Page 1

User Manual
System
Page 2
i
Hologic Inc.
250 Campus Drive
Marlborough, MA 01752 USA
TEL: For Technical Support (US A a nd Cana da)
1-888-PRETERM (1-888-773-8376)
1-800-442-9892
FAX: 1-508-263-2967
TEL: For Technical Support (Outside the USA and Canada)
Asia +852 3526 0718
Australia: +61 2 9888 8000
Austria: 0800 291 919
Belgium: 0800 773 78
Denmark: 8088 1378
Finland: 0800 114 829
France: 0800 913 659
Germany: 0800 183 0227
Ireland (Rep): 1 800 554 144
Italy: 800 786 308
Netherlands: 0800 022 6782
Norway: 800 155 64
Portugal: 800 841 034
Spain: 900 994 197
South Africa: 0800 980 731
Sweden: 020 797 943
Switzerland: 0800 298 921
UK: 0800 032 3318
Rest of the world: 0041.21.633.39.26
Intl Fax number: 0041.21.633.39.10
2013 Hologic Inc. All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any
form or by any means in whole or in part without the prior written permission of Hologic Inc.
The TLi
IQ
System is covered by U.S. patent numbers 6,267,722 and 6,394,952. The TLiIQ Analyzer is covered by U.S. patent number
Des. 434,153. The Cassette Housing is covered by U.S. patent number Des. 432,244.
The Hologic logo, TLiIQ, and TLiIQ QCette are registered trademarks of Hologic Inc.
Printed in the USA 06023-001 Rev 001
Hologic UK
Link 10 Napier Way
Crawley, West Sussex
R
H10 9RA UK
+44 (0) 1293 522 080
IMPORTANT: Read the entire manual before operating the TLi
IQ
®
System.
Page 3

TLiIQ System User Manual ii
If this equipment is used in a manner not specified by the manufacturer, then the protection provided by the equipment
may be impaired.
FCC Notice:
This equipment has been tested and found to comply with the limits of a Class B digital device, pursuant to Part 15 of the
FCC Rules. These limits are designed to provide reasonable protection against harmful interference in a residential
installation. This equipment generates, uses and can radiate radio frequency energy and, if not installed and used in
accordance with the instructions, may cause harmful interference to radio communications. However, there is no
guarantee that interference will not occur in a particular installation. If this equipment does cause harmful interference to
radio or television reception, which can be determined by turning the equipment off and on, the user is encouraged to try
to correct the interference by one or more of the following measures:
Reorient or relocate the receiving antenna.
Increase the separation betw een the equ ipment and receiver.
Connect the equipment into an outlet on a circuit different from that to which the receiver is connected.
Consult the dealer or an experienced radio/television technician for help.
FCC Warning:
Changes or modification not expressly approved by the manufacturer responsible for compliance could void the user's
authority to operate the equipment.
Note: The use of a non-shielded interface cable with this equipment is prohibited.
Page 4

TLiIQ System User Manual iii
CE Notice:
This equipment has been tested and found to be in compliance with the following standards per the IVD Directive:
EN61326-1 Electrical Equipment for Measurement, Control and Laboratory Use
EN55011 Radiated and Conducted Emissions
EN61010-1 Safety Requirements
EN61000-3-2 Harmonic Emissions
EN61000-3-3 Voltage Fluctuations
EN61000-4-2 Electrostatic Discharge
EN61000-4-4 Electrical Fast Transients
EN61000-4-5 Voltage Surges
EN61000-4-6 Conducted Immunity
EN61000-4-11 Voltage Interrupts
Page 5

TLiIQ System User Manual iv
Disposal of Electrical & Electronic Equipment
Waste Electrical and Electronic Equipment (WEEE)
Hologic is dedicated to meeting country specific requirements associated with the environmentally sound treatment of our
products. Our objective is to reduce the waste arising from our electrical and electronic equipment. Hologic realizes the
benefits of subjecting such WEEE equipment to potential reuse, treatment, recycling or recovery to minimize the amount
of hazardous substances entering the environment.
Your Responsibility
As a Hologic c us tomer, you are responsible for ensuring that devices marked with the symbol shown below are not placed
into the municipal waste system unless authorized to do so by the authorities in your area. Please contact Hologic (see
below) prior to disposing any electrical equipment provided by Hologic.
Symbol Used on the Instrument
The following symbol is used on this instrument:
Reclamation
Hologic will provide for the collection and proper reclamation of electrical devices we provide to our customers. Hologic
strives to reuse Hologic devices, subassemblies, and components whenever possible. When reuse is not appropriate,
Hologic will ensure the waste material is properly disposed of.
Do not dispose in municipal waste.
Contact Hologic (see below) for information
regarding proper disposal.
Page 6
TLiIQ System User Manual v
Hologic Contact Information
Corporate Headquarters
HOLOGIC INC.
250 CAMPUS DRIVE
MARLBOROUGH, MA 01752 USA
TEL: (USA and Canada)
1-888-PRETERM (1-888-773-8376)
1-800-442-9892
FAX: 1-508-263-2967
Authorized
Representative - Europe
HOLOGIC (UK) LIMITED
UNIT 2, LINK 10
NAPIER WAY
CRAWLEY, WEST SUSSEX RH10 9RA
UNITED KINGDOM
Tel: +44 1293 522080
FAX: +44 1293 528010
Page 7
TLiIQ System User Manual vi
Symbols Used on the Instrument
The following symbols are used on this instrument:
Warning, refer to
accompanying documents.
Manufactured by
Waste Electrical and Electronic
Equipment - contact Hologic for
disposal of the instrument.
Authorized Representative in
the European Community
Lot
Store between 18°C and 30°C
Catalog number
For in vitro diagnostic testing
Page 8

TLiIQ System User Manual vii
Table of Contents
TABLE OF CONTENTS
Section Page
I Introduction
• Intended Use 1-1
• General Description 1-1
• Components of the Analyzer 1-2
• Keypad 1-3
• Keypad Functions 1-3
• Keypad Entries 1-6
• Cassette Insertion Site 1-11
• Displayed/Printed Results 1-12
• Specifications 1-17
• Cautions and Warnings 1-19
2 Installation
• General 2-1
• Environmental Factors 2-1
• Unpacking 2-2
• System Setup 2-4
Page 9

TLiIQ System User Manual viii
Table of Contents
Section Page
• Getting Started 2-5
• Setting the Date and Time 2-6
• TLi
IQ
QCette® Setup 2-7
• Factory Default Settings 2-8
3 General Operating/Testing Instructions
• Starting the System 3.1
• Set Calibration 3-2
• Test Patient 3-4
• Daily QC 3-7
• Liquid Controls 3-9
• Viewing Results On-Screen 3-12
• Incubation Mode 3-13
• Internal Mode 3-13
• External Mode 3-14
4 Software Functions – Detailed Descriptions
• Startup Screen 4-1
• Main Menu 4-2
Page 10

TLiIQ System User Manual ix
Table of Contents
Section Page
• Set Calibration 4-3
• Test Patient 4-5
• Daily QC 4-10
• Liquid Controls 4-14
• Access Data 4-19
• View/Print Data 4-19
• Data Transfer 4-21
• View Setup 4-23
• Change Setup 4-24
• Date/Time 4-24
• Autoprint 4-27
• Incubation Mode 4-28
• TLi
IQ
QCette® Setup 4-29
• Test Counts 4-33
5 Care of the Analyzer
• General Cleaning 5-1
• Cleaning of Cassette Insertion Site 5-1
• Cleaning Agents Approved for Use 5-1
Page 11

TLiIQ System User Manual x
Table of Contents
Section Page
6 Printer
• Loading Printer Labels 6-1
• Removing an Empty Label Roll 6-4
• Clearing Label Jams 6-5
7 Troubleshooting
• General Information 7-1
• Troubleshooting Table 7-1
• Error/Invalid Codes 7-7
8 Service and Warranty
• Technical Service 8-1
• Contact Information - Technical Service 8-1
• Replacement Parts 8-2
• Contact Information - fFN Customer Service 8-2
• Warranty 8-3
Page 12

TLiIQ System User Manual 1 – 1
Section 1 – Introduction
SECTION I – INTRODUCTION
For In Vitro Diagnostic Use Only
To be used by trained laboratory personnel
INTENDED USE
The Hologic TLi
IQ
®
System is intended to be used in conjunction with the Rapid fFN Cassette, the Rapid fFN Control Kit,
and the TLi
IQ
QCette® for the detection of fetal fibronectin in cervicovaginal secretions. Refer to the directional insert for
the Rapid fFN Cassette for detailed intended use information.
GENERAL DESCRIPTION
The TLi
IQ
®
Analyzer is an electronic optical reflectance device that converts a colorimetric reaction from a cassette into a
digitized format. The data are analyzed using multiple parameters, including a comparison of sample data to calibration
data. The analyzer provides one of three possible patient test results: Positive, Negative, or Invalid.
The result is positive if the signal intensity derived from the patient sample is greater than or equal to the reference
calibration value sp ecified by the calibration code. The result is negative if the signal intensity derived from the p atient
sample is less than the reference calibration va lue specified by the calibrat ion code. The result is reported as invalid if
specific internal test criteria have not been met.
Page 13

TLiIQ System User Manual 1 – 2
Section 1 – Introduction
COMPONENTS OF THE ANALYZER
The major components of the analyzer are the display screen, the keypad, and the cassette insertion site.
Display screen
Cassette
insertion site
Keypad
Page 14

TLiIQ System User Manual 1 – 3
Section 1 – Introduction
KEYPAD
Numeric – Use keypad to enter numerical characters from 0 to 9.
Alpha – Use keypad to enter alpha characters from A to Z.
KEYPAD FUNCTIONS
(Vertical Scroll )
Alpha characters - Use ↑ ↓ to navigate through the alphanumeric keys when selecting
an alpha character.
Scrolling through Data Records - Use ↑ ↓ when scrolling through data records in
ACCESS DATA mode.
Menu Screens - Some menus require up to three screens to display all of the options.
Use
↑ ↓
to go to the next or previous screen of the menu.
Page 15

TLiIQ System User Manual 1 – 4
Section 1 – Introduction
(Left Arrow Key)
Previous Page - Use ← to go to the previous page within a data record.
Delete - Use DELETE to delete characters to the left of the cursor.
(Right Arrow Key)
Next Page - Use → to go to the next page within a data record.
Space - Use SPACE to enter a space in the position of the cursor.
Page 16

TLiIQ System User Manual 1 – 5
Section 1 – Introduction
(Print/Enter Key)
Accept/Confirm - Press ENTER to accept or confirm an entry in any data entry field.
Print - Press PRINT to print a data record.
This print function is only active when a data record is on the display screen.
(Escape Key)
Press ESC to return to the most recent Menu screen, unless otherwise specified. If ESC
is pressed in any screen requiring data entry, all entries will revert to the previous setting.
Page 17

TLiIQ System User Manual 1 – 6
Section 1 – Introduction
KEYPAD ENTRIES
Entries of numerical characters require pressing the appropriate numeric key.
Entries of alpha characters require pressing the numeric key containing the alpha character and the ↑ or ↓ arrows (scroll
keys).
Page 18
TLiIQ System User Manual 1 – 7
Section 1 – Introduction
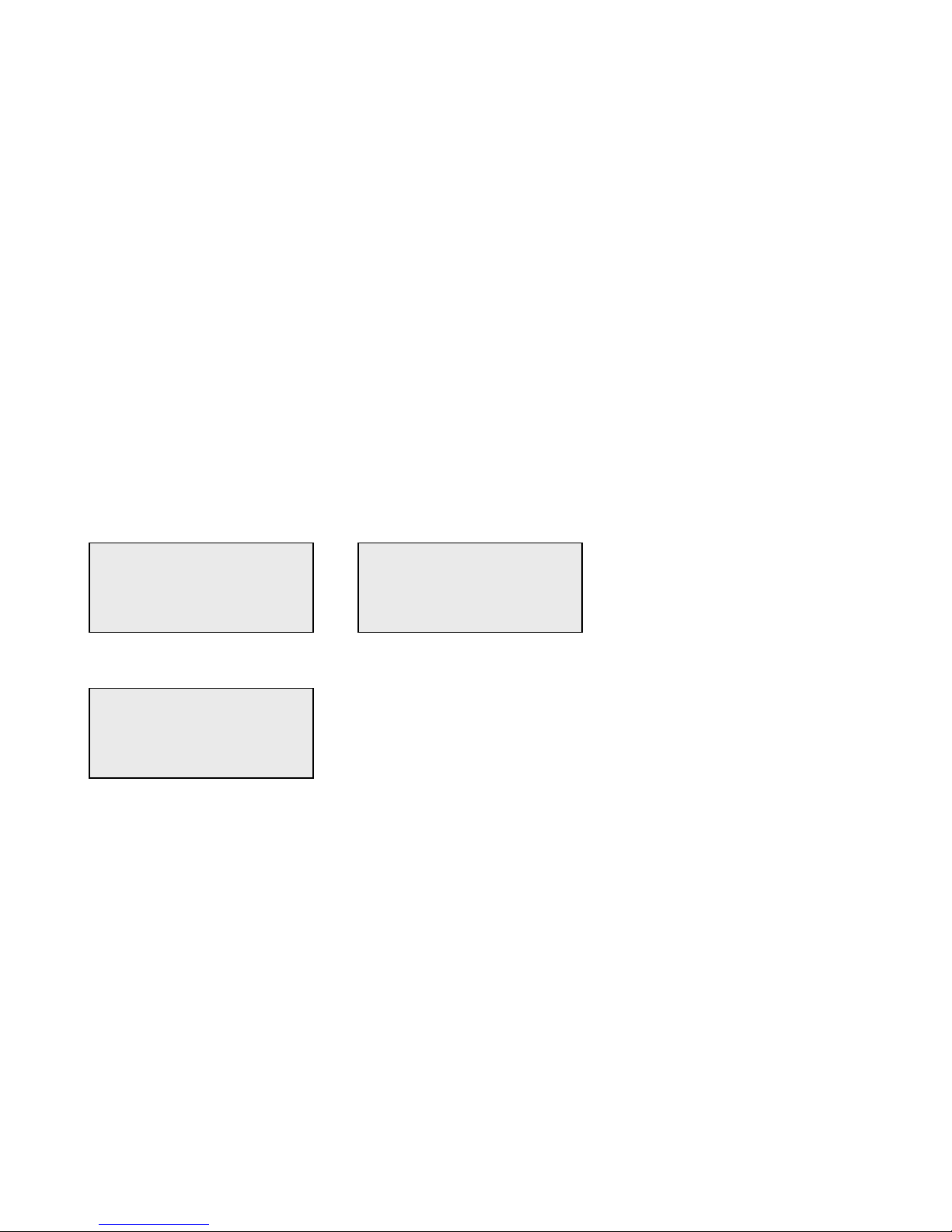
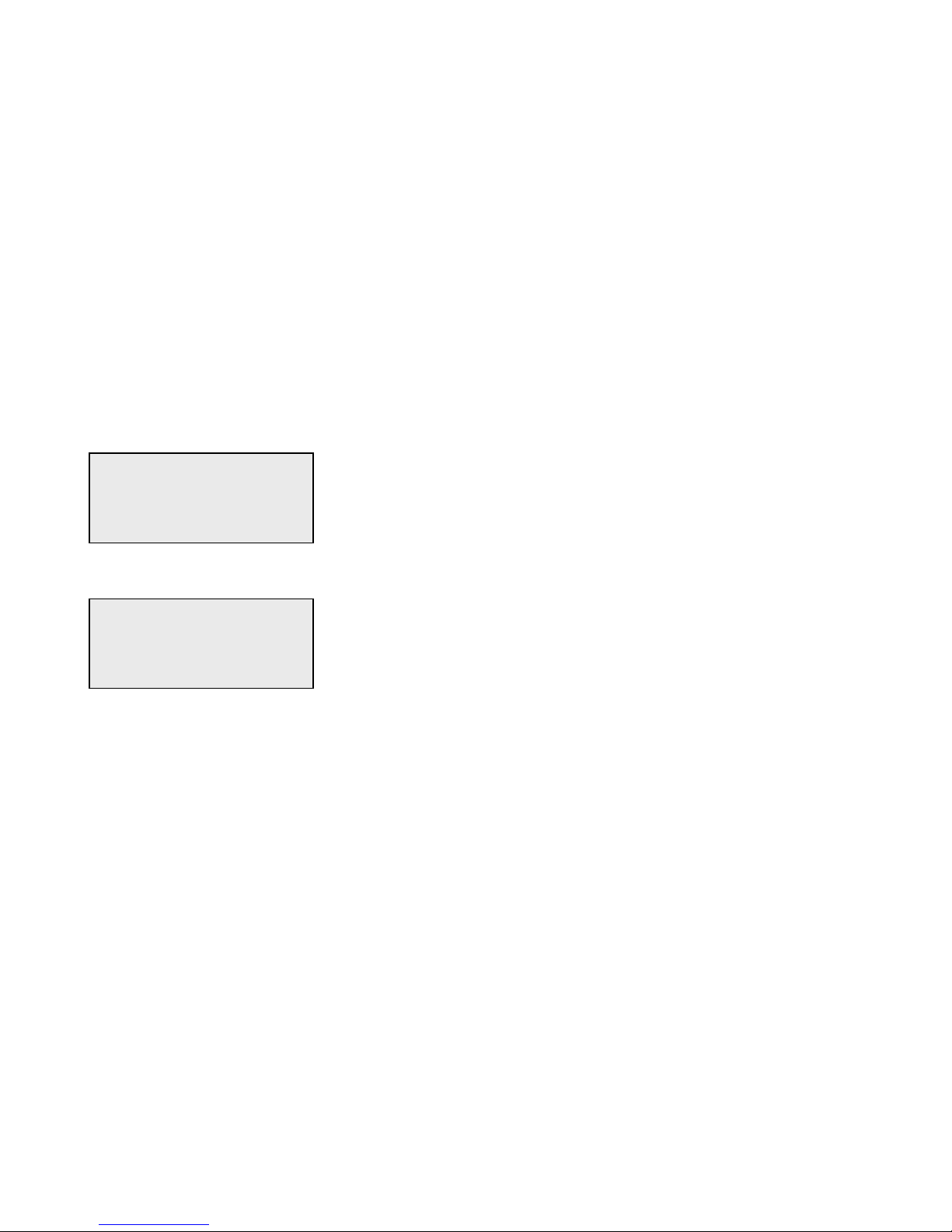
Example - to enter Cassette Lot Number C9123.
1 - Press 2. Use ↑ arrow until C appears on the display screen.
NOTE: The ↑ arrow will scroll repetitively through the characters 2-A-B-C. The ↓ arrow will scroll repetitively through the
characters 2-C-B-A.
2 - Press 9.
CASSETTE LOT #
>2
ENTER - ACCEPT
CASSETTE LOT #
>C
ENTER - ACCEPT
CASSETTE LOT #
>C9
ENTER - ACCEPT
Page 19
TLiIQ System User Manual 1 – 8
Section 1 – Introduction
3 - Press each subsequent number 1, 2, 3.
4 - Press ENTER after all entries have been made.
CASSETTE LOT #
>C912_
ENTER - ACCEPT
CASSETTE LOT #
>C9123
ENTER - ACCEPT
Page 20
TLiIQ System User Manual 1 – 9
Section 1 – Introduction
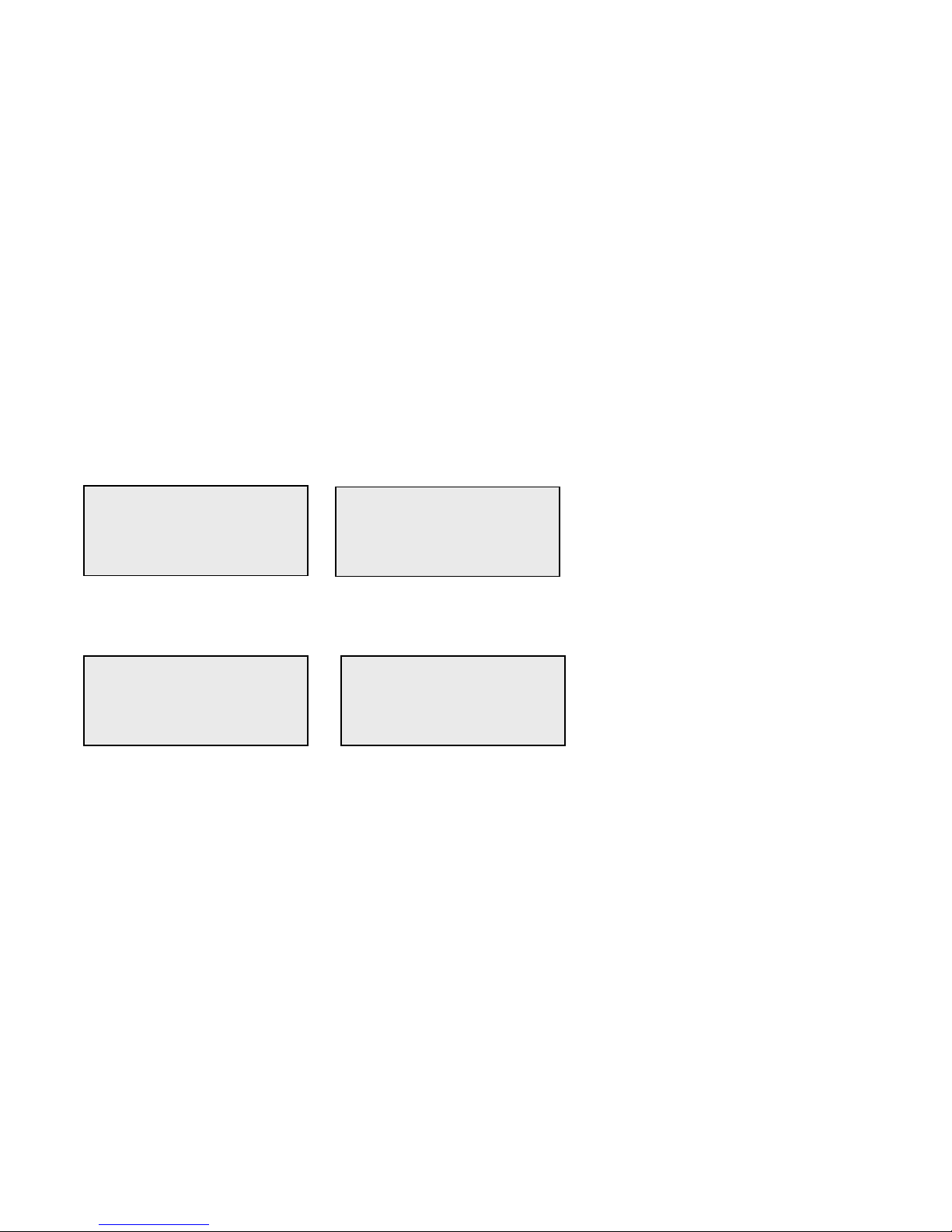
Example - to enter Patient Name ABE
1 - Press 2. Use ↑ or ↓ arrow until A appears on the display screen.
2 - Press 2 again. Use ↑ or ↓ arrow until
B appears on the display screen.
PATIENT ID
>2
ENTER - ACCEPT
PATIENT ID
>A
ENTER - ACCEPT
PATIENT ID
>A2
ENTER - ACCEPT
PATIENT ID
>AB
ENTER - ACCEPT
Page 21

TLiIQ System User Manual 1 – 10
Section 1 – Introduction
3 - Press 3 to enter the next letter. Use ↑ or ↓ arrow until E appears on the display screen.
4 - Press ENTER after all entries have been made.
PATIENT ID
>AB3
ENTER - ACCEPT
PATIENT ID
>ABE
ENTER - ACCEPT
Page 22

TLiIQ System User Manual 1 – 11
Section 1 – Introduction
CASSETTE INSERTION SITE
The Cassette Insertion Site contains a slightly concave trough designed to capture any fluids that may have been spilled
while applying sample to the Rapid fFN Cassette. This area of the instrument should be cleaned regularly (see Section 5).
Page 23
TLiIQ System User Manual 1 – 12
Section 1 – Introduction
DISPLAYED/PRINTED RESULTS
Each test result is displayed on the analyzer display screen. A test result requires three screens to display all of the data
associated with the result. With AUTOPRINT ON, the test result is autom atic all y printed. Eac h print ed result requires one
printer label. Results can be printed from any data record screen either immediately after a test or in DATA ACCESS
mode.
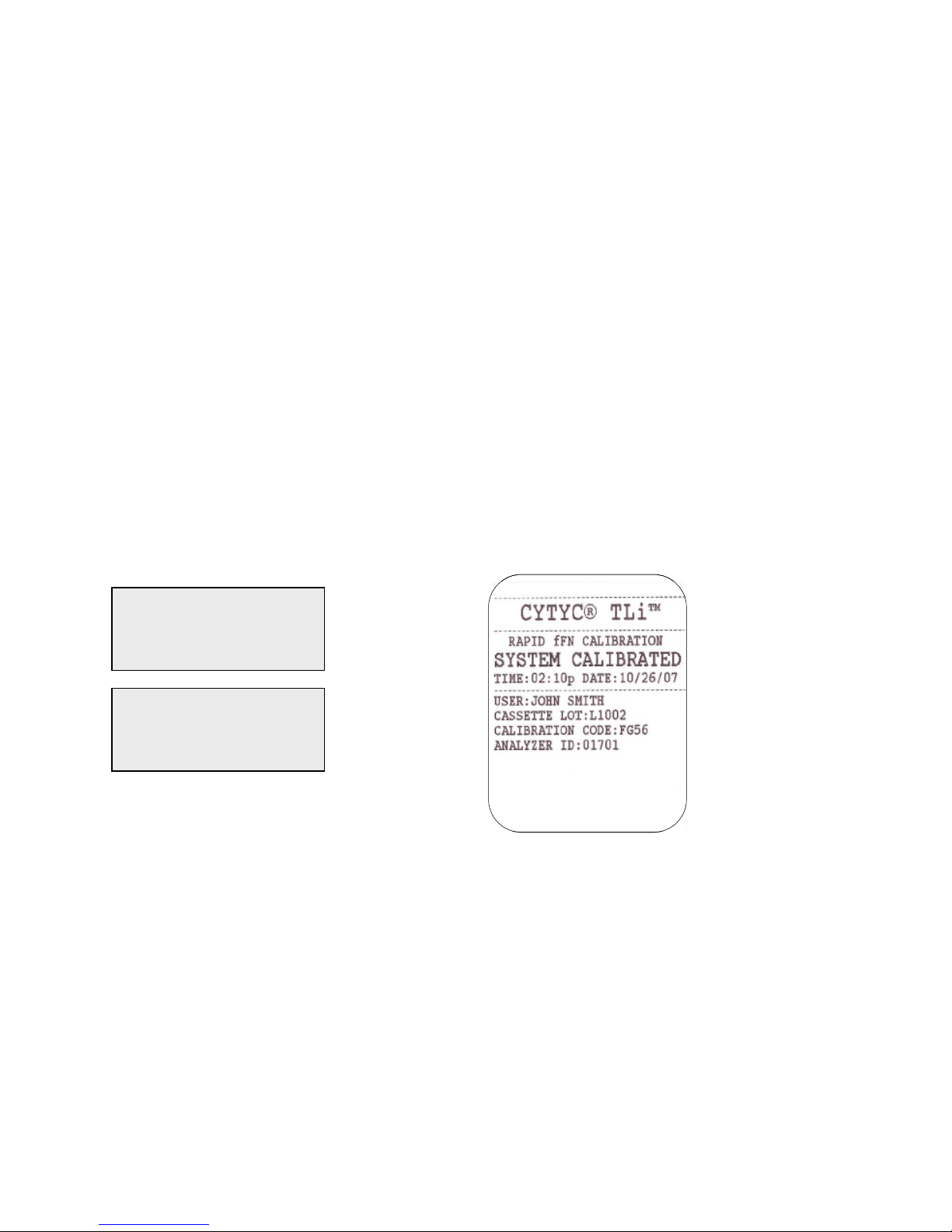
Example: Displayed/Printed Results of Calibration Record
Displayed Printed
CAL CODE:FG56
CASSETTE LN:L1002
ANALYZER ID:01701
ESC-MAIN MENU ←
FETAL FIBRONECTIN
02:10 PM 10/26/07
SYSTEM CALIBRATED
USER:XXXXXXXXXXXX→
Page 24
TLiIQ System User Manual 1 – 13
Section 1 – Introduction
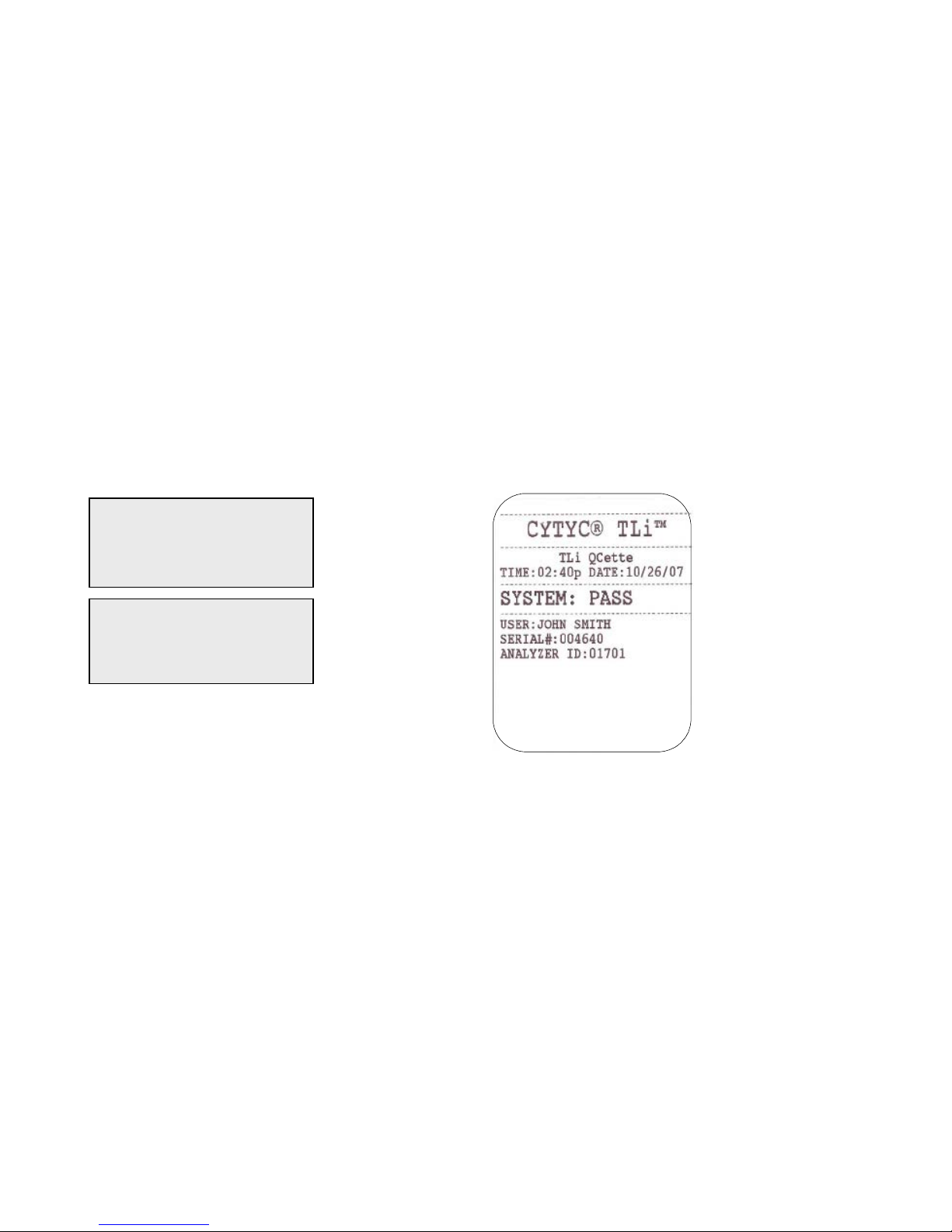
Example: Displayed/Printed Re sults of TLi
IQ
QCette®
Displayed Printed
QCette
02:40 PM 10/26/07
SYSTEM: PASS
USER:XXXXXXXXXXXX →
CAL CODE:FG56
QCette SN:004640
ANALYZER ID:01701
ESC-MAIN MENU ←
Page 25

TLiIQ System User Manual 1 – 14
Section 1 – Introduction
Example: Displayed/Printed Re sults of Negative Control Record
Displayed Printed
CAL CODE:FG56
CASSETTE LN:L1002
ANALYZER ID:01701
ESC-MAIN MENU ←
INTERNAL CONTROLS
USER:XXXXXXXXXXXXXX
ANALYZER: PAS S
CASSETTE: PASS ← →
FETAL FIBRONECTIN
02:45 PM 10/26/07
NEG CTL:M1023
RESULT:PASS →
Page 26
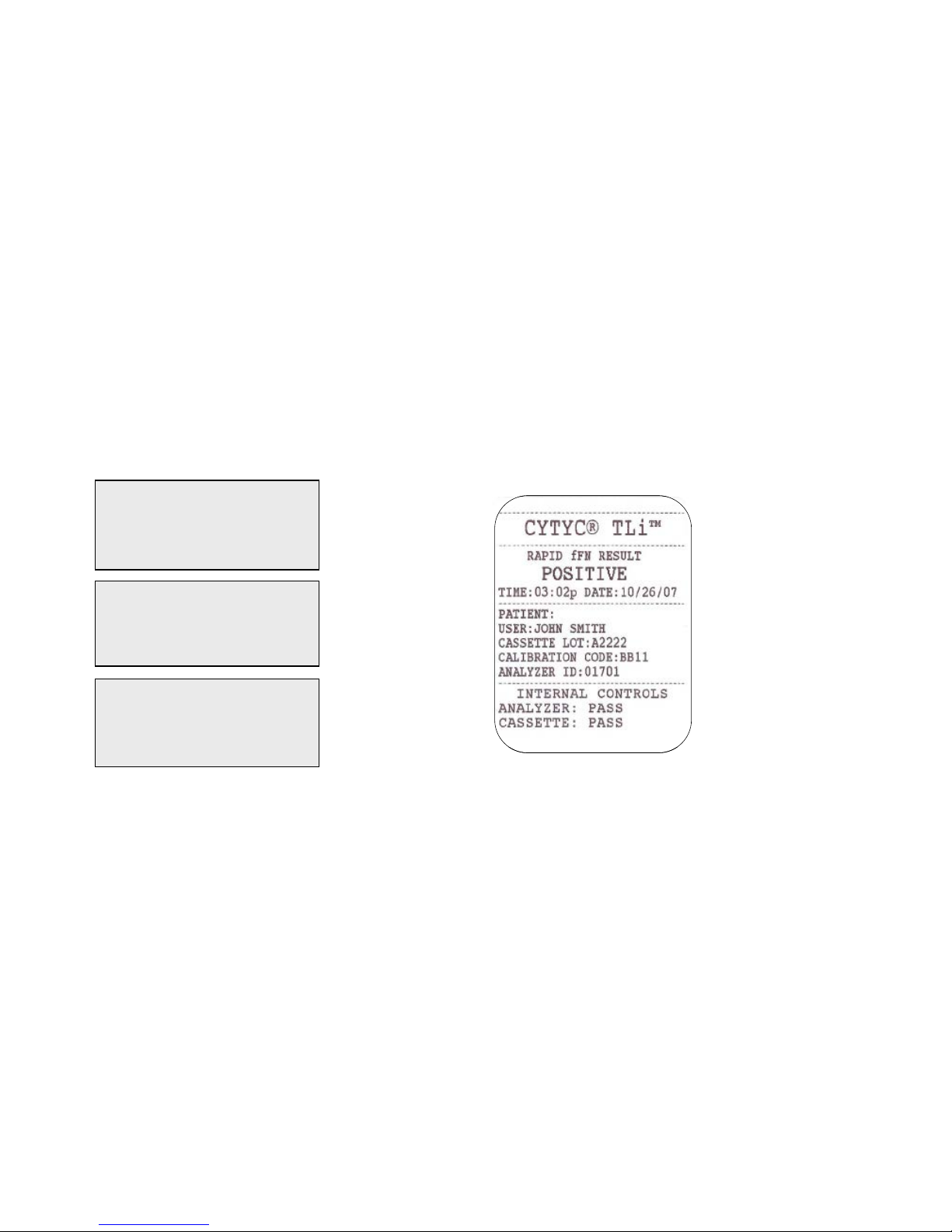
TLiIQ System User Manual 1 – 15
Section 1 – Introduction
Example: Displayed/Printed Re sults of Patient Record
Displayed Printed
CAL CODE:BB11
CASSETTE LN:A2222
ANALYZER ID:01701
ESC-MAIN MENU ←
USER:XXXXXXXXXXXX
INTERNAL CONTROLS
ANALYZER: PASS
CASSETTE: PASS ← →
FETAL FIBRONECTIN
03:02 PM 10/26/07
PT:XXXXXXXXXXXXXXXX
RESULT:POSITIVE →
Page 27
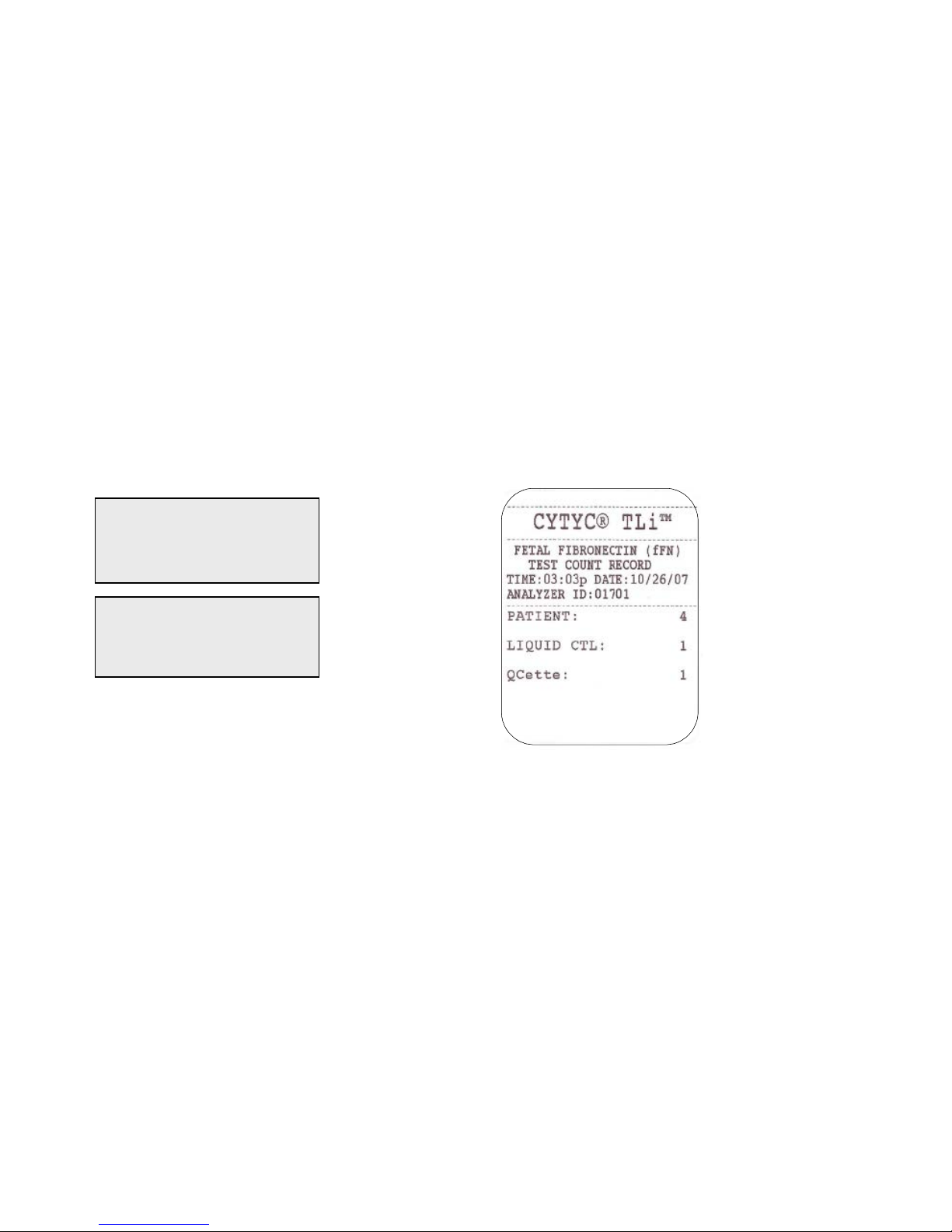
TLiIQ System User Manual 1 – 16
Section 1 – Introduction
Example: Displayed/Printed Re sults of Test Counts Record
Displayed Printed
PATIENT:4
CONTROL:1
QCette:1
FETAL FIBRONECTIN
TEST COUNTS
03:03 PM 10/26/07
ANALYZER:01701 →
Page 28
TLiIQ System User Manual 1 – 17
Section 1 – Introduction
SPECIFICATIONS
Power Supply UL 12 VDC listed power supply
Memory Capacity
50 Calibration Records
50 QCette Records
50 Control Records
50 Patient Records
Display
4 lines
20 characters per line
alphanumeric 5 x 8 matrix Supertwist LCD
Black characters with gray background
Keypad
3.5 x 4.5 inches
tactile membrane
alphanumeric keys
Page 29
TLiIQ System User Manual 1 – 18
Section 1 – Introduction
Dimensions
Length - 8.9 inches
Width - 6.9 inches
Height - 1.0 to 3.0 inches
Weight - 1.2 pounds
Operating Temperature
18° to 30°C
64° to 86°F
A.C. Supply 120VAC / 60Hz / 16W
or
220VAC / 50Hz / 16W
Input Connector Coaxial power plug with positive center conductor
Output Connectors 9 pin male and 9 pin female data connectors
Page 30

TLiIQ System User Manual 1 – 19
Section 1 – Introduction
CAUTIONS AND WARNINGS
There are no known hazards associated with the TLi
IQ
System when it is operated in accordance with the instructions in
this manual. However, you should be aware of situations that can result in serious injury.
WARNING! Ensure that the analyzer power adapter is connected to a grounded AC electrical outlet that
provides voltage and current specified by Hologic. Use of an incompatible power receptacle can cause shock
and fire hazard.
CAUTION! Use only the power adapter supplied by Hologic. Use of an incompatible power adapter can damage the
internal components.
CAUTION! Always turn off the power and unp lug the power adapter before c leaning the exterior of the analyzer. Fluid
can damage internal components. DO NOT clean the power adapter.
CAUTION! Extreme heat can damage the display and other electronic components.
Page 31

TLiIQ System User Manual 1 – 20
Section 1 – Introduction
WARNING! Never apply cleaning reagents by spray as the liquid may leak into the analyzer causing damage
to the electrical components or possibly electrical shock to the user.
CAUTION! Do not immerse the analyzer in liquid. Fluid can damage internal components.
CAUTION! Do not clean the keypad with undiluted bleach solution or other solvents. Caustic cleaning solutions can
damage the keypad.
CAUTION! Use appropriate laboratory procedures for handling biohazardous materials.
Page 32

TLiIQ System User Manual 2 – 1
Section 2 – Installation
SECTION 2 – INSTALLATION
GENERAL
This section provides detailed installation instructions for the TLi
IQ
System. Follow installation steps carefully to insure
proper installation and operatio n.
ENVIRONMENTAL FACTORS
The TLi
IQ
System has been designed to be safe under the following conditions: Indoor use; Altitudes up to 2000m;
Maximum relative humidity of 80% for temperatures up to 31°C decreasing linearly to 50% relative humidity at 40°C; Main
supply voltage fluctuations not to exceed ± 10% of the nominal; transient overvoltages according to Installation Category
and Pollution Degree 2. However, as with all electronic instruments, prolonged exposure to high temperature and humidity
should be avoided. The operating temperature should be held relatively constant. The optimum operating temperature is
18° to 30°C (64° to 86°F). Before operating, allow the instrument to equilibrate to room temperature.
Place the instrument where it will not be subjected to extreme temperature variations (e.g., near open windows, ovens,
hot plates, radiators, direct sunlight).
Page 33

TLiIQ System User Manual 2 – 2
Section 2 – Installation
UNPACKING
TLi
IQ
Analyzer
_______________________
Carefully remove the analyzer and accessories from the shipping carton. Inspect the carton and the analyzer for visible
signs of damage. If the analyzer is damaged, immediately contact the carrier and Hologic Customer Service.
The carton should contain the following
parts/accessories:
• TLi
IQ
Analyzer
• Power Ad apt er
• User Manual
• TLi
IQ
QCette®
NOTE: Retain the shipping carton for future use. If the
analyzer needs to be shipped, use the original shipping
carton.
Page 34

TLiIQ System User Manual 2 – 3
Section 2 – Installation
Printer
Carefully remove the printer and accessories from the shipping carton. Inspect the carton and the printer for visible signs
of damage. If the printer is damaged, immediately contact the carrier and Hologic Customer Service.
The printer carton should contain the following parts/accessories:
• Printer
• Printer Labels (2 rolls)
• Power Cor d
• Printer Cable
NOTE: Retain the shipping carton for future use.
If the printer needs to be shipped, use the
original shipping carton.
Page 35

TLiIQ System User Manual 2 – 4
Section 2 – Installation
SYSTEM SETUP
1. The Analyzer and Printer should be placed on a flat, level surface.
2. Connect the 9-pin connector of the printer cable to the analyzer and the modular jack to the printer.
System Printer Analyzer
3. Connect the small connector of the analyzer power adapter to the analyzer and the larger power connector to a
grounded AC electrical outl et. Caution: Only power connectors provided with the TLi
IQ
analyzer and printer may be
used. Any substitutions can result in damage to the TLi
IQ
analyzer and printer.
4. Connect the small connector of the printer power cord to the printer and the larger connector of the power cord to a
grounded AC electrical outl et.
Page 36

TLiIQ System User Manual 2 – 5
Section 2 – Installation
GETTING STARTED
Turn the analyzer power switch to the ON position. The power switch is located on the left side of the instrument. (If the
analyzer does not turn on, see Section 6, Troubleshooting.)
The analyzer will perform SYSTEM DIAGNOSTICS (a s elf -test of the
analyzer components).
If there is a problem after the self-test, a beep will sound to indicate an
error and an error code will be displayed. If an error code is displayed,
refer to the troubleshooting section of the manual.
Once SYSTEM DIAGNOSTICS is complete, the display will change to the software VERSION, DATE, and TIME for five
seconds, and then to the fFN Main Menu. The date and time may need to be reset for your time zone.
HOLOGIC TLi SYSTEM
VERSION 2.0
SYSTEM DIAGNOSTICS
IN PROCESS
HOLOGIC TLi SYSTEM
VERSION 2.0
03:00 PM 10/26/07
Page 37

TLiIQ System User Manual 2 – 6
Section 2 – Installation
SETTING THE DATE AN D TIME
1. From the Main Menu, select CHANGE SETUP by pressing the ↓ to
get to the second page of the Main Menu. Press 6 for CHANGE
SETUP. This will display the SETUP MENU.
2. From the SETUP MENU, press 1 to display DATE/TIME and follow
the prompts.
For more details about setting the date and time, see Section 4, Software Functions – Detailed Descriptions.
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
SETUP MENU
1-DATE/TIME
2-AUTOPRINT
3-INCUBATION MODE ↓
Page 38

TLiIQ System User Manual 2 – 7
Section 2 – Installation
TLi
IQ
QCette® SETUP
1. From the Main Menu, select CHANGE SETUP by pressing the ↓ to
get to the second page of the Main Menu. Press 6 for CHANGE
SETUP. This will display the SETUP MENU.
2. From the SETUP MENU, press the ↓ or 4 to display QCette SETUP
and follow the prompts.
For more details about setting up the TLi
IQ
QCette, see Section 4, Software Functions – Detailed Descriptions.
SETUP MENU
4-QCette SETUP
↑
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
SETUP MENU
1-DATE/TIME
2-AUTOPRINT
3-INCUBATION MODE ↓
Page 39

TLiIQ System User Manual 2 – 8
Section 2 – Installation
FACTORY DEFAULT SETTINGS
The TLi
IQ
System uses the following default settings. To customize the unit to your laboratory requirements, refer to
Section 4, Software Functions – Detailed Descriptions.
The Default Settings are as follows:
AUTOPRINT
Factory setting is Autoprint ON. After every result, the printer will generate a printed result.
INCUBATION MODE
Factory setting is INTERNAL Mode. The incubation mode refers to the timing of the incubation process and the initiation
of the cassette analysis.
In the INTERNAL mode, the analyzer will time the incubation and automatically start the analysis when the incubation is
complete.
In the EXTERNAL mode, the user will be responsible for timing the incubation and for starting the analysis.
NOTE: The INTERNAL mode is recommended by the manufacturer to ensure proper timing of the assay.
Page 40

TLiIQ System User Manual 3 − 1
Section 3 – General Operating/Testing Instructions
SECTION 3 – GENERAL OPERATING / TESTING INSTRUCTIONS
After instrument installation, the TLi
IQ
analyzer can be operated on a day-to-day basis by using the following procedures.
Read Section 4 for detailed descriptions of displays, prompts and operating sequences.
STARTING THE SYSTEM
1. Turn on the analyzer using the on/off switch located on the left side
of the analyzer.
The screen will display SYSTEM DIAGNOSTICS. If the analyzer fails
the self-test, two beeps will sound; otherwise the analyzer will go to the
next screen.
Once SYSTEM DIAGNOSTICS is complete, the display will change to
the software VERSION and the DATE and TIME for five seconds and
then to the Main Menu. Verify the date and time are correct.
See Section 4 for setting Date/Time.
2. Turn on the printer using switch at rear and ensure labels
are in the printer. See Section 6 for loading printer labels.
HOLOGIC TLi SYSTEM
VERSION 2.0
SYSTEM DIAGNOSTICS
IN PROCESS
HOLOGIC TLi SYSTEM
VERSION 2.0
03:00 PM 10/26/07
Page 41

TLiIQ System User Manual 3 − 2
Section 3 – General Operating/Testing Instructions
SET CALIBRATION
NOTE: Calibration must be set when changing cassette lots.
1. From the Main Menu, press 2 to select SET CALIBRATION.
2. Enter USER ID and press ENTER.
3. Enter the CASSETTE LOT# (on cassette pouch) and press ENTER.
The lot number must be entered to proceed to the next step.
fFN MAIN MENU
1-TEST PATIENT
2-SET CALIBRATION
3-DAILY QC ↓
SET CALIBRATION
USER ID
>JOHN SMITH
ENTER-CONFIRM
SET CALIBRATION
CASSETTE LOT#
>L1002
ENTER-ACCEPT
Page 42

TLiIQ System User Manual 3 − 3
Section 3 – General Operating/Testing Instructions
4. Enter the CALIBRATION CODE# (on cassette box label) and press
ENTER. The code number must be entered to proceed to the next
step.
NOTE: The calibration code is established by Hologic for each lot of
Rapid fFN Cassettes.
5. When calibration is complete, the system will automatically display
and print the result if AUTOPRINT is set to ON, or it may be
printed/reprinted by pressing the PRINT/ENTER key.
6. Press ESC to return to the Main Menu.
FETAL FIBRONECTIN
03:00 PM 10/26/07
SYSTEM CALIBRATED
USER:JOHN SMITH →
SET CALIBRATION
CALIBRATION CODE#
>FG56
ENTER-ACCEPT
Page 43

TLiIQ System User Manual 3 − 4
Section 3 – General Operating/Testing Instructions
TEST PATIENT (Internal Incubation Mod e)
1. From the Main Menu, press 1 to select TEST PATIENT.
2. Enter USER ID and press ENTER.
3. Enter the last two digits of the CASSETTE LOT# (on cassette
pouch) and press ENTER. The lot number must be entered to
proceed to the next step.
NOTE: The analyzer will automatically compare the CASSETTE LOT#
used to set calibration with the cassette lot number used for
patient testing. If the lot numbers do not match, the analyzer will
request the user to recalibrate the system. The cassette lot
number used for calibration will be displayed on the third line.
fFN MAIN MENU
1-TEST PATIENT
2-SET CALIBRATION
3-DAILY QC ↓
TEST PATIENT
USER ID
>JOHN SMITH
ENTER-CONFIRM
TEST PATIENT
CASSETTE LOT#
>L1002
ENTER-ACCEPT
Page 44

TLiIQ System User Manual 3 − 5
Section 3 – General Operating/Testing Instructions
4. Enter up to 16 alphanumeric characters for a PATIENT ID and press
ENTER.
5. Insert cassette and press ENTER.
6. Add sample and immediately press ENT ER.
PATIENT ID
>JANE DOE 123
ENTER-ACCEPT
INTERNAL INCUBATION
INSERT CASSETTE
PRESS ENTER
TO CONTINUE
ADD SAMPLE
AND IMMEDIATELY
PRESS ENTER
Page 45

TLiIQ System User Manual 3 − 6
Section 3 – General Operating/Testing Instructions
7. The analyzer will begin a 20-minute incubation countdown.
8. Following incubation, the analyzer will begin analysis of the cassette.
9. When testing is complete, the system will automatically display and
print the result if AUTOPRINT is set to ON, or it may be
printed/reprinted by pressing the PRINT/ENTER key.
10. Press ESC to return to the Main Menu.
FETAL FIBRONECTIN
03:00 PM 10/26/07
PT:JANE DOE 123
RESULT:POSITIVE →
TEST IN PROCESS
DO NOT REMOVE
CASSETTE
19 MIN 56 SEC
TEST IN PROCESS
ANALYZING
DO NOT REMOVE
CASSETTE
Page 46

TLiIQ System User Manual 3 − 7
Section 3 – General Operating/Testing Instructions
DAILY QC
NOTE: TLi
IQ
QCette SETUP must be performed PRIOR to running the
QCette as a quality control device. See Section 4 for TLi
IQ
QCette
SETUP.
1. From the Main Menu, press 3 to select DAILY QC.
2. Enter USER ID and press ENTER.
3. Enter the QCette SN (on QCette plastic housing) and press ENTER.
The serial number must be entered to proceed to the next step. The
correct format is 6 numbers (e.g., 001004). Enter all leading zeros.
NOTE: The serial number entered at daily QC must be identical to the
serial number entered at TLi
IQ
QCette setup.
fFN MAIN MENU
1-TEST PATIENT
2-SET CALIBRATION
3-DAILY QC ↓
QCette
ENTER QCette SN
>001004
ENTER-ACCEPT
QCette
USER ID
>JOHN SMITH
ENTER-CONFIRM
Page 47

TLiIQ System User Manual 3 − 8
Section 3 – General Operating/Testing Instructions
4. Insert the QCette and press ENTER. The analyzer will begin the
analysis of the QCette.
5. When testing is complete, the system will automatically display and
print the result if AUTOPRINT is set to ON, or it may be
printed/reprinted by pressing the PRINT/ENTER key.
6. Press ESC to return to the Main Menu.
INSERT QCette
ENTER-CONTINUE
TEST IN PROCESS
ANALYZING
DO NOT REMOVE
CASSETTE
QCette
03:00 PM 10/26/07
SYSTEM: PASS
USER:JOHN SMITH →
Page 48

TLiIQ System User Manual 3 − 9
Section 3 – General Operating/Testing Instructions
LIQUID CONTROLS (Internal Incubati on Mod e)
1. From the Main Menu, press 8 to select LIQUID CONTROLS.
2. Enter USER ID and press ENTER.
3. Enter the CASSETTE LOT# (on cassette pouch) and press ENTER.
The lot number must be entered to proceed to the next step.
RUN CONTROL
CASSETTE LOT#
>L1002
ENTER-ACCEPT
fFN MAIN MENU
7-TEST COUNTS
8-LIQUID CONTROLS
↓
RUN CONTROL
USER ID
>JOHN SMITH
ENTER-CONFIRM
Page 49

TLiIQ System User Manual 3 − 10
Section 3 – General Operating/Testing Instructions
4. Select Negative or Positive Control.
5. Enter the CONTROL LOT# (on bottle label) and press ENTER.
6. Insert cassette and press ENTER.
CONTROL TEST MENU
1-NEGATIVE CONTROL
2-POSITIVE CONTROL
NEGATIVE CTL LOT#
>M1023
ENTER-CONFIRM
INTERNAL INCUBATION
INSERT CASSETTE
PRESS ENTER
TO CONTINUE
Page 50

TLiIQ System User Manual 3 − 11
Section 3 – General Operating/Testing Instructions
7. Add sample and immediately press ENT ER.
8. The analyzer will begin a 20-minute incubation countdown.
9. Following incubation, the analyzer will begin analysis of the cassette.
10. When testing is complete, the system will automatically display and
print the result if AUTOPRINT is set to ON, or it may be
printed/reprinted by pressing the PRINT/ENTER key.
11. Press ESC to return to the Main Menu.
FETAL FIBRONECTIN
03:00 PM 10/26/07
NEG CTL:M1023
RESULT:PASS →
ADD SAMPLE
AND IMMEDIATELY
PRESS ENTER
TEST IN PROCESS
DO NOT REMOVE
CASSETTE
19 MIN 56 SEC
TEST IN PROCESS
ANALYZING
DO NOT REMOVE
CASSETTE
Page 51

TLiIQ System User Manual 3 − 12
Section 3 – General Operating/Testing Instructions
VIEWING RESULTS ON SCREEN
Upon completion of every test, the analyzer will automatically display the
results on up to three screens. Each screen can be accessed by using
the ← and → keys. To print the result record, press the ENTER/PRINT
key from any screen.
NOTE: Internal controls are performed automatically during each Rapid
fFN test. These internal controls check for (1) a threshold level of
signal at the procedural control line, (2) proper sample flow
across the Rapid fFN Cassette, (3) absence of conjugate
aggregation, and (4) proper functioning of the TLi
IQ
analyzer
hardware.
FETAL FIBRONECTIN
03:00 PM 10/26/07
PT:JANE DOE 123
RESULT:POSITIVE →
CAL CODE:FG56
CASSETTE LN:L1002
ANALYZER ID:00426
ESC-MAIN MENU ←
INTERNAL CONTROLS
USER:JOHN SMITH
ANALYZER: PAS S
CASSETTE: PASS ← →
Page 52

TLiIQ System User Manual 3 − 13
Section 3 – General Operating/Testing Instructions
INCUBATION MODE
The incubation mode may be Internal (incubation timed by analyzer) or External (incubation timed by user). The user
prompts are the same for both modes until the analyzer reaches the “INSERT CASSETTE” screen.
NOTE: The INTERNAL mode is recommended by the manufacturer to ensure proper timing of the assay.
INTERNAL MODE – In this mode, pressing ENTER will prompt the user
to add the sample and the analyzer will automatically complete the test.
If the sample is not added within 2 minutes, the test is invalidated.
INTERNAL INCUBATION
INSERT CASSETTE
PRESS ENTER
TO CONTINUE
TEST IN PROCESS
DO NOT REMOVE
CASSETTE
19 MIN 56 SEC
ADD SAMPLE
AND IMMEDIATELY
PRESS ENTER
Page 53

TLiIQ System User Manual 3 − 14
Section 3 – General Operating/Testing Instructions
EXTERNAL MODE – In this mode, the user is responsible for timing the
incubation and starting the analysis. Upon completion of 20 minute
incubation, insert cassette and press ENTER. The analyzer will
automatically complete the test. If additional cassettes are run, wait at
least 5 minutes before adding sample to the next cassette.
EXTERNAL INCUBATION
WHEN TIME COMPLETE
INSERT CASSETTE
AND PRESS ENTER
TEST IN PROCESS
ANALYZING
DO NOT REMOVE
CASSETTE
Page 54

TLiIQ System User Manual 4 − 1
Section 4 − Software Functions - Detailed Descriptions
SECTION 4 − SOFTWARE FUNCTIONS - DET AILED DESCRIPTIONS
STARTUP SCREEN
When the analyzer is turned on, the screen will display HOLOGIC TLi
SYSTEM and the software version, while performing an internal self-test
(SYSTEM DIAGNOSTICS).
Following the self-test, the analyzer displays the software version and
the current date and time for five seconds before displaying the Main
Menu.
HOLOGIC TLi SYSTEM
VERSION 2.0
SYSTEM DIAGNOSTICS
IN PROCESS
HOLOGIC TLi SYSTEM
VERSION 2.0
03:00 PM 10/26/07
Page 55

TLiIQ System User Manual 4 − 2
Section 4 − Software Functions - Detailed Descriptions
MAIN MENU
The Main Menu, displayed over three screens, consists of Test Patient,
Set Calibration, Daily QC, Access Data, View Setup, Change Setup, Test
Counts, and Liquid Controls. Selecting the number in front of each option
initiates that procedure or displays a submenu.
fFN MAIN MENU
1-TEST PATIENT
2-SET CALIBRATION
3-DAILY QC ↓
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
fFN MAIN MENU
7-TEST COUNTS
8-LIQUID CONTROLS
↑
Page 56

TLiIQ System User Manual 4 − 3
Section 4 − Software Functions - Detailed Descriptions
SET CALIBRATION
Option 2 on the Main Menu screen allows the user to set the calibration
on the analyzer. Follow the analyzer prompts. Calibration must be set
when changing cassette lots.
NOTE: If the calibration has not been set, menu option 2 will flash.
Calibration must be set before the analyzer can be used for testing.
The most recent USER ID is always displayed. Press ENTER to accept
the ID, or enter a new User ID. This field will accept 15 alpha or numeric
characters. To leave this field blank, delete the information using the ←
key.
SET CALIBRATION
USER ID
>JOHN SMITH
ENTER-CONFIRM
fFN MAIN MENU
1-TEST PATIENT
2-SET CALIBRATION
3-DAILY QC ↓
Page 57

TLiIQ System User Manual 4 − 4
Section 4 − Software Functions - Detailed Descriptions
The CASSETTE LOT# must be entered to proceed to the next step. The
CASSETTE LOT# is located on the cassette pouch. The software
requires that the lot number is entered in the correct format: one alpha
character followed by four numeric characters (e.g., L1002).
The CALIBRATION CODE# must be entered to proceed to the next step.
The CALIBRATION CODE# is located on the cassette box. The software
requires that the code number is entered in the correct format: two alpha
characters followed by two numeric characters (e.g., FG56).
NOTE: The calibration code is established by Hologic for each lot of
Rapid fFN Cassettes.
Calibration Data Record - This record is displayed over two screens.
Each screen can be accessed by using the → and ← keys. The
complete record will be printed automatically if AUTOPRINT is set to ON,
or it may be printed/reprinted by pressing the PRINT/ENTER key.
SET CALIBRATION
CALIBRATION CODE#
>FG56
ENTER-ACCEPT
FETAL FIBRONECTIN
03:00 PM 10/26/07
SYSTEM CALIBRATED
USER:JOHN SMITH →
CAL CODE:FG56
CASSETTE LN:L1002
ANALYZER ID:00426
ESC-MAIN MENU ←
SET CALIBRATION
CASSETTE LOT#
>L1002
ENTER-ACCEPT
Page 58

TLiIQ System User Manual 4 − 5
Section 4 − Software Functions - Detailed Descriptions
TEST PATIENT (Internal Incubation Mode)
Option 1 on the Main Menu screen allows the user to test a patient
sample. Follow the analyzer prompts.
The most recent USER ID is always displayed. Press ENTER to accept
the ID, or enter a new User ID. This field will accept 15 alpha or numeric
characters. To leave this field blank, delete the information using the ←
key.
The CASSETTE LOT# must be entered to proceed to the next step. For
convenience, the last 2 numbers only can be entered if the lot has not
changed. The CASSETTE LOT# is located on the cassette pouch. The
software requires that the lot number is entered in the correct format: one
alpha character followed by four numeric characters (e.g., L1002).
TEST PATIENT
USER ID
>JOHN SMITH
ENTER-CONFIRM
TEST PATIENT
CASSETTE LOT#
>L1002
ENTER-ACCEPT
fFN MAIN MENU
1-TEST PATIENT
2-SET CALIBRATION
3-DAILY QC ↓
Page 59

TLiIQ System User Manual 4 − 6
Section 4 − Software Functions - Detailed Descriptions
The analyzer automatically compares the CASSETTE LOT# used to set
calibration with the cassette lot number used for patient testing. If the lot
numbers do not match, the test process cannot continue. When this
occurs, the cassette lot number used for calibration will be displayed,
and the user is prompted to recalibrate the system.
Enter up to 16 alphanumeric characters for a PATIENT ID and press
ENTER.
This message will be displayed if a cassette is present in the analyzer
prior to reaching the next screen. Remove cassette and press ENTER.
PATIENT ID
>JANE DOE 123
ENTER-ACCEPT
CASSETTE LOT CHANGED
CALIBRATE SYSTEM
L1002
ESC-MAIN MENU
REMOVE CASSET TE
PRESS ENTER
TO CONTINUE
Page 60

TLiIQ System User Manual 4 − 7
Section 4 − Software Functions - Detailed Descriptions
The analyzer then prompts the user to insert cassette and press ENTER.
This message will be displayed if a cassette is not inserted. Press
ENTER to return to the previous screen.
A two-minute timer starts during which time this message flashes and the
analyzer beeps. Add patient sample and immediately press ENTER.
INTERNAL INCUBATION
INSERT CASSETTE
PRESS ENTER
TO CONTINUE
ADD SAMPLE
AND IMMEDIATELY
PRESS ENTER
CASSETT NOT
INSERTED
ENTER-CONTINUE
CASSETTE NOT
INSERTED
ENTER-CONTINUE
Page 61

TLiIQ System User Manual 4 − 8
Section 4 − Software Functions - Detailed Descriptions
If the patient sample is not added and ENTER not pressed within allotted
time, the test process cannot continue. This message will be displayed.
Press ESC to return to the Main Menu. No record of the test will be held
in memory.
Once the sample is added, the analyzer will begin a 20-minute
incubation countdown. To abort the test, press ESC. Pressing ESC will
terminate the test and the data will be lost.
Upon completion of the incubation period, the analyzer will begin the
analysis of the cassette. Do not disturb the analyzer until the results are
displayed. The analysis will take approximately 2 - 3 minutes.
SAMPLE NOT ADDED
WITHIN ALLOTTED TIME
ESC-MAIN MENU
TEST IN PROCESS
ANALYZING
DO NOT REMOVE
CASSETTE
TEST IN PROCESS
DO NOT REMOVE
CASSETTE
19 MIN 56 SEC
Page 62

TLiIQ System User Manual 4 − 9
Section 4 − Software Functions - Detailed Descriptions
TEST WARNING: This mes s age will be disp layed if ESC was pressed
during testing. Lines 1 and 2 will flash prompting the user to select
ENTER to continue test, or ESC to end test. This message will hold for 5
seconds and then revert back to its respective screen. If the test is
cancelled, a new cassette will be required to repeat the test.
Patient Data Record – This record is displayed over three screens. Each
screen can be accessed by using the ← and → keys. The complete
record will be printed autom aticall y if AUTOPRINT is set to ON, or it may
be printed/reprinted by pressing the PRINT/ENTER key.
Patient results are POSITIVE, NEGATIVE, or INVALID.
An INVALID result should be repeated. (See Section 7, Item 13.)
Invalid results will not be stored in memory.
NOTE: Internal Controls are performed automatically during each Rapid
fFN test. These internal controls check for (1) a threshold level of
signal at the procedural control line, (2) proper sample flow
across the Rapid fFN Cassette, (3) absence of conjugate
aggregation, and (4) proper functioning of the TLi
IQ
analyzer
hardware.
FETAL FIBRONECTIN
03:00 PM 10/26/07
PT:JANE DOE 123
RESULT:POSITIVE →
INTERNAL CONTROLS
USER:JOHN SMITH
ANALYZER: PAS S
CASSETTE: PASS ← →
ARE YOU SURE YOU
WANT TO CANCEL TEST
ENTER-CONTINUE
ESC-MAIN MENU
CAL CODE:FG56
CASSETTE LN:L1002
ANALYZER ID:00426
ESC-MAIN MENU ←
Page 63

TLiIQ System User Manual 4 − 10
Section 4 − Software Functions - Detailed Descriptions
DAILY QC
Prior to running the TLi
IQ
QCette® for the first time, QCette SETUP must
be performed. See page 4-29. Refer to the TLi
IQ
QCette directional insert
for more information.
Option 3 on the Main Menu screen allows the user to run the QCette.
The most recent USER ID is always displayed. Press ENTER to accept
the ID, or enter a new User ID. This field will accept 15 alpha or numeric
characters. To leave this field blank, delete the information using the ←
key.
The QCette SN must be entered to proceed to the next step. The serial
number is printed on the QCette plastic housing. The software requires
that the serial number is entered in the correct format: six numeric
characters (e.g., 001004). Enter al l leadin g zeros .
QCette
ENTER QCette SN
>001004
ENTER-CONFIRM
QCette
USER ID
>JOHN SMITH
ENTER-CONFIRM
fFN MAIN MENU
1-TEST PATIENT
2-SET CALIBRATION
3-DAILY QC ↓
Page 64

TLiIQ System User Manual 4 − 11
Section 4 − Software Functions - Detailed Descriptions
This message will be displayed if the QCette serial number entered is not
identical to the serial number entered at the time of QCette setup.
This message will be displayed if a cassette is present in the analyzer
prior to reaching the next screen. Remove cassette and press ENTER.
The analyzer then prompts the user to insert the QCette and press
ENTER.
This message will be displayed if the QCette is not inserted. Press
ENTER to continue.
INSERT QCette
ENTER-CONTINUE
CASSETTE # CHANGED
NNNNNN
SETUP ANALYZER CTL
ESC-MAIN MENU
CASSETTE NOT
INSERTED
ENTER-CONTINUE
REMOVE CASSET TE
PRESS ENTER
TO CONTINUE
Page 65

TLiIQ System User Manual 4 − 12
Section 4 − Software Functions - Detailed Descriptions
A two-minute timer starts during which time this message flashes and the
analyzer beeps. Insert the QCette and pres s ENTER.
The analyzer will read the QCette. Do not disturb the analyzer until the
results are displayed. The analysis will take approximately 2-3 minutes.
TEST IN PROCESS
ANALYZING
DO NOT REMOVE
CASSETTE
INSERT QCette
ENTER-CONTINUE
Page 66

TLiIQ System User Manual 4 − 13
Section 4 − Software Functions - Detailed Descriptions
TEST WARNING: This message will be displa yed if ESC was pres sed
during testing. Lines 1 and 2 will flash prompting the user to select
ENTER to continue test, or ESC to end test. This message will hold for
5 seconds and then revert back to its respective screen.
QCette Data Record – This record will be displayed on two screens.
Each screen can be accessed by using the ← and → keys. The
complete record will be printed automatically if AUTOPRINT is set to ON,
or it may be printed/reprinted by pressing the PRINT/ENTER key.
QCette results are SYSTEM PASS, SYSTEM FAIL, or INVALID.
A FAIL or INVALID result should be repeated. (See Section 7, Items 9
and 10.)
Invalid results will not be stored in memory.
ARE YOU SURE YOU
WANT TO CANCEL TEST
ENTER-CONTINUE
ESC-MAIN MENU
QCette
03:00 PM 10/26/07
SYSTEM: PASS
USER:JOHN SMITH →
CAL CODE:FG56
QCette SN:00104
ANALYZER ID:00426
ESC-MAIN MENU ←
Page 67

TLiIQ System User Manual 4 − 14
Section 4 − Software Functions - Detailed Descriptions
LIQUID CONTROLS (Internal Incubati on Mod e)
Option 8 on the Main Menu screen allows the user to run the LIQUID
CONTROLS.
The most recent USER ID is always displayed. Press ENTER to accept
the ID, or enter a new User ID. This field will accept 15 alpha or numeric
characters. To leave this field blank, delete the information using the ←
key.
The CASSETTE LOT# must be entered to proceed to the next step. For
convenience, the last 2 numbers only can be entered if the lot has not
changed. The CASSETTE LOT# is located on the cassette pouch. The
software requires that the lot number is entered in the correct format: one
alpha character followed by four numeric characters (e.g., L1002).
RUN CONTROL
USER ID
>JOHN SMITH
ENTER-CONFIRM
fFN MAIN MENU
7-TEST COUNTS
8-LIQUID CONTROLS
↓
RUN CONTROL
CASSETTE LOT#
>L1002
ENTER - ACCEPT
Page 68

TLiIQ System User Manual 4 − 15
Section 4 − Software Functions - Detailed Descriptions
The analyzer automatically compares the CASSETTE LOT# used to set
calibration with the cassette lot number used for testing controls. If the lot
numbers do not match, the test process cannot continue. When this
occurs, the cassette lot number used for calibration will be displayed,
and the user is prompted to recalibrate the system.
From the CONTROL TEST MENU, select 1-NEGATIVE CONTROL or 2POSITIVE CONTROL.
The most recent CONTROL LOT# is always displayed. Press ENTER to
accept the lot number, or enter a new control lot number. This field will
accept up to 12 alphanumeric characters.
CONTROL TEST MENU
1-NEGATIVE CONTROL
2-POSITIVE CONTROL
NEGATIVE CTL LOT#
>M1023
ENTER-CONFIRM
CASSETTE LOT CHANGED
CALIBRATE SYSTEM
L1002
ESC-MAIN MENU
Page 69

TLiIQ System User Manual 4 − 16
Section 4 − Software Functions - Detailed Descriptions
This message will be displayed if a cassette is present in the analyzer
prior to reaching the next screen. Remove cassette and press ENTER.
The analyzer then prompts the user to insert the cassette and press
ENTER.
This message will be displayed if a cassette is not inserted. Press
ENTER to return to the previous screen.
INTERNAL INCUBATION
INSERT CASSETTE
PRESS ENTER
TO CONTINUE
REMOVE CASSET TE
PRESS ENTER
TO CONTINUE
CASSETTE NOT
INSERTED
ENTER-CONTINUE
Page 70

TLiIQ System User Manual 4 − 17
Section 4 − Software Functions - Detailed Descriptions
A two-minute timer starts during which time this message flashes and the
analyzer beeps. Add control sample and immediately press ENTER.
If the sample is not added and ENTER not pressed within allotted tim e,
the test process cannot continue. This message will be displayed. Press
ESC to return to the Main Menu. No record of the test will be held in
memory.
Once the sample is added, the analyzer will begin a 20-minute
incubation countdown. To abort the test, press ESC. Pressing ESC will
terminate the test and the data will be lost.
Upon completion of the incubation period, the analyzer will begin the
analysis of the cassette. Do not disturb the analyzer until the results are
displayed. The analysis will take approximately 2-3 minutes.
TEST IN PROCESS
DO NOT REMOVE
CASSETTE
19 MIN 56 SEC
TEST IN PROCESS
ANALYZING
DO NOT REMOVE
CASSETTE
ADD SAMPLE
AND IMMEDIATELY
PRESS ENTER
SAMPLE NOT ADDED
WITHIN ALLOTTED TIME
ESC-MAIN MENU
Page 71

TLiIQ System User Manual 4 − 18
Section 4 − Software Functions - Detailed Descriptions
TEST WARNING: This message will be displa yed if ESC was pres sed
during testing. Lines 1 and 2 will flash prompting the user to select
ENTER to continue test or ESC to end test. This message will hold for 5
seconds and then revert back to its respective screen. If the test is
cancelled, a new cassette will be required to repeat the test.
Liquid Control Data Record - This record is displayed over three screens.
Each screen can be accessed by using the ← and → keys. The
complete record will be printed automatically if AUTOPRINT is set to ON,
or it may be printed/reprinted by pressing the PRINT/ENTER key.
Control results are PASS, FAIL, or INVALID.
A FAIL or INVALID result should be repeated (See Section 7, Items 11
and 12.)
Invalid results will not be stored in memory.
FETAL FIBRONECTIN
03:00 PM 10/26/07
NEG CTL:M1023
RESULT:PASS →
INTERNAL CONTROLS
USER:JOHN SMITH
ANALYZER: PAS S
CASSETTE: PASS ← →
ARE YOU SURE YOU
WANT TO CANCEL TEST
ENTER–CONTINUE
ESC–MAIN MENU
CAL CODE:FG56
CASSETTE LN:L1002
ANALYZER ID:00426
ESC-MAIN MENU ←
Page 72

TLiIQ System User Manual 4 − 19
Section 4 − Software Functions - Detailed Descriptions
ACC ESS DATA - VIEW/PRINT DATA
Option 4 on the Main Menu screen allows the user to ACCESS DATA
stored in the analyzer.
Select option 1 on the ACCESS DATA MENU for VIEW/PRINT DATA.
Select the category of data records to view/print. The categories are
displayed over two screens. Each screen can be accessed by using ↓
and ↑ keys.
ACCESS DATA MENU
1-VIEW/PRINT DATA
2-DATA TRANSFER
ACCESS DATA MENU
1-PATIENT
2-QCette
3-CONTROLS ↓
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
ACCESS DATA MENU
4-CALIBRATION
↑
Page 73

TLiIQ System User Manual 4 − 20
Section 4 − Software Functions - Detailed Descriptions
The most recent record for the category of data records selected will be
displayed. PATIENT was chosen for this example. Use the ↑ and ↓ keys
to view other records in the category.
Use the ← and → keys to view pages within a record.
Printing the record – The record displayed may be printed by pressing
the ENTER/PRINT key while in any of the three pages of the record. The
full record will be printed on a single label.
Most Recent Patient
Record N
↓
Most Recent Patient
Record N-1
Earliest Patient Record
↑
Pg 1 of record
→
Pg 2 of record
← →
Pg 3 of record
←
Page 74

TLiIQ System User Manual 4 − 21
Section 4 − Software Functions - Detailed Descriptions
ACC ESS DATA - DATA TRANSFER
Option 4 on the Main Menu screen allows the user to ACCESS DATA
for data transfer to a computer via an RS232 port.
Select option 2 on the ACCESS DATA MENU for DATA TRANSFER.
Connect the appropriate end of the interface cable to the RS232 port
(labeled DATA) of the analyzer. Connect the other end of the interface
cable to the appropriate port of the laboratory computer.
NOTE: Data transferred to a computer is in ASCII format. Capture and organization of the transferred data is done at the
discretion of the user. Hologic Inc. DOES NOT provide software or technical support relating to the manipulation of data
once it has left the analyzer.
ACCESS DATA MENU
1-VIEW/PRINT DATA
2-DATA TRANSFER
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
Page 75

TLiIQ System User Manual 4 − 22
Section 4 − Software Functions - Detailed Descriptions
This message will be displayed while the data transfer is in process.
This message will be displayed if a computer is not attached. Press ESC
to return to the ACCESS DATA MENU.
PLEASE WAIT
COMPUTER NOT PRESENT
ESC-MENU
Page 76

TLiIQ System User Manual 4 − 23
Section 4 − Software Functions - Detailed Descriptions
VIEW SETUP
Option 5 on the Main Menu screen allows the user to view current
settings without editing them.
VIEW SETUP is displayed over two screens. Each screen can be
accessed by using ↓ and ↑ keys.
VIEW SETUP
CAL CODE:FG56
DATE:10/26/07
TIME:03:00 PM ↓
VIEW SETUP
INCUBATION:INTERNAL
AUTOPRINT:ON
↑
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
Page 77

TLiIQ System User Manual 4 − 24
Section 4 − Software Functions - Detailed Descriptions
CHANGE SETUP – DATE/TIME
Option 6 on the Main Menu screen allows the user to change the
DATE/TIME, Autoprint, or Incubation Mode, or to perform QCette Setup
from the SETUP MENU.
The SETUP MENU is displayed over two screens. Each screen can be
accessed by using ↓ and ↑ keys.
Select option 1 on the SETUP MENU to change the DATE/TIME.
SETUP MENU
1-DATE/TIME
2-AUTOPRINT
3-INCUBATION MODE ↓
SETUP MENU
4- QCette SETUP
↑
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
Page 78

TLiIQ System User Manual 4 − 25
Section 4 − Software Functions - Detailed Descriptions
Enter the date at the cursor position on the SET DATE display.
Single digit months and days must be preceded by a zero (e.g.,
September 9, 2007 will be entered as 09/09/07). Use the ← key to delete
incorrect entries. Press ENTER to accept.
Select 1 for 12 HOUR (AM/PM) format or 2 for 24 HOUR (Military Time)
format on the SET TIME display.
SET DATE
MM/DD/YY
> / /
ENTER-ACCEPT
SET TIME
1-12 HOUR
2-24 HOUR
SET DATE
MM/DD/YY
>09/09/07
ENTER-ACCEPT
Page 79

TLiIQ System User Manual 4 − 26
Section 4 − Software Functions - Detailed Descriptions
12 Hour (AM/PM) Format
Enter the time at the cursor position on the TIME display.
Single digit hours or minutes must be preceded by a zero (e.g., 9:09am
must be entered as 09:09AM). Use the ← key to delete incorrect entries.
Use the ↑↓ keys to choose AM/PM. Press ENTER to accept and return
to the SETUP MENU.
24 Hour (Military Time) Format
Enter the time at the cursor position on the TIME display.
Single digit hours or minutes must be preceded by a zero (e.g., 9:09am
must be entered as 09:09). Use the ← key to delete incorrect entries.
Press ENTER to accept and return to the SETUP MENU.
TIME
_ : AM
↑↓ AM/PM
ENTER-ACCEPT
TIME
HH:MM
> :
ENTER-ACCEPT
TIME
09:09AM
↑↓ AM/PM
ENTER-ACCEPT
TIME
HH:MM
>09:09
ENTER-ACCEPT
Page 80

TLiIQ System User Manual 4 − 27
Section 4 − Software Functions - Detailed Descriptions
CHANGE SETUP – AUTOPRINT
Option 6 on the Main Menu screen allows the user to change the
Date/Time, AUTOPRINT, or Incubation Mode, or to perform QCette
Setup from the SETUP MENU.
Select option 2 on the SETUP MENU to change AUTOPRINT.
Autoprint automatically prints test results when set in the ON position.
When set in the OFF position, printouts may be obtained by pressing the
PRINT/ENTER key.
The current setting will be flashing. Select 1-ON or 2-OFF. Press ENTER
to accept and return to the SETUP MENU.
AUTOPRINT
1-ON
2-OFF
ENTER-ACCEPT
SETUP MENU
1-DATE/TIME
2-AUTOPRINT
3-INCUBATION MODE ↓
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
Page 81

TLiIQ System User Manual 4 − 28
Section 4 − Software Functions - Detailed Descriptions
CHANGE SETUP – INCUBATION MODE
Option 6 on the Main Menu screen allows the user to change the
Date/Time, Autoprint, or INCUBATION MODE, or to perform QCette
Setup from the SETUP MENU.
Select option 3 on the SETUP MENU to change INCUBATION MODE.
In the Internal Mode, the analyzer times the incubation and starts the
analysis. External Mode requires the user to manually time the
incubation and start the analysis.
The current setting will be flashing. Select 1-INTERNAL or 2EXTERNAL. Press ENTER to accept and return to the SETUP MENU.
INCUBATION MODE
1-INTERNAL
2-EXTERNAL
ENTER-ACCEPT
SETUP MENU
1-DATE/TIME
2-AUTOPRINT
3-INCUBATION MODE ↓
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
Page 82

TLiIQ System User Manual 4 − 29
Section 4 − Software Functions - Detailed Descriptions
CHANGE SETUP – TLiIQ QCette® SETUP
Option 6 on the Main Menu screen allows the user to change the
Date/Time, Autoprint, or Incubation Mode, or to perform QCette SETUP
from the SETUP MENU.
The SETUP MENU is displayed over two screens. Each screen can be
accessed by using ↓ and ↑ keys.
The QCette SETUP initializes the QCette for use in evaluating the
performance of the analyzer. During the initialization process, the
performance criteria of the analyzer are established. QCette SETUP
must be performed PRIOR to running the QCette as a quality control
device.
Select option 4 on the SETUP MENU to begin QCette SETUP.
fFN MAIN MENU
4-ACCESS DATA
5-VIEW SETUP
6-CHANGE SETUP ↑↓
SETUP MENU
1-DATE/TIME
2-AUTOPRINT
3-INCUBATION MODE ↓
SETUP MENU
4-QCette SETUP
↑
Page 83

TLiIQ System User Manual 4 − 30
Section 4 − Software Functions - Detailed Descriptions
The most recent USER ID is always displayed. Press ENTER to accept
the ID, or enter a new User ID. This field will accept 15 alpha or numeric
characters. To leave this field blank, delete the information using the ←
key.
The QCette SN must be entered to proceed to the next step. The serial
number is printed on the QCette plastic housing. The software requires
that the serial number is entered in the correct format: six numeric
characters (e.g., 001004). Enter al l leadin g zeros .
This message will be displayed if a cassette is present in the analyzer
prior to reaching the next screen. Remove cassette and press ENTER.
QCette SETUP
ENTER QCette SN
>001004
ENTER-CONFIRM
QCette SETUP
USER ID
>JOHN SMITH
ENTER-CONFIRM
REMOVE CASSET TE
PRESS ENTER
TO CONTINUE
Page 84

TLiIQ System User Manual 4 − 31
Section 4 − Software Functions - Detailed Descriptions
The analyzer then prompts the user to insert the QCette and press
ENTER. A two-minute timer starts during which time this message
flashes and the analyzer beeps. Insert the QCette and press ENTER.
This message willI be displayed if the QCette is not inserted. Press
ENTER to continue. Then insert the QCette and press ENTER.
The analyzer will begin initializing the QCette. Do not disturb the
analyzer until the results are displayed. The initialization process will take
approximately 12-15 minutes. Initialization can be terminated by pressing
ESC.
INSERT QCette
ENTER-CONTINUE
INITIALIZING QCette
DO NOT REMOVE
CASSETTE
CASSETTE NOT
INSERTED
ENTER-CONTINUE
Page 85

TLiIQ System User Manual 4 − 32
Section 4 − Software Functions - Detailed Descriptions
TEST WARNING: This message will appear if ESC was press ed dur ing
testing. Lines 1 and 2 will flash prompting the user to select ENTER to
continue test, or ESC to end test. This message will hold for 5 seconds
and then revert back to its respective screen.
Upon completion of the QCette Setup, this message will be displayed.
SETUP COMPLETE indicates that the performance criteria of the
analyzer have been established.
This message will be displayed if the QCette Setup is not completed.
SETUP ERROR indicates that the performance criteria of the analyzer
have not been established. Please refer to Section 7, Troubleshooting.
ARE YOU SURE YOU
WANT TO CANCEL TEST
ENTER-CONTINUE
ESC-MAIN MENU
QCette
SETUP COMPLETE
ESC-MAIN MENU
QCette
SETUP ERROR
NNNN
ESC-MAIN MENU
Page 86

TLiIQ System User Manual 4 − 33
Section 4 − Software Functions - Detailed Descriptions
TEST COUNTS
Option 7 on the Main Menu screen allows the user to view the number of
tests by category that wer e per f orm ed on the anal yzer and automatically
print a Test Counts Report (TCR).
TEST COUNTS are displayed over two screens. Each screen can be
accessed by using the ← and → keys.
TEST COUNTS
PATIENT:96
CONTROL:8
QCette:30 ←
FETAL FIBRONECTIN
TEST COUNTS
03:00 PM 10/26/07
ANALYZER:00426 →
fFN MAIN MENU
7-TEST COUNTS
8-LIQUID CONTROLS
↑
Page 87

TLi
IQ
System User Manual 5 − 1
Section 5 − Care of the Analyzer
SECTION 5 − CARE OF THE ANALYZER
GENERAL CLEANING
Keep the analyzer free of dust. If needed, clean the exterior with a damp cloth and mild detergent.
WARNING: Liquids MUST NOT be allowed to seep into the analyzer. Keep the analyzer dry at all
times.
Liquids leaking into the analyzer may cause damage to the electrical components or possibly electrical shock
to the user.
CAUTION: DO NOT use solvents of any type on any part of the analyzer. Solvents can damage the display and keypad.
CLEANING OF CASSETTE INSERTION SITE
The cassette insertion site can come into contact with biological fluids and should be cleaned regularly.
CAUTION: Use appropriate laboratory procedures for handling biohazardous materials.
CLEANING AGENTS APPROVED FOR USE
Reagents not listed below may cause discoloration to the analyzer case and membrane keypad.
The following cleaning agents may be a p p lied with a cloth or lab wiper only. NEVER apply agents by spray.
• 10% Bleach
• 75% Isopropyl Alcohol
• BacDown (disinfectant)
Page 88

TLi
IQ
System User Manual 6 – 1
Section 6 – Printer
SECTION 6 – PRINTER
LOADING PRINTER LABELS
1. Open the printer cover for access to the interior of the printer. Remove any packing material.
2. Remove the label spool from the printer.
3. Notice that the label spool has distinct LEFT and RIGHT sides. Refer to the illustration on each piece for correct
assembly. The right side slides in and out and can be removed entirely to load label rolls. The adjustable spool
design holds labels of any width.
Page 89

TLi
IQ
System User Manual 6 – 2
Section 6 – Printer
4. Remove the RIGHT SIDE of the spool by sliding it off the right end.
5. Remove the tape from the end of a new roll of labels. Cut the lead label in half to create a clean straight edge.
The printer feeds a straight edge much easier than a rough edge.
6. Refer to Figure 1 while following these instructions: Slide the roll of
labels over the spool from right to left as shown in Figure 1(a). Then
reattach the right side of the spool and push it firmly against the label
roll as shown in Figure 1(b). The labels will feed from the bottom of
the roll.
7. Ensure the power cord is connected. Turn on the printer (On/Off
switch is located at the back of the printer ). T he green po wer li ght wil l
flash and the printer motor will run as it looks for labels to feed.
Figure 1
b
a
Figure 1
Page 90

TLi
IQ
System User Manual 6 – 3
Section 6 – Printer
8. Holding the spool of labels in one hand, use the other hand to feed the free end of the roll into the feed slot on the
inside of the printer, as shown in Figure 2. (If it is easier, rest the labels on the top edge of the printer, freeing both
hands to feed the labels.)
9. Push the end into the slot until a slight resistance is felt. Continue
pushing gently. The label feed motor will feed the end and carry the
labels through the printer and out the exit slot. The printer will stop
feeding automatically at the end of the first label. If the motor stops
running while still in the process of loading labels, press the form feed
button to get it started again. (To protect itself the motor stops running
every few seconds.)
10. Insert the label spool into the printer. The spool will fit into the raised
shoulder slots in the printer.
11. Close the cover and the printer is ready to print labels.
Figure 2
Page 91

TLi
IQ
System User Manual 6 – 4
Section 6 – Printer
REMOVING AN EM PT Y LABEL ROLL
When the printer is out of labels, the green power light will flash.
1. Leave the printer turned on and open the cover. The last label on the roll may be connected to the corrugated core by
a piece of tape. If it is, use scissors to cut the label between the roll and the label feed slot. Remove the label spool
from the printer.
2. Press the Form Feed button on the printer's front panel to eject the remaining label stock from the printer.
3. Slide off the right side of the spool and remove the corrugated core.
4. Load a new roll of labels (see Loading Printer Labels for instructions).
Page 92

TLi
IQ
System User Manual 6 – 5
Section 6 – Printer
CLEARING LABEL JAMS
If the labels jam in the printer, follow these steps to remove them.
1. Open the printer cover and use scissors to cut the label between the feed slot and the roll of labels.
2. Press the Form Feed button on the printer's front panel to advance the label through the printer. Reload the labels
(see Loading Printer Labels for instructions).
3. If the label will not come through the form feed slot, remove the label spool from the printer. Pull the jammed label
gently back out of the printer through the feed slot.
Page 93

TLiIQ System User Manual 7 – 1
Section 7 – Troubleshooting
SECTION 7 − TROUBLESHOOTING
GENERAL INFORMATION
The TLi
IQ
analyzer software is designed for easy troubleshooting. Always heed the beep tones and follow the display
screen prompts to obtain the best performance from your System. The following table lists potential problems, sources of
trouble, and recommended solutions. Call Hologic Inc. Technical Service for any questions related to the performance of
your TLi
IQ
System.
ITEM PROBLEM
SOURCE
SOLUTION
1
Analyzer display
screen is blank.
Analyzer Power Cord
and Adapter
ON/OFF Switch
Ensure analyzer power cord is firmly connected to
analyzer.
Ensure analyzer power adapter is plugged into a
grounded AC electrical outl et.
Ensure analyzer ON/OFF switch is in ON position.
2
Error Code is
displayed when
analyzer is first
turned on.
Analyzer
Turn analyzer off and back on to reinitialize the system.
If the Error Code persists, refer to Error/Invalid Code table.
Page 94

TLiIQ System User Manual 7 – 2
Section 7 – Troubleshooting
ITEM PROBLEM
SOURCE
SOLUTION
3
Analysis process is
interrupted and/or
unusual characters
appear on display
screen, and the
analyzer does not
respond to keypad
inputs.
Electrostatic Discharge
Turn analyzer off and back on to reinitialize the system.
Proceed with testing.
4
Printer fails to print.
Printer Power Cord
ON/OFF Button
Printer Cable
Printer Labels
Ensure printer power cord is firmly connected to printer.
Ensure printer power cord is plugged into a grounded AC
electrical outlet.
Ensure green light is lit. This indicates printer is on.
Ensure printer cable is connected to the printer and the
analyzer.
Ensure printer is not out of printer labels. To order printer
labels, contact Hologic Inc.
Page 95
TLiIQ System User Manual 7 – 3
Section 7 – Troubleshooting
ITEM PROBLEM
SOURCE
SOLUTION
5
Printer not on when
test was run.
Printer
Turn printer on. Recall the test result on the analyzer
display screen. Press PRINT/ENTER on the analyzer to
print the result.
6
Printer output in
unusual font.
Printer
Turn analyzer and printer off and back on.
7
Analyzer turned off
after calibration, or
power failure
occurred after
calibration.
Power
The calibration remains in memory. Reset calibration only
if prompted by analyzer.
8
Cassette cannot be
removed.
Analyzer
Turn analyzer off and back on to reinitialize the system.
Page 96

TLiIQ System User Manual 7 – 4
Section 7 – Troubleshooting
ITEM PROBLEM
SOURCE
SOLUTION
9
TLiIQ QCette failed
to complete setup.
TLiIQ QCette
Analyzer
Ensure QCette is clean and not damaged. Repeat TLiIQ
QCette setup as described in Section 4. If the QCette
setup fails a second time, call technical service.
Turn analyzer off and back on to reinitialize the system.
Repeat TLi
IQ
QCette setup as described in Section 4. If
the QCette setup fails a second time, call technical
service.
10
TLiIQ QCette failed
during daily quality
control.
TLiIQ QCette
Analyzer
Ensure QCette is clean and not damaged. Repeat TLiIQ
QCette daily quality control as described in Section 4. If
the QCette testing fails a second time, call technical
service.
Turn analyzer off and back on to reinitialize the system.
Repeat TLi
IQ
QCette daily quality control as described in
Section 4. If the QCette testing fails a second time, call
technical service.
Page 97

TLiIQ System User Manual 7 – 5
Section 7 – Troubleshooting
ITEM PROBLEM
SOURCE
SOLUTION
11
Liquid control
failed.
Liquid Control
Review the control procedures and repeat the test.
Verify the control has not expired, and is neither cloudy
nor discolored.
If the control fails a second time, call technical service.
12
Invalid liquid control
test result.
Analyzer Internal
Controls: Analyzer
Fail/Cassette Pass
Analyzer Internal
Controls: Analyzer
Pass/Cassette Fail
Refer to Error/Invalid Code table.
Run TLi
IQ
QCette to verify analyzer is functioning properly.
Do not bump or jar analyzer during the test.
Review the Rapid fFN Cassette Kit directional insert to
ensure the correct procedure was followed.
Examine the cassette. Cassette imperfection may cause
an invalid test result. Rerun control on a fresh cassette.
Page 98

TLiIQ System User Manual 7 – 6
Section 7 – Troubleshooting
ITEM PROBLEM
SOURCE
SOLUTION
13
Invalid patient test
result.
Analyzer Internal
Controls:
Analyzer Fail/Cassette
Pass
Analyzer Internal
Controls:
Analyzer Pass/Cassette
Fail
Refer to Error/Invalid Code table.
Run TLi
IQ
QCette to verify analyzer is functioning.
Do not bump or jar analyzer during the test.
Review the Rapid fFN Cassette Kit directional insert to
ensure the correct procedure was followed.
Examine the cassette. Viscous patient samples that flow
slowly may require testing by an alternate format such as
the fFN Enzyme Immunoassay.
Examine the cassette. Cassette imperfection may cause
an invalid test result. Rerun patient sample on a fresh
cassette.
14
Computer not
present message
appears.
Data Transfer
Refer to Section 4, pp. 21, 22.
Page 99

TLiIQ System User Manual 7 – 7
Section 7 – Troubleshooting
ERROR/INVALID CODES
ERROR CODE
DEFINITION
TROUBLE SHOOTING PROCEDURE
720, 721
Possible Motor Problem
Turn analyzer off and back on to
reinitialize the system. If error code
persists, call technical service.
621, 622
Possible Optics Problem
Turn analyzer off and back on to
reinitialize the system. If error code
persists, call technical service.
Other Codes
Call technical service
Page 100

TLi
IQ
System User Manual 8 – 1
Section 8 – Service and Warranty
SECTION 8 – SERVICE AND WARRANTY
TECHNICAL SERVICE
Analyzer
The TLiIQ analyzer is a self-contained instrument. There are no user-serviceable parts. With proper care and use, the
analyzer should operate reliably with minimal attention. If a problem should occur, refer to Section 7, Troubleshooting.
For analyzer service, call Hologic Technical Service.
Printer
The printer is a self-contained instrument. If a problem should occur, refer to Section 7, Troubleshooting. For printer
service, call Hologic Technical Service.
CONTACT INFORMATION
Hologic Inc.
250 Campus Drive
Marlborough, MA 01752 USA
www.Hologic.com
 Loading...
Loading...