Page 1

GEHealthcare
X-rayBoneDensitometerwithenCOREv17
software-UserManual
Thismanualsupportsthefollowingproducts:LunariDXA
Series,LunarProdigySeries,LunarDPXSeries
X-rayBoneDensitometerwithenCORE
v17software-UserManual
LU43616EN-2ENRevision19(September
2017)
©2017GEHealthcare
Page 2

Page 3

1Safety............................................................................................................25
PrecautionsforStandardOperatingProcedures........................................25
OperatorSafety.............................................................................................26
PersonnelMonitors.....................................................................................26
X-RayandShutterGraphics.......................................................................27
X-RayShutter...............................................................................................27
X-RayPowerSupply....................................................................................27
PatientSafety................................................................................................27
MechanicalSafety.........................................................................................29
Symbols.........................................................................................................29
SampleLabels...............................................................................................30
FailsafeCircuit...............................................................................................35
X-RayShieldingRequirements.....................................................................35
ElectricalSafety.............................................................................................36
PeripheralCongurations...........................................................................36
ScatterRadiation..........................................................................................37
2ProductInformation....................................................................................39
IntendedUse.................................................................................................39
IndicationsforUse........................................................................................39
CautionsforDXADeterminations................................................................41
DeviceDescriptions......................................................................................42
ScannerTableAssembly...............................................................................47
TrainingInformation.....................................................................................50
Classications................................................................................................50
InstallationandOperation............................................................................50
SoftwareInstallation.....................................................................................50
Features.........................................................................................................51
HardwareFeatures.....................................................................................51
SoftwareFeatures.......................................................................................51
QualityAssurance(QA)Features................................................................52
UserInformation.........................................................................................52
Options.........................................................................................................52
3DailyUse.......................................................................................................55
3
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 4

DailyUse........................................................................................................55
ArchiveExamFiles.......................................................................................55
SafeUseGuidelines......................................................................................56
EmergencyStopButton................................................................................57
TestEmergencyStopButton......................................................................57
CleanScannerTableEnvironment...............................................................57
AnnualMaintenance.....................................................................................57
X-RayTubeandLaserAssemblyMaintenance...........................................58
4QualityAssurance(QA)...............................................................................59
DailyQualityAssuranceProcedure..............................................................59
PrecisionandAccuracy................................................................................60
QAControls....................................................................................................61
QATrendReportingOptions.........................................................................61
MeasuretheSpinePhantom........................................................................63
5MeasurementandAnalysis........................................................................67
Measurement:OverviewandWarnings......................................................67
MeasurementModes..................................................................................68
MeasurementProcedures:Overview..........................................................72
SelectExistingPatientRecord....................................................................72
RecordNewPatientInformation................................................................73
SelectMeasurementSite............................................................................75
AbortMeasurement....................................................................................75
OneVision.....................................................................................................76
QuickView....................................................................................................77
AnalysisProcedures:Overview....................................................................77
SelectImage................................................................................................77
AdjustImage................................................................................................78
Advanced:AdjustROIs................................................................................79
Advanced:AdjustPointTyping...................................................................79
ExamineResults..........................................................................................80
OneScan........................................................................................................85
TurningOneScanOnandOff......................................................................85
OneScanMeasurement..............................................................................85
APSpineMeasurementandAnalysis..........................................................86
4
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 5

APSpineMeasurement...............................................................................86
APSpineAnalysis........................................................................................89
Femur/DualFemurMeasurementandAnalysis..........................................91
Femur/DualFemurMeasurement..............................................................91
AtypicalFemurFractureMeasurement.....................................................93
Femur/DualFemurAnalysis........................................................................96
ForearmMeasurementandAnalysis.........................................................110
ForearmMeasurement.............................................................................110
ForearmAnalysis.......................................................................................113
TotalBodyMeasurementandAnalysis.....................................................114
TotalBodyMeasurement..........................................................................114
TotalBodyAnalysis...................................................................................116
BodyCompositionMeasurementandAnalysis........................................121
BodyCompositionMeasurement.............................................................122
BodyCompositionAnalysis......................................................................124
Sarcopenia(MuscleLosswithAging).........................................................139
LateralSpineMeasurementandAnalysis.................................................143
LateralSpineMeasurement.....................................................................143
LateralSpineAnalysis...............................................................................146
LVAMorphometryMeasurementandAnalysis.........................................147
LVAMorphometryMeasurement..............................................................147
LVAMorphometryAnalysis.......................................................................149
LVASpineGeometryMeasurementandAnalysis.....................................155
LVASpineGeometryMeasurement..........................................................155
LVASpineGeometryAnalysis...................................................................157
APVAMorphometryMeasurementandAnalysis......................................158
APVAMorphometryMeasurement...........................................................158
APVAMorphometryAnalysis....................................................................160
APVASpineGeometryMeasurementandAnalysis..................................160
APVASpineGeometryMeasurement.......................................................160
APVASpineGeometryAnalysis................................................................162
DualVAMeasurementandAnalysis..........................................................163
PediatricMeasurementandAnalysis........................................................163
PediatricMeasurement.............................................................................164
5
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 6

PediatricAnalysis......................................................................................165
HandMeasurementandAnalysis..............................................................167
HandMeasurement..................................................................................167
HandAnalysis............................................................................................169
OrthopedicHipMeasurementandAnalysis..............................................170
OrthopedicHipMeasurement..................................................................170
OrthopedicHipAnalysis............................................................................172
OrthopedicKneeMeasurementandAnalysis...........................................174
OrthopedicKneeMeasurement...............................................................174
OrthopedicKneeAnalysis.........................................................................176
SmallAnimalMeasurementandAnalysis.................................................177
SmallAnimalMeasurement......................................................................177
SmallAnimalAnalysis...............................................................................178
CustomAnalysis..........................................................................................180
CustomAnalysisToolbar..........................................................................180
PrecisionCalculator....................................................................................181
PrecisionWizard........................................................................................182
CustomReferencePopulation....................................................................182
CreateaNewReferencePopulation........................................................183
EditaCustomReferencePopulation.......................................................183
DeleteaCustomReferencePopulation...................................................183
ScanCheck...................................................................................................183
ScanCheckChecklist.................................................................................184
AdjustingScanCheckThresholds.............................................................184
6DirectoryManagement.............................................................................187
MoveScans.................................................................................................187
CopyExamFiles..........................................................................................187
EmailImageFiles........................................................................................187
EditPatientsorExams................................................................................188
DeletePatients,Exams,orImages.............................................................188
ChangeImageType....................................................................................189
BatchExamFileOperations.......................................................................189
7Reporting....................................................................................................191
Reports........................................................................................................191
6
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 7

DXAReports...............................................................................................191
ComposerReports....................................................................................192
ReportCenter..............................................................................................193
CreateaReport.........................................................................................193
SelectAdditionalReports..........................................................................195
ChangeanAssessmentforaReport.......................................................196
CongureRulestoAutomaticallySelectReports....................................197
OptimizingtheReportCenter...................................................................198
StyleSheets.................................................................................................199
CreateaNewStyleSheet.........................................................................200
Subreports...................................................................................................201
CreateaNewSubreport...........................................................................201
AddaSubreporttoaStyleSheet.............................................................201
CongureaSubreporttoAppearOnlyUnderCertainConditions...........202
AssessmentEditor.......................................................................................202
ComposerDatabase...................................................................................205
CreateaNewDatabase............................................................................205
ChangetheActiveDatabase....................................................................206
PracticeManagementTools.......................................................................206
AvailableReports.......................................................................................206
AddaQuery...............................................................................................209
EditaQuery...............................................................................................211
DeleteaQuery...........................................................................................211
BMDSite/RegionFilters.............................................................................211
HistoryCatalog..........................................................................................212
8DatabaseMaintenance.............................................................................213
DatabaseMaintenance..............................................................................213
CompressDatabase...................................................................................213
DeleteDatabase.........................................................................................214
EditDatabase..............................................................................................214
ExportDatabase.........................................................................................216
NewDatabase.............................................................................................216
Archive.........................................................................................................217
RestoreBackup...........................................................................................218
7
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 8

RebuildDatabase........................................................................................218
ImportEntireDatabases.............................................................................219
ImportDatabaseManually.........................................................................220
SupportedImportOptions..........................................................................220
TaskScheduler............................................................................................221
SQLServerDatabaseInterface..................................................................221
ExternalUSBHardDrive.............................................................................222
9Troubleshooting.........................................................................................225
Troubleshooting..........................................................................................225
10ScreensandToolbars................................................................................227
ScreensandToolbars.................................................................................227
UsingScreens............................................................................................227
UsingToolbars...........................................................................................227
PatientBlock..............................................................................................227
HelpText....................................................................................................227
MainScreen.................................................................................................227
CommonToolbar.......................................................................................229
NewMeasurementScreen.........................................................................229
NewMeasurementToolbar......................................................................230
AnalyzeWhenDoneOption......................................................................230
HomeScannerArm...................................................................................230
ParkScanner.............................................................................................231
AnalyzeScreen............................................................................................231
AnalyzeToolbar........................................................................................231
ResultsTabs...............................................................................................232
DirectoryScreen..........................................................................................233
Search........................................................................................................233
DirectoryToolbar.......................................................................................234
PatientListandExamList.........................................................................234
DatabaseSidebar......................................................................................235
QualityAssuranceScreen...........................................................................235
QualityAssuranceToolbar........................................................................236
SystemStatus............................................................................................236
Options........................................................................................................236
8
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 9

UserOptions..............................................................................................236
ConnectivityOptions.................................................................................250
ErrorLog......................................................................................................252
11Security.......................................................................................................255
Introduction.................................................................................................255
SecurityFeatures........................................................................................255
AccessControls.........................................................................................255
WindowsUserAccountRequirements....................................................256
ApplicationSecuritySettings....................................................................256
CreateWindowsUserGroups................................................................256
AddUserstoWindowsGroups...............................................................257
CongureUserAccountsforElectronicSignatures................................257
CongureApplicationFunctionsAvailabletoGroups.............................257
Authentication...........................................................................................258
Authorization.............................................................................................259
AuditControls............................................................................................259
MaliciousSoftwareProtection..................................................................261
WorkstationSecurity.................................................................................263
DataProtection.........................................................................................263
SecurityOperations....................................................................................264
NetworkSecurity.......................................................................................264
BusinessContinuity...................................................................................264
MediaAccessControlPoints....................................................................264
RemoteService...........................................................................................265
NetworkInterfaceSpecicationsandRiskManagement........................266
UsingtheGEHCProductSecurityDatabase.............................................268
ASpecications.............................................................................................271
SystemsSpecications...............................................................................271
PhysicalSpecications................................................................................272
OperationalEnvironmentSpecications...................................................273
StorageandTransportEnvironmentSpecications.................................276
SpaceRequirements...................................................................................276
LeakageCurrent..........................................................................................279
InputPower.................................................................................................279
9
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 10

FuseCapability............................................................................................280
CollimatorSpecications............................................................................280
X-RayGeneratorTechnicalSpecications................................................281
X-RayTubeHeadAssembly.......................................................................289
X-RayTubeTechnicalInformation...........................................................290
AnodeHeating/CoolingCurves................................................................293
FilamentEmissionCharacteristics...........................................................295
X-RayTubeAssemblyHeating/CoolingCurves.......................................296
MaximumScanArea(LongXTransverse)...............................................297
ScatterRadiationDiagrams.......................................................................298
CurrentandTypicalDoseTables................................................................307
IECandUL/CSACertication......................................................................320
ElectromagneticInterference....................................................................320
ElectromagneticCompatibility(EMC)Performance..................................320
EMCEnvironmentandGuidance..............................................................321
DeclarationsofImmunityandEmissions................................................321
MinimumPCRequirements......................................................................323
BReferenceData..........................................................................................329
enCOREReferenceData.............................................................................329
UsingtheReferencePopulationComparison...........................................329
ChoosingReferencePopulationOptions.................................................330
ConguringtheComparisontoReferenceGraph...................................331
ReferenceDataPopulations.......................................................................332
CAdultReferenceData................................................................................339
BoneMineralDensity(BMD).......................................................................339
%YoungAdult..............................................................................................340
%Age-Matched...........................................................................................341
%Age-Matched:WeightAdjustment.......................................................342
%Age-MatchedEthnicityAdjustment......................................................343
%Age-MatchedNationalityReferenceDatabase...................................344
ReferenceGraph:FemaleandMale..........................................................344
ReferenceGraphs:OtherSites...................................................................345
BoneMineralDensityReferencePopulations...........................................346
ComparisontoYoungAdult......................................................................347
10
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 11

ComparisontoAge-Matched...................................................................347
EffectofPostmenopausalYears..............................................................350
ReferencePopulationDatabase...............................................................350
AgeAdjustment.........................................................................................350
WeightAdjustment...................................................................................358
LateralSpineMorphometryReferenceValues..........................................359
HipAxisLength............................................................................................362
References...................................................................................................363
DPediatricReferenceData..........................................................................371
BoneMineralDensity(BMD).......................................................................371
%Age-Matched...........................................................................................372
%Age-MatchedEthnicityAdjustment......................................................373
%Age-MatchedNationalityReferenceDatabase...................................374
ReferenceGraph:FemaleandMale..........................................................374
ReferenceGraphs:OtherSites...................................................................375
BoneMineralDensityReferencePopulations...........................................376
ComparisontoAge-Matched...................................................................377
ReferencePopulationDatabase...............................................................380
AgeAdjustment.........................................................................................380
GrowthIndices............................................................................................394
References...................................................................................................401
EBodyCompositionReferenceData..........................................................403
Introduction.................................................................................................403
AndroidandGynoidRegionsofInterest....................................................403
ReferencePopulationsthatSupportTotalBodyComposition
ReferenceData...........................................................................................404
BodyCompositionReferenceValuesforFemalePercentFat..................404
BodyCompositionReferenceDataforMalePercentFat.........................406
BodyCompositionPercentFatReferenceData.....................................406
References...................................................................................................409
FUSA(NHANES1999-2004)TotalBodyReferenceData..........................411
Introduction.................................................................................................411
NHANES1999-2004ReferencePopulation...............................................414
GAFFPhantomStudyResults......................................................................457
11
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 12

Introduction.................................................................................................457
AccuracyofBeakSize.................................................................................457
ReproducibilityofBeakSize........................................................................458
BeakSizeDependenceonPositioning.......................................................458
12
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 13
ContactInformation
www.gehealthcare.com
Headquarters/Legal
Manufacturer
GEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLC.
Streetaddress:
3030OhmedaDr .
Madison,WI53718
USA
Mailingaddress:
P.O.Box7550
Madison,WI53707-7550
USA
Phone:+1(800)437–1171
China
No.19ChangjiangRoad
Wuxi,Jiangsu,214028
GEMedicalSystemsSCS
283ruedelaMinière
78530BUC,France
France
24Avenuedel'Europe-CS20529
78457VELIZY
Germany
BeethovenStr.239
D-42655Solingen
Germany
Phone:+49–212–2802–0
Fax:+49–212–2802–390
Asia/Pacic
4–7–127Asahigaoka
Hino-shi,Tokyo191–8503
P.R.C.
Phone:+86–510–85225888
Fax:+86–510–85226688
PhysicalManufacturerAddress
GEMEDICALSYSTEMSMONTERREY,
MEXICOS.A.DEC.V.
CalleEspañaNo300,Parque
IndustrialHuinalá,Apocada
Phone:+33–1–34–49–5365
Fax:+33–1–34–49–5406
Turkey
GEMedicalSystemsTürkiyeLtd.Şti
EsentepeMah.HarmanSok.No:8
34394ŞişliİstanbulTürkiye
Japan
Phone:+81–42–585–5111
Fax:+81–42–585–3077
NuevoLeonCP66645MEXICO
GEMedicalSystemsUltrasound&PrimaryCareDiagnostics,LLC,aGeneralElectriccompany,doingbusinessasGEHealthcare/GE
SantéauQuébec.
13
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 14

Preface
Thismanualprovidesinstructionsforoperatingthesoftwareandscantable,safetyandmaintenanceinformation,andtechnical
specicationsforyourbonedensitometer.
Theinformationinthismanualissubjecttochangewithoutnotice.Youmayuseorcopythesoftwaredescribedinthismanualonlyin
accordancewiththetermsofyoursoftwarelicense,productwarranty,orservicecontractagreements.
Nopartofthispublicationmaybereproducedforanypurposewhatsoever,storedinaretrievalsystem,ortransmittedinanyformorbyany
means,mechanical,photocopying,recordingorotherwise,withouttheexpresswrittenpermissionofGEHealthcare.
Anyreproduction,photocopyingandrecordinginwholeorpartisprohibited.Anyinformationcontainedhereinshallnotbedisclosedtoany
companyviewedasacompetitortoGEHealthcare.
GEHealthcaremakesnowarrantyofanykindwithregardtothismaterial,andshallnotbeheldliableforerrorscontainedhereinorfor
incidentalorconsequentialdamagesinconnectionwiththefurnishingsoruseofthismanual.
TheinformationcontainedinthemanualiscondentialandproprietarytoGeneralElectric.Thisinformationisprovidedonlytoauthorized
representativesofGEHealthcarecustomerssolelyforthepurposeoffacilitatingtheuseofGEHealthcareproducts.Noinformation
containedhereinmaybedisclosedtoanyunauthorizedpersonforanypurposewhatsoeverwithoutpriorwrittenconsentofGEHealthcare.
Copyright©2007-2017
GEHealthcare,Madison,Wisconsin.Allrightsreserved.
FirstyearofCEMark:2007
Readthismanualthoroughlybeforeusingthesystemorattemptingtoserviceanycomponents.Unauthorizedservicemayvoidsystem
warrantiesorservicecontracts.ConsulttheGEHealthcareCustomerServiceDepartmentbeforeattemptinganyservice:800-437-1171
(U.S.A).
UnitedStatesFederallawrestrictsthisdevicetosalebyorontheorderofalicensedphysician.
Thisisrequiredper21CFR801.109(CodeofFederalRegulations).
LunariDXA,Prodigy,DPX,andCoreScanaretrademarksorregisteredtrademarksofGeneralElectricCompany.Allotherproductandbrand
namesareregisteredtrademarksortrademarksoftheirrespectivecompanies.
Thedevicesforwhichthismanualisusedmayalsobemarketedunderthefollowingnames:
LunariDXA
iDXA
iDXAAdvance
iDXAPro
iDXAForma
LunariDXA
LunarProdigy*Series
ProdigyAdvance
ProdigyAdvanceCompact
ProdigyPrimo
ProdigyPrimoCompact
ProdigyPro
ProdigyProCompact
ProdigyForma
Primo
PrimoCompact
Prodigy
ProdigyCompact
LunarDPX*Series
DPX-NT
DPX-MD+
DPXBravo
DPXDuo
DPXPro
MD+
Bravo
Duo
*
aretrademarksofGeneralElectricCompany.
*
Series
14
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 15

Throughoutthismanualtheterm“image”isusedtoindicateaDual-energyX-rayAbsorptiometry(DXA)image,whichisconstructedfrom
low-energyandhigh-energysignals.Dependingontheintendeduse,whenaDXAimageisdisplayedforaquantitativeapplicationsuchas
SpineorFemurBMD,theimageislabeled“ImageNotforDiagnosis.”
ForapplicationssuchasLateralVertebralAssessment(LVA)runningonProdigyoriDXA,theimageislabeled“ImageforSpineMorphometry
AssessmentOnly.”ForapplicationssuchasAtypicalFemurFracture(AFF)runningonProdigyoriDXA,theimageislabeled“Imagefor
atypicalfemurfractureassessmentonly”.
Thesimpleterm“image”isusedthroughoutthemanualforreadability.
15
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 16

LicenseandWarrantyInformation
PleasecarefullyreadthefollowingtermsandconditionsbeforeinstallingoroperatingtheGEHealthcareSoftware("Software").Byinstalling
orusingtheSoftwareinYourGEHealthcareproduct,Youindicateyouracceptanceofthesetermsandconditions.IfYoudonotagreewith
thetermsandconditions,donotinstalloroperatetheSoftwareandreturnittoGEHealthcare.
TheSoftwarehasbeenprovidedtoYouforuseonaspecicGEHealthcareproduct.TheSoftwareisprovidedunderthetermsofthis
agreementandislicensedtoYou,notsold.YourrightstousetheSoftwarearesubjecttothetermsandconditionscontainedwithinthis
LicenseAgreementandGEHealthcarereservesanyrightsnotexpresslygrantedtoYou.Thislicenseisnon-exclusiveandanon-transferable
licensetousetheGEHealthcareSoftware.Re-distributionofSoftwareoranydocumentationprovidedtoYoubyGEHealthcareisstrictly
prohibited.
ThisproductincludessomesoftwarecomponentsthatarelicensedundertheGNUGeneralPublicLicense(GPL).SourcecodeforGPL
componentsisavailableuponrequest.
ThetermsandconditionsofthisLicenseAgreementandLimitedSoftwareWarrantyareasfollows:
1.LICENSE.
ThisLicenseallowsYouto:
(a)usetheSoftwareonaproductinaccordancewiththeaccompanyingdocumentation.To"use"theSoftwaremeansthattheSoftware
iseitherloadedinthetemporarymemoryofacomputerorinstalledonanypermanentmemoryormediaofacomputer(e.g.,hard
disk,CD-ROM,opticaldisk,zipdisk,andthelike);
(b)makeone(1)copy,inmachine-readableform,oftheSoftwareasprovidedtoYousolelyforthepurposesofbackup;providedthatsuch
copyincludesthereproductionofanycopyrightnoticeorotherproprietarynoticeappearinginoronsuchSoftware.
2.LICENSERESTRICTIONS.
(a)YOUMAYNOT,EXCEPTASEXPRESSLYPROVIDEDFORINTHISLICENSE:(i)DECOMPILE,DISASSEMBLE,ORREVERSEENGINEERTHE
SOFTWARE(excepttotheextentapplicablelawsspecicallyprohibitsuchrestriction);(ii)COPY,MODIFY,ADAPT,TRANSFER,TRANSLATE,RENT,
LEASE,GRANTASECURITYINTERESTIN,ORLOANTHESOFTWAREORANYPORTIONTHEREOF;(iii)CREATEDERIVATIVEWORKSBASEDUPON
THESOFTWAREORANYPORTIONTHEREOF;OR(iv)REMOVEANYCOPYRIGHTORPROPRIETARYNOTICESORLABELSINORONTHESOFTWARE.
(b)YouunderstandthatGEHealthcaremayupdateorrevisetheSoftware,andinsodoingincurnoobligationtofurnishsuchupdatestoYou
underthisLicense.GEHealthcarehasnoobligationtoimprove,updateorsupporttheSoftwareinthefuture.
(c)IntheeventtheinstrumentorproductdesignatedfortheSoftwareissoldorotherwisetransferredtoathirdparty,thatpartyisnot
authorizedtousetheSoftwareunlesstheyrstpaytoGEHealthcaretheapplicablelicensefeeandagreetothetermsandconditionsofa
SoftwareLicenseAgreement.UpontransferoftheSoftwareoranycopythereof,theLicensegrantedhereundershallterminateimmediately.
3.TERMANDTERMINA TION.
ThisLicenseiseffectiveuntilterminated.ThisLicensewillterminateimmediatelywithoutnoticefromGEHealthcareorjudicialresolutionif
YoufailtocomplywithanyprovisionoftheLicense.UponanyterminationofthisLicense,YouagreetoreturnordestroytheSoftware,all
accompanyingwrittenmaterialsandallcopiesthereofinanyform.Section5willsurviveanytermination.
4.EXPORTLAW.
YouagreethatneithertheSoftwarenoranydirectproductthereofisbeingorwillbeshipped,transferredorre-exported,directlyorindirectly
intoanycountryprohibitedunderUnitedStateslaworregulationspromulgatedthereunder.
5.WARRANTY.
GEHealthcarewarrantsthat,tothebestofourknowledge,thesoftwareprovidedwiththisLicensewillperformasdescribedinthe
product'soperator'smanualandthetechnicalspecicationforthisSoftware.Thislimitedwarrantyiscontingentuponproperuseofthe
SoftwareanddoesnotcoveranySoftwarewhichhasbeenmodied,subjectedtomaliciouslogic,unusualphysicalorelectricalstress,or
usedoncomputerequipmentnotspeciedbyGEHealthcare.
GEHealthcaredoesnotwarrantthatthefunctionscontainedinthisSoftwarewillmeetyourrequirements,orthattheoperationofthe
Softwarewillbeuninterruptedorerror-free.StatementsmadeaboutthisSoftwaredonotconstitutewarrantiesandshallnotbereliedupon
byYouindecidingwhethertopurchasetheGEHealthcareproductorusetheSoftware.INNOEVENTSHALLGEHealthcareBELIABLETO
YOUFORANYDAMAGESARISINGOUTOFTHEUSEORINABILITYTOUSESUCHSOFTWARE.
THESOLEANDEXCLUSIVEREMEDYINTHEEVENTOFDEFECTISEXPRESSLYLIMITEDTOTHEREPLACEMENTOFTHESOFTWAREPROVIDED.IF
FAILUREOFTHESOFTWAREHASRESULTEDFROMACCIDENTORABUSE,GEHEALTHCARESHALLHAVENORESPONSIBILITYTOREPLACE
THESOFTWARE.
GEHealthcarewillconsiderthiswarrantytobevoidifYoufailtocomplywiththetermsintheSoftwareLicenseAgreement.
16
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 17

6.TITLE.
Title,ownershiprights,andintellectualpropertyrightsintheSoftwareshallremainwithGEHealthcare.ThisSoftwareisprotectedbythe
copyrightlawsandtreaties.
7.MISCELLANEOUS.
ThisAgreementrepresentsthecompleteagreementconcerningthisLicenseandmaybeamendedonlybyawritingexecutedbyboth
parties.TheLicenseisgovernedbythelawsoftheStateofWisconsin,U.S.A.withoutregardtoitsconictoflawsprinciples.Ifanyprovision
ofthisAgreementisheldbyacourtofcompetentjurisdictiontobeunenforceable,thatprovisionshallbeenforcedtothemaximumextent
permissibleand/orreformedonlytotheextentnecessarytomakeitenforceable,andtheremainingprovisionsofthisAgreementwillnot
beaffectedorimpairedinanyway.IfanylegalactionorproceedingisbroughtfortheenforcementofthisAgreement,orbecauseof
anyallegeddispute,breach,defaultormisrepresentationinconnectionwithanyoftheprovisionsofthisAgreement,thesuccessfulor
prevailingpartyshallbeentitledtorecoverreasonableattorneys'feesandothercostsincurredinsuchactionorproceeding,inadditionto
anyotherrelieftowhichsuchpartymaybeentitled.
17
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 18

Registration
Governmenthealthdepartmentscanrequiremedicalfacilitiestoregisterdiagnosticx-rayequipment.Manymunicipalandstatehealth
agenciesrequiremedicalhealthfacilitiestoemploycertiedradiologictechnologiststooperatediagnosticx-raydevices.Contactyourlocal
regulatoryauthoritiesorGErepresentativeforregistrationguidelinesandregulationcompliance.
18
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 19
DisposalofMaterials
Thescannercontainslead(forx-rayshielding)andoneofthefollowing:sodiumiodide,cadmiumtelluride,LutetiumYttriumSilicon
Dioxide(L YSO),orcadmiumzinctelluride(usedforx-raydetection).
WEEELabel
Thissymbolindicatesthatthewasteofelectricalandelectronicequipmentmustnotbedisposedasunsortedmunicipalwasteandmustbe
collectedseparately.Pleasecontactanauthorizedrepresentativeofthemanufacturerforinformationconcerningthedecommissioningof
yourequipment.
IfyoucontractwithGEHealthcareforthedisposalofyourscanner,GEHealthcarewillproperlydisposeofthesematerials.Ifyouchoose
todisposeofyourscanneryourself,bothsubstancesmustbedisposedofinaccordancewithlocalregulations.ContactyourlocalGE
representativeforWEEEinformation.
19
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 20
FDACertiedComponents
LunariDXASeries
ThefollowingtablegivescomponentscertiedtotheFDAforusewithLunariDXAseriesscanners.Thetablesareupdatedperiodically.
ContactGEHealthcareforacurrentlistingofcompatiblecomponents.
ComponentDescriptionGEModel
Tubehead
assembly
GEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLCiDXA
40782
SeriesX-RayTubeHeadAssembly
X-raycontrollerGEMedicalSystemsUltrasound&
41718
PrimaryCareDiagnostics,LLCiDXA
SeriesX-rayController
CollimatorGEMedicalSystemsUltrasound&
42129
PrimaryCareDiagnostics,LLCiDXA
SeriesCollimatorAssembly
PRODIGYAdvance301000andhigher,PRODIGY301000andhigher
ThefollowingtablesgivecomponentscertiedtotheFDAforusewithProdigyseriesscanners.Thetablesareupdatedperiodically.Contact
GEHealthcareforacurrentlistingofcompatiblecomponents.
ComponentDescriptionGEModel
X-raycontrollerGEMedicalSystemsUltrasound&
41170
PrimaryCareDiagnostics,LLCsingle
boardcontroller
Highvoltagepower
supplies
GEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLCModel:
2907
7681
7681
GEMedicalSystemsUltrasound
&PrimaryCareDiagnostics,LLC
Model:SBD40PN280X2890or
SBD40PN280X4445
Tubehead
assembly
GEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLCX-Ray
8743or45645
TubeHeadAssembly
CollimatorGEMedicalSystemsUltrasound
8915
&PrimaryCareDiagnostics,LLC
PRODIGYCollimatorAssembly
20
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 21
PRODIGYAdvance40000-141999,PRODIGY13000-13999
ComponentDescriptionGEModel
X-raycontrollerGEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLCsingle
boardcontroller
Highvoltagepower
supplies
GEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLCModel
2907
GEMedicalSystemsUltrasound
&PrimaryCareDiagnostics,LLC
ModelSBD40PN280X2890or
SBD40PN280X4445
Tubehead
assembly
GEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLCX-Ray
TubeHeadAssembly
CollimatorGEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLCProdigy
CollimatorAssembly
7635
7681
7681
8743or45645
8915
DPX-NT/PRO/MD+/72000andhigher/90000andhigher
ThefollowinggivecomponentscertiedtotheFDAforusewithDPX-NT/PRO/MD+scanners.Thetablesareupdatedperiodically.Contact
GEHealthcareforacurrentlistingofcompatiblecomponents.
ComponentDescriptionGEModel
X-raycontrollerGEMedicalSystemsUltrasound&
7634
PrimaryCareDiagnostics,LLCsingle
boardcontroller
Highvoltagepower
supplies
GEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLCModel
2907
7681
7681
GEMedicalSystemsUltrasound
&PrimaryCareDiagnostics,LLC
ModelSBD40PN280X2890or
SBD40PN280X4445
21
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 22
ComponentDescriptionGEModel
DPXDuo,DPXBravo
TubeheadassemblyGEMedicalSystemsUltrasound&
8548or45649
PrimaryCareDiagnostics,LLCX-Ray
TubeHeadAssembly
CollimatorGEMedicalSystemsUltrasound&
7767
PrimaryCareDiagnostics,LLCDEXA
CollimatorAssembly
ComponentDescriptionGEModel
X-raycontrollerGEMedicalSystemsUltrasound&Primary
CareDiagnostics,LLCsingleboardcontroller
Highvoltagepower
supplies
GEMedicalSystemsUltrasound&
PrimaryCareDiagnostics,LLCModel
SBD40PN280X2890orSBD40PN280X4445
41500
7681
TubeheadassemblyGEMedicalSystemsUltrasound&Primary
CareDiagnostics,LLCX-RayTubeHead
Assembly
CollimatorGEMedicalSystemsUltrasound&Primary
CareDiagnostics,LLCDEXACollimator
Assembly
8548or45649
7767
22
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 23

OperatorProle
TheintendedusersoftheDXAscanneraremedicalprofessionalswithknowledgeandexperiencerequiredtoworkwithx-rayequipment.
23
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 24

24
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 25

Safety
PrecautionsforStandardOperatingProcedures
Useofcontrolsoradjustmentsorperformanceoftheproceduresotherthan
thosespeciedhereinmayresultinhazardous(laserorx-ray)radiation
exposure.
1.Donotattempttooperatethex-raybonedensitometerwithoutrstreading
thismanual.
2.Donotremovetheassemblypanelsorattemptanyrepairswithoutprior
instructionsfromauthorizedpersonnel.
3.PerformtheQualityAssuranceprocedureeachmorning.Ifanytestfails,check
thepositionofthecalibrationblockandreruntheQAprocedure.Ifatestfails
again,contactGESupport.Also,callGESupportifmorethantwofailuresoccurin
aone-weekperiod.Iftheroomtemperaturechangesmorethan5°Cduringthe
day,performanotherdailyQA.
4.Ifthepatientisormightbepregnant,alwayscontactthepatient'sphysician
beforeperformingascan.
5.Remaininvisualcontactwiththepatientwhileascanisinprogress.Ensurethat
thepatientdoesnotmoveduringthemeasurement.Minimizetheamountoftime
thepatientliesatonthescantable.
6.Restrictaccesstotheroomtoauthorizedpersonnel.
7.Donotattempttoserviceanyofthesystem'selectricalcomponentswhilethe
x-raybonedensitometeristurnedON.Highvoltageisusedtoproducex-rays.
8.Radiationsafetyinformationislocatedwithinthismanualyoureceivedwithyour
system.Reviewthisinformationbeforeoperation.
9.Tostopthex-raybonedensitometerinanemergency,presstheemergency
stopbuttononthescanarm.DONOTusetheemergencystopbuttontoroutinely
abortascan.
10.Immediatelyremoveanyuidsspilledonthepadoranysurfaceoftable.
11.Allsurfacesshouldbecleanedtomeetsite'sguidelinesforhandlingbloodand
bodyuids.Padmaterialmaybedamagedbycertainchemicals.Useappropriate
hospitalgradedisinfectant(forexample:Cidex®,HBQuat,Precise®,PDI)followed
bymilddetergent.
12.Donotgeneratex-raysthroughtheuseofremoteapplications.
13.Protectthecomputeragainstmaliciouslogicandunauthorizednetwork
access.Onlyallowauthorizeduseraccess.Preventvirusattacksbyusing
1
25
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 26
Safety
OperatorSafety
rewalls,anti-virussoftwareandsoftwarepatchupdates.ContactyourlocalGE
representativeformoreinformation.
14.DPXDuo:Extendthestepthefulldistancetoprovidemaximumsurfaceareafor
thepatienttogetonandoffthetablewithoutriskofinjury.
15.DPXDuo:Donotplaceanexcessiveloadonfootreststirrup(maximumload
is60pounds),drawers(maximumloadis100pounds),orlegextensiontable
(maximumloadis300pounds).
16.DPXDuo:Donotsitonlegextensiontable.
BecausetheDXAhastwocontrolpoints(PCandfrontpanel),theoperatorshould
visuallyensurethatnopersonisnearmovingparts,pinchpoints,orthex-raybeam
beforestartingascan.Theoperatormustunderstandtheuseoftheemergencystop
buttononthefrontpanel.SeeEmergencyStopButton(57).
DPXNT/MD,DPXDuo/Bravo,andProdigyscanners:Toavoidscatterradiation,the
operatorshouldremainatleast3feet(1meter)awayfromthecenterofthescanner.
iDXAscanners:Toavoidscatterradiation,theoperatorshouldremainatleast6feet(2
meters)awayfromthecenterofthescanner.
Maximizingthedistancefromthepatientwilldecreasetheoperator’sexposure
toscatterradiation;however,theoperatorshouldmaintainvisualorvoicecontact
withthepatientatalltimes.Optionalprotectiveequipmentwillfurtherreducethe
operator’sexposuretoscatterradiation.
PersonnelMonitors
Personnelmonitorsarenotnecessarytooperatethescanner.
Itisnotlikelythatyoucanreceivemorethan25%ofthemaximumpermissiblex-ray
dosefromthescanner.However,somefacilitieschoosetousepersonnelmonitors.
Refertoyourcity,countyorstateHealthDepartmentorRadiationSafetyOfcer
foryourfacility'spolicy.
Filmbadgesandthermalluminescentdosimeter(TLD)badgesareobtainedfroma
supplieraccreditedbytheNationalVoluntaryLaboratoryAccreditationProgramfor
personneldosimetryprocessing.
ThefollowingisasamplesituationforaclinicmeasuringanAPspineandDualFemur
on5subjectsperdaywithanexposurerateof0.18mR/hratadistanceof2meters
estimatedfromtheiDXAisodosecurves.
SampleCalculationforEstimatedExposureperYearfromScatterwithiDXA
Densitometer
ScanType
APSpine
DualFemurStandard
2.5mAScanTimeperDay(sec)
2.5mAScanTimeperDay(hours)
Mode
Standard
Average
Scans/Day
5
5535535
Scan
Time/Day
(sec/day)
260260
Equivalent
2.5mAScan
Time/day
(sec/day)
795
0.221
26
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 27
Safety
X-RayandShutterGraphics
ScanType
2.5mAScanTimeperWeek(hours)
2.5mAScanTimeperYear(hours)
2.5mAExposurefromIsodosePlots(mR/hr)
TotalExposurefor1Year(mR)
TotalAbsorbedDosefor1Year(mRad)0.92Rad/R
DuringameasurementorQualityAssuranceprocedure,x-rayandshuttergraphics
areshownonthecomputermonitor .Thegraphicsaregreentoindicatex-raysareoff
andtheshutterisclosed,andyellowtoindicatex-raysareonandtheshutterisopen.
X-raysoffandshutterclosed(green)
Mode
Average
Scans/Day
Scan
Time/Day
(sec/day)
Equivalent
2.5mAScan
Time/day
(sec/day)
1.11
57.5
0.18
10.3
9.5
X-RayShutter
X-RayPowerSupply
PatientSafety
PinchPoints
X-raysonandshutteropen(yellow)
WhenpowertothescannerisinterruptedduringameasurementorQualityAssurance
procedure,theshutterclosesandthex-raytubestopsgeneratingx-radiation.
Thex-raytubeassemblyuseshighvoltagetogeneratex-rays.DONOTtouch
internalcomponents.DONOTattempttoserviceinternalcomponents.
Thislabelidentiesthelocationofpossiblepinchpoints.
27
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 28

Safety
Whenthescannerarmisinmotion,makesurepossiblepinchpointareasareclearat
alltimes.Patientlimbsmustremaininsidetheboundariesofthetabletop.Apinch
pointispossiblebetweenthescannerarmandtable.
LaserSafety
DONOTSTAREINTOTHELASERBEAMduringpatientpositioningandQuality
Assuranceprocedures.Thislabelislocatedunderthescannerarmandshowsthe
locationofthelaseraperture:
RadiationSafety
Thelaserapertureislocatedontheundersideofthescannerarm,facingthepatient.
Keepthelaserapertureawayfromthepatient’seyesduringpatientpositioning.
X-rayexposure:Thesystemmakesradiationwhenelectricvoltageissuppliedto,and
currentowsthrough,thex-raytube.Duringameasurement,theshutteropenstolet
abeamofradiationpassthroughthescannertableandpatient.
ForiDXAsystems,thenominalradiationeldatthescannertabletopis18.4mmx
3.3mm.
ForProdigysystems,thenominalradiationeldatthescannertabletopis19.5mmx
3.4mm.
ForDPXsystems,thenominalradiationeldatthescannertabletopis2mm.
Leadoxideshieldingsurroundsthex-raytubeinsertinsidethetubehousingassembly
andreducesradiationlevelsaroundthescannertable.
Leakageradiation:<0.4mR/hrat1meter .
28
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 29
SkinEntranceDose
MechanicalSafety
Safety
RefertoCurrentandTypicalDoseTables(307)forirradiationtimesandskinentrance
doses.AVictoreenmodel530PrecisionElectrometer/DosemeterwithaModel660-5
IonChamberwasusedtomeasuretheX-rayentrancedose.
Thescannerarmmovesdowntheentirelengthofthescannertable.Makesurethe
patientdoesnotinterferewiththemovementofthescannerarmtopreventpossible
injury.Inaddition,makesurethattherearenoobjectsbehindthescannertablethat
mightobstructmovementofthescannerarm.
iDXAscanners:Weightappliedtothescantablebedmustnotexceed204kg(450lb).
DPXNT/MD+scanners:Weightappliedtothescantablebedmustnotexceed136
kg(300lb).
DPXDuo/BravoandProdigyscanners:Weightappliedtothescantablebedor
footstep(DPXDuoonly)mustnotexceed159kg(350lb).
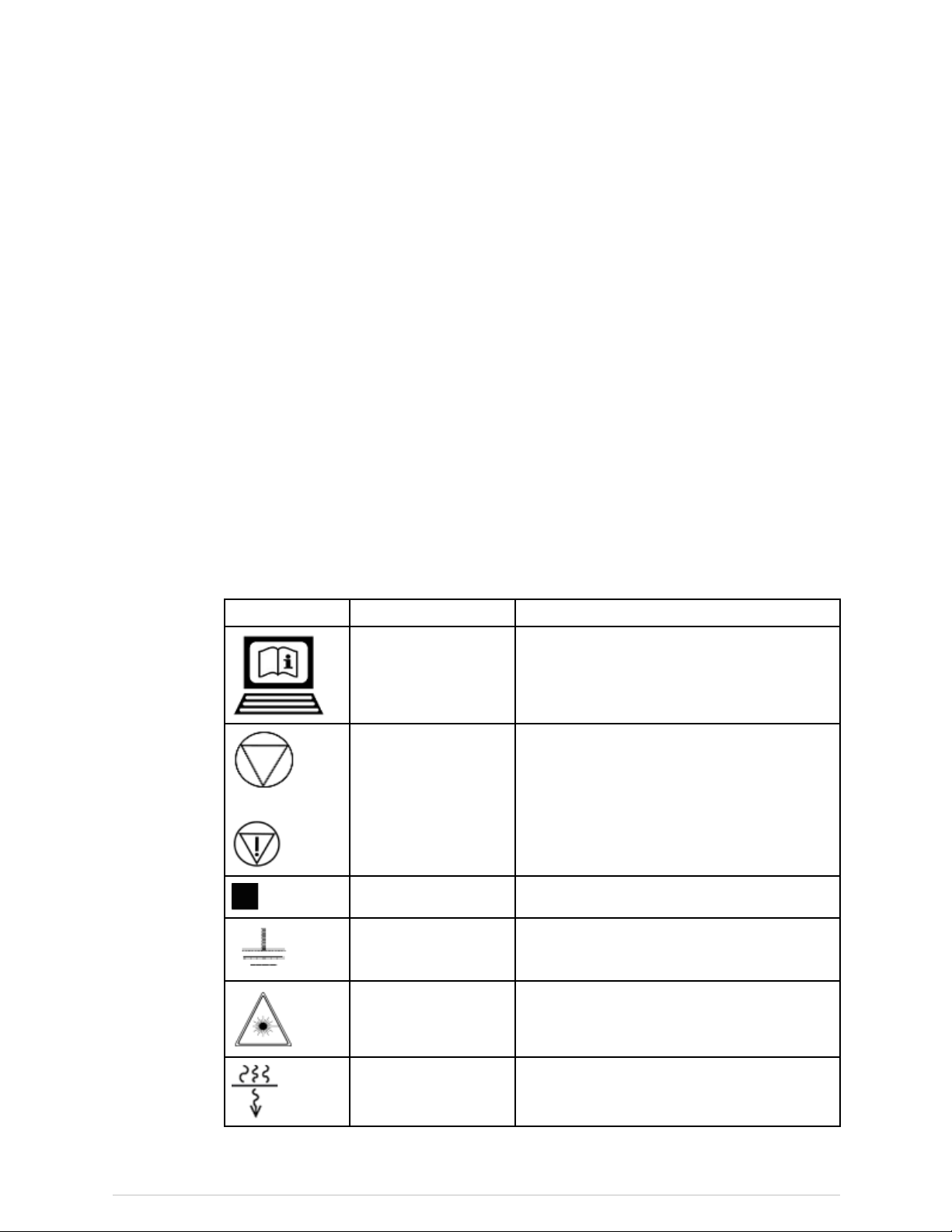
Symbols
Symbol
or
Name
ElectronicInstructions
forUse
EmergencyStop
FocalPointSymbolfromEN60417-1,5327
FunctionalEarthShowslocationofFunctionalEarthterminal
Description
SymbolindicatingthattheInstructionsforUse
aresuppliedinelectronicform
Showsthelocationoftheemergencystop
button
LaserOn
PermanentFiltrationSymbolfromEN60417-1,5381
29
X-rayBoneDensitometerwithenCOREv17software-UserManual
ShowsthelocationoftheLaserOnindicator.
Page 30
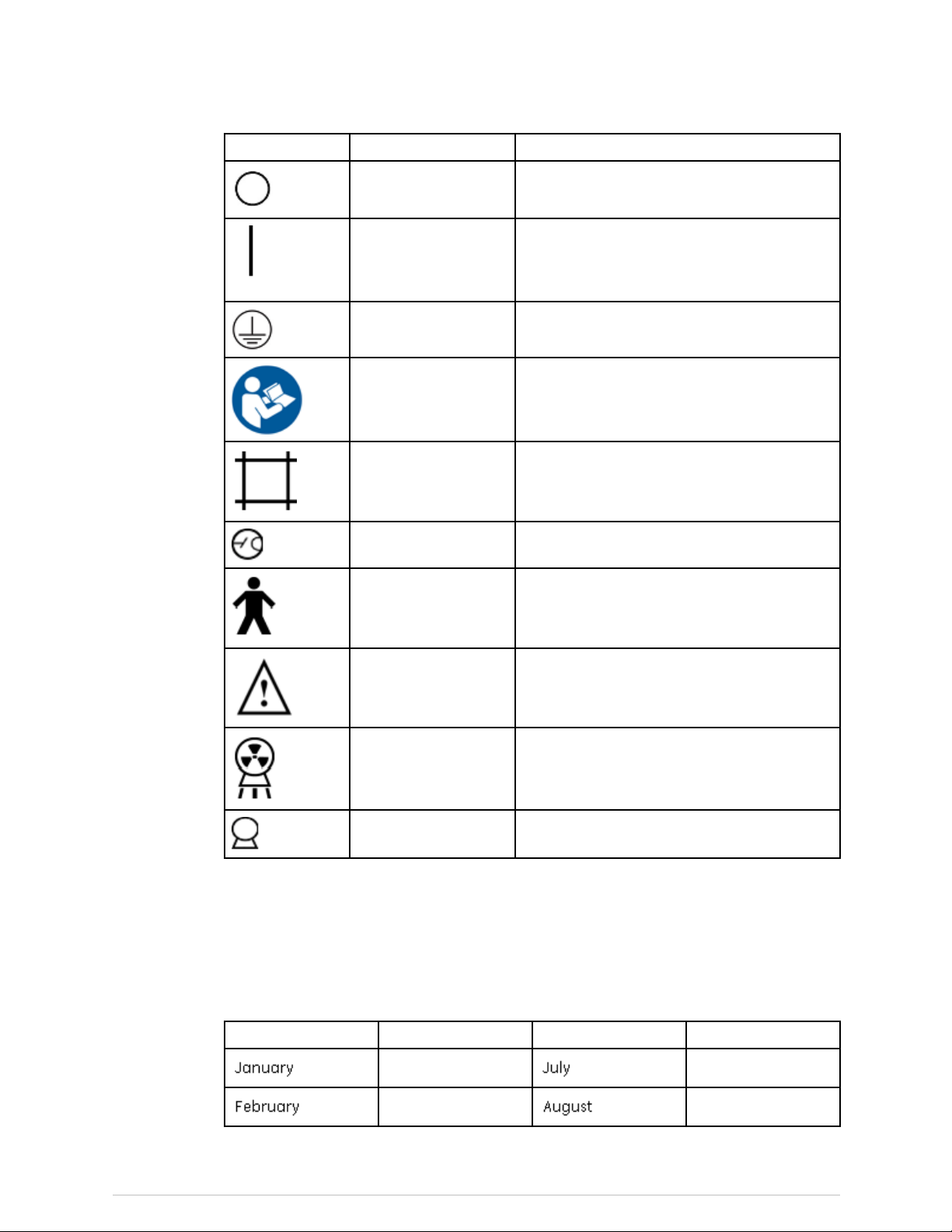
Safety
Symbol
Name
PowerOffShowstheswitchpositionforPowerOff
PowerOn
ProtectiveEarthShowslocationofProtectiveEarthterminal
RefertoInstruction
Manual
ShutterOpenShowsthelocationoftheShutterOpen
TubeInsertSymbolfromEN60417-1,5337
TypeBEquipment
Description
ShowsthelocationofthePowerOnindicator
andtheswitchpositionforPowerOn
AlertstheuserthattheUserManualcontains
importantsafetyinformation
indicator
ShowsthatthescannerhasTypeBprotection
againstelectricalshock
SampleLabels
Warning
X-rayOn
X-raySource
Showsimportantsafetywarnings,suchasthe
locationofpinchpoints
ShowsthelocationoftheX-rayOnindicator
SymbolfromEN60417-1,5338
Actuallabelappearancemayvaryfromthesamplesdisplayed
inthissection.
Forlabelsshowingcerticationto21USCFRSubchapterJ,themonthinthe
Manufacturedeldistranslatedbelow.
EnglishTranslationEnglishTranslation
January
February
July
August
30
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 31

EnglishTranslationEnglishTranslation
MarchSeptember
AprilOctober
Safety
May
June
LaserCautionandIonizingRadiationLabel
November
December
ThislabelshowsthatthescannerusesaClassIIlaserandproducesionizingradiation
(x-rays).
LaserRadiation.Donotstareintobeam.Class2LaserProduct.Laserwavelengthis
655nm.
31
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 32

Safety
TubeHousingAssemblyLabel
Thislabelgivestubeassemblyandx-raysourcecharacteristicsinformation.Itis
locatedonthetubeheadassembly(insidethescanner)andonthefootpanelofthe
scanner.
SystemLabel
Thislabelgivessysteminputpowerrequirementsandcomplianceinformation.Itis
locatedonthefootpanelofscanners.
32
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 33

TheRefertoInstructionManualsymbolindicatesaneedtoreadaccompanying
documents.
ThePersonsymbolreferstoTypeBappliedparts(anyexposedsurfaceofthescanner
tableassembly)fordegreeofelectricshockprotectionperEN60601-1.
TheFansymboldenotesthationizingradiationisgenerated.
TheGOSTsymbolshowscompliancewithRussianregulations.
TheCEmarkshowscompliancewiththeMedicalDeviceDirective93/42/EEC.
TheETLmarkshowscompliancetoANSI/AAMIES60601-1andCAN/CSAC22.2No.
60601-1.
TheEACsymbolshowsthatthisproductpassedallconformityassessment(approval)
proceduresthatcorrespondtotherequirementsofapplicabletechnicalregulations
oftheEurasianCustomsUnion.
TheWasteReceptaclemarkindicatesthatthewasteofelectricalandelectronic
equipmentmustnotbedisposedasunsortedmunicipalwasteandmustbecollected
separately.Pleasecontactanauthorizedrepresentativeofthemanufacturerfor
informationconcerningthedecommissioningofyourequipment.
Safety
HighVoltagePowerSupplyLabel
Thislabelgiveshighvoltagepowersupply(x-raygenerator)information.Itislocated
onthehighvoltagepowersupply(insidethescanner)andonthefootpanelofthe
scanner.
33
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 34

Safety
X-rayControllerLabel
Thislabelshowsx-raycontrollercompliance.Itislocatednearthex-raycontroller
(insidethescanner)andonthefootpanelofthescanner.
CollimatorAssembly
Thislabelgivescollimatorassemblyinformation.Itislocatedonthecollimator(inside
thescanner)andonthefootpanelofthescanner.
WarningandRadiationSymbolLabel
Thislabelshowsthatthesystemusesionizingradiation.Itisfoundonlyonsystems
deliveredintheUnitedStates.Alwaysobeyinstructionsforsafeoperation.
34
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 35

GroundingReliabilityLabel
Thislabelstatesthatgroundingreliabilitycanbemaintainedonlywhenusinga
"HospitalGrade"or"HospitalOnly"receptacle.Itisfoundonallpowercordsof
systemsdeliveredintheUnitedStates.
Safety
UniqueDeviceIdenticationLabel
Everymedicaldevicehasauniquemarkingforidentication.TheUDImarking
appearsonthedevicelabeling.
FailsafeCircuit
Duringoperation,thescannerisconstantlymonitoredfordiagnosticfailures.Ifa
diagnosticfailureoccurs,thefailsafecircuitstopspowertothescannermotorsand
disablesthex-raysystem.Amessageisshownonthecomputerthatdescribesthe
failure.CallGESupportoryourGEdistributorandprovidethefailuredescription.
X-RayShieldingRequirements
InstallaCaution:X-Radiationsignintheareaorroomwherethesystemisoperated.
Becauseoflowleakagelevelsofradiationfromthex-raytubeassembly,additional
shieldinginthewalls,oor,orceilingisnotnecessary.However,callyourstateorlocal
healthandradiationsafetydepartmentsforshieldingrequirements.
ThisisonlyanexampleofaUDImarking.
35
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 36

Safety
ElectricalSafety
PeripheralCongurations
InsulatepatientfromanymetalassociatedwiththeDPXDuobyusinga
non-conductivematerialduringcauterizationorsimilartreatmentstoavoid
shockorburns.
Donotplugadditionaloutletstripsorextensioncordsintopowersource
connectedtoscanner.
Toavoidriskofelectricshock,thisequipmentmustbeconnectedonlytoa
supplymainswithprotectiveearth.Scannerpowercordmustbeconnected
directlytothewalloutletortoaredundantlygroundedUPS.Neverpowerthe
scannerviaanoutletstrip.
Thecorrectconnectionofthecomputerandallperipheralsisnecessaryto
maintainelectricalsafety.Thesignalcableofthescannerisintendedonlyfor
connectiontoanapprovedcomputer .CallGESupportoryourGEdistributor
beforeaddingperipherals.
StandardRoomConguration
SmallRoomConguration
Operatorshallnottouchpatientandcomputerorperipheralssimultaneously.
Failuretouseoutletstripsproperlycancausemedicalelectricalsystem
leakagecurrentsinexcessof100microamperes.Formoreinformationon
medicalelectricalsystems,refertoIEC60601-1.
Thecomputer,peripherals,andallotherequipmentmustbelocatedmorethan1.5m
fromthescanner.Ifanoutletstripisusedtopowerthecomputer,itmustbemounted
offtheoorsothatitdoesnottouchotherequipment.
Amodemand/ornetworkconnectioncanbemadeatanytimeifyouareusingthe
standardroomconguration.
Youmustpowerthecomputer,peripherals,andallotherequipmentwithanisolating
transformeriftheroomistoosmalltomaintainatleast1.5mofseparationbetween
thescannerandallotherequipment.
TheisolationtransformersuppliedbyGEHealthcarehasamaximumoutput
of400/500VA.Becausethetransformerincludesamultiplesocketoutlet,only
system-relatedequipmentshallbepoweredbytheisolationtransformer.
Amodemand/ornetworkconnectioncanonlybemadeinthesmallroom
congurationifallexposedmetalsurfacesofthecomputerandperipheralsareoutof
thepatientenvironment.
36
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 37

Safety
ScatterRadiation
RefertoScatterRadiationDiagrams(298)toseeisodosediagramsofscannerscatter
radiation.
37
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 38

Safety
38
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 39

ProductInformation
IntendedUse
Thebonedensitometerisdesignedtoestimatethebonemineraldensityandbody
composition(leanandfattissuemass)ofpatientswhenmedicallyindicatedbytheir
physicians.
Thismanualprovidesinstructionsforoperatingthesoftwareandscantable,system
information,andmaintenanceinformation.
UnitedStatesFederalLawrestrictsthisdevicetothesale,distribution,andusebyor
ontheorderofaphysician(USAonly).
VariablesAffectingScanResults
Scanresultscanbeaffectedbyoperatortechniqueandpatientvariability.
Operatortechniquereferstopatientpositioningandscananalysis.Tominimize
techniquevariables:
2
●Establishconsistentpositioningandscananalysisroutinesbyusinganatomical
landmarkswhenpositioningpatients.
●Duringanalysis,manipulaterawscandataonlywhenabsolutelynecessary.
Patientvariabilityreferstochangesinthepatient'smedicalhistory,metabolism,and
diet.Italsoreferstodiagnosticproceduresthatinvolveradionuclideuptakeand
medicaltreatment,andthepresenceofexternalradiation(particularlytheuseof
otherradiation-generatingdevicesinthevicinityofthesystem).Tominimizepatient
variability:
●Thoroughlyfamiliarizeyourselfwiththepatient'shistory.
●Installthescannerinanenvironmenteffectivelyshieldedfromothersourcesof
externalradiation.
IndicationsforUse
Thex-raybonedensitometersupportsthefollowingindicationsforuse:
Providesanestimateofbonemineraldensityatvariousanatomicalsites(Spine,
Femur,TotalBody,andForearm).Thesevaluescanthenbecomparedtoanadult
referencepopulationatthesolediscretionofthephysician.
Providesanassessmentofrelativefractureriskbasedonthepatient'sT-scorevalue
usingthecategoriesoffractureriskdenedbytheWorldHealthOrganization(WHO).
Providesanassessmentof10-yearfractureriskusingWHOFRAXmodel.
39
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 40

ProductInformation
Providesastandardizedbonedensityreportusingdatafromthedensitometerand
physician-generatedassessmentsbasedonthepatient'sdemographics,whichcan
assistthephysicianincommunicatingscanresultstothepatientandthepatient's
referringphysician.
OptionalHandBMDsoftwareestimatestheBMDatthehand.
OptionalDual-EnergyVertebralAssessmentsoftwareprovidesanx-rayimageofthe
spineforqualitativevisualassessmentinordertoidentifyvertebraldeformationsand
estimatevertebralheights(morphometry).
OptionalOrthopedicsoftwareestimatesperiprostheticBMDofanorthopedichip
orkneeimplant(pre-andpost-surgery).
DPXonly:OptionalPediatricsoftwareoptionexpandstherangeofbonedensitometry
referencedatatoincludeages5through19years.Thesoftwareprovidesa
comparisonofmeasurevariablesobtainedbydualenergyx-rayabsorptiometryto
adatabaseofreferencevalues.Thesedatacanbeusedforcomparativepurposes
atthesolediscretionofthephysician.
OptionalCompletePediatricsoftwareoptionmeasuresbonemineralcontent(BMC),
bonemineraldensity(BMD)andbodycomposition(leanbodymassandfatmass)
inpatientsfrombirthto20yearsofage.Thesoftwareprovidesacomparisonof
measuredvariablesobtainedbydualenergyx-rayabsorptiometrytoadatabase
ofreferencevaluesforpatients5-19yearsofage.Thesedatacanbeusedfor
comparativepurposesatthesolediscretionofthephysician.Thesoftwaredoes
notprovideareferencepopulationforcomparativepurposesforpatientsyounger
than5yearsofage.
OptionalBodyCompositionsoftwaremeasurestheregionalandwholebodybone
mineraldensity(BMD),leanandfattissuemass,andcalculatesotherderivativevalues
whichcanbedisplayedinuser-denedstatisticalformatsandtrends,andcompared
toreferencepopulationsatthesolediscretionofthehealthcareprofessional.Someof
thediseases/conditionsforwhichbodycompositionvaluesareusefulincludechronic
renalfailure,anorexianervosa,obesity,AIDS/HIV,andcysticbrosis.
TheMirrorImagefunctionusedontheGELunarDXAbonedensitometerscanbe
usedtoestimatethetotalbodycompositionandbonemineraldensity(BMD)when
regionsofthebodyareoutsideofthescanwindowbyusingscanneddatafromthe
correspondingregion(s)ontheoppositehalfofthebody.
OptionalCoreScan
*
softwareestimatestheVisceralAdiposeTissue(VAT)content
withintheandroidregioninamaleorfemalepopulationbetweentheagesof18
and90withaBMIbetween18.5and40,excludingpregnantwomen.Thecontent
thatisestimatedistheVATMassandVATVolume.Thevaluescanbedisplayedin
user-denedstatisticalformatsandtrends.Someofthediseases/conditionsforwhich
VATestimationcanbeusefulincludehypertension,impairedfastingglucose,impaired
glucosetolerance,diabetesmellitus,dyslipidemiaandmetabolicsyndrome.
OptionaltotalbodycompositionsoftwareestimatestheRestingMetabolicRate(RMR)
inthemaleorfemalepopulationage18andolder.Thedatacanbedisplayedin
user-denedstatisticalformatsandtrends.
OptionaltotalbodycompositionsoftwareestimatestheRelativeSkeletalMuscleIndex
(RSMI)inthemaleorfemalepopulationage18andolder .Thedatacanbedisplayedin
user-denedstatisticalformatsandtrends.
OptionalAdvancedHipAssessment(AHA)softwareprovidesameasurementofhip
axislength(HAL)andameanvalueofHALforCaucasianandAsianfemaleson
40
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 41

femurimages.Italsocalculateshipgeometryvaluesusedtoevaluatethestructural
propertiesofthehip.
TheDPX-Duohasspecialmechanicalfeaturesincludingstirrups,storagedrawers,and
patientsteptoallowuseasanexamtablewhenbonedensitometryisdisabledand
thescanarmisrotatedandlockedparalleltothetable.
OptionalAtypicalFemurFracture(AFF)softwareusesfemurimagestovisualizefocal
reactionorthickeningalongthelateralcortexofthefemoralshaftwhichmaybe
accompaniedbyatransverseradiolucentline.Thissoftwareprovidesmeasurements
ofthelateralandmedialcortexwidthandquantiesfocalthickeningofthelateral
cortexalongthefemoralshaft.Thebeakingindexcanbedisplayedandtrended
acrossserialscans.
Optionalsarcopeniasoftwarecalculatesvaluesbasedonpublisheddenitionsand
thresholdsusingmeasuredappendicularleanmassincombinationwithpatient
demographicsandenteredvaluesofmusclestrengthandphysicalperformance.
Thesevaluesmaybeusefultohealthcareprofessionalsintheirmanagementof
sarcopenia.
CautionsforDXADeterminations
ProductInformation
Youshouldbeawareofthefollowingfactorswhichmayaffecttheclinicalaccuracyof
DXAspineestimates:markeddistortionsofskeletalarchitecture(e.g.,osteophytes,
degenerativediscdisease,spinalarthritis,spondylolisthesis,kyphoscoliosis,and
vertebralfractures)andsignicantcalciumdepositsintheaortacanfalselyelevate
spinebonemineralvalues.Regionsthatcontainthesedystrophiccalcicationscanbe
excludedfromthescananalysisinsomecases.Thescannercanbeusedtomonitor
changesinbonemineralovertimeinpatientswiththesedisorders,butcautionmust
betakenininterpretation.UseDXAestimatesasanaidtoothermethodsinthe
evaluationofpatientbonemineralstatusintheclinicalsetting.
Inaddition,spineestimateswillbedifculttointerpretforpatientswithorthopedic
metaldevicesandprevioussurgicalinterventions,suchasbonegrafts.Radiographic
contrastmaterialandradiopharmaceuticalsusedformyelograms,bariumenemas,
andotherdiagnostictestspreventaccurateestimates.Bariumclearsthebodywithin
afewdays,buttheoil-baseddyesusedinmyelogramsseveralyearsagomayremain
withinthebodyforyears.Athree-daywaitingperiodissufcienttimeforbarium
andmostradiopharmaceuticalstobecompletelydischargedfromthebody.DXA
measurementswillbedifculttointerpretforpatientstakingStrontiumorStrontium
ranelatebecauseDXAoverestimatestheactualbonemassduetothehigheratomic
numberofStrontiumcomparedtoCalcium.
Femurestimateswillbedifculttointerpretforpatientswithorthopedicmetaldevices
andprevioussurgicalinterventions.Themostcommoncomplicatingfactorsforfemur
estimatesareprostheticdevicesandsurgicalimplantsintheregionofthebonescan.
Resultsmaybeadverselyaffectedifthepatienthasdifcultywiththedesired25°
inwardrotationofthelegorwithmaintainingthispositionwithoutmovement.
TotalBodyestimatesrequireconsistentpatientpositioningforaccurateresultsand
willbedifculttointerpretforpatientswithorthopedicmetaldevicesandprevious
surgicalinterventions.Theoperatorshouldpayparticularattentiontothelocationof
thepatient'sarms,keepingthepositioningthesameforeachscan.Resultsmaybe
affectedifthepatientmovesduringthescan.
41
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 42

ProductInformation
DeviceDescriptions
Structure
Thedeviceincludesthefollowingbasiccomponents:
1.Anx-raysourcewithappropriateltrationtoformawell-deneddual-energy
beam,
2.Anx-raydetectorcapableofmeasuringtheattenuatedbeamattwoenergylevels,
3.Asupportforholdingthesubjectbetweenthesourceanddetector,
4.Amechanicalmeanstomovethesourceanddetectorinarectilinearscanofa
selectedareaofthesubject’sbody,and
5.Softwareandelectroniccontrolsforthepreviouslymentionedcomponents.
6.Whereapplicable,phantomsandpositioningblocksareusedwiththesystem.
Dependingupontheenabledfeatures,thesecomponentsmayvaryormaynot
berequired.
7.Optionalsystemcomponentsincludethesmallroomkit,whichisusedwhen
thePCisclosetothetable;encapsulatedphantom,whichisanencapsulated
aluminumspineinanacrylicblock;andanuninterruptiblepowersource(UPS).
Thebonedensitometerisdividedintoascannerandacomputer.Thescanner
comprisesthex-raysourceanddetector,thepatienttable,themechanicaldrive
system,andthelowestlevelportionsofthecontrolsystem.Thescannerisin
communicationwiththecomputer,whichisastandardPC.Thecomputerrunsthe
enCOREsoftware,andthuscontrolsthescanner,acquiresscandatafromthescanner,
storesandanalyzesthedata,andinteractswiththehumanoperator.
DPX-BravoandDPX-Duo
TheDPX-BravoandDPX-Duomodelsusepencilbeamtechnologywithasingle-crystal
channelNaIdetectorandhaveacompacttabledesigntoprovidespaceefciency.
TheDPX-DuoandtheDPX-Bravocomeequippedwithascanarmthatswingstothe
sideofthetablewhennotinuseasadensitometerandtofacilitatepatientloading.
X-rayscanningisnotpossibleuntilthescanarmislockedintothescanposition.A
handlereleasesthescanarminterlockandallowsoperatortomovethescanarm
forpatientloading.Oncethepatientisloadedonthetable,theoperatormovesthe
scanarmbacktoscanningpositionandthearmlocksintoscanposition.Ifascan
isattemptedwithoutthescanarmlockedintoposition,thefollowingerrorwillbe
displayed:
ErrorDescription:
Swingarmnotlockedinscanningposition.Pleaselockbeforecontinuing.
CorrectiveAction:
Pleasetryagain.Iftheproblempersists,contactGELunarSupportforassistance.
Toretractthescanarmoncethescaniscompleted,homethescanarm.
42
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 43

ProductInformation
Pulltheleveronthefrontofthescanarmtowardsyouandpushthescanarmtothe
leftuntilitrestsalongthebackofthescanningtable.Thepatientcanthensitupand
thetableisfreeofobstruction.
Thepowerswitchislocatedattheheadofthetable.Thereisalsoarollattheheadof
thetabletostoreupto21”x3”(53.34cmx7.62cm)exampaper.Thetableweight
limitis159kg(350pounds).
DPX-Bravo
Item
1
2
3
4
Description
Tablepad
Swingscanarm
Scanarmcontrolpanel
Exampaperrollandpowerswitch(headofscanner–notshown)
TheDPX-Duomodelalsohasmechanicalfeaturesincludingstirrups,procedure
drawer,storagedrawers,andpatientsteptoallowuseasanexamtablewhenbone
densitometryisdisabledandthescanarmisrotatedandlockedparalleltothetable.
43
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 44

ProductInformation
DPX-Duo
DPX-NT/Pro/MD+
Item
1
2
3
4
5
6
7
8
Description
Patientstep
Storagedrawers
Stirrups
Tablepad
Swingscanarm(inscanningposition)
Scanarmcontrolpanel
Exampaperrollandpowerswitch(headoftable–notshown)
Proceduredrawer
TheDPX-NT,DPX-Pro,andDPX-MD+modelscomeinfullandcompactsizesanduse
pencilbeamtechnologywithasingle-crystalchannelNaIdetector.Thepowerswitch
islocatedonthelowerfrontpanel.Thetableweightlimitis136kg(300lbs).
44
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 45

ProductInformation
DPX-NT
Item
1
2
3
4
Description
Powerswitch
Tablepad
Scanarm
Scanarmcontrolpanel
Prodigy/ProdigyPrimo/ProdigyAdvance/Prodigy
Pro/ProdigyForma
Prodigymodelscomeinfullandcompactsizesandusefanbeamtechnologywitha
16-channeldetector.Thepowerswitchislocatedatthefootofthescanner.Thetable
weightlimitis159kg(350lbs).
ProdigySeries
Item
1
2
45
X-rayBoneDensitometerwithenCOREv17software-UserManual
Description
Powerswitch
Tablepad
Page 46

ProductInformation
iDXA/iDXAForma
Item
3
4
Description
Scanarm
Scanarmcontrolpanel
TheLunariDXAusesfanbeamtechnologywitha64-channeldetector,isascanner
designedforoptimalimagequality,andsupportspatient'sweightsto204kg(450lbs).
Thepowerswitchandexampaperrollislocatedattheheadofthescanner.
LunariDXASeries
Item
1
2
3
4
5
Description
Exampaperroll
Tablepad
Scanarm
Scanarmcontrolpanel
Powerswitch(headofscanner–notshown)
TheWarninglabelidentiesthelocationofpossiblepinchpoints.Whenthescanner
armisinmotion,makesurepossiblepinchpointareasareclearatalltimes.The
technologistmustkeepbothfeetawayfromthemovingcarriage.Patientlimbsmust
remaininsidetheboundariesofthetabletoptoavoidapinchbetweenthescanner
armandtable.
46
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 47

ScannerTableAssembly
ScannerTable
ProductInformation
Theterm“scanner”isequalto“x-raybonedensitometer.”
NomodicationoftheScannerTableassemblyisallowed.Forservice,please
callGESupportoryourGEdistributor.
Thescannertableisusedtosupportthepatientduringameasurementorgeneral
examination(DPXDuoonly).Inaddition,thex-raysourceassemblyandother
electronicsarecontainedinsidethescannertable.
ScannerArm
Thelaserlight,emittedfromanapertureonthescannerarm,helpsyoulocatethe
measurementstartposition.Positioningswitchesletyoumovethescannerarmuntil
thelaserlightislocatedatthecorrectstartposition.Thestartpositionisdifferentfor
eachmeasurementtype.
OnDPXDuo/Bravoscanners,thescanarmhasareleaseandlockingmechanism
allowingtheupperarmtoswivelwhenthescannerisidle.Thescanarmmustbein
thelockedpositionoverthescannertabletoperformameasurement.
ScanArmControlPanel
Scanarmcontrolpanel:DPXBravo/Duo
47
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 48

ProductInformation
1Alarmindicatorlights
2Levertolock/unlockswingarm
3Positioningswitches
4Emergencystopbutton
Scanarmcontrolpanel:DPXNT/MD+/Prodigy/ProdigyPrimo/ProdigyAdvance
1
2
3
Alarmindicatorlights
Emergencystopbutton
Positioningswitches
Scanarmcontrolpanel:iDXA
1
2
Alarmindicatorlights
Emergencystopbutton
48
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 49

ProductInformation
3
4
Thefollowingtabledescribestheindicatorslocatedonthescanarmcontrolpanel.
Thex-ray,shutter,andlaserindicatorsareconsideredlowpriorityalarmconditionsas
denedinIEC60601-1-8.Thepowerindicatorisnotconsideredanalarmcondition.
SymbolIndicator
Positioningswitches
Startscanbutton
Status(on)
Green(power)
Yellow(x-ray)X-raytubeassemblyissupplyingx-rays
Yellow(shutter)Shutterisopen
Amber(laser)
Powerissuppliedtothescannertable
Laserison
EmergencyStopButton
Pushtheredemergencystopbuttontostopthescannerarmandimmediatelyshut
downx-raysinanemergency.Donotusetheemergencystopbuttontoroutinely
stopthescannerduringnormaloperation.
PositioningSwitches
Thepositioningswitchesmovethescannerarmanddetectortothemeasurement
startposition(thelaserlightindicatesthepositionofthedetector).TheBack/Front
switchmovesthedetectoracrossthewidthofthescannertable.TheLeft/Rightswitch
movesthescannerarmdownthelengthofthescannertable.
SwingArmPositionSensingSwitches
OnDPXDuo/Bravoscanners,theswingarmpositionsensingswitchesdetectthe
lockingstatusoftheswingarmandtheswingarmlatch.Theswingarmlatchmust
belockedandtheswingarmmustbeinthelockedpositionoverthescantable
beforeameasurementcanbeperformed.Releaseoftheswingarmlatchduringa
measurementwillabortthescanandthemeasurementdatawillbelost.
StartScanButton
OniDXAscanners,thestartscanbuttoninitiatesthepatientmeasurement.Thestart
scanbuttonislocatedonthedisplaypanelnearthepositioningswitches.
49
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 50

ProductInformation
TrainingInformation
GEHealthcareoritsauthorizeddistributorsprovideindividual,hands-ontrainingas
partoftheinstallationprocedureforyoursystem.(GEdistributorsprovidetraining
forsystemsinstalledoutsidetheUnitedStates.)AnApplicationsSpecialistprovides
informationonsoftwareandhardwareoperations,andreviewsthewarningsand
cautionsinthemanuals.
Onlytrainedtechnologistsshouldoperatethesystem.New
technologistsshouldreceivetrainingpriortounsupervised
operationofthesystem.Additionaltrainingsessions
areavailableonrequestforanominalfee.Formore
information,contacttheGECustomerServiceDepartmentat
888-281-4947,oryourlocalGErepresentative.
Classications
Protectionagainstelectricshock:ClassI,TypeB
Protectionagainstwater:IPX0
InstallationandOperation
SoftwareInstallation
Operationmode:Continuousoperation
Thedevicecanneitherbeusedinammableanestheticmixturewithairnor
non-ammableanestheticmixturewithoxygenornitrousoxide.
OnlyindividualstrainedbyGEHealthcareshouldserviceorinstallthex-raybone
densitometer.DonotattempttoservicetheX-rayBoneDensitometer.CallGEService
oryourGEdistributorforsupport.
Beforeoperatingthex-raybonedensitometer,reviewSafety(26).
Ifloadingsoftware,youwillbeaskedforyoursystemnumberandfeaturecodeduring
theinstallationprocedure.Thesenumbersareprintedonasheetincludedwiththe
software.
1.PuttheDVDintheDVDdrive.
2.WhentheInstallationwindowappears,selecttheproductsoftwareoption.
Iftheinstallationdoesnotautomaticallystart,select
MyComputer ,selecttheDVDdrive,anddouble-clickthe
softwareinstallationicon.
3.Followthescreenpromptstoinstalltheprogram.
TheenCOREinstallationprogramautomaticallyappliesWindows732-bitService
Pack1andvalidatedMicrosoftSecurityupdatesonEnglishoperatingsystems,
ifnotalreadyinstalled.Onceallupdatesareapplied,thesystemautomatically
restartsandcontinueswiththeinstallationoftheenCOREproductsoftware.This
processmaytakeupto90minutes.
50
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 51

Features
Dependingonthecountry,scannermodel,andnumberofoptionsyoupurchased,not
allofthefeatureslistedbelowmaybeincludedwithyourscanner:
HardwareFeatures
●DPXsystems:Pencilbeamtechnology.
●ProdigyandiDXAsystems:Narrow-anglefanbeamtechnologywithMulti-View
imagereconstruction.
●DPXsystems:Single-elementNaIdetector.
●ProdigyandiDXAsystems:Multi-elementdetector .Imagingperformancemeetsor
exceeds1.5mmholepairsandislimitedbytheProdigydetectorpitchof3.2mm.
●iDXAandDPX-Duo/Bravosystems:Paperrolldispenser.
●DPX-Duo/Bravosystems:Swingarm.
●DPX-Duosystems:Footrests,patientstep,twostoragedrawers,proceduretray.
SoftwareFeatures
ProductInformation
Notallfeaturesareavailableonallmodels.
●QuickViewmeasurementapplication
●Totalbodymeasurementandanalysis
●Pediatricspinemeasurementandanalysis
●Pediatrictotalbodymeasurementandanalysis
●Pediatricfemurmeasurementandanalysis
●Pediatricassessmentsofgrowthanddevelopmentincludingheightforage,BMC
forbonearea,boneareaforheight,leanbodymassforheight,andBMCforlean
bodymass
●Lateralspinemeasurementandanalysis
●Dual-energyvertebralassessment(lateralandAP)
●Spinegeometry
●Handmeasurementandanalysis
●Smallanimaltotalbodymeasurementandanalysis
●ClearViewlterforenhancingimagesbyreducingsofttissuenoiseandimproving
boneedges(useradjustable)
●Totalbody%fatandandroid/gynoid%fatestimation
●RestingMetabolicRate(RMR)estimation
●RelativeSkeletalMuscleIndex(RSMI)estimation
●Sarcopeniacalculator
●CoreScan–VisceralAdiposeTissue(VAT)estimation
●Bodycompositioncolorcodingandmapping
●APspinemeasurementandanalysis
●OneScanmeasurementapplication
●Femurmeasurementandanalysis
●Atypicalfemurfracturemeasurementandanalysis
51
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 52

ProductInformation
●DualFemurmeasurementandanalysis
●Totalbodyandregionaltissuequantitation
●AndroidandgynoidROIanalysisonbodycomposition
●Forearmmeasurementandanalysis
●Orthopedichipmeasurementandanalysis(withextendedGruenanalysis)
●Orthopedickneemeasurementandanalysis
●ScanCheck(formerlyknownasComputerAidedDensitometry,orCAD)
●Composerreportingtools
●PracticeManagementtools
●DICOMandHL7interfacecapability
●SQLServerapplication
●TeleDensitometry
●Multi-userdatabasecapability
●OneVisioncapability
●AdvancedHipAssessment(AHA)hipstrengthanalysis
●Customregionofinterest(ROI)analysis
●Previousscanimagecomparison
●Automaticmetaldetection
●HIPAASecureView
●Patient/examdirectorywithmultipledatabases
●GlobalUIwithmultiplelanguages,regionalsettings
●SmartScanforscanwindowoptimizationanddosereduction
●Automaticscanmodeselection
●Autoanalysis
●ROIcomparison(copy)
●BMDorSBMD,BMCandareameasurements
●Referencedata:Lunar,NHANES,manyregionalpopulations,andcustomoptions
●ReferencedatacomparisonsT/ZscoresandpercentYA/AM
●FRAX10-YearFractureRisk
●Trending
QualityAssurance(QA)Features
●QAautomatedtestprogram
●QAtrending
●Six-pointcalibration
●APspinephantom
UserInformation
●UserManual
Options
●Uninterruptiblepowersupply(UPS)
52
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 53

●SmallRoomkit
●Encapsulatedphantom
●PCcart
●Positioners
●Washabletablepad
ProductInformation
53
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 54

ProductInformation
54
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 55

DailyUse
DailyUse
3
1.Qualityassurance:Everymorning,beforeyoustartpatientmeasurements,
completethedailyQualityAssuranceprocedure.RefertoQualityAssurance(59).
Makesureyousaveyourprintedresultsforfuturereference.
2.Measurepatients:Iftimeallows,enterthePrimary,Secondary,andAdditional
dataforthepatientsyouexpecttomeasureduringtheday.RefertoMeasurement
(72).
3.Analyzeresults:Analyzeandprintresultsimmediatelyaftereachpatient
measurementiftimeallows.Otherwise,analyzeallofthepatientlesafterthe
lastpatienthasbeenmeasured.RefertoAnalysis(77).
4.Archiveexamles:Archiveyourexamlesbeforeyouleavefortheday.Inthe
unlikelyeventofacomputermalfunction,itisveryimportantthatyouhave
archivedlesofallofyourpatientmeasurementstorebuildyourdatabase.Refer
toArchiveExamFiles(55)forarchiveprocedures.
ArchiveExamFiles
5.Shutdowncomputer:Attheendoftheday,selectExitfromtheMainscreen,
selectShutDownfromtheClosewindow,andclickOKtoclosetheprogram.
Donotturnoffthescannerattheendofthedayfor
stationarysystems.
Eachday,archivenewexamlesfromyourcomputerharddrivetoanarchivedisk
orexternalharddrive.
Theprogramidentiesarchivedlesbylabelingthemwiththedrivelocationandthe
numberofthearchivedisk.Forexample:thethirdarchivedisklocatedindriveAis
labeledA:A3.LabelsforarchivedisksareshownintheLabelcolumnoftheImage
lelistontheDirectoryscreen.
Itisimportantthatyouwritethearchivenumberoneacharchivedisk.Ifitisnecessary
torestorearchivedlestotheharddriveorrebuildyourdatabase,theprogram
requiresthatyouusetheappropriatearchivediskaccordingtoitslabel.
1.SelectDirectoryfromtheMainscreenortheCommontoolbar.
55
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 56

DailyUse
2.Completeoneoftheseprocedures:
Archiveallexamsforall
patients
Archiveallexamsforall
patientsinthecurrent
searchresults
Archiveallexamsfor
selectedpatient
Archiveselectedexam1.SelectapatientfromthePatientlist.
1.SelectArchivefromtheDirectorytoolbar .
2.Inthedialogbox,selectArchiveallexamsforall
patients.
1.Selectasearcheldfromthedropdownmenu.
2.Entersearchcriteriaintheeldprovided.
3.ClicktheSearchbutton.
4.SelectArchivefromtheDirectorytoolbar .
5.Inthedialogbox,selectArchiveallexamsforall
patientsinthecurrentsearchresults.
1.SelectapatientfromthePatientlist.
2.SelectArchivefromtheDirectorytoolbar .
3.Inthedialogbox,selectArchiveallexamsforselected
patient.
2.Selectthepatientexamleyouwanttoarchive.
3.SelectArchivefromtheDirectorytoolbar .
4.Inthedialogbox,selectArchiveselectedexam.
3.SelectOK.
Theprogramarchivestheexamlesfromthecomputerharddrivetothearchive
diskorexternalharddrive.ThearchivenumberfortheleisshownintheLabel
columnoftheImagelist.
4.Ifanarchivestoragesourceneedstobeinitiated,theprogrampromptsyouto
insertalabeledarchivediskintheappropriatediskdrive.Insertadiskasdirected.
SafeUseGuidelines
Obeythesesafetyguidelinesatalltimes.
●Readthemanualbeforeyouoperatethescanner.
●Thetechnologistoperatingthescannermustremaininvisualcontactwiththe
patientduringthemeasurement.
●Donotattempttoservicethescanner.CallGESupportoryourGEDistributor.
●Whenthescannerisnotinuse,makesuretheShutterOpen,X-ray,andLaser
lightsareoff.
●Donotputexcessivepressureonthescannerarm.
●Usethescannertableforpatientmeasurementsandexaminations(DPXDuo)only:
donotsit,standorlieonthetableforotherpurposes.
●Donotletliquidstouchthecomputerorscannertablemechanicsandelectronics.
56
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 57

EmergencyStopButton
Bepreparedtoabortthescanintheunlikelyeventarmmotionstopswith
thex-rayson.
Theemergencystopbuttonistheround,redbuttonlocatedonthescannercontrol
panel.
Donotusetheemergencystopbuttontoroutinelystopthescannerduringnormal
operation.
1.PushtheEmergencyStopbuttontostopameasurementinanemergency.
Powertothescannertablemotors,x-raytubehead,shutter ,andlaseristurnedoff.
2.SelectOKinthemessagewindowonthecomputerscreen.
TestEmergencyStopButton
DailyUse
Ifthereisahardwareproblem,DONOTtrytomeasurea
patient.CallGESupportoryourGEdistributor.
Testtheemergencystopbuttononceamonth,asfollows:
1.StartastandardAPSpinemeasurement.Donothaveapatientonthetable.
2.Pushtheemergencystopbutton.
MakesuretheX-rayandShutterlightsareoffandthatamessageonthe
computermonitorindicatestheemergencystopbuttonisactivated.
3.OnDPXNT/MD,DPXDuo/Bravo,andProdigysystem:Pushtheemergencystop
buttonagaintoresetthesystem.
4.Donotsavethepatientmeasurement.
Iftheemergencystopproceduredoesnotwork,callGESupportoryourGEdistributor.
CleanScannerTableEnvironment
Vacuumanddustthesystemsiteweekly.Dustthesurfaceofthesystemregularly
andusenonabrasivecleanerstoremovedirt.Donotletliquidspenetrateinsidethe
scannertable.
Donotconnectavacuumcleanertothesameelectricaloutletasthescanner.
Propercleaningandhandlingproceduresmustbefollowedtopreventthe
possibilityofcross-infectionsbetweensubjectsscannedonthesamesystem.
Cleananddisinfectthesystemaccordingtoyourlocalandcountryspecic
hygienicregulations.
AnnualMaintenance
57
GEHealthcarerecommendsthatyouscheduleannualpreventivemaintenanceby
aGE-authorizedserviceengineerafteryourwarrantyperiodexpires.ContactGE
SupportoryourGEdistributor.
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 58

DailyUse
X-RayTubeandLaserAssemblyMaintenance
ThereareNOUSER-SERVICEABLECOMPONENTSinsidethex-raytubeheadand
laserassemblies.
DONOTattempton-siteservicing.CallGESupportoryourGEdistributorimmediately
ifthesystemmalfunctions.
DONOTattempttomaintainorrepairthecomponentsandscannertable.Doingso
voidsallcurrentwarrantyandservicecontracts.
58
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 59

QualityAssurance(QA)
DailyQualityAssuranceProcedure
CompleteQualityAssuranceproceduresdaily.MakesureeachQAprocedurepasses.
Ifyoursystemdoesnotpassatest,checkthepositionofthecalibrationblockand
completetheQualityAssuranceprocedureagain.Iftheprocedurefailsasecondtime,
callGESupportoryourGEdistributor.
CompleteaQualityAssurance(QA)testeachmorningbeforeyoumeasureapatient.If
theroomtemperaturechangesmorethan5°Cduringtheday,thenperformanother
DailyQA.Thisprocedurecalibratesandveriesfunctionalityaswellastheaccuracy
andprecisionofthedensitometer.TheQAprocedureshouldbeperformedaminimum
ofonceaweekifthescannerisnotbeingused.SaveallQAprintoutsforoneyear.
UsetheblackcalibrationblocktocompleteaQAtest(thecalibrationblockconsists
oftissue-equivalentmaterialwiththreebone-simulatingchambersofknownbone
mineralcontent).LeavethepadonthescannertableduringtheQAprocedure.
1.SelectQualityAssurance(F5)fromtheMainscreenorselectQAfromthe
Commontoolbar.
4
2.SelectStart.
Amessageinstructsyoutopositionthecalibrationblock.
3.Putthecalibrationblockonthepadsothatthelaserlightrestsinthecenterofthe
cross-hairlabelonthecalibrationblock,andthebrassisonthebottom.
QAcalibrationblock
1
2
Laser
Brassonbottom
59
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 60

QualityAssurance(QA)
PrecisionandAccuracy
Precision
4.SelectOK,andfollowthescreenpromptstocompletetheQAprocedure.
IftheQAtestdidnotpass,repositionthecalibrationblockandrepeatthe
procedure.
Iftheprocedurefailsasecondtime,callGESupportforassistance.
5.ToprinttheQAresults(iftheautoprintoptionisnotset)selectReport.Keep
theQAprintoutforoneyear.
6.TotrendtheQAresults,selectSettings,chooseTableorGraph,andselectthe
resultthatyouwouldliketotrend.SelectOK.
Failuresarerepresentedbyreddotsonthegraph.
PrecisionistheabilitytorepeatedlyobtainthesameBMDvalue.Precisionerrormay
bereportedasastandarddeviation(SD)ing/cm
2
orasacoefcientofvariation(%CV)
whichisdenedastheSD/MeanBMDinpercent.
ThefollowingtabledenestheexpectedprecisionerrorformostcommonDXA
measurements.
Site
APspineBMD
FemurBMD
TotalbodyBMD
Precision(g/cm
0.010g/cm
0.010g/cm
0.010g/cm
2
)
2
2
2
Precision(%CV)
1.0%L1-L4orL2-L4
1.0%
1.0%
RegionofInterest
DualFemurTotal
Total
Precisionforothersiteswillrange1-3%.Precisionmaybeaffectedbyoperator
techniqueandothervariablesassociatedwithpatientanatomy.SeeCautionsforDXA
Determinations(41
)andPrecisionCalculator(181)foradditionaldetails.
APSpineThicknessDependence
TheprecisionofBMDmeasurementsoverasofttissuethicknessrangefrom15cmto
25cmislessthanorequalto2%(%CV).
APSpineDependenceonHeightaboveTabletop
TheprecisionofBMDmeasurementsovera5cmchangeinobjectplaneislessthan
orequalto2%(%CV).
TheprintedDXAreportsdisplayafootnoteindicatingtheprecisionerrorassociated
withameasurement:
1–Statistically68%ofrepeatscansfallwithin1SD(±0.010g/cm
2
forAPSpineL1-L4)
Accuracy
Accuracyindicateshowcloseameasuredvalueistotheactualvalue.
TheDailyQAfeaturemeasuresastandardblackblockwhichissuppliedwith
eachscanner.TheblockconsistsofthreechamberssimulatingBMDvaluesof
60
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 61

QualityAssurance(QA)
QAControls
approximately0.500g/cm
2
,1.000g/cm
2
,and1.500g/cm
2
.DuringDailyQA,the
BMDforeachchamberismeasuredandcheckedthatitiswithin0.030g/cm
itsexpectedvaluewhichensuresthelinearcorrelationforBMD(R≥0.99).Atthe
endofQA,theentireblockisscannedtosimulateanAPspinemeasurementand
theaccuracyischeckedagain.
NavigatetoQA>QATrendGraphstomonitorQAtrendsforkeyvalues.
FormoreinformationabouttheQAGUI,refertoQualityAssuranceScreen(235)and
QualityAssuranceToolbar(236).
ForinformationaboutcustomizingtheQAoptions,refertoQATab(249)intheOptions
section.
IconItem
StartupTest
MechanicalTest
Description
●DatabaseValidation
●ScannerSelf-test
●QABlockSearch
●Peaking
●BeamStop
●TransverseDistance
●LongitudinalDistance
2
of
ClicktheTrendtooltoexittheQAprocessscreen.
QATrendReportingOptions
OntheQAscreen,clickSettings.ThesettingsscreenprovidesmanyoptionsforQA
trendreportingcomponents.
IntheupperrightpanelareoptionsfortheQAReportType:
X-ray/Detector
Calibration●BMDvaluesofHigh,Mediumand
PhantomBMD,BMC,Area,Edgedetection
●SpectrumSpillover
●ReferenceCounts
●DetectorStatus
Lowblockchambers
●TissuevaluesofLean,Normaland
Fatblockvalues
●Trendanalysis
●QAPhantomReport
●Ancillarypage
61
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 62

QualityAssurance(QA)
●LegacyQAReport
QAPhantomReport
QAAncillarypage
62
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 63

LegacyQAReport
QualityAssurance(QA)
MeasuretheSpinePhantom
TheenCORE-basedscannerdoesnotrequireaseparateQualityControltoberun
inadditiontoQualityAssurance.ThedailyQAprocedurerunonthescannerboth
calibratesthemachineandhas“bone”chambersthatareusedforQualityControl
measurements.Thisremovesthenecessityofrequiringaphantomtobemeasured
bytheuserforseparatecontrolmeasures.Thephantomisconsideredaservice
tool.Everysystemincludesanaluminumspinephantomandwatercontainer.An
encapsulatedphantomisavailableforpurchase.
Watercontainer(left)andencapsulatedphantom(right)
Aspinephantombaselinewasperformedwiththeinstallationofyourscanner.Thisis
foundinthepatientdatabase.Forgeneraluse,usethesamepatientinformationthat
wasestablishedwiththespinephantombaselinescan.
63
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 64

QualityAssurance(QA)
Fordetailsonperformingthescan,refertoAPSpineMeasurementandAnalysis(86).
1.Put15cmofwaterintheplasticcontainerandpositionthealuminumphantom
inthemiddleoftheplasticcontainer.
2.PositionthephantomsothatL5istowardthefootofthescanner.
3.FromtheMainscreen,selectMeasure(F2).
4.Haveyoumeasuredthephantombefore?
a.Ifyes,selectthephantomfromthepatientlistandcontinuetostep7.
b.Ifno,continuetostep5and6torecordtheinformationforthespinephantom.
5.Recordtheprimaryinformationinthedialogbox:
Field
FirstnameSpine
Middleinitial
LastName
BirthDateRecordthecurrentdateminus40years.
Height67inchesor170centimeters
Weight154poundsor70kilograms
Sex
EthnicGroupWhite
Entry
None
Phantom
Forexample,iftoday'sdateisSeptember
28,2011,type09/28/1971.Donot
changethisdateforfuturespinephantom
measurements.
Male
6.Selectthesecondarytabandrecordthefollowinginformation:
Field
Comments
Entry
Recordthephantomnumbergivenon
theL5regionofthespinephantom.Also
recordyourSystemIDnumber.This
numberislocatedinTools>UserOptions
>Systemtab.
7.SelectPositionfromthetoolbar.
Agraphicisshownillustratingthecorrectpatientandlaserpositionforthescan
type.
8.Positionthelasercross-hairontheletter“R”intheword“LUNAR”ontheL5
vertebralbodyofthephantom.
64
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 65

9.Startthescan.
QualityAssurance(QA)
10.WhenapproximatelyhalfofT12isimaged,selectAbortfromthetoolbar.
11.ChooseSavemeasurementfromtheSavedialogboxandselectOKifthe
measurementwasperformedcorrectly.
12.Forspinephantomanalysis,itisnecessarytoverifyandadjustaccordinglyfor
thefollowingvertebralheights:
L2:3.00cm+/-.02cm
L3:3.50cm+/-.02cm
L4:4.00cm+/-.02cm
L2-L4regionheightshouldbe10.5cm
13.OntheAnalysisscreen,selecttheROItooltoviewthisinformation.
65
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 66

QualityAssurance(QA)
66
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 67

MeasurementandAnalysis
Measurement:OverviewandWarnings
PatientConsiderations
Obeythesepatientconsiderationsbeforeyoustartapatientmeasurement:
●Clothingrestrictions:Makesurethepatientremovesitemsthatcanattenuate
thex-raybeam,suchasclothingwithzippers,snaps,buckles,andbuttons.Ask
patientstowearajoggingsuittotheexamorgivethemanexaminationgown
whentheyarrive.
●Radionuclideandradiopaqueagents:Makesurethepatienthasnotingestedor
beeninjectedwithradionuclidesorradiopaqueagentsinthepast3to5days.If
thepatienthastakenteststhatusesuchagents,postponethemeasurementuntil
alltracesoftheelementhaveleftthepatient’sbody.A72-hourwaitingperiodis
usuallylongenoughformostagentstoleavethepatient’sbody.However ,consult
yourradiationsafetyofcer(RSO).
●Pregnancyrestrictions:Ifitisnecessarytomeasureapregnantpatient,thefetus
couldbeexposedtosmallamountsofradiation.Postponethemeasurementuntil
theendofpregnancyifclinicalmanagementisnotaffected.Thedecisionto
subjectafetustoradiationexposuremustbemadebythereferringphysician,
notingthat1)bonequalityformostpatientsdoesnotsignicantlychangeduring
pregnancyand2)intheadvancedstagesofpregnancy,thefetus’mineralizedbone
caninterferewithmeasurementsofthemother’sspineandfemur .
●Metaldevices:BMDestimatesaredifculttointerpretforpatientswithorthopedic
metaldeviceswithinthescaneld.
5
MeasurementWarnings
67
EachscannerisequippedwithaClassIILaserthatislessthan1milliwattin
strength.DONOTST AREINTOTHEBEAM.
Removefoamlegblockpriortopositioningscanarmoverthepatientand
immediatelyaftercompletinganAPSpinescan.
Verifythatthepatient'shead,arms,knees,andanyotherbodypartsarenot
indirectpathofamovingscanarm.
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 68

MeasurementandAnalysis
MeasurementModes
Patientthicknessdeterminestheappropriatemeasurementmode.Theprogram
defaultstotheappropriatemodebasedonthepatient'sheightandweight.Default
scanparametersarerecommendationstobeapplieddirectlytoallowoptimized
operation.Theoperatormakesthenaldecisionbasedonpatientthicknessbefore
startingtheexam.Theselectionofmeasurementmodesinuencesthepatient
radiationexposure.Formoreinformationaboutradiationexposureandmeasurement
modes,refertothesectionCurrentandTypicalDoseTables(307).
iDXA:PatientThicknessLimitsforenCOREMeasurementModes
iDXAiDXAForma
Site
APSpine
Femur
TotalBody
Mode
Thick
Standard
Thin
QuickView
Thick
Standard
Thin
QuickView
Thick-AFF
Standard-AFF
Thin-AFF
Thick
Standard
Thin
Patient
thicknessor
weight
>25cm>25cm
13-25cm13-25cm
<13cm<13cm
>13cm
>25cm>25cm
13-25cm13-25cm
<13cm<13cm
>13cm
>25cm>25cm
13-25cm13-25cm
<13cm<13cm
>25cm>25cm
16-25cm16-25cm
<16cm<16cm
Patientthickness
orweight
NA
NA
Small<9kg(20lb)<9kg(20lb)
APVA
OrthopedicHip
LVA
LateralStandardAllthicknessesAllthicknesses
68
X-rayBoneDensitometerwithenCOREv17software-UserManual
Thick
Standard
Thin
Thick
Standard
Thin
Standard
Thin
>25cm>25cm
13-25cm13-25cm
<13cm<13cm
>25cm>25cm
13-25cm13-25cm
<13cm<13cm
>13cm>13cm
<13cm<13cm
Page 69

iDXAiDXAForma
MeasurementandAnalysis
Site
Mode
Patient
thicknessor
weight
Forearm
StandardAllthicknessesAllthicknesses
HandStandardAllthicknessesAllthicknesses
OrthopedicKneeStandardAllthicknessesAllthicknesses
iDXA:SmallAnimalWeightLimitsforenCOREMeasurementModes
iDXAiDXAForma
ModePatientWeightPatientWeight
SmallAnimal
Large
>20kg>20kg
Medium2.0-20.0kg2.0-20.0kg
Small<2.0kg<2.0kg
Prodigy:PatientThicknessLimitsforenCOREMeasurementModes
Prodigy,
Prodigy
Pro,Prodigy
Advance
Patientthickness
orweight
ProdigyPrimo,
ProdigyForma
Site
Mode
Patient
thicknessor
Patientthickness
orweight
weight
APSpine
Thick
Standard
Thin
QuickView
Femur
Thick
Detail
Standard
Thin
QuickView
Thick-AFF
Standard-AFF
Thin-AFF
>25cm>25cm
13-25cm13-25cm
<13cm<13cm
>13cm
NA
>25cm>25cm
>13cm
NA
13-25cm13-25cm
<13cm<13cm
>13cm
>25cm
13-25cm
<13cm
NA
NA
NA
NA
69
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 70

MeasurementandAnalysis
Site
TotalBody
APVA
OrthopedicHip
LVA
Prodigy,
Prodigy
Pro,Prodigy
Advance
Mode
Patient
thicknessor
weight
Thick
Standard
Thin
>25cm>25cm
16-25cm16-25cm
<16cm<16cm
Small<27kg(60lb)
Thick
Standard
Thin
Thick
Standard
Thin
Standard
>25cm>25cm
13-25cm13-25cm
<13cm<13cm
>25cm>25cm
13-25cm13-25cm
<13cm<13cm
>13cm>13cm
ProdigyPrimo,
ProdigyForma
Patientthickness
orweight
<27kg(60lb)(N/A
onPrimo)
LateralStandardAllthicknessesAllthicknesses
Forearm
StandardAllthicknessesAllthicknesses
HandStandardAllthicknessesAllthicknesses
OrthopedicKneeStandardAllthicknessesAllthicknesses
Prodigy:SmallAnimalWeightLimitsforenCOREMeasurementModes
Prodigy,
Prodigy
Pro,Prodigy
Advance
ModePatientWeightPatientWeight
SmallAnimal
Large
Medium2.0-20.0kg2.0-20.0kg
Small<2.0kg<2.0kg
>20kg>20kg
(N/AonPrimo)
ProdigyPrimo,
ProdigyForma
70
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 71

DPX-Bravo/Duo:PatientThicknessLimitsforenCOREMeasurementModes
MeasurementandAnalysis
Site
APSpine
Femur
OrthopedicHip
ModePatientthickness
Thick>25cm
Standard15-25cm
Thin<15cm
Thick>25cm
Detail>15cm
Standard15-25cm
Thin<15cm
Thick>25cm
Standard15-25cm
Thin<15cm
LateralStandardNA
ForearmStandardAllthicknesses
DPX-NT/MD+:PatientThicknessLimitsforenCOREMeasurementModes
Site
APSpine
Femur
ModePatientthickness
Thick
Standard
Thin
Thick
Detail
Standard
Thin
>25cm
15-25cm
<15cm
>25cm
>15cm
15-25cm
<15cm
71
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 72

MeasurementandAnalysis
Site
TotalBody
OrthopedicHip
LateralStandardAllthicknesses
Forearm
ModePatientthickness
Thick
Standard
Thin
Thick
Standard
Thin
StandardAllthicknesses
MeasurementProcedures:Overview
Thissectiondescribesthebasicstepsnecessarytocompleteapatientmeasurement.
Thesestepsmustbecompletedintheordergiven.Reviewthestepsbeforeyoustarta
patientmeasurement.
1.Recordorselectpatientinformation:
●Recordinformationforanewpatient(73),or
●Selectapatientrecordfromthedatabase(72)
2.Selectthemeasurementsite:
>25cm
15-25cm
<15cm
>25cm
15-25cm
<15cm
●APSpine(86
●Femur/DualFemur(91)
●Forearm(110)
●TotalBody(114
●LateralSpine(143)
●LVAMorphometry(147)
●LVASpineGeometry(155
●APVAMorphometry(158)
●APVASpineGeometry(160)
●DualVA(APVAandLVAinonescan)(163)
●Pediatric(163
●Hand(167)
●OrthopedicHip(170
●OrthopedicKnee(174)
●SmallAnimal(177
)
)
)
)
)
Aftermeasurement,continuewithanalysis(77).
SelectExistingPatientRecord
)
SelectapatientforanewmeasurementfromeithertheMainscreenortheDirectory
screen.UsetheSearchoption(233)tondthepatient,ifnecessary.
FromtheDirectoryscreen
72
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 73

RecordNewPatientInformation
MeasurementandAnalysis
1.InthePatientlist,highlightthepatientandselectMeasurefromtheCommon
toolbar.
TheNewMeasurementscreenisshown.
FromtheMainscreen
1.SelectMeasure.
2.InthePatientInformationdialogbox,selectFind.
3.Double-clickthepatientinthePatientlist.
4.InthePatientInformationdialogbox,verifythatthepatientinformationiscorrect,
andthenselectOK.
TheNewMeasurementscreenisshown.
1.SelectMeasurefromtheMainscreenorNewfromtheDirectorytoolbar.
73
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 74

MeasurementandAnalysis
2.RecordthenecessaryinformationinthethreetabsinthePatientInformation
dialogbox.
PatientInformationdialogbox
●Primarytab:Youmustrecordthepatient’sname,birthdate,height,and
weighttocompleteapatientmeasurement.Changethedefaultgenderor
ethnicityintheTools>UserOptions>Directorytab>DirectoryRulesand
Defaultsbutton.
●Secondarytab:TheSecondarytabletsyourecordcommentsand
administrativeinformationthatisnotrequiredtocompleteapatient
measurement.IftheISCDguidelinesareturnedonunderTools>UserOptions
>Systems,itisimportanttoenteramenopauseageforpostmenopausal
womenbecausetheWHOcriteriaareonlyappliedtopostmenopausalwomen
andmenage50andolder.
●Additionaltab:TheAdditionaltabletsyourecordfracture,indication,and
treatmentinformationforthepatient.ThetabalsoallowsyoutoassignICD-9
orICD-10codestoeachfracture,indication,andtreatmentappliedtoall
patients.Inaddition,youcanenterthepatient’sinsuranceinformation.This
informationisnotrequiredtocompleteameasurement.
74
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 75

3.SelectOKwhenyouhavenishedrecordingthepatientinformation.
WhenyouattempttoaddanewpatienttotheDirectory,thesystemchecks
whetherthepatientalreadyexists.Ifthesystemndspotentialduplicates,you
arepromptedtoeitherselectanexistingpatientortocreateanewpatient.
Preventingduplicatepatientrecordsisimportantbecauseasinglepatientrecord
isneededtotrendapatient’sexamdataovertime.
Youcancongureyoursystemtoevaluateeitherthepatientname,birthdateand
patientIDoronlythepatientIDwhencheckingforduplicatesusingTools>User
Options>Directorytab>DirectoryRulesandDefaultsbutton.Pleaseseethe
DirectoryTab(241
IfyoustartedfromtheMainscreen,theNewMeasurementscreenisshown.
Proceedwiththemeasurement.
IfyoustartedfromtheDirectoryscreen,highlightthepatient'snameandselect
MeasurefromthetoolbartoopentheNewMeasurementscreen,andthen
proceedwiththemeasurement.
SelectMeasurementSite
MeasurementandAnalysis
)sectionformoreinformation.
AbortMeasurement
TheNewMeasurementscreenshowsaskeletalimageofthesitesyoucanselectto
measure.
NewMeasurementscreen
1.OntheNewMeasurementscreen,clickthesiteyouwanttomeasure.
ThesiteyouselectishighlightedintheExamlist.
2.Followthemeasurementprocedureforthesiteyouselected.
RefertoMeasurementModes(68)formoreinformation.
Iftheimageisnotcorrect,orifyoudeterminethatasufcientareaofthe
measurementhasbeenobtained,selectAbort(F5)fromtheNewMeasurement
toolbar.
WhenyouselectAbort,themeasurementstopsautomaticallywhenthedetector
reachestheedgeofthescanwindow.Amessageshowstheseoptions:
●ResumeMeasurementSelectthisoptiontocontinuethemeasurement.
●SaveMeasurementSelectthisoptiontosavethecurrentmeasurement.
75
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 76

MeasurementandAnalysis
OneVision
●RepositionthismeasurementanddonotsavetheabortedmeasurementSelect
thisoptiontostartthemeasurementagainusingthesamesettings.Theboxthat
isshownaroundtheimageshowsthemeasurementarea.Usethearrowkeys
tomovetheboxandrepositionthemeasurement.SelectStartfromtheNew
Measurementtoolbartorestartthemeasurement.
●"SetupanewmeasurementanddonotsavetheabortedmeasurementSelect
thisoptiontochangethesettingsforthemeasurement.
TheOneVisionfeatureallowsyoutosetupmultiplemeasurementsinoneexam.This
eliminateskeystrokesandimprovesthroughputforcustomersthatroutinelyperform
multiplemeasurementsoneachpatient.OneVisionisrequiredforDICOMorHL7
reportinginterfaces.Bydefault,theenCOREsoftwareincludestheexamcombinations
ofAPSpine+DualFemur,APSpine+DualFemur+LVA,andDualVA(LVA+APVA).Exam
combinationsarefoundatthetopoftheExamlistontheNewMeasurementscreen.
Theimagesincludedintheexamaredisplayedinatabview.
WhenscanningwithaseriesofOneVisionscantypes,selectNexttoproceedtothe
nextimagesiteintheexam,orRepeatthecurrentmeasurement.RefertoBasic
MeasurementProcedures(72)forinformationaboutobtainingappropriateimage
measurements.
CreateExamProtocols
YoucancreateyourownexamprotocolswiththeOneVisionfeature.
1.SelectMeasure>CreateExams.
2.Tocreateanexamprotocol,selectNew.
Youcanalsodelete,rename,oreditexistingexamprotocolsfromtheCreate
Examdialog.
76
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 77

MeasurementandAnalysis
3.Enteraprotocolnameintheboxprovided,thenselectOK.
Itisrecommendedthatyouenteranamethatdescribeseithertheimages
includedintheexamoraspecicdescriptionoftheexam.
Aftertheprotocolnameisentered,denethemeasurementsitesincludedinthe
examandthesequenceofthemeasurementsintheexam.
4.Selecttheimagesitefromtheavailablesitesontheleft,andselectAddtoaddthe
imagesitetotheexam.
5.UsetheUpandDownbuttonstomodifythesequenceofimagemeasurements
intheexam.
6.Whennished,clickOK.
QuickView
QuickViewoffersafast,10secondspineorfemurscan.Measurementandanalysis
proceduresarethesameasotherscanmodes.
ThelargerpixelwidthofQuickViewresultsinreducedimageresolution.
Standardscanmodesprovideoptimalprecisionandarerecommendedforfollow-up
scanstomonitorchangesinBMD.
Formoredetailsonthescanmodespecications,refertoMeasurementModes(68
AnalysisProcedures:Overview
Thissectiondescribesthebasicstepsnecessarytoperformanalysisofacompleted
patientmeasurement(72).Thesestepsmustbecompletedintheordergiven.Review
thestepsbeforeyoustartanalysis.
TheresultstabsforAPSpine,Femur,Forearm,andTotalBody
imagesincludeaScanCheck(183).UsethelistofYes/No
questionstoassistinanalysis.Thereisaspaceforcomments.
YoucanprintthechecklistbyselectingtoprintScanCheckin
theReportCenter.
1.Selectanimage(77)
2.Adjusttheimage(78)
●Advanced:AdjustROIs(79)
●Advanced:Adjustpointtyping(79)
3.Completeanalysisfortheselectedsite.
SelectImage
Thesebasicstepsapplytoallimages.Forspecicinstructionsaboutsiteanalysis,
refertothetopicforthespecicsite(forexample,APSpineAnalysis(89)orFemur/Dual
FemurAnalysis(96)).
).
1.FromtheMainscreen,clickAnalyze,selecttheimagetoanalyze,andclickOK.
Or,fromtheDirectoryscreen,selectthepatientfromthePatientlist,selectthe
imagetoanalyze,andclickAnalyze.
YoucanusetheSearchoptiontolocateapatientinalargedatabase.
77
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 78

MeasurementandAnalysis
AdjustImage
2.Adjusttheimageasneeded.
enCOREtypicallyperformstheanalysisautomatically.DonotchangeROIsor
pointtypingunlesstheanalysisshowsanobviousneedforcorrections.
Afterselectinganimage(77),youcanadjustitasneeded.
1.SelectImagingfromtheAnalyzetoolbartoadjusttheimage:
TheImageToolswindowisshown.
2.Usethiswindowtochangethegraylevelsoftheimageandzoomtheimage.
TheImageToolswindowshowsaboneproleandgivestheseoptions:
●Brightness:Toadjustthebrightnessfortheimage,clickanddragthe
brightnessscrollbarrightorleft.
●Contrast:Toadjustthecontrastfortheimage,clickanddragthecontrast
scrollbarrightorleft.
●ClearView:ForLVAandiDXAAPSpine,Femur,andForearmimages,youcan
adjustthesharpness.Toincreaseordecreasethesharpnessoftheimage,
movethearrowupordowntheClearViewscale.
●Zoom:Tozoomtheimage,usethebartoscrollthroughthepercentagevalues.
UsethePantooliftheimageislargerthanthewindowareaontheAnalyze
screen.
3.ClicktheDialogSizeiconinthelowerleftandselectAdvancedtoadjust
Threshold,Range,Low/HighControls,orImageTypeandtoresetcontrast.
4.Usethesetools(shownontheAnalyzescreen(231))tomagnifyanimageduring
analysis:
Icon
Tool
ResetModeSelecttodeactivatetheZoomandPan
ZoomImage
PanImage
ZoomSliderUsetheZoomSlidertozoomtheimage
Description
Imagetools.
SelecttheZoomImagetooltozoomin
oroutontheimage.Clicktheimageto
zoomin,orclicktozoomout.
Ifyouzoomtheimagelargerthanthe
viewableareaonthescreen,usethe
PanImagetooltoviewhiddenareas
oftheimage.Clicktheimageanddrag
thecursortopantheimage.
inorout
78
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 79

Advanced:AdjustROIs
ROIsdonotneedtobeadjustedinmostcircumstances.Theproceduresforadjusting
ROIpositionarespecictoeachmeasurementsite.
SomeROIadjustmentswillrendertheresultsunreliable.
Seethespecicimagetyperecommendationsforanalysis.
Advanced:AdjustPointTyping
Pointtypingisatoolthatletsyouviewhowtheprogramclassiedthesamplepoints,
andchangetheclassicationifnecessary.Thepointtypingdeterminestheplacement
ofboneedges.
enCOREanalysisautomaticallyassignspointtypingtoanimageandusuallyrequires
noadjustment.Signicantchangestopointtypingwillaffectboththeresultsand
reproducibilityofanimage.
MeasurementandAnalysis
Theproceduresthatfollowgiveinstructionstoexamineandadjustpointtypingfor
animage.
Donotadjustthepointtypingunlesstheprogramhasmadeobviouserrors.Change
thepointtypingonlyiftheareathatneedstobechangedislargerthanthedefault
cursorsize.ItisrecommendedthatchangesbelimitedtoBoneandNeutralpoint
typing.
1.SelectPointsfromtheAnalyzetoolbar.
ThePointTypewindowisshown.Theprogramautomaticallydeterminesifa
sampleisbone,tissue,neutral,air,orartifact:
●Bone:VerifythattheboneistypedasBone.
●Artifact:Foreignmaterialtobeexcludedinanalysis.
●Tissue:Tissuepointtypingisspecictoeachmeasurementsite.
●Neutral:SelecttheNeutralbrushtypeandverifythatathinborderofneutral
samplesisshownaroundthebone.
NeutralpointtypingisnotavailableonTotalBodyscans.
2.Toadjustpointtyping,selectabrushtype(BoneorNeutral)andabrushsize.
3.Clickontheimagetomakeyourchanges.
4.Ifnecessary,selecttheArtifactbrushtopointtypeanartifactintheimage.
●Toreturntheimagetoitsoriginalstate,selectReset.
●Tocorrecterrorsyoumakewhileadjustingthepointtyping,selectUndo.
79
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 80

MeasurementandAnalysis
ExamplesofCorrectBonePointTyping
APSpinecorrectbonepointtypingFemurcorrectbonepointtyping
ForearmcorrectbonepointtypingLateralspinecorrectbonepointtyping
ExamineResults
ReferenceGraph
80
TheResultstabsontheAnalyzescreenletyoureviewBMD,reference,trend,and
compositionresults.
Tochangedefaultsettingsforgraphs,referencedata,andresultstables,referto
Options(243).
Thereferencegraphprovidesavisualrepresentationoftheexamresultscompared
toareferencepopulation.Apatient'sBMD,expressedingramspercentimeter
squared,isplottedagainsthisorherage.Thesquarewithablackdotrepresentsthe
patient.Eachcoloredbarbelowthedarkgreensectionrepresentsonestandard
deviationbelowtheYoungAdultvalue.Thethinlineseparatingthedarkgreenfrom
thelightgreenindicatesaT-Scoreof–1.Followingacross,thepatient’sT-Scorecan
bedetermined.
TheT-Scoreindicateshowmanystandarddeviationsapatient'sBMDisfromthe
meanBMDvalueofthehealthyYoungAdultreferencepopulation.AnegativeT-Score
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 81

MeasurementandAnalysis
indicatesthepatient'sBMDisbelowtheYoungAdultvalue.ApositiveT-Score
indicatesthepatient'sBMDisabovetheYoungAdultvalue.
T-Score
ThebluebardisplaystheAgeMatchedReferenceanddemonstratesthechange
inbonedensityassociatedwithaging.Thecenterlineofthebluebariscalleda
regressionline.TheregressionlineshowstheexpectedBMDatdifferentagesfora
particularmeasurementsite.ThebluebarrepresentstheexpectedAge-MatchedBMD
±1standarddeviationforagivenpatient.
Age-MatchedReference
enCOREfactorsintwovariableswhichaffecttheregressionline:
●WeightAdjustment:BodyweightismoderatelyassociatedwithBMD(r=~0.3).
Asweightincreasesordecreases,bonedensitygenerallyincreasesordecreases
proportionally.Foreverykilogramofweightaboveorbelowtheaverageweight
formen(78kg)andwomen(65kg),theexpectedBMDisadjustedby0.004forAP
spineBMDand0.003forfemurBMD.Thisweightadjustmentisappliedforweights
between25and100kg.Weightadjustmentsareappliedtoage-matchedvalues
(Z-Score)only.Youngadult(T-Score)valuesarenotaffected.Ifthesamepatient
weighs90kginsteadof45kg,theblueregressionbarshiftsupwardwhilethe
patient'sBMDisunchanged.Inotherwords,theage-matchedweightadjustment
increasedthepatient'sexpectedBMD.TheWeightAdjustmentoptionisturnedon
oroffinTools>UserOptions>ReferenceData.
●EthnicAdjustment:TheEthnicAdjustment,liketheWeightAdjustment,only
affectstheage-matchedregressionbar .Youngadultvaluesarenotaffected.The
ethnicadjustmenttakesethnicoriginintoconsiderationforthenalage-matched
comparison.Theprogramautomaticallyshiftstheage-matchedregressionbar
upordownaccordingtoethnicoriginifthisoptionisturnedonoroffinTools>
UserOptions>ReferenceData.
81
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 82

MeasurementandAnalysis
ReferenceResults
enCOREmaybeconguredtodisplaytheWorldHealthOrganization(WHO)or
JapaneseSocietyforBoneandMineralResearch(JSBMR)barsinTools>UserOptions
>ResultsDisplaytab>ReferenceGraphOptions.Thisoptionlabelsthegraphwith
thedifferentWHOorJSBMRclassications;Normal,Osteopenia,andOsteoporosis.
Useofthereferencepopulationcomparisonsisfullyatthediscretionoftheclinician.
TheprogramdoesNOTshowthecomparativereferencevalueswhenshippedfrom
GEHealthcare.
Referenceresultsincludethereferencegraphandreferenceresultstable:
●Referencegraph:SelecttheDensitometryResultstabtoviewthereferencegraph.
TheReferencegraphshowsthepatient'sT-ScoreandZ-Scoreresults.
●Referenceresultstable:TheresultstableshownbelowtheReferencegraph
givestheresultsforeachregionanalyzed.Toviewtheresultsforaregiononthe
Referencegraph,highlighttheregion.
BMDvaluesshouldbeconsideredtogetherwithotherriskfactors(lowbody
weight,fracturehistory,corticosteroiduse,useoflong-actingtranquilizers,history
offalling)inthepatientevaluation.Inparticular,patientswithapriorhistoryof
osteoporoticfractureshouldbeconsideredtohavedoubletheriskoffuture
fractureatanydensitylevel.
82
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 83

PatientTrending
TrendingGraphs
MeasurementandAnalysis
enCOREprovidesamonitoringtooltoviewchangesinapatient'sBMDovertime.To
viewtrendingresults,1)allofthetrendedmeasurementsmustbefromthesamesite,
and2)eachtrendedmeasurementmustbeanalyzed.
Scanswithmixedpositioningand/oranalysismaynotbetrendedtogetheronall
graphs.
SelecttheTrendResultstabtoviewpatienttrendinginformation.
Eachmeasurementisshownasaseparatesquareonthegraph.Adotisshown
inthesquarethatrepresentsthecurrentimage.Trendgraphscanbecongured
aspercentchangeorreference.
Tochangethegraphdisplay,gotoTools>UserOptions>Trendingtab.Referto
Options(236)forinformationaboutconguringUserOptions.
Scanswithmixedpositioningand/oranalysismaynotbetrendedtogetheronall
graphs.ReferenceOnlyTrendGraphs(enabledinTools>UserOptions>Results
Displaytab>ReferenceGraphOptionsbutton>Graphsection)allowmixedanalysis
orpositionerexamsonthesametrendgraph(noBMDdependency).
PercentChangeTrendGraph
83
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 84

MeasurementandAnalysis
ReferenceTrendGraph(BMDandreference)
ReferenceOnlyTrendGraph(noBMDdependency)
TrendingResultsTable
Thetrendingresultstableisshownbelowthetrendinggraph.Thetablegivesthe
dateofthemeasurement,thepatient'sage,theBMDforthemeasurement,and
thechangeinBMD.
TrendinginformationisshownfortheregionhighlightedintheDensitometrytable.To
changetheregion,highlightanewregionandselecttheTrendtab.
84
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 85

OneScan
OneScanperformsanAPSpineandDualFemurexamwithoutrepositioningbetween
scans.OneScandoesnotusethefoamlegblockpositionerforspinepositioning.
TurningOneScanOnandOff
TheOneScanoptioncanbedefaultedonoroffthroughTools>UserOptions>
Measuretab.
Apausemaybeenabledtooccurbetweenfemurs(DualFemur)orbetweenAPSpine
andFemurscans.SeeOneScanMeasurement(85).
ThePositionscreenalsoincludesaOneScancheckbox.Ifthisisselected,andthe
patienthashadapreviousscan,thesoftwarewillauto-selectthematchingOneScan
optionfortrending.
DetermineiftheOneScanfeaturewasonoroffthroughexaminationoftheanalysis
screenundertheInformationtab.
OneScanMeasurement
MeasurementandAnalysis
ThepositioninggraphicsaretieddirectlytothecongurationoftheOneScancheck
box.
OneScanisintendedtobeusedwithoutthelegblock.
Thescreenchangesslightly,dependinguponwhethertheOneScanboxischecked.
OneScanon
IfyouareusingOneScan,positionthepatientasfollows:
1.Helpthepatientontothescannertableandpositionthepatientinthecenter
ofthescantable.
OneScanoff
2.Usethecenterlineonthetableasareferencetoalignthepatient.Thepatient's
armsshouldbecrossedoverthechest,awayfromthesideofeachhip.
3.Alignthecenterlineonthescannertablewiththeguideonthebaseofthefoot
brace.
85
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 86

MeasurementandAnalysis
4.Internallyrotatethepatient'slegs,andsecurethepatient'sfeettothefootbrace.
Itissuggestedthatyoudonotremovethepatient’sshoes.
DuringacombinedAPSpineandDualFemur(orsinglefemur)measurement,the
softwareimmediatelyproceedstothefemursetup.WiththeOneScanfeature
enabled,thesoftwareproceedsdirectlytothepositioningscreenforadjustment
ofthelaserlightposition.TheOneScanfeatureeliminatesthispause,asthe
patientisalreadypositionedwiththeirfeetintheDualFemurbraceforfemur
measurements.
Apausemaybeenabledbetweenscans.ThisoptionisfoundinTools>User
Options>Measuretab.Checkthedesiredoptions.
APSpineMeasurementandAnalysis
APSpinemeasurementandanalysisprovidesanestimateofbonemineraldensity
forthelumbarspine.Thesevaluescanthenbecomparedtoanadultreference
populationatthediscretionofthephysician.
APSpineMeasurement
ThepositioningrequirementsforanAPSpinemeasurementdependonwhetheryou
havechosenOneScaninTools>UserOptions>Measuretab.
●IfOneScanischeckedforaspecicmeasurement,donotusethefoamblock
●IfOneScanisnotcheckedforaspecicmeasurement,besuretousethefoam
ForOneScanmeasurements,theT-Scorecalculationassumesthefoamblockisnot
used.
1.Positionthepatient.
positioner.
blockpositioner.
Ifyouareusingthefoamlegblockpositioner:
a.Helpthepatientontothescannertableandpositionthepatientinthecenter
ofthescantable.
b.Usethecenterlineonthetableasareferencetoalignthepatient.
Thepatient'sarmsshouldbeonthescannertable,alongsidethepatient's
body.
86
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 87

MeasurementandAnalysis
Removethefoamlegblockpriortopositioningthescanarmand
immediatelyaftertheAPSpinescan.
c.SelectPositionfromtheNewMeasurementtoolbar.
Thescannerarmmovestotheapproximatestartposition,andagraphicis
shownthatgivesthecorrectpatientpositionandmeasurementstartposition.
d.Usethefoamlegblocktoelevatethepatient'slegs.Makesurethepatient's
thighsforma60°to90°anglewiththetabletop.Thisstephelpsseparate
vertebraeandattenthelowerback.
Ifyouarenotusingthefoamlegblockpositioner(OneScan):
a.Helpthepatientontothescannertableandpositionthepatientinthecenter
ofthescantable.
b.Usethecenterlineonthetableasareferencetoalignthepatient.
Thepatient'sarmsshouldbecrossedoverthechest,awayfromthesideof
eachhip.
c.SelectPositionfromtheNewMeasurementtoolbar.
Thescannerarmmovestotheapproximatestartposition,andagraphicis
shownthatgivesthecorrectpatientpositionandmeasurementstartposition.
d.Usethecenterlineonthescannertableasareferencetomakesurethefoot
braceiscentered.
e.Alignthecenterlinewiththeguideonthebaseofthefootbrace.Internally
rotatethepatient'slegs,andsecurethepatient'sfeettothefootbrace(itis
recommendedthatyoudonotremovethepatient'sshoes).
87
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 88

MeasurementandAnalysis
2.SelecttheappropriatescanmodebasedonthethicknessoftheAPSpinearea.
ThescanmodefortheAPSpinemaybedifferentthanthescanmodeusedfor
thefemur,basedonthepatient'sweightdistribution.
3.Adjustthepositionofthelaserlight.Positionthelaserlightapproximately5cm
belowthepatient'snavelandinthesamelongitudinalplaneasthepatient's
midline.
4.Tostartthemeasurement,selectStartfromtheNewMeasurementtoolbar
5.Monitortheimagetomakesureitiscorrect.Makesurethat:
●Thespineisinthecenteroftheimage
●(1)AllofL4(1)isshown
●(2)ThetopofL5(2)isshownintherst1-2sweepsforiDXAandProdigy
scanners
●(2)ThetopofL5(2)isshownintherst5-15scanlinesforDPXscanners
●(3)Approximately1/2ofT12isshown
88
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 89

MeasurementandAnalysis
6.Iftheimageisnotcorrect,selectAbort,repositionthelaserlight,andrestart
themeasurement.
7.Tocompleteanothermeasurementforthepatient,selectSetUpfromtheNew
Measurementtoolbar.
8.Ifyouhavecompletedmeasurementsforthepatient,selectHometomovethe
scannerarmtotheHomeposition
9.SelectAnalyzetoproceedtoAnalysis,orClosetoexittheMeasurementscreen.
APSpineAnalysis
1.Ifanimageisnotalreadyopen,selectanimageleforanalysis.
TheresultsmayincludeaScanChecktab.UseScanCheck
(183)toassistintheanalysisoftheimageandtohelpyou
makecorrectionswherenecessary.
2.Ifnecessary,selectImagingfromtheAnalysistoolbartoadjusttheimage.
3.Ifnecessary,selectROIsfromtheAnalyzetoolbartoadjusttheROIs.
enCOREsoftwarewillplacetheROIscorrectlymostofthetime.Donotmake
adjustmentstotheanalysisunlessthereisanobviousadjustmentneeded.
Makesurevertebraearecorrectlyidentiedandintervertebral(IV)markersare
betweenthevertebralbodies(1)andlocatedatthelowestpointofbonedensity
asindicatedontheboneprole(2).
Analysisresults
4.IfyouneedtoadjustROIsforanAPSpineimage,usethesetools:
Icon
Tool
AddROISelectAddROItoaddanROIduringAPSpine
Description
analysis.WhenyouaddanewROI,itisinserted
belowtheROIthatisselectedontheimage.
SelectLabelROItolabeltheROIsaccordingly.
DeleteROISelectDeleteROItoremoveanROIduring
APSpineanalysis.ClicktheROI,thenselect
DeleteROI.SelectLabelROItolabeltheROIsif
necessary.
89
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 90

MeasurementandAnalysis
Icon
Tool
MoveROI
RotateROI
LabelROIsSelectLabelROIstorelabelROIsafteryouhave
ExcludeROIsThistoolletsyouremoveROIsfromAPspine
Show/HideScanCheck
Markers
Description
SelectMoveROItomoveROIs.
SelectRotateROItorotateanROI.
addedordeletedanROIfromanimage.
results.
SelectExcludeROI,andthenselecttheROIs
youwanttoexcludefromanalysis.Parentheses
appeararoundtheROIlabelsofexcludedROIs.
ResultsforindividualROIsareshownevenifthe
ROIsareexcludedfromanalysis.ExcludedROIs
arenotincludedintheresultsforcombinations
ofvertebrae.
Thistoolletsyoushow/hideScanCheckmarkers
thatindicateapossiblehighdensityarea,such
asanartifactorosteophyte.
ScanCheckMarkersisavailableonlyifScanCheck
isenabled.
5.IfyouadjustROIs,selectResultstoviewtheanalysisresults.
6.Ifyouneedtoadjustpointtyping,selectPointsfromtheAnalyzetoolbar.
Donotadjustthepointtypingunlesstheprogrammadeobviouserrors.
7.Ifyouadjustpointtyping,selectResultstoviewthenewanalysisresultsbased
onyourchanges.
Bonepoints
Neutralpoints
Tissuepoints
8.SelectSavetosaveyourchanges,orselectClosethenNoifyoudonotwant
tosaveyourchanges.
RefertoCustomAnalysisProcedures(180)andEstimatedTotalBodyFatand
Android/GynoidFat(129)foradditionalanalysisinformation.
90
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 91

Femur/DualFemurMeasurementandAnalysis
Femur/DualFemurmeasurementandanalysisprovidesanestimateofbonemineral
densityfortheproximalfemur.Thesevaluescanthenbecomparedtoanadult
referencepopulationatsolediscretionofthephysician.
Femur/DualFemurMeasurement
1.Helpthepatientontothescannertableandpositionthepatientinthecenterof
thescantable.Usethecenterlineonthetableasareference.
Thepatient'sarmsshouldbecrossedoverthechest,awayfromthesideof
eachhip.
MeasurementandAnalysis
2.SelectPositionfromtheNewMeasurementtoolbar.
Thescannerarmmovestotheapproximatestartposition,andagraphicisshown
thatgivesthecorrectpatientpositionandmeasurementstartposition.
3.Usethecenterlineonthescannertableasareferencetomakesurethefootbrace
iscentered.Alignthecenterlinewiththeguideonthebaseofthefootbrace.
Internallyrotatethepatient'slegs,andsecurethepatient'sfeettothefootbrace
(itisrecommendedthatyoudonotremovethepatient'sshoes).
4.Selecttheappropriatescanmodebasedonthethicknessofthefemurarea.
Thescanmodeforthefemurmaybedifferentthanthescanmodeusedforthe
APSpine,basedonthepatient'sweightdistribution.
91
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 92

MeasurementandAnalysis
5.Adjustthepositionofthelaserlight.Positionthelaserlightapproximately7-8cm
belowthegreatertrochanterwherethetransverse(PubicSymphysis)andmidline
ofthefemurintersect.
IfyouareperformingaDualFemurmeasurement,positionthelaserlightfor
theleftfemurrst.
6.Tostartthemeasurement,selectStartfromtheNewMeasurementtoolbar
7.Monitortheimagetomakesureitiscorrect.Makesurethat:
●TheFemurimageshowsthegreatertrochanter(1),femoralneck(2),and
ischium(3).
●Aminimumofthreecentimetersoftissueshouldbeshownabovethegreater
trochanterandbelowtheischium.
8.Iftheimageisnotcorrect,selectAbort,repositionthelaserlight,andrestart
themeasurement.
9.IfyouareperformingaDualFemurmeasurement:
DualFemurletsyoumeasurethepatient'sleftandrightfemurinanautomatic
sequence.Aftertheprogramhasmeasuredtheleftfemur,thescanarmmoves
totheapproximatestartpositionfortherightfemur.
Checkthestartpositionand,ifnecessary,adjustthemeasurementstartposition
fortherightfemur.
10.DPX-DuoandDPX-Bravohaveanarrowscanregion.Therefore,repositioningthe
patientforthecontra-lateralfemurmaybenecessary.
11.Tocompleteanothermeasurementforthepatient,selectSetUpfromtheNew
Measurementtoolbar.
12.Ifyouhavecompletedmeasurementsforthepatient,selectHometomovethe
scannerarmtotheHomeposition
13.SelectAnalyzetoproceedtoAnalysis,orClosetoexittheMeasurementscreen.
92
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 93

AtypicalFemurFractureMeasurement
IfyoupurchasedtheAtypicalFemurFracture(AFF)optionforyourbonedensitometer,
AFFfeaturesappearintheenCOREsoftware.
AFFmeasurementandanalysisprovidesanx-rayimageoftheentirefemurforboth
qualitativevisualassessmentandquantitativemeasuresinordertoidentifyareasof
focalthickeningalongthelateralcortexofthefemoralshaft.Youcanalsogetbone
mineraldensityvaluesfortheproximalfemuroftheAFFmeasurement.
1.Helpthepatientontothescannertableandpositionthepatientinthecenterof
thescantable.Usethecenterlineonthetableasareference.
Thepatient'sarmsshouldbecrossedoverthechest,awayfromthesideof
eachhip.
MeasurementandAnalysis
2.SelectPositionfromtheNewMeasurementtoolbar.
Thescannerarmmovestotheapproximatestartposition,andagraphicisshown
thatgivesthecorrectpatientpositionandmeasurementstartposition.
3.Usethecenterlineonthescannertableasareferencetomakesurethefootbrace
iscentered.Alignthecenterlinewiththeguideonthebaseofthefootbrace.
Internallyrotatethepatient'slegs,andsecurethepatient'sfeettothefootbrace
(itisrecommendedthatyoudonotremovethepatient'sshoes).
4.Selecttheappropriatescanmodebasedonthethicknessofthefemurarea.
Thescanmodeforthefemurmaybedifferentthanthescanmodeusedforthe
APSpine,basedonthepatient'sweightdistribution.
93
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 94

MeasurementandAnalysis
5.Adjustthepositionofthelaserlight.Thelaserlightpositionforafemur
measurementdependsonwhetheryouhavechosentoperformdistaland
proximalscansforAFF.
ForAFFmeasurementsofboththedistalandproximalfemur,positionthelaser
lightsoitiscenteredonthepatella.
IfyouareperformingaDualFemurmeasurement,positionthelaserlightforthe
leftfemurrst.
6.Tostartthemeasurement,selectStartfromtheNewMeasurementtoolbar
7.Monitortheimagetomakesureitiscorrect.Makesurethat:
●TheFemurimageshowsthegreatertrochanter(1),femoralneck(2),and
ischium(3).
94
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 95

MeasurementandAnalysis
●Aminimumofthreecentimetersoftissueshouldbeshownabovethegreater
trochanter.
8.Iftheimageisnotcorrect,selectAbort,repositionthelaserlight,andrestart
themeasurement.
9.AFFmeasurementsareperformedasasequenceoftwoexposures.Afterthe
programhasmeasuredthedistalfemur,apromptisdisplayedtostartthe
measurementoftheproximalfemur.
10.IfyouareperformingaDualFemurmeasurement:
DualFemurletsyoumeasurethepatient'sleftandrightfemurinanautomatic
sequence.Aftertheprogramhasmeasuredtheleftfemur,thescanarmmoves
totheapproximatestartpositionfortherightfemur.
Checkthestartpositionand,ifnecessary,adjustthemeasurementstartposition
fortherightfemur.
11.Tocompleteanothermeasurementforthepatient,selectSetUpfromtheNew
Measurementtoolbar.
12.Ifyouhavecompletedmeasurementsforthepatient,selectHometomovethe
scannerarmtotheHomeposition
95
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 96

MeasurementandAnalysis
13.SelectAnalyzetoproceedtoAnalysis,orClosetoexittheMeasurementscreen.
Femur/DualFemurAnalysis
1.Ifanimageisnotalreadyopen,selectanimageleforanalysis.
2.Ifnecessary,selectImagingfromtheAnalyzetoolbartoadjusttheimage.
3.Ifnecessary,selectPointsfromtheAnalyzetoolbartoadjustpointtyping.
WhenyouopenaDualFemurimageforanalysis,theleftandrightfemurimages
arebothshown.Theactivefemurhasablueboxarounditsimagewindow.Click
insideanimagewindowtomakethatfemurimagetheactiveimage.Theresults
includeBMDvaluesforeachregionofeachfemurandaveragesanddifferences
betweenfemurs.Referencedataandtrendingareavailable.
TheresultsmayincludeaScanChecktab.UseScanCheck
(183)toassistintheanalysisoftheimageandtohelpyou
makecorrectionswherenecessary.
Bonepoints
Neutralpoints
Tissuepoints
Donotadjustthepointtypingunlesstheprogrammadeobviouserrors.
4.Ifyouadjustpointtyping,selectResultstoviewtheanalysisresultsbasedon
yourchanges.
5.Generally,noadjustmentsarenecessarytoROIplacement.
Donotadjust(move,rotate,orsize)theNeckROIunlessitisobviouslyincorrect.
TheNeckROIshouldbepositionedasfollows:
●TheNeckROIincludesnopartofthegreatertrochanter
●TheNeckROIincludessofttissueoneithersideoftheneck
●TheNeckROIisperpendiculartothefemoralneck
●TheNeckROIcontainslittleornoischium(iftheischiumisincludedintheNeck
ROI,theprogramautomaticallyassignsthebonewithintheischiumasNeutral)
96
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 97

ItisnotrecommendedthatyouadjusttheNeckROI.
MeasurementandAnalysis
a.Ifitisnecessarytoperformfurtheradjustments,selecttheROItoolfromthe
Analysistoolbartocompletetheseprocedures:
●SelecttheSearchtooltopositiontheneckROIcorrectly.Searchlocatesthe
regionofthelowestBMDandmostnarrowareaoftheneck.
●Move:UsethecursortoselectandmovetheNeckROIandtheNeckAxis.
●Rotate:UsethecursortoselectandrotatetheNeckROIandtheNeckAxis.
●Size:SelecttheSizetool.Usethecursortoincludetissueoneithersideof
theneckifnoneispresent.NeveredittheNeckROIwidth.
6.SelectResultstoviewtheanalysisresults.
7.SelectSavetosavechanges.
RefertoEstimatedTotalBody%FatandAndroid/Gynoid%Fat(129),Advanced
HipAnalysis(106),orFRAX10-YearFractureRisk(97)foradditionalanalysis
information.
FRAX10-YearFractureRisk
FRAX10-YearFractureRiskprovidesanestimateof10-yearprobabilityofhipfracture
and10-yearprobabilityofamajorosteoporoticfracture(clinicalspine,forearm,hip,or
shoulderfracture)formenandpost-menopausalwomenages40-90years.
Thisestimateisbasedonthepatient’sage,gender,population,ethnicity,height,
weight,femurneckBMDT-score,andthepresenceorabsenceofseveralriskfactors.It
97
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 98

MeasurementandAnalysis
iscomputedusingtheFRAXmodelendorsedbytheWorldHealthOrganization(WHO).
T-scoresarebasedontheNHANESreferencevaluesforwomenaged20-29years.
Thephysicianshouldreviewlocalclinicalguidelines,andthendeterminetheoptimal
FRAXconguration.
FRAXisatrademarkoftheUniversityofShefeldMedicalSchool,CentreforMetabolic
BoneDiseases,AWorldHealthOrganization(WHO)CollaboratingCentre.
EnablingandConguringFRAX
ToenableFRAX,selectTools>UserOptions>SystemtabandclickFRAX.
FRAXdialog
CongureFRAXaccordingtositeneeds.Youcanchooseto:
●Alwayscalculateapatient’sfracturerisk(checkEnableFRAX)
●CalculateFRAXaccordingtoNOF/ISCDrecommendations(checkEnableFRAXand
ApplyU.S.NOF/ISCDFRAXRecommendations)
●NevercalculateFRAX(clearbothcheckboxes)
Thedefaultisbothboxesunchecked.
FRAX10-YearFractureRiskCalculation
TheFRAXtabisavailableduringLeftFemur,RightFemur,andDualFemuranalysis.
RefertothisgurewhenfollowingthestepsbelowtocalculateFRAX10-Year
ProbabilityofFracture.
98
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 99

FRAXriskcalculation
1.Checktheappropriateriskfactors,accordingtotheseguidelines:
MeasurementandAnalysis
RiskFactorsSelectriskfactorifthepatient:
Alcohol(3ormoreunits
perday)
Takes3ormoreunitsofalcoholdaily.Thisis
equivalenttoastandardglassofbeer(285ml),a
singlemeasureofspirits(30ml),amedium-sized
glassofwine(120ml),or1measureofanaperitif
(60ml)
FamilyHist.(Parenthip
fracture)
Glucocorticoids
(Chronic)
Hasahistoryofhipfractureinthepatient's
motherorfather
Isexposedtooralglucocorticoidsorhasbeen
exposedtooralglucocorticoidsformorethan3
monthsatadoseofprednisoloneof5mgdailyor
more(orequivalentdosesofotherglucocorticoids)
HistoryofFracture
(Adult)
Hasapreviousfractureinadultlifeoccurring
spontaneously,orafracturearisingfromtrauma
which,inahealthyindividual,wouldnothave
resultedinafracture
SecondaryOsteoporosisHasadisorderstronglyassociatedwith
osteoporosis.TheseincludetypeI(insulin
dependent)diabetes,osteogenesisimperfectain
adults,untreatedlong-standinghyperthyroidism,
hypogonadismorprematuremenopause(<45
years),chronicmalnutrition,ormalabsorptionand
chronicliverdisease
RheumatoidArthritisHasaconrmeddiagnosisofrheumatoidarthritis
TobaccoUser(Current
Currentlysmokestobacco
Smoker)
99
X-rayBoneDensitometerwithenCOREv17software-UserManual
Page 100

MeasurementandAnalysis
2.ChecktheappropriateNOF/ISCDFilters,accordingtotheseguidelines:
NOF/ISCDFilters
OnTreatment
PreviousFracture(Hip
orSpine)
Description
Examplesof“OnTreatment”patientsinclude:
●Estrogen/HormoneTherapyorSERMwithinthe
pastoneyear
●Calcitoninwithinthepastoneyear
●ParathyroidHormone(PTH)withinthepastone
year
●Denosumabwithinthepastoneyear
●Bisphosphonatewithinthepasttwoyears
(unlessitisanoraltakenfor<2months)
Calciumandvitamin
DdoNOTconstitute
“OnTreatment”inthis
context.
Priorhiporvertebralfracture(clinicalor
morphometric).
3.DualFemurmeasurementsautomaticallyselecttheregion(LeftorRight)withthe
lowestfemoralNeckBMD(lowest).Youcanchangethisselectionbyclickingon
theregionofchoice(Left,Right,orMean).
4.SelecttheappropriateFRAXPopulationfromthedropdownmenu.Ifthepatient's
countryisnotrepresented,selectthecountryforwhichtheepidemiologyof
osteoporosismostcloselyapproximatesthepatient'scountry.
BasedontheFRAXmodel,examplesofhighriskcountriesareDenmarkand
Sweden.LowriskcountriesincludeLebanonandChina.
5.ClicktheCalculatebutton.
The10-yearprobabilityofhipfractureand10-yearprobabilityofamajor
osteoporoticfracturearedisplayed.
FRAX10-YearFractureRiskReports
ThefollowingFRAX10-YearFractureRiskreportsareavailable:
Composer
DXAreports
FRAX
DualFemurFRAX,LeftFemurFRAX,andRightFemurFRAX
(availabilitybasedonopenexam).
RefertoCreatingaReport(193)andDXAResultsReports(191)formoreinformation
ongeneratingreports.
AtypicalFemurFractureAnalysis
AtypicalFemurFractures(AFFs)arestressfracturesthatoccurinthefemurshaftand
maybeaccompaniedbyafocalordiffuseperiostealreactionofthelateralcortex
surroundingtheregionwherethefractureinitiated.Anareaofcorticalthickeningis
calleda“beak”,andtheprocessofthethickeningiscalled“beaking”.
AtypicalFemurFracture(AFF)Analysisdisplaysanimageoftheentirefemurtoallow
theusertovisualizefocalreactionorthickeningofthelateralcortex.Inaddition,the
100
X-rayBoneDensitometerwithenCOREv17software-UserManual
 Loading...
Loading...