Human HumaClotDuo User manual

HumaClot Duo
| User Manual
plus
|
Cat.No. 15650/1


Revision List of the Manual
No. DATE / Rev. REVISION DESCRIPTION
01 01/2008-03 First edition
02 02/2008-10 Adaptation for software version C5.15
03 03/2009-11 D-Dimer added
04 04/2010-07 Corrections (typographical errors, no. of figures)

2/2

1 INTRODUCTION
This manual is considered as a part of the instrument; it has to be at the operator’s hand as well as at the
maintenance operator’s availability. For accurate installation, use and maintenance, please read the following
instructions carefully. In order to avoid instrument or personal damages, carefully read the ”GENERAL SAFETY
WARNINGS”, describing the suitable operating procedures. In case of breakdowns or any troubles with the
instrument, apply to the local Technical Service.
2 USER WARRANTY
HUMAN warrants that instruments sold by one of its authorised representatives shall be free of any defect in
material or workmanship, provided that this warranty shall apply only to defects which become apparent within
one year from the date of delivery of the new instrument to the purchaser.
The HUMAN representative shall replace or repair any defective item at no charge, except for transportation
expenses to the point of repair.
This warranty excludes the HUMAN representative from liability to replace any item considered as expendable in
the course of normal usage, e.g.: lamps, valves, syringes, glassware, fuses, diskettes, tubing etc.
The HUMAN representative shall be relieved of any liability under this warranty if the product is not used in
accordance with the manufacturer's instructions, altered in any way not specified by HUMAN, not regularly
maintained, used with equipment not approved by HUMAN or used for purposes for which it was not designed.
HUMAN shall be relieved of any obligation under this warranty, unless a completed installation / warranty
registration form is received by HUMAN within 15 days of installation of this product.
This warranty does not apply to damages incurred in shipment of goods. Any damage so incurred shall be re-ported
to the freight carrier for settlement or claim.
3 INTENDED USE OF THE INSTRUMENT
[IVD]
The instrument has to be used for the expected purposes and in perfect technical conditions, by qualified
personnel, in working conditions and maintenance operations as described in this manual, according to the
GENERAL SAFETY WARNINGS. This manual contains instructions for professional qualified operators.
4 GENERAL SAFETY WARNINGS
Use only chemical reagents and accessories specified and supplied by HUMAN and/or mentioned in this manual.
Place the product so that it has proper ventilation.
The instrument should be installed on a stationary flat working surface, free from vibrations.
Do not operate in area with excessive dust.
Work at room temperature and humidity, according to the specifications listed in this manual.
Do not operate this instrument with covers and panels removed.
Only use the power cord specified for this product, with the grounding conductor of the power cord connected to
earth ground.
Use only the fuse type and rating specified by the manufacturer for this instrument, use of fuses with improper
ratings may pose electrical and fire hazards.
To avoid fire or shock hazard, observe all ratings and markings on the instrument.
Do not power the instrument in potentially explosive environment or at risk of fire.
Prior to cleaning and/or maintaining the instrument, switch off the instrument and remove the power cord.
For cleaning use only materials specified in this manual, otherwise parts may become damaged.
It is recommended always to wear protective apparel and eye protection while using this instrument.
Respective warning symbols, if appearing in this manual, should be carefully considered.
I

5 DISPOSAL MANAGEMENT CONCEPT
The currently valid local regulations governing disposal must be observed. It is in the responsibility of the user to
arrange proper disposal of the individual components.
All parts which may comprise potentially infectious materials have to be disinfected by suitable validated
procedures (autoclaving, chemical treatment) prior to disposal. Applicable local regulations for disposal have to be
carefully observed.
The Instruments and electronic accessories (without batteries, power packs etc.) must be disposed of according to
the regulations for the disposal of electronic components.
Batteries, power packs and similar power source have to be dismounted from electric/electronic parts and disposed
off in accordance with applicable local regulations.
6 INSTRUMENT DISINFECTION
Analytical instruments for in vitro diagnostic involve the handling of human samples and controls which should be
considered at least potentially infectious. Therefore every part and accessory of the respective instrument which
may have come into contact with such samples must equally be considered as potentially infectious.
Before doing any servicing on the instrument it is very important to thoroughly disinfect all possibly contaminated
parts. Before the instrument is removed from the laboratory for disposal or servicing, it must be
decontaminated/disinfected. Decontamination/disinfection should be performed by a authorised well-trained
personnel, observing all necessary safety precautions. Instruments to be returned have to be accompanied by a
disinfection certificate completed by the responsible laboratory manager. If a disinfection certificate is not
supplied, the returning laboratory will be responsible for charges resulting from non-acceptance of the instrument
by the servicing centre, or from authority’s interventions.
7 NOTICE
Every effort has been made to avoid errors in text and diagrams, however, HUMAN GmbH assumes no
responsibility for any errors which may appear in this publication. It is the policy of HUMAN GmbH to improve
products as new techniques and components become available. HUMAN GmbH therefore has to reserve the right
to change specifications if necessary in the course of such improvements.
II

NOTICE
Analytical instruments for in vitro diagnostic application involve the handling of human samples and controls
which should be considered at least potentially infectious. Therefore every part and accessory of the respective
instrument which may have come into contact with such samples must equally be considered as potentially
infectious.
BIOHAZARD
The „BIOHAZARD“ warning label must be affixed to instrument prior to first use with biological material !
Servicing Note:
Before doing any servicing on the instrument it is very important to thoroughly disinfect all possibly contaminated
parts. Before the instrument is removed from the laboratory for disposal or servicing, it must be decontaminated.
Decontamination should be performed by authorised well-trained personnel only, observing all necessary safety
precautions. Instruments to be returned have to be accompanied by a decontamination certificate completed by
the responsible laboratory manager. If a decontamination certificate is not supplied, the returning laboratory will
be responsible for charges resulting from non-acceptance of the instrument by the servicing centre, or from
authority’s interventions.
HUMAN
Gesellschaft für Biochemica und Diagnostica mbH
| Max-Planck-Ring 21 · 65205 Wiesbaden · Germany
| Tel.: +49 61 22/99 88-0 · Fax: +49 61 22/99 88-100
| e-Mail: tech-support@human.de · www.human.de
a

b

Contents
Symbols 3
1 Safety information 3
2 General 4
2.1 Intended Purpose 4
2.2 Installation 5
2.3 Technical Data 5
3 Instrument Components 5
3.1 Incubator Block 5
3.2 Control Panel 7
3.3 Rear View of the Instrument 9
3.4 AutoHumaPette (optional) 9
3.5 Thermal-Printer (optional) 9
3.6 Barcode Scanner (optional) 9
4 THEORY OF OPERATION 10
4.1 Clotting Assay (CLOT) 11
4.2 Derived Fibrinogen (CLOT + FIB) 11
4.3 Chromogenic Assay (KINETIC) 12
4.4 Chromogenic Ecarin Assay (100 mOD) 12
4.5 Immunoturbidimetric Assay (IMMUNO) 13
5 Operating Instructions 14
5.1 Setup System 14
5.1.1 Language 14
5.1.2 Fibrinogen Concentration Units 14
5.1.3 Temperature Control 14
5.1.4 Signal 14
5.1.5 Autostart 15
5.1.6 Contrast of the LCD (Liquid Crystal Display) 15
5.1.7 Speed of the Mixer 15
5.1.8 Patient Identification 15
5.2 Setup Test 17
5.2.1 Setup Test 17
5.2.2 Units 17
5.2.3 Standard Curve 18
5.2.4 Correlation Factor (linearity index for calibration data) 18
5.2.5 Store Data 18
5.2.6 Print Test 18
5.2.7 Autostart 18
5.3 Test Analysis 20
5.3.1 Test Selection 20
5.3.2 Optic Activation & Entering Patient Identification Numbers 21
5.3.3 Duplicate testing 22
5.3.4 Starting the Analysis 22
5.3.5 Display during measuring 22
5.3.6 Manual Stop during Measurement 23
5.3.7 Return to Main Menu 23
5.3.8 Unit Key Functions 23
5.3.9 Stopwatch Functions 23

5.3.10 Result Warning Messages 23
5.4 Instrument Settings 25
5.4.1 Set System to Default 25
5.4.2 Change Temperature Adjustment 25
5.4.3 Set All Test Calibration Points to Zero 26
5.4.4 Set OD Correction 26
5.4.5 Set COAG CORRECTION 26
6 Service Menu 28
6.1 System Analysis 28
6.2 Optic Values 29
6.3 Print Sys-ID 29
7 Troubleshooting 31
8 MAINTENANCE 33
8.1 Recommended Maintenance 33
8.2 Temperature Adjustment 33
8.3 Cleaning procedures 33
9 APPLICATIONS 34
9.1 Test Overview 34
9.2 Prothrombin Time 35
9.3 Derived Fibrinogen 36
9.4 Clauss Fibrinogen Assay 36
9.5 Thrombin Time Assay 37
9.6 D-Dimer 38
9.7 APTT & APC Resistance 39
9.8 PT-Based Factor Assays (II, V, VII, X) 40
9.9 APTT-Based Factor Assays (VIII, IX, XI, XII) 41
10 SPECIAL FUNCTIONS 42
10.1 Software Upgrading 42
10.2 Interface Protocol (uni-directional) 43
11 PRODUCT CATALOGUE 43
2/44
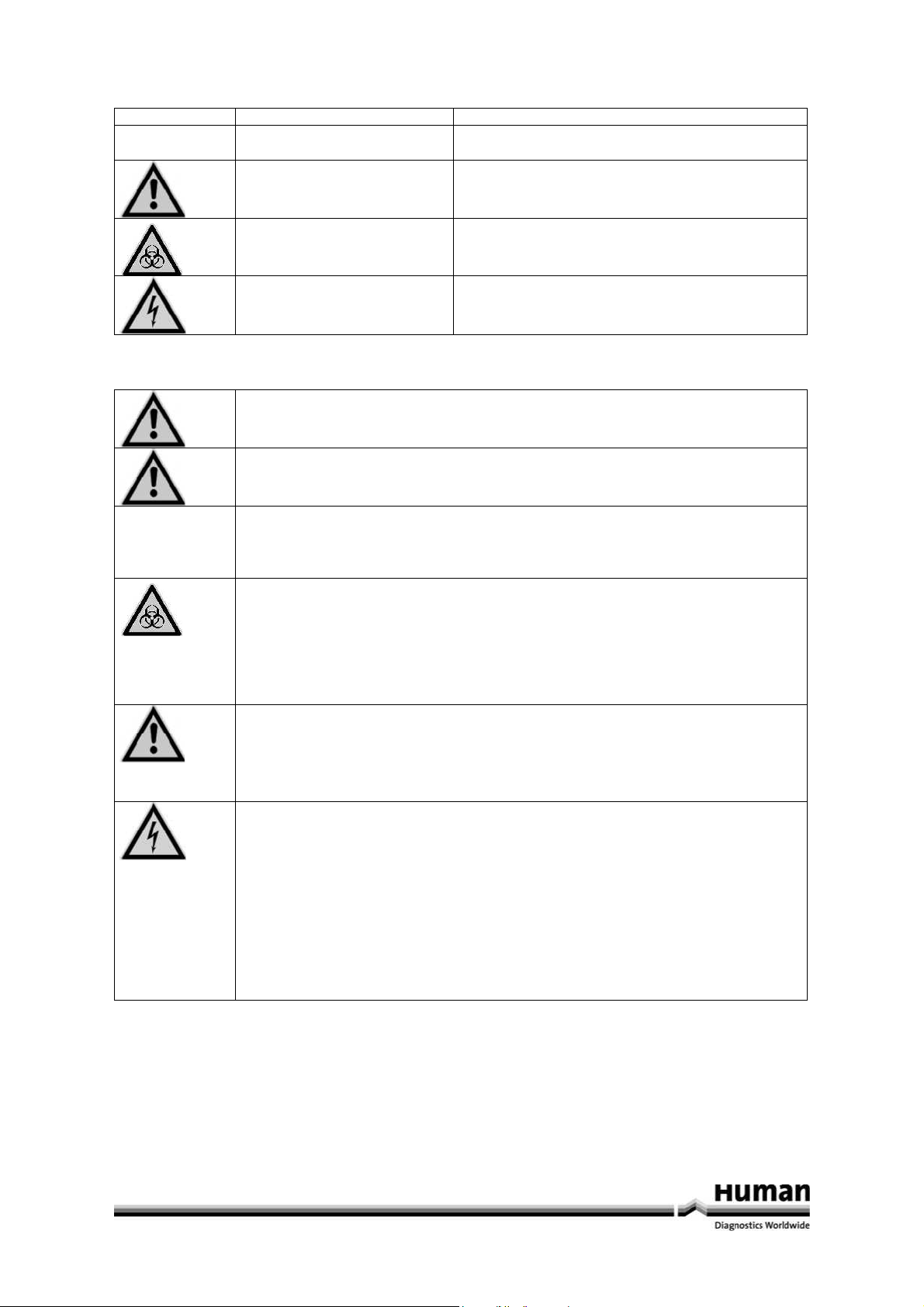
Symbols
Symbol Meaning Explanation
Advice Indicates important information and tips.
Ö
Warning! Risk of possibly serious health hazard or damage to
equipment if warning is not heeded.
1 Safety information
‰
Biohazard! Danger of infection from the samples and reagents
used.
Danger! Risk to operating personnel or equipment due to electric
shock.
Recommend materials
Use only original disposables.
Use only manufacturer-approved materials.
Do quality control
Carry out control measurement runs at regular intervals to ensure that the analyzer
continues to function properly.
Waste cuvettes
The cuvette blocks are intended as single-use items only.
Infectious Material
Avoid direct contact with samples and sample residues in the used cuvettes.
Infectious material such as cuvette waste and liquid waste must be disposed in compliance
with local regulations governing for infectious materials.
Wear medical infection grade protective gloves for all cleaning and maintenance work
involving potential contact with infectious liquids. Use each pair of gloves once only.
Use approved disinfectants to disinfect your hands after completion of the work.
Environmental conditions
Ambient temperature must be 18...25°C.
Humidity must be below 70%.
Avoid any vibrations or impacts to analyzer.
Do not use the analyzer in the presence of explosive or inflammable gas.
Electrical Safety
Make sure the operating voltage setting is correct before connecting the device to the power
mains.
Use only grounded electrical outlets.
Use only grounded extension cords in perfect condition. Damaged cords must be replaced
immediately.
Never interrupt protective ground contacts.
Never remove housing elements, protective covers or secured structural elements, since so
doing could expose parts carrying electric current.
Make sure surfaces such as the floor and workbench are dry while work is being done on the
device.
3/44 Human HumaClot Duo
plus
User Manual

2 General
plus
The HumaClot Duo
immunoturbidimetric testing capabilities. The HumaClot Duo
is a manual 2-channel photo-optical instrument that offers clotting, chromogenic &
plus
can be used for a wide variety of coagulation and
fibrinolysis tests such as:
- Prothrombin Time (PT)
- Activated Prothrombin Time (aPTT)
- Thrombin Time (TT)
- Venom Time (VT)
- Fibrinogen (FIB)
- Factors (FII - FXII)
- Antithrombin (AT3)
- Heparin (HEP)
- D-Dimer (DD)
- Protein C (PC)
- Protein S (PS)
- von Willebrand Factor (VWF)
- Ecarin Chromogenic Assay Thrombin (ECAT)
- Ecarin Chromogenic Assay Hirudin (ECAH)
- Plasminogen (PLG)
- α2-Antiplasmin (A2AP)
- Activated PC resistance (APCR)
- Lupus Anticoagulant (Screen, Confirm)
FEATURES:
- Coagulation analyzer for turbidimetric, chromogenic and immunoturbidimetric assays.
- Highly reliable, durable and nearly service-free system
- Autosensing optics to eliminate interference like bilirubin.
- Approved clotting algorithm for all kind of samples and reagents. Biphasic aPTT curve detection
- Low fibrinogen curve detection
- Fibrinogen concentration can also be derived from PT results. In addition, the standard CLAUSS method is
available.
- Calculation in % activity, INR, Ratio, g/l, ng/ml or mg/dl
- Every test is programmable with up to 5 calibration points
- Correlation analysis of calibration curves
- 2 independent stop-watch functions
- Multi-language software
- (German, English, Spanish, French, Italian, Portuguese)
- Patient identification (autoseries, manual input, barcode)
- Duplicate-test mode
- Profile testing (PT, aPTT, FIB)
- APC-R with automatic ratio calculation
- DRVVT with automatic ratio calculation
- Microvolume testing (total of 60...75 μl)
- Reagent stirring with magnetic bars
- Routines for self-checks (trouble-shooting)
- Routines for printouts (result, calibration, service, system)
- AutoHumaPette with electronically triggered start
- Automatic start triggered by reagent addition
- Optional data management and research software
- Optional printer
- Optional barcode scanner for patient identification
- Easy software update
- Interface for Laboratory Information & Management Systems (LIMS)
- Compact design and light weight
2.1 Intended Purpose
plus
The HumaClot Duo
is designed to carry out coagulometric tests such as PT, PTT, TT, fibrinogen, single factor tests,
chromogenic and immunoturbidimetric tests (e.g., D-dimer, antithrombin III, etc.).
Use only citrate plasma for test analysis runs: Mix 9 parts venous blood with 1 part 3.2% (0.109 M) sodium citrate
and centrifuge the mixture at 1,500 g for approx. 15 minutes. Plasma must be used within 2 hours.
4/44

Do not use plasma with more than 25 mg/dl bilirubin concentration
Ö
The HumaClot Duo
also have received training on this instrument and have read and understood this user manual.
2.2 Installation
No special precautions are necessary when starting up the HumaClot Duo
recommended:
- Place on a level surface in an area free from excessive temperature fluctuations.
- Avoid vibrations during measurement.
- Protect the instrument from direct sunlight, moisture and dust.
- Ensure that the mains supply conforms to the voltage and frequency rating on the instrument’s identification
plate before plugging in the instrument for the first time.
2.3 Technical Data
Instrument:
Boards SMD (Small Mounted Device) based
Microprocessor NEC V25
Flash-EPROM 128 KByte
RAM 128 KByte
EEPROM 2 KByte
AD-Converter 18 Bit (16 bit used)
Optics 2 LED’s ultra bright, pulse modulation control
RS 232 9600 Bd, 8 Data, 1 Stop, no parity (uni-directional)
Keyboard:
3x 8 matrixes foil keyboard, with test, function and numerical keys
with green temperature LED, indicating 37°C + 0.5°C
Display:
4 line x 20 character LCD (Liquid Crystal Display)
Incubation block:
12 cuvette pre-warming positions,
5 reagent positions
2 measuring positions
Dimensions:
WxDxH: 290x205x80 mm
Weight:
1.41 kg
Ambient temperature/humidity range for operation:
18...25°C / < 70°rH
Power Supply:
external, 42 W max
Input voltages 100 VAC to 240 VAC / 47 to 63 Hz
Output voltages +5Vdc/5A; +15Vdc/2A; -15Vdc/0.8A
Do not use plasma with more than 1,000 mg/l hemoglobin concentration
plus
must be operated by a specialist trained in clinical laboratory techniques. The operator must
plus
. However, the following is
The instrument must be connected to the power supply by the mains cable supplied. If
obvious damage has occurred during shipping, do not use. Contact your local HUMAN
distributor for replacement or repair.
3 Instrument Components
3.1 Incubator Block
The incubator block is made from aluminium, which ensures equal distribution of heat. The temperature of the
incubator block is regulated to 36.5°C - 37.5˚C
5/44 Human HumaClot Duo
plus
User Manual
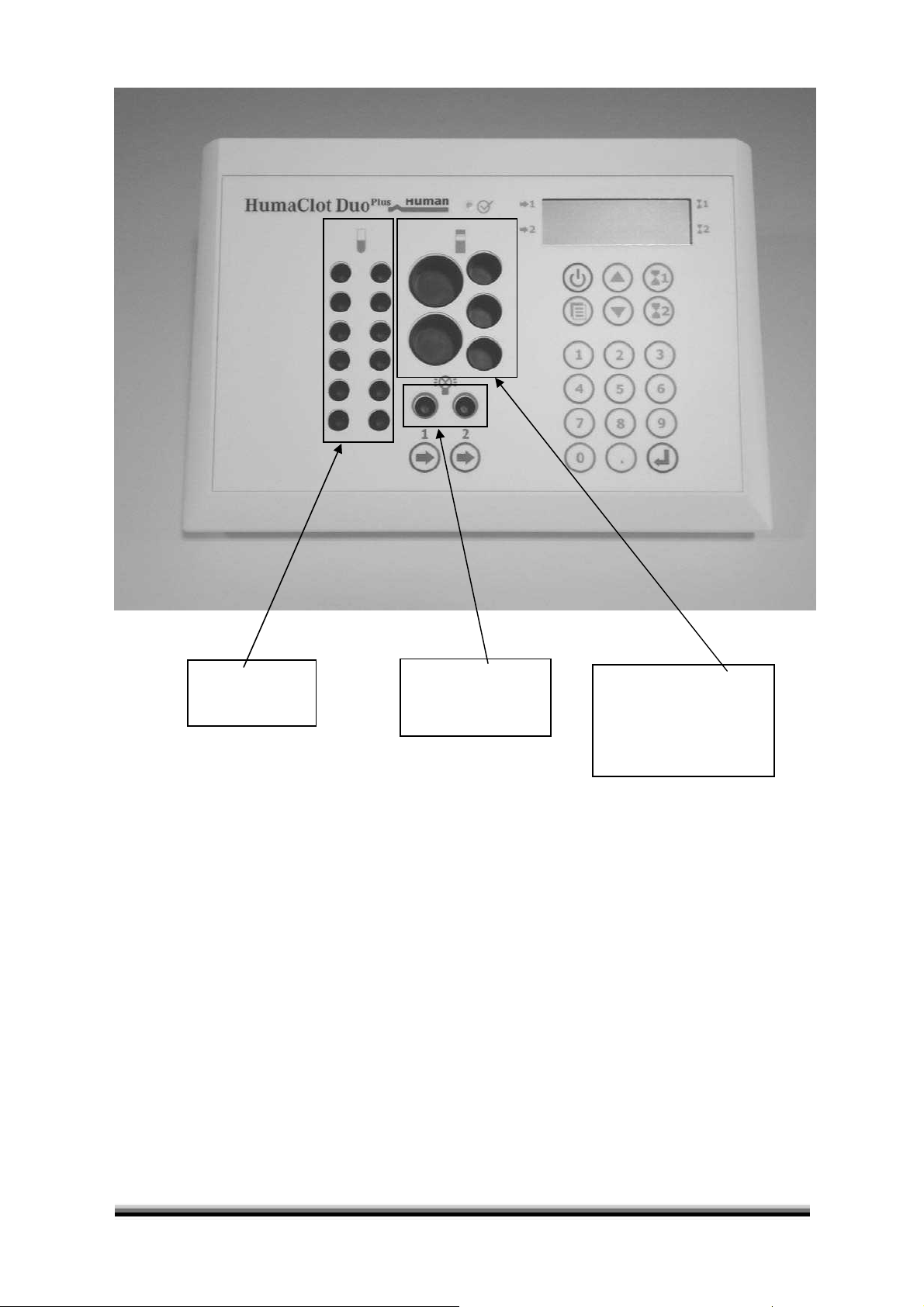
12 positions for
prewarming or
incubation
Figure 1 Incubator Block
Measuring positions
right = Optic 2
left = Optic 1
For reagent vials,
containers or tubes:
Ø 15mm
Ø 23,5mm, stirred
Ø 24,2mm
6/44
3.2 Control Panel
Figure 2 Control Panel
1
2
1
2
1
2
1
2
1
4
7
0
2
5
8
.
3
6
9
7/44 Human HumaClot Duo
plus
User Manual
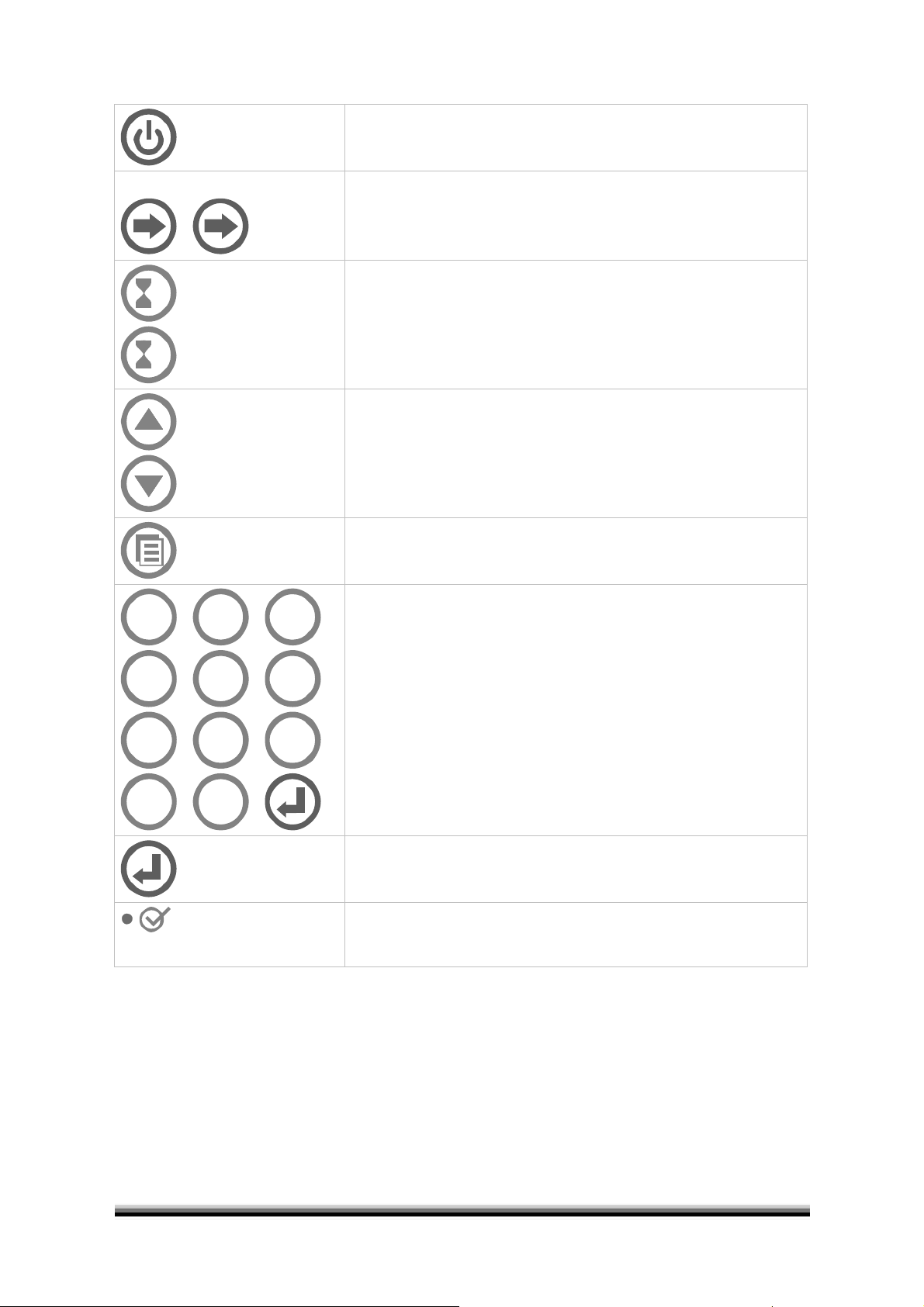
Control panel in detail
1
2
1
2
ON / OFF switches the unit on and off
Optics 1 / 2 activates channel 1 and/or 2
Timer 1 / 2 activate timer function 1 and/or 2
Cursor up/down line up/down, select setup parameters
Menu go back to main menu or next entry
1
4
7
0
2
5
8
.
3
6
9
Numeric keys for input of calibration values and
Patient identification or
selection of submenu and
selection of test no.
Confirm with red enter key
Enter confirm entry, jump to next entry
Temperature indicates temperature is within the allowed range
of 37°C ± 0.5°C
8/44

3.3 Rear View of the Instrument
Figure 3 Rear of Equipment
DC IN: Connection to Power Supply
Pipette: Connection to AutoHumaPette
Printer: Connection to Thermal Printer
Service: Connection to PC (Firmware upgrading, LIMS) or to barcode scanner
3.4 AutoHumaPette (optional)
Optional accessory tool for automatic test start. The pipette supports four volumes (25 to 200 μl)
3.5 Thermal-Printer (optional)
Optional accessory tool for automatic print-out. The thermal-printer must support a serial interface, set to 9600
Baud, 8 Data, 1 Stop, No parity. When the Thermal printer is connected with printer-port of the HumaClot Duo
Plus
,
following data will be printed automatically:
- Result Print-Out
- Test Parameter Print-Out
- Service-Report Print-Out
- System-Identification Print-Out
3.6 Barcode Scanner (optional)
Optional accessory tool for easy handling of patient identification. Up to 20 characters can be read.
Barcodes with more information will cut off at the maximum length. The barcode scanner must support a serial
interface, set to 9600 Bd, 8 Data, 1 Stop, No parity.
Warning: The barcode scanner is powered with 5V over PIN 9 of the RS232
Ö
Interface of the analyzer. Do only use scanners with that feature.
9/44 Human HumaClot Duo
plus
User Manual
 Loading...
Loading...