Page 1
QUICK REFERENCE GUIDE
Defi nity™ Cervical Dilator
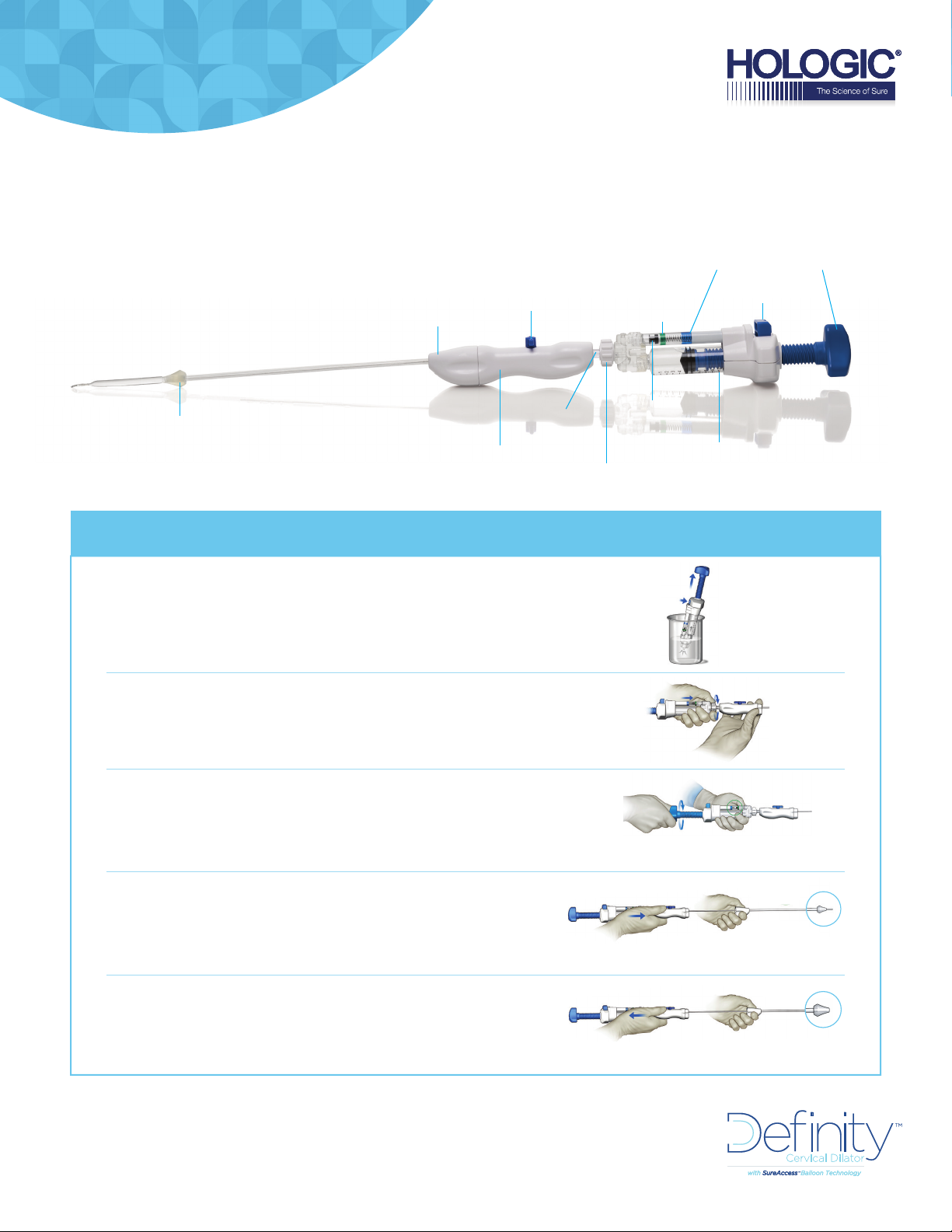
SYSTEM OVERVIEW
ACORN TIP
DILATION QUICK SETUP | Preparing the Dilator
FILL the in ation device with 10cc of sterile saline
DISTAL HANDLE
PROXIMAL HANDLE
STOPCOCK
CONNECTOR
LUER
LOCKING NUT
CHARGE ZONE
(GREEN)
PRESSURE
INDICATOR
10CC MARK
RELEASE
BUTTON
PLUNGERDILATION ZONE
CONNECT the inflation device to the dilator catheter
CHARGE the outer balloon by rotating the plunger
clockwise until the pressure indicator is
within the charge zone (green)
PREPARE the outer balloon by pushing
the proximal handle forward
1-2 cm
RETRACT the outer balloon and now
you’re ready for dilation
Page 2
QUICK REFERENCE GUIDE
Defi nity Cervical Dilator
SYSTEM OVERVIEW
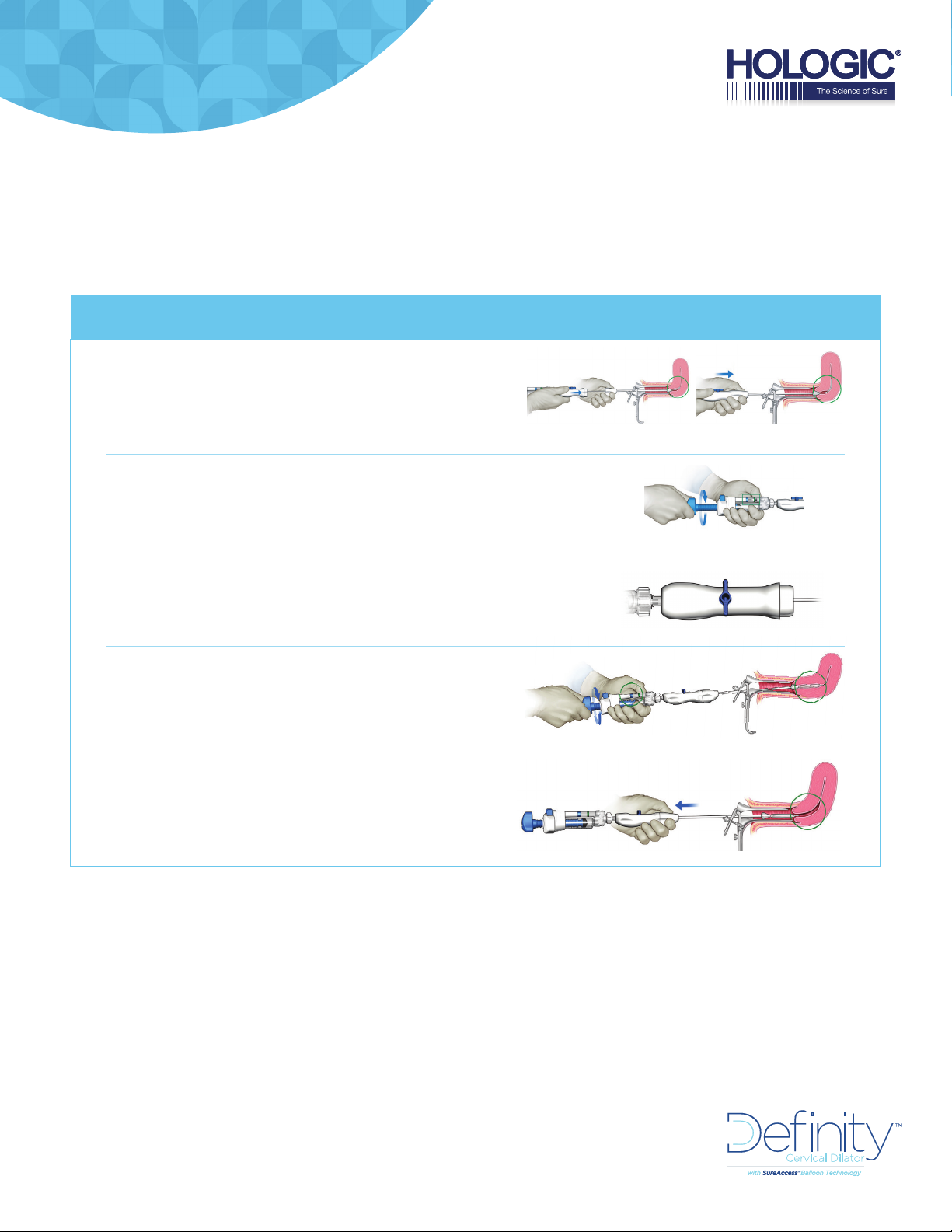
DILATION QUICK SETUP | Performing the Dilation
INSERT the Definity cervical dilator
ensuring the Acorn tip is snug
against the cervix
DEPLOY the inner balloon by rotating the plunger
until the pressure indicator drops below the
charge zone (green)
ROTATE the stopcock 90 degrees
DILATE by slowly turning the plunger
until the pressure indication is
in the dilation zone (blue)
COMPLETE the dilation and remove
This Quick Reference Guide is designed to be used in conjunction with, not to replace, the Defi nity cervical dilator Instructions for
Use. Prior to performing the dilation, the physician must review and be familiar with the full operating instructions for the Defi nity
cervical dilator, as well as any warnings, contraindications, and safety information.
IMPORTANT SAFETY INFORMATION
The Defi nity™ cervical dilator catheter system is intended to be used whenever cervical softening and dilation is desired.
Some examples are: treatment of cervical stenosis, IUD placement and removal, placement of instruments for intrauterine
radiotherapy, endometrial biopsy, global endometrial ablation, uterine tissue removal, uterine curettage, diagnostic
hysteroscopy, operative hysteroscopy. This device is not intended for use in the induction of labor. Use of the Defi nity
cervical dilator catheter system is contraindicated in patients with: an active genital tract infection such as genital
herpes, pelvic structure abnormality that prevents passage of the device, or invasive cervical cancer. This device is also
contraindicated for the induction of labor.
MISC-06164-001 Rev. 001 ©2019 Hologic, Inc. Hologic, Defi nity, SureAccess, The Science of Sure, and associated logos
are trademarks or registered trademarks of Hologic, Inc. and/or its subsidiaries in the United States and/or other countries.
 Loading...
Loading...