Page 1

Instruction Manual
HI 3820
Acidity Test Kit
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product. Please read the
instructions carefully before using the chemical test kit. It
will provide you with the necessary information for correct
use of the kit.
SPECIFICATIONSSPECIFICATIONS
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage, notify your Dealer or the nearest Hanna office
immediately.
Each kit is supplied with:
• Dechlorinating reagent, 1 bottle with dropper (10
• Bromophenol Blue Indicator, 1 bottle with dropper
• Phenolphtalein Indicator, 1 bottle with dropper (10
• HI 3820-0, 1 bottle (120 mL);
• 2 calibrated vessels (10 and 50 mL);
• 1 calibrated syringe.
Note: Any damaged or defective item must be returned in
Range 0 to 100 mg/L (ppm) CaCO
Smallest Increment 1 mg/L [in the 0-100 mg/L range]
Analysis Method Base titration using phenolphthalein
Sample Size 5 mL and 25 mL
Number of Tests 110 (average)
Case Dimensions 260x120x60 mm (10.2x4.7x2.4")
Shipping Weight 910 g (34.0 oz.)
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
mL);
(10 mL);
mL);
its original packing materials.
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
0 to 500 mg/L (ppm) CaCO
5 mg/L [in the 0-500 mg/L range]
and bromphenol blue indicators
3
3
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
Acidity is the quantitative capacity of a water sample to
neutralize a base to a set pH. Therefore, the greater
acidity, the more potentially corrosive the water. Acidity can
be caused by mineral acids, organic acids and carbon
dioxide in the form of carbonic acid. Today, our water
supplies are becoming more contaminated with corrosive
chemicals from industrial dumping or acid rain. Therefore,
acidity measurements are an essential monitoring device to
define and control pollution in sewers, lakes and rivers.
Acidity of water is equally important to monitor in soils and
fish farming to maximize the growing environment.
The Hanna Acidity Test Kit is equipped with all you need to
determine acidity of water. The kit is quick, easy to use and
portable. This makes it practical for field as well as laboratory use. The design makes the kit easy to handle and,
except for HI 3820-0, practically prevents accidental injury
or damage due to spills.
Note: mg/L is equivalent to ppm (parts per million).
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
Strong acids (such as mineral acids) and organic acids can
contribute to the acidity of a water sample. With the use of
diluted sodium hydroxide as the titrant and bromphenol
blue or phenolphthalein indicators, the contribution of
strong or organic acids can be determined. The measurement of the strong acid contribution to the sample acidity is
known as methyl orange acidity. This is carried out by
titrating with sodium hydroxide until the solution turns from
yellow to green/blue (pH endpoint about 4.5). The total
acidity caused by both mineral and organic acids is determined by titrating to an endpoint pH of 8.3, using
phenolphthalein as an indicator. This is known as phenolphthalein acidity.
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
READ ALL THE INSTRUCTIONS BEFORE USING THE TEST KIT
LOOK AT THE BACK PAGE FOR THE ILLUSTRATED PROCEDURE
Note: Push and twist pipet tip onto tapered end of syringe
ensuring an air tight-fit.
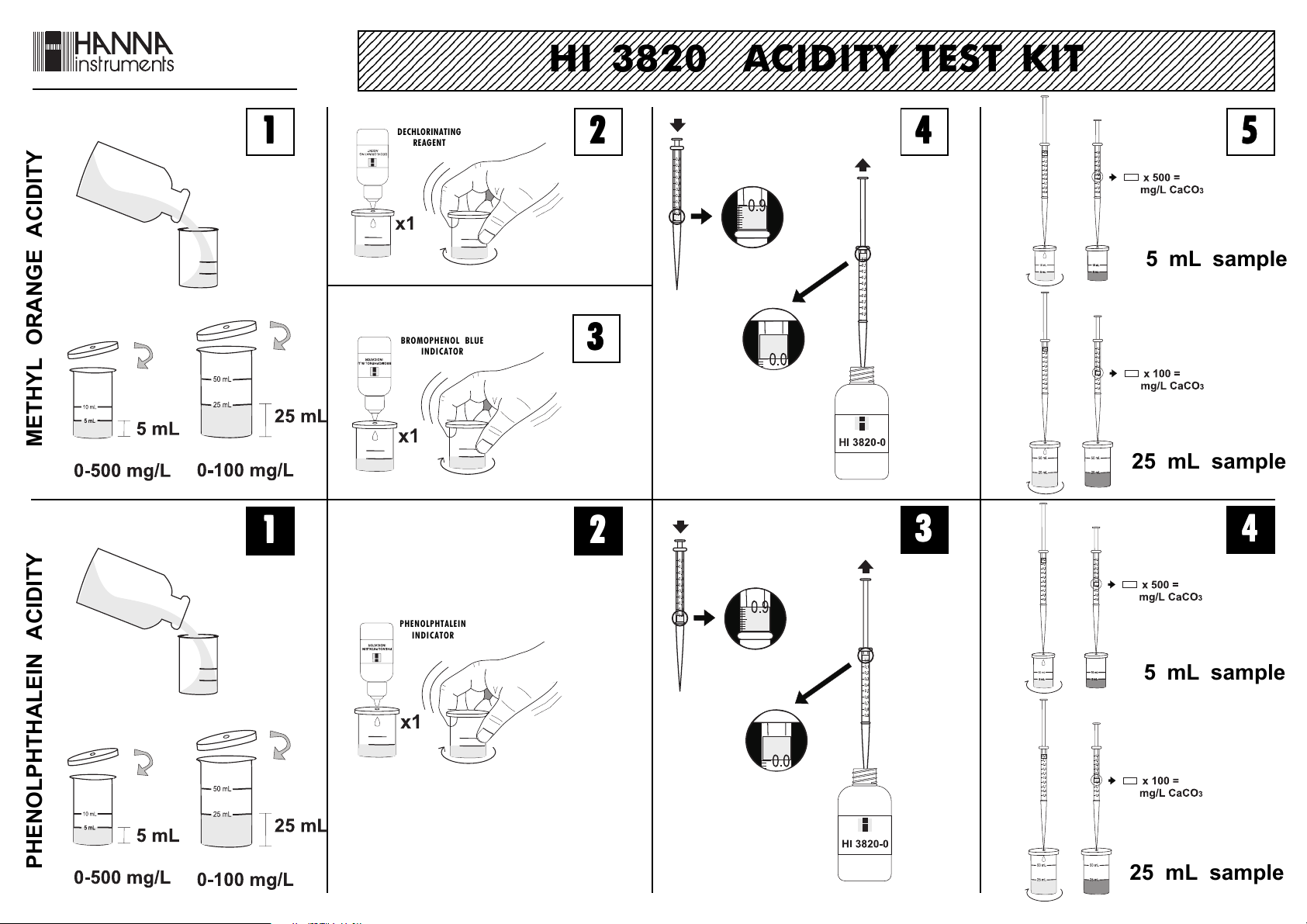
Determination of Methyl Orange Acidity
• Remove the cap from the small plastic vessel. Rinse the
vessel with water sample, fill to the 5
mL mark and replace the cap.
• Add 1 drop of Dechlorinating reagent
through the cap port and mix by
ISTR3820R2 12/01
carefully swirling the vessel in tight circles.
• Through the cap port, add 1 drop of Bromophenol Blue
indicator and mix. If the solution is green or blue, then
record the methyl orange acidity as zero. Proceed with
procedure for the determination of phenolphthalein acidity.
If the solution is yellow proceed with the next step.
• Take the titration syringe and push the plunger
completely into the syringe. Insert tip into HI
3820-0 solution and pull the plunger out until
the lower edge of the plunger seal is on the
0 mL mark of the syringe.
• Place the syringe tip into the cap port of the
plastic vessel and slowly add the titration
solution drop-wise, swirling to mix after each
drop. Continue adding titration solution until
the solution in the plastic vessel changes from
yellow to green.
• Read off the milliliters of titration solution from the syringe
scale and multiply by 500 to obtain mg/L (ppm) CaCO3.
x 500 = mg/L CaCO
Determination of Phenolphthalein Acidity
• Remove the cap from the small plastic
vessel. Rinse the vessel with water
sample, fill to the 5 mL mark and
replace the cap.
• Through the cap port, add 1 drop of Phenolphtalein
indicator and mix. If the solution turns red or pink,
then the solution is alkaline and an alkalinity test must
be carried out (see Hanna Alkalinity Test Kit – HI
3811). If the solution remains colorless, proceed to next
step.
• Take the titration syringe and push the
plunger completely into the syringe. Insert tip
into HI 3820-0 solution and pull the plunger
out until the lower edge of the plunger seal is
on the 0 mL mark of the syringe.
• Place the syringe tip into the cap port of the
plastic vessel and slowly add the titration
solution dropwise, swirling to mix after each
drop. Continue adding titration solution until
the solution in the plastic vessel turns pink.
• Read off the milliliters of titration solution from the syringe
scale and multiply by 500 to obtain mg/L (ppm) CaCO3.
x 500 = mg/L CaCO
Low Range Determinations
If result is lower than 100 mg/L, the precision of the test
can be improved.
• Remove the cap from the large
plastic vessel. Rinse the vessel with
water sample, fill to the 25 mL
mark and replace the cap.
• Proceed with the test as explained for high range
measurements.
• To obtain the results for both methyl orange and
phenolphthalein acidity, multiply the read off the syringe by 100.
3
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
1987 Annual Book of ASTM Standard, Volume 11.01
Water (1), pages 151-158.
Official Methods of Analysis, A.O.A.C., 14thEdition, 1984,
page 618.
Standard Methods for the Examination of Water and Wastewater, 18thEdition, 1992, pages 2-23, 2-24.
ACCESSORIESACCESSORIES
ACCESSORIES
ACCESSORIESACCESSORIES
HI 3820-100 Spare reagents (100 tests)
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this test kit may be hazardous if
improperly handled. Read Health and Safety Data Sheets
before performing the test.
x 100 = mg/L CaCO
3
3
Page 2
234567890123456789012345678901212345678901234567890123456789012123456789012345678901234567890121234567890123456
7
7
7
7
7
7
234567890123456789012345678901212345678901234567890123456789012123456789012345678901234567890121234567890123456
234567890123456789012345678901212345678901234567890123456789012123456789012345678901234567890121234567890123456
234567890123456789012345678901212345678901234567890123456789012123456789012345678901234567890121234567890123456
234567890123456789012345678901212345678901234567890123456789012123456789012345678901234567890121234567890123456
234567890123456789012345678901212345678901234567890123456789012123456789012345678901234567890121234567890123456
HI 3820 ACIDITY TEST KIT
METHYL ORANGE ACIDITYPHENOLPHTHALEIN ACIDITY
11
1
11
11
1
11
DECHLORINATING
REAGENT
BROMOPHENOL BLUE
INDICATOR
22
2
22
33
3
33
22
2
22
44
4
44
33
3
33
55
5
55
5 mL sample
25 mL sample
44
4
44
PHENOLPHTALEIN
INDICATOR
5 mL sample
25 mL sample
 Loading...
Loading...