Page 1
QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Technical Specifications
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 1
HP Healthcare Edition HC270cr QHD Clinical Review Monitor
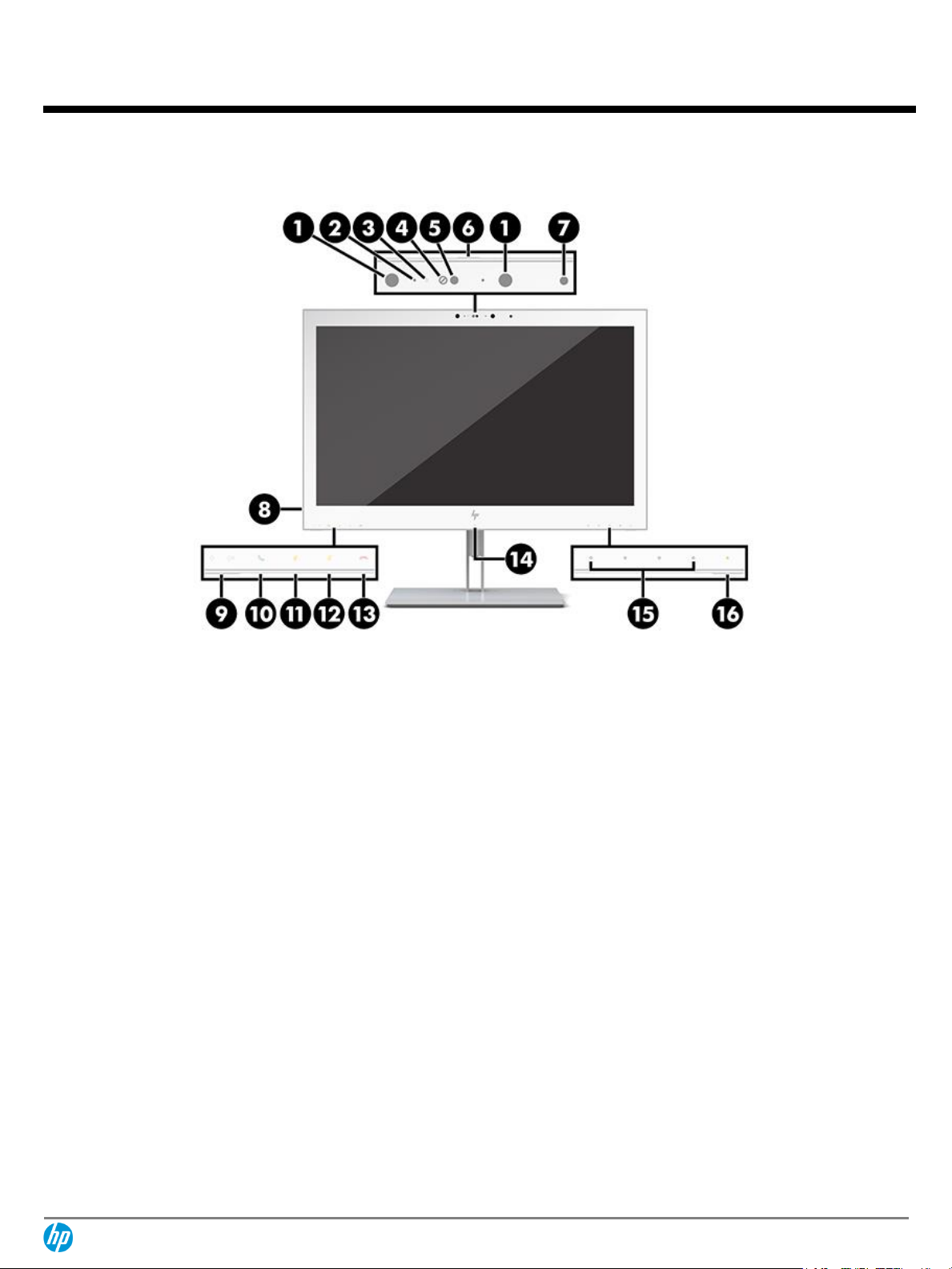
Front
1.
Infrared light
9.
Audio Volume Control
2.
Camera microphones
10.
Answer/Call button
3.
Camera light
11.
Mute microphone button
4.
IR camera lens
12.
Mute speaker button
5.
Webcam lens
13.
Reject/Hang up button
6.
Camera shutter
14.
RFID sensor
7.
Ambient light sensor
15.
OSD buttons
8.
Audio-out (headphone) connector
16.
Power light
Page 2
QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Technical Specifications
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 2
HP Healthcare Edition HC270cr QHD Clinical Review Monitor
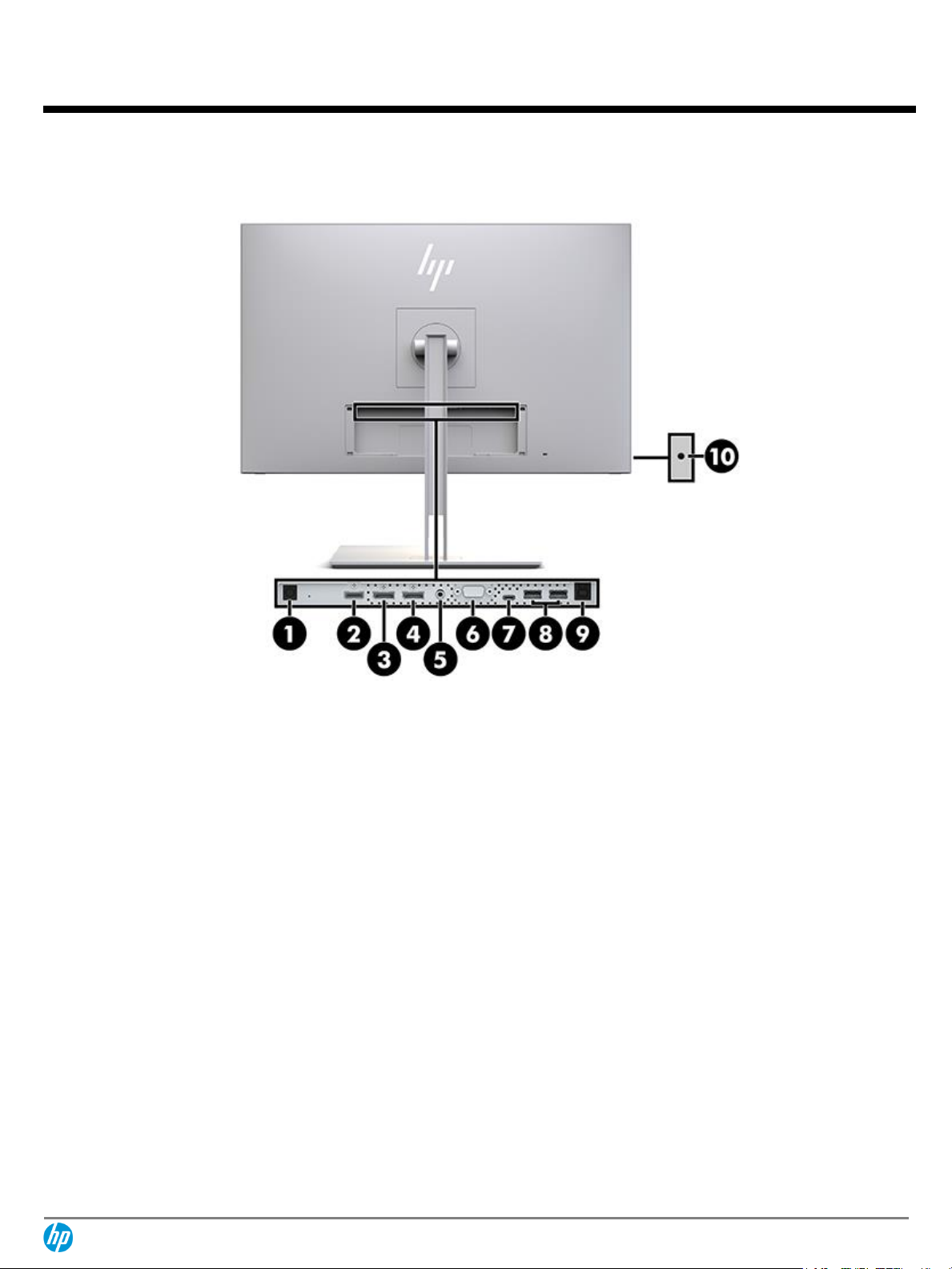
Rear
1.
Power connector
6.
VGA
2.
HDMI
7.
USB Type-C™
3.
DisplayPort™ IN
8.
USB 3.0 Type-A
downstream ports (2)
4.
DisplayPort™ OUT
9.
USB 3.0 Type-B upstream
5.
Audio-IN
10.
Headphone (audio OUT)
Page 3

QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Technical Specifications
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 3
Model: 3XE86AA
Panel Specifications*
Display Size (Diagonal)
27-in
Display Panel Type
AHVA
Native Resolution/Timing
2560×1440 @ 60 Hz
NOTE: DisplayPort 1.2 or HDMI 1.4 required to drive panel at its native resolution
Panel Bit Depth
10-bit
Aspect Ratio
16:9
Brightness - Typical
350 cd/m² (310 cd/m2 with cover glass)
Static Contrast Ratio - Typical
1000:1
Static Contrast Ratio - Minimum
600:1
Dynamic Contrast Ratio (DCR)
3,000,000:1
Pixel Pitch
0.233 x 0.233 mm
Pixels Per Inch (PPI)
108.8
Backlight Lamp Life (to half brightness in hours)
30k minimum
Anti-Glare Panel
Yes
Response Time – Typical
Level 1 (Default, 12ms Gray-to-Gray)
Level 2 xxms (Gray-to-Gray)
Level 3 xxms (Gray-to-Gray)
Level 4 xxms (Gray-to-Gray)
Level 5 xxms (Gray-to-Gray)
Horizontal Viewing Angle
(typical CR>10)
178°
Vertical Viewing Angle (typical CR>10)
178°
Panel Active Area (w x h)
596.74 x 335.66 mm
Preset Graphic Modes/Supported
Resolutions
640x480@60Hz, 720x400@70Hz
800x600@60Hz, 1024x768@60Hz
1280x720@60Hz, 1280x1024@60Hz
1366x768@60Hz, 1680x1050@60Hz
1440x900@60Hz, 1600x900@60Hz
1600x1200@60Hz, 1920x1080@60Hz
1920x1200@60Hz 2560×1440 @60Hz
Maximum Resolution
2560×1440 @ 60 Hz
Recommended Resolution
2560×1440 @ 60 Hz
Vertical Scan Range
50 - 60 Hz
Horizontal Scan Range
30 -90 kHz
Default Color Space
DICOM (0.0 ALC)
Maximum Pixel Clock Speed
241.5Mhz
*All performance specifications represent the typical specifications provided by HP's component
manufacturers; actual performance may vary either higher or lower.
Color Gamut Coverage
sRGB
100%
Color Management
Factory Color Calibrated
Yes (DICOM (0.0 ALC), DICOM (2.2 ALC), sRGB)
Factory Color Presets
sRGB (D65), DICOM (0.0 ALC), DICOM (2.2 ALC), Neutral,
Cool, Viewing Modes, Cusom RGB
Color Space / Subsampling Support
RGB 4:4:4, YCBCR 4:4:4, YCBCR 4:2:2
Default Color Temperature
DICOM (0.0 ALC)
Low Blue Light Modes
Yes RGB Channel Adjust
Yes Gain
User Calibration Support
Yes External Calibration Instrument Support
X-Rite i1Display Pro
Page 4

QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Technical Specifications
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 4
Monitor Specifications
Exterior Color of Monitor Bezel and
Stand
Ceramic white, Pike Silver
Plug & Play
Yes Tilt
Yes, –5° to +25°
Swivel
Yes, +-45°
Pivot
Yes, +-90°
Height Adjustment Range
Yes, 110 mm
VESA Mounting
Yes, 100 mm x 100mm
Security Lock Ready
Yes Detachable Base
Yes, ships detached
Multimedia
Webcam
720p
Infra-Red Sensors
Yes Windows Hello Compatible
Yes Microphone
Yes Speakers (eg 4 speakers)
Yes, 2 speakers
Speakers Output Power
2 W per channel
Conferencing Hot-Keys
Yes, (Start Call, End Call, Mute mIc, Mute speakers)
Skype for Business Certified
VIDYO ™ Tested
Yes
Yes
On Screen Display
(OSD)
On Screen Display User Controls
Brightness, Color Control, Input Control, Image Control, PIP
Control, Power Control, Menu Control, Sanitization,
Management, USB Host Selection
User Programmable Modes
Yes, 20
Monitor Control Buttons or Switches
Menu, Minus ("-"), Plus ("+"), Input Control, Power
User-Assignable Function Buttons
Yes, 3 (8 options)
Audio Controls
Yes, Volume
Languages
10 (English, Spanish, German, French, Italian, Netherlands,
Portuguese, Japanese, T-Chinese and S-Chinese)
Connector Types
DisplayPort™
QTYx2 (DP in and DP out)
HDMI
QTYx1
USB Type-C™
QTYx1
VGA
QTYx1
HDCP support
Yes, (DisplayPort and HDMI)
Audio Output
QTYx1, Analog - earphone
Audio Input
QTYx1, Analog - line in
USB Port
Specifications
USB Version
3.0
USB Hub
Yes Downstream Ports
2 Ports
Upstream Ports
1 Port (USB Type-B)
Power Output Maximum
Standard 4.5W for each downstream port.
Page 5

QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Technical Specifications
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 5
USB-Type C™ Port
Specifications
USB-Type C™ Version
USB 3.1 Gen1 / 5G bps
USB-Type C™ Hub
Yes Upstream USB-Type C™ Ports
1 Port
Video Support
USB-C Alt mode – DisplayPort™ 1.2
Power Output
5V/3A
9V/3A
10V/5A
12V/5A
15V/4.33A
20V/3.25A
Power Output Maximum
65W Max
Special Features
Picture-in-Picture, Picture-by-Picture
Yes Ambient Light Sensor
Yes
Power & Operating
Specs
Power Supply
External
Power Source
100 – 240 VAC 50/60 Hz
Power Consumption - Maximum
180W
Energy Saving / Standby Mode
1W Power Consumption - Typical
65W
Power cable length
1.8 m
Operating Conditions
Operating Temperature - Celsius
5o – 35oC
Operating Temperature - Fahrenheit
41o – 95oF
Non-operating Temperature - Celsius
–20° – 60°C
Non-operating Temperature Fahrenheit
29° – 140°F
Operating Humidity
20 – 80% Relative Humidity (non-condensing)
Non-operating Humidity
5 – 95%
Operating Altitude
0 – 2,000 m for FSP.
Non-operating Altitude
0 – 12.192 m (40,000 ft.)
RFID Card Reader
Operating Frequencies
125 kHz, 13.56 MHz
Read Height
≤ 2cm (Typical)
Device Class
HID Protocol
USB HID
NFC RF Standards (in reading CSN)
ISO/IEC 14443 A, ISO/IEC 14443 B
ISO/IEC 15693
NFC Forum Support
Tag Type 1, Type 2, Type 3, and Type 4
in reading CSN
Raw RF Data Rates:
Low-frequency Cards: 1,2050 to 7,812 bps
High-frequency Cards: 106 to 848 bps
Page 6

QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Technical Specifications
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 6
RFID Card Reader
Card Types Supported – 125 kHz
AWID (RDR-698x Compatible)
Cardax UID (RDR-6C8x Compatible)
CASI-RUSCO (GE Security, UTC) (RDR-628x Compatible)
CDVI
COTAG
Deister UID
DIGITAG
Dimpna UID
EM 410x (RDR-6E8x Compatible)
EM 410x Alternate
Farpointe Data (Pyramid) PSC-1 26 Bit ( RDR-647x
Compatible)
Farpointe Data (Pyramid) UID
GProx-II ID
GProx-II UID (IRDR-6G8x Compatible)
HID Prox (RDR-608x Compatible)
HID Prox UID
Hitag 1 & S (RDR-6H8x Compatible)
Hitag 1 & S Alternate
Hitag 2 (RDR-6H8x Compatible)
Hitag 2 Alternate
ID Teck (RDR-6A8x Compatible)
ID Teck Alternate (128 bits)
Indala ASP 26 bit (Motorola) (RDR-638x Compatible)
Indala ASP UID (Motorola)
Indala ASP+ UID (Motorola)
IO Prox (Kantech) (RDR-678x Compatible)
Isonas
Keri NXT UID
Keri PSC-1 26 Bit: (RDR-647x Compatible)
Keri UID (RDR-6K8x Compatible)
Nedap
NexKey, Quadrakey, KeyMate, 2SmartKey (Honeywell)
Nexwatch (Honeywell) (RDR-6N8x Compatible)
Paradox
Postech
Pyramid (Farpointe Data) PSC-1 26 Bit
Pyramid (Farpointe Data) UID
Radio Key (Secura Key -02) RKCx-02 (RDR-6Z8x
Compatible)
ReadyKey Pro UID RDR-6R8x Compatible)
Rosslare
Russwin UID
Secura Key -01 RKCx-01
Card Types Supported – 13.56 kHz
CEPAS
e-TAG
Felica (NFC Type 3)
HID iCLASS
I-Code
ISO 14443B
I-tag
Legic Advant
MIFARE Classic (32 bits)
MIFARE DESFire
MIFARE DESFire EV1
MIFARE Plus
Page 7

QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Technical Specifications
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 7
MIFARE Ultralight
my-d CSN (Infinion)
NFC Type 1
NFC Type 2
NFC Type 4
NTWare
Oyster
RDR-758x Equivalent (iCLASS, ISO 14443A, ISO 15693)
Tag-It (Texas instruments)
Environmental
Features and
Certifications
Low Halogen
Yes
NOTE: External power supplies, power cords, cables and peripherals are not Low Halogen. Service parts
obtained after purchase may not be Low Halogen.
Agency Approvals and Certifications
CE, CB (IEC 60950 /IEC 60601-1-2: 2015/IEC
62368)/KCC/ICE/ISO 9241-307/Low Blue
Light/UL/CCC/CEL/CECP/SEPA Certified
Edge/VCCI/FCC/BSMI/WEEE/Skype for Business Certified.
Agency approvals and certification of RF:
1. CE: EN 300 330 V2.1.1 (2017-02)
2. CE EMF - EN62311:2008
3. ACMA - AS/NZS: 4268:2017
4. ACMA EMF Radiocommunications (Electromagnetic
Radiation-Human Exposure) Standard 2014 AS/NZS
2772.2: 201
5.FCC - Part15.225 part 15C
6.NCC-- LP0002
7.Japan-- JRF ARIB-T66
8.Korea—MIC
9.China-SRRC
China Energy Label
CEL Grade 2
SmartWay Transport Partnership - NA
only
Yes (NA SKU)
Unit Product/Package
Specifications
Product Dimensions
Unpacked with stand (W x D x H)
65.06 x 24.69 x 55.49 cm
25.61 x 9.72 x 21.85 in
Product Dimensions
Packed (W x D x H)
76.8 x 30.5 x 52.7cm
30.24 x 12.01 x 20.75 in
Display Head Dimensions
Unpacked without stand (W x D x H)
65.06 x 4.372 x 41.68 cm
25.61 x 1.72 x 16.41 in
Base Area Footprint
(W x D x H)
246.99 x 329.99 cm
9.72 x 12.99 in
Product Weight
Unpacked with sand
8.81kg
19.38 lb
Product Weight
Packed
12.45kg
27.39 lb
Product Weight
Head only
5.45 kg
11.99 lb
Page 8

QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Technical Specifications
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 8
Pallet Information
Pallet Dimensions (L x W x H)
930 x 780 x 120 mm
1230 x 780 x 120 mm
36.6 x 30.7 x 4.7 in
48.4 x 30.7 x 4.7 in
Pallet Total Weight
149.4 kg/199.2 kg
328.68 lbs/438.24lbs
Pallet Layers
3(4) Pallet Product per Layer
4 Total Products per Pallet
12(16)
Container Load, 20-Foot
196 units
Container Load, 40-Foot
420 units
Container Load, 40-Foot HighQ
420 units
Software Included
HP Image Auto Rotate Utility, HP Healthcare Edition DICOM Calibration Tool (end user can download
from website)
Accessories Included
What's in the box?
AC power cord (1.8m) 6.2 ft, VGA cable (1.8m) 5.9 ft, HDMI
cable (1.8m) 5.9 ft, USB A to B cable (1.8m), DP Cable
(1.8m), USB C to C cable (1.8m)
User Guide / Warranty
Languages
English, Arabic, S. Chinese, T. Chinese, Czech, Danish, Dutch, Finnish, French, German, Greek, Hungarian,
Italian, Japanese, Kazakh, Korean, Norwegian, Polish, Brazilian Portuguese, Russian, Slovenian,
Spanish, Swedish and Turkish.
Page 9

QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Technical Specifications
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 9
© Copyright 2018 HP Development Company, L.P. The information contained herein is subject to change without notice. The only
warranties for HP products and services are set forth in the express warranty statements accompanying such products and
services. Nothing herein should be construed as constituting an additional warranty. HP shall not be liable for technical or editorial
errors or omissions contained herein.
ENERGY STAR® and the ENERGY STAR® mark are registered trademarks of the U.S. Environmental Protection Agency. DisplayPort™
and the DisplayPort™ logo are trademarks owned by the Video Electronics Standards Association (VESA®) in the United States and
other countries. USB Type-C™ is a trademark of USB Implementers Forum.
EPEAT® registered where applicable. EPEAT registration varies by country. See www.epeat.net for registration status by country.
See HP’s 3rd party option store for solar energy accessory at www.hp.com/go/options
Page 10

QuickSpecs
HP Healthcare Edition HC270cr QHD Clinical Review
Monitor
Summary of Changes
c05992641 — DA16233 — Worldwide — Version 1 — April 27, 2018
Page 10
Change Log
V1 to V2
 Loading...
Loading...