
EQUIVITAL™ EQ-01 VITAL SIGNS
MONITOR
HEALTH CARE PRACTIONER GUIDE
Draft: A Date: 28th April 2006
For Support or further information on this product contact:
Customer Support
Hidalgo Limited
Stable Block at The Grange
20 Market Street
Cambridge
CB4 5QG
United Kingdom
www.hidalgo.co.uk
Tel: +441954 233430
email: equivitalsupport@hidalgo.co.uk
Stable Block at The Grange, 20 Market Street, Swavesey, Cambridge, CB4 5QG
Tel: +44 (0) 1954 233430 Fax: +44 (0) 1954 233431
www.hidalgo.co.uk

HEALTH CARE PRACTIONER GUIDE
CONTENTS
Equivital Monitor Intended Use: ................................................................................................................ 6
Contraindications ................................................................................................................................... 7
Technical Description :............................................................................................................................... 8
System Overview..................................................................................................................................... 8
System Components ................................................................................................................................ 10
Hardware: .............................................................................................................................................. 10
Chest Belt ........................................................................................................................................... 10
The Sensor Electronics Module “SEM” .............................................................................................11
Battery Charger ................................................................................................................................. 12
Software : ............................................................................................................................................... 12
SEM Customisation Utility .................................................................................................................. 12
SEM Viewer ......................................................................................................................................... 13
System Integration Kit........................................................................................................................ 13
Sensor Overview ....................................................................................................................................... 14
Block Diagram ....................................................................................................................................... 14
ECG and Heart Rate Derivation.......................................................................................................... 15
Breathing Frequency ............................................................................................................................ 17
Skin Temperature ............................................................................................................................... 18
Body Position and Motion................................................................................................................. 19
Indications and Alerts............................................................................................................................... 20
ECG Indications..................................................................................................................................... 20
Respiration Effort Indications ............................................................................................................... 20
Combined Indication ....................................................................................................................... 21
Threshold Exception Condition: ....................................................................................................... 23
Single Alarm Exception Condition:.................................................................................................. 23
Multiple Alarm Exception Condition ............................................................................................... 24
Sensor Error Condition: ...................................................................................................................... 24
Sensor Self Check Okay:................................................................................................................... 25
Exception Cleared Condition: ......................................................................................................... 25
Confidence Derivation : ................................................................................................................... 26
VSDS Confidence Rate..................................................................................................................... 27
Secondary Confirmation: ................................................................................................................. 27
Connecting and Using the Sensor – Model Number EQ01-002/012 (Type 0 Low Power PAN
Interface) 29
Sensor Activation .................................................................................................................................. 29
Sensor Range......................................................................................................................................... 29
Protocol Overview ................................................................................................................................ 29
Sensor Diagnostics ................................................................................................................................ 30
Connecting to and Using the Sensor– Model Number EQ01-001....................................................... 31
(Type 1 Bluetooth™) ................................................................................................................................ 31
Bluetooth™ Connection Information ............................................................................................... 31
Sensor Data Security............................................................................................................................. 31
Application Protocol Overview ........................................................................................................... 31
Summary Disclosure .......................................................................................................................... 31
Full Disclosure ..................................................................................................................................... 32
Sensor Configuration Data ...................................................................................................................... 34
Indication Rate Limits and Time Thresholds........................................................................................ 34
Power On Defaults ............................................................................................................................ 36
Bluetooth Link Parameter ................................................................................................................. 37
Belt Construction....................................................................................................................................... 38
Accessories................................................................................................................................................ 39
Repair and Service ................................................................................................................................... 39
Technical Specifications .......................................................................................................................... 39
FCC Compliance and Advisory Notice (US Markets) .......................................................................... 42
Draft: A 28th April 2006 Page: 2 of 42

HEALTH CARE PRACTIONER GUIDE
Explanation of Symbols
Within this manual the following symbols are used to indicate warnings and precautions a user or
operator should take when using this device:
A warning is shown within a highlighted box and includes a symbol
Failure to follow the warning may comprise the safe operation of the device and can lead to a
risk of injury.
A precaution is noted in a highlighted text. Failure to follow a precaution may lead to a
reduction in performance of the device when in use.
Purpose of this guide
This guide is intended for use by trained Health Care Practitioners using the Equivital™
physiological monitoring device to monitor individuals vitals signs and who are familiar with
cardio respiratory monitoring terminology and practise.
It also provides information for system integrators who wish to use Equivital™ as element of a
broader healthcare monitoring system.
A separate user guide is available for use by patients and users of the device. Consult this
manual to find information on:
Warnings and Cautions a user/patient should know before wearing the device
•
How to fit the device to the body
•
• How to switch on the device
• How to change batteries and recharge the device
Draft: A 28th April 2006 Page: 3 of 42
HEALTH CARE PRACTIONER GUIDE
Before using Equivital™
Please observe the following warnings and precautions:
Warnings
• THE EQUIVITAL™ DEVICE MAY BE USED TO MONITOR INDIVIDUALS IN THE
WORKPLACE. WHEN USED IN THIS MANNER THE DEVICE MUST BE INTEGRATED INTO
THE OPERATING ORGANISATIONS SAFETY AND RISK MANAGEMENT PROCEDURES.
USERS SHOULD RECEIVE APPROPRIATE TRAINING FROM YOUR ORGANISATION
BEFORE USING THIS DEVICE
USE OF THE DEVICE DOES NOT JUSTIFY THE USER OR ORGANISATION TO TAKE
•
ADDITIONAL SAFETY RISKS OR TO REDUCE LEVEL OF CARE.
• THIS DEVICE DOES NOT AUTOMATICALLY CALL EMERGENCY SERVICE ASSISTANCE.
• THE DEPLOYING ORGANISATION IS RESPONSIBLE TO ENSURE THAT THESE WARNINGS
ARE UNDERSTOOD AND FOLLOWED.
• THE EQUIVITAL DEVICE SHOULD NOT BE USED FOR SURGICAL PROCEDURES, TO
PERFORM SYNCRONISED CARDIOVERSION OR INTRACARDIAC MONITORING, OR
WHEN PERFORMING EXTERNAL PACING.
• THE DEVICE SHOULD NOT BE APPLIED TO USERS WHO HAVE EXISTING SIGNS OF SKIN
IRRITATION AND DAMAGE AT THE SITES THE DEVICE IS LOCATED ON.
• USERS WHO EXPERIENCE IRRITATION AND RASHING SHOULD BE ADVISED TO
DISCONTINUE USE IMMEDIATELY.
THE DEVICE SHOULD NOT BE USED ON PATIENTS WITH IMPLANTED DEFIBRILLATORS
•
OR PACEMAKERS.
DO NOT ATTEMPT TO CONNECT ANY CABLES TO THE EQUIVITAL DEVICE WHEN
•
WORN ON BODY- THIS INCLUDES HIDALGO SUPPLIED BATTERY CHARGING DEVICE.
ALWAYS REMOVE THE DEVICE BEFORE CHARGING.
• USE ONLY THE HIDALGO PROVIDED BATTERY CHARGER AND BELT ASSEMBLIES WITH
THE DEVICE. THE SAFE USE OF THE DEVICE IS ONLY GUARANTEED WITH THESE
ACCESSORIES. A FULL LIST OF ACCESSORIES IS PROVIDED AT THE REAR OF THIS
DOCUMENT
Draft: A 28th April 2006 Page: 4 of 42
HEALTH CARE PRACTIONER GUIDE
Precautions
ADVISE THE USER NOT TO USE LOTIONS, OILS, PERFUMES, DEODORANT OR POWDER ON THE AREA
•
WHERE THE SENSOR BELT IS BEING APPLIED
• EACH TIME YOU ISSUE THE SENSOR INSPECT THE BELT AND CASE UNIT FOR SIGNS OF DAMAGE (TEARS/
CRACKS ETC). IF ANY DAMAGE IS IDENTIFIED DO NOT USE THE SENSOR UNTIL THE DAMAGED PART
HAS BEEN REPLACED
TO GET MAXIMUM PERFORMANCE FROM THE SYSTEM YOU SHOULD REPLACE THE BELT HARNESS AFTER
•
25 WASHES.
Draft: A 28th April 2006 Page: 5 of 42

HEALTH CARE PRACTIONER GUIDE
Equivital Monitor Intended Use:
The Equivital™ Vital Signs Physiological Monitor is a non-invasive ambulatory wireless
telemetry device intended to allow monitoring of a users vital signs physiology in
environment’s where access to traditional clinical care facilities may be limited or
impractical (for example in the workplace or outdoors) and justification exists for such
monitoring.
The device maybe used by persons operating in circumstances where an increased risk of
physical trauma exists due to the environment in which the user is placed. Typically, these
environments may be found within personnel working in the military, public safety and
hazardous plant workplaces.
The device may also be used as a general cardio respiratory monitor, in particular, where
the compact and ambulatory characteristics of the device are advantageous. In
addition, the device may also be used for the collection of ambulatory physiology for
general research purposes in application such as sports or human performance medicine
and research.
The device offers continuous monitoring of two views of the user’s heart electrical activity
(ECG) and respiratory breathing frequency inferred from thoracic cavity movement and
uses this data to derive a Heart and Breathing Effort Rate.
The sensor also provides additional information:
• Physiological waveforms.
an indication of the users activity level (none, low or high) derived from a
•
movement detection sensor.
body orientation.
•
• chest skin surface temperature.
• alternate secondary measurement of heart rate based on the detection of the
users R wave using a separate hardware processing function.
• alternate secondary measurement of respiration effort using thoracic impedance
pneumography.
• indications and alerts if physiology exceeds predefined boundaries.
The wireless data provided by the sensor may be viewed using a standalone PC based
viewing application, or integrated into a broader care monitoring application. In the later
application the system integrator is responsible for the end to end performance of the
system and appropriate regulatory compliance.
It use within occupational welfare monitoring is intended as an addition to the deploying
organisations established risk assement and welfare management procedures. The device
is not intended to replace the need for such assessments to have occurred and
appropriate procedures to be put in place.
Draft: A 28th April 2006 Page: 6 of 42

HEALTH CARE PRACTIONER GUIDE
Contraindications
The device is not intended to replace the need for appropriate medical supervision and
safe practise to be provided to users by an operating organisation.
The device is not intended for use by children less than 16 years or adults over 65 years.
The device is not intended for use as apnea monitor within a clinical context.
The device is not intended for surgical use or as part of life support systems within a clinical
context.
Draft: A 28th April 2006 Page: 7 of 42
HEALTH CARE PRACTIONER GUIDE
Technical Description :
System Overview
Personal
Area
Network
Telephone or
Mobile Data Wide
Area Network
Remote Monitoring Station
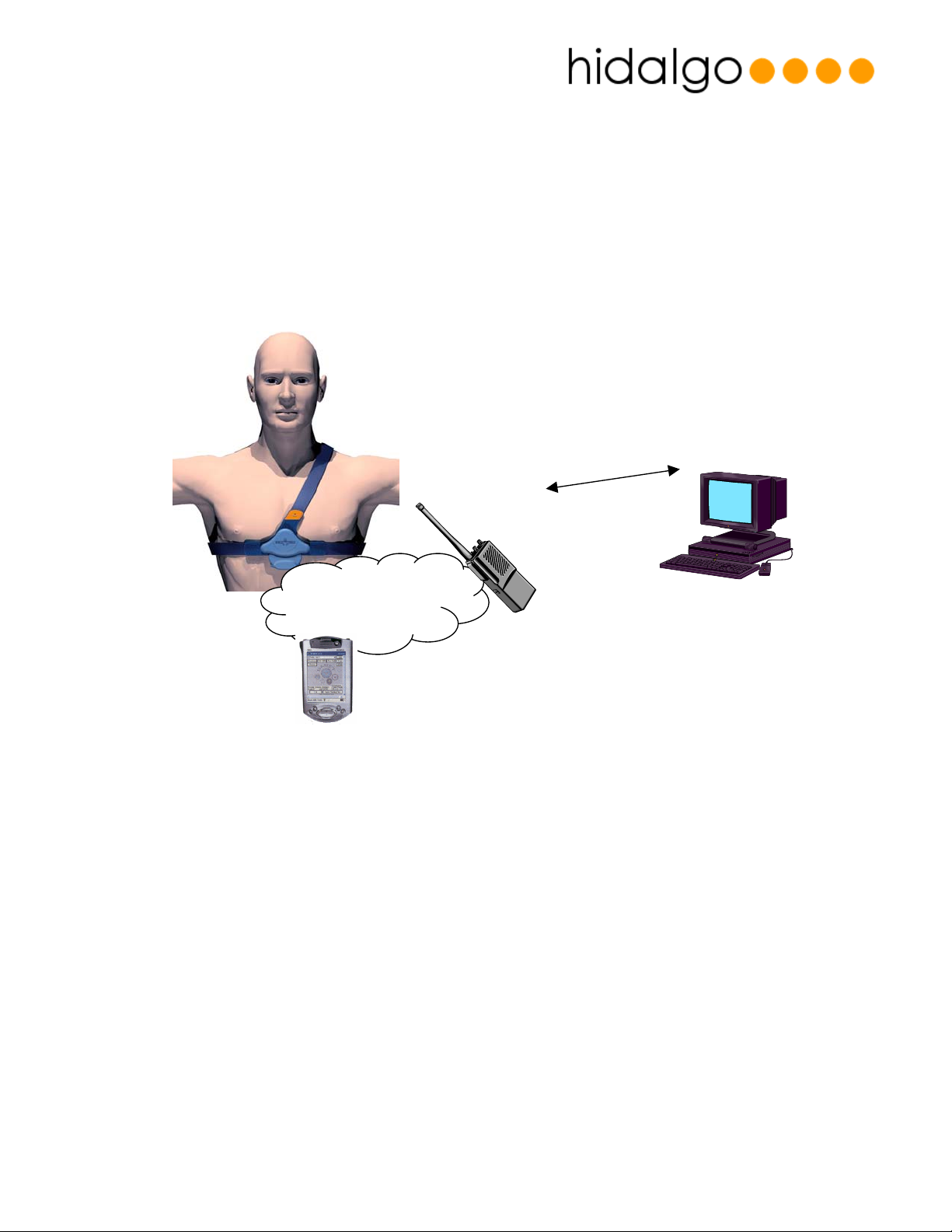
Figure 1 - Sensor Application Diagram
Figure 1 shows an application diagram for the Equivital sensor. The sensor is worn by a user and
records the user’s physiology in real time.
The Equivital sensor comprises of two main elements:
• A passive chest belt containing three conductive sensor electrodes and a expansion
strain gauge.
• A sensor electronics module (or “SEM”) which contains power, processing electronics
and a wireless transmitter.
The sensor transmits this data on its personal area network radio to a wireless receiving device.
The choice of radio technology used by the device is selectable at the point of manufacture
and currently the following options can be supplied:
Draft: A 28th April 2006 Page: 8 of 42
HEALTH CARE PRACTIONER GUIDE
• Type 0 radio interface – A low power packet radio interface designed for use in
applications within the United States Military.
Type 1 radio interface – Bluetooth™ Transceiver supporting the Bluetooth™ defined
•
Serial Port Profile (SPP).
The sensor can communicate with a wireless receiving device which may either record and
display the data locally (e.g. on a PC which contains an integral wireless transceiver) or may
relay the data over further wide area communications networks before it is displayed on a
remote monitoring station.
The wireless receiving device used is dependant on the type of radio interface ordered. For the
Type 0 interface, the sensor is designed to use a protocol suitable to send data to a US
Department of Defense developed receiver/data logger know as a Hub. The same interface is
also supported by the MiniMitter/Respironics Vitalsense monitor.
The Type 1 interface is designed to allow flexibility in choice of receiving device and hence uses
the open communications standard Bluetooth™ to provide the data communications path.
Hence the device may be used with any Bluetooth™ compliant receiver.
The system is provided with a Window s based viewing application “Equivital Viewer” which
enables the data from a sensor to be displayed and recorded. It is expected that some
deploying organisations will wish to develop specific applications which integrate the sensor into
a larger scale monitoring system (eg: including location and mapping functions).
• ORGANISATIONS INTEGRATING THE EQUIVITAL SENSOR INTO LARGER SCALE
SYSTEM APPLICATIONS ARE RESPONSIBLE FOR ENSURING AND VALIDATING THE
SAFETY, EFFICACY AND REGULATORY COMPLIANCE OF THE RESULTING SYSTEM
A system integrators toolkit is available which provides information on the wireless protocol and
a test application to assist development of such applications.
Draft: A 28th April 2006 Page: 9 of 42
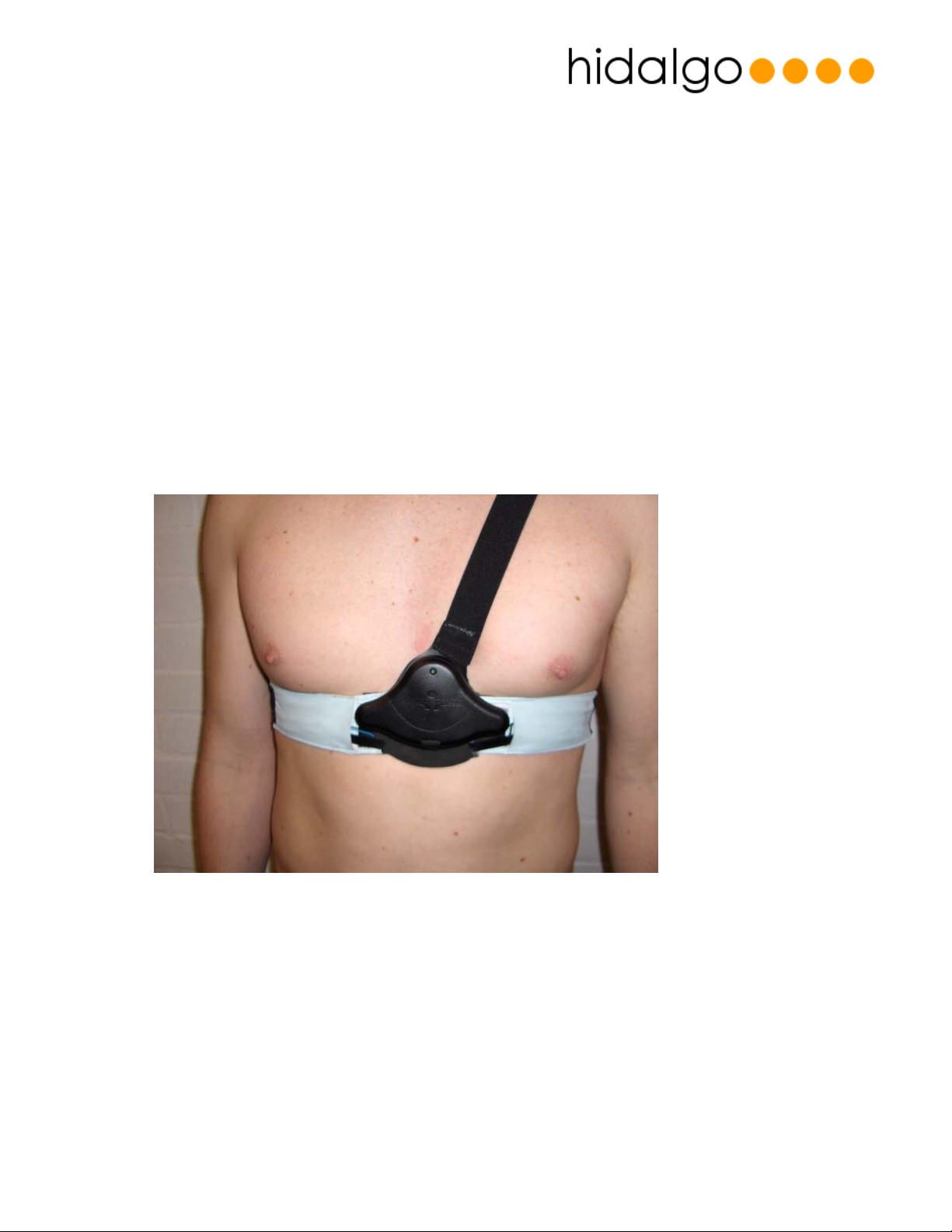
HEALTH CARE PRACTIONER GUIDE
System Components
Hardware:
The following sections provide an overview of each of the main elements needed to use the
Equivital sensor:
Chest Belt
The chest belt comprises a horizontal band which goes around the users chest beneath the
pectorals. Within this band three fabric based silver coated electrodes are located which
connect to the body in order to capture ECG signals and also measure thoracic cavity
impedance changes from the user. In addition a strain gauge is also contained to measure the
expansion of the thoracic cavity, associated with breathing effort.
Figure 2 - VSDS Chest Belt
The horizontal strap is tensioned by a belt adjuster provided at the rear of the belt. Details on
how to fit the belt correctly are provided in the Equivital User Application Guide.
A elasticated shoulder strap is provided which acts to minimise the belt slipping down the torso
during exercise.
The chest belt contains a center piece which sits beneath the sternum and provides five snap
connectors which are used to connect the electronics module (“SEM”) to the belt.
The chest belt is available in a variety of sizes to match differing users – See for details
Draft: A 28th April 2006 Page: 10 of 42

HEALTH CARE PRACTIONER GUIDE
The materials selection used to make the belt is provided in Section tbd.
The Sensor Electronics Module “SEM”
The Sensor Electronics Module “SEM” device contains all of the processing electronics , software
and communications circuits needed to operate the sensor.
The SEM may be ordered from the factory in two power supply variants;
• 2 x AAA/LR03 primary (non rechargeable) cells
• Rechargeable Li-ION battery
Figure 3 - Sensor Electronics Module Front and Rear
The rear of the SEM unit provides the mating connectors needed to attach the device to the
chest belt. In addition, a battery door is also provided to allow batteries to be changed.
On the front of the unit is an external interface connector which normally has a protective bung
connected.
External
Interface
Draft: A 28th April 2006 Page: 11 of 42
HEALTH CARE PRACTIONER GUIDE
This interface is used to:
• Recharge the Li-ION Cell
• Customise the SEM with specific information
• Perform system maintenance functions (by the factory)
NOTE THE DEVICE WILL NOT FUNCTION IF THE BUNG IS NOT FITTED
Battery Charger
The battery charger is a medically approved recharging device and needs to be connected to
the SEM to recharge the SEM.
The battery charger will charge a flat battery in 2.5 hours.
USE ONLY PRIMARY NON-RECHARGABLE (LR03 AAA) ALKALINE CELLS.
•
RECHARGEABLE AAA CELLS SHOULD NOT BE USED.
• DO NOT ATTEMPT TO CONNECT ANY CABLES TO THE EQUIVITAL DEVICE WHEN
WORN ON BODY- THIS INCLUDES HIDALGO SUPPLIED BATTERY CHARGING DEVICE.
ALWAYS REMOVE THE DEVICE BEFORE CHARGING.
• USE ONLY THE HIDALGO PROVIDED BATTERY CHARGER AND BELT ASSEMBLIES WITH
THE DEVICE. THE SAFE USE OF THE DEVICE IS ONLY GUARANTEED WITH THESE
ACCESSORIES. A FULL LIST OF ACCESSORIES IS PROVIDED AT THE REAR OF THIS
DOCUMENT
Software :
SEM Customisation Utility
This is a Windows based application which allows health care professional to configure certain
thresholds and features within the Sensor Electronics Module.
Minimum system and operating system requirements are provided with distribution discs
containing this application.
Draft: A 28th April 2006 Page: 12 of 42
HEALTH CARE PRACTIONER GUIDE
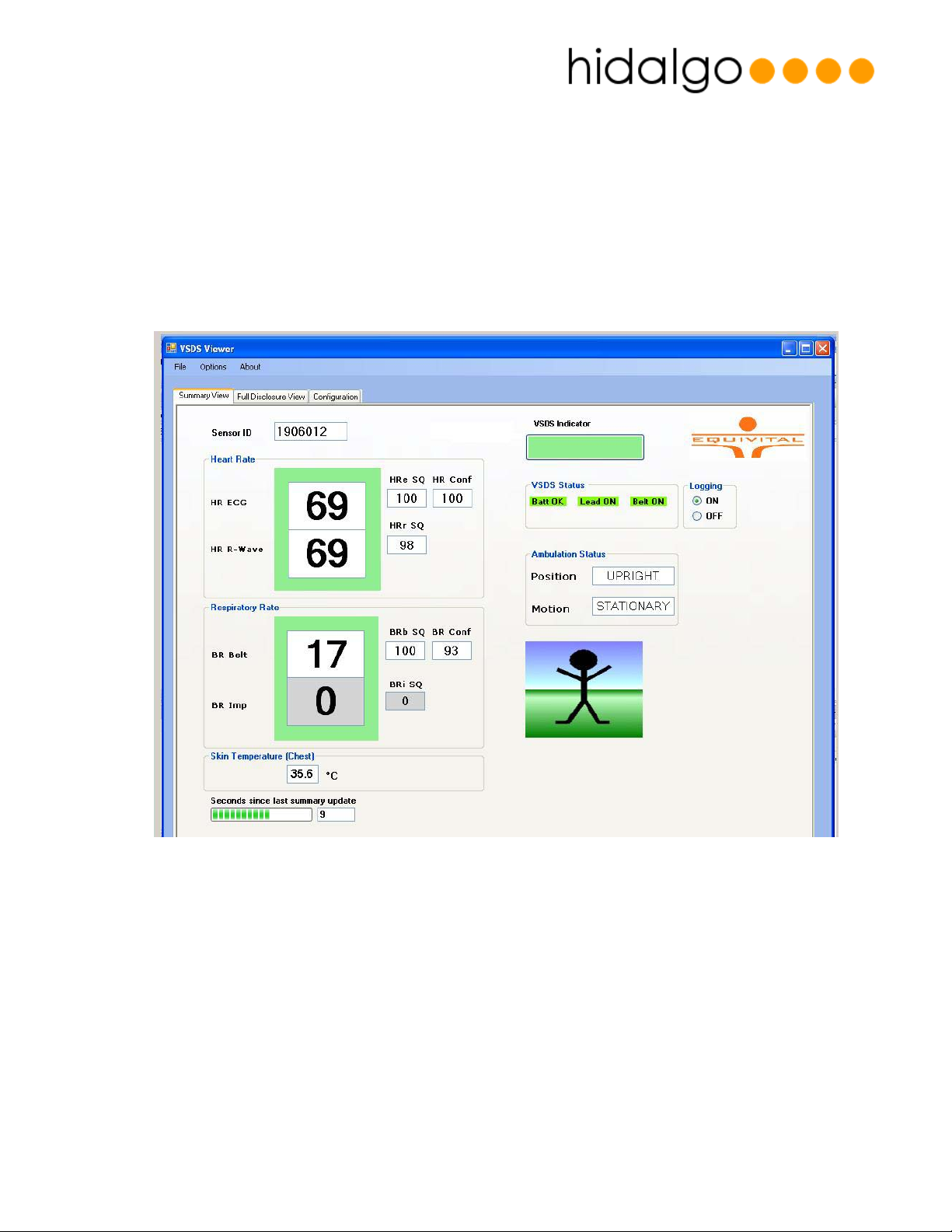
SEM Viewer
The SEM Viewer is a Windows application which allows a remote user to display
Heart Rate
•
• Heart Rate Indications
• Respiration Effort Rate
• Skin Temperature
• Body Position
• Motion
• Sensor information and diagnostics
Figure 4 - SEM Viewer Application
System Integration Kit
For system integrators a special development kit is available which includes protocol interfacing
information and test applications to ease integration and application development. Contact
Hidalgo for further details.
Draft: A 28th April 2006 Page: 13 of 42

HEALTH CARE PRACTIONER GUIDE
Sensor Overview
Block Diagram
Figure 5 - Sensor Electronics Module Block Diagram
Figure 5 shows the outline block diagram of the sensor.
Three ECG electrodes from the belt provide the on-body electrical connection to the sensor
providing two alternate views of the users ECG. Two of the ECG electrodes are also used to
measure thoracic impedance variations of the user associated with respiration effort. The
primary chest expansion sensor, contained in the belt, is also connected to the SEM unit.
The core of the sensor electronics module comprises signal filtering and conditioning circuitry for
each of the sensor parameters and also for internally contained sensors to measure skin surface
temperature (sensed by a probe connected to the right hand front ECG electrode) , motion
and body position sensor. The signal conditioning circuitry also contains a software controller
which digitises the sensor data waveforms, processes the data to derive measures such as rates ,
and transfers the data periodically to the wireless transceiver for onward transmission from the
sensor.
An external interface port is also provided which can be used to configure operational
information the SEM uses, to charge the unit and also to connect external sensors.
Draft: A 28th April 2006 Page: 14 of 42

HEALTH CARE PRACTIONER GUIDE
ECG and Heart Rate Derivation
The sensor provides two leads of ECG sharing a common reference electrode (Left Hand Front
location).
The electrode locations within the sensor belt are shown in Figure 6 . These provide two nonstandard views of the hearts electrical activity.
The ECG is samples at 256Hz with a 10 bit resolution. The signal bandwidth is switchable between
ambulatory monitoring mode (5Hz – 85Hz) and diagnostic quality (0.05Hz – 85Hz) settings under
software control. The sensor defaults to ambulatory monitoring mode unless a command is sent
to switch it into diagnostic mode. The ambulatory filtering chosen for the device is designed to
optimise detection reliability under high activity and removes significant amounts of the low
frequency elements of the ECG waveform not needed to achieve this.
Figure 7 provides an example of the same ECG views in both filtering modes for comparison.
The sensor also contains an alternate means to measure heart rate using an analogue heartbeat detector circuit (commonly known as an “R Wave Detector) using the same view as the
ECG 1 electrode. This measure is provided for redundancy purposes and also can be used to
increase battery life in circumstances where the HCP does not require digitised ECG.
ECG 1
ECG 2
Figure 6 - ECG Electrode Locations
Draft: A 28th April 2006 Page: 15 of 42

HEALTH CARE PRACTIONER GUIDE
1400
1200
1000
800
600
ECG Views
ECG1
ECG2
400
200
0
1 28 55 82 109 136 163 190 217 244 271 298 325 352 379 406 433 460
Figure 7 - ECG Waveform examples
Heart rate is calculated as a 60 second rolling average reported every 15 seconds. The rates are
identified as HRe (Heart Rate – ECG derived) and HRr (Heart Rate – R wave(hardware) derived)
A signal quality indicator is also provided with each rate in the range 0-100 (100 = best).
The
This provides an indicator which will reduce when noise peaks occur within the ECG trace.
The HRr signal quality is calculated as follows:
signal quality is calculated as follows:
HRe
(No of confirmed beats/No of candidate beats) * 100
(Mean IBI/ Mean IBI of lowest 8 inter beat intervals) * 100
Draft: A 28th April 2006 Page: 16 of 42

HEALTH CARE PRACTIONER GUIDE
This provides an indicator which will reduce if extra or missed hardware pulse are decoded by
the R wave detection circuit.
Note: Irregular rhythms will also act to reduce the signal quality indicator for HRr.
An overall HR confidence figure is also provided in the range 0-100 (100=best)
This indicator is meant to assist a HCP viewing remotely to a potentially noisy trace and hence
the increased risk of inaccuracy (See Confidence Derivation :)
• THE EQUIVITAL DEVICE SHOULD NOT BE USED FOR SURGICAL PROCEDURES, TO
PERFORM SYNCRONISED CARDIOVERSION OR INTRACARDIAC MONITORING, OR
WHEN PERFORMING EXTERNAL PACING.
The radio system used by the sensors offers a variable delay (in the order of milliseconds) in the
transmission of the ECG and hence the device waveforms cannot be assumed to be truly real
time synchronised to the users ECG.
Breathing Frequency
The primary means of deriving breathing frequency is via a resistive expansion sensor contained
within the belt as shown in Figure 8;
Figure 8 - Respiration Sensor Location
As the users thoracic cavity expands and contracts with respiration effort the sensors resistance
decreases and increases respectively and when fed into appropriate circuitry can be
converted into a respiration effort rate waveform. The belt sensor is sampled at 25.6Hz at a
resolution of 10bits. The signal is filtered to reject noise and then digitised. The overall gain in the
circuit is switched between ambulatory mode and high sensitivity mode. The later mode is used
Draft: A 28th April 2006 Page: 17 of 42

HEALTH CARE PRACTIONER GUIDE
on static, non – upright users to increase breathing sensitivity to shallow breathing patterns as
may be found on resting or sleeping individuals.
In addition respiration effort may also be measure using a thoracic impedance pnuemography
technique. In this technique a high frequency (50kHz) constant current signal is passed through
the body. This signal is modulated by the varying impedance of the thoracic cavity due to
breathing effort and the resultant signal may be processed to provide a breathing frequency.
Thoracic impedance pneumography is known to suffer from significant motion artefact and
should only be used on static patients.
The Respiration effort frequency is calculated as a 60 second rolling average reported every 15
seconds. The rates are identified as BRb (Breathing Rate Belt) and BRi (Breathing Rate
Impedance)
A signal quality indicator is also provided with each indicator in the range 0-100. The signal
quality is calculated as follows:
(No of sensor peaks meeting peak threshold/No of sensor peaks) * 100
This indicator is meant to assist a HCP viewing remotely to a potentially noisy trace and hence
reducing accuracy.
An overall BR confidence figure is also provided in the range 0-100 (100=best)
This indicator is meant to assist a HCP viewing remotely to a potentially noisy trace and hence
the increased risk of inaccuracy (See Confidence Derivation :)
Right
Left Lung
Heart
Skin Temperature
Skin temperature is measured by a thermistor contained in the sensor module adjacent to the
right hand front ECG electrode. Skin temperature is calculated to a resolution of 0.1°C every 15
seconds.
Because of the mass of the sensor, an initial settling time is needed from when the device is
placed on body. This is typically 15 minutes.
Draft: A 28th April 2006 Page: 18 of 42

HEALTH CARE PRACTIONER GUIDE
Body Position and Motion
Body Position and motion are calculated using orthogonal three accelerometer channels and
are reported as follows:
• Prone (lying down - face down)
• Supine (lying down -face up)
• Upright
• Side (lying down – right or left side)
• Inverted (upside down)
Motion is reported as:
None (stationary)
•
Low (ie: walking)
•
High (ie: running)
•
• MOTION DETECTION CAN ERROR DUE TO EXTERNAL INFLUENCES. FOR EXAMPLE:
CERTAIN VEHICLES AND TERRAINS MAY PRODUCE PATTERNS SIMILAR TO
AMBULATORY ACTIVITY. FOR THIS REASON THE PRESENCE OF MOTION SHOULD BE
USED AS A SUPPLEMENTAL INDICATION AND NOT AS A SOLE MEANS TO DETERMINE
THE WELFARE OF A USER.
In addition the raw accelerometer waveforms may also be transmitted from the sensor.
Draft: A 28th April 2006 Page: 19 of 42

HEALTH CARE PRACTIONER GUIDE
Indications and Alerts
Indications are sent by the sensor once is determines certain conditions have been exceeded
within the measured physiology:
ECG Indications
Three indications are provided:
Heart Rate High (Tachycardia) Indication:
The measured heart rate exceeds the customised tachycardia threshold in the sensor (See
Sensor Configuration Data)
Heart Rate Low (Bradycardia) Indication:
The measured heart rate exceeds the customised bradycardia threshold in the sensor (See
Sensor Configuration Data)
Short Term Heart Rate Alert (Cardiac Standstill)
The sensor has not detected a heart beat for a defined time window. This window is
normally set to be shorter than the normal heart rate window in order to provide early
indication of possible cardiac standstill. The time window may be customised in the SEM
(See Sensor Configuration Data)
Respiration Effort Indications
Three indications are provided:
Breathing Rate High Indication:
The measured heart rate exceeds the customised high breathing rate threshold in the
sensor (See Sensor Configuration Data)
Breathing Rate Low Indication:
The measured heart rate exceeds the customised low breathing rate threshold in the
sensor (See Sensor Configuration Data)
Short Term Breathing Rate Alert
The sensor has not detected a breath for a defined time window. This window is normally
set to be shorter than the normal breathing rate window in order to provide early
indication of possible respiratory distress. The time window may be customised in the SEM
(See Sensor Configuration Data)
Draft: A 28th April 2006 Page: 20 of 42

HEALTH CARE PRACTIONER GUIDE
Combined Indication
Because the device may be used remotely to monitor users, an additional alert/indication is
provided which is an aggregation of the earlier thresholds measured by the device.
This may assist the HCP in more rapid detection of users displaying unexpected physiology or
multiple threshold exceptions.
The combined indication uses a colour coded scheme:
• Red = Alarm - high risk physiology requiring immediate review
• Yellow = Indication that physiology is outside expected boundaries and requires closer
scrutiny.
Grey = Absence of detectable physiological signals for a sustained time period.
•
Green = Normal – Physiology is within expected boundaries.
•
Blue = Device in an inoperative or inconclusive state.
•
The boundaries are also defined by configurable levels within the VSDS (See Sensor
Configuration Data).
These shall be set by the managing health care professional and will be occupationally/activity
dependent. A set of default setting are provided to assist the HCP with a defined starting point.
The setting of the indication output is defined as per the state transition diagram shown in Figure
9 overleaf.
As well as using the current physiology values to determine the output value of this indicator the
sensor also uses the confidence values associated with these measures (See Technical
Description :) to determine the signal quality being measured by the sensor in order to assess if
the noise level are likely to be within a range where reliable indication can be generated. This
allows the sensor to reduce false indications by avoiding raising indications and alerts if the
underlying cardio respiratory data may be noisy due to external influences. Note that in this case
the sensor state output indicates this is the case and does not indicate a normal (none alerting)
state so the monitoring personnel can take appropriate action to investigate the cause.
Draft: A 28th April 2006 Page: 21 of 42

g
S
HEALTH CARE PRACTIONER GUIDE
4
Threshold Exception
Condition and Time
Threshold 1 Exceeded
YELLOW
13 Sensor Error
TART
5
Singe Alarm Exception
Condition and Time
Threshold 2 exceeded
6,7
Exception Condition
Cleared and Time
Threshold 1 exceeded
9 HR and BR =0
16 BR<=2 and
Threshold Time 1
exceeded
BLUE
2 - Sensor Self Check OK AND
VSDS Algorithm Confidence > Min
Confidence Threshold and Time
Threshold 5 exceeded
GREEN
8 Multiple Alarm
Condition
HRrst and BRbst =0
RED
14 Sensor Error
Figure 9 - Combined Indication States
3 Sensor Error OR VSDS
Al
orithm Confidence <
Min Confidence
Threshold and Time
Threshold 1 exceeded
10
HR and BR=0 and
Threshold Time 4
Exceeded
12 Ambulation
Detected
GREY
15 Sensor Error
Draft: A 28th April 2006 Page: 22 of 42

HEALTH CARE PRACTIONER GUIDE
Threshold Exception Condition:
The threshold exception condition is intended to identify vital signs cardio respiratory physiology
which is outside expected values (but is not for example absent all together).
The condition is present if either of the following criteria are met for Time Threshold 1:
Heart Rate
R Wave derived Heart Rate (HRr) is > HR Hi Threshold (Tachycardia) AND Heart Rate
confidence HR Conf is > HR Confidence Threshold
OR
ECG derived Heart Rate (HRe) is is > HR Hi Threshold (Tachycardia) AND Heart Rate
confidence HR Conf is > HR Confidence Threshold
OR
R Wave derived Heart Rate (HRr) is < HR Lo Threshold (Bradycardia) AND Heart Rate
confidence HR Conf is > HR Confidence Threshold.
OR
ECG derived Heart Rate (HRe) is is < HR Lo Threshold (Bradycardia) AND Heart Rate
confidence HR Conf is > HR Confidence Threshold
Breathing Frequency Rate
Chest expansion derived Breathing Rate (BRb) is > BR Hi Threshold AND Breathing Rate
confidence BR Conf is > BR Confidence Threshold
OR
Belt derived Breathing Rate (BRb) is < BR Lo Threshold AND Breathing Rate confidence BR
Conf is > BR Confidence Threshold.
Single Alarm Exception Condition:
The single alarm exception condition is intended to identify the absence of detectable vital
signs cardio respiratory physiology:
The condition is present if the following criteria is met for Time Threshold 2:
Heart Rate
R Wave derived short term Heart Rate (HRrst) is = 0 AND Heart Rate confidence HR Conf is
> HR Confidence Threshold
OR
Draft: A 28th April 2006 Page: 23 of 42

HEALTH CARE PRACTIONER GUIDE
ECG derived short term Heart Rate (HRest) is = 0 AND Heart Rate confidence HR Conf is >
HR Confidence Threshold
Breathing Frequency Rate
Belt derived Short Term Breathing Rate (BRbst) is = 0 AND Breathing Rate confidence BR
Conf is > BR Confidence Threshold.
Multiple Alarm Exception Condition
The multiple alarm exception condition is intended to identify the cessation of all measured vital
signs cardio respiratory physiology.
The condition is present if the following criteria is met:
Heart Rate
R Wave derived short term Heart Rate (HRrst) is = 0 AND Heart Rate confidence HR Conf is
> HR Confidence Threshold
OR
ECG derived short term Heart Rate (HRest) is = 0 AND Heart Rate confidence HR Conf is >
HR Confidence Threshold
AND
Belt derived Short Term Breathing Rate (BRbst) is = 0 AND Breathing Rate confidence BR
Conf is >
BR Confidence Threshold.
Sensor Error Condition:
A sensor error condition is detected if the following criteria is met:
Failure of power on self checks (the SEM unit or belt is faulty)
OR
Failure of in operation self checks (the SEM or belt has developed a fault)
OR
ECG Lead Off detection
OR
Respiration expansion belt off detection
OR
VSDS Algorithm Confidence is < VSDS Min Operational Confidence
Draft: A 28th April 2006 Page: 24 of 42

HEALTH CARE PRACTIONER GUIDE
Sensor Self Check Okay:
A sensor self check okay condition is detected if the following criteria is met:
Successful power on self checks
AND
ECG Lead On Detection
AND
Respiration expansion belt On detection
AND
VSDS Algorithm Confidence is > VSDS Min Operational Confidence for Time Threshold 1.
Exception Cleared Condition:
The exception cleared condition is intended to identify vital signs cardio respiratory physiology
which is inside expected values :
The condition is present if the following criteria is met for Time Threshold 1:
Heart Rate
R Wave derived Heart Rate (HRr) is < HR Hi Threshold (Tachycardia) AND Heart Rate
confidence HR Conf is > HR Confidence Threshold
AND
ECG derived Heart Rate (HRe) is < HR Hi Threshold (Tachycardia) AND Heart Rate
confidence HR Conf is > HR Confidence Threshold
AND
R Wave derived Heart Rate (HRr) is > HR Lo Threshold (Bradycardia) AND Heart Rate
confidence HR Conf is > HR Confidence Threshold.
AND
ECG derived Heart Rate (HRe) is is > HR Lo Threshold (Bradycardia) AND Heart Rate
confidence HR Conf is > HR Confidence Threshold
Breathing Frequency Rate
Belt derived Breathing Rate (BRb) is < BR Hi Threshold AND Breathing Rate confidence BR
Conf is > BR Confidence Threshold
AND
Belt derived Breathing Rate (BRb) is > BR Lo Threshold AND Breathing Rate confidence BR
Conf is > BR Confidence Threshold.
Draft: A 28th April 2006 Page: 25 of 42

HEALTH CARE PRACTIONER GUIDE
Confidence Derivation :
Heart Rate
The methods to derive Heart Rate signal quality are specified in Sensor Overview.
An overall Heart Rate confidence is provided by the following rule:
A comparison of the two Heart Rates (HRe and HRr) is defined as the percentage error
(unsigned) between the primary measure (HR ECG) and R waved derived Heart Rate (HRr)
Heart rate confidence is then computed as a weighted average as follows:
HR Conf = ( (w*HRe Signal Quality)+(x* HRr Signal Quality)+(y*HR Correlation))/3
The weighting factors are defined to bias the confidence to be higher if the two measures are
enabled and reporting values
If at the point of computation only a single measure is enabled then confidence will be simply
be:
Weighting factor * HRx Signal Quality
The weighting factors are w, x = 0.9 , y = 1.2. The weighting factors are defined to bias the
confidence to be higher if the two measures are enabled and reporting values.
Breathing Rate
The methods to derive Breathing Rate signal quality are specified in Sensor Overview.
BR Correlation is defined as the percentage error (unsigned) between the primary measure
(BRb) and Impedance derived Heart Rate (BRi)
Breathing rate confidence is computed as a weighted average as follows:
BR Conf = ( (w*BRb Signal Quality)+(x* BRi Signal Quality)+(y*BR Correlation))/3
The weighting factors are defined to bias the confidence to be higher if the two measures are
enabled and reporting values.
If at the point of computation only a single measure is enabled then confidence will be simply:
Weighting factor * BRx Signal Quality
The weighting factors are w, x = 0.9 , y = 1.2
Draft: A 28th April 2006 Page: 26 of 42

HEALTH CARE PRACTIONER GUIDE
VSDS Confidence Rate
The VSDS confidence value is derived as follows:
VSDS Confidence = ( (w*HR Conf)+(x* BR Conf))/2 )
The weighting factors are defined to bias the confidence measure towards higher respiration
confidence considering its primary importance in vital signs assement compared to heart rate
variation (excluding the cardiac arrest case)
The weighting factors are w= 0.5 , x =1.5.
Secondary Confirmation:
Secondary confirmation is used to improve the confidence in a vital sign parameter by
comparing it to an alternate measure of the same parameter and measuring the error between
the two. If the error is close then confidence can be increased that the sensor is operating
correctly. This is then included in the confidence measure which is used to determine the state
transition validity.
The following rules are used to determine when a state change dependent secondary
confirmation is undertaken:
Heart Rate
• By Customisation:
If by customisation the device is configured to enable both means of Heart Rate
measurement all the time then by default secondary confirmation is used as part of
the confidence computation.
• Dependent on the Current Active Measure Signal Quality
If the current heart rate derivation has a high signal quality (> HR Signal Quality
Threshold )then the device will not need the secondary confirmation before making a
transition. The secondary measure however will be enabled for future measurements
however.
Respiration Rate
• By Customisation
If by customisation the device is configured to enable both means of Respiration Rate
measurement then by default secondary confirmation is used as part of the
confidence computation only when the subject is static. This reflects that the
secondary respiration means can only be considered reliable when the subject is not
moving.
• Dependent on the Current Active Measure Signal Quality
If the current respiration rate derivation has a high signal quality (> BR Signal Quality
Threshold )then the device will not need the secondary confirmation before making a
transition. The secondary measure however will be enabled for future measurements
Draft: A 28th April 2006 Page: 27 of 42

HEALTH CARE PRACTIONER GUIDE
however. If the signal quality is less than the signal quality threshold then the device will
perform a secondary confirmation if the subject is not moving.
Draft: A 28th April 2006 Page: 28 of 42

HEALTH CARE PRACTIONER GUIDE
Connecting and Using the Sensor – Model Number EQ01-002/012
(Type 0 Low Power PAN Interface)
The Type 0 radio interface is intended for use by the following defined receiver modules:
1. Mini Mitter/Respironics Vital Sense (K)
2. A US DoD re-packaged variant of the above monitor (restricted to the US Military)
Consult the user instructions for the receiver module for more details on how to operate the
receiver unit and recover the sensor data.
Sensor Activation
The protocol used requires sensors to be optically activated by receiver module before use. This
is achieved by operating the activation command on the receiver, and holding the activation
sensor on the receiver close to the SEM activation window shown below:
Activation
Window
Sensor Range
The sensor range is intentionally low and the device should be kept within 1 meter of the receiver
unit to guarantee reliable data transmission.
Protocol Overview
The protocol employed by the system uses repeated transmissions of a data packet sent
approximately every 15 seconds. The transmission rate has been chosen to provide some
redundancy if a packet is not received due to noise on the radio channel. The sensor will
continue to transmit data once activated and hence a sensor which is not operational, has
stopped working , or has gone out of range, can be detected rapidly by the absence of a valid
data packet over a fixed time window (eg: 1 minute)
Draft: A 28th April 2006 Page: 29 of 42

HEALTH CARE PRACTIONER GUIDE
The data packet contains:
• Sensor Transmitter Serial Number
Heart Rate and Confidence Value
•
Respiration Effort Rate and Value
•
• Skin Temperature (Chest)
• Motion
• Body Position
The packet is protected by a cyclic redundancy code to detect transmission errors and reject
corrupted data.
The data is transferred in a binary formatted, non-text format and hence is not directly readable
without the packet data specification.
Sensor Diagnostics
The Sensor also provides the following diagnostics
1. Low Battery – The batteries should be changed as soon as possible.
2. Sensor INOP – The sensor has detected an internal problem and has become
inoperative. If this message is seen persistently (more than 3 sucessive transmissions) the
device should be returned for service
3. Sensor Lead Off – The ECG or Belt Sensor have become detached from the SEM. Check
the belt location and that the SEM is clipped to the belt correctly. Other causes may be
excessive skin impedance (dirt , dead skin) or a damaged belt.
Draft: A 28th April 2006 Page: 30 of 42

HEALTH CARE PRACTIONER GUIDE
Connecting to and Using the Sensor– Model Number EQ01-001
(Type 1 Bluetooth™)
Bluetooth™ Connection Information
The Type 1 version of the sensor complies with the Bluetooth™ protocol specification V1.1 and
can be used with a Bluetooth™ certified transceiver which supports the serial port profile. This
profile allows serial data to be passed over the Bluetooth™ radio and to be presented at the
receiver end as a serial data stream communications port.
The SEM sensor appears as a “discoverable slave” device which means it can be located and
connected to by the receiving unit. Consult the manual for your Bluetooth™ receiver for
instruction on how to do initate the connection.
Sensor Data Security
The Bluetooth™ protocol implements both encryption and a pass key access.
The pass key for the sensor should be programmed into the unit by the SEM customise utility (See
Sensor Configuration Data)
• Failure to set a unique Bluetooth™ pass key may compromise the security of the
device and may make it easier for other persons to connect to the sensor.
Application Protocol Overview
Summary Disclosure
Summary disclosure is transferred every 15 seconds. The transfer of data is defined in [4].
The data sent is as follows:
• Respiration Band Rate (if configured)
• Respiration Band Signal Quality (if configured)
• Impedance Rate (if configured)
• Respiration Rate Confidence
• EDR Rate (if configured)
• EDR Quality (if configured)
• Skin Temperature
Body Orientation
•
Motion Classification
•
• Combined Vital Signs Indication and Confidence (if configured)
• ECG Heart Rate (if configured)
Draft: A 28th April 2006 Page: 31 of 42

HEALTH CARE PRACTIONER GUIDE
• ECG Signal Quality (if configured)
• R Wave Heart Rate (if configured)
• R Wave Signal Quality (if configured)
• Heart Rate Confidence
The following indications are sent every 5 seconds:
ECG Indications:
•
Low Heart Rate
o
High Heart Rate
o
• Respiration Indications
Low Breathing Rate
o
o High Breathing Rate
• Sensor Fault Codes
Full Disclosure
When full disclosure is selected, then in addition to the Summary disclosure, the following data
are transmitted:
• Raw Waveform Data
o ECG1 and 2
o Impedance Trace
o Respiration Belt Trace
o Accelerometer Traces
Battery voltage sensor value
o
A four bit sequence number is transmitted with the ECG1 and 2 waveform data. The lower two
bits of the sequence number are transmitted in the most significant two bits of the 12 bit
message data of ECG1. The upper two bits are transmitted in the most significant two bits of the
12 bit message data of ECG2. The sequence number is incremented every time both messages
have been transmitted. The sequence number will cycle every 16 transmissions, allowing the
detection of dropouts of up to 16/256 s or 62.5 ms.
The protocol uses a non human readable tag based method to transfer the data
All application layer messages are 3 characters long and consist of a single character message
type followed by two characters of data, regardless of the need for this amount of data. This is
illustrated in Figure 10.
Message Type Data 1 Data 2
Figure 10 - Message Structure
When the data is carrying a single unsigned integer value (the normal case) the data is
encoded as follows:
The maximum size of unsigned integer value is taken to be 12 bits (the A/D converter has a
maximum resolution of 10 bits so this allows some flexibility).
The first transmitted data character is formed by taking the least significant 6 bits of the 12 bits
and adding 32 decimal (20 Hex) to avoid non-printing characters. Similarly the second
transmitted data character is formed by taking the most significant 6 bits of the 12 bits and
adding 32 decimal.
Draft: A 28th April 2006 Page: 32 of 42

HEALTH CARE PRACTIONER GUIDE
A copy of the interface protocol is available as part of the system integrators kit provided by
Hidalgo.
Draft: A 28th April 2006 Page: 33 of 42

y
HEALTH CARE PRACTIONER GUIDE
Sensor Configuration Data
Indication Rate Limits and Time Thresholds
Parameter Parameter Name Description Range Default
Tachycardia limit HR High Threshold Tachycardia limit for heart rate 0: No Limit
1-255 bpm
Bradycardia limit HR Low Threshold Bradycardia limit for heart rate 0: No Limit
1-255 bpm
Upper Respiration Limit BR High Threshold Upper respiration limit for respiration rate 0: No Limit
1-255 bpm
Lower Respiration Limit BR Low Threshold Lower respiration limit for respiration rate 0: No Limit
1-255 bpm
Threshold Exception Time Time Threshold 1 Time required for an out of threshold rate
to exist before an indication is raised
Short term Heart Rate
Window
HR(st)TimeWindow Time period over which a short term heart
rate is measured in order to provide an
earl
indication of failure to detect heart
0: Infinite
1 – 255
minutes
0: None
1-255
200 bpm
30 bpm
0 (i.e. no limit)
5 bpm
2 minutes
10 seconds
Draft: A 28th April 2006 Page: 34 of 42

HEALTH CARE PRACTIONER GUIDE
Parameter Parameter Name Description Range Default
beats seconds
Short term Breathing Rate
window
Time to alert – cardiac
alarm
Time to alert – breathing
alarm
Heart Rate Confidence
Threshold
Breathing Rate Confidence
Threshold
Breathing Rate Secondary
Confirmation Threshold
Heart Rate Secondary
Confirmation Threshold
BR(st)TimeWindow Time period over which a short term
breathing rate is measured in order to
provide an early indication of failure to
detect respiration effort
Time Threshold 2 Time period when HR(st) = 0 before an
indication is raised
Time Threshold 3 Time period that BR(st) = 0 before an
indication is raised
HR Confidence
Threshold
BR Confidence
Threshold
Minimum confidence in Heart Rate signal
needed to make a alarm or alert condition
Minimum confidence in Breathing Rate
signal needed to make a alarm or alert
condition
BR Signal Quality
Threshold
Minimum signal quality threshold needed
to allow a state change to be made
without a secondary confirmation
measurement being made
HR Signal Quality
Threshold
Minimum signal quality threshold needed
to allow a state change to be made
without a secondary confirmation
measurement being made
0: None
1-255
seconds
0-255
seconds
0-255
seconds
0-100%
0-100%
0-100%
0-100%
20
Seconds
0 seconds
0 seconds
80%
80%
85%
85%
Combined indication
operational confidence
threshold
VSDS Minimum
Operational
Confidence
Minimum VSDS combined algorithm
confidence below which the output is
considered unreliable and should be set to
Draft: A 28th April 2006 Page: 35 of 42
0-100%
65%

HEALTH CARE PRACTIONER GUIDE
Parameter Parameter Name Description Range Default
unknown or inoperative
Time Threshold – Sustained
absence of cardio
respiratory signals
Time Threshold – Sensor
Initialisation
Time Threshold 4 Time period to transition from red to grey
state when no vital sign signals are being
measured.
Time Threshold 5 Time period to transition from BLUE state to
GREEN state once VSDS algorithm
confidence is above minimum threshold.
0-255mins 5 mins
0-255mins 3 mins
Power On Defaults
Parameter Parameter Name Description Range Default
Power on disclosure control Full Disclosure Whether the sensor will power up and send
physiological waveforms as well as
summary data (applies to the Type 1 radio
interface only)
Heart Rate and ECG
Control
Heart Rate – R Wave
Control
Whether the sensor will power up with its
ECG derived heart rate (HRe) enabled
Whether the sensor will power up with its
hardware derived heart rate (HRr)
enabled
On
Off
On
Off
On
Off
On
On
On
Breathing Rate – Chest
Expansion Belt Control
Draft: A 28th April 2006 Page: 36 of 42
Whether the sensor will power up with its
thoracic expansion belt derived respiration
rate (BRb) enabled
On
Off
On

HEALTH CARE PRACTIONER GUIDE
Parameter Parameter Name Description Range Default
Breathing Rate – Thoracic
Impedance Control
Combined Indication
Control
Whether the sensor will power up with its
thoracic impedance derived respiration
rate (BRi) enabled
Whether the sensor will power up with its
combined indication algorithm enabled
On
Off
On
Off
Off
On
Bluetooth Link Parameter
Parameter Parameter Name Description Range Default
Bluetooth Access Code Pass key used to allow connection to the
sensor
4 digit
numeric
1111
Draft: A 28th April 2006 Page: 37 of 42

HEALTH CARE PRACTIONER GUIDE
Belt Construction
The chest belt is made of the following materials:
4% Polyurethane
36% Polyamide
9% Lycra
15% Neoprene
21% Polyester
The following sizing chart provides a guideline on how to select the correct belt size to use
Belt Size Shirt Size Chest Circumference
S S 33-37
M M 37-41
L L 41-45
XL XL 45-49
Some variation may exist for uses at the size boundaries in which case it is recommended to go
for the larger sized belt.
Draft: A 28th April 2006 Page: 38 of 42

HEALTH CARE PRACTIONER GUIDE
Accessories
• USE ONLY THE FOLLOWING ACCESSORIES.
Battery Charger
Hidalgo Part Number:
EQ-ACC-01 - Mascot 2240 LI(UK Plug)
EQ-ACC-02 - Mascot 2240 LI (US Plug)
EQ-ACC-03 - Mascot 2240 LI (EU Plug)
Chest Belt
Hidalgo Part Number:
EQ01-020 (/S/M/L/XL)
Repair and Service
• THERE ARE NO USER SERVICEABLE PARTS IN THE DEVICE. SHOULD YOU REQUIRE
Technical Specifications
SERVICE OR REPAIR PLEASE CONTACT HIDALGO
Device Classification
Shock Protection : Type BF Applied Part , Internally Powered Equipment
Enviromental Protection: IPx0 (unprotected)
Flammable Gas Protection: Unprotected
Mode of Operation: Continuous
Draft: A 28th April 2006 Page: 39 of 42

HEALTH CARE PRACTIONER GUIDE
FDA Device Classification: Class II
EU Device Classification: Class IIb
Chest Harness /Belt
Size (circumference):
Small: 40mm x 108mm
Medium: 40mm x 116mm
Large: 40mm x 124mm
Extra Large:40mm x 131mm
Weight: 80g
Operating temperature: -10°C to +55°C
Operating Humidity: 10% to 75% RH Non-Condensing
Storage Temperature: -20°C to +65°C
Storage Humidity: 5% to 90% RH Non-Condensing
Air Pressure: 570hPA – 1060hPA
Sensor Electronics Module
• General
Size (overall dimensions): 123mm x 75mm x 14mm
Weight: 75g
Power: 2 x 1.5v AAA LR03 Alkaline cells or
3.7V 740mA Li-ION rechargeable cell
Operating temperature: -10°C to +55°C
Operating Humidity: 0% to 95% RH Non-Condensing
Storage Temperature: -20°C to +65°C
Storage Humidity: 0% to 95% RH Non-Condensing
• ECG
No of leads: 2
Sampling frequency: 256 Hz
Resolution: 10 bits
Voltage range: +/- 5mV
CMRR: >85 dB
Frequency Range: Diagnostic Setting : 0.05 – 85 Hz (3dB points)
Monitor/Ambulation Setting : 5Hz – 85Hz (3dB points)
Heart Rate Calculation
Frequency: 15 seconds
• Impedance Respiration Effort
Measurement type: Bipolar
Sampling frequency: 25.6 Hz
Resolution: 10 bits
Modulation: 50 KHz
Drive Current: 200 µA
Frequency Range: 0.05 – 7 Hz
Draft: A 28th April 2006 Page: 40 of 42

HEALTH CARE PRACTIONER GUIDE
Respiration Rate Reporting
Frequency: 15 seconds
• Chest Expansion Respiration Effort
Measurement type: Resistive strain gauge
Sampling frequency: 25.6 Hz
Resolution: 10 bits
Frequency Range: 0.05 – 7 Hz
Respiration Rate Reporting
Frequency: 15 seconds
• Temperature
Sampling frequency: 0.25Hz
Resolution: 10 bits
Range: 10°C - 45°C
Sensor Accuracy: <35.8C and > 41C +/- 0.3 C
35.8C to 37C +/-0.2C
37 to 39 C +/- 0.1C
39C to 41 C +/- 0.2 C
Measurement type: Thermistor
Temperature Reporting
Frequency: 15 seconds
Electromagnetic Compatibility
This device has been designed to meet the relevant radio and electromagnetic interference
standards for the countries it is used in.
A risk remains however, as for all radio based devices, that interference may occur either from or
to the device.
If you experience unwanted interference increase the physical separation between the devices.
We recommend a separation of 0.5 meter or greater between the SEM device and other
wireless devices.
If you have specific concerns about the devices compatibility or experience problems which
cannot be resolved by increasing separation of the devices, please contact Hidalgo.
• IF YOU ARE ENTERING A FACILITY WHERE INTERFERENCE MAY BE A PARTICULAR
CONCERN (EG: A HOSPITAL OR HAZARDOUS PLANT ENVIROMENT ) CONTACT THE
PERSON IN CHARGE OF THE FACILITY TO CHECK IF ANY SPECIAL PRECAUTIONS
NEED TO BE TAKEN.
Draft: A 28th April 2006 Page: 41 of 42

HEALTH CARE PRACTIONER GUIDE
FCC Compliance and Advisory Notice (US Markets)
This equipment has been tested and found to comply with the limits for a Class B digital device,
pursuant to Part 15 of the FCC Rules. These limits are designed to provide reasonable protection
against harmful interference in a residential installation. This equipment generates, uses and can
radiate radio frequency energy and, if not installed and used in accordance with the
instructions for use, may cause harmful interference to radio communications.
However, there is no guarantee that interference will not occur in a particular installation. If this
equipment does cause harmful interference to radio or television reception which can be
determined by turning the equipment off and on, the user is encouraged to try to correct the
interference by one or more of the following measures:
• Reorient or relocate the receiving antenna.
Increase the separation between the equipment and the receiver.
•
• Connect the equipment into an outlet on a circuit different from that to which the receiver is
connected.
• Consult the dealer or an experienced radio/television technician for help.
This device complies with Part 15 of the FCC Rules. Operation is subject to the following two
conditions: (1) this device may not cause harmful interference, and (2) these devices must
accept any interference received, including interference that may cause undesired
operation.
Type 0 Radio Interface:
FCC ID:
Type 1 Radio Interface:
FCC ID:
Draft: A 28th April 2006 Page: 42 of 42
 Loading...
Loading...