Page 1
Instruction Manual
HI 3822
Sulfite Test Kit
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product. Please read the
instructions carefully before using the chemical test kit. It
will provide you with the necessary information for correct
use of the kit.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
• Sulfamic Acid Solution, 1 bottle with dropper (30 mL);
• EDTA Reagent, 1 bottle with dropper (30 mL);
• Sulphuric Acid solution, 1 bottle with dropper (15 mL);
• Starch Indicator, 1 bottle with dropper (10 mL);
• HI 3822-0 Reagent Titrant Solution, 1 bottle (120
mL);
• 2 calibrated vessels (20 & 50 mL);
• 1 calibrated syringe with tip.
Note: Any damaged or defective item must be returned in
its original packing materials.
SPECIFICATIONS
Range 0 to 20 mg/L (ppm) Na2SO
0 to 200 mg/L (ppm) Na2SO
Analysis Method Iodometric method – Titration
Sample Size 5 mL & 50 mL
Number of Tests 110 (average)
Case Dimensions 260x120x60 mm (10.2x4.7x2.4")
Shipping Weight 910 g (34.0 oz.)
SIGNIFICANCE AND USE
There are many reasons to monitor sulfite concentration in
water. In industrial applications, a sulfite concentration of
approximately 20 mg/L must be mantained to prevent pitting and oxidation of metal components as in boiler feed
and effluent waters. A high level of sulfite results in a
lowered pH, thus promoting corrosion. The monitoring of
sulfite is important in environmental control. Sulfite ions are
toxic to aquatic lifeforms and their ability to remove dissolved oxygen in water will destroy the delicate balance of
ecology of lakes, rivers and ponds.
The Hanna Sulfite Test Kit makes monitoring easy, quick
and safe. The compact size gives the user the versatility to
use the kit practically anywhere. The design of the kit
makes it practically impossible to spill the reagents, thereby
reducing the possibility of injury or damage to property.
Note: mg/L is equivalent to ppm (parts per million).
CHEMICAL REACTION
A iodometric method is used. Iodide ions react with iodate
ions in the presence of sulfuric acid to form iodine (Step 1).
The sulfite present in the water sample then reduces the
iodine back to iodide (Step 2). An excess of iodate ions will
generate additional iodine, which will form a blue complex
with starch. This color change determines the end point of
this titration.
Step 1: KIO3+ 5KI + 3H2SO4 →3I2 + 3K2SO4 + 3H2O
Step 2: SO
ISTR3822R2 04/02 PRINTED IN ITALY
2–
+ I2 +H2O → SO
3
2–
+ 2HI
4
INSTRUCTIONS
3
3
READ ALL THE INSTRUCTIONS BEFORE USING THE TEST KIT
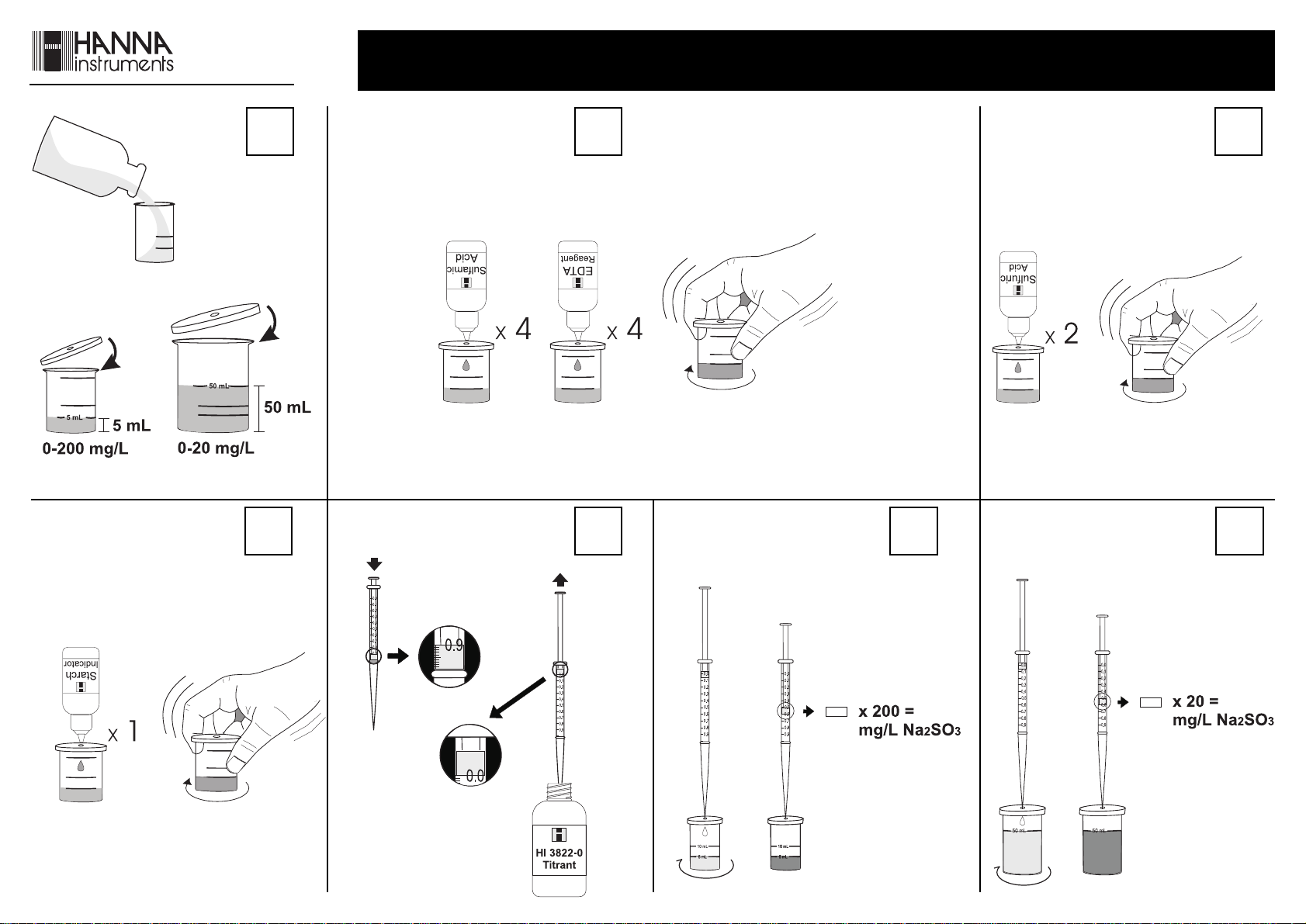
LOOK AT THE BACK PAGE FOR THE ILLUSTRATED PROCEDURE
Note: Push and twist pipet tip onto tapered end of syringe
ensuring an air tight-fit.
HIGH RANGE – 0 to 200 mg/L Na2SO
3
• Remove the cap from the small
plastic vessel. Rinse the plastic vessel
with water sample, fill to the 5 mL
• Place the syringe tip into the cap port
of the plastic vessel and slowly add the
titration solution dropwise, swirling to
mix after each drop. Continue adding
titration solution until the solution in
the plastic vessel changes from colorless
to blue.
• Read off the milliliters of titration solution from the syringe
scale and multiply by 200 to obtain mg/L (ppm) sodium
sulfite.
mark and replace the cap.
x 200 = Na2SO
3
• Add 4 drops each of Sulfamic Acid Solution and EDTA
Reagent through the cap port and mix by carefully
swirling the vessel in tight circles.
LOW RANGE – 0 to 20 mg/L Na2SO
3
• If results are lower than 20 mg/
L, the precision of the test can be
improved as follows.
Remove the cap from the large
plastic vessel. Rinse the vessel with
• Add 2 drops of Sulfuric Acid Solution through the cap
port and mix as described before.
the water sample, fill to the 50
mL mark and replace the cap.
Proceed with the test as described
before and multiply the values on the syringe scale by 20
to obtain mg/L sodium sulfite in the sample.
x 20 = Na2SO
3
• Add 1 drop of Starch Indicator through the cap port
and mix.
REFERENCES
1987 Annual Book of ASTM Standard, Volume 11.01 Water
(1), pages 732-736.
Standard Methods for the Examination of Water and Wastewater, 20th Edition, 1998, page 4-173.
• Take the titration syringe and push the
plunger completely into the syringe.
Insert tip into HI 3822-0 Reagent Titrant
Solution and pull the plunger out until
the lower edge of the plunger seal is on
the 0 mL mark of the syringe.
The chemicals contained in this test kit may be hazardous if
improperly handled. Read Health and Safety Data Sheets
before performing the test.
HEALTH AND SAFETY
Page 2
HI 3822 SULFITE TEST KIT
1
4
2 3
5 7
5 mL sample 50 mL sample
6
 Loading...
Loading...