Page 1

1
ENGLISH
U.S. FEDERAL LAW RESTRICTS THIS DEVICE TO
SALE BY OR ON THE ORDER OF A PHYSICIAN
Read these instructions completely prior to using the Omni Hysteroscope.
These instructions describe the Omni Hysteroscope, associated sheaths, and outflow channels:
Catalogue
number
Product Catalogue
number
Product Hystero-
scope
working
channel
diameter
Outflow
channel
part
number
Compatible MyoSure Tissue
Removal Devices
Note: All Models may not be
available in all regions. Contact your
Hologic Representative for a list of
models available in your region.
60-200
Omni
Hysteroscope
60-201
Omni 3.7mm
Diagnostic
Sheath
Not
Applicable
Not
Applicable
Not Applicable
60-202
Omni
5.5mm
Operative
Sheath
3mm
40-201
MyoSure Tissue Removal Device
10-401
MyoSure LITE Tissue Removal Device
30-401LITE
MyoSure REACH Tissue Removal Device
10-401FC
MyoSure Manual Tissue Removal Device
20-401ML
60-203
Omni
6mm
Operative
Sheath
4mm
50-201XL
MyoSure Tissue Removal Device
10-401
MyoSure LITE Tissue Removal Device
30-401LITE
MyoSure REACH Tissue Removal Device
10-401FC
MyoSure Manual Tissue Removal Device
20-401ML
MyoSure XL Tissue Removal Device
50-501XL
MyoSure FMS-XL Tissue Removal Device
50-601XL
TABLE1.
InstructionsforUse
Page 2

2
ENGLISH
Device Description
The Omni Hysteroscope is intended for use in visualizing the
uterine cavity and performing operative hysteroscopy procedures
including use with the MyoSure Tissue Removal Device. The Omni
Hysteroscope system includes a base scope with compatible
sheaths of varying working channel size. The removable outflow
channels are intended to be used to provide a fluid outflow lumen
for use with Omni 5.5mm and 6mm Operative Sheaths. The
removable outflow channel includes a sealed entry port to permit
the introduction of instrumentation.
The reusable rod lens hysteroscope utilizes rod lenses for
visualization and fibers for illumination. The hysteroscope includes
Omni 5.5mm and 6mm Operative Sheaths to accommodate the
respective MyoSure Tissue Removal Device. (See Table1.)
The operative hysteroscopy system can be combined with a
hysteroscopic fluid management system to provide continuous flow
hysteroscopy capability. The hysteroscope is normally coupled to a
camera and video display unit for visualization.
Indications for Use
The Omni Hysteroscope is used to provide viewing of the cervical
canal and the uterine cavity for the purpose of performing
diagnostic and surgical procedures.
Diagnostic Hysteroscopy
• Abnormal uterine bleeding
• Infertility and pregnancy wastage
• Evaluation of abnormal hysterosalpingogram
• Intrauterine foreign body
• Amenorrhea
• Pelvic Pain
Operative Hysteroscopy
• Directed biopsy
• Removal of submucous fibroids and large polyps
• Submucous Myomectomy (see Contraindications)
• Transection of intrauterine adhesions
• Transection of intrauterine septa
Contraindications
• Acute pelvic inflammatory disease
Hysteroscopy may also be contraindicated by the following
conditions, depending on their severity or extent:
• Inability to distend uterus
• Cervical stenosis
• Cervical/vaginal infection
• Uterine bleeding or menses
• Known pregnancy
• Invasive carcinoma of the cervix
• Recent uterine perforation
• Medical contraindication or intolerance to anesthesia
Contraindications to Hysteroscopic Myomectomy
Hysteroscopic myomectomy should not be undertaken without
adequate training, preceptorship, and clinical experience. The
following are clinical conditions that can significantly complicate
hysteroscopic myomectomy:
• Severe anemia
• Inability to circumnavigate a myoma due to myoma size (e.g.,
predominantly intramural myomas with small submucous
components).
Warnings
• For use only by physicians trained in hysteroscopy
• Suspicion of pregnancy should suggest a pregnancy test
before the performance of diagnostic hysteroscopy.
• The Omni Hysteroscope set is only to be used in conjunction
with accessories that comply with the following safety
standards: National/Regional versions of IEC 60601-1, the
general safety requirements for medical devices; and, as
applicable, IEC 60601-2-18, particular safety requirements
for endoscope equipment and accessories; and IEC 606012-2, particular safety requirements for High Frequency
(HF) surgical equipment and accessories. Before using any
accessory, be sure to follow the instructions provided with
the accessory, including in the case of a HF electrode, the
maximum recurring peak voltage rating.
• When using HF surgical equipment, keep the working part of
the active electrode in the field of view to avoid accidental
burns.
• The hysteroscope, sheath(s), outflow channel(s) and
accessory components are shipped non-sterile. They must
be thoroughly cleaned and sterilized before each use.
• If each scope light post adapters have been used, they need
to be disassembled, cleaned, and sterilized before every
subsequent use.
• Uterine perforation can result in possible injury to bowel,
bladder, major blood vessels, and ureter.
• High energy radiated light emitted from illuminating fiber
at the distal end of the scope may give rise to temperatures
exceeding 106°F/41°C (within 8mm in front of the scope).
Do not leave tip of scope in direct contact with the patient
tissue or combustible materials, as burns may result. Lower
the light source output when working in close proximity to
the object.
• The hysteroscope light post and adapter may exceed
temperatures of 41°C. Hysteroscopes should not be placed
on the patient or on combustible materials, as burns may
result.
• To prevent potential safety hazard to the patient caused
by accidental loss of function of the device (i.e., front end
damage by surgical instruments) it is recommend to have
an additional sterile “stand-by” device during surgical
procedures.
• When scopes are used with laser equipment, appropriate
filtering spectacles must be worn by the operating team.
In some cases, a specific filter must be put between the
scope and camera head to prevent camera damage by highpower laser radiation. Contact your laser supplier for details.
To prevent scope damage by high-power laser radiation,
always ensure that the laser delivery fiber is seen through
the scope and not directed at the scope before energizing
the laser.
For Continuous Flow Hysteroscopy:
• If liquid distension medium is used, strict fluid intake and
output surveillance should be maintained. Intrauterine
Page 3
3
ENGLISH
instillation exceeding 1liter should be followed with care
due to the possibility of fluid overload.
Potential Complications of Continuous Flow Hysteroscopy:
• Hyponatremia
• Hypothermia
• Uterine perforation resulting in possible injury to adjacent
anatomy
• Pulmonary edema
• Cerebral edema
Precautions
• Vaginal ultrasonography before hysteroscopy may identify clinical
conditions that will alter patient management.
• Intrauterine distension can usually be accomplished with
pressures in the range of 35–75mmHg. Unless the systemic
blood pressure is excessive, it is seldom necessary to use
pressures greater than 75–80mmHg.
• Do not use the seals if the sterile package is open or appears
compromised. Do not use the device if damage is observed.
• Avoid exposing the scope to sudden temperature changes. Do
not immerse hot scopes into cold water or liquid.
• Any mechanical manipulation of the eyepiece may result in seal
breakage, therefore do not attempt to remove the eyepiece.
• Avoid contact with metal parts of the scope and other conductive
accessories by ensuring before activation of the HF output that
the active electrode is at a sufficient distance from the tip of the
scope.
• To avoid perforation, do not use the scope tip as a probe and
exercise caution when the scope is being inserted through the
cervix and when the scope tip is near the uterine wall.
Inspection Prior to Use
Prior to each use, the outer surface of the insertion portion of the
hysteroscope, sheath(s) and outflow channel(s) should be inspected
to ensure there are no unintended rough surfaces, sharp edges or
protrusions. Check that both the hysteroscope and outflow channel
contain seals.
Hysteroscope System Set-up Instructions
The Omni Hysteroscope consists of a base scope (60-200),
compatible sheaths including an Omni 3.7mm Diagnostic Sheath
(60-201), Omni 5.5mm Operative Sheath (60-202) and Omni 6mm
Operative Sheath (60-203), and Removable Outflow Channels (40201 and 50-201XL) as shown in Figure 1.
FIGURE1. REPRESENTATIVE HYSTEROSCOPE &
OUTFLOW CHANNEL
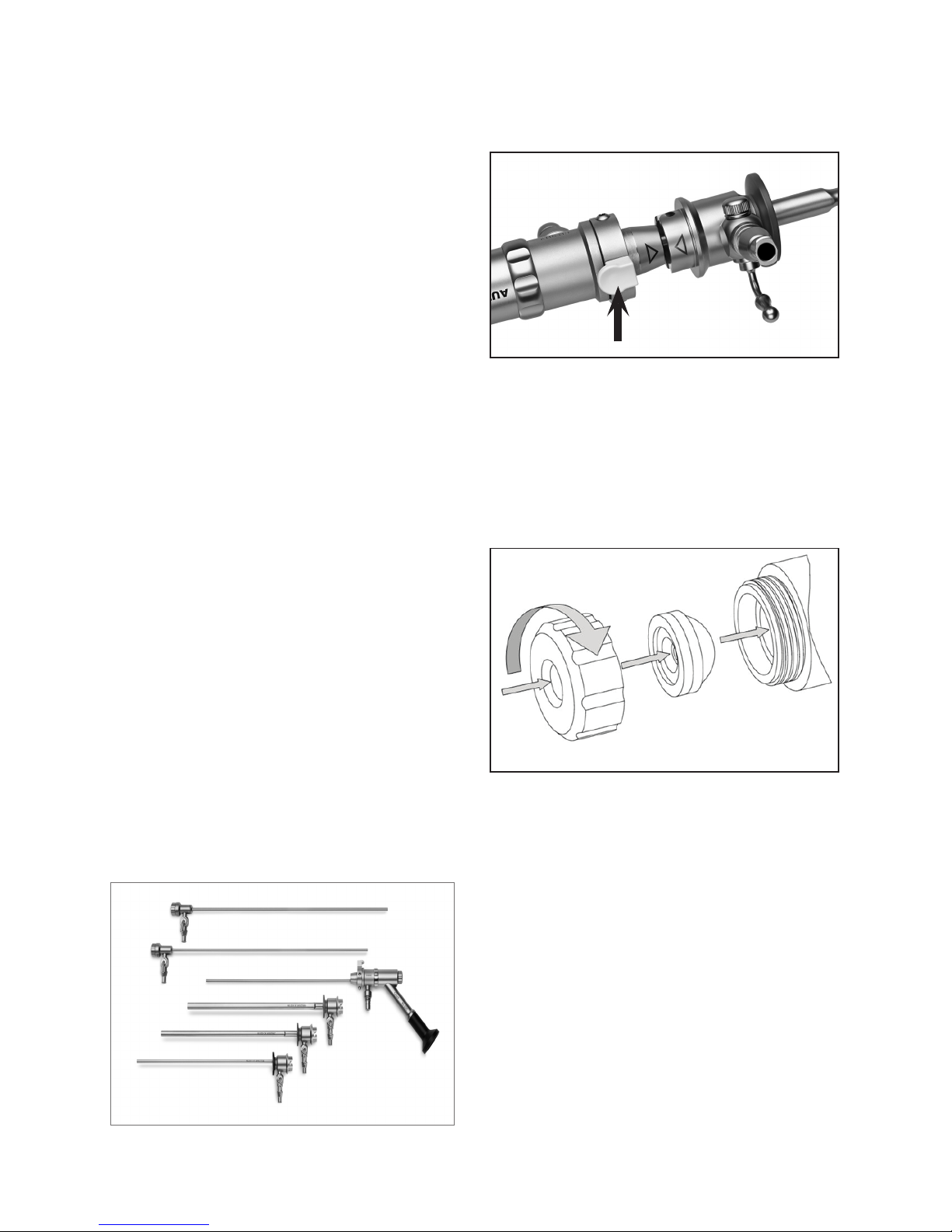
To place compatible sheath over base hysteroscope
Using the arrows for orientation, slide the sheath over the exposed
rod lens until the end of the sheath engages with the base of the
scope and is secure as seen in Figure 2. To release the sheath,
push the locking mechanism pin at the base of the scope.
FIGURE2. SECURE SHEATH
To Insert Sterile Single-Use Seal (40-902):
Both the hysteroscope and the outflow channel contain single-use
seals for their working channels. Figure3 below illustrates the
installation of the seals.
Caution: To ensure proper performance of the system and prevent
leaks, install new seals in the hysteroscope and the outflow
channel prior to use.
FIGURE3. SEAL INSTALLATION
To place removable outflow channel into hysteroscope:
Insert the removable outflow channel into the proximal seal of the
hysteroscope working channel. Reverse this process to remove the
outflow channel.
To Attach Fluid Connections:
The Omni 3.7mm Diagnostic Sheath, Omni 5.5mm Operative
Sheath and Omni 6mm Operative Sheath accept a standard male
luer connection for fluid inflow. The Removable Outflow Channel
includes a universal stopcock for both luer and friction connections.
Needed Equipment for Indicated Procedures
Fiber optic light source, fiber optic light guide (not supplied with
this product)
Hysteroscope Assembly/Disassembly Instructions
The Omni Hysteroscope is compatible with Metal-Halide and Xenon
light sources with up to 300watts of power.
Place the correct adapter on the light post of the fiber optic scope
and on the instrument end of the light guide. Adapters are available
Seal Cap
Seal
6.0mm Sheath
5.5mm Sheath
3.7mm Diagnostic Sheath
Hysteroscope
Removable Outflow Channels
Push to Release
Page 4

4
ENGLISH
for connection to Storz, Olympus, Dyonics, Wolf, and ACMI light
sources as shown in Figure4.
FIGURE4. LIGHT POST ADAPTERS
The light post threads may be lubricated as needed, being sure to
remove any excess lubricant as required. Make sure that the fiber
optic surface remains free of foreign matter. Do not use tools to
tighten the adapters – hand tighten only.
Directions for Use
The surgeon may look through the direct-view hysteroscope (with
eyepiece) directly with his or her eye. If a video system is being
connected to the scope, thread a camera coupler onto the camera
head and then insert the eyepiece into the camera coupler.
Plug the video cable into the camera control unit (CCU).
Turn on the power to the monitor, CCU, and light source. Adjust the
video system components per the manufacturer’s instructions. The
system is now ready to use.
Hysteroscope Cleaning Instructions—General
The device should not be allowed to dry after procedure before
cleaning to ensure effective removal of contaminant material.
• If still inserted, separate the removable outflow channel from the
hysteroscope.
• If still attached, separate the sheath from the hysteroscope using
the locking mechanism at the base of the hysteroscope.
• Light post Adapters must be removed prior to cleaning and
sterilization.
• Remove single-use seals and seal caps from hysteroscope and
removable outflow channel(s).
• Warning: Failure to remove the single-use seals from
hysteroscope and removable outflow channels will affect
proper cleaning and sterilization of the product.
• Open the stopcocks on the sheath(s) and removable outflow
channel(s).
• Flush all lumens of the hysteroscope, sheath(s) and removable
outflow channel(s) with warm tap water.
• Scrub the hysteroscope, sheath(s), and removable outflow
channel(s) using a nylon-bristled brush that is suitable to contact
the full interior dimensions (diameter and length) of the lumens.
Scrub all surfaces, crevices, interior cavities of the stopcock and
lumens to remove any visible debris. Do not scratch any of the
optical surfaces.
• The following brushes dimensions are recommended
• For the Omni 5.5mm and 6mm Operative Sheaths, a nylon-
bristled brush with bristle area length of 2” (50mm), bristle
diameter of 0.315” (8mm) and an overall length of 14” (35cm)
is recommended
• For the Omni 3.7mm Diagnostic Sheath and all other lumens,
a nylon-bristled brush with bristle area length of 2” (50mm),
bristle diameter of 0.197” (5mm) and an overall length of 14”
(35cm) is recommended.
• Utilizing the stopcocks, flush the lumens of the sheath(s) and
removable outflow channel(s) with an enzymatic, neutral pH
cleaner a minimum of three (3) times ensuring that no air
remains within the lumen.
• Flush the lumen of the hysteroscope with enzymatic, neutral
pH cleaner a minimum of three (3) times ensuring that no air
remains within the lumen.
• Hysteroscope, sheath(s), removable outflow channel(s) and
accessory components should be soaked in an enzymatic,
neutral pH cleaner in accordance with cleaning solution
instructions.
• Thoroughly rinse the hysteroscope, sheath(s), and removable
outflow channel(s), including flushing all lumens, and accessory
components to completely remove the cleaning solution.
• Dry the hysteroscope, sheath(s), removable outflow channel(s)
and accessory components with a lint free soft cloth or filtered
compressed air.
• Visually check all surfaces, crevices, interior cavities of the
stopcock and lumens.
• Check visually for cleanliness to ensure that all debris have been
removed. If not visually clean, repeat the reprocessing steps until
the device is visually clean.
Hysteroscope Cleaning Instructions—
Optical Surfaces
Due to insufficient cleaning or foreign matter contamination,
deposits may develop on the three optical surfaces of the
hysteroscope as shown below.
These are:
• The distal tip
• The proximal window or eyepiece
• The fiber optic light post
To remove these deposits, a tube of bio-compatible polishing paste
is enclosed with each hysteroscope.
To remove the deposits, dab some polishing paste onto a clean,
cotton-tipped swab. Gently press the swab onto the optical surface
to be cleaned and scrub the surface with a circular motion. Rinse
the optical surface with water to remove any remaining polishing
paste.
NOTE: Cleaning should only be performed when the image as
viewed through the scope is cloudy, and not as part of your
routine cleaning procedures.
NOTE: Do not use any ultrasonic cleaning methods. The energy
transmitted through fluid cavitations will damage seals and
optical surfaces and will void the warranty.
Storz/Olympus
ACMI
Dyonics/Wolf
Page 5

5
ENGLISH
NOTE: Foreign matter remaining on the fiber surface of the
light post after cleaning may tend to burn and discolor the
surface when exposed to a high intensity light source.
Sterilization
The hysteroscope, sheath(s), removable outflow channel(s), and
accessory components should be sterilized prior to use according
to the parameters listed below. Be sure the distal working length
does not experience any undue forces or stress which can damage
the delicate internal optics.
Sterrad®— (100NX Systems – Standard Cycle Setting, 100S
System - Short Cycle Setting) Device meets guidelines for Sterrad
100S, and 100NX systems and requires the use of a Sterradcompatible tray or container system (APTIMAX® tray REF: 13831
or equivalent). Refer to manufacturer’s Instructions for Use for
more information. Trays should be wrapped with two layers of
a sterilization wrap that is cleared by the FDA for the indicated
sterilization cycle (Halyard Health H400 or equivalent).
Steam Autoclave— Prior to sterilization, the hysteroscope,
sheath(s), removable outflow channel(s) and accessory components
should be prepared in the following configuration:
• Double pouched in sterilization pouches that are cleared by the
FDA for the indicated sterilization cycle (Cardinal Health pouch
CAT #T90009 or equivalent).
Follow standard hospital procedures:
Pre-vacuum method: 132° C (270° F) for 4 minutes and 35 minutes dry time
IMPORTANT: It is recommended that the institution employ
procedures which include the use of biological indicators in order
to determine the effectiveness of the sterilization process.
Maintenance
We recommend that you inspect the hysteroscope, sheath(s) and
outflow channel(s) carefully before and after the procedure for
possible signs of damage.
First, check the image quality of the scope by viewing the monitor.
If image quality is impaired:
• Check the distal and proximal lenses of the hysteroscope for
cracked or scratched lenses.
• Check the surface cleanliness of the distal and proximal lenses. A
foggy or cloudy image can be the result of moisture entering the
optical system or lack of cleanliness of exterior surfaces. When
viewing reflected light, the surfaces should appear smooth and
shiny.
As a second step, check the illumination system of the scope.
Reduced brightness can result from fiber damage:
• Check for fiber optic damage in the scope by holding the distal
end of the scope toward a low power light and observing the
light post on the hub. The center of the light post should appear
clear or white. Noticeable black spots indicate serious damage
to the fiber illumination bundle in the scope. This will affect light
transmission and the brightness of the image viewed on the
monitor.
• Check the light cable for damaged fibers by holding one end
of the cable toward a low power light and observing the other
end. Broken fiber will appear as black spots in the light field. A
damaged light cable will affect its ability to transmit light and the
brightness of the image viewed on the monitor.
Storage
The Omni Hysteroscope System should be stored either in their
shipping box or in a sterilization tray. In either case, proper care
should be taken to ensure that the base hysteroscope, sheath(s)
and outflow channel(s) are immobile to prevent any damage.
Service - Accessories
The following are replacement/service parts for the Omni
Hysteroscope System:
REF Description
40-201 Replacement MyoSure Outflow
Channel
50-201XL Replacement MyoSure XL Outflow
Channel
ASY-04996 Hysteroscope Light Source Adapters -
1each: Wolf and Storz
40-902 MyoSure Single Use Seal Set - 10per
box
40-904 MyoSure Hysterscope and Outflow
Channel Seal Cap
60-201 Omni 3.7mm Diagnostic Sheath
60-202 Omni 5.5mm Operative Sheath
60-203 Omni 6mm Operative Sheath
WARRANTY, SERVICE, AND REPAIR
WARRANTIES
Except as otherwise expressly stated in the Agreement: i)
Equipment manufactured by Hologic is warranted to the original
Customer to perform substantially in accordance with published
product specifications for one (1) year starting from the date of
shipment, or if Installation is required, from the date of Installation
(“Warranty Period”); ii) digital imaging mammography x-ray tubes
are warranted for twenty-four (24) months, during which the x-ray
tubes are fully warranted for the first twelve (12) months and are
warranted on a straight-line prorated basis during months 13-24;
iii) replacement parts and remanufactured items are warranted
for the remainder of the Warranty Period or ninety (90) days
from shipment, whichever is longer; iv) consumable Supplies are
warranted to conform to published specifications for a period
ending on the expiration date shown on their respective packages;
v) licensed Software is warranted to operate in accordance
with published specifications; vi) Services are warranted to be
supplied in a workman-like manner; vii) non-Hologic Manufactured
Equipment is warranted through its manufacturer and such
manufacturer’s warranties shall extend to Hologic’s customers,
to the extent permitted by the manufacturer of such non-Hologic
Manufactured Equipment. Hologic does not warrant that use of
Products will be uninterrupted or error-free, or that Products will
operate with non-Hologic authorized third-party products.
These warranties do not apply to any item that is: (a) repaired,
moved, or altered other than by Hologic authorized service
personnel; (b) subjected to physical (including thermal or electrical)
abuse, stress, or misuse; (c) stored, maintained, or operated in
any manner inconsistent with applicable Hologic specifications
or instructions, including Customer’s refusal to allow Hologic
Page 6

6
ENGLISH
recommended Software upgrades; or (d) designated as supplied
subject to a non-Hologic warranty or on a pre-release or “as-is”
basis.
TECHNICAL SUPPORT AND PRODUCT RETURN INFORMATION
Contact Hologic Technical Support if the Omni Hysteroscope
System fails to operate as intended. If product is to be returned
to Hologic for any reason, Technical Support will issue a Returned
Materials Authorization (RMA) number and biohazard kit if
applicable. Return the Omni Hysteroscope System according to the
instructions provided by Technical Support. Be sure to clean and
sterilize the product before returning it and include all accessories
in the box with the returned unit.
Return used or opened product according to the instructions
provided with the Hologic-supplied biohazard kit.
For More Information
For technical support or reorder information in the United States,
please contact:
Hologic, Inc.
250 Campus Drive
Marlborough, MA 01752 USA
Phone: 1.800.442.9892 (toll-free)
www.hologic.com
International customers, contact your distributor or local Hologic
Sales Representative:
European Representative
Hologic Ltd.
Heron House Oaks Business Park, Crewe Road
Wythenshawe, Manchester. M23 9HZ, UK
Phone: +44 (0)161 946 2206
Symbols Used on Labeling
Authorized Representative in the European Community
Batch code
Catalogue number
Use by
Manufacturer
Patient contact parts do not contain phthalate
DEHP
Serial number
U.S. federal law restricts this device to sale by or on the order of
a physician
Do not re-use
Do not resterilize
Sterilized using irradiation
Consult instructions for use
Contents
Non-sterile
Hologic, Omni, and MyoSure are associated logos are registered
trademarks of Hologic, Inc. and/or its subsidiaries in the United
States and other countries. All other trademarks, registered
trademarks, and product names are the property of their respective
owners.
© 2018 Hologic, Inc.
AW-17817-002 Rev. 006
 Loading...
Loading...