Page 1

Coatings for Extreme Applications
Perma-Z
Hi-Pro-Z
July
2008
Page 2
Page Header
The Zinc Advantage
In 1972, Greenheck took the lead as the first
commercial and industrial fan manufacturer to
introduce electrostatic powder coatings. Today,
Greenheck continues to lead by being the first to
offer a superior zinc-rich powder basecoat and
powder coating finish.
This zinc-rich basecoat technology is used
extensively outside the HVAC industry to protect
bridge beams, automotive components and other
heavy-gauge steel products. Now, this advanced
technology is exclusively available on Greenheck
welded steel products.
Greenheck’s coating process starts with a minimum
of five wash stages to treat all components prior
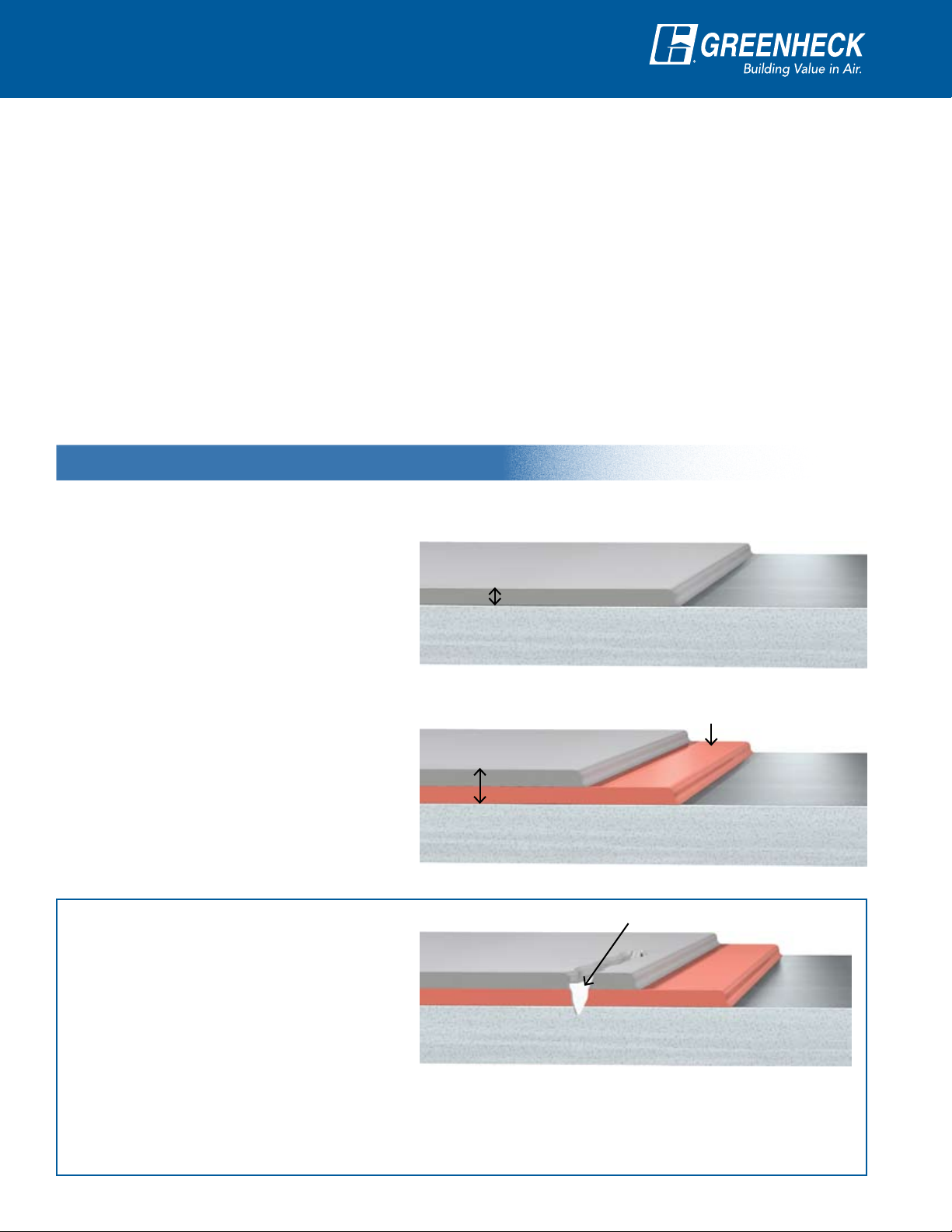
Two Coat System
When compared to a traditional single coat
application, the benefits of the two coat
system include:
An automatic powder coat application •
produces uniform coverage and unmatched
paint quality.
One Coat Process
to painting. Cleaner parts result in better coating
adhesion and durability. We then use an advanced
two coat powder application method that includes
a basecoat of zinc-rich powder and a topcoat of
Greenheck’s Permatector™ or Hi-Pro Polyester. The
combination of these two topcoats over our new zincrich basecoat results in the two new coatings Perma-Z
and Hi-Pro-Z. These oven cured coatings provide
superior corrosion resistance along with a tough,
uniform finish to combat the most extreme conditions.
To help determine what process and coating may
be right for your specific environment, refer to the
performance tested guide provided on page three.
Topcoat
Standard
2-3 mils
Base Steel
Surface Preparation
The double coat thickness provides superior •
durability and protection from air and water.
The zinc-rich basecoat includes an epoxy •
component that provides additional corrosion
protection.
The zinc-rich basecoat provides chemical •
protection of exposed steel to prevent
corrosion.
The Zinc Advantage
The zinc-rich basecoat actively and
passively protects the base steel if the
coating becomes damaged and the
steel is exposed to air and water.
The zinc-rich basecoat has a lower
electrochemical potential than the base
steel. As a result, the steel is actively held in a neutral state when exposed to a corrosive environment—the
driving force of corrosion is halted. A protective layer forms over the damaged surface as a by-product of
the chemical reaction and passively protects the exposed steel from further corrosion due to air and water.
Two Coat Process
Topcoat
4-6 mils Total
Base Steel
Protective Layer
Damaged Surface
If scratched or chipped the zinc donates electrons
to the iron, producing immediate corrosion protection.
Zinc-rich Primer
(70% zinc)
Surface Preparation
Advanced
2
Page 3

Page Header
Performance Tested
When selecting a powder coating finish for heavy-gauge welded steel fans, critical information
such as environment, moisture, exposure, abrasives, and chemicals should be considered.
Powder coatings are the best choice for most extreme applications. Major advantages over
most vendor-applied liquid coatings include:
Superior finish with uniform coverage and thickness.•
Environments
A better coating provides better protection.•
The process is environmentally friendly.•
Unequaled value.•
Coatings Color Coating Specifications
Permatector™
Standard coating for steel products in
both indoor and outdoor applications
Thickness: 2.0 - 3.0 mils
Polyester urethane
powder coating
CHEMICAL *EXTREME WEATHER
X X
ABRASIVE PARTICLES
CLEAN AIR
COASTAL
Light Gray
Hi-Pro Polyester
Formulated for exterior durability,
One coat Process
color and gloss retention. Excellent for
chemical applications
Perma-Z
Two coat powder paint coating provides
outstanding corrosion protection in
many extreme applications
Hi-Pro-Z
Two coat powder paint coating is
Two coat Process
resistant to saltwater, chemical fumes
and moisture in corrosive environments
Note: Perma-Z and Hi-Pro-Z are not available on aluminum. *Chemical-Resistant Rating Below
Thickness: 2.0 - 3.0 mils
High performance
polyester urethane
Dark Gray
powder coating
Thickness: 4.0 - 6.0 mils
Permatector™ topcoat
with zinc-rich, epoxy
Light Gray
basecoat
Thickness: 4.0 - 6.0 mils
Hi-Pro Polyester
topcoat with zinc-rich,
Dark Gray
epoxy basecoat
X X X
X X X X X
X X X X X X
SUN-UV
Test Data
Salt Spray ASTM B117
Hours 1000 2000 3000 4000
Permatector™
Hi-Pro Poly
Perma-Z
Hi-Pro-Z
Chemical Resistance Ratings
*
Chemical
Permatector™ 0 1 2 2 0 —
Hi-Pro Poly 0 0 0 1 0 —
Perma-Z 0 1 2 2 0 2
Hi-Pro-Z 0 0 0 1 0 1
RATING
DESCRIPTIONS
Bleach
0 - No effect 1- Slight change in gloss or color
2- Surface etching, severe staining, but film integrity remains
3- Significant pitting, cratering, swelling, or erosion with obvious surface deterioration
Sulfuric
Acid (10%)
HCI (10%) MEK
Durability
Pencil
Hardness
ASTM D3363
3H No Failure
2H No Failure
3H No Failure
2H No Failure
Chlorine
(0.1%)
Cross-Hatch
Adhesion
ASTM D3359-B
NaOH (20%)
Salt Spray ASTM B117 is a
comparative test that indicates
the corrosion resistance of
powder paint coatings.
Pencil Hardness and Cross-Hatch
Adhesion tests determine the
durability of a coating to withstand
scratches, nicks and chips.
Chemical Resistance Ratings
provide information on how each
coating option will hold-up in
certain chemical environments.
3
Page 4

Warranty Plus
Greenheck’s two coat powder paint system provides unparalleled corrosion protection
in the most extreme conditions. Test data demonstrates our two coat paint system
offers three and four times the corrosion resistance of other coatings commonly
Coating Warranty
Coating Type
Permatector™, Hi-Pro 1 Year
Perma-Z 2 Years
Hi-Pro-Z 2 Years
From Ship
Specifications
Date
available within the fan industry. The value
of this unmatched corrosion protection is
realized by our industry-leading coating
warranty.
For detailed warranty and limitation
information contact your area representative.
Multi-Stage Wash
All carbon steel components shall be cleaned and
chemically treated by multi-stage processes that
shall include alkaline cleaner to remove oil and
film; oxide removal to eliminate oxide formed on
laser cut components; iron phosphate coating to
increase corrosion protection and paint bond; and
seal rinse to seal pores in iron phosphate coating
for optimum corrosion protection.
Two Coat Electrostatic Powder System
Fan components shall be coated with a two coat
powder coating consisting of a zinc-rich powder
basecoat electrostatically applied and gelled.
Minimum dry film thickness to be 2-3 mils. The
Building Value in Air
basecoat shall consist of 70 percent zinc and shall
be formulated with an epoxy binder.
Greenheck delivers value
to mechanical engineers by
A polyester urethane powder topcoat shall be
helping them solve virtually
electrostatically applied at a minimum dry film
thickness of 2-3 mils atop the basecoat and baked
any air quality challenges
simultaneously with the zinc-rich basecoat. The
their clients face with a
total dry film thickness of the two coat powder
comprehensive selection of
coating system shall be a minimum of 4-6 mils.
top quality, innovative air-
related equipment. We offer
extra value to contractors
by providing easy-to-install,
competitively priced, reliable
products that arrive on time.
Available Coatings
Perma-Z shall exceed
3,000-hour salt spray under
ASTM B117 test method. Finish
color shall be industrial (light) gray.
Hi-Pro-Z shall exceed 4,000-hour salt spray under
ASTM B117 test method. Finish color shall be
charcoal (dark) gray.
Specify Perma-Z and Hi-Pro-Z for your extreme
applications or for any environment where physical
appearance and corrosion protection is essential.
As a result of our commitment to continuous improvement,
Greenheck reserves the right to change specifications
without notice.
And building owners and
occupants value the energy
efficiency, low maintenance
and quiet dependable operation
they experience long after the
construction project ends.
Our Warranty
Going Green
For decades, Greenheck has focused on the
environmental side of the building industry—
developing reliable, energy-efficient products
and systems to promote
occupants’ health and
comfort. As one of the
first manufacturers of air
state-of-the-art, two coat powder paint system.
Our coating system includes pretreatment of
the multi-stage washer waste prior to release to
the municipal water treatment facilities, a 100%
powder paint reclaim system, and infrared curing
booster ovens with energy savings approaching
50% when compared to typical convection ovens.
movement and control
equipment to join the U.S.
Green Building Council,
Prepared to Support
Green Building Efforts
P.O. Box 410 • Schofield, WI 54476-0410 • Phone (715) 359-6171 • greenheck.com
P.O. Box 410 • Schofield, WI 54476-0410 • Phone (715) 359-6171 • greenheck.com
our desire for sustainable
buildings has extended to our
We help engineers, architects, contractors, and
owners succeed in their green initiatives, on any
project. Going green? Go Greenheck - visit our
Web site or contact your area representative.
4
Prepared to Support
Green Building Efforts
Coatings for Extreme Applications Rev. 1 July 2008 RG
Copyright © 2007 Greenheck Fan Corp.
Copyright © 2008 Greenheck Fan Corp.
Catalog Rev. # Month Year
 Loading...
Loading...