Page 1
Form ENG-0034-T01, Rev. 005
TITLE
DOCUMENT NUMBER
REV
TEXT, MYOSURE XL MODIFIED FOR
FLUENT, TEXT, EN OUS,
AW-15026-002
010
SIZE A
SHEET 1 OF 1
REV AUTHORED BY
DATE
S VAILLANCOURT
03/21/19
REV DRAFTED BY
DATE
S VAILLANCOURT
03/21/19
PROPRIETARY: This document contains
proprietary data of Hologic, Inc. No
disclosure, reproduction or use of any
part thereof may be made except by
written permission from Hologic.
REV. RELEASE DATE:
15 MAY 2019
Artwork consists of:
• Nine 8.25-inch x 11-inch sheets attached.
Artwork master contains the following file(s):
File Name
Description
AW-15026-002_010_02.zip
Source and supplier print file
AW-15026-002_010_02.pdf
View file
Artwork prints black and white.
Page 2

MyoSure® XL Tissue Removal Device for Fluent
Instructions for Use
Please read all information carefully.
Description
The MyoSure XL Tissue Removal Device for Fluent is a sterile, hand-held tissue removal device used to remove intrauterine tissue. It is connected
via a flexible drive shaft to the Fluent Fluid Management System. A foot pedal allows the user to control the tissue removal device by turning the
motor in the Fluent Fluid Management System on and off.
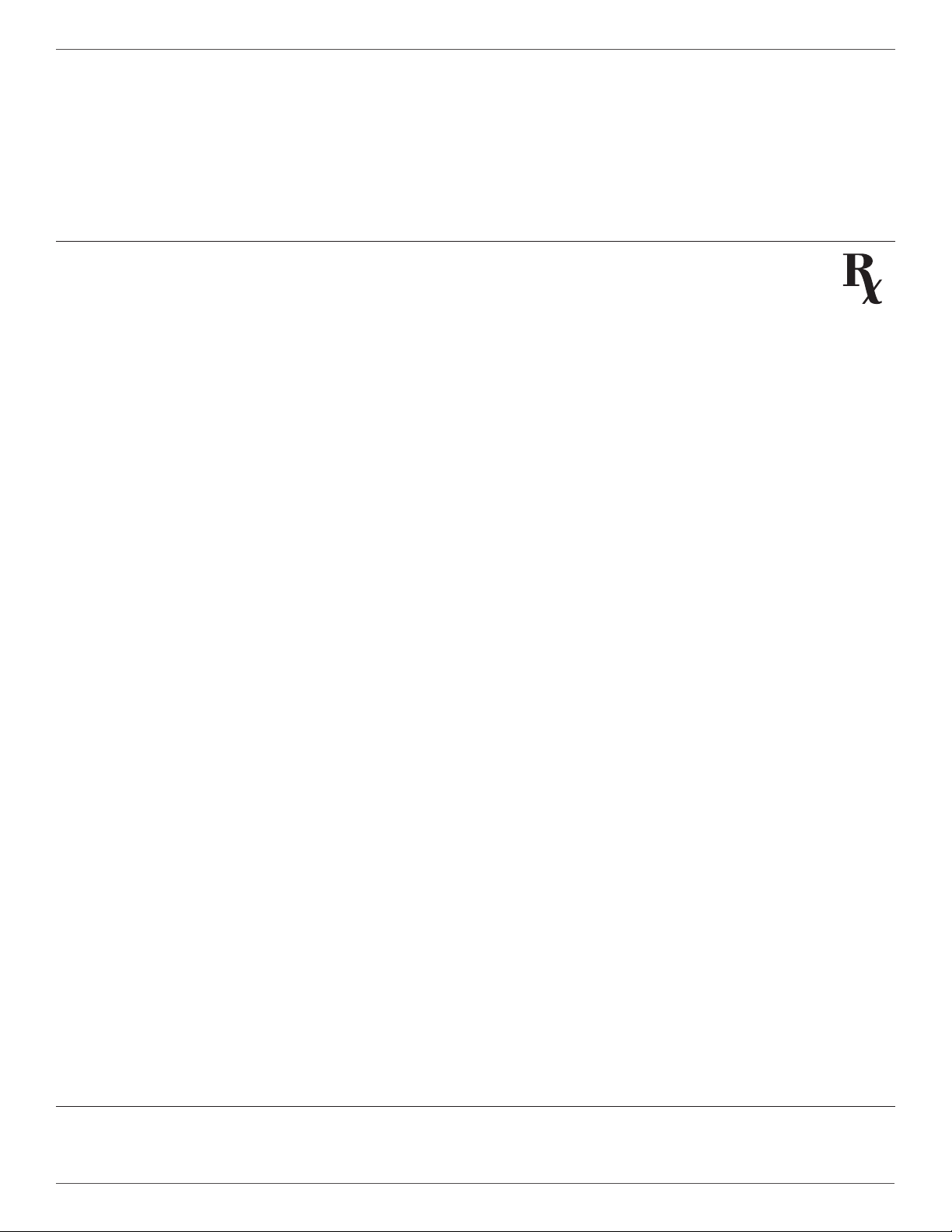
RX ONLY (U.S.) Federal law restricts this device to sale by or on the order of a physician pursuant to 21 CFR 801.109(b)(1).
ONLY
Indications for Use
The MyoSure XL Tissue Removal Device for Fluent is intended for intrauterine use by trained gynecologists to hysteroscopically resect and remove
tissue such as: Submucous myomas, Endometrial Polyps, and Retained products of conception.
Contraindications
The MyoSure XL Tissue Removal Device for Fluent should not be used with pregnant patients or patients exhibiting pelvic infection, cervical
malignancies, or previously diagnosed uterine cancer.
Warnings and Precautions
The brief operating instructions in this guide will make the system easier to use. As with any surgical instrument, there are important health and
safety considerations. These are as follows:
• Warning: Please consider pre-operative imaging prior to the procedure to assess the patient for evidence of placental invasion of the
myometrium. In the immediate postpartum phase, removal of retained products of conception (RPOC) in the setting of known or suspected
placenta acreta, placenta increta or placenta percreta poses a risk of significant and potentially life threatening bleeding.
• Before using the MyoSure XL Tissue Removal Device for Fluent for the first time, please review all available product information.
• Before using the MyoSure XL Tissue Removal Device for Fluent, you should be experienced in hysteroscopic surgery with powered
instruments. Healthy uterine tissue can be injured by improper use of the tissue removal device. Use every available means to avoid such
injury.
• The MyoSure XL Tissue Removal Device for Fluent is only compatible with the Fluent Fluid Management System. Use of any other motorized
power source or fluid management system may fail to operate the device or lead to patient or physician injury.
• If visualization is lost at any point during a procedure, stop cutting immediately.
• Periodic irrigation of the tissue removal device tip is recommended to provide adequate cooling and to prevent accumulation of excised
materials in the surgical site.
DANGER: Risk of explosion if used in the presence of flammable anesthetics.
WARNING: Exercise extreme caution when resecting tissue in patients who have implants that extend into the uterine cavity.
• Do not use the MyoSure XL Tissue Removal Device for Fluent to resect tissue that is adjacent to an implant. When resecting tissue in patients
that have implants, assure that:
• the device’s cutting window is facing away from (i.e., 180° opposite) the implant;
• the visual field is clear; and
• the device’s cutting window is engaged in tissue and is moved away from the implant as tissue resection proceeds.
• In the event an implant becomes entangled with a MyoSure cutter, the following steps are recommended:
• cease cutting immediately;
• kink the device’s outflow tube to prevent a loss of uterine distension;
• disconnect the device’s drive cable from the Fluent Fluid Management System;
• grasp the end of the device drive cable with a hemostat or other clamping device;
• hold the drive cable hub and tissue removal device to prevent twisting;
• open the tissue removal device cutting window by manually twisting the hemostat; and
• gently pull the device into the hysteroscope to detach the MyoSure XL Tissue Removal Device for Fluent from the implant.
• If this unit is configured as part of a system, the entire system should be tested for compliance with IEC 60601-1-1.
English 1
Page 3
MyoSure® XL Tissue Removal Device for Fluent
• If the leakage current of the configured system exceeds the limits of IEC 60601-1-1, install an appropriately rated UL 2601-1/IEC 60601-1
approved isolation transformer and retest the system.
• The use of accessory equipment in the patient vicinity not complying with the equivalent medical safety requirements of this equipment
may lead to a reduced level of safety of the resulting system. The use of accessory equipment outside the patient vicinity not complying with
medical or otherwise appropriate safety requirements may lead to a reduced level of safety of the resulting system.
• Use of an accessory, transducer, or cable other than those specified by Hologic may result in increased emissions or decreased immunity of
the Fluent Fluid Management System or the MyoSure XL Tissue Removal Device for Fluent.
Precautions
Federal law restricts this device to sale by or on the order of a physician.
• The tissue removal device should be stored at room temperature, away from moisture and direct heat.
• Do not use after expiration date.
• Do not use the device if the sterile package is open or appears compromised. Do not use the device if damage is observed.
• To assure optimal performance, replace the tissue removal device after 2 hours of cutting time.
• The tissue removal device is intended for single use only. Do not re-sterilize. Discard tissue removal device assembly after use.
• Do not lubricate tissue removal device.
• Use of a reprocessed, single-use tissue removal device may permanently damage, impede performance, or cause failure of the Fluent Fluid
Management System. Use of such products may render any warranties null and void.
• DO NOT attempt to sharply bend the flexible drive cable in a diameter of less than 8 inches (20 centimeters). A sharply bent or kinked drive
cable may cause the Fluent Fluid Management System to overheat and stop. During a procedure, a minimum distance of 5 feet (1.5 meters)
should be maintained between the Fluent Fluid Management System and the tissue removal device to allow the drive cable to hang in a large
arc with no bends, loops, or kinks.
• DO NOT rotate the tissue removal device >180° if the tissue removal device is not running. The cutting window may open up which will lead to
the inability to maintain distension. If this situation occurs, tap the foot pedal once or twice to run the tissue removal device; the cutting
window will then close automatically.
• If it appears that the tissue removal device’s cutter blade has stopped rotating during a procedure, check to ensure that the tissue removal
device is properly connected to the Fluent Fluid Management System, all cables are secure, and that the drive cable has not
wrapped into a loop.
• Exercise care when inserting or removing the device from the MyoSure Hysteroscope. Insertion and removal of the device should be performed
under direct visualization at all times.
• To avoid perforation, keep the device tip under direct visualization and exercise care at all times when maneuvering it or cutting tissue close to
the uterine wall. Never use the device tip as a probe or dissecting tool.
• Excessive bending of the device distal tip can cause the tissue removal device’s cutter to come out of the cutting window. If such damage
occurs, replace the device immediately.
• Do not allow the rotating portion of the tissue removal device to touch any metallic object such as a hysteroscope or sheath. Damage to both
instruments is likely. Damage to the tissue removal device can range from a slight distortion or dulling of the cutting edge to actual fracture
of the tip in vivo. If such contact does occur, inspect the tip. If you find cracks, fractures, or dulling, or if you have any other reason to suspect a
tissue removal device is damaged, replace it immediately.
• Do not operate the tissue removal device in the open air for an extended period, as the lack of irrigation may cause the tissue removal device
to overheat and seize.
• Excessive leverage on the tissue removal device does not improve cutting performance and, in extreme cases, may result in wear, degradation,
and seizing of the inner assembly.
• Do not cool the tissue removal device by immersing it in cold water.
• Electrical safety testing should be performed by a biomedical engineer or other qualified person.
• This equipment contains electronic printed circuit assemblies. At the end of the useful life of the equipment it should be disposed of in
accordance with any applicable national or institutional related policy relating to obsolete electronic equipment.
Electromagnetic Safety
The MyoSure XL Tissue Removal Device for Fluent is only to be used with the Fluent Fluid Management System. The Fluent Fluid Management
System needs special precautions regarding electromagnetic safety.
English2
Page 4

MyoSure® XL Tissue Removal Device for Fluent
Impact of Mobile and Portable HF Communication Devices
The emission of high frequency energy by mobile communication devices may impact the function of the electrical medical device. Operating
such devices (e.g., cell phones, GPS phones) in the proximity of the electrical medical device is prohibited.
Electrical Connections
Do not touch electrical connections identified with this warning label.
Do not establish a connection between these plugs and sockets without first implementing precautionary ESD (electrostatic discharge) measures.
The following are ESD precautionary measures:
• Apply potential equalization (PE), if available on your equipment, to all devices to be connected.
• Use only the listed equipment and accessories.
Employees have to be informed about and trained in ESD precautionary measures.
Guidelines and Manufacturer’s Statement
Electromagnetic Emissions
The FLUENT FLUID MANAGEMENT SYSTEM is intended for use in the electromagnetic environment specified below. The customer or the user of
the FLUENT FLUID MANAGEMENT SYSTEM should assure that it is used in such an environment.
Emissions test Compliance Electromagnetic environment - guidance
RF emissions
CISPR 11
RF emissions
CISPR 11
RF emissions
CISPR 11
Harmonic emissions
IEC 61000-3-2
Voltage fluctuations / flicker emissions
IEC 61000-3-3
Group 1 The FLUENT FLUID MANAGEMENT SYSTEM uses RF energy only for its internal
function. Therefore, its RF emissions are very low and are not likely to cause
interference in nearby electronic equipment.
Group 2 The FLUENT FLUID MANAGEMENT SYSTEM must emit electro-magnetic energy
in order to perform its intended function. Nearby electronic equipment may be
affected.
Class A The FLUENT FLUID MANAGEMENT SYSTEM is suitable for use in all establish-
ments other than domestic and those directly connected to the public low-volt-
Complies
Complies
age power supply network that supplies buildings used for domestic purposes.
NOTE: The EMISSIONS characteristics of this equipment make it suitable
for use in industrial areas and hospitals (CISPR 11 class A). If it is used in a
residential environment (for which CISPR 11 class B is normally required) this
equipment might not offer adequate protection to radio-frequency communication services. The user might need to take mitigation measures, such as
relocating or re-orienting the equipment.
Guidance and Manufacturer’s Declaration
Electromagnetic Immunity
The FLUENT FLUID MANAGEMENT SYSTEM is intended for use in the electromagnetic environment specified below. The customer or the user of
the FLUENT FLUID MANAGEMENT SYSTEM should assure that it is used in such an environment.
Immunity test IEC 60601
test level
Electrostatic discharge (ESD)
IEC 61000-4-2
Electrical fast transients / bursts
IEC 61000-4-4
Surge
IEC 61000-4-5
English 3
± 8 kV contact
± 15 kV air
± 2 kV for power supply
lines
± 1 kV for input / output
lines
± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
Compliance level Electromagnetic environment - guidance
± 8 kV contact
± 15 kV air
± 2 kV for power supply
lines
± 1 kV line(s) to line(s)
± 2 kV line(s) to earth
Floors should be wood, concrete or ceramic tile.
If floors are covered with synthetic material, the
relative humidity should be at least 30%.
Mains power quality should be that of a typical
commercial or hospital environment.
Mains power quality should be that of a typical
commercial or hospital environment.
Page 5

MyoSure® XL Tissue Removal Device for Fluent
Immunity test IEC 60601
test level
Voltage dips, short interruptions and
voltage variations on power supply
input lines
IEC 61000-4-11
0% UT (100% dip in the
UT) for ½ cycle at 0°,
45°, 90°, 135°, 180°,
225°, 270°, and 315°
0% UT (100% dip in the
UT) for 1 cycle and 70%
(30% dip in the UT) UT
for 25/30 cycles at 0°
0% UT (100% dip in the
UT) for 250/300 cycles
Power frequency (50/60 Hz)
30 A/m 30 A/m Power frequency magnetic fields should be at
magnetic field
IEC 61000-4-8
Conducted RF
IEC 61000-4-6
3 Vrms
150 kHz to 80 MHz
6 Vrms in ISM Bands
between 150 kHz and
80 MHz
Radiated HF interference quantities
according to IEC 61000-4-3
3 V/m
80 MHz to 2.7 GHz
Compliance level Electromagnetic environment - guidance
0% UT (100% dip in the
UT) for ½ cycle at 0°,
45°, 90°, 135°, 180°,
225°, 270°, and 315°
0% UT (100% dip in the
UT) for 1 cycle and 70%
(30% dip in the UT) UT
for 25/30 cycles at 0°
Mains power quality should be that of a typical
commercial or hospital environment.
If the user of the /operator of FLUENT FLUID MANAGEMENT SYSTEM requires continued operation
during power mains interruptions, it is recommended that the FLUENT FLUID MANAGEMENT
SYSTEM be powered from an uninterruptible power
supply or battery.
0% UT (100% dip in the
UT) for 250/300 cycles
levels characteristic of a typical location in a typical
commercial or hospital environment.
3 Vrms
150 kHz to 80 MHz
6 Vrms in ISM Bands
between 150 kHz and
80 MHz
Portable and mobile RF communications equipment used no closer to any part of the FLUENT
FLUID MANAGEMENT SYSTEM, including cables,
than the recommended separation distance calculated from the equation applicable to the frequency
of the transmitter.
Recommended safety distance:
3 V/m
80 MHz to 2.7 GHz
d = 1.2√P for 150 KHz to 80 MHz
d = 1.2√P for 80 MHz to 800 MHz
d = 2.3√P for 800 MHz to 2.7 GHz
Where P is the maximum output power rating
of the transmitter in watts [W] according to
the transmitter manufacturer, and d is the
recommended separation distance in meters [m].
Field strengths from fixed RF transmitters, as
determined by an electromagnetic site survey a,
should be less than the compliance level in each
frequency range.
b
Interference may occur in the vicinity of equipment
marked with the following symbol:
Note *: UT is the AC mains voltage prior to application of the test level.
Note 1: At 80 MHz and 800 MHz, the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection of structures,
objects, and people.
a) Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio,
AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due
to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the FLUENT FLUID MANAGEMENT SYSTEM is used exceeds the applicable compliance level above, the FLUENT FLUID MANAGEMENT SYSTEM should
be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as re-orienting or
relocating the changing orientation or the location of the FLUENT FLUID MANAGEMENT SYSTEM.
b) Over the frequency range of 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
English4
Page 6

MyoSure® XL Tissue Removal Device for Fluent
Recommended Separation Distances
The following lists the recommended separation distances between portable and mobile RF communications equipment and the FLUENT FLUID
MANAGEMENT SYSTEM.
Recommended Separation Distances
The FLUENT FLUID MANAGEMENT SYSTEM is intended for use in an electromagnetic environment in which radiated RF disturbances are con-
trolled. The customer or the user of the FLUENT FLUID MANAGEMENT SYSTEM can help prevent electromagnetic interference by maintaining a
minimum distance between portable and mobile RF communications equipment (transmitters) and the FLUENT FLUID MANAGEMENT SYSTEM as
recommended below, according to maximum output power of the communications equipment.
Rated maximum output power of transmitter
[W]
0.01 0.12 0.12 0.23
0.1 0.38 0.38 0.73
1 1.2 1.2 2.3
10 3.8 3.8 7.3
100 12 12 23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated by
using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts [W]
according to the transmitter manufacturer.
Note 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
Note 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures,
objects, and people
Separation distance according to frequency of transmitter [m]
150 kHz to 80 MHz
d = 1.2√P
80 MHz to 800 MHz
d = 1.2√P
800 MHz to 2.7 GHz
d = 2.3√P
Tissue Removal Device for Fluent: 50-601XL/50-603XL
The MyoSure XL Tissue Removal Device for Fluent is shown in Figure 1. It is a hand-held
unit which is connected to the Fluent Fluid Management System via a 6-foot (1.8-meter)
flexible drive cable and to the Out-FloPak via a 10-foot (3-meter) suction tube. Cutting action
is activated by a foot pedal. The tissue removal device is a single-use device designed to
hysteroscopically remove intrauterine tissue.
The flexible drive cable is inserted into the drive cable adapter on the front panel of the Fluent
Fluid Management System.
The proximal end of the suction tube is connected to the Out-FloPak of the Fluent Fluid
Management System.
The suction pressure draws fluid and resected tissue through the tissue removal device’s
cutting window.
Figure 1. MyoSure XL Tissue Removal Device
for Fluent
Set-up
The tissue removal device is EtO sterilized. Verify that the tissue removal device is sterile prior
to use.
Do not use if the package is opened or damaged. Discard all opened, unused devices.
CAUTION: The tissue removal device is intended for single use only. DO NOT RE-
STERILIZE. DO NOT REUSE. Discard tissue removal device after use. Dispose of
the tissue removal device and packaging according to your facility’s policies
and procedures concerning biohazardous materials and sharps waste.
WARNING - DANGER: Risk of explosion if used in the presence of flammable
anesthetics.
1. Review the System Configuration Diagram in Figure 2 for set-up outline.
2. Refer to the Fluent Fluid Management System Operator’s Manual for instructions on how to set up the Fluent Fluid Management System.
3. Connect the foot pedal tube to the connector on the front of the Fluent Fluid Management System console.
English 5
Figure 2. System Configuration
Page 7

MyoSure® XL Tissue Removal Device for Fluent
Connecting the Tissue Removal Device to the Fluent Fluid Management System
1. Remove the tissue removal device (REF 50-601XL/50-603XL) from the sterile package.
2. Sterile person hands the flexible drive cable and suction tube to the non-sterile person.
3. Non-sterile person inserts the flexible cable into the corresponding adapter on the Fluent
Fluid Management System as shown in Figure 3.
4. The tissue removal device flexible drive cable has a keyed feature that serves to align the
handpiece cable to the Fluent Fluid Management System connector. The metal tab on the
connector is pushed down, the flexible cable inserted and then the tab is released.
CAUTION: DO NOT attempt to sharply bend the flexible drive cable in a diameter of less
than 8 inches (20 centimeters). A sharply bent or kinked drive cable may
cause the Fluent Fluid Management System motor to overheat and stop. During
a procedure, a minimum distance of 5 feet (1.5 meters) should be maintained
between the Fluent Fluid Management System and the tissue removal device to allow the drive cable to hang in a large arc
with no bends, loops, or kinks.
5. Non-sterile person attaches the tissue removal device suction tube to the corresponding fitting on the Out-FloPak.
Figure 3. Insert drive cable and foot pedal into
the Fluent Fluid Management System.
Operation
1. Set up the Fluent Fluid Management System for a MyoSure tissue removal procedure per the
instructions in the Fluent Fluid Management System Operating Manual.
2. The foot pedal activates tissue removal device operation. The foot pedal turns the motor ON
and OFF. Once the foot pedal is depressed, the tissue removal device accelerates and rotates
to the set speed and continues until the foot pedal is released.
3. Press the foot pedal and observe the tissue removal device action to verify that the motor
runs and that the cutting window is closed as shown in Figure 4.
WARNING: Periodic irrigation of the tissue removal device tip is recommended to provide
adequate cooling and to prevent accumulation of excised materials in the
surgical site.
4. Introduce the tissue removal device through the straight 4 mm working channel of a
hysteroscope.
5. Under direct hysteroscopic visualization, position the tissue removal device’s side facing
cutting window against target pathology.
Figure 4. Closed Tissue Removal Device
cutting window on left
CAUTION: Excessive leverage on the tissue removal device does not improve cutting performance and, in extreme cases, may result in
wear, degradation, and seizing of the cutter assembly.
6. Press the foot pedal to activate the tissue removal device’s cutting blade.
7. The tissue removal device’s reciprocating action alternately opens and closes the device’s cutting window to the outflow pump of the Fluent
Fluid Management System thereby drawing tissue into the cutting window.
8. Cutting takes place when the tissue removal device cutting edge rotates and translates through the tissue removal device’s cutting window.
CAUTION: If it appears that the blade has stopped rotating during a procedure, check to ensure that the tissue removal device is
properly connected to the Fluent Fluid Management System, all cables to the Fluent Fluid Management System are secure,
and that the drive cable has not wrapped into a loop.
NOTE: If the Fluent Fluid Management System powers down unexpectedly, leave it off for 15 seconds, restart the system and follow
on-screen prompts.
English6
Page 8

MyoSure® XL Tissue Removal Device for Fluent
Storage
The tissue removal device should be stored at room temperature, away from moisture and direct heat. Do not use after expiration date.
Sterility
The tissue removal device is EtO sterilized. DO NOT RE-STERILIZE. DO NOT REUSE. Do not use if package is opened or damaged. Discard all
opened, unused devices
Disposal
Disconnect the tissue removal device from the Fluent Fluid Management System. Dispose of the tissue removal device and packaging according
to your facility’s policies and procedures concerning biohazardous materials and sharps waste.
CAUTION: The tissue removal device contains electronic printed circuit assemblies. At the end of the useful life of the equipment
it should be disposed of in accordance with any applicable national or institutional related policy relating to obsolete
electronic equipment.
Troubleshooting
If the device does not operate, check the following:
1. The Fluent Fluid Management System is plugged into a wall outlet.
2. The wall outlet has power.
3. The power cord is attached to the rear of the Fluent Fluid Management System.
4. The foot pedal is connected to the front of the Fluent Fluid Management System.
5. The suction tubing is connected.
If excess bending force is applied to the MyoSure XL Tissue Removal Device for Fluent, the system may temporarily stop to prevent further
damage.
NOTE: If the Fluent Fluid Management System powers down unexpectedly, leave it off for 15 seconds, restart the system and follow
on-screen prompts.
Technical Specifications
Tissue Removal Device: 50-601XL/50-603XL
Sterile, single use device
Working Length: 12.6” / 32.0 cm
OD: 4 mm
WARRANTY, SERVICE, AND REPAIR
Warranty
Except as otherwise expressly stated in the Agreement: i) Equipment manufactured by Hologic is warranted to the original Customer to perform
substantially in accordance with published product specifications for one (1) year starting from the date of shipment, or if Installation is required,
from the date of Installation (“Warranty Period”); ii) digital imaging mammography x-ray tubes are warranted for twenty-four (24) months, during
which the x-ray tubes are fully warranted for the first twelve (12) months and are warranted on a straight-line prorated basis during months 1324; iii) replacement parts and remanufactured items are warranted for the remainder of the Warranty Period or ninety (90) days from shipment,
whichever is longer; iv) consumable Supplies are warranted to conform to published specifications for a period ending on the expiration date
shown on their respective packages; v) licensed Software is warranted to operate in accordance with published specifications; vi) Services are
warranted to be supplied in a workman-like manner; vii) non-Hologic Manufactured Equipment is warranted through its manufacturer and such
manufacturer’s warranties shall extend to Hologic’s customers, to the extent permitted by the manufacturer of such non-Hologic Manufactured
Equipment. Hologic does not warrant that use of Products will be uninterrupted or error-free, or that Products will operate with non-Hologic
authorized third-party products.
These warranties do not apply to any item that is: (a) repaired, moved, or altered other than by Hologic authorized service personnel; (b)
subjected to physical (including thermal or electrical) abuse, stress, or misuse; (c) stored, maintained, or operated in any manner inconsistent
with applicable Hologic specifications or instructions, including Customer’s refusal to allow Hologic recommended Software upgrades; or (d)
designated as supplied subject to a non-Hologic warranty or on a pre-release or “as-is” basis.
English 7
Page 9

MyoSure® XL Tissue Removal Device for Fluent
Technical Support and Product Information
Contact Hologic Technical Support if the MyoSure XL Tissue Removal Device for Fluent fails to operate as intended. If product is to be returned
to Hologic for any reason, Technical Support will issue a Returned Materials Authorization (RMA) number and biohazard kit if applicable. Return
the MyoSure XL Tissue Removal Device for Fluent according to the instructions provided by Technical Support. Be sure to clean and sterilize the
product before returning it and include all accessories in the box with the returned unit.
Return used or opened product according to the instructions provided with the Hologic-supplied biohazard kit.
For More Information
For technical support or reorder information in the United States, please contact:
Hologic, Inc., 250 Campus Drive
Marlborough, MA 01752 USA
Phone: 1.800.442.9892 (toll-free)
www.hologic.com
International customers, contact your distributor or local Hologic Sales Representative:
European Representative
Hologic BVBA
Da Vincilaan 5
1930 Zaventem
Belgium
Phone: +32 2 711 46 80
Symbols Definitions
Authorized Representative in the European Community
Batch code, Lot code
Catalogue number, Part number, or reorder number
Consult instructions for use
Contents
Do not re-use
Follow instructions for use
Use by
Manufacturer
ON
Main electrical power on.
English8
Page 10

MyoSure® XL Tissue Removal Device for Fluent
Symbols Definitions
OFF
Main electrical power off.
Patient contact parts not made with DEHP
Serial number
Sterilized using ethylene oxide
U.S. federal law restricts this device to sale by or on the order of a physician
Do not resterilize
Do not use if package is damaged
Hologic, MyoSure, and associated logos are registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and other countries.
All other trademarks, registered trademarks, and product names are the property of their respective owners.
©2017-2019 Hologic Inc.
AW-15026-002 Rev. 010
English 9
 Loading...
Loading...