Page 1
Instruction Manual
HI 38086
Calcium
Test Kit for
Irrigation Water
www.hannainst.com
Dear Customer,
Thank you for choosing a Hanna Product.
Please read the instruction sheet carefully before using the
test kit. It will provide you with the necessary information
for correct use of the kit. If you need additional information,
do not hesitate to e-mail us at tech@hannainst.com.
Remove the test kit from the packing material and examine
it carefully to make sure that no damage has occurred
during shipping. If there is any noticeable damage, notify
your Dealer or the nearest Hanna office immediately.
Each kit is supplied with:
Buffer Reagent, 1 bottle with dropper (30 mL);
•
Oxalate Reagent, packet powder (100 pcs);
•
• Deionized Water, 1 bottle (500 mL)
• 1 long glass test tube (50 mL);
•1 calibrated plastic vessel (50 mL);
•1 long plastic pipette (1 mL);
•1 plastic spoon;
1 graduated card;
•
• 1 line card.
Note: Any damaged or defective item must be returned in
its original packing materials.
;
SPECIFICATIONSSPECIFICATIONS
SPECIFICATIONS
SPECIFICATIONSSPECIFICATIONS
Range 0 to 125 mg/L (ppm) as Ca
0 to 250 mg/L (ppm) as Ca
Smallest Increment 1 mg/L [in the 0-125 mg/L range]
2 mg/L [in the 0-250 mg/L range]
Analysis Method Turbidimetric
Sample Size 50 mL or 25 mL
Number of Tests 100
Case Dimensions 235x175x115 mm (9.2x6.9x4.5")
Shipping Weight 950 g (33.5 oz.)
SIGNIFICANCE AND USESIGNIFICANCE AND USE
SIGNIFICANCE AND USE
SIGNIFICANCE AND USESIGNIFICANCE AND USE
Calcium presence in water supplies results from passage over
deposits of limestone, dolomite, gypsum and gypsiferous
shale. Its concentration may extend from 0 to several
hundred milligrams per liter, depending on its source and
treatment. Calcium is necessary in plant and animal nutrition since it is an essential constituent of bones, shells and
plant structures.
Calcium in water as carbonate is one of the primary
components of water hardness which can cause pipe or tube
scaling.
Note: mg/L is equivalent to ppm (parts per million).
CHEMICAL REACTIONCHEMICAL REACTION
CHEMICAL REACTION
CHEMICAL REACTIONCHEMICAL REACTION
The Hanna Test Kit determines Calcium in irrigation water
via a turbidimetric method. Hanna reagents react selectively with calcium to form a white suspension. The developed
turbidity is proportional to calcium concentration.
INSTRUCTIONSINSTRUCTIONS
INSTRUCTIONS
INSTRUCTIONSINSTRUCTIONS
READ THE ENTIRE INSTRUCTIONS BEFORE USING THE KIT
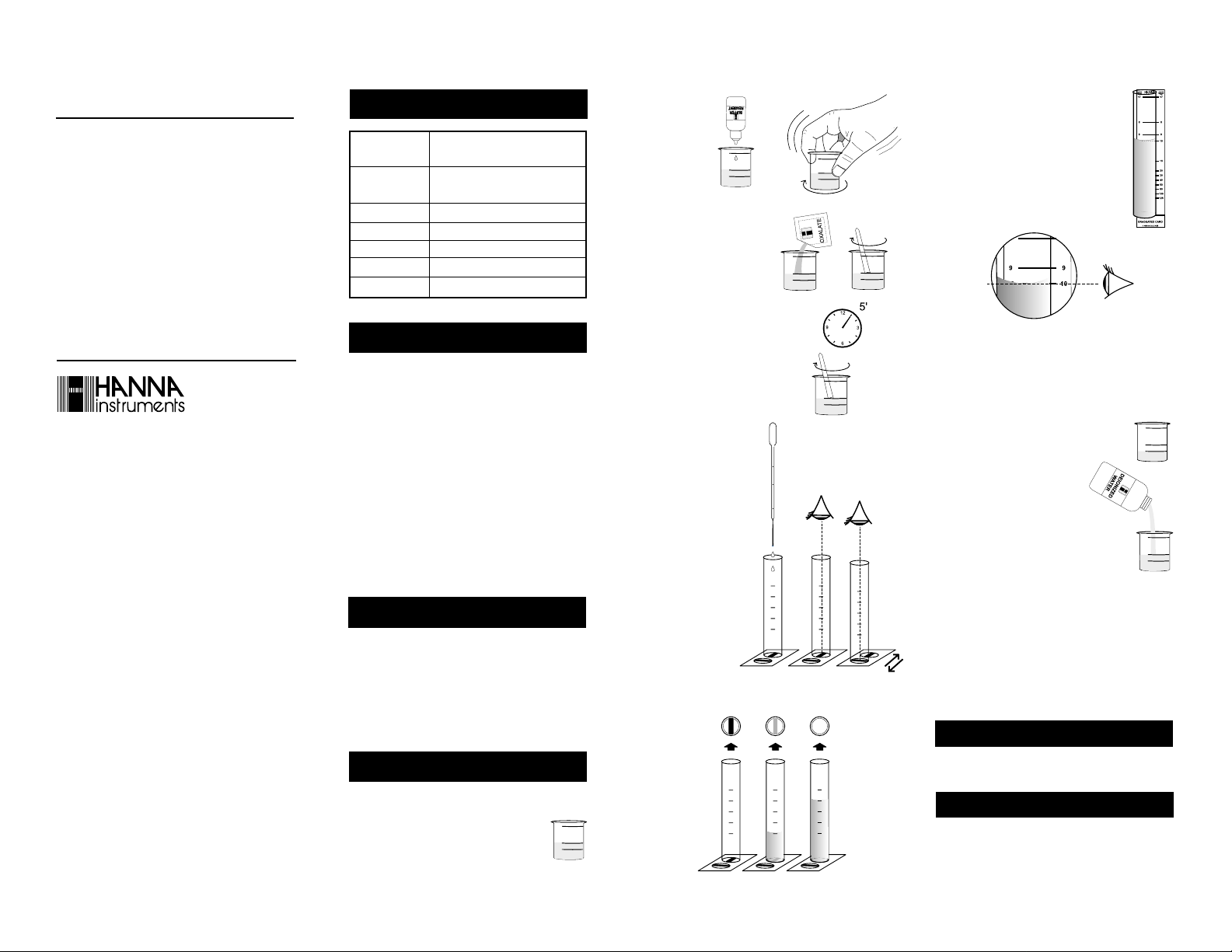
1- Fill the plastic vessel with 50 mL
ISTR38086 02/00 PRINTED IN ITALY
of water sample (up to the mark).
2- Add 5 drops
of Buffer Reagent and
swirl to mix.
3- Add 1 packet of Oxalate
Reagent and mix for 30
seconds with the plastic
spoon. Some deposits may
remain, but they do not
affect the measurement.
4- Wait for 5 minutes to allow reaction
to complete. If Calcium is present,
the solution will become turbid.
5- Using the spoon, swirl gently the
reacted sample.
6- Place the glass test
tube on the Line
card and look from
the top of the tube
at one of the
black stripes on
the Line card. Use
the pipette to fill
the tube with the
reacted sample
until the black
stripe completely
disappears. To
help in detecting
the endpoint,
move the test tube from one black stripe to the other
until they are both no longer visible.
x5
7- Hold the tube close to the Graduated
card as shown in the figure.
8- Read directly from the Graduated card
the concentration in mg/L (ppm) of calcium that corresponds to the level of the
liquid in the test tube.
9- In case the black stripe on the Line card disappears
with the liquid level under the 125 ppm mark, the
calcium concentration is higher than 125 ppm and the
original sample needs to be diluted. In this case perform
the test as follows.
10- Fill the plastic vessel with water
sample up to the 25 mL mark.
11- Add Deionized Water up to the
50 mL mark.
12-Follow the instructions from step
2 to step 7.
13- Read from the Graduated card the value in correspon-
dence of the level of the liquid in the test tube and
multiply it by 2 to obtain the concentration in mg/L
(ppm) of calcium.
14- To convert the reading in mg/L of CaCO
mg/L of calcium by 2.5.
15- Rinse all labware with demineralized water after each
analysis and shake dry.
REFERENCESREFERENCES
REFERENCES
REFERENCESREFERENCES
Vogel's, Textbook of Quantitative Chemical Analysis, 5th Ed.,
Longman Scientific & Technical.
HEALTH AND SAFETYHEALTH AND SAFETY
HEALTH AND SAFETY
HEALTH AND SAFETYHEALTH AND SAFETY
The chemicals contained in this kit may be hazardous if
improperly handled. Read the relevant Health and Safety
Data Sheet before performing this test.
25 mL
, multiply the
3
 Loading...
Loading...