Page 1
HI 3812 HARDNESS TEST KIT
2 3 41
5 mL sample
x5
50 mL sample
x1
5
Page 2
Instruction Manual
HI 3812
Hardness Test Kit
SPECIFICATIONS
Range 0 to 30 mg/L (ppm) CaCO
0 to 300 mg/L (ppm) CaCO
Smallest Increment 0.3 mg/L [in the 0-30 mg/L range]
3 mg/L [in the 0-300 mg/L range]
Analysis Method EDTA titration
Sample Size 5 mL and 50 mL (average)
Number of Tests 100 (average)
Case Dimensions 200x120x60 mm (7.9x4.7x2.4")
Shipping Weight 460 g (1 lb.)
3
3
SIGNIFICANCE AND USE
INSTRUCTIONS
READ ALL THE INSTRUCTIONS BEFORE USING THE TEST KIT
LOOK AT THE BACK PAGE FOR THE ILLUSTRATED PROCEDURE
Note: push and twist pipet tip into tapered end of syringe
ensuring an air-tight fit.
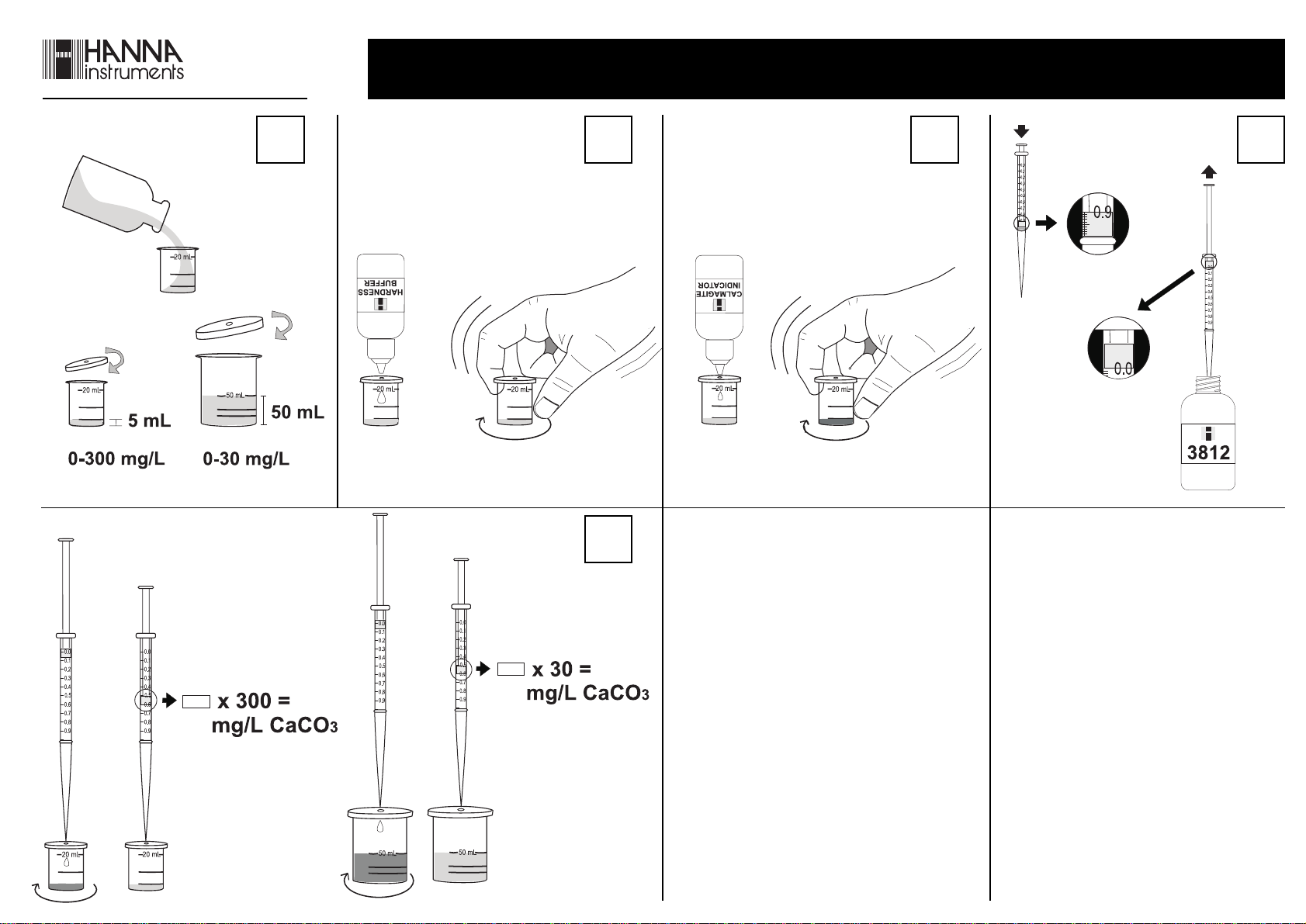
HIGH RANGE – 0 to 300 mg/L CaCO
• Remove the cap from the small plastic
vessel. Rinse the plastic vessel with
the water sample, fill to the 5 mL
mark and replace the cap.
• Add 5 drops of Hardness Buffer through the cap port
and mix carefully swirling the vessel in tight circles.
3
• Continue adding the titration solution
until the solution becomes purple, then
mix for 15 seconds after each additional
drop until the solution turns blue.
15"
• Read off the milliliters of titration solution from the syringe
scale and multiply by 300 to obtain mg/L (ppm) CaCO3.
x 300 = CaCO
3
SPECIFICATIONS
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instructions carefully before using the chemical
test kit. It will provide you with the necessary information for
correct use of the kit.
Remove the chemical test kit from the packing material and
examine it carefully to make sure that no damage has
occurred during shipping. If there is any noticeable damage,
notify your Dealer or the nearest Hanna office immediately.
Each kit is supplied with:
Hardness Buffer, 1 bottle with dropper (30 mL);
•
Calmagite Indicator, 1 bottle with dropper (10 mL);
•
HI 3812-0 EDTA Solution, 1 bottle (120 mL);
•
1 calibrated plastic vessel (20 mL) with cap;
•
1 calibrated plastic vessel (50 mL) with cap;
•
1 syringe (1 mL) with tip.
•
Note: any damaged or defective item must be returned in its
original packing materials.
In history, water hardness was defined by the capacity of
water to precipitate soap. The ionic species in the water
causing the precipitation was later found to be primarily
calcium and magnesium. In the present, therefore, water
hardness is actually a quantitative measure of these ions in
the water sample. It is also now known that certain other
ion species, such as iron, zinc and manganese, contribute to
the overall water hardness. The measure and subsequent
control of water hardness is essential to prevent scaling and
clogging in water pipes. The Hanna Hardness Test Kit makes
monitoring easy, quick and safe. The compact size provides
the versatility to use the kit anywhere. The design of the kit
makes it easy to handle.
CHEMICAL REACTION
The hardness level as mg/L (ppm) calcium carbonate is determined by an EDTA (ethylene-diamine-tetraacetic acid) titration.
The solution is first adjusted to a pH of 10 using a buffer
solution. The indicator chelates with metal ions such as magnesium or calcium to form a red colored complex. As EDTA is
added, metal ions complex with it. After all the free metal ions
have been complexed, an excess EDTA removes the metal ions
complexed with the indicator to form a blue colored solution.
This color change from red to blue is the endpoint of the
titration.
ISTR3812R3 03/02
x 5
• Add 1 drop of Calmagite Indicator through the cap port
and mix as described above. The solution becomes a redviolet color.
x 1
• Take the titration syringe and
push the plunger completely into
the syringe. Insert tip into HI
3812-0 EDTA Solution and pull
the plunger out until the lower
edge of the seal is on the 0 mL
mark of the syringe.
• Place the syringe tip into the
cap port of the plastic vessel
and slowly add the titration
solution dropwise, swirling to
mix after each drop.
LOW RANGE – 0 to 30 mg/L CaCO
If result is lower than 30 mg/L, the precision of the test can
be improved by following the procedure below.
• Remove the cap from the large
plastic vessel. Rinse it with the
water sample, fill to the 50 mL
mark and replace the cap.
• Proceed with the titration as for the high range test.
• Read off the milliliters of titration solution from the syringe
scale and multiply by 30 to obtain mg/L (ppm) CaCO3.
3
x 30 = CaCO
REFERENCES
Standard Methods for the Examination of Water and
Wastewater.
Annual Book of ASTM Standard, vol. 11.01, Water (I).
HEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read Health and Safety Data Sheet
before performing this test.
3
 Loading...
Loading...