Page 1

Technical Publications
Vscan Extend™
Version 1.0
User Manual
5721203-100 — English
Rev. D
Operating Documentation
Copyright © 2016 By General Electric Co.
Page 2

Regulatory requirement
This product complies with regulatory requirements of the following European
Directive 93/42/EEC concerning medical devices.
This manual is a reference for the Vscan Extend. It applies to all versions of the 1.0
software for the Vscan Extend ultrasound system.
Manufacturer:
GE VINGMED ULTRASOUND A/S
Strandpromenaden 45
3191 Horten, Norway
Tel.: (+47) 3302 1100 Fax: (+47) 3302 1350
Page 3

Revision History
Reason for Change
DATE
REV
Rev. A 2016/04/05 Initial draft
Rev. B 2016/05/19 Included External Battery charger label
Rev. C 2016/06/01 Removed breast and testes from indication of use
Rev. D 2016/08/18 Included IP33, Ophthalmic and Aorta presets, IEC
(YYYY/MM/DD)
Included Power button under device labels
Included revised rating label
Included sections in Privacy and Security chapter
statement
Included the revised rating label, battery label and
external charger label
Included the scope for all the standards in Table i-1
60601-11 and 12 standards, Tricefy App
REASON FOR CHANGE
Please verify that you are using the latest revision of this document. Information
pertaining to this document is maintained on ePDM (GE electronic Product Data
Management). If you need to know the latest revision, contact your distributor, local GE
Sales Representative or in the USA call the GE Ultrasound Clinical Answer Center at
1 800 682 5327 or 1 262 524 5698.
Vscan Extend – User Manual i-1
5721203-100 Rev. D
Page 4

This page intentionally left blank.
i-2 Vscan Extend – User Manual
5721203-100
Rev. D
Page 5

Regulatory Requirements
Conformance Standards
The GE product families are tested to meet all applicable
requirements in relevant EU Directives and European/
International standards. Any changes to accessories, peripheral
units or any other part of the system must be approved by the
manufacturer: GE Medical Systems. Ignoring this advice may
compromise the regulatory approvals obtaine d for the product.
This product complies with the regulatory requirement of the
following:
Table i-1: Regulatory Requirements
Standard/Directive Scope
93/42/EEC Medical Devices Directive (MDD)
2007/47/EC (MDD amendment)
Directive 2011/65/EU RoHS
2002/96/EC WEEE
The CE label affixed to the product testifies compliance to
the Directive. The location of the CE marking is shown in the
Safety chapter of this manual.
EN55011 Industrial, scientific and medical equipment -
Radio-frequency disturbance characteristics - Limits and
methods of measurement
IEC* 60601-1
CAN/CSA-C22.2 No 601.1
IEC* 60601-2-37 Medical electrical equipment - Part 2-37. Particular
IEC* 60601-1-2 Medical Electrical Equipment - part 1-2. Collateral standard:
IEC* 60601-1-4 Medical Electrical Equipment - part 1-4. Collateral standard:
IEC* 60601-1-6 Medical Electrical Equipment - part 1-6. Collateral standard:
NEMA/AIUM UD-3 Standard for real-time display of thermal and mechanical
Medical Electrical Equipment, Part 1; General Requirements
for Safety
requirements for the safety of ultrasonic medical diagnostic
and monitoring equipment
Electromagnetic compatibility - Requirements and tests.
Programmable electrical medical systems
Usability.
acoustic output indices on diagnostic ultrasound equipment.
Vscan Extend – User Manual i-3
5721203-100 Rev. D
Page 6

Table i-1: Regulatory Requirements (Continued)
Standard/Directive Scope
ISO10993-1 Biological evaluation of medical devices
EN 300 328 Electromagnetic compatibility and Radio spectrum Matters
ISO 14971 Medical devices - Application of risk management to medical
IEC* 62304 Medical device software - Software life-cycle processes
IEC* 62366 Medical devices - Application of usability engineering to
IEC* 60601-1-11 Requirements for medical electrical equipment and medical
IEC* 60601-1-12 Requirements for medical electrical equipment and medical
* including national deviations
(ERM); Wideband transmission systems
devices
medical devices
electrical systems used in the home healthcare environment
electrical systems intended for use in the emergency
medical services environment
Certifications
• GE Vingmed Ultrasound is ISO 13485 certified.
i-4 Vscan Extend – User Manual
5721203-100
Rev. D
Page 7

Classifications
Class II Equipment
The following classifications are in accordance with the IEC/
EN 60601-1.
Type and degree of protection against electric shock:
• Vscan Extend has an internal battery which allows the
operation during AC power absence.
• The AC adapter is Class II.
• Vscan Extend has type BFApplied Part.
Vscan Extend main unit is rated IP33:
• 3: Protected against solid foreign objects of 2,5 mm Ø and
greater.
• 3: Protected against spraying water.
Vscan Extend probe (immersible portion) is IPX7.
EQUIPMENT in which protection against electric shock does not
rely on BASIC INSULATION only, but in which additional safety
precautions such as DOUBLE INSULATION or REINFORCED
INSULATION are provided, there being no provision for
protective earthing or reliance upon installation conditions.
Type BF Applied part
TYPE BF APPLIED PART providing a specified degree of
protection against electric shock, with particular regard to
allowable LEAKAGE CURRENT.
Table i-2: Leakage Current
Normal mode Single fault condition
Patient leakage current <100 microA <500 microA
Original Documentation
• The original document was written in English.
Vscan Extend – User Manual i-5
5721203-100 Rev. D
Page 8

Country Specific Approval
• USA AND TERRITORIES
DANGER
• JAPAN
DANGER
• CHINA
DANGER
Importer Information
Turkey
The following optional feature IS NOT available in the USA
and its territories:
– Ophthalmalic
The following optional feature IS NOT available in JAPAN:
– Ophthalmalic
The following optional feature IS NOT available in CHINA:
– Ophthalmalic
i-6 Vscan Extend – User Manual
5721203-100
Rev. D
Page 9

Conformance Standards - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - i-3
Certifications - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - i-4
Classifications - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - i-5
Class II Equipment - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - i-5
Type BF Applied part - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - i-5
Original Documentation- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - i-5
Country Specific Approval - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - i-6
Importer Information - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - i-6
Table of Contents
Chapter 1 — Introduction
Overview
Attention - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-2
General description- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-2
Principles of operation- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-3
Safety - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-3
Intended use - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-3
Indications for use (for all countries except USA, China and Japan)- - - - 1-4
Indications for use (for USA, China and Japan) - - - - - - - - - - - - - - - - - - 1-5
Contraindication for use (for USA, China and Japan) - - - - - - - - - - - - - - 1-5
Intended users - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-6
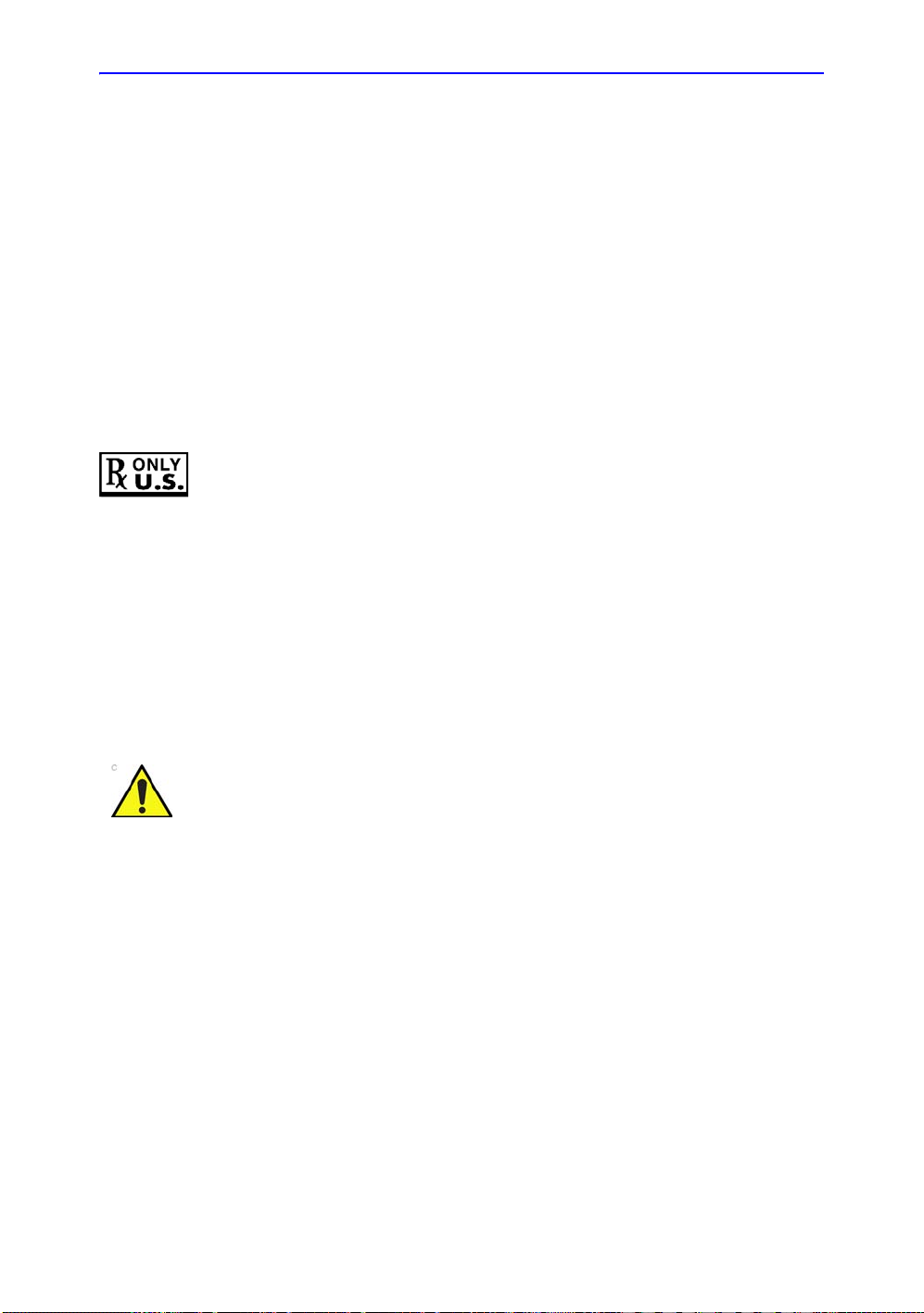
Prescription Device- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-6
Operator profile - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-6
Warnings
Important Safety Considerations - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-7
Contact Information
Contacting GE Ultrasound - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-10
Global ultrasound support center phone numbers - - - - - - - - - - - - - - - 1-11
Manufacturer - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 1-14
Chapter 2 — Safety
Introduction
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-2
Owner responsibility
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-3
Notice against user modification- - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-4
Important safety considerations
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-5
Patient Safety- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-5
Diagnostic information- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-6
Table of Contents
Vscan Extend – User Manual i-7
5721203-100 Rev. D
Page 10

General precautionary advice for the use of diagnostic ultrasound in
combination with ultrasound contrast agents - - - - - - - - - - - - - - - - - 2-6
Mechanical hazards - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-6
Electrical hazard- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-7
Personnel and equipment safety - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-7
Explosion hazard - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-7
Electrical hazard- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-8
Electromagnetic Compatibility (EMC) - - - - - - - - - - - - - - - - - - - - - - - - 2-10
Electromagnetic emissions - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-14
Electromagnetic immunity - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-14
Separation distances- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-18
Essential Performance - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-18
Acoustic output- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-19
Environmental protection- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-23
Maximum probe temperature
Device labels and symbols
Vscan Extend Labels (Example)- - - - - - - - - - - - - - - - - - - - - - - - - - - - 2-25
Explanation of the Pollution control label for China- - - - - - - - - - - - - - - 2-29
Chapter 3 — Preparing Vscan Extend for Use
Package contents
Vscan Extend package contents - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-2
Environmental requirements
Environmental requirements for the device - - - - - - - - - - - - - - - - - - - - - 3-3
System description
System overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-4
Accessories and Configurations- - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-7
External battery charger compartment (option) - - - - - - - - - - - - - - - - - 3-11
Display screens - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-12
Vscan Extend Battery
Battery- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-15
Initial use
First time use - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-22
Power on/off- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-22
Vscan Extend activation - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 3-24
Chapter 4 — Vscan Extend Settings
Settings
Scan Settings- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-2
Server Settings- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-3
Diagnostics - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-12
System Settings - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-13
Administrator Access - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-15
Storage Access - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-17
Wi-Fi - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-21
Configuring Wi-Fi - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-23
MDM Installation Procedures (Optional) - - - - - - - - - - - - - - - - - - - - - - 4-24
Certificate Authority- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-28
GE Marketplace - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 4-31
i-8 Vscan Extend – User Manual
5721203-100
Rev. D
Page 11

Chapter 5 — Using Vscan Extend
Scanning
General scanning recommendations- - - - - - - - - - - - - - - - - - - - - - - - - - 5-2
Black and white imaging - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-17
Color imaging- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-20
Auto freeze - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-23
AutoCycle - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-23
Use of sterile sheath - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-23
Measurements
Taking measurements- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-24
Review and recall of stored data
Backups Recommended - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-26
Reviewing and recalling images or videos from an exam list- - - - - - - - 5-26
Deletion of data - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-28
Data Export - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-30
Backup - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-42
Restore - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-45
Using Vscan Extend Apps
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-47
Bladder Volume - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-47
Lung Protocol- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-54
Tricefy - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 5-62
Chapter 6 — Vscan Extend Maintenance
System care and maintenance
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-2
Inspection - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-3
Cleaning and disinfection
Cleaning the device - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-4
Method of cleaning: Manual- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-6
Disinfecting the device - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-7
Disinfecting the probe - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-9
Rinsing and Lubricating Agents - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-11
Upgrade software
Scanner software - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-12
Troubleshooting
Vscan Extend troubleshooting - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-14
System Warning Messages - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 6-16
Chapter 7 — Appendix
Specifications
Dimension and weight- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-2
Phased array transducer for deep scanning - - - - - - - - - - - - - - - - - - - - 7-2
Linear transducer for shallow scanning- - - - - - - - - - - - - - - - - - - - - - - - 7-2
Acoustic Output Reporting Tables
Definitions, symbols and abbreviations - - - - - - - - - - - - - - - - - - - - - - - - 7-3
Acoustic Output Reporting Tables for Track 3/EN/IEC 60601-2-37 - - - - 7-6
Measurement accuracy
Basic Measurements- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 7-12
Vscan Extend – User Manual i-9
5721203-100 Rev. D
Page 12

Chapter 8 — Privacy and Security
Introduction
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-2
How to contact GE - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-2
Privacy and Security Environment - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-3
Network Connectivity
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-6
System interconnections - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-6
Network Requirements - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-8
Network Protocols- - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-8
Information Protection
Overview - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-9
Network Security - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-9
System Protection
Vscan Extend – system protection - - - - - - - - - - - - - - - - - - - - - - - - - - 8-16
Personal Information Collected by the Product
Information collection and use - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-17
Manual information collection - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-18
Information disclosure - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - - 8-19
Retention and destruction of personal information - - - - - - - - - - - - - - - 8-19
Potential Hazardous Situations Resulting From Failures of the IT Network- -
8-21
Index
i-10 Vscan Extend – User Manual
5721203-100
Rev. D
Page 13

Chapter 1
Introduction
Contents:
‘Overview’ on page 1-2
‘Warnings’ on page 1-7
‘Contact Information’ on page 1-10
Vscan Extend – User Manual 1-1
5721203-100 Rev. D
Page 14

Introduction
Attention
Overview
This manual covers the following two configurations of the
Vscan Extend. Refer to the relevant section based on the
configuration purchased.
• Vscan Extend
deep scanning (holding the phased array transducer G3S)
• Vscan Extend
deep and shallow scanning (holding the phased array
transducer G3S and the linear array transducer G8L)
Vscan Extend is a trademark of General Electric Company.
TM
configured with a sector probe allowing
TM
configured with a Dual Probe allowing
General description
Vscan Extend is a pocket-sized, battery powered general
purpose diagnostic ultrasound system. The system consists of a
handheld unit with a 5 inch touch screen display and a
permanently attached probe.
The battery can be charged either in the system or alone. The
system is capable of transferring images wirelessly to a DICOM
server or via Windows Share. Data can also be exported to a
standard PC using a wired USB export.
Capabilities also include access to GE Marketplace, which
allows the user to download Bladder Volume, Lung Protocol,
Barcode Reader and Interface to Case Exchange Software
Apps.
1-2 Vscan Extend – User Manual
5721203-100
Rev. D
Page 15

Principles of operation
Medical ultrasound images are created by computer and digital
memory from the transmission and reception of mechanical
high-frequency waves applied through a probe. The mechanical
ultrasound waves spread through the body, producing an echo
where density changes occur. The echoes return to the probe
where they are converted back into electrical signals.
These echo signals are amplified and processed by several
analog and digital circuits having filters with many frequency and
time response options, transforming the high-frequency
electrical signals into a series of digital image signals which are
stored in memory. Once in memory, the image can be displayed
in real-time on the image monitor.
A probe is an accurate, solid-state device, providing multiple
image formats. The digital design and use of solid-state
components provides highly stable and consistent imaging
performance with minimal required maintenance.
Safety
Overview
Intended use
Read and understand all instructions in the User's Manual
before attempting to use the ultrasound unit. Keep the manual
with the equipment at all time. Periodically review the
procedures for operation and safety precautions.
Vscan Extend is a general purpose diagnostic ultrasound
imaging system for use by qualified and trained healthcare
professionals enabling visualization and measurement of
anatomical structures and fluid.
Vscan Extend – User Manual 1-3
5721203-100 Rev. D
Page 16

Introduction
Indications for use (for all countries except USA, China and Japan)
Vscan Extend is a general purpose diagnostic ultrasound
imaging system for use by qualified and trained healthcare
professionals enabling visualization and measurement of
anatomical structures and fluid. It's pocket-sized portability and
simplified user interface enables integration into examination
and training sessions indoors and in other environments
described in the user manual. The information can be used for
basic/focused assessments and adjunctively wi th othe r m ed ica l
data for clinical diagnosis purposes during routine, periodic
monitoring, and triage.
With the phased array transducer on the sector probe, the
specific clinical applications and exam types include: Cardiac;
Abdominal; Renal; OB/GYN; Urology; Fetal, Evaluation of
Presence of Fluid; Imaging Guidance for Needle/Catheter
Placement (e.g. paracentesis, pericardiocentesis, thoracentesis,
amniocentesis); Peripheral Vascular Imaging (e.g. arteries and
veins); Thoracic/Lung (e.g. pleural motion/sliding, line artifacts);
Adult Cephalic; and Pediatrics.
With the addition of the linear array transducer on the single
dual headed probe solution, the specific clinical applications and
exam types are expanded to include: Peripheral vascular
imaging (e.g. lower extremity, carotid); Procedure Guidance for
Arterial or Venous Vessels (e.g. central lines, upper extremity);
Small Organs (e.g. thyroid); Musculoskeletal (Long Bone; Hip,
shoulder, elbow and Knee Joints); Evaluation of Presence of
Fluid; Thoracic/Lung (e.g. pleural motion/sliding, line artifacts);
Ophthalmic*; and Pediatrics.
WARNING
*Ophthalmic use is provided as an option. Ophthalmic
scanning MUST only be used with the linear functionality of th e
Dual Probe.
If the Vscan Extend purchased does NOT have the Ophthalmic
preset option, DO NOT use for ophthalmic use or any use
causing the acoustic beam to pass through the eye.
1-4 Vscan Extend – User Manual
5721203-100
Rev. D
Page 17

Indications for use (for USA, China and Japan)
Vscan Extend is a general purpose diagnostic ultrasound
imaging system for use by qualified and trained healthcare
professionals enabling visualization and measurement of
anatomical structures and fluid. It's pocket-sized portability and
simplified user interface enables integration into examination
and training sessions indoors and in other environments
described in the user manual. The information can be used for
basic/focused assessments and adjunctively wi th othe r m ed ica l
data for clinical diagnosis purposes during routine, periodic
monitoring, and triage.
With the phased array transducer on the sector probe, the
specific clinical applications and exam types include: Cardiac;
Abdominal; Renal; OB/GYN; Urology; Fetal, Evaluation of
Presence of Fluid; Imaging Guidance for Needle/Catheter
Placement (e.g. paracentesis, pericardiocentesis, thoracentesis,
amniocentesis); Peripheral Vascular Imaging (e.g. arteries and
veins); Thoracic/Lung (e.g. pleural motion/sliding, line artifacts);
Adult Cephalic; and Pediatrics.
With the addition of the linear array transducer on the single
dual headed probe solution, the specific clinical applications and
exam types are expanded to include: Peripheral vascular
imaging (e.g. lower extremity, carotid); Procedure Guidance for
Arterial or Venous Vessels (e.g. central lines, upper extremity);
Small Organs (e.g. thyroid); Musculoskeletal (Long Bone; Hip,
shoulder, elbow and Knee Joints); Evaluation of Presence of
Fluid; Thoracic/Lung (e.g. pleural motion/sliding, line artifacts);
and Pediatrics.
Overview
Contraindication for use (for USA, China and Japan)
The Vscan Extend ultrasound device is not intended for
ophthalmic use or any use causing the acoustic beam to pass
through the eye.
Vscan Extend – User Manual 1-5
5721203-100 Rev. D
Page 18
Introduction
Intended users
Prescription Device
Operator profile
Vscan Extend is intended to be used by qualified and trained
healthcare professionals that are legally authorized by law in the
country, state or other local municipality in which he pr actice s to
use the device. The list of the potential users includes but is not
limited to (based on title/geographical location): primary care
physicians, point-of-care users, sonographers, medical
healthcare technicians, nurses, midwives, paramedics, nurse
practitioner, physician assistants, medical students.
The users may or may not be working under supervision or
authority of a physician.
For USA only:
CAUTION: Federal law restricts this device to sale by or on the
order of a physician.
CAUTION
Qualified and trained healthcare professionals with at least a
basic level of general ultrasound training that includes limited
image acquisition techniques and interpretation (i.e. po sition the
probe correctly on the patient and determine at least normal vs.
abnormal anatomy views during scanning).
The operator must read and understand the user manual.
Contact GE sales representative fo r product training assistance
and visit the Vscan web portal for reference materials.
1-6 Vscan Extend – User Manual
5721203-100
Rev. D
Page 19

Important Safety Considerations
To prevent damage of the equipment or injury to your self or
others, read the following safety warnings before using Vscan
Extend.
Warnings
Warnings
WARNING
• Vscan Extend is a precision instrument. Handle Vscan
Extend and its accessories with care. Do not subject Vscan
Extend to mechanical shock or impact.
• Do not attempt to disassemble or alter any part of the unit
including the probe, the battery, the AC/DC adapter and
accessories. Disassembly or modification may result in
electrical shock.
• Stop using the unit if it emits smoke or noxious fumes.
Failure to do so may result in electrical shock or fire.
• Stop using the unit if the casing is damaged, including the
probe. Failure to do so may result in electrical shock.
• Do not use the device if the gorilla glass is broken.
• Do not use the AC/DC adapter if showing visible damages.
• Use only the designated power accessories (battery and
charger). Failure to do so may result in electrical shock or
fire.
• Do not place the battery near a heat source or expose it to
direct flame. Such exposure may lead to corrosive liquid
leakage, electrical shock or fire.
• To reduce risk for electrical shock, do not plug or unplug
the AC/DC adapter from mains socket with wet hands.
• Avoid dropping or subjecting the unit, including the probe,
the battery and accessories to severe impacts. This could
result in electrical shock, corrosive liquid leakage and
injury.
• Keep good hand contact with Vscan Extend during
scanning to avoid heating up of the unit and termination of
scan due to built-in temperature limits.
• At times, user may be required to enter PIN to save patient
data. It is extremely important to remember this PIN in
order to avoid loss of patient data in case of entering wrong
PIN multiple times or if the user forgets the PIN.
Vscan Extend – User Manual 1-7
5721203-100 Rev. D
Page 20

Introduction
Important Safety Considerations (continued)
WARNING
WARNING
• Do not short-circuit the battery terminal with metallic
objects. This may result in overheating and burns.
• Do not store or carry a battery loosely with metallic
devices.
• Disconnect the battery charger when not in use to avoid
fire hazard.
• Keep the charger dry. Failure to observe this precaution
may result in fire and electric shock
• Keep this unit out of reach of children. Strangulation
resulting from baby or child entanglement in probe cable
may occur.
Before charging or using a battery it is important that you read
and understand the battery safety and environment information.
• Do not damage the rechargeable battery. A damaged
battery can cause an explosion or fire, and can re su lt in
personal injury and/or property damage. To prevent injury
or damaged do not use or charge the battery if it appears to
be damaged. Signs of damage include, but are not limited
to, discoloration, warping, and leaking batt er y fluid . Do no t
expose the battery to fire, high temperature, or direct
sunlight. Do not immerse or expose the battery to water.
Do not use or store the battery inside a vehicle during hot
weather. Do not drop or puncture the battery. Do not open
the battery or short-circuit its contacts.
WARNING
Avoid contact with the rechargeable battery if it appears to be
leaking. Battery fluid is corrosive, and contact with it can result
in personal injury and/or property damage.
To prevent injury or damage:
• If the battery leaks, avoid contact with the battery fluid. If
any liquid from the battery should come in contact with the
eye, immediately wash the eye with plenty of water and
seek medical advice as soon as possible. Do not rub your
eyes!
• If battery fluid gets onto your skin or clothing, immediately
use clean water to wash off the battery fluid.
1-8 Vscan Extend – User Manual
5721203-100
Rev. D
Page 21

Important Safety Considerations (continued)
Warnings
WARNING
CAUTION
Charge and use the rechargeable battery only in strict
accordance with the instructions. Charging or using the battery
in unauthorized equipment can cause an explosion or fire, and
can result in personal injury and/or equipment damage.
To prevent injury or damage:
• Do not charge or use the battery if it appears to be
damaged or leaking.
• Charge the battery only in a Vscan Extend device or in the
Vscan Extend battery charger. Be sure to follow all
instructions that are provided with the battery charger.
• Discontinue charging a battery that gives off extreme heat
or a burning odor.
• Use the battery only in the Vscan Extend.
• Use the battery only for its intended use and according to
the instructions in the product documentation.
• Ensure to backup data regularly and to erase the data
before sending the Vscan Extend for service.
• To access or add patient information, a device pin is
required. Ensure not to lose the pin.
CAUTION
Vscan Extend – User Manual 1-9
5721203-100 Rev. D
Vscan Extend cannot be sterilized. Apply a sterile sheath if the
device is going to be used in an application where a higher
degree of cleanliness is required. The sheath should cover the
probe and any part of the probe cable that might come in
contact with the area requiring higher cleanliness.
Page 22

Introduction
Contacting GE Ultrasound
For additional information or assistance, please contact your
local distributor or the appropriate support resource listed on the
following pages:
Internet
https://vscan.gehealthcare.com
http://www.gehealthcare.com
USA
Contact Information
Clinical Questions
Service Questions
TEL: (1) 800-437-1171
Ultrasound Service Engineering
9900 Innovation Drive
Wauwatosa, WI 53226
Please contact your local Applications or Sales Representative.
For service contact your local Service Representative.
1-10 Vscan Extend – User Manual
5721203-100
Rev. D
Page 23

Accessories Catalog Requests
To request the latest GE Accessories catalog or equipment
brochures in the United St ates, call the Response Center
TEL: (1) 800-643-6439
In other locations, contact your local Applications, Sales or
Service Representative.
Placing an order
To place an order, order supplies or ask an accessory-related
question in the United States, call the GE Access Center
TEL: (1) 800-472-3666
In other locations, contact your local Applications, Sales or
Service Representative.
Global ultrasound support center phone numbers
For countries not listed in the tables below, please contact the
local distributor.
Contact Information
When contacting Support you will have to provide your system
ID. If the system ID is unknown, please give the Temporary
System ID “Vscan Extend” to be properly routed for support.
Americas
Table 1-1: Americas
Region Telephone
United States
Canada 800-668-0732
Mexico 0800 9043400
Puerto Rico 0800 4371171
Brazil 0800 122345
Argentina 0800 3331984
Chile 0800 367000
1
For USA only: when contacting GE CARES you will have to provide your system ID. If system ID is
unknown, please give the Temporary System ID “Vscan Extend” to be properly routed for support.
1
800-437-1171
Vscan Extend – User Manual 1-11
5721203-100 Rev. D
Page 24

Introduction
Europe, Middle East and Africa
Table 1-2: Europe, Middle East and Africa
Region Telephone
Algeria +21321484612
Andorra 902 400 246
Austria 0800244260
Belgium Dutch +32 262 638 38
Belgium French +32 262 638 39
Bulgarian +35929712040
Denmark 80404944
Egypt +202 19434 [hot line]
Finland 0981710182
France 0800139140
G. D. Luxembourg 080022973
Germany 08004373784
Greece 302109690660
Holy See 800 827168
Hungary +36-23-410-510
Ireland 1800992557
Israel Contact local distributor
Italy Central 800 827168
Italy North-East 800 827166
Italy North-West 800 827164
Italy South 800 827170
Liechtenstein 0041 44 809 9293
Monaco 0800139140
Netherlands 8000994442
Northern Ireland 08000720248
Norway 80062043
Portugal 800 834 004
Russia +7 495 739 69 75
San Marino 800 827168
1-12 Vscan Extend – User Manual
5721203-100
Rev. D
Page 25

Table 1-2: Europe, Middle East and Africa (Continued)
Region Telephone
Saudi Arabia 800 1243002
South Africa 800 111 671
Spain 902 400 246
Sweden 0201201436
Switzerland 0800556958
Turkey Contact local distributor
UAE 800 3646
UK 0845 8503392
Ukraine +38 044 391 37 56 (57)
Contact Information
Vscan Extend – User Manual 1-13
5721203-100 Rev. D
Page 26

Introduction
Asia and Pacific
Table 1-3: Asia and Pacific
Region Telephone
China 8008108188
Hong Kong (852) 21006288
Taiwan 0800-021-770
India (91) 1800-425-8025
Singapore (65) 62773444
Australia 1-800-659-465
New Zealand 0800 65 94 65
Japan 0120-055-919
Korea (82) 2-1544-6119
Bangladesh (880) 29135488
Sri Lanka (94) 114891178
Bhutan Contact GE India
Maldives Contact GE India
Nepal Contact local distributor
Malaysia 1800 88 3911
Thailand (66) 26248400
Vietnam Contact local distributor
Philippines Contact local distributor
Indonesia Contact local distributor
Laos Contact local distributor
Brunei Darussalam Contact local distributor
Cambodia Contact local distributor
Manufacturer
GE VINGMED ULTRASOUND A/S
Strandpromenaden 45
3191 Horten, Norway
TEL: (+47) 3302 1100; FAX: (+47) 3302 1350
1-14 Vscan Extend – User Manual
5721203-100
Rev. D
Page 27

Chapter 2
Safety
Contents:
‘Introduction’ on page 2-2
‘Owner responsibility’ on page 2-3
‘Important safety considerations’ on page 2-5
‘Maximum probe temperature’ on page 2-24
‘Device labels and symbols’ on page 2-25
Vscan Extend – User Manual 2-1
5721203-100 Rev. D
Page 28

Safety
Overview
Introduction
This chapter describes the important safety measures which
should be taken before operating the Vscan Extend. Procedures
for simple care and maintenance of the Vscan Extend are also
described.
Various levels of safety precautions may be foun d on the
equipment, and different levels of severity are identified by one
of the following icons that precede precautionary statements in
the text.
The following icons are used to indicate pr ec au tio ns :
DANGER
WARNING
CAUTION
Indicates that a specific hazard is known to exist which through
inappropriate conditions or actions will cause:
• Severe or fatal personal injury
• Substantial property damage.
Indicates that a specific hazard is known to exist which through
inappropriate conditions or actions may cause:
• Severe personal injury
• Substantial property damage.
Indicates that a potential hazard may exist which through
inappropriate conditions or actions will or can cause:
• Minor injury
• Property damage.
2-2 Vscan Extend – User Manual
5721203-100
Rev. D
Page 29

Overview
Owner responsibility
Owner responsibility
It is the responsibility of the owner to ensure that anyone
operating Vscan Extend reads and understands this section of
the manual. However, there is no representation that the act of
reading this manual renders the reader qualified to operate,
inspect, test, align, calibrate, troubleshoot, repair or modify the
system. The owner should make certain that only properly
trained, fully-qualified service personnel undert ake maintenance
of the equipment. There are no user serviceable parts in the
system or accessories. If servicing is required, contact GE. See
‘Contact Information’ on page 1-10 for more information.
The owner of Vscan Extend should ensure that only properly
trained, fully qualified personnel are authorized to operate the
system. Before authorizing anyone to operate the system, it
should be verified that the person has read, and fully
understands, the operating instructions contained in this
manual. It is advisable to maintain a list of authorized operators.
Should the system fail to operate correctly, or if Vscan Extend
does not respond to the commands described in this manual,
the operator should contact the nearest field GE Ultrasound
Service Office.
For information about specific requirements and regulations
applicable to the use of electronic medical equipment, consult
the local, state and federal agencies.
Vscan Extend – User Manual 2-3
5721203-100 Rev. D
Page 30

Safety
Overview (continued)
The owner of Vscan Extend MUST be aware of the data
protection policies while configuring the DICOM and Windows
Share servers. GE is not responsible for data sharing. Bef ore
configuring the servers, the owner of the Vscan Extend MUST
abide by the applicable privacy acts specific to their region or
country.
CAUTION
CAUTION
For USA only:
Federal law restricts this device to sale by or on the order of a
physician.
Vscan Extend should be used in compliance with law. Some
jurisdictions restrict certain uses, such as gender
determination.
Notice against user modification
Never modify this product, including system components,
cables, and so on. User modification may cause safety hazards
and degradation in system performance. All modification must
be done by a GE qualified person.
Software upgrade following GE recommendations can be done
by the user.
2-4 Vscan Extend – User Manual
5721203-100
Rev. D
Page 31

Overview
Important safety considerations
Important safety considerations
This section includes considerations for the following:
• Patient safety
• Personnel and equipment safety
The information contained in this section is intended to
familiarize the user with the hazards associated with the use of
Vscan Extend, and to alert them to the extent to which injury and
damage may occur if the precautions are not observed.
Users are obligated to familiarize themselves with these safety
considerations and to avoid conditions that could result in injury
or damage.
Patient Safety
Patient identification
WARNING
CAUTION
The concerns listed in this section can seriously affect the
safety of the patient undergoing a diagnostic ultrasound
examination.
Always include proper identification with all patient data.
Identification errors could result in an incorrect diagnosis.
If the Vscan Extend needs to be sent for repair, ensure that the
patient information is backed up and confirm backup was
successful. The patient information MUST be erased from
internal memory (See ‘Backup’ on page 5-42 for more
information.) before shipping.
To access or add patient information, a device pin is required.
Retain the 4-digit pin in a safe location to prevent loss of the
pin. Avoid sharing the PIN. See ‘Administrator Access’ on
page 4-15 for more information.
Vscan Extend – User Manual 2-5
5721203-100 Rev. D
Page 32

Safety
Diagnostic information
The images and calculations provided by the system, including
bladder volume and lung protocol, are intended for use by
competent users, as a diagnostic tool. They are not to be
explicitly regarded as the sole, irrefutable basis for clinical
diagnosis. Users are encouraged to study the literature and
reach their own professional conclusions regarding the clinical
use of the system.
The user should be aware of the product specifications and of
the system accuracy and stability limitations. These limitations
must be considered before making any decisio n ba sed on
quantitative values. If in doubt, the nearest GE Ultrasound
Service Office should be consulted.
Equipment malfunction or incorrect settings can result in
measurement errors or failure to detect detail s in the image. The
user must become thoroughly familiar with the operation of the
Vscan Extend in order to optimize its performance and to
recognize possible malfunctions.
CAUTION
Avoid reflections from windows/lamps/direct sunlight on the
display. Avoid analyzing data from small viewing angles.
General precautionary advice for the use of diagnostic ultrasound in
combination with ultrasound contrast agents
CAUTION
The Vscan Extend is not intended to be used with a contrast
agent. Cardiac rhythm disturbances during cardiac studies
using gas ultrasound contrast agents have been observed in
the diagnostic range of Mechanical Index (MI) values. See the
specific package insert for the contrast agent being used for
further details.
Mechanical hazards
A damaged probe may result in injury or increased risk of
contamination. Inspect the probe frequently for sharp, pointed or
rough surface damage that could cause injury or tear protective
barriers (gloves and sheaths).
2-6 Vscan Extend – User Manual
5721203-100
Rev. D
Page 33

Electrical hazard
A damaged probe may increase the risk of electric shock if
conductive solutions come in contact with internal electronics.
Inspect the probe often for cracks or openings in the housing
and holes in and around the acoustic lens, or other damag e that
could allow moisture to enter. Become familiar with the probe's
care precautions outlined in ‘Inspecting the Vscan Extend’ on
page 6-3.
Personnel and equipment safety
Important safety considerations
DANGER
Explosion hazard
The hazards listed below can seriously affect the safety of
personnel and equipment during a diagnostic ultrasound
examination.
Never operate the equipment in the presence of flammable or
explosive liquids, vapors or gases. Malfunctions in the Vscan
Extend, or sparks, can electrically ignite these substances.
Operators should be aware of the following points to prevent
such explosion hazards.
• If flammable substances are detected in the environment,
do not plug in or turn on the system.
• If flammable substances are detected after the system has
been turned on, do not attempt to turn of f the Vscan Exten d,
or to unplug it.
• If flammable substances are detected, evacuate and
ventilate the area before turning off Vscan Extend.
Vscan Extend – User Manual 2-7
5721203-100 Rev. D
Page 34

Safety
Electrical hazard
WARNING
NOTE: Any rest energy within our scanners or their components will be
To avoid injury
Pacemaker hazard
The internal circuits of the AC/DC adapter use high voltages,
capable of causing serious injury or death by electrical shock.
below 60 V DC or 2 mJ.
• Do not remove the Vscan Extend’s protective covers. No
user-serviceable parts are inside. If servicing is required,
contact GE service.
• Conductive fluids seeping into the active circuit components
may cause short circuiting, which could result in an electrical
fire.
The possibility of the system interfering with pacemakers is
minimal. However, as this system generates high frequency
electrical signals, the operator should be aware of the potential
hazard this could cause.
Electrical Safety
Device classifications
Vscan Extend is an internally powered device, type BF.
The AC/DC adapter is Class II.
2-8 Vscan Extend – User Manual
5721203-100
Rev. D
Page 35

External Connection
Important safety considerations
CAUTION
1. Patient environment
Connection to a PC can be done when the PC is in compliance
with the EN/IEC 60950 (Data processing equipment).
The computer connected to Vscan Extend must be kept
outside the patient environment (refer to local regulation and
EN/ES/IEC 60601-1).
Figure 2-1. Patient environment
Allergic reactions to latex-containing medical devices
Due to reports of severe allergic reactions to medical devices
containing latex (natural rubber), the FDA advises healthcare
professionals to identify latex-sensitive patients, and be
prepared to treat allergic reactions promptly. Latex is a
component of many medical devices, including surgical and
examination gloves, catheters, incubation tubes, anesthesia
masks and dental dams. Patient reaction to latex has ranged
from contact urticaria, to systemic anaphylaxis.
For more details regarding allergic reaction to latex, refer to FDA
Medical Alert MDA91-1, March 29.
Vscan Extend – User Manual 2-9
5721203-100 Rev. D
Page 36

Safety
Electromagnetic Compatibility (EMC)
NOTE: This unit carries the CE mark. It complies with regulatory
requirements of the European Directive 93/42/EEC concerning
medical devices. It also complies with emission limits for a
Group 1, Class B Medical Device as stated in EN/
IEC 60601-1-2.
Electrical medical equipment needs special precautions
regarding EMC and needs to be installed and put into service
according to the EMC information provided in this manual.
AII types of electronic equipment may characteristically cause
electromagnetic interference with other equipment, transmitted
either through air or connecting cables. The term
Electromagnetic Compatibility (EMC), indicates the capability of
the equipment to curb electromagnetic influence from other
equipment, while at the same time not affecting oth er equipment
with similar electromagnetic radiation.
Radiated or conducted electromagnetic signals can cause
distortion, degradation, or artifacts in the ultrasound image
which may impair the ultrasound unit’s essential performance
(see ‘Electrical Safety’ on page 2-8).
There is no guarantee that interference will not occur in a
particular installation. If this equipment is found to cause or
respond to interference, attempt to correct the problem by one
or more of the following measures:
• Re-orient or re-locate the affected device.
• Increase the separation between the unit and the affected
device.
• Power the equipment from a source other than that of the
affected device.
• Consult the service representative for further suggestions.
The manufacturer is not responsible for any interference or
responses caused by the use of interconnecting cables other
than those recommended, or by unauthorized changes or
modifications to this unit. Unauthorized changes or
modifications could void the user's authority to operate the
equipment.
To comply with the regulations on electromagnetic interference,
all interconnecting cables to peripheral devices must be
shielded and properly grounded. Use of cable s no t pr op er ly
shielded and grounded may result in the equipment causing or
responding to radio frequency interference, in violation of the
European Union Medical Device Directive and FCC regulations.
2-10 Vscan Extend – User Manual
5721203-100
Rev. D
Page 37

Important safety considerations
Electromagnetic Compatibility (EMC) (continued)
Devices which intrinsically transmit radio waves such as cellular
phones, radio transceivers, mobile radio transmitters,
radio-controlled toys, and so on, should preferably not be
operated near the unit. See ‘Electromagnetic Compatibility
(EMC)’ on page 2-10 about the recommended minimum
separation distances between portable and mobile RF
communications equipment and th e ult ra so un d unit.
Any electrical device can unintentionally emit electromagnetic
waves. However, minimum device separation distances cannot
be calculated for such unspecified electromagnetic radiation.
When the ultrasound unit is used adjacent to or in close
proximity to other equipment the user shou ld be att en tiv e to
unexpected device behavior which may be caused by such
electromagnetic radiation.
The ultrasound unit is intended for use in the electromagnetic
environment specified in the tables below.
The user of ultrasound unit should assure that the device is
used in such an environment.
FCC compliance statements
1. This device may not cause harmful interference, and
2. This device must accept any interference received,
including Interference that may caus e un de sir ed op er at i on .
FCC Caution!!!
• Any changes or modifications not expressly approved by the
party Responsible for compliance could void the user's
authority to operate this Equipment
• This equipment should be installed and operated with
minimum distance 20 cm between the radiator & your body.
Part 15B compliance statements for digital devices:
NOTE: This equipment has been tested and found to comply with the
limits for a Class B digital device, pursuant to part 15 of the FCC
Rules. These limits are designed to provide reasonable
protection against harmful interference in a residential
installation.
Vscan Extend – User Manual 2-11
5721203-100 Rev. D
Page 38

Safety
FCC compliance statements (continued)
This equipment generates, uses and can radiate radio
frequency energy and, if not installed and used in accordance
with the instructions, may cause harmful interference to radio
communications. However, there is no guarantee that
interference will not occur in a particular installation. If this
equipment does cause harmful interference to radio or television
Reception, which can be determined by turning the equipment
off and on, the user is encouraged to try to correct th e
interference by one or more of the following measures:
• Increase the separation between the equipment and
receiver.
• Connect the equipment into an outlet on a circuit different
from that to which the receiver is connected
• Consult the dealer or an experienced radio/TV technician for
help.
This Class B digital apparatus complies with Canadian
ICES-003.
This device complies with Industry Canada license-exempt RSS
standard(s). Operation is subject to the following two conditions:
1. This device may not cause interference, and
2. This device must accept any interference, including
interference that may cause undesired operation of the
device.
FCC compliance statements for Canada (French translation)
Le présent appareil est conforme aux CNR d'Industrie Canada
applicables aux appareils radio exempts de licence.
L'exploitation est autorisée aux.
deux conditions suivantes:
1. l'appareil ne doit pas produire de brouillage, et
2. l'utilisateur de l'appareil doit accepter tout brouillage
adioélectrique subi, même si le brouillage est susceptible
d'en compromettre le fonctionnement.
2-12 Vscan Extend – User Manual
5721203-100
Rev. D
Page 39

Important safety considerations
FCC compliance statements for Canada (French translation) (continued)
This equipment complies with radio frequency exposure limit s
set forth by Industry Canada for an uncontrolled Environment.
This equipment should be installed and operated with minimum
distance 20 cm between the device and the user or
bystanders.Cet équipement est conforme aux limites
d'exposition aux radiofréquences
définies par Industrie Canada pourun environnement non
contrôlé. Cet équipement doit être installé et utilisé avec un
minimum de 20 cm de distance entre le dispositif et l'utilisateur
ou des tiers.
un appareil numérique, en vertu de l’article 15 de la
réglementation de la FCC. Ces limites ont été instaurées pour
fournir une protection raisonnable contre toute interférence
nuisible dans une installation résidentielle. Cet équipement
génère, utilise et peut émettre de l’énergie radiofréquence. S’il
n’est pas installé et utilisé conformément aux instructions, il peut
provoquer des interférences sur les communications radio.
Cependant, il n’est pas garanti que des interférences ne se
produiront pas dans certaines installations. Si cet équipement
cause des interférences à la réception radio ou télévisée (ce qui
peut être vérifi é en éteignant l’appareil puis en le remettant
sous tension), l’utilisateur peut tenter de les résoudre en suivant
une ou plusieurs des mesures ci-après:
• Réorienter ou déplacer l’antenne réceptrice. Augmenter
l’espace entre
• l’appareil et le récepteur. Brancher l’appareil à une prise de
courant différente de celle sur laquelle le récepteur est
branché.
Pour obtenir de l’aide, contacter le vendeur ou un technician
radio/télévision expérimenté.
REMARQUE : Toute modifi cation non autorisée expressément
par le fabricant responsable de la conformité peut annuler le
droit de l’utilisateur à faire fonctionner le produit.
Vscan Extend – User Manual 2-13
5721203-100 Rev. D
Page 40

Safety
Electromagnetic emissions
Table 2-1: Electromagnetic emissions
Guidance and manufacturer’s declaration – electromagnetic emissions.
Emissions test Compliance Electromagnetic environment - guidance
RF emission
EN55011
RF emission
EN55011
Harmonic emission
EN 61000-3-2
Voltage fluctuations/flicker
emissions
EN/IEC 61000-3-3
Group 1
Class B
Group 1
Class B
Complies
Complies
The Vscan Extend uses RF energy only for its internal
function. Therefore, its RF emissions are very low and
are not likely to cause any interference in nearby
electronic equipment.
The Vscan Extend is suitable for use in all
establishments, including domestic establishments and
those directly connected to the public low-voltage power
supply network that supplies buildings used for domestic
purposes.
Electromagnetic immunity
Table 2-2: Electromagnetic immunity (Part 1)
Guidance and manufacturer’s declaration – electromagnetic immunity.
The Vscan Extend is intended for use in the electromagnetic environment below. The
customer or the user of the Vscan Extend should assure that it is used in such an
environment.
Electromagnetic
Immunity test
EN 60601
test level Compliance level
environment –
guidance
Electrostatic discharge
(ESD)
EN 61000-4-2
Electrical fast transient/
burst
EN 61000-4-4
Surge
EN 61000-4-5
±6 kV contact
±8 kV air
±2 kV for power-supply
lines
±1 kV for input/output
lines
±1 kV line(s) to line(s) ±1 kV line(s) to
±6 kV
±8 kV
±2 kV for
power-supply lines
±1 kV for input/
output lines
line(s)
Floors should be wood,
concrete or ceramic tile. If
floors are covered with
synthetic material, the
relative humidity should be
at least 30%.
Mains power quality should
be that of a typical
commercial or hospital
environment.
Mains power quality should
be that of a typical
commercial or hospital
environment.
2-14 Vscan Extend – User Manual
5721203-100
Rev. D
Page 41

Important safety considerations
Table 2-2: Electromagnetic immunity (Part 1) (Continued)
Guidance and manufacturer’s declaration – electromagnetic immunity.
The Vscan Extend is intended for use in the electromagnetic environment below. The
customer or the user of the Vscan Extend should assure that it is used in such an
environment.
Electromagnetic
Immunity test
EN 60601
test level
Compliance level
environment –
guidance
Voltage dips, short
interruptions and
voltage variations on
power supply input lines
EN 61000-4-11
Power frequency (50/
60 Hz) magnetic field
EN/IEC 61000-4-8
NOTE: U
is the a. c. mains voltage prior to application of the test level.
T
5% U
(>95% dip in
T
) for 0,5 cycle
U
T
40% UT
(60% dip in U
cycles
70% U
(30% dip in
T
) for 25 cycles
U
T
) for 5 s
T
(>95% dip in
T
< 5% U
U
) for 5
T
(>95% dip in
5% U
T
) for 0,5 cycle
U
T
40% UT
(60% dip in U
cycles
70% U
(30% dip in
T
) for 25 cycles
U
T
< 5% U
in U
(>95% dip
T
) for 5 s
T
) for 5
T
Mains power quality should
be that of a typical
commercial or hospital
environment. If the user of
the ultrasound unit requires
continued operation during
power mains interruptions, it
is recommended that the
ultrasound unit is powered
from an uninterruptible
power supply or a battery.
3 A/m 3 A/m Power frequency magnetic
fields should be at levels
characteristic of a typical
location in a typical
commercial or hospital
environment.
Vscan Extend – User Manual 2-15
5721203-100 Rev. D
Page 42

Safety
Electromagnetic immunity (continued)
Table 2-3: Electromagnetic immunity (Part 2)
Guidance and manufacturer ’s declaration – electromagnetic immuni ty – for all medical
electrical equipment and medical electrical systems that not life-supporting
The Vscan Extend is intended for use in the electromagnetic environment below. The
customer or the user of the Vscan Extend should assure that it is used in such an
environment.
Immunity test
Conducted RF
EN 61000-4-6
Radiated RF
EN 61000-4-3
IEC 60601 test
level
3Vrms
150 kHz to 80 MHz
3 V/m
80 Mhz to 2,5 GHz
Complianc
e level
3V
3 V/m
Electromagnetic
environment – guidance
Portable and mobile RF communications
equipment should be used no closer to any
part of the ultrasound unit, including cables,
than the recommended separation distance
calculated from the equation applicable to
the frequency of the transmitter.
Recommended separation distance
80 MHz to 800 MHz
800 MHz to 2.5 GHz
where p is the maximum output power rating
of the transmitter in watts (W) according to
the transmitter manufacturer and d is the
recommended separation distance in metres
(m).
Field strengths from fixed RF transmitters,
as determined by an electromagnetic site
a
survey,
should be less than the compliance
level in each frequency range.
Interference may occur in the vicinity of
equipment marked with the following symbol
c
b
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
2-16 Vscan Extend – User Manual
5721203-100
Rev. D
Page 43

Important safety considerations
Table 2-3: Electromagnetic immunity (Part 2) (Continued)
Guidance and manufacturer ’s declaration – electromagnetic immuni ty – for all medical
electrical equipment and medical electrical systems that not life-supporting
The Vscan Extend is intended for use in the electromagnetic environment below. The
customer or the user of the Vscan Extend should assure that it is used in such an
environment.
IEC 60601 test
Immunity test
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and
land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted
theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an
electromagnetic site survey should be considered. If the measured field strength in the location in which the
ultrasound unit is used exceeds the applicable RF compliance level above, the ultrasound unit should be
observed to verify normal operation. If abnormal performance is observed, additional measures may be
necessary, such as re-orienting or relocating the ultrasound unit.
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
level
Complianc
e level
Electromagnetic
environment – guidance
c
Vscan Extend – User Manual 2-17
5721203-100 Rev. D
Page 44

Safety
Separation distances
Table 2-4: Separation distances
Recommended separation distances between portable and mobile RF communications
equipment and the ultrasound unit
The ultrasound unit is intended for use in an electromagnetic environment in which radiated RF
disturbances are controlled. The customer or the user of the ultrasound unit can help prevent
electromagnetic interference by maintaining a minimum distance between portable and mobile RF
communications equipment (transmitters) and the ultrasound unit as recommended below, according to the
maximum output power of the communications equipment.
Separation distance according to frequency of transmitter (m)
Rated maximum output
of transmitter W
0,01 0,12 0,12 0,23
0,1 0,38 0,38 0,73
1 1,2 1,2 2,3
10 3,8 3,8 7,3
100 12 12 23
For transmitters rated at a maximum output power not listed above the recommended separation distance d
in metres (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is
the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by
absorption and reflection from structures, objects and people.
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2,5 GHz
Essential Performance
The essential performance of the Vscan Extend is:
• The ability to display physiological images as input for
diagnosis by qualified and trained healthcare professionals.
• The ability to display quantified data as input for diagnosis
by qualified and trained healthcare professionals.
• The display of ultrasound indexes as aid for safe use of the
Vscan Extend.
2-18 Vscan Extend – User Manual
5721203-100
Rev. D
Page 45

Acoustic output
Definition of the acoustic output parameters
Thermal Index
TI is an estimate of the temperature increase of soft tissue or
bone. There are three thermal index categories:
• TIS: Soft tissue thermal index. The main TI category. Used
for applications that do not image bone.
• TIB: Bone thermal index (bone located in a focal region).
Used for fetal application.
• TIC: Cranial bone thermal index (bone located close to the
surface). Used for transcranial application.
Reference to calculation of TI can be found in:
• NEMA Standards Publication UD 3: “Standard for Real-T ime
Display of Thermal and Mechanical Acoustic Output Indices
on Diagnostic Ultrasound Equipment”, Revision 2
• EN/IEC 60601-2-37. Medical electrical equipment.
Part 2-37: Particular requirements for the safety of
ultrasonic medical diagnostic and monitoring equipment
Important safety considerations
Mechanical Index
Ispta
MI is the estimated likelihood of tissue damage due to cavitation.
The absolute maximum limits of the MI is 1.9 as set by the FDA
guidance of September 9, 2008 for diagnostic ultrasound
systems and transducers.
The Ispta is the Spatial Peak Temporal Average Intensity. The
absolute maximum limit of Ispta is 720 mW/cm
FDA guidance of September 9, 2008 for diagnostic ultrasound
systems and transducers.
2
as set by the
Vscan Extend – User Manual 2-19
5721203-100 Rev. D
Page 46

Safety
Acoustic output and display on Vscan Extend
MI and TI values are displayed on the scanning screen.
The maximum possible MI and Ispta on the Vscan Extend is
within the limits set in Track 3 in the FDA guidance of
September 9, 2008 for diagnostic ultrasound systems and
transducers, MI <1.9 and Ispta <720 mW/cm
Display Accuracy and Acoustic Measurement Uncertainties
The display accuracy and measurement precision of the output
display are summarized in the table below. Accuracy of the
output display (TI, MI) parameters depends on the
measurement system precision, the acoustic model used to
calculate the parameters and variation in the acoustic output of
probes and systems. The measurement precision and overall
accuracy of the measurements have been as ses se d by
determining both the random and the systematic uncertainties
and given in percent at 95% confidence level.
Table 2-5: Display accuracy
2
.
Parameter Estimated accuracy
Pressure, MI ±25% ±15%
Power, TI ±50% ±40%
a
a. Accuracy = (Measured value - displayed value)/displayed
value * 100%
Measurement precision
black and white/color
2-20 Vscan Extend – User Manual
5721203-100
Rev. D
Page 47

System controls affecting acoustic output
The operator controls that directly affect the acoustic output are
discussed in the Acoustic Output Data Tables (See ‘Acoustic
Output Reporting Tables’ on page 7-3 for more information.).
These tables show the highest possible acoustic intensity for a
given mode, obtainable only whe n the maxim um combination of
control settings is selected. Most settings result in a much lower
output. It is important to note the following:
• The duration of an ultrasound examination is as important
as the acoustic output, since patient exposure to output is
directly related to the exposure time.
• Controls can help to reduce patient exposure, even though it
may not directly affect acoustic output.
The British Medical Ultrasound Society has suggested some
maximum scanning times relative to displayed TI as follows:
Table 2-6: Maximum scanning times
General Abdominal, *Peripheral
Vascular, Adult cephalic,
Musculoskeletal, cardiac and other
Obstetric scanning
Important safety considerations
scanning
TI time TI time Note
0.0–0.7 Unlimited 0.0–1.0 Unlimited Monitor TI
0.7–1.0 < 60 min 1.0–1.5 < 120 min
1.0–1.5 < 30 min 1.5–2.0 < 60 min
1.5–2.0 < 15 min 2.0–2.5 < 15 min
2.0–2.5 < 4 min 2.5–3.0 < 4 min
2.5–3.0 < 1 min
*Peripheral vascular is applicable for only dual probe
References
• The British Medical Ultrasound Society. Guidelines for the safe use of diagnostic ultrasound equipment.
• American Institute of Ultrasound in Medicine Consensus Report on Potential Bioeffects of Diagnostic
Ultrasound.
Vscan Extend – User Manual 2-21
5721203-100 Rev. D
Page 48

Safety
Application selection
Selecting the application appropriate to a particular ultrasound
examination automatically provides acoustic output limits within
FDA guidances for that application. Other parame te rs which
optimize performance for the selected application are also set
automatically, and should assist in reducing the patient
exposure time.
Changing imaging modes
Acoustic output depends on the imaging mode selected. This
greatly affects the energ y absorbed by the tissue (see ‘Acoustic
Output Reporting Tables’ on page 7-3 for TI and MI values in
black and white or color imaging).
ALARA
Ultrasound procedures should be performed using output levels
and exposure times As Low Reasonably Achievable (ALARA)
while acquiring clinical information.
During a diagnostic ultrasound examination, high frequency
sound penetrates and interacts with tissue in and around the
area of anatomy to be imaged. Only a small portion of the sound
energy is reflected back to the probe for use in constructing the
image while the remainder is dissipated within the tissue. The
interaction of sound energy with tissue at sufficiently high levels
can produce biological effects (aka bioeffects) of either a
mechanical or thermal nature. Bioeffect is ge nerally undesired in
diagnostic application and may be harmful in some conditions.
ALARA training is provided in the Medical Ultrasound Safety
booklet, published by AIUM (American Institute of Ultrasound in
Medicine) provided on the Documentation CD. The ALARA
education program for the clinical end-user covers basic
ultrasound principles, possible biological effects, the derivation
and meaning of the indices, ALARA principles, and examples of
specific applications of the ALARA principle.
2-22 Vscan Extend – User Manual
5721203-100
Rev. D
Page 49

ALARA (continued)
Training
Important safety considerations
To contact the AIUM concerning their publications:
• In the USA, by telephone at 1-800-638-5352
• To write them, use the following address:
AIUM
14750 Sweitzer Lane
Suite 100
Laurel, MD, USA 20707-5906
In addition, the sections ‘Acoustic output and display on Vscan
Extend’ on page 2-20 and ‘System controls affecting acoustic
output’ on page 2-21 should be studied carefully in order to
implement ALARA.
During each ultrasound examination the user is expected to
weigh the medical benefit of the diagnostic information that
would be obtained against the risk of potential harmful effects.
Once a diagnostic image is achieved, prolonging the exposure
cannot be justified. It is recommended that all users receive
proper training in applications before performing them in a
clinical setting.
Environmental protection
System disposal
The equipment must not be disposed of as unsorted municipal
waste nor be destroyed by incineration.
It must be collected separately. Please contact an authorized
representative of the manufacturer for information concerning
the decommissioning of your equipment.
Vscan Extend – User Manual 2-23
5721203-100 Rev. D
Page 50

Safety
Maximum probe temperature
The table below indicates the maximum probe temperature.
Table 2-7: Maximum probe temperature
Probe
Vscan Extend – configured with Sector probe:
Phased array transducer (G3S) for Deep scanning
Vscan Extend – configured with Dual probe:
Phased array transducer (G3S) for Deep scanning
Vscan Extend – configured with Dual probe:
Linear array transducer (G8L) allowing shallow
scanning
NOTE: Measurement uncertainty and probe variation: 2 °C.
NOTE: Lens temperature measured under following conditions per EN/
IEC 60601-2-37:
• Thermocouple was placed at the geometric center of the
lens.
• Thermal phantom at 33 °C (or 23 °C) for external probes.
(Temperature rise is measured and added to 33 °C if the
phantom is at 23 °C).
NOTE: Thermal phantom made with tissue-mimicking material as
referenced in EN/IEC 60601-2-37.
• Probe placed upright in contact with above thermal
phantom.
• Auto-freeze capability is disabled.
• Lens temperature is monitored for 30 minutes.
Max Temp in deg C
(Simulated use)
39.7 43.1
41 42.6
39.1 42.3
Max Temp in deg C
(Still air)
2-24 Vscan Extend – User Manual
5721203-100
Rev. D
Page 51

Device labels and symbols
Vscan Extend Labels (Example)
Device labels and symbols
Figure 2-2. Vscan Extend rating label
1. Rating label
2. Inner label
3. UDI label - contains UDI information
4. Serial number label - contains device serial number
NOTE: The label shown in Figure 2-2 is a sample. The label varies
country to country based on country requirements.
Vscan Extend – User Manual 2-25
5721203-100 Rev. D
Page 52

Safety
Vscan Extend Labels (Example) (continued)
Figure 2-3. Vscan Extend battery label
Figure 2-4. Vscan Extend AC/DC adapter label
Figure 2-5. Vscan Extend external battery charger label
2-26 Vscan Extend – User Manual
5721203-100
Rev. D
Page 53

Vscan Extend Labels (Example) (continued)
The following table describes the purpose of safety labels and
other important information provided on the equipment.
Table 2-8: Label Icons
Label Purpose Location
CE mark • Vscan Extend system
Device labels and symbols
• Vscan Extend External
Battery charger
This symbol indicates that the waste of
electrical and electronic equipment must
not be disposed as unsorted municipal
waste and must be collected separately.
Please contact the manufacturer or other
authorized disposal company to
decommission your equipment.
Follow instructions for use. Read and
understand all instructions in the User's
Manual before attempting to use the
ultrasound unit.
TÜV mark Vscan Extend system
Prescription device statement for the USA
only:
Caution: Federal law restricts this device to
sale by or on the order of a physician.
Type BF Applied Part symbol (see
‘Classifications’ on page i-5)
Keep dry Vscan Extend system
• Vscan Extend system
• Vscan Extend battery
• Vscan Extend External
battery charger
• Vscan Extend system
• Vscan Extend External
Battery charger
Vscan Extend system
Vscan Extend system
Input, use only Vscan Extend charger. Vscan Extend system
Vscan Extend External Battery
charger
Rechargeable, use only Vscan Extend
battery.
Input to Vscan Extend battery Vscan Extend External Battery
Vscan Extend system
Vscan Extend External Battery
charger
charger
Vscan Extend – User Manual 2-27
5721203-100 Rev. D
Page 54

Safety
Table 2-8: Label Icons (Continued)
Label Purpose Location
Assembled in Romania
(Romania is a country
name)
Manufacturer name and address • Vscan Extend system
Manufacturing date (year-month) • Vscan Extend system
Part number • Vscan Extend system
Serial number • Vscan Extend system
Unique Device Identification (UDI). Every
system has a unique marking for
identification. Scan or enter the UDI
information into the patient health record
according to governing laws.
Refer label inside Vscan Extend system
Identify the customs country of origin of the
materials.
• Vscan Extend External
Battery charger
• Vscan Extend External
Battery charger
• Vscan Extend External
Battery charger
• Vscan Extend External
Battery charger
Vscan Extend system
Vscan Extend system
Push button (power switch) Vscan Extend system
NOTE: As a safety precaution, scanning is not possible when charging
the battery.
2-28 Vscan Extend – User Manual
5721203-100
Rev. D
Page 55

Device labels and symbols
Explanation of the Pollution control label for China
The following product pollution control information is provided
according to SJ/T11364-2014 Marking for Restriction of
Hazardous Substances caused by electrical and electronic
products
Table 2-9: Label for China
Label Description
This symbol indicates the product contains hazardous materials in excess of
the limits established by the Chinese standard GB/T 26572 Requirements of
concentration limits for certain restricted substances in electrical and
electronic products. The number in the symbol is the Environment-friendly
Use Period (EFUP), which indicates the period during which the hazardous
substances contained in electrical and electronic products will not leak or
mutate under normal operating conditions so that the use of such electrical
and electronic products will not result in any severe environmental pollution,
any bodily injury or damage to any assets. The unit of the period is “Year”.
In order to maintain the declared EFUP, the product shall be operated
normally according to the instructions and environmental conditions as
defined in the product manual, and periodic maintenance schedules
specified in Product Maintenance Procedures shall be followed strictly.
Consumables or certain parts may have their own label with an EFUP value
less than the product. Periodic replacement of those consumables or parts
to maintain the declared EFUP shall be done in accordance with the
Product Maintenance Procedures.
This product must not be disposed of as unsorted municipal waste, and
must be collected separately and handled properly after decommissioning.
Table 2-10: Hazardous substances
Hazardous Substances' Name
Component Name
Probe & Cable X OOOOO
Main unit OOOOOO
O: Indicates that hazardous substance contained in all of the homogeneous materials for this part is below
the limit requirement in GB/T 26572.
X: Indicates that hazardous substance contained in at least one of the homogeneous materials used for this
part is above the limit requirement in GB/T 26572.
• Data listed in the table represents best information available at the time of publication.
• Applications of hazardous substances in this medical device are required to achieve its intended clinical
uses, and/or to provide better protection to human beings and/or to environment, due to lack of reasonably
(economically or technically) available substitutes.
Pb Hg Cd Cr
6+
PBB PBDE
Vscan Extend – User Manual 2-29
5721203-100 Rev. D
Page 56

Safety
2-30 Vscan Extend – User Manual
5721203-100
Rev. D
Page 57

Chapter 3
Preparing Vscan Extend for
Use
Contents:
‘Package contents’ on page 3-2
‘System description’ on page 3-4
‘Battery’ on page 3-15
‘First time use’ on page 3-22
‘Activation’ on page 3-24
Vscan Extend – User Manual 3-1
5721203-100 Rev. D
Page 58

Preparing Vscan Extend for Use
Vscan Extend package contents
Make sure all items listed below are included in the package.
Package contents
Figure 3-1. Vscan Extend package contents
1. Vscan Extend softcase with
device and probe
2. USB cable
3. Gel bottle location
4. Region-specific plugs
5. AC/DC adapter
6. Battery
Table 3-1: Package content
Item Description
Vscan Extend Quick Card
Vscan Extend User Manual
Service contact information
3-2 Vscan Extend – User Manual
5721203-100
Rev. D
Page 59

Environmental requirements
Environmental requirements
Environmental requirements for the device
Table 3-2: Environmental requirements
Description Operational Non operational Storage and transport
Temperature 0 °C to + 40 °C - 40 °C to + 70 °C - 40 °C to + 70 °C
Humidity 90%, non-condensing 15–90% 90%, non-condensing
Air pressure 620 hPa to 1060 hPa 620 hPa to 1060 hPa 620 hPa to 1060 hPa
Transient operating conditions
NOTE: Permissible transient environmental operating conditions:
• Temperature range of 0-40 degrees
• Relative humidity range of 15 to 90% non-condensing
NOTE: Avoid exposing the unit to saline moisture. In case of exp osu r e
to saline moisture, clean the unit as described on ‘Cleaning and
disinfection’ on page 6-4.
Refer to Table 3-2 above for additional information regarding
storage of the battery.
Image display on the Vscan Extend is dependent on ambient
light; avoid direct sunlight or reflections from other light sources
on the display when scanning and reviewing images. The
display viewing angle should be as small as possible.
If you are having difficulty seeing the image due to the lighting
conditions try to change brightness (see ‘Scan Settings’ on
page 4-2) or change your position/location of use.
The time required for the Vscan Extend to warm from minimum
storage between uses until the device is rea dy fo r use at 20
degrees ambient is approximately 1 minute.
The time required for the Vscan Extend to cool from minimum
storage between uses until the device is rea dy fo r use at 20
degrees ambient is approximately 1 minute.
The Vscan Extend is transportable in a road a mbulance or fixe d
/rotary wing aircraft.
Vscan Extend – User Manual 3-3
5721203-100 Rev. D
Page 60

Preparing Vscan Extend for Use
System description
System overview
Vscan Extend configured with a Sector probe for deep scanning
Figure 3-2. Vscan Extend with a Sector probe
1. Display
2. Sector probe
3. Serial port (For Service ONLY)
NOTE: Green LED indicates Vscan Extend is fully charged and an
amber LED indicates it is charging.
4. Power button
5. LED indicates charging status
6. USB port and Power connector
3-4 Vscan Extend – User Manual
5721203-100
Rev. D
Page 61

System description
Vscan Extend configured with a Dual probe holding both a phased array
and linear array transducer for deep and shallow scanning
Figure 3-3. Vscan Extend with Dual probe
1. Display
2. Dual probe
3. Serial port (For Service ONLY)
NOTE: Green LED indicates Vscan Extend is fully charged and an
amber LED indicates it is charging.
4. Power button
5. LED indicates charging status
6. USB port and Power connector
Vscan Extend – User Manual 3-5
5721203-100 Rev. D
Page 62

Preparing Vscan Extend for Use
Vscan Extend Dual Probe
Figure 3-4. The dual prob e
1. Phased array transducer (deep scanning)
2. Linear array transducer (shallow scanning)
3-6 Vscan Extend – User Manual
5721203-100
Rev. D
Page 63

Accessories and Configurations
Standard and optional accessories
Table 3-3: Standard accessories
Accessory Figure
1. Global AC adapter with
interchangeable region-specific
plugs
2. One rechargeable battery
3. USB cable
4. MicroSD memory card (For
service personnel only. Not for
customer use.*)
System description
5. Soft case
6. User Manual
7. Hardcopy Quick Card
* The microSD card provided in the device captures error logs
and is strictly for Service personnel only. The user must
remove this microSD card and insert a blank one when
performing data backup. The microSD card that captures error
log files is used for troubleshooting by the Repair Depot.
** Not available in all countries
Vscan Extend – User Manual 3-7
5721203-100 Rev. D
Page 64

Preparing Vscan Extend for Use
Accessory Figure
8. Gel (60g bottle)
* The microSD card provided in the device captures error logs
and is strictly for Service personnel only. The user must
remove this microSD card and insert a blank one when
performing data backup. The microSD card that captures error
log files is used for troubleshooting by the Repair Depot.
** Not available in all countries
Table 3-3: Standard accessories
**
3-8 Vscan Extend – User Manual
5721203-100
Rev. D
Page 65

Standard and optional accessories (continued)
Table 3-4: Optional accessories
Accessory Figure
1. Additional Soft case
2. Robust case to carry
complete Vscan Extend set
3. Robust case to carry only
scanner, gel, and potential
extra battery
4. External Battery charger
System description
5. Additional Battery
6. Additional AC adapter
7. Hardcopy User manual
Vscan Extend – User Manual 3-9
5721203-100 Rev. D
Page 66

Preparing Vscan Extend for Use
Connectivity Configurations
Vscan Extend is available in three different connectivity
configurations:
1. USB configuration
2. Wi-Fi Access configuration
3. DICOM configuration
The table below describes the standard and connectivity
configurations.
Table 3-5: Configurations
USB
Description
Generic image format (jpg, mp4) for
data stored on device or exported to PC
Image transfer to PC via USB cable A A A
Manual labeling of exam data with
patient ID
FIPS compliant data encryption A A A
Data backup capability on microSD card A A A
Wireless image transfer to shared
network folders
Enterprise grade wireless encryption
standards including EAP and WPA2
(PSK)
Mobile Device Management client
support
Access to GE marketplace to selectively download and install Vscan Extend apps
Bladder Volume A A
Lung Protocol A A
Tricefy Uplink A A
Wireless reading DICOM Modality
Worklist
Wireless image export in DICOM format A
Access to reference materials on Vscan
web portal
configuration
AAA
AAA
AAA
Wi-Fi Access
configuration
AA
AA
AA
DICOM
configuration
A
* The Vscan Extend app represents only the interface to the cloud-ba sed case
exchange solution which is separately provided by GE
A: Available Blank cells indicates: Not Available
3-10 Vscan Extend – User Manual
5721203-100
Rev. D
Page 67

External battery charger compartment (option)
The external battery charger compartment is used to charge the
battery outside the Vscan Extend.
NOTE: The Vscan Extend battery can either be charged in the main un it
by directly plugging the AC/DC adapter or externally by placing
the Vscan Extend battery on the external battery charger.
NOTE: Scanning is disabled during charging if charged in the main unit.
Figure 3-5. External battery charger compartment
The table below lists the units or parts that can be replaced by
the user (CRU - Customer Replacable units)
System description
Table 3-6: CRU
Description Part number
Battery 5693456
AC/DC adapter 5693487
Application Software SD
Card
External Battery Charger 5716861
5716858
Vscan Extend – User Manual 3-11
5721203-100 Rev. D
Page 68

Preparing Vscan Extend for Use
Display screens
Figure 3-6. Scanning screens (Black and white mode)
Phased array transducer
1. Header
• Menu icon
• Patient information
• Exam number
• Battery level indicator
• MI and TI values
2. Depth scale
3. Footer
• Color icon
• Gain
• Depth
• Store
Linear array transducer
1. Header
• Menu icon
• Patient information
• Exam number
• Battery level indicator
• MI and TI values
2. Depth scale
3. Footer
• Color icon
• Gain
• Depth
• Store
NOTE: The screen graphics in this manual are only for illustrational
purposes. Actual screen output or graphics may vary.
3-12 Vscan Extend – User Manual
5721203-100
Rev. D
Page 69

Display screens (continued)
System description
Figure 3-7. Scanning screens (Color mode)
Phased array transducer
1. Header
• Menu icon
• Patient information
• Exam number
• Battery level indicator
• MI and TI values
2. Depth scale
3. Footer
• Color icon
• Gain
• Depth
• Store
4. Color bar
5. Color ROI (Region of Interest)
Linear array transducer
1. Header
• Menu icon
• Patient information
• Exam number
• Battery level indicator
• MI and TI values
2. Depth scale
3. Footer
• Color icon
• Gain
• Depth
• Store
4. Color bar
5. Color ROI (Region of Interest)
Vscan Extend – User Manual 3-13
5721203-100 Rev. D
Page 70

Preparing Vscan Extend for Use
Display screens (continued)
Figure 3-8. Menu screen
3-14 Vscan Extend – User Manual
5721203-100
Rev. D
Page 71

Battery
Vscan Extend Battery
Vscan Extend Battery
The Vscan Extend system is powered by a Li ION battery
(Model - U80395). The battery is not fully charged prior to
shipment. To maximize time of use, it is recommended to
recharge the battery before use for at least 1.5 hours. Establish
a routine for charging the battery to maximize system
availability.
The battery must be charged while housed in the Vscan Extend
or in the external battery charger.
The Li ION battery, used in Vscan Extend, with the battery code
is displayed below.
Figure 3-9. Vscan Extend battery
Battery specification
Table 3-7: Battery specification
Items Unit Value Description
Basic Voltage mV 7400 MAX
Current mA 1150 Avg 1C
Vscan Extend – User Manual 3-15
5721203-100 Rev. D
Page 72

Preparing Vscan Extend for Use
Battery (continued)
WARNING
CAUTION
Use only the AC adapter provided with the Vscan Extend.
The Vscan Extend cannot be charged via the USB cable when
connected to a PC.
Figure 3-10. Vscan Extend AC adapter
The AC adapter must be kept outside the patient environment
(refer to local regulation and EN 60601-1).
Figure 3-11. Patient environment
1. Patient environment
WARNING
Do NOT simultaneously touch the patie nt and the char ger plu g
on the AC adapter.
3-16 Vscan Extend – User Manual
5721203-100
Rev. D
Page 73

Power plugs
Vscan Extend Battery
1. Select the country specific plug.
Figure 3-12. Power plugs
Voltage requirement s
1. North America, Japan
2. China
3. Australia, New Zealand
4. UK, Hong Kong, Singapore
5. Continental Europe and Korea
(for unearthed electrical outlet)
2. Insert the relevant plug into the adapter.
Figure 3-13. Insert the plug
The AC/DC adapter will function on voltage from 100 to
240 VAC and 50/60 Hz.
CAUTION
Only use mains power of 100 – 240 VAC. Voltage outside this
range can cause a malfunction or destroy the AC/DC adapter.
Vscan Extend – User Manual 3-17
5721203-100 Rev. D
Page 74

Preparing Vscan Extend for Use
Charging the battery
1. Insert the battery into the device.
Figure 3-14. Insert the ba tte ry
2. Plug the AC/DC adapter into the electrical outlet.
Charging the battery using the optional External battery charger
compartment
1. Place the external battery charger on a flat surf ac e.
2. Insert the battery in the compartment.
NOTE: Do not apply excessive pressure to the battery while
inserting it into the compartment. There is no snap p ro vided
on the external battery charger. The battery fits smoothly
into the compartment without a click.
3. Plug the charger plug into the charger connector on the
external battery charging compartment.
3-18 Vscan Extend – User Manual
5721203-100
Rev. D
Page 75

Battery level indicator
The battery level indicator is displayed on the screen. The
following icons are displayed.
Vscan Extend Battery
Table 3-8: Battery level indicator
Icon Description
Battery fully charged (>=95%).
Battery 75% charged (>=70% and <95%).
Battery 50% charged (>=45% and <70%).
Battery critical, or low Battery (<45%).
Prepare to recharge the battery or have a spare battery
available.
Battery charging.
Vscan Extend – User Manual 3-19
5721203-100 Rev. D
Page 76

Preparing Vscan Extend for Use
Inserting/removing the battery
To insert the battery
1. Insert the battery in the compartment until the lid clicks in
place.
To remove the battery
1. Power off the Vscan Extend.
Figure 3-15. Inserting ba tt er y
CAUTION
Do not attempt to remove the battery without powering off
the Vscan Extend.
2. Push the button on the battery compartment lid and lift the
battery.
Figure 3-16. Removing battery
3-20 Vscan Extend – User Manual
5721203-100
Rev. D
Page 77

Battery specifications
Table 3-9: Battery specification
Item Specification
Charging time at 90% About 75 minutes
Capacity About 1 hour while continuously scanning
Lifetime At least 300 charges
In order to get maximum charging capacity with your Vscan
Extend battery, you should initially allow the battery to be fully
charged and then fully discharged at least three times. Perform
normal operation during these cycles. Once the initial charging/
discharging cycles are performed, the following is applicable
without reducing the lifetime of the battery:
• It is not necessary to completely discharge the battery
before re-charging.
• It is possible to stop charging the battery before it is fully
charged, but the battery will then be discharged more
rapidly.
• It is possible to charge the battery several times each day, if
needed.
Vscan Extend Battery
When storing the Vscan Extend for a period longer than three
months, remove the battery from the Vscan Extend main unit.
Recharge the battery every three months to maintain battery
performance. When the battery is stored separately, storage can
be at least one year before recharging the battery is needed.
NOTE: Make sure to remove the battery from the device before
shipping from one place to another.
To minimize battery performance degradation, avoid prolonged
storage of the battery outside the Vscan Extend operational
temperature range (see ‘Environmental requir ements for the
device’ on page 3-3).
Vscan Extend – User Manual 3-21
5721203-100 Rev. D
Page 78

Preparing Vscan Extend for Use
First time use
Before Vscan Extend can be used, the following steps must be
done:
1. Install the battery. (See ‘To insert the battery’ on page 3-20
for more information.)
2. Press the Power button to power on Vscan Extend.
3. Activate Vscan Extend. (See ‘Vscan Extend activation’ on
page 3-24 for more information.)
Power on/off
Initial use
To power on the Vscan Extend
Press the Power button.
Device boots up and scan screen displays within 45 seconds.
NOTE: The Scan screen displays only when the device has been
activated. See ‘Activation’ on page 3-24 for more information.
To power off the Vscan Extend
1. Press the Power button.
A pop-up displays prompting to Power off OR Restart.
2. Select Power off.
3-22 Vscan Extend – User Manual
5721203-100
Rev. D
Page 79

To restart Vscan Extend
1. Press and hold the Power button.
2. Select Restart to restart the device.
Standby Mode
Vscan Extend enters standby mode when the Power bu tton is
quickly pushed. In this mode, Vscan Extend will not have power
to the probe or any display.
OR
Vscan Extend enters standby mode automatically while in
freeze mode for more than 10 minutes.
Push the Power button again once to resume from standby
mode. The device is ready to scan within a second.
NOTE: When resuming the device from standby mode, a new exam is
created and the previous exam data is lost, if not previously
saved.
Initial use
Sleep Mode
NOTE: When resuming the device from sleep mode, a new exam is
Startup procedure
Vscan Extend enters sleep mode if it continues to be in standby
mode for more than 3 hours.
Push the Power button again to resume from sleep mode. The
device is ready to scan within approximately 5 seconds.
created and the previous exam data is lost, if not previously
saved.
The approximate time taken for the device from switching on
until the device is ready for normal use is 40 seconds.
Vscan Extend – User Manual 3-23
5721203-100 Rev. D
Page 80

Preparing Vscan Extend for Use
Vscan Extend activation
Activation
There are three possible scenarios to activate Vscan Extend:
1. Scenario 1: Activation using Wi-Fi on the Vscan Extend
device.
2. Scenario 2: Manual activation - activate Vscan Extend by
visiting the url: https://vscanactive.gehealthcare.com from a
PC.
3. Scenario 3: Activation without internet access. If there is no
internet access, contact GE Service (see page 1-8 for
phone numbers, then see ‘Offline activation’ on p age 4-12)
to activate Vscan Extend.
Activation using Wi-Fi
The activation process includes steps to register the Vscan
Extend device.
NOTE: When Wi-Fi is enabled the device can be activated directly.
3-24 Vscan Extend – User Manual
5721203-100
Rev. D
Page 81

Set Language, Date and Time
1. Connect Vscan Extend to a power source.
2. Press the Power button to power on Vscan Extend.
The Startup screen displays, followed by the Language
screen.
NOTE: The default language is set to English.
3. To choose another language, press Change Language.
Initial use
Figure 3-17. Select language
4. Choose the desired language. The screen changes back to
the Language screen.
5. Press Next.
Vscan Extend – User Manual 3-25
5721203-100 Rev. D
Page 82

Preparing Vscan Extend for Use
Set Language, Date and Time (continued)
6. The Date and Time screen displays.
NOTE: The current date and time is set by default.
7. Press Change Date and Time to change the date and time,
if needed.
Figure 3-18. Date and Time
Choose either the automatic date and time format which
sets the date and time by synchronizing through the
internet.
OR
Set the date and time manually.
8. Press Next.
3-26 Vscan Extend – User Manual
5721203-100
Rev. D
Page 83

Connecting Wi-Fi
NOTE: If Wi-Fi is not detected, configure Wi-Fi. See ‘Configuring Wi-Fi’
Initial use
on page 4-23 for more information.
1. Select your Wi-Fi network and enter your Wi-Fi password.
(All non hidden Wi-Fi networks are displayed.)
Figure 3-19. Wi-Fi settings
2. Press Next.
NOTE: Ensure that the Vscan Extend is connected to a non-clinical
Wi-Fi hotspot to start activation.
NOTE: A non-clinical Wi-Fi has access to the public internet, which is
not part of the hospital internet. If you are unable to view the
non-clinical Wi-Fi or do not have the password, contact your
system administrator.
NOTE: If you do not have access to public Wi-Fi, proceed with manual
activation of the device. See ‘Manual activation’ on page 3-35
for more information.
NOTE: If the hospital network does not have an internet connection,
proceed with manual activation of the device. See ‘Manual
activation’ on page 3-35 for more information.
Vscan Extend – User Manual 3-27
5721203-100 Rev. D
Page 84

Preparing Vscan Extend for Use
Connecting Wi-Fi (continued)
1. Enter the authentication details to connect to the network.
Figure 3-20. Authentication code
2. Once the device connects to the network, press the Back
arrow to exit the Settings screen.
The screen changes back to the Wi-Fi screen (Figure 3-19
on page 3-27.)
3. Press Next.
3-28 Vscan Extend – User Manual
5721203-100
Rev. D
Page 85

Registration
First time registrants
Initial use
Registering the device creates a Vscan Extend account which
enables access to the GE Marketplace and other features.
1. First time registrants, press Register to create an account.
Enter the required details.
Figure 3-21. Registration details
NOTE: If you have previously registered a Vscan Extend device,
press Already Registered using your registered email. See
‘Registered users’ on page 3-31 for more information.
HINTS
Press the Arrow key twice to get the letters in all caps and
press the number key to toggle between numbers and
alphabet.
Figure 3-22. Keyboard tips
Vscan Extend – User Manual 3-29
5721203-100 Rev. D
Page 86

Preparing Vscan Extend for Use
First time registrants (continued)
2. Check the desired options to either participate in
collaborative GE activities or to receive special offers and
promotions.
Press Register.
3. An email is sent to the email address used during
registration.
NOTE: The email has a link requiring you to change your password
for security purposes.
4. Press Activate my device.
Figure 3-23. Choose an option
5. Vscan Extend has been activated. Press Start Using
Device.
Figure 3-24. Start using Vscan Extend
3-30 Vscan Extend – User Manual
5721203-100
Rev. D
Page 87

Registered users
NOTE: Ensure Vscan Extend is connected to a non-clinical Wi-Fi.
Initial use
1. Press Already Registered.
2. Enter your email ID and password.
Figure 3-25. Already Register ed
NOTE: If you log in with a wrong email ID, a pop-up message
displays: “Login error: Your email ID is not confirmed yet”.
Try again with a valid email ID.
Vscan Extend – User Manual 3-31
5721203-100 Rev. D
Page 88

Preparing Vscan Extend for Use
Registered users (continued)
3. While the activation process is in progress, the wireless is
connecting to the GE Store and the activation key is being
entered automatically.
4. Press Activate my device.
5. Vscan Extend has been activated. Start using the device.
Figure 3-26. Start using Vscan Extend
3-32 Vscan Extend – User Manual
5721203-100
Rev. D
Page 89

Already registered users - Forgot password
1. If you forgot your password, press Forgot Password.
2. An email is sent to your confirmed email address with a link
to reset the password.
Figure 3-27. Forgot password
3. Click on the link on a personal computer to reset the
password.
4. Enter your email ID and new password. The Vscan Extend
is activated and ready to use.
Initial use
Figure 3-28. Reset password and start using Vscan Extend
Vscan Extend – User Manual 3-33
5721203-100 Rev. D
Page 90

Preparing Vscan Extend for Use
Network access failed
1. If you are unable to connect to a Wi-Fi network, press Retry
and try to activate the device again.
2. If the problem persists, contact your internet service
provider and GE at help@gehc.com to connect to the
network.
3. If the network is not accessible or you are unable to connect
to the network, you can activate Vscan Extend manually.
See ‘Manual activation’ on page 3-35 for more information.
Figure 3-29. Retry
3-34 Vscan Extend – User Manual
5721203-100
Rev. D
Page 91

Manual activation
NOTE: The PC should have internet access. If there is no internet
Initial use
To activate Vscan Extend without Wi-Fi connection, follow the
steps below.
1. Open the URL (https://vscanactive.gehealthcare.com) in the
internet browser on your PC.
access, call GE Service center or contact your local Sales
representative.
2. Follow the directions provided on the site to generate the
activation key for Vscan Extend.
The following information is required:
• The Vscan Extend serial number written on the rea r
label of the system.
3. User information as per the figure below.
Figure 3-30. User Information
4. Enter other necessary details.
Figure 3-31. Opt-in checkbox
Figure 3-32. Opt-in comm u nic at ion
Vscan Extend – User Manual 3-35
5721203-100 Rev. D
Page 92

Preparing Vscan Extend for Use
Manual activation (continued)
5. Press Submit to initiate registration.
6. The Activation key is generated. Store the key in a
convenient location to enter into the device and for future
reference.
Figure 3-33. Submit
Figure 3-34. Activation key details
3-36 Vscan Extend – User Manual
5721203-100
Rev. D
Page 93

Activation key
Initial use
The Activation key screen is displayed on the Vscan Extend.
1. Enter the activation key generated while registering on the
portal (https://vscanactive.gehealthcare.com)
Figure 3-35. Enter activation key
HINTS
Press the Backspace key if a mistake occurred while entering
the activation key.
2. Press Activate.
Figure 3-36. Start using the device
3. Vscan Extend has been activated. Start using the device.
Vscan Extend – User Manual 3-37
5721203-100 Rev. D
Page 94

Preparing Vscan Extend for Use
3-38 Vscan Extend – User Manual
5721203-100
Rev. D
Page 95

Chapter 4
Vscan Extend Settings
Contents:
‘Settings’ on page 4-2
‘Scan Settings’ on page 4 - 2
‘Server Settings’ on page 4-3
‘Diagnostics’ on page 4-12
‘Wi-Fi’ on page 4-21
‘MDM Installation Procedures (Optional)’ on page 4-24
‘Certificate Authority’ on page 4-28
‘GE Marketplace’ on page 4-31
Vscan Extend – User Manual 4-1
5721203-100 Rev. D
Page 96

Vscan Extend Settings
The following settings and functions are available under the
main Settings menu.
Scan Settings
Press Menu -> Settings
Settings
Figure 4-1. Settings
• Brightness - adjusts the screen brightness of the device
Swipe the brightness level indicator to the left to decrease
the brightness and swipe to the right to increase the
brightness.
4-2 Vscan Extend – User Manual
5721203-100
Rev. D
Page 97

Scan Settings (continued)
Auto Freeze (Probe) - set the auto freeze time.
1. Press Auto Freeze (Probe)
2. Choose the desired value
3. Press OK to set the value
Video Duration - set duration of the captured Cine loops.
1. Press Video Duration
2. Choose the desired value
3. Press OK to set the value
Unit of Measurement - set unit of measurement
1. Press Unit of Measurement
2. Choose the desired unit
3. Press OK to set the value
Set current preset as default
1. Press Set Current Preset as Default
2. Press Yes to set the displayed preset as default.
Settings
Server Settings
Allows to configure the DICOM servers.
Figure 4-2. Server settings
Worklist Server - gets patient and intended study information
Image Server - remote store location for media (video/images)
DICOM Encoding - Choose the desired encoding type and press
OK.
Vscan Extend – User Manual 4-3
5721203-100 Rev. D
Page 98

Vscan Extend Settings
Server Settings (continued)
To configure the Worklist server:
HINTS
Press the title bar (the entire top section on the screen) or
swipe left most side of the screen to access the Menu.
1. Press Menu -> Settings
Figure 4-3. Settings
2. Scroll down to Server Settings. Press Worklist Server.
Figure 4-4. Worklist server
4-4 Vscan Extend – User Manual
5721203-100
Rev. D
Page 99

Configure Worklist Server
Connect Vscan Extend to the hospital wireless network. See
‘Activation using Wi-Fi’ on page 3-24 for more information.
1. Enter all fields to add a new Worklist server.
Settings
Figure 4-5. Configure Worklist Server
NOTE: Swipe upwards to get the full screen and enter all details.
Contact the hospital IT administrator for details.
Vscan Extend – User Manual 4-5
5721203-100 Rev. D
Page 100

Vscan Extend Settings
Configure Worklist Server (continued)
NOTE: By default, DICOM is listed while configuring the Worklist
Server.
Figure 4-6. DICOM listed
2. Enter any unique name for Calling AE Title (e.g. Nixon
Building Schedule).
3. Press Add Server to add the Worklist Server.
4. Press Verify to verify communication with the Worklist
Server.
Figure 4-7. Verify Worklist Server
4-6 Vscan Extend – User Manual
5721203-100
Rev. D
 Loading...
Loading...