINIVIEW™ is a registered trademark/ a
common law trademark of Instrumentarium Dental,
PaloDEx Group Oy.
U.S. patents US6731717, US6829326 and USRE41197.
Finnish patents 114383.
Documentation, trademark and the software are
copyrighted with all rights reserved. Under the copyright
laws the documentation may not be copied, photocopied,
reproduced, translated, or reduced to any electronic
medium or machine readable form in whole or part, without
the prior written permission of Instrumentarium Dental.
The original language of this manual is English.
Instrumentarium Dental reserves the right to make
changes in specification and features shown herein, or
discontinue the product described at any time without
notice or obligation. Contact your Instrumentarium Dental
representative for the most current information.
Manufacturer Instrumentarium Dental, PaloDEx Group Oy
Nahkelantie 160 (P.O. Box 20)
FI-04300 Tuusula
FINLAND
Tel. +358 10 270 2000
Fax. +358 10 270 2230
For service, contact your local distributor.
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

ORTHOPANTOMOGRAPH® OP300
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

Table of Contents
1 Introduction..................................................................................................................1
1.1 ORTHOPANTOMOGRAPH® OP300 .................................................................... 1
1.2 References............................................................................................................ 2
1.3 Intended use ......................................................................................................... 2
1.4 Associated documentation .................................................................................... 3
1.5 Abbreviations used in this manual ........................................................................ 3
1.6 Warnings and precautions .................................................................................... 3
1.6.1 Warnings for cross infection....................................................................... 3
1.6.2 Warnings to be observed during installation and service...........................4
1.6.2.1 Cautions for Electrostatic discharge ............................................ 5
1.6.3 General warnings .......................................................................................6
1.7 Unauthorized Modifications................................................................................... 9
1.8 Disclaimer ............................................................................................................. 9
1.9 Disposal ................................................................................................................ 9
2 Unit description ......................................................................................................... 11
2.1 Main parts and controls....................................................................................... 11
2.2 Patient positioning lights ..................................................................................... 13
2.3 Patient positioning panel .....................................................................................16
2.3.1 Cephalometric unit (optional) ................................................................... 16
2.4 Main control panel............................................................................................... 17
2.5 Unit identification labels ...................................................................................... 18
2.6 Unit movements ..................................................................................................19
2.7 Finger guards ...................................................................................................... 21
2.8 Emergency stop switch ....................................................................................... 22
3 Electrical description ................................................................................................ 23
3.1 Circuit boards ......................................................................................................23
3.2 Electrical component location ............................................................................. 25
3.3 List of circuit boards and electric devices............................................................ 28
3.4 Schematic diagrams............................................................................................ 31
3.5 Circuit board descriptions.................................................................................... 45
3.5.1 R3210 CPU (Central Processing Unit)..................................................... 45
3.5.2 CEPH R3210............................................................................................ 46
3.5.3 R3300 IO1 (Input/Output 1)...................................................................... 47
3.5.4 R3400 NGEO IO2 (Input/Output 2 Board) ...............................................48
3.5.5 R3700 X-Ray power supply ..................................................................... 50
3.5.6 R3800 X-Ray generator ........................................................................... 51
3.5.7 R5100 3-Phase stepper driver ................................................................. 53
3.5.8 R5300 Panel raw power supply ............................................................... 54
3.5.9 EA100 Power distribution board............................................................... 55
3.5.10 EA200 Connector board........................................................................... 57
3.5.11 EA300 Connector board........................................................................... 59
3.5.12 EB100 Rotator opto board ....................................................................... 59
3.5.13 EB200 Head support board ..................................................................... 61
3.5.14 EC100 Pan sensor connector board ........................................................ 61
3.5.15 ED100 Pan sensor connector board ........................................................ 63
3.5.16 ED200 Ceph sensor connector board...................................................... 64
rev i
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

3.5.17 EE100 Ceph encoder opto board............................................................. 65
3.5.18 EE200 Ceph sensor connector board ...................................................... 66
3.5.19 EG100 Dual opto board ........................................................................... 67
3.5.20 EG200 Interface board............................................................................. 68
3.5.21 EG400 Panel power supply...................................................................... 70
3.5.22 Panoramic sensor (CMOS) ...................................................................... 71
3.5.23 3D sensor (CMOS)................................................................................... 71
3.5.24 Ethernet switch/router .............................................................................. 72
3.5.25 EC400 Collimator opto board (MFOV only) ............................................. 73
3.6 Replacing the boards ..........................................................................................74
3.7 Modification of external warning light circuitry for 230V ...................................... 76
4 Firmware description ................................................................................................ 79
4.1 Overview .............................................................................................................79
4.2 Detailed View ......................................................................................................80
4.3 Operating modes................................................................................................. 81
4.4 Firmware states................................................................................................... 82
5 Hardware operation................................................................................................... 87
5.1 Emergency stop ..................................................................................................87
5.2 Movements.......................................................................................................... 88
5.3 Rotation movement .............................................................................................88
5.4 X-movement........................................................................................................89
5.5 Y-movement........................................................................................................90
5.6 Z-movement ........................................................................................................91
5.7 Chin rest movement ............................................................................................ 92
5.8 Collimator movement ..........................................................................................93
5.9 Collimator MFOV movement............................................................................... 94
5.10 Pan sensor movement ........................................................................................ 95
5.11 Secondary collimator movement......................................................................... 96
5.12 Ceph sensor movement ...................................................................................... 97
5.13 Positioning lights ................................................................................................. 98
5.14 Positioning lights MFOV...................................................................................... 99
5.15 Imaging chain.................................................................................................... 100
5.15.1 3D imaging chain ................................................................................... 100
5.15.2 Panoramic imaging chain....................................................................... 101
5.15.3 Cephalometric imaging chain................................................................. 102
5.15.4 Exposure control .................................................................................... 103
5.15.5 Exposure warning light........................................................................... 104
6 The s2terminal and service functions ................................................................... 105
6.1 Opening the s2Terminal.................................................................................... 106
6.2 s2terminal commands ....................................................................................... 107
6.3 Unit state commands ........................................................................................ 109
6.4 Unit service commands..................................................................................... 111
7 Error Codes and Trouble Shooting........................................................................113
7.1 Initial actions ..................................................................................................... 113
7.2 Checking the unit .............................................................................................. 113
7.3 Reporting problems to technical support........................................................... 116
7.4 Error code descriptions ..................................................................................... 119
ii
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

7.4.1 Error 4 Rotator home position was not found......................................... 119
7.4.2 Error 5 Exposure was taking too long and a timeout occured................ 120
7.4.3 Error 6 Generator failure ........................................................................120
7.4.4 Error 9 R3300 IO1 board is not functioning ........................................... 121
7.4.5 Error 12 R3400 IO2 board is not functioning ......................................... 122
7.4.6 Error 13 Configuration data was lost ..................................................... 122
7.4.7 Error 21 Calibration data receive timeout............................................... 123
7.4.8 Error 22 Reading data from the 3D sensor failed................................... 123
7.4.9 Error 23 Configuration data is invalid ..................................................... 124
7.4.10 Error 24 Voltage (kV) failure in R3800 X-ray generator ......................... 124
7.4.11 Error 25 Current (mA) failure in R3800 X-ray generator ........................ 124
7.4.12 Error 26 Preheat failure in R3800 X-ray generator ................................125
7.4.13 Error 27 Rotation encoder failure ........................................................... 125
7.4.14 Error 29 Error in sensor clocking signal ................................................. 125
7.4.15 Error 30 CPU firmware is corrupted ....................................................... 126
7.4.16 Error 31 CPU board’s memory test failed ..............................................126
7.4.17 Error 32 Movement of Z-motor failed ..................................................... 126
7.4.18 Error 33 Movement of C (collimator) motor failed .................................. 127
7.4.19 Error 34 Movement of D (sensor) motor failed....................................... 128
7.4.20 Error 35 Movement of J (chin support) motor failed............................... 129
7.4.21 Error 36 Movement of N motor failed ..................................................... 130
7.4.22 Error 37 Movement of R (Rotation) motor failed .................................... 131
7.4.23 Error 38 Movement of X motor failed ..................................................... 132
7.4.24 Error 39 Movement of Y motor failed ..................................................... 133
7.4.25 Error 40 Movement of S (secondary collimator) motor failed................. 134
7.4.26 Error 41 Movement of T (cephalostat sensor) motor failed.................... 135
7.4.27 Error 42 Ceph IO board is not functioning.............................................. 136
7.4.28 Error 43 Serial IO board error ................................................................ 137
7.4.29 Error 45 No X-rays detected on cephalostat sensor .............................. 137
7.4.30 Error 46 Emergency stop button is pressed........................................... 138
7.4.31 Error 47 Button stuck ............................................................................. 139
7.4.32 Error 48 Internal failure .......................................................................... 139
7.4.33 Error 49 Reading panoramic sensor data failed..................................... 140
7.4.34 Error 50 Reading cephalostat sensor data failed ................................... 141
7.4.35 Error 51 Cephalostat sensor missing ..................................................... 142
7.4.36 Error 52 Movement of L-motor failed .....................................................143
7.4.37 Error 100 Internal communication bus error........................................... 144
7.4.38 Error 101 Incorrect internal message format.......................................... 144
7.4.39 GUI Error 103 External GUI not connected............................................ 145
7.4.40 GUI Error 104 Failed to validate calibration data ................................... 145
7.5 Warnings and notifications ................................................................................ 146
7.6 Hardware trouble shooting ................................................................................ 152
7.6.1 Internal and external LANs .................................................................... 152
7.6.1.1 No Ethernet connection between the unit and the workstation 152
7.6.1.2 Connection between the workstation and
X-ray unit is lost from time to time ...........................................153
7.6.1.3 No connection to R3300, R3400 and GUI PC ........................ 153
7.6.1.4 No connection to the R3300 .................................................... 153
7.6.1.5 No connections to the R3400 .................................................. 154
7.6.2 Power distribution (mains) ..................................................................... 154
7.6.2.1 Nothing happens when the X-ray unit is switched on .............. 154
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved
iii

7.6.2.2 No X-rays................................................................................. 155
7.6.3 Power distribution (low voltages) .......................................................... 155
7.6.3.1 Nothing happens when the X-ray unit is switched on .............. 155
7.6.3.2 X-, Y- or rotator not working..................................................... 156
7.6.3.3 N-, Collimator- or sensor swap not working............................. 157
7.6.3.4 CEPH sensor or secondary collimator not working .................158
7.6.3.5 The panorama imaging not working ........................................ 158
7.6.3.6 The 3D imaging not working.................................................... 158
7.6.3.7 The CEPH imaging not working............................................... 159
7.6.3.8 R3210, R3300 or R3400 does not operate.............................. 159
7.6.4 3D imaging ............................................................................................159
7.6.4.1 3D sensor is not recognized .................................................... 159
7.6.4.2 Generally poor image quality................................................... 160
7.6.4.3 Horizontal bars in projection image ......................................... 160
7.6.4.4 Reduced gray scale................................................................. 160
7.6.4.5 Shadows on the projection image............................................ 160
7.6.5 Panorama imaging ................................................................................160
7.6.5.1 PAN sensor is not recognized ................................................. 160
7.6.5.2 Image is messy........................................................................161
7.6.5.3 Image is too dark or light ......................................................... 161
7.6.6 CEPH imaging ....................................................................................... 161
7.6.6.1 CEPH sensor is not recognized...............................................161
7.6.6.2 Image is messy........................................................................161
7.6.6.3 Image is too dark or light ......................................................... 161
7.6.7 Exposure control ...................................................................................161
7.6.7.1 Exposure does not start when the exposure switch is pressed161
7.6.7.2 Exposure sequence starts but no X-rays are generated .........163
7.6.8 X-ray generation .................................................................................... 164
7.6.8.1 No X-rays generated................................................................ 164
7.6.8.2 Images too dark or too light (Wrong dose generated) ............. 165
7.6.9 Motion control ........................................................................................167
7.6.10 Z-Motion control .................................................................................... 168
7.6.10.1 Main support up/down (Z) movement does not work............... 168
7.6.10.2 Main support movement only works in one direction............... 169
7.7 Calibration program numbers............................................................................ 171
7.8 Imaging program numbers ................................................................................ 172
8 Maintenance procedure .......................................................................................... 175
iv
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

1 Introduction
1.1 ORTHOPANTOMOGRAPH® OP300
INSTRUMENTARIUM DENTAL™
ORTHOPANTOMOGRAPH® OP300 x-ray unit (hereafter
called “OP300”) is a dental x-ray system for producing high
quality digital images of dentition, TM-joints and skull. In
order to take images with OP300 you need a suitable PC
hardware connected to the OP300 unit and CLINIVIEW™
software (or suitable third party software via TWAIN driver)
to capture and manage images.
OP300 performs the following procedures:
Panoramic
• Standard panoramic
• Pediatric panoramic
• Wide arch panoramic
• Bitewing
• TMJ, PA projection
• Ortho TMJ, axial corrected lateral projection
• Maxillary sinus
• Ortho Zone enhanced panoramic
• Orthogonal panoramic
Cephalometric (optional)
• Cephalometric lateral projection
• Cephalometric pediatric lateral projection
• Cephalometric postero-anterior (PA) projection
• Reverse Towne projection
• Waters view
• Carpus program (optional) (Not available in USA
and Canada)
210986 rev 2 Instrumentarium Dental 1
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

1 Introduction
3D SFOV (optional) H x W
• 61x41 mm Field of View
• 61x78 mm Field of View
3D MFOV (Maxio) (optional) H x W
• MFOV (Maxio) 50 x 50 mm Field of View
• MFOV (Maxio) 61 x 78 mm Field of View
• MFOV (Maxio) 78 x 78 mm Field of View
• MFOV (Maxio) 78 x 150 mm Field of View
• MFOV (Maxio) 130 x 150 mm Field of View
(optional)
1.2 References
The following instructions are delivered with in the
OP300 installation manual:
• Firmware update instructions
• Calibration instructions
• Cephalostat upgrade instructions
• Cephalostat side changing instructions
The following instructions are separate and can be ordered
from customer service:
• 3D upgrade instructions are delivered with the 3D
upgrade kit.
1.3 Intended use
OP300 must only be used and operated by dentist and
other qualified professionals. OP300 must only be used to
take panoramic, cephalometric and 3D images of the
dento-maxillofacial complex of the human skull. It must not
be used to take images of any other part of the human
body.
Panoramic and 3D exposures should not be used if
conventional intraoral radiographic images (bitewing
exposures) would be sufficient.
Cone beam computed tomography images are not
adequate for the analysis of soft tissue.
2 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

CAUTION! USA only: Federal law restricts this device to
sale by or on the order of a dentist or other qualified
professional.
1.4 Associated documentation
• OP300 user manual
• OP300 installation manual
• The CLINIVIEW™ software user manual
• The CLINIVIEW™ software installation manual
• The user manual supplied with the dental imaging
software
• The installation manual supplied with the dental
imaging software
• The user manual supplied with the 3D imaging
software
1 Introduction
• The installation manual supplied with the 3D
imaging software
1.5 Abbreviations used in this manual
FOV = Field Of View. The cylindrical 3D volume that is
reconstructed by the system.
ROI = Region Of Interest. The anatomical area or region of
the patient that you are interested to examine.
FH = Frankfort-Horizontal
H = Horizontal
ADC = Automatic Dose rate Control
1.6 Warnings and precautions
1.6.1 Warnings for cross infection
Always use available disposable protective covers with the
patient positioning accessories:
• Bite fork cover
• Chin support cover
• Head support cover
• Nose support cover
• Ear holder cover
210986 rev 2 Instrumentarium Dental 3
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

1 Introduction
1.6.2 Warnings to be observed during installation and service
Before attempting to service the device make sure that you
know how to operate it. Read the user’s manual.
Read and familiarize yourself with the warnings and
precautions listed in the user’s manual.
Only use original spare parts from the manufacturer when
repairing the device or replacing parts.
Warning - Radiation Safety
Before servicing the unit familiarize yourself with local and
national radiation safety standards and requirements
relating to dental x-ray equipment.
Warning - Electrical Safety
Disconnect the unit from the main power supply before
removing any covers.
Disconnect the unit from the main power supply before
repairing or replacing mechanical parts or installing
accessories.
Be careful when operating the unit not to get body parts or
clothing trapped between moving parts.
AES exposure settings shall be checked by the operator
before taking exposures.Disconnect the unit from the
mains power connection before servicing the unit, e.g.
replacing circuit boards or other electrical components.
If there are capacitors on a circuit board or electrical device
wait ten (10) minutes, after disconnecting the unit from the
power supply, before handling the board or device.
If you have to leave the unit unattended with covers
removed during servicing or maintenance, disconnect the
unit from main power supply so that anyone who
inadvertantly touches the unit does not receive an electric
shock.
This unit should only be used in areas that are provided
with a protective earth connection to ensure an
equipotential ground connection.
4 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

1 Introduction
Warning - Explosion hazard
Some disinfectants and cleaning agents may vaporize to
form an explosive vapour. If such disinfectants and
cleaning agents are used the vapour should be allowed to
disperse before switching the unit on.
Warning - Cleaning the unit
Switch the unit off and disconnect it from the main power
supply before cleaning or disinfecting the unit.
The aperture plate and the tube housing are made of lead
(Pb), which is a toxic material. Do not touch it with your
bare hands.
The installer must ensure that:
• The fixing screws are suitable for the wall material.
• The wall for fixing the unit is strong enough for attaching the unit. It must withstand loads of 5000N
or more.
• Pull out strength of the screws is 5000N or more.
• The wall fixing screws are adequately tightened.
To avoid the unit from tipping over, fix the unit with floor
bolts appropriate to the surface the unit is mounted on. The
bolts and the floor material must endure force of 5000 N.
The installer must ensure that the upper shelf attachment
screws are tightened.
The unit should be installed in a place with enough space
for safe operation. See the unit installation manual for
recommended minimum site dimensions. It is the
responsibility of the customer to ensure that the site is
large enough for the patients.
Be aware of hot surfaces when removing covers during
installation and maintenance.
When installing a dental X-ray unit always observe local
and national safety, radiation control and electrical
regulations.
1.6.2.1 Cautions for Electrostatic discharge
Electrostatic Discharge (ESD) can damage or destroy
electronic components.
210986 rev 2 Instrumentarium Dental 5
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

1 Introduction
When servicing the device take precautions to avoid
electrostatic build up and discharge (ESD). Follow the
recommendations for the prevention of ESD that are used
in the country in which you are working. If no
recommendations are available follow the guide lines
below.
Leave all new or replacement circuit boards and electrical
parts in their protective packaging until the boards are
needed.
Before handling circuit boards and electrical parts make
sure that any static electricity charge that has built up in
you body is discharged.
When handling circuit boards hold them by their edges and
do not touch any connectors or components.
When examining and checking circuit boards use an
elasticated wrist wrap which is connected to a ground point
through a 1 Mohm current limiting cable. For a ground
point use water pipes, radiators or other objects that are
known to be connected to the ground. Also use a cable to
connect the x-ray unit to the same ground potential as the
wrist wrap.
If an antistatic mat is used, connect the wrist wrap to the
mat and the mat to the ground potential.
Wash the wrist wrap and check that it is in good condition
frequently.
1.6.3 General warnings
Personnel operating the device must be adequately trained
with respect to the technological principles of operation
and radiation protection when using cone beam computed
tomography (CBCT) imaging.
This unit complies with the EMC (Electromagnetic
Compatibility) according to IEC 60601-1-2. Radio
transmitting equipment, cellular phones etc. shall not be
used in close proximity of the unit as they could influence
the performance of the unit.
6 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

1 Introduction
The correct software and settings in the workstation are
essential to the performance of the unit. Consult technical
support to ensure correct setup.
Danger: Explosion hazard - do not use in the presence of
flammable anesthetics, gases or vapors.
The unit is factory set to operate using a 230-240 ±10 VAC
power supply. Never connect the unit to a power supply
different to the voltage marked on the unit.
The site must fulfill the environmental requirements in the
installation manual chapter technical specifications.There
should be free space around the unit for safe operation.To
maintain patient safety it is mandatory to use an
unshielded CAT6 Ethernet cable between the unit and the
network or workstation, so that multiple chassis are not
connected. Non-medical grade PC should not be used in
patient environment.This product itself complies with IEC
60601-1 medical safety standard but in order to the system
incorporating also a PC to comply the standard, EITHER
the PC has to be a medical PC OR the PC has to be
located over 1,5 meters apart from the unit. The installer
and the user of the system shall confirm that at least one of
the above requirements is fulfilled. A PC is a medical one if
it complies IEC 60601-1 standard and that is indicated in
the accompanying documents of the PC. See chapter
Technical specifications, Minimum PC Requirements, in
user manual.
All service operations must be made by authorized service
personnel only.
The annual service as described in manual is mandatory
for the correct and safe operation of the unit.When taking
exposures, operators and service personnel must protect
themselves from radiation and remain at least two meters
(six feet) away from the unit during exposure. Protect the
patient from scattered radiation by placing a protective lead
apron over the patient.The unit must be installed and
serviced according to the unit Installation & adjustments
manual by a qualified technician.Only personnel trained
and approved by the manufacturer of the unit are allowed
to service the unit.
3D should not be used for routine or screening
examinations in which a radiograph is taken regardless of
the presence or absence of clinical signs and symptoms.
3D imaging examinations must be justified for each patient
to demonstrate that the benefits outweigh the risks.
210986 rev 2 Instrumentarium Dental 7
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

1 Introduction
Where it is likely that evaluation of soft tissues will be
required as part of the patient’s radiological assessment,
the imaging should be done using conventional medical CT
or MR, rather than 3D imaging using Cone Beam
technology.
Make sure that patient’s thyroid glands are protected by a
lead apron during the exposure.
The place where the unit is to be installed and the position
from where the user will take exposures must be correctly
shielded from the radiation that is generated when the unit
is operated. Ensure to fulfill or exceed the requirements of
your local regulations.
The unit or its parts must not be changed or modified in
any way without approval and instructions from the
manufacturer.
When servicing use only approved replacement parts
supplied by the manufacturer.
The use of accessories not complying with the equivalent
safety requirements of this equipment may lead to a
reduced level of safety of the resulting system.
If this device is used with 3rd party imaging application
software not supplied by the manufacturer, the 3rd party
imaging application software must comply with all local
laws on patient information software. This includes the
Medical Device Directive 93/42/EEC and/or relevant legal
requirements in the USA.
Do not connect any equipment to the unit that has not been
supplied with the unit or that is not recommended by the
manufacturer. The use of accessory equipment not
complying with the equivalent safety requirements of this
equipment may lead to a reduced level of safety of the
resulting system.
All protective covers must be properly installed before
handing unit to the user or when operating the unit.
Correct sharp layer should be chosen when using
multilayer PAN images. See user manual chapter
Multilayer PAN images for correct procedure.
8 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

1.7 Unauthorized Modifications
Unauthorized changes or modifications to any part of the
unit or its equipment can have hazardous consequences.
Changes or modifications must not be made unless
specifically authorized by the manufacturer. When properly
assembled with a compatible beam limiting device, the
diagnostic source assembly will fully meet the United
States of America Federal Performance Standards for
Diagnostic X-Ray Systems and Their Components (21
CFR 1020. 30-32) provided. No parts may be removed
from the unit and no unauthorized adjustments are made to
the beam limiting device or tube housing assembly.
Never remove or remanufacture any part of the tubehead
assembly or beam limiting device. Never adjust any part of
the beam limiting device unless under the direction of the
manufacturer or their authorized distributor.
1 Introduction
1.8 Disclaimer
The manufacturer shall have no liability for consequential
damages, personal injury, loss, damage or expense
directly or indirectly arising from the use of its products. No
agent, distributor or other party is authorized to make any
warranty or other liability on behalf of the manufacturer with
respect to its products.
1.9 Disposal
The device, its spare parts, its replacement parts and its
accessories may include parts that are made of or include
materials that are non-environmentally friendly or
hazardous. These parts must be disposed of in
accordance with all local, national and international
regulations regarding the disposal of non-environmentally
friendly or hazardous materials.
Unit has at least the following parts that should be
regarded as non-environmental friendly waste products:
■ Tubehead (Pb, oil)
■ Collimator (Pb)
■ All electronic circuits, electronic boards inside
■ Sensor covers (EMC painted)
210986 rev 2 Instrumentarium Dental 9
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

1 Introduction
10 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved
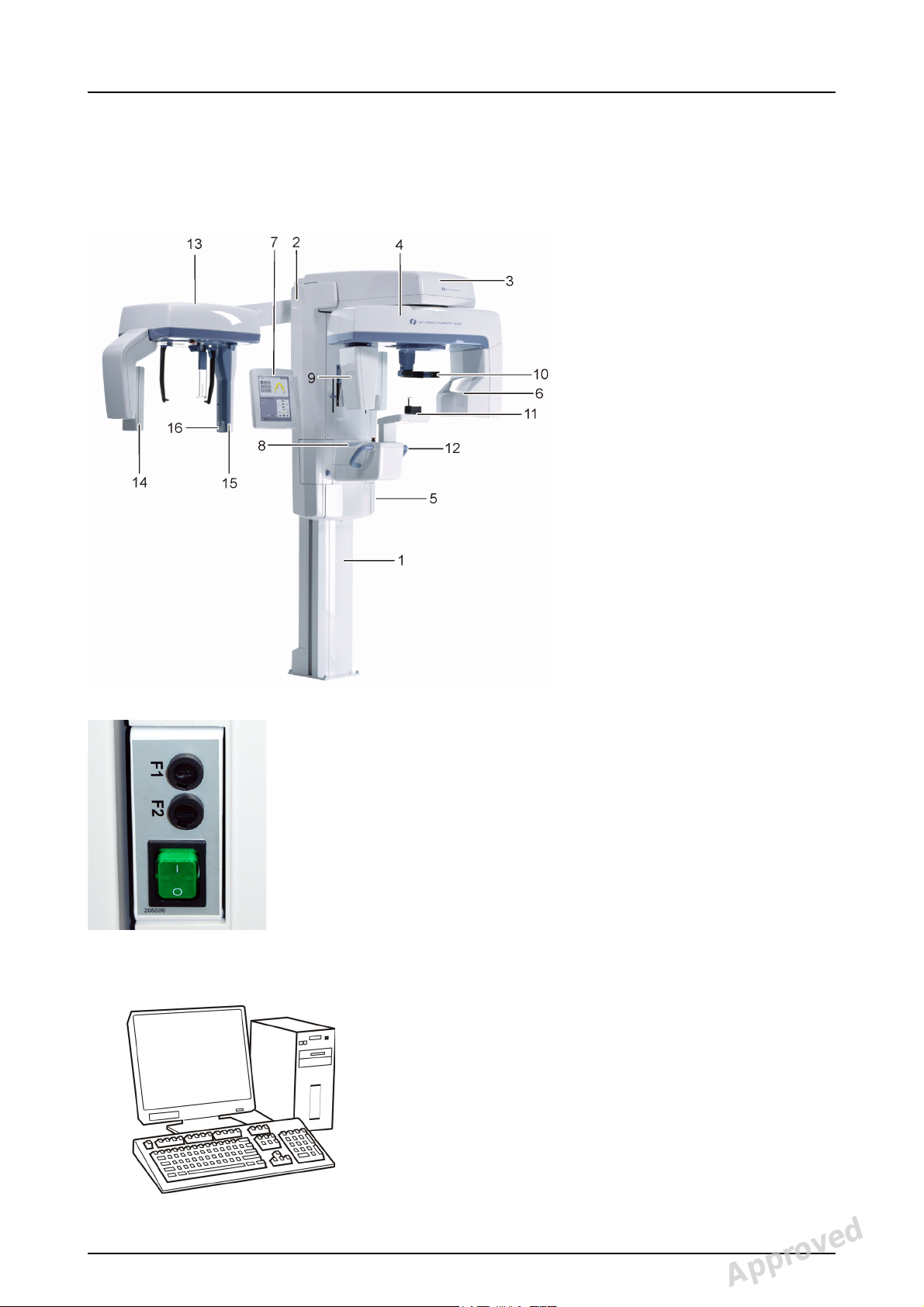
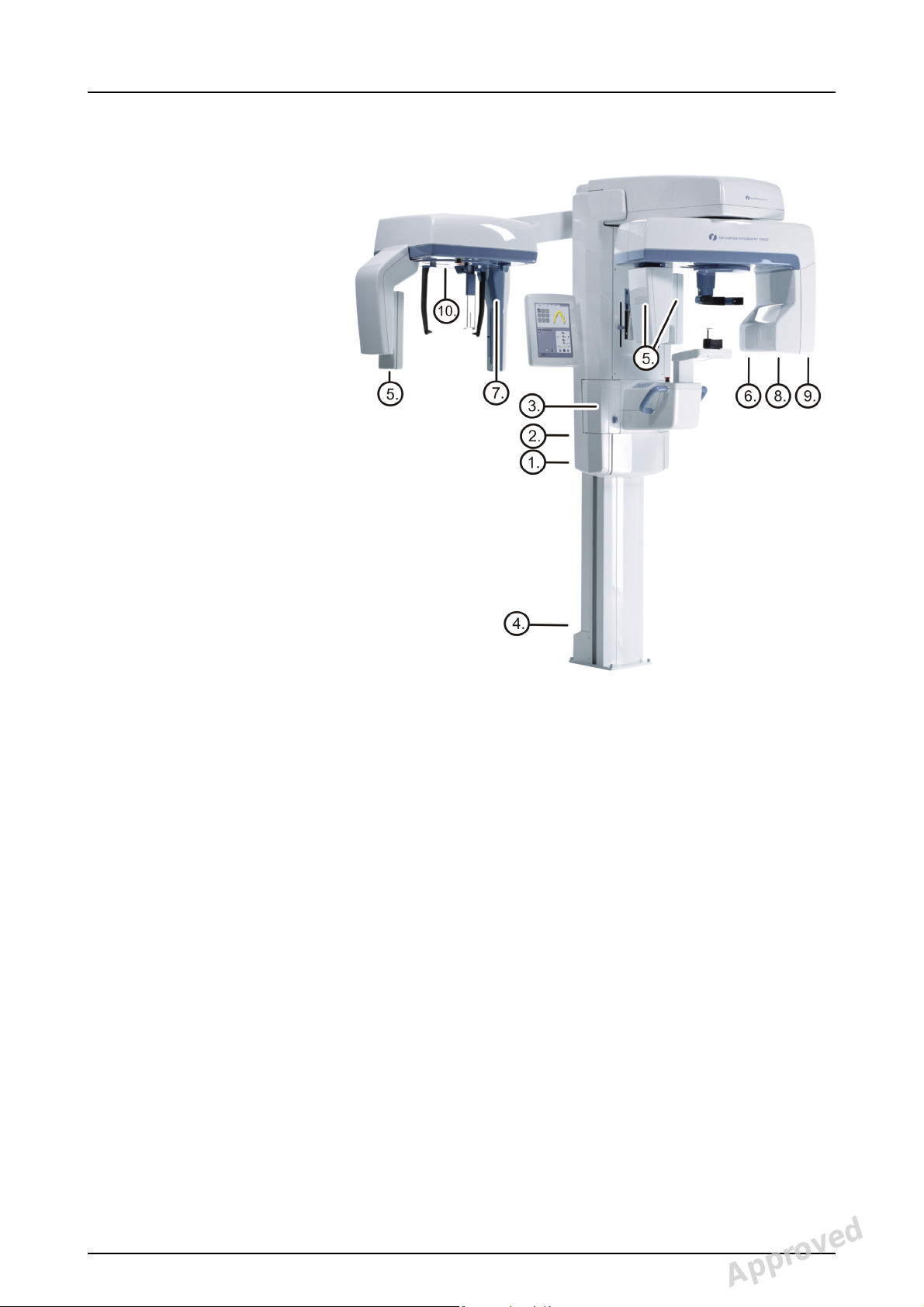
2 Unit description
1. Column
2. Carriage
3. Main support
4. Rotating unit
5. On/off switch (rear of carriage)
and main fuses
6. Tubehead assembly
7. Touch screen display
8. Positioning panel
9. Sensor head
10.Head support
11.Chin rest
12.Handles
13.Cephalostat unit
14.Cephalostat sensor
15.Secondary collimator
16.Positioning panel
Fig 2.1. On/off switch and main fuses
2.1 Main parts and controls
PC with MDD approved dental imaging software and 3D
viewing software (not included).
All software must conform to the MDD and the relevant
legal requirements in the USA.
The PC must conform to all the unit and dental imaging
software requirements.
210986 rev 2 Instrumentarium Dental 11
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

2 Unit description
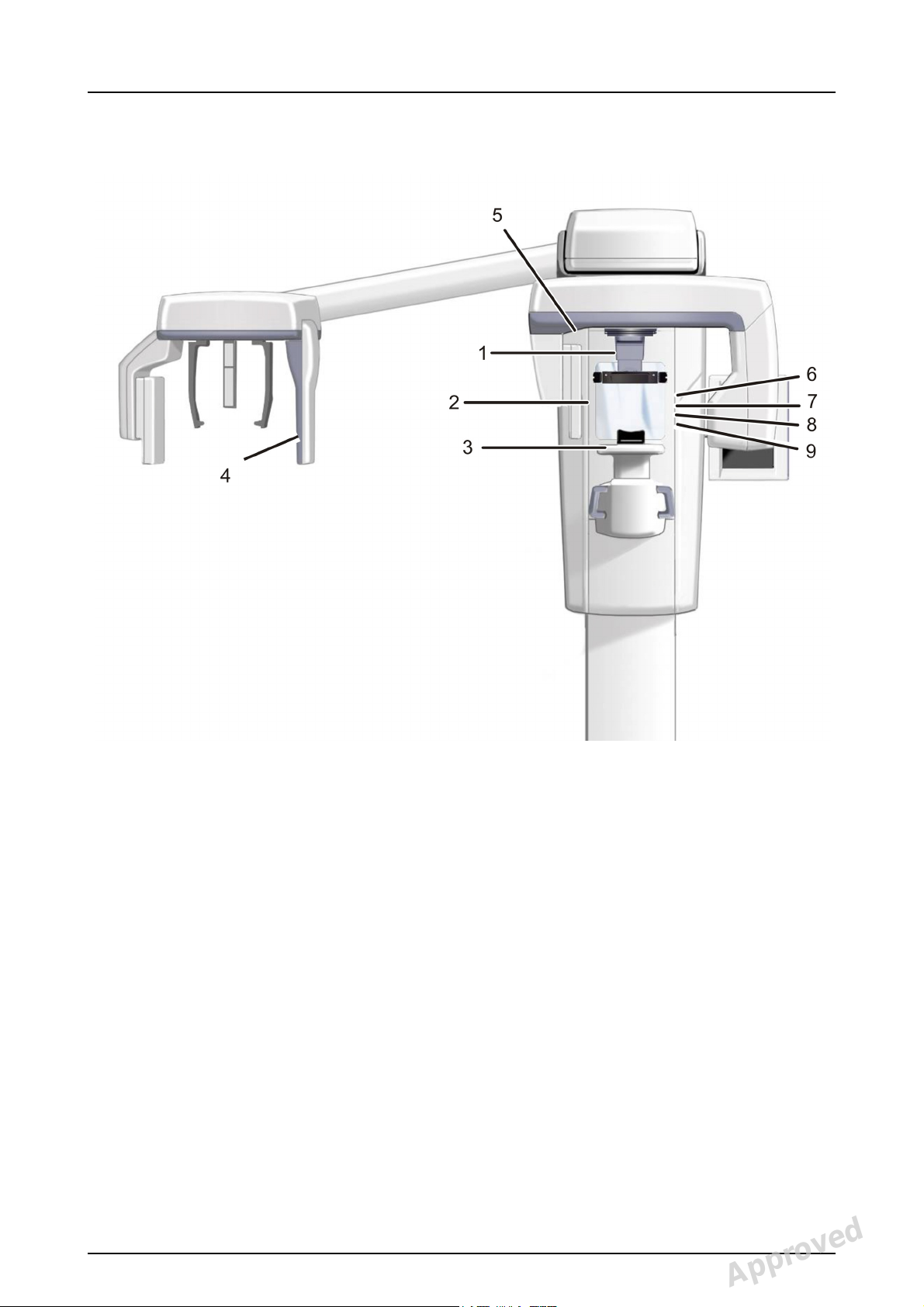
1. Sensor holder (units without 3D option)
2. Panoramic sensor
1. 3D sensor (units with 3D option)
2. Panoramic sensor
12 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved
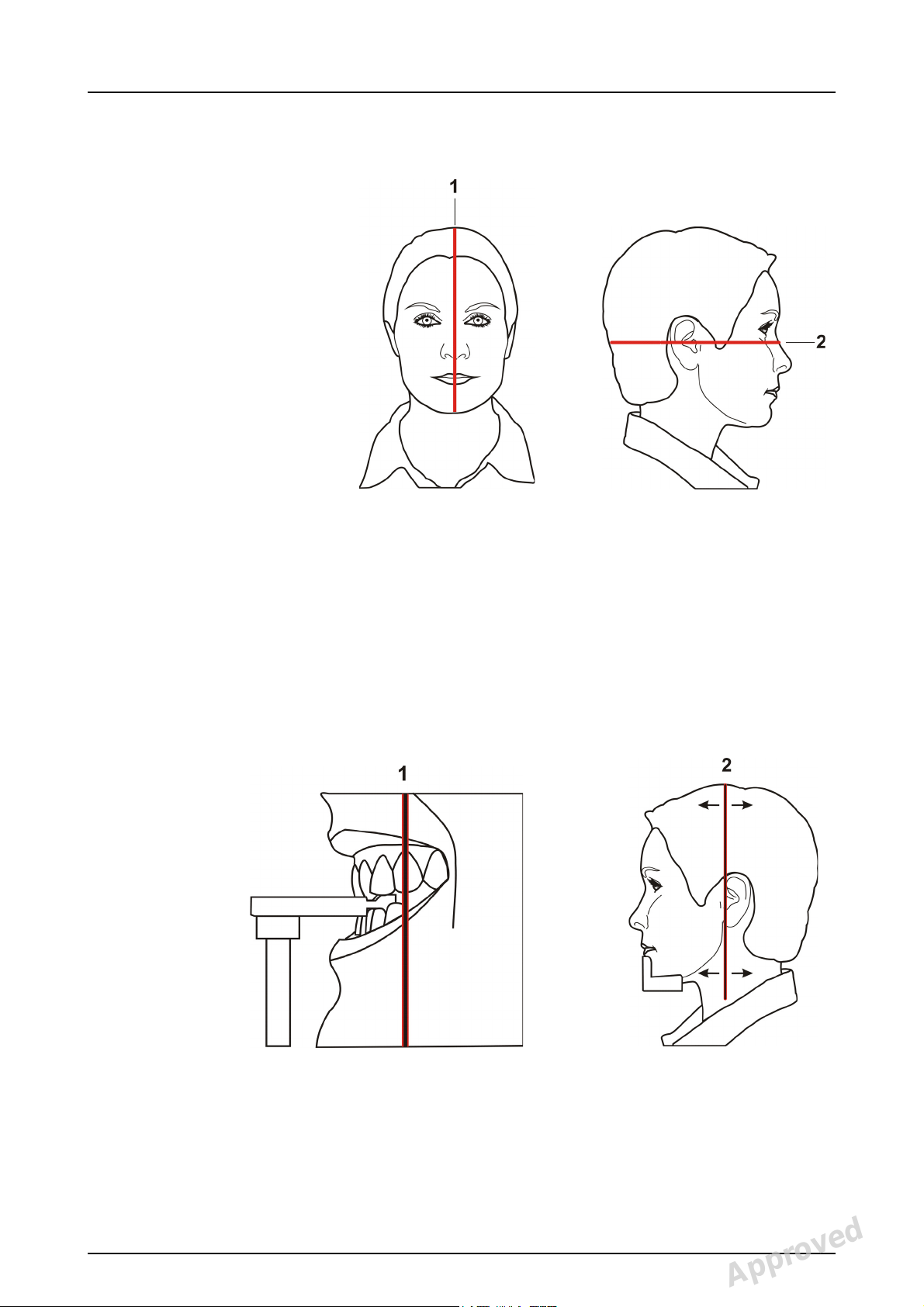
2.2 Patient positioning lights
2 Unit description
1. Midsagittal light
2. Frankfort horizontal (FH) light /
Horizontal light, top of 130 mm high FOV (3D MFOV
(Maxio) option only)
3. Image layer light
4. Cephalometric FH light
5. TMJ light
6. Horizontal light, top of 78 mm high FOV
(3D MFOV (Maxio) option only)
7. Horizontal light, top of 61 mm high FOV
(3D option only)
8. Horizontal light, top of 50 mm FOV
(3D MFOV (Maxio) option only)
9. Horizontal light, bottom of FOV (3D option only)
210986 rev 2 Instrumentarium Dental 13
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved
2 Unit description
Fig 2.1.
Fig 2.2.
Panoramic lights
1. Midsagittal light
2. FH light
1. Image layer light
2. TMJ light
14 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

Cephalometric lights
Fig 2.3.
Fig 2.4.
1. FH light
2 Unit description
3D lights (optional)
Note! Appropriate lasers are turned automatically on
based on selected FOV.
1. Midsagittal light
2. Horizontal light, top of FOV
Note! With 3D MFOV (Maxio) option height 130 mm is
indicated with Frankfort horizontal (FH) light. Move FH
light to 130 mm position.
3. Horizontal light, bottom of FOV
210986 rev 2 Instrumentarium Dental 15
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved
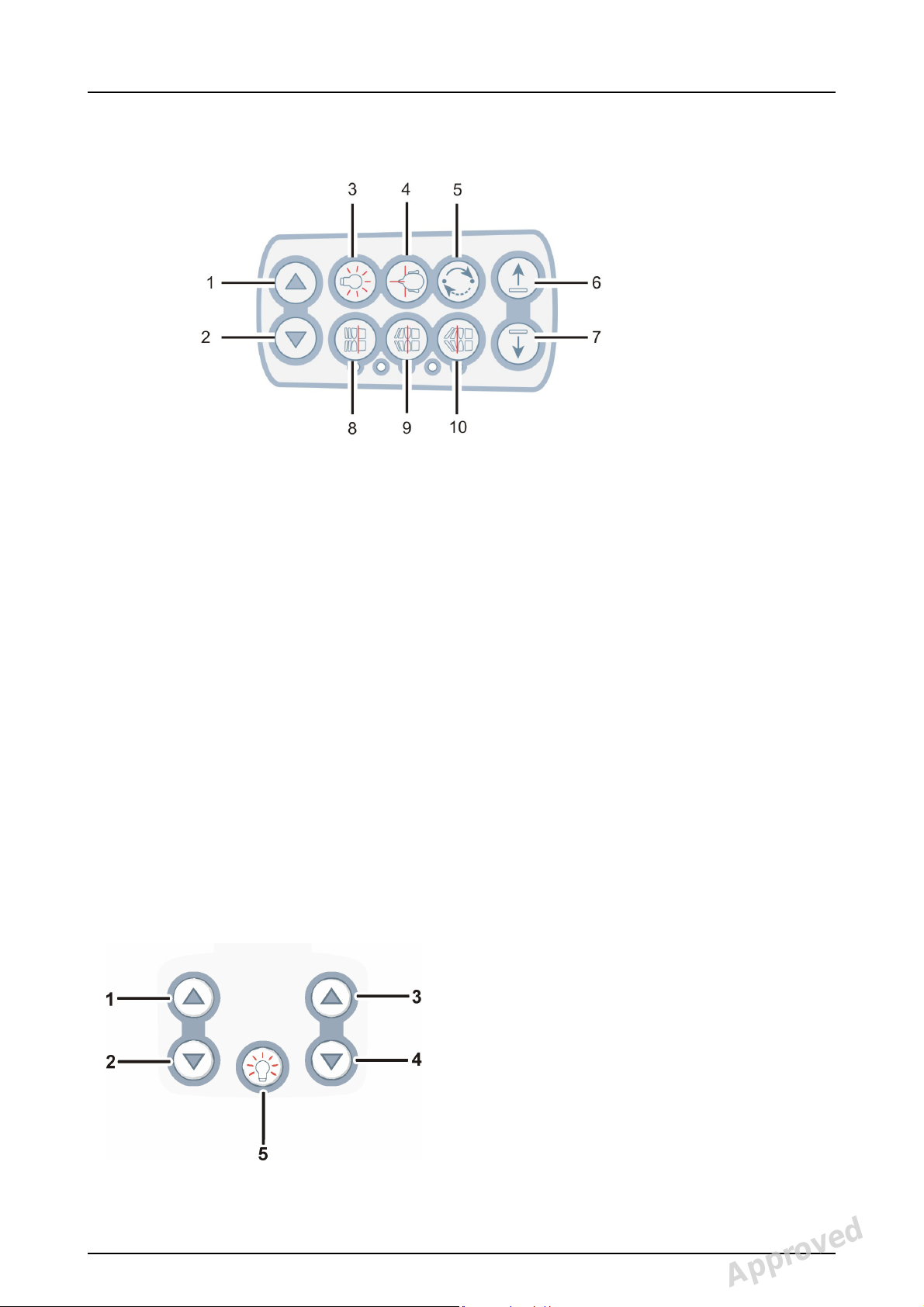
2 Unit description
2.3 Patient positioning panel
1. Carriage UP
2. Carriage DOWN
3. Positioning lights ON/OFF
4. Patient positioning
5. Start positioning
6. Chin support UP
7. Chin support DOWN
8. Move the image layer anterior before exposure 3 mm,
with sinus program 10 mm
9. Normal occlusion/ reset position
10.Move the image layer posterior before exposure 3 mm,
with sinus program 10 mm
2.3.1 Cephalometric unit (optional)
1. Carriage UP
2. Carriage DOWN
16 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
3. Carriage UP
4. Carriage DOWN
5. Positioning lights ON/OFF
Approved
D511961, 2
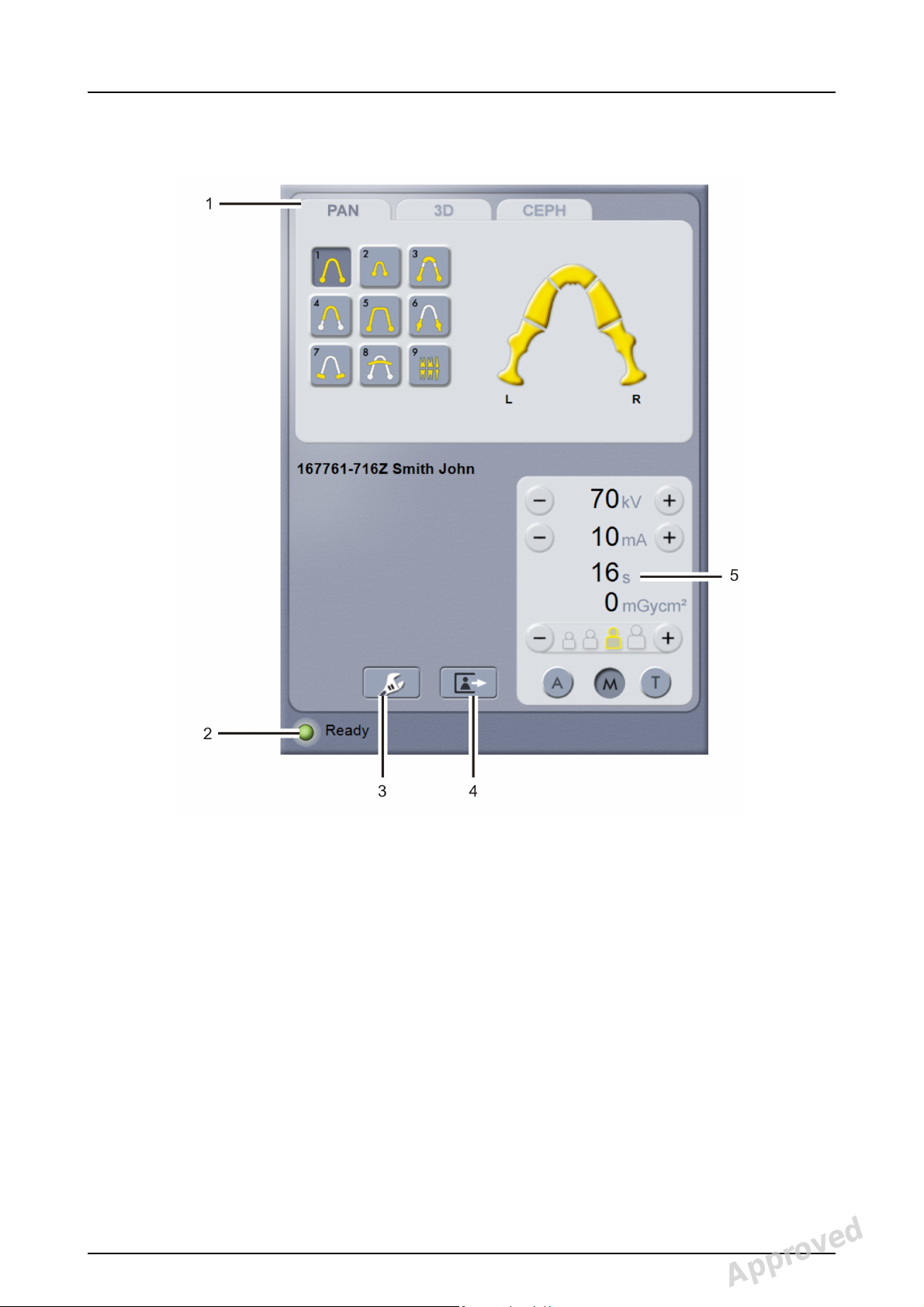
2.4 Main control panel
2 Unit description
1. Modality / imaging program section
2. Status of the unit
3. Settings
4. End examination
5. Exposure settings
210986 rev 2 Instrumentarium Dental 17
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved
2 Unit description
Fig 2.6.
2.5 Unit identification labels
1. Main label
2. 10A & 15A Fuse labels (next to the fuse holder)
3. Laser class 1 warning label IEC 60825-1:2007
4. Ethernet label
5. Sensors
6. (Primary) collimator label
7. (Secondary) cephalostat collimator label
8. Tubehead label
(on the tubehead and on the tubehead cover)
9. Warning label for deadly voltages
(inside the tubehead cover)
10.Cephalostat main label
18 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved
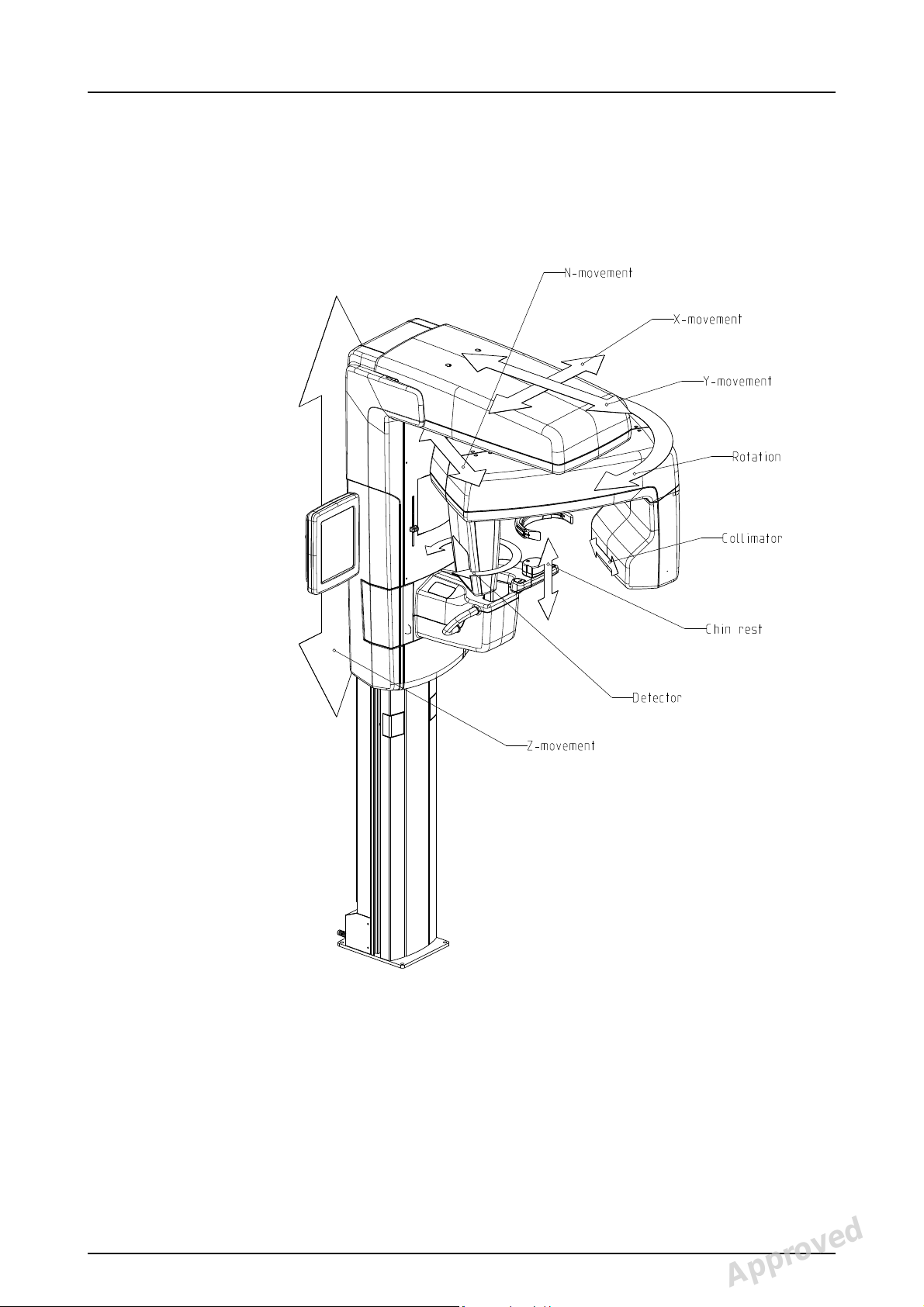
2.6 Unit movements
(R)
(C)
(J)
(D)
Fig 2.7.
Panoramic unit movements
2 Unit description
210986 rev 2 Instrumentarium Dental 19
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved
2 Unit description
Fig 2.8.
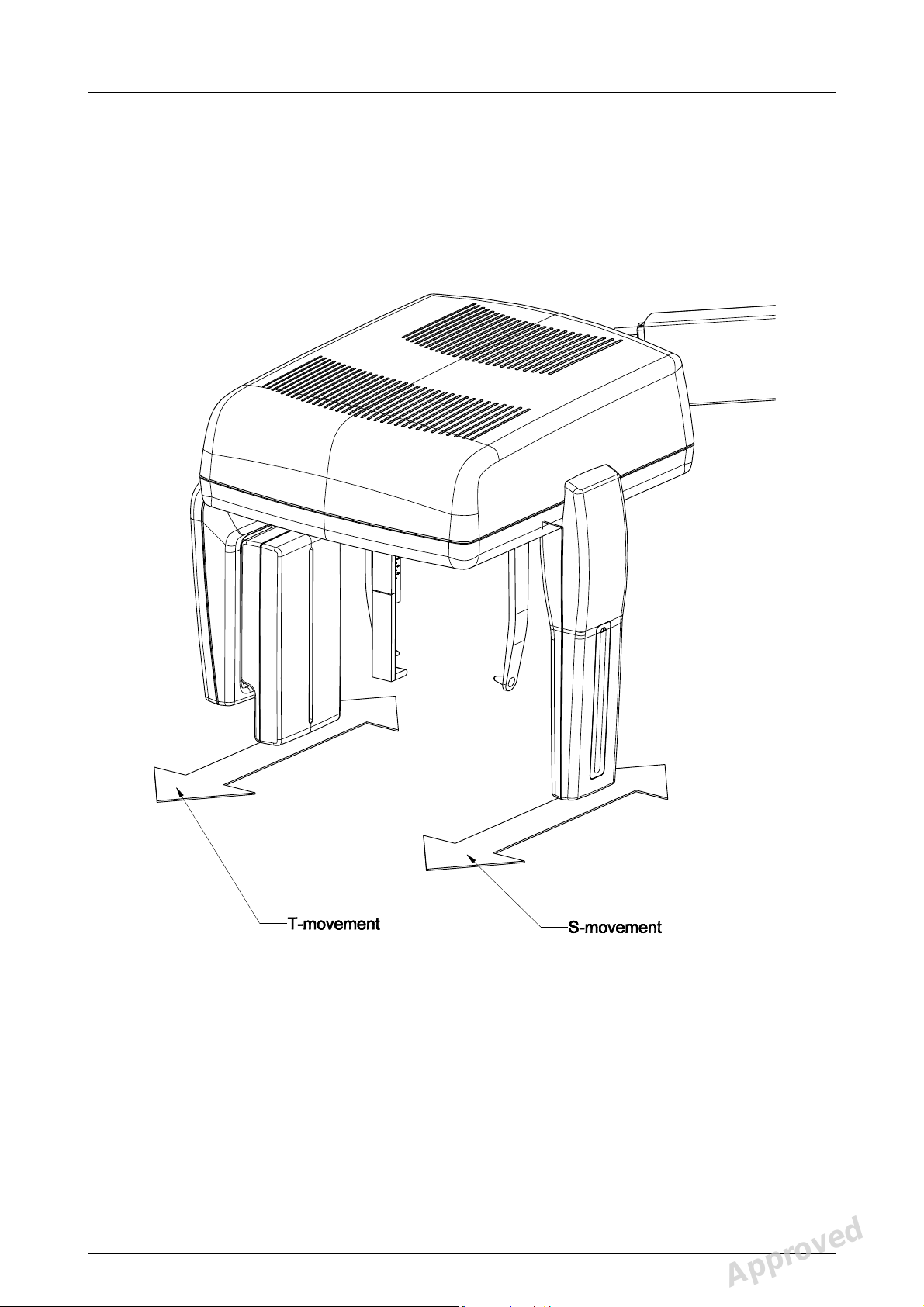
Cephalometric unit movements
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
20 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
D511961, 2
Approved
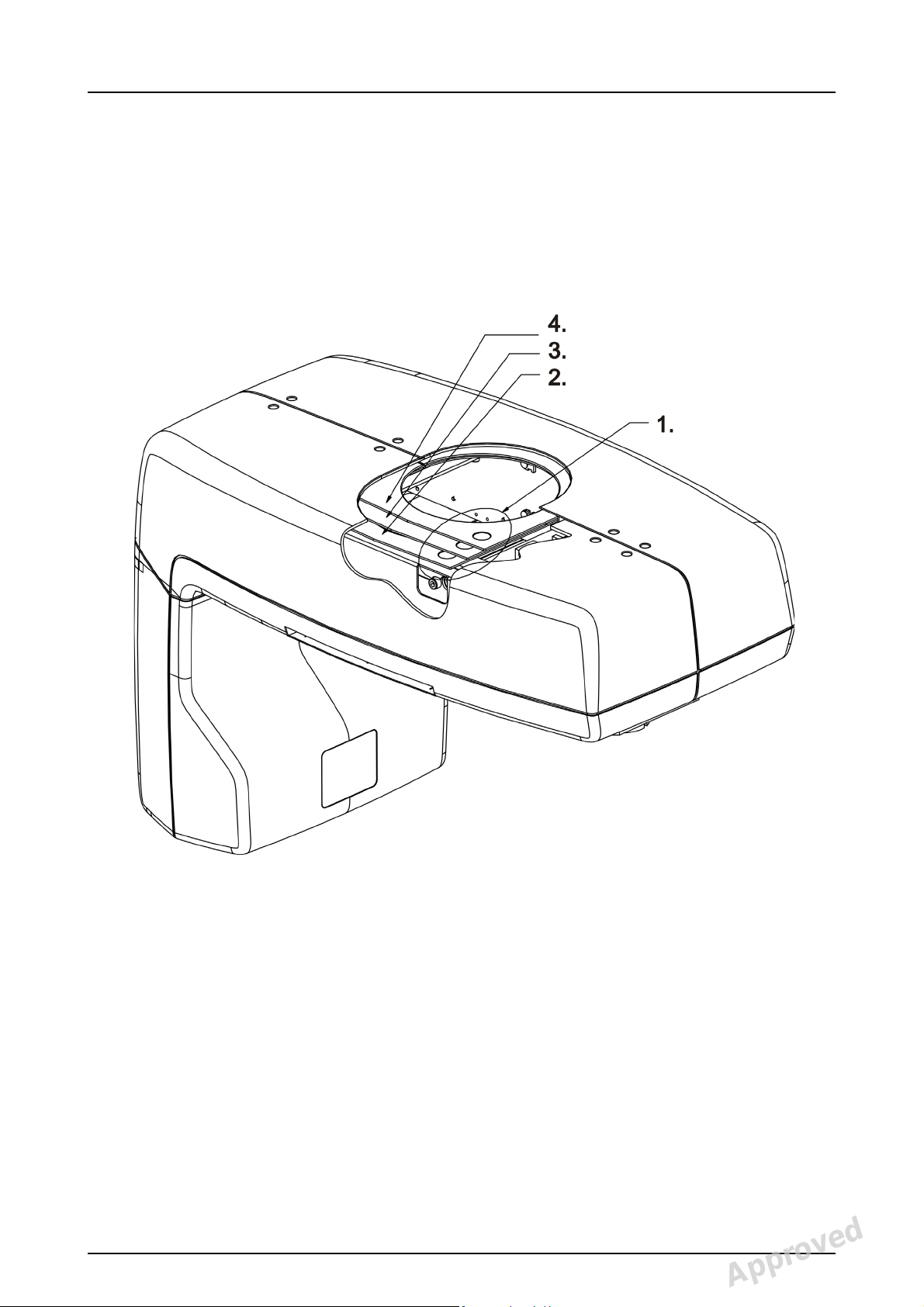
2.7 Finger guards
If finger guards are installed incorrectly during the service,
unit may get stuck in some extreme positions in
N-movements.
Correct installing of finger guards:
2 Unit description
1. These marks should be in this corner facing up.
2. part number 204373 / SP00374
3. part number 204374 / SP00374
4. part number 204375 / SP00374
210986 rev 2 Instrumentarium Dental 21
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved

2 Unit description
Fig 2.9.
Fig 2.10.
2.8 Emergency stop switch
In case of malfunction of the exposure button or other
protective devices of the unit, an emergency stop switch is
provided near the handles and on the roof of the
cephalostat head so that the patient can reach it.
If the emergency stop switch is pressed during an
exposure, the exposure is terminated immediately and the
x-ray unit is completely stopped. An interrupted exposure
cannot be continued later, but has to be retaken from the
beginning.
Press to stop the unit, rotate to release.
22 Instrumentarium Dental 210986 rev 2
Reviewed: Linteri Päivi 2014-07-11 13:33
Approved: Ristisuo Hanna 2014-07-14 13:37
See PDM system to determine the status of this document. Printed out: 2014-08-25 16:05:07
Copyright © 2014 by PaloDEx Group Oy. All rights reserved.
D511961, 2
Approved
 Loading...
Loading...