Page 1
Instruction Manual
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
• Mix by carefully swirling the cube in tight circles.
HI 3831
Chlorine
Test Kit
ISO 9000 Certified
Company since 1992
http://www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product. Please read the
instructions carefully before using the chemical test kit. It
will provide you with the necessary information for a correct
use of the kit.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
• Calibrated vessel (50 mL);
• Reagent 1 (30 mL);
• Reagent 2 (30 mL);
• Reagent 3 (30 mL);
• Color Comparison Cube.
Note: Any damaged or defective item must be returned in
Range 0 to 2.5 mg/L (ppm) Chlorine
Analysis Method Colorimetric
Sample Size 5 mL
Number of Tests 110 (average)
Case Dimensions 200x120x60 mm (7.9x4.7x2.4")
Shipping Weight 460 g (1 lb.)
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
its original packing materials.
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
In pools and drinking water supplies, chlorination serves to
kill or deactivate disease-producing microorganisms. It can
also improve water quality by reacting with ammonia, iron,
sulfide and some organic substances. However, an excessive
concentration of chlorine in water can produce adverse conditions, such as formation of carcinogenic chloroform or
other toxins. To maximize the purpose for chlorination and
minimize any adverse effects, it is essential to monitor the
chlorine levels closely.
The Hanna Chlorine Test Kit determines the Chlorine concentration in water via a color cube. This makes the test kit
practical for field use. No iodine or bromine can be present
for this test to work properly.
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
The addition of chlorine to water produces hydrochloric and
hypochlorous acids. The hypochlorous acid acts as the disinfectant and bleaching agent. These are known as free
chlorine. The formation of chloroamines and nitrogen trichloride will occur if ammonia is present. These are known as
bound chlorine. Both, free and total chlorine are measured
by a colorimetric method. The sample is initially treated
with a phosphate buffer to a pH of approximately 6.3 pH.
The addition of DPD (N,N-diethyl-p-phenylenediamine) is
immediately oxidized by chlorine producing a reddish color.
The color intensity of the solution determines the free chlorine concentration. The total chlorine determination requires
an excess addition of iodide ions to the solutions. The iodide
ions react with chloroamines and form iodine. The iodine
readily oxidizes the DPD, thus adding to the red color.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
Standard Methods for the Examination of Water and Waste-
th
water, 16
Edition, 1985, pages 309-310.
ACCESSORIESACCESSORIES
ACCESSORIES
ACCESSORIESACCESSORIES
HI 3831-100 Spare reagents (100 tests)
ISTR3831R1 02/98 PRINTED IN ITALY
READ ALL THE INSTRUCTIONS BEFORE USING THE TEST KIT
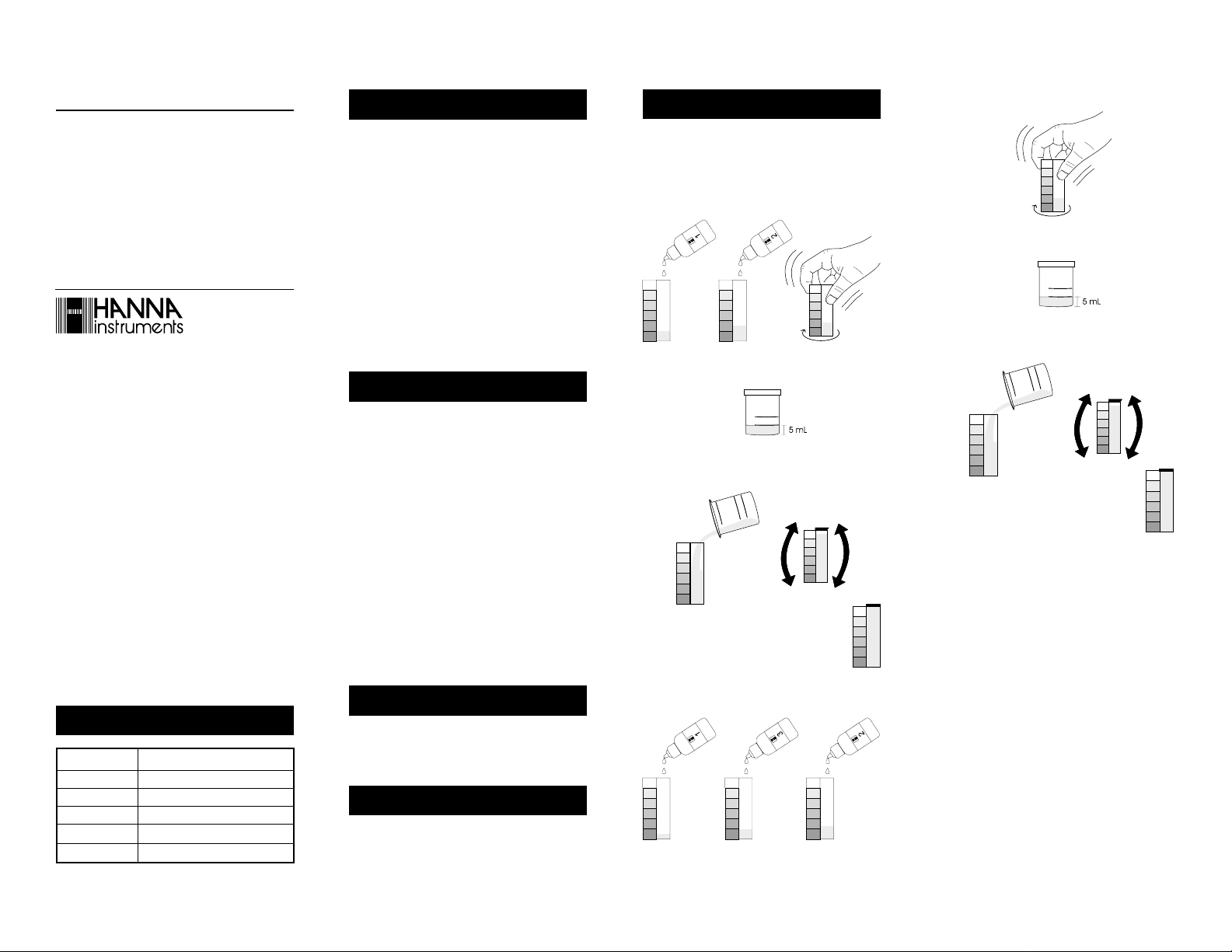
Free Chlorine
• Add 5 drops of Reagent 1 and 3 drops of Reagent 2 to
the color comparison cube and mix by carefully swirling
the cube in tight circles.
5
• Remove the cap from the plastic vessel. Rinse the plastic
vessel and fill to the 5 mL mark with water sample.
• Transfer the 5 mL water sample from the plastic vessel
into the color comparison cube. Replace the cap and
mix it by inverting several times.
• Determine which color band best matches the
solution in the vessel and record the results in
mg/L (ppm) free chlorine.
Total Chlorine
• Add 5 drops of Reagent 1, 2 drops of Reagent 3 and 3
drops of Reagent 2 to the color comparison cube.
3
532
• Remove the cap from the plastic vessel. Rinse the plastic
vessel and fill to the 5 mL mark with water sample.
• Transfer the 25 mL water sample from the plastic vessel
into the color comparison cube. Replace the cap and
mix it by inverting several times.
• Determine which color band best matches the
solution in the vessel and record the results in
mg/L (ppm) total chlorine.
Bound Chlorine
• The concentration of bound chlorine in the sample is
determined by subtracting the free chlorine result from
the total chlorine result.
 Loading...
Loading...