horiba Micros 60 User Manual

ABX Micros 60
User manual
HORIBA ABX
Rue du caducée - Parc Euromédecine
34184 MONTPELLIER Cedex 4 - FRANCE
P/n: RAB042FEN

ABX Micros 60
Introduction
1. Revisions
Tab. 1: User Manual revisions
Technical
Index
note
Ba RAH911AA V1.4 All 05/23/02
Bb RAH911AA V1.4 1,3,6 11/04/02
Ca RAH939AA V1.6 All 12/10/02
Cb ECR1354 V1.6 UL correction 1 01/09/03
Da RAH986AA V1.6 CE IVD Norms Intro, 1 16/09/03
EEN RAN153AA V1.6 HORIBA ABX All 09/03/05
FEN RAN252AA V1.7.0
◆ This document applies to the latest higher software version.
◆ When a subsequent software version changes the information in this document, a new issue will
be released.
Software
revision
Modifications Section Date
see 10.1. Corrections associated to
V1.7.0. Software version, page Intro-
duction-16
All 15/06/06
HORIBA ABX
B.P. 7290
Rue du caducée
Parc Euromédecine
34184 MONTPELLIER Cedex 04 - FRANCE
Tel: (33) 4 67 14 15 16
Fax: (33) 4 67 14 15 17
2 - RAB042FEN - User Manual - ABX Micros 60

Introduction
1.1. Declaration of conformity
◆ Latest version of the CE declaration of conformity for this instrument is available on www.horiba-
abx.com
1.2. Notice of liability
The information in this manual is distributed on an "As Is" basis, without warranty. While every precaution has been taken in the preparation of this manual, HORIBA ABX will not assume any liability
to any persons or entities with respect to loss or damage, caused or alleged to be caused directly or
indirectly by not following the instructions contained in this manual, or by using the computer software and hardware products described herein in a manner inconsistent with our product labeling.
1.3. Trademarks
Other product names mentioned within this publication may be trademarks or registered trademarks
of other companies.
1.4. Copyright ® 2005 by HORIBA ABX
All rights reserved. No part of this book may be reproduced or transmitted in any form or by any
means, electronic, mechanical, photocopying, recording, or otherwise, without the prior written permission of HORIBA ABX.
1.5. Potential hazards
To alert the operator of potentially hazardous conditions, one of the bold captioned headings which
are described below is provided wherever necessary throughout this text.
WARNING: Flags a procedure that if not followed properly, can prove to be extremely hazardous to either the operator or the environment or both.
CAUTION: Emphasizes an operating procedure that must be followed to avoid
possible damage to the instrument or erroneous test results.
NOTA: Emphasizes the important information especially helpful to the operator
before, during or after a specific operational function.
ABX Micros 60 - User Manual - RAB042FEN - 3

ABX Micros 60
2. Warning and precautions
◆ Work safety reliability and general characteristics are guaranteed by HORIBA ABX under the fol-
lowing conditions:
- User manual must be enterely read and personnel trained by HORIBA ABX before attempting
to operate instrument.
- The user always operates with full knowledge and appreciation of instrument warnings, alarms
and flags.
- Always refer to labeling and HORIBA ABX instructions in order to avoid to compromise system
integrity.
◆ The instrument must be operated as instructed in the user manual. Any other use might compro-
mise system integrity and might be hazardous for the operator.
◆The reagents and accessoiries stipulated by HORIBA ABX have been validated
in accordance with the European Directive for in-vitro medical devices (98/
79/CE).
◆The use of any other reagents and accessoiries may place at risk the perfor-
mance of the instrument, engaging the users responsability. In this case,
HORIBA ABX takes no responsability for the device nor for the results rendered.
◆Disposal gloves, eyes protection and lab coat must be worn by the operator.
◆Local or national regulations must be applied in all the operations.
◆Portable/mobile telephones should not be used in proximity of the instru-
ment.
◆All peripheral devices should be IEC compatible.
2.1. Limited guarantee
The duration of guarantee is stipulated in the sales conditions associated with the purchase of this
instrument. To validate the guarantee, ensure the following is adhered to:
1- The system is operated under the instructions of this manual.
2- Only software or hardware specified by HORIBA ABX is installed on the instrument. This software
must be the original copyrighted version.
3- Services and repairs are provided by a HORIBA ABX authorized technician, using only HORIBA
ABX approved spare parts.
4- The electrical supply of the laboratory adheres to national or international regulations.
5- Specimens are collected and stored in normal conditions.
6- Reagents used are those specified in this user manual.
7- Proper tools are used when maintenance or troubleshooting operations are performed.
If this instrument has been supplied to you by anyone other than HORIBA ABX
or an authorised representative, HORIBA ABX cannot guarantee this product in
terms of specification, latest revision and latest documentation. Further information may be obtained from your authorised representative.
4 - RAB042FEN - User Manual - ABX Micros 60
2.2. Safety Precautions
Electronic and moving parts:
The following parts must not be handled or checked by the user:
- Electrical power supply.
- Electronic circuit boards.
◆Operator injury may occur from an electric shock. Electronic components can
shock and injure the user. Do not tamper with the instrument and do not remove any components (covers, doors, panels and so on) unless otherwise instructed within this document.
◆Moving parts: It is strictly forbidden to disable sensors as it may cause op-
erator injuries. Protection covers must not be opened during instrument operations.
◆The battery may explode if it is not replaced correctly! Replace only with the
same or equivalent type recommanded by the manufacturer. Dispose of used
batteries according to the manufacturer’s instructions.
Introduction
Working conditions
2.3. Biological risks
Consider all specimens, reagents, calibrators, controls, etc… that contain human blood or serum as potentially infectious ! Use established, good laboratory working practices when handling specimens. Wear protective gear, gloves,
lab coats, safety glasses and/or face shields, and follow other bio-safety practices as specified in OSHA Blood Borne Pathogens Rule (29 CFR part 1910.
1030) or equivalent bio-safety procedures.
HORIBA ABX uses disinfectant product for instrument decontamination and highly recommends it
to decontaminate your instrument (See Section 6, 1.1.3. General Cleaning of the Instrument,
page 6-3)
3. Working conditions
3.1. Environment
◆ The operation of the ABX Micros 60 should be restricted to indoor location use only. Op-
eration of the instrument at altitudes of over 2000 Meters (6000 feet) is not recommended.
◆ The ABX Micros 60 is designed for safety from voltages surges according to INSTALLATION
CATEGORY II and POLLUTION DEGREE 2 (IEC 61010-1) Please contact your local HORIBA ABX representative for information regarding operation locations, when it does not comply with the recommended specifications.
3.2. Location
◆ The ABX Micros 60 should be placed on a clean and leveled table or workbench.
◆ Please note that the ABX Micros 60, printer and reagents weigh approximately 30 kilo-
grams (66 lbs).
◆ Avoid exposure to sunlight.
◆ Place your instrument where it is not exposed to water or vapor.
ABX Micros 60 - User Manual - RAB042FEN - 5

ABX Micros 60
◆ Place your instrument where it is free from vibration or shock
◆ Place your instrument where an independent power receptacle can be used.
◆ Use a receptacle different from the one used by a device that easily generate noise such as a cen-
trifuge, etc...
◆ Provide a space of at least 20 cm (8 inches) at the back of the instrument for arranging the power
cable and tubings.
The power switch and input voltage supply connection should always be accessible. When positioning the system for operational use, leave the required
amount of space for easy accessibility to these items
3.3. Grounding
Proper grounding is required when installing the system. Check the wall outlet ground (earth) for
proper grounding to the facilities electrical ground. If you are unsure of the outlet grounding, contact your facilities engineer to verify the proper outlet ground.
3.4. Humidity/temperature conditions
The ABX Micros 60 must operate between temperatures of 18 to 32°C (65 to 90°F). Maximum relative humidity 80% for temperatures up to 31°C (88°F). If it is stored at a temperature less
than 10°C (50°F), the instrument should stand for 1 hour at the correct room temperature before
use.
3.5. Electromagnetic environment check
◆ The ABX Micros 60 has been designed to produce less than the accepted level of electro-
magnetic interference in order to operate in conformity with its destination, allowing the correct
operation of other instruments also in conformity with their destination.
◆ In case of suspected electromagnetic noise, check that the instrument has not been placed in the
proximity of electromagnetic fields or short wave emissions, (i. e. Radar, X-rays, Scanners, Cell
phones, etc...)
3.6. Environmental protection
3.6.1. Disposal used accessories and consumables:
Must be collected by a laboratory specialized in elimination and recycling of this kind of material
according to the local legislation.
3.6.2. Disposal ABX Micros 60 instrument:
It should be disposed of, in accordance with local legislation, and should be treated as being contaminated with blood. The appropriate biological precautions should be taken.
If any doubt, please contact your HORIBA ABX representative service department.
6 - RAB042FEN - User Manual - ABX Micros 60
Introduction
Working conditions
▼ European Legislation
In accordance with the European Directive (2002/96/CE, known also as W.E.E.E) instruments having
the above symbol, and sold into a European country by HORIBA ABX or an authorised representative
must be disposed of and recycled correctly at the end of its useful life.
Due to the local changing regulations in each country, please contact your local representative for detailed and upto date information on how to appropriately dispose of the instrument.
3.7. Transportation and storage conditions
◆ Condition for storage and transportation: Temperature from -20°C to +50°C
Prior to the shipping of an instrument by transporter, whatever the destination, an external decontamination of the instrument must be carried out.
ABX Micros 60 - User Manual - RAB042FEN - 7
ABX Micros 60
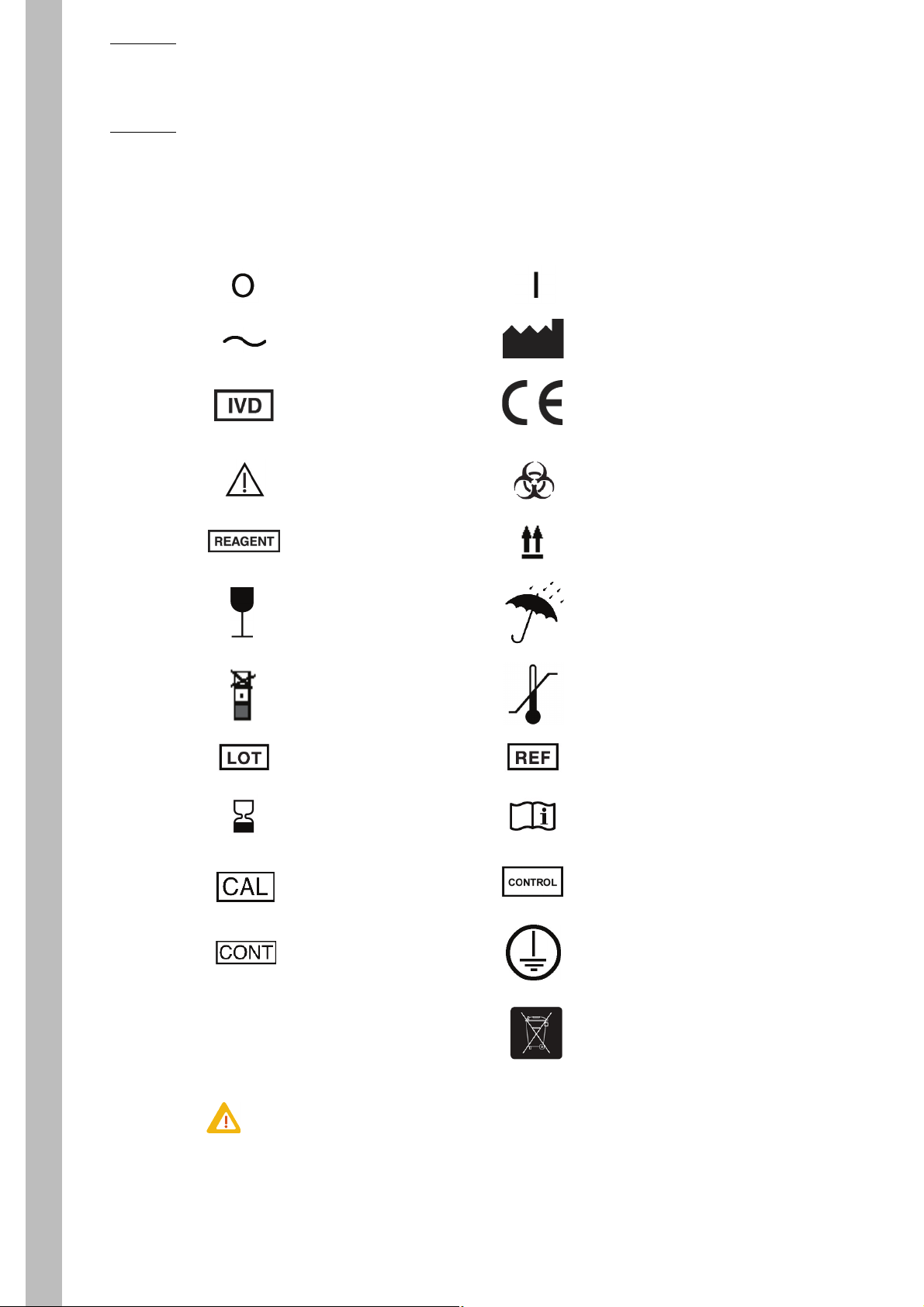
4. Graphics and symbols
Switch off position Switch on position
Alternating current Manufacturer
In Vitro Diagnostic Medical
Device
This product conforms to the
EEC Standards and Directives named in the Declaration of
Conformity.
Caution, consult accompanying
documents
Reagent Up
Fragile, handle with care Keep dry
Do not stack Temperature limitation
Batch code Catalogue number
Use by Consult instructions for use
Calibrator Control
Biological risks
Content Ground
All graphics including screens and printout, photographs are for illustrations
purposes only and are not contractual.
8 - RAB042FEN - User Manual - ABX Micros 60
This product should be disposed
of and recycled at the end of the
useful life in accordance with
the WEEE Directive (2002/96/
CE)

Introduction
5. Labels
5.1. Main power label
In order to replace the 2x1A Fuses located under the power plug connection on the back of the analyzer, carry out the following procedure:
- Do Not remove the instrument protection cover.
- Power OFF the analyzer.
- Disconnect the main power cable from the back power cord receptacle on the analyzer.
- Pull the little flap marked «250V fuse».
- Remove the fuses from their holding receptacle
- Check for the correct ohms on each fuse.
- Use only «Slow-blow» internal fuses.
- Use only fuses having the following characteristics:
◆ for 100/120Vac supply:1A 250V SB
◆ for 220/240Vac supply:1A 250V SB
Labels
5.2. Input/Output Label
Waste: Connect the waste output line to the (Waste position) fitting. Note the Waste label for Waste
output «Only»
Diluent: Connect the Diluent input line to the (Diluent position) fitting. Note the Diluent label for
Diluent input «Only»
Lyse: Connect the Clear tubing marked with a «MINILYSE» label to a straw and place it into the Lyse
reagent container.
Miniclean: Connect the Blue tubing marked with a «MINICLEAN» label to a straw and place it into
the Miniclean reagent container.
RS-232 output connection: Used only by HORIBA ABX qualified Engineers.
Printer connection: Do not connect any printer which has not been recommended by a HORIBA ABX
qualified Engineer.
5.3. «Biological risks» label
This label is located on the left side cover
Fig. 1: Biological risks
ABX Micros 60 - User Manual - RAB042FEN - 9

ABX Micros 60
Consider all specimens, reagents, calibrators, controls, etc… that contain human blood or serum as potentially infectious ! Use established, good laboratory working practices when handling specimens. Wear protective gear, gloves,
lab coats, safety glasses and/or face shields, and follow other bio-safety practices as specified in OSHA Blood Borne Pathogens Rule (29 CFR part 1910.
1030) or equivalent bio-safety procedures.
5.4. Internal label
This label is located on the WBC chamber cover (main covers must be opened to reach it)
Fig. 2: ESD label
Electrostatic Sensitive Device (ESD).
The reagent and chambers are susceptible to cause ESD damage to the instrument motherboard when they are handled without ESD safe handling tools.
Make contact with the instrument ground (cove r s crew fo r example) before proceeding on the chambers in order to prevent electrostatic discharges
10 - RAB042FEN - User Manual - ABX Micros 60

5.5. Identification Label
Introduction
Intended use
Fig. 3: Identification label
6. Intended use
The ABX Micros 60 OS/OT is a fully automated (Microprocessor controlled) Hematology an-
alyzer used for in-vitro diagnostics testing of Whole Blood specimens and Whole Blood component
concentrates.
Important: When analyzing Whole Blood component concentrates, you must
consider the Linear Range of the component parameter and its associated parameters if any. These concentrates may prematurely pollute the counting aperture when analyzing them. It is suggested that you perform 3 Backflushes
and/or a Concentrated Cleaning after analyzing the concentrates.
The ABX Micros 60 OS/OT is available in 5, 8, 16, and 18 parameters. These parameters are
noted according to the system setup (See Section 1, 1.1. Parameters, page 1-3)
The Rate of determination is approximately 60 samples per hour in the optimum configuration. The
system is totally automated,including an internal dilution system, and a Graphic printer for recording all test results including flags and graphics.
7. Presentation
The ABX Micros 60, which is small in size, has 8 main parts.
1- The Electrical supply.
2- The Electronic Main board.
3- The Dilution Pneumatics.
4- The Control panel, including a key pad and LCD screen.
5- A Reagent compartment.
ABX Micros 60 - User Manual - RAB042FEN - 11

ABX Micros 60
6- A Printer that prints out results and Distribution curves.
7- A Smart Card Reader (optional) for Quality Control result records and Patient result records.
8- A Barcode reader (optional) for a direct entry of the Alphanumerical identifications.
ABX Micros 60 Models Available:
This instrument is available in the following different models:
◆ TheABX Micros 60-OT: This model is an «Open Tube» unit «Without» a Smart Card reader.
The operator must remove the cap from the blood collection tube before analyzing any sample.
◆ The ABX Micros 60-OS: This model is an «Open Tube» unit «With» a Smart Card reader.
The Smart Card reader gives the operator the ability to record results and perform automated Quality Control. The operator must remove the cap from the blood collection tube before analyzing any
sample.
OT and OS are indicated in the instrument serial number that identifies the
unit and model.
12 - RAB042FEN - User Manual - ABX Micros 60

Introduction
8. Installation
An HORIBA ABX representative will install your instrument, software, and printer.
8.1. Package contents
A thorough inspection is carried out on the ABX Micros 60 before sending it. We, nev-
ertheless, recommend checking the total system as soon as it is received to report any anomalies to
the carrier.
Verify that all of the parts from the package list are present:
ABX Micros 60-OS/OT boxes contain the following parts :
The
◆ Instrument
◆ Printer
◆ User manual CDROM: RAX056A
◆ Reagents, controls & calibrators CD ROM: RAX055A
◆ Daily guide : RAB180
◆ Power cable (european) : DAC011A or power cable (US) : DAC012A
◆ Installation kit (Bottle or Pack)
Installation
8.1.1. «Bottle» Installation kit
The ABX Micros 60-OT «bottle» installation kit (XEA 314 B) includes :
Tab. 2: Bottle installation kit
DESIGNATION PART NUMBER QTY
Reagent straw L=270 mm XEA017A 3
Sampling needle GBC069AS 1
Diluent/Waste male connector EAC019A 2
Cristal tube 3x6 EAE011A 4
O’ ring 6x1.5 FAA036A 1
O’ ring 1.4x1.25 FAA0053A 2
Rubber stopper 2 holes FBL001A 1
Reagent bottle stopper XDA566A 2
Common installation kit XEA312B 1
8.1.2. «Pack» installation kit
The ABX Micros 60-OT «pack» installation kit (XEA 332 B) includes :
Tab. 3: Pack installation kit
DESIGNATION PART NUMBER QTY
Sampling needle MICROS 60-CT GBC069AS 1
O’ ring 6x1.5 FAA036A 2
O’ring 0.74x1.02 FAA053A 2
Common installation kit XEA312B 1
ABX Micros 60 - User Manual - RAB042FEN - 13

ABX Micros 60
8.2. Reagent connections
(See Section 6, 1.2. Reagent connections, page 6-4)
14 - RAB042FEN - User Manual - ABX Micros 60

Introduction
User manual contents
9. User manual contents
Introduction .............................................................. Introduction-2
Warning and precautions.......................................................................... Introduction-4
Working conditions ................................................................................. Introduction-5
Graphics and symbols .............................................................................. Introduction-8
Labels ................................................................................................... Introduction-9
Intended use........................................................................................ Introduction-11
Presentation ........................................................................................ Introduction-11
Installation.......................................................................................... Introduction-13
User manual contents ............................................................................ Introduction-15
User manual modifications ..................................................................... Introduction-16
Section 1. Specifications .............................................................1-1
Technical Specifications (V1.7.0)............................................................................... 1-3
Physical Specifications............................................................................................. 1-6
Summary of Performance Data .................................................................................. 1-8
Limitations .......................................................................................................... 1-13
Reagent Specifications........................................................................................... 1-19
Reagent Consumption ............................................................................................ 1-20
Waste Handling Procedure ...................................................................................... 1-20
Section 2. Description & technology ..............................................2-1
Description ............................................................................................................ 2-2
Technology ........................................................................................................... 2-3
Section 3. Workflow ....................................................................3-1
Startup checks........................................................................................................ 3-2
Daily Quality Control & Calibration verification ........................................................... 3-4
Sample selection and identification .......................................................................... 3-5
Running Samples ................................................................................................... 3-7
Results ................................................................................................................. 3-9
Flags................................................................................................................... 3-14
Section 4. Quality Assurance ........................................................4-1
Quality Control ...................................................................................................... 4-2
Calibration .......................................................................................................... 4-11
Section 5. Setup .........................................................................5-1
Setup overview ....................................................................................................... 5-2
Results Options ..................................................................................................... 5-3
Laboratory Limits ................................................................................................... 5-7
Special Functions ................................................................................................. 5-10
Date and Time ..................................................................................................... 5-14
Host Computer Options ......................................................................................... 5-15
Barcode Setup ..................................................................................................... 5-17
Memory Card ....................................................................................................... 5-17
Section 6. Maintenance & Troubleshooting .....................................6-1
Maintenance .......................................................................................................... 6-2
Troubleshooting.................................................................................................... 6-18
Menu/Overview..................................................................................................... 6-25
ABX Micros 60 - User Manual - - 15

ABX Micros 60
10. User manual modifications
10.1. Corrections associated to V1.7.0. Software version
V1.7.0 Version # update .................................................................... Chap Introduction-2
declaration of conformity removed...................................................... Chap Introduction-3
weee directive ................................................................................. Chap Introduction-8
Identification Label modified............................................................Chap Introduction-11
PRP specification removed................................................................Chap Introduction-11
Installation procedure removed (technical manual) ..............................Chap Introduction-13
Package content modification...........................................................Chap Introduction-13
addition alphalyse 360...................................................................................... Chap 1-5
Link CD Reagent leaflets.................................................................................... Chap 1-5
Heat Output addition........................................................................................ Chap 1-6
CEIvd approved reagents correction .................................................................. Chap 1-19
Addition CD ROM reagent leaflets RAX055 ......................................................... Chap 1-19
Startup Background limits management on HGB.................................................... Chap 3-3
2SD (standard deviation) replaced by SD in QC ..................................................... Chap 4-9
Calibration general recommendations addition ................................................... Chap 4-11
16 - RAB042FEN - User Manual - ABX Micros 60

Specifications
Section 1. Specifications
Contents
1. Technical Specifications (V1.7) .......................................................................1-3
1.1. Parameters .............................................................................................. 1-3
1.2. Throughput Analysis ................................................................................. 1-4
1.3. Memory Capacity (Smart Cards) .................................................................. 1-5
1.4. Statistics and Quality Control ..................................................................... 1-5
1.5. Reagents ................................................................................................ 1-5
1.6. Calibration .............................................................................................. 1-5
1.7. Measurements and Computation ................................................................. 1-5
1.8. Outputs .................................................................................................. 1-5
1.9. Display ................................................................................................... 1-6
1.10. Barcode Reader Options .......................................................................... 1-6
2. Physical Specifications ...................................................................................1-6
2.1. Power Requirements ................................................................................. 1-6
2.2. Operating Temperature/Humidity ................................................................ 1-6
2.3. Dimensions and Weight ............................................................................. 1-6
2.4. Wastes ................................................................................................... 1-6
2.5. Minimum Sample Volume ........................................................................... 1-6
2.6. Dilution Ratios ........................................................................................ 1-7
2.7. Counting Aperture Diameter ....................................................................... 1-7
2.8. Hemoglobin Measurement ......................................................................... 1-7
3. Summary of Performance Data .......................................................................1-8
3.1. Precision (Reproducibility)* ...................................................................... 1-8
3.2. Precision Claims* ..................................................................................... 1-9
3.3. Precision (Repeatability) ......................................................................... 1-10
3.4. Linearity* ............................................................................................. 1-10
3.5. Carry-over ............................................................................................. 1-11
3.6. Normal Ranges ....................................................................................... 1-11
3.7. Accuracy ............................................................................................... 1-12
3.8. Leucocytes Differential count ................................................................... 1-12
3.9. Sample stability study ............................................................................ 1-12
4. Limitations .................................................................................................1-13
4.1. Maintenance ......................................................................................... 1-13
4.2. Blood Specimens .................................................................................... 1-13
4.2.1. Sample collection and mixing ........................................................... 1-13
4.2.2. Sample Stability ............................................................................. 1-13
4.2.3. Anti-coagulants and their effects (on whole blood) ............................. 1-14
4.3. Known Interfering Substances .................................................................. 1-14
4.3.1. HCT (Hematocrit) ........................................................................... 1-14
4.3.2. RBC Red Blood Cells (Erythrocytes) .................................................... 1-14
4.3.3. WBC White Blood Cells (Leukocytes) .................................................. 1-15
4.3.4. HGB (Hemoglobin) .......................................................................... 1-16
4.3.5. MCV (Mean Corpuscular Volume) ........................................................ 1-16
4.3.6. MCH (Mean Corpuscular Hemoglobin) ................................................. 1-16
4.3.7. MCHC (Mean Corpuscular Hemoglobin Concentration) ........................... 1-16
4.3.8. RDW (Red cell Distribution Width) ..................................................... 1-16
4.3.9. PLT (Platelets) ............................................................................... 1-17
4.3.10. MPV (Mean Platelet Volume) ........................................................... 1-17
4.3.11. LYM # (Lymphocyte count Absolute Number) ..................................... 1-18
4.3.12. LYM % (Lymphocyte Percentage) ..................................................... 1-18
4.3.13. MON # (Monocyte count Absolute Number ) ...................................... 1-18
ABX Micros 60 - User Manual - RAB042FEN - 1

ABX Micros 60
4.3.14. MON % (Monocyte Percentage) ........................................................1-18
4.3.15. GRA # (Granulocyte count Absolute Number) ..................................... 1-18
4.3.16. GRA % (Granulocyte Percentage ) .................................................... 1-18
5. Reagent Specifications ................................................................................. 1-19
6. Reagent Consumption .................................................................................. 1-20
7. Waste Handling Procedure ............................................................................ 1-20
2 - RAB042FEN - User Manual - ABX Micros 60

1. Technical Specifications (V1.7.0)
◆WBC, RBC, and PLT Histograms
◆Quantitative Flags
◆Parameter selection by choice of Software
Note: The ABX Micros 60 performs automated blood counts and requires no man-
ual operations for aspirating blood, dilutions, measuring, calculations, print-outs, and
computer transfer of data. The parameters are given according to the Internal Setup
1.1. Parameters
Specifications
Technical Specifications (V1.7.0)
Tab.1-1:
Tab.1-2: ABX Micros 60 - 8 parameters
ABX Micros 60 - 5 parameters
5 parameters
WBC White Blood Cells
RBC Red Blood Cells
HGB Hemoglobin
HCT Hematocrit
MPV Mean Platelet Volume
RBC Distribution Curve
8 parameters
WBC White blood cells
RBC Red blood cells
HGB Hemoglobin
HCT Hematocrit
MCV Mean Corpuscular Volume
MCH Mean Corpuscular Hemoglobin
MCHC Mean Corpuscular Hemoglobin Concentration
PLT Platelets
RBC and PLT Distribution Curves
Tab.1-3: ABX Micros 60 - 16 parameters
16 parameters
WBC White blood cells
LYM % Lymphocyte Percentage
LYM # Lymphocyte Absolute number
MON % Monocyte Percentage
MON # Monocyte Absolute number
GRA % Granulocyte Percentage
GRA # Granulocyte Absolute number
ABX Micros 60 - User Manual - RAB042FEN - 3

ABX Micros 60
Tab.1-3: ABX Micros 60 - 16 parameters
16 parameters
RBC Red blood cells
HGB Hemoglobin
HCT Hematocrit
MCV Mean Corpuscular Volume
MCH Mean Corpuscular Hemoglobin
MCHC Mean Corpuscular Hemoglobin Concentration
RDW Red cell Distribution Width
PLT Platelets
MPV Mean Platelet Volume
WBC, RBC, and PLT Distribution Curves
Tab.1-4: ABX Micros 60 - 18 parameters
18 Parameters
WBC White blood cells
LYM % Lymphocyte Percentage
LYM # Lymphocyte Absolute number
MON % Monocyte Percentage
MON # Monocyte Absolute number
GRA % Granulocyte Percentage
GRA # Granulocyte Absolute number
RBC Red blood cells
HGB Hemoglobin
HCT Hematocrit
MCV Mean Corpuscular Volume
MCH Mean Corpuscular Hemoglobin
MCHC Mean Corpuscular Hemoglobin Concentration
RDW Red cell Distribution Width
PLT Platelets
MPV Mean Platelet Volume
PDW Platelet Distribution Width
PCT Plateletcrit
WBC, RBC, and PLT Distribution Curves
PCT and PDW have not been established as indications for this product, in the United
States. The use of PCT and PDW should be restricted to Research Use Only.
1.2. Throughput Analysis
◆Approximately 60 Samples/ hour.
4 - RAB042FEN - User Manual - ABX Micros 60
1.3. Memory Capacity (Smart Cards)
◆Last sample «ONLY».......................Internal Memory capacity
◆60 samples...................................Memory Smart Card option
◆99 samples...................................Quality Control Smart Card option
1.4. Statistics and Quality Control
◆Extended Quality Control package (Optional).
◆Quality Control Smart Card option.
1.5. Reagents
3 Reagents or 1 Pack of Reagents
Specifications
Technical Specifications (V1.7.0)
◆Diluent: ......................................ABX MINIDIL LMG (10L)
◆Cleaner: ......................................ABX MINICLEAN (1L)
◆Lyse: ..........................................ABX MINILYSE (1L) or
..........................................ABX MINILYSE LMG (1L) or
..........................................ABX ALPHALYSE 360 (0.36L) or
..........................................ABX LYSEBIO(0.4L or 1L)
◆all reagents: .................................ABX MINIPACK LMG (4.2L)
The CD ROM RAX055 delivered with your instrument provides Reagents, Controls and
Calibrators leaflets/msds. Latest versions of these documents are available on www.horiba-abx.com/documentation.
1.6. Calibration
◆Automatic Calibration procedure.
◆Direct entering of Calibration Coefficients.
1.7. Measurements and Computation
◆Impedance change for WBC, RBC, PLT
◆Spectrophotometry for HGB
◆Impedance change for LYM%, MON%, GRA%
◆Computation from stored Data that was directly measured for MCV, MCH, MCHC, RDW, MPV,
LYM#, MON#, GRA#
1.8. Outputs
◆Hard Copy printing
◆External output (RS232)
ABX Micros 60 - User Manual - RAB042FEN - 5

ABX Micros 60
1.9. Display
◆LCD Screen: ................................. 2 Lines of 40 characters, backlighted
1.10. Barcode Reader Options
◆EAN 8, EAN 13, C 39, C 128, ITF (2of5), CODABAR, STF, and
◆C 93 with or without Checksum.
2. Physical Specifications
2.1. Power Requirements
◆Power supply: .............................. 100V, 240V (+/- 10%)
.................................................... 50/60Hz
◆Power Consumption:...................... Maximum: 150VA (-30%, +10%)
.................................................... In use: 110VA (-30%, +10%)
.................................................... Stand-by mode: 35 VA (-30%, +10%)
◆Heat output................................. 197Kj/h (187BTU/h)
2.2. Operating Temperature/Humidity
◆18 to 32°C (65 to 90°F)
◆Maximum relative Humidity, 80% for temperatures up to 31°C (88°F) decreasing linearly to
50% relative humidity at 40°C (104°F).
◆Avoid exposure to direct sunlight.
◆Avoid exposure to air conditioning and or heating ducts.
2.3. Dimensions and Weight
◆Height: ....................................... Approximately 440mm (16.5 inches)
◆Width: ........................................ Approximately 360mm (14.2 inches)
◆Depth: ........................................ Approximately 330mm (12.6 inches)
◆Weight:....................................... Approximately 14Kgs (31 lbs)
2.4. Wastes
◆Automatic disposal.
◆Waste handling according to Local/National regulations.
2.5. Minimum Sample Volume
◆Minimum blood sample requirement:. .......... 50µl
◆Analyzer sample volume:................. .......... 10µl
6 - RAB042FEN - User Manual - ABX Micros 60

2.6. Dilution Ratios
◆WBC: ...........................................Approximately 1/250
◆RBC/PLT: ......................................Approximately 1/15000
2.7. Counting Aperture Diameter
◆WBC: ...........................................80µm
◆RBC:............................................50µm
2.8. Hemoglobin Measurement
◆Performed in the WBC/HGB Chamber
◆Light source: LED (Light Emiting Diode) at wavelength 550nm
Specifications
Physical Specifications
ABX Micros 60 - User Manual - RAB042FEN - 7
ABX Micros 60
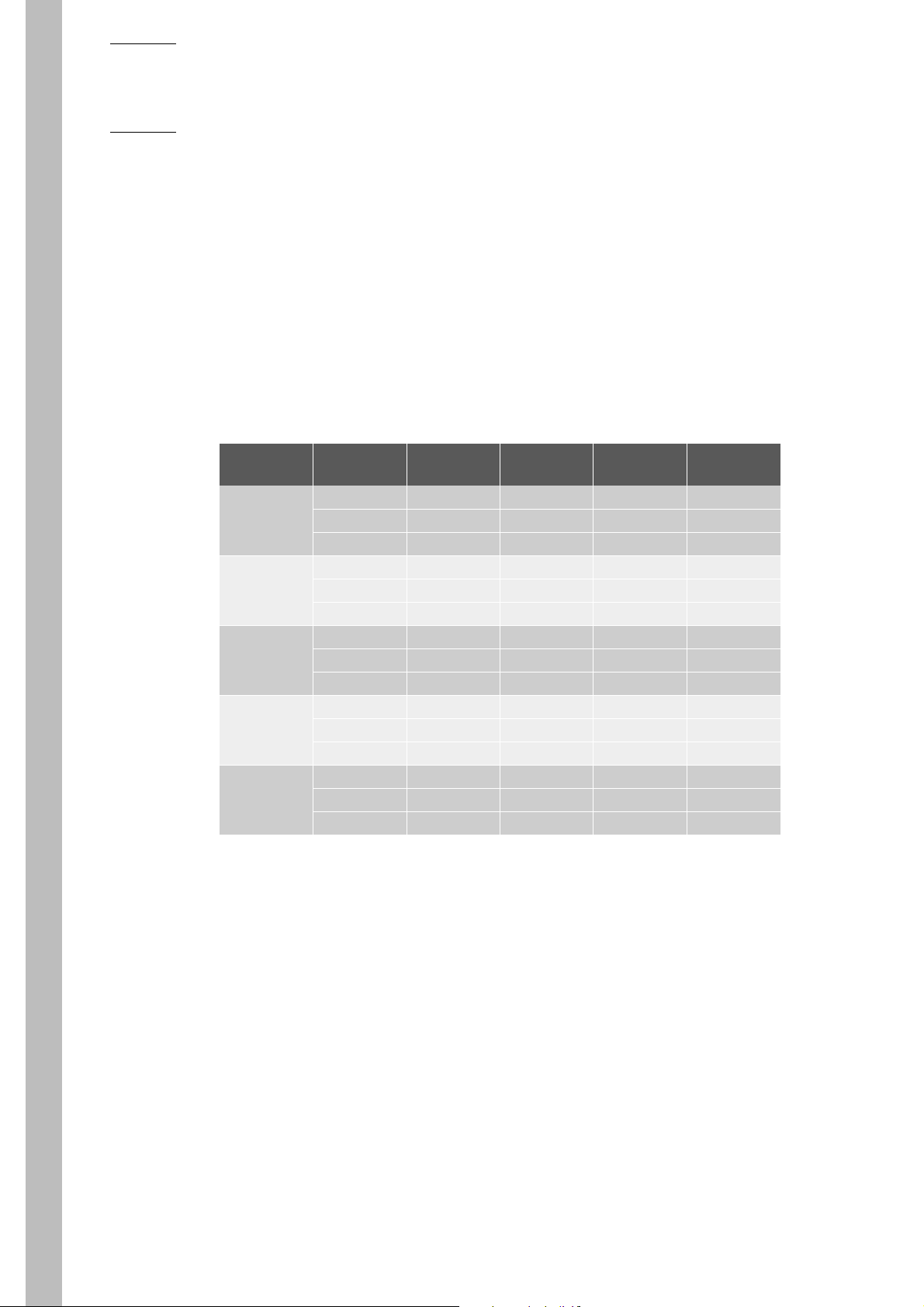
3. Summary of Performance Data
3.1. Precision (Reproducibility)*
The ABX Micros 60 was initially calibrated with Minocal Calibrator (Lot N° MCAL 325 Expiry
Date: 11-05-2002).
Three levels of ABX Minotrol 16 control material (Lot N°: M243) were run in duplicate twice daily
for 20 days. The results were used to quantify within run precision, SD of the run means, SD of the
daily means, and the Total Imprecision with the NCCLS EP-5 Guidelines.
.
Tab.1-5:
Parameter
WBC M243 Normal
RBC M243 Normal
M243 Low
HGB M243 Normal
HCT M243 Normal
PLT M243 Normal
M243 Low
MINOTROL 16
Control
M243 High
M243 Low
M243 High
M243 High
M243 Low
M243 High
M243 Low
M243 High
Within RunSDSD of Runs
SD of Daily
Means
0.23 0.20 0.19 0.29
0.10 0.08 0.11 0.14
0.06 0.03 0.04 0.06
0.05 0.04 0.04 0.06
0.05 0.04 0.04 0.06
0.04 0.03 0.03 0.04
0.31 0.23 0.40 0.49
0.12 0.16 0.43 0.45
0.09 0.22 0.29 0.33
0.52 0.79 0.96 1.17
0.56 0.51 0.74 0.91
0.26 0.26 0.32 0.42
13.71 6.49 9.07 14.04
9.17 7.23 5.87 10.13
5.83 3.78 3.69 4.97
Means
Total imprecision (SD)
8 - RAB042FEN - User Manual - ABX Micros 60

Tab.1-6:
Parameter
WBC M243 Normal
RBC M243 Normal
M243 Low
HGB M243 Normal
HCT M243 Normal
PLT M243 Normal
M243 Low
MINOTROL 16
Control
M243 High
M243 Low
M243 High
M243 High
M243 Low
M243 High
M243 Low
M243 High
Specifications
Summary of Performance Data
Within run
CV%
1.19 1.01 0.96 1.46
1.39 1.11 1.46 1.93
3.06 1.68 1.93 3.13
0.9 0.62 0.78 1.1
1.02 0.88 0.8 1.24
1.46 1.07 1.15 1.72
1.72 1.28 2.23 2.7
0.85 1.17 3.17 3.33
1.49 3.5 4.65 5.37
1.06 1.62 1.96 2.39
1.51 1.38 2 2.47
1.6 1.6 1.96 2.53
2.89 1.37 1.91 2.96
9.17 7.23 5.87 10.13
5.14 6.87 6.70 9.04
CV% of run
means
CV% of Daily
means
Total imprecision (CV%)
Evaluation of Precision Performance of Clinical Chemistry Devices; Approved Guidelines, NCCLS document EP-5 (ISBN 1-56238-145-8) 1999
*Source 510K submission # K030799
3.2. Precision Claims*
Tab.1-7:
Parameters %CV Nominal Values
WBC <2.5% 10.0 x 103 /µL
RBC <2.0% 5 x 106 /µL
HGB <1.5% 15 g /dL
HCT <2.0% 45 %
PLT <5.0% 300 x 103 /µL
*Source 510K submission # K030799
ABX Micros 60 - User Manual - RAB042FEN - 9

ABX Micros 60
3.3. Precision (Repeatability)
Based on 20 consecutive samplings from 1 Fresh Normal Whole Blood, without any alarms.
Tab.1-8:
Precision Table: N = 20
Parameters
WBC <2.5% 10.0 x 103 /µL
RBC <2.0% 5 x 106 /µL
HGB <1.5% 15 g /dL
HCT <2.0% 45 %
PLT <5.0% 300 x 103 /µL
%CV Test Level
3.4. Linearity*
◆Linearity range: The Manufacturer’s tested linearity zone of the instrument using linearity kits
and/or human blood.
◆Linearity limits: Maximum and minimum values within the instrument returns no dilution
alarm.
◆Visible Range: Range values given by the instrument. These values (above linearity limits)
are given as an indication. They are given associated with a «D» flag. This Visible range is outside Manufacturer’s range.
◆Linearity Kits: Linearity was tested using available «Low Range» and «Full Range» Linearity
Test kits. The Test kits were analyzed and data was computed according to the Manufacturer’s
instructions.
◆Human Blood: Linearity was also obtained on human blood, using a minimum of 5 dilution
point. The results of this study are as followed
Tab.1-9:
Parameters Linearity Range Linearity limits Visible Range
WBC (103/mm3 ) 0.5 - 122 0 - 100 100 - 150 ± 0.3 ± 5 %
RBC (106/mm3 ) 0.2 - 8.7 0 - 8 8 - 18 ± 0.07 ± 3 %
PLT (103/mm3 ) (A) 10 - 2327 0 - 2200 2200 - 6000 ± 10 ± 10%
PLT (103/mm3 ) (B) 25 - 4990 0 - 4000 4000 - 6000 ± 10 ± 10%
HGB (g/dL) 2.0 - 27 0 - 26 26 - 30 ± 0.3 ± 3 %
HCT (%) 1.8 - 82.3 0 - 80 80 - 90 ± 2.0 ± 3 %
*Source 510K submission # K030799
A: for HGB> 2 g/dL and RBC>0.5x10
B: for HGB< 2 g/dL and RBC>0.5x106/mm
6
/mm
3
3
10 - RAB042FEN - User Manual - ABX Micros 60
Error limit
(Which ever is greater)

Specifications
Summary of Performance Data
3.5. Carry-over
Carry-over was tested by analyzing samples with «High Concentrations» of WBC’s, RBC’s, HGB, and
PLT’s. Each sample was analyzed in triplicate, followed by 3 background cycles. The % Carry-over is
calculated by using the following formula:
Carry-over = (Background1 - Background3)/(Sample3 - Background3) X 100
Parame
Tab.1-10:
Parameters units WBC (103/mm3 ) RBC (106/mm3 ) HGB (g/dL) PLT (103/mm3 )
Blood count Level 63.0 7.58 23.4 988
% Carry-over (Actual) 0.3 0.00 0.0 0.0
% Carry-over (Claimed) < 0.5% <0.5% <0.5% <0.5%
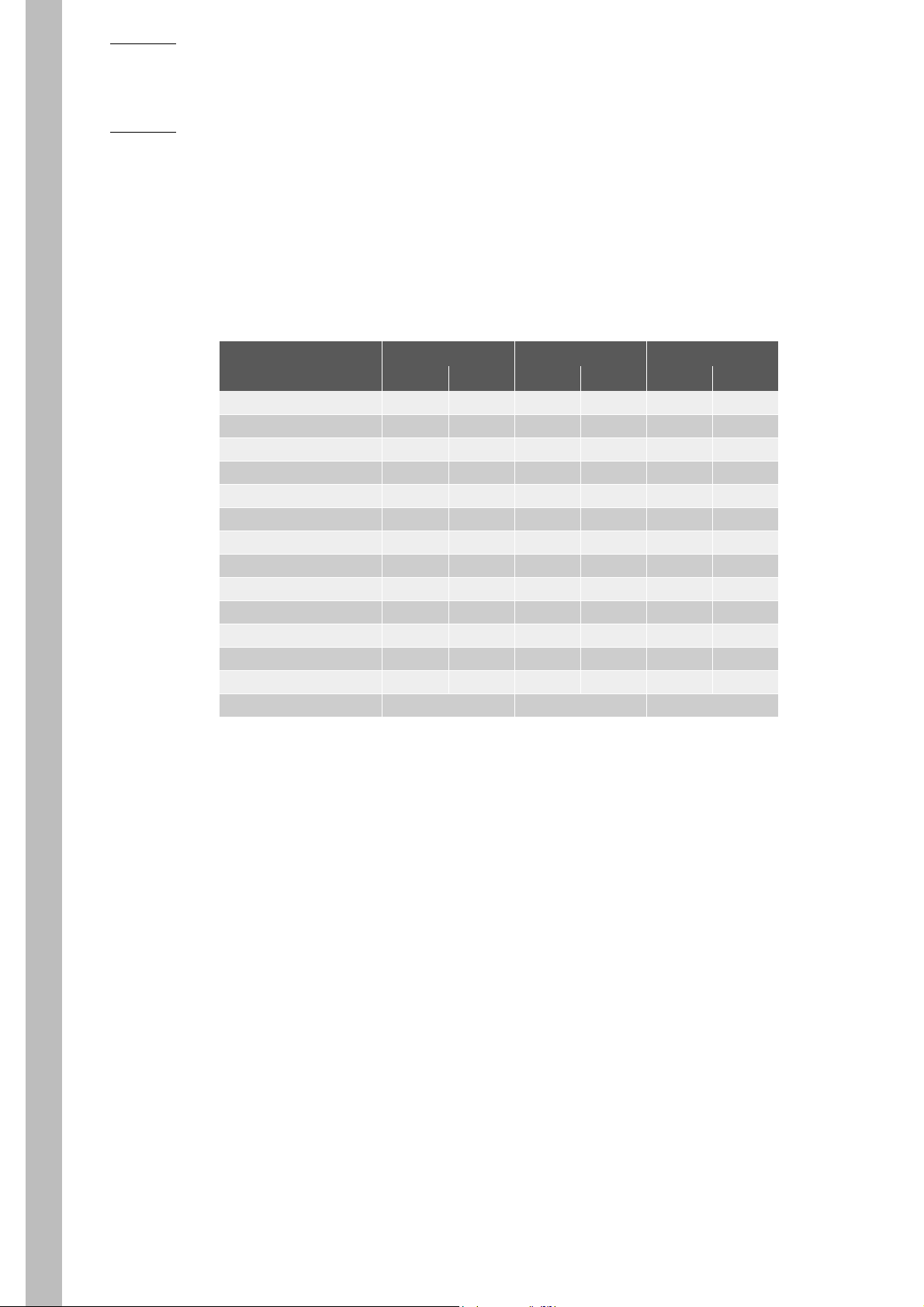
3.6. Normal Ranges
These Normal ranges were established from a study performed in Somerville, NJ. (U.S.A.) This study
encompasses the central 95% of the values, in the distribution of 43 Normal, Healthy, and Drug Free
individuals. These Ranges are as followed:
)
Tab.1-11:
Parameters Male (N=21) Female (N=22)
WBC (103/mm3) 4.7 - 9.6 4.9 - 12.3
Lymphocytes (%) 23 - 47 19 - 41
Monocytes (%) 3 - 6 2 - 6
Granulocytes (%) 49 - 74 53 - 79
RBC (106/mm3) 4.37 - 5.63 3.90 - 5.10
HGB (g/dl) 13.5 - 16.5 12.0 - 15.0
HCT (%) 41 - 50 37 - 45
MCV (µm3) 83 - 101 84 - 96
MCH (pg) 26 - 34 27 - 34
MCHC (g/dl) 32 - 35 32 - 35
RDW (%) 12 - 16 12 - 14
PLT (103/mm3) 145 - 355 150 - 330
MPV (µm3) 7.3 - 9.0 8 - 10
PCT and PDW have not been established as indications for this product, in the United States. The
use of PCT and PDW should be restricted to Research Use Only.
Expected values will vary with sample population and/or geographical location. It is
highly recommended that each laboratory establish its own Normal ranges based upon
the local population!
ABX Micros 60 - User Manual - RAB042FEN - 11
ABX Micros 60
3.7. Accuracy
The Accuracy performance was proven by analyzing approximately 200 patient specimens on the
ABX Micros 60 along with a commercially available Reference Analyzer, located in 3 differ-
ent locations throughout the United States. The following table summarizes the data:
Tab.1-12:
Parameters n R
WBC (103/mm3) 198 0.992 209 0.997 203 0.995
RBC (106/mm3) 198 0.995 212 0.995 204 0.990
HGB (g/dl) 188 0.994 212 0.998 204 0.985
HCT (%) 198 0.980 212 0.994 204 0.982
MCV (µm3) 198 0.988 212 0.987 204 0.980
MCH (pg) 188 0.969 212 0.962 204 0.962
MCHC (g/dl) 188 0.311 212 0.654 204 0.471
RDW (%) 198 0.950 212 0.944 204 0.895
PLT (103/mm3) 169 0.994 201 0.981 198 0.926
MPV (µm3) 191 0.639 204 0.709 203 0.863
Lymphocytes (%) 98 0.975 110 0.991 119 0.461
Monocytes (%) 98 0.552 104 0.787 119 0.461
Granulocytes (%) 98 0.969 105 0.990 119 0.968
Reference Analyzers Baker System 9000 Coulter S Plus IV Coulter JT
Site 1 Site 2 Site 3
2
n R
2
n R
2
n: Number of specimens analyzed
2
: Correlation coefficient from the regression curve Reference/ABX Micros 60
R
As mentioned above, this clinical study was performed at 3 different sites throughout the United
States.
3.8. Leucocytes Differential count
Not available at the time of publication
3.9. Sample stability study
Not available at the time of publication
12 - RAB042FEN - User Manual - ABX Micros 60

Specifications
4. Limitations
Whilst every effort is taken by HORIBA ABX to investigate and indicate all known interference’s, it is by no means possible to guarantee that all interference’s have been identified. At all times, results should be validated and communicated only once all
information relating to the patient has been assessed and taken into account.
4.1. Maintenance
In this Manual, specific Start-up, Shutdown, and Maintenance procedures are listed. The Maintenance procedures listed in this manual are mandatory for the proper use and operation of the ABX
Micros 60.
Failure to execute any of these maintenance procedures may result in «Decreased Reliability» of the system. High emphasis on maintaining the system is strongly suggested.
Limitations
4.2. Blood Specimens
All Blood samples should be collected using proper technique.
Consider all Specimens, Reagents, Calibrators, Controls, etc... that contain Human blood
or serum as potentially infectious.
Use established, good laboratory working practices when handling specimens. Wear protective gear, gloves, lab coats, safety glasses or face shields, and follow other biosafety
practices as specified in OSHA Bloodborne Pathogen Rule (29 CFR Part 1910, 1030) or
other equivalent biosafety procedures.
4.2.1. Sample collection and mixing
When collecting blood specimens, venous blood is recommended, but arterial blood may also be used
in extreme cases. Blood collection must be placed in vacuum or atmospheric sample collection tubes.
(For USA Only). For additional information on collecting venous and capillary samples,
refer to NCCLS document H3-A4 and NCCLS document H4-A4 (sept.1999).
The sample collection tube must be filled to the exact quantity of blood indicated on
the tube itself. Any incorrectly measured blood sample collections will show a variation
in results.
4.2.2. Sample Stability
Fresh Whole Blood specimens are recommended. The ICSH (International Committee for Standardization in Hematology) defines a Fresh blood specimen as «One processed within 4 hours after
collection».
Well mixed Whole Blood specimens, collected in EDTA anti-coagulant and run within eight hours after collection, provide the most accurate results for all parameters. The white cell size distribution
may shift when specimens are assayed between 5 and 20 Minutes after collection and more than 8
hours after collection.
ABX Micros 60 - User Manual - RAB042FEN - 13

ABX Micros 60
4.2.3. Anti-coagulants and their effects (on whole blood)
This is a list of commonly used anti-coagulants used for whole blood collections:
◆Heparin: Causes an increase in cell clumping, (WBC’s and PLT’s) and modifies cytoplasmic color
with Romanowsky staining (Blue background). An increase in HCT and MCV with high heparin
concentrations > 7.5UL /capillary tube.
◆Trisodium Citrate: Because the anti-coagulant is liquid, it includes a dilution estimated at
10/11 when filling 5ml tubes with whole blood. This anti-coagulant is used in coagulation. It
is sometimes used in hematology whe n an EDTA - induc ed Pseudothrombocytopenia is suspected.
◆Acid Citrate Dextrose (ACD) and Citrate Phosphate Dextrose with Adenine (CPDA): The
most commonly used anti-coagulants for cell concentrates (in particular Platelet concentrates)
is normally not used for cell counting. There is no seriously known interference.
Anti-Coagulants used in blood collection may vary in the effects of changing the characteristics of the blood components. Caution is advised when selecting an anti-coagulant for analysis on the ABX Micros 60.
◆EDTA: Amoung the EDTA salts, EDTA K
times NA2 EDTA are used. EDTA K2 and EDTA K3 are the most frequently used anti-coagulants for
Hematology testing Worldwide. Mainly because they have been recommended by ICSH since
1993. The other EDTA salts are acceptable as well. EDTA can include Pseudothrombocytopenia (estimated frequency: 1/800) through Platelet clumping.
◆Fluoride: Was used before EDTA replaced it. No side affects as known so far.
(USA and Japan), EDTA K3 (USA and Europe), and some-
2
4.3. Known Interfering Substances
Verification of any «Abnormal» test result, (including Flagged results or results Outside
their normal range) should be performed using reference methods or other standard lab-
oratory procedures for the conclusive verification of these abnormal results. The section
below starts the list of the known limitations of automated blood cell counters which
use the principle of impedance.
4.3.1. HCT (Hematocrit)
Red Blood cell Agglutination - May produce erroneous HCT and MCV values. Red blood cells agglutination may be detected by observing abnormal MCH and MCHC values, as well as examination of a
stained blood smear in such cases. Manual Laboratory methods may be required to obtain an accurate HCT value.
4.3.2. RBC Red Blood Cells (Erythrocytes)
The Red blood cell dilution contains all the formed elements in the blood: Erythrocytes, Leukocytes,
and Platelets. During the counting of the RBC’s, Platelets are below the RBC size minimum threshold,
therefore they are not counted as RBC’s.
Leukocytes - (White Blood cells) on the other hand, are included in the RBC count.However,since
the normal ratio between Red blood cells and White blood cells is so extreme, the influence of counting the WBC’s during the RBC count is negligible.
High WBC’s - In rare cases where the WBC’s are extremely high, the RBC count may be corrected,
especially if the RBC count is extremely low in comparison to the high WBC count.
14 - RAB042FEN - User Manual - ABX Micros 60
 Loading...
Loading...