Page 1
DOC022.53.90072
BODTrak™ II
USER MANUAL
02/2010,
Edition 2
© Hach Company, 2008, 2010. All rights reserved. Printed in China.
Page 2

Page 3

Table of contents
Section 1 Specifications .................................................................................. 5
Section 2 General information ....................................................................... 7
2.1 Safety information .......................................................................................7
2.1.1 Use of hazard information ................................................................ 7
2.1.2 Precautionary labels ......................................................................... 7
2.2 Theory of operation ..................................................................................... 8
2.2.1 Oxygen transfer to sample ............................................................... 8
2.2.2 Pressure sensor function .................................................................. 9
2.2.3 Removing carbon dioxide ................................................................. 9
Section 3 Installation ...................................................................................... 11
3.1 Component list .......................................................................................... 11
3.2 Electrical installation ................................................................................. 12
Section 4 Operation ........................................................................................13
4.1 Operational controls .................................................................................. 13
4.1.1 Channel selection keys ................................................................... 14
4.1.2 The arrow keys ...............................................................................14
4.1.3 The ON key .................................................................................... 14
4.1.4 The OFF key ................................................................................... 14
4.2 Bottle connections .................................................................................... 14
4.3 Setting the clock ....................................................................................... 15
4.4 RS232 Interface ........................................................................................ 15
4.5 Downloading test results ........................................................................... 16
4.5.1 Import data ..................................................................................... 16
4.5.2 Data format ..................................................................................... 17
4.5.3 Printing test results ......................................................................... 18
Section 5 BODTrak™ II procedures .......................................................... 19
5.1 General Information .................................................................................. 19
5.2 Simplified procedure .................................................................................20
5.3 Hach GGA (glucose/glutamic acid) procedure ..........................................22
5.4 Hach Standard Method procedure ............................................................ 24
5.5 Completion steps for all procedures ......................................................... 27
5.5.1 Determination of results .................................................................. 30
5.6 Typical curves ........................................................................................... 32
5.7 Special considerations .............................................................................. 33
5.7.1 Sample dilution ...............................................................................33
5.7.2 Sample seeding .............................................................................. 33
5.7.3 Sample temperature ....................................................................... 33
5.7.4 Toxic materials ...............................................................................33
5.7.5 Chlorine .......................................................................................... 34
5.7.6 pH effect .........................................................................................34
5.7.7 Supersaturation .............................................................................. 34
Section 6 Maintenance .................................................................................. 35
6.1 Cleaning the instrument ............................................................................ 35
6.1.1 Sample bottles ................................................................................ 35
3
Page 4

Table of contents
6.1.2 Stir bars and seal cups ....................................................................35
6.1.3 Bottle fences ...................................................................................35
Section 7 Troubleshooting ............................................................................37
7.1 High oxygen demand .................................................................................38
7.2 Nitrification .................................................................................................38
7.3 Excessive time lag .....................................................................................38
7.4 Sample temperature ..................................................................................38
7.5 Bottle leak ..................................................................................................39
Section 8 Replacement parts and accessories ......................................41
8.1 Replacement parts ....................................................................................41
8.2 Reagents ...................................................................................................41
8.3 Optional reagents ......................................................................................41
8.4 Accessories ...............................................................................................42
Section 9 Contact Information ......................................................................45
4
Page 5

Section 1 Specifications
Specifications are subject to change without notice.
Table 1 Specifications
General
Range Selectable, 0 to 35, 0 to 70, 0 to 350, 0 to 700 mg/L
3
Dimensions 28.9 x 26 x 9.8 cm (11
External power supply
Capacity Six 492 mL bottles
Shipping weight 4 kg (8.8 lb)
Operating temperature 20 ºC (68 ºF)
Storage temperature 0 to 40 ºC (104 ºF)
Input: 110 to 240 V, 50/60 Hz, Output: 24 V, UL CSA, and
TUV approved
Method performance specifications
On a standard containing 150 mg/L each of glucose and
glutamic acid, a single analyst using 6 BODTrak™ II
Precision
Drift Less than 3 mg/L BOD in 5 days
Resolution 1 mg/L BOD
instruments and testing 44 samples obtained a mean of
235 mg/L BOD with a 95% confidence limit of distribution
of 11 mg/L or a range of
224 to 246 mg/L BOD.
/8 x 10 ¼ x 3 7/8 inches)
Table 2 Certification
Certification
Hach Company certifies this instrument was tested thoroughly, inspected and found to meet
its published specifications when it was shipped from the factory. The BODTrak II has been
tested and is certified as indicated to the following instrumentation standards:
FCC Part 15, Sub-Part B, Class A Limits: Supporting test records by Intellistor, certified
compliance by Hach Company
Canadian Interference-Causing Equipment Regulation, ICES-003, Class A: Supporting
test records by Intellistor, certified compliance by Hach Company
EN 55011/CISPR 11(EMI) “B” Limits per 89/336/EEC EMC: Supporting test records by
Intellistor, certified compliance by Hach Company
EN 50082-1 (Immunity) per 89/336/EEC EMC: Supporting test records by Hach Company,
certified compliance by Hach Company. Standards include:
• IEC 801-2 and EN 61000-4-2 (ESD)
• IEC 801-3 and EN V50140 (RF & EM Field)
• IEC 801-4 and EN 61000-4-4 (Fast Transient)
• EN 61000-4-5 (Surge)
Warranty: US 1 year; EU 2 year
5
Page 6

Specifications
Table 2 Certification (continued)
Radio frequency interference
This Class A digital apparatus meets all requirements of the Canadian Interference-Causing
Equipment Regulations. This device complies with Part 15 of the FCC Rules. Operation is
subject to the following two conditions:
(1) This device may not cause harmful interference, and (2) this device must accept any
interference received, including interference that may cause undesired operation.
Warning
Changes or modifications to this unit not expressly approved by the party
responsible for compliance could void the user’s authority to operate the equipment.
This equipment has been tested and found to comply with the limits for a Class A digital
device, pursuant to Part 15 of the FCC Rules. These limits are designed to provide
reasonable protection against harmful interference when the equipment is operated in a
commercial environment. This equipment generates, uses and can radiate radio frequency
energy and, if not installed and used in accordance with the instruction manual, may cause
harmful interference to radio communications. Operation of this equipment in a residential
area is likely to cause harmful interference, in which case the user will be required to correct
the interference at their own expense. Shielded cables must be used with this unit to ensure
compliance with the Class A FCC limits. Because this instrument operates on and
generates radio frequency energy, interference to radio and television reception may occur.
If such interference does occur, the operator should take the necessary steps to correct the
interference. The following techniques of reducing the interference problems are easily
applied:
• Disconnect power from the BODTrak II instrument to verify the instrument is
the source of the interference.
• If the BODTrak II is plugged into the same outlet as the device with which it is
interfering, try another outlet.
• Move the BODTrak II away from the device receiving the interference.
• Reposition the receiving antenna for the device receiving the interference.
• Try combinations of the above.
6
Page 7
Section 2 General information
2.1 Safety information
Please read this entire manual before unpacking, setting up or operating this
equipment. Pay attention to all danger and caution statements. Failure to do so
could result in serious injury to the operator or damage to the equipment.
Make sure that the protection provided by this equipment is not impaired, do not
use or install this equipment in any manner other than that specified in this manual.
2.1.1 Use of hazard information
DANGER
Indicates a potentially or imminently hazardous situation which, if not
avoided, will result in death or serious injury.
WARNING
Indicates a potentially or imminently hazardous situation which, if not
avoided, could result in death or serious injury.
CAUTION
Indicates a potentially hazardous situation that may result in minor or
moderate injury.
Important Note: Indicates a situation which, if not avoided, may cause damage to
the instrument. Information that requires special emphasis.
Note: Information that supplements points in the main text.
2.1.2 Precautionary labels
Read all labels and tags attached to the instrument. Personal injury or damage to
the instrument could occur if not observed. A symbol, if noted on the instrument, will
be included with a danger or caution statement in the manual.
This symbol, if noted on the instrument, references the instruction manual for
operation and/or safety information.
Electrical equipment marked with this symbol may not be disposed of in
European public disposal systems after 12 August of 2005. In conformity with
European local and national regulations (EU Directive 2002/96/EC), European
electrical equipment users must now return old or end-of life equipment to the
Producer for disposal at no charge to the user.
Note: For return for recycling, please contact the equipment producer or supplier
for instructions on how to return end-of-life equipment, producer-supplied
electrical accessories, and all auxiliary items for proper disposal.
7
Page 8

General information
2.2 Theory of operation
Respirometric Biochemical Oxygen Demand (BOD) is a test done at 20 °C (68 °F)
in a controlled environment. The test period can be 5, 7 or 10 days, contingent on
the analysis or protocol. The BOD test measures the quantity of oxygen consumed
by bacteria that oxidize organic matter in a water sample. The test is used to
measure waste loadings at wastewater treatment plants and to examine the
efficiency of wastewater treatment.
BOD test results help find general oxygen uptake patterns. This lets operators
estimate plant operating efficiency and find correct treatment procedures.
Advantages to the BODTrak™ II as an alternative to the dilution method are:
• Minimal time to prepare a sample.
• Decreased total test time.
• The BODTrak II method gives results comparable to the diluton method (BOD5)
in 2 to 3 days.
• Calibration and dissolved oxygen measurement are not necessary.
• The BODTrak II test is easy to monitor.
• The sample is stirred constantly and kept in natural conditions. This makes the
BODTrak II results similar to occurrences found in a natural environment. The
dilution method supplies no additional oxygen to the sample. This causes a
higher percentage of oxygen depletion and possible retardation of biochemical
reactions.
• The BOD can be monitored at any time because the instrument continuously
shows the BOD result. Pressure changes in the closed BODTrak II system are
shown graphically in milligrams per liter (mg/L) on an LCD. The system supplies
360 uniform data points over the selected time period.
• The BODTrak II system continuously removes carbon dioxide from the system
so that the pressure difference monitored is proportional to the quantity of
oxygen used.
• Degassing can cause negative errors when heat is applied to a sample to
achieve experimental temperature. The BODTrak II adjusts for this occurrence.
The BODTrak II does not start the test until the temperature gets to equilibrium.
2.2.1 Oxygen transfer to sample
Bacteria in the sample use oxygen while consuming organic matter in the sample
bottles. The air in the bottle above the sample contains 21% oxygen and
replenishes the dissolved oxygen used by the bacteria. During the test period, stir
bars continually mix the sample in each bottle. This moves oxygen from the air to
the sample and helps simulate natural conditions.
8
Page 9

General information
2.2.2 Pressure sensor function
The BODTrak II is sealed to prevent external atmospheric pressure changes in the
test bottle. Pressure sensors monitor air pressure in the sample bottles. When
oxygen is consumed, the pressure in the bottle head space drops. The pressure
drop correlates directly to BOD.
2.2.3 Removing carbon dioxide
Carbon dioxide is made when microorganisms oxidize organic matter in the
sample. The carbon dioxide must be removed from the system so it does not
interfere with the measurement. Potassium hydroxide pellets put in the seal cup of
each sample bottle before the test remove the carbon dioxide.
9
Page 10

10
Page 11

Section 3 Installation
3.1 Component list
Compare each item below to the items in the shipment. If an item is missing or
damaged, refer to the manufacturer.
• BODTrak™ II instrument
• A UL/CSA approved 115 VAC power cord with a NEMA 5-15P style plug
• A 230 VAC harmonized power cord with a continental European plug
• Power supply, auto–switching between 115 V and 230 V
• 6 seal cups
• 6 BODTrak II amber sample bottles
• 6 BODTrak II magnetic stir bars
• Spatula scoop
• One package nutrient buffer solution pillows
• One container potassium hydroxide pellets
11
Page 12
Installation
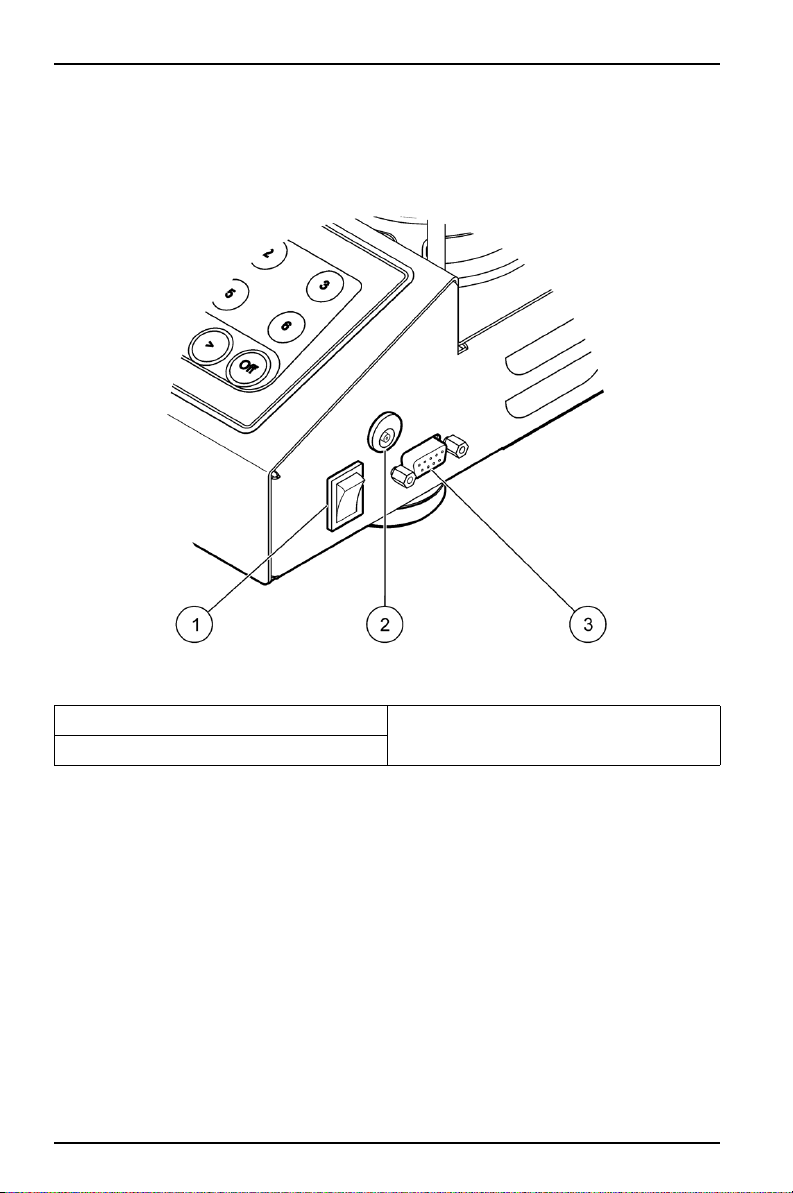
3.2 Electrical installation
The power adaptor supplies AC power to the IEC universal connector (Figure 1).
The power switch powers the instrument on and off.
Figure 1 External connections
1 Power switch 3 RS232 connector
2 IEC universal connector
12
Page 13
Section 4 Operation
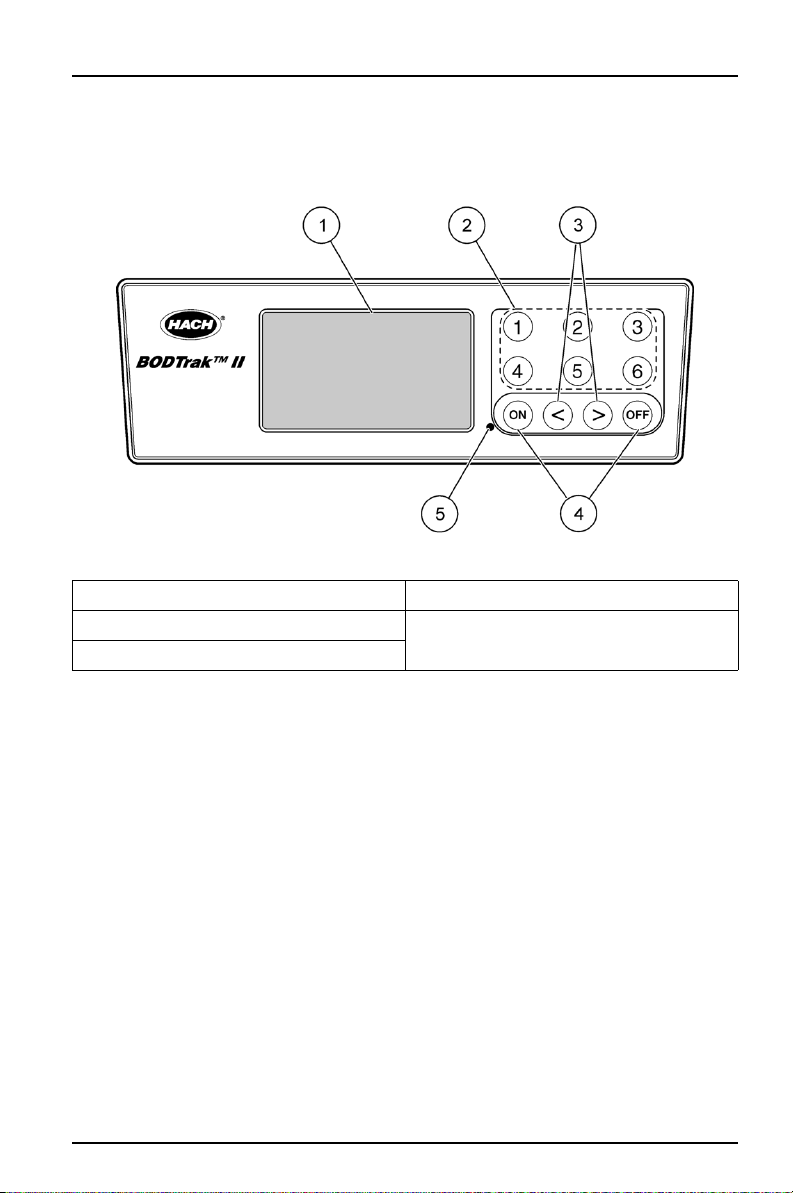
4.1 Operational controls
The BODTrak™ II operator controls are shown in Figure 2.
Figure 2 Operational controls
1 Display 4 ON/OFF keys
2 Channel selection keys 5 Power indicator
3 Arrow keys
1
The ON/OFF keys start and stop a test. They do not power the instrument on and off.
1
13
Page 14
Operation
4.1.1 Channel selection keys
Push the related channel selection key to show data for one of the 6 bottles.
The channel selection keys are also used in the instrument setup menu to choose a
parameter to be edited (Table 3).
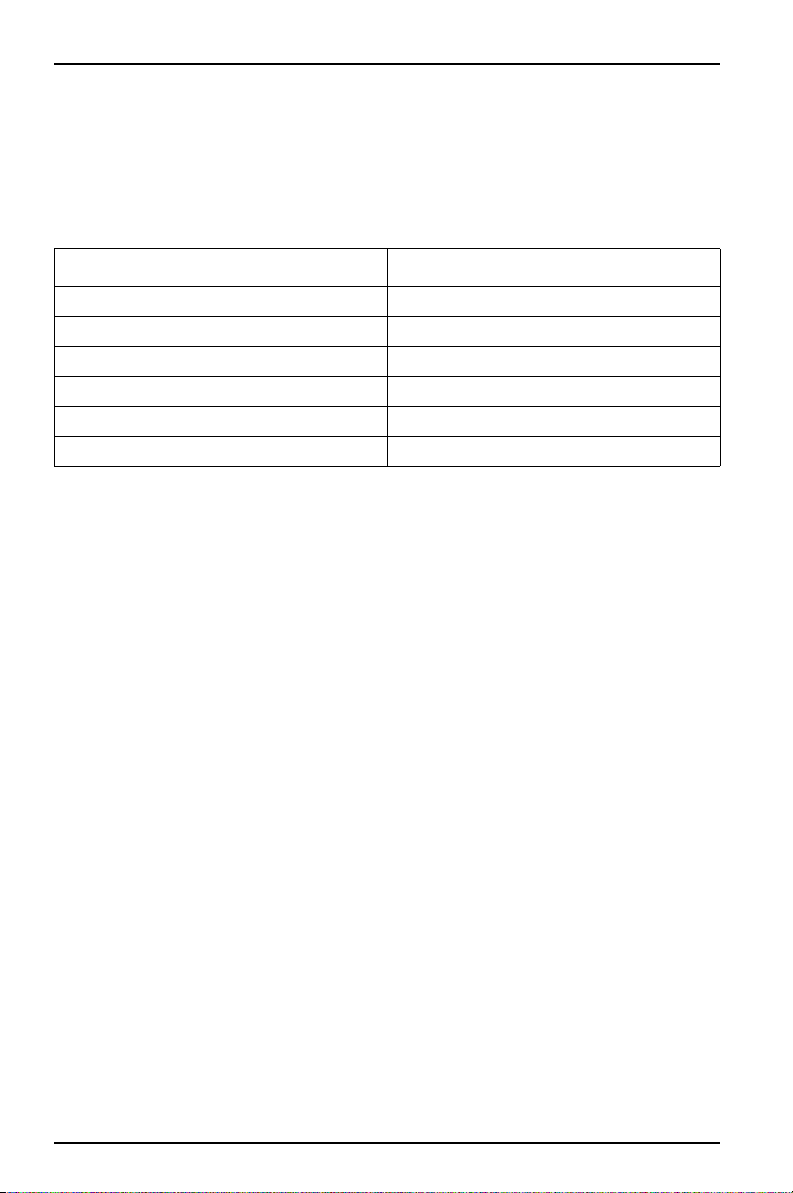
Table 3 Channel key setup parameters
Channel Parameter
1 Year (0-99)
2 Month (1-12)
3 Day (1-31)
4 Hour (0-24)
5 Minute (0-59)
6 Test Length (5, 7, or 10 days)
4.1.2 The arrow keys
The display shows a graph of BOD values on the vertical axis and time in days on
the horizontal axis. Push the left and right arrow keys to move the cursor along the
BOD curve to show the approximate coordinates (time, BOD) of the selected data
point.
The time interval and BOD value of the data point are shown in the lower right of
the display. The cursor is automatically placed at the most recently collected data
point in a channel display.
Push and hold the two arrow keys at the same time to go into the instrument setup
menu. The arrow keys are also used to change the time, date, test length and
range.
4.1.3 The ON key
To access the range selection menu, push the ON key from a channel display
screen. Then push and hold the ON key to start the test for the selected channel.
4.1.4 The OFF key
When a test is in DELAY or RUN modes, pushing and holding the OFF key
manually ends the test. The instrument will display END. The OFF key is also used
to exit the instrument setup menu or range selection menu. Any changes made
prior to exit will be saved.
4.2 Bottle connections
Each bottle position/channel has the applicable tube numbered with a plastic
sleeve. The bottle positions are numbered 1 though 6 with number 1 in the back left
corner of the chassis. Use the channel selection keys as a guide.
14
Page 15

Operation
4.3 Setting the clock
All channels must show END or CLEAR before the clock can be set. Push and hold
the two arrow keys at the same time until the instrument setup menu is shown.
Select the clock parameter to be adjusted by pushing the applicable channel key
(Table 3 on page 14). Use the arrow keys to edit the chosen parameter. Adjust
each parameter in the same manner. When all time adjustments are complete,
push the OFF key to save and go back to the data display screen.
4.4 RS232 Interface
All RS232 connections are made through the Serial I/O port (Figure 1 on page 12).
Connect the 9-pin D connector of a computer interface cable to the Serial I/O port
on the instrument. Connect the opposite end of the cable to the computer Serial I/O
port (Com 1 or Com 2).
The BODTrak II instrument is equipped as Data Communication Equipment (DCE).
The BODTrak II operates at 9600 baud with 8 data bits, no parity and one stop bit.
The computer or printer will not receive complete transmissions if the device cannot
continuously receive at 9600 baud.
Note: Use of the specified cable or an equivalent shielded cable is mandatory to meet Radio
Frequency Emissions requirements.
15
Page 16

Operation
4.5 Downloading test results
To transfer test results to a PC:
1. Choose
2. In the Connection Description window, type in a name for the connection and
choose an icon to represent it. Click
3. In the Connect To window, use the drop-down menu to choose the COM port
connected to the BODTrak II instrument. Click
4. Configure the COM port properties:
BPS = 9600, Data Bits = 8, Parity = None, Stop Bits = 1,
Flow Control = None.
5. Click
6. Choose
7. In the Capture Text window, click
Name the file and click SAVE.
8. In the Capture Text window click START.
9. Power on the BODTrak II. Push the applicable channel key for the data to be
downloaded.
10. Type GA in the HyperTerminal window, then push
complete when the screen stops adding new data.
11. Choose
12. Choose
13. To end the HyperTerminal session, choose FILE, EXIT.
14. Click
PROGRAMS, ACCESSORIES, COMMUNICATIONS, HYPERTERMINAL.
OK.
OK.
OK. The connect indicator will be shown.
TRANSFER, CAPTURE TEXT.
BROWSE to choose a specific save location.
ENTER. The transfer is
TRANSFER, CAPTURE TEXT, STOP.
CALL, DISCONNECT. The disconnected indicator will be shown.
YES to save the session and all instrument/port configuration settings.
4.5.1 Import data
To import the data from the captured text file:
1. Open a new or existing spreadsheet. Choose DATA, IMPORT EXTERNAL DATA,
IMPORT DATA.
2. Select the text file captured in HyperTerminal. Click
3. In the Text Import Wizard, choose Delimited as the file type, the start row in
the spreadsheet, and Windows (ANSI) as the file origin. Click NEXT.
4. Check the Space delimiter and Treat consecutive delimiters as one check
boxes. Click
NEXT.
5. Choose General as the Column data format then click
6. In the Import Data window, choose Existing worksheet. Choose the starting cell
then click
OK. The data will appear in your spreadsheet.
7. Choose File, Save As to save the spreadsheet.
The spreadsheet data cannot be edited or formatted in HyperTerminal or with the
BODTrak II.
16
IMPORT.
FINISH.
Page 17

Operation
4.5.2 Data format
When a result array is downloaded to HyperTerminal, all data from the test are sent
without pause. The data flow cannot be stopped or paused.
Figure 3 shows channel number, start date, start time, and the format of the
downloaded data. BOD values in mg/L follow. Only the first data points, of a
maximum of 360 equal distance points, are shown in this example. Each line ends
with a carriage return and a line feed. The end of the data stream is shown by a
message such as “Test Run to Completion” and a dollar symbol ($).
If small negative BOD values are seen at the start of a test, refer to Troubleshooting
on page 37.
BOD Log for Ch 1
Status: END
Full Scale: 700 mg/L
Tst length: 7 days
Start Date: 3/3/08
Time: 13:04
Days, Reading (mg/L)
0.00, 0
0.05, 10
0.11, 12
0.16, 12
0.22, 14
0.27, 14
0.33, 12
0.38, 8
0.44, 10
0.50, 12
0.55, 12
0.61, 14
-
-
Test Run to Completion
$
Figure 3 Downloaded test data
17
Page 18

Operation
4.5.3 Printing test results
The BODTrak II is compatible with the Citizen PD-24 printer, which is available as
an optional accessory (Section 8 on page 41). Connect the printer cable to the
serial port on the BODTrak II using the gender adapter provided with the printer.
Make sure the printer interface settings are correct (section 4.4 on page 15).
Power on the BODTrak II instrument. Push and hold the applicable channel number
for approximately 5 seconds at any time during a test. This moves the test results
from the BODTrak II to the printer. The instrument will send a copy of the graphical
display and a truncated data stream (127 data points).
18
Page 19

Section 5 BODTrak™ II procedures
5.1 General Information
There are three BODTrak II procedure variations. Choose the procedure that meets
the application requirements.
The Simplified procedure (section 5.2 on page 20) is recommended when sample
seeding, extra nutrients or buffers are not necessary. It is also recommended when
accuracy requirements are not stringent.
The Hach GGA (glucose/glutamic acid) procedure (section 5.3 on page 22) is
recommended for all accuracy and performance checks using seeded GGA. It is
also recommended when test accuracy is important.
The Hach Standard Method procedure (section 5.4 on page 24) is recommended
when samples are seeded or extra nutrients or reagents are added. Use this
procedure when following Standard Methods for the Examination of Water and
Wastewater, 21st Edition, Method 5210 D Respirometric Method.
All procedure variations are followed by completion steps for all procedures
(section 5.5 on page 27). It is possible to use a combination of these procedures
with one instrument, but in different bottles. Only one test length can be chosen.
Before starting the test:
Use the applicable sample volume tables for each procedure.
If power is interrupted when the instrument is in DELAY status, the test will stop and the
status will change to CLEAR when power returns. Start the test again.
If power is interrupted when the instrument is in RUN status, the test will resume when
power returns.
Keep deionized water overnight in an incubator at 20 ºC. Shake the deionized water to
saturate with air.
Settle the seed overnight in the BOD incubator at a temperature of 20 ºC. Be careful not to
disturb the settled solution. Pipet seed solution from the top.
Dilution is necessary if samples have BOD values more than 700 mg/L (5.7 on page 33).
At elevations higher than 5000 feet above sea level the 0 to 35 mg/L BOD range is
decreased to 0 to 25 mg/L BOD. Adjustment is not necessary for other test ranges.
Refer to section 5.7 on page 33 for special considerations including sample seeding and
pretreatment.
Use only BODTrak II stir bars and bottles. They are designed specifically for use with the
BODTrak II.
19
Page 20

BODTrak™ II procedures
5.2 Simplified procedure
Required apparatus:
BODTrak II bottle
Thermometer
Blender (optional)
Graduated cylinder
Required reagents:
1 nutrient buffer pillow
Table 4 Simplified sample volumes
BOD range mg/L Sample volume mL
0 to 35 420
0 to 70 355
0 to 350 160
0 to 700 95
1. Heat or cool the
sample to 19 to 21 ºC
(66 F to 70 ºF).
20
2. Homogenize the
sample in a blender if it
contains large settleable
or floatable solids.
3. Choose the correct
sample size for the
sample range (Table 4).
Measure the sample into a
graduated cylinder.
Page 21

BODTrak™ II procedures
4. Add the contents of 1
nutrient buffer pillow to the
graduated cylinder.
5. Transfer the contents of
the graduated cylinder to
a BODTrak II bottle.
Repeat steps 1 to 5 for
additional samples.
6. Continue to the
completion steps for all
procedures (section 5.5
on page 27).
21
Page 22

BODTrak™ II procedures
5.3 Hach GGA (glucose/glutamic acid) procedure
Required apparatus:
BODTrak II bottle
Graduated cylinder
Volumetric pipet and pipet filler
Tensette® pipet and pipet tips
Wash water bottle
Ampule breaker
Required reagents:
Deionized water
Hach GGA solution
1 nutrient buffer pillow
Before starting the test:
Use Hach BOD Standard Solution Ampules for Manometric Method (3000 mg/L Glucose,
3000 mg/L Glutamic acid).
On a standard containing 150 mg/L each of glucose and glutamic acid, a single analyst
using 6 BODTrak II instruments and testing 44 samples obtained a mean of 235 mg/L BOD
with a 95% Confidence Limit of Distribution of 11 mg/L or a range of 224 to 246 mg/L BOD
after 5 days.
Always prepare the seed blank before the GGA samples. Use the same amount of seed for
all GGA samples and seed blank.
Refer to section 5.7 on page 33 for special considerations.
Prepare seed blank
Use steps 1, 3 to 7.
Prepare sample
Use steps 1 to 7.
22
Page 23

BODTrak™ II procedures
Table 5 GGA sample volumes
BOD range
(mg/L)
0 to 350 8.0 10 to 35 160
Note: If seed strength is unknown, use 20 mL. Adjust seed volume as necessary to achieve
optimum GGA results. Use the same amount of seed for all GGA samples and seed blank.
GGA volume
(mL)
Seed volume
(mL)
Final Volume
(mL)
1. Add approximately
30 mL of deionized water
to a 200 mL graduated
cylinder.
4. Use a tensette pipet to
add the correct quantity of
seed to the graduated
cylinder (Table 5).
7. Continue to the
completion steps for all
procedures (section 5.5
on page 27).
2. Use a volumetric pipet
to transfer 8.0 mL of Hach
GGA solution to the
graduated cylinder.
Note: Skip this step when
preparing the seed blank.
5. Dilute to the sample to
160 mL using a deionized
water wash bottle.
3. Add the contents of 1
nutrient buffer pillow to the
graduated cylinder.
6. Transfer the prepared
sample from the
graduated cylinder to a
BODTrak II bottle.
Note: For additional GGA
samples, repeat steps 1 to 6.
23
Page 24

BODTrak™ II procedures
5.4 Hach Standard Method procedure
Required apparatus:
Thermometer
BODTrak II bottle
Blender (optional)
Graduated cylinder
Tensette pipet and pipet tips
Wash water bottle
Required reagents:
1 nutrient buffer pillow
Additional nutrient or buffer (optional)
Deionized water
Before starting the test:
Use the sample volume table to choose the correct sample size (Table 6).
If seeding samples, prepare a seed blank before preparing a sample. Treat the seed blank
the same as any other sample and omit step 5.
Refer to section 5.7 on page 33 for special considerations.
Table 6 Hach Standard Method sample volumes
BOD range
(mg/L)
Sample
volume (mL)
Seed volume
(mL)
Final volume
(mL)
Dilution
factor
0 to 35 370 10 to 35 420 1.14
0 to 70 305 10 to 35 355 1.16
0 to 350 110 10 to 35 160 1.45
0 to 700 45 10 to 35 95 2.11
Note: If seed strength is unknown, use 20 mL. Adjust seed volume as necessary to achieve
optimum results.
24
Page 25

BODTrak™ II procedures
1. Heat or cool the
sample to 19 to 21 ºC
(66 to 70 ºF).
4. Add the contents of 1
nutrient buffer pillow to the
graduated cylinder.
2. Homogenize the
sample in a blender if it
contains large settleable
or floatable solids.
5. If seeding the sample,
use a tensette pipet to add
the correct quantity of
seed to the graduated
cylinder (Table 6 on page
24).
3. Choose the correct
sample size for the
sample range (Table 6 on
page 24). Measure the
sample into a graduated
cylinder.
6. If necessary, add more
nutrient or buffer. Do not
add more than a total
volume of 50 mL (seed,
nutrient, buffer).
25
Page 26

BODTrak™ II procedures
7. Fill to the final test
range volume, if
necessary, with a
deionized wash water
bottle (Table 6 on page
24).
8. Transfer the prepared
sample from the
graduated cylinder to a
BODTrak II bottle.
Note: Repeat steps 1 to 8 for
additional samples.
9. Continue to the
completion steps for all
procedures (section 5.5
on page 27).
26
Page 27

BODTrak™ II procedures
5.5 Completion steps for all procedures
Required apparatus:
BODTrak II
Spatula scoop
BOD incubator
Seal cup
Stir bar
Required reagents:
2 potassium hydroxide pellets
1. Put a BODTrak II stir
bar into the bottle.
2. Put a seal cup into the
neck of the bottle.
3. Use a spatula scoop
to add 2 potassium
hydroxide pellets to the
seal cup. Repeat steps
1 to 3 for each sample
bottle.
27
Page 28

BODTrak™ II procedures
4. Put the bottles on the
BODTrak II chassis.
Connect the applicable
tube to the sample bottle
and tighten the cap.
7. Push and hold the left
and right arrow keys at
the same time to access
the instrument setup
menu.
Note: Set the time and date,
if necessary (section 4.3 on
page 15).
5. Put the instrument in
the incubator. The
incubator temperature
must be 20 ± 1 ºC
(68 ± 1 ºF).
Note: Instrument
performance has not been
tested at other
temperatures.
8. Push the Channel 6
key to access the test
length parameter. Use
the arrow keys to choose
a 5, 7 or 10 day test.
Note: The selected test
length is for all 6 channels.
6. Plug in and power on
the instrument. Make
sure all stir bars are
rotating. If not, lift the
bottle up and set down
again.
9. Push OFF to save
selections and exit the
menu.
28
Page 29

BODTrak™ II procedures
10.To start the test, push
the channel number
applicable to the bottle.
13.Push and hold the ON
key to start a test. A
graph will be displayed.
Note: To cancel a test push
and hold the OFF key.
Note: There is a built-in
1 hour instrument/sample
equilibration period before
data collection. The display
will show DELAY during this
period.
11.Push the ON key. The
range selection menu is
shown.
14.Do steps 10 through
13 again to set the test
range and start each of
the 6 channels. It is not
necessary to operate all
6 channels if less than 6
samples are available.
12.Use the arrow keys to
choose the test range.
Note: Use the left arrow key
for the 0 to 35 and
0 to 70 mg/L ranges. Use
the right arrow key for the
0 to 350 and 0 to 700 mg/L
ranges.
29
Page 30

BODTrak™ II procedures
5.5.1 Determination of results
After the end of the chosen test period (5, 7 or 10 days), END is shown on the
display. The procedure that is done dictates the determination of the results. The
results are determined based on the selected procedure: Simplified, Hach GGA or
Hach Standard Method.
5.5.1.1 Simplified sample results
The Simplified Procedure results are shown on the BODTrak II display. Push the
applicable channel selection key to show the results.
Note: If the sample was pre-diluted, apply a dilution factor to the instrument reading
(section 5.7.1 on page 33).
5.5.1.2 Hach GGA (glucose/glutamic acid) results
The seed blank and seeded GGA sample results are necessary for the Hach GGA
procedure results.
1. Push the channel selection key for the seed blank bottle. The results are
shown.
2. Push the channel selection key for the seeded GGA sample bottle. The results
are shown.
3. Calculate the results:
BOD mg/L = seeded GGA sample result - seed blank result
5.5.1.3 Hach Standard Method results
1. Push the channel selection key for the Hach Standard Method sample bottle.
The results are shown.
Note: Treat the seed blank the same as all other samples.
Note: If the sample was pre-diluted, apply a dilution factor to the instrument reading
(section 5.7.1 on page 33).
2. Find the dilution factor based on the selected range (Table 6 on page 24).
Example: If the sample range selected was 0 to 350 mg/L BOD, the dilution
factor is 1.45.
3. Calculate the corrected results:
BOD mg/L = BOD mg/L (instrument reading) x dilution factor
Example:
Instrument Reading = 200 mg/L, BOD dilution factor = 1.45
200 mg/L x 1.45 = 290 mg/L BOD (Corrected Result)
30
Page 31

BODTrak™ II procedures
BOD mg/L()ABx
SA
SB
--------
⎝⎠
⎛⎞
–=
BOD mg/L()290mg/L 120mg/L x
20mL
110mL
-------------------
⎝⎠
⎛⎞
–=
4. When samples are seeded, calculate the results using this equation and the
corrected results:
Where:
A = corrected BOD of the seeded sample
B = corrected BOD of the seed blank
SA = volume of seed in sample (sample can also be GGA)
SB = volume of seed in seed blank
Example:
A= 290 mg/L BOD
B= 120 mg/L BOD
SA= 20 mL
SB= 110 mL
BOD mg/L = 268 mg/L
31
Page 32

BODTrak™ II procedures
5.6 Typical curves
Typical curves through a 10 day test period are shown in Figure 4. For incorrect
curves refer to Figure 5 on page 37.
Figure 4 Typical curves
1 Typical with substrate variation 3 Typical with time lag
2 Typical
32
Page 33

BODTrak™ II procedures
5.7 Special considerations
5.7.1 Sample dilution
Unknown sample BOD effluent is typically in the 0 to 70 mg/L range. Unknown
sample BOD influent is typically in the 0 to 700 mg/L range. When the oxygen
requirement of a sample is more than 700 mg/L, dilute the sample with high-quality
distilled or deionized water.
Calculate the results to include the additional dilution factor. Example: If the BOD of
the sample is 1000 mg/L, dilute the sample 1:1 with distilled or deionized water. The
estimated BOD is now 500 mg/L. Use the sample volume specified in the table for
the 0 to 700 mg/L range of the chosen procedure. Multiply the instrument reading
result by 2. If using the Hach Standard Method procedure, continue with remaining
calculations.
5.7.2 Sample seeding
Some types of BOD samples do not contain sufficient bacteria to oxidize the
organic matter in the sample. Many industrial wastes are of this type. Some sewage
treatment plant effluents are chlorinated and essentially sterile. A BOD test cannot
be done in the absence of viable bacteria. To test such samples, seed each bottle
from a source known to contain a viable bacterial population.
Settled domestic wastewater plant influent or primary clarifier effluent are the
preferred sources of seed for most samples. Mixed liquor or undisinfected effluent
can be used for seed, but it is recommended to include a nitrification inhibitor.
Commercial seed sources are sometimes suitable. To prepare, see the instructions
from the manufacturer.
5.7.3 Sample temperature
Standard Methods for the Examination of Water and Wastewater, 21st Ed., 2005,
5210 D recommends an incubation temperature of 20 ±1 °C (68 °F) for the BOD
test. Put the BODTrak II instrument in an incubator that is adjusted to 20 ±1 °C. An
applicable BOD incubator is available from Hach (section 8.1 on page 41). Warm or
cool samples to 20 ± 1 °C.
Note: Instrument performance has not been validated at temperatures other than 20 °C.
5.7.4 Toxic materials
Industrial and chlorinated samples often contain toxic substances and require
special considerations when running BOD tests. Toxic materials in the sample will
cause decreased BOD values. Dilute the sample to minimize the toxic materials or
their effects. Refer to Standard Methods for the Examination of Water and
Wastewater, 21st edition, 5210 D.
33
Page 34

BODTrak™ II procedures
mL of Sodium Thiosulfate
mL used()mL sample to be dechlorinated()
100
-------------------------------------------------------------------------------------------------------------------
=
5.7.5 Chlorine
Any chlorine in the sample must be removed prior to testing. Keep the sample at
room temperature for 1 to 2 hours before a test to dissipate low chlorine
concentrations. If any chlorine remains after sitting for 2 hours or if the chlorine
concentration is high, add sodium thiosulfate to remove the chlorine:
1. In a 250 mL erlenmeyer flask add 100-mL of sample.
2. Add 10 mL of 100 g/L potassium iodide solution and
10 mL of 0.02 N sulfuric acid standard solution to the erlenmeyer flask.
3. Add 3 droppers of starch indicator solution and swirl to mix.
4. Titrate from dark blue to colorless with 0.025 N Sodium Thiosulfate standard
solution.
5. Calculate the quantity of sodium thiosulfate standard solution necessary to
dechlorinate the remaining sample:
6. Add the necessary quantity of 0.025 N sodium thiosulfate standard solution to
the sample and mix fully. After 10 to 20 minutes, do the BOD test.
5.7.6 pH effect
Low BOD test results occur when sample pH is outside the range of 6 to 8. Keep
this pH to simulate source sample conditions or adjust the pH to neutrality (buffered
at pH 7). Use 1.0 N (or weaker) sulfuric acid to neutralize caustic samples. Use 1.0
N (or weaker) sodium hydroxide to neutralize acidic samples. When samples are
pH adjusted, they should also be seeded.
5.7.7 Supersaturation
Equilibrate supersaturated cold samples (containing more than 9 mg/L dissolved
oxygen at 20 °C) to saturation:
1. Heat or cool the sample temperature to approximately 20 °C.
2. Half fill a sample bottle with sample.
3. Shake for 2 minutes or aerate with filtered compressed air for 2 hours.
34
Page 35

Section 6 Maintenance
DANGER
Only qualified personnel should conduct the tasks described in this section
of the manual.
6.1 Cleaning the instrument
Clean spills on the BODTrak™ II instrument with a soft cloth which has been
dampened with deionized or distilled water.
6.1.1 Sample bottles
After each test, empty the sample bottles and flush them thoroughly with hot water.
Use a brush, hot water and soap to remove residue. Residue creates a BOD. Flush
the bottles thoroughly with tap water and finally with distilled or deionized water to
remove all detergent.
6.1.2 Stir bars and seal cups
Clean the stir bars with hot water and soap. Use a brush to remove deposits. Flush
with tap water and finally with distilled or deionized water to remove all detergent.
Carefully empty and rinse the seal cups with water. Invert to dry.
6.1.3 Bottle fences
The bottle fences prevent tipping of the bottles and provide tubing management
during storage. For storage, put the tubing into the opening in the bottle fence.
Wind the tubing counter-clockwise and secure the bottle cap inside the fence.
35
Page 36

36
Page 37

Section 7 Troubleshooting
Incorrect BOD curves through a 10 day test period are shown in Figure 5. For
typical curves refer to Figure 4 on page 32.
Figure 5 Incorrect BOD curves
1 High oxygen demand 4 Initial sample temperature below
20 ºC or supersaturated with
oxygen
2 Nitrification 5 Bottle leak
3 Excessive time lag
37
Page 38

Troubleshooting
7.1 High oxygen demand
Samples that are above range (for example, a BOD over 350 mg/L when a 160-mL
sample is taken) will cause results as shown in Curve 1 (Figure 5 on page 37).
Dilute the sample (section 5.7 on page 33) or use a higher BOD range and a
different sample volume (Table 4 on page 20, Table 5 on page 23 or Table 6 on
page 24).
When the BOD range of a sample is unknown:
• Use the results from the Chemical Oxygen Demand (COD) test. An estimated
BOD value can be obtained by multiplying the COD by 0.68.
• Use the results from a series of BOD tests using the same sample but different
volumes.
• Or use dilution ratios to choose an applicable BOD range.
Typically, effluent is in the 0-70 mg/L range while influent is in the 0-700 mg/L
range. When the BOD of the sample is more than 700 mg/L, prepare a sample
dilution (section 5.7 on page 33).
7.2 Nitrification
The condition shown by Curve 2 is an example of nitrification (Figure 5 on page
37). Deviation from the typical curve (shown as the dashed line) is apparent by the
concave increase near the end of the test period.
Biological oxidation of organic nitrogen usually occurs after 5 days with typical
domestic waste. Nitrifying bacteria develop more slowly than other types of
bacteria.
However, some samples contain a high concentration of nitrifying bacteria and
nitrification results can occur sooner. Control nitrification problems with Hach
Nitrification Inhibitor. Dispense the inhibitor powder into an empty sample bottle and
then add the sample. With the Hach Dispenser cap, dispense 6 shots
(approximately 0.48 grams) into the empty bottle. Refer to replacement parts and
accessories (Section 8 on page 41).
7.3 Excessive time lag
Curve 3 (Figure 5 on page 37) shows a test that did not start with sufficient bacteria
during the incubation period. To do a test on a sample without sufficient bacteria,
seed the sample (section 5.7.2 on page 33).
Bacteria acclimation also causes conditions that could cause Curve 3. This
sometimes occurs with standards and added seed. Add more seed or choose a
different seed source.
7.4 Sample temperature
The initial negative results of Curve 4 (Figure 5 on page 37) show that the initial
sample temperature was below the specified range of 20 ± 1 ºC. A sample
supersaturated with oxygen will also display this type of curve (section 5.7.3 on
page 33 and section 5.7.7 on page 34).
38
Page 39

Troubleshooting
7.5 Bottle leak
Curve 5 (Figure 5 on page 37) shows a bottle leak. A bottle leak can also cause no
response from the system. If such a response occurs, check the seal cup and bottle
cap for contamination or damage.
39
Page 40

40
Page 41

Section 8 Replacement parts and
accessories
8.1 Replacement parts
Description Quantity Item Number
BODTrak™ II Instrument, 115/230 VAC 1 2952400
Bottle, BODTrak II, amber (6x) 1 714421
Power cord, 18/3 SVT 7.5’, 10A-125 VAC for North
American 115 VAC use
Power Cord, 8’, with continental European plug for 230
VAC u se
Power Supply 1 2952500
Computer cable for data transfer to PC 1 2959300
Seal Cup 1 2959500
Spatula scoop 1 1225700
Stir Bar, magnetic, BODTrak II 1 2959400
8.2 Reagents
Description Quantity Item Number
Respirometric BOD nutrient buffer pillows 1 2962266
Potassium hydroxide pellets 1 31425
1 2959200
1 2959100
8.3 Optional reagents
Description Quantity Item Number
Nitrification inhibitor, 35 g 1 253335
Dispenser cap for 35 g bottle (for use with nitrification
inhibitor)
Polyseed Inoculum (50x) 1 2918700
Potassium iodide solution, 100 g/L, 500 mL 1 1228949
Sodium Hydroxide standard solution, 1.0 N,
900 mL
Sodium Thiosulfate standard solution, 0.025 N, 1000
mL
Starch indicator solution, dropping bottle,
100 mL MDB
Sulfuric acid, ACS, 500 mL 1 97949
1 45901
1 104553
1 35253
1 34932
41
Page 42

8.3 Optional reagents (continued)
Description Quantity Item Number
Sulfuric acid, 0.02 N standard solution, 1000 mL 1 20353
Sulfuric acid, 1.0 N standard solution,
1000 mL
Voluette ampule standard for BOD, 3000 mg/L for
manometric, 10-mL/ampule, 16 ampules
1 127053
1 1486610
8.4 Accessories
Description Quantity Item Number
Ampule breaker kit for voluette ampules 1 2196800
Bottle, wash, 500 mL 1 62011
Bottle, polyethylene, with spigot, 4 L 1 1486817
Brush, cylinder, size 2 1 68700
Buret, straight stopcock, Teflon plug, 25 mL 1 1405940
Clamp, buret, double 1 32800
Cylinder, graduated, 10 mL 1 50838
Cylinder, graduated, 25 mL 1 50840
Cylinder, graduated, 50 mL 1 50841
Cylinder, graduated, 100 mL 1 50842
Cylinder, graduated, 250 mL 1 50846
Cylinder, graduated, 500 mL 1 58049
Cylinder, graduated, 1000 mL 1 50853
Flask, erlenmeyer 1 50546
Incubator, BOD, Model 205, 110 V 1 2616200
Incubator, BOD, Model 205, 220/240 V 1 2616202
Pipet, Tensette®, 0.1 to 1.0 mL 1 1970001
Pipet, Tensette, 1 to 10 mL 1 1970010
Pipet tips, 0.1 to 1.0 mL (50x) 1 2185696
Pipet tips, 0.1 to 1.0 mL (1000x) 1 2185628
Pipet tips, 1 to 10 mL (50x) 1 2199796
Pipet tips, 1 to 10 mL (250x) 1 2199725
Pipet filler, 3 valve 1 1218900
Pipet, serological, glass, 10 mL 1 53238
Printer, Citizen PD-24 with cable 1 2960100
Standard Methods for the Examination of Water and
Wastewater
Support stand, buret 1 32900
1 2270800
42
Page 43

Replacement parts and accessories
8.4 Accessories (continued)
Description Quantity Item Number
Thermometer, Mercury, -20 to 110 °C 1 56601
Thermometer, non-Mercury, -20 to 110 °C 1 2635702
Water Still, 120 V 1 2615900
Water Still, 220 V 1 2615902
Water System, Ultrapure, Millipore Direct -Q 3 1 2512100
DQ3 purification pack 1 2512201
43
Page 44

44
Page 45

Section 9 Contact Information
HACH Company World
Headquarters
P.O. Box 389
Loveland, Colorado
80539-0389 U.S.A.
Tel (800) 227-HACH
(800) 227-4224
(U.S.A. only)
Fax (970) 669-2932
orders@hach.com
www.hach.com
Repair Service in Latin
America, the Caribbean,
the Far East, Indian
Subcontinent, Africa,
Europe, or the Middle
East:
Hach Company World
Headquarters,
P.O. Box 389
Loveland, Colorado,
80539-0389 U.S.A.
Tel +001 (970) 669-3050
Fax +001 (970) 669-2932
intl@hach.com
HACH LANGE LTD
Unit 1, Chestnut Road
Western Industrial Estate
IRL-Dublin 12
Tel. +353(0)1 46 02 5 22
Fax +353(0)1 4 50 93 37
info@hach-lange.ie
www.hach-lange.ie
HACH LANGE FRANCE
S.A.S.
33, Rue du Ballon
F-93165 Noisy Le Grand
Tél. +33 (0)1 48 15 68 70
Fax +33 (0)1 48 15 80 00
info@hach-lange.fr
www.hach-lange.fr
HACH LANGE APS
Åkandevej 21
DK-2700 Brønshøj
Tel. +45 36 77 29 11
Fax +45 36 77 49 11
info@hach-lange.dk
www.hach-lange.dk
Repair Service in the
United States:
HACH Company
Ames Service
100 Dayton Avenue
Ames, Iowa 50010
Tel (800) 227-4224
(U.S.A. only)
Fax (515) 232-3835
HACH LANGE GMBH
Willstätterstraße 11
D-40549 Düsseldorf
Tel. +49 (0)2 11 52 88-320
Fax +49 (0)2 11 52 88-210
info@hach-lange.de
www.hach-lange.de
HACH LANGE GMBH
Hütteldorferstr. 299/Top 6
A-1140 Wien
Tel. +43 (0)1 9 12 16 92
Fax +43 (0)1 9 12 16 92-99
info@hach-lange.at
www.hach-lange.at
HACH LANGE SA
Motstraat 54
B-2800 Mechelen
Tél. +32 (0)15 42 35 00
Fax +32 (0)15 41 61 20
info@hach-lange.be
www.hach-lange.be
HACH LANGE AB
Vinthundsvägen 159A
SE-128 62 Sköndal
Tel. +46 (0)8 7 98 05 00
Fax +46 (0)8 7 98 05 30
info@hach-lange.se
www.hach-lange.se
Repair Service in
Canada:
Hach Sales & Service
Canada Ltd.
1313 Border Street, Unit 34
Winnipeg, Manitoba
R3H 0X4
Tel (800) 665-7635
(Canada only)
Tel (204) 632-5598
Fax (204) 694-5134
canada@hach.com
HACH LANGE LTD
Pacific Way
Salford
GB-Manchester, M50 1DL
Tel. +44 (0)161 872 14 87
Fax +44 (0)161 848 73 24
info@hach-lange.co.uk
www.hach-lange.co.uk
DR. BRUNO LANGE AG
Juchstrasse 1
CH-8604 Hegnau
Tel. +41(0)44 9 45 66 10
Fax +41(0)44 9 45 66 76
info@hach-lange.ch
www.hach-lange.ch
DR. LANGE NEDERLAND
B.V.
Laan van Westroijen 2a
NL-4003 AZ Tiel
Tel. +31(0)344 63 11 30
Fax +31(0)344 63 11 50
info@hach-lange.nl
www.hach-lange.nl
HACH LANGE S.R.L.
Via Riccione, 14
I-20156 Milano
Tel. +39 02 39 23 14-1
Fax +39 02 39 23 14-39
info@hach-lange.it
www.hach-lange.it
45
Page 46

Contact Information
HACH LANGE S.L.U.
Edif. Arteaga Centrum
C/Larrauri, 1C- 2ª Pl.
E-48160 Derio/Vizcaya
Tel. +34 94 657 33 88
Fax +34 94 657 33 97
info@hach-lange.es
www.hach-lange.es
HACH LANGE S.R.O.
Lešanská 2a/1176
CZ-141 00 Praha 4
Tel. +420 272 12 45 45
Fax +420 272 12 45 46
info@hach-lange.cz
www.hach-lange.cz
HACH LANGE
8, Kr. Sarafov str.
BG-1164 Sofia
Tel. +359 (0)2 963 44 54
Fax +359 (0)2 866 04 47
info@hach-lange.bg
www.hach-lange.bg
ΗΑCH LANGE E.Π.Ε.
Αυλίδος 27
GR-115 27 Αθήνα
Τηλ. +30 210 7777038
Fax +30 210 7777976
info@hach-lange.gr
www.hach-lange.gr
HACH LANGE LDA
Av. do Forte nº8
Fracção M
P-2790-072 Carnaxide
Tel. +351 214 253 420
Fax +351 214 253 429
info@hach-lange.pt
www.hach-lange.pt
HACH LANGE KFT.
Hegyalja út 7-13.
H-1016 Budapest
Tel. +36 (06)1 225 7783
Fax +36 (06)1 225 7784
info@hach-lange.hu
www.hach-lange.hu
HACH LANGE SU
ANALİZ SİSTEMLERİ
LTD.ŞTİ.
Hilal Mah. 75. Sokak
Arman Plaza No: 9/A
TR-06550 Çankaya/ANKARA
Tel. +90 (0)312 440 98 98
Fax +90 (0)312 442 11 01
bilgi@hach-lange.com.tr
www.hach-lange.com.tr
HACH LANGE E.P.E.
27, Avlidos str
GR-115 27 Athens
Tel. +30 210 7777038
Fax +30 210 7777976
info@hach-lange.gr
www.hach-lange.gr
HACH LANGE SP.ZO.O.
ul. Opolska 143 a
PL-52-013 Wrocław
Tel. +48 (0)71 342 10-83
Fax +48 (0)71 342 10-79
info@hach-lange.pl
www.hach-lange.pl
HACH LANGE S.R.L.
Str. Leonida, nr. 13
Sector 2
RO-020555 Bucuresti
Tel. +40 (0) 21 201 92 43
Fax +40 (0) 21 201 92 43
info@hach-lange.ro
www.hach-lange.ro
HACH LANGE D.O.O.
Fajfarjeva 15
SI-1230 Domžale
Tel. +386 (0)59 051 000
Fax +386 (0)59 051 010
info@hach-lange.si
www.hach-lange.si
46
 Loading...
Loading...