Page 1

Operator's Manual
BM/Hitachi 917
S
V 2.1 – Operator's Manual
Page 2
BM/Hitachi 917
The contents of this manual, including all drawings and tables, are the property of
BOEHRINGER MANNHEIM GmbH. They may not be copied or duplicated without prior
written consent, or used in whole or in parts for the manufacture or sale of items.
BOEHRINGER MANNHEIM GmbH reserves the right to make changes to their manual
without being obliged to give notice of this.
No responsibility is accepted by BOEHRINGER MANNHEIM GmbH for any errors this manual
may contain.
This manual describes the BM/Hitachi 917 Software Version 2.
Version Published Software affected
V 1.0 January 1995 V 1.03
V 2.0 June 1996 V 2
V 2.1 March 1997 V 2
First Edition – March, 1997
Boehringer Mannheim GmbH, Mannheim
Production: P. Hirn, D.H. Jung, B. Koroknay, J. Lutzke, I. Reinhardt, J. Westera
Layout: Scriptor Dokumentations Service GmbH, Bielefeld
V 2.1 – Operator's Manual
Page 3

Contents
Contents
V 2.1 – Operator's Manual 1
Page 4

BM/Hitachi 917
0. Potential Hazards and Safety Precautions 0-1
1. Introduction 1-1
1.1 Manual Outline 1-2
2. Daily Routine 2-1
2.1 Introduction 2-2
2.2 Daily Maintenance - Outline 2-3
2.3 System Start 2-6
2.3.1 Introduction 2-6
2.3.2 Power On 2-6
2.3.3 Automatic Start Procedure 2-7
2.3.4. Log On/Log Off 2-8
2.4 Daily Start Up 2-10
2.4.1 Checking the Reagents REQUIREMENTS Screen 2-11
2.4.2 Reconstituting Calibrators and Controls 2-13
2.4.3 Air Purge (Photometric System) 2-15
2.4.4 ISE Prime, Conditioning, Calibration 2-16
2.5 Calibration Test Selection 2-18
2.5.1 Automatic Calibration 2-18
2.5.2 Manual Control Test Selection (from Sample Disk 2) 2-24
2.6 Initiate Run with Routine Patient Test Selections 2-25
2.6.1 Routine Patient Test Selection 2-25
2.6.2 Initiate Run Procedure 2-29
2.6.3 Entering Non-Barcoded or Unreadable Barcoded Samples
(in the Barcode Mode) 2-34
2.7 Sample Tracking 2-36
2.8 Measurement of Additional Routine Samples 2-37
2.9 STAT Test Selections 2-38
2 V 2.1 – Operator's Manual
Page 5

Contents
2.10 Processing of Rerun Samples 2-41
2.10.1 Automatic Rerun 2-42
2.10.2 Manual Rerun 2-43
2.11 Adding Reagent During a Run 2-45
2.12 Patient Reports 2-47
2.12.1 Selecting the Patient Report Format for Real Time Printing 2-47
2.12.2 Printing Patient Reports in Batch 2-48
2.13 Data Management 2-49
2.13.1 Reviewing Data 2-49
2.13.2 Editing Data 2-50
2.13.3 Deleting Functions 2-55
2.14 Quality Control Procedures 2-58
2.14.1 Selecting Controls for Real Time QC 2-59
2.14.2 Individual QC List 2-60
2.14.3 Setting of Controls in the Individual QC Chart 2-61
2.14.4 Validation of Individual QC with Real Time QC
(Rejection of Single Test Couplings) 2-63
2.15 QC File Maintenance 2-65
2.15.1 Accumulate QC Data 2-65
2.15.1 Deleting QC Data 2-66
2.15.3 Cumulative QC List 2-67
2.15.4 Validation of Cumulative QC 2-68
2.15.5 Printing the Cumulative QC List and Chart 2-69
2.15.6 Deleting the Cumulative QC 2-70
2.16 System Shutdown 2-71
2.16.1 Instrument Shutdown 2-71
2.16.2 Activating the SLEEP Mode 2-72
V 2.1 – Operator's Manual 3
Page 6

BM/Hitachi 917
3. Maintenance and Daily Care 3-1
3.1 Introduction 3-2
3.1.1 Necessary Material and Safety Precautions 3-2
3.1.2 Automatic System Cleaning (Daily) 3-5
3.1.3 ERGO Console Cleaning 3-6
3.1.4 Maintenance and Care Schedule 3-7
3.2 Daily System Maintenance 3-9
3.2.1 Emptying the Waste Container 3-9
3.2.2 Checking/Replacing the Detergent 3-10
3.2.3 Checking the Detergent/Replenishing the Bottles 3-12
3.2.4 Cleaning the Reagent Probes and Sample Probe 3-13
3.2.5 Cleaning the Stirring Unit 3-14
3.2.6 Checking the Paper Supply 3-15
3.2.7 Cleaning the Instrument Surfaces 3-15
3.2.8 Cleaning the Nozzles of the Cell Rinse Unit 3-15
3.2.9 Priming, Conditioning and Calibrating the ISE Unit 3-16
3.2.10 Cleaning the ISE Unit with ISE Wash Solution 3-17
3.2.11 Checking the Temperature of the Incubation Bath 3-17
3.2.12 Performing a Photometer Check 3-18
3.3 Weekly System Maintenance 3-19
3.3.1 Cleaning the Reaction Cells 3-19
3.3.2 Performing the Cell Blank Measurement 3-20
3.3.3 Cleaning the Rinse Bath for the Sample Probe, Reagent Probes,
and the Stirring Paddle 3-21
3.4 Monthly System Maintenance 3-22
3.4.1 Replacing the Reaction Cell Segments 3-22
3.4.2 Cleaning the Incubation Bath and the Incubation Bath Drain Filter 3-24
3.4.3 Cleaning the Inside of the Sample and Reagent Disks 3-28
3.4.4 Cleaning the Air Filter 3-30
3.4.5 Replacing the ISE Pinch Valve Tubing 3-32
4 V 2.1 – Operator's Manual
Page 7

Contents
3.5 Quarterly System Maintenance 3-33
3.5.1 Cleaning the Inlet Water Filter 3-33
3.5.2 Replacing the Syringe Seals 3-34
3.6 Six Monthly System Maintenance 3-42
3.6.1 Replacing the ISE Reference Electrode 3-42
3.6.2 Replacing the Teflon Block (Nozzle Tip) 3-44
3.7 Analyzer Maintenance As Required 3-45
3.7.1 Emptying the Vacuum Tank 3-45
3.7.2 Replacing the Photometer Lamp 3-47
3.7.3 Cleaning/Replacing Clogged Probes 3-52
3.7.4 Checking the Alignments 3-54
3.7.5 Cleaning Clogged Rinse Nozzles 3-63
3.7.6 Replacing the Stirring Paddles 3-65
3.7.7 Replacing the ISE Measurement Electrodes (Na+, K+, Cl-) 3-67
3.8 Printer Maintenance 3-70
3.8.1 Start-Up 3-70
3.8.2 Replacing the Ink Ribbon Cartridge 3-71
3.8.3 Loading Continuous-Feed Paper 3-74
3.8.4 Inserting Single Sheets 3-78
3.8.5 Setting the Paper Thickness 3-80
V 2.1 – Operator's Manual 5
Page 8

BM/Hitachi 917
4. Operation Support 4-1
4.1 Adding a New Application 4-2
4.1.1 Loading New Applications from Disk 4-3
4.1.2 Loading New Applications from a Barsheet 4-5
4.1.3 Adding of User-Definable Applications (901 to 905) 4-9
4.1.4 Key Setting 4-11
4.1.5 Assigning the Print Order 4-12
4.1.6 Reagent Registration 4-13
4.1.7. Programming Preset K Factors for Assays 4-13
4.2 Loading of a Serum Index Application 4-14
4.3 Special Wash Programming 4-16
4.3.1 Reagent Probe Wash 4-16
4.3.2 Sample Probe Wash 4-19
4.3.3 Cell Wash 4-22
4.4 Calculated and Compensated Tests 4-25
4.4.1 Calculated Tests 4-25
4.4.2 Compensated Tests 4-30
4.5 Loading Calibrator Information 4-33
4.5.1 Manual Setting of Calibrator 4-33
4.5.2 Loading Calibrator Setpoints from a Barsheet 4-34
4.5.3 Assigning a Calibrator Position 4-35
4.5.4 Deleting a Calibrator 4-36
4.5.5 Deleting a Calibrator Position 4-37
4.6 Loading Control Information 4-38
4.6.1 Manual Setting of Controls 4-38
4.6.2 Loading Control Setpoints from a Barsheet 4-40
4.6.3 Assigning a Control Position 4-42
4.6.4 Deleting a Control 4-43
4.6.5 Deleting a Control Position 4-44
4.6.6 Activating Controls 4-45
4.6.7 Assigning Key Settings for Controls 4-46
4.6.8 Manual Control Test Selections (Sample Disk 2) 4-47
4.6.9 Setting Controls for the QC 4-48
6 V 2.1 – Operator's Manual
Page 9

Contents
4.7 Defining Profiles 4-50
4.7.1 Adding a Profile 4-50
4.7.2 Deleting a Profile / Deleting a Test from a Profile 4-52
4.7.3 Default Profiles 4-54
4.8 Operator-ID / Password Management 4-55
4.8.1 Assigning an Operator ID 4-56
4.8.2 Delete Operator ID 4-57
4.8.3 Password Registration 4-58
4.9 Disk Management 4-59
4.9.1 Writing System Parameters (Application Settings) on a Floppy
Disk 4-59
4.9.2 Reading System Parameters from a Floppy Disk 4-60
4.9.3 Formatting a Floppy Disk 4-61
4.9.4 Archive Data 4-62
4.9.5 Saving QC Data 4-63
4.10 Define Maintenance Frequency and Maintenance Item 4-64
4.10.1 Defining the Maintenance Frequency 4-64
4.10.2 Defining the Maintenance Item 4-66
V 2.1 – Operator's Manual 7
Page 10

BM/Hitachi 917
5. Printouts 5-1
5.1 MEASUREMENT DATA Printout 5-2
5.1.1 REPORT Format 5-2
5.1.2 MONITOR Format 5-5
5.2 REACTION MONITOR Printout 5-6
5.3 REQUISITION LIST Printout 5-8
5.3.1 Requisition List Without Sample Barcode 5-8
5.3.2 Requisition List With Sample Barcode 5-9
5.4 RERUN LIST Printout 5-10
5.5 PRECISION CHECK Printout 5-12
5.6 PROFILING LIST Printout 5-13
5.7 SYSTEM REQUIREMENTS Printout 5-14
5.8 REAGENT STATUS Printout 5-15
5.9 CALIBRATION MONITOR Printout 5-17
5.9.1 CALIBRATION MONITOR Photometry 5-18
5.9.2 CALIBRATION MONITOR ISE 5-22
5.10 REACTION MONITOR Printout 5-23
5.10.1 The Primary and Secondary Wavelengths Option 5-24
5.10.2 The Primary Minus Secondary Wavelengths Option 5-25
5.11 CALIBRATOR LOAD LIST Printout 5-26
5.12 CALIBRATION TRACE Printout 5-27
5.12.1 CALIBRATION TRACE Photometry, Rate 5-28
5.12.2 CALIBRATION TRACE Photometry, Endpoint 5-30
5.12.3 CALIBRATION TRACE ISE 5-31
5.13 INDIVIDUAL QC CHART Printout 5-32
5.14 INDIVIDUAL QC LIST Printout 5-34
5.15 CUMULATIVE QC CHART Printout 5-35
5.16 CUMULATIVE QC LIST Printout 5-37
5.17 ALARM TRACE Printout 5-38
5.17.1 DAILY ALARM TRACE 5-38
5.17.2 CUMULATIVE ALARM TRACE 5-39
8 V 2.1 – Operator's Manual
Page 11

Contents
5.18 SYSTEM COMMUNICATION TRACE Printout 5-40
5.19 OPERATOR ID TRACE Printout 5-42
5.20 CUMULATIVE OPERATION LIST Printout 5-43
5.21 MAINTENANCE REPORT Printout 5-45
5.22 REPORT EXAMPLE Printout 5-46
5.22.1 Setting ONE REPORT/PAGE 5-47
5.22.2 Setting TWO REPORTS/PAGE 5-49
5.23 CELL BLANK MEASUREMENT Printout 5-51
5.24 PHOTOMETER CHECK Printout 5-52
5.25 CELL BLANK MEASUREMENT Printout 5-53
5.26 PRINTER CHECK Printout 5-55
5.27 BARCODE READER CHECK Printout 5-56
5.28 ISE CHECK Printout 5-57
5.29 HD CHECK Printout 5-59
5.30 FD CHECK Printout 5-61
V 2.1 – Operator's Manual 9
Page 12

BM/Hitachi 917
6. Troubleshooting 6-1
6.1 Overview 6-2
6.2 General Troubleshooting Strategy 6-6
6.2.1 Troubleshooting Conditions at power up 6-8
6.2.2 Test troubleshooting 6-9
6.2.3 Preparation of reagents, calibrators, controls 6-10
6.2.4 High test results 6-11
6.2.5 Low test results 6-12
6.2.6 Erratic test results 6-13
6.2.7 Problems with a single sample or control 6-15
6.2.8 Problems with a single test 6-16
6.2.9 Problems with tests with one calibration set point 6-17
6.2.10 Problems with all tests with more than one calibration set point 6-18
6.2.11 Problems with multiple tests (photometrics only) 6-19
6.2.12 Problems with all tests, including ISEs 6-20
6.2.13 Problems with ISE, all results are erratic,
excessive air in sipper syringe 6-21
6.2.14 Erratic ISE results 6-22
6.2.15 Problems with ISE, high internal standard values 6-24
6.2.16 Problems with low sodium, potassium and chloride values 6-25
6.2.17 Problems with a biased enzymes 6-26
6.3 Data Alarms 6-27
6.3.1 Calculation disabled 6-27
6.3.2 Absorbance over 6-28
6.3.3 Absorbance maximum over (non-linear calibration) 6-29
6.3.4 ADC abnormal 6-29
6.3.5 Calculation test error 6-30
6.3.6 Calibration result abnormal 6-30
6.3.7 Calibration error 6-31
6.3.8 Cell blank abnormal 6-32
6.3.9 Test-to-test compensation error 6-33
6.3.10 Test-to-test compensation disabled 6-34
6.3.11 Duplicate error 6-34
6.3.12 Edited test 6-36
6.3.13 Outside of reference value 6-36
10 V 2.1 – Operator's Manual
Page 13

Contents
6.3.14 Internal standard concentration abnormal 6-37
6.3.15 Level error 6-38
6.3.16 Technical value limit over 6-40
6.3.17 Reaction limit over at all points (Limt0) 6-41
6.3.18 Reaction limit over at 1 point (Limt1) 6-42
6.3.19 Reaction limit over (Limt2) 6-43
6.3.20 Linearity abnormal (Lin.) 6-44
6.3.21 Linearity abnormal (Lin.8) 6-46
6.3.22 ISE Preparation abnormal 6-48
6.3.23 Noise error 6-49
6.3.24 Overflow 6-50
6.3.25 Prozone error 6-50
6.3.26 QC error 1 6-51
6.3.27 QC error 2 6-52
6.3.28 Random error 6-53
6.3.29 Reagent short 6-54
6.3.30 Value outside repeat limit 6-55
6.3.31 Sample value abnormal 6-55
6.3.32 Sample short 6-56
6.3.33 SD error 6-57
6.3.34 Sensitivity error 6-58
6.3.35 Slope abnormal 6-59
6.3.36 Standard error 6-60
6.3.37 Systematic error 1 6-61
6.3.38 Systematic error 2 6-62
6.3.39 Systematic error 3 6-63
6.3.40 Systematic error 4 6-64
6.3.41 Systematic error 5 6-65
6.3.42 Systematic error 6 6-66
6.3.43 Standard 1 absorbance abnormal 6-67
7. Appendix
A.1 Glossary A-2
A.2 Index A-13
V 2.1 – Operator's Manual 11
Page 14

BM/Hitachi 917
12 V 2.1 – Operator's Manual
Page 15

Potential Hazards and Safety Precautions
0. Potential Hazards and Safety Precautions
V 2.1 – Operator's Manual 0 - 1
Page 16
BM/Hitachi 917
❚ Safety Warnings
Before putting the analyzer into operation, you should become acquainted with all
precautions and regulations concerning the handling of the analyzers electrical and
mechanical components in order to rule out any potentially hazardous situations to
yourself and your colleagues.
All safety warnings that the operator must consider in this manual are classified as
below. Acquaint yourself with the following labels and pictures.
WARNING
If these instructions are not strictly adhered to, there is a risk of fatal injury e.g. from
power supplies or infection.
CAUTION
If these instructions are not strictly adhered to, there is an increased risk of injury and/or
serious functional disruptions to the analyzer.
ATTENTION
Identifies all other situations where increased attention is necessary (e.g. to avoid
damage to the analyzer).
Note
Indicates information to be considered e.g. remarks, etc.
is the international symbol for caution
0 - 2 V 2.1 – Operator's Manual
Page 17
❚ Safety Precautions
WARNING
Electrical Safety
Never open the back or side panels when the analyzer is connected to the
mains supply (wall socket)! Pull the plug out beforehand!
You can receive an electric shock by touching supply-carrying components.
CAUTION
Flammable and Explosive Materials
Avoid using flammable materials around the analyzer.
Electrical sparks can cause fires and explosions.
Potential Hazards and Safety Precautions
CAUTION
Analyzer in Operation
Never touch the sample pipetter, reagent pipetters, stirrer, rinse units and
all other moving components when the analyzer is operating.
There is a risk of injury from the moving components and the analyzer can
also be damaged.
WARNING
Photometer Lamp
Never look for long periods into the light of the photometer lamp without
eye protection.
If this warning is not heeded, damage to the eyes can occur. Wear
darkened protective goggles to protect yourself from ultra violet light,
when you must look at the light transmitted from the photometer lamp for
long periods.
V 2.1 – Operator's Manual 0 - 3
Page 18
BM/Hitachi 917
WARNiNG
CAUTION
Samples
1. Avoid direct contact with samples that contain bacteria or are poten-
tially infectious.
If sample material comes in contact with the analyzer surface, clean it
up immediately with a towel.
2. Ensure that the sample contains no fibrin clots, dust or other insoluble
contaminations.
If the sample contains insoluble material, false results can be produced. This applies especially when fibrin clots block the sample
pipetter.
Liquid Waste
1. Potentially infectious waste must be disposed of according to the legal
regulations.
There is a risk of contamination from potentially infectious waste.
2. Contact the manufacturer of the reagent, if you require more informa-
tion about its environmental compatibility.
CAUTION
Correctness/Precision of the Measurements
To ensure that the analyzer operates correctly, controls must be measured
and the functions must be monitored.
Erroneous measurements can lead to a false diagnosis resulting in an
incorrect therapy that puts the patient at risk.
0 - 4 V 2.1 – Operator's Manual
Page 19
Potential Hazards and Safety Precautions
❚ Safety Precautions During Operation
CAUTION
Correct Usage
1. The analyzer was designed for clinical-chemical analyses, electrolyte analyses,
immunological tests and medical analyses using water-soluble samples and reagents.
Comply with the manufacturer regulations of the reagent when using the tests on the
analyzer.
2. If reagents are used other than those developed by Boehringer Mannheim, then
Boehringer Mannheim guarantees the technical specifications of the analyzer but
takes no responsibility for the measured results.
CAUTION
Operator Qualifications
1. The operation of the analyzer should only be performed under the control of a
qualified person, who has taken part in a recognized training from Boehringer
Mannheim.
2. For clinical tests, the analyzer should only be controlled by a practitioner or a
laboratory doctor.
CAUTION
Operation and Maintenance
1. The operation and maintenance of the analyzer may only be performed according to
the described procedures. No other components other than those specified may be
touched.
2. Never open the screwed down analyzer covers when the analyzer is connected to a
power supply. Contact with the circuit boards can damage the electronic components.
V 2.1 – Operator's Manual 0 - 5
Page 20
BM/Hitachi 917
3. Never touch the sample pipetter, reagent pipetter, stirrer, rinse units and all other
moving components of the analyzer when it is in operation.
If this precaution is not adhered to, damage or interruption to the analyzer can occur.
4. Never touch the reaction disk, sample disk and reagent disk when they are rotating.
If this precaution is not adhered to, damage or interruption to the analyzer can occur.
CAUTION
Environment Conditions (Installation Conditions)
Consider the specified installation conditions. If the analyzer is to be located somewhere else, contact Boerhinger Mannheim customer support.
CAUTION
Limitations for Samples and Reagents
1. The reaction cells, sample cups and the tubing for liquid waste are not impervious to
organic solutions. Never use organic solutions.
2. Never use samples or reagents that can stick to the sample probe, reagent probes
or the reaction cells. This also applies to samples and reagents that may block the
sample probe or reagent probes. False measurements may be produced.
CAUTION
Loading Samples and Reagents
Only load samples and reagents into the intended positions on the analyzer. Spilt
samples and reagents can cause disruptions to the analyzer. Only load reagents when
the analyzer is in the "stand-by" mode, or when the reagent stop function permits
loading.
If this precaution is not adhered to, damage or interruption to the analyzer can occur.
0 - 6 V 2.1 – Operator's Manual
Page 21
Potential Hazards and Safety Precautions
CAUTION
Analyzer Cover
The analyzer cover should always be closed during operation. Only open the cover to
load samples, etc.
CAUTION
Sample Disk
Never load new samples or change the sample disk when the LEDs, indicating that the
disk is rotating, are lit or blinking.
LED 1 indicates that sample disk 1 is rotating.
LED 2 indicates that sample disk 2 is rotating.
If this precaution is not adhered to, the analyzer can be damaged.
CAUTION
Reaction Disk
1. Never touch the reaction disk when the analyzer is operating.
2. Always follow the corresponding instructions when removing, loading or replacing
the reaction disk or the reaction cell segments.
If this precaution is not adhered to, damage or interruption to the analyzer can occur.
CAUTION
Reagent Disk
Only open the reagent disk cover in order to replace reagents.
If this precaution is not adhered to, the cooling can be affected thus causing the
reagents to expire. Opening the cover during an analysis causes an analyzer alarm.
V 2.1 – Operator's Manual 0 - 7
Page 22
BM/Hitachi 917
CAUTION
Reaction Cells
If a reaction cell dries out after use, cracks can occur and contaminations may not be
able to be removed. Therefore, the reaction cells must be stored after use in deionized
water. If the analyzer is not going to be used for more than three days, the reaction cells
must be removed from the reaction disk and must be stored in a 2% solution of
Hitergent.
CAUTION
Switching the Analyzer On
Never switch the analyzer off and then immediately back on. Wait at least 30 seconds
before switching back on.
If this is not adhered to, the analyzer may be damaged.
CAUTION
Handling Detergents
Never touch detergents with bare fingers, this can cause skin damage. Wear rubber
gloves when handling detergents.
CAUTION
Photometer Lamp
Do not touch the hot lamp or lamp housing, there is a risk of burns.
CAUTION
Stirrer
Do not bend the stirring paddle. Bent stirring paddles can cause false measurements.
0 - 8 V 2.1 – Operator's Manual
Page 23

1. Introduction
Manual Outline
V 2.1 – Operator's Manual 1 - 1
Page 24

BM/Hitachi 917
1.1 Manual Outline
The Operator’s Manual is a part of the documentation for the analyzer BM/Hitachi 917,
which additionally includes the volume System Description and the Short Guide.
The volume Operator’s Manual offers a detailed overview of all processes concerned with
the daily operation and those processes required fro the daily routine of the analyzer.
– In chapter 0 potentially hazardous sources are brought to light as well as safety notes
and precautionary measures.
– Chapter 2 provides detailed information about the daily routine on the analyzer.
– Procedures for analyzer care and maintenance are described in detail in chapter 3.
– Chapter 4 deals with operation support that could be useful when tuning the analyzer
to the laboratory requirements.
– In chapter 5 all printouts are described that can be requested from the analyzer.
– The 6th and last chapter describes errors and what measures must be taken to cure
them.
– The Appendix consists of a Glossary and an Index, allowing you quick access to any
required information.
1 - 2 V 2.1 – Operator's Manual
Page 25

2. Daily Routine
Introduction
V 2.1 – Operator's Manual 2 - 1
Page 26

BM/Hitachi 917
2.1 Introduction
In this chapter you find a description how the daily routine is prepared, performed and
shut down. Step by step you will get to know how to switch on the analyzer, how to
access the menus, how to determine the type and number of samples, how to allocate
tests, how to start the analysis, and how to obtain results at the end of the analysis.
Your service person at Boehringer Mannheim
Name:
Address:
Tel.:
Fax.:
2 - 2 V 2.1 – Operator's Manual
Page 27

Daily Maintenance - Outline
2.2 Daily Maintenance - Outline
Perform all daily operational checks prior to beginning the first run of the day. Each daily
operational check should be logged on the MAINTENANCE LOG sub menu. Default daily
maintenance items include:
1. Empty waste container
2. Check / replace cell detergent supply
3. Check / replace detergent supplies (1D1 - 2D3)
4. Check W1/W2 positions on sample disk 2
5. Clean sample and reagent probes
6. Clean stirrer paddles
7. Check paper supplies
8. Clean instrument surfaces
9. Clean cell rinse unit nozzles
10. Wash ISE unit with ISE Wash Solution (in position W2)
11. ISE prime/condition/calibrate
12. Check incubation bath temperature
13. Perform photometer check
14. Update Maintenance Log
V 2.1 – Operator's Manual 2 - 3
Page 28
BM/Hitachi 917
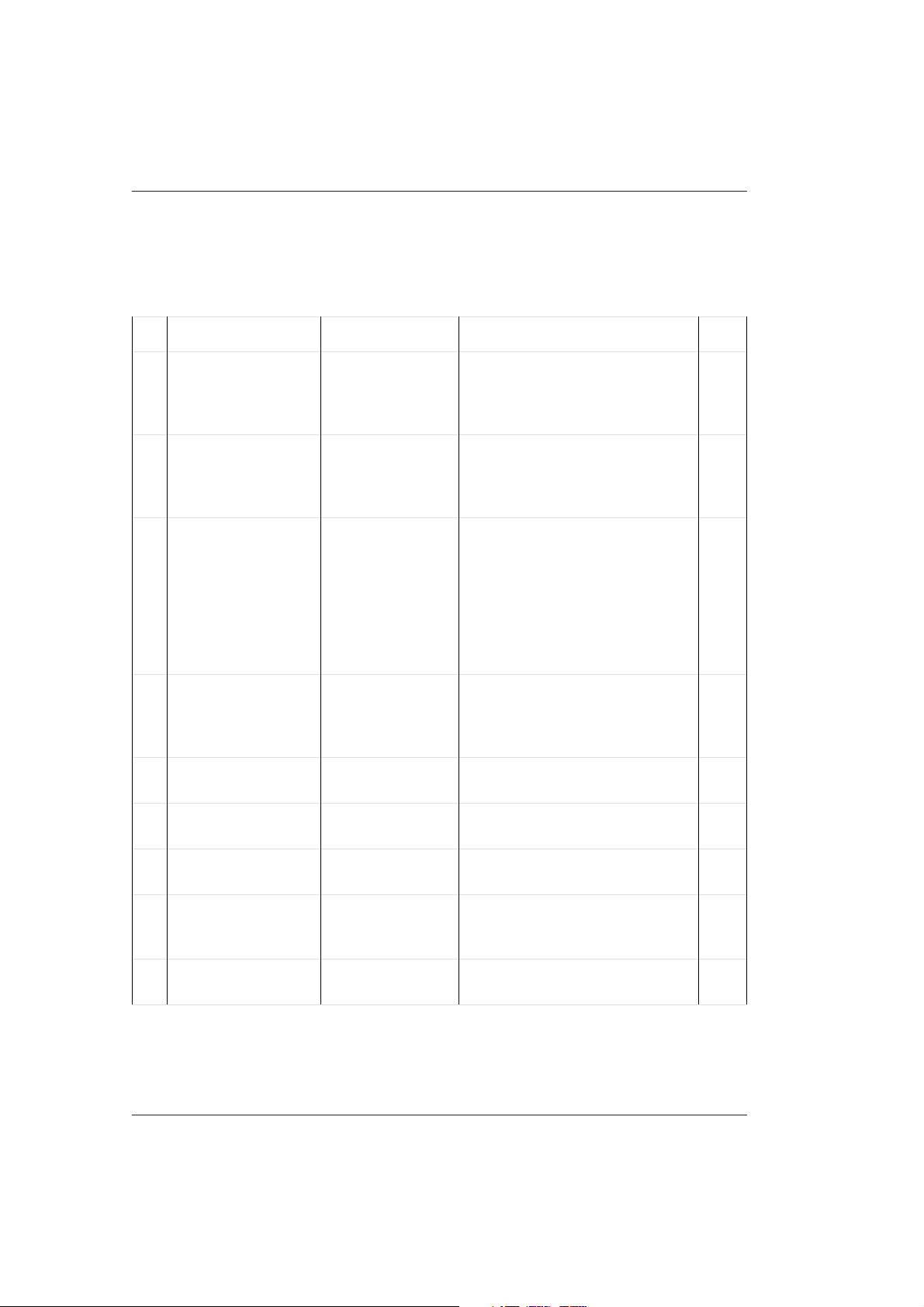
The following table displays an overview of the above maintenance procedures. More
detailed information can be found in chapter 3 Maintenance and Daily Care of the
Operator's Manual.
No. Maintenance Item Material required Note Details Page
1 Empty waste
container
2 Check / replace cell
detergent supply
3 Check / replace
detergent supplies
(1D1 - 2D3)
4 Check W1, W2
position on sample
disk 2
5 Clean sample and
reagent probes
disinfecting solution, water for
rinsing, paper
towels
Detergent 1
(NaOH-D)
fresh detergent Hitergent is used for position
NaOH-D
ISE wash solution
alcohol, lint-free
cloth
Content of the waste container is
potentially hazardous. Wear
disposable gloves.
Place both tube filters in the first
NaOH-D bottle. Execute a Cell
detergent prime, before running
analysis.
1D1 and 2D1.
NaOH-D (70 mL bottle) is used
for position 1D2 and 2D2.
Detergent for additional rinsing is
used for position 1D3 and 2D3.
NaOH-D is used for position W1.
ISE wash solution is used for
position W2.
Switch off the instrument to
move the pipettors.
3-9
3-10
3-12
3-5
3-13
6 Clean stirrer
paddles
7 Check paper supply printer paper Load new printer paper, if
8 Clean instrument
surfaces
9 Clean nozzles of the
cell rinse unit
alcohol, lint-free
cloth
disinfecting
solution, water,
lint-free cloth
alcohol, lint-free
cloth
Switch off the instrument to
move the stirrer paddles.
necessary.
Clean all surfaces with a lint-free
cloth and water or disinfecting
solution, if needed.
Instrument must be in Standby
or OFF.
3-14
3-15
3-15
3-15
2 - 4 V 2.1 – Operator's Manual
Page 29

Daily Maintenance - Outline
No. Maintenance Item Material required Note Details Page
10 Wash ISE Unit with
ISE wash solution (in
position W2)
11 ISE prime/condition/
calibration
12 Check incubation
bath temperature
13 Perform photometer
check
14 Update
Maintenance log
ISE wash solution ISE maintenance has to be
performed once a day. Ensure
that ISE conditioning and
calibration are performed prior to
the start of the next routine.
ISE reagents,
human sample,
calibrators
Perform an ISE prime and
calibration once a day.
Check the incubation bath
temperature displayed on the
SAMPLE TRACKING global
menu screen (Tolerance: 37°C ±
0.2 °C)
Check that the results do not
exceed 16000
in the MAINTE LOG sub menu
(MAINT/UTILITY main menu)
3-17
3-16
3-17
3-18
V 2.1 – Operator's Manual 2 - 5
Page 30

BM/Hitachi 917
2.3 System Start
2.3.1 Introduction
There are two ways to start the routine operation - by pressing the main switch of the
analyzer (POWER ON) on the right side of the front panel, or by waking up the instrument
from the SLEEP mode.
2.3.2 Power On
1. If necessary, turn on the external water supply and open the tap, before starting the
daily routine operation.
2. Switch on the analyzer using the main switch (on the right side of the front panel). The
computer, screen, and the printer are also switched on, if the main switches of these
components are permanently left in the ON position.
2 - 6 V 2.1 – Operator's Manual
Page 31

System Start
2.3.3 Automatic Start Procedure
If the automatic start procedure is selected, the instrument initializes at a set time and
performs the startup maintenance functions. Then it is ready for operation. The SLEEP
mode can be canceled at any time by pressing the displayed EXECUTE button. If the
SLEEP mode is automatically or manually canceled, the LOG ON screen is displayed again.
After the initialization phase, the LOG ON screen is displayed.
Note
If the analyzer is automatically initialized (“waking up” from the SLEEP mode), a photometer check is automatically performed. If the instrument is manually started, a photometer
check has to be requested manually in the MAINTE LOG sub menu (MAINT/UTILITY main
menu).
V 2.1 – Operator's Manual 2 - 7
Page 32

BM/Hitachi 917
2.3.4 Log On/Log Off
Your service person activates the LOG ON function during installation.
❚ Log On
1. Switch on the instrument. If the screen saver is on, just touch the screen to open the
LOG ON screen.
2. When the analyzer is in STANDBY mode, enter your operator ID and password in the
corresponding fields. If the entries are correct, you can now open the individual
software menus.
Note
A supervisor must assign operator IDs and the operator level from the MAINT/UTILITY
main menu, SYSTEMS sub menu, LOG ON window.
If the Log On mode is not activated, the LOG ON screen is not displayed.
2 - 8 V 2.1 – Operator's Manual
Page 33

System Start
❚ Log Off
1. When you have finished your routine work on the analyzer so that you are ready to
log off, touch the START button to display the START CONDITIONS global menu.
2. Touch the LOG OFF button to display the LOG OFF window.
3. Touch YES to log off the analyzer.
4. The system returns to the LOG ON screen so that next operator can log on the
system.
V 2.1 – Operator's Manual 2 - 9
Page 34

BM/Hitachi 917
2.4 Daily Start Up
The automated start up procedures require minimal operator involvement. Do not omit
any of the described procedures from your daily routine. Only touchscreen instructions
for the following procedures are given. Most of the procedures may also be performed
using the keyboard. Keyboard equivalents of the touchscreen navigation methods are
described in the guidance field in the upper right-hand corner of the screen.
Safety Precautions:
While the analyzer is in operation, follow these precautions:
❚ Keep the top cover of the analyzer closed.
❚ Do not place, replace or remove samples while the sample disk is rotating.
❚ Avoid touching the sample probe, reagent probes, stirring paddles, and other moving
parts.
❚ Do not remove or replace the reagent disk covers.
❚ Do not place reagent or sample containers on the cover of the analyzer.
2 - 10 V 2.1 – Operator's Manual
Page 35

Daily Start Up
2.4.1 Checking the Reagents REQUIREMENTS Screen
1. Touch WORKPLACE followed by REQUIREMENTS to display the REQUIREMENTS sub
menu.
2. The screen is divided into two list boxes. The list box on the left displays the
corresponding test.
Different highlight colors in the columns REAG, CALIB and QC are used to indicate the
current status, e.g. which method requires reagent or if a calibration or control failure
occurred.
The list box on the right displays detailed information about the specific highlighted
test in the left list box:
For photometric reagents:
Red highlight indicates that there is no reagent volume or that the reagent bottle is
missing. The bottle is canceled and the barcode cannot be reused.
Yellow highlight indicates that the remaining reagent volume is less than the reagent
level defined for the analyzer. This number is defined on the MAINT/UTILITY, SYSTEM
screen.
Purple highlight indicates that the remaining reagent volume is less than the reagent
level check volume defined for that test. This number is defined in the REAGENT CHECK
window of the REAGENTS main menu and can be different for each reagent. This
number should be entered to reflect your workload needs.
V 2.1 – Operator's Manual 2 - 11
Page 36

BM/Hitachi 917
For ISE reagents:
Red highlight indicates < 10 mL of any ISE reagent remains.
Yellow highlight indicates that < 50 mL of Internal Standard solution or < 30 mL of
either KCl or diluent remain.
For calibrations:
Red highlight indicates that a calibration is required or that a calibration has failed.
For controls:
Red highlight indicates that a QC violation occurred and a random or system alarm
was issued or that the control is not on the analyzer.
3. To print a copy of the list, if desired, touch the PRINT button to open the corresponding
global menu. Touch SYSTEM REQUIREMENTS in the displayed list, then touch the PRINT
button.
4. Put the required reagents into the corresponding reagent disks (see package insert for
preparation details).
2 - 12 V 2.1 – Operator's Manual
Page 37

Daily Start Up
2.4.2 Reconstituting Calibrators and Controls
Quality control products are used to verify calibration as well as the precision and
accuracy of the instrument. Controls should be performed after every calibration, or
should be measured according to the corresponding legal guidelines. Additional control
runs should be established by your laboratory, based upon its needs.
1. Reconstitute all calibrators and control materials according to the instructions provided with each kit of material or according to legal regulations.
2. Print out a calibrator load list.
V 2.1 – Operator's Manual 2 - 13
Page 38

BM/Hitachi 917
3. Touch QC, followed by CONTROLS to display the position list of the controls and
calibrators in sample disk 2.
4. Put calibrators and controls in the indicated positions.
2 - 14 V 2.1 – Operator's Manual
Page 39

Daily Start Up
2.4.3 Air Purge (Photometric System)
The air purge procedure occurs automatically when the instrument is powered ON or after
Wake-Up. If you have not assayed any samples within an eight-hour period and (instrument in Stand-by), perform an air purge to ensure that there is no air in the hydraulic
tubing between the probes (photometric reagent and sample) and their respective pipettors. Air in the syringes or tubings can result in imprecise pipetting. This procedure
replaces the hydraulic line water with freshly degassed, DI water and takes approximately
one minute to complete. This procedure does not purge the ISE system pipettors.
1. Touch MAINT/UTILITY.
2. Touch MAINTENANCE.
3. Touch AIR PURGE.
4. Touch SELECT.
5. Touch EXECUTE.
6. DI water will be flushed through the pipettor. Check the water jet ejecting from the
pipettors. Check for leaks and excess air. Check the syringes for air bubbles.
7. Repeat steps 1-5, if necessary, to remove all air.
V 2.1 – Operator's Manual 2 - 15
Page 40

BM/Hitachi 917
2.4.4 ISE Prime, Conditioning, Calibration
Perform an ISE prime if you have not assayed any samples within an eight-hour period
(instrument in Stand-by) to ensure that there is no air in the hydraulic tubings. Replace
used up reagent bottles by new one. If you are replacing ISE reagents, reset the reagent
volumes by pressing the green ISE reset buttons (to the right of the ISE compartment) or
enter the volume in the REAGENTS main menu. Never fill old reagent into the new bottles,
which may result in bacterial growth.
1 Touch MAINT/UTILITY, followed by MAINTENANCE to open the corresponding sub
menu. Touch ISE PRIME and SELECT to display the corresponding window. Select the
ALL option and touch EXECUTE.
2. Condition the electrodes by performing an ISE measurement of 10 human serum
samples.
2 - 16 V 2.1 – Operator's Manual
Page 41

Daily Start Up
3. Calibrate the ISE unit. Open the STATUS sub menu (CALIBRATION main menu) and
touch the REPEAT CALIB button. Select the ISE item from the displayed list and touch
FULL.
4. Touch the EXECUTE button in the STATUS sub menu to initiate the full ISE calibration. If
the instrument is in Stand-by, touch the EXECUTE button in the START CONDITIONS
global menu.
V 2.1 – Operator's Manual 2 - 17
Page 42

BM/Hitachi 917
2.5 Calibration Test Selection
Your 917 analyzer offers the opportunity to select between the automatic calibration
feature and a manually requested calibration. To ensure proper operation of your 917
analyzer, calibrate each Boehringer Mannheim assay at the recommended interval specified on the APPLICATION sub menu (MAINT/UTILITY main menu).
2.5.1 Automatic Calibration
Calibrations are recommended to be performed when the calibration expires (time out
calibration) or a new bottle or lot is used up. Each Boehringer Mannheim test can be
automatically calibrated. Controls are automatically run for each requested test following
calibration, if requested.
❚ Time Out Calibration
1. Touch CALIBRATION, followed by STATUS to display the STATUS sub menu. Then
touch REPEAT CALIB.
2. The second number is the defined time interval. The remaining time is displayed as
the first number, e.g. the 1 in 1/48. This means that during the next hour an automatic calibration is executed, and the time interval is 48 hours. After the calibration
the time display is updated to 48/48.
2 - 18 V 2.1 – Operator's Manual
Page 43

Calibration Test Selection
3. All tests requiring calibration within a defined time period (e.g. 5 hours) are highlighted. The tests have to be marked before. Then, press the TIME OUT button.
Define the time period of all tests within this time period (yellow highlight). Tests
with the remaining time interval zero are not displayed in yellow. They are automatically calibrated if the START button is pressed. Press EXECUTE to start the calibration. If you want to delete already entered control requests, touch the corresponding calibration type button (BLANK, 2 POINT, FULL or SPAN). The yellow highlight
disappears again.
V 2.1 – Operator's Manual 2 - 19
Page 44

BM/Hitachi 917
❚ Start Up Calibration
Use the START UP CALIB button to display a list from which you can select tests for
start up calibration. A start up calibration performs the calibration for the requested
tests at the beginning of a run and can be initiated in Stand-by only.
1. Touch CALIBRATION, followed by STATUS to display the STATUS sub menu.
2. Touch START UP CALIB.
3. Touch the tests in the list box which are to be calibrated.
4. Touch BLANK, 2 POINT, FULL or SPAN to choose a calibration option.
IF a calibrator load list is desired,
THEN proceed to step 5.
IF no calibrator load list is desired,
THEN proceed to step 8.
5. Touch PRINT to display the PRINT global menu.
2 - 20 V 2.1 – Operator's Manual
Page 45

Calibration Test Selection
6. Touch CALIB LOAD LIST, then touch SELECT.
7. Touch START UP, then touch PRINT to print the calibrator load list.
8. Load calibrators and controls on sample disk 2 according to the printed load list.
9. Touch START, to display the START CONDITIONS global menu. Touch CALIBRATION
START UP
and EXECUTE to request a start up calibration. Press the START button in
the START CONDITIONS global menu to activate the analyzer.
10. Verify that no alarms exist on the calibration and that the QC results are in range.
V 2.1 – Operator's Manual 2 - 21
Page 46

BM/Hitachi 917
❚ Repeat Calibration
Use the REPEAT CALIB button to request tests for a repeat calibration. Any failed
calibrations are automatically put on the repeat list. A repeat calibration can either be
performed in Stand-by or Operate mode.
1. Touch CALIBRATION, followed by STATUS to display the STATUS sub menu.
2. Touch REPEAT CALIB.
3. Touch the tests in the list box for which you want a repeat calibration.
4. Touch BLANK, 2 POINT, FULL or SPAN to choose a calibration option.
IF a calibrator load list is desired,
THEN proceed to step 5.
IF a calibrator load list is not desired,
THEN proceed to step 8.
5. Touch PRINT to display the PRINT global menu.
2 - 22 V 2.1 – Operator's Manual
Page 47

Calibration Test Selection
6. Touch CALIBRATION, followed by CALIB LOAD LIST, then touch SELECT.
7. Touch RE CALIB, then touch PRINT to print out the calibrator load list.
8. Load calibrators and controls on sample disk 2 according to the printed list of the
required Standards.
9. If the analyzer is in operation, touch EXECUTE, followed by YES to start the repeat
calibration.
If the analyzer is in Stand-by mode, touch EXECUTE, followed by YES and the START
button in the START CONDITIONS global menu to start the analyzer.
10. Verify that no alarms exist on the calibration and that the QC results are in range.
V 2.1 – Operator's Manual 2 - 23
Page 48

BM/Hitachi 917
2.5.2 Manual Control Test Selection (from Sample Disk 2)
Independent from calibrator or control intervals, controls can be requested and measured
manually on sample disk 2 during operation.
1. Touch WORKPLACE, followed by TEST SELECTION. Touch the CONTROL button.
2. Touch the S. DISK 2 button only if the barcode reader is activated. Then open the
CONTROL/LOT assist box.
3. Select the desired control from the list and press ENTER. The available tests for this
control appear in the TEST KEY matrix field.
4. Select the desired test and press ENTER.
5. Touch the EXECUTE button to integrate the control test request in the processing run. If
the analyzer is in Stand-by mode, the analyzer has to be started first.
6. The results are transferred to the INDIVIDUAL QC list. An automatic data evaluation in
the REAL TIME QC is only possible, if the specifically defined controls are requested
together.
Note
If the EXECUTE button is pressed several times, the control is nevertheless measured only
once.
2 - 24 V 2.1 – Operator's Manual
Page 49

Initiate Run with Routine Patient Test Selections
2.6 Initiate Run with Routine Patient Test Selections
Routine patient test selections can be made when the instrument is in Stand-by, Stop,
Operate or Sample Stop mode. It is also possible to enter patient test selections manually
with or without barcode reader in use.
Patient test selections can either be entered manually or downloaded from a host
computer with or without barcode reader in use.
Use this procedure to enter test selections manually for barcoded samples that are not
downloaded from the host and for non-barcoded samples. The procedure varies slightly
depending on whether:
❚ sample barcode reader is on or off
❚ host communication is on or off
2.6.1 Routine Patient Test Selection
1. Touch WORKPLACE, followed by TEST SELECTION to display the TEST SELECTION sub
menu.
2. Touch ROUTINE to enter routine patient test selections.
V 2.1 – Operator's Manual 2 - 25
Page 50

BM/Hitachi 917
3. For non-barcode mode:
The SAMPLE NO. field is highlighted. Enter the first sample number, then press ENTER.
Enter the sample disk number in the second field, followed by ENTER. Then proceed
with entering the sample disk position number and confirm with ENTER. The cursor
advances to the PATIENT ID text box. Type the PATIENT ID number, if required, then
press ENTER.
For barcode mode:
The PATIENT ID field is highlighted. Enter the barcode number of the sample, then
press ENTER.
4. To change the default setting for the sample type, move the cursor to the SAMPLE
assist box. Open the box and touch the desired sample type. The assist box
TYPE
closes and the selected sample type is displayed.
5. To change the default setting for the sample cup, move the cursor to the SAMPLE CUP
assist box. Open the box and touch the desired sample cup. The assist box closes
and the selected sample cup is displayed.
6. To change the default setting for the sample volume, touch STANDARD, DEC or INC to
choose standard sample volume, decreased sample volume or increased sample
volume respectively.
7. Touch the DEMOGRAPHIC button to display the DEMOGRAPHICS window. Enter all
desired demographic information about the sample. Then, touch the ENTER button.
2 - 26 V 2.1 – Operator's Manual
Page 51

Initiate Run with Routine Patient Test Selections
8. Touch the desired test keys or profile keys on the test key matrix at the lower portion
of the screen. Selecting a test individually or by profile key, results in the test key(s) on
the screen turning white in color. When all desired tests for the sample have been
selected, touch the ENTER button. The test selections are stored and the white color
disappears.
❚ If a yellow dot appears on the test key matrix for a specific test, this indicates that
the test was masked by the operator in the START CONDITIONS global menu.
Masked tests are not be run.
❚ If a red line appears on the test key matrix for a specific test, this indicates that the
test is masked by the analyzer. Masked tests are not be run. Check the REAGENTS
main menu to resolve the situation that caused the test to be automatically
masked.
9. The repeat function can be used only if the sample barcode reader is OFF. The repeat
function is used for batch programming. It is available only after test selections are
made for the first sample in the batch. Touch the REPEAT button to display the REPEAT
window. Type the number of the last sample you want to be processed with the
repeated test selections. Touch the ENTER button. (The sample number increments
automatically.)
V 2.1 – Operator's Manual 2 - 27
Page 52

BM/Hitachi 917
10. If you want to print out a load list of the test selections, touch the PRINT button to open
the corresponding global menu. Touch WORKPLACE, followed by REQUISITION LIST.
Then touch the SELECT button to display the REQUISITION LIST window. Enter the first
and last sample number and then touch the PRINT button.
2 - 28 V 2.1 – Operator's Manual
Page 53

Initiate Run with Routine Patient Test Selections
2.6.2 Initiate Run Procedure
After the patient test selections have been made, touch the START button to display the
START CONDITIONS global menu and to define the settings for the subsequent run.
1. Touch the START button to open the START CONDITIONS global menu. Depending on
barcode mode and host mode, entries vary as follows:
Sample
barcode reader
no no À
yes yes Á
yes no
no yes Â
Host
communication
START SAMPLE
see
➀ If the analyzer is being operated without sample barcode reader and there is no
host connection, then only 1 field is displayed. The sequence number that is used
for the run start must be entered here.
➁ If the sample barcode reader is switched on, 3 fields appear, independent of
whether there is a host connection present or not. The sequence number, disk
number and disk position number that are used for the run start must be entered
here.
V 2.1 – Operator's Manual 2 - 29
Page 54

BM/Hitachi 917
➂ If the analyzer is being operated without sample barcode reader but with host, then
5 fields appear. Sequence number, disk number, disk position number of the start
sample, the last disk and last disk position number must be entered here.
2. You may request a start up calibration. Touch the START UP EXECUTE button to
perform a start up calibration. A control measurement of all installed and active tests
is automatically performed after the start up calibration.
3. ISE maintenance should not be performed during daily routine. Touch the NO button. If
YES is pressed, the analyzer performs after each run an automatic ISE maintenance
with ISE wash solution on disk 2. Conditioning and ISE calibration is necessary after
each ISE maintenance.
4. Check the following mode settings to ensure parameters are set correctly for the run
you are starting. Open the RERUN MODE window by touching the corresponding
button in the START CONDITIONS global menu.
To change the routine rerun setting there are two options available in the opened
window. If the AUTOMATIC option is selected, all tests with faulty results (outside the
limits) are automatically rerun. Two modes are available:
REAL TIME: If this button is activated, this sample will be measured again during the
current run.
AFTER 1ST: If this button is activated, the rerun will be carried out at the end of the run.
2 - 30 V 2.1 – Operator's Manual
Page 55

Initiate Run with Routine Patient Test Selections
If the RERUN ONLY option is activated, samples that were scheduled for the rerun can
be measured again. The system marks results in the DATA REVIEW sub menu (WORK-
PLACE
main menu) that are outside the limits defined for the applications. These
samples are scheduled for a rerun.
If the STAT RERUN option is activated, a rerun of STAT samples will be performed
automatically under certain conditions.
If the results of a STAT sample are outside the limits, the system will automatically
measure that sample again. If the NO button is activated, the STAT sample will not be
rerun.
5. To change the REAL TIME DATA PRINT and REAL TIME CALIB PRINT setting, touch the
PRINT/HOST button in the START CONDITIONS global menu. Touch the print options
you prefer. If the button MONITOR is activated, the results will be printed out in the
monitor format. if REPORT is selected, the results will be printed out in the more
extensive report format. If NO PRINT is activated, the results will not be printed out, but
can be checked in the DATA REVIEW sub menu (WORKPLACE main menu) and printed
out via the PRINT global menu.
To activate the HOST COMMUNICATION setting, touch the PRINT/HOST button. Touch
ON-LINE or OFF-LINE to set the desired host communication mode. Then, touch the
CLOSE button.
V 2.1 – Operator's Manual 2 - 31
Page 56

BM/Hitachi 917
6. To change any test masking, touch the MASKING button to open the corresponding
window. A list of all tests appears in the list box. A masked test has an asterisk (*)
following the test name. To mask a test, touch the test name in the list box and then
the MASKED button. An asterisk (*) appears following the test name.
To unmask a test, touch first the test name in the list box and then the MASKED button.
The asterisk following the test name disappears.
Touch the ENTER button to save any changes to test masking.
7. Touch the CLOSE button to close the window. Touching CLOSE does not change any
modifications you previously made to the START CONDITIONS global menu.
2 - 32 V 2.1 – Operator's Manual
Page 57

Initiate Run with Routine Patient Test Selections
Before you start the analysis:
8. Results of up to 10,000 samples are stored on the hard disk for later retrieval. You
must ensure that each sequence number is used only once before archiving data and
then clearing the data from the hard disk. The data with the same sequence number
will be overwritten. If the sequence number reaches 10,000, the following sequence
number (i.e. 1) will be overwritten.
9. Before you start the analysis, check the ALARM global menu for the presence of any
alarms (as indicated by the ALARM button turning red). Review the alarm(s) and correct
the condition(s) before continuing.
10. Place all patient samples, controls, and calibrators in their appropriate positions on
the sample disks 1 and 2. Place a sample cup with a recommended wash solution in
the ”W” positions on sample disk 2.
11. Touch the START button when you are ready to initiate the run.
V 2.1 – Operator's Manual 2 - 33
Page 58

BM/Hitachi 917
2.6.3 Entering Non-Barcoded or Unreadable Barcoded Samples (in the Barcode Mode)
1. Touch WORKPLACE, followed by TEST SELECTION. Touch ROUTINE to enter routine
patient test selections. The cursor will highlight the PATIENT ID text box.
2. To change the default setting for the sample type (e.g. serum/plasma, urine), touch the
SAMPLE TYPE assist key. Touch the desired sample type in the displayed list. The
assist box closes and the selected sample type is displayed.
3. To change the default setting for the sample cup, touch the SAMPLE CUP assist key.
Touch the desired sample cup in the displayed list. The assist box closes and the
selected sample cup is displayed.
4. To change the default setting for the sample volume, touch the desired SAMPLE VOL
button, STANDARD, DEC or INC, to choose standard sample volume, decreased
sample volume or increased sample volume respectively.
5. Enter the patient ID in the PATIENT ID text box and press ENTER. Touch the DEMO-
GRAPHIC
button to display the DEMOGRAPHICS window. Enter all desired demograph-
ic information about the sample. Touch the ENTER button. If there are no requests
from the host, touch the desired test key or profile key on the keyboard matrix.
Selecting a test individually or by profile key results in the test key or keys on the
screen turning white in color. When all desired tests for the sample have been
selected, press ENTER.
2 - 34 V 2.1 – Operator's Manual
Page 59

Initiate Run with Routine Patient Test Selections
6. Touch the NON-ID ASSIGNMENT button to display the NON-ID ASSIGNMENT window.
The NON-ID ASSIGNMENT function can be used if a barcode is damaged and cannot
be read by the barcode reader. Enter a vacant, not registered, disk position number in
the POSITION text box and press ENTER. Enter the patient ID number. Touch the
REGIST button, and then the CLOSE button. The POS and patient ID number is
displayed in the list box on the left side.
V 2.1 – Operator's Manual 2 - 35
Page 60

BM/Hitachi 917
2.7 Sample Tracking
Use the SAMPLE TRACKING global menu to monitor the progress of samples through
sample processing. Information about any sample currently being processed is displayed
inside the sample disk ring on the screen. Samples can be searched by ID or by name.
The sample ring has individual circles that represent each sample disk position.
These positions are highlighted as follows:
indicates a completed sample
indicates a sample that is in process
indicates an open position
indicates that the sample needs further attention from the operator and that the
sample has to be measured again (rerun).
1. Touch SAMPLE TRACK, to display the SAMPLE TRACKING global menu.
2. The sample position and test currently being processed is automatically displayed in
the text fields inside the graphic display of the sample disk.
3. To find a sample of the current run on the disk, you can use the SEARCH function, as
indicated in the upper left corner of the screen. Touch ROUTINE or STAT and enter the
PATIENT ID number or the SAMPLE number that you want to find. If the sample is
found, it is marked with a "▼". A comment is also displayed for this particular sample.
4. In the RERUN list all reruns of the current run are displayed. Touch DISPLAY to display:
the position, sample type, sample number and ID of the rerun. The rerun list selection
lists contains all samples highlighted in red.
2 - 36 V 2.1 – Operator's Manual
Page 61

Measurement of Additional Routine Samples
2.8 Measurement of Additional Routine Samples
Additional routine samples may be requested at any time. Follow the procedure for
programming routine samples. If the analyzer is not in operation, for example in Sampling
Stop or Stand-by mode, you must touch START in the START CONDITIONS global menu to
begin the run.
V 2.1 – Operator's Manual 2 - 37
Page 62

BM/Hitachi 917
2.9 STAT Test Selections
STAT patient test selections can be made at any time, independent of the instrument
mode. In the barcode mode STAT samples can be put in every position on the sample
disk. In the non-barcode mode STAT samples can only be placed in the reserved
positions. STAT samples are pipetted with the highest priority and are processed during
the pipetting of routine samples.
1. Touch WORKPLACE, followed by TEST SELECTION. Touch STAT to enter STAT patient
test selections.
2. The cursor highlights the POSITION text box, if the barcode reader is off.
The cursor highlights the POSITION test box, if the barcode reader is on. Enter the
position number and/or the patient ID number.
2 - 38 V 2.1 – Operator's Manual
Page 63

STAT Test Selection
3. To change the default setting for the sample type (e.g. to urine or CSF), touch the
SAMPLE TYPE assist box. Touch the desired sample type in the displayed list. The
assist box closes and the selected sample type is displayed.
4. To change the default setting for the sample cup, touch the SAMPLE CUP assist box.
Touch the desired sample cup in the displayed list. The assist box closes and the
selected sample cup is displayed.
5. To change the default setting for the sample volume, touch STANDARD, DEC or INC to
choose standard sample volume, decreased sample volume or increased sample
volume respectively.
6. Touch the DEMOGRAPHIC button to display the DEMOGRAPHIC window. Enter all
desired demographic information about the sample. Touch the ENTER button. Request the desired tests or profiles by pressing the corresponding test keys and
confirming with ENTER.
7. If there are no requests from the host, touch the desired test key or profile key on the
keyboard matrix. Selecting a test individually or by profile key results in the test key or
keys on the screen turning white in color. When all desired tests for the sample are
selected, press ENTER.
If a yellow dot appears on the test key matrix for a specific test, this indicates that the
test is masked by the operator from the START CONDITIONS global menu.
If a red bar appears on the test key matrix for a specific test, this indicates that the test
is masked by the analyzer. Masked tests may be requested, but are however not
processed.
V 2.1 – Operator's Manual 2 - 39
Page 64

BM/Hitachi 917
❚ Change Routine Sample to STAT Sample (in barcode mode only)
You may change a sample that has been programmed as a routine sample to a STAT
sample. This enables the sample to be processed as a STAT, i.e. before any remaining
routine samples.
1. Touch WORKPLACE, followed by TEST SELECTION and ROUTINE.
2. Type the PATIENT ID number and press ENTER.
3. Touch the CHANGE TO STAT button to display the CHANGE TO STAT window.
4. Enter the sample position number, corresponding to the sample’s position on the
sample disk, then press ENTER.
5. The sample located in this position will be processed as a STAT sample.
2 - 40 V 2.1 – Operator's Manual
Page 65

Processing of Rerun Samples
2.10 Processing of Rerun Samples
You may process rerun samples in two different ways, as automatic reruns or as manual
reruns, requested by the operator.
All tests for a sample with results that do not meet the defined criteria are placed on a
rerun list independent of the run mode.
In the START CONDITIONS global menu, two different modes are selectable - REAL TIME
and AFTER 1ST.
❚ REAL TIME: The sample is rerun without delay during the current run.
❚ AFTER 1ST: The rerun is performed at the end of the current analytical run.
The RERUN ONLY option can be used to request manually rerun samples. The instrument
highlights in the DATA REVIEW sub menu (WORKPLACE main menu) all result that are
outside the limits that are specified in the applications. These samples can be defined for
a rerun.
V 2.1 – Operator's Manual 2 - 41
Page 66

BM/Hitachi 917
2.10.1 Automatic Rerun
1. Touch the START button to display the START CONDITIONS global menu.
2. Touch the RERUN button to display the RERUN MODE window.
3. Touch AUTOMATIC to request that reruns be processed without operator intervention.
4. Touch REAL TIME if you want rerun samples to be processed during the current routine
run. Touch AFTER 1ST if you want reruns processed at the end of the current run.
5. Touch the ENTER button to save the rerun settings.
2 - 42 V 2.1 – Operator's Manual
Page 67

Processing of Rerun Samples
2.10.2 Manual Rerun
1. Touch the START button to display the START CONDITIONS global menu.
2. Touch the RERUN button to display the RERUN MODE window.
3. Touch NO in the ROUTINE RUN area to request that reruns are not processed during or
after routine runs.
4. Touch the ENTER button to save the rerun settings.
5. After the analytical run is finished: Touch WORKPLACE and DATA REVIEW to display the
DATA REVIEW sub menu.
Sample barcode reader on Sample barcode reader off
Samples that have incomplete results are marked with an I (left column on the lefthand side). Touch a sample you wish to review and the individual test results are
displayed in the list on the right. Rerun tests are marked with a symbol The symbol
indicates the sample volume for rerun (■: normal, ▲: increased, ▼: decreased). If you
want an other than the recommended volume, touch the respective buttons: Touch
the NORMAL button to select tests for rerun with normal sample volumes. Touch the
INCREASE button to select tests for rerun with in-creased sample volumes. Touch the
DECREASE button to select test for rerun with decreased sample volumes. For each
sample, additional test requests can be made in Stand-by mode. Touch the bars “- -
-” at the end of the test result list and select the desired VOLUME. A window is
displayed in which you can select a test for an additional rerun. Then, touch SELECT.
V 2.1 – Operator's Manual 2 - 43
Page 68

BM/Hitachi 917
6. Repeat step 5 for any other samples that have to be rerun.
7. Touch the START button to display the START CONDITIONS global menu.
8. Touch the RERUN button to display the RERUN MODE window.
9. Touch RERUN ONLY to request rerun processing, then touch ENTER.
10. Enter the start sample number for the rerun in the START CONDITIONS global menu.
11. Press the START button.
2 - 44 V 2.1 – Operator's Manual
Page 69

Adding Reagent During a Run
2.11 Adding Reagent During a Run
Use the REQUIREMENTS sub menu (WORKPLACE main menu) to check the levels of
reagents.
If reagent levels are highlighted in yellow (REAG column) for any reagent, you may need to
add reagent during the run. A reagent is highlighted in yellow when the defined number of
remaining tests in the bottle reaches or falls below the limit that is set in the MAINT/UTILITY,
SYSTEM, ALARM SETTING, REAGENT CHECK LEVEL screen.
If reagents must be added during a running analysis, perform the following steps:
V 2.1 – Operator's Manual 2 - 45
Page 70

BM/Hitachi 917
1. Touch REAGENTS, followed by INTERRUPT to display the REAGENT INTERRUPT window in which the time remaining for the reload interrupt is specified. Then touch
EXECUTE.
Press EXECUTE after adding reagent. The time remaining before you can add reagent
counts down on the screen. After reaching zero, a message (“Place reagent replace
reagent tray lid. Then press EXECUTE for reagent registration”) is displayed in the
window.
Place the reagent bottles into their assigned positions and touch the EXECUTE button.
2. The system automatically performs a reagent registration and resumes the current
run.
2 - 46 V 2.1 – Operator's Manual
Page 71

Patient Report
2.12 Patient Reports
There are two patient result printout formats: MONITOR and REPORT.
❚ The MONITOR format is a shorter report format giving each test result. In the MONITOR
format date and time, sample type, sequence number, ID number and comment 1 is
printed out for each sample. The results are printed out next to each other with data
flags.
❚ The REPORT format additionally gives the header, patient demographic information,
results, units and expected values. Choose your format on the START CONDITIONS
global menu, PRINT/HOST window.
You can request patient report print-outs automatically or manually. The report format
can be customized to fit your laboratory needs (MAINT/UTILITY main menu, REPORT
FORMAT
2.12.1 Selecting the Patient Report Format for Real Time Printing
1. Touch START followed by PRINT/HOST to display the PRINT/HOST window.
2. From the REAL TIME DATA PRINT selections, touch MONITOR to select the monitor
3. Touch CLOSE to save the print settings.
4. The patient reports will print in real time, when all results for the patient sample are
sub menu).
format. Touch REPORT to select the report format. Touch NO PRINT to get no real time
print.
available.
V 2.1 – Operator's Manual 2 - 47
Page 72

BM/Hitachi 917
2.12.2 Printing Patient Reports in Batch
1. Touch START followed by PRINT/HOST to display the PRINT/HOST window.
2. From the REAL TIME DATA PRINT selections, touch NO PRINT to select no real time
report printing.
3. Touch CLOSE to save the print settings.
4. To print results at a later time, touch WORKPLACE, DATA REVIEW to display the DATA
REVIEW
sub menu.
5. Mark the data with the marking key M or R on the scrollbar. The scroll bars on the right
side of each box have an R at the top and an M at the bottom. When R is highlighted,
a consecutive range of samples may be selected by touching the first and last sample
in the desired range. When M is highlighted, multiple, non-consecutive samples may
be selected. If neither is highlighted, only one sample at a time may be selected.
6. Touch the PRINT button to display the PRINT global menu.
7. Select MEASUREMENT DATA from the list box and touch SELECT. Touch MONITOR or
REPORT to choose the report format.
8. Touch ALL or EDITED to print all results or only results that have been edited.
9. Touch PRINT to print out the patient reports.
2 - 48 V 2.1 – Operator's Manual
Page 73

Data Management
2.13 Data Management
The way results are documented, depends on the mode settings selected from the START
CONDITIONS
Data is saved on the hard drive but can also be saved on a floppy disk.
You may edit and delete data as necessary. Edited data can be printed out as a patient
report or be transmitted to the host.
2.13.1 Reviewing Data
Use the DATA REVIEW sub menu and the steps below to review patient data:
global menu, PRINT/HOST window.
1. Touch WORKPLACE, followed by DATA REVIEW to display the DATA REVIEW sub menu.
2. Touch SEARCH to display the SEARCH CRITERIA window. Select the desired search
criteria and touch the ENTER button.
3. All samples meeting the selected search criteria are displayed in the left box. If control
is selected as the search criterion, all other search criteria have to be deactivated.
V 2.1 – Operator's Manual 2 - 49
Page 74

BM/Hitachi 917
2.13.2 Editing Data
❚ Overwrite First Result with Rerun Result
1. Touch the sample that needs to be edited in the left box. Details of the test
information appear in the right box, including first run results and rerun results.
2. Touch the page key -1- to access the second window level and then the EDIT
button to display the EDIT DATA window.
3. Touch the REPLACE button if you only need to replace first run results with rerun
results. The replace function automatically overwrites all tests of this sample with
the rerun result.
4. Touch the CLOSE button to close the window and save the edits.
5. Repeat steps 1-4 for all samples where the first result has to be overwritten by the
rerun result.
2 - 50 V 2.1 – Operator's Manual
Page 75

Data Management
❚ Overwrite Results
1. Touch the sample that needs to be edited in the left box. Details of the test
information appear in the right box, including first run results and rerun results.
2. Touch the page key -1- to access the second window level and then the EDIT
button to display the EDIT DATA window.
3. Touch the test name in the list box if you need to edit manually the result. The first
run result and rerun result appear in the EDIT DATA text boxes. Enter the new
results.
4. Touch the CLOSE button to close the window and save the edits.
5. Repeat steps 1-4 for all samples where the first result has to be overwritten by the
rerun result.
V 2.1 – Operator's Manual 2 - 51
Page 76

BM/Hitachi 917
❚ Sending Edited Data to the Host
1. Mark the data on the DATA REVIEW sub menu with the marking keys R or M. The
scroll bars on the right side of the sample list box have an R at the top and an M at
the bottom. When R is highlighted, a consecutive range of samples may be
transmitted by touching the first and last sample in the desired range. When M is
highlighted, multiple, non-consecutive samples may be transmitted. If neither is
highlighted, only one sample at a time may be transmitted.
2. Touch the SEND TO HOST button to display the SEND TO HOST window.
3 Touch ALL or EDITED to select which data is sent to the host. Touch EDITED to send
only edited data to the host.
4. Touch RESULT to send the results to the host. Touching ABS. sends the absorbance readings.
5. Touch ENTER to begin the data transmission to the host.
6. Touch QUIT to stop host transmission.
2 - 52 V 2.1 – Operator's Manual
Page 77

Data Management
❚ Printing Edited Data
1. Touch WORKPLACE, followed by DATA REVIEW to display the DATA REVIEW sub
menu.
2. Mark the data in the DATA REVIEW sub menu with the marking keys M or R. The
scroll bars on the right side of the sample list box have an R at the top and an M at
the bottom. When R is highlighted, a consecutive range of samples may be printed
by touching the first and last sample in the desired range. When M is highlighted,
multiple, non-consecutive samples may be printed. If neither is highlighted, only
one sample at a time may be printed.
3. Touch the PRINT button to display the PRINT global menu.
4. Touch WORKPLACE and select the MEASUREMENT DATA report from the list box.
Touch SELECT to display the MEASUREMENT DATA window.
V 2.1 – Operator's Manual 2 - 53
Page 78

BM/Hitachi 917
5. Touch MONITOR or REPORT to choose the report format.
6. Touch ALL or EDITED to print all results or only results that have been edited.
7. Touch PRINT to print out the patient reports.
2 - 54 V 2.1 – Operator's Manual
Page 79

Data Management
2.13.3 Deleting Functions
❚ Deleting Samples
1. Touch WORKPLACE, then DATA REVIEW to display the DATA REVIEW sub menu.
With the left list box selected:
2. Select the sample(s) to be deleted. You can delete a single sample, a range or all
samples. The scroll bars on the right side of the sample list box have an R at the
top and an M at the bottom. When R is highlighted, a consecutive range of samples
may be deleted by touching the first and last sample in the desired range. When M
is highlighted, multiple, non-consecutive samples may be deleted. If neither is
highlighted, only one sample at a time may be deleted.
If you want to delete samples with all corresponding data:
3. Touch the page key -1 - to access the second window level and then DELETE to
open the corresponding window.
4. Touch YES to delete the highlighted samples. Note that the test selection is deleted
together with the results.
V 2.1 – Operator's Manual 2 - 55
Page 80

BM/Hitachi 917
❚ Deleting Single Tests
1. Touch WORKPLACE and DATA REVIEW to display the DATA REVIEW sub menu.
2. Select the test(s) to be deleted in the left list box. You can delete a single test, a
range of tests or all tests.
3. The scroll bars on the right side of the test list box have an R at the top and an M at
the bottom. When R is highlighted, a consecutive range of tests for the selected
sample may be deleted by touching the first and last test in the desired range.
When M is highlighted, multiple, non-consecutive tests for the selected sample
may be deleted. If neither is highlighted, the whole sample is deleted.
4. Touch DELETE to open the DELETE RECORD window.
5. Touch YES to delete the selected test. Note that only the single test result but not
the test selection is deleted.
2 - 56 V 2.1 – Operator's Manual
Page 81

Data Management
❚ Deleting Sample Types (Batch Delete)
To delete all samples of a sample type:
1. Touch WORKPLACE, DATA REVIEW to display the DATA REVIEW sub menu. Touch
the BATCH DELETE button to display the BATCH DELETE window.
2. Touch STAT to delete all STAT sample data.
Touch ROUTINE to delete all routine sample data.
Touch CONTROL to delete all control sample data.
Touch ALL to delete all data.
3. Touch ENTER, followed by YES to delete the selected data.
4 Touch CANCEL to cancel the deletion of the selected data.
Note
This procedure deletes also the test selections.
V 2.1 – Operator's Manual 2 - 57
Page 82

BM/Hitachi 917
2.14 Quality Control Procedures
During routine operation, the instrument compares paired (X) and (Y) control values
against the mean and standard deviation entered for each control in the REAL TIME QC
window (INDIVIDUAL QC sub menu). The REAL TIME QC screen evaluates quality control
results by a multi-rule Shewhart method. The rules are selected by the operator.
Each set of control results is either acceptable or causes a random, system, or QC error.
If a random, systematic, or QC alarm occurs, an alarm message appears on the ALARM
global menu.
In addition, an audible alarm occurs when an error of this type is detected. Consult the
above screens during a test run to ensure that patient results are properly controlled.
2 - 58 V 2.1 – Operator's Manual
Page 83

Quality Control Procedures
2.14.1 Selecting Controls for Real Time QC
1. Touch QC, followed by INDIVIDUAL and REAL TIME QC to display the REAL TIME QC
window. Daily QC results can be reviewed and checked in this window.
2. Touch the TEST assist box to display the list of tests. Touch the name of the test you
want to review and press ENTER.
3. Touch the SELECT button to display the SELECT CONTROL window to choose the
controls you wish to review.
4. Touch the control in the list, followed by AXIS X to assign a control to the X-axis.
5. Touch the control in the list, followed by AXIS Y to assign a control to the Y-axis. Touch
ENTER to display the graph.
6. Touch RULES if you wish to change the rules by which the QC data are evaluated. The
SELECT RULES window is displayed. Touch the rules you want used in the evaluation,
followed by ENTER. The previously measured controls are not redrawn according to
the rule selection.
7. The graph shown displays all of the QC results for the specified test and control levels.
Random, System and QC errors are displayed along with normal QC data.
V 2.1 – Operator's Manual 2 - 59
Page 84

BM/Hitachi 917
2.14.2 Individual QC List
1. Touch QC, followed by INDIVIDUAL to display the INDIVIDUAL QC LIST sub menu.
2. All daily QC results for the selected test that have not been accumulated are displayed, even if the analyzer is powered off and powered on again.
2 - 60 V 2.1 – Operator's Manual
Page 85

Quality Control Procedures
2.14.3 Setting of Controls in the Individual QC Chart
1. Touch QC, followed by INDIVIDUAL and CHART to display the INDIVIDUAL QC CHART
window.
2. Touch the TEST assist box. Touch the test you want to review and press ENTER.
3. Touch SELECT to display the SELECT CONTROL window. Touch the control name
followed by the PLOT button. This assigns the selected control to the selected plot.
The selected control appears next to the PLOT button. Repeat the procedure for the
other two controls you want displayed. Touch ENTER.
V 2.1 – Operator's Manual 2 - 61
Page 86

BM/Hitachi 917
❚ Validation of Controls with the Individual QC Chart
1. Touch QC, followed by INDIVIDUAL and CHART to display the INDIVIDUAL QC CHART
window.
2. Touch the TEST assist box. Touch the test you want to review and press ENTER.
3. Up to three different controls can be displayed in the chart with three different
symbols. Controls can be displayed (or not displayed) by pressing the corresponding control button. A yellow bar indicates that the controls are accumulated.
4. If you wish to exclude a control from the statistical evaluation, use the scrollbar and
use the arrow keys to move the cursor to the desired control symbol. Then touch
COMMENT. Enter the required comment into the comment line and confirm with
ENTER. The excluded control is displayed as a non-filled-out symbol.
2 - 62 V 2.1 – Operator's Manual
Page 87

Quality Control Procedures
2.14.4 Validation of Individual QC with Real Time QC (Rejection of Single Test Couplings)
1. Touch QC, followed by INDIVIDUAL and REAL TIME QC to display the REAL TIME QC
window.
2. Touch the TEST assist box to display the list of tests. Touch the name of the test you
want to review and press ENTER.
3. Touch the SELECT button to display the SELECT CONTROL window.
Select the controls you wish to review. Previously defined controls are displayed on
the X-axis and Y-axis.
V 2.1 – Operator's Manual 2 - 63
Page 88

BM/Hitachi 917
4. Touch REJECT to display the REJECT DATA window.
Touch the data type you want to reject (RANDOM, SYSTEM, QC or ALL). Then touch
ENTER, followed by YES.
2 - 64 V 2.1 – Operator's Manual
Page 89

QC File Maintenance
2.15 QC File Maintenance
Use the following procedures to accumulate and delete QC data on a regular basis:
2.15.1 Accumulate QC Data
1. Touch QC, followed by INDIVIDUAL to display the INDIVIDUAL QC LIST sub menu.
2. Touch the test(s) in the left list box which you want to accumulate.
The scroll bars on the right side of the list box have an R at the top and an M at the
bottom. When R is highlighted, a consecutive range data may be accumulated by
touching the first and last result in the desired range. When M is highlighted, multiple,
non-consecutive data for the selected test may be accumulated.
3. Touch the ACCUMULATE button to display the ACCUMULATE DATA window.
4. Enter the control number (see guidance box) to accumulate the selected data.
Accumulating the data deletes the data from the INDIVIDUAL QC sub menu.
V 2.1 – Operator's Manual 2 - 65
Page 90

BM/Hitachi 917
2.15.2 Deleting QC Data
1. Touch QC, followed by INDIVIDUAL to display the INDIVIDUAL QC LIST sub menu.
2. Touch the name of the test you want to delete.
The scroll bars on the right side of the list box have an R at the top and an M at the
bottom. When R is highlighted, a consecutive range data may be deleted by touching
the first and last result in the desired range. When M is highlighted, multiple, nonconsecutive data for the selected test may be deleted. If neither is highlighted, only
one set of data may be deleted at a time.
3. Touch the DELETE DATA button to display the corresponding window. Then touch YES,
to delete the selected data.
2 - 66 V 2.1 – Operator's Manual
Page 91

QC File Maintenance
2.15.3 Cumulative QC List
1. Touch QC, followed by CUMULATIVE, to display the CUMULATIVE QC LIST sub menu.
2. Touch the TEST button. The CUMULATIVE QC list is sorted by test in alphabetical order.
If you touch the CONTROL button, the CUMULATIVE QC list is sorted by control in
alphabetical order.
3. The statistics e.g. number of accumulations, mean, SD, CV is displayed.
V 2.1 – Operator's Manual 2 - 67
Page 92

BM/Hitachi 917
2.15.4 Validation of Cumulative QC
1. Touch QC, CUMULATIVE, CHART to display the CUMULATIVE QC CHART window.
2. Touch the TEST assist box. Touch the test you want to activate.
3. Up to three different controls can be displayed in the chart with three different
symbols. Controls can be displayed (or not displayed) by pressing the corresponding
control button.
Touch select to display the CHART SELECT window. Touch the control name followed
by the PLOT button. This assigns the selected control to the selected plot. Repeat this
steps for the other two controls you want to be displayed. Then touch ENTER.
2 - 68 V 2.1 – Operator's Manual
Page 93

QC File Maintenance
2.15.5 Printing the Cumulative QC List and Chart
1. Touch QC, followed by CUMULATIVE to display the CUMULATIVE QC LIST sub menu.
2. Touch the name of the test you want to print out.
The scroll bars on the right side of the list box have an R at the top and an M at the
bottom. When R is highlighted, a consecutive range data may be printed by touching
the first and last result in the desired range. When M is highlighted, multiple, nonconsecutive data for the selected test may be printed. If neither is highlighted, only
one set of data may be printed at a time.
3. Touch the PRINT button, followed by QC and CUMULATIVE QC CHART or LIST. The
CUMULATIVE QC CHART window or the CUMULATIVE QC LIST window opens. Press the
PRINT button to print out the cumulative QC chart or cumulative QC list.
If the cumulative QC chart is printed out, the selected controls are printed out as well,
together with statistic data.
V 2.1 – Operator's Manual 2 - 69
Page 94

BM/Hitachi 917
2.15.6 Deleting the Cumulative QC
1. Touch QC, followed by CUMULATIVE to display the CUMULATIVE QC LIST sub menu.
2. Touch the name of the test you want to review. The scrollbars on the right side of the
list box have an R at the top and an M at the bottom. When R is highlighted, a
consecutive range data may be deleted by touching the first and last result in the
desired range. When M is highlighted, multiple, non-consecutive data for the selected
test may be deleted. If neither is highlighted, only one set of data may be deleted at a
time.
3. Touch the DELETE DATA button to display the DELETE DATA window.
4. Touch YES to delete the selected data.
2 - 70 V 2.1 – Operator's Manual
Page 95

System Shutdown
2.16 System Shutdown
There are two different ways to terminate the analyzer operation: Firstly, the instrument
can be completely shut down. Secondly, the SLEEP mode can be activated which helps to
save time when the analyzer is restarted.
2.16.1 Instrument Shutdown
Perform the following steps to shut down completely the analyzer:
1. Push the POWER switch (on the right of the front panel of the analyzer) to OFF position.
The analyzer is now switched off. The computer, screen, and the printer are also
switched off, if the main switches of these components are permanently left in the ON
position.
2. Remove the patient samples from the sample disk 1.
3. Remove the calibrators/controls which are no longer needed from the sample disk 2.
4. Switch off the external water supply.
V 2.1 – Operator's Manual 2 - 71
Page 96

BM/Hitachi 917
2.16.2 Activating the SLEEP Mode
Perform the following steps to activate the SLEEP mode:
1. The “wake-up” time of the instrument for a specific day can be set in the SLEEP
window in the START CONDITIONS global menu.
Press the EXECUTE button to activate the timer. The instrument is automatically
initialized at the specified time.
2. Remove the patient samples from the sample disk 1.
3. Remove the calibrators/controls which are no longer needed from the sample disk 2.
4. Switch off the external water supply.
Note
The SLEEP mode can be interrupted at any time by pressing the CANCEL button in the
displayed SLEEPING window.
2 - 72 V 2.1 – Operator's Manual
Page 97

3. Maintenance and Daily Care
Introduction
V 2.1 – Operator's Manual 3 - 1
Page 98

BM/Hitachi 917
3.1 Introduction
As with all precision instruments the BM/Hitachi 917 requires preventative care and
maintenance measures to ensure trouble-free functionality which in turn ensures correct
results and error-free operation.
As well as the detailed instructions given in the maintenance schedule of this chapter, you
should heed the following guidelines to avoid irregularities and any potential malfunctions
resulting from the latter.
Comply with the maintenance and care schedule described below in this chapter. A
trouble-free and long term operation of the analyzer can only be guaranteed, if the
periodic maintenance measures are adhered to. Regular care and maintenance of the
system prevents time-intensive repairs.
Inform your Boehringer Mannheim service representative about any unusual occurrences.
3.1.1 Necessary Material and Safety Precautions
At the beginning of each procedure in this chapter, the necessary materials and safety
precautions are listed. Please comply with this information!
Warning
Strictly comply with the normal laboratory safety precautions and the safety precautions
that are specified in the following instructions.
Extreme caution is necessary when handling supply-carrying, sharp-edged or pointed
analyzer components. Risk of injury!
Wear protective gloves when handling potentially infectious materials and analyzer components that come in contact with these materials. Health risk!
3 - 2 V 2.1 – Operator's Manual
Page 99

Introduction
Tools and Accessories
Phillips screwdriver for 2 mm and 4 mm screws (for removing covers)
Stainless steel wire with diameters of 0.2 mm and 0.5 mm (for cleaning probes)
Special syringe wrench (for syringes and seals)
Deionized water (conductivity < 1 µS/cm)
Lint-free towels (cleaning)
Vacuum cleaner, brush (for the dust filter of the cooling unit)
Tweezers (accessories)
A glass (500 mL)
Bucket
Commercial, disposable plastic syringe (50 mL or 100 mL)
Detergents
Hitergent
Load the bottles (70 mL) in positions 1D1 and 2D1 (compartments) next to reagent disks.
Hitergent is added after a water replacement in the incubation bath.
Detergent 1 (NaOH-D)
Place a container with this detergent in the appropriate position behind the front door.
Load the bottles (70 mL) containing this detergent in positions 1D2 and 2D2 in the marked
compartments adjacent to the reagent disks. The detergent is used for cleaning the
reaction cells and reagent probes.
Detergent 2
As an option, a second detergent can be loaded in the appropriate position behind the
front door.
Warning
Detergents can cause skin rashes. Please wear rubber gloves.
V 2.1 – Operator's Manual 3 - 3
Page 100

BM/Hitachi 917
Spare Parts and Consumables
Part/Nomenclature Part Number BM Order Number
Halogen lamp 705–0840
Set of reaction cells (32 segments) 714–0650 156 8132
Hitergent (12 x 70 mL bottle) 155 5448
NaOH-D (70 mL bottle) 155 5430
NaOH-D (2 L bottle) 155 1540
Hitachi sample cup (quantity: 5000) 039 4246
Hitachi Micro Cup (quantity: 100) 122 9290
Upper seal for sample syringe 714–1360 156 8477
Spacer for sample syringe 714–1282 156 8523
Upper seal for sample syringe 714–1361 156 8485
Upper seal for reagent syringe 714–1362 156 8493
Spacer for reagent syringe 714–1291 156 8531
Lower seal for reagent syringe 714–1363 156 8507
Lower O-ring for sample/reagent syringe L 456006 098 9142
Upper seal washer for syringes L 443085 068 5917
Teflon block (nozzle tip) 714–2403 156 6466
Seal for ISE syringes L 172108 082 5344
Lower O-ring for ISE syringe 737–1629 085 5766
Serum probe 714–0575 156 6083
Reagent probe 714–0570 156 6075
Stirrer paddle 714–0602 156 6105
Set of cleaning wires (0.5 mm und 0.2 mm) 705–0516 064 1766
Pinch valve tubing 140 2650
Na electrode 082 5468
K electrode 082 5441
Cl electrode 106 9004
ISE reference electrode 140 3826
Printer paper 082 5506
Printer ink ribbon cartridge 122 9346
3 - 4 V 2.1 – Operator's Manual
 Loading...
Loading...