Page 1

Page 2

ThinPrep® 3000 Processor
Operator’s Manual
HOLOGIC, INC.
250 C
AMPUS DRIVE
MARLBOROUGH, MA 01752 USA
T
EL: 1-800-442-9892
1-508-263-2900
F
AX: 1-508-229-2795
W
EB: WWW.HOLOGIC.COM
For Use With Version 1.x.y Software
MAN-02586-001
Page 3

Caution:
Federal law restricts this device to sale by or on the order of a physician, or any other
practitioner licensed by the law of the State in which the practitioner practices to use or order the use
®
of the device and are trained and experienced in the use of the ThinPrep
3000 processor.
Preparation of microscope slides using the ThinPrep 3000 processor should be performed only by
personnel who have been trained by Hologic or by organizations or individuals designated by
Hologic.
Evaluation of microscope slides produced with the ThinPrep 3000 processor should be performed
only by cytotechnologists and pathologists who have been trained to evaluate ThinPrep-prepared
slides by Hologic or by organizations or individuals designated by Hologic.
© Hologic, Inc., 2017. All rights reserved. No part of this publication may be reproduced,
transmitted, transcribed, stored in a retrieval system, or translated into any language or computer
language, in any form, or by any means, electronic, mechanical, magnetic, optical, chemical, manual,
or otherwise, without the prior written permission of Hologic, Inc., 250 Campus Drive, Marlborough,
Massachusetts, 01752, United States of America.
Although this guide has been prepared with every precaution to ensure accuracy, Hologic assumes
no liability for any errors or omissions, nor for any damages resulting from the application or use of
this information.
This product may be covered by one or more U.S. patents identified at
http://hologic.com/patentinformation
Hologic, CellFyx, PreservCyt, and ThinPrep are trademarks or registered trademarks of Hologic, Inc.
and/or its subsidiaries in the United States and/or other countries. All other trademarks, registered
trademarks, and product names are the property of their respective owners.
Caution: Changes or modifications to this unit not expressly approved by the party responsible
for compliance could void the user’s authority to operate the equipment.
This equipment has been tested and found to comply with the limits for a Class A digital device,
pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable protections
against harmful interference when the equipment is operated in a commercial environment. This
equipment generates, uses, and can radiate radio frequency energy; and if not installed and used
in accordance with the instruction manual, may cause harmful interference to radio
communications. Operation of this equipment in a residential area is likely to cause harmful
interference, in which case the user will be required to correct the interference at his own expense.
For Use with Model: ThinPrep® 3000
Document Number: AW-07494-001 Rev. 006
Page 4

The ThinPrep
Processor
®
®
Processor
The ThinPrep
Page 5

Instructions for Use
MAN-03939-001 Rev. 004 page 1 of 13
Page 6

INTENDED USE
The ThinPrep® 3000 Processor (TP-3000) is a device that produces cytologic preparations
on glass microscope slides from gynecologic (cervical) samples, and is intended for use in
cervical cytologic examinations of material collected for the ThinPrep Pap Test. TP-3000
prepared microscope slides are examined by trained cytotechnologists and pathologists for
the presence of atypical cells, cervical neoplasia, including its precursor lesions (Low
Grade Squamous Intraepithelial Lesions, High Grade Squamous Intraepithelial Lesions),
and carcinoma as well as all other cytologic criteria as defined by The Bethesda System for
Reporting Cervical/Vaginal Cytologic Diagnoses
1
(Bethesda System).
SUMMARY AND EXPLANATION OF THE SYSTEM
The ThinPrep process begins with the patient’s gynecologic sample being collected by the clinician,
which is then immersed and rinsed in a PreservCyt
vial is then capped, labeled, and sent to a laboratory equipped with a TP-3000.
At the laboratory, the PreservCyt sample vial is bar-coded along with the test request form to
establish a sample chain of custody and is placed into a TP-3000. A gentle dispersion step mixes
the cell sample by currents in the fluid that are strong enough to separate debris and disperse mucus,
but gentle enough to have no adverse effect on cell appearance.
The cells are then captured on a Gynecological ThinPrep Pap Test Filter that is specifically designed
to collect cells. The TP-3000 constantly monitors the rate of flow through the ThinPrep Pap Test
Filter during the collection process in order to prevent the cellular presentation from being too scant
or too dense. The TP-3000 will label the glass slide with the sample identification number read from
the bar-code on the sample vial. A thin layer of cells is then transferred to a glass slide in a 20 mmdiameter circle. The slide is completed when its cells are fixed in place by a fixative solution
(CellFyx
™
Solution) that is applied automatically by the processor.
The ThinPrep Pap Test Slide Preparation Process
®
Solution sample vial. The PreservCyt sample
1. Dispersion 2. Cell Collection 3. Cell Transfer
(1) Dispersion (2) Cell Collection (3) Cell Transfer
The cell sample is mixed by currents created
in the preservation fluid that are strong
enough to separate debris and disperse mucus,
but gentle enough to have no adverse effect
on cell appearance.
A gentle vacuum is applied to the ThinPrep
Pap Test Filter to collect cells.
The ThinPrep Pap Test Filter is gently pressed
against the ThinPrep Microscope Slide.
Positive pressure applied to the inside of the
filter assists in transferring the cells from the
filter membrane to the surface of the slide.
MAN-03939-001 Rev. 004 page 2 of 13
Page 7

As with conventional Pap smears, slides prepared with the TP-3000 are examined in the
context of the patient’s clinical history and information provided by other diagnostic
procedures such as colposcopy, biopsy, and human papillomavirus (HPV) testing, to
determine patient management.
The PreservCyt
collection and transport medium for gynecologic specimens tested with the Cervista
®
Solution component of the ThinPrep 2000 System is an alternative
®
HPV HR Test, the Cervista® HPV 16/18 Test, the Roche cobas® HPV Test and the
Digene Hybrid Capture System HPV DNA. Refer to the respective manufacturer’s
package inserts for instructions for using PreservCyt Solution for collection, transport,
storage, and preparation of specimens for use in those systems.
The PreservCyt Solution component of the ThinPrep 2000 System is an alternative
collection and transport medium for gynecologic specimens tested with the Hologic
APTIMA COMBO 2
Assay, and the BD ProbeTec
®
CT/NG Assays, the Hologic APTIMA® Trichomonas vaginalis
™
CT Qx Amplified DNA Assay. Refer to the respective
manufacturer’s package inserts for instructions for using PreservCyt Solution for
collection, transport, storage, and preparation of specimens for use in those systems.
The PreservCyt Solution component of the ThinPrep 2000 System is also an alternative
collection and transport medium for gynecologic specimens tested with the Roche
Diagnostics COBAS AMPLICOR
TM
CT/NG assay. Refer to Hologic’s labeling
(Document #MAN-02063-001) for instructions for using PreservCyt Solution for
collection, transport, storage, and preparation of specimens and to the Roche Diagnostics
COBAS AMPLICOR CT/NG package insert for instructions for use of that system.
LIMITATIONS
Gynecologic samples collected for the TP-3000 should be collected using a broom-
Preparation of slides on the TP-3000 should be performed only by personnel who
The staining procedure using the CellFyx
Evaluation of slides prepared on the TP-3000 should be performed only by
Supplies used for TP-3000 gynecologic slide preparations are those designed by
All supplies, with the exception of CellFyx Fixative Solution, are single-use
The performance of HPV DNA and CT/NG testing on reprocessed sample vials has
type or endocervical brush/plastic spatula combination collection devices. Refer to
the instructions provided with the collection device for warnings, contraindications,
and limitations associated with specimen collection.
have been trained by Hologic or by organizations or individuals designated by
Hologic.
®
Fixative Solution has been demonstrated
for Papanicolaou stain only.
cytotechnologists and pathologists who have been trained to evaluate ThinPrep Pap
Test slides by Hologic or by organizations or individuals designated by Hologic.
Hologic specifically for use on the instrument. These supplies include PreservCyt
®
Solution vials for use with the ThinPrep Pap Test, ThinPrep Pap Test Filters,
ThinPrep Microscope Slides, and CellFyx Fixative Solution. For proper performance
of the system these supplies cannot be substituted. After use, supplies should be
disposed of in accordance with local, state, and federal regulations.
disposable items and cannot be reused.
not been evaluated.
MAN-03939-001 Rev. 004 page 3 of 13
Page 8

CONTRAINDICATIONS
Chlamydia trachomatis and Neisseria gonorrhoeae testing using the Roche
Diagnostics COBAS AMPLICOR and Gen-Probe APTIMA COMBO 2
assays should not be performed on a sample that has already been processed using
the ThinPrep 3000 processor.
WARNINGS
For In Vitro Diagnostic Use.
Danger. PreservCyt Solution contains methanol. Toxic if swallowed. Toxic if
inhaled. Causes organ damage. Keep away from heat, sparks, open flames and hot
surfaces. Other solutions must not be substituted for PreservCyt Solution. PreservCyt
Solution should be stored and disposed of in accordance with local, state, and federal
regulations.
PRECAUTIONS
A TP-3000 generates, uses and can radiate radio frequency energy, and if not
installed and used in accordance with the Operator’s Manual, may cause interference
to radio communications. Operation of this equipment in a residential area is likely
to cause harmful interference, in which case, the user will be required to correct the
interference at his/her own expense.
PreservCyt Solution with cytologic sample intended for ThinPrep Pap testing must be stored
between 15
PreservCyt Solution with cytologic sample intended for CT/NG testing using the Roche
Diagnostics COBAS AMPLICOR CT/NG test must be stored between 4
o
(77
F) and tested within 6 weeks of collection.
Excessively bloody samples may result in a higher unsatisfactory
oC
(59oF) and 30oC (86oF) and tested within 6 weeks of collection.
1
rate.
®
CT/NG
o
C (39oF) and 25oC
MAN-03939-001 Rev. 004 page 4 of 13
Page 9
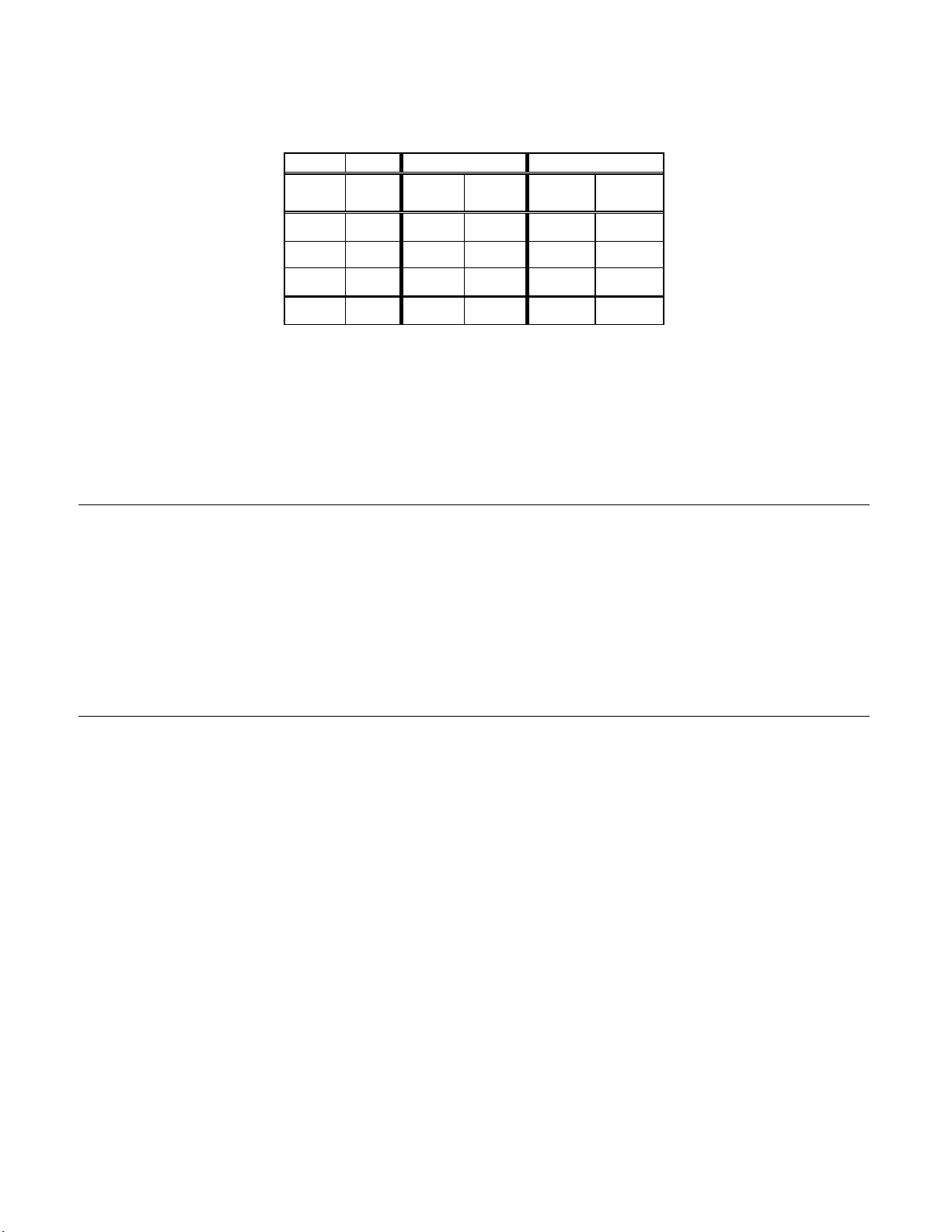
PreservCyt Solution was challenged with a variety of microbial and viral organisms.
The following table presents the starting concentrations of viable organisms, and the
number of viable organisms found after 15 minutes in the PreservCyt solution. The
log reduction of viable organisms is also presented. As with all laboratory
procedures, universal precautions should be followed.
Organism Initial Concentration
Candida albicans 5.5 x 105 CFU/mL >4.7
Aspergillus niger* 4.8 x 105 CFU/mL 2.7
Escherichia coli 2.8 x 105 CFU/mL >4.4
Staphylococcus aureus 2.3 x 105 CFU/mL >4.4
Pseudomonas aeruginosa 2.5 x 105 CFU/mL
Mycobacterium tuberculosis** 9.4 x 105 CFU/mL 4.9
Rabbitpox virus 6.0 x 106 PFU/mL 5.5***
HIV-1 1.0 x 10
* After 1 hour >4.7 log reduction
** After 1 hour >5.7 log reduction
*** Data is for 5 minutes
Log Reduction after
15 min.
>4.4
7.5
TCID50/mL 7.0***
PERFORMANCE CHARACTERISTICS: REPORT OF CLINICAL
STUDIES
A prospective multi-center clinical study was conducted at three sites to evaluate the
performance of the TP-3000 in direct comparison to the ThinPrep
2000). The objective of this clinical study was to demonstrate that gynecologic specimens
prepared using both instruments were equivalent when used for the detection of atypical
cells and cervical cancer or its precursor lesions in a variety of patient populations. In
addition, an assessment of specimen adequacy was performed.
The initial clinical study protocol was a single-masked, direct-to-vial, matched-pair study,
for which the order of preparation for each instrument was randomized. At the laboratory,
the PreservCyt sample vial was placed into both a TP-3000 and a TP-2000 and two slides
were prepared (one per instrument) from the patient’s sample. All slides were examined
and diagnosed independently. The same cytotechnologist and pathologist (if referred)
reviewed each matched-paired slide set. To minimize slide recognition bias there was a
minimum one-day lag between the cytotechnologist and pathologist review of all slides
from a matched-pair set. Reporting forms containing patient history as well as a checklist
of all possible categories of the Bethesda System were used to record the results of the
screening. A panel of three independent pathologists adjudicated all discordant cases (a
one-grade or higher cytologic difference) in a masked fashion to determine a consensus
diagnosis.
®
2000 Processor (TP-
MAN-03939-001 Rev. 004 page 5 of 13
Page 10
LABORATORY AND PATIENT CHARACTERISTICS
The cytology laboratories participating in the study were comprised of one referral center
(designated as S1), one screening/referral center (designated as S2) and one screening
center (designated as S3).
The screening center in the study served patient populations (screening populations) with
rates of abnormality (Low-grade Squamous Intraepithelial Lesion [LSIL] and more severe
lesions) similar to the United States average of less than 5%.
study served a high risk referral patient population (referral populations) characterized by
high rates (>10%) of cervical abnormality. The screening/referral center’s abnormality rate
was a combination of the two previously mentioned rates. Table
laboratories and the patient populations.
Table 1: Site Characteristics
Laboratory Characteristics Clinical Study Demographics
Site Type of Patient
Population
S1 Referral 44,709 1188 18-85 11.8 51.8 35.2
Screening/Refer
S2
ral
Laboratory
Volume -
Smears per
Year
62,195 1141 18-77 6.0 21.8 15.1
Cases Patient
Age Range
Post
Menopausal
%
3
The referral center in the
Previous
Abnormal
Pap Smear
%
1 describes the
Con-current
Infection
%
S3 Screening 90,639 1198 18-82 12.5 22.7 10.2
Cases with patient’s age less than 18 years or patients with a hysterectomy were
excluded from this analysis.
CLINICAL STUDY RESULTS
The diagnostic classes of the Bethesda System are used to present the comparison between
the TP-3000 and TP-2000 findings from all of the clinical trial sites.
Three independent pathologists served as an adjudication panel for the three clinical sites.
The panel reviewed all discordant cases (a one-grade or higher cytologic difference) for
descriptive diagnosis and specimen adequacy. Since a true reference cannot be determined
in such studies and therefore true sensitivity cannot be calculated, the use of an independent
adjudicated review provides an alternative to histologic confirmation by biopsy or human
papillomavirus (HPV) testing as a means for determining the reference diagnosis.
Consensus was determined when a minimum of 2 out of 3 independent pathologists
rendered an equivalent diagnosis. If a majority vote could not be obtained, a consensus was
achieved during a review by all three pathologists at a multi-headed scope.
Table 2 shows the unadjudicated descriptive diagnosis results from all sites for the TP3000 and TP-2000. Of the 3,527 total patients enrolled in the study, 3,224 were included
in the descriptive diagnosis analysis after all data integrity sorting was applied.
Few cases of cervical cancer were represented in the clinical study, as is typical in the
United States patient population.
4
MAN-03939-001 Rev. 004 page 6 of 13
Page 11
Table 2: Unadjudicated 7 x 7 Classification Table, All Categories
TP-3000
TP- NEG 2570
2000 ASCUS
AGUS
LSIL
HSIL
SQ CA
GL CA
TOTAL
Abbreviations for Diagnoses: NEG = Normal or negative, ASCUS = Atypical
Squamous Cells of Undetermined Significance, AGUS = Atypical Glandular Cells of
Undetermined Significance, LSIL = Low-grade Squamous Intraepithelial Lesion, HSIL
= High-grade Squamous Intraepithelial Lesion, SQ CA = Squamous Cell Carcinoma,
GL CA = Glandular Cell Adenocarcinoma
NEG ASCUS AGUS LSIL HSIL SQ CA GL CA TOTAL
104 6 26 3 0 0 2709
119
4 1
17 29 1
0 10 0 17
0 0 0 0 0
0 0 0 0 0 0
2710 234 7 198 73 2 0
90
0 23 6 0 0 238
0
0 0 0 0 5
132
10 0 0 189
54
0 0 81
2
0 2
0
0
3224
Tables 3 - 9 show the adjudicated descriptive diagnosis results from all sites for the TP3000 and TP-2000.
Table 3: Adjudicated 7 x 7 Diagnostic Classification Table, All Categories (Includes adjudicated cases only)
TP-3000
TP- NEG 258
2000 ASCUS
AGUS
LSIL
HSIL
SQ CA
GL CA
TOTAL
Abbreviations for Diagnoses: NEG = Normal or negative, ASCUS = Atypical
Squamous Cells of Undetermined Significance, AGUS = Atypical Glandular Cells of
Undetermined Significance, LSIL = Low-grade Squamous Intraepithelial Lesion, HSIL
= High-grade Squamous Intraepithelial Lesion, SQ CA = Squamous Cell Carcinoma,
GL CA = Glandular Cell Adenocarcinoma
NEG ASCUS AGUS LSIL HSIL SQ CA GL CA TOTAL
25 0 5 1 0 0 289
29
0 0
6 9 0
1 2 0 3
0 0 0 0 0
0 0 0 0 0 0
294 47 0 29 6 0 0
11
0 11 0 0 0 51
0
0 0 0 0 0
10
2 0 0 27
3
0 0 9
0
0 0
0
0
376
The diagnostic data analysis from all sites is summarized in Table 4 for adjudicated
cytologic results of LSIL+.
Table 4: Adjudicated Two-Category Diagnostic Classification Table, LSIL and More Severe Lesions
(Includes adjudicated cases only)
TP-3000
NEG/ASCUS/
AGUS
TP- NEG/ASCUS/AGUS 323 17 340
2000 LSIL+ 18 18 36
TOTAL 341 35 376
The diagnostic data analysis from each site is summarized in Table 5 for adjudicated
cytologic results of LSIL+. When the p-value is significant (p < 0.05), the method
favored is indicated in the tables.
LSIL+ TOTAL
MAN-03939-001 Rev. 004 page 7 of 13
Page 12
Table 5: Adjudicated Results by Site, LSIL and More Severe Lesions (Includes adjudicated cases only)
Site
S1
S2
S3
For LSIL and more severe lesions, the adjudicated
diagnostic comparison was statistically equivalent at all
sites.
Cases TP-3000
LSIL+
240 13 15 0.791 Neither
65 16 16 1.000 Neither
71 6 5 1.000 Neither
TP-2000
LSIL+ p-Value
Method
Favored
The diagnostic data analysis from all sites is summarized in Table
6 for adjudicated
cytologic results of HSIL+.
Table 6: Adjudicated Two-Category Diagnostic Classification Table, HSIL and More Severe Lesions
(Includes adjudicated cases only)
TP-3000
NEG/ASCUS/
AGUS/LSIL
TP-
2000 HSIL+ 6 3 9
TOTAL 370 6 376
NEG/ASCUS/
AGUS/LSIL
364 3 367
HSIL+ TOTAL
The diagnostic data analysis from each site is summarized in Table 7 for adjudicated
cytologic results of HSIL+. When the p-value is significant (p < 0.05), the method favored
is indicated in the tables.
Table 7: Adjudicated Results by Site, HSIL and More Severe Lesions (Includes adjudicated cases only)
Site
S1
S2
S3
Cases TP-3000
HSIL+
240 1 1 1.000 Neither
65 3 5 0.625 Neither
71 2 3 1.000 Neither
For HSIL and more severe lesions, the adjudicated
diagnostic comparison was statistically equivalent at all sites.
TP-2000
HSIL+
p-Value Method
Favored
MAN-03939-001 Rev. 004 page 8 of 13
Page 13

Table 8 below shows the summary of the Bethesda System categories of the
unadjudicated descriptive diagnosis data for all sites.
Table 8: Unadjudicated Summary of Descriptive Diagnosis
Descriptive Diagnosis TP-2000 TP-3000
Number of Patients: 3224
Benign Cellular Changes:
Infection:
Trichomonas Vaginalis
Candida spp.
Coccobacilli
Actinomyces spp.
Herpes
Other
Reactive Cellular Changes
Associated with:
Inflammation
Atrophic Vaginitis
Radiation
IUD
Other
Epithelial Cell Abnormalities:
Squamous Cell:
ASCUS (combined)
Favor reactive
Favor neoplastic
Undetermined
LSIL
HSIL
Carcinoma
Glandular Cell:
Benign Endometrial cells in
Postmenopausal Women
AGUS (combined)
Favor reactive
Favor neoplastic
Undetermined
Note: Some patients had more than one descriptive diagnosis subcategory.
ASCUS=Atypical Squamous Cells of Undetermined Significance
AGUS=Atypical Glandular Cells of Undetermined Significance
N % N %
903
69
208
346
313
16
89
526
239
82
81
76
189
81
11
28.0
2.1
6.5
10.7
0
2
7
1
0
2
6
2
0
4
0.0
0.1
0.2
9.7
0.5
0.0
0.0
2.8
16.3
7.4
2.5
2.5
2.4
5.9
2.5
0.1
0.3
0.2
0.1
0.0
0.1
848
67
193
347
292
16
72
525
236
73
69
94
198
73
11
23.6
1
2
2
0
0
2
8
2
1
5
2.1
6.0
10.8
0.0
0.1
0.1
9.1
0.5
0.0
0.0
2.2
16.3
7.3
2.3
2.1
2.9
6.1
2.3
0.1
0.3
0.3
0.1
0.0
0.2
MAN-03939-001 Rev. 004 page 9 of 13
Page 14

Table 9 shows the summary of the Bethesda System categories of the adjudicated
descriptive diagnosis data for all sites.
Table 9: Adjudicated Summary of Descriptive Diagnosis
(Includes adjudicated cases only)
Descriptive Diagnosis TP-2000 TP-3000
Number of Patients: 376
Benign Cellular Changes:
Infection:
Trichomonas Vaginalis
Candida spp.
Coccobacilli
Actinomyces spp.
Herpes
Other
Reactive Cellular Changes
Associated with:
Inflammation
Atrophic
Vaginitis
Radiation
IUD
Other
Epithelial Cell Abnormalities:
Squamous Cell:
ASCUS (combined)
Favor reactive
Favor neoplastic
Undetermined
LSIL
HSIL
Carcinoma
Glandular Cell:
Benign Endometrial cells in
Postmenopausal Women
AGUS (combined)
Favor reactive
Favor neoplastic
Undetermined
Note: Some patients had more than one descriptive diagnosis subcategory.
ASCUS=Atypical Squamous Cells of Undetermined Significance
AGUS=Atypical Glandular Cells of Undetermined Significance
N % N %
163
35
62
89
88
87
33
45
27
43.4
8
0
0
2
1
0
0
4
9
9
0
1
0
0
0
0
2.1
9.3
16.5
0.0
0.0
0.5
23.7
0.3
0.0
0.0
1.1
23.4
23.2
2.4
8.8
12.0
7.2
2.4
0.0
0.3
0.0
0.0
0.0
0.0
174
11
30
72
96
82
78
31
40
29
46.3
0
0
0
0
0
1
0
7
6
0
0
0
0
0
0
2.9
8.0
19.1
0.0
0.0
0.0
25.5
0.0
0.0
0.3
0.0
21.8
20.7
1.9
8.2
10.6
7.7
1.6
0.0
0.0
0.0
0.0
0.0
0.0
The Bethesda System delineates specimen adequacy in three categories: satisfactory,
satisfactory but limited by (SBLB) and unsatisfactory. Of the 3,527 total patients enrolled
in the study, 3,489 were included in the specimen adequacy analysis after all data integrity
sorting was applied.
MAN-03939-001 Rev. 004 page 10 of 13
Page 15

Tables 10 and 11 show the summary of the Bethesda System categories of the unadjudicated and
adjudicated specimen adequacy data for all sites.
Table 10: Unadjudicated Summary of Specimen Adequacy Results
Specimen Adequacy TP-2000 TP-3000
Number of Patients: 3489
N % N %
Satisfactory 2985 85.6 2951 84.6
Satisfactory for Evaluation but Limited by:
Air-Drying Artifact
Thick Smear
Endocervical Component A bsent
Scant Squamous Epithelial Component
Obscuring Blood
Obscuring Inflammation
No Clinical History
Cytolysis
Other
Unsatisfactory for Evaluation:
Air-Drying Artifact
Thick Smear
Endocervical Component A bsent
Scant Squamous Epithelial Component
Obscuring Blood
Obscuring Inflammation
No Clinical History
Cytolysis
Other
385
244
125
22
15
119
109
20
11.0
0
0.0
1
0.0
7.0
3.6
0.6
0.4
0
0.0
1
0.0
0
0.0
3.4
0
0.0
0
0.0
2
0.1
3.1
0.6
3
0.1
0
0.0
0
0.0
0
0.0
398
237
122
29
24
140
126
36
11.4
1
0.0
2
0.1
6.8
3.5
0.8
0.7
2
0.1
4
0.1
0.1
2
4.0
0
0.0
0
0.0
3
0.1
3.6
1.0
5
0.1
0
0.0
0
0.0
1
0.0
Note: Some patients had more than one subcategory.
Table 11: Adjudicated Summary of Specimen Adequacy Results
(Includes adjudicated cases only)
Specimen Adequacy TP-2000 TP-3000
Number of Patients: 57
N % N %
Satisfactory 12 21.1 9 15.8
Satisfactory for Evaluation but Limited by:
Air-Drying Artifact
Thick Smear
Endocervical Component A bsent
Scant Squamous Epithelial Component
Obscuring Blood
Obscuring Inflammation
No Clinical History
Cytolysis
Other
Unsatisfactory for Evaluation:
Air-Drying Artifact
Thick Smear
Endocervical Component A bsent
Scant Squamous Epithelial Component
Obscuring Blood
Obscuring Inflammation
No Clinical History
Cytolysis
Other
24
24
21
13
21
42.1
0
0
6
0
1
0
0
0
0.0
0.0
10.5
42.1
0.0
1.8
0.0
0.0
0.0
36.8
0
0
0
1
0
0
0
0.0
0.0
22.8
36.8
0.0
1.8
0.0
0.0
0.0
18
18
30
30
10
31.6
0
0
4
1
3
0
0
0
0.0
0.0
7.0
31.6
1.8
5.3
0.0
0.0
0.0
52.6
0
0
9
3
0
0
0
0.0
0.0
15.8
52.6
17.5
5.3
0.0
0.0
0.0
Note: Some patients had more than one subcategory.
Table 12 shows the adjudicated specimen adequacy results, respectively, from all sites for the
TP-3000 and TP-2000.
Table 12: Adjudicated Two-Category Diagnostic Classification Table, Specimen Adequacy Results
(Includes adjudicated cases only)
TP-3000
SBLB/SAT UNSAT TOTAL
TP-2000 SBLB/SAT 23 13 36
UNSAT 4 17 21
TOTAL 27 30 57
MAN-03939-001 Rev. 004 page 11 of 13
Page 16
The adjudicated specimen adequacy results from each site are presented in Table 13 as SAT/SBLB
versus UNSAT.
Table 13: Adjudicated Specimen Adequacy Results by Site (Includes adjudicated cases only)
SAT/SBLB UNSAT*
Site
S1
Cases
TP-3000
Cases
50 24 33 26 17
TP-2000
Cases
TP-3000
Cases
TP-2000
Cases
S2
S3
All Sites
*Note: Excessively bloody samples may result in a higher unsatisfactory rate.
1 0 0 1 1
6 3 3 3 3
57 27 36 30 21
The TP-3000 provides similar results to the TP-2000 System in a variety of patient populations.
The TP-3000 may be used as a replacement for the TP-2000 System in the preparation of cervical
cytology samples on glass microscope slides used in the detection of atypical cells, cervical cancer,
or its precursor lesions, as well as all other cytologic categories as defined by The Bethesda System.
TECHNICAL SERVICE AND PRODUCT INFORMATION
For technical service and assistance related to use of the ThinPrep® 3000 Processor, contact
Hologic:
Telephone: 1-800-442-9892
Fax: 1-508-229-2795
For international or toll-free blocked calls, please contact 1-508-263-2900.
Email: info@hologic.com
REQUIRED MATERIALS
The TP-3000 consists of the following components:
The ThinPrep
®
3000 Processor (Model TP-3000)
ThinPrep 3000 Processor Operator’s Manual
Power Cord
Program Memory Card
Staining Rack Adapters
Accessory Kit
MATERIALS REQUIRED BUT NOT PROVIDED
Slide staining system
Coverslips and mounting media
®
20 ml PreservCyt
Solution vials
ThinPrep Pap Test Filters
CellFy x™ Fixative Solution
ThinPrep Microscope Slides
MAN-03939-001 Rev. 004 page 12 of 13
Page 17

STORAGE
Store PreservCyt Solution between 15°C (59°F) and 30°C (86°F). Do not use beyond the
expiration date printed on the container.
Store PreservCyt Solution with cytologic sample intended for ThinPrep Pap testing between 15°C
(59°F) and 30°C (86°F) for up to 6 weeks.
Store PreservCyt Solution with cytologic sample intended for CT/NG using the Roche
Diagnostics COBAS AMPLICOR CT/NG test testing between 4°C (39°F) and 25°C (77°F) for
up to 6 weeks.
Store CellFyx Solution between 15C and 30C. Do not use beyond the expiration date printed
on the container.
CellFyx Solution preserves cells on slides up to 5 days at 15C to 30C prior to staining.
BIBLIOGRAPHY
1. Kurman RJ, Solomon D. The Bethesda System for Reporting Cervical/Vaginal Cytologic
Diseases, Springer-Verlag, New York 1994.
2. United States Pharmacopeia (U.S.P.), Preservative Antimicrobial Effectiveness Test, U.S.P. XXII
(51).
3. Jones HW. Impact of The Bethesda System, Cancer 77 pp. 1914-1918, 1995.
4. American Cancer Society. Cancer Facts and Figures, 1995.
Hologic, Inc.
250 Campus Drive
Marlborough, MA 01752, USA
1-800-442-9892
www.hologic.com
2017 Hologic, Inc. All rights reserved.
AW-07101-001 Rev. 006
MAN-03939-001 Rev. 004 page 13 of 13
Page 18

Table of Contents
Table of Contents
Page 19

Table of Contents
Chapter One
INTRODUCTION
TABLE OF CONTENTS
SECTION A:
SECTION B:
SECTION C:
SECTION D:
SECTION E:
SECTION F:
SECTION G:
Chapter Two
THINPREP 3000 INSTALLATION
SECTION A:
SECTION B:
SECTION C:
SECTION D:
Overview and Function of the
ThinPrep® 3000 Processor 1.1
Overview of Instrument Systems 1.4
Material Requirements 1.8
ThinPrep 3000 Processor
Technical Specifications 1.9
Internal Quality Control 1.12
ThinPrep 3000 Processor Hazards 1.12
Disposal 1.17
General 2.1
Action Upon Delivery 2.1
Preparation Prior to Installation 2.3
Storage and Handling Post Installation 2.3
SECTION E:
SECTION F:
SECTION G:
SECTION H:
SECTION I:
SECTION J:
Connect Power 2.4
How to Turn the Processor On/Off 2.4
System Startup 2.6
Setting the Time and Date 2.7
Setting the Slide Printer Output 2.10
Setting the Audible Key Press 2.11
ThinPrep® 3000 Processor Operator’s Manual
i
Page 20

TABLE OF CONTENTS
Chapter Three
PRESERVCYT AND CELLFYX SOLUTIONS
SECTION A:
SECTION B:
SECTION C:
Introduction 3.1
PreservCyt® Solution 3.2
CellFyx™ Solution 3.5
Chapter Four
GYNECOLOGIC SAMPLE COLLECTION AND PREPARATION
SECTION A:
SECTION B:
SECTION C:
SECTION D:
SECTION E:
Introduction 4.1
Specimen Collection 4.2
Special Precautions 4.4
Specimen Processing 4.4
Sample Processing Troubleshooting 4.6
Chapter Five
INSTRUMENT OPERATION
SECTION A:
SECTION B:
Chapter Overview 5.1
Optional Instructions for Ancillary Testing 5.2
SECTION C:
SECTION E:
SECTION E:
SECTION F:
SECTION G:
SECTION H:
SECTION I:
ii
ThinPrep® 3000 Processor Operator’s Manual
Instrument Doors 5.4
Items Required to Begin Batch Processing 5.5
Begin Batch Processing 5.14
Completing a Batch 5.18
Reading the Batch Report 5.20
Pausing a Batch in Process 5.22
Canceling a Batch in Process 5.23
Page 21

Chapter Six
INSTRUMENT MAINTENANCE
TABLE OF CONTENTS
SECTION A:
SECTION B:
SECTION C:
SECTION D:
SECTION E:
SECTION F:
SECTION G:
SECTION H:
SECTION I:
SECTION J:
SECTION K:
SECTION L:
SECTION M:
SECTION N:
SECTION O:
Recommended Maintenance Schedule 6.2
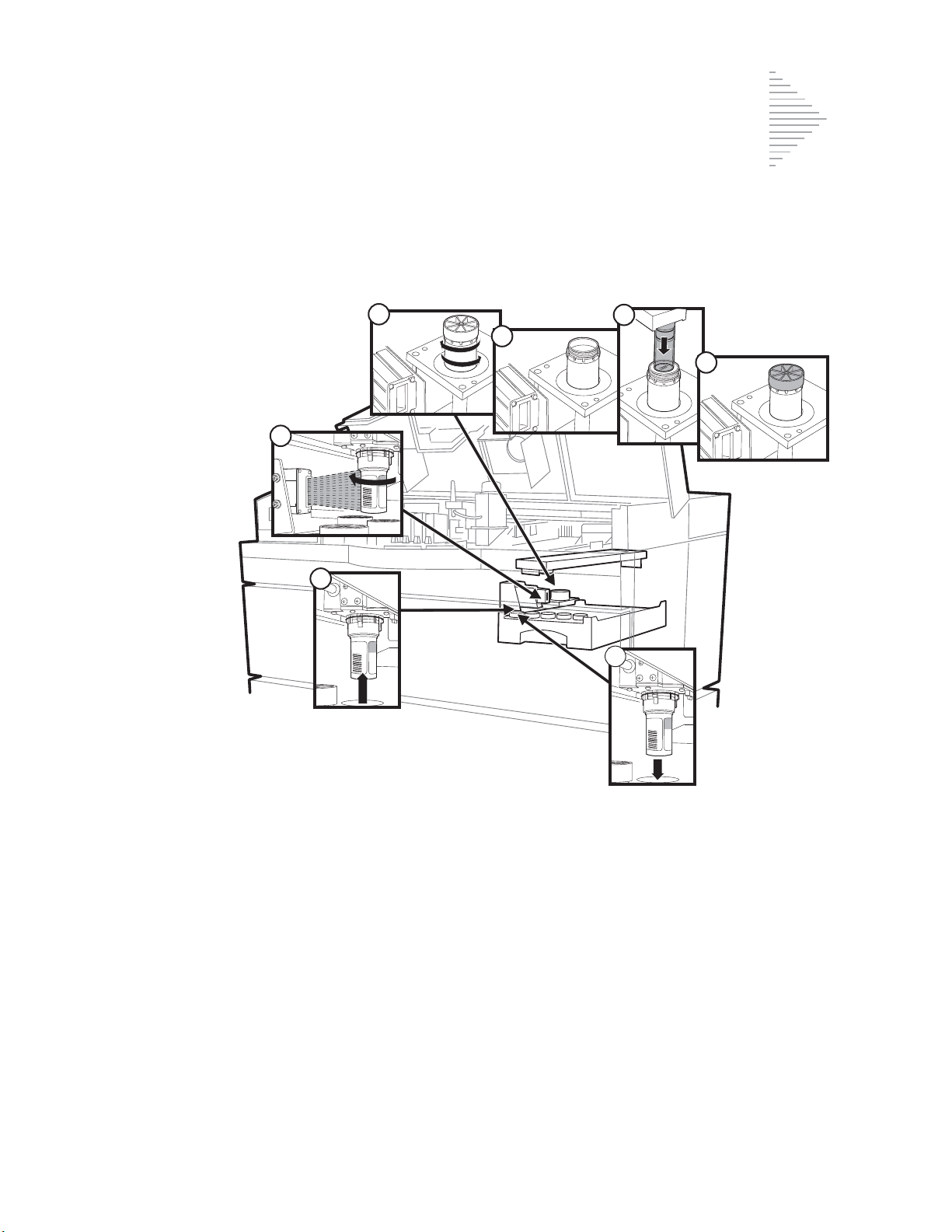
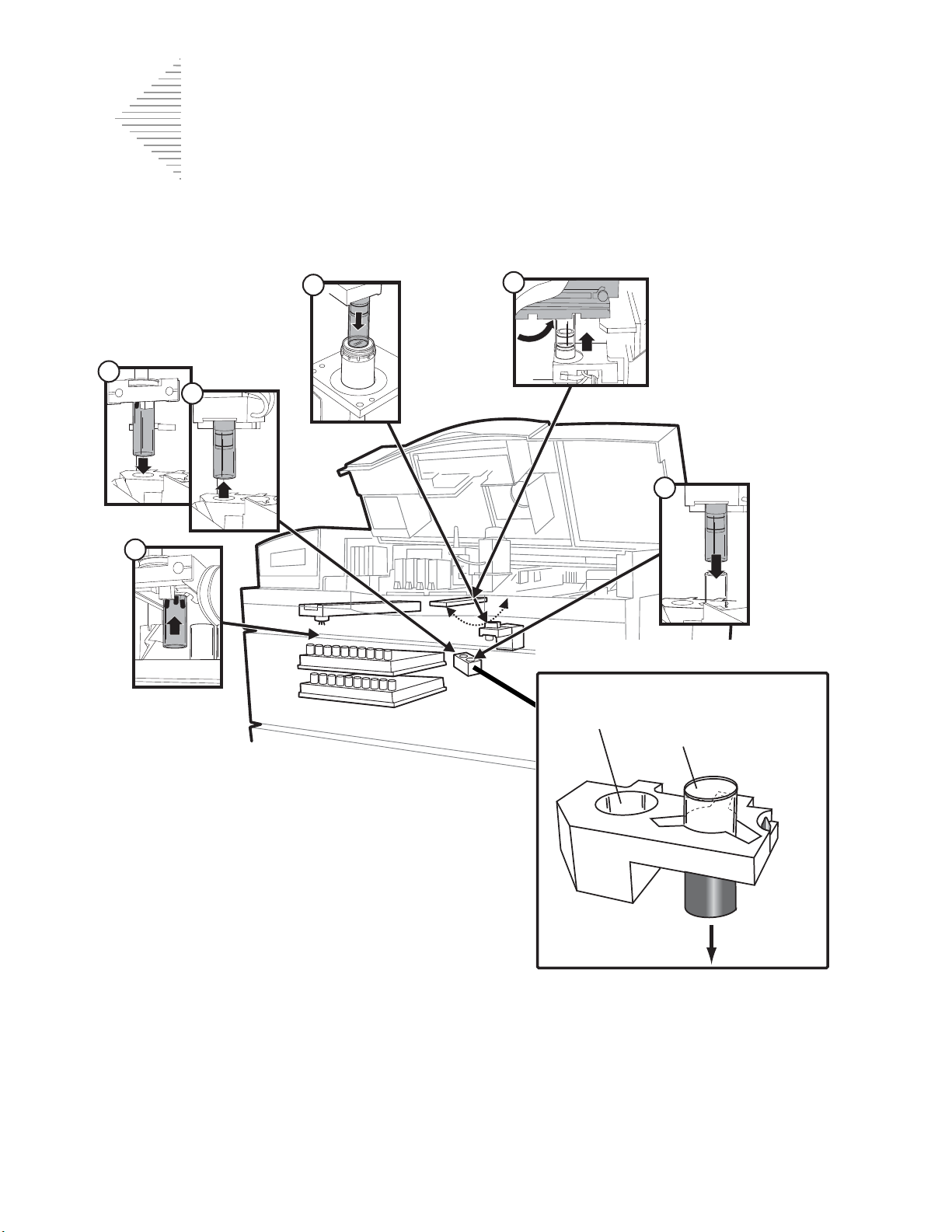
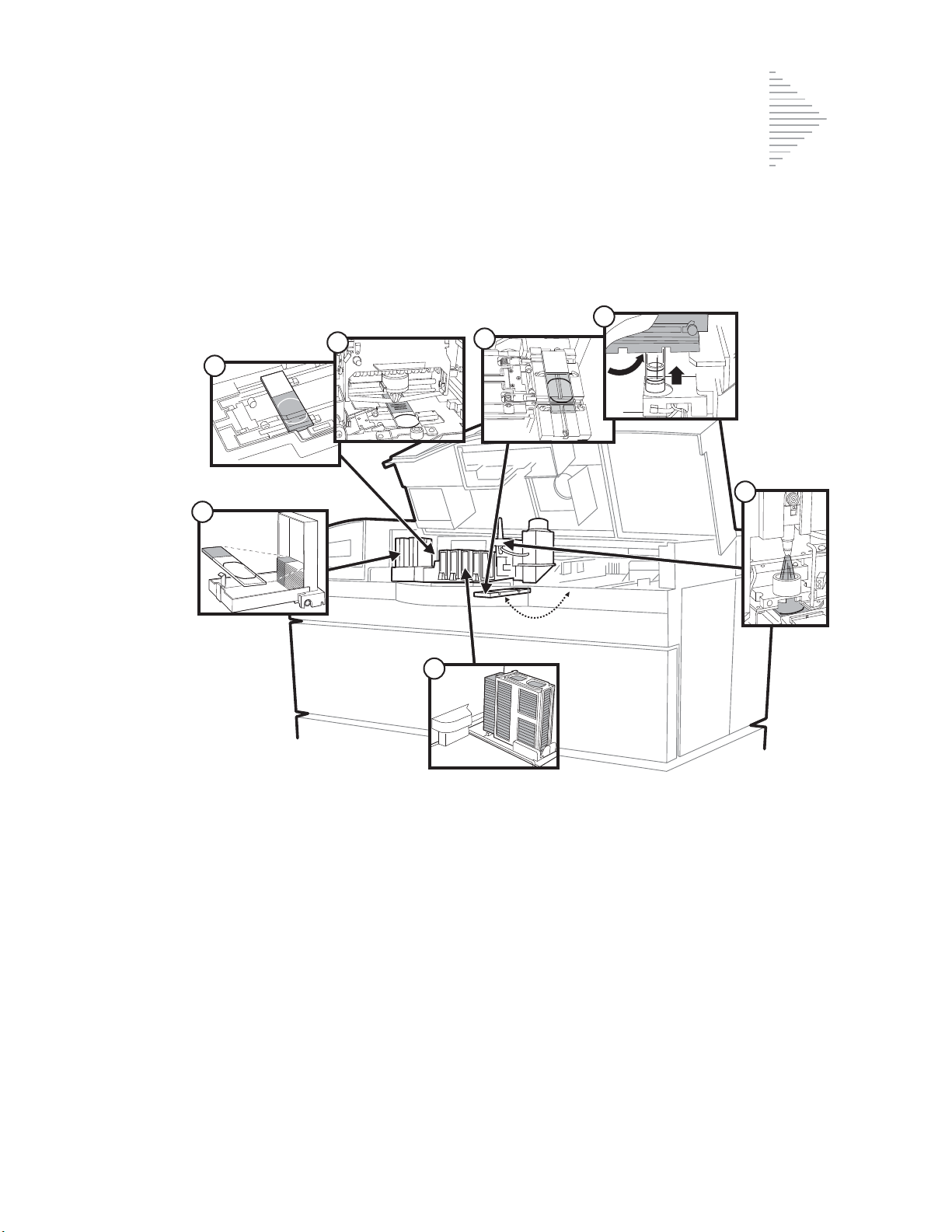
Fixative System Preventative Maintenance 6.5
Emptying the Slide Waste Bin 6.9
Lubricating the
Sample Processing Arm O-Rings 6.10
Pneumatic System Testing 6.13
Cleaning the Slide Path 6.17
Replacing the Fixative Shield 6.23
General Cleaning 6.24
Pinch Valve Tubing Replacement 6.26
Waste System Maintenance 6.28
Emptying the Filter Waste Box 6.31
Replenishing CellFyx™ Solution 6.32
Replacing the Slide Printer Ribbon 6.34
Replacing the User-Accessible Fuses 6.36
Replacing the Results Printer Paper 6.38
Chapter Seven
TROUBLESHOOTING
SECTION A:
SECTION B:
SECTION C:
SECTION D:
Chapter Overview 7.1
Sample Errors 7.2
System Fault Errors, Reprocessing Required 7.30
‘’Batch Canceled Due To’ Reporting 7.53
Chapter Eight
STAINING AND COVERSLIPPING
SECTION A:
SECTION B:
SECTION C:
SECTION D:
Introduction 8.1
Recommended Staining Guidelines 8.2
Coverslipping Recommendations 8.4
Common Artifacts 8.5
ThinPrep® 3000 Processor Operator’s Manual
iii
Page 22

TABLE OF CONTENTS
Chapter Nine
THINPREP PAP TEST TRAINING PROGRAM 9.1
Chapter Ten
USER INTERFACE SCREENS
SECTION A:
SECTION B:
Overview of the User Interface Screens 10.1
Menu Trees 10.1
INDEX INDEX.1
SERVICE INFORMATION SERVICE.1
O
RDERING INFORMATION
O
RDERING
MATERIAL SAFETY DATA SHEETS SERVICE.1
PreservCyt Solution
CellFyx Solution
Versa-Clean™ Solution
APPENDIX
ThinPrep 3000 Processor: Laboratory Flow
.1
Barcode Label Specifications for the ThinPrep 3000 Processor
Sample Vial Label Application Guide
ThinPrep 3000 Processor Quick Reference Guide
iv
ThinPrep® 3000 Processor Operator’s Manual
Page 23

1. Introduction
1. Introduction
Page 24
1
Chapter One
SECTION
A
Introduction
CONTENTS
INTRODUCTION
SECTION A:
SECTION B:
SECTION C:
SECTION D:
SECTION E:
SECTION F:
SECTION G:
Overview and Function of the
ThinPrep® 3000 Processor 1.1
Overview of Instrument Systems 1.4
Material Requirements 1.8
ThinPrep 3000 Processor
Technical Specifications 1.9
Internal Quality Control 1.12
ThinPrep 3000 Hazards 1.12
Disposal 1.17
OVERVIEW AND FUNCTION OF THE THINPREP® 3000 PROCESSOR
The ThinPrep 3000 processor (refer to Figure 1-1) automates key steps in the batch processing of
®
fluid-based gynecologic specimens for use with the ThinPrep
processed, transferred and fixed onto microscope slides in preparation for staining, cover slipping
and screening. Key system components include The ThinPrep 3000 processor, sample vials of
PreservCyt
use, CellFyx™ Solution, and ThinPrep microscope slides.
®
Solution for use with the ThinPrep Pap test, ThinPrep Pap test filters for gynecologic
Pap test. The samples are collected,
ThinPrep® 3000 Processor Operator’s Manual
1.1
Page 25

1
INTRODUCTION
ThinPrep microscope slides
ThinPrep Pap
test filters
Gynecologic samples in
ThinPrep Pap test
PreservCyt Solution
ThinPrep 3000 processor
CellFyx Solution
Figure 1-1 The ThinPrep 3000 Processor
The ThinPrep® Pap Test
The ThinPrep Pap test is a fluid-based method for the collection and preparation of gynecologic
samples.
The ThinPrep Pap test begins at the physician’s office where, using a broom-type collection device or
endocervical brush/plastic spatula, cervical cells are collected from the patient. Rather than
smearing the patient’s sample directly onto a microscope slide, the collection device is immediately
immersed and rinsed in a vial of PreservCyt Solution for use with the ThinPrep Pap test.
The sample vial is then capped and tightened. Patient information is recorded onto the vial of
solution containing the sample and forwarded to a laboratory equipped to process the ThinPrep Pap
test.
At the laboratory, matching barcoded labels are applied to the sample vial and accompanying test
request form. The sample vial is then placed in a sample vial tray and loaded into the ThinPrep 3000
processor.
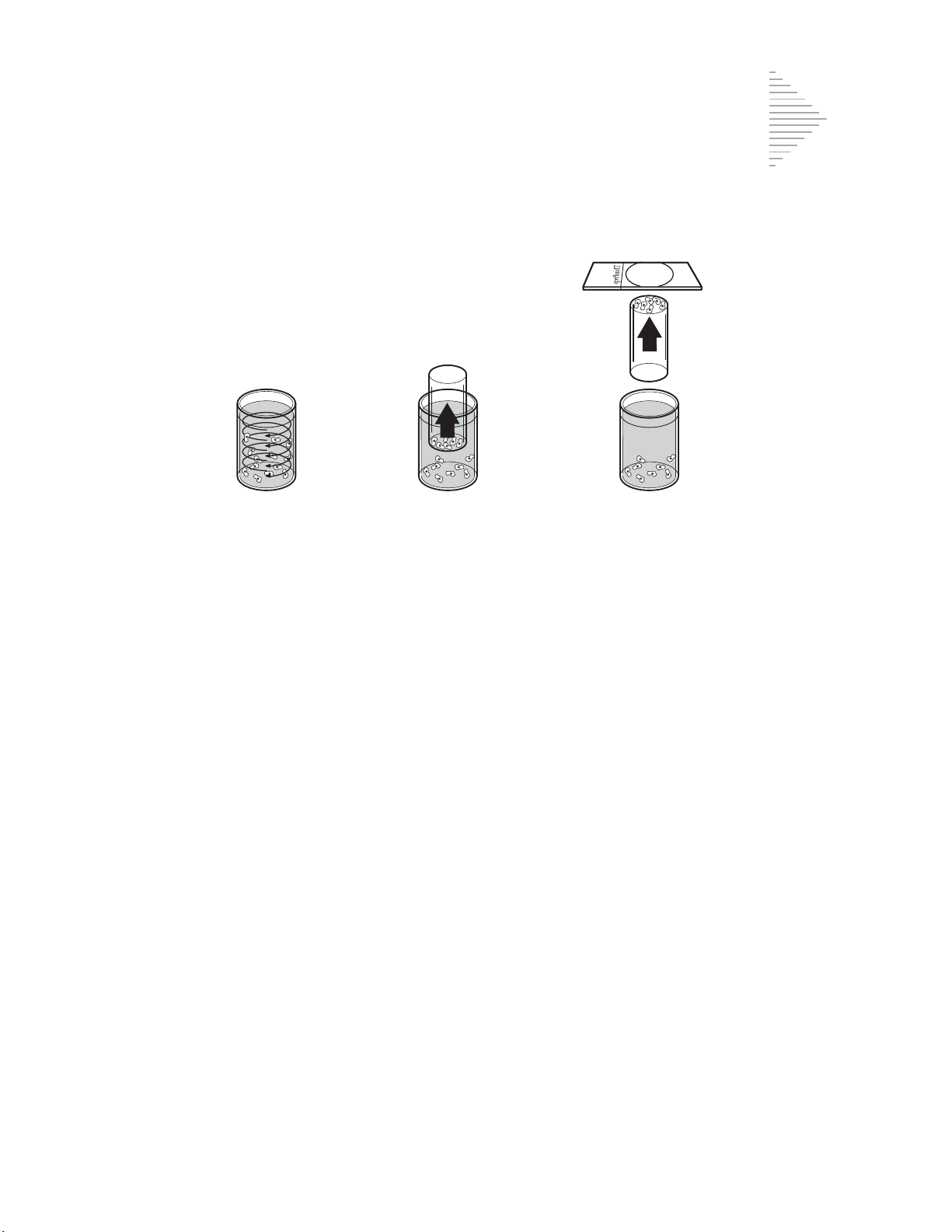
(Refer to Figure 1-2.) During the slide preparation process, a gentle dispersion step breaks up blood,
mucus and non-diagnostic debris and thoroughly mixes the cell sample. The cells are then collected
onto a ThinPrep Pap test filter. A thin layer of cells is then transferred to a ThinPrep microscope slide.
The ThinPrep 3000 processor then applies CellFyx Fixative Solution to the slide, after which the slide
is delivered to a staining rack.
1.2
ThinPrep® 3000 Processor Operator’s Manual
Page 26
INTRODUCTION
1
Dispersion
The sample vial is
rotated, dispersing
debris and mucus
while thoroughly
mixing the cell
sample.
Cell Collection
A gentle vacuum is
created in the
ThinPrep Pap test
filter, which collects
cells on the exterior
surface of the
membrane.
Cell Transfer
The ThinPrep Pap test
filter is inverted and
the collected cells are
gently and evenly
transferred onto the
ThinPrep microscope
slide in a defined area.
Figure 1-2 The ThinPrep Sample Preparation Process
ThinPrep® 3000 Processor Operator’s Manual
1.3
Page 27
1
INTRODUCTION
SECTION
B
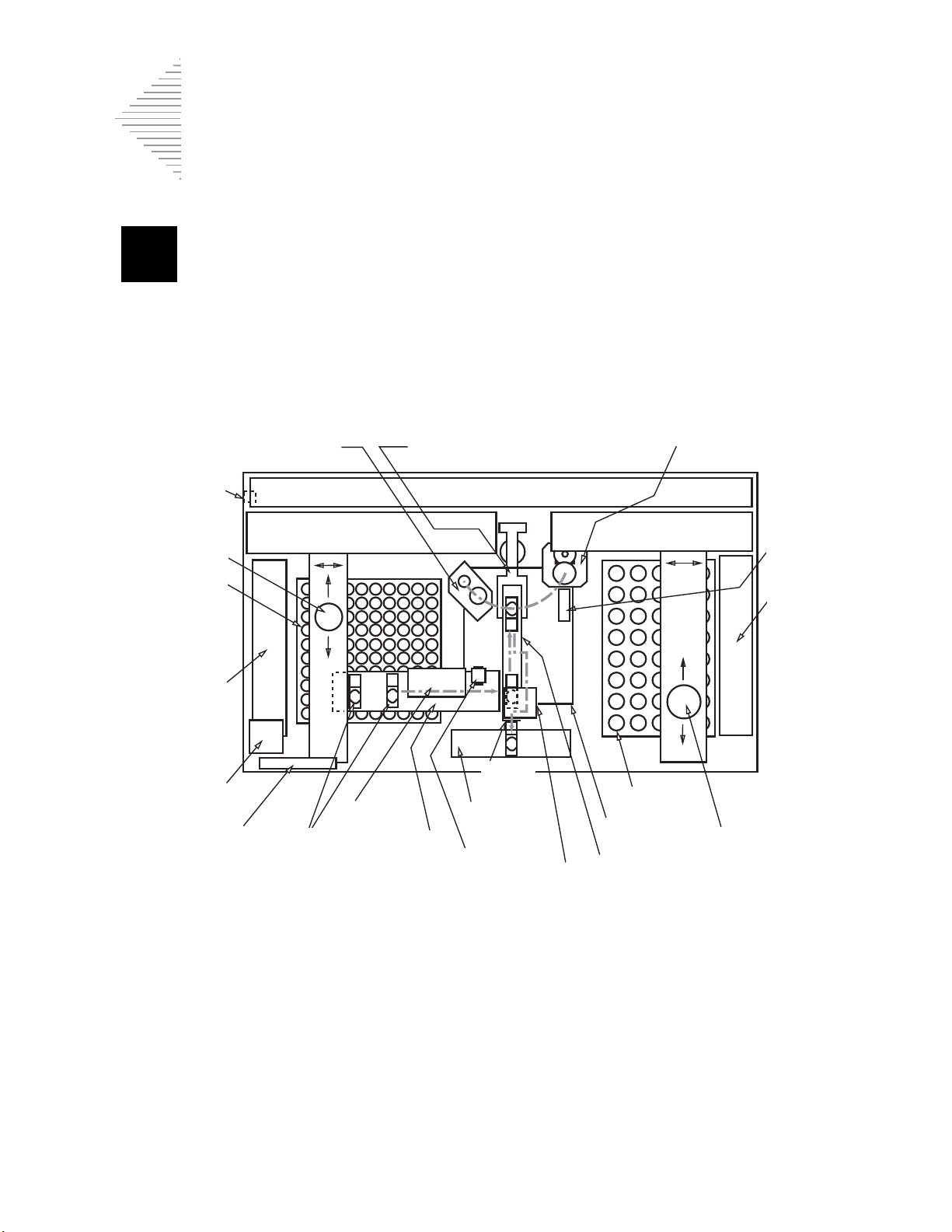
Sample processing armFilter elevator
(TOP VIEW)
Power cord
connection
Filter gripper
Filters
Filter tray
handler
Results printer
Display & keypad
Slide
cartridges
Slide
translator
Slide printer
Slide
ejector
Slide output
system
OCR
reader
Fixative
dispenser
Slide cell transfer arm
Filter waste bin
Sample vials
Sample vial
gripper
Vial tray
handler
Barcode
reader
Sample processing station
THINPREP 3000 PROCESSOR
(FRONT OF INSTRUMENT)
Electronic and pneumatic systems
Filter robot Sample vial robot
OVERVIEW OF INSTRUMENT SYSTEMS
Figure 1-3 Overview of Instrument Systems
1.4
ThinPrep® 3000 Processor Operator’s Manual
Page 28
1
Vial Handling System
005532
1
005532
2
3
4
5
6
005532
7
INTRODUCTION
Figure 1-4 Vial Handling System
1. The sample vial is picked from the tray.
2. The barcode is scanned and read.
3. The sample vial is delivered to the sample processing station and the sample is dispersed by
spinning the vial.
4. The vial is uncapped.
5. The filter is introduced for cell collection.
6. The vial is recapped.
7. The sample vial is returned to the sample tray.
ThinPrep® 3000 Processor Operator’s Manual
1.5
Page 29
1
INTRODUCTION
1
4
5
6
2
3
Disposal chute
Filter elevator (detail)
New filter
position
Used filter
position
Filter Handling System
Figure 1-5 Filter Handling System
1. A ThinPrep Pap test filter is picked from the tray.
2. The filter is delivered to the filter elevator.
3. The sample processing arm retrieves the filter.
4. The filter is brought to the sample processing station and placed in the vial for sample collection.
5. The sample processing arm rotates and precisely meets with the slide cell transfer arm, bearing a
slide. Cell transfer occurs.
6. The used filter is returned to the filter elevator for disposal.
1.6
ThinPrep® 3000 Processor Operator’s Manual
Page 30
1
Slide Handling System
ThinPrep
Slide
1
7
2
3
6
4
5
INTRODUCTION
Figure 1-6 Slide Handling System
1. Slide cartridge(s) loaded with slides are placed in the instrument.
2. The slide translator picks a slide from the cartridge and carries it to the slide printer.
3. The barcode number scanned from the sample vial is printed onto the slide, along with the time,
date and facility name (optional).
4. The slide translator hands the slide off to the slide cell transfer arm.
5. The slide cell transfer arm pivots to convey the slide to meet the ThinPrep Pap test filter for cell
transfer.
6. The slide cell transfer arm delivers the slide to the fixative dispenser, where fixative is applied.
7. The prepared slide is placed into a staining rack.
ThinPrep® 3000 Processor Operator’s Manual
1.7
Page 31

1
INTRODUCTION
SECTION
C
1
2
5
6
7
9
10
12
00001168
4
!
MAX
M
A
X
3
CellFyx
SOLUTION
ª
8
11
ThinPre
p
M
icro
sc
o
p
e
S
lid
e
s
MATERIAL REQUIREMENTS
Components of the ThinPrep 3000 Processor
1. Slide printer ribbon 7. Waste bottle assembly
2. Staining racks 8. Filter waste box
3. CellFyx Solution 9. ThinPrep Pap test filter trays
4. Sample vials (with barcode) 10. Results printer paper
5. Sample vial trays 11. ThinPrep microscope slides
6. Slide waste bin 12. Slide cartridges
Figure 1-7 Material Requirements
1.8
ThinPrep® 3000 Processor Operator’s Manual
Page 32

1
THINPREP 3000 PROCESSOR TECHNICAL SPECIFICATIONS
SECTION
D
CYTYC
49 in.
(124 cm)
29 in.
(74 cm)
57 in.
(145 cm)
Approximate weight: 700 lb/318 kg.
ThinPrep 3000 Processor Dimensions
Note:
All measurements in this manual are rounded to the nearest whole number.
Figure 1-8 Dimensions
INTRODUCTION
ThinPrep® 3000 Processor Operator’s Manual
1.9
Page 33

1
INTRODUCTION
67 in.
(170 cm)
29 in.
(74 cm)
17 in.
(43 cm)
49 in.
(124 cm)
9 in.
(23 cm)
C
Y
T
Y
C
Shown with service
door open
ThinPrep 3000 Processor Clearances
A minimum installation clearance of 1 foot (30.5 cm) at the rear, top, and sides of the instrument is
required for ventilation. (Shown with service cover open.)
Figure 1-9 Clearances
ThinPrep® 3000 Processor Operator’s Manual
1.10
Page 34

1
Environmental
INTRODUCTION
Operating1 Temperature Range:
60–90°F/16–32°C
Non-Operating2 Temperature Range:
-20–122°F/ -29–50°C
Operating Humidity Range:
20–90% RH, non-condensing
Non-Operating Humidity Range:
15–95% RH, non-condensing
Pollution Degree:
II, in accordance with IEC 664.
The ThinPrep 3000 processor is for indoor use only in an office or a clean laboratory
environment.
Power
Voltage:
100–240~ (Volts Alternating Current, no selection required)
Mains supply voltage not to exceed ± 10% of the nominal voltage
Frequency:
47–63Hz
Current:
Heat Generated:
WARNING:
Fusing: ( )
4A maximum; 500W maximum
Approximately 1100BTU/HR
Instrument Fusing
External
Internal
supply (non-operator accessible)
1. Operating: The ThinPrep 3000 processor is plugged in and turned on.
2. Non-Operating: The ThinPrep 3000 processor may be plugged in, but is not turned on.
– 2 X T6.3AL 250V 5 x 20mm; 6.3A time delay low break capacity
– 1 X 15A 250V 3AB; 15A fast blow high break capacity fuse internal to system power
ThinPrep® 3000 Processor Operator’s Manual
1.11
Page 35

1
INTRODUCTION
SECTION
E
SECTION
F
Safety, EMI and EMC Standards
The ThinPrep 3000 processor has been tested and certified by a U.S. nationally recognized testing
laboratory (NRTL) to comply with Safety, Electro-Magnetic Interference (EMI) and Electro-Magnetic
Compatibility (EMC) standards.
INTERNAL QUALITY CONTROL
Power On Self Test (POST)
At the time the ThinPrep 3000 processor is powered on (refer to page 2.4) the system goes through a
self-diagnostic test. Electrical, mechanical and software systems are tested to confirm each performs
properly. The operator is alerted to any malfunction via a message on the user interface. If the
instrument does not function or there are persistent errors, contact Hologic Technical Support.
THINPREP 3000 PROCESSOR HAZARDS
The ThinPrep 3000 processor is intended to be operated in the manner specified in this manual. Be
sure to review and understand the information listed below in order to avoid harm to operators and/
or damage to the instrument.
If this equipment is used in a manner not specified by the manufacturer, then the protection provided
by the equipment may be impaired.
Warnings, Cautions and Notes
The terms
•
•
•
WARNING, CAUTION
A
WARNING
injury or death.
A
CAUTION
produce inaccurate data or invalidate a procedure, although personal injury is unlikely.
A
Note
provides useful information within the context of the instructions being provided.
advises against certain actions or situations that could result in personal
advises against actions or situations that could damage equipment,
and
Note
have specific meanings in this manual.
1.12
ThinPrep® 3000 Processor Operator’s Manual
Page 36

INTRODUCTION
1
Fixative reservoi r
Slide cartridges
Tray latches
Slide waste bin
Tray latches
Warning Symbol Used on the Instrument
The ThinPrep 3000 processor has the exclamation mark within a triangle symbol placed on it
specifically to warn the operator to refer to the operator’s manual. (Refer to the illustration below.) Be
sure to review and understand the warnings listed below in order to avoid damage to the instrument
and any harm to operators. One or more of the warnings may be pertinent to the area marked.
Warnings Used in this Manual
WARNING
Service Installation Only
This instrument is to be installed and/or moved by trained Hologic personnel only.
CAUTION
There is a miniature barcode laser scanner embedded inside this product for identifying samples.
There is no intended access to this laser source for operators. Use of controls, adjustments or
procedures other than those specified in this manual may result in hazardous radiation exposure.
WARNING
Instrument Fusing
For continued protection against fire, replace only with fuses of the specified type and current rating.
Refer to “Instrument Maintenance” on page 6.1 for instructions on replacing user accessible fuses.
ThinPrep® 3000 Processor Operator’s Manual
1.13
Page 37

1
INTRODUCTION
WARNING
Flammable Liquid and Vapor
Flammable liquid and vapor. Keep away from heat, sparks, open flames and hot surfaces.
WARNING
Strong oxidizers, such as bleach, are incompatible with PreservCyt Solution and therefore should not
be used to clean the waste bottle.
WARNING
Toxic Mixture
Refer to Safety Data Sheets (SDS) at www.hologicsds.com for safe handling instructions. Wear
personal protective laboratory gear.
WARNING
Moving Parts
The instrument contains many internal moving parts. Keep hands, loose clothing, jewelry, etc., clear.
Do not operate with the doors open.
WARNING
Power Connection
To ensure safe operation, the instrument must be connected to a three-wire grounded receptacle.
WARNING
Sharp Edges/Hot Surfaces
The instrument contains sharp edges and hot surfaces. Use extreme caution when handling items
near these areas. Allow hot surfaces to cool before handling.
WARNING
Glass, Sharp Edges
The instrument uses glass microscope slides, which have sharp edges. In addition, the slides may be
broken in their packaging or in the machine. Use caution when handling glass slides and when
cleaning the instrument.
WARNING
Protective Clothing
Wear protective clothing in accordance to universal precautions1 when operating the instrument.
1. US Department of Health and Human Services, Biosafety in Microbiological and Biomedical Labora-
tories, 2nd Edition, May 1988. pp 109-111.
1.14
ThinPrep® 3000 Processor Operator’s Manual
Page 38

INTRODUCTION
1
Slide insertion label Printer ribbon label
Printer roll label
Model/rating label
Serial numberLaser source (inside)
WARNING
Waste Removal
Use caution when emptying the waste bottle. Refer to “Emptying the Waste Bottle” on page 6.28 for
instructions on removing and emptying the waste bottle.
WARNING
Do not process a cerebral spinal fluid (CSF) specimen or other sample type that is suspected of
possessing prion infectivity (PrPsc) derived from a person with a TSE, such as Creutzfeldt-Jakob
disease, on a ThinPrep processor. A TSE-contaminated processor cannot be effectively
decontaminated and therefore must be properly disposed of in order to avoid potential harm to users
of the processor or service personnel.
Location of Instrument Labels
The
model/rating
serial number
The
A label illustrating how to insert a batch
batch printer.
A label illustrating how to orient and insert the
label for the instrument is located on the left side of the machine.
label is located inside the flip-down center door.
printer roll
is located on the inside of the cover over the
slide cartridges
ThinPrep® 3000 Processor Operator’s Manual
is located inside the top cover.
1.15
Page 39

1
INTRODUCTION
A label illustrating how to remove and replace the
hatch.
Attention
list of warnings on the previous pages.
- labels are located at different parts of the instrument. Refer to the explanation and
slide printer ribbon
is located inside the top
1.16
ThinPrep® 3000 Processor Operator’s Manual
Page 40

1
DISPOSAL
SECTION
G
Disposal of Consumables
•
Used filters. Dispose of as regular waste.
•
Swabs and towels for instrument cleaning. Dispose of as regular waste.
•
Waste bottle contents. Dispose of all solvents as hazardous waste. Follow local, state,
provincial and federal or country guidelines. As with all laboratory practices, universal
precautions should be followed.
•
PreservCyt Solution. Follow local, state, provincial and federal or country guidelines.
Dispose of all solvents as hazardous waste.
•
CellFyx Solution. Follow local, state, provincial and federal or country guidelines.
Dispose of all solvents as hazardous waste.
INTRODUCTION
•
Versa-Clean Solution. Follow local, state, provincial and federal or country guidelines.
Dispose of all solvents as hazardous waste.
•
Pinch valve tubing. Dispose of as regular waste.
•
Broken glass. Dispose of in a Sharps container.
•
Super Lube. Dispose of as regular waste.
Disposal of the Instrument
Do not dispose in municipal waste.
Contact Hologic for information regarding proper disposal.
HOLOGIC, INC.
250 C
AMPUS DRIVE
MARLBOROUGH, MA 01752 USA
T
EL: 1-800-442-9892
1-508-263-2900
F
AX: 1-508-229-2795
W
EB: WWW.HOLOGIC.COM
ThinPrep® 3000 Processor Operator’s Manual
1.17
Page 41

1
INTRODUCTION
This page intentionally left blank
1.18
ThinPrep® 3000 Processor Operator’s Manual
Page 42

2. ThinPrep 3000
Installation
Installation
2. ThinPrep 3000
Page 43

THINPREP 3000 INSTALLATION
2
SECTION
A
SECTION
B
Chapter Two
ThinPrep 3000 Installation
CONTENTS
SECTION A:
SECTION B:
SECTION C:
SECTION D:
SECTION E:
SECTION F:
SECTION G:
SECTION H:
SECTION I:
SECTION J:
General 2.1
Action Upon Delivery 2.1
Preparation Prior to Installation 2.3
Storage and Handling Post Installation 2.3
Connect Power 2.4
How to Turn the Processor On/Off 2.4
System Startup 2.6
Setting the Time and Date 2.7
Setting the Slide Printer Output 2.10
Setting the Audible Key Press 2.11
GENERAL
The ThinPrep® 3000 Processor must be installed by Hologic service personnel. When installation is
complete, the service personnel trains the operator(s), using the operator’s manual as the training
guide. In the event the instrument must be moved after installation, please contact Hologic Technical
Support.
CAUTION:
Vibrating machinery, such as centrifuges, should not be installed near the instrument.
ACTION UPON DELIVERY
Inspect the packing carton(s) for damage. Report any damage immediately to the shipper and/or
Hologic Technical Support as soon as possible.
Leave the equipment in the packing carton for Hologic service installation.
Store the equipment in a suitable environment until installation (cool, dry, vibration-free area).
ThinPrep® 3000 Processor Operator’s Manual
2.1
Page 44

2
THINPREP 3000 INSTALLATION
Shipping Container Checklist
You will receive the following items when the ThinPrep® 3000 processor is delivered for installation.
(These items may vary according to your order.)
•
ThinPrep 3000 processor 1
• ThinPrep 3000 Processor Operator’s Manual 1
•Power cord 1
• Program Memory Card 1
• Staining rack adapters 4
• Installation Kit, including: 1
Filter waste box 1
Fixative shield mount 1
Pinch valve tubing 4 pieces
Pneumatic test vial (in protective case) 1
Sample vial trays (
Slide cartridges 2
Staining racks (
Slide waste bin 2
Transport cover for the waste bottle 1
Aerosol spray bottle 1 bottle
(
Consumable Items
Filter waste box liner 5
Slide printer ribbon 2
Results printer paper (
ThinPrep 3000 Maintenance Kit 1 kit
Ver s a- C le a n ™ S o lut i on 1 b o tt l e
4 per package
4 per package
):
5 rolls per box
)2 packages
)2 packages
)1 box
2.2
ThinPrep® 3000 Processor Operator’s Manual
Page 45

THINPREP 3000 INSTALLATION
2
SECTION
C
SECTION
D
PREPARATION PRIOR TO INSTALLATION
Pre-Installation Site Assessment
A pre-installation site assessment is performed by Hologic service personnel. Be sure to have
prepared any and all site configuration requirements as instructed by the service personnel.
Location and Configuration
Locate the ThinPrep 3000 processor near a three-wire grounded power outlet that is free of voltage
fluctuations and power surges. Vibrating equipment, such as vortexors or centrifuges should not be
installed near the instrument.
Refer to Figure 1-9‚ Clearances, for space needed for installation.
STORAGE AND HANDLING - POST INSTALLATION
Be sure to clean and maintain the ThinPrep 3000 processor as instructed in this manual. Refer to
“Instrument Maintenance” on page 6.1.
If the instrument is to be moved after installation, please contact Hologic Technical Support.
ThinPrep® 3000 Processor Operator’s Manual
2.3
Page 46

2
THINPREP 3000 INSTALLATION
SECTION
E
CYTYC
SECTION
F
CONNECT POWER
Plug the IEC receptacle end of the power cord (provided with the processor) into the socket, located
beneath the power switch (Figure 2-1). Plug the other end of the power cord into the wall outlet. To
ensure safe operation of the instrument, use a three-wire grounded outlet.
Figure 2-1 Connect Power to the Instrument
WARNING: Grounded Outlet
Serial port*
Rocker switch
Power On position
IEC receptacle end
Power cord
*Serial port: For use by Hologic personnel only.
HOW TO TURN THE PROCESSOR ON/OFF
Turn the Processor On
To turn the ThinPrep 3000 processor on, press the rocker switch, located by the power cord, as shown
below.
2.4
ThinPrep® 3000 Processor Operator’s Manual
Page 47

THINPREP 3000 INSTALLATION
2
Instrument On
Instrument Off
Rocker switch
Turn the Processor Off
To turn the instrument off, press the rocker switch to the opposite position. To completely remove
power from the instrument, unplug the power cord from the wall outlet.
Note:
The ThinPrep 3000 processor is intended to remain on.
Extended Shutdown (Taking the Instrument Out of Service)
If the instrument is to be shut down for an extended time, turn it off as instructed above.
Remove and safely store any patient slides and sample vials that may be on-board the instrument.
Empty the waste bottle, the slide waste bin and the filter waste box (refer to Instrument Maintenance
for each of these items).
Close all of the doors and unplug the power cord from the wall socket.
ThinPrep® 3000 Processor Operator’s Manual
2.5
Page 48

2
THINPREP 3000 INSTALLATION
SECTION
G
Message screen
User interface
Prompt keys
ThinPrep 3000
TP3 xx.xxxxxx
CYTYC
Corporation
Self Test in Progress
System Ready: Start Batch
Load Samples and
Supplies Menu
Select Start Batch
08:25:00 AM 04/28/00 Print Results
SYSTEM STARTUP
Upon startup, the instrument conducts an initial self test for several minutes. During this time,
Test in Progress
At the conclusion of the self test, the main menu screen is displayed:
•
Select Start Batch to begin processing samples.
•
Select Menu to access the Utility menu: Status, Maintenance, Setup and Test menus.
•
Select Print Results to print the batch report from the previous batch of samples
processed.
and a progress bar are displayed on the message screen:
Self
2.6
ThinPrep® 3000 Processor Operator’s Manual
Page 49

THINPREP 3000 INSTALLATION
2
SECTION
H
Set Time More
Set Date Main Menu
Slide Printer Output Back
Select Date Format More
Select Time Format Main Menu
Audible Key Press Back
Select Time Format
AM/PM Main Menu
24 HR Back
SETTING THE TIME AND DATE
Setting the Time
In this procedure, first select the desired time format, then set the time.
1. In the main menu screen, select
2. Select
3. Select
4. Press
5. Select a time format:
Setup
.
More
(option at the upper right of the display).
Select Time Format
.
AM/PM
or
Menu
24 HR
.
.
Note:
Time format is for the user interface display only. All reporting (slide printing and batch
reports) will automatically have 24-hour formats on them.
ThinPrep® 3000 Processor Operator’s Manual
2.7
Page 50

2
THINPREP 3000 INSTALLATION
Hour Set
1:05 PM Minute Set
Set Back
Set Time More
Set Date Main Menu
Slide Printer Output Back
Select Date Format More
Select Time Format Main Menu
Audible Key Press Back
6. Press
7. To set the hour, press the
8. To set minutes, press the
9. Select
Note:
Back
to return to the Set Time display screen. Select
Set
to save.
Selecting
Hour Set
Minute Set
Back
at any time prior to selecting
previous screen.
Setting the Date
1. In the main menu screen, select
2. Select
3. Select
Setup
.
More
(option at the upper right of the display).
Set Time
.
prompt key until the correct hour appears.
prompt key until the correct minutes appears.
Set
will delete all new entries and return to the
Menu
.
4. Press
5. Select a date format:
2.8
Select Date Format
MM/DD/YY
ThinPrep® 3000 Processor Operator’s Manual
.
or
DD.MM.YY
.
Page 51

THINPREP 3000 INSTALLATION
2
Select Date Format
MM/DD/YY Main Menu
DD.MM.YY Back
Year Set Month Set
04/28/00 Date Set
Set Back
6. Press
7. To set the year, press the
8. To set the month, press the
9. To set the day, press the
10. Select
Note:
Back
to return to the Set Date display screen. Select
Set
to save.
Selecting
previous screen.
Back
at any time prior to selecting
Year Set
Date Set
Set Date.
prompt key until the correct year appears.
Month Set
prompt key until the correct month appears.
prompt key until the correct day appears.
Set
will delete all new entries and return to the
ThinPrep® 3000 Processor Operator’s Manual
2.9
Page 52

2
THINPREP 3000 INSTALLATION
SECTION
I
1
2
3
Date/Time Stamp Enable
Facility Name Disable
Enter Name AAAAAAAAAAAAAA
Set Back
4
5
6
SETTING THE SLIDE PRINTER OUTPUT
The sample barcode number automatically prints on each slide. To record the date, time, and your
facility’s name on the ThinPrep microscope slide, you must enter this information using this setup
utility.
1. In the main menu screen, select
2. Select
3. Select
Note:
4. To activate the date/time feature, press prompt key #1 until the highlighted box appears next to
5. Press prompt key #4 or #5 until
6. To activate the facility’s name, press prompt key #1 until the highlighted box appears next to
7. Press prompt key #4 or #5 until
8. To input your facility’s name, press prompt key “1” until the highlighted box appears next to
9. Use prompt keys #4 (steps from A to Z) and #5 (steps from Z to A) to move one letter at a time
10. After selecting a letter, press prompt key #2 to move to the next letter space.
Note:
Setup
.
Slide Printer Output
Selecting
previous screen
Date/Time Stamp
ture.)
Facility Name
Enter Name
through the alphabet.
The field for the facility name is 14 characters’ long, all capital letters.
Back
at any time prior to selecting
.
.
.
.
Menu
Enable
Enable
.
Set
will delete all new entries and return to the
appears. (Selecting
appears.
Disable
deactivates the date/time fea-
11. Select
2.10
Set
to save.
ThinPrep® 3000 Processor Operator’s Manual
Page 53

THINPREP 3000 INSTALLATION
2
SECTION
J
Select Date Format More
Select Time Format Main Menu
Audible Key Press Back
Audible Key Press Enable
Main Menu
Set Back
SETTING THE AUDIBLE KEY PRESS
This is an option that sounds a ‘beep’ every time a key on the user interface panel is pressed.
1. In the main menu screen, select
2. In the next display, select
3. In the
Setup
menu, select
Setup
More
Menu
.
. Select
.
Audible Key Press
.
4. The key will toggle between the Enable and Disable choices for this function.
• To produce an audible beep with each key press, highlight
• To turn off the audible beep (if it has already been set on), highlight
Enable
ThinPrep® 3000 Processor Operator’s Manual
and then press
Disable
and press
Set
.
Set
.
2.11
Page 54

2
THINPREP 3000 INSTALLATION
This page intentionally left blank
2.12
ThinPrep® 3000 Processor Operator’s Manual
Page 55

3. PreservCyt and
CellFyx Solutions
CellFyx Solutions
3. PreservCyt and
Page 56

PRESERVCYT® AND CELLFYX™ SOLUTIONS
3
SECTION
A
Chapter Three
PreservCyt® and CellFyx™ Solutions
CONTENTS
SECTION A:
SECTION B:
SECTION C:
Introduction 3.1
PreservCyt® Solution 3.2
CellFyx™ Solution 3.5
INTRODUCTION
The following sections describe the function and specifications of Hologic cytologic preservative
fluid, PreservCyt Solution and fixative fluid, CellFyx Solution.
ThinPrep® 3000 Processor Operator’s Manual
3.1
Page 57

3
PRESERVCYT® AND CELLFYX™ SOLUTIONS
SECTION
B
PRESERVCYT® SOLUTION
PreservCyt Solution is a methanol-based, buffered solution designed to preserve cells during trans-
®
port and slide preparation on the ThinPrep
PreservCyt Solution is optimized for the ThinPrep processor slide preparation process and cannot be
substituted with any other reagents.
Packaging
Please refer to the
regarding the ordering of solutions and supplies for the ThinPrep 3000 processor.
Vials (20 mL) of PreservCyt Solution are contained in each ThinPrep Pap Test Kit.
Ordering Information
Composition
PreservCyt Solution contains buffered methanol. It contains no reactive ingredients. It contains no
active ingredients.
3000 processor.
in this manual for part numbers and detailed information
WARNING:
To xi c
Flammable
WARNING:
swallowed. Toxic if inhaled. Causes damage to organs. Cannot be made nonpoisonous. Keep away from heat, sparks, open flames and hot surfaces. Other
solutions cannot be substituted for PreservCyt Solution.
Danger. PreservCyt Solution contains methanol. Toxic if
3.2
ThinPrep® 3000 Processor Operator’s Manual
Page 58

PRESERVCYT® AND CELLFYX™ SOLUTIONS
3
WARNING: See Package Insert Before
SPecimen Collection.
NAME:
ID#:
3256723-46
Line on cap and
line on vial should
meet or slightly
overlap. If the cap
on the vial does
not have a line,
ensure the cap is
tightened
securely.
Storage Requirements
• Store PreservCyt Solution between 15°C (59°F) and 30°C (86°F). Do not use beyond the expiration date printed on the container.
• Store PreservCyt Solution
with
cytologic sample intended for ThinPrep Pap testing between
15°C (59°F) and 30°C (86°F) for up to 6 weeks.
• Store PreservCyt Solution
with
cytologic sample intended for CT/NG testing using the Roche
Diagnostics COBAS AMPLICOR CT/NG test between 4°C (39°F) and 25°C (77°F) for up to
weeks.
6
Transportation
When transporting a PreservCyt Solution vial containing cells, make sure the vial is tightly sealed. To
prevent leakage, align the mark on the cap with the mark on the vial as shown in the figure below.
Figure 3-1 PreservCyt Solution Vial
The shipping category for PreservCyt Solution is
The shipping category for PreservCyt Solution containing cells is “diagnostic sample.”
Please refer to the Shipping Requirements and Recommendations guide at the end of this chapter.
Stability
Do not use PreservCyt Solution after the expiration date on the container label. Expired vials should
be discarded using appropriate laboratory procedures. Also, refer to storage requirements above for
cell preservation limits.
“flammable liquids, n.o.s. (methanol)” (USA only)
“flammable liquids, toxic, n.o.s. (methanol)” (outside the USA)
ThinPrep® 3000 Processor Operator’s Manual
3.3
Page 59

3
PRESERVCYT® AND CELLFYX™ SOLUTIONS
Handling/Disposal
Handle all chemical-containing materials carefully in accordance with safe laboratory practices.
When required by reagent composition, additional precautions are marked on the reagent containers
or in the instructions for use.
Dispose of PreservCyt Solution according to the guidelines for disposing of hazardous waste.
PreservCyt Solution contains methanol.
PreservCyt Solution was challenged with a variety of microbial and viral organisms. The following
table presents the starting concentrations of viable organisms and the number of viable organisms
found after 15 minutes in the PreservCyt Solution. The log reduction for viable organisms is also presented. As with all laboratory procedures, universal precautions should be followed.
Organism Initial Concentration
Candida albicans
Aspergillus niger*
Escherichia coli
Staphylococcus aureus
Pseudomonas aeruginosa
Mycobacterium tuberculosis**
Rabbitpox virus
HIV-1
* After 1 hour >4.7 log reduction
** After 1 hour >5.7 log reduction
*** Data is for 5 minutes
5.5 x 10
4.8 x 10
2.8 x 10
2.3 x 10
2.5 x 10
9.4 x 10
6.0 x 10
1.0 x 10
5
CFU/mL
5
CFU/mL
5
CFU/mL
5
CFU/mL
5
CFU/mL
5
CFU/mL
6
PFU/mL
7.5
TCID50/mL
Log Reduction after
15 min.
>4.7
2.7
>4.4
>4.4
>4.4
4.9
5.5***
7.0***
Interfering Substances
The use of lubricants (e.g., KY Jelly) should be minimized prior to specimen collection. Lubricants
can adhere to the filter membrane and may cause poor cell transfer to the slide.
3.4
ThinPrep® 3000 Processor Operator’s Manual
Page 60

PRESERVCYT® AND CELLFYX™ SOLUTIONS
3
SECTION
C
CELLFYX
CellFyx Solution is a methanol-based, fixative solution designed to preserve morphologic features of
cells for up to 5 days at room temperature. CellFyx Solution is optimized for the ThinPrep 3000 processor slide preparation process and cannot be substituted with any other reagents.
Packaging
Please refer to the
regarding the ordering of solutions and supplies for the ThinPrep 3000 processor.
Composition
CellFyx Solution contains methanol. It contains no reactive ingredients.
™
SOLUTION
Ordering Information
in this manual for part numbers and detailed information
WARNING:
Causes damage to organs. Cannot be made non-poisonous. Keep away from heat, sparks, open
flames and hot surfaces. Other solutions cannot be substituted for CellFyx Solution.
Storage Requirements
• The storage condition for CellFyx Solution is up to two years from date of manufacture at
15°C to 30°C.
• Slides fixed with CellFyx are preserved for 5 days at room temperature.
Stability
Do not use CellFyx Solution after the expiration date on the container label. Expired bottles should
be discarded using appropriate laboratory procedures.
Handling/Disposal
Handle all chemical-containing materials carefully in accordance with safe laboratory practices.
When required by reagent composition, additional precautions are marked on the reagent
containers.
Dispose of CellFyx Solution according to the guidelines for disposing of hazardous waste. CellFyx
Solution contains methanol.
Danger. CellFyx Solution contains methanol. Toxic if swallowed. Toxic if inhaled.
ThinPrep® 3000 Processor Operator’s Manual
3.5
Page 61

3
PRESERVCYT® AND CELLFYX™ SOLUTIONS
This page intentionally left blank
3.6
ThinPrep® 3000 Processor Operator’s Manual
Page 62

ThinPrep®Solutions
(1)
StorageGuide
(2)
The National Fire Protection Association (NFPA) is the expert aut hority that local fire departments and fire safety code
enforcement authorities look to for fire safety standards and codes. Their codes are developed through a consensus standards
development process approved by the American National Standards Institute. The NFPA codes are used as guidelines by most
fire code enforcement agencies. Since these codes are guidelines, your local Authority Having Jurisdiction (AHJ) for fire code
enforcement may make the final determination. The summary chart below is based upon guidelines for facilities protected by
standard sprinkler systems.
The ThinPrep products NFPA ratings are listed in a table below this chart.
Use this chart to help you determine your maxi mum storage limits for flammable and combustible liquids.
Maximum Quantities of Flammable and Combustible Liquids in Laboratory Units Outside of Inside Liquid Storage Areas
Lab Unit Fire
Hazard Class
A (High)
(6)
B
(Moderate)
(7)
C
(Low)
(7)
D
(Minimal)
General Warehouse
Liquid Warehouse
Office, to include Exam Rooms 30-2015 10 38 1900
Maximum allowable storage per ft2 in an inside storage room that is smaller than 150ft2 in size. 30-2015 5 19 950
Maximum allowable storage per ft2 in an inside storage room that is larger than 150ft2 and less than
(1) Solution classifications: PreservCyt – Class IC; CytoLyt – Class II; CellFyx – Class IB
(2) This information is Hologic’s summary of the various regulations. To view the codes in their entirety, please refer to NFPA 30 and NFPA 45.
(3) A Liquid Warehouse shall have a sprinkler system that complies with the appropriate system indicated in NFPA 30.
(4) An Inside Liquid Storage Area is a storage room totally enclosed within a building and having no exterior walls.
(5) A Laboratory Unit is the area surrounded by firewalls per NFPA 30 Flammable and Combustible Liquids Code.
(6) Reduce quantities by 50% for B laboratory units located above the 3
(7) Reduce quantities by 25% for C and D laboratory units located on the 4
(8) 20ml PreservCyt vials.
(9) A Fire Area is the area of a building separated from the remainder of the building by construction having a fire resistance of at least 1-hour and having all
Flammable
&
Combustible
Liquid Class
I 45-2015 10 38 1900 480 1820 91,000 20 76 3800 480 1820 91,000
I, II, IIIA 45-2015 20 76 3800 800 3028 151,400 40 150 7500 1600 6060 303,000
I 45-2015 5 19 950 300 1136 56,800 10 38 1900 480 1820 91,000
I, II, IIIA 45-2015 10 38 1900 400 1515 75,750 20 76 3800 800 3028 151,400
I 45-2015 2 7.5 375 150 570 28,500 4 15 750 300 1136 56,800
I, II, IIIA 45-2015 4 15 750 200 757 37,8520 8 30 1500 400 1515 75,750
I 45-2015 1 4 200 75 284 14,200 2 7.5 375 150 570 28,500
I, II, IIIA 45-2015 1 4 200 75 284 14, 200 2 7.5 375 150 570 28,500
Maximum Quantities of PreservCyt Solution (Class IC) That Can Be Stored per Fire Area
(10)(12)(13)
30-2015 120 460 23,000
(3,11)
30-2015 Unlimited Unlimited Unlimited
th
floor
6
communicating openings properly protected by an assembly having a fire resistance rating of at least 1-hour per NFPA 30 Flammable and Combustible Liquids Code.
(3)
Quantities in Use Quantities in Use and Storage
NFPA
Code
Max per 100ft2 (9.2m2) of
Lab Unit
(5)
Gallons Liters Vials
Max Quantity per Lab
Unit
(8)
Gallons Liters Vials
Max per 100ft
(8)
Gallons Liters Vials
(9)
Outside a Safety Flammable Cabinet
2
Lab Unit
(9.2m2) of
(5)
(8)
Gallons Liters Vials
Location NFPA Code Gallons Liters Vials
Allowable Quantities of PreservCyt Solution That Can Be Stored in a Liquid Storage Room
Location NFPA Code Gallons Liters Vials
500ft
2
in size.
30-2015 10 38 1900
rd
floor.
th-6th
floors of a building and reduce quantities by 50% for C and D laboratory units above the
(4)
Max Quantity per Lab Unit
(8)
(8)
(8)
AW-13654-001 Rev. 001
Page 63

ThinPrep®Solutions
(1)
StorageGuide
(2)
(10) Allowable quantities in a warehouse can be increased with a sprinkler system rated higher than standard systems.
(11) A Liquid Warehouse is a separate, detached building or attached building used for warehousing-type operations for liquids.
(12) Quantities are permitted to be increased 100% where stored in approved flammable liquids storage cabinets.
(13) Quantities are permitted to be increased 100% in buildings equipped throughout with an automatic sprinkler system installed in accordance tiwh NFPA13, Standard
for the Installation of Sprinkler Systems.
This table lists the NFPA ratings for all the ThinPrep products.
ThinPrep Product Health Hazard Flammability Hazard Instability Hazard Specific Hazard
ThinPrep PreservCyt Solution 2 3 0 N/A
ThinPrep CytoLyt Solution 2 2 0 N/A
ThinPrep CellFyx Solution 2 3 0 N/A
ThinPrep Rinse Solution 0 0 0 N/A
ThinPrep Bluing Solution 0 0 0 N/A
ThinPrep Rinse II Solution 2 3 0 N/A
ThinPrep Bluing II Solution 0 0 0 N/A
ThinPrep Stain EA Solution 2 3 0 N/A
ThinPrep Stain Orange G Solution 2 3 0 N/A
ThinPrep Nuclear Stain 2 0 0 N/A
AW-13654-001 Rev. 001
Page 64

ThinPrep® Solutions Shipping Requirements *
Scope:
These requirements include shipping:
Biological specimens (patient specimens) in ThinPrep® solutions
Biological specimens in solutions other than ThinPrep
Biological specimens not in solutions
®
ThinPrep
ThinPrep
PreservCyt™ Solution without biological specimens
®
CytoLyt™ Solution without biological specimens
Note: Shippers of Hazardous Materials or Dangerous Goods must be trained according to the
various Hazardous Materials/Dangerous Good regulations
A. Shipping Requirements when shipping patient samples in ThinPrep PreservCyt Solution only –
Ambient Temperature:
1. Patient samples / biological substances (pathogens) contained ThinPrep PreservCyt Solution
are neutralized or inactivated by the solution and as such no longer pose a health risk. (For
further information regarding this, refer to the ThinPrep 2000 or ThinPrep 5000 Operators’
Manual).
2. Materials that have been neutralized or inactivated are exempt from the Category B Class 6,
Division 6.2 requirements.
3. Solutions that contain neutralized or inactivated pathogens, and meet the criteria of one or
more of the other hazards risks, must be shipped according to the shipping requirements for
that hazard risk(s).
®
solutions
4. ThinPrep PreservCyt Solution is a Flammable liquid when shipped domestic or international
Therefore, follow the instructions in Section C below, Shipping ThinPrep® PreservCyt™
Solution Only (such as from a laboratory to a physician).
B Shipping Biological Specimens in Solutions (other than ThinPrep PreservCyt Solution) or
Without Solutions
Notes:
When biological specimens are shipped in a solution of a quantity of 30 ml or less and are
packed in accordance with these guidelines, no further requirements in the Hazardous
Materials (Dangerous Goods) Regulations need be met. However, training is re commended.”
Definitions:
Biological Substance, Category B: Materials containing or suspected to contain infectious
substances that do not meet Category A criteria. IATA Dangerous Goods regulations were
revised with an effective date of January 1, 2015. Note: The term “diagnostic specimen” has
been replaced with “biological substance, Category B”
Exempt specimens: Specimens that with the minimal likelihood that pathogens are present
(fixed tissue, etc.)
* These instructions are Hologic’s interpretation of the various regulations as of the effective date.
However, Hologic will not be responsible for any non-conformance to the actual regulations.
1
Page 1 of 7
AW-13653-001 Rev. 001
Page 65

Shipping Requirements Category B or Exempt
1. Packaging must consist of three components
a. a primary receptacle, leak proof
b. secondary packaging, leak proof
c. a rigid outer packaging
NOTES:
FedEx will not accept clinical samples or diagnostic specimens packaged in
FedEx envelopes, FedEx tubes, FedEx Paks, or FedEx Boxes, Styrofoam boxes,
plastic bags, or paper envelopes.
FedEx will accept clinical samples in FedEx Clinical Paks, FedEx Medium Clinical
Boxes or FedEx Large Clinical Boxes.
2. The primary receptacle cannot contain more that 1L of a liquid substance (500 ml if using
FedEx).
3. If multiple fragile primary receptacles are placed in a single secondary packaging, they
1
– Ambient Temperature:
2
must be either individually wrapped or separated to prevent contact between them.
4. Absorbent material must be placed between the primary receptacle and the secondary
packaging. The absorbent material (cotton balls, cellulose wadding, absorbent packets, paper
towels) must be in sufficient quantity to absorb the entire contents of the primary receptacle(s)
so that any release of the liquid substance will not compromise the integrity of the cushioning
material or the outer packaging.
5. The outer packaging must not contain more than 4L or 4kg of material. This quantity excludes
ice, dry ice, or liquid nitrogen when used to keep specimens cold.
6. An itemized list of contents must be enclosed between the secondary packaging and the outer
packaging.
7. The packaging must successfully pass a 4 ft. drop test (Section 6.6.1 IATA regulations).
8. The UN3373 mark must be displayed on the external surface of the outer packaging (one
surface of the outer packaging must have a minimum dimension of 100 mm x 100 mm FedEx
minimum is 7”x 4”x 2”) on a background of a contrasting color and must be clearly visible and
legible. The mark must be in the form of a diamond with each side having a length of at least 50
mm. Lettering must be at least 6mm high.
9. The proper shipping name “Biological Substance, Category B” in letters at least 6mm high must
be marked on the outer package adjacent to the diamond shaped UN3373 mark.
Page 2 of 7
AW-13653-001 Rev. 001
Page 66

10. If using FedEx, the FedEx USA Airbill, Section 6, Special Handling must be completed with
dangerous goods/dry ice information:
Does this shipment contain dangerous goods?
YES- Shipper’s Declaration not required
11. The outer container of all diagnostic/clinical specimen packages must display the following:
a. Sender’s name and address
b. Recipient’s name and address
c. The words “Biologi cal Substance, Category B”
d. The UN 3373 label
Shipping Requirements Category B or Exempt
NOTE: FedEx defers to IATA regulations for the shipping of refrigerated or frozen diagnostic specimens.2
1
– Frozen or Refrigerated Specimens:
Follow all packaging directions for Category B or Exempt – Ambient Temperature plus:
1. Place ice or dry ice outside of the secondary packaging. Interior supports must be provided to
secure the secondary packaging in the original position after the ice or dry ice has dissipated. If
ice is used, the outside packaging or overpack must be leak proof. If dry ice is used, the
packaging must be designed and constructed to permit the release of CO
2
gas to prevent a
buildup of pressure that could rupture the packaging.
2. Always affix the Class 9, UN 1845 dry ice label as well as the UN 3373, Biological Substance,
Category B label to these shipments
3. If using FedEx, the FedEx USA Airbill, Section 6, Special Handling must be completed with
dangerous goods/dry ice information:
Does this shipment contain dangerous goods?
YES- Shipper’s Declaration not required
Enter kg of dry ice used (if applicable)
4. The outer container of all diagnostic/clinical specimen packages must display the following:
a. Sender’s name and address
b. Recipient’s name and address
c. The words “Biologi cal Substance, Category B”
d. The UN 3373 label
e. Class 9 label, including UN 1845, and net weight if packaged with dry ice
C Shipping ThinPrep
Domestic Ground Shipments - Limited Quantities:
AW-13653-001 Rev. 001
®
PreservCyt™ Solution Only (such as from a laboratory to a physician)
Page 3 of 7
Page 67

Notes:
ThinPrep
®
PreservCyt™ Solution is classified as a Class 3 Flammable liquid, assigned to Packing
Group III (PG III).
49 CFR 173.150 (Limited Quantities) allows ThinPrep
®
PreservCyt™ Solution in vials to be shipped
in Limited Quantities when shipped via ground transportation in a sturdy box. The total volume in
a package cannot exceed 5 liters or weigh more than 30 kg (66 lbs). Limited Quantities are
exempt from labeling requirements.
Limited Quantity domestic ground shipping recommendations:
1. ThinPrep
®
PreservCyt™ Solution must be shipped in the vials.
2. Place the vials in a good quality cardboard box, such as the ThinPrep® box that holds 250 vials.
Pack vials in a manner (adding protective packing material as necessary) as to limit movement of
individual vials.
3. Mark the package as “Flammable liquids, n.o.s., (Methanol Solution), 3, UN1993, Ltd. Qty.” add
orientation arrows on the ends, and the Limited Quantity label:
4. Print “UN1993, Flammable liquids, n.o.s., (Methanol Solution), 3, PG III, Ltd. Qty.” on the
Shipping papers.
Domestic Ground Shipments - Other than Limited Quantities:
When shipping packages in excess of “Limited Quantity” amounts:
1. Do not include “Ltd Qty” in the wording on the package or on the Shipping papers as
indicated in c and d above.
2. Affix a Class 3 “Flammable Liquid” hazard label to the outer package in close proximity of the
wording described in “C” above. See the example of the label on the last page of these
recommendations.
3. Mark the package as “Flammable liquids, n.o.s., (Methanol Solution), 3, UN1993, Net Qty.”
Domestic Air Shipments:
In addition to 1 and 2 above in Domestic Ground Shipments – Other than Limited Quantities, the
following are recommendations for domestic air shipments:
3. Maximum allowable package sizes are:
i. Sixty (60) liters (3000-vials) for passenger aircraft, and
ii. Two hundred twenty (220) liters (11,000-vials) for cargo aircraft.
Page 4 of 7
AW-13653-001 Rev. 001
Page 68

4. Single packages containing more than sixty (60) liters (3000-vials) of total product must be
clearly marked “FOR CARGO AIRCRAFT ONLY”.
5. The vials must be shipped in United Nations (UN) certified 4G packaging for any quantity in
an aircraft. (e.g., ThinPrep
®
PreservCyt™ Solution 250-vial box or equivalent.)
6. A Class 3 “Flammable Liquid” label must be affixed to the outer package near the words
“Flammable liquids, n.o.s., (Methanol Solution)”.
All Domestic Shipments:
The following are recommendations for all domestic ground and air shipments:
1. If the ThinPrep
material, the hazardous material must be listed first, or be printed in a contrasting color (or
highlighted) to differentiate it from the non-hazardous material.
2. The total volume of ThinPrep® PreservCyt™ Solution and the number of vials must appear on
the shipping papers.
International Ground Shipments - Limited Quantities:
When shipping internationally, ThinPrep
Class 3 (Flammable Liquid), and with a secondary hazard of Class 6.1 (Toxic). It is assigned to PG
III.
The reference used for the international ground recommendations is the ADR - European Agreement
Concerning the International Carriage of Dangerous Good by Road (United Nations). A “Limited
Quantity” is defined as a package containing a maximum net quantity of 5-liters and not weighing
more than 20 kg (40 lbs). The recommendations for international ground shipments are as follows:
1. ThinPrep® PreservCyt™ Solution must be shipped in the vials.
2. Place the vials in a good quality cardboard box, such as the Cytyc box that holds 250 vials.
Pack vials in a manner (adding protective packing material as necessary) as to limit
movement of individual vials.
3. Mark the package with “UN1992, Flammable liquids, toxic, n.o.s., (Methanol Solution), 3, 6.1,
PGIII Ltd. Qty” orientation arrows on the ends and the Limited Quantity label that has a “Y” on
it.
®
PreservCyt™ Solution is shipped in a package also containing non-hazardous
®
PreservCyt™ Solution is classified with a primary hazard of
4. The shipping papers should include all the information indicated in “3” above.
International Ground Shipments – Other then Limited Quantities:
Page 5 of 7
AW-13653-001 Rev. 001
Page 69

1. Do not include “Ltd Qty” in the wording on the package or on the Shipping papers as
indicated in c and d above.
2. Affix both a Class 3 “Flammable Liquid” label and a secondary Class 6.1 “Toxic” label to the
package adjacent to the markings. (Copies of the labels can be found on the last page of this
document.)
Class 6.1 “Toxic” secondary hazard label.
3. Mark the package with “UN1992, Flammable liquids, toxic, n.o.s., (Methanol Solution), 3, 6.1,
PG III, Net Qty”.
International Air Shipments:
The references used for the International Air recommendations are: In addition to a and b above
in International Ground Shipments, the following are the recommendations for international air
shipments:
1. Maximum allowable package sizes are:
i. Sixty (60) liters (3000-vials) for passenger aircraft, and
ii. Two hundred twenty (220) liters (11,000-vials) for cargo aircraft.
2. Packages containing more than sixty (60) liters of product must be clearly marked “FOR
CARGO AIRCRAFT ONLY”
3. The vials must be shipped in United Nations (UN) certified 4G packaging for any quantity in
an aircraft. (e.g., ThinPrep
®
PreservCyt™ Solution 250-vial box or equivalent.) Pack vials in a
manner (adding protective packing material as necessary) as to limit movement of individual
vials.
4. Limited Quantity exemption can only be used if the package has a maximum net quantity of
2-liters.
5. Packaging manufacturer’s specifications markings are not required when shipping Limited
Quantity.
6. Mark the package with “UN1992, Flammable liquids, toxic, n.o.s., (Methanol Solution), 3, 6.1,
PGIII, Net. Qty”.
7. When a “Cargo Aircraft Only” marking is required, it must be affixed on the same package
surface and near the hazard labels.
8. The shipper is responsible for the completion of a “Shipper’s Declaration for Dangerous
Goods” form.
D. Shipping ThinPrep® CytoLyt™ Solution Only (such as from a laboratory to a physician)
Domestic Ground Shipments:
Page 6 of 7
AW-13653-001 Rev. 001
Page 70

ThinPrep® CytoLyt™ Solution has a flash point of 109° F. For domestic ground transportation only, a
flammable liquid with a flashpoint at or above 100° F that does not meet the definition of any other
hazard class may be reclassed as a combustible liquid. As such, ThinPrep
shipped via ground, is exempt from the requirements of the DOT Hazardous Materials Regulations.
®
CytoLyt™ Solution,
Domestic Air Shipments:
When shipping ThinPrep® CytoLyt™ Solution via air, follow the Domestic Air Shipments
®
recommendations for Shipping ThinPrep
PreservCyt™ Solution Only that can be found in Section C
of this document.
International Ground and Air shipments:
When shipping ThinPrep® CytoLyt™ Solution via ground or air, follow the International Ground or Air
®
Shipments recommendations for Shipping ThinPrep
PreservCyt™ Solution Only guidelines that can
be found in Section C of this document.
E. Shipping ThinPrep® CytoLyt™ Solution With Patient Sample (such as from a physician to a
laboratory)
Domestic Shipments:
ThinPrep® CytoLyt™ Solution containing a patient sample is classified as a Biological Substance,
Category B. Follow the recommendations in Section B of this document.
International Shipments:
ThinPrep® CytoLyt™ Solution containing a patient sample is classified as a Biological Substance,
Category B. Follow the recommendations in Section B of this document.
References:
49 CFR 100 to 185, Transportation
th
Dangerous Goods Regulati ons, 56
Edition, 2015, International Air Transportation Association
(IATA)
International Civil Aviation Organization’s (ICAO) Technical Instructions for the Safe Transport of
Dangerous Goods by Air
Foot Notes:
1. See Packing Instruction 650 in the IATA Dangerous Goods
Regulations
2. FedEx Document 33539PL: “Packaging Clinical Samples” and “Packaging UN 3373 Shipments”
Page 7 of 7
AW-13653-001 Rev. 001
Page 71

4. Sample Collection
and Preparation
and Preparation
4. Sample Collection
Page 72

GYNECOLOGIC SAMPLE COLLECTION AND PREPARATION
4
SECTION
A
Chapter Four
Gynecologic Sample Collection and
Preparation
CONTENTS
SECTION A:
SECTION B:
SECTION C:
SECTION D:
SECTION E:
Introduction 4.1
Specimen Collection 4.2
Special Precautions 4.4
Specimen Processing 4.4
Sample Processing Troubleshooting 4.6
INTRODUCTION
The ThinPrep® 3000 processor prepares microscope slides from ThinPrep Pap test gynecologic samples. The collected specimen includes cell samples from the ectocervix and the endocervix.
ThinPrep® 3000 Processor Operator’s Manual
4.1
Page 73

4
GYNECOLOGIC SAMPLE COLLECTION AND PREPARATION
SECTION
B
SPECIMEN COLLECTION
Collect Gynecologic Sample Using the Broom-Like Device
Physician/clinician instructions for collecting gynecologic samples.
1.
Obtain
device. Insert the central bristles of the broom into the endocervical
canal deep enough to allow the shorter bristles to fully contact the ectocervix. Push gently, and rotate the broom in a clockwise direction five
times.
2.
Rinse
vial by pushing the broom into the bottom of the vial 10 times, forcing
the bristles apart. As a final step, swirl the broom vigorously to further
release material. Discard the collection device.
an adequate sampling from the cervix using a broom-like
the broom as quickly as possible into the PreservCyt® Solution
Tighten
3.
torque line on the vial.
Record
4.
Record
request form.
Note:
If the sample is to be sent elsewhere for processing, continue with the
next step.
Place
5.
oratory.
the cap so that the torque line on the cap meets or passes the
the patient’s name and ID number on the vial.
the patient information and medical history on the cytology
If the sample is to be processed immediately, allow the sample to
stand in the PreservCyt Solution vial for at least 15 minutes before
processing.
the vial and requisition in a specimen bag for transport to the lab-
Refer to the instructions provided with the collection device for warnings, contraindications, and
limitations associated with specimen collection.
4.2
ThinPrep® 3000 Processor Operator’s Manual
Page 74

GYNECOLOGIC SAMPLE COLLECTION AND PREPARATION
4
Collect Gynecologic Sample, Using the Endocervical Brush/Spatula Device
Physician/clinician instructions for collecting gynecologic samples.
1.
Obtain
ula.
Rinse
2.
vial by swirling the spatula vigorously in the vial 10 times. Discard the
spatula.
an adequate sampling from the ectocervix using a
the spatula as quickly as possible into the PreservCyt Solution
plastic
spat-
Obtain
3.
cal brush device. Insert brush into the cervix until only the bottom-most
fibers are exposed. Slowly rotate ¼ or ½ turn in one direction. DO NOT
OVER-ROTATE.
Rinse
4.
rotating the device in the solution 10 times while pushing against the
PreservCyt vial wall. Swirl vigorously to further release material. Discard the brush.
5.
Tighten
torque line on the vial.
6.
Record
Record
requisition form.
Note:
an adequate sampling from the endocervix using an endocervi-
the brush as quickly as possible in the PreservCyt Solution by
the cap so that the torque line on the cap meets or passes the
the patient’s name and ID number on the vial.
the patient information and medical history on the cytology
If the sample is to be processed immediately, allow the sample to
stand in the PreservCyt Solution vial for at least 15 minutes before
processing.
If the sample is to be sent elsewhere for processing, continue with the
next step.
Place
7.
Refer to the instructions provided with the collection device for warnings, contraindications, and
limitations associated with specimen collection.
the vial and requisition in a specimen bag for transport to the lab-
oratory.
ThinPrep® 3000 Processor Operator’s Manual
4.3
Page 75

4
GYNECOLOGIC SAMPLE COLLECTION AND PREPARATION
SECTION
C
SECTION
D
SPECIAL PRECAUTIONS
PreservCyt® Solution
After sample transfer to the PreservCyt Solution vial, the sample should stand for at least 15 minutes
before processing.
For more information on PreservCyt Solution, refer to Chapter 3, “PreservCyt® and CellFyx™
Solutions”.
Interfering Substances
The use of lubricants (e.g., KY Jelly) should be minimized prior to specimen collection. Lubricants
can adhere to the filter membrane and may cause poor cell transfer to the slide.
Handling/Disposal
Handle all chemical-containing materials carefully in accordance with safe laboratory practices.
When required by reagent composition, additional precautions are marked on the reagent containers
or the instructions for use.
Dispose of PreservCyt Solution according to the guidelines for disposing of hazardous waste.
PreservCyt Solution contains methanol.
SPECIMEN PROCESSING
Materials Required
Refer to Materials Required in “MATERIAL REQUIREMENTS” on page 1.8 and “ITEMS REQUIRED
TO BEGIN BATCH PROCESSING” on page 5.5 for a list and explanation of materials required.
Specimen Preparation
•
The gynecologic sample should be deposited in the PreservCyt Solution immediately
upon collection.
4.4
ThinPrep® 3000 Processor Operator’s Manual
Page 76

GYNECOLOGIC SAMPLE COLLECTION AND PREPARATION
4
ID#
00001168
Frosted area
•
The PreservCyt sample vial fluid level should be within the frosted area of the sample
vial.
Figure 4-1 PreservCyt Sample Vial Fluid Level
Specimen Stability
• Store PreservCyt Solution
15°C (59°F) and 30°C (86°F) for up to 6
with
cytologic sample intended for ThinPrep Pap testing between
weeks.
Run on ThinPrep 3000 Processor
Process samples on the ThinPrep 3000 processor. Refer to “Instrument Operation” on page 5.1.
ThinPrep® 3000 Processor Operator’s Manual
4.5
Page 77

4
GYNECOLOGIC SAMPLE COLLECTION AND PREPARATION
SECTION
E
SAMPLE PROCESSING TROUBLESHOOTING
REPROCESSING A THINPREP PAP TEST SAMPLE VIAL FOLLOWING AN UNSATISFACTORY
RESULT
Note:
Laboratory personnel may reprocess ThinPrep Pap test specimens where slides have been interpreted as inadequate (“Unsatisfactory for Evaluation”) for diagnosis following cytotechnologist
screening. The instructions below must be followed in order to properly reprocess these specimens.
This section specifically addresses reprocessing ThinPrep Pap test samples following an
“unsatisfactory for evaluation” result. The ‘reprocessing’ of samples described in this section
should not be confused with the reprocessing related to specimen sample errors outlined in
Chapter 7 of this manual. Reprocessing as described in Chapter 7 refers to the re-running of a
specimen.
Note:
Note:
Reprocessing a ThinPrep Pap test specimen may only be performed once.
Good laboratory practices should be followed to avoid introducing contaminants into the
PreservCyt Solution sample vial.
Reprocessing Protocol
1 Prepare a wash solution of sufficient volume to add 30 mL to every Thin-
Prep Pap test specimen being reprocessed. The wash solution is made by
mixing 9 parts CytoLyt Solution with 1 part glacial acetic acid.
2 Prior to performing this step, assure there is sufficient volume in the Thin-
Prep Pap test specimen to result in a pellet, following centrifugation. Pour
the contents of the ThinPrep Pap test specimen into a centrifuge tube
appropriately labeled to maintain chain of custody. Retain the vial.
3 Pellet the contents of the centrifuge tube by centrifugation at 1200 x
5 minutes.
Note:
g
for
Once centrifugation is complete, the cell pellet should be clearly visible but the cells may not be tightly packed together (the pellet may
appear fluffy).
4.6
ThinPrep® 3000 Processor Operator’s Manual
Page 78

GYNECOLOGIC SAMPLE COLLECTION AND PREPARATION
4
30 ml
4 a. Carefully pour off the supernatant from the centrifuge tube to avoid
loss of cells. Dispose of according to local regulations.
b. Vortex the centrifuge tube briefly.
c. Pour 30 mL of the CytoLyt Solution and 10% glacial acetic acid mixture
into the centrifuge tube and cap securely.
d. Invert the centrifuge tube by hand several times to mix.
g
5 Pellet the cells again by centrifugation—1200 x
6 a. Carefully pour off the supernatant from the centrifuge tube to avoid
loss of cells. Dispose of according to local regulations.
b. Vortex the centrifuge tube briefly.
for 5 minutes.
7 a. Using the volume markings on the centrifuge tube, pour the necessary
quantity of unused (i.e., containing no patient specimens) PreservCyt
Solution to the cells and fill to a final volume of 20 mL. Secure the cap
tightly.
b. Invert the centrifuge tube several times to mix and transfer the sample
back into the retained specimen vial.
8 Process the specimen using a ThinPrep 3000 processor according to the pro-
cedure for running gynecologic specimens. Evaluate the resultant slide
according to
Diagnosis
with the clinical impression, a new specimen may be necessary.
The Bethesda System for Reporting Cervical/Vaginal Cytologic
. If after reprocessing, negative results from specimen do not fit
ThinPrep® 3000 Processor Operator’s Manual
4.7
Page 79

4
GYNECOLOGIC SAMPLE COLLECTION AND PREPARATION
This page intentionally left blank
4.8
ThinPrep® 3000 Processor Operator’s Manual
Page 80

5. Instrument
Operation
Operation
5. Instrument
Page 81

5
Chapter Five
SECTION
A
Instrument Operation
CONTENTS
INSTRUMENT OPERATION
SECTION A:
SECTION B:
SECTION C:
SECTION D:
SECTION E:
SECTION F:
SECTION G:
SECTION H:
SECTION I:
Chapter Overview 5.1
Optional Instructions for Ancillary Testing 5.2
Instrument Doors 5.4
Items Required to Begin Batch Processing 5.5
Begin Batch Processing 5.14
Completing a Batch 5.18
Reading the Batch Report 5.20
Pausing a Batch in Process 5.22
Canceling a Batch in Process 5.23
CHAPTER OVERVIEW
Routine instrument operation consists of loading supplies, starting the batch process and unloading
the prepared slides and processed sample vials when the batch is complete. A batch report (or
‘results printout’) is automatically generated at the completion of each batch. Any samples that may
require further operator attention are flagged on the batch report, along with a description of the
type of process or sample error. The action of pausing or canceling a batch is available at any time
during processing.
ThinPrep® 3000 Processor Operator’s Manual
5.1
Page 82

5
INSTRUMENT OPERATION
SECTION
B
OPTIONAL INSTRUCTIONS FOR ANCILLARY TESTING
Testing for certain sexually transmitted diseases (STD) and for Human Papilloma Virus (HPV) in
conjunction with cytology may be enabled by the removal of an aliquot of up to 4 mL (Aliquot
Removal) from the PreservCyt sample vial before preparing the ThinPrep Pap test slide.
Laboratory personnel must follow the specific instructions in this section to appropriately remove
the desired aliquot volume and prepare the PreservCyt sample vial for the ThinPrep Pap test. Adherence to these instructions must be maintained to ensure there is no adverse effect on the ThinPrep
Pap test result.
Because cytology/HPV testing and STD testing address different clinical questions, Aliquot Removal
may not be suitable for all clinical situations. Physicians and other persons responsible for ordering
clinical tests should be familiar with the following:
• There is no evidence of degradation of cytology results by Aliquot Removal, however, this
cannot be ruled out for all specimens. As with any subsampling step in anatomic pathology,
chance misallocation of diagnostic cells may occur if they are very rare. If negative results
from the specimen do not fit with the clinical impression, a new specimen may be necessary.
• Aliquot Removal from low-cellularity specimens may leave insufficient material in the
PreservCyt sample vial for preparation of a satisfactory ThinPrep Pap test slide.
• Aliquot Removal may leave insufficient material In the PreservCyt sample vial for performance of ancillary testing (e.g., reflexive HPV testing) using the residual specimen following
preparation of a ThinPrep Pap test slide.
• Co-collection of separate samples for the ThinPrep Pap test and STD testing may be considered in lieu of Aliquot Removal.
• When opting for concurrent cytologic and STD testing, providers should consider risk and
clinical history (e.g., disease prevalence, patient age, sexual history or pregnancy) as well as
specimen suitability (e.g., exudates or bleeding) that can impact diagnostic reliability.
Sexually Transmitted Diseases Treatment Guidelines 2002 (Centers for Disease Control and Prevention, MMWR 2002: 51(No. RR-6)) provides clinical guidance for the management and treatment of
individual patients, including use of Pap testing.
It is contraindicated to perform
the Roche Diagnostics COBAS AMPLICOR CT/NG test, if the sample has already been processed
using the ThinPrep 3000 processor.
Chlamydia trachomatis
and
Neisseria gonorrhoeae
testing, using
5.2
ThinPrep® 3000 Processor Operator’s Manual
Page 83

INSTRUMENT OPERATION
5
Removing an Aliquot (of up to 4 mL) from the PreservCyt Sample Vial prior to
Performing the ThinPrep Pap Test
Note:
Only one aliquot may be removed from the PreservCyt sample vial prior to performing the
ThinPrep Pap test, regardless of the volume of the aliquot (maximum aliquot
volume = 4 mL).
Note:
1. Vortex the vial at high speed for 8 to 12 seconds.
CAUTION:
homogeneity of the sample.
2. Carefully remove the vial cap.
3. Using a pipetting device, withdraw an aliquot of up to 4 mL from the vial. Take care to avoid
4. Dispense the aliquot into a suitably sized and labeled polypropylene tube and close tightly to
5. Store the aliquot under conditions appropriate for ancillary test(s). Refer to manufacturer or lab-
6. Dispose of the pipetting device in accordance with local, state, and federal regulations.
7. Using a new pipetting device, withdraw a quantity of unused PreservCyt Solution from its con-
8. Transfer the volume of unused PreservCyt Solution to the vial from which the aliquot was
9. Secure the vial cap. (The line on the cap and line on the vial should meet or slightly overlap.)
10. Dispose of the pipetting device in accordance with local, state, and federal regulations.
11. Refer to the remaining steps in this chapter to complete the ThinPrep Pap test.
Good laboratory practices should be followed to avoid introducing contaminants into either
the PreservCyt sample vial or the aliquot. It is recommended to use powder-free gloves and
an individually wrapped, disposable pipetting device with an aerosol barrier tip that is sized
appropriately for the volume being withdrawn and dispensed. You should not use serological
pipettes. In order to minimize the potential for cross-contamination, aliquot removal should
be performed in an appropriate location outside an area where amplification is performed.
The desired aliquot must be removed immediately after vortexing the vial to ensure
contaminating gloves with solution. If gloves should become contaminated, replace with a clean
pair before proceeding to the next specimen.
prevent leakage/evaporation.
oratory instructions for performing ancillary test(s) on the aliquot.
tainer that is equal in volume to that of the aliquot removed from the vial in step 3.
removed in step 3.
ThinPrep® 3000 Processor Operator’s Manual
5.3
Page 84
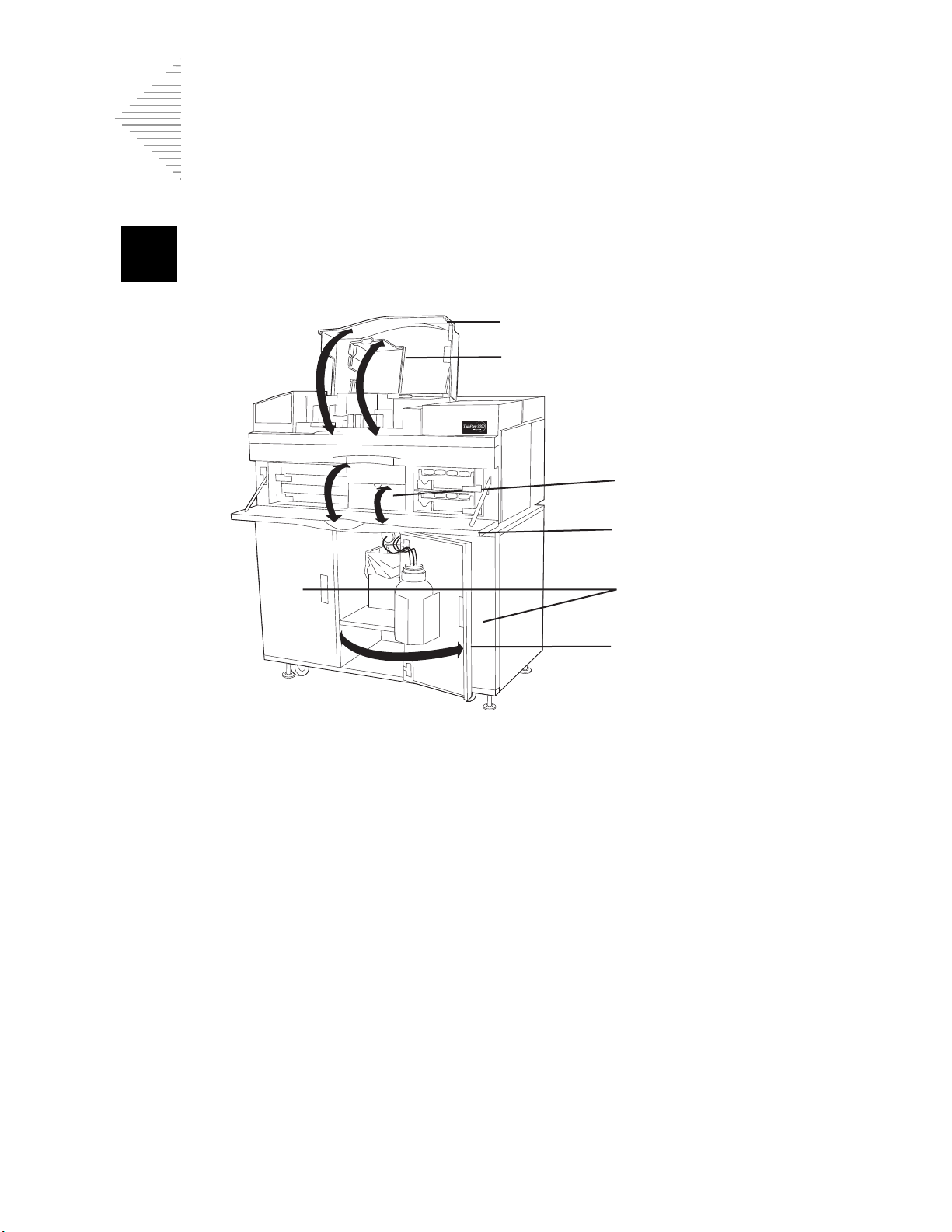
5
INSTRUMENT OPERATION
SECTION
C
Top door
Slide printer cover
Center door
Front door
Center cart door
Left & right
cart doors
INSTRUMENT DOORS
•
Top d o or
dispense area and the CellFyx
batch processing or when the processor is moving mechanisms.
•
Slide printer cover
•
Center door
tubing. The instrument serial number is located here.
•
Front door
trays and area. This door locks with the top door during batch processing or when the proces
sor is moving mechanisms.
•
Left and right cart doors
5.4
ThinPrep® 3000 Processor Operator’s Manual
provides access to the slide cartridges, slide printer cover, staining racks, fixative
provides access to the slide printer ribbon and the slide printer assembly.
flips down to access the slide waste bin and for replacement of the pinch valve
provides access to the filter trays and area, the center door and the sample vial
provide access to storage for accessories and consumables.
™
Solution reservoir. This door locks with the front door during
-
Page 85

INSTRUMENT OPERATION
5
SECTION
D
3
5
1
2
00001168
4
ThinPre
p
Microscope
Slides
ITEMS REQUIRED TO BEGIN BATCH PROCESSING
To begin batch processing, it is necessary to have the following items ready for use:
1. Trays of Gyn ThinPrep® Pap test filters
2. ThinPrep microscope slides and slide cartridges
3. Staining racks
4. Barcoded ThinPrep Pap test sample vials
5. Sample vial trays
ThinPrep® 3000 Processor Operator’s Manual
5.5
Page 86

5
INSTRUMENT OPERATION
Tray latch
closed
Tray latch
open
Gyn ThinPrep Pap Test Filters
Gyn ThinPrep® Pap test filters are disposable, clear plastic cylinders used in the collection and transfer of cells from gynecologic specimens onto ThinPrep microscope slides.
The filters come in trays of 100. Two trays can be loaded into the instrument. (A minimum of one is
required.)
Loading the Gyn ThinPrep Pap test filters
CAUTION:
1. Open the tray latches as shown below.
2. Remove any empty filter trays, if present.
3. Remove and discard the clear plastic cover from the new tray(s) of filters.
4. Insert the new tray(s) of filters. Slide the tray all the way to the back of the shelf until it hits the
stops at the rear.
5. Secure the trays by closing their respective latches.
Note:
Do not handle the filters directly unless necessary. Only touch the outer surface. Do not touch
the membrane on the bottom.
Only replace filter trays if they are empty. Partially loaded trays should remain in place.
Note:
5.6
The top tray is designated tray A and the bottom tray is tray B. Raised letters on the instrument cover near the latches help identify each tray.
ThinPrep® 3000 Processor Operator’s Manual
Page 87

INSTRUMENT OPERATION
5
ThinPrep Microscope Slides and Slide Cartridges
ThinPrep microscope slides are specially designed glass slides for use with the ThinPrep processor.
Only ThinPrep slides are to be used.
WARNING:
Two metal slide cartridges are used to insert the ThinPrep microscope slides into the processor. Each
slide cartridge holds approximately 100 slides, or one package of ThinPrep microscope slides.
Clean the slide cartridge prior to loading slides
Before loading a cartridge with microscope slides wipe the inside of the cartridge with a lint-free
cloth to remove glass dust. Clean the bottom surface where the slides sit, to ensure proper slide picking. (Refer to “CLEANING THE SLIDE PATH” on page 6.17.)
Loading the ThinPrep microscope slides into the slide cartridge
Note:
The slides must be oriented correctly to work with the automated functions of the
ThinPrep 3000 processor.
ensure accurate loading of slides. A visual check of the slides after being loaded into the
cartridge is recommended.
Sharp Edges. Glass
The microscope slide package and the slide cartridge are keyed to
1. Remove the cover of the slide package.
2. Fold back the cellophane wrapper.
3. Hold the package of slides horizontally in your hand as shown.
ThinPrep® 3000 Processor Operator’s Manual
5.7
Page 88

5
INSTRUMENT OPERATION
4. Place an open slide cartridge over the slide package so that its metal grooves fall into the cutout
slots.
5. Holding them together, gently flip the slide cartridge and slide package over.
6. After the slides are transferred into the slide cartridge, remove and discard the wrapper and plastic package.
5.8
ThinPrep® 3000 Processor Operator’s Manual
Page 89

INSTRUMENT OPERATION
5
Clasp
7. Tilt the cartridge up slightly and make sure the slides are aligned squarely, evenly and are sitting
flat on the bottom of the cartridge. Confirm correct orientation of the slides. Gently close the slide
cartridge door over the slides. The latch will click when the cartridge is completely closed.
Loading the slide cartridges into the processor
CAUTION:
a partially filled cartridge.
With the slide cartridge clasp up and facing as shown, slide it into slot “A” or “B”.
Only reload slide cartridges when prompted by the instrument. Do not manually load
Staining Racks
Staining racks are plastic frames that hold the processed ThinPrep microscope slides. The slides
remain in the staining racks until batch processing ends.
The processor can hold up to four staining racks. Each rack has a capacity of 20 processed ThinPrep
slides.
Loading the empty staining racks
1. Pull the lever back with one hand.
2. With the other hand, insert the staining rack in the direction of arrow as shown below, releasing
the lever to secure it. Make sure it sits flat on the cradle.
ThinPrep® 3000 Processor Operator’s Manual
5.9
Page 90

5
INSTRUMENT OPERATION
Incorrect
Correct
Cradle
Lever
3. Place an empty staining rack into each of the four cradles.
5.10
ThinPrep® 3000 Processor Operator’s Manual
Page 91

INSTRUMENT OPERATION
5
Removing the staining racks
1. Positioning the thumb and index finger of one hand, grasp the rack as shown below.
CAUTION:
2. With the other hand, pull the lever down.
CAUTION:
racks.
Avoid touching the slides with your fingers when removing the staining racks.
Slides are not securely held in the staining rack. Use caution when removing the
3. Gently slide the rack toward the lever.
4. Tilt the staining rack as shown above.
5. Carefully lift out the staining rack. Avoid dislodging any processed slides.
ThinPrep® 3000 Processor Operator’s Manual
5.11
Page 92

5
INSTRUMENT OPERATION
00001168
Torque features
Barcoded Sample Vials
WARNING:
Flammable Liquid and Vapor
The PreservCyt® Solution sample vials for use with the ThinPrep Pap test contain the collected cervical cell samples in a preservative solution. During processing, the ThinPrep 3000 processor collects
the cells from the vials.
Vial preparation
See the Sample Vial Label Application Guide in the Appendix for recommended barcode label
application.
Toxic Mixture
Place the barcode labels
shown below. During application, avoid placing the barcode label over patient information, multiple
labels, or on the torque features. Sticking labels on incorrectly can cause a failure to read the barcode
or a failure of the instrument removing the vial from the tray.
The uncovered strip of the sample vial allows you to see the frosted band which indicates the maximum/minimum acceptable fluid fill range for a sample. Make sure the fluid level is within this
range. Refer to Chapter 7, “Troubleshooting”, if the level is too high or too low.
Additionally, check to make sure there is no foreign matter in the vial. Refer to Chapter 7, “Troubleshooting”, if an object is in the sample vial.
Inserting vials into the sample vial trays
Place sample vials into the numbered holes of the sample vial tray (the tray does not have to be full
to run a batch; the vials can be in any order). The ThinPrep 3000 processor can process up to
80 samples, (two full sample vial trays) per batch.
vertically
on the ThinPrep Pap test label, using the edge for alignment, as
5.12
ThinPrep® 3000 Processor Operator’s Manual
Page 93
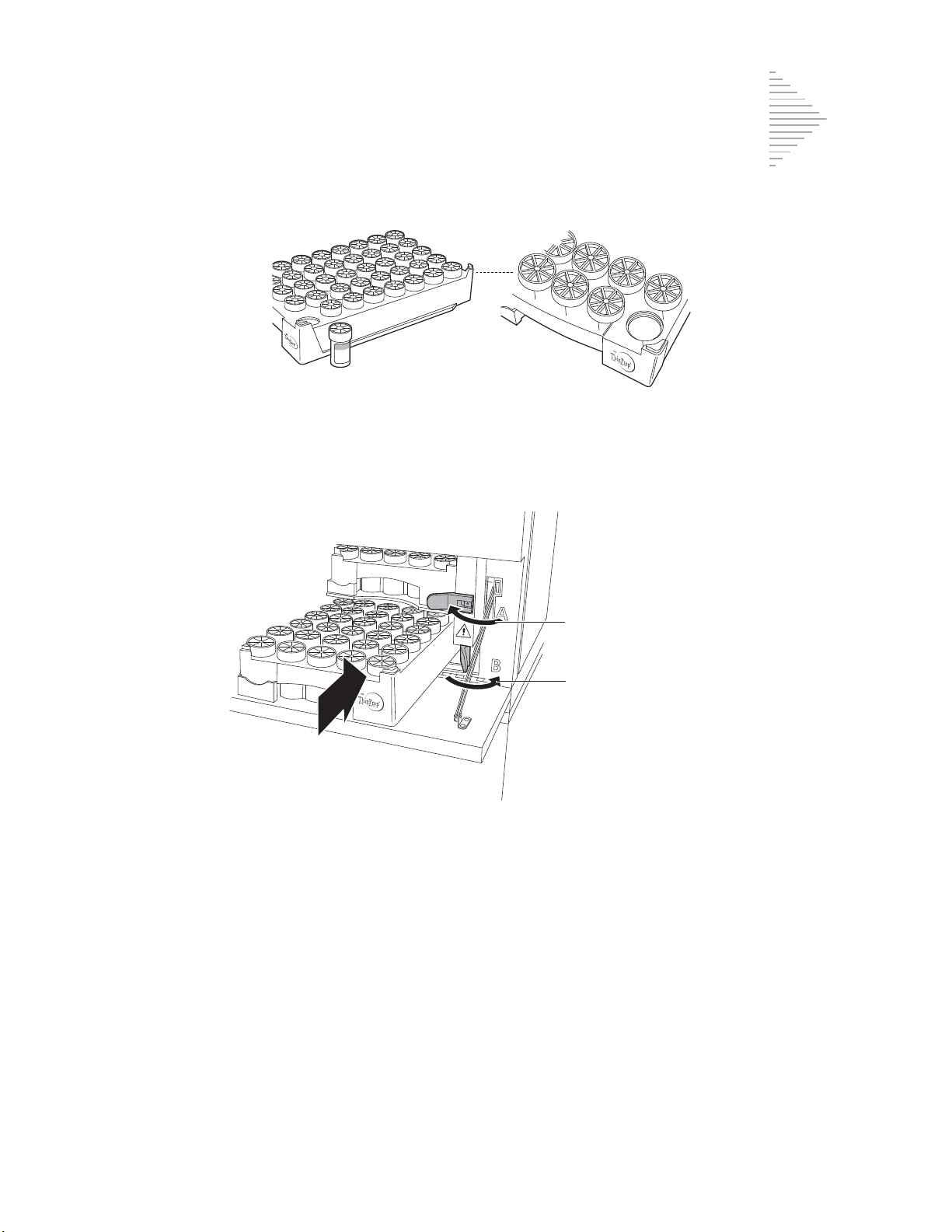
5
Loading the sample vial trays
40
39
38
37
35
34
34
33
Tray latch closed
Tray latch open
1. Open the tray latches as shown below.
INSTRUMENT OPERATION
WARNING:
Sharp Edges & Hot Surfaces.
Moving Parts
2. Remove any previously processed sample vial trays, if present.
3. Load the sample vial trays into the empty slots so that the ThinPrep Pap test logo on the tray is
facing outward. (The trays only fit in one way.) Be sure to slide each tray fully into the instrument
until it hits the stops at the rear.
4. Secure the trays by closing their respective latches.
Refer to “READING THE BATCH REPORT” on page 5.20 for batch report information.
Note:
The top tray is designated tray A and the bottom tray is tray B. Raised letters on the instrument cover near the latches help identify each tray. A results report for each tray, A and B,
prints out at the conclusion of the batch. It is important to correlate the report with the correct
tray.
ThinPrep® 3000 Processor Operator’s Manual
5.13
Page 94

5
INSTRUMENT OPERATION
SECTION
E
System Ready Start Batch
Load Samples and
Supplies Menus
Select "Start Batch"
Print Results
08:25-AM 11/09/99
Checking Consumables
Please Wait
Batch Status Pause
Samples In Process
Processed
00
BEGIN BATCH PROCESSING
1. Confirm that the main menu, shown below, is displayed. If this menu is not displayed, follow the
1
prompts
2. In the main menu, follow the screen prompt to load samples and supplies. In order to begin processing, the following items must be properly loaded:
• Sample vial tray(s) containing barcoded sample vials
to return to the main menu.
• Filter tray(s) of ThinPrep Pap test filters
• Slide cartridge(s) containing ThinPrep microscope slides
• Empty staining racks
3. Close both the front and top door of the instrument. Processing will not begin if they are not completely shut.
4. Press
Start Batch
to begin batch processing.
5. The instrument performs a check of supplies needed to process a batch.
6. Processing begins when the following screen is displayed:
1. Prompt: Text that appears on the display next to the prompt keys.
5.14
ThinPrep® 3000 Processor Operator’s Manual
Page 95

INSTRUMENT OPERATION
5
Items Must Be Addressed:
Filters
Slides
Staining Racks Continue
Sample Tray
Add Fixative Fluid
Empty Waste Bottle and Waste Bins Cancel
Insert Clean Fixative Shield
If the instrument does not detect materials necessary to process a batch, the “Items Must Be
Addressed” message screen appears.
Items Must Be Addressed
Listed on the screen are the specific materials to be addressed before beginning a batch. Only the
items that need attention will be listed. You may see a display with some or all of the items shown
above.
No batch will be processed until these items are addressed.
1. Press
2. Press
Cancel
to cancel the batch entirely.
Continue
to attend to the required items. Wait for the doors to unlock.
Requirements for any items appearing on this display are listed below:
Refer to “ITEMS REQUIRED TO BEGIN BATCH PROCESSING” on page 5.5 for instructions on
loading items.
Filters
The processor does not detect at least one tray of Gyn ThinPrep Pap test filters.
• A minimum of one tray of filters must be loaded before batch processing can begin. Empty filter trays must be removed.
Slides
The processor does not detect at least one slide cartridge containing slides.
• A minimum of one slide cartridge containing slides must be inserted before batch processing
can begin.
• If slide cartridges are present, check that
both
contain slides and that
neither
has a slide jam,
which would prevent slide picking.
Staining racks
The processor does not detect any empty staining racks.
• At least one empty staining rack must be loaded before processing can begin.
• Be sure to remove any staining racks with slides from previous batches.
• If this message occurs during a batch, remove any staining racks containing processed slides
and replace them with empty ones
before
pressing
Continue
ThinPrep® 3000 Processor Operator’s Manual
.
5.15
Page 96

5
INSTRUMENT OPERATION
Sample trays
The processor does not detect at least one tray of sample vials.
• A minimum of one sample tray must be loaded before batch processing can begin.
CAUTION:
ative reservoir. Do not overfill.
Refer to “REPLENISHING CELLFYX SOLUTION” on page 6.32, for filling the fixative reservoir.
Fixative
The fixative reservoir is low on CellFyx Solution.
• The fixative reservoir must be filled with
begin.
Fixative cap
The processor does not detect the cap on top of the fixative reservoir.
• The cap must be tightly screwed onto the top of the fixative reservoir before processing can
begin.
Empty waste bottle and waste bins
The level of waste fluid within the waste bottle is above the maximum level. Make sure the slide
waste bin is present and loaded correctly.
• The waste bottle must be emptied before processing can begin.
• Empty the filter waste box, and slide waste bin at this time.
• The slide waste bin must be properly loaded before processing can begin.
Never add fixative unless prompted by the instrument. Only add one bottle to the fix-
one
bottle of CellFyx Solution before processing can
Insert clean fixative shield
At the time the instrument prompts for CellFyx Solution replenishment, it will also display a message to insert a clean fixative shield. This is important in preventing unwanted fixative buildup.
Refer to “REPLACING THE FIXATIVE SHIELD” on page 6.23.
After addressing items and closing all doors on the instrument, press
If materials cannot be loaded at this time, press
main menu.
5.16
ThinPrep® 3000 Processor Operator’s Manual
Cancel
to end batch processing and return to the
Continue
.
Page 97

INSTRUMENT OPERATION
5
Please Address Items:
Filters
Slides
Staining Racks Load
Sample Trays
Continue
Close Doors and Press Continue
Continue
Please Address Items
This message notifies you that these items are low in supply. Although the processor can start batch
processing, the full batch of sample vials may not be completed. The following items may appear on
this screen:
Filters
Slides
Staining Racks
Sample Trays
•Select
Load
to address the listed item(s). If supplies are loaded, the “Checking Consumables
Please Wait” screen is displayed.
•Select
Note:
Continue
to bypass loading item(s) at this time, beginning batch processing.
If the user does not respond to this message within five minutes, the batch will start by itself.
Close Doors and Press Continue
This message notifies you that an instrument door is open.
• Confirm that both the front door and top door are closed.
• After closing any open doors, select
Continue
to begin batch processing.
ThinPrep® 3000 Processor Operator’s Manual
5.17
Page 98

5
INSTRUMENT OPERATION
Batch Status Pause
Samples In Process
Processed
00
SECTION
F
Batch Complete
Please Unload Staining Racks,
Remove Vial Trays
Empty Waste Bin
Address Samples with Processing Errors
Continue
Processing begins when the following screen is displayed:
COMPLETING A BATCH
1. At the completion of batch processing, “Batch Complete, Unload Staining Racks & Remove Vial
Trays” is displayed on the message screen. If a sample error occurred, a bottom line reads
“Address Samples with Processing Errors”. A batch report will print from the results printer.
Note:
The instrument will beep once per minute to indicate the batch is complete. If an error
occurred, it will beep three times per minute. The beeps cease when the operator presses
tinue
. The display transitions to the System Ready screen.
Con-
2. Carefully unload the staining racks containing processed slides from the instrument as described
in
“Removing the staining racks” on page 5.11.
3. Tear off the printed batch report by pulling it toward you, against the edge of the paper opening.
4. Separate tray “A” (top) batch report from tray “B” (bottom) batch report. Match them with the
correct sample tray. (The tray slots are marked A and B on the instrument near the tray latches.)
Refer to “READING THE BATCH REPORT” on page 5.20 for batch report information.
5. Review the batch report for sample vials not processed. They will be marked with an asterisk and
listed in the Reprocessing Required field. Refer to
Required” on page 7.30, for follow-up action if sample vials were not processed.
5.18
ThinPrep® 3000 Processor Operator’s Manual
“SYSTEM FAULT ERRORS Reprocessing
Page 99

INSTRUMENT OPERATION
5
Routine Maintenance Required
Please Clean Slide Path
Please Run Pneumatic Test Continue
6. Fold and insert the appropriate batch report into its corresponding sample vial tray for identification.
Note:
Place the report so that the time and date can be seen. This will aid in locating a sample if necessary.
7. Remove the processed sample vial trays.
8. Remove the sample vials not processed from the tray(s). Address the issue, if possible. Refer to
Chapter 7. Include these sample vials in another batch for reprocessing.
Routine Maintenance Required
Every 800 slides (10 full batches) the instrument will prompt for Operator Maintenance:
CAUTION:
instrument operation.
Press
Continue
SLIDE PATH” on page 6.17 for instructions.
It is important to perform these maintenance tasks at this time, to ensure proper
. Refer to “PNEUMATIC SYSTEM TESTING” on page 6.13, and “CLEANING THE
ThinPrep® 3000 Processor Operator’s Manual
5.19
Page 100

5
INSTRUMENT OPERATION
SECTION
G
TP 3000 Batch Result
04/28/00 07:35
TP3 XX.XXXXXXXX
Serial No.: 00001A00A0
Tray A
Reprocessing Required
Reason Position
Slide Misprint A 5
A13
Sample ID# Position
000000010526 A 1
000000010527 A 2
000000010528 A 3
000000010529 A 4
000000010530 A 5*
000000010531 A 6
000000018376 A 7
000000018377 A 8
000000018378 A 9
000000018379 A10
000000018380 A11
000000018381 A12
A13*
000000018383 A14
000000010525 A15
000000010532 A16
000000010533 A17
000000010534
000000010535
000000010536 A20
000000010537 A21
000000010538 A22
000000010539 A23
000000010540 A24
000000010541 A25
000000018384
518
Total Samples Processed
------------
No Barcode
Date
Instrument/batch
identification
Software
revision
Time batch was starte d
Identifies sample
error by tray location
Error reporting
Lists reason
for error
Processed sample
identification
Sample ID
(barcode)
Total samples
processed tally
Sample tray
Instrument serial
number
READING THE BATCH REPORT
A sample batch report is shown. The samples needing operator attention are marked with an
asterisk.
5.20
ThinPrep® 3000 Processor Operator’s Manual
 Loading...
Loading...