Page 1

User Manual
Software Version 8.5
PN MAN-00709 Rev 001
R2™DM
Page 2

Page 3

Mammography
R2™ DM User Manual
Software Version 8.5
PN MAN-00709 Rev 001
Page 4

Technical Support
For support in North America contact:
Toll Free: +1 866.243.2533 (866.CHECKED)
Email: techsupport@r2tech.com
Hours: Monday – Friday, 6:00 AM – 5:00 PM, PT (GMT –8:00)
Website: www.r2tech.com
For support in Europe, South America, or Asia, contact your local dealer or distributor.
© 2007, Hologic, Inc. All rights reserved. Duplication or distribution without written permission
is prohibited. Hologic reserves the right to revise this manual. Issued March 2007.
Protected by one or more of the following U.S. Patents: 4907156, 5133020, 5452367, 5491627,
5537485, 5622171, 5657362, 5673332, 5729620, 5732697, 5740268, 5815591, 5828774,
5832103, 5917929, 6014452, 6035056, 6075879, 6078680, 6185320, 6198838, 6263092,
6266435, 6301378, 6404908, 6434262, 6477262, 6574357, 6580818, 6640001, 6628815,
6909795, 7054473, 7072498, 7146031
Hologic, the Hologic logo, CheckMate, DigitalNow, Earlier. Smarter. Better, ELC, EmphaSize,
Gold Standard CAD, GreenLight, ImageChecker, Malc, PeerView, R2, R2 Technology, and the
R2 Logo are trademarks or registered trademarks of Hologic, Inc. in the USA. Microsoft, and
Windows are registered trademarks of Microsoft Corporation. MagView is a registered
trademark of MagView Corporation. MergeCOM-3 is a trademark of Merge Healthcare. MRS is
a registered trademark of Mammography Reporting System, Inc. PenRad is a registered
trademark of PenRad Technologies, Inc.
Hologic Inc.
35 Crosby Drive
Bedford, MA 01730-1401 USA
Tel: +1.781.999.7300
Sales: +1.781.999.7453
Fax: +1.781.280.0668
www.hologic.com
Asia Pacific
Room 302, Hung Kei Building
5-8 Queen Victoria Street
Central, Hong Kong
Tel: +852.3102.9200
Hologic N.V.
Authorized Representative
Leuvensesteenweg 250A
1800 Vilvoorde, Belgium
Tel: +32.2.711.4680
Fax: +32.2.725.2087
MergeCOM-3 Advanced Integrator’s
Tool Kit is a product of Merge
Healthcare.
ii R2 DMax User Manual – PN MAN-00709 Rev 001
Page 5

Contents
Part 1: Introduction....................................................... 1
1.1. Available Resources ................................................... 1
1.2. How to Use this Manual ................................................ 2
1.3. Warnings and Cautions ................................................ 4
Manual Organization .................................................. 2
Conventions Used in this Manual ......................................... 2
Symbols Used ........................................................ 3
Radiological Interpretation .............................................. 4
Warnings: System Operation............................................. 5
Warnings: Maintenance ................................................ 5
Cautions: System Operation ............................................. 6
Cautions: Installation and Maintenance .................................... 7
Part 2: Description........................................................ 9
2.1. Overview ........................................................... 9
2.2. System Components ................................................. 12
2.3. Overview of Operation ................................................ 14
Studies Screen Overview ............................................... 14
Scanning and Processing ............................................... 16
System Alerts........................................................ 17
Controls Screen ...................................................... 19
2.4. System Features..................................................... 20
Feature Licensing Flexibility ............................................ 20
Other System Features................................................. 22
2.5. ImageChecker Film-Screen CAD......................................... 23
Intended Use ........................................................ 23
CAD Marks ......................................................... 23
EmphaSize.......................................................... 23
CAD Operating Points ................................................ 24
Number of CAD Marks ................................................ 25
2.6. System Inputs and Outputs ............................................ 26
CAD-Supported Views ................................................ 26
View Modifiers ...................................................... 27
Digital Image Output Options........................................... 28
R2 DMax User Manual – PN MAN-00709 Rev 001 iii
Page 6

Contents
Part 3: Studies........................................................... 29
3.1. Working with Films .................................................. 30
Film Requirements ................................................... 30
R2-Supported Lead Markers ............................................ 30
Lead Marker Placement................................................ 31
Separator Sheets ..................................................... 31
Scanning Protocols ................................................... 32
3.2. Preparing Cases to Be Scanned ......................................... 34
3.3. Scanning with the Auto Protocol ........................................ 35
3.4. Scanning with a Manual Protocol ....................................... 37
3.5. Scanning with the Any Protocol......................................... 40
3.6. Adding Cases to the Stack ............................................. 42
3.7. Stopping Scanning and Removing a Case ................................. 43
3.8. Viewing Study Information ............................................ 44
3.9. Verifying Scanned Cases .............................................. 46
3.10. Correcting Scanned Images ............................................ 47
3.11. Printing CAD Results ................................................. 50
3.12. Searching for a Study ................................................. 51
3.13. Using Queue Manager ................................................ 52
Part 4: Alerts ............................................................ 55
4.1. Alerts Listed Alphabetically ............................................ 56
Archiving was cancelled for case (3045).................................... 56
Cannot process image successfully (1063) .................................. 56
Cannot process this case (1062).......................................... 56
Could not connect to reporting system database (3503) ....................... 56
ImageChecker attempted but could not send case (3016, 3018, 3031, 3032) ........ 56
ImageChecker attempted but could not print reimbursement report (2008)........ 57
ImageChecker cannot contact the scanner (1010) ............................ 57
ImageChecker cannot identify the lead markers (3023)........................ 57
ImageChecker cannot read the barcode (1041) .............................. 57
ImageChecker is busy (3012, 1029, 1030) .................................. 57
ImageChecker license not found (1024) ................................... 58
ImageChecker could not print a CAD report (2007) .......................... 58
ImageChecker could not send results (2005) ................................ 58
Images didn’t transfer (1031) ........................................... 58
Image transfer was interrupted (3013)..................................... 58
Missing or invalid barcode at top of stack (1016) ............................ 59
No output jobs scheduled for case (3054) .................................. 59
No patient record information is associated with barcode (3501) ................ 59
Not all films in case are specified by scanning protocol (3024) .................. 59
Printer not found (3014)............................................... 59
iv R2 DMax User Manual – PN MAN-00709 Rev 001
Page 7

Contents
Rebooting the operating system, please wait (5021) .......................... 60
Reporting system query error (3502)...................................... 60
Restarting R2 software, please wait (5019).................................. 60
Scanner lock lever is raised (3051)........................................ 60
Scanner not ready! (5002) .............................................. 60
Scanner test failed (3025) .............................................. 60
Scanning is disabled, remote maintenance in progress (5010, 5059) .............. 61
Scheduled reimbursement report (5050)................................... 61
Shutting down the operating system, please wait (5020) ....................... 61
Shutting down the R2 software, please wait (5018) ........................... 61
The same barcode has been assigned to multiple patients (3500) ................ 61
The scanner is not responding (1007) ..................................... 62
The scanner might be empty (3033) ...................................... 62
X films were found when Y were expected. (1017, 1018) ....................... 62
4.2. Common Questions .................................................. 63
CAD does not run on views with implants ................................. 63
CAD marks do not appear on thumbnails .................................. 63
Case icons become yellow alerts; there are no images ......................... 63
Film is ‘missing’ inside the scanner ....................................... 63
Image is missing from a case ............................................ 63
Images are reversed in the thumbnails..................................... 63
Images at PACS/workstation are of poor quality ............................. 63
Images didn’t print out ................................................ 64
Images do not appear on my display unit .................................. 64
Monitor isn’t working ................................................. 64
Main screen is frozen and I can’t do anything ............................... 64
Patient ID flash on the printout is backwards ............................... 64
Radiologist needs CAD quickly .......................................... 65
Scanned case before entering patient information ............................ 65
Yellow ‘X’ appears on my image thumbnails ................................ 65
Part 5: Controls.......................................................... 67
5.1. About Option ....................................................... 68
5.2. Maintenance Options ................................................. 69
5.3. Shutdown and Reboot Options ......................................... 70
5.4. Outputs Options ..................................................... 71
5.5. Patient ID Option .................................................... 72
5.6. Performance Options ................................................. 73
Ejecting Films ....................................................... 74
Resetting the Clean Scanner Timer ....................................... 74
Reviewing System Verification Results .................................... 74
5.7. Printing Options ..................................................... 75
R2 DMax User Manual – PN MAN-00709 Rev 001 v
Page 8

Contents
Configuring CAD Results Printing ....................................... 76
Setting When the Reimbursement Barcode Report will be Printed ............... 76
Printing a Reimbursement Barcode Report ................................. 76
5.8. Scanning Protocols................................................... 78
Changing Scanning Protocol Order....................................... 79
Changing the Default Scanning Protocol................................... 79
Deleting a Scanning Protocol ........................................... 79
Creating Scanning Protocols ............................................ 80
Editing Scanning Protocols ............................................. 81
5.9. Service Utilities...................................................... 82
Calibrating the Touch Screen ........................................... 82
Connecting to the Service Tool .......................................... 82
Part 6: Maintenance ..................................................... 83
6.1. Maintenance Reminders............................................... 83
Diagnostic tests failed ................................................. 83
Reminder: Please clean the scanner ....................................... 83
Test Films: Needed ................................................... 83
Preventive maintenance is required....................................... 83
6.2. Cleaning the Scanner ................................................. 84
6.3. Power-Cycling the System ............................................. 86
6.4. Running the Weekly Tests ............................................. 88
6.5. Resetting the Handheld Barcode Scanner ................................. 89
Part 7: Using DigitalNow and the R2 Patient ID System .................. 91
7.1. Overview .......................................................... 91
7.2. DigitalNow Archiving ................................................. 92
7.3. Using the R2 Patient ID System ......................................... 93
Querying an External Database .......................................... 95
7.4. Assigning R2 Barcodes in Other Databases ................................ 97
Assigning a Barcode in PenRad .......................................... 97
Assigning a Barcode in MagView......................................... 97
Assigning a Barcode in MRS ............................................ 97
Index .................................................................... 99
vi R2 DMax User Manual – PN MAN-00709 Rev 001
Page 9

Part 1: Introduction
f 1.1. Available Resources
f
1.2. How to Use this Manual
f
1.3. Warnings and Cautions
Part 1 provides a brief description of resources available from Hologic | R2,
information about using this manual, and safety information.
1.1. Available Resources
When you are working with the R2 DM system, the following resources are available
from Hologic | R2:
• Onscreen Messages: The R2 GreenLight™ user interface is designed to guide you
as you use the system. Onscreen messages appear when appropriate and provide
friendly instructions.
• Technical Support and Service: For contact information, see ‘Technical Support’
on the back of the title page of this manual.
•
User Manuals: This user manual describes the use of the R2 DM system and
provides maintenance and troubleshooting instructions in a simple, streamlined
format. You can purchase additional copies of the user manual through your
Hologic | R2 Account Manager.
•
Customer Bulletins: Hologic | R2 is committed to making our products safe and
easy to use. If an issue arises or new features become available, you will receive a
Customer Bulletin.
•
Training: On-line training materials are available through the R2 Member Center,
which is available at http://www.r2tech.com/main/member_login.php. Simply
register for access to a wide range of training courses on v8.5 software. In addition,
the Hologic | R2 Applications team is available to train your staff, should you feel
they need additional training. Contact your Hologic | R2 Account Manager if you
want personalized instruction.
R2 DMax User Manual – PN MAN-00709 Rev 001 1
Page 10

Part 1: Introduction
1.2. How to Use this Manual
This manual was written to ensure safe and proper use of the system. Before use, read
this manual carefully in order to realize the full capabilities of the system. If
something is unclear during daily use or if a problem occurs, please refer to this
manual.
Manual Organization
This manual is organized as follows:
f
Part 1: Introduction provides a list of resources available from Hologic | R2,
information about using this manual, and safety information.
f
Part 2: Description provides background information on the system, component and
feature descriptions, an overview of operation, and CAD processing information.
f
Part 3: Studies provides instructions for working with films, scanning them,
displaying study results, verifying and labeling images, and searching for a study.
f
Part 4: Alerts provides a listing of alert conditions with instructions for resolving the
alerts and diagnosing and solving common problems.
f
Part 5: Controls provides instructions for using the system configuration and
maintenance options that appear on the Controls screen.
f
Part 6: Maintenance provides instructions for cleaning the system and running the
weekly tests.
f
Part 7: Using DigitalNow and the R2 Patient ID System provides information about
archiving images with the R2 DigitalNow™ feature and the R2 Patient ID system.
Conventions Used in this Manual
This manual uses the following conventions to provide technical and safety
information of special interest.
Note: Background information provided to clarify a particular step or procedure.
Important: An instruction provided to ensure correct results and optimal
performance.
Caution: An instruction that, if not followed, can result in damage to the system.
WARNING! An instruction that, if not followed, can result in a hazardous condition.
Also note the following conventions:
• Italic and
engage,’ ‘
• When this manual directs you to touch a button on the touch-screen monitor, the
name of the button is shown in boldface type; for example, ‘Touch
boldface typefaces are used for emphasis. Examples: ‘Press carefully to
Do not press down.’
Start’.
• When this manual directs you to press a key on the computer keyboard, the key
names are outlined; for example, ‘Press
• If you are reading this manual online, click on the blue hyperlinked text to jump to
the referenced section.
2 R2 DMax User Manual – PN MAN-00709 Rev 001
[×Enter]’.
Page 11
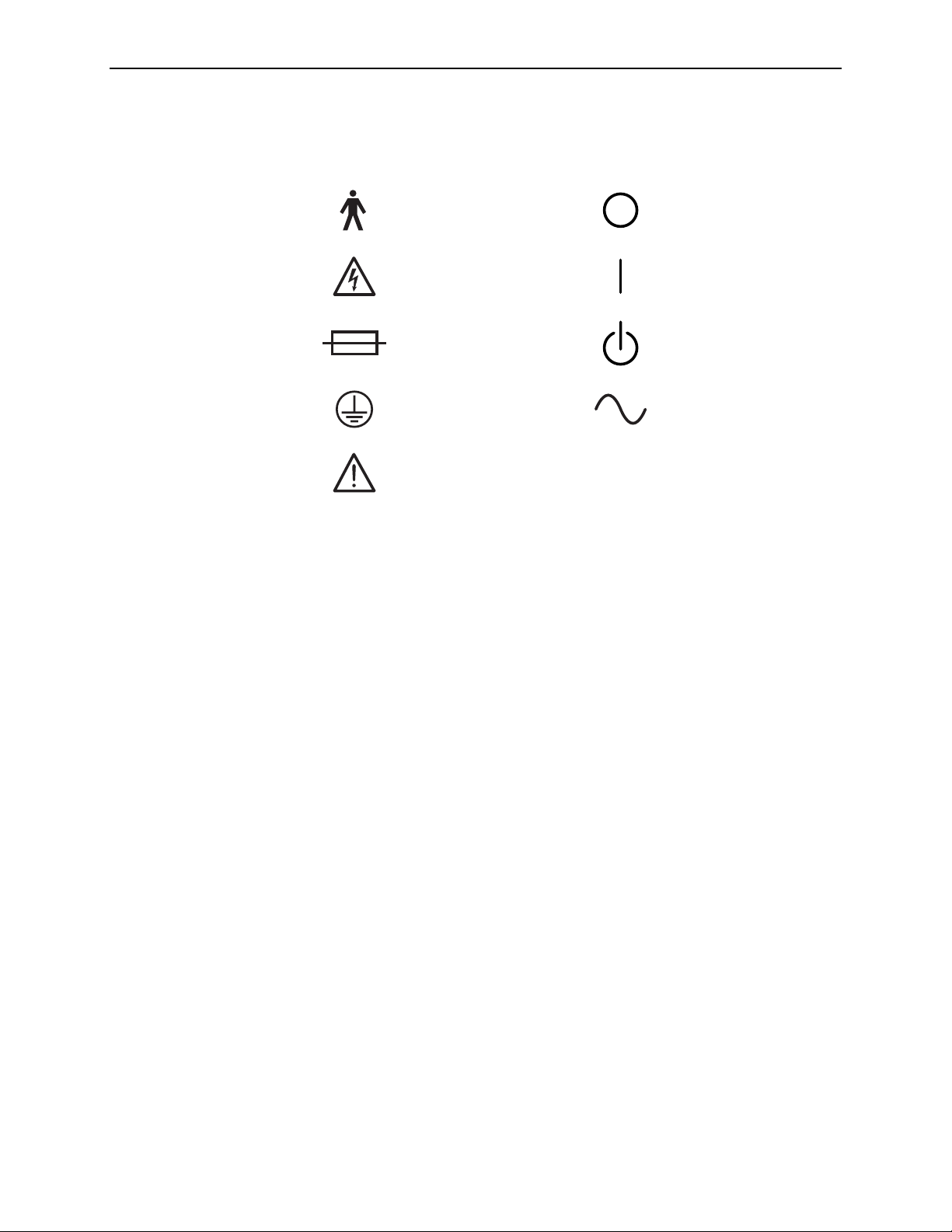
Symbols Used
The following internationally recognized symbols may be used in this manual and on
R2 products.
1.2. How to Use this Manual
Type B Equipment Off
OnDangerous Voltage
StandbyFuse
Alternating CurrentProtective Earth Ground
Attention: Consult accompanying documents, or
pay special attention to the note next to the symbol.
Common Symbols
R2 DMax User Manual – PN MAN-00709 Rev 001 3
Page 12

Part 1: Introduction
1.3. Warnings and Cautions
Radiological Interpretation
• The radiologist should base interpretation only on the original images and not
depend on the CAD marks for interpretation.
• The device is a detection aid, not an interpretative aid. Activate the CAD marks
only after the first reading.
• The device marks calcification features with triangles (Calc marks) and mass
features with asterisks (Mass marks). Where mass and calcification features
overlap within a specified distance (10-mm default), the device marks overlapping
features with pointed crosses (Malc™ marks). These features may not represent
cancer, and the skill of the user is still required for proper interpretation of areas
marked by the device.
• EmphaSize (variable-size) marks – Sites may choose to display prominence detail,
in which case the size of a Calc, Mass, or Malc mark is proportional to the ranking
of the feature by the algorithm. The marked features may not represent cancer,
and the skill of the user is still required for proper interpretation of areas marked
by the device.
• For proper system operation, the technical quality of the original films or images
(e.g., contrast) should meet relevant MQSA standards (or the appropriate national
standards) and be acceptable to the mammographer.
• The use of digitized images (scanned film images) for primary reading has not
been approved by MQSA. Digitized film images retrieved from archive should be
used only for the purpose of comparison with digital images meeting the current
standard.
• The device does not identify all areas that are suspicious for cancer.
– Some lesions are not marked by the device and a user should not be dissuaded
from working up a finding if the device fails to mark that site.
– The device is not designed to detect changes from prior mammograms.
– The device is not designed to detect skin thickening or nipple retractions.
– Conditions of the breast that diminish mammographic sensitivity, such as
density of normal tissue, also diminish the sensitivity of the device.
– The device is more sensitive for detection of calcifications than masses, and the
sensitivity depends on the site-specific operating points chosen. For sensitivity
values, see ‘
masses, the algorithm has a lower sensitivity for masses greater than 2.5 cm in
diameter.
– Individual practice patterns may influence results obtained when using this
device. Therefore, each facility and radiologist should carefully monitor the results
that this device has on their practice of mammography in order to optimize its
effectiveness.
• Safety and effectiveness have not been established for analyzing mammograms
from patients with breast implants. For patients with breast implants, only
CAD Operating Points’ on page 24. In addition to not marking all
4 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 13

Implant-Displaced Views can be processed by the system and only in cases with a
maximum of 2.5 cm (1 in) of the breast implant appearing on the image.
• The performance of the system has not been characterized for special diagnostic
views (e.g., magnified views or spot-compressed views). Process only full-view
diagnostic images.
• The performance of the system has not been characterized for segmented views of
the breast (e.g., ‘mosaic’ views) where there is not a clear breast border. Process
only views with breast borders.
Warnings: System Operation
• Use the system only outside the patient environment. The system is rated for use
only in an office environment.
• Remove all potentially obstructive jewelry and clothing before loading films in the
processing unit, to minimize the possibility of injury due to moving parts, or
damage to the system.
• The symbol next to the power connector indicates a potential shock hazard.
Ensure that the system is connected to a power receptacle that is properly
grounded and provides voltage and current within the specifications of the system
to reduce the likelihood of electrical shock or fire hazard.
1.3. Warnings and Cautions
• There is a small risk of electrostatic discharge (static electricity). It is mostly a
nuisance and not likely to cause harm. However, a shock can cause accidental
injury, if, for example, you suddenly withdraw your arm and hit it against
something. To minimize possibility of shock, maintain greater than 40% relative
humidity, use shoes with natural-material soles, place equipment on nonpolymer
floors such as concrete, wood, or floors treated with static dissipative, and/or
routinely touch metal with a metal object, such as a key, to painlessly discharge the
charge from your body.
• Do not place liquid containers on the device. In the event of a spill, shut down
power to all components prior to cleaning to minimize the possibility of electrical
shock. Do not operate the device if internal components are exposed to liquid.
Contact your service representative.
Warnings: Maintenance
• Barcode scanners used with the system are Class I Laser/LED products – do not
stare into the beam.
• Before cleaning the computer, always shut down the system according to the
shutdown procedures in this user manual and disconnect the power cord to
prevent the possibility of electrical shock. Never use alcohol, benzene, thinner, or
other flammable cleaning agents.
• Never use a two-prong plug or extension cord to connect primary power to the
system. Use of a two-prong adapter disconnects the utility ground and creates a
severe shock hazard.
• The R2 scanners are heavy. It is recommended that two people participate when
moving equipment.
R2 DMax User Manual – PN MAN-00709 Rev 001 5
Page 14

Part 1: Introduction
Cautions: System Operation
• Operators should review this user manual and receive training before using the
system.
• To ensure optimal system performance, perform the system verification tests and
scanner maintenance procedures as instructed in this user manual.
• To ensure proper system operation, use only separator sheets and barcodes
provided by Hologic | R2.
• To properly identify patients in the archive system, be sure to enter the patient
information and the barcode from the separator sheet into the patient
identification system correctly.
• For sites with multiple processing systems: When entering data into the R2 Patient
ID web page, check the name of the processing unit on the web page to ensure that
you are entering the patient information into the processing unit that you will use
to scan the case.
• For the display images to correspond correctly to the film position at the display
unit, be sure to orient and order the films correctly when scanning, as described in
this user manual.
• Ensure that the case retrieved from the archive to be used for comparison is from
the same patient as the current case under review. It is recommended that you use
the patient flash for this confirmation.
• Use only standard mammographic film: 18 × 24 cm or 24 × 30 cm.
• Do not attempt to scan films that have labels or tape with edges within 1 mm of
the edge of the film, or labels that are not pressed flat, or have curled corners, as
they may jam.
• Do not attempt to scan bent, damaged, or curled films, as they may jam.
• Always shut down the system according to the procedures provided in this user
manual. Failure to shut the system down properly can cause loss of data or damage
to the computer operating system.
• This equipment has been tested and found to comply with the limits for a Class A
digital device, pursuant to Part 15 of the FCC Rules. These limits are designed to
provide reasonable protection against harmful interference when the equipment is
operated in a commercial environment. This equipment generates, uses, and can
radiate radio frequency energy and, if not installed and used in accordance with
the instruction manual, may cause harmful interference to radio communications.
Operation of this equipment in a residential area is likely to cause harmful
interference, in which case the user will be required to correct the interference at
her or his own expense.
Note: The device design and mode of operation are consistent with current standard
mammography clinical practices, as governed by the requirements of the Mammography
Quality Standards Act (MQSA) of 1992. Users are cautioned to comply with the MQSA or
the appropriate national standards, when implementing R2 systems in clinical protocols.
6 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 15

Cautions: Installation and Maintenance
This product contains no user-serviceable parts. To prevent damage to the system:
• Maintain equipment in a well-ventilated, air-conditioned environment.
• When necessary, shut down the system according to the procedures recommended
in this user manual.
• Do not attempt to install or repair the R2 system. Only trained personnel,
authorized by Hologic | R2, are qualified to install or repair the system.
For service training, contact your technical service representative or call Hologic | R2
Technical Support (see ‘
manual).
Technical Support’ on the back of the title page of this
1.3. Warnings and Cautions
R2 DMax User Manual – PN MAN-00709 Rev 001 7
Page 16

Part 1: Introduction
8 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 17

Part 2: Description
f 2.1. Overview
f
2.2. System Components
f
2.3. Overview of Operation
f
2.4. System Features
f
2.5. ImageChecker Film-Screen CAD
f
2.6. System Inputs and Outputs
Part 2 provides an overview of the system, a description of system components, an
overview of system operation and features, and information about CAD processing.
2.1. Overview
The R2 DM system is a full-featured mammography film-scanning platform designed
to:
• Scan mammography x-ray films and convert them into digital image files
• Analyze the images using R2 Technology’s proprietary ImageChecker® CAD
algorithm to detect regions of the breast that may be cancerous
• Allow users to review the scanned images, and to reorient, label, and reprocess the
images as needed
• Interface with mammography reporting systems to retrieve patient information,
which can then be integrated with the study images
• Transmit the study results to an output device such as an R2 CheckMate™ Ultra
display unit or PACS where they can be viewed or archived
Note: This manual uses the following terms to describe mammography images and
media:
• Case: a series of x-ray films associated with a particular patient.
• Stack: a group of cases including a separator sheet on top of each case.
• Study: a series of digitized images associated with a particular patient.
R2 DMax User Manual – PN MAN-00709 Rev 001 9
Page 18
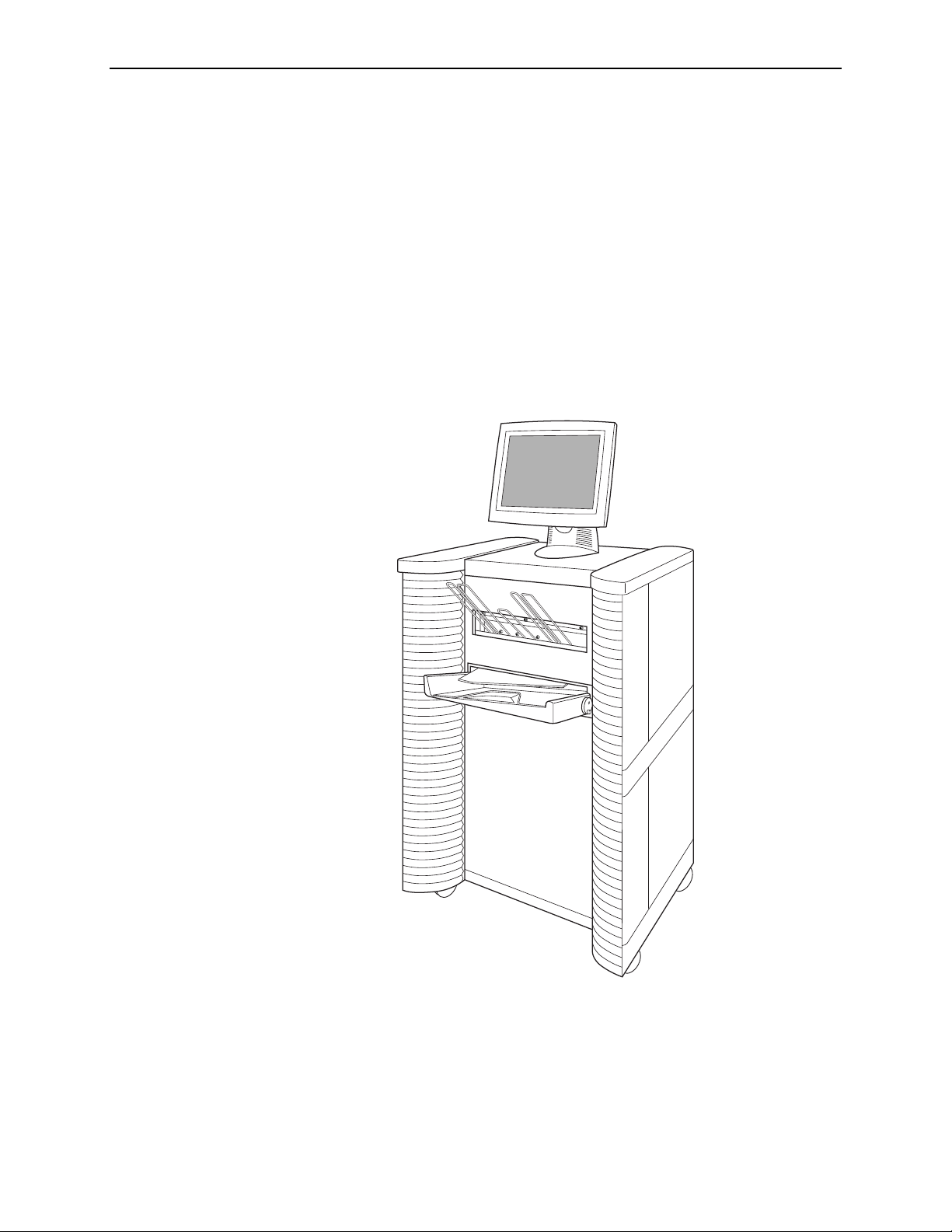
Part 2: Description
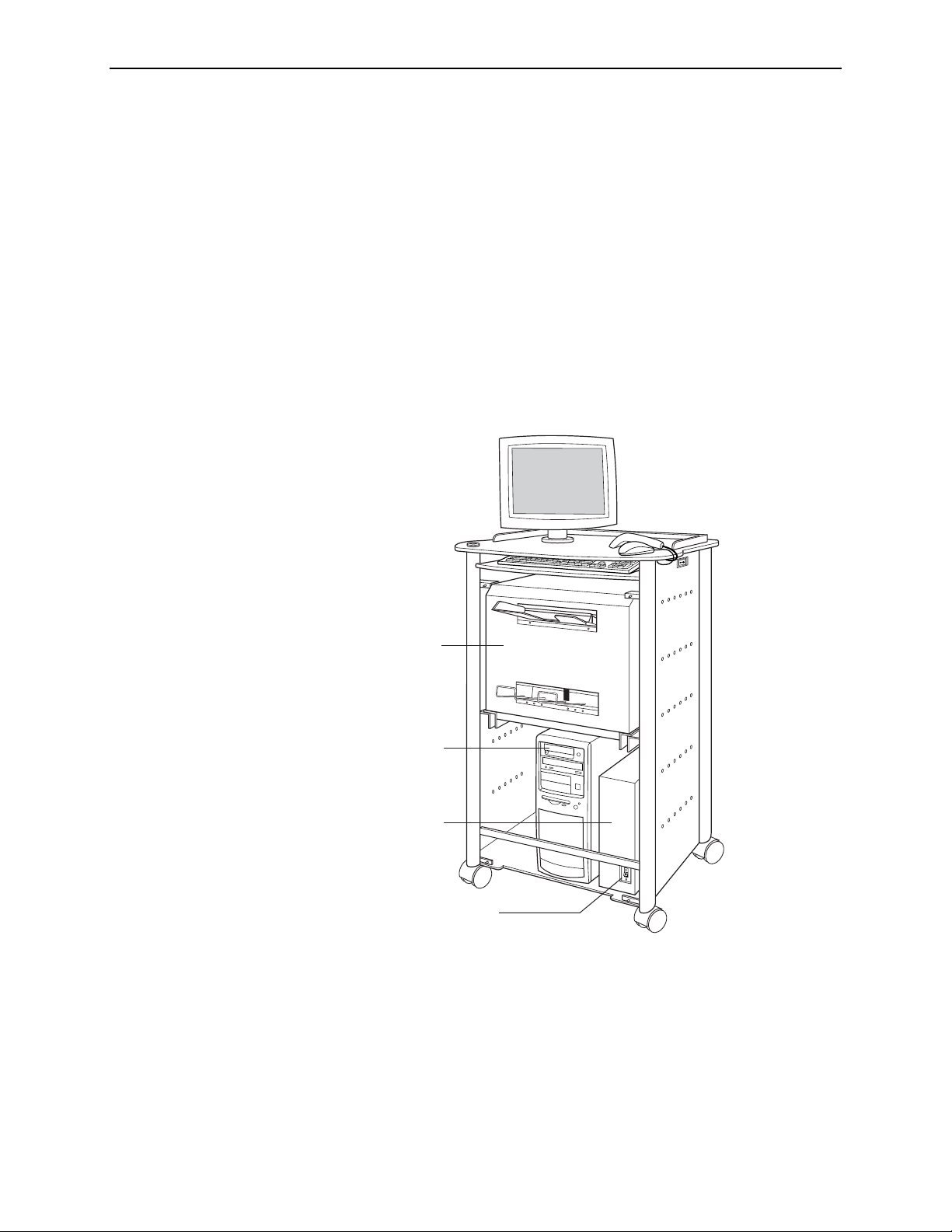
The R2 DM system consists of a touch-screen monitor, a handheld barcode scanner, a
keyboard, a film scanner, and a computer that performs the ImageChecker CAD and
DigitalNow processing, all housed in a movable cart.
When using the system, the technologist loads a stack of cases into the film scanner.
Each case can include from 1 to 24 x-ray mammography films. The technologist
inserts a barcoded R2 separator sheet before each case. When the technologist touches
the Start button on the touch screen, the system begins scanning the films, converting
each film into a digital image, and starting a new case each time it encounters a
separator sheet. After each case is scanned, the technologist has the option to review
the case and verify that the films were scanned correctly. Then the system sends the
images or CAD results to output devices such as display units, PACS or softcopy
review workstations.
10 R2 DMax User Manual – PN MAN-00709 Rev 001
R2 DM System
Page 19
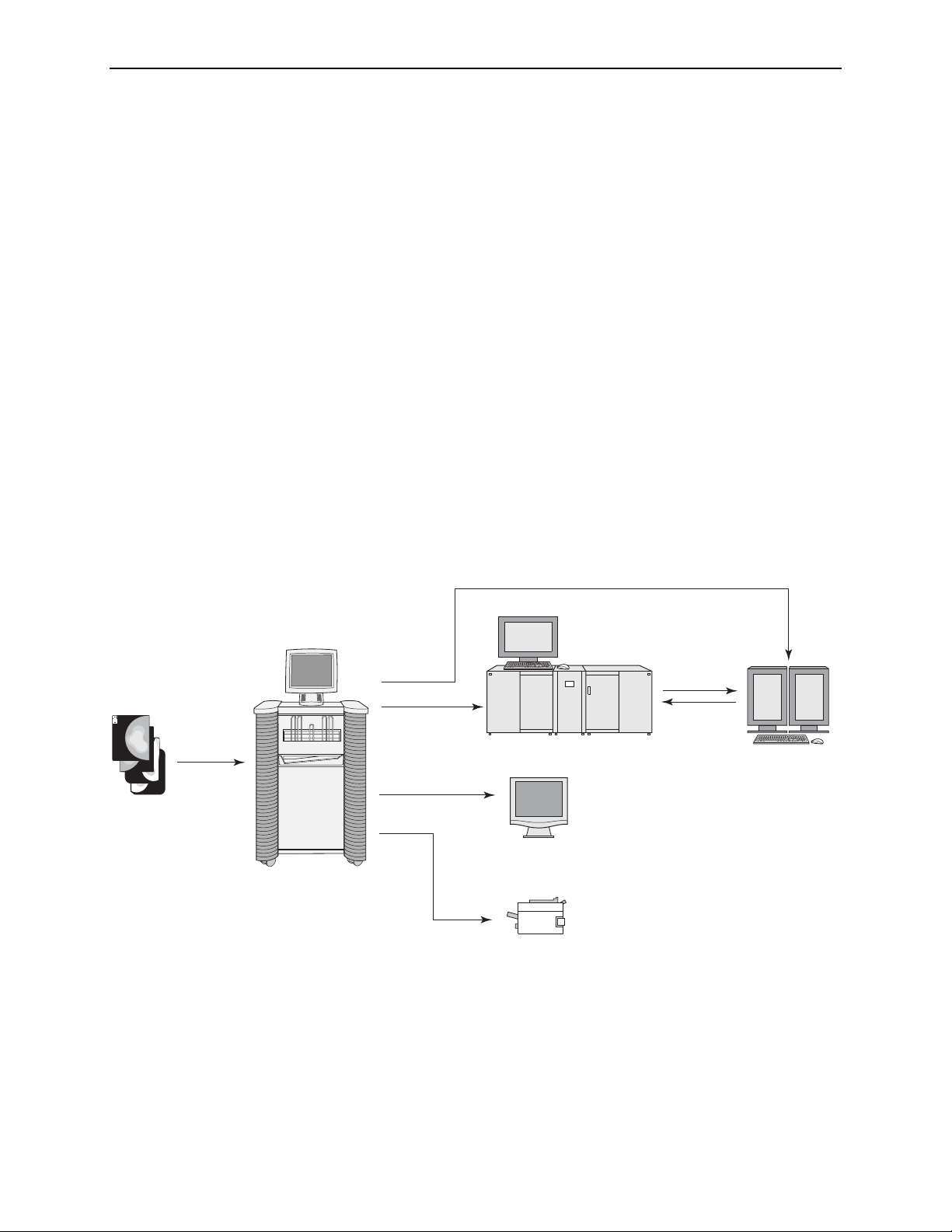
2.1. Overview
If the system is configured with the ImageChecker CAD license, it analyzes the
scanned images with a proprietary algorithm (i.e., a set of criteria) that identifies
regions of interest, which can include clusters of bright spots (suggestive of
calcification clusters), and dense regions with or without radiating lines (suggestive of
masses or architectural distortions). The system generates CAD marks identifying the
regions of interest. Images with CAD results can be sent to an R2 display unit, PACS,
review workstation, or printer.
After making an initial interpretation from the original mammograms, the radiologist
displays the CAD marks and chooses whether or not to reinspect the marked regions
on the original mammogram. The ImageChecker algorithm marks visually
perceptible structures that have some of the generally accepted geometric
characteristics of calcifications or masses. The marked areas may be something other
than an actual abnormality, which is generally recognized by the radiologist upon a
second review of the original mammogram.
R2 software version 8.5 now includes the ability to send images and/or CAD results to
virtually any DICOM-conformant workstation and/or archive (using R2
Technology’s DigitalNow feature), allowing CAD results to be displayed, stored, and
managed universally.
Films
For more information about the CAD marks and the ImageChecker algorithm, see
‘
2.5. ImageChecker Film-Screen CAD’.
PACS
Review Workstation
CheckMate Ultra
R2 DM
Film-Scanning Platform
Printer
System Diagram
R2 DMax User Manual – PN MAN-00709 Rev 001 11
Page 20
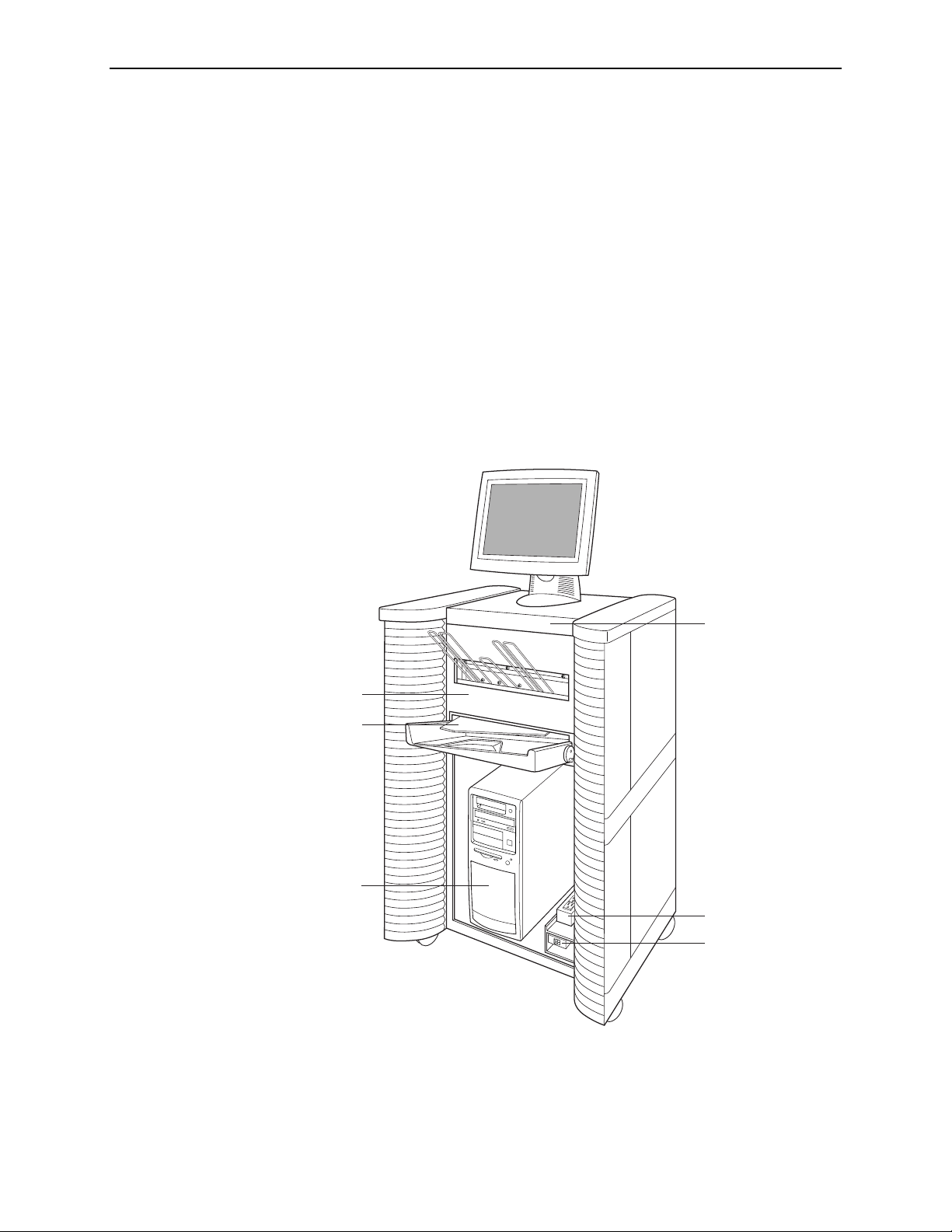
Part 2: Description
2.2. System Components
This section describes the major system components.
•
Touch-Screen Monitor: Use to control and monitor the system (start scanning,
select studies, view system status, etc.).
Scanner: Use to convert the mammogram films to digital images. You stack the
•
films and place them in the input tray. After the scanner digitizes the images, it
stacks the films in the output tray. The DM system scans each film in about
60 seconds.
•
Barcode Scanner: Use to select a study to display by scanning a barcoded patient
ID or the barcode on a separator sheet.
•
Keyboard: Use to type search criteria when searching for studies, and to enter
patient information.
•
Mouse: Use to select screen objects as an alternative to the touch screen.
Scanner
Interlock
Computer
Scanner
Control Panel
(closed)
Power Strip
Power Switch
Exterior Components
12 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 21
2.2. System Components
• Separator Sheets: The separator sheet provides a unique R2 ID number (a 10-
digit barcode) that the system reads and uses to identify each case and associate the
scanned images with the patient’s films. Each case fed into the R2 system has its
own separator sheet.
•
Test Sheets and Films: Use for the weekly tests to ensure optimal system
performance. For more information, see ‘
•
Lead Markers: Read by the R2 system to identify standard views automatically. For
more information on lead markers, see ‘
6.4. Running the Weekly Tests’.
R2-Supported Lead Markers’ on page 30.
The following components are located behind the front cover. You’ll only need to
access these components for certain maintenance procedures.
•
Computer: Processes the scanned films and images.
•
Scanner Power Supply: Provides power to the scanner.
Scanner
Computer
Scanner
Power Supply
Scanner Power Switch
Interior Components
R2 DMax User Manual – PN MAN-00709 Rev 001 13
Page 22
Part 2: Description
t
t
2.3. Overview of Operation
The system’s touch-screen interface provides quick access to all system functions. The
GreenLight user interface features three main screens –
– plus a common area with film scanning and search options at the right. You can go
to each screen by touching one of the tabs at the bottom of the display.
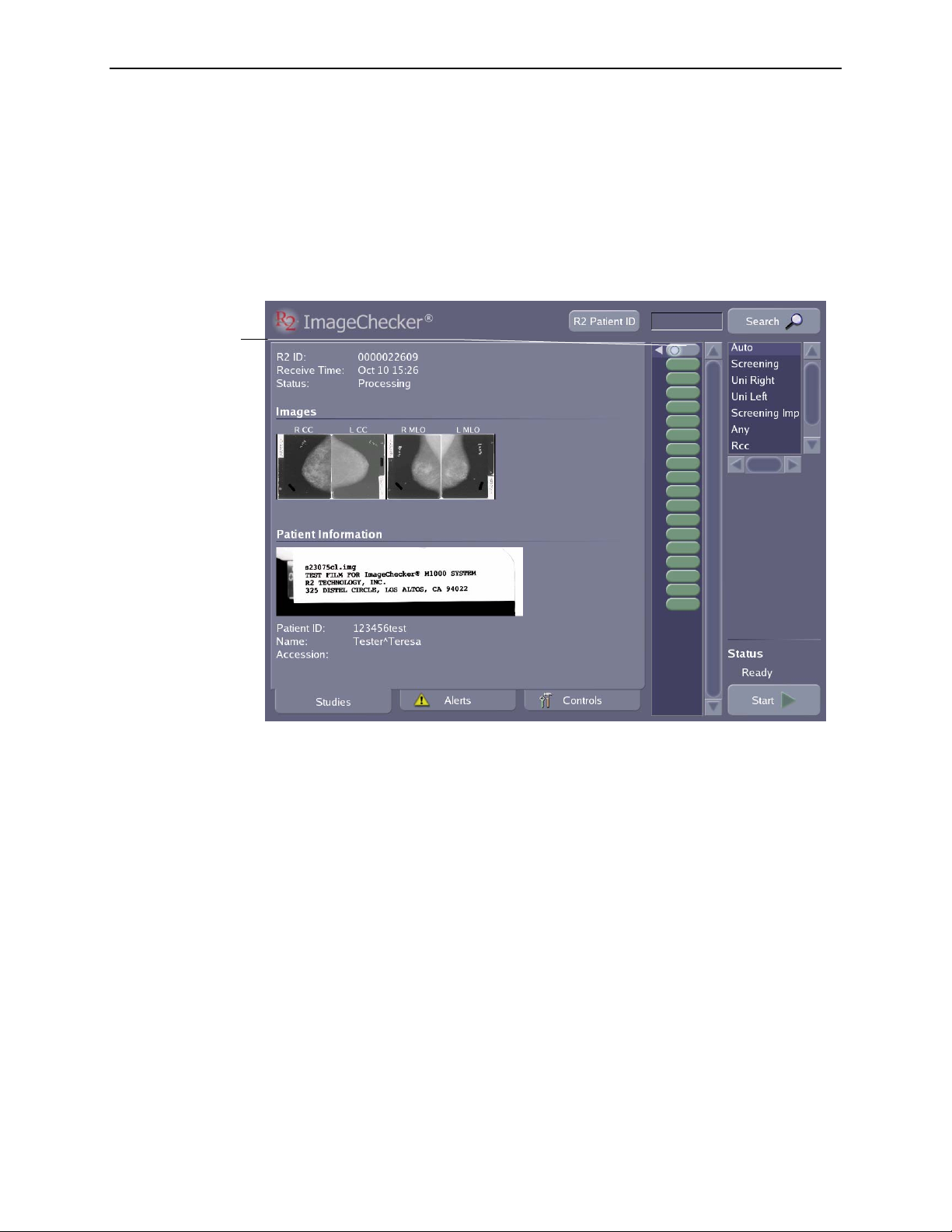
Studies Screen Overview
When scanning films, you will use the Studies screen, which is shown below:
The Study icon at
he top represents
the study currently
being processed or
he study most
recently processed.
The Study icons
below represent
studies processed
earlier.
The white triangle
indicates the study
icon linked to the
study information
currently on-screen.
Studies, Alerts, and Controls
The following items appear on the Studies screen:
•
Study icons each represent one study. Scroll the study icon list to view additional
studies. To display the results of any completed study, simply touch the icon for
the study. The appearance of the study icon indicates the status of the case; the
various icons that may appear are shown in the table on page
•
Study Information appears for each case of films as they are scanned and
16.
processed. The Study Information includes the R2 ID, Receive Time (the time
scanning began for the study), and current Status, as well as thumbnail images of
the films and patient information discussed below.
• Thumbnail film
Images appear as they are scanned and can be viewed at any time
after the system scans the films.
•
Patient Information includes the patient flash from the scanned film and other
data associated with each study (in particular, data imported from a
mammography reporting system).
• The
R2 Patient ID button appears only if your system is configured to use the
R2 Patient ID system. Click the button to access the R2 Patient ID database. For
more information see ‘
7.3. Using the R2 Patient ID System’.
14 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 23

2.3. Overview of Operation
• The Search field allows you to type patient data or a barcode number and quickly
find the patient record. You can also activate the Search field with the handheld
barcode scanner.
• The
Scanning Protocol list allows you to select a scanning protocol that matches
the number and types of films in your cases. Includes R2 standard scanning
protocols and any custom protocols created on site.
• The
Start button is used to begin scanning and processing a stack of films. When
scanning begins, the button changes to Stop.
• The
Status field, which appears above the Start button, indicates the scanner
status, for example, ‘
Ready’, ‘Scanning’, etc.
R2 DMax User Manual – PN MAN-00709 Rev 001 15
Page 24
Part 2: Description
Scanning and Processing
To scan a case, you must first create a stack of films. The case at the top of the stack is
scanned first. You select a scanning protocol based on the type of cases you will be
scanning (see ‘
To scan films you simply:
• Arrange the films with a separator sheet on top of each case.
• Organize films and separator sheets in a stack.
• Place the stack in the film input tray.
3.1. Working with Films’ for more information).
• Touch the
Start button.
The scanner picks up the first separator sheet followed by the first film in the stack. A
new study icon appears at the top of the list, and a white circle on the icon flashes to
indicate that the system is scanning or processing the films. Each separator sheet
includes a barcode that the system uses to organize the results. When the system scans
the barcode on the separator sheet, the barcode number appears on the Studies screen
as the ‘R2 ID’. As the films are scanned, the system displays thumbnail images, the
patient flash, and any patient information linked to the R2 ID.
When the system finishes processing the case, the Status field changes to ‘Completed’.
A white arrow on the study icon flashes to indicate that the system is sending study
results to the display unit, PACS/workstation, or printer on your network.
Note: Before the study results are sent to the display/archive device, you may be
required to ‘verify’ that the system scanned the films correctly. This option is configured
on the Controls Outputs screen. For more information, see ‘
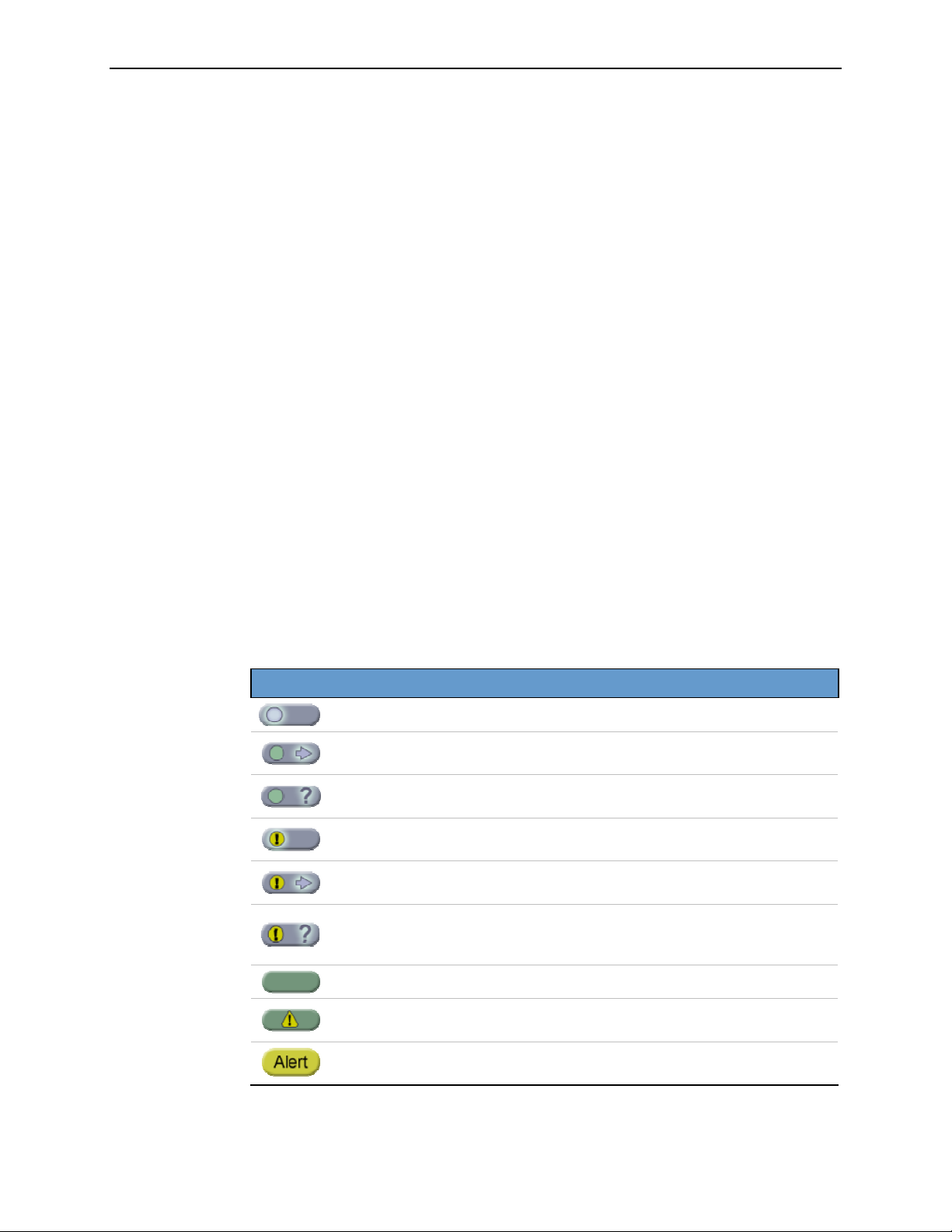
The appearance of the study icon indicates the status of the case:
Study Icon Status
(blinking circle)
(blinking arrow)
(blinking question mark)
(blinking exclamation point)
(blinking arrow)
(blinking question mark)
Case films are being scanned or processed.
Case results are being sent to a display or
archive device.
Scanned images for the case require
verification.
Case films are being scanned or processed, but
a non-fatal fault condition has occurred.
Case results are being transmitted, but a nonfatal fault condition has occurred.
Scanned images for the case require
verification, and a non-fatal fault condition
has occurred.
5.4. Outputs Options’.
For more about using the Studies screen, see ‘
16 R2 DMax User Manual – PN MAN-00709 Rev 001
Case is complete, results have been sent.
Case is complete, but a fault condition has
occurred that may require user intervention.
Case has failed. A fault condition has occurred
that requires user intervention.
Part 3: Studies’.
Page 25
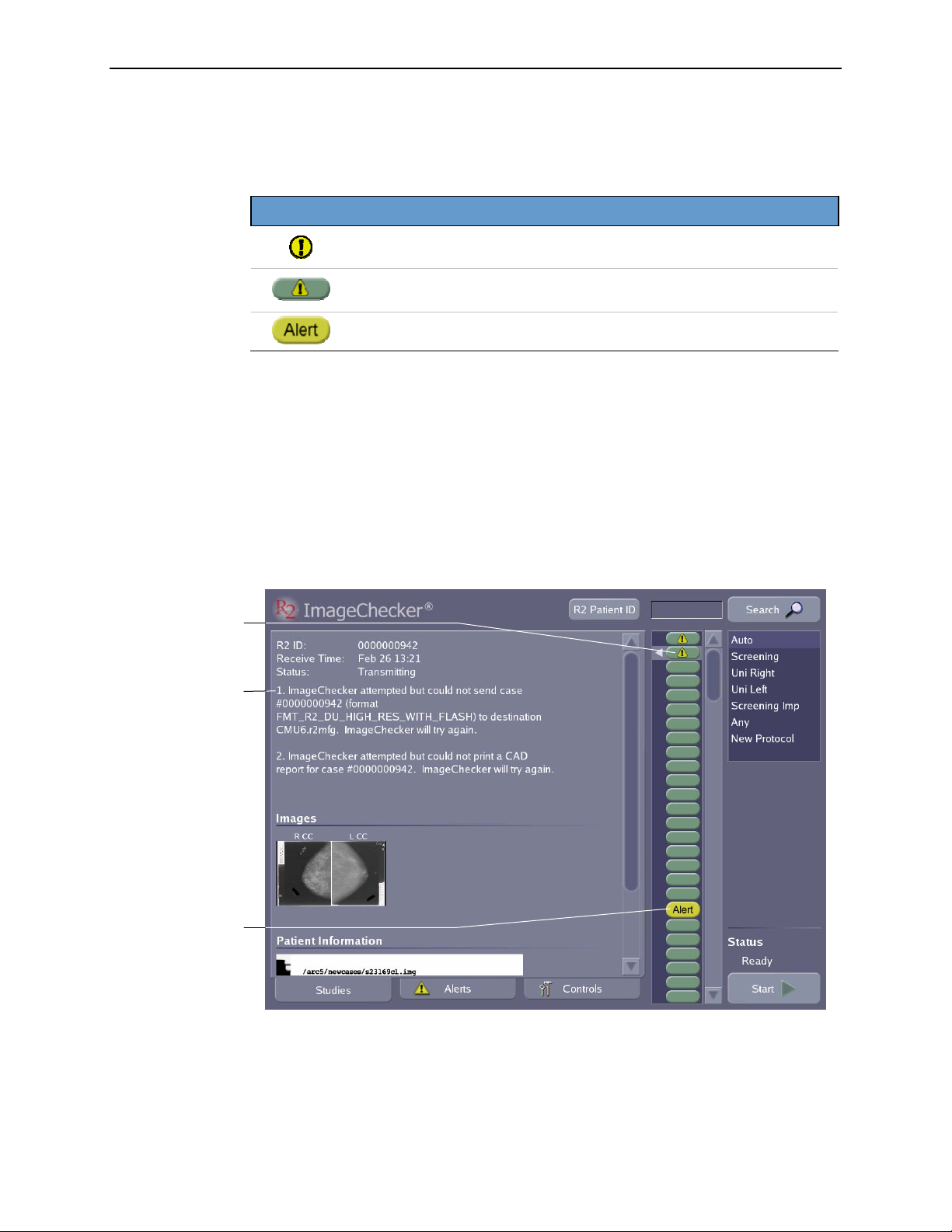
System Alerts
2.3. Overview of Operation
If a problem occurs during scanning or processing a case, or while transmitting case
results, the system issues an alert. There are three general types of alerts:
Alert Icon Status
A non-fatal fault condition has occurred. This alert appears on the case
study icons (see previous table).
The software has processed the images successfully, but a fault
condition has occurred that may require user intervention.
Case has failed, results have not been sent.
A description of the alert condition appears for the selected study in the area of the
screen below the study’s R2 ID.
The outcome of any alert condition depends upon the nature of the problem the
system has encountered. In the example shown below, the system is experiencing
problems as it attempts to send case results to a CheckMate Ultra display unit and to
a printer. If after repeated attempts it cannot transmit the case results, the system may
issue a ‘case-failed’ alert and change the study icon accordingly.
This study
generated a
non-fatal alert.
Alert description
This study
generated a
‘case-failed’ alert.
The system produces alerts for a variety of reasons. When necessary, the alert
description provides instructions for resolving the problem. In some cases you will
want to refer to ‘
Part 4: Alerts’ for more information about a particular alert.
R2 DMax User Manual – PN MAN-00709 Rev 001 17
Page 26
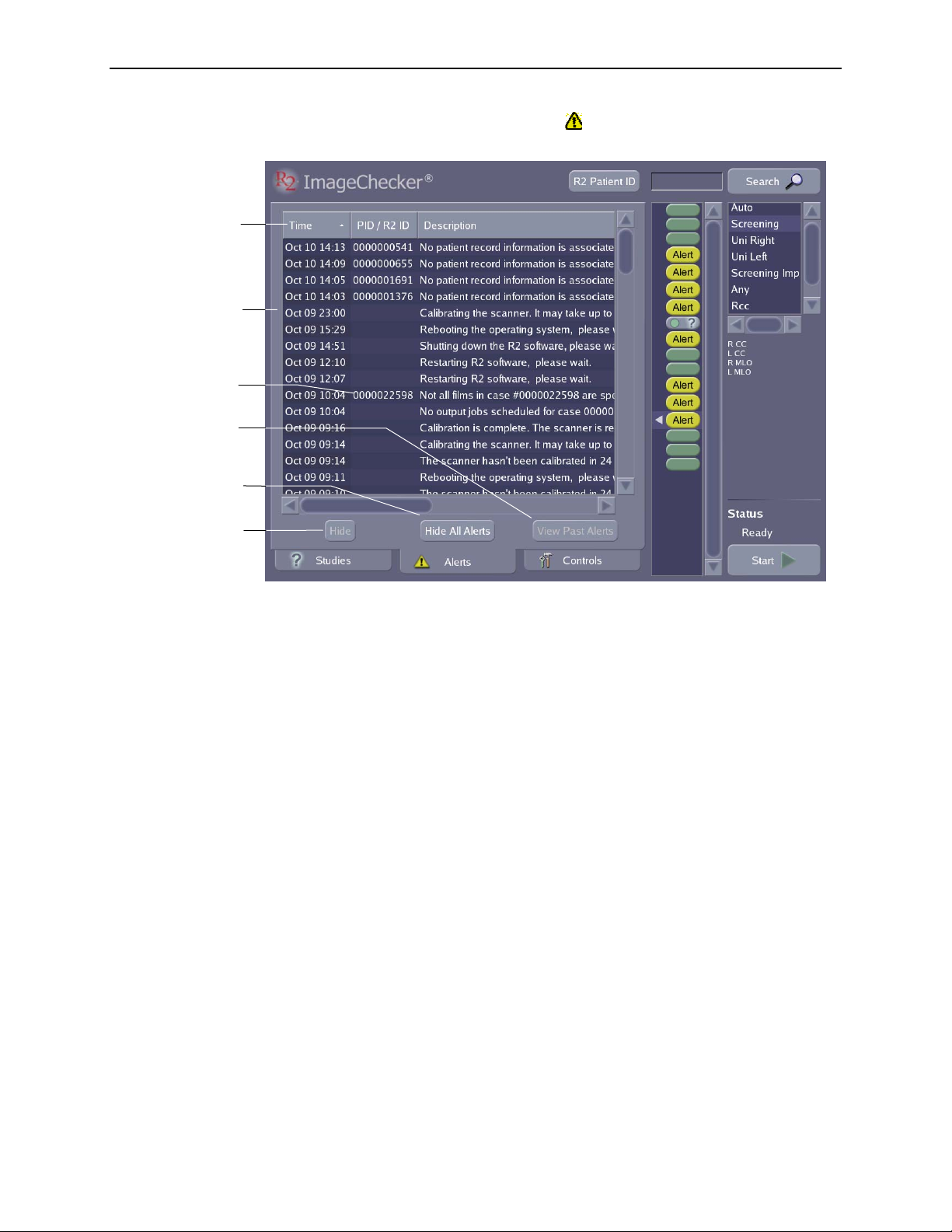
Part 2: Description
Touch to sort alerts
by Time, R2 ID, or
alert Description
Date and time alert
was generated
R2 ID
Touch to view or
hide alerts you’ve
hidden.
Touch to hide
all alerts.
When an alert is issued, the yellow alert icon ( ) appears on the Alerts tab at the
bottom of the screen. If you touch the
Alerts tab, you can see recent system alerts.
Touch to hide a
selected alert.
When reviewing alerts, you can use the Alerts screen to sort alerts by time issued,
R2 ID, or by description. You can also hide one or more alerts.
18 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 27
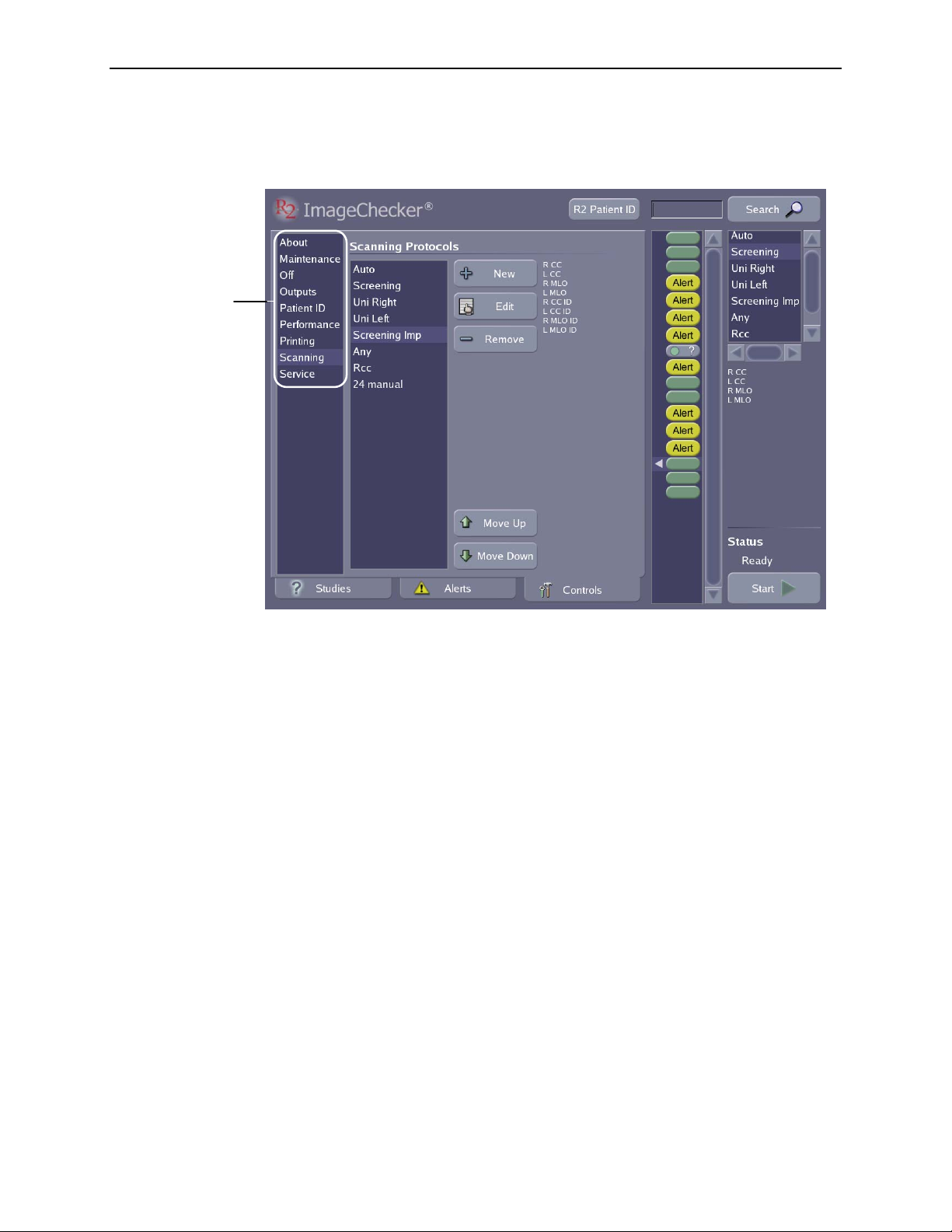
Controls Screen
Touch a screen
name to display its
contents.
2.3. Overview of Operation
The Controls screen is used to configure system settings to meet your particular
needs, run maintenance procedures, and display system information.
For details about the settings, utilities, and information on the Controls screen, see
‘
Part 5: Controls’.
R2 DMax User Manual – PN MAN-00709 Rev 001 19
Page 28
Part 2: Description
2.4. System Features
This section describes the principal features of the R2 DM system.
Feature Licensing Flexibility
The system features are determined by the licensed options selected by each site,
allowing you to invest only in the features you want, as well as add features at a later
date. The following features can be obtained by purchasing the appropriate license:
•
ImageChecker Film-Screen CAD
•
DigitalNow DICOM Export
•
Reporting System Interface
•
PeerView and PeerView Digital
These features are described further in the following sections.
ImageChecker Film-Screen CAD
The ImageChecker license provides R2 Technology’s Gold Standard CAD™
algorithm, which provides the medical-imaging industry’s highest sensitivity at any
given false-mark rate. The algorithm offers three operating points to accommodate
differing radiologist preferences. The performance of the system for each of the three
operating points as measured on R2 Technology’s film database is summarized below:
When your system is installed, you can select different operating points for
calcifications and masses. For more information, see ‘
CAD
’.
Note: Every time films are rescanned, the image created is subtly different. This
difference is of no consequence to a human observer, but slight rotation, shift, and
electronic noise differences will cause some CAD marks to vary with each rescan. This is
expected behavior.
Note: If the ImageChecker software has been upgraded to a newer revision since the
mammograms were first processed, the resulting CAD marks may be different if a newer
algorithm is used to process the cases.
Operating Point 0 1 2
Calcification Sensitivity 95% 96% 97%
Mass Sensitivity 83% 88% 90%
False Marks per Case 1.0 1.5 2.0
2.5. ImageChecker Film-Screen
DigitalNow DICOM Export
R2 film-scanning platforms can send digitized film images to a DICOM-conformant
archiving system for future review. Sites using full-field digital mammography can
scan prior studies and compare those priors with the current digital study on a
softcopy review workstation. Sites planning to move to digital in the near future can
plan ahead and send the digitized images to an archive system now, and retrieve them
for comparison later to current digital studies.
R2 systems configured with DigitalNow utilize the separator sheet barcode (‘R2 ID’)
for identifying the patient associated with the digitized film images. This allows for
20 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 29

2.4. System Features
the proper identification, storage, and retrieval of digitized film images in the archive
system.
The system can be configured to generate 50 or 100 micron output images, or both.
For digitized film images, the processing units perform dynamic window leveling on
each image, as well as blacken image areas that lie outside the film edges.
DigitalNow enables sites with ImageChecker CAD to archive CAD marks to PACS, if
desired. The CAD marks can also be printed and archived with other paper-based
patient records.
Important! From time to time, be sure to check images received at the review
workstation/PACS to confirm that films continue to be properly scanned and digitized.
Reporting System Interface
R2 DM systems can interface with PenRad, MRS, and MagView mammography
reporting systems. The R2 software retrieves patient information from the reporting
system database – such as the patient name, medical record number, and birth date –
and links this information to the patient images. Patient information can be printed
on the
(see page
CAD Results Report (see page 50), and on the Reimbursement Barcode Report
22).
To link reporting system records to R2 Technology images, you use a keyboard or
barcode scanner to enter the R2 ID from the separator sheet that will be used when
the system scans the films.
Important! Be sure to enter the R2 ID into the reporting system before entering it on
the R2 film-scanning platform.
PeerView and PeerView Digital
PeerView™ and PeerView™ Digital are optional features that help radiologists better
understand why a region of interest was marked. PeerView and PeerView Digital
display a close-up, high-resolution section of the image and highlight physical
features found by the algorithm, facilitating the radiologist’s reassessment of the
mammograms.
• PeerView is licensed on CheckMate Ultra display units and Mammolux motorized
viewers. When licensed, PeerView asks the R2 DM system to send the additional
high-resolution CAD information to the display unit.
• PeerView Digital is licensed on the DM system. When licensed, PeerView Digital
creates extra CAD information in the Mammography CAD SR output, viewable on
some softcopy review workstations. It displays a close-up, high-resolution section
of the image, highlights physical features found by the algorithm, and produces
measurements of the physical features.
R2 DMax User Manual – PN MAN-00709 Rev 001 21
Page 30

Part 2: Description
Other System Features
In addition to the licensed features, the R2 DM system includes the following
standard features:
•
Establish Patient Identification
•
Automatic Film Marker Identification
•
Reimbursement Barcode Report
These features are described further below.
Establish Patient Identification
Each case fed into the R2 film-scanning system must have its own separator sheet.
The R2 system uses the barcoded R2 ID on the separator sheet to associate the patient
films with the images in the system.
When processing a patient’s films, you write the R2 ID from the separator sheet on
the patient file. If using a mammography reporting system, you also enter the R2 ID
into the appropriate field in the patient’s reporting system record.
Important! It is a good idea to record the R2 ID on the outside of the patient film
jacket. In addition, it is critical that ImageChecker CAD users keep the separator sheet
with the films until after the radiologist has read the films and CAD results.
Automatic Film Marker Identification
If your four, standard-view films were processed using R2-supported lead markers,
and the markers are well-placed, you can scan the films using Auto film detection
mode. This means your cases can consist of any number of films (up to four), and
you can place them in the scanner in any orientation (e.g., rotated or emulsion side
up or down) and in any order.
This timesaving feature enables sites to scan as many cases as possible in as short a
time as possible. The lead markers must be a supported type, they must not be
attached with putty or other opaque substances, and they must be placed properly (as
per MQSA standards). R2-suported lead markers are available from Hologic | R2,
Livingston Products, Siemens, Techno-Aide, or All-Craft Wellman. For the supported
marker types, see ‘
R2-Supported Lead Markers’ on page 30.
Reimbursement Barcode Report
The Reimbursement Barcode Report is a listing of all separator sheet barcode
numbers scanned in a specific time period. You can schedule it to run monthly,
weekly, or on demand for a specified date range.
The report lists the date, the cases processed on that date, and the time the case was
processed. This can be correlated to the patient using the R2 barcode number written
in the patient file, thereby completing the reimbursement audit trail.
The report lists only cases that were successfully. When using a mammography
reporting system, additional patient information can be listed with each report entry.
For an example printout, see ‘
Printing a Reimbursement Barcode Report’ on page 76.
22 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 31

2.5. ImageChecker Film-Screen CAD
When configured with the ImageChecker CAD license, the R2 DM system applies
R2 Technology’s Gold Standard CAD software algorithm to mammography images in
order to detect regions of the breast that may be cancerous. ImageChecker CAD
identifies and marks regions of interest that may warrant a second review by the
radiologist, thereby helping to minimize observational oversights.
R2 Technology’s ImageChecker CAD software was the first computer-aided detection
(CAD) software approved by the FDA for full-view diagnostic and screening
mammograms. Since that first approval, the ImageChecker software has been refined
many times, resulting in an algorithm that is highly sensitive with few false marks.
This section describes features available for systems configured with the
ImageChecker Film-Screen CAD license.
Intended Use
The R2 DM system, when configured with the ImageChecker Film-Screen CAD
license, identifies and marks regions of interest on routine screening and diagnostic
mammograms to bring them to the attention of the radiologist after the initial
reading has been completed. The system assists the radiologist in minimizing
observational oversights by identifying areas on the original mammogram that may
warrant a second review.
2.5. ImageChecker Film-Screen CAD
CAD Marks
EmphaSize
The ImageChecker software provides three types of CAD marks (Mass, Calc, and
Malc) that can appear in the results. Each mark identifies a region of interest for the
radiologist to review.
Calc – Marks regions suggestive of calcifications.
Mass – Marks regions suggestive of masses or architectural distortions.
Malc – Composite mark indicates an overlap of Calc and Mass marks.
ImageChecker CAD also includes EmphaSize™ variable-size marks. The feature
allows the display unit or review workstation to display marks of variable size that
correlate to feature significance. When the algorithm determines that a region is more
significant, the CAD mark appears larger, indicating that the region should receive
more emphasis from the radiologist.
R2 DMax User Manual – PN MAN-00709 Rev 001 23
Page 32
Part 2: Description
CAD Operating Points
The ImageChecker software allows each site to choose between three different
operating points (i.e., CAD algorithm thresholds): operating point 2, which
emphasizes sensitivity, operating point 0, which emphasizes a low false-mark rate,
and operating point 1, which is an intermediate point.
The table below gives the sensitivity and false-mark rate values for the three operating
points for version 8 ImageChecker applications, as measured on R2 Technology’s
large film test database of biopsy-proven malignancies and confirmed normal cases
for the four standard views (RCC, LCC, RMLO, and LMLO).
Operating Point: 2 1 0
Calcification Cases (n = 588)
Mean Sensitivity1
95% Confidence Interval
97%
95.5–98.3%
96%
94.0–97.2%
95%
93.1–96.7%
Mass Cases (n = 767)
Mean Sensitivity
95% Confidence Interval
Overall (n = 1355)
Mean Sensitivity
95% Confidence Interval
False-Positive Marks2
Calcification FP/Image
Mass FP/Image
Total False-Positive Marks/Case
Specificity2
95% Confidence Interval
1
1
90%
87.7–92.0%
93%
91.5–94.3%
0.16
0.35
2.0
24.5%
20.5–28.5%
88%
85.6–90.2%
91%
89.7–92.7%
0.12
0.25
1.5
35.1%
30.6–39.5%
83%
79.8–85.2%
88%
86.2–90.0%
0.09
0.17
1.0
48.3%
43.7–53.0%
1. ‘Sensitivity’ refers only to the sensitivity of the CAD algorithm, not to the sensitivity of the
radiologist using the device.
2. ‘False-Positive’ and ‘Specificity’ data were developed from a review of 445 confirmed
normal cases
refers to the average number of false-positive marks per image measured on normal cases.
‘Specificity’ refers to the percentage of normal cases that, when processed, show no CAD
marks.
(defined as those with a subsequent normal screening study). ‘FP/Image’
• Operating point 2 focuses on sensitivity. It gives the best performance for marking
regions of interest (i.e., CAD sensitivity), with a higher false-mark rate. It is suited
for sites that want the system to be as sensitive as possible, regardless of the higher
false-positive mark rate.
•
Operating point 1 represents an intermediate point. Improvements in the algo-
rithm have allowed points 0 and 2 to be set quite far apart, and some sites may
want intermediate behavior from the CAD algorithm.
•
Operating point 0 trades off a lower false-mark rate for a lower overall sensitivity.
This operating point is suited for sites that prefer the system to display the fewest
false marks.
You can choose a different operating point for calcifications than for masses. For
example, if you want high sensitivity for calcifications but a moderate balance
between sensitivity and false positives for masses, choose operating point 2 for
calcifications and operating point 1 for masses.
24 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 33
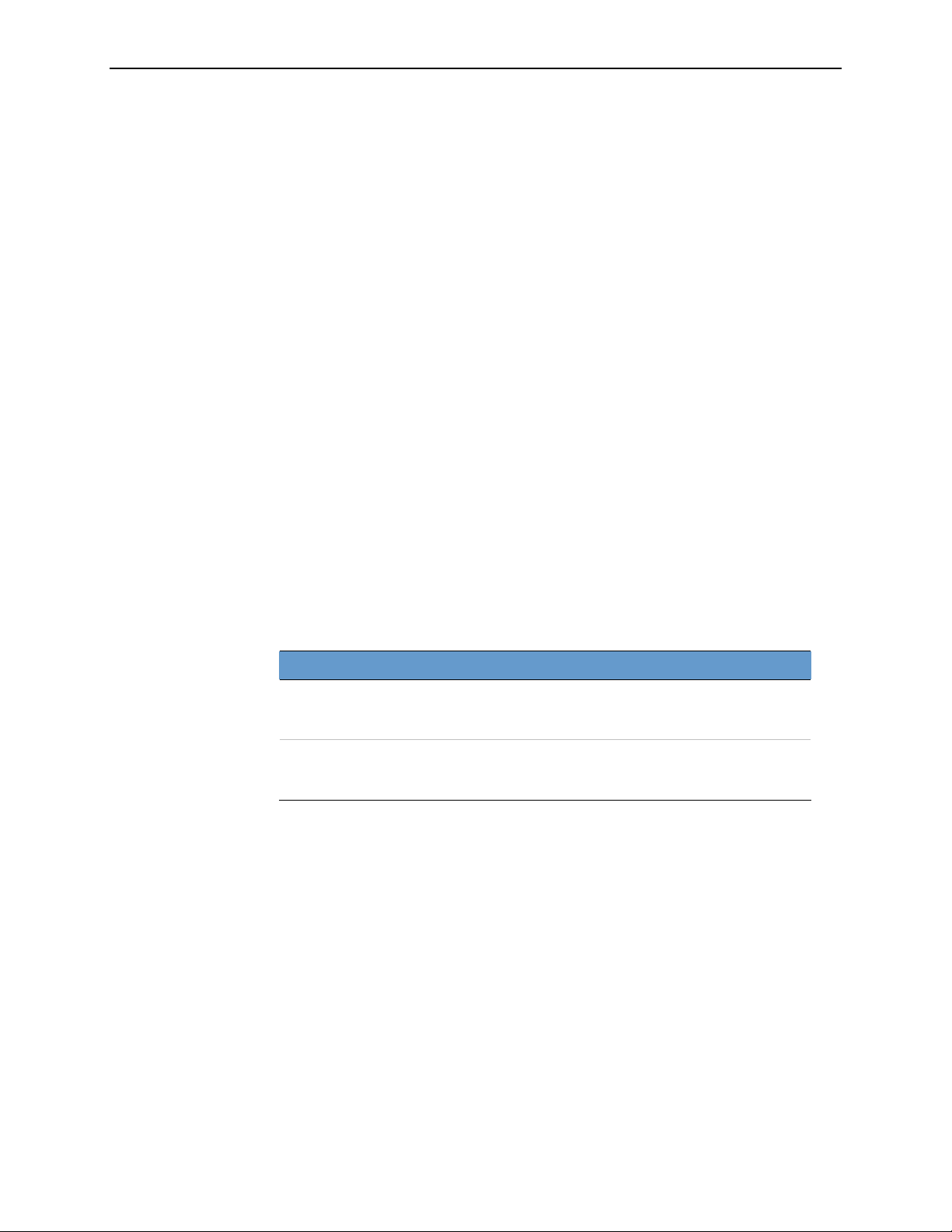
2.5. ImageChecker Film-Screen CAD
Each system is shipped with a default set of operating points for both masses and
calcifications. You can discuss the choices that are right for your site with your
Applications Specialist or, if you would like to change configurations, contact your
Hologic | R2 Technical Service Representative.
Note: To determine false-mark rates, R2 Technology processes normal screening
cases (defined as those with a subsequent normal screening study) through the
ImageChecker software and measures the number of false marks per image. More
extraordinary screening cases, such as those with additional views, and diagnostic
studies, may have a very different make-up of images and, as such, may produce results
that fall outside the measured normal case mark rate. Since the mark rate is measured
per image, a larger number of images in a case should correspond on average with a
higher total mark count for that case. However, while clinical experience demonstrates
some variation in mark rates, R2 Technology has not found the false-mark rate to vary
dramatically when averaged over a large number of cases.
Note: A recent revision to the DICOM standard has resulted in a nomenclature
change. The terms, ‘thresholds A, B, and C’, used in previous R2 Technology products
(software version 8.1 and earlier), have been replaced by ‘operating points 2, 1, and 0’.
Also note that earlier versions of the ImageChecker application (before version 8)
produce slightly different performance results. For further information, see the manuals
provided with those systems.
Number of CAD Marks
The software limits the number of CAD marks for each image and case. The actual
number of CAD marks produced depends upon the individual case and the operating
point selected for the CAD algorithm. Note that the specificity of the device at the
most sensitive operating point is no lower than 24.5% (24.5% of normal cases show
no marks).
Per Image Limit Per Case Limit
Standard four views
(RCC, LCC, RMLO, LMLO)
Extra views
*For cases with more than four views, the maximum number of marks per case
depends upon the number of images in the case (up to 24 images per case).
3 Calc marks
2 Mass marks
2 Malc marks
3 Calc marks
2 Mass marks
2 Malc marks
8 Calc marks
4 Mass marks
4 Malc marks
Dependent upon the
number of images*
R2 DMax User Manual – PN MAN-00709 Rev 001 25
Page 34
Part 2: Description
2.6. System Inputs and Outputs
R2 software version 8.5 supports up to 24 images per case. All scanned film images
can be viewed at a digital review workstation and/or archived on a PACS. Other
devices, such as the CheckMate Ultra display unit, can display up to four views per
case, if the views are supported by the display unit.
CAD-Supported Views
If your system has the ImageChecker license, all images are CAD-processed if the
views and any modifiers are supported by the software. The four ‘standard views’
processed automatically by the ImageChecker software are:
• LCC – Left Cranio-Caudal
• LMLO – Left Medio-Lateral Oblique
The Four Standard Views
• RCC – Right Cranio-Caudal
• RMLO – Right Medio-Lateral Oblique
In addition, systems with ImageChecker CAD can process the ‘equivalent’ and
‘reversed equivalent’ views, as long as they do not include unsupported view
modifiers (see next page). However, CAD results for certain views and view modifiers
cannot be displayed or printed on some output devices. The following table shows the
CAD-supported views and the output devices that can accept these views.
CAD-Supported Views
Standard View View Label
Cranio-Caudal CC z z
Medio-Lateral Oblique MLO z z
Equivalent View
Medio-Lateral ML z z
Exaggerated Cranio-Caudal XCC z z
Cranio-Caudal Exaggerated Laterally XCCL z z
Cranio-Caudal Exaggerated Medially XCCM z z
Reversed Equivalent View
Latero-Medial LM z —
Latero-Medial Oblique LMO z —
Cranio-Caudal From Below FB z —
Superolateral to Inferomedial Oblique SIO z —
z = Output supported to this device — = Output not supported to this device or format
DICOM
Workstation,
PACS
Display Unit
Printer,
CAD SC
26 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 35

2.6. System Inputs and Outputs
In the preceding table, ‘Display Unit’ refers to a CheckMate Ultra display unit or
Mammolux motorized viewer. These devices (and the R2 postscript printer) can
display (or print) no more than four views per case.
• When multiple images of the same view are present in a case, the last image of a
specific view will be sent to the display unit or printer.
• When a case includes multiple equivalent views, the system chooses the four views
sent to the display unit and printer based on the following preference order:
Preference
Order
1 CC MLO
2 FB* ML
3 XCC LM*
4 XCCL LMO*
5 XCCM SIO*
*Indicates a reversed view, not available for display units,
printouts, or CAD SC output.
CC Equivalent MLO Equivalent
View Modifiers
View modifiers can be added to describe any supported view, however, some
modifiers are not supported for CAD processing. Using a non-supported modifier
will prevent CAD processing a supported view. The following table lists the supported
DICOM view modifiers and indicates which are supported for CAD processing.
View Modifier Label View Modified CAD Support
Axillary Tail AT MLO z
Cleavage CV CC
Partial View (none) Any
Rolled Lateral …RL Any z
Rolled Medial …RM Any z
Rolled Inferior …RI Any z
Rolled Superior …RS Any z
Magnification M… Any
Spot Compression S Any
Implant Displaced* ID Any z
Implant Present (none) Any
Tangential TAN Any z
* Implant-displaced views with a maximum of 2.5 cm (1 in) of implant imaged.
Note: The Implant Present and Partial View modifier labels are NOT added to the view
description, but are present in the DICOM header.
R2 DMax User Manual – PN MAN-00709 Rev 001 27
Page 36
Part 2: Description
Digital Image Output Options
The R2 DM system can send files with any of the following formats to a PACS or
review workstation.
•
DigitalNow MG Image: This is a traditional mammography image format where
the image is comprised of Natural Pixel Values, the values actually created by the
film scanner.
•
DigitalNow ELC™ Enhanced MG Image: This image has been processed using R2’s
proprietary ELC algorithm to enhance contrast within local regions of the images.
This format was developed specifically to increase the conspicuity of low-contrast
microcalcification clusters.
•
Mammography CAD SR: This is the DICOM standard format for CAD results.
CAD SR files are generally useful only when viewed with the images on an
advanced FFDM or PACS workstation. PACS support for storing Mammography
CAD SR objects is increasing, and IHE support for Mammography CAD SR is
beginning to result in more PACS workstations being able to display
Mammography CAD SR content.
•
CAD SC Image: This is a Secondary Capture image object that includes the CAD
results burned into a montage of low-resolution images, similar to the images that
R2 sends to motorized viewers or CheckMate display units. This form of output is
useful for PACS that do not support CAD SR. Not supported for reversed views.
DigitalNow images can be created as either 50- or 100-micron image files. The file
sizes can depend upon the film size and resolution.
Film Size 50µ Image 100µ Image
18 × 24 cm 35 MB 8.6 MB
24 × 30 cm 50 MB 14.4 MB
28 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 37
Part 3: Studies
f 3.1. Working with Films
f
3.2. Preparing Cases to Be Scanned
f
3.3. Scanning with the Auto Protocol
f
3.4. Scanning with a Manual Protocol
f
3.5. Scanning with the Any Protocol
f
3.6. Adding Cases to the Stack
f
3.7. Stopping Scanning and Removing a Case
f
3.8. Viewing Study Information
f
3.9. Verifying Scanned Cases
f
3.11. Printing CAD Results
f
3.12. Searching for a Study
f
3.13. Using Queue Manager
Part 3 provides instructions for working with films, scanning cases, working with
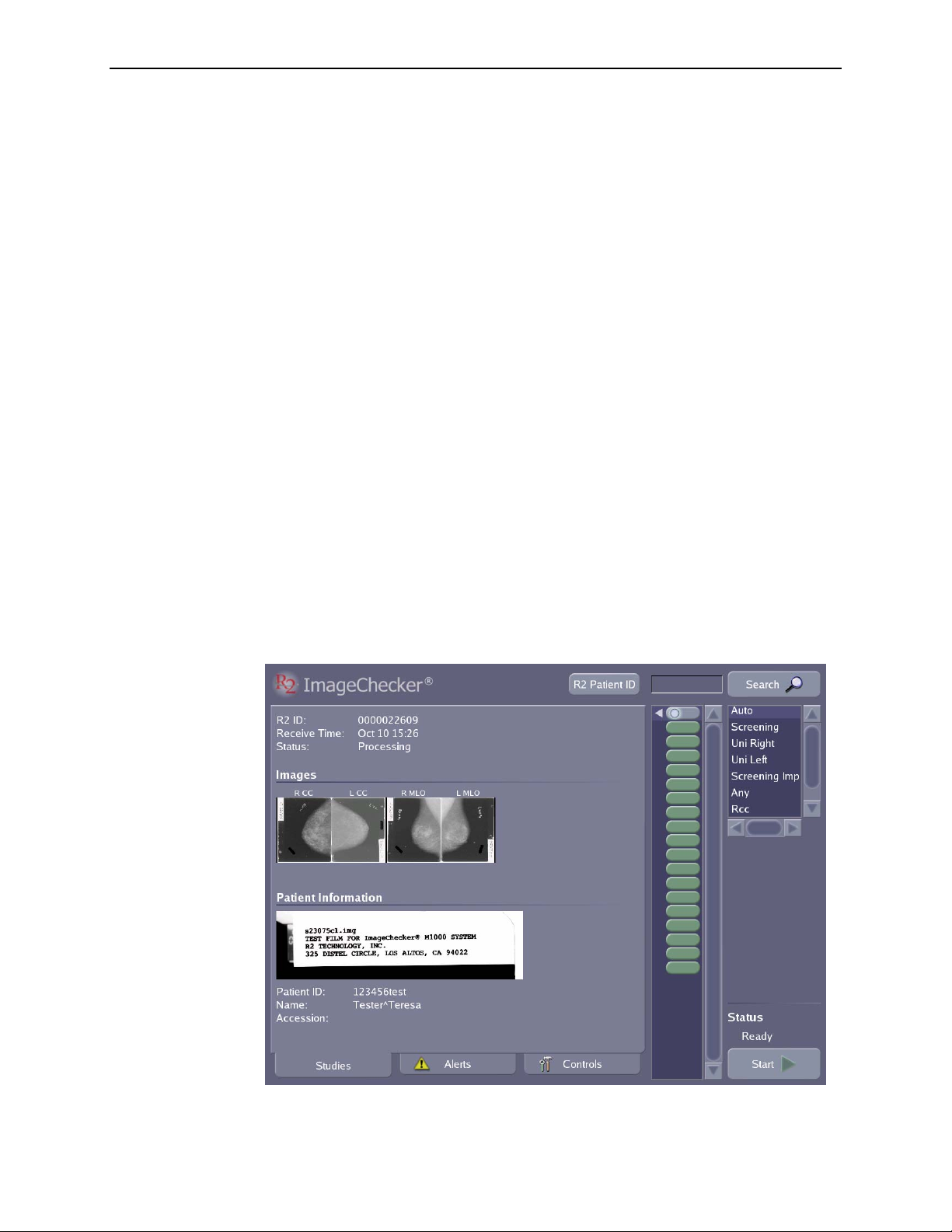
scanned images, displaying study results, and searching for a study.
For most operations you will be using the
Studies screen, which is shown below:
R2 DMax User Manual – PN MAN-00709 Rev 001 29
Page 38
Part 3: Studies
3.1. Working with Films
To use the system successfully, please read and understand the film requirements and
modes used to scan the films.
Film Requirements
All films must meet the following requirements:
• Original films only – no copies
• No bent, damaged, or curled films
• 18 × 24 cm or 24 × 30 cm standard full-field views
• No sticky residue, wax or grease pencil marks on films
• No labels over the edges of films, or curled labels
• MQSA or other required standards
Because the scanner uses suction cups to lift each film into the scanner, anything that
interferes with the suction cups, such as a patient label placed improperly or a wax
pen mark, may cause the scanner to drop films.
R2-Supported Lead Markers
The R2 film-scanning platform can scan any film-based mammograms that meet the
requirements listed above. However, when you use R2-supported lead markers with
the four standard views, there is an additional degree of convenience: you can load
the films in any orientation and in any order. (For more information, see ‘
Protocols
R2-supported lead markers are available from Livingston Products, Siemens, TechnoAide, All-Craft Wellman, and Hologic | R2. An illustration of the supported type from
each company is shown below.
’ on page 32.)
Livingston –
www.livingstonproducts.com
Siemens –
Scanning
www.siemens.com
30 R2 DMax User Manual – PN MAN-00709 Rev 001
Techno-Aide –
All-Craft Wellman – www.all-craftwellman.com
Hologic | R2 – www.r2tech.com
www.technoaide.com
Page 39
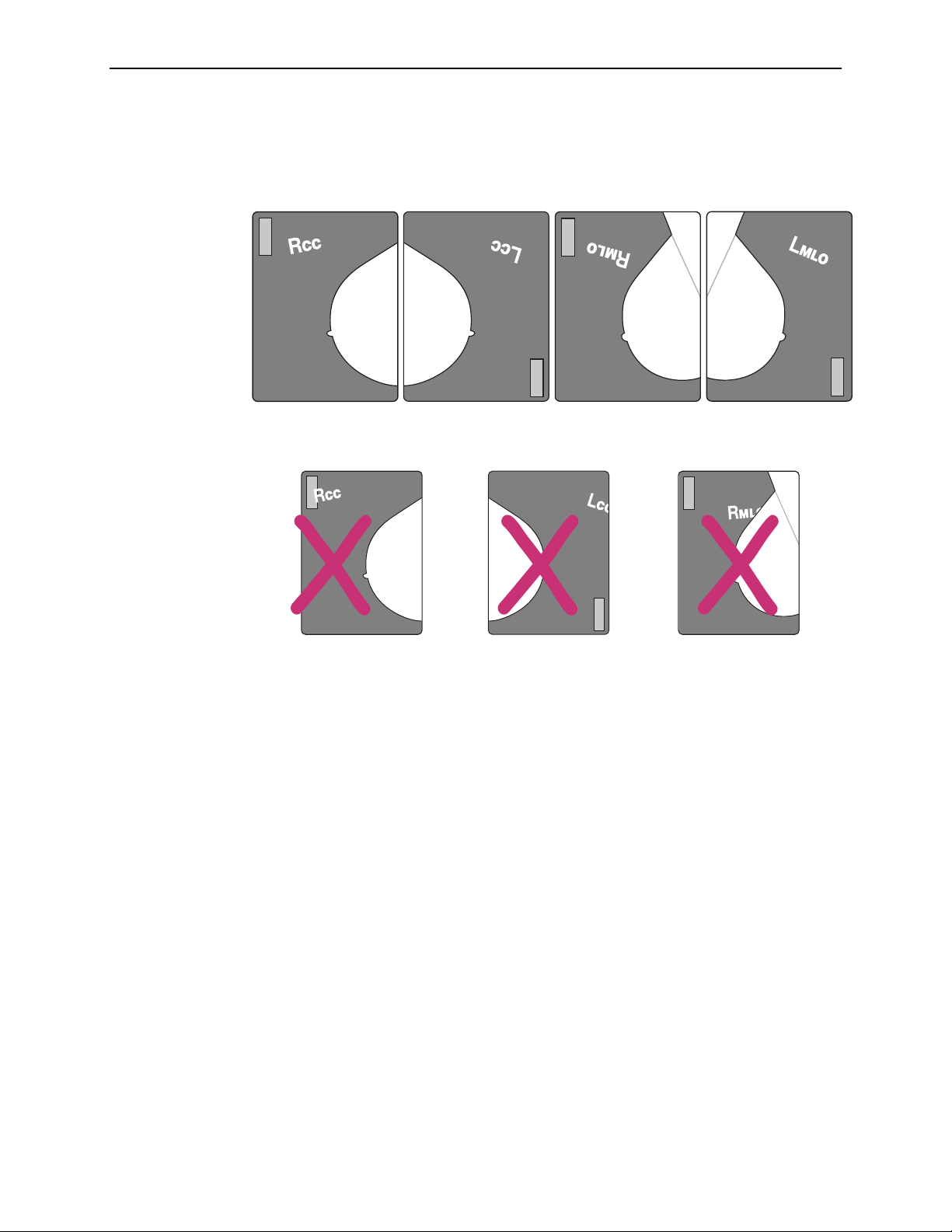
Lead Marker Placement
Correct placement of the lead markers is essential for optimal results. The following
examples show lead markers that are easy for the system to read, and that are not
touching the patient ID label, the breast tissue, or the film edge.
The following lead markers are incorrectly placed, as explained below:
3.1. Working with Films
Correct Auto Protocol Lead Marker Placement
Patient ID Label Edge of Film Breast
Separator Sheets
The R2 DM system is shipped with 1,000 separator sheets. Run through all sheets on a
rotating basis to ensure that patient barcode numbers are not reused before the
images are deleted from the system.
The separator sheets are sturdy and able to withstand a great deal of use. However, be
sure to store them flat, do not write on them with anything greasy or waxy, do not put
tape on the edges, and keep them clean. Most importantly, do not obscure the
barcode or the barcode number.
To order additional separator sheets, contact your Hologic | R2 Account Manager or
Technical Support.
Incorrect Auto Protocol Lead Marker Placement
R2 DMax User Manual – PN MAN-00709 Rev 001 31
Page 40

Part 3: Studies
Scanning Protocols
The system includes six preconfigured scanning protocols: Auto, Screening,
Uni Right, Uni Left, Screening Imp, and Any. You can also create your own
site-specific scanning protocols, see ‘
The default (i.e., preselected) scanning protocol is Auto. You can change the default
scanning protocol using the Controls Scanning screen (as described on page
Auto Protocol
When using the Auto protocol, the system recognizes the lead markers and uses them
to properly identify, orient, and process films with the four standard views.
You can load the films for each case in any order (e.g., RMLO, LCC, RCC, LMLO)
and in any orientation (e.g., rotated 180°, emulsion side up or down). The system
automatically scans all films in the stack. Each case in the stack can have any number
of films up to four. The system automatically adjusts if there are fewer than four
films. However, in Auto mode, the system will not process duplicate views within the
same case (e.g., two RCC films).
After you touch the Start button, the system processes all cases in the stack, up to a
maximum of 20 cases with four films per case.
5.8. Scanning Protocols’.
79).
Manual Protocols
R2 provides four preconfigured Manual scanning protocols: Screening, Uni Right,
Uni Left and Screening Imp. Each protocol requires you to load films with specific
views in a predefined sequence. For the views used with each predefined scanning
protocol, see ‘
Manual scanning protocols for your site, or alter the included protocols as needed.
When using a Manual scanning protocol, the system does not recognize the lead
markers. Instead, it relies on you loading the films:
• With the views specified by the selected scanning protocol.
• In the correct order – defined by the selected protocol.
• In the correct orientation – emulsion (dull) side down, landscape for small films,
portrait for large films.
• With the patient flash in alternating opposite corners – if the protocol specifies
alternating sides (upper right and lower left for small films, upper left and lower
right for large films).
The system also assumes that each study includes the same film views (as defined by
the selected protocol). If you are using a Manual protocol (because, for example, you
don’t use R2-supported lead markers) and the cases to be scanned include different
numbers of films or different views, create stacks of like cases and scan them
separately using the appropriate scanning protocol.
3.4. Scanning with a Manual Protocol’. You can also define custom
When using a Manual scanning protocol, as with the Auto protocol, the system
automatically scans all cases in the stack. When scanning is complete, the system
switches back to the default scanning protocol, as configured on the Controls
Scanning screen.
32 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 41
Any Protocol
This special scanning protocol allows you to scan any number of films (up to 24 per
case) in any order and orientation. When using the Any protocol, you must identify
all views manually after the case is scanned.
Choosing a Scanning Protocol
The scanning protocol you choose depends on the views present in the case, and the
condition of the films. In general, you will want to select a scanning protocol that
suits about 90% of the cases in the stack, which will minimize the adjustments that
you have to make after scanning. However, sometimes it may be faster simply to scan
with the Any protocol and correct the images afterwards.
Important! Many scanning errors, including scanning films in the wrong order or
scanning a film in the wrong orientation, can be overcome using the GreenLight Image
Correction feature. When in doubt, scan the films as best you can and fix any problems
after scanning. However, note that you cannot use GreenLight to correct images
produced from films scanned from different patients under a single separator sheet;
those films will have to be re-scanned using the appropriate number of separator sheets.
The following table provides basic guidelines for selecting a scanning protocol.
If your films ... Then see ...
• Are the four standard screening views (RCC, LCC, RMLO,
LMLO) and
• Were taken using an R2-supported lead marker (those shown
on page
• Have well-placed lead markers
In all cases include the same number and type of views, and:
• Have unsupported, missing, or bad lead markers, or
• Have incorrectly placed lead markers (e.g., over a breast
image, under an ID label, or only partly on film, as shown on
page
• Have non-standard views or views with modifiers, or
• Have more than four views per case
• Include views that do not conform to any available scanning
protocols
30) and
31), or
3.1. Working with Films
3.3. Scanning with
the Auto Protocol
3.4. Scanning with a
Manual Protocol
3.5. Scanning with
the Any Protocol
R2 DMax User Manual – PN MAN-00709 Rev 001 33
Page 42

Part 3: Studies
3.2. Preparing Cases to Be Scanned
Use this procedure to create a stack of cases to be scanned.
1 Place a separator sheet on top of each film case.
2 Write the last four digits of the separator sheet barcode number on the patient’s file.
Write the last four digits
of the separator sheet
0000009896
barcode number on each
patient’s file.
Place small films (18 × 24)
in portrait orientation,
large films (24 × 30) in
landscape.
Creating a Stack of Cases
Create a neat stack of films and separator sheets. Observe the following guidelines.
3
• Put small-film cases at the top of the stack, mixed-film cases in the middle, and
large-film cases on the bottom.
• If the number of films in the case does not match any of the defined scanning
protocols, use the Any protocol.
• Use the correct number of films per case, as specified by the selected scanning
protocol.
• If you plan to scan with the Auto protocol, you can use from one to four films, as
long as they have supported lead markers and are from the four standard views
with no duplicate views. You can arrange the films in any order and in any
orientation. See ‘
3.3. Scanning with the Auto Protocol’. Load only the four
standard views with no duplicates.
• If you plan to scan with a Manual protocol, you must arrange the films exactly as
defined by the desired scanning protocol. See ‘
Protocol
’.
3.4. Scanning with a Manual
• If you plan to use the Any protocol to scan a special case (one that doesn’t
conform to any available scanning protocol), then see ‘
Protocol
Note: If you make a mistake when preparing a case for scanning, you can sometimes
correct it after scanning is complete via the Image Correction screen. See ‘
Scanned Cases
’.
’ for more information.
3.5. Scanning with the Any
3.9. Verifying
34 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 43

3.3. Scanning with the Auto Protocol
Use this procedure to scan cases using R2 Technology’s Automatic Film Marker
Identification (AFMI) feature. The Auto protocol accepts up to four films per case.
You can scan with the Auto protocol if all cases in the stack include only the four
standard mammography views (RCC, LCC, RMLO, LMLO), and were processed
using a supported lead markers (those shown on page
• For cases that do not meet these criteria, but conform to a different scanning
protocol, see ‘
3.4. Scanning with a Manual Protocol’.
3.3. Scanning with the Auto Protocol
30) that were well-placed.
• For cases which do not confirm to any defined scanning protocol, see ‘
Scanning with the Any Protocol
Important! The Auto protocol does not support duplicate views (for example, two
RCC films or two LMLO films). Use a Manual protocol or the Any protocol for cases with
duplicate views.
X To scan with the Auto protocol
1
Prepare the cases to be scanned as described in ‘3.2. Preparing Cases to Be Scanned’.
’.
You can arrange the films of the four standard views in any order and in any
orientation.
2 Load the stack into the input tray. Insert the stack with:
• The arrow on the top separator sheet pointing into the scanner.
• The leading edges of the films and separator sheets aligned flush.
You can load up to 100 sheets – including films and separator sheets – up to 20 fourfilm cases.
3.5.
Loading Films
Verify that Auto is selected in the Scanning Protocol list on the Studies screen.
3
Note: You cannot change the scanning protocol if a case is currently being scanned.
Touch Start. The system status changes from ‘Ready’ to ‘Scanning’, and the Start
4
button becomes a
R2 DMax User Manual – PN MAN-00709 Rev 001 35
Stop button.
Page 44

Part 3: Studies
After the system scans the first film, a new study icon appears at the top of the study
list, and a white circle on the icon flashes to indicate that the films are being
processed. If you touch this icon, you can monitor the progress of the study as the
films are processed. There are three possible outcomes:
•
Success: If the system processes the case successfully and verification of cases is
not required, then the system automatically sends the study results to the output
device (display unit, printer and/or PACS archive). An arrow on the icon flashes to
indicate that the system is sending the results. After the system sends the results,
the icon appears solid green.
•
Verification Required: If verification of cases is required, then you must review the
scanned image thumbnails and patient information and confirm that the results
are acceptable. If no Alert has been issued, then the Done button appears on the
Studies screen. When you touch Done, the system sends the results to the output
device (display unit, printer and/or PACS archive).
•
Alerts: If the study icon is replaced with an Alert icon, you may be required to take
corrective action. In many situations instead of rescanning the case, you can often
correct the alert and reprocess the images via the Image Correction screen.
For more information, see ‘
messages, see ‘
5 When the cases have been scanned successfully, put the films and corresponding
Part 4: Alerts’.
3.9. Verifying Scanned Cases’. For information on alert
separator sheets together in the patient folders for hanging.
Note: If the system has been configured to use a different scanning protocol by
default (on the Controls Scanning screen), then after scanning a stack of films, the
system returns to the default protocol.
36 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 45

3.4. Scanning with a Manual Protocol
When you use a Manual scanning protocol, each case in the stack must include the
correct number and type of film views, as defined in the protocol. You may also need
to use a Manual protocol if films:
• Have unsupported, missing, or bad lead markers, or
• Have incorrectly placed lead markers (e.g., over a breast image, under an ID label,
or only partly on film, as shown on page
• Have non-standard views or views with modifiers, or
• Have more than four views per case
If one of the cases in the stack has any of the characteristics listed above and does not
have the number of film views specified in any existing Manual protocol, use
procedure ‘
The system ships with four predefined Manual scanning protocols.
Your system may have additional user-created scanning protocols specific to your
site. For more information, see ‘
3.5. Scanning with the Any Protocol’ for that case.
Predefined Manual
Scanning Protocols Views Included
Screening
Uni Right
Uni Left
Screening Imp
3.4. Scanning with a Manual Protocol
31), or
RCC
LCC
RMLO
LMLO
RCC
RMLO
RXCC
LCC
LMLO
LXCC
RCC
LCC
RMLO
LMLO
RCC ID
LCC ID
RMLO ID
LMLO ID
5.8. Scanning Protocols’.
For a list of supported views and modifiers, see ‘
R2 DMax User Manual – PN MAN-00709 Rev 001 37
CAD-Supported Views’ on page 26.
Page 46

Part 3: Studies
X To scan with a Manual protocol
Orientation
• Emulsion (dull)
side down
• Small: landscape
• Large: portrait.
1
Prepare the cases to be scanned as described in ‘3.2. Preparing Cases to Be Scanned’.
2 Arrange the films and their separator sheets in the order shown on the screen below
the selected scanning protocol, and with the orientation shown below.
24 × 30
18 × 24
Important! When using a Manual scanning protocol, use the same film views for all
cases in the stack, and arrange the films in the order defined by the selected protocol.
Load the stack into the input tray. Insert the stack with:
3
• The arrow on the top separator sheet pointing into the scanner.
• The leading edges of the films and separator sheets aligned flush.
You can load up to 100 sheets – including films and separator sheets – up to 20 fourfilm cases.
Loading Films
38 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 47

3.4. Scanning with a Manual Protocol
f
4 Touch the desired scanning protocol. In the example shown at left,
Screening is selected. The views defined for the selected protocol are
displayed below the protocol list.
Note: You cannot change the scanning protocol while a case is being
scanned.
Touch Start. The system status changes from ‘Ready’ to ‘Scanning’, and
5
the
Start button changes to Stop.
6 A new study icon appears at the top of the study list, and a white circle on
the icon flashes to indicate that the films are being processed. If you touch
this icon, you can monitor the progress of the study as the films are
processed. There are three possible outcomes:
•
Success: If the system processes the case successfully and verification o
cases is not required, then the system automatically sends the study
results to the output device (display unit, printer and/or PACS
archive). An arrow on the icon flashes to indicate that the system is
sending the results. After the system sends the results, the icon appears
solid green.
•
Verification Required: If verification of cases is required, then you must
review the scanned image thumbnails and patient information and
confirm that the results are acceptable. If no Alert has been issued, then
the Done button appears on the Studies screen. When you touch Done,
the system sends the results to the output device (display unit, printer
and/or PACS archive).
•
Alerts: If the study icon is replaced with an Alert icon, you may be
required to take corrective action. In many situations instead of
rescanning the case, you can often correct the alert and reprocess the
images via the Image Correction screen.
For more information, see ‘
on alert messages, see ‘
7 When the cases have been scanned successfully, put the films and
3.9. Verifying Scanned Cases’. For information
Part 4: Alerts’.
corresponding separator sheets together in the patient folders for hanging.
Note: If the system has been configured to use a different scanning
protocol by default (on the Controls Scanning screen), then after scanning a
stack of films, the system returns to the default protocol.
R2 DMax User Manual – PN MAN-00709 Rev 001 39
Page 48
Part 3: Studies
3.5. Scanning with the Any Protocol
Use the Any protocol to scan cases that do not conform to an existing scanning
protocol, for example, if the case has missing films, extra films, or views not included
in any existing scanning protocol.
The Any protocol contains no predefined views. This allows you to scan any number
of films (up to 24) with any type of view and later identify the views manually once
the case has been scanned.
A neat and ordered stack can save time spent adjusting the image orientation after
scanning. Keep the following in mind:
• Remove films from the stack that would normally be discarded rather than saved
(for example, technical retakes).
• Orienting the films correctly in the stack saves time adjusting the orientation later
via the Image Correction screen.
X To scan with the Any protocol
1
Prepare the case to be scanned as described in ‘3.2. Preparing Cases to Be Scanned’.
2 Place the separator sheet on top of the stack for each case.
You can arrange the films in any order, although you may wish to use a sequence
defined by your site (for example, the four standard views first, followed by additional
or equivalent views). As needed, turn films a quarter turn to match the orientation
shown on the separator sheet.
If you load films in a random order as shown below, you will have to reorient the
images later via the Image Correction screen.
3
Load the stack into the input tray. Insert the stack with:
• The arrow on the top separator sheet pointing into the scanner.
• The leading edges of the films and separator sheets aligned flush.
40 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 49

3.5. Scanning with the Any Protocol
You can load up to 100 sheets – including films and separator sheets.
4 Select the Any protocol by touching Any.
Loading Films
Note: You cannot change the scanning protocol while a case is being
scanned.
Touch Start. The system status changes from ‘Ready’ to ‘Scanning’, and
5
the
Start button changes to Stop.
A new study icon appears at the top of the study list. As soon as the first
film is scanned, the icon displays the yellow alert symbol. The case status
shows ‘Failed’ with an explanation that the image contains an unknown
view. After the entire case has been scanned, you must identify all image
views via the Image Correction screen and reprocess the case. To do so:
6 Touch an image to display the Image Correction screen. See ‘3.9. Verifying
Scanned Cases
’ for further information on reorienting and labeling
images. Identify each image and correct the orientation as needed.
7 When done, touch Reprocess on the Image Correction screen to reprocess
the images. The system returns to the Studies screen, and a white circle on
the study icon flashes to indicate that the system is processing the views.
When processing is complete, a white arrow on the icon flashes to indicate
that the results are being transmitted to a display unit, printer, and/or
archive system. Once the study has been transmitted, the icon appears
solid green.
8 When the case has finished scanning, put the films and separator sheet
together in the patient folder for hanging.
Note: After scanning the case, the system returns to the default protocol
configured on the Controls Scanning screen.
R2 DMax User Manual – PN MAN-00709 Rev 001 41
Page 50

Part 3: Studies
3.6. Adding Cases to the Stack
You can add one or more cases anywhere in the stack. If you’re not in a hurry for the
case, add it to the bottom. If you’re in a hurry, add it to the top. (Cases at the top are
processed first.)
Note: This procedure can produce an alert indicating there were too few films for the
previous case. However, it is often faster to remove the films and produce the alert than
to wait for the films to be scanned and processed.
Important: Verify that cases you add to the stack conform to the selected scanning
protocol, or select the desired protocol before scanning the case.
X To add cases to the stack
1
Prepare the cases to be scanned as described in ‘3.2. Preparing Cases to Be Scanned’.
2 If the system is currently scanning, touch Stop. The scanner will stop after it finishes
the current film.
Important: When you touch Stop, the system always resets to the default scanning
protocol. If the system generates an alert, touch OK in the pop-up window.
3
Remove the films and separator sheets from the feeder. Rebuild the stack in the order
you want to run the cases. If you want to run the new cases first, add them at the top
of the stack.
Note: Be sure the films and separator sheets are in the correct order. If the system
did not scan all the films from the last case, take the scanned films from the output tray
and put them together with the films that were not scanned.
4
Insert the new stack of cases into the feeder.
5 If necessary, select the scanning protocol you want to use.
6 Touch Start to resume scanning.
42 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 51

3.7. Stopping Scanning and Removing a Case
3.7. Stopping Scanning and Removing a Case
You cannot ‘cancel’ a study, but if you don’t want to continue scanning the current
set of films, you can stop the system and remove any remaining films from the input
tray (as described below).
Note: This procedure can cause the system to produce an alert indicating there were
too few films. However, it is often faster to remove the films and produce the alert than
to wait for the films to be scanned and processed.
Important: If you changed the scanning protocol from its default setting before you
started scanning (e.g., if the default scanning protocol on the Controls Scanning screen is
Auto and you touched Screening before touching Start), the system resets the scanning
protocol to its default when you touch Stop in step 1 of the procedure below. If you
want to resume scanning with the non-default protocol after removing one or more
cases, you’ll need to select that scanning protocol again before touching Start in step 6.
X To stop scanning
1
Touch Stop. The scanner will stop after it finishes scanning the current film.
Note: If the system generates an alert, touch OK in the pop-up window.
2 Remove the entire stack from the input tray.
3 Remove the remaining films for the cancelled case.
4 As desired, remove any other case, that is, the separator sheet and the films associated
with it.
5 Place the remaining cases back in the input tray.
Important! Be sure that the next item to be scanned is the separator sheet for the
next case.
Select the desired scanning protocol, if not already selected.
6
7 Touch Start to resume scanning.
R2 DMax User Manual – PN MAN-00709 Rev 001 43
Page 52
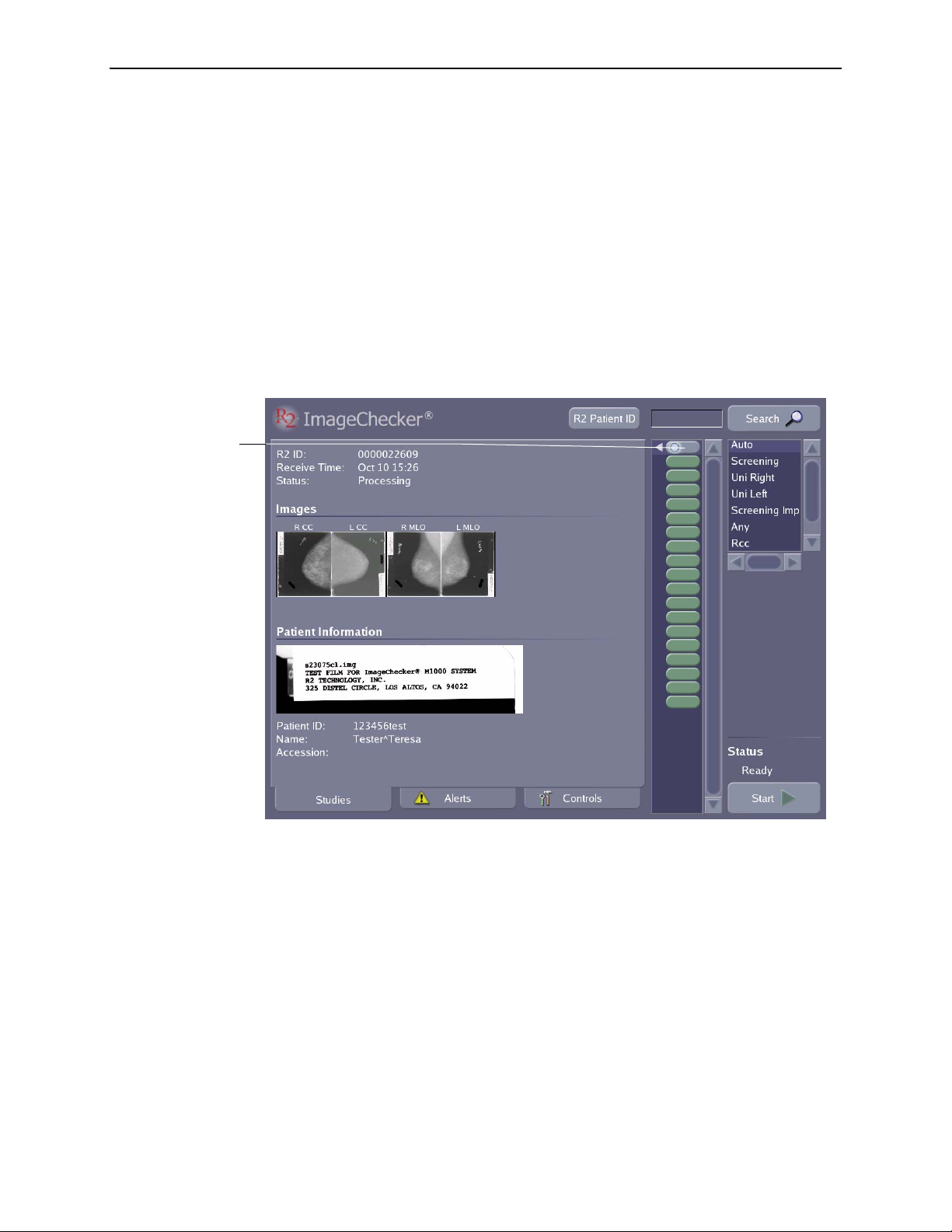
Part 3: Studies
t
t
3.8. Viewing Study Information
As each study is processed, the system displays the study information on the Studies
screen. The results include the image thumbnails, patient flash (if present), and
patient information.
You can display study information in one of three ways:
• You can touch the study icon in the study list.
• You can type the patient identification, name, or R2 ID (separator sheet barcode
number) and press
• You can use the handheld barcode scanner to scan in the patient identification or
R2 barcode number.
[×Enter] (or touch Search).
The Study icon at
he top represents
the study currently
being processed or
he study most
recently processed.
The Study icons
below represent
studies processed
earlier.
For more information about searching, see ‘
3.12. Searching for a Study’.
For any scanned case, the system displays the following:
• The
• The
• The study
R2 ID, which is the barcode number from the study’s separator sheet
Receive Time, which is the time film scanning began for the selected study.
Status. Depending upon the case, the Status field can display a variety
of messages (see table, next page).
• Thumbnail
Images of films. These images appear as they are processed and can be
viewed at any time after processing is complete. Touch any thumbnail image to
open the Image Correction screen.
•
Patient Information shows the patient flash from the scanned film (if present),
followed by the Patient ID, Name, and Accession number from the reporting
system or R2 Technology’s patient identification system. The patient flash image
appears for each film as it is scanned. After the system scans the last film for a
44 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 53

3.8. Viewing Study Information
study, it retains the patient flash image from that film as part of the study
information.
Note the following:
• The image thumbnails always appear in film scanning order. Once the films have been
scanned, you cannot change the order.
• The thumbnails are always shown in anatomic orientation (e.g., RCC nipple points
left), and this orientation cannot be changed.
• CAD marks do not appear on the touch-screen monitor. The CAD marks appear only
on the output devices (display units, review workstations, printouts, etc.).
The following table shows the Status conditions that the system can display for a case.
Case Status Message Meaning
Processing The system is scanning and processing the selected case.
Needs Verification
Transmitting
Completed The system has finished transmitting the study.
Failed
If one or more images in the study could not be processed for any reason, a yellow ‘X’
appears on the image. However, all scanned images will still be sent to the PACS if
your system is configured for archiving. See ‘
alert messages.
The system requires you to review and ‘verify’ the case
images before it will send the study results to a display
device or archive.
The system is transmitting the scanned images to a
display device or archive.
The system was unable to complete the processing or
study transfer and has issued an alert.
Part 4: Alerts’ for more information on
Note: The message shown in the example above indicates that the images were
archived using Natural Pixel Values instead of in the DigitalNow ELC Enhanced MG image
format. For more information, see ‘
R2 DMax User Manual – PN MAN-00709 Rev 001 45
Digital Image Output Options’ on page 28.
Page 54

Part 3: Studies
3.9. Verifying Scanned Cases
At some facilities, the system will require you to ‘verify’ scanned cases before you
archive them. The verification step is configured by selecting the ‘On with
verification’ setting on the Controls Outputs screen (see ‘5.4. Outputs Options’).
Important! Hologic | R2 recommends that facilities using DigitalNow DICOM Export
always verify the images before archiving them to reduce the chance of improperly
oriented or identified images being sent to PACS.
If your system has been configured to require verification, then after the system
processes the cases it displays a blinking question mark on each study icon. The
question mark also appears on the Studies screen tab at the lower left of the screen.
X To verify cases
1
Touch the study icon for the case you want to verify. The system displays the results
for the selected study.
2
Review the case results. Check to see that the images are oriented correctly and that
patient was retrieved successfully from the archive.
3 Touch the Done button or make corrections.
• If the results are acceptable, touch the Done button. In response, the system
transmits the results to the archive or other output device.
• If the results are not acceptable, or if the system produced an alert for the case, you
may need to take corrective action. In some situations, you may need to reorient
or label images by using the Image Correction screen. For more information, see
‘3.10. Correcting Scanned Images’.
4 Repeat these steps for each case needing verification.
46 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 55
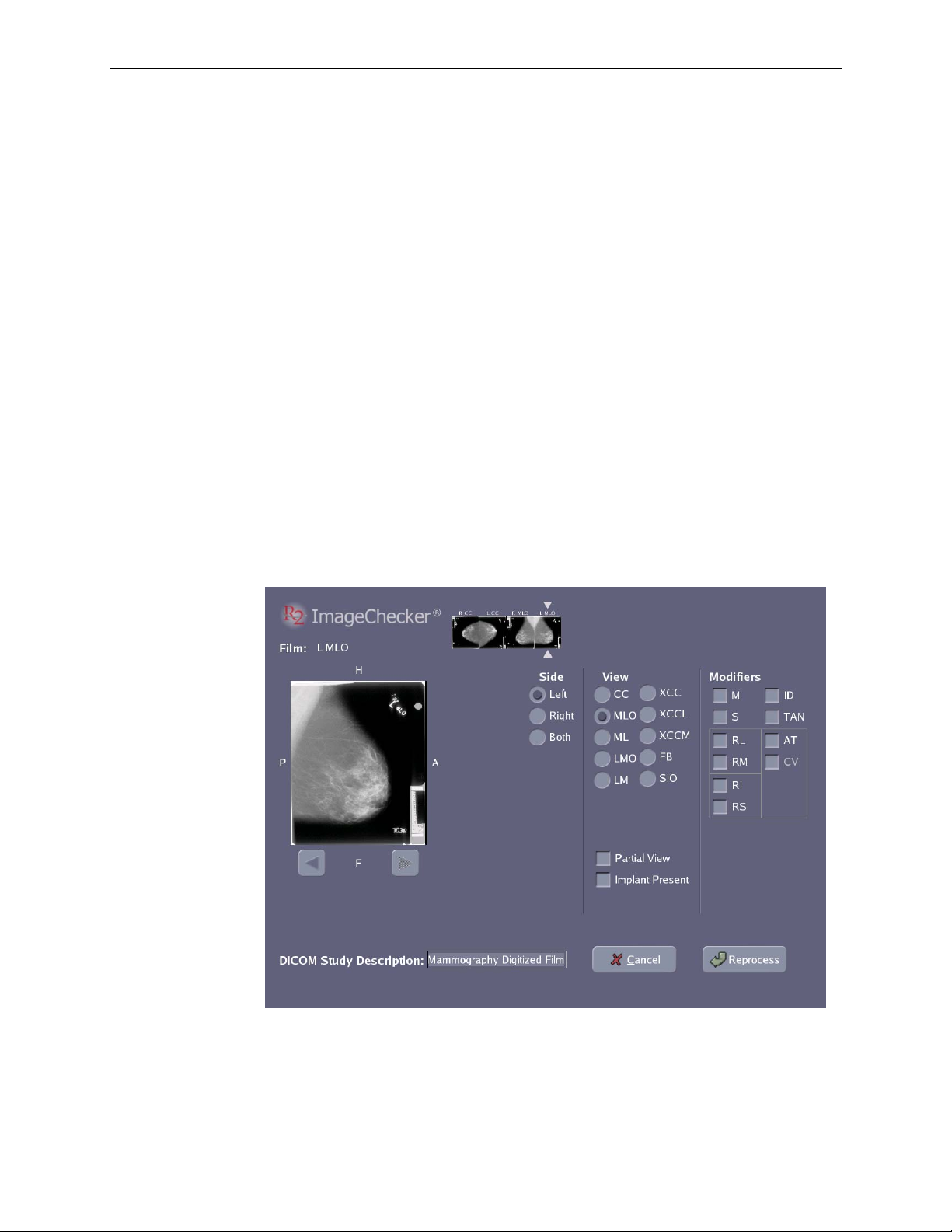
3.10. Correcting Scanned Images
After you scan a case, you use the Image Correction screen to reorient images, change
film view labels and modifiers, or add a DICOM study description. For example, you
must correct the scanned images if a case includes one or more non-standard views or
view modifiers, or if the system is unable to identify a lead marker. In addition, you
must use the Image Correction screen for all cases scanned with the Any protocol.
In many cases, the system issues an alert to let you know that it could not completely
process the case. However, you can use the Image Correction screen even with cases
that have been completely processed without alerts.
The Image Correction screen allows you to:
• Reorient scanned images (flip or rotate them)
• Change the film view labels and modifiers (e.g., RCC to LCC)
• Edit the DICOM study description for the case
Note: If films for two or more patients were scanned under a single separator sheet,
you must rescan the case.
X To correct scanned images
3.10. Correcting Scanned Images
1 Open the Image Correction screen.
Touch the study icon for the study you want to
work with. Then touch one of the thumbnail images. The Image Correction screen
opens as shown in the following example:
The image thumbnails for the case appear at the top of the screen. The image you
selected appears enlarged at left. To navigate through the study images, touch the
arrow buttons below the enlarged image, or touch any of the image thumbnails.
Note: If the displayed images and their labels and DICOM Study Description are all
correct, then simply touch Cancel to return to the Studies screen.
R2 DMax User Manual – PN MAN-00709 Rev 001 47
Page 56
Part 3: Studies
2 Reorient image, as needed.
a Touch the enlarged thumbnail at the left of the screen. Each time you touch the
image, it flips alternately along the vertical and horizontal axes. Flipping the image
a total of four times restores the original orientation.
b Check the image orientation with the indicators on the four sides of the image
(Left, Right, Anterior, Posterior, Head, Foot). When the orientation is correct, go
on to step
3.
48 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 57

3.10. Correcting Scanned Images
3 Modify the view label, as needed.
To define or change the view label, build it from
left to right: select a side, a view, and then modifiers (as needed).
a
Touch the proper Side selection: Left, Right, or Both (for cleavage view).
b Touch the proper View selection. Note the two additional selections below the
view area for Partial View and Implant Present.
c Touch to select Modifiers as needed.
As you build the view label, invalid selections are eliminated. For example, if you
choose Both for the side, the system automatically sets the view to CC and the
modifier to CV. You can chain valid modifiers together as needed.
Some modifiers (for example, M and S) appear before the view designation; other
modifiers (such as ID and TAN) appear after the view designation.
Note: You must select a view when using the TAN modifier, as TAN can be used with
either the CC or MLO view.
See ‘CAD-Supported Views’ on page 26 for a complete list of views and modifiers.
4 Repeat steps 2 and 3 for each image.
5 Modify the DICOM study description, as needed. If desired, touch the DICOM Study
Description field and use the keyboard to change the DICOM study description for
the case. The default description is ‘R2 Mammography Digitized Film’.
Note: All images in the case will contain the same DICOM Study Description;
modifying it in one image changes it for all images in the case.
6 Reprocess the case.
Once the images and their labels are correct, touch Reprocess.
The system cancels the old case (removes the old study icon), reprocesses the case (all
images), and creates a new study icon at the top of the study icon list. If the system
does not issue an alert, it automatically sends the results to the output devices (PACS,
display unit, printer, etc.).
R2 DMax User Manual – PN MAN-00709 Rev 001 49
Page 58

Part 3: Studies
3.11. Printing CAD Results
If you have configured the system to print CAD results for every study (as described
in ‘
5.7. Printing Options’), the CAD results are automatically printed when
processing is complete.
The following is an example of a CAD Results Report:
If you want to create an additional paper printout of the CAD results, touch any of
the thumbnail images for the case and reprocess it. Because the films are not
rescanned, the results for the case will be identical to the original.
50 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 59

3.12. Searching for a Study
You can search for and display the information from any previously processed study
using any of the following study data:
• R2 ID (from the separator sheet barcode)
• Patient ID (from the reporting system)
• Name (from the reporting system)
The search is limited to the 200 most recently processed studies (or fewer, depending
on the available disk space in the processing unit’s computer).
X To search for a study and display its results
3.12. Searching for a Study
1 Type the data
for the study you want to find. You can just start typing; you don’t
need to touch the Search field first. You can also enter data by using the handheld
scanner to scan a barcode from a patient record or separator sheet.
Note: The system does not accept (or need) wildcard characters (e.g., *). The search
function will find any data that partially or completely matches the text you enter. If you
leave the search field blank, the search returns all studies on the system.
2
Touch Search. The search results appear:
• If only one study matches the search criteria, the system displays the study
information automatically.
• If multiple matches occur, the system displays the Search Results window, as
shown in the following example.
Note: To move the window, touch and drag the Search Results title bar.
If more than one study appears, touch the study you want to see. The Search Results
window closes and the results of the study you selected appear on the Studies tab.
R2 DMax User Manual – PN MAN-00709 Rev 001 51
Page 60

Part 3: Studies
3.13. Using Queue Manager
Queue Manager enables you to monitor and manage the queue of cases being
processed by the R2 system.
X To enter Queue Manager
1
Using any web browser on the same network as the R2 system, in the Address box
type the IP address for the processing unit followed by /qman, and press
example:
http://12.345.67.89/qman
The browser will display the log-in window.
[×Enter]. For
2
Enter your Queue Manager database password (h0spital), and click login.
52 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 61

3.13. Using Queue Manager
The system displays the Queue Manager screen, showing the list of cases and the
status of each case. Click Help at the upper-right corner for detailed instructions.
• You can filter the list of cases by entering search criteria in any of the fields in the
Search panel at the top of the screen and then clicking the search button.
• You can sort the list of cases by clicking on any of the column headers. Clicking it
again reverses the sort order.
• When you click on a patient name, the information associated with the study is
shown in the Description panel at the bottom of the screen.
3 Select one or more cases using the checkboxes on the left side of the queue.
4 Use the run next, delete, and stop buttons to move the selected case(s) to the top of
the queue (run next), delete them, or stop processing them.
5 When you are finished, exit the web browser.
R2 DMax User Manual – PN MAN-00709 Rev 001 53
Page 62

Part 3: Studies
54 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 63

Part 4: Alerts
f 4.1. Alerts Listed Alphabetically
f
4.2. Common Questions
Part 4 provides a listing of alert conditions with instructions for resolving the alerts
and diagnosing and solving common problems.
The Alerts are designed to help you and Hologic | R2 service personnel diagnose
problems when they arise. When they occur, the alerts appear in two places:
Touch to sort alerts
by Time, R2 ID, or
alert Description
Date and time alert
was generated
R2 ID
Touch to view or
hide alerts you’ve
hidden.
Touch to hide
all alerts.
Touch to hide a
selected alert.
• On the
• On the
Studies screen, just below the Status field for the study currently displayed.
Alerts screen, where you can view all recent alerts.
Also, whenever the system generates a new alert, an alert icon ( ) appears on the
Alert tab.
R2 DMax User Manual – PN MAN-00709 Rev 001 55
Page 64

Part 4: Alerts
4.1. Alerts Listed Alphabetically
The alert messages are presented here in alphabetical order, with instructions for
addressing each problem.
Archiving was cancelled for case (3045)
The barcode has no associated patient information in the reporting system database.
Enter the patient information into the reporting system database, including the R2
barcode on the separator sheet scanned with the case, then reprocess the case via the
Image Correction screen. See ‘
Cannot process image successfully (1063)
The system is unable to perform CAD processing or image enhancement on the
image due to poor film quality. A yellow ‘X’ appears on the image thumbnails on the
Studies tab. Even though no processing was possible, the scanned images are archived
as usual.
• Verify that the images have the correct view labels, if not correct the view labels on
the Image Correction screen. See ‘
information.
3.9. Verifying Scanned Cases’ for more information.
3.9. Verifying Scanned Cases’ for more
• Verify that the image views and view modifiers are supported for CAD processing.
See ‘
2.6. System Inputs and Outputs’ for more information.
Cannot process this case (1062)
The R2 software cannot work with the images as they are.
• Correct the images as necessary via the Image Correction screen, and reprocess the
study.
• If films for two or more patients were scanned under a single separator sheet,
rescan the case.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
Could not connect to reporting system database (3503)
The system is unable to retrieve a patient record from the reporting system. The
reporting system may be off-line or the R2 system may not be set up properly to
connect to the reporting system.
• Verify that the reporting system server is up and running.
• Check network cables to confirm that they are properly connected.
• Consult with the reporting system administrator at your facility.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
ImageChecker attempted but could not send case (3016, 3018, 3031, 3032)
The system is trying to transfer the images but cannot find the specified output device
(display unit, PACS, etc.). The system will try repeatedly to send the images. Usually,
it is either a display unit problem or your computer network is down. To fix:
• Make sure the display unit is up and running. If this was the problem, the studies
should transfer within about ten minutes.
56 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 65

4.1. Alerts Listed Alphabetically
• If this isn’t the problem, check to be sure all cables between the processing unit
and the display unit are connected and that the computer network is up.
• If your network is up, all cables are connected, and the display unit is in working
condition, call Hologic | R2 Technical Support.
ImageChecker attempted but could not print reimbursement report (2008)
The system has canceled the print request for the study. You will only see this alert
after several ‘Printer not found...’ alerts. The system tries repeatedly to print and
displays this message when it gives up. To fix:
• Verify that the printer is on, on-line, and has enough paper. After correcting any of
these problems, ensure that printing is set to ‘on’ and reprocess the study in the
Image Correction screen.
• If the ‘Printer not found...’ or similar alert appears again, check that all cables
between the printer and the processing unit are connected. Check with your
system administrator that your network is up. Reprocess the study in the Image
Correction screen and print.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
ImageChecker cannot contact the scanner (1010)
This message may occur during system start-up, or if the scanner was inadvertently
turned off. The system does not allow scanning films until the problem is fixed.
• If the system is rebooting, wait about 5 minutes.
• Power-cycle the system. See ‘
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
6.3. Power-Cycling the System’.
ImageChecker cannot identify the lead markers (3023)
This message appears when scanning with the Auto protocol. It means that the system
did not recognize the lead markers. Either they are not one of the supported types or
there is a problem with marker position and/or placement on the film(s). To fix:
• Identify and label the views on the Image Correction screen and reprocess the
study. If this doesn’t fix the problem, call Hologic | R2 Technical Support.
ImageChecker cannot read the barcode (1041)
The scanner is not able to read the barcode on the separator sheet.
• Ensure that the separator sheet is in the proper position (e.g., is not half-fed in the
feeder) and is not damaged
• Reload the stack and restart scanning.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
ImageChecker is busy (3012, 1029, 1030)
The images for the study have not yet been sent.
• Wait about ten minutes – the problem should resolve itself.
• If it doesn’t, ensure the destination (i.e., display unit or printer) is up and ready to
receive images.
R2 DMax User Manual – PN MAN-00709 Rev 001 57
Page 66

Part 4: Alerts
• Check that all cables between the processing unit and the destination are
connected and that the network is up.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
ImageChecker license not found (1024)
This message means the system could not verify a valid license.
• Ensure that the license dongle is present and properly connected. If this does not
solve the problem, call Hologic | R2 Technical Support.
ImageChecker could not print a CAD report (2007)
The system has canceled the print request for the study. You will only see this alert
after several ‘Printer not found...’ alerts. The system tries repeatedly to print and
displays this message when it gives up. To fix:
• Verify that the printer is on, on-line, and has enough paper. After correcting any of
these problems, ensure that printing is set to ‘on’ and reprocess the study in the
Image Correction screen.
• If the ‘Printer not found...’ or similar alert appears again, check that all cables
between the printer and the processing unit are connected. Check with your
system administrator that your network is up. Reprocess the study in the Image
Correction screen and print.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
ImageChecker could not send results (2005)
The system has tried to send the results to the desired output destination, but the
time limit to retry has expired without successfully sending the results.
• Reprocess the images via the Image Correction screen. See ‘
Cases
’ for more information.
• If the problem persists, call Hologic | R2 Technical Support.
Images didn’t transfer (1031)
Images couldn’t be sent to the output device (display unit, PACS, etc.) and the
transfer was canceled by the system. You will see this alert only after several
‘ImageChecker … could not send case #...’ alerts (#3016) have appeared. The system
tries repeatedly to send the studies and displays this message when it gives up trying.
• Be sure the display unit is up and running.
• Check cable connections between the processing unit and the display unit (i.e., the
network cable from the system to the wall and the display unit network cable to
the wall). Reprocess the study in the Image Correction screen.
• If this doesn’t fix the problem, ask your system administrator if the network is up.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
3.9. Verifying Scanned
Image transfer was interrupted (3013)
The system is trying to transmit results but cannot find the specified output device
(display unit, PACS, etc.). The system will try repeatedly to send the results. To fix:
58 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 67

• Make sure the destination device on. If this was the problem, the results should
appear within about ten minutes.
• Check that all cables between the processing unit and the destination device are
connected and that the network is up.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
Missing or invalid barcode at top of stack (1016)
The scanner cannot read the barcode at the top of the stack.
• Check that the separator sheet is oriented correctly in the scanner input tray. See
diagram on separator sheet.
• If the separator sheet is oriented correctly and the problem persists, call
Hologic | R2 Technical Support.
No output jobs scheduled for case (3054)
The R2 system was not configured with any output destinations for CAD results.
If your system is set up to output to the printer only verify that printing is turned on
at the Controls screen. See ‘
5.7. Printing Options’ for more information
4.1. Alerts Listed Alphabetically
No patient record information is associated with barcode (3501)
This alert appears when you are using the mammography reporting system and
attempting to transmit the study results. It means that there is no patient record in
the reporting system database with that specific barcode. When this alert is issued, the
results will not be archived. Although you can print the results for the study, any
patient information in the reporting system will not be listed on the printout.
The images in the R2 system are linked to the patient record in the reporting system
by the separator sheet barcode number (R2 ID). When you scan the films, the R2
system searches for the patient record in the reporting system database so it can
acquire the patient information.
Always enter the R2 barcode number into the patient record in the reporting system
database before you scan the films.
If there is no patient information associated with the R2 ID, you can either:
• Enter the information into the R2 Patient ID system and reprocess the case, or
• Enter the information into PenRad, MRS or MagView and reprocess the case (to
requery from their database)
Not all films in case are specified by scanning protocol (3024)
There are more films in the case than defined by the selected scanning protocol.
Identify the film views via the Image correction screen and reprocess. See
Verifying Scanned Cases
for more information.
3.9.
Printer not found (3014)
The processing unit is attempting to send the study results to the printer, but the
printer is not responding. The printer may be unable to respond, or there may be a
network or configuration problem. The system will continue to try to send the study
results.
R2 DMax User Manual – PN MAN-00709 Rev 001 59
Page 68

Part 4: Alerts
• Verify that the printer is running.
• Check all cable connections between the processing unit and the printer (i.e., the
network cable from the processing unit to the wall and the printer network cable
to the wall). Sometimes a cable becomes loose. If so, wait about ten minutes, the
system will resend the study results.
• Ask your system administrator if the network is down. If it is, and it is down long
enough, the system will finally give up, and you will have to reprocess the study
with printing turned on.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
Rebooting the operating system, please wait (5021)
You’ll see this message when the system is rebooting (starting up again after being
shut down and turned off).
• Wait about 10 minutes. Once the system comes back up, you will see the Ready
message above the Start button.
• If the system doesn’t come back up after 15 minutes, call Hologic | R2 Technical
Support.
Reporting system query error (3502)
The R2 system could not communicate with the reporting system database, and
possibly the system is not set up correctly. Contact Hologic | R2 Technical Support
and they will diagnose and fix the problem.
Restarting R2 software, please wait (5019)
• Wait about 10 minutes. Once the system comes back up, you will see the ‘Ready’
message above the Start button.
• If the system doesn’t come back up after 15 minutes, call Hologic | R2 Technical
Support.
Scanner lock lever is raised (3051)
This usually happens when there are too many films in the feeder. Remove cases and
touch OK to continue.
Scanner not ready! (5002)
For some reason, the scanner did not turn on properly.
• Power-cycle the system. See ‘
• If this doesn’t fix the problem, call Hologic | R2 Technical Support.
6.3. Power-Cycling the System’.
Scanner test failed (3025)
This message appears when you run the weekly test films and the scanner test fails.
Usually you can clear the alert by rerunning ALL tests again.
• First, make sure the test films and separator sheets are not bent or damaged in any
way. If they are, call Hologic | R2 Technical Support for replacements.
• Rerun all test films. See ‘
problem.
60 R2 DMax User Manual – PN MAN-00709 Rev 001
6.4. Running the Weekly Tests’. Usually, this fixes the
Page 69

4.1. Alerts Listed Alphabetically
• If any tests fail again, power-cycle the system (see ‘6.3. Power-Cycling the System’)
and rerun the tests again.
• If any tests fail at this point, call Hologic | R2 Technical Support. Hologic | R2 does
not recommend that you use the system if any of the tests failed.
Scanning is disabled, remote maintenance in progress (5010, 5059)
Hologic | R2 Technical Support is working on your system, and you’ll need to wait
until they are done.
• Wait two minutes. The Ready message will appear above the Start button.
• If your Hologic | R2 Technical support is done and the message does not go away,
power-cycle the system. See ‘
• If this doesn’t fix the problem, call Hologic | R2 Technical Support.
6.3. Power-Cycling the System’.
Scheduled reimbursement report (5050)
Appears when the system prints a reimbursement report automatically (weekly or
monthly, depending upon the system configuration). This is normal. The message
does not appear when you print a reimbursement report manually. See ‘
Options
’ for more information.
5.7. Printing
Shutting down the operating system, please wait (5020)
This means the system is shutting down.
• Let the system shut down. Usually, it shuts down only when instructed to do so.
• If this happened on its own, power-cycle the system to bring the system back up.
See ‘
6.3. Power-Cycling the System’.
• If the alert continues to appear, contact Hologic | R2 Technical Support.
Shutting down the R2 software, please wait (5018)
This means the system is shutting down. It will shut down the R2 Technology
software, then the operating system.
• Let the system shut down. Usually, it shuts down only when instructed to do so.
• If this happened on its own, power-cycle the system to bring the system back up.
See ‘
6.3. Power-Cycling the System’.
• If it happens again, contact Hologic | R2 Technical Support.
The same barcode has been assigned to multiple patients (3500)
This alert appears when you are using the mammography reporting system. It means
you assigned the same R2 Technology barcode number to two different patients
within the reporting system.
The images in the R2 system are associated to the patient record in the reporting
system by the barcode number on the separator sheet. When you scan the studies, the
R2 system searches for the patient record in the reporting system database that has
that barcode and associates the images to that patient record. If there is more than
one patient record with the same barcode, the system does not know which patient
the images belong to.
R2 DMax User Manual – PN MAN-00709 Rev 001 61
Page 70

Part 4: Alerts
• Access the reporting system database and check the patient records.
• Replace the duplicate barcode number with a new barcode number. Use this new
sheet to scan the case.
The scanner is not responding (1007)
The internal scanner software is not responding to signals from the processing unit.
By power-cycling the system, you can reset the internal scanner software.
• Power-cycle the system. See ‘
• If this doesn’t fix the problem, call Hologic | R2 Technical Support.
6.3. Power-Cycling the System’.
The scanner might be empty (3033)
The scanner is not detecting any films in the scanner.
• Ensure that the films and separator sheets are properly loaded (e.g., are not half fed
in the feeder).
• Reload the case and restart scanning.
• If these steps do not solve the problem, call Hologic | R2 Technical Support.
X films were found when Y were expected. (1017, 1018)
This usually happens when (1) a separator sheet is missing from the stack or is upside
down, (2) the system failed to read the barcode for the next case in the stack (the
barcode is obscured, smudged, or damaged), or (3) a case did not have the number of
films specified by protocol.
• Check to see if the next case is missing its separator sheet or if the barcode is
obscured somehow. Add a separator sheet, or clean the separator sheet. If films for
two or more patients were scanned under a single separator sheet, rescan the case.
• In many situations, you can simply correct the image labels and reprocess the
images via the Image Correction screen. See ‘
more information.
3.9. Verifying Scanned Cases’ for
• If these steps do not fix the problem, call Hologic | R2 Technical Support.
62 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 71

4.2. Common Questions
This Section provides common issues and solutions for R2 systems.
CAD does not run on views with implants
CAD does not run on images with the Implant Present modifier enabled, even if
Implant Displaced is also enabled. If it is necessary to run CAD on Implant Displaced
images, do not enable the Implant Present modifier either on the Image Correction
screen or in scanning protocols.
CAD marks do not appear on thumbnails
Marks do not display on the thumbnails. They only appear on the display unit, print
out, and/or archive system (if configured).
Case icons become yellow alerts; there are no images
Typically, an alert will be generated when an image fails to process. If it did, follow the
instructions in the alert, or turn to the Alerts tab and look for that alert.
If an alert did not pop up, and there are no alerts in the Alerts screen, rescan the case
using the appropriate Manual scanning protocol.
4.2. Common Questions
Film is ‘missing’ inside the scanner
If a film jams in the scanner, see ‘Ejecting Films’ on page 74.
Image is missing from a case
Occasionally, the film scanner reads the separator sheet barcode through the last film
of a case. Although extremely rare, this can result in a case where the last image is
missing. Sometimes an alert will appear (‘X films were found when Y were expected’),
but not always. This situation is most likely to occur with an LMLO image from a film
with a light pectoral muscle, or with any film that has an extremely light area in the
upper-left corner.
If you suspect that the film scanner has read a separator sheet barcode through a film,
then reorient the film and rescan the case. As an alternative, run the case at the
bottom of the next stack of cases.
Images are reversed in the thumbnails
Typically, this happens either when you are scanning with a Manual protocol and did
not stack the films in the order expected by the R2 system or when you are scanning
with the Auto protocol and the lead markers are bad or obscured.
• Use the Image Correction screen to flip the images to the correct orientation and
reprocess. See ‘
• If the problem persists, contact Hologic | R2 Technical Support.
3.9. Verifying Scanned Cases’.
Images at PACS/workstation are of poor quality
Contact Hologic | R2 Technical Support. In some cases, a service representative may
need to adjust the contrast and brightness settings for your system. Also note that
some DigitalNow ELC Enhanced MG images will not be rendered well, in particular,
if (1) a film is very dark, (2) a normal-view image has a large, bright artifact, or (3) a
R2 DMax User Manual – PN MAN-00709 Rev 001 63
Page 72

Part 4: Alerts
special-view image has a very dense region (i.e., as bright as regions with implants
elsewhere in the image).
Images didn’t print out
• Make sure the study processed successfully, or that there were no printer alerts. If a
case did not scan and/or process successfully, it will not print out.
• If the system couldn’t find the printer, it will generate an alert. To find out if there
was an alert, check the Alerts screen.
• If the study processed correctly, check to make sure that printing is enabled. See
‘
Configuring CAD Results Printing’ on page 76 for information on turning
printing on/off. If printing is turned off, turn it on and reprocess the study in the
Image Correction screen.
• If none of these steps fixes the problem, contact Hologic | R2 Technical Support.
Note: As of version 8.5, not all scanned images are printed. Only four images appear
on the printout. Images which have not been processed by CAD, and reversed views do
not print.
Images do not appear on my display unit
Typically, this happens when there is a delay on the processing unit.
• Check the processing unit for alerts.
• If there were no alerts, wait ten minutes, check the display unit again.
• Ask your system administrator if there have been network problems.
• If after ten minutes the images don’t display and your network is up, contact your
Hologic | R2 Service Representative.
Note: R2 display units do not display all scanned images. For more information, see
‘
2.6. System Inputs and Outputs’.
Monitor isn’t working
The monitor power switch may have been accidentally turned off. The processor unit
is probably still working. Switch the monitor back on. If this doesn’t work, verify that
the monitor power cable is securely fastened.
Main screen is frozen and I can’t do anything
Contact Hologic | R2 Technical Support. They will log in remotely and troubleshoot
the system. DO NOT just turn off the system; if you do, you will not allow the
software to shut down properly, which can result in other problems.
Patient ID flash on the printout is backwards
• Typically, this happens when you scan the case with a Manual protocol and loaded
the stack or case upside down. See ‘
on adjusting the image orientation.
3.9. Verifying Scanned Cases’ for instructions
• This problem can also occur if the service configuration is incorrect. If the
problem persists, contact Hologic | R2 Technical Service.
64 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 73

Radiologist needs CAD quickly
For sites that archive images to PACS, a radiologist may request a quick turnaround
for CAD results from certain films without waiting for the system to scan all films.
Splitting the films into two cases may be undesirable because that produces separate
studies at the PACS for the case. In such a situation, you can turn off archiving (see
5.4. Outputs Options), scan and process the requested views, and print the results for
the radiologist. Then, turn archiving back on, scan and process all films for the case,
and send the all results for the case to the PACS.
Scanned case before entering patient information
You can enter the patient information after scanning and reprocess the study to link
the images to the proper patient. You must include the barcode from the separator
sheet.
4.2. Common Questions
• See ‘
• See ‘
Part 7: Using DigitalNow and the R2 Patient ID System’ for more information
on entering patient information.
3.9. Verifying Scanned Cases’ for information on reprocessing a study.
Yellow ‘X’ appears on my image thumbnails
The yellow ‘X’ icon indicates that CAD processing could not be performed on an
image.
• Verify that the images have the correct view labels. If they do not, correct the view
labels on the Image Correction screen. See ‘
information.
• Verify that the image views and view modifiers are supported for CAD processing.
See ‘
2.6. System Inputs and Outputs’ for more information.
Even though no processing was possible, the scanned images are archived as usual.
3.9. Verifying Scanned Cases’ for more
R2 DMax User Manual – PN MAN-00709 Rev 001 65
Page 74

Part 4: Alerts
66 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 75

Part 5: Controls
f 5.1. About Option
f
5.2. Maintenance Options
f
5.3. Shutdown and Reboot Options
f
5.4. Outputs Options
f
5.5. Patient ID Option
f
5.6. Performance Options
f
5.7. Printing Options
f
5.8. Scanning Protocols
f
5.9. Service Utilities
Part 5 provides instructions for using the system configuration and maintenance
options that appear on the
system settings to meet your particular needs, run maintenance utilities, and display
system information.
Controls screen. The Controls screen is used to configure
Touch a screen
name to display its
contents.
Note: The Outputs, Patient ID, and Printing options appear only if they were
configured by the Hologic | R2 service engineer when the system was installed.
R2 DMax User Manual – PN MAN-00709 Rev 001 67
Page 76

Part 5: Controls
5.1. About Option
The About option on the Controls screen displays information about the
configuration of your R2 film-scanning system on the network. This information is
configured by service personnel when the system is installed. Normally, you will not
need to refer to this information.
X To display the About screen
Touch the Controls tab and then select About (if the About screen does not appear).
68 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 77

5.2. Maintenance Options
The Maintenance option on the Controls screen allows you to review whether
scheduled maintenance is needed.
X To display the Maintenance screen
Touch the Controls tab and then select Maintenance.
5.2. Maintenance Options
The screen identifies any required maintenance procedures with a yellow alert icon.
For further information, see ‘
Part 6: Maintenance’.
R2 DMax User Manual – PN MAN-00709 Rev 001 69
Page 78

Part 5: Controls
5.3. Shutdown and Reboot Options
The Off option on the Controls screen allows you to:
• Reboot (restart) the system
• Shut down the system
X To display the Off screen
Touch the Controls tab and then select Off.
Use the Shutdown option whenever you need to power-cycle the system. Normally,
you will shut the system down on a weekly basis and sometimes during troubleshooting. For further information, see ‘
6.3. Power-Cycling the System’.
When you touch Reboot or Shutdown, a confirmation window appears which
requires you to either confirm or cancel the power down command.
70 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 79

5.4. Outputs Options
The Outputs option on the Controls screen allows you to specify whether you want
R2 Technology’s archiving feature turned on.
Note: This option appears only if it was configured by the Hologic | R2 service
engineer when the system was installed.
In order for Patient ID and Archiving to function, first a Hologic | R2 service engineer
must configure your system to communicate to a patient database and the external
storage provider (i.e., archive), respectively. The Archiving settings are available only
when this feature has been licensed and properly configured on your system.
X To display the Outputs screen
Touch the Controls tab and then select Outputs.
5.4. Outputs Options
• To enable or disable this feature, touch On or Off, as desired.
• To verify each study before it is archived, touch
On with verification.
Important: Archiving will function only if the reporting system is licensed and
configured properly.
Note: Switching Archiving to ‘On’ has no effect on studies currently waiting to be
verified. You must still manually verify each case.
R2 DMax User Manual – PN MAN-00709 Rev 001 71
Page 80
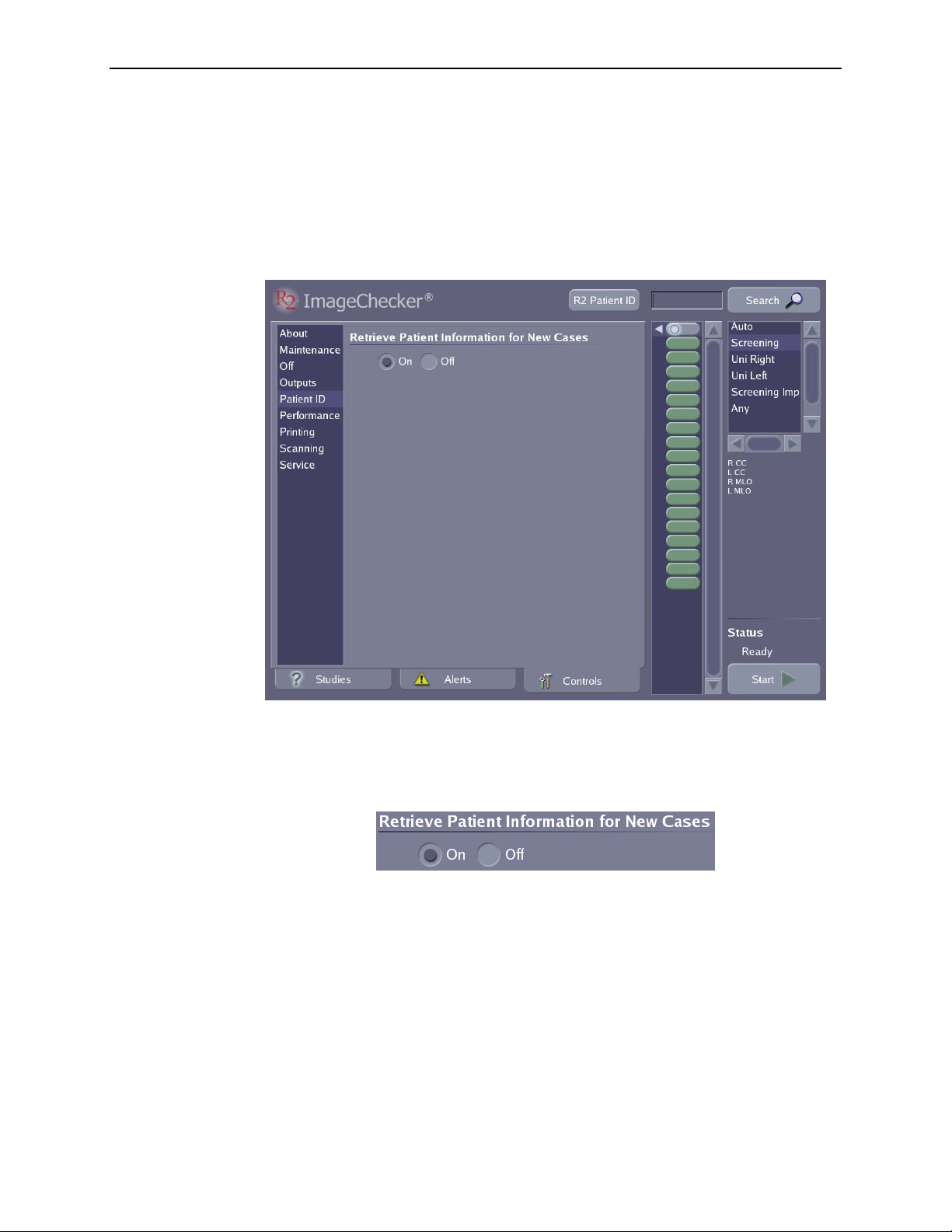
Part 5: Controls
5.5. Patient ID Option
The Patient ID option on the Controls screen allows you to specify whether the
R2 system should retrieve patient information from your reporting system or patient
information database. If set up properly, when you scan the R2 ID, the R2 system will
request that the reporting system transmit the patient information associated with the
case. This option only appears if your site has licensed the reporting system
integration feature.
A Hologic | R2 service engineer must first configure the R2 system to communicate
with the reporting system. Once configured, you can enable or disable the data
transfer using the Retrieve Patient Information setting on the Patient ID screen.
To enable or disable this feature, simply touch On or Off, as desired.
72 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 81
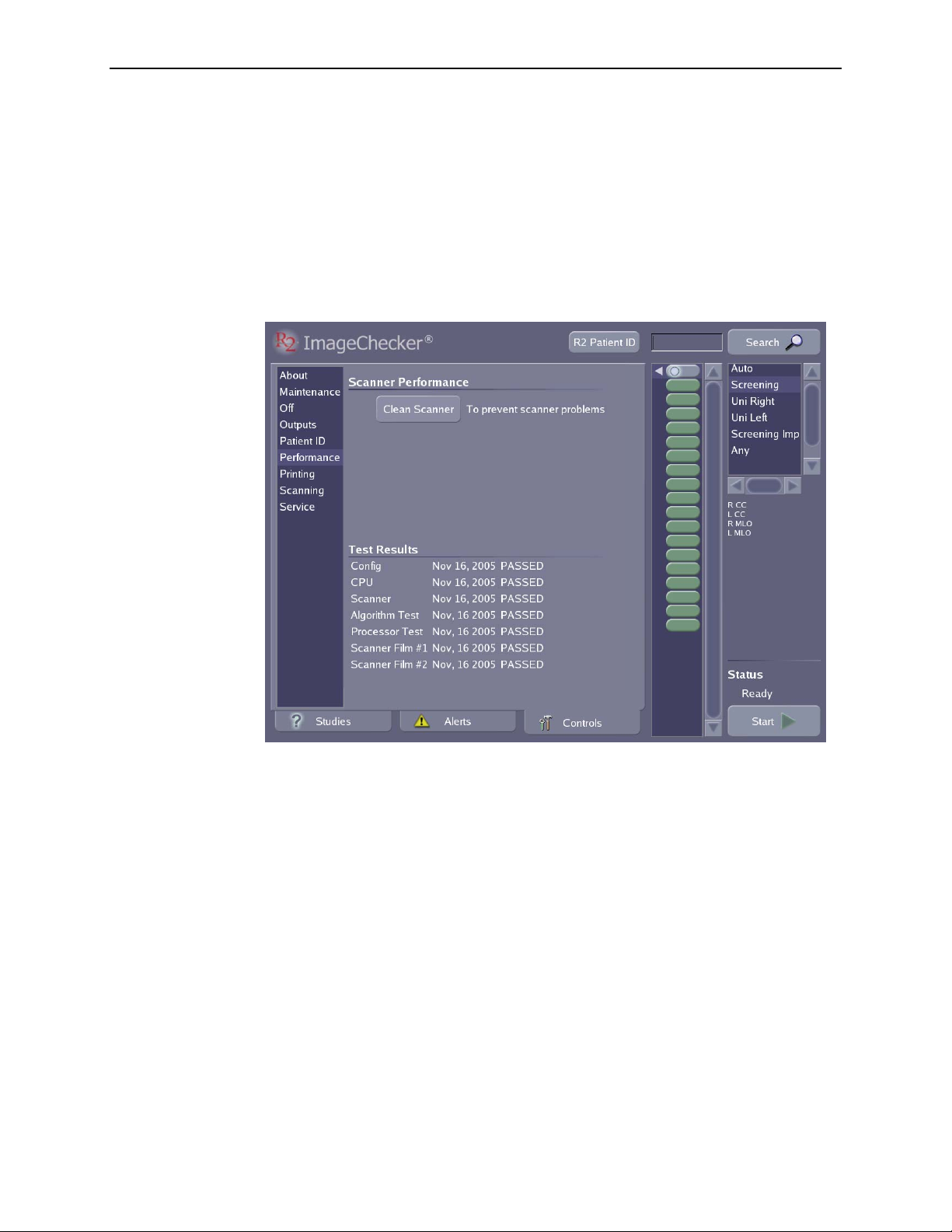
5.6. Performance Options
The Performance option on the Controls screen provides access to the:
• Film ejection procedure
• Scanner cleaning procedure
• Results of system verification testing
X To display the Performance screen
Touch the Controls tab and then select Performance.
5.6. Performance Options
R2 DMax User Manual – PN MAN-00709 Rev 001 73
Page 82
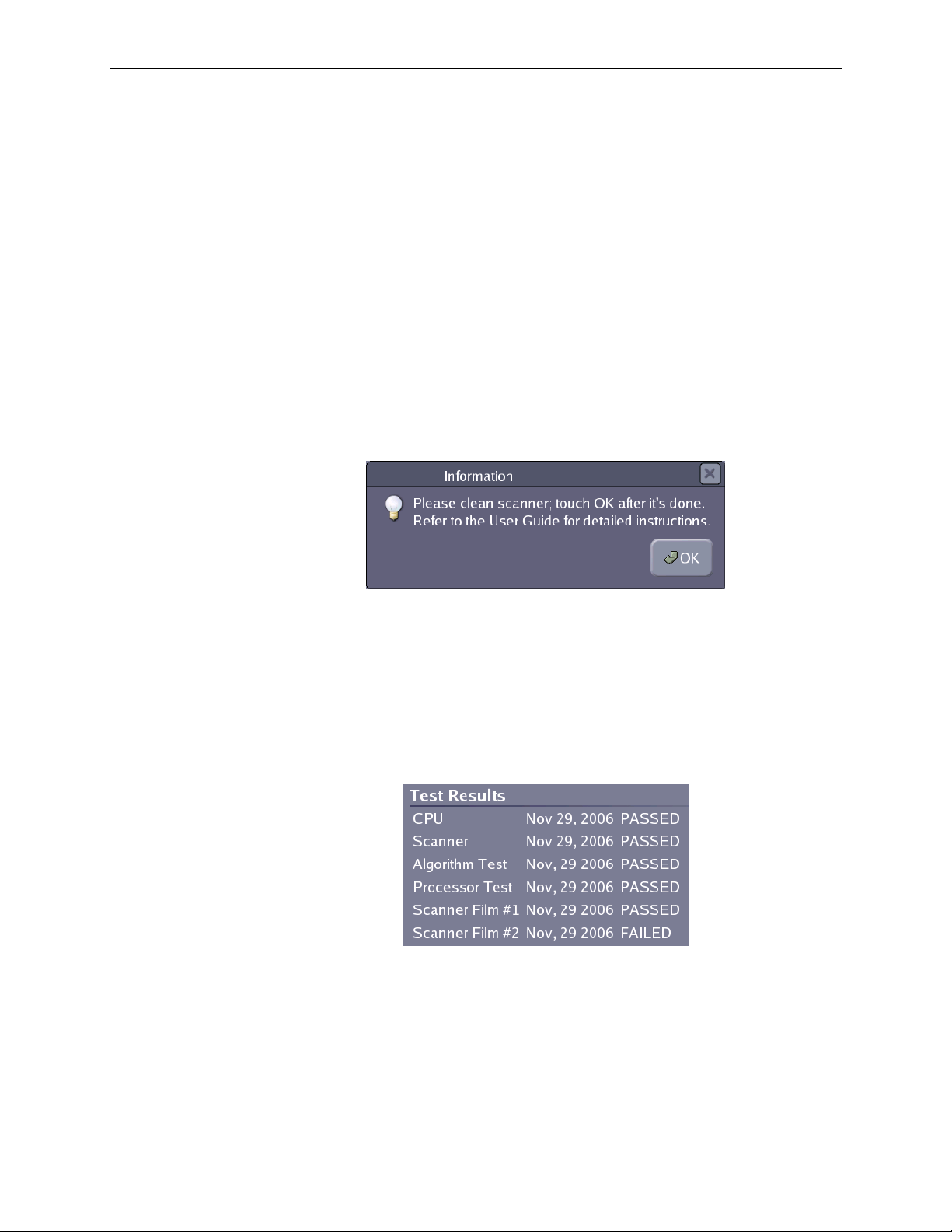
Part 5: Controls
Ejecting Films
Use the following procedure if a film seems to be jammed inside the scanner.
X To remove a jammed film
1
Lift up the interlock (the protective shield on the input tray). If you can see the film,
gently pull it out, using both hands and pulling gently but firmly.
2 If you can’t see the film, or can’t get it out, lift up the scanner control panel cover.
Press the UP and DOWN arrows at the same time and hold them down for 3 seconds.
The film should come out.
3 If the preceding step doesn’t eject the film, power-cycle the system. See ‘6.3. Power-
Cycling the System
4 If the preceding steps do not fix the problem, call Hologic | R2 Technical Support.
Resetting the Clean Scanner Timer
When you touch the Clean Scanner button, the system displays:
’.
Clean the scanner as instructed in ‘Part 6: Maintenance’. When you finish cleaning
the scanner, touch the
OK button shown above. When you touch OK, the Scanner
Cleaning message on the Maintenance screen changes from ‘Needed’ to ‘OK’.
If you do not clean the scanner, touch the ‘X’ shown above to cancel the timer reset.
Reviewing System Verification Results
The Test Results portion of the Performance screen provides the results of the most
recent system verification testing, including the scanner test.
For more information on the tests, see ‘6.4. Running the Weekly Tests’.
74 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 83
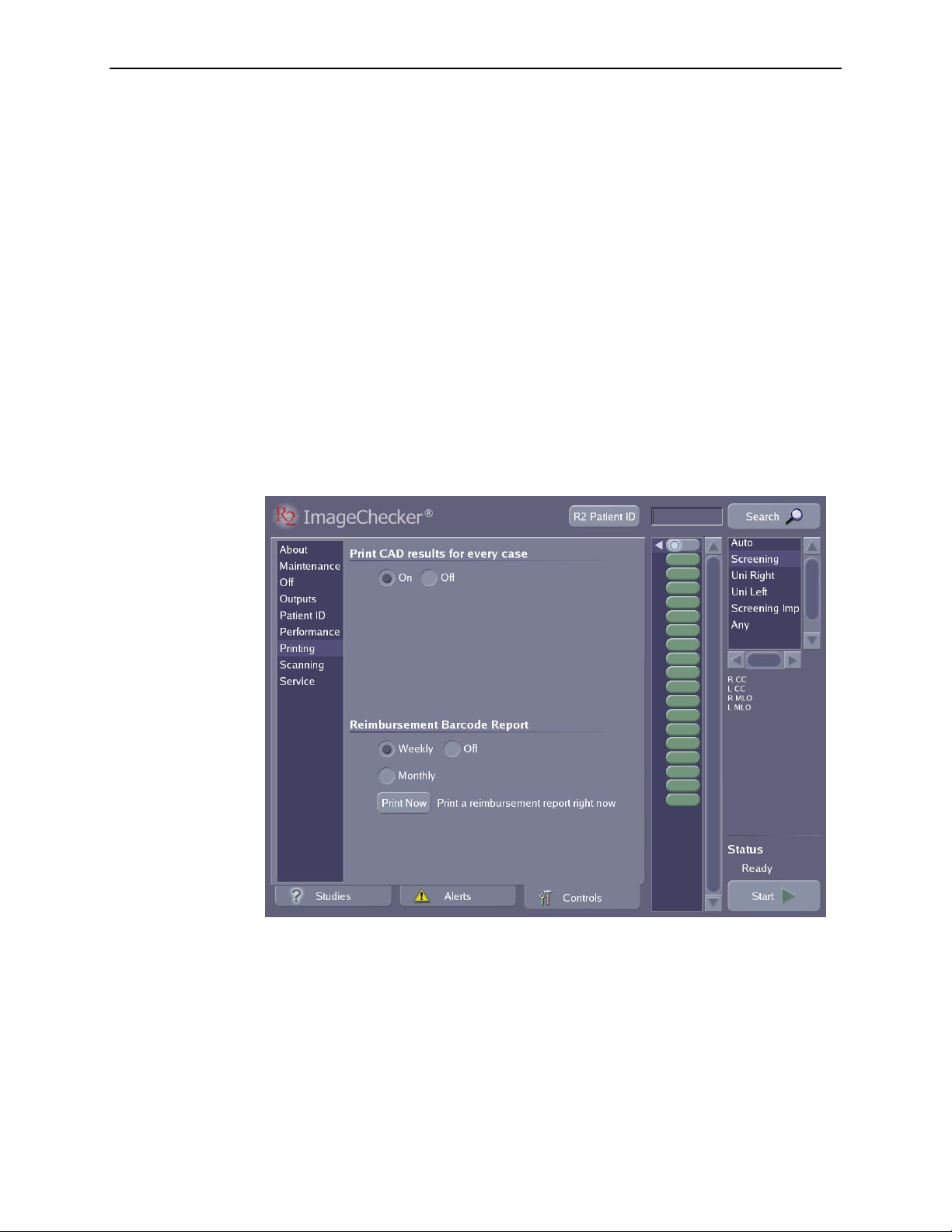
5.7. Printing Options
The Printing option on the Controls screen allows you to:
• Configure the system to send images and CAD results to a printer on your
network. See ‘
• Configure the system to print the barcode reimbursement report on a weekly or
monthly basis (or not at all).
5.7. Printing Options
Configuring CAD Results Printing’ on page 76.
• Print the barcode reimbursement report. See ‘
Barcode Report will be Printed
’ on page 76.
Setting When the Reimbursement
Note: Printing options appear only if configured by the Hologic | R2 service engineer
when the system was installed.
Note: Not all scanned images are sent to the printer. For more information, see ‘2.6.
System Inputs and Outputs
Once configured, you can use this screen to decide how you want to use the printer.
X To display the Printing screen
Touch the Controls tab and then select Printing.
’.
R2 DMax User Manual – PN MAN-00709 Rev 001 75
Page 84

Part 5: Controls
Configuring CAD Results Printing
You can configure the system so that it
• Prints images with CAD results for every case, or
• Does not print at all.
To enable or disable this feature, touch
On or Off, as desired.
Setting When the Reimbursement Barcode Report will be Printed
You can choose to print a Reimbursement Barcode Report either automatically on a
weekly or monthly basis, or not at all.
• To specify automatic printing of the reimbursement report, touch
Monthly.
• To disable automatic printing of the reimbursement report, touch
Weekly or
Off.
Printing a Reimbursement Barcode Report
X To print a reimbursement report
1
Touch Print Now. A calendar window appears.
2 Specify the date range and touch Print.
76 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 85

5.7. Printing Options
An example of a Reimbursement Report is shown below. The clinic information in
the header can be configured by a Hologic | R2 service engineer. For each case, the
report prints the R2 ID from the separator sheet, or the patient information, if the
system was able to retrieve the patient information from the reporting system.
CASE HISTORY REPORT: Mar 07 2007 - Mar 13 2007
Mar 07 2005 Wed
Barcode:
0000095458
Case Time:
17:38:53
Barcode:
0000095460
Case Time:
17:41:09
Barcode:
0000095461
Case Time:
17:43:26
Name:
Sellers, Patricia
Case Time:
17:45:32
Name:
Eldridge, Jessica
Case Time:
17:47:53
Name:
Binder, Taylor
Case Time:
17:49:59
Name:
Keefer, Ruby
Case Time:
17:52:08
ImageChecker
Computer Aided Detection
ID: Birthdate:
ID:
ID:
ID:
406893192
ID:
289450104
ID:
338495028
ID:
029478850
®
Westside Clinic
1234 Eastside Drive
Northside, CA 95014
United States
Birthdate:
Birthdate:
Birthdate:
19510501
Birthdate:
19180702
19530401
Birthdate:
19261209
Birthdate:
R2 DMax User Manual – PN MAN-00709 Rev 001 77
Page 86
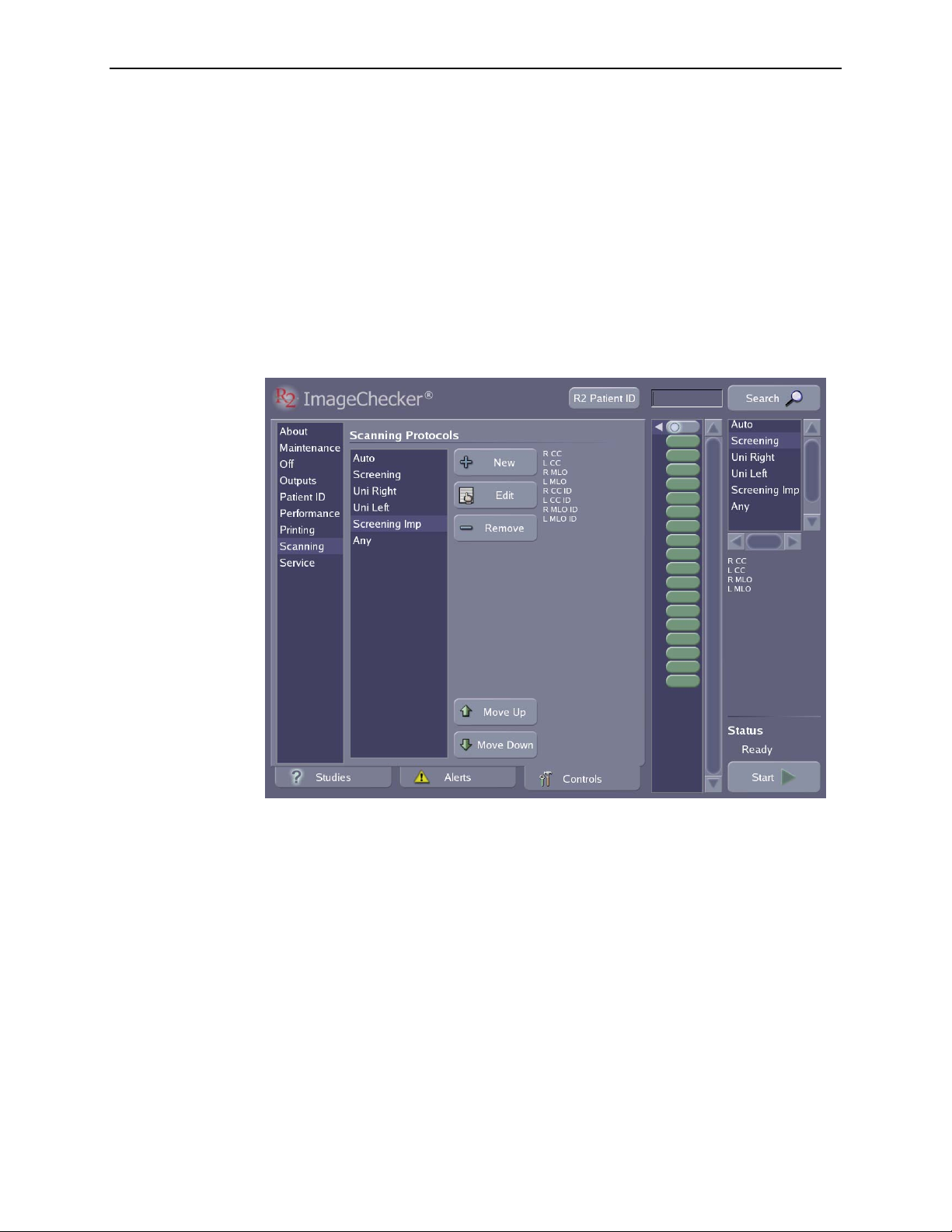
Part 5: Controls
5.8. Scanning Protocols
The Scanning option allows you to perform the following procedures:
• ‘
Changing Scanning Protocol Order’ on page 79
• ‘
Changing the Default Scanning Protocol’ on page 79
Deleting a Scanning Protocol’ on page 79
• ‘
• ‘
Creating Scanning Protocols’ on page 80
• ‘
Editing Scanning Protocols’ on page 81
X To display the Scanning screen
Touch the Controls tab and then select Scanning.
78 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 87

Changing Scanning Protocol Order
You can change the order in which the scanning protocols appear in the scanning
protocol list on the Studies screen.
X To change the scanning protocol order
1
Touch a scanning protocol in the list at the left of the screen.
2 Touch the Move Up and Move Down buttons to move the selected scanning protocol
up or down in the list.
Changing the Default Scanning Protocol
The default scanning protocol appears at the top of the scanning protocol list.
X To change the default scanning protocol
1
Touch the desired default scanning protocol in the list at the left of the screen.
2 Touch the Move Up button to bring the desired protocol to the top of the list.
Deleting a Scanning Protocol
You can delete unwanted scanning protocols.
5.8. Scanning Protocols
X To delete the default scanning protocol
1
Touch the desired scanning protocol in the list at the left of the screen.
2 Touch Remove.
3 Confirm that you want to delete the protocol.
Note: You can delete any scanning protocol except the Auto protocol.
R2 DMax User Manual – PN MAN-00709 Rev 001 79
Page 88
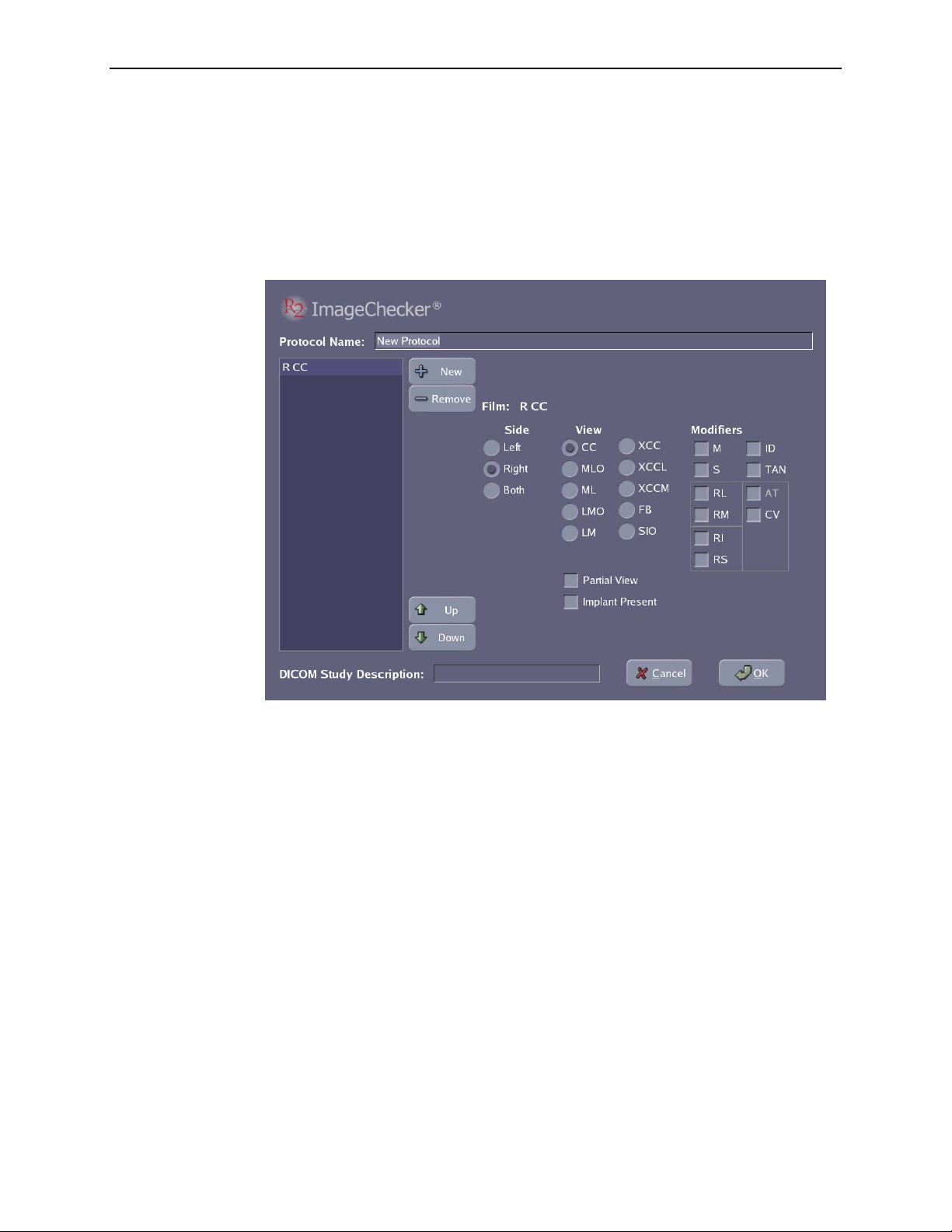
Part 5: Controls
Creating Scanning Protocols
The Scanning option on the Controls screen allows you to create custom scanning
protocols, and edit or delete existing protocols.
X To create a new scanning protocol
1
Touch New on the Controls Scanning screen to display the Edit Protocol Screen.
2 Touch (or click in) the Protocol Name field, and enter a name for your new protocol.
3
Touch New to add a film view to the protocol. Build the film view from left to right:
select a side, a view, and any modifiers.
a Touch the proper Side selection: Left, Right, or Both (if cleavage view).
b Touch to select the View. Note the two additional selections below the view area
for Partial View and Implant Present.
c Touch to select any modifiers needed.
Note: As you build the film view, invalid selections are eliminated. For example if you
choose Both for the side, the view is set to CC and the modifier is set to CV. Valid
modifiers can be chained together as needed. Some modifiers (for example, M and S) are
added before the view designation, other modifiers (such as ID and TAN) are added after.
For a list of supported views see ‘
4
Repeat step 3 for each film view you wish to add to the scanning protocol.
• To move a view, touch a view name in the list at left and then touch
• To remove the view from the scanning protocol, touch a view name in the list at
left and then touch
Remove.
• To enter a study description, touch the
5 Touch OK when finished.
CAD-Supported Views’ on page 26.
Up or Down.
DICOM Study Description field.
80 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 89

Editing Scanning Protocols
You can edit scanning protocols from the Controls Scanning screen.
X To edit a scanning protocol
1
Touch a scanning protocol in the list at the left of the screen.
2 Touch Edit to open the Edit Protocol screen.
3 Edit the Protocol. You have the following options:
5.8. Scanning Protocols
• To change the view order, touch a view name in the list at the left, then touch
or
Down as needed.
Up
• To remove the view from the scanning protocol, touch the view name, then touch
Remove.
• To enter a new name for the protocol, touch the Protocol name, then enter a new
name.
• To add a new view to the protocol, touch
New. Build the view from left to right
starting with the side, then a view, then modifiers.
• To add or change the DICOM study description, touch the field and edit the study
description as desired.
4 Touch OK when finished, or touch Cancel to cancel changes to the scanning protocol.
Note: You can edit any scanning protocol except the Auto protocol. If you delete all
views from a Manual protocol, the protocol will be equivalent to the Any protocol.
R2 DMax User Manual – PN MAN-00709 Rev 001 81
Page 90

Part 5: Controls
5.9. Service Utilities
The Service option on the Controls screen allows:
• Calibrating the touch screen
• Access to the service tools (for service personnel only)
X To display the Service screen
Touch the Controls tab and then select Service.
Calibrating the Touch Screen
Calibrating the touch screen can make it easier to precisely touch the buttons and
other controls.
To calibrate, first touch Calibrate Touch Screen, and then follow the simple onscreen instructions. When you finish, you will have to restart the system to save the
new settings.
Connecting to the Service Tool
Access to the service tool is password protected and intended solely for use by
authorized Hologic | R2 service personnel.
82 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 91

Part 6: Maintenance
f 6.1. Maintenance Reminders
f
6.2. Cleaning the Scanner
f
6.3. Power-Cycling the System
f
6.4. Running the Weekly Tests
f
6.5. Resetting the Handheld Barcode Scanner
Part 6 provides maintenance procedures that must be performed
optimal system performance. Hologic | R2 strongly recommends you follow all
maintenance instructions given in this manual.
6.1. Maintenance Reminders
weekly to ensure
The system automatically issues a reminder message whenever a required
maintenance procedure must be performed. The reminder messages are listed below.
Diagnostic tests failed
The scanner diagnostic tests failed. Each time the system starts up, the scanner
performs tests to make sure all internal systems are working properly. You cannot
scan until the scanner passes its tests. To fix the problem, power-cycle the system. See
‘
6.3. Power-Cycling the System’. If this doesn’t resolve the problem, call Hologic | R2
Technical Support.
Reminder: Please clean the scanner
Clean the scanner regularly in order to keep the system performing optimally. For
instructions on cleaning the scanner, see ‘
Test Films: Needed
The system test films have not been run, are overdue, or have failed. To fix the
problem, see ‘
6.4. Running the Weekly Tests’. (More information about individual
film tests is available on the Controls Performance page; see ‘
Options
’.)
Preventive maintenance is required
Your system is due for its quarterly preventive maintenance servicing. Call
Hologic | R2 Technical Support to schedule an appointment. In the meantime, you
can continue scanning. If the system is not serviced within 180 days, the alert will
appear continuously.
6.2. Cleaning the Scanner’.
5.6. Performance
R2 DMax User Manual – PN MAN-00709 Rev 001 83
Page 92

Part 6: Maintenance
6.2. Cleaning the Scanner
To ensure optimum scanner performance, at least once each day clean the scanner
diffuser, and then clean the scanner’s suction cups using alcohol and the cleaning
swabs provided with the system.
Note: If you are running less than 80 cases daily, you can clean the scanner once
each day. If you are running 80 or more cases daily, Hologic | R2 recommends that you
clean the scanner twice each day.
To clean the scanner diffuser
1
Place the diffuser cleaner in the input tray, with the cleaning strip facing up, closest to
you.
Control Panel
Diffuser Cleaner
Cleaning Strip
Diffuser Cleaner
2
Lift the cover so you can see the scanner control panel.
3 On the scanner control panel:
a Press QUIT.
b Press MENU until ‘4 CLEANING MODE’ appears on the scanner control panel
display, and then press ENTER.
c Press the UP/DOWN button until ‘DIFFUSER CLEANING’ appears on the
display, and then press ENTER.
d Press START/STOP. The scanner loads the diffuser cleaner.
e When you see ‘DIFFUSER CLEANING PULL OUT CLN_FILM’ on the display,
pull out the cleaner sheet, gently but firmly.
4 Repeat steps 1–3 two more times.
Note: If an error occurs, run the diffuser cleaner through the system six times, and
then shut down and restart the system.
Store the diffuser cleaner in a safe, clean location.
5
84 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 93

6.2. Cleaning the Scanner
X To clean the suction cups
1
Place about 5 drops of 70% isopropyl alcohol onto each pad of the suction cup
cleaning plate, and rub it in gently with your finger.
Note: Use isopropyl alcohol regardless of any instructions on the cleaning plate.
2
Place the suction cup cleaner in the input tray, adjacent to the left side of the tray.
Control Panel
Fabric Pads
Suction Cup
Cleaner
Suction Cup Cleaning Plate
Lift the cover so you can see the scanner control panel.
3
4 On the scanner control panel:
a Press QUIT.
b Press MENU until ‘4 CLEANING MODE’ appears on the control panel display,
and then press ENTER.
c ‘SUCTION PAD CLEANING’ appears on the display. Press ENTER to continue.
d When ‘SET CLN_PLATE & PUSH <START/STOP>’ appears on the display, press
START/STOP.
5 Repeat step 4 two more times.
6 Remove the suction cup cleaning plate from the tray and store it in a safe, clean
location along with the diffuser cleaner.
Caution: After cleaning the suction cups, wait at least five minutes before scanning
any films. Residual alcohol can damage the films!
7
After cleaning the suction cups, touch the Controls tab and then Performance.
8 Touch the Clean Scanner button. The system displays:
9
Touch OK. When you touch OK, the Scanner Cleaning message on the Maintenance
screen changes from ‘Needed’ to ‘OK’.
R2 DMax User Manual – PN MAN-00709 Rev 001 85
Page 94

Part 6: Maintenance
6.3. Power-Cycling the System
‘Power-cycling’ means to shut down the computer and then switch it back on. By
power-cycling once a week, you will help keep the processing unit computer
operating properly.
X To power-cycle the system
1
Touch the Controls tab and then select Off.
2 Touch the Shutdown button. After the software application shuts down, the monitor
displays ‘Power down…’
3 Release the front cover as shown below.
Front Cover Removal
WARNING! In the next step, be careful when lifting the cover; it’s heavy.
4 Lift the cover up to free its top lip from the frame on which it hangs, and then set it
aside.
86 R2 DMax User Manual – PN MAN-00709 Rev 001
Magnetic Strips (2)
Under Cover
Page 95

6.3. Power-Cycling the System
5
Switch off the system using the power switch beneath the power strip.
Power Strip
System
Power Switch
Shutting Off the System
Wait ten (10) seconds. Switch on the system using the power switch. When the
6
Studies screen appears, the system will be ready for use.
R2 DMax User Manual – PN MAN-00709 Rev 001 87
Page 96
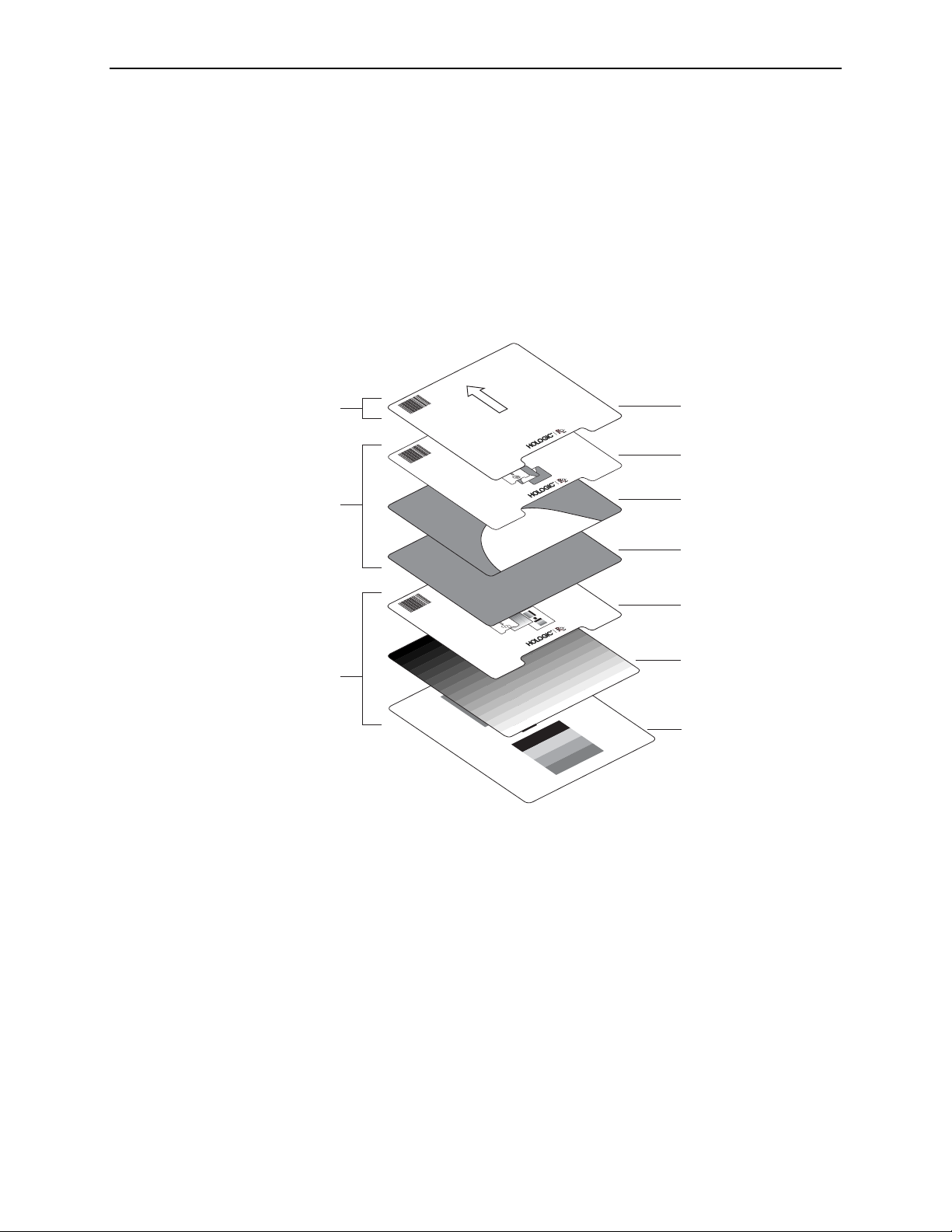
Part 6: Maintenance
6.4. Running the Weekly Tests
Three tests must be run weekly to ensure optimal system performance. Always run
the tests together and in this order: Algorithm on top, then Processor, then Scanner –
think ‘APS’!
X To run the weekly tests
1
Stack the test sheets and films as shown. Note that the test films are ordered as if they
were ordinary films.
Important! If your system does not have the CAD license, do not run the Algorithm
and Processor tests. Run only the Scanner test films.
Algorithm
Processor
9999999906
9999999905
9999999901
No films required
Test #1- Algorithm
Test #2- Proc
P/N 10743 Rev B
est #3- Scanner
T
essor
Pr
Proc
ocessor
essor
Test 1
T
est 2
1
2
Sheet #1 Algorithm
Sheet #2 Processor
Film #1 Processor
Film #2 Processor
Sheet #3 Scanner
Film #1 Scanner (Step)
Scanner
Film #2 Scanner (MTF)
2
Place the stack in the input tray and touch Start. The computer recognizes the test
film barcode numbers and automatically changes to Test mode. The test requires
about five minutes to complete.
3 When done, review the results by touching the Controls tab and then selecting the
Performance option.
4 Verify that all test results read ‘PASSED’.
• If all tests pass, you’re done for the week.
• If any test reads FAILED, rerun ALL the tests. Be sure that the test sheets and films
are loaded in the correct order. If any test fails again, power-cycle the system (see
‘
6.3. Power-Cycling the System’) and rerun all the tests.
• If any test fails again, call your Hologic | R2 service representative.
88 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 97

6.5. Resetting the Handheld Barcode Scanner
6.5. Resetting the Handheld Barcode Scanner
Occasionally it is necessary to reset the handheld barcode scanner software to its
default settings. If the barcode scanner stops reading barcodes properly, use the
barcode labels provided in this section to reset it.
X To reset the handheld barcode scanner
1
Scan the ‘Enter/Exit Configuration Mode’ barcode shown below. When you scan the
barcode, the scanner should beep three times.
2
Scan the ‘Recall Defaults’ barcode. When you scan the barcode, the scanner should
beep once. This erases all previous settings and returns the barcode scanner to its
factory default communications settings.
R2 DMax User Manual – PN MAN-00709 Rev 001 89
Page 98

Part 6: Maintenance
90 R2 DMax User Manual – PN MAN-00709 Rev 001
Page 99

Part 7: Using DigitalNow and the R2 Patient ID System
f 7.1. Overview
f
7.2. DigitalNow Archiving
f
7.3. Using the R2 Patient ID System
f
7.4. Assigning R2 Barcodes in Other Databases
Part 7 provides information about DigitalNow, which provides archiving features and
the R2 Patient ID system.
7.1. Overview
The R2 DM system supports the archiving of digitized film images and CAD results
for supported DICOM views. You can enable and disable the archiving features by
means of the Outputs option on the Controls screen. At the time of installation, the
features are set either to On or Off, based on your preference.
The Archiving feature requires a patient information database from which to draw
the necessary patient information. This ensures that the images and CAD results are
stored properly in the archive. Therefore, the Patient ID feature must be enabled and
the system configured to communicate with a reporting system (e.g., R2 Technology
Patient ID, MagView, MRS, or PenRad) in order for the Archiving feature to
function. In addition, the R2 system must be configured to communicate with the
archive system.
If both Patient ID and Archiving are enabled, the images and any CAD results are sent
to the archive system after the images are processed.
These archived images and CAD results, like any other record in the archive system,
can then be retrieved and viewed on softcopy review workstations and/or printed.
R2 DMax User Manual – PN MAN-00709 Rev 001 91
Page 100

Part 7: Using DigitalNow and the R2 Patient ID System
7.2. DigitalNow Archiving
The workflow for DigitalNow archiving is as follows:
1 At any point prior to scanning film mammograms for a case, you must enter the R2
barcode on the separator sheet used for the case into the record for the appropriate
patient in the patient information database.
2 Then, the films can be scanned. The system retrieves the patient information from the
patient database, and transfers the images and CAD results to the archive system. The
images and CAD results are also sent to the display units to which the R2 system is
configured to send.
If images are scanned with a Manual protocol, and archiving is set to ‘On with
verification’, you must verify the images are correct before they can be sent to the
archive. For more information, see ‘
Once the images and CAD results are stored in the archive, they can be manipulated
like any other record in the archive system. You can send them to the softcopy digital
review workstation or printer. Keep in mind that once film mammograms are
digitized and archived, they are treated like digital images and will not be sent to
display units other than a softcopy digital review workstation or printer.
3.9. Verifying Scanned Cases’.
If archiving is enabled, and no record in the patient database matches the R2
Technology barcode, the system does not process the case. You can correct this by
entering patient information in the database after the case is scanned and
reprocessing the case.
92 R2 DMax User Manual – PN MAN-00709 Rev 001
 Loading...
Loading...