IDEMIA Identity and Security France MPHAC005A User Manual

MorphoAccess® VP MD
Installation Guide
COPYRIGHT© 2018 IDEMIA
Osny, France
MorphoAccess® VP MD – Installation Guide December 2018
2019_200000XXXX-V0

MorphoAccess® VP MD – Installation Guide
Warning
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
2
December 2018
Warning
COPYRIGHT© 2018 IDEMIA. All rights reserved.
Information in this document is subject to change without notice and do not represent
a commitment on the part of IDEMIA.
This document contains information of a proprietary nature to IDEMIA and is submitted
in confidence for a specific purpose. The recipient assumes custody and control and
agrees that this document will not be copied or reproduced in whole or in part, nor its
contents revealed in any manner or to any person except to meet the purpose for
which it was delivered.
This caveat is applicable to all the pages of this document.
This manual makes reference to names and products that are trademarks of their
respective owners.

MorphoAccess® VP MD – Installation Guide
Revision History
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
3
December 2018
Version
Date
Reference
Description
01
April 2019
2019_000000XXXX-V0
Document creation
Revision History
The table below contains the history of changes made to the present document.

MorphoAccess® VP MD – Installation Guide
Table of Content
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
4
December 2018
Table of Content
Warning ........................................................................................................................................ 2
Revision History ............................................................................................................................ 3
Section 1 : Introduction ................................................................................................................ 9
MorphoAccess® VP MD terminals ........................................................................................... 10
Scope of the document ............................................................................................................ 11
Safety Instructions ................................................................................................................... 12
Wiring Recommendations ................................................................................................... 13
Regulatory, safety and Environmental notices ....................................................................... 14
European Union (CE) regulatory notices ............................................................................. 14
USA (FCC) regulatory notices ............................................................................................... 15
Canada (IC) regulatory notices ............................................................................................ 16
Others recommendations .................................................................................................... 17
Recommendations for terminal implementation .................................................................... 18
Section 2 : General Description .................................................................................................. 21
Box opening ............................................................................................................................. 22
Components of the initial package ......................................................................................... 23
Terminal's front view description ............................................................................................ 24
Terminal's rear view description ............................................................................................. 25
MorphoAccess® VP MD Technical Characteristics .................................................................. 26
Section 3 : Installation Procedure ............................................................................................... 28
Before proceeding to the installation ..................................................................................... 29
Installation .............................................................................................................................. 30
Required tools (not supplied)............................................................................................... 30
Equipment from the initial package to use ......................................................................... 30
Step by step procedure ........................................................................................................ 31
Section 4 : Electrical Interface .................................................................................................... 35
Wiring overview ...................................................................................................................... 36
Power Supply ........................................................................................................................... 37
External Power supply ......................................................................................................... 37
POE (Power Over Ethernet) ................................................................................................. 37
Output Relay ........................................................................................................................... 38
Nominal characteristics of relay .......................................................................................... 38
Example of connection for electrical door locks .................................................................. 38
Tamper Switch ......................................................................................................................... 39

MorphoAccess® VP MD – Installation Guide
Table of Content
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
5
December 2018
Operating principle for the switch ....................................................................................... 39
Nominal characteristics of switch block .............................................................................. 39
Wiegand port wiring ............................................................................................................... 40
Wiegand input wiring .......................................................................................................... 40
Wiegand output wiring ....................................................................................................... 41
The controller supports neither LED1 nor LED2 signals ....................................................... 42
The controller supports only LED1 signal ............................................................................ 42
The controller supports LED1 and LED2 signals .................................................................. 42
Data Clock Input .................................................................................................................. 43
Data Clock Output ............................................................................................................... 43
RS-485 port wiring ................................................................................................................... 44
GPIO wiring ............................................................................................................................. 45
Single Door Access Control (SDAC) implementation ............................................................... 46
Ethernet connection ................................................................................................................ 47
Default Ethernet configuration ........................................................................................... 47
Recommendations for RJ45 wiring ...................................................................................... 48
External USB connection ......................................................................................................... 49
Wi-Fi™ dongle installation ...................................................................................................... 50
Section 5 : User Interface ........................................................................................................... 51
Modes for controlling access rights ........................................................................................ 52
Introduction ......................................................................................................................... 52
Identification mode ............................................................................................................. 52
Authentication (verification) mode ..................................................................................... 52
Multi-factor mode ............................................................................................................... 53
Proxy mode .......................................................................................................................... 53
External database mode (also called polling mode) ........................................................... 53
Configuring the terminal ......................................................................................................... 55
These Anti-tamper / anti-pulling switches .............................................................................. 56
Section 6 : Accessories, Software Licenses and PC Applications ................................................ 57
Compatible Accessories, Licenses and Software ..................................................................... 58
Compatible PC applications .................................................................................................... 59
Section 7 : Recommendations .................................................................................................... 60
Global warning .................................................................................................................... 61
General precautions ............................................................................................................ 61
Areas containing combustibles ............................................................................................ 61
Specific precautions for terminals equipped with a contactless smartcard reader ............ 61

MorphoAccess® VP MD – Installation Guide
Table of Content
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
6
December 2018
Ethernet connection ............................................................................................................ 61
Date / Time synchronization ............................................................................................... 61
Cleaning precautions ........................................................................................................... 62
Main principles ........................................................................................................................ 64
Most useful areas for biometric data...................................................................................... 65
Position of finger ..................................................................................................................... 66
Correct position ................................................................................................................... 66
Bad finger position .............................................................................................................. 67
Troubleshooting ...................................................................................................................... 68
How to get the latest versions of documents ......................................................................... 70
Documents concerning the MorphoAccess® terminal ............................................................ 71
Troubleshooting ...................................................................................................................... 73
The terminal IP address is unknown or it is not possible to connect to the terminal.......... 73
The sensor is switched off .................................................................................................... 73
The terminal returns erratic responses to Ping commands ................................................ 73
Technical Support and Hotline ................................................................................................ 74
North America ..................................................................................................................... 74
South America ..................................................................................................................... 74
South Africa ......................................................................................................................... 74
India: .................................................................................................................................... 74
Europe and rest of the world: .............................................................................................. 74
Web site ............................................................................................................................... 74

MorphoAccess® VP MD – Installation Guide
Table of Content
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
7
December 2018
Annex 1 : Finger Placement Recommendations ...................................................................... 63
Annex 2 : Bibliography ............................................................................................................. 69
Annex 3 : Support .................................................................................................................... 72

MorphoAccess® VP MD – Installation Guide
Table of Figures
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
8
December 2018
Table of Figures
Figure 1: Implementation Recommandations ............................................................................. 19
Figure 2: Box Opening .................................................................................................................. 22
Figure 3: Box Content .................................................................................................................. 23
Figure 4: MorphoAccess® VP MD terminal front view ................................................................ 24
Figure 5: MorphoAccess® VP MD terminal rear view ................................................................. 25
Figure 6: Removing the top cover ............................................................................................... 29
Figure 7: Drilling template ........................................................................................................... 32
Figure 8: Wall plate fixing ............................................................................................................ 33
Figure 9: Terminal’s Body fixation ............................................................................................... 33
Figure 10: Assembling top and bottom covers ............................................................................ 34
Figure 11: Cabling layout ............................................................................................................. 36
Figure 12: Power supply wiring ................................................................................................... 37
Figure 13: Output relay wiring ..................................................................................................... 38
Figure 14: Example of electric door strike connection ................................................................ 38
Figure 15: Tamper switch wiring ................................................................................................. 39
Figure 16: Wiegand input wiring ................................................................................................. 40
Figure 17: Wiegand output wiring ............................................................................................... 41
Figure 18: Wiegand port wiring – DataClock ............................................................................... 43
Figure 19: Wiegand port wiring – Data Clock .............................................................................. 43
Figure 20: RS-485 port wiring – RS485 ........................................................................................ 44
Figure 21: GPIO wiring ................................................................................................................. 45
Figure 22: SDAC wiring................................................................................................................. 46
Figure 23: Direct Point to Point Ethernet Connection ................................................................. 48
Figure 24: RJ45 wiring .................................................................................................................. 48
Figure 25: External USB connection ............................................................................................ 49
Figure 26: Wi-Fi™ dongle installation .......................................................................................... 50
Figure 27: Most useful areas for biometric data ......................................................................... 65
Figure 28: Recommended finger positions .................................................................................. 66
Figure 29: Incorrect finger positions ........................................................................................... 67

MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
9
December 2018
Section 1 : Introduction

MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
10
December 2018
MorphoAccess® VP MD terminals
Congratulations for selecting the MorphoAccess® VP MD, first ever Physical Access
Control terminals to integrate the state of the art multimodal technology combining
finger vein and fingerprint biometrics.
This terminal brings to access control systems the strong assets of the finger
vein/fingerprint multimodality:
the capability to address those individuals who usually experience difficulties to use
mono-modal biometric devices,
an excellent FRR@FAR ratio, which allows a high security level without affecting
comfort of use,
an enhanced resistance to spoofing (by combining the protection mechanisms
intrinsic to each technology and also by making the most of the new characteristics
resulting from the fusion of the two biometrics),
while offering the same easiness of use which makes finger biometrics-based systems
quickly adopted by end-users.
In addition, the MorphoAccess® VP MD offers the following advantages:
high quality optical sensor (IQS quality sensor),
supports multiple input/output interfaces used in the physical access control
industry,
Local Area Network interface for easy interaction with other host systems ; LAN and
WLAN possibilities (Wi-Fi™ as an option),
practicality at installation and connection, as illustrated by this installation guide.
We definitely believe that our MorphoAccess® VP MD will come up to the expectations
of our faithful and most demanding partners, as the ultimate solution for Security,
Accuracy and Performance of their equipments!
To ensure the most effective use of your MorphoAccess® VP MD terminal, we
recommend that you read this Installation Guide completely and attentively.
MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
11
December 2018
MA VP DM
Marketing Name
Multimodal
finger vein/ fingerprint
biometrics
MIFARE®/DESFire®
Contactless
smartcard readert
Water
Resistant
Regulatory
Model Number
(*)
MorphoAccess VP MD
MPH-AC005A
Scope of the document
This guide deals with the installation of MorphoAccess® VP MD, which is made up of
following list of products:
(*) The Regulatory Model Number is the main product identifier in the regulatory
documentation and test reports associated to the product
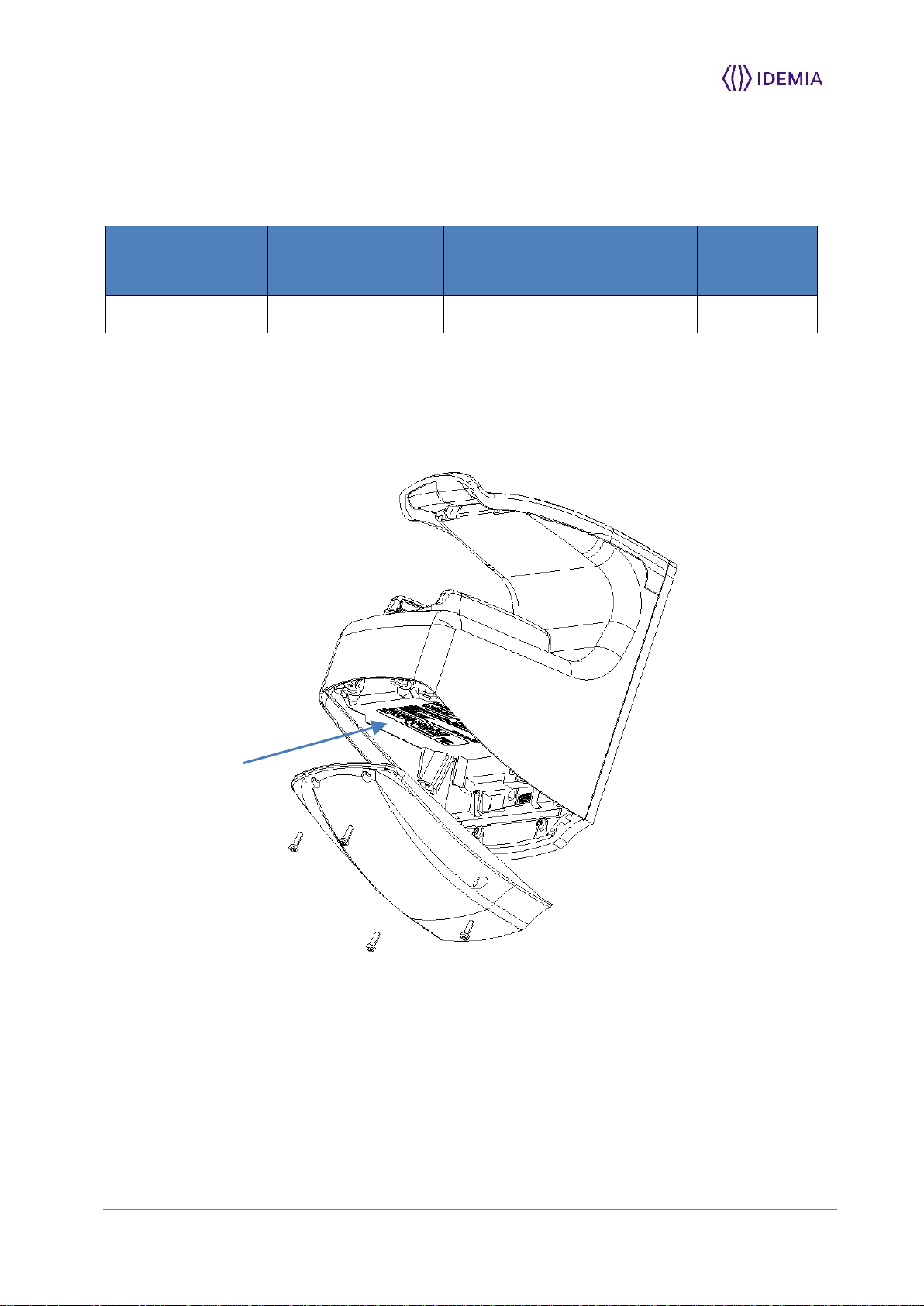
The Regulatory Model Number and other product identification informations can be found on
the stickers at the bottom of the product after removing bottom cover :

MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
12
December 2018
Safety Instructions
means Direct Current (DC)
The installation of this product should be made by a qualified service Person and should
comply with all local regulations.
It is strongly recommended to use a class II power supply at 12VDC 1A minimum in
conformity with Safety Electrical Low Voltage (SELV). The power supply cable length
should not exceed 10 meters.
This system must be installed in accordance with the National Electrical Code (NFPA
70), and the local authority having jurisdiction.
This product is intended to be installed with a power supply complying with IEC60950-
1, in accordance with the NEC Class 2 requirements; or supplied by a listed IEC60950-1
external Power Unit marked Class 2, Limited Power source, or LPS and rated 12VDC, 1A
minimum or 24VDC, 0,5A minimum.
In case of building-to-building connection (power source in a building, and terminal in
another building), it is recommended to connect the 0V of the power supply to the
earthing system of the building. And the terminal block Power Ground must be
connected with the earthing system of the other building.
Note that all connections of the MorphoAccess® VP MD terminal described hereafter
are of SELV (Safety Electrical Low Voltage) type.
MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
13
December 2018
Gauge AWG
Diameter
(mm)
Maximum drop
voltage @ 1m
(V)
Maximum drop
voltage @ 5m (V)
Maximum drop
voltage
@ 10m (V)
18
1.02
0.02
0.11
0.21
20
0.81
0.03
0.17
0.33
22
0.64
0.05
0.26
0.53
24
0.51
0.08
0.42
0.84
V1
V2
L
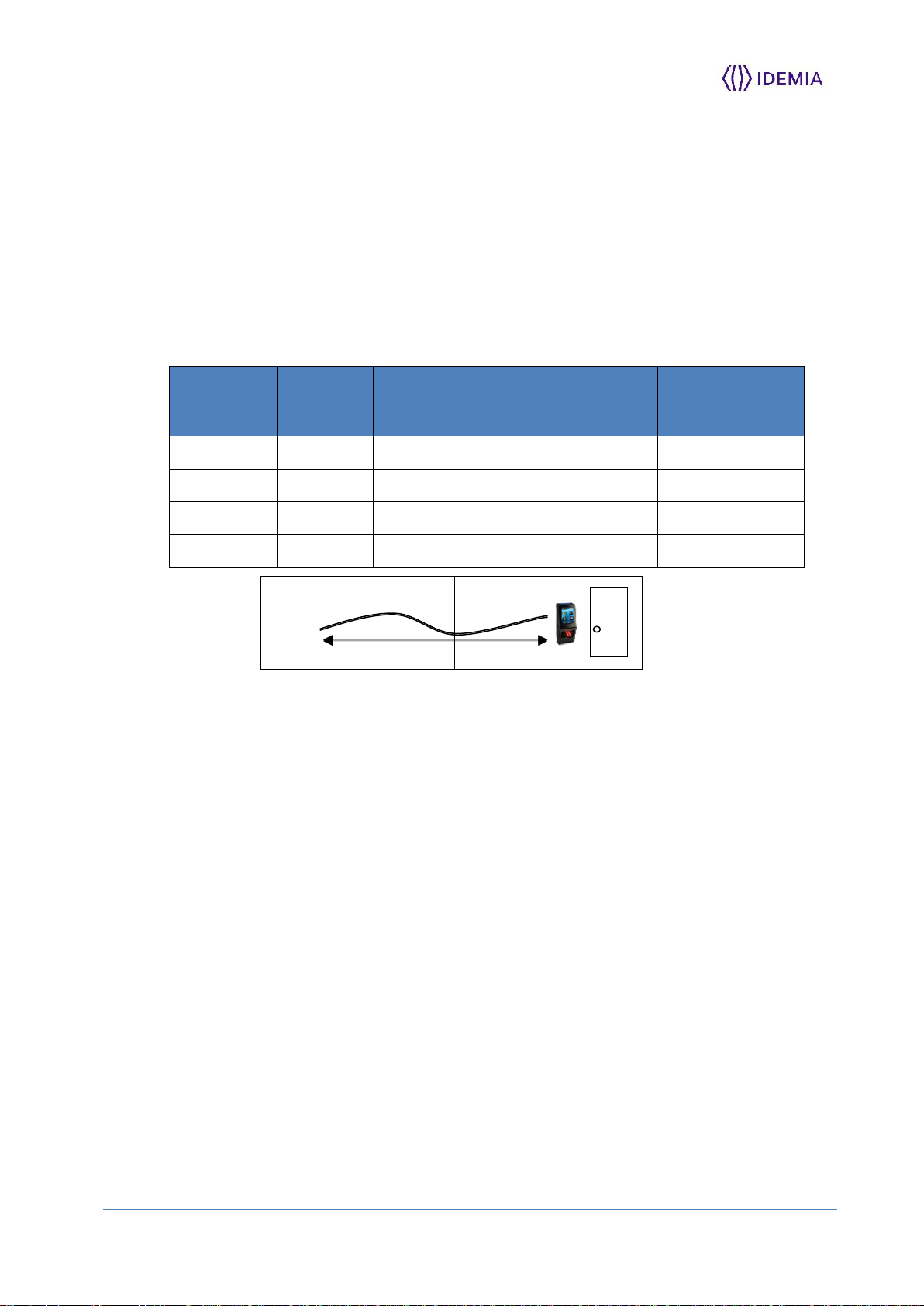
Wiring Recommendations
IDEMIA recommends using a gauge AWG20 for 12VDC power supply (when the
terminal is not powered using POE feature).
The voltage must be measured on the product block connector and must be equal to
12VDC-24VDC (-15% / +10%)
For information, this table shows the maximum drop voltage observed on the terminal
MorphoAccess® VP MD, depending on the length of the cable:
Drop voltage = loss of power due to wire resistance and its length:
V2 = V1 – Drop voltage

MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
14
December 2018
Regulatory, safety and Environmental notices
European Union (CE) regulatory notices
Declaration of Conformity
Products bearing the CE marking comply with one or more of the following EU
Directives as may be applicable:
EMC Directive 2014/30/EU.
RED Directive 2014/53/EU.
ROHS Directive 2011/65/EU.
Compliance with these directives is assessed using applicable European Harmonised
Standards.
MorphoAccess® VP MD is intended to be used for professional application only
(buildings, airport...).
This product may cause interference if used in residential areas. Such use must be
avoided unless the user takes special measures to reduce magnetic emissions to
prevent interference to the reception of radio and television broadcast.
The full Declaration of Conformity is available on demand to your reseller. Please,
provide him the product model name or its Regulatory Model Number (Model on the
label).
Products with wireless features (EMF)
This product meets the provisions of the EU's Council recommendation 1999/519/EC
on the limitation of the exposure of the general public to electromagnetic fields (0 Hz
to 300 GHz).

MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
15
December 2018
This device complies with part 15 of the FCC Rules. Operation is subject to
the following two conditions: (1) this device may not cause harmful
interference, and (2) this device must accept any interference received,
including interference that may cause undesired operation.
USA (FCC) regulatory notices
Changes or modifications not expressly approved by the party responsible for
compliance could void the user's authority to operate the equipment.
Responsible Party:
IDEMIA Identity & Security France
2, place Samuel de Champlain
92400 Courbevoie – France
NOTE: This equipment has been tested and found to comply with the limits for a Class B digital
device, pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential installation. This equipment generates,
uses and can radiate radio frequency energy and, if not installed and used in accordance with
the instructions, may cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this equipment does
cause harmful interference to radio or television reception, which can be determined by turning
the equipment off and on, the user is encouraged to try to correct the interference by one of the
following measures:
Reorient or relocate the receiving antenna.
Increase the separation between the equipment and receiver.
Connect the equipment into an outlet on a circuit different from that to which the
receiver is connected.
Consult the dealer or an experienced radio/TV technician for help.
Shielded cables must be used with this unit to ensure compliance with category B FCC
restrictions.
MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
16
December 2018
Canada (IC) regulatory notices
WARNING TO USERS IN THE CANADA / ATTENTION POUR LES UTILISATEURS AU
CANADA
This device complies with Industry Canada licence-exempt RSS standard(s). Operation is subject
to the following two conditions: (1) this device may not cause interference, and (2) this device
must accept any interference, including interference that may cause undesired operation of the
device.
Under Industry Canada regulations, this radio transmitter may only operate using an antenna of
a type and maximum (or lesser) gain approved for the transmitter by Industry Canada.
To reduce potential radio interference to other users, the antenna type and its gain should be so
chosen that the equivalent isotropically radiated power (e.i.r.p.) is not more than that necessary
for successful communication.
Le présent appareil est conforme aux CNR d’Industrie Canada applicables aux appareils radio
exempts de licence. L’exploitation est autorisée aux deux conditions suivantes: (1) il ne doit pas
produire de brouillage, et (2) l’utilisateur du dispositif doit être prêt a accepter tout brouillage
radioélectrique reçu, même si ce brouillage est susceptible de compromettre le fonctionnement
du dispositif.
Conformément à la réglementation d’Industrie Canada, le présent émetteur radio peut fonctionner
avec une antenne d’un type et d’un gain maximal (ou inférieur) approuvé pour l’émetteur par
Industrie Canada.
Dans le but de réduire les risques de brouillage radioélectrique à l’ intention d’autres utilisateurs,
il faut choisir le type d’antenne et son gain de sorte que la puissance isotrope rayonnée
équivalente (p.i.r.e.) ne dépasse pas l’intensité nécessaire à l’établissement d’une communication
satisfaisante.
.
Note : UL LLC has not verified this product for compliance in respect to Canadian
standards.

MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
17
December 2018
This device complies with Industry Canada licence-exempt RSS standard(s). Operation is subject to the
following two conditions: (1) this device may not cause interference, and (2) this device must accept any
interference, including interference that may cause undesired operation of the device.
Under Industry Canada regulations, this radio transmitter may only operate using an antenna of a type
and maximum (or lesser) gain approved for the transmitter by Industry Canada.
To reduce potential radio interference to other users, the antenna type and its gain should be so chosen
that the equivalent isotropically radiated power (e.i.r.p.) is not more than that necessary for successful
communication.
Others recommendations
The MorphoAccess® VP MD incorporates a capacitive sensor for contactless finger
presence detection.
In the presence of electromagnetic disturbances, this function can be activated
inadvertently. This behavior remains normal (the electromagnetic field modifies the
capacity of the sensor) and has no impact for the user (the access control remains
functional) outside the light signal. To avoid the light signal, Idemia recommends the
use of an EMI filter on the power lines and recommends, as far as possible, to install
the MorphoAccess® VP MD at a sufficient distance from the antennas of potential RF
transmitters.
Potential safety conditions notice
If you notice any of the following conditions (or if you have other safety concerns), do
not use the product: crackling, hissing, or popping sound, or a strong odor or smoke
coming from the product. It is normal for these conditions to appear when an internal
electronic component fails in a safe and controlled manner. However, these conditions
may also indicate a potential safety issue. Do not assume it is a safe failure. Turn off
the product, disconnect it from its power source, and contact technical support for
assistance.
Disposal of waste equipment by users
This symbol means do not dispose of your product with your other household waste.
Instead, you should protect human health and the environment by handing over your
waste equipment to a designated collection point for the recycling of waste electrical
and electronic equipment.

MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
18
December 2018
Recommendations for terminal implementation
Every installation is unique. Sometimes the issues are well defined and can be handled
in a standard fashion; sometimes the issues are very specific and may not be
immediately recognizable.
IDEMIA recommends following these steps for a successful installation:
Plan the installation - Choose the type of hardware required, decide if a network is
required, and decide on the location and number of required terminals.
Unpack all items - Unpack all items and check against the packing list.
Install network hardware components - Install the cabling and components
needed to run the system.
Install software - Install the software needed to set up the terminals.
Pre-configure device - Connect the terminals to the Ethernet, supply power to the
terminals, and pre-configure the terminals.
Mount devices - Mount the terminals in their final locations
Power distribution and device hook up - Connect the terminals wiring via the back
panel.
Power-up procedure - Check the power connections, and then start the system
safely.
MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
19
December 2018
ACCESS CONTROL
SYSTEM
(*) OR EQUIVALENT
NON SECURED
AREA
ACCESS
CONTROLLER
TCP/IP, Wiegand, Dataclock, RS485
ELECTRIC
LATCH (*)
SECURED
AREA
Dry Contact
MorphoAccess
®
VP MD Terminal
Dry Contact
ALARM
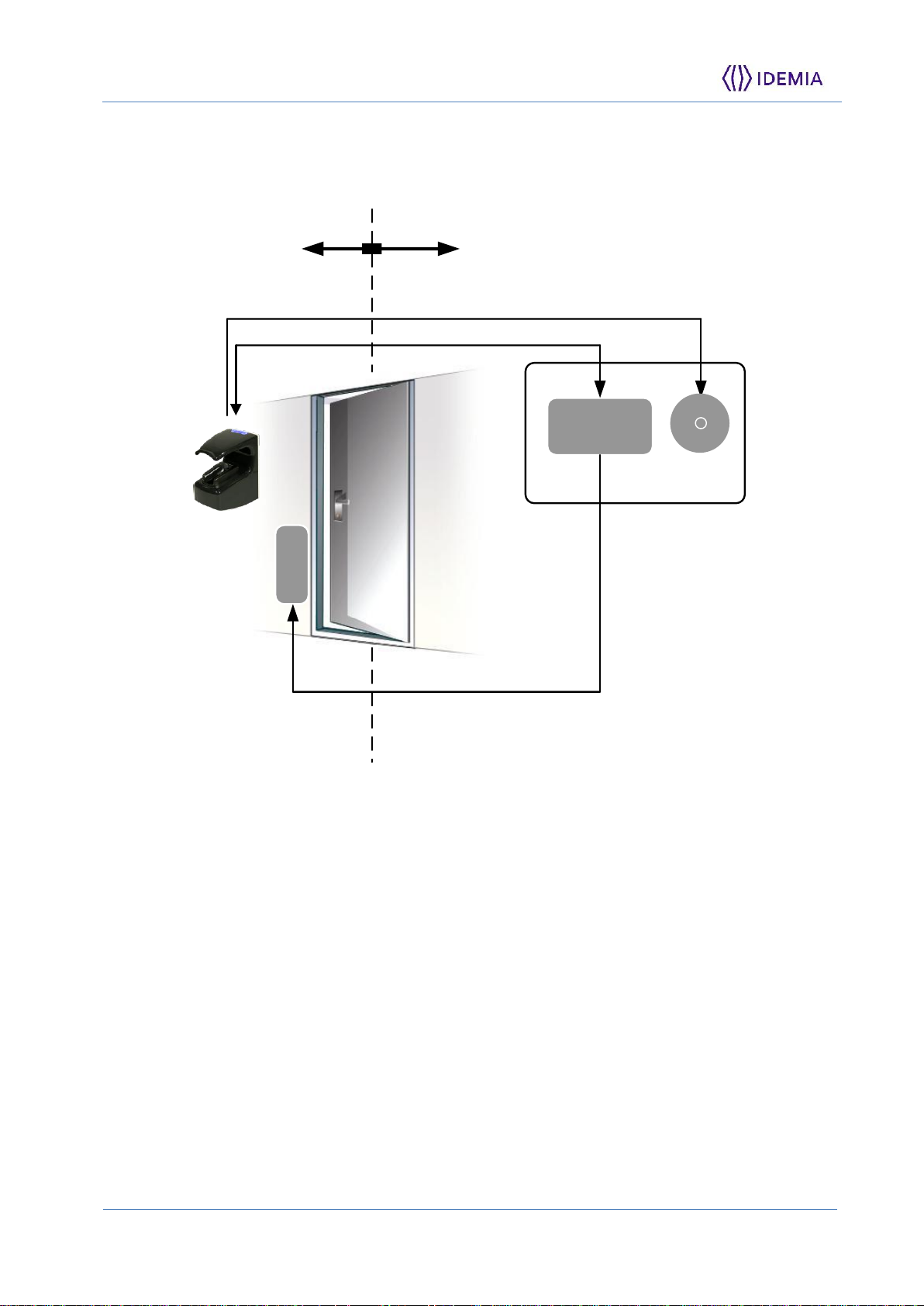
To secure properly an access, IDEMIA recommends installing the MorphoAccess® VP
MD terminal as a part of the typical Access Control environment, as the one described
in the figure below.
Figure 1: Implementation Recommandations
This environment comprises:
The MorphoAccess® VP MD terminal itself
Its role is to perform one-to-many multimodal biometric identification or one-to-one
multimodal biometric verification, i.e. to identify the individual who is presenting his
finger on the terminal sensor by comparing his biometric data with the references
previously stored in the terminal database (in the form of multimodal biometric
templates) or to verify his identity using the reference stored in a contactless card
presented to the terminal.
An Access Controller (3rd party product)
The Controller is the element which controls the access rights of the individuals to the
secured area. For that reason, it must be located in the secured area.

MorphoAccess® VP MD – Installation Guide
Section 1 :Introduction
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
20
December 2018
The individuals who are authorized to access the secured area have their User ID listed
in a so-called "authorized user List" (in contrast with a banned card list).
The MorphoAccess® VP MD terminal and the Controller are communicating using one
of the available protocol (TCP/IP, Wiegand, Dataclock, or RS485). The typical process is
described below:
After access request, and local checks, the MorphoAccess® VP MD terminal sends
the User ID, and the result of local checks, to the Controller
The Controller performs additional checks, and sends its decision to the
MorphoAccess® VP MD terminal (which displays GREEN light if access is granted or
RED light if denied), and to the electric latch of the door (though a door controller)
if access is granted to the user
The MorphoAccess® VP MD terminal sends an alarm signal to the Controller as soon
as a malicious operation is detected (terminal pulled out from the wall or opened);
please refer to the paragraph dealing with anti-pulling and anti-tamper switches for
more explanations.
The Controller is part of the global Access Control System of the secured area, which
can provide useful features such as manage:
authorized user lists (i.e. for VIP),
banned card lists (i.e. for lost user cards),
an access request log (who and when, access granted or denied,..),
an event log (i.e. tamper detection, access control for evacuation of the
building,...).
The MorphoAccess® terminal is able to work alone, without Controller, but the
protection level of the secured area is lower.
An Alarm (3rd party product)
This element is connected to the MorphoAccess® VP MD terminal through a dry
contact.
The MorphoAccess® VP MD terminal sends the command to activate the Alarm as soon
as a malicious operation (terminal pulled out from the wall or having its bottom cover
opened out of maintenance operations) is detected; please refer to the paragraph
dealing with anti-pulling and anti-tamper switches for more explanations.
A Electric door strike or equivalent (3rd party product)
The activation of this element, open the door or the gate, to provide the physical access
to the protected area. The Controller is the one which sends the command to activate
the strike if access is granted (i.e. if the individual's User ID is listed in the Controller
authorized user List). Connection between these two elements is done through a dry
contact.

MorphoAccess® VP MD – Installation Guide
Section 2 :General Description
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
21
December 2018
Section 2 : General
Description

MorphoAccess® VP MD – Installation Guide
Section 2 :General Description
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
22
December 2018
Box opening
At the box opening, components shall be extracted from the protection casing as
depicted in the pictures below.
Extract the bottom cover and the wall plate (which are not screwed to the terminal)
and keep them separate until the installation of the terminal is completed. The
screwing of the bottom cover is the last stage of the installation.
Do not forget to withdraw the sachet of screws, bolts and connectors from the white
protection casing.
Figure 2: Box Opening
MorphoAccess® VP MD – Installation Guide
Section 2 :General Description
2019_200000XXXX-V0
IDEMIA DOCUMENT - REPRODUCTION AND DISCLOSURE PROHIBITED
23
December 2018
( X4)
K25-8
Torx 8 Print
( X4)
M3-10
Torx 10 Print
1 2 3
4 6 5
7
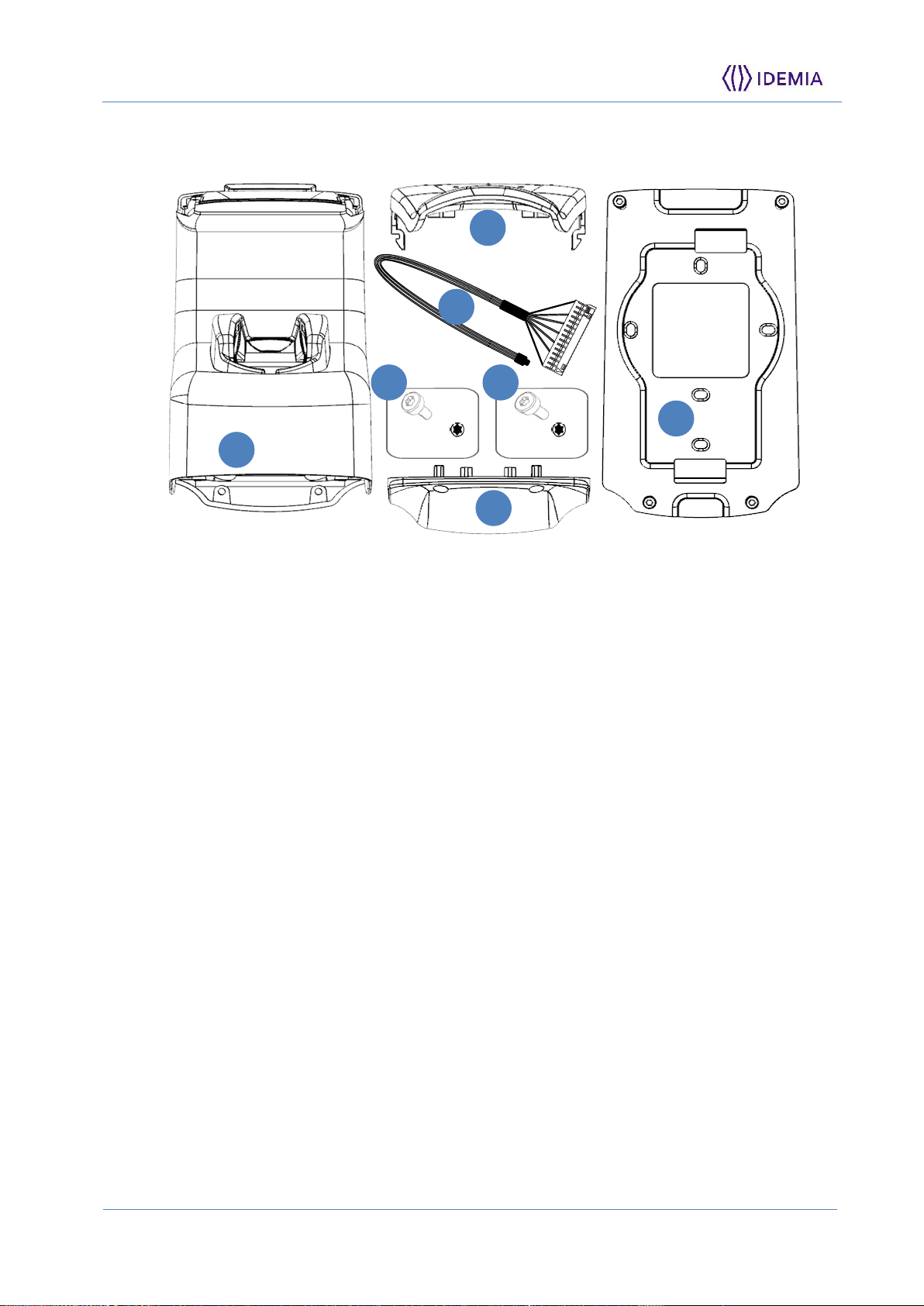
Components of the initial package
Figure 3: Box Content
1. One (1) Terminal’s body
2. One (1) top cover fixed at the body of the terminal
3. One (1) bottom cover
4. Four (4) M3X10 Screws (for terminal fixing to the wall plate) – T10 Torx and 4mm
flat print
5. Four (4) K25x8 Screws (Bottom cover fixing) – T8 Torx Print
6. One (1) Connection cable
7. One (1) Wall frame
 Loading...
Loading...