Page 1

1
Monica Novii® Wireless Patch System
Instructions For Use
107-PT-005-EN rev P
Information contained in this IFU manual corresponds to Novii Interface firmware ver 2.71 and POD firmware ver 2.54.
© Copyright Monica Healthcare Ltd 2017. All rights are reserved worldwide. Reproduction in whole or part is strictly prohibited
without prior consent of the copyright holder.
Page 2

2
Declaration
The information and descriptions contained in this Instruction For Use are the property of Monica
Healthcare Ltd and may not be copied, reproduced, disseminated, or distributed without written
permission from Monica Healthcare Ltd.
Information in this Instruction For Use is believed to be accurate and reliable, but the information
contained in this document is subject to change without notice. However, Monica Healthcare Ltd
assumes no responsibility for its use, or any infringements of patents or other rights of third parties that
may result from its use. No license is granted by implication or otherwise under any patent or patent
rights of Monica Healthcare.
This Instruction For Use is intended for trained medical personnel (including obstetricians, midwives,
nurses, and physicians) who are familiar with obstetric procedures.
Monica Healthcare only considers itself responsible for any effects on safety, reliability and
performance of the equipment if:
1. Assembly operations, re-adjustments, modifications or repairs are carried out by persons
authorized by Monica Healthcare, and
2. The electrical installation complies with national standards, and
3. The equipment is used in accordance with the Instructions For Use
Indications For Use
The Monica Novii POD is an intrapartum Maternal/Fetal Monitor that non-invasively measures
and displays fetal heart rate (FHR), uterine activity (UA) and maternal heart rate (MHR). The
Novii POD acquires and displays the FHR tracing from abdominal surface electrodes that pick
up the fetal ECG (fECG) signal. Using the same surface electrodes, the POD also acquires and
displays the UA tracing from the uterine electromyography (EMG) signal and the MHR tracing
from the maternal ECG signal (mECG). The POD is indicated for use on women who are at >36
completed weeks, in labor, with singleton pregnancies, using surface electrodes on the maternal
abdomen.
The Novii Patch is an accessory to the Novii POD that connects directly to the Novii POD and
contains the surface electrodes that attach to the abdomen.
The Novii Interface is an accessory to the Novii POD which provides a means of interfacing the
wireless output of the Novii POD to the transducer inputs of a Maternal/Fetal Monitor. The Novii
Interface enables signals collected by the Novii POD to be printed and displayed on a
Maternal/Fetal Monitor and sent on to a central network, if connected.
The Novii POD maternal-Maternal/Fetal Monitor and its accessories are intended for use by
healthcare professionals in a clinical setting
Page 3

3
Conventions Used in This Operator Manual
WARNING: A warning alerts you to a potential serious outcome, adverse event, or safety hazard.
Failure to observe a warning may result in death or serious injury to the user or patient.
CAUTION: A caution alerts you to situations where special care is necessary for the safe and
effective use of the product. Failure to observe a caution may result in minor or moderate
personal injury or damage to the product or other property, and possibly in a remote risk
of more serious injury.
On your monitor, this sign indicates that there is detailed information in this book, which
you must read before proceeding with your task.
Monica and Novii are registered trademarks of Monica Healthcare Ltd in the USA,
EU, China and Japan
Other brand names and product names are trademarks or registered trademarks of their
respective holders.
Numbers in brackets ( ) refer to the key number in Figure 1.
CAUTION: US law restricts this device to sale by, or on the order of, a physician.
Page 4

4
Contents
Symbols & Standards ............................................................................................................ 6
1.1 Symbols ....................................................................................................................................................................................................... 6
1.2 Standards .................................................................................................................................................................................................... 7
Safety ...................................................................................................................................... 8
2.1 Indications for Use ....................................................................................................................................................................................... 8
2.2 Contraindications ......................................................................................................................................................................................... 8
2.3 Warnings and Cautions ............................................................................................................................................................................... 9
2.4 Electromagnetic Compatibility (EMC) ........................................................................................................................................................ 15
2.5 Electrostatic Discharge (ESD) precautions ............................................................................................................................................... 19
2.6 Magnetic Resonance Environment (MRE) ................................................................................................................................................ 20
Unpacking ............................................................................................................................. 21
Product Description ............................................................................................................. 23
4.1 General description ................................................................................................................................................................................... 23
4.2 Data processing ........................................................................................................................................................................................ 23
4.3 Data viewing .............................................................................................................................................................................................. 24
4.4 Data accuracy ........................................................................................................................................................................................... 24
4.5 Classification of Medical equipment and marking ..................................................................................................................................... 24
4.6 Wireless Technology ................................................................................................................................................................................. 24
4.7 FCC Information (USA) ............................................................................................................................................................................. 27
Installation & Settings ......................................................................................................... 29
5.1 Initial Screen, Device Registration ............................................................................................................................................................ 30
5.2 Cable Connection ...................................................................................................................................................................................... 32
5.3 Accessing Settings .................................................................................................................................................................................... 33
5.4 Maternal Movement Alert using the UA trace ............................................................................................................................................ 35
5.5 Monica Identifier ........................................................................................................................................................................................ 36
5.6 Low UA Sensitivity ..................................................................................................................................................................................... 36
5.7 High UA Sensitivity .................................................................................................................................................................................... 36
5.8 TEST function ............................................................................................................................................................................................ 37
Operating Novii .................................................................................................................... 39
6.1 Introduction ................................................................................................................................................................................................ 39
6.2 Screen Format ........................................................................................................................................................................................... 40
6.3 Initial Screen and Standby Screen ............................................................................................................................................................ 41
6.4 Start Screen: ............................................................................................................................................................................................. 42
6.5 To Start Monitoring .................................................................................................................................................................................... 42
6.6 Novii Interface Icons and Status Controls/Messages ................................................................................................................................ 47
6.7 Novii Interface Monitoring alert/help messages ........................................................................................................................................ 49
6.8 How to continue monitoring when the Low Battery alert is activated ........................................................................................................ 52
6.9 Placing/Removing PODs from the Novii Interface Charging Wells ........................................................................................................... 52
Page 5

5
6.10 Monitoring Alert priority ............................................................................................................................................................................. 54
6.11 Turning Off the Interface ........................................................................................................................................................................... 54
6.12 Novii FHR, MHR, UA synchronization & mixed modality monitoring ......................................................................................................... 54
6.13 The two blue LED lights on the POD ......................................................................................................................................................... 55
Interface Visual Alerts ......................................................................................................... 57
7.1 Return POD to charging bay visual alert ................................................................................................................................................... 57
7.2 POD removed from Patch visual alert ....................................................................................................................................................... 57
7.3 POD left in Patch without responding to skin/electrode problems............................................................................................................. 58
7.4 A non-Monica Patch is detected at the start of monitoring visual alert...................................................................................................... 58
7.5 A non-Monica Patch is detected during monitoring ................................................................................................................................... 58
Help icon ............................................................................................................................... 60
Cleaning ................................................................................................................................ 61
9.1 Cleaning (Patch is single used and should be disposed of as hazardous waste) ..................................................................................... 61
Accessories & Part Numbers ............................................................................................ 62
10.1 Interface Cables ........................................................................................................................................................................................ 62
Patch Specification ............................................................................................................ 63
Interface Specification ....................................................................................................... 64
POD Specification .............................................................................................................. 66
Fault Finding ...................................................................................................................... 69
FHR Gaps Troubleshooting Table .................................................................................... 74
Allergic Reaction to Patch ................................................................................................ 76
16.1 Overview ................................................................................................................................................................................................... 76
16.2 Guidelines ................................................................................................................................................................................................. 76
16.3 Treatment .................................................................................................................................................................................................. 77
Servicing ............................................................................................................................. 78
Maintenance & Fault Reporting ........................................................................................ 79
18.1 Maintenance .............................................................................................................................................................................................. 79
18.2 Calibration ................................................................................................................................................................................................. 79
18.3 Firmware version for Novii Interface and Pod ........................................................................................................................................... 79
18.4 Disposal of Product Waste ........................................................................................................................................................................ 79
Page 6
6
Symbols & Standards
This section describes symbols used in this Instructions For Use and the safety precautions that
appear as symbols or labels on the Novii Wireless Patch System itself and the standards that it
complies with.
1.1 Symbols
Consult Instructions for use
Do Not Use If Package is Damaged
Use by
Batch code
Manufacture date
Manufacturer
ESD - Static sensitive device
WEEE logo:
This symbol indicates that the waste of electrical and electronic equipment including
battery must not be disposed as unsorted municipal waste and must be collected
separately. Please contact an authorized representative of the manufacturer for
information concerning the decommissioning of your equipment.
Include RF transmitters
Class II Insulation
TYPE BF EQUIPMENT: Type BF equipment is suitable for intentional external and internal
application to the patient, excluding direct cardiac application. Type BF equipment has an
F-type applied part.
The applied Parts of the Novii System are the five electrodes of the Novii Patch that are
placed on the patient abdomen. This applied part connects to the pins at the bottom of the
Novii POD.
Do not reuse
LOT
Page 7
7
No Latex used
No PVC used
Temperature limitation
The Novii system is not to be taken into a Magnetic Resonance (MR) environment
FCC ID: YOM-
6961-MON
Federal Communication Commission identification number. Complies with United States
Radio communication requirements.
1.2 Standards
The Monica Novii Interface complies with the following safety standards
Standard
Description
IEC 60601-1:2005 +A1: 2012 incl.
USA deviations
Medical Electrical Equipment
Part 1: General requirements for basic safety and essential
performance
IEC 60601-1-2:2007
Medical Electrical Equipment Part 1-2
General Requirements for basic safety and essential performance –
Collateral standard: Electromagnetic compatibility – requirements and
tests
EN ISO14971: 2012
Medical Devices – Application of risk management to medical
devices (ISO 14971:2007, Corrected version 2007-10-01)
ANSI /AAMI EC12
Disposable ECG electrodes
EN 62133: 2nd Edition 2012
Secondary cells and batteries containing alkaline or other
non-acid electrolytes – Safety requirements for portable sealed
secondary cells, and for batteries made from them, for use in portable
applications
EN ISO 10993
Biological evaluation of medical devices
FCC CFR 47: Part 15.107 & 15.109
Title 47--Telecommunication
Chapter I – Federal Communications Commission 15 – Radio
Frequency devices
EN 60529:1992 +A2:2013
Specification for degrees of protection provided by enclosures
(IP code)
EN ISO 15223-1:2012
Graphical Symbols for use in the labelling of medical devices
Page 8

8
Safety
2.1 Indications for Use
The Monica Novii POD is an intrapartum Maternal/Fetal Monitor that non-invasively measures
and displays fetal heart rate (FHR), uterine activity (UA) and maternal heart rate (MHR). The
Novii POD acquires and displays the FHR tracing from abdominal surface electrodes that pick
up the fetal ECG (fECG) signal. Using the same surface electrodes, the POD also acquires and
displays the UA tracing from the uterine electromyography (EMG) signal and the MHR tracing
from the maternal ECG signal (mECG). The POD is indicated for use on women who are at >36
completed weeks, in labor, with singleton pregnancies, using surface electrodes on the maternal
abdomen.
The Novii Patch is an accessory to the Novii POD that connects directly to the Novii POD and
contains the surface electrodes that attach to the abdomen.
The Novii Interface is an accessory to the Novii POD which provides a means of interfacing the
wireless output of the Novii POD to the transducer inputs of a Maternal/Fetal Monitor. The Novii
Interface enables signals collected by the Novii POD to be printed and displayed on a
Maternal/Fetal Monitor and sent on to a central network, if connected.
The Novii POD maternal-Maternal/Fetal Monitor and its accessories are intended for use by
healthcare professionals in a clinical setting
2.2 Contraindications
The Novii Interface is contraindicated for use in preterm gestation (≤36 completed weeks). The
uterine contraction trace generated by the Novii POD and monitored by the Maternal/Fetal
Monitor via the Novii Interface may show deflections from baseline that do not represent uterine
contractions. These deflections from baseline may represent electrical activity in the
myometrium that is not sufficiently organized to cause the uterine smooth muscle to contract. In
the context of a preterm pregnancy, clinical misinterpretation of the uterine tracing may lead to
unnecessary intervention, such as tocolysis, diagnostic procedures, and/or preterm delivery.
IMPORTANT NOTE: The Monica Novii system is contra-indicated for use with: Magnetic
Resonance Imaging (MRI) scanners, Computer Tomography (CT) scanners, Diathermy / electro
surgery, Metal Detectors, Transcutaneous Electrical Nerve Stimulation (TENS) machines,
Cardiac Pacemakers, Cardiac Defibrillators.
This symbol is displayed on the Novii Interface, Novii POD packaging and Novii
Patch Packaging labels to indicate the Novii system is not to be taken into a
Magnetic Resonance (MR) environment.
Page 9

9
2.3 Warnings and Cautions
2.3.1 Clinical
WARNING: The Novii Wireless Patch does not replace observation and evaluation of the
mother and fetus at regular intervals, by a qualified care provider, who will make
diagnoses and decide on treatments and interventions. Clinical assessment of the
Maternal/Fetal Monitor’s display or trace when using the Novii Wireless Patch
solution must be combined with knowledge of patient history and risk factors to
properly care for the mother and fetus.
WARNING: If you are concerned with the clinical data provided by Monica it should be verified
by an alternative method, such as palpation of the maternal pulse to exclude
MHR/FHR confusion or hand held Doppler to confirm the FHR.
WARNING: The safety and effectiveness of Novii FHR, MHR and UA have NOT been cleared
by the FDA for the following patient populations:
• Preterm gestation (i.e. ≤ 36 completed weeks gestation)
• Antepartum (i.e. at term, but not in labor)
• Multiple gestations
WARNING: A labor monitor is intended for use by clinical professionals who are trained in the
medical procedures, practices, and the terminology required when monitoring
obstetric patients. The monitor is only one clinical indicator of labor progress and
fetal/maternal well-being. The monitor is designed to assist the clinical staff in
assessing the status of the patient and her unborn baby.
WARNING: Monica Healthcare recommends establishing the presence of the fetal heartbeat
by auscultation before starting continuous monitoring by either using a Pinard
stethoscope or hand held Doppler.
WARNING: If the signal quality indicator on the Novii Interface display is red for an extended
period, use an alternative method to confirm FHR.
WARNING: Monica UA provides information on the frequency and timing of the contraction
peak. Interpretation of the Monica UA pattern should be done in the clinical context
of the patient. It is always good practice to use manual palpation, maternal
perception of UA and observation in conjunction with the UA trace. It is important
to note that there will be a delay of 10 seconds or more from maternal perception
and/or manual palpation when compared to the display on the Maternal/Fetal
Monitor and trace paper.
Page 10
10
WARNING: MHR/FHR confusion. When the FHR is tracking close to the MHR you should
always confirm the FHR using another modality.
WARNING: Monica does not recommend or support mixing Novii UA with US/FSE FHR
monitoring.
There is a 10-second delay (5mm on the tracing) in the Novii UA trace with respect
to the US/FSE FHR trace; late decelerations could appear as early decelerations
masking a potential fetal compromise.
Using the US transducer in addition to Novii FHR, MHR and UA to confirm the
FHR, for short periods, during gaps or suspected artifact can be used, but the
potential for missing a fetal compromise remains, due to US FHR and Novii UA
desynchronization.
WARNING: Monica does not recommend or support mixing Novii FHR/MHR with
TOCO/IUPC UA monitoring.
If the Novii UA cable is disconnected and the TOCO/IUPC is used (against this
recommendation), it is clinically important to understand that the FHR/MHR shift
will have changed from a 10 second to a 6 second delay (3 mm). Early
decelerations may appear as ‘subtle’ late decelerations. This could lead to an
unnecessary intervention.
CAUTION: The 10 second (or 6 second, if the Novii UA cable is disconnected) MHR delay
should be taken into consideration when monitoring the patient’s response to a
test dose during epidural placement. There is a 6 or 10 second MHR delay in
reporting the MHR with respect to real time events.
CAUTION: The 10 second (or 6 second, if the Novii UA cable is disconnected) FHR shift
should be taken into consideration during prolonged FHR decelerations when
resuscitative measures are being used, the impact of any maneuver will not be
seen for 10 seconds.
CAUTION: The 10-second UA delay should be taken into consideration when coaching
patients to push during the second stage. The patient may sense the contraction
before it appears on the monitor tracing- the contraction has already been building
for 10 seconds.
CAUTION: When the patient is moving and/or the fetus is active caution should be exercised
in interpreting the UA trace. If the interpretation of uterine contractile pattern(s) is
uncertain, another modality to monitor uterine contractions should be considered
and clinical management of the patient adjusted appropriately. The Novii POD
Page 11

11
monitors uterine activity by measuring the electrical signals (EMG) generated by
the uterine muscle when it contracts, as opposed to the tocodynamometer (TOCO
transducer) which monitors uterine activity as measured by the displacement of a
plunger or button with respect to a guard ring caused by the tightening of the uterus
during a contraction. Small relative changes in the electrode positions used to
monitor the uterine EMG resulting from maternal or fetal movement cause
electrical signals that can look like uterine activity.
CAUTION: The Novii POD when attached to the Novii Patch can remain on the patient while
taking a bath or shower (rated IP57), but monitoring will not work when the woman
is in the bathtub and the POD is fully submerged under water (restricting the
Bluetooth signal) and cannot be guaranteed during a shower. However, the POD
needs to remain attached to the patch while exposed to water to maintain the
integrity of the Patch.
CAUTION: We recommend that the Novii fetal/maternal ECG waveform is not displayed on
Coro 259 series monitor by manually turning this option off. No diagnostic
information can be inferred from waveform sent from Novii Interface to the
Maternal/Fetal Monitor. It is a pulse that can be used by the monitor to accurately
calculate the FHR and MHR.
CAUTION: Only touch the UA zero reference button on the Maternal/Fetal Monitor when
prompted by the Novii Interface at the start of the monitoring. Do not touch the UA
reference button during a monitoring session since it could result in masking
contractions, unless it is confirmed by palpation of the uterus that no contraction is
present.
CAUTION: If the Maternal/Fetal Monitor UA reference button is accidently touched during
monitoring wait until you are confident the woman is not having a contraction (by
using palpation) and then re-touch the UA reference button on the Maternal/Fetal
Monitor.
CAUTION: Any unexpected data from the Novii Interface as shown on the Maternal/Fetal
Monitor display or trace must result in further examination of the mother and fetus
in a hospital environment.
CAUTION: The Novii POD transmits FHR, UA and MHR data to the Maternal/Fetal Monitor
with a short delay of 10 seconds. Data is synchronized allowing accurate
interpretation of decelerations in relation the peak of contractions. Duration of Novii
Wireless Patch contractions can be shorter than mechanical contractions, hence
when palpating the uterus there will be a delay between manual detection of a
contraction and the display of the contraction on the Maternal/Fetal Monitor.
Page 12
12
CAUTION: It may prove difficult to use the Novii UA to coach patients to commence
contraction pain coping strategies or actively push in the second stage of labor. Its
value lies in providing an accurate picture of the pattern of uterine contractions
over time.
CAUTION: High and Low UA sensitivity gives the user the choice to best conform with the
clinical situation; the Low UA sensitivity setting is less sensitive to UA and removes
some of the small deflections that may represent artifacts or inconsequential
contractions. It is, however, important to switch to High sensitivity once the patient
is in established labor. Novii will automatically switch back to High UA sensitivity
after 60 min of Low UA sensitivity monitoring. No warning is given.
CAUTION: Prior to the connection of the Novii POD, the Novii Patch must not come in contact
with water since any water trapped in the POD connection area may damage the
POD. An example of this situation could be when a bed bath is given after the
Patch has been fitted, but before the POD has been connected.
2.3.2 Uterine EMG Activity; Potential Problems with Clinical Interpretation
WARNING: The Novii POD may monitor UA deflections from baseline that do not represent
uterine contractions that cause an increase in intra-uterine pressure. These
deflections from baseline may represent electrical activity in the myometrium that
is not sufficiently organized to cause the uterine smooth muscle to contract. When
this occurs, the “false contraction” often does not attain the amplitude of true
uterine contractions. If the interpretation of uterine contractile pattern(s) is
uncertain, another modality to monitor uterine contractions should be considered
and clinical management of the patient adjusted appropriately.
WARNING: The Novii POD monitors uterine contractions by measuring electrical activity
(EMG) of the uterus as opposed to a tocodynamometer (TOCO transducer) which
monitors uterine activity as measured by the movement of a button with reference
to a guard-ring. The button is pressed in by a tightening of the uterine muscle as
measured on the abdominal wall. Occasionally, low amplitude electrical activity
insufficient to cause a contraction detected by a TOCO transducer is displayed as
a deflection above baseline on the Novii Interface Maternal/Fetal Monitor tracing.
These deflections from baseline may represent electrical activity in myometrium
that is not sufficiently organized to cause the uterine smooth muscle to contract.
Thus, caution should be used in interpreting as contractions deflections from
baseline that have relatively lower amplitude compared to contractions
characteristic of the overall uterine activity pattern. False positive UC could also
occur from maternal activity or vigorous fetal movement. Any movement that
changes the maternal abdominal surface contours can produce, what appears on
the trace to be, a UC. This is caused by small changes in the electrode positions
in relation to each other and to the underlying skin. This may create confusion
Page 13
13
particularly during early induction monitoring, when regular true contractions are
not present. Before any definitive clinical interpretation of UC information
generated by Novii is made, ensure, if possible that the patient is not moving and
is in a comfortable and relaxed position. If there is concern about false positive
contractions during early labor or induction, it can be helpful to have the patient
use the event marker on the GE Corometrics 259 Series Maternal/Fetal Monitor to
indicate when she feels a contraction and/or the fetus move.
Irregular high amplitude ‘ragged’ looking contractions that are coincidental with
fetal or maternal movements with no other clinical indication of UC should be
discounted. They are unlikely to be real contractions. As such, they should not
influence medical intervention unless corroborated by another device or clinical
assessment.
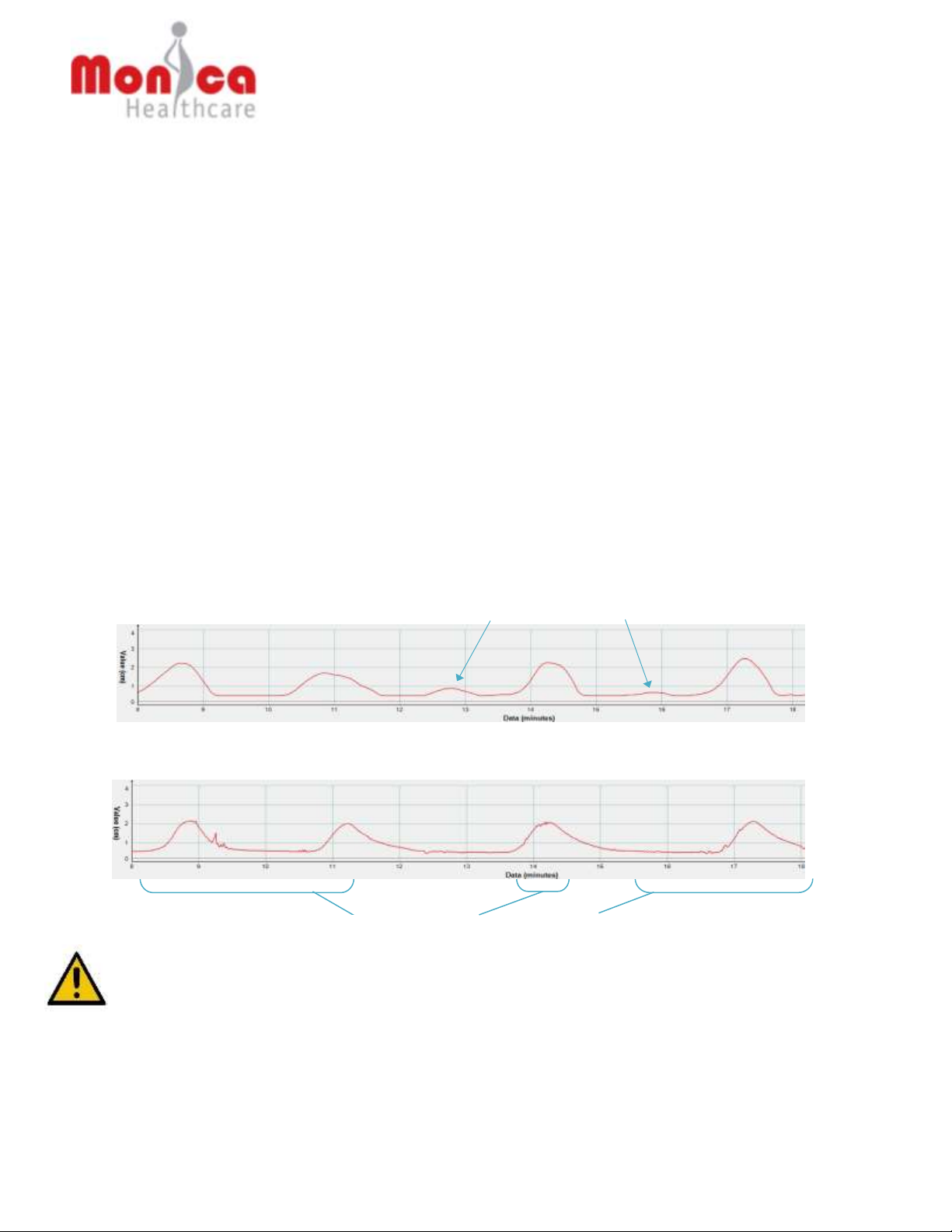
For example, in the following sample Maternal/Fetal Monitor tracing using uterine
EMG, there are deflections above the baseline in the tracing that does not
correspond to uterine contractions in a the simultaneously monitored IUPC tracing
(e.g., deflections identified by arrows). IUPC is considered the gold standard for
monitoring uterine contractions.
WARNING: Users should not use the low sensitivity setting during active labor; the onset of the
contraction trace will be further delayed and the amplitude will be reduced. The
peak will remain synchronized with the FHR trace.
Deflections do not correspond to a uterine contraction as monitored by IUPC
Deflections corresponding to ‘true’ uterine contractions
Uterine EMG trace:
IUPC trace:
Page 14

14
2.3.3 Safety
WARNING: Only use the Novii Interface with the GE Corometrics 259 Series Maternal/Fetal
Monitor with the specific interface cable for that monitor, see Section 10.1.
WARNING: Do not position the Novii Interface so as to make it difficult to disconnect its AC/DC
adapter. Position the Interface on a stable surface more than 20 cm from the
patient or user during normal use.
WARNING: The Novii Interface power cable and other interconnecting cables must be
positioned and/or restrained to avoid users and patients tripping over them.
WARNING: The operator should not touch the unearthed metal parts of the Novii Interface and
the patient at the same time. In particular do not touch the metal shielding of the
connectors at the back of the Novii Interface and the patient at the same time.
WARNING: The Monica Novii is not suitable for use in an Oxygen rich environment
WARNING: The Novii Interface is not explosion-proof and must not be used in the presence of
flammable anesthetic gases.
WARNING: SHOCK HAZARD. Do not attempt to connect the power cable with wet hands.
Make certain that your hands are clean and dry before touching a power cable or
plug.
WARNING: Use only the power supply supplied with the device.
WARNING: Unplug the Novii Interface from the AC power supply before cleaning. Do not
immerse the unit in water or allow liquids to enter the case.
WARNING: Examine the Novii Interface and accessories periodically to ensure that the cables,
connectors and the device itself do not have visible evidence of damage that may
affect performance. The recommended inspection interval is once per week or
less. Do not use the device if there is any visible sign of damage.
WARNING: Do not attempt to service the Novii Interface. Only Monica approved and qualified
service personnel should attempt any necessary internal servicing.
WARNING: The Novii Interface is not specified or intended for operation in conjunction with
any other type of monitoring equipment except the specific devices that have been
identified for use in this Instruction For Use.
WARNING: Novii should not be used for primary monitoring in applications where any loss of
the FHR and UA signal is unacceptable.
WARNING: No Modification of this equipment is allowed.
WARNING: Do not use a new Novii Patch if the Package is damaged or open.
WARNING: The Novii POD contains a Li-ion battery. Do not throw the Novii POD into a fire or
other heat source. Do not put the Novii POD into any liquid such as water or
Page 15
15
gasoline (except when attached to the Patch and used during a shower or bath).
Do not put the Novii POD into a pocket or bag without adequate protection. Do not
disassemble the Novii POD. Do not crush or pierce the Novii POD. Do not leave
the Novii POD close to a fire or heat source above 30 °C. Do not use the Novii
POD if there are any signs of visible damage. Do not discharge the Novii POD in
any way other than it’s intended use.
Do not use the Novii POD if there is any discoloration, unusual heat, odor or
discharge. Do not put the Novii POD into a microwave or pressurized container.
If liquid leaks from the Novii POD onto your clothes or skin wash well immediately
with fresh water.
If liquid leaks from the Novii POD and comes into contact with your eye, do not rub
your eye, wash well with clean edible oil and see a doctor immediately.
WARNING: Do not charge the Pods on an external wireless charger, only charge via the Novii
Interface
CAUTION: Keep the operating environment free of dust, vibrations, corrosive, or flammable
materials, and extremes of temperature. The Novii Interface and all cable
connectors should be kept clean and free of electrode gel and other substances.
CAUTION: The Novii Interface is rated IPX0. Do not operate the Novii Interface if it is damp or
wet because of condensation or spills. Avoid using the equipment immediately
after moving it from a cold environment to a warm, humid location.
CAUTION: The Novii POD on its own is rated IPX0. The Novii POD is rated IP57 only when
mated with the Novii Patch. Do not submerse the Novii POD in any liquid if not
mated to a patch.
CAUTION: Never use sharp or pointed objects to operate the touch screen display. Do not
exert excessive pressure when operating the touch screen.
CAUTION: The POD gold connection pins need to be kept clean, and should be protected at
all times; only keep your PODs in the Interface charging bays or clipped to a Patch.
Placing it down anywhere else could result in damage to the gold pins.
2.4 Electromagnetic Compatibility (EMC)
2.4.1 Electromagnetic Interferences
The Novii System has been designed to minimize the impact of electromagnetic interference
from other electrical equipment and also to minimize the interference caused to other electrical
equipment by the Novii System. The Novii system has been tested and found to comply with the
Medical Electrical Equipment - General Requirements for Safety-Collateral Standard:
Electromagnetic Compatibility, EN60601-1-2:2007, and FFC CRF47 Parts 15.107 & 15.109,
Class A limits. However because of proliferation of radio-frequency transmitting equipment and
Page 16
16
other sources of electrical noise in the health-care environments, it is possible that high levels
of such interference due to proximity or strength of the source may result in the disruption of
performance of the Novii system.
Risks and Characterization associated with Electro Magnetic Interferences:
Risk
EMI characterization
High EMI interrupting
the Bluetooth
transmission
between the Novii
POD and Novii
Interface
This will present as a simultaneous gap in the FHR, MHR and UA
data to the user
The Bluetooth connection can be interrupted intermittently or
constantly. The Bluetooth communication interruptions will create
gaps on the tracing of the Maternal/Fetal Monitor attached to the Novii
System. In the event of such interference these gaps will typically
occur simultaneously on the FHR, MHR and Uterine Activity tracing
even if the patient is in close proximity of the Novii Interface.
High EMI present on
the inputs of the
Novii POD
This will present to the user as gaps in FHR data only
On some occasions, the electromagnetic interference will not disrupt
the Bluetooth transmission of all signals simultaneously, but gaps will
occur in the FHR tracing only since the Novii System will stop
detecting the FHR if the noise in the abdominal recording is too high
to detect signals accurately.
Electrostatic
Discharge (ESD)
present on the Novii
System (either POD
or Interface)
ESD present on the Novii System could create artifacts. Specifically,
this artifact will present as transient changes to the FHR trace,
appearing as deflections on the FHR trace of 35 BPM maximum (e.g.
from a reading of 120 BPM down to 85 BPM). These FHR deflections
are very short in duration and would appear to the user as a spike on
the FHR trace.
Once the source of ESD interference has been removed the Novii
System will go on working as normal, there will be no permanent
damage to the system.
If you suspect your Novii System is affected by electromagnetic interference from another
electrical device, it may be necessary to take mitigation measures, such as re-orienting or
relocating the Novii Interface or the device creating the interference. In general, the further away
the Novii System is from the interfering device, the lower the interference will be (please follow
guide lines of Warning G below for minimum distances with other electrical equipment). If the
device creating interference is not in use, it is advised to turn it off. Turning equipment in the
vicinity off and on can help to isolate the offending equipment.
WARNING: A) The Novii system is medical electrical equipment and needs special precautions
regarding EMC: it needs to be installed and put into service according to the EMC
information provided in this section.
WARNING: B) Portable and mobile RF communications equipment can affect medical electrical
equipment.
Page 17
17
WARNING: C) Use of accessories and cables other than those specified in Section 10.1 of this
manual may result in increased EMC emissions and/or decreased immunity of the Novii system
to other electrical equipment.
The cables listed in Section 10.1 are to be used exclusively with the Monica Novii Interface and
Monica IF24. If these cables are used with systems other than the Novii Interface and IF24, it
may result in an increase of emissions or decrease in the immunity of that system.
WARNING: D) The Novii Interface connects to a Maternal/Fetal Monitor; hence it will be adjacent
to, or stacked on top of, a Maternal/Fetal Monitor. It should be verified that the Novii Interface is
correctly calibrated with the Maternal/Fetal Monitor it is connected to and the operation is normal
and as expected in the configuration in which it will be used. To confirm correct calibration the
TEST function of the Novii Interface should be used. The equipment or system (e.g. the
Maternal/Fetal Monitor) should be observed to verify normal operation in the configuration in
which it will be used.
WARNING: E) For Electromagnetic Compatibility the Novii Interface has been tested to IEC EN
60601-1-2. The Essential Performance for that test is the Recording Mode when the Novii
Interface collects via Bluetooth the patient data from a Novii POD and transfers the data to a
Maternal/Fetal Monitor through the connecting cables. Essential performance in Transmission
Mode was defined as “no FHR/UA gaps greater than 30s, no FHR error greater than 15 BPM
for 15s, no UA error larger than 20% of full scale for more than 30s and no interruption of the
transmission mode”.
WARNING: F) This equipment/system is intended for use by healthcare professionals only. This
equipment/ system may cause radio interference or may disrupt the operation of nearby
equipment. It may be necessary to take mitigation measures, such as re-orienting or relocating
the Novii or shielding the location.
WARNING: G) The Novii Interface may be interfered with by other equipment, even if that other
equipment complies with CISPR emission requirements.
Guidance and manufacturer’s declaration – electromagnetic emissions
Table 1 of EN60601-1-2
The Novii system is intended for use in the electromagnetic environment specified below.
The customer or the user of Novii system should assure that it is used in such an environment.
Emissions test
Compliance
Electromagnetic environment – guidance
RF emissions
CISPR 11
Group 1
The Novi™ system uses RF energy only for its internal function.
Therefore, its RF emissions are very low and are not likely to cause any
interference in nearby electronic equipment.
RF emissions
CISPR 11 Class A
The Novi™ system is suitable for use in all establishments other than
domestic and those directly connected to the public low-voltage power
supply network that supplies buildings used for domestic purposes.
Harmonic emissions
IEC 61000-3-2
Class A
Voltage fluctuations/
flicker emissions
IEC 61000-3-3
Complies
Page 18
18
Guidance and manufacturer’s declaration – electromagnetic immunity
Table 2 of EN60601-1-2
The Novi™ system is intended for use in the electromagnetic environment specified below. The customer or the user of the
Novi™ Interface should assure that it is used in such an environment.
IMMUNITY test
IEC 60601 test level
Compliance level
Electromagnetic environment – guidance
Electrostatic
discharge (ESD)
IEC 61000-4-2
± 6 kV contact
± 8 kV air
± 6 kV contact
± 8 kV air
Floors should be wood, concrete or
ceramic tile. If floors are covered with
synthetic material, the relative humidity
should be at least 30 %.
Transient/burst
IEC 61000-4-4
± 2 kV for power supply lines
± 1 kV for input/output lines
± 2 kV for power
supply lines
± 1 kV for input/output lines
AC power should meet the standards of a
typical commercial or hospital
environment.
Surge
IEC 61000-4-5
± 1 kV line(s) to
line(s)
± 2 kV line(s) to earth
± 1 kV line(s) to
line(s)
± 2 kV line(s) to earth
AC power should meet the standards of a
typical commercial or hospital
environment.
IMMUNITY test
IEC 60601 test level
Compliance level
Electromagnetic environment – guidance
Voltage dips, short
interruptions and
voltage variations
on power supply
input lines
IEC 61000-4-11
<5 % UT (>95 % dip in UT)
for 0,5 cycle
40 % UT (60 % dip in UT) for
5 cycles
70 % UT (30 % dip in UT) for
25 cycles
<5 % UT (>95 % dip in UT)
for 5 s
<5 % UT (>95 % dip in UT)
for 0,5 cycle
40 % UT (60 % dip in UT)
for 5 cycles
70 % UT (30 % dip in UT)
for 25 cycles
<5 % UT (>95 % dip in UT)
for 5 s
AC power should meet the standards of a
typical commercial or hospital
environment. If the user of the Novii
system requires continued operation
during power mains interruptions, it is
recommended that the Novii Interface be
powered from an uninterruptible power
supply or a battery.
Power frequency
(50/60 Hz)
magnetic field
IEC 61000-4-8
3 A/m
3 A/m
Power frequency magnetic fields should be
at levels characteristic of a typical location
in a typical commercial
or hospital environment.
NOTE UT is the AC mains voltage prior to application of the test level.
Guidance and manufacturer’s declaration – electromagnetic immunity
Table 4 of EN60601-1-2
Novi™ system is intended for use in the electromagnetic environment specified below. The customer or the user of the Novi™
Interface should assure that it is used in such an environment.
IMMUNITY test
IEC 60601 TEST LEVEL
Compliance
level
Electromagnetic environment – guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms
150 kHz to 80 MHz
3 V/m
80 MHz to 2,5 GHz
3 V
3 V/m
Portable and mobile RF communications
equipment should be used no closer to any
part of Novi™ system, including cables,
than the recommended separation distance
calculated from the equation applicable to
the frequency of the transmitter.
Recommended separation distance:
d = 1.2√P 150 kHz to 80 MHz
d= 1.2 √P 80MHz to 800MHz
d = 2.3 √P 800MHz to 2.5GHz
Where P is the maximum output power
rating of the transmitter in watts (W)
according to the transmitter manufacturer
and d is the recommended separation
distance in meters (m).
Field strengths from fixed RF transmitters,
as determined by an electromagnetic site
survey, a should be less than the
compliance level in each frequency range.b
Page 19
19
Interference may occur in the vicinity of
equipment marked with the following
symbol:
NOTE 1 At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection
from structures, objects and people.
a
Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios,
amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the
electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured
field strength in the location in which the Novi™ system is used exceeds the applicable RF compliance level above, the Novi™
system should be observed to verify normal operation. If abnormal performance is observed, additional measures may be
necessary, such as re-orienting or relocating the Novi™ system
b
Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
Recommended separation distances between portable and mobile RF communications equipment and the Novi™ system
Table 6 of EN60601-1-2
The Novi™ system is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The
customer or the user of the Novi™ system can help prevent electromagnetic interference by maintaining a minimum distance
between portable and mobile RF communications equipment (transmitters) and the Novi™ system as recommended below,
according to the maximum output power of the communications equipment.
Rated
maximum
output power
of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz
d = 1.2√P
80 MHz to 800 MHz
d= 1.2 √P
800 MHz to 2,5 GHz
d = 2.3 √P
0.01
0.12
0.12
0.23
0.1
0.38
0.38
0.73
1
1.20
1.20
2.3
10
3.80
3.80
7.3
100
12
12
23
For transmitters rated at a maximum output power not listed above, the recommended separation distance d in meters (m) can
be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of
the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1 At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2 These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection
from structures, objects and people.
WARNING: H) The Novii system may be interfered with Radiofrequency identification (RFID)
systems (tag and reader). Ensure RFID reader is placed as far as possible from
the Novii Interface. If an RFID tag is placed on the Novii POD or Novii Interface
and you experience poor quality data (Data transmission loss, gaps in FHR data,
Gaps in MHR data, uninterpretable uterine activity) please remove the RFID tag
and RFID reader and check again the Novii System data quality. If the presence
of the RFID correlates with the poor performance of the Novii System, please
report the issue to your distributor or to Monica Healthcare and do not use the
RFID system in conjunction with the Novii System.
2.5 Electrostatic Discharge (ESD) precautions
Page 20
20
The symbol on the Novii system indicates that it is a Static sensitive device.
The Novii POD pins and the Novii Interface connectors are extremely static sensitive and should
be handled using electrostatic discharge precautions.
ESD present on the Novii System could create artifacts. Specifically, this artifact will present as
transient changes to the FHR trace, appearing as deflections on the FHR trace of 35 BPM
maximum (e.g. from a reading of 120 BPM down to 85 BPM). These FHR deflections are very
short in duration and would appear to the user as a spike on the FHR trace.
Once the source of ESD interference has been removed the Novii System will go on working as
normal, there will be no permanent damage to the system.
WARNING: A) Although precautions have been taken to ensure otherwise, static electricity could
cause damage to the pins of the Novii POD or the pins of all three connectors located at the
back of the Novii Interface and render the system inoperable. Pins of the Novii POD or pins of
the Novii Interface connectors should not be touched, and connection to these connectors
should not be made unless ESD precautionary measures are used.
WARNING: B) ESD precautionary measures should be taken to minimize the risk of damage to
the Novii system. More specifically:
• The pins of all connectors at the back of the Novii Interface and the pins of the Novii POD
should not be touched by any part of the body, including the fingers.
• Always connect the interface cables first to the Maternal/Fetal Monitor, and then to the
Novii Interface.
• If the interface cables are not connected to the Maternal/Fetal Monitor, disconnect the
interface cables from the Novii Interface.
• Do not touch any metallic parts of the Novii Interface and the patient at the same time.
WARNING: C) Staff who uses the Novii system should receive an explanation of the ESD
warning symbol and training in ESD precautionary procedures.
WARNING: D) The minimum contents of ESD precautionary procedure training should be the
explanation of the ESD symbol and the understanding of the principles listed in
warning B.
2.6 Magnetic Resonance Environment (MRE)
WARNING: The Novii Wireless Patch System cannot be used or placed in a MR Environment.
This could result in serious injuries and death of patients and other individuals.
Page 21

21
Unpacking
The box should contain (but not limited to) the following items:
• x1 Monica Novii Interface device
• x1 Power Supply for Interface device
• x3 Cables to connect the Novii Interface to your GE Corometrics Fetal Monitor (FECG,
TOCO and MECG input cables).
Some package variations exclude the MECG cable
• x2 Monica Novii PODs
Some package variations include an additional POD as a backup/replacement device for
loss, damage or breakdown. This spare POD should remain in the box and placed in a
secure location that does not see extremes in temperature e.g. a locked cabinet/drawer
in the nurse Manager’s office
• x1 3M red Dot 2236 skin prep tape
• x1 Getting Started / Registration card (Novii Wireless Patch System requires one time
registration before use, see Section 5.1.2)
• CD containing Instructions For Use and support materials
Check that you can identify all the items in the box.
(2) Novii Pod charging
bays, shown with two
docked Novii Pods
(1) Touch Screen
Display
Fig 1a - Monica Novii Interface, front view; showing the start screen, with a
POD in each of the two charging bays. Numbers in brackets ( ) in this user
manual refer to the key numbers in this figure
Page 22
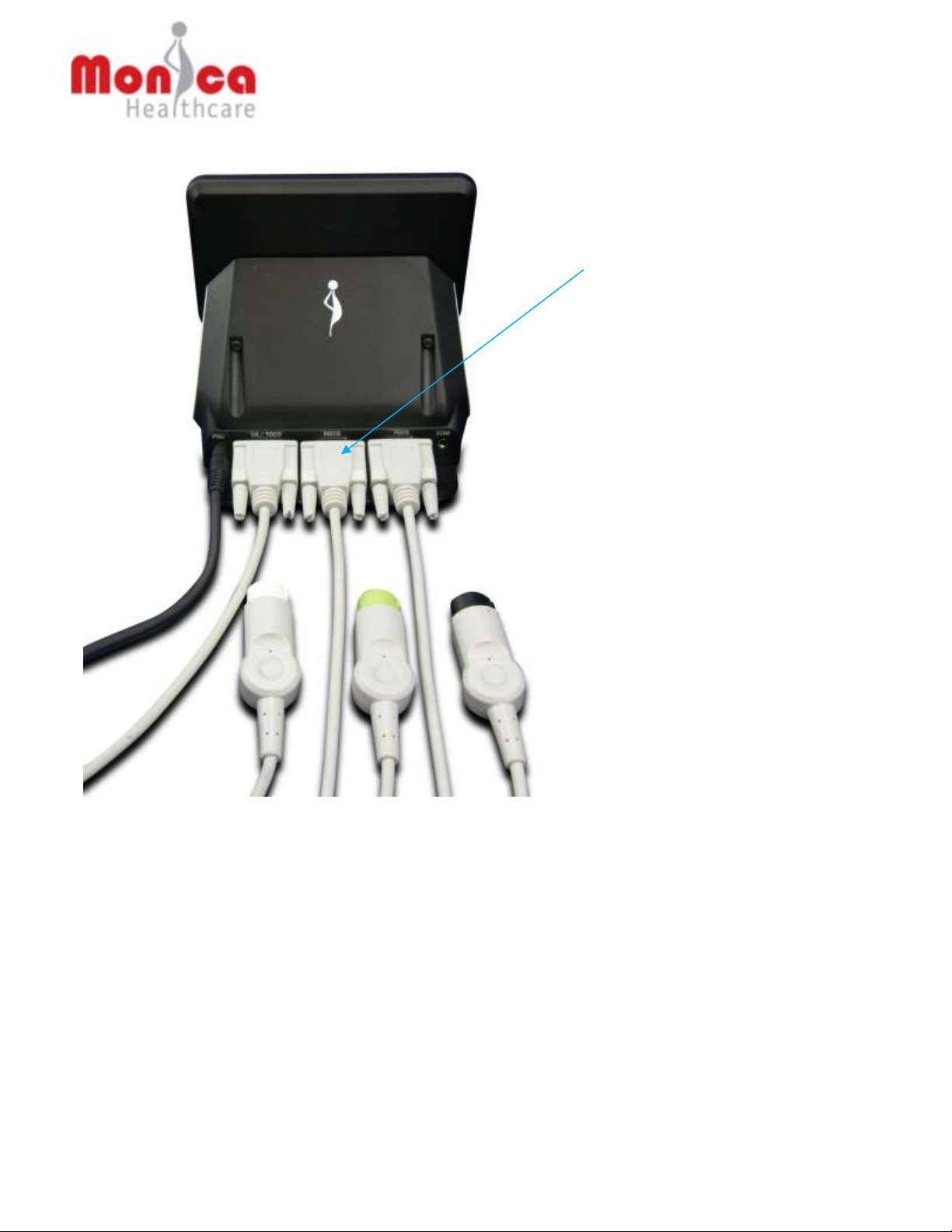
22
(3) Port connectors for the three
cables specific to the GE
Corometrics 259 monitor,
shown connected. The cables
connect to the UA/TOCO,
MECG and FECG ports on the
GE Corometric 259 monitor.
The DC power input socket is
on left of UA/TOCO socket.
The serial port to the right of
the FECG socket is only used
for maintenance by an
employee of Monica
Healthcare or by a Monica
trained and nominated person
Fig 1b - Monica Novii Interface, rear view, showing the three cables that connect
to the GE Corometrics 259 monitor and powers supply cable. Numbers in
brackets ( ) in this user manual refer to the key numbers in this figure.
Page 23

23
Product Description
4.1 General description
The Monica Novii Interface is a device that allows a Novii POD to send fetal, maternal and UA
data to the GE Corometrics 259 Series Maternal/Fetal Monitor. The Monica Novii POD is a
wearable, battery-powered device for surveillance of fetal and maternal well-being. The Novii
POD is designed to passively monitor Fetal Heart Rate (FHR), Uterine Activity (UA) and Maternal
Heart Rate (MHR) during pregnancy. The Novii Wireless Patch system is cleared for use from
36 completed week’s gestation for intrapartum use in singleton pregnancies. The Novii POD is
attached via a magnetic clip directly on to the Novii Patch which locates 5 ECG electrodes on
the abdomen of a pregnant woman, using the umbilicus as reference location point (when the
umbilicus has been displaced the midpoint between the fundus and the edge of symphysis pubis
should be used, see Section 6.5.2). The Novii POD then monitors the electrical signals present
at the electrode sites: fetal ECG, maternal ECG and Uterine EMG (Electromyography) plus noise
and interference signals. The acquired signals are then converted by the Novii POD into a digital
format and processed in real-time to extract clinically relevant information, such as Fetal Heart
Rate, Uterine Activity and Maternal Heart Rate.
The Novii POD sends the FHR, UA and MHR data along with maternal movement from the onboard three axis accelerometer, signal quality and POD battery status signals to the Novii
Interface. This digital data is sent wirelessly via Bluetooth. The Novii Interface receives the
Bluetooth data and converts the FHR, MHR and UA data into an analogue signal before feeding
it to a Maternal/Fetal Monitor via the external DECG (FHR), TOCO (UA) and MECG inputs
(analogue signals). The plugs and cables are specific to the Maternal/Fetal Monitor being
connected. The Maternal/Fetal Monitor will display, print, and connect to a central station the
data from the Novii Interface as if it was acquired from traditional transducers.
The Novii POD has no controls only an LED to indicate when it is on and working. Placing the
POD in a free Novii Interface charging bay that is switched on will allow it to wirelessly connect
with the Novii Interface and for its battery to be charged inductively. The POD will then be
automatically activated when removed from the charging bay. Set-up and operation instructions
are communicated to the user via the Novii Interface display as described in Section 6.
On dispatch the Interface and all PODs making up one Novii Wireless Patch System are
‘locked’ i.e. cannot be used until they have been registered, see Section 5.1.2.
4.2 Data processing
Digital data from the Novii POD is received by the Novii Interface by a Bluetooth wireless
connection; fetal heart rate (FHR), uterine contraction (UA) and maternal heart rate (MHR)
signals are then converted to analogue signals in real-time by the Novii Interface for transfer to
Page 24
24
the Maternal/Fetal Monitor. The Novii POD generates a rolling two second average FHR and
MHR updated every ¼ second. The UA resolution is 1 step out of the 255 steps full range i.e.
1/255 and the amplitude is updated every ¼ second from a low pass filtered signal.
4.3 Data viewing
No data is stored by the Novii Interface; the screen provides user feedback on the signal quality,
Bluetooth status and other settings with help information when appropriate. There is an option
to display a digital value of the maternal heart rate when MECG is not available as a monitoring
option on the Maternal/Fetal Monitor or the MHR cable has not been connected, see Section
5.3.2.
4.4 Data accuracy
The FHR and MHR output to the Maternal/Fetal Monitor is within 1 BPM (Beat Per Minute) of
the data received from the Novii POD. The UA resolution is 1 step out of the 255 full range i.e.
1/255.
4.5 Classification of Medical equipment and marking
Protection against Electrical Shock
Novii Interface: Class II ME Equipment
Novii POD: Internally Powered ME
Equipment with Type BF applied parts.
IP rating
The Novii Interface is rated IPX0
The Novii POD and Patch are rated IPX0
when not connected together and IP57
when connected together
Method of sterilization
Not intended to be sterilized. See Section 9
for cleaning instructions
Suitability for use in an OXYGEN RICH
ENVIRONMENT
Not suitable for use in an oxygen rich
environment
Mode of Operation
Continuous Operation
4.6 Wireless Technology
The Monica Novii System uses Wireless Technology to perform four main functions, specifically:
▪ to communicate patient monitoring data from the POD/Patch to the Interface via
Bluetooth, and;
▪ to charge the battery in the Novii PODs when docked to the Interface using wireless
induction charging (WPC 1.1). The Interface has two charging bays allowing two PODs
to be charged at the same time
Page 25

25
▪ to authenticate the Bluetooth communication between the POD and Interface using
wireless infrared communication (IrDA).
4.6.1 Novii Bluetooth wireless characteristics:
During patient monitoring the Novii Interface and POD communicate wirelessly via two Bluetooth
Transceivers. Bluetooth uses a radio technology called frequency-hopping spread spectrum,
which chops up the data being sent and transmits chunks of it on up to 79 frequency bands of 1
MHz each in the range 2,400-2,483.5 GHz (allowing for guard bands). This helps to ensure the
performance and accuracy of transmitted data. The Bluetooth module is Class 1.5 (with transmit
power control) with a maximum transmit power of 10.5dBm. The power is controllable by
software and is typically 4dBM.
The Bluetooth set up and configuration is fully automatic and does not require any user set up
(Bluetooth Address and Pin are automatically exchanged via an IrDA connection which is
initiated by a POD proximity detector, see Section 4.6.2. A key characteristic of the Novii wireless
system is that it uses a very low power transmission setting (100 times less than a mobile phone)
to mitigate any risks from harmful radio frequencies. Another key characteristic of the Novii
system is that it is designed to communicate over a short distance and if the patient goes out of
range (typically greater than 100 feet) there will be a visual alert.
The Novii Interface can only connect to a POD that is placed in the charging bay.
The Bluetooth characteristics of the Novii system are as follow:
FFC ID of Novii POD and
Interface
T7V1315
Radio Technology
Bluetooth: Frequency-hopping spread spectrum
RF frequencies
79 bands (1 MHz each; centered from 2.402 to 2.480 GHz) in the
range 2,400-2,483.5 GHz (allowing for guard bands).
Bluetooth Class / Power
Class 1.5 Bluetooth module. Software controllable power. Max
power 10.5 dBm. Typical power 4dBm
Bluetooth specification
v2.1 + EDR (Enhanced Data Rate)
Sensitivity
-93 dBm
Data rate
Up to 2,178 kilo bit per second (kbps). The Novii POD sends data by
packet of 80 bytes every 2 seconds
Protocol
Bluetooth HCI via ACL data packets including Forward Error
Correction scheme. CRC mechanism for error detection
Data Encryption / Security
The Bluetooth link between the Novii POD and Novii Interface is
encrypted (128 bit private key link). The Novii POD and Interface are
not discoverable
Distance
Up to 30 meters line of sight
Alert
Bluetooth out of range alert on the Novii Interface
Pairing process
Automatic pairing process using a separate IrDA to transmit the
POD Bluetooth address and pin to the Interface. This is initiated
Page 26

26
only when prior to monitoring the POD is placed in an Interface
charging bay
Quality of service
The Novii Interface and Novii POD do not allow multiple connections
to the Bluetooth Interface. The connection between the POD and
Interface is one to one and the full bandwidth is dedicated to
transmitting the patient data. The Bluetooth interface allow data
transmission up to 2,178 kilo bit per second(kbps). However only a
bandwidth of 320kbps is required to transmit the patient data (80
bytes every 2 seconds)
4.6.2 Wireless charging technology characteristics:
The charging of the Novii PODs on the interface uses ‘Qi Near Field Magnetic Induction’. The
wireless charging is compliant to WPC 1.1. The wireless charging is only activated when a Novii
POD is detected on one of the two charging bays of the Novii Interface. Detection is made via
polarized Hall effect sensors. The Novii Interface and POD are fitted with magnets so that when
the POD is placed on the charging bay, the POD is automatically positioned correctly. The
wireless induction charger also features a Foreign Object Detection (FOD) scheme to protect
the Interface from overheating in the presence of a metallic foreign object.
The wireless charging characteristics of the Novii system are as follow:
Wireless Induction
technology
Conforms to WPC 1.1 "Qi" near-field magnetic induction. Closed-Loop
Power Transfer Control with full bridge inverter
Power
Max transmitted power on POD: 5W: 5V/1A
Protection
Over temperature protection and proprietary FOD
Proprietary Foreign Object Detection
RF frequencies
Power transfer by modulating the switching frequency of the full-bridge
inverter from 110kHz to 205kHz at a fixed 50% duty cycle specified by the
WPC specification
Communication
protocol
Proprietary Back-Channel Communication (transmitted alongside the
WPC Message Packets). CRC mechanism for error detection
Quality of service
One to one connection. The full bandwidth is dedicated to transmitting the
pairing data.
4.6.3 Wireless infrared communication (IrDA) characteristics:
The Novii Pod and Interface are each fitted with an Infrared Transceiver complaint with the IrDA
physical layer IrPHY 1.4. Before an active Bluetooth communication between the Pod and the
Interface can be established, an authentication process is carried out using the IrDA wireless
protocol to transmit the Pods Bluetooth address and security PIN to the Interface. The IrDA
communication is only initiated once the Pod is placed on the Interfaces charging bay. This forms
the automatic pairing process required before any other Bluetooth communication can take place
between the Pod and Interface.
The wireless infrared communication characteristics of the Novii system are as follows:
Wireless infrared
communication
specification
Conforms to the IrDA® specification.
Power
Low power IrDA. MAX. 150 mW/sr
Page 27

27
Data Rate
Up to 115 kilo bit per second (kbps). The Novii system utilizes 9600 kilo bit
per second.
Distance
Up to 30 cm/20 cm. The Novii Pod transceiver is tuned so that it can
only be detected 1 cm away from it.
Quality of service
The IrDA transceivers of the Novii Pod continuously send the
Bluetooth address when placed on the Interface charging bay up
until the Interface can connect to the Pod via Bluetooth before the
transceiver turns off.
4.7 FCC Information (USA)
4.7.1 FCC Rules Compliance
FCC ID
Novii Pod – YOM-6960-MON
Novii Interface – YOM-6961-MON
FCC Rules Compliance
This device complies with Part 15 & Part 18 of the FCC Rules.
Operation is subject to the following two conditions:
1. This device may not cause harmful interference, and
2. This device must accept any interference received, including interference that may cause undesired
operation.
FCC Service Information
Changes or modifications not expressly approved by the party responsible for compliance could void the
user’s authority to operate the equipment.
FCC Interference Statement
This equipment has been tested and found to comply with the limits for a Class B digital device, pursuant
to Part 15 of the FCC Rules. These limits are designed to provide reasonable protection against harmful
interference in a residential installation. This equipment generates uses and can radiate radio frequency
energy and, if not installed and used in accordance with the instructions, may cause harmful interference
Page 28

28
to radio communications. However, there is no guarantee that interference will not occur in a particular
installation. If this equipment does cause harmful interference to radio or television reception, which can
be determined by turning the equipment off and on, the user is encouraged to try to correct the
interference by one of the following measures:
o Reorient or relocate the receiving antenna
o Increase the separation between the equipment and receiver
o Connect the equipment into an outlet on a circuit different from that to which the receiver
is connected
o Consult the dealer or an experienced radio/TV technician for help
Page 29

29
Installation & Settings
Installation of the Novii Wireless Patch System should be performed by a trained healthcare
professional.
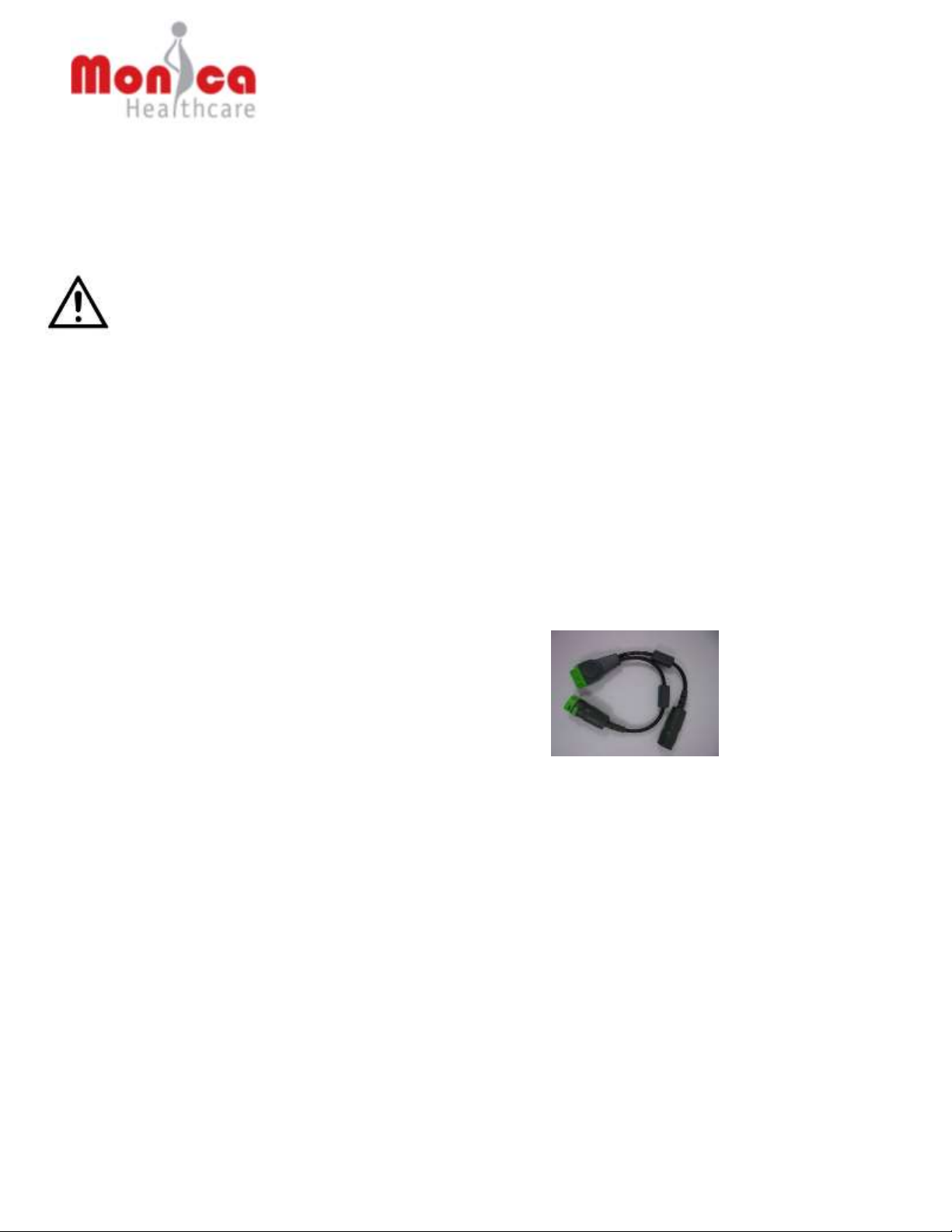
Novii Interface settings allow the audio alerts and MHR display to be adjusted to the hospital
requirements.
Factory default settings are:
• Language –English
• Display MHR on Novii Interface - Disabled
• Audio alerts - Disabled
In a typical situation:
• The Novii Interface will be located on the same cart or furniture as the Maternal/Fetal
Monitor (either using a VESA mount or on the top of the cart) allowing the operator to use
both devices conveniently. Cable connection of the Novii Interface to the Maternal/Fetal
Monitor and to the AC power supply is described below, Section 5.2.
• After setup and the Patient is wearing the Novii POD and Patch, the patient can be
positioned anywhere within the room and, depending on the construction of the L&D floor
and interference from other Bluetooth and Wi-Fi transmitting devices, can be up to 100 feet
away (the Bluetooth Class 1.5 connection allows distances up to 100 feet between patient
and the Novii Interface under ideal line of sight situations).
Page 30

30
5.1 Initial Screen, Device Registration
5.1.1 Power on/off
When the Novii Interface is switched on, by connecting the power supply (there is no on/off
switch) the following splash display will be shown, indicating the Interface program version
number, for around 5 seconds while the device starts and internal checks are performed.
If this is the first time the Interface has been switched on the following language selection screen
will appear. Select your language by touching the SELECT LANGUAGE bar then press the
forward arrow key to save and exit.
Page 31
31
5.1.2 Device Registration
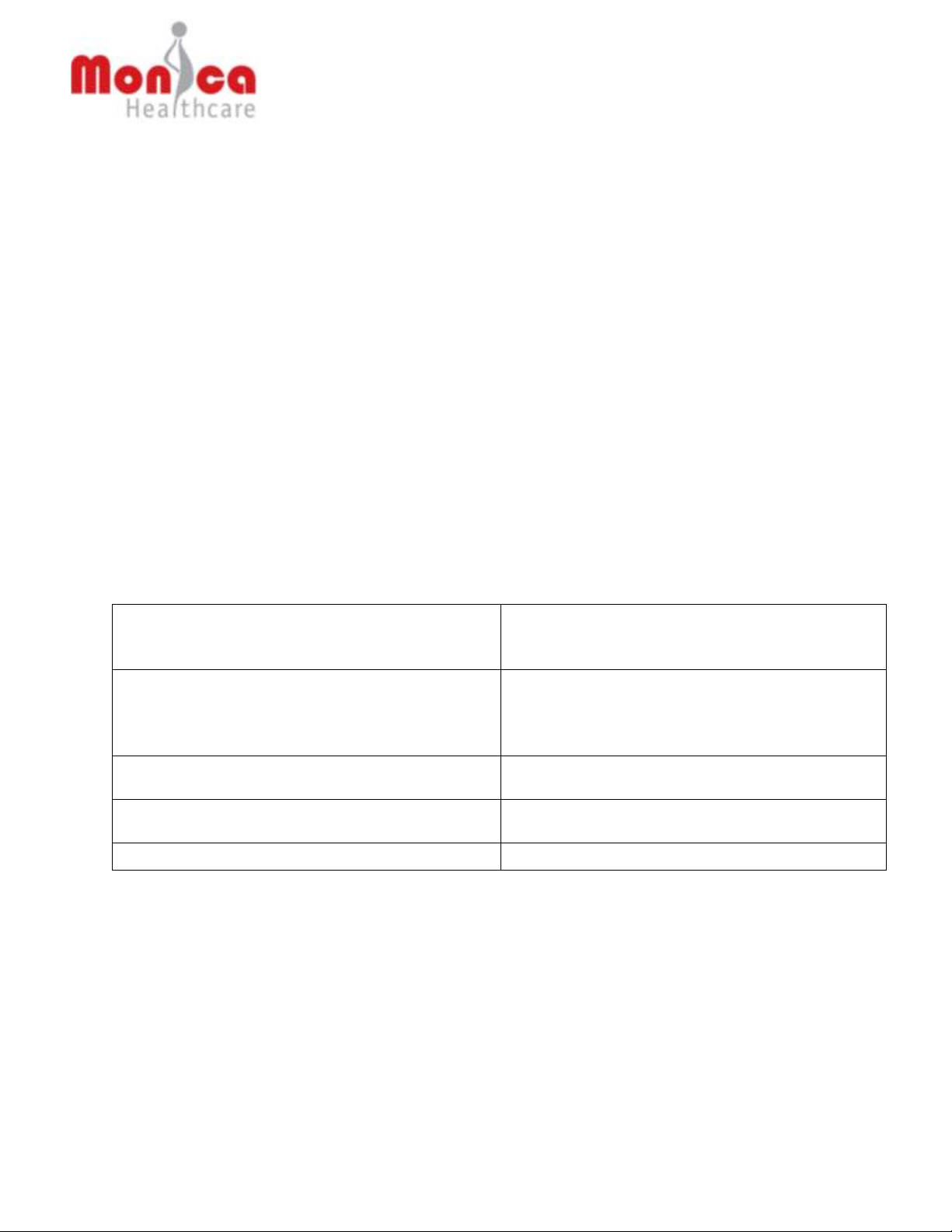
The Novii Interface and PODs cannot be used until they have been registered with Monica
Healthcare Ltd. The screen below will only be seen when the Interface or a POD placed in the
charging bay has yet to be registered:
Warranty will start from the date registered and the data you provide will be used to keep you
informed of software updates and key device critical information. Any information entered will be
treated as confidential and will not be circulated to third parties. Once the device has been
registered the pass code will be provided.
The Interface and the PODs are effectively locked until the correct pass code is entered via the
numeric key pad on the display. The back arrow can be used to delete the last number(s)
entered if a mistake has been made. The Interface and each POD has to be registered
separately starting with the Interface:
To register the Novii Interface and PODs:
1. You will need a computer or notebook PC with Internet access
2. Go to www.monicahealthcare.com/support
3. You will need to Login to your Monica Healthcare account. If you do not have an account
with Monica Healthcare you will need to create one by entering your name and email
address under the section headed Register. You will then be sent a password to the
email address entered which will allow you to Login. Your user name is your email
address.
4. Once you Login select ‘Register Novii Device’ from the menu and follow the screen
instructions.
Page 32
32
5. Once you have completed the registration process you will be given a pass code to enter
on the Interface display.
6. Once the Interface has been unlocked any un-registered POD placed in the Interface
charging bay will bring up the Registration display and the process will need to be
repeated to unlock the POD(s).
CAUTION: To avoid any confusion register one POD at any time, by placing the POD in
the left charging bay only.
CAUTION: The Novii warranty registration process should only be carried out by a
Hospital bio-med engineer or other competent person.
CAUTION: If for any reason the registration process fails the Interface should be
disconnected from the power and re-started.
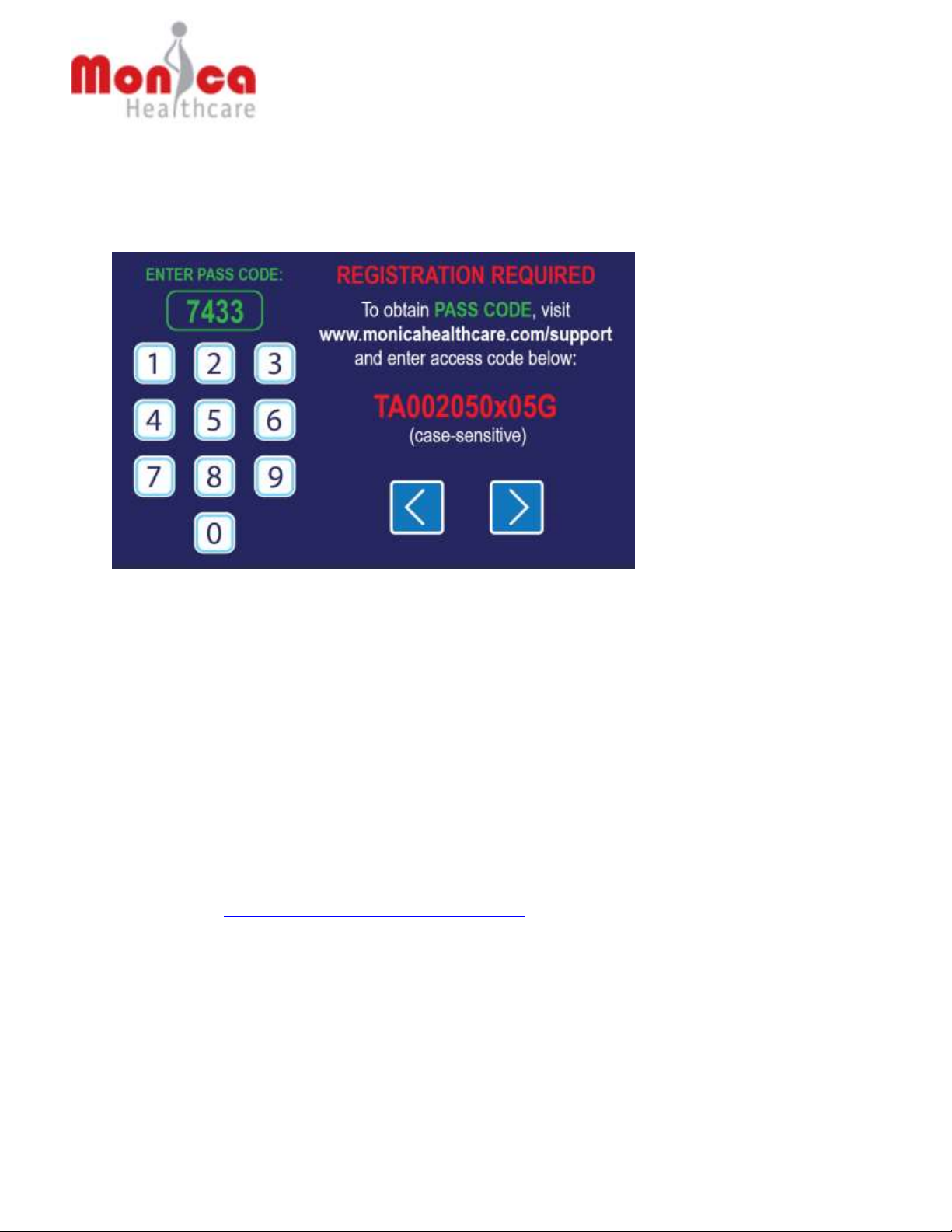
5.2 Cable Connection
1. The Novii Interface will be supplied with specific interface cables and calibrated only for use with
GE Corometrics 259 Maternal/Fetal Monitors.
The GE Corometrics 259 Maternal/Fetal Monitor must be equipped with GE Y-adapter cable
(part# 1442AA0), shown below
2. Refer to Section 10.1 to confirm that you are using the correct cables for your Corometrics
Maternal/Fetal Monitor before beginning the set up.
3. The Interface Cables are permanently connected by using a screwdriver to secure them to the
back of the Interface. Cable Connection is as follows:
a. Connect Novii FECG interface cable to the FECG (Fetal Scalp Electrode) port on the
Fetal Monitor first (using the already connected GE Y adaptor if using the Corometrics
259), then into the port labelled FECG (3) on the rear of the Novii Interface, tighten screw
with a screwdriver.
b. Connect Novii UA interface cable to the TOCO port on the Fetal Monitor first and then
into the port labelled TOCO (3) on the rear of the Novii Interface, , tighten screw with a
screwdriver.
c. If available on the Maternal/Fetal Monitor being used, connect Novii MHR interface cable
to the MECG port on the Maternal/Fetal Monitor first (using the already connected GE Y
Page 33
33
adaptor if using the Corometrics 259) and then into the port labelled MECG (3) on the
rear of the Novii Interface, tighten screw with a screwdriver.
4. Connect the cable of the Novii power supply (107-PT-002) to the power socket on the rear of
the Novii Interface (socket labelled PSU), and then connect the power supply to the AC power
source.
5. The Power Supply of the Novii Interface is regarded as part of the Medical Electrical Equipment.
CAUTION: It is important to run the Novii TEST sequence after installation to ensure that the
Interface, cables, Y’ cable adaptor and Maternal/Fetal Monitor are working
correctly, Section 5.8. It is important that during the test the ‘Y’ cable is moved
around to ensure there are no intermittent connection problems. If you see FHR or
MHR errors please quarantine the ‘Y’ cable and advise your GE Healthcare
representative,
5.3 Accessing Settings
From the Start screen, Section 6.4, enter set up by selecting the SETUP icon
.
There is only one ‘SETUP’ screen, touch ‘NEXT/EXIT’ forward arrow key to accept changes if
any made and exit.
Touching the item ‘bar’ will scroll the user through the available options or take the user to
another screen with a list to select from or more information/options e.g. ABOUT
5.3.1 SELECT LANGUAGE
Touching this item ‘bar’ will provide a list of available languages to choose from.
Page 34

34
5.3.2 DISPLAY MHR ON INTERFACE
Touching this item ‘bar’ will Enable or Disable the MHR display on the Novii Interface.
Selecting to display the MHR on the Novii Interface will automatically turn on the “MHR/FHR
coincidence Alert”. The default is not to display the MHR on the Novii Interface. As well as a
visual alert there is also an audio alert and this will be enabled if the AUDIO ALERTS are turned
ON, see Section 5.3.3 below.
5.3.3 AUDIO ALERTS
The factory default is AUDIO ALERTS DISABLED and can only be changed in the SETUP. By
touching the AUDIO ALERTS item ‘bar’ in SETUP the audio alerts can be ENABLED, providing
an audible alert to supplement the visual alert for the following situations:
i. Low POD battery - Audio alert is always enabled
ii. POD not returned to Interface charging bay - Audio alert is always enabled
iii. MHR coincident with FHR (only if the DISPLAY MHR ON INTERFACE has been
Enabled) and Audio Alerts have been enabled
iv. Electrode(s) detached from abdomen. Audio alerts need to be enabled
v. Patch not genuine - Audio alert is always enabled
Once an alert sounds it can be silenced by touching the SOUND icon which will be flashing or
by following on screen instructions. If the alert condition continues the alert will repeat according
to the schedule below:
Alert Condition
Initial Alert Condition
Once acknowledged Audio Alert
will repeat if the condition does
not resolve after
Low battery
Up to 60 minutes battery life left
15 minutes
MHR coincident with FHR
MHR is within ±10 bpm of FHR for
60 seconds
60 minutes
POD left in Patch and Novii
Patch electrode/skin
preparation check is not
passed or bypassed
After 10 min
Will not be repeated once alert has
been cancelled
POD not returned after
removed from Patch
2 minutes after end of 2 minute
count down
Will not repeat after POD is docked
or alarm condition is acknowledged
on display screen
POD not attached to Patch
After 2 minute count down has
finished
Will not repeat after POD is docked
or alarm condition is acknowledged
on display screen
Electrode(s) detached from
abdomen
When electrode(s) detached
Will not repeat after audio alert has
been silenced
Page 35

35
5.3.4 ABOUT
Touching the About item ‘bar’ will display the Novii Interface firmware version and serial number
along with the firmware version and serial number of any PODs docked and the Monica contact
details.
5.3.5 UPGRADE INTERFACE
A confirmation screen shows that the Novii Interface is in Bluetooth upgrade mode with
instructions. This should only be carried out by a trained bio-med engineer or a trained Monica
authorized person, who has access to the upgrade instructions.
5.3.6 UPGRADE POD
A confirmation screen shows that the Novii POD placed in right or left hand charging bay is in
Bluetooth upgrade mode with instructions. This should only be carried out by a trained bio-med
engineer or a trained Monica nominated person, see the service manual for instructions.
5.4 Maternal Movement Alert using the UA trace
This feature is always enabled.
Following a 20 second period of consistent maternal movement (identified by the accelerometer
in the Novii POD), the UA trace printed by the Maternal/Fetal Monitor will be thickened to alert
the user that caution needs to be taken when interpreting the trace 20 seconds before the start
of the alert and for as long as it is visible on the trace, see example below. Maternal movement
can cause UA artifact to be displayed and or compromise the FHR extraction.
UA Alert –
Trace thickening
Page 36
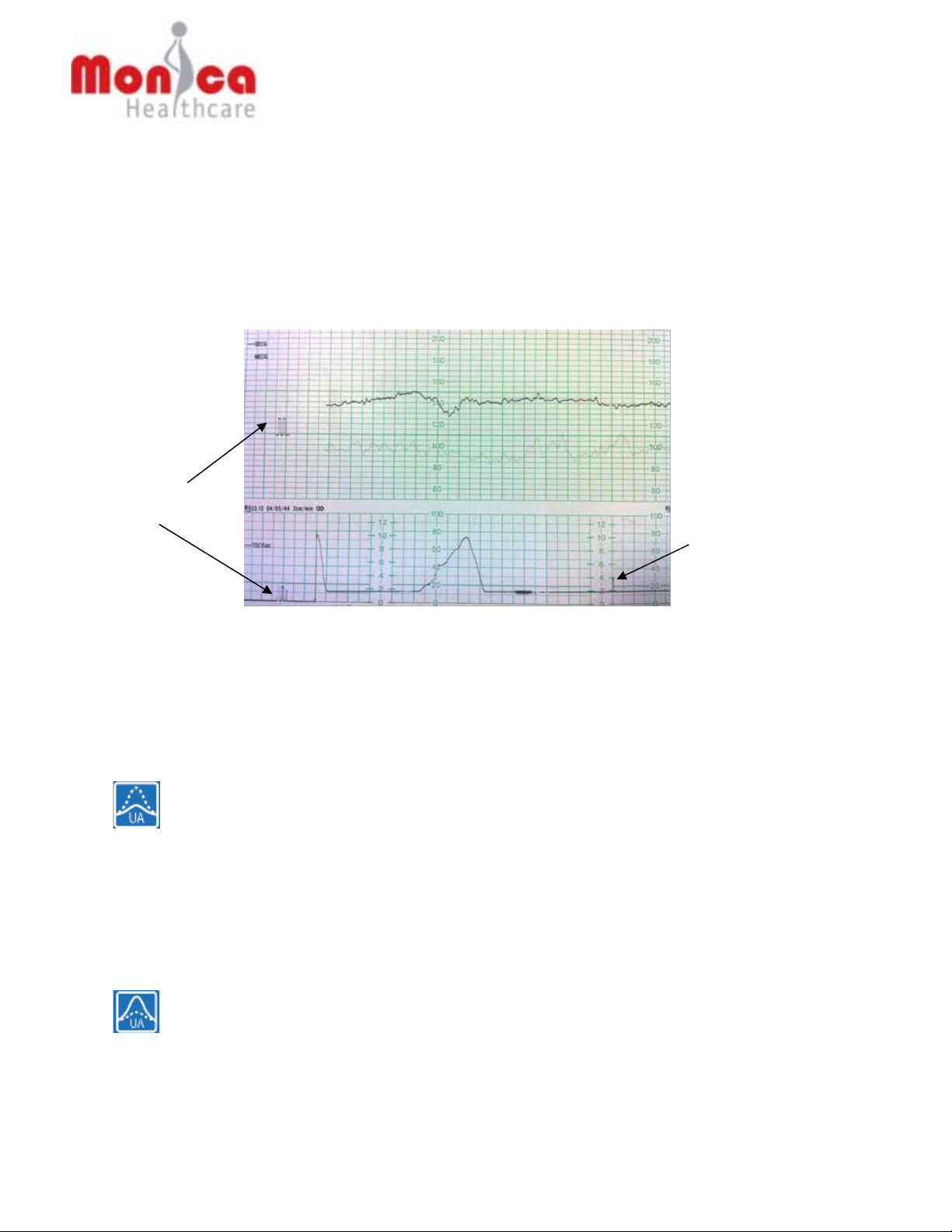
36
5.5 Monica Identifier
This feature is always enabled.
The Maternal/Fetal Monitor will print a Monica Identifier (a small identifying spike) on the UA
trace every 5 minutes and, during the first 10 seconds prior to the start of a new Novii monitoring
episode, a Monica Mark resembling an M will be sent to both the UA and FHR Maternal/Fetal
Monitor inputs.
Printing of the Monica Identifier and Mark on the trace ensures during retrospective viewing and
viewing of data on a central monitoring system, the user knows that Monica Novii was/is being
used. The height of the Monica Identifier mark is determined by the UA sensitivity setting. Mark
height reduces by 50% when Low UA sensitivity is set.
5.6 Low UA Sensitivity
When selected, it is set to a suitable level for pre and early induction patients to reduce
artifact from maternal/fetal movement and other sources. It can be changed at any time during
the monitoring episode by the user. The default start-up setting is high UA Sensitivity. When
Low UA Sensitivity selected the Interface will automatically switch it back to High UA sensitivity
after 60 min.
5.7 High UA Sensitivity
When selected, it sets the UA to a suitable level for established labor patients. It can be
changed at any time during the monitoring episode by the user.
Monica Identifier
Every 5 minutes.
Height of spike is set
by UA Sensitivity
setting
Monica Mark
Start of monitoring
Page 37

37
5.8 TEST function
To confirm that the Novii Interface, Maternal/Fetal Monitor, GE Y adaptor and cables work
correctly, touch the TEST icon from the Start screen. A signal will be sent to the Maternal/Fetal
Monitor to check correct functionality. Monica recommends that whenever the user requires
evidence to demonstrate the correct operation of the Interface and Maternal/Fetal Monitor e.g.
after installation, or to confirm that there are no breaks in the cables or a fault has developed;
the TEST icon on the Start screen should be used. The GE Y adaptor should always be moved,
shaken, to ensure there are no intermittent problems.
After touching the TEST icon the user will be asked to zero the TOCO on the Maternal/Fetal
Monitor– see below, and confirm using the forward arrow key.
Once confirmed, a test FHR, MHR and UA signal will be sent from the Novii Interface to the
Maternal/Fetal Monitor. The FHR, UA and MHR (if connected) values displayed on the
Maternal/Fetal Monitor digital display should match the FHR, MHR and UA numbers displayed
on the Novii Interface display:
Page 38
38
The test values shown on the digital Maternal/Fetal Monitor display should be continuous and
stable. If not, check the GE Y adaptor and if faulty, quarantine and contact your local GE
Healthcare representative.
The FHR digital display should read 120±1bpm, the MHR digital display should read 70±1bpm
and the TOCO should read 105±10% full scale deflection. If the FHR or MHR are not within +/1bpm and/or the UA is not ±10% of this expected value please contact your Monica Distributor
and do not use this Novii Interface until the problem has been resolved.
Answering YES will end the TEST process and take the user back to the Start screen, (Section
6.4). If the user answers NO the following instruction will be displayed:
Page 39

39
Operating Novii
6.1 Introduction
To help set-up the Novii Interface and provide status information of how the POD and Patch are
operating; a touch color screen is used. There is no on/off switch; the Novii Interface will always
be on when connected to a live AC power source. The Novii Interface follows a number of simple
rules and convections:
Warning and Alerts:
Are always displayed in ORANGE
Touch Icons:
Active controls to change the status of a function or select a new
function are displayed with a white icon in a blue box showing the
status or function. For example:
Set-Up Icon
Novii POD Status:
The battery charging levels and status of a Novii POD placed in the
right or left charging bay (2) is shown in lower left or right of the
display.
Page 40
40
1
6.2 Screen Format
The screen on the Novii Interface guides the user when starting a monitoring session and then
helps the user achieve the best signal quality, through status alerts and control options. The
format of the main monitoring screen is shown below:
Novii POD status when placed and removed from right/left Novii Interface charging bays
below display.
This area reserved for help/support information, alert messages and Novii MHR display
when enabled
User controls: SETUP, HELP, TEST; (these three are not available during monitoring),
SOUND (on/off) and UA SENSITIVITY (high/low); these two are only shown during
monitoring. Touching these icons will toggle between the two states.
During monitoring this area provides Novii POD performance/status information: Battery
life, fECG signal quality and serial number of the monitoring POD. When not
monitoring, this area is combined with area 2 to extend region for help/support
information/messages.
4
1
2 3 4
3
2
Page 41

41
6.3 Initial Screen and Standby Screen
6.3.1 Power on/off
When Novii Interface is switched on, by connecting the power supply (there is no on/off switch)
the following splash display will be shown, indicating the Interface program version number, for
around 5 seconds while the device starts and internal checks are performed.
If the device has not been registered it will ask for the language to be selected. It will then go to
the registration screen, please refer to Section 5.1.2.
If the Novii Interface has been inactive for 10 minutes and there is no monitoring, no Bluetooth
connection nor other event activity, the Standby screen below will be displayed:
Page 42

42
Touching the Standby icon, or removing and redocking a POD will take the user to the
‘start-screen‘, Section 6.4.
6.4 Start Screen:
The Start Screen will be displayed if the following conditions are met:
Novii Interface and PODs have been registered
One or more PODs have been placed in the charging bays
A POD has sufficient battery life (>4.0hrs) to commence monitoring (it takes up to 2hrs
to fully charge a POD from empty):
6.5 To Start Monitoring
6.5.1 Instruction 1: Place Patch on Abdomen
Place the Novii Patch as described below or refer to the picture instructions on the Patch pouch.
The Novii Patch can be left on the patient’s skin for up to 48 hours. Do not place the Novii Patch
on skin with any lesions.
1. Check the expiry date and confirm the Pouch has not been opened.
2. Wash any cream/oil/gel from abdomen and ensure the area is dry.
To check the connection to the
monitor touch the TEST
button, Section 5.8
To access the settings touch
the SETUP icon.
The status of the Novii Pod(s)
placed in the two charging
bays is shown here
For additional support and help
touch the HELP icon
Page 43
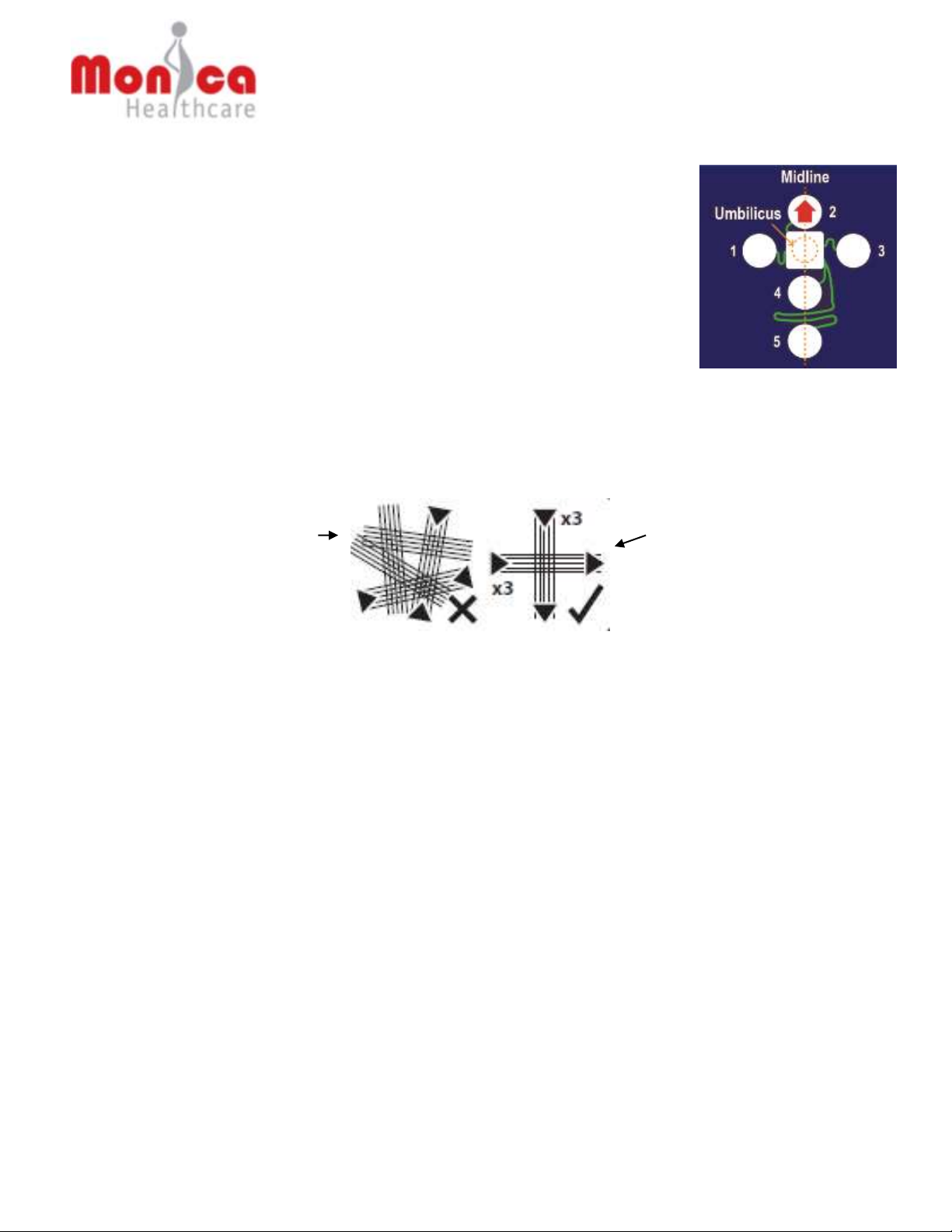
43
3. Remove the backing from the sticky central area of the Patch under the POD clip.
4. The POD clip should be placed on the midline over the center of
the uterus. For most patients the umbilicus is a good anatomical
reference. See below for women with a displaced umbilicus.
Ensure the Patch is placed correctly as shown in Fig. 2, with the
three central electrodes #2, 4 and 5, aligned along the patient’s
mid-line and the red arrow at top, pointing towards the head, then
stick down.
5. Lift up one of the electrodes around the Novii POD clip (electrode
#1, 2, 3 or 4); focus on the small area of the skin where the center
of the electrode will be placed.
6. Using about 1”/2cm strip of the 3M skin prep tape to exfoliate the skin in one direction only,
with a deliberate but gentle stroke, lifting finger after each stroke; 3 strokes each in 2
perpendicular directions to create an X or cross pattern. The center of the electrode needs
to match up with center of the skin prep area.
7. Once done, remove the electrode backing and stick down firmly, trying to avoid pressing
the central gel area of electrode
8. Repeat for the remaining 3 electrodes around the clip
9. For the last remaining electrode attached to the long flexible cable, electrode #5, Fig 2:
a. Remove the electrode backing first ready to stick down
b. Prepare the skin (see 6 above) so that the center of the electrode will be positioned
on the midline approximately 2.4”/6cm above the rim of the symphysis pubis
c. Stick down precisely over the center of prepared area
10. In patients with a displaced umbilicus: Where the umbilicus is displaced downwards by
more than 3cm from the center of the uterus, with the patient supine or semi supine, you
will need to estimate where the center of the uterus is, following one of the following
approaches:
a. Position POD clip along the mid-line where it intersects the horizontal line passing
over the iliac crests
b. Position POD clip along the mid-line at the mid-point between the fundus and
symphysis pubis).
Don’t do this
Instead, DO this!
Fig. 2
Page 44
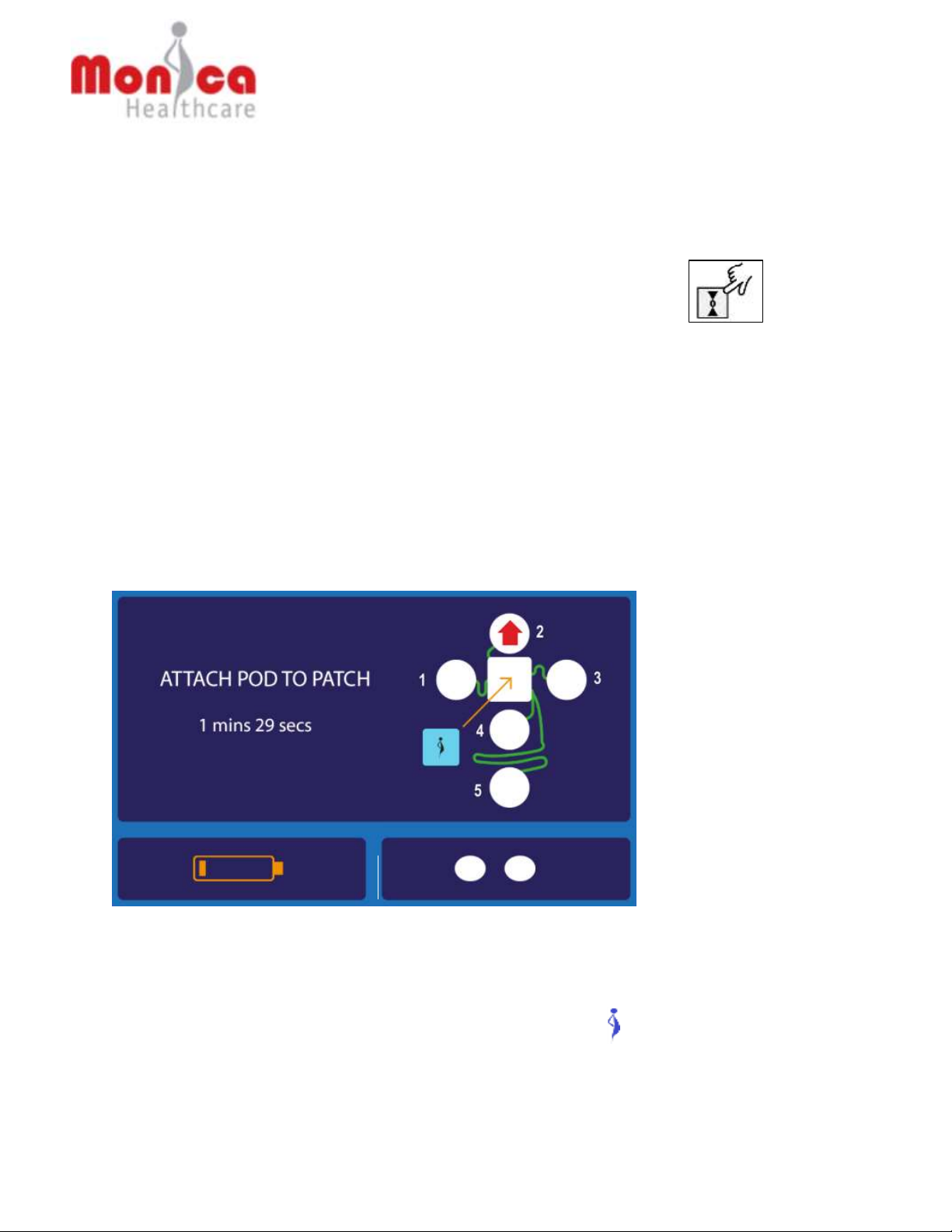
44
c. Position POD clip so that the top edge of electrode #2 is 5”/10-12cm below the
fundus.
The electrode on the flexible cable, electrode #5, Fig. 2, should be placed on top of
pannus approximating to the point 2.4”/6cm horizontally from the symphysis pubis looking
vertically down. This is difficult to estimate and if the FHR signal is poor, reposition this
electrode lower down on abdomen to maximize FHR signal and consider
placing under the pannus just below turn ensuring the electrode is
not folded.
6.5.2 Instruction 1: Zero UA on Maternal/Fetal Monitor
Press the UA zero reference button on theFetal Monitor.
6.5.3 Instruction 3: Select a Charged POD
1. Remove any Novii POD from one of the Novii Interface charging bays as long as the POD battery
status icon is GREEN. Once it is removed the blue lights on the front of the Novii POD will flash
alternately, to indicate that the POD is now ‘active’ and paired to the Novii Interface.
2. The Interface display will change to a countdown as shown below. The Novii POD must now
be clipped to the Patch, within 2 minutes.
3. The battery charging icon on the Interface will be replaced by a ‘busy’ icon ( 1, 2, 3 white dots),
indicating that the POD is preparing to commence monitoring. The busy icon will remain until
the MHR is detected when it will stop.
4. The POD is attached to the Patch with the Monica symbol facing up. Magnets in both the
Patch clip and POD ensure correct placement and, no force is required.
Page 45

45
5. If the POD is not attached to the Novii Patch within the 2-minute countdown it will switch off and
the blue lights will go out and an audio/visual alert will be generated immediately after the
countdown finishes.
6. If the 2nd POD is removed from the charging bay whilst the 1st POD is monitoring a patient, it will
not turn on.
7. Once the POD is attached to the Patch, an electrode check screen will appear indicating if the
skin preparation at each electrode site has been successful. If there is a skin/electrode problem
the screen shown below will be displayed:
CAUTION: If the POD is removed before the TOCO zero on the Maternal/Fetal Monitor has
been pressed; the user will have either re-dock the POD and start again, or, palpate the uterus
and when confident that the patient is not having a contraction press the zero TOCO icon on
the Maternal/Fetal Monitor.
9. A diagram of the Patch is displayed, as shown above, to the right of the screen with a key to the
symbols shown on each of the electrodes to the left. There are three electrode states which are:
a. If an orange circle or red cross is shown on an electrode corresponding to the
electrode site, more skin preparation is required. Lift up the electrode, dry the skin and
repeat skin-prep instructions above (Section 6.5.1). Just one orange circle or red cross
will prevent the monitoring from starting. Following one repreparation attempt and green
checks not achieved the user can choose to bypass the skin/electrode check by
touching the forward arrow icon. Accuracy of the fetal heart rate should not be affected,
but fetal heart rate detection may be lower.
b. When there are 5 green check marks the monitoring screen shown below will be
automatically displayed (MHR Interface display disabled).
Page 46

46
10. If you need to end the setup, or monitoring session, remove the POD from the Patch and return
it to the charging bay on the Novii Interface that it came from.
11. The monitoring screen helps the user achieve the best signal quality, control the monitoring
mode, view status alerts and if enabled display the MHR. The format of the display is shown
above.
12. Once the POD identifies and extracts the MHR both blue lights will flash together every 2
seconds.
13. FHR, MHR and UA monitoring should commence within one minute, once the monitoring screen
above is displayed.
14. FHR, MHR and UA data is collected wirelessly from the Novii POD and sent to the Novii Interface
and then on to the Maternal/Fetal Monitor via the Novii Interface cables. The Maternal/Fetal
Monitor acts as if a FECG scalp cable, MECG cable and a TOCO/IUPC transducer cable are
connected and will display and print the FHR, MHR and UA. The user can swap from one or
more of the Monica monitoring modalities to another method e.g. Monica UA, simply by removing
the Novii UA interface plug and replacing with the TOCO UA transducer plug.
WARNING: This is not supported or recommended because the US FHR and TOCO/IUPC UA
are not synchronized with the Novii FHR, MHR and UA, see Section 6.12. It is
recommended that a note is made on the trace or patient notes. Please refer to
the Maternal/Fetal Monitor manufactures instructions for more details on the
display and printing options available.
15. If ‘Display MHR on Interface’ option is enabled in the settings even if the mECG cable supplied
with the Novii Interface is connected to the Maternal/Fetal Monitor, a digital display of MHR will
be shown on the Novii Interface.
Page 47

47
6.6 Novii Interface Icons and Status Controls/Messages
Symbol
Description
Digital display of the maternal heart rate (MHR).
Needs to be enabled in the settings, Section 5.3.2. Please note – MHR is
not shown when alert or help messages are being shown
When the MHR is shown on the Novii screen. This alert symbol is displayed
when the MHR and FHR are within 10 bpm of each other for longer than 60
seconds. If enabled, an audible alert will also be heard until the user silences it
by touching the audio alert sound icon which will be flashing. The audio alert
will be silenced for 60 minutes. The visual alert will disappear when the FHR
and MHR diverge with a greater than 10 bpm difference for a cumulative time
of 60 seconds.
Novii POD fECG signal quality indicator, is indicated by the color and number
of squares in the indicator bar.
• x3 green squares indicates a good mECG/fECG, which should provide good
FHR extraction.
• x2 orange squares indicates that the FHR extraction may be compromised
e.g. maternal movement, poor signal to noise, and the user should be
cautious in accepting the FHR trace and seek confirmation.
• x1 red square indicates there is no FHR extraction because the abdominal
maternal/fetal ECG is poor, the noise levels are high or there is a fault
condition preventing FHR extraction.
Novii Pod battery status, consisting of 8 charge levels.
A green battery icon showing all 8 segments indicates the Novii Pod has a
battery life of up to 11 hours.
A orange battery icon with only one of eight segments showing indicates that
the POD battery life has dropped to around 60 minutes and the user should be
prepared to replace the POD. When this occurs an alert/help message will be
displayed, see Section 6.7.2.
A orange battery icon with no segments indicates an empty POD battery
Uterine Activity is set high and this is the correct setting for active Labor.
Touching the icon will change the mode to low sensitivity as shown below. The
default start-up setting is high.
Page 48

48
Uterine Activity is set low and many users find this low sensitivity setting better
for pre/early induction Labor. In low sensitivity artifact produced by fetal and
maternal movement is suppressed. Touching the icon will change the mode to
high sensitivity as shown above, which is the default start-up setting.
When using the Low UA sensitivity setting the Interface will automatically switch
back to High UA sensitivity after 60 min. There is no audio or visual alert/help
message, other than a change to the UA sensitivity icon when this happens.
Symbol
Description
Sound alerts enabled.
Factory default setting is OFF
During an audio alert, touching the SOUND ON icon will disable audio.
All sound alerts disabled except for Battery Low, Return POD to charging bay
and Patch not genuine
Touching this icon will initiate the SET UP options which allows system
defaults to be changed and access to other functions. Please refer to
Section 5.3.
Touching this icon will send FHR, MHR and UA reference signal to the
Maternal/Fetal Monitor. Please refer Section 5.8.
Used as a next/exit instruction
Used as back/cancel instruction
Help icon: Provides advice where the User Manual can be located
Page 49
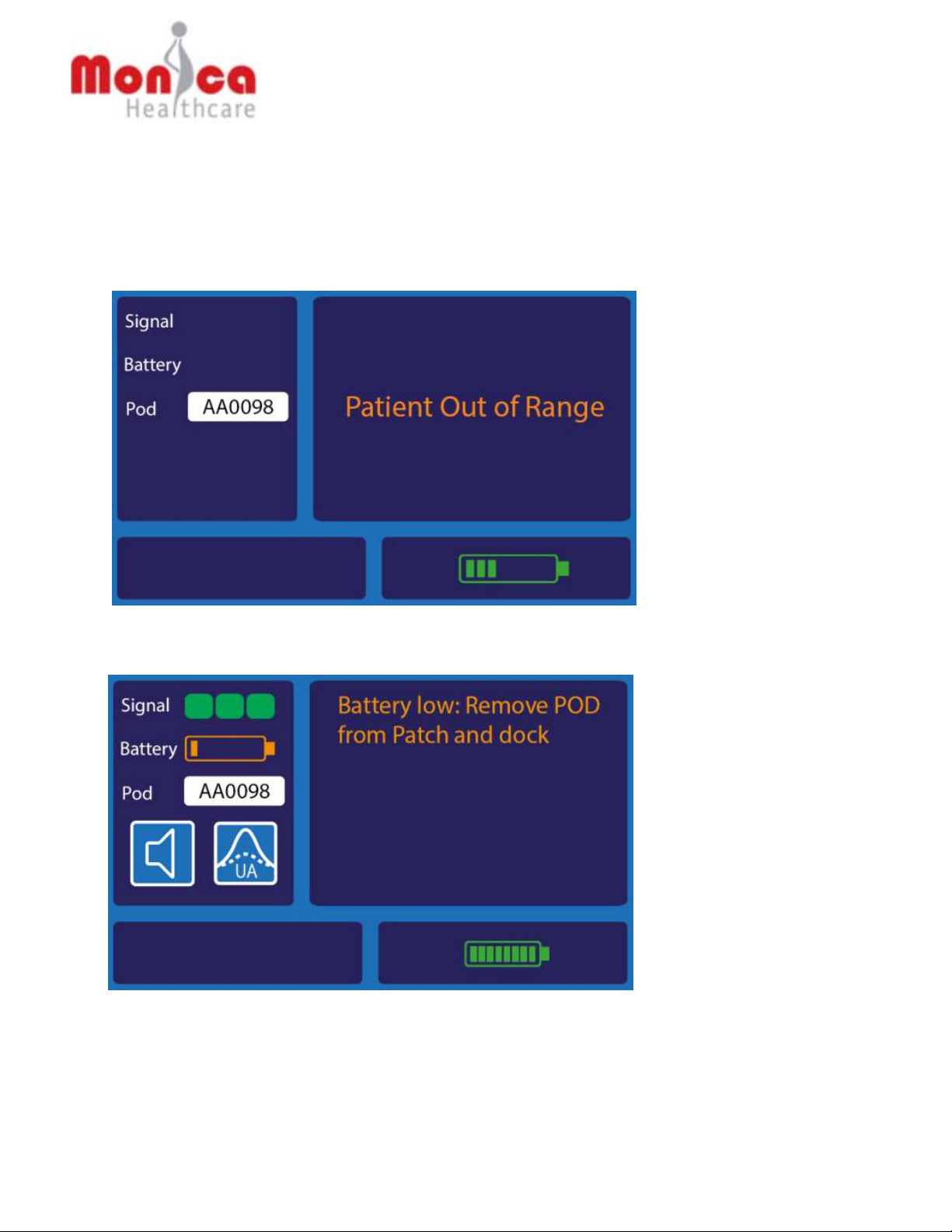
49
6.7 Novii Interface Monitoring alert/help messages
To help the user the Novii Interface provides a number of help/alert messages or displayed
symbols during monitoring. The messages are dynamic. These alerts/help messages are
shown below.
6.7.1 Patient out of Bluetooth range
6.7.2 Low battery
Low battery: Battery low alert
message is in orange and it will
flash and audio alert will sound (if
sound is off will show on until
silenced) when only one segment
(around 60 minutes is remaining)
Patient is out of wireless range and
the Interface cannot pick up the
Bluetooth signal. Message will
flash. Note loss of signal and
battery information..
Patient out of range
Page 50

50
6.7.3 Lost FHR
When using the Monica Novii Interface always use its signal quality indicator and not the quality
indicator on the Maternal/Fetal Monitor, If signal quality turns red or orange:
Refer to the FHR Gaps Troubleshooting Table, Section 15, in summary:
a) Stop the patient ambulating
b) Make the patient more comfortable so as to relax abdominal muscles to improve the
signal to noise e.g. place a pillow to support the patient’s back
c) Change the patient position so as to change the conduction pathway between the fetal
heart and abdomen, by moving the fetus in relation to the abdomen e.g. ask patient to
lie on her left or right side
d) If the abdomen is mobile, or patient position has changed use a rolled blanket/towel or
pillow to support abdomen so as to keep the Patch centered on the uterus
e) In a women with a pannus, remove the lower electrode and re-position on the midline 1-
2”/3-5cm below the original placement or on the underside of the pannus just below the
turn.
f) You can use the US transducer to provide an FHR during a gap, as long as Novii has
not been removed and you understand the impact of the 5mm time shift. The US FHR
and TOCO/IUPC UA are not synchronized with the Novii FHR, MHR and UA, see
Section 6.12.
If the situation persists change to another modality e.g. FSE/US FHR or TOCO/IUPC UA and
discontinue the use of Novii.
Unacceptable FHR quality (red):
No message is displayed when
fECG signal quality is poor and the
FHR cannot be extracted. No alert
sound.
Page 51
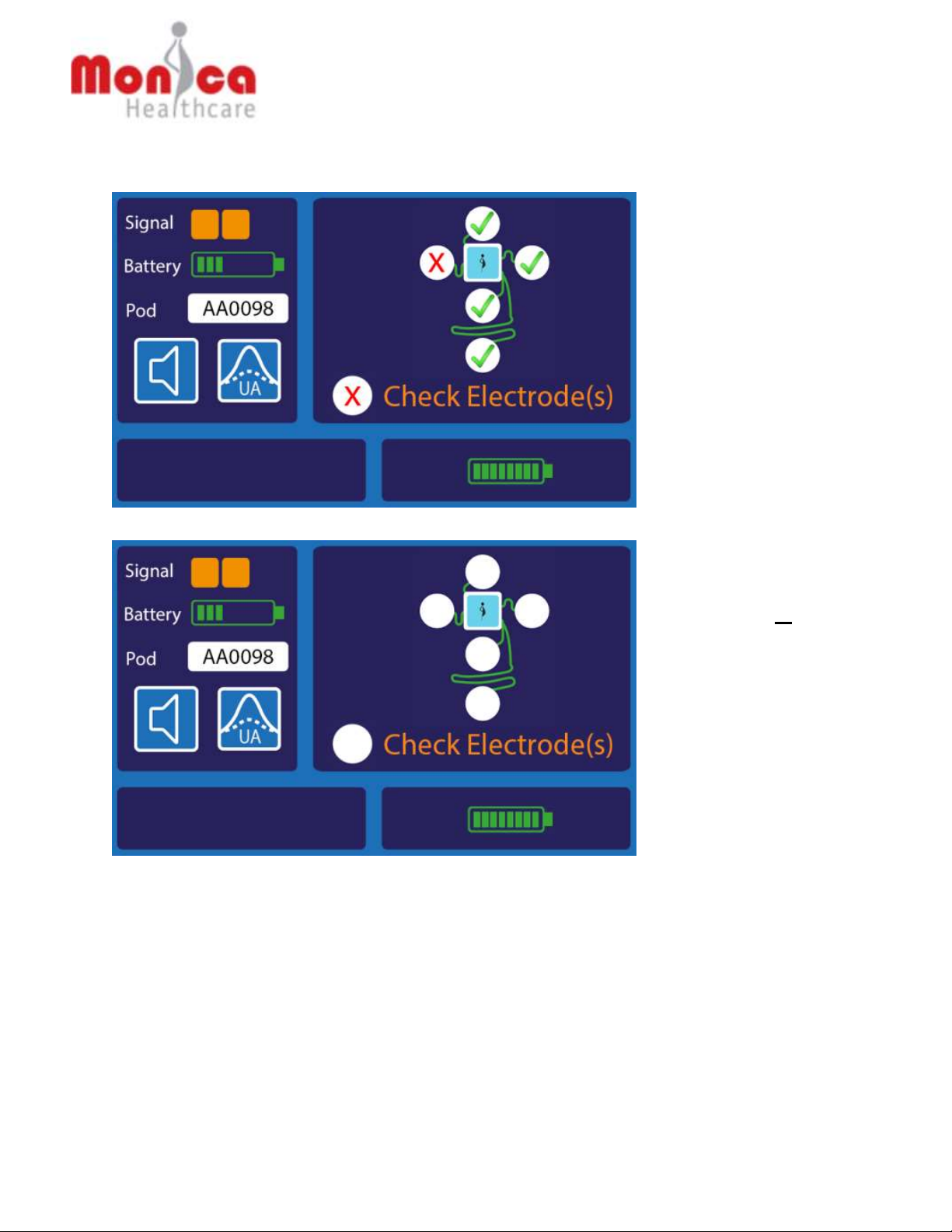
51
6.7.4 Electrode disconnection:
The Novii Interface will create a priority visual alert if an electrode has become disconnected.
Electrode disconnection: The
Novii Interface will create a priority
visual alert if an electrode has
become disconnected. If only one
electrode has become disconnected
then the display will indicate the
electrode to check. Reattach the
highlighted electrode to the skin, if
required micropore tape can be
used to ensure the electrode is held
in place.
If more than one electrode has
become disconnected, this display
will be shown and all electrodes
should be checked to ensure a
good contact with the skin.
Micropore tape can be used to
ensure the electrodes are held in
place.
Page 52
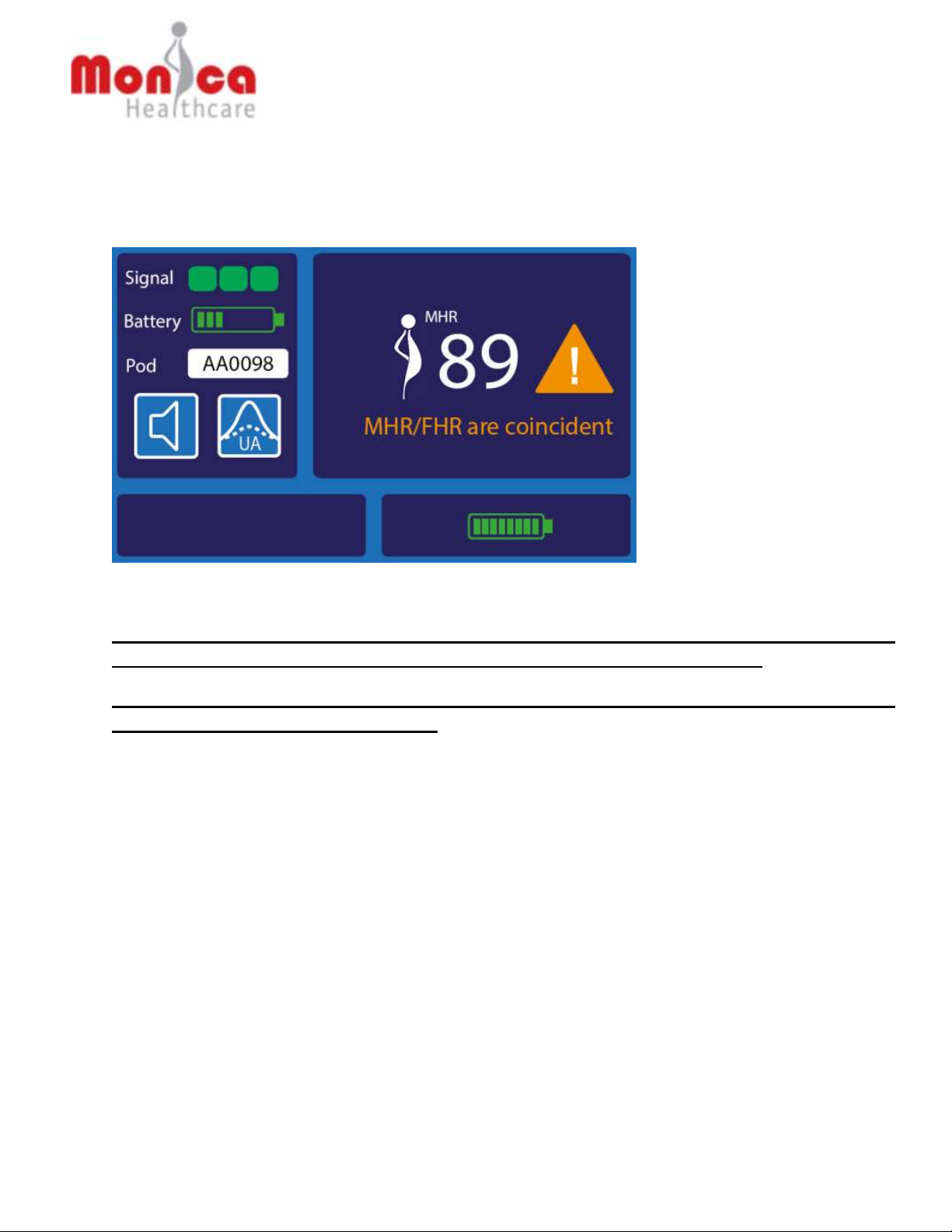
52
6.7.5 MHR/FHR coincidence:
The Novii Interface will create an audio/visual alert if the MHR and FHR are coincident (+/10BPM for more than 60s). This visual alert is available only when Display MHR on Interface
option is enabled.
6.8 How to continue monitoring when the Low Battery alert is activated
When the battery is low the monitoring session must first be ended before taking a
charged POD to connect to the Patch to continue the monitoring session.
To end a monitoring session the POD must be removed from the Patch and placed in an
empty charging bay on the Interface.
If possible the POD should be cleaned before it is returned to Interface, preferably as soon as it
is removed from Patch
6.9 Placing/Removing PODs from the Novii Interface Charging Wells
The lower section of the screen shows the charging status of a Novii POD placed in the right
and/or left Novii Interface charging bays.
While the POD is charging one of the blue lights on the POD will flash slowly. When the POD
is fully charged it will turn off.
When a POD is monitoring a patient the charging bay from where it was taken should not
be used if possible. It is ‘locked’ and a POD placed in this charging bay during a monitoring
session will not be recognized by the Interface. It will charge, but because it is not recognized
by the Interface no battery charge icon, will be displayed, nor will the blue lights on the POD
In this example an audio alert will
be heard. The audio alert will be
silenced for 60 minutes by touching
the 'SOUND IS ON' icon. The alert
will disappear if the coincidence
disappears.
Page 53

53
flash slowly to indicate that the POD is charging. The charging bay will be ‘un-locked’ when the
monitoring session is ended.
The color of the battery icon indicates if the docked POD has sufficient charge to start a
monitoring session. Green means yes, orange means no. If a POD is removed from the
charging bay showing an orange battery shaped icon, the blue lights on the POD will not turn
on. The POD is off and cannot be used to connect to a Patch. This is because the battery has
yet to reach a minimum battery charge level to give at least 240 mins of monitoring.
There are 6 possible status messages/displays for each charging bay – shown below for left
bay:
1. POD in charging bay with low charge <4 hrs i.e. battery icon is orange – POD will not switch
on if removed.
2. POD in charging bay is not recognized e.g. wrong firmware or communication fault. The
interface will automatically try to initialize communication again, but if message remains
contact your local distributor / GE sales representative to arrange service request.
3. POD in charging bay has a battery fault, contact your local distributor / GE sales
representative to arrange service request.
4. POD in charging bay is charged and can be used to monitor a patient.
5. When a POD is placed or removed from a charging bay a waiting icon (1, 2, 3 white dots)
may appear. This indicates that the Interface has recognized the POD placement or removal
but is waiting for internal checks to be completed.
6. POD is missing from charging bay
Page 54

54
6.10 Monitoring Alert priority
Priority order is:
1. PATIENT OUT OF RANGE
2. CHECK ELECTRODES for a possible disconnection
3. BATTERY LOW
4. MHR/FHR COINCIDENCE (only if MHR is displayed on Interface)
5. POD not returned
6.11 Turning Off the Interface
There is no power button on the Novii Interface, removing the power supply will turn the Interface
off. Once the PODs are fully charged, the Interface can be turned off. If the Interface if switched
on and there has been no activity for 10 minutes, the Interface will go into the ‘power-save’
standby mode, this will allow the POD(s) to fully charge and then automatically turn off when full,
with minimal power consumption.
6.12 Novii FHR, MHR, UA synchronization & mixed modality monitoring
The Novii UA, FHR and MHR traces are all synchronized, but shifted in relation to real-time
events by around 5mm (10 seconds) on the trace. This is due to the time it takes to extract,
send and confirm the Novii FHR, MHR, UA from the abdominal electrical signals. In normal
operation this will have no impact on the management of the patient or the interpretation of the
trace with the following exceptions:
WARNING: Monica does not recommend or support mixing Novii UA with US/FSE FHR
monitoring.
There is a 10-second shift (5mm on the tracing) in the Novii UA trace with respect
to the US/FSE FHR trace such that late decelerations could appear as early
decelerations masking a potential fetal compromise.
Using the US transducer in addition to Novii FHR, MHR and UA to confirm the
FHR, for short periods, during gaps or suspected artifact can be used, but the
potential for missing a fetal compromise remains, due to US FHR and Novii UA
desynchronization.
WARNING: Monica does not recommend or support mixing Novii FHR/MHR with TOCO/IUPC
UA.
Page 55

55
If the Novii UA cable is disconnected and the TOCO/IUPC is used (against this
recommendation), it is clinically important to understand that the FHR/MHR shift
will have changed from 5 mm to 3 mm ( 6 seconds). Early decelerations may
appear as ‘subtle’ late decelerations. This could lead to an unnecessary
intervention.
WARNING: DO NOT USE THE NOVII MHR TO MONITOR THE PATIENTS RESPONSE TO
A TEST DOSE DURING EPIDURAL PLACEMENT. There is a 10 second MHR
shift in reporting the MHR with respect to real time events when the Novii UA
Interface is connected to the Maternal/Fetal Monitor (reduced to 6 seconds if the
UA Interface cable is not connected). To avoid this problem, disconnect the Novii
MHR lead from the Maternal/Fetal Monitor. If the GE Corometrics 259 Series
Maternal/Fetal Monitor display MHR has been set to automatic (default) removing
the maternal ECG input will default the MHR display to use the SpO2 input for
MHR. Replace the Novii MHR lead when epidural placement has been completed.
CAUTION: The 10 second FHR shift should be taken into consideration during prolonged FHR
decelerations when resuscitative measures are being used, the impact of any
manoeuvre will not be seen for 10 seconds.
CAUTION: The 10-second UA shift should be taken into consideration when coaching patients
to push during the second stage. The patient may sense the contraction before it
appears on the monitor tracing - the contraction has already been building for 10
seconds.
CAUTION: When the patient is moving and/or the fetus is active caution should be exercised
in interpreting the UA trace. If the interpretation of uterine contractile pattern(s) is
uncertain, another modality to monitor uterine contractions should be considered
and clinical management of the patient adjusted appropriately. The Novii POD
monitors uterine activity by measuring the electrical signals (EMG) generated by
the uterine muscle when it contracts, as opposed to the tocodynamometer (TOCO
transducer) which monitors uterine activity as measured by the displacement of a
plunger or button with respect to a guard ring caused by the tightening of the uterus
during a contraction. Small relative changes in the electrode positions used to
monitor the uterine EMG resulting from maternal or fetal movement cause
electrical signals that can look like uterine activity.
6.13 The two blue LED lights on the POD
These are used to indicate the status of the POD:
1. Charging: Upper LED (head of pregnant Monica i, flashes slowly when charging and both
LEDs will turn off when fully charged
Page 56

56
2. POD is ‘on/active’ when removed from charging bay: if and only if LEDs flash alternately
on/off
3. Connected to Patch: Both LEDs are on continuously when connected to patch and waiting
for monitoring to start
4. Monitoring/MHR detected: Both LEDs flash slowly together
5. If both LEDs are off when removed from Interface, POD is off and should be returned to
Interface for storage and charging.
6. If both LEDs are off when POD is on the Interface, POD is fully charged.
Page 57

57
Interface Visual Alerts
7.1 Return POD to charging bay visual alert
If a POD is removed from a charging bay when no monitoring session is in progress and POD
has sufficient charge there will be an audio and visual alert after 2 mins if it has not been placed
in a Patch or re-docked. The following alert message will be displayed:
The alert shown above can be cancelled by touching the forward/exit arrow button and it will
not be repeated or by returning the POD to charging bay.
7.2 POD removed from Patch visual alert
During monitoring if a POD is removed from Patch. The following 2-minute count-down
message will be displayed.
Page 58

58
If the POD has not been re-attached to the Patch or placed in charging bay at the end of the 2
minutes countdown, the monitoring session ends. The POD switches off and the Interface will
return to the Start Screen. The return POD to charging bay audio/visual alert, Section 7.1, will
appear after 2 minutes if the Pod is not returned to a charging bay.
7.3 POD left in Patch without responding to skin/electrode problems
If a monitoring POD is left on Patch and skin/electrode problems have been detected, but no
action taken (bypass or repeat exfoliation). After 10 minutes the return POD to charging bay
audio/visual alert, Section 7.1, will appear.
7.4 A non-Monica Patch is detected at the start of monitoring visual alert
When the POD is first connected to the Patch, it will read the security chip embedded in the
Patch. If the Patch is not recognized the following message will be displayed:
If back arrow button is pressed, the POD will turn off, but if the Pod is not placed in a charging
bay within 2 mins, the return POD to charging bay alert triggers (Section 7.1)
7.5 A non-Monica Patch is detected during monitoring
During monitoring the POD will periodically read the security chip and if the Patch is not
recognized (non-genuine) the monitoring session will end and the POD will switch off. The
Interface will show the following display with an audio alert for 5 minutes until the Pod is
returned to a charging bay on the Interface:
Page 59

59
Page 60

60
Help icon
When the help icon is selected from the start screen, the user will be guided on how to
access further support and instructions.
Page 61

61
Cleaning
9.1 Cleaning (Patch is single used and should be disposed of as hazardous
waste)
To avoid damage to any parts of the Novii system, clean and disinfect only according to the
following instructions. Care MUST be taken to preserve labels on the Novii POD, Novii Interface
and the Maternal/Fetal Monitor cables.
CAUTION: Disconnect Novii Interface from the AC power supply before cleaning.
CAUTION: The POD gold connection pins need to be kept clean, and should be protected at all
times; only keep your PODs in the Interface charging bays or clipped to a Patch.
Placing it down anywhere else could result in damage to the gold pins.
CAUTION: Do not remove, conceal or deface the labels.
CAUTION: Do not autoclave the Novii Interface or Novii POD or any accessories. Do not gas
sterilize.
CAUTION: Do not immerse the device or any accessories in liquid and do not expose any
connector pin to the cleaning solution. Do not apply oil at any point.
CAUTION: Do NOT use strong oxidants such as bleach.
CAUTION: Do NOT use bleaches containing sodium hypochlorite or any other cleaning solution
other than those recommended here, Table 2, because permanent damage to the
Novii Interface, Novii POD and cables could occur.
CAUTION: The water temperature must not exceed 40°C (104°F). Do not use chlorine bleach.
CAUTION: Take extra care when cleaning the touch screen display, which is sensitive to rough
handling.
Clean - Wipe the Novii Interface, Novii POD and Interface cables with a soft non-abrasive cloth
or disposable wipe soaked in aqueous detergent/ disinfectant or other solution such as 70%
isopropyl alcohol. Do not use aerosol preparations since they might contain organic solvents.
Do not pour fluids directly on the unit and its accessories. Wipe the exterior of the Novii Interface,
Novii POD and Interface cables three times. Prepare the detergent according to the
manufacturer’s recommendations. If necessary scrub the Novii Interface, Novii POD and cables
with the solution using a soft bristled brush for five minutes.
Wash off & Dry - When using solutions, use sterile wipes or gauze to avoid pouring fluids directly
on the unit and its accessories. Wipe the Novii Interface, Novii POD, and cables three times with
sterile or distilled water to remove cleaning solution residue. Dry the Novii Interface, Novii POD,
connector and cables thoroughly with a sterile soft towel or gauze surgical sponge.
Page 62

62
Accessories & Part Numbers
Part No.
Description
107-PT-001
Novii Interface
107-PT-003
Novii Pod
107-PT-002-US
Novii Interface Power Cable (US)
107-PT-002
Novii Interface Power Cable (UK,EU,AU)
107-PT-004-10
Novii Patch (box of 10)
107-PT-004-50
Novii Patch (box of 50)
100-PT-007
3M red Dot 2236 skin prep tape
100-PT-025
Monica User Manual CD (includes promotional video and
other support material)
10.1 Interface Cables
Input
Description
Part #
Plug Color
FECG
Monica Interface CTG Cable - GE Corometrics
DECG round grey connector
105-PT-102
UA
Monica Interface CTG Cable - GE Corometrics UA
round white connector
105-PT-106
MECG
Monica Interface CTG Cable - GE Corometrics
MECG round green connector (requires GE ‘Y’
adaptor cable, part # 1442AA0)
105-PT-104
Page 63

63
Patch Specification
General Information
This symbol on your device indicates that you
should consult information contained in this
book
Manufacturer
Monica Healthcare, Unit 8, Interchange 25
Business Park, Nottingham, NG10 5QG, UK,
Phone: +44 115 949 6960
Model
Single Patch 107-PT-004
Box (10 patches) 107-PT-004-10
Box (50 patches) 107-PT-004-50
Input
Electrophysiological signals picked up from the skin surface via the
5 ECG Electrode contact areas integrated into the patch
Output
Electrical signals collected in a central area for input to the Novii Pod.
The patch is passive.
Encryption
Microchip containing factory pre-set code (SHA_256 encryption)
Weight
12g
Dimensions
190mm x 155mm x 12mm (including clip)
IP rating
IP57 (when attached to patient) only when mated to the Novii Pod,
otherwise IPX0
Shelf Life
12 months (from Date of Manufacture)
Latex & PVC Free
Yes
Packaging
Individual foil pouches & transportation cards
Operating
Temperature
+10°C to +30°C
Storage Temperature
+10°C to +30°C
Page 64

64
Interface Specification
General Information
This symbol on your device indicates that you
should consult information contained in this
book
Manufacturer
Monica Healthcare, Unit 8, Interchange 25
Business Park, Nottingham, NG10 5QG, UK,
Phone: +44 115 949 6960
Model
107-PT-001
Software revision
level
Select 'About' in the Set-Up menu of the
Interface to display software version, see
Section 5.3.4
Mode of operation
Continuous use
Data I/O
Bluetooth Wireless
input
Protocol
Range
Output
Bluetooth V2.1 + EDR Class 1.5, from Novii
Interface.
Modified Series 50 protocol.
30m (line of sight)
Real-time to Maternal/Fetal Monitor via
Interface cables, comprising:
• Direct fetal ECG pulse (for FHR)
• MECG pulse (for MHR)
• Uterine Activity waveform (for UA)
User Interface
Capacitive Touch
screen LCD
display
Alert Buzzer
Resolution 800 x 400 resolution (RGB 65K
colors)
Viewing Area: 108mm x 65mm.
Touch panel durability (tap test): 1 Million
Frequency: 3.4kHz ± 0.5kHz
Charging Bays
2x wireless charging bays for Novii PODs (with magnetic location)
Charge Time for 2x fully discharged pods – up to 2hrs
Uses IrDA to facilitate automatic pairing with the Pod
Page 65
65
Power Supply
Monica reference
Input
Output
Dimensions
Weight
107-PT-002-US
100V~ to 240V~, 50Hz to 60Hz, 400mA
5V DC / 3000mA
152 mm x 137 mm x 150 mm
688g
IP rating
IPX0
Accessories
Interface Connection Cables for GE Corometrics 259 Series
Maternal/Fetal Monitor: FHR (105-PT-102); MHR (105-PT-104) UA
(105-PT-106)
Novii Interface Power Cable (107-PT-002-US)
Operating Temp
+10 deg C to +30 deg C
Storage Temp
+10 deg C to +30 deg C
Page 66

66
POD Specification
General Information
This symbol on your device indicates that you
should consult information contained in this
book
Manufacturer
Monica Healthcare, Unit, 8, Interchange 25
Business Park, Nottingham, NG10 5QG, UK,
Phone: +44 115 949 6960
Model
107-PT-003
Software revision
level
Select 'About' in the options of the Interface to
display software version (see Section 5.3.4)
Mode of
operation
Real-Time / Continuous use
TYPE BF EQUIPMENT: Type BF equipment is
suitable for intentional external and internal
application to the patient, excluding direct
cardiac application. Type BF equipment has an
F-type applied part.
Applied Parts:
The applied Parts of the Novii POD are the five
electrodes of the Novii Patch that are placed on
the patient abdomen. This applied parts
connect to the pins at the bottom of the Novii
POD
User Interface
LED
FHR
Range
Resolution
Accuracy
60-240 beats per minute
Resolution: 1/4 BPM produced 4 time per
second from a rolling 2s average Bland Altman
versus AN24 predicate: 7.1 BPM rms (95%
limit of agreement: -13.7 to 14.1 BPM). Bias:
0.194 BPM, see Figure 2 and Figure 3 below
MHR
Range
Resolution
Accuracy
40-240 beats per minute
Resolution: 1/4 BPM Produced 4 time per
second from a rolling 2s average Bland Altman
versus AN24 predicate: 5.3 BPM rms (95%
limit of agreement: -10.4 to 10.5 BPM). Bias:
0.035 BPM See Figure 4 and Figure 5 below
Page 67
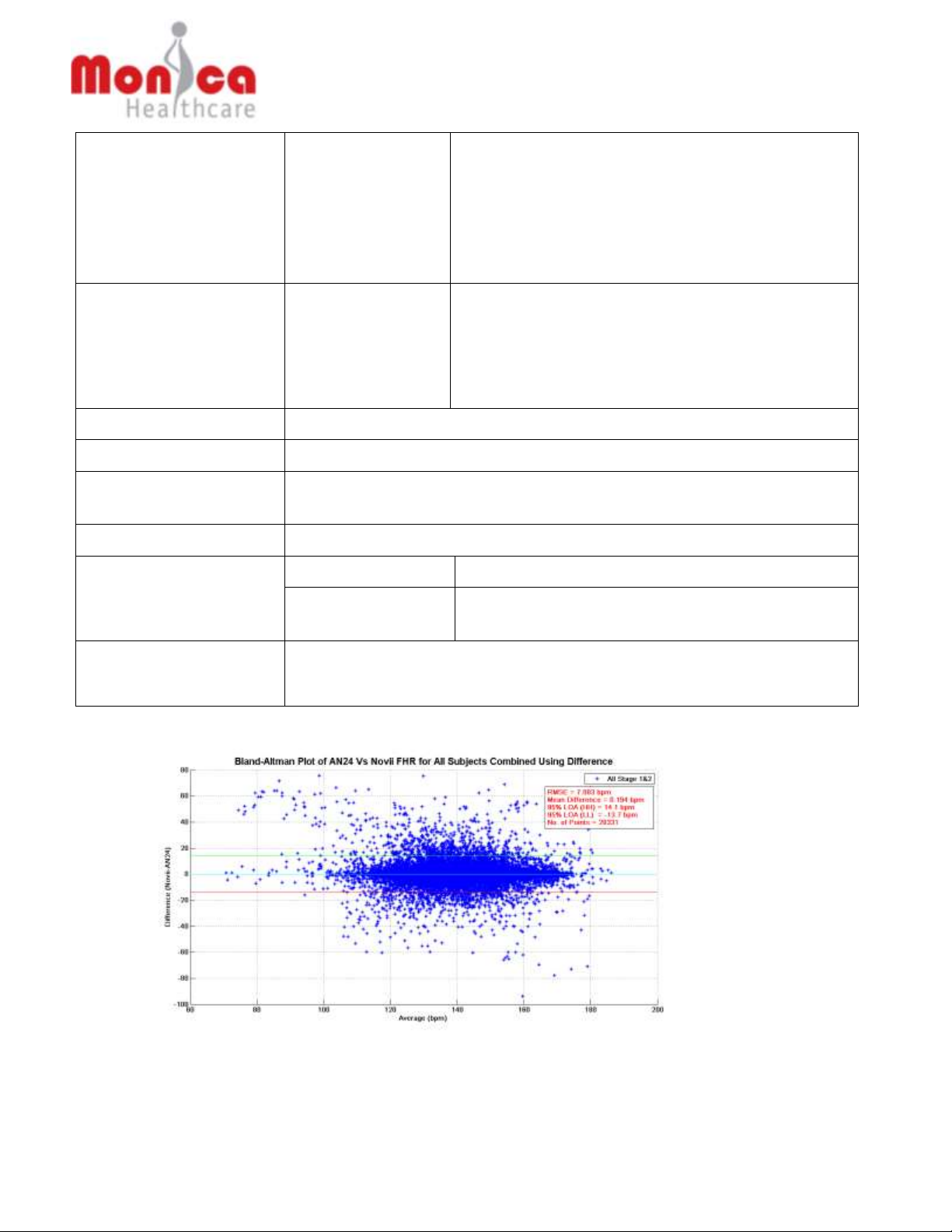
67
UA
Range
Resolution
Accuracy
0-500 microvolts
0-255 levels representing 100% of full scale
Produced 4 time per second from a rolling 2s
average
98% percent agreement (95% confidence limit:
96.6%), 86.05% Positive Percent Agreement
(95% confidence limit 81.9%)
Power
Battery
Battery Life
Battery Charging
Rechargeable lithium polymer 3.7V 750mAh
80% capacity after 475 charges cycles
Up to 11 hours battery life
Contactless charging with the Novii Interface
(107-PT-001)
Dimensions
45mm x 39mm x 20mm (including contact pins)
Weight
40g
IP rating
The Novii POD is rated IP57 only when mated to a Novii Patch. If
not mated to a Novii Patch the rating is IPX0
Accessories
Single Use Monica Novii Patch: 107-PT-004
Environmental
conditions of use
Normal use
+10°C to +30°C
Transport and
storage
+10°C to +30°C
Type
Type BF Equipment (applied part is the Novii patch, which
connects to the pod via the spring contact pins at the bottom of the
pod)
Figure 2: FHR Bland Altman Novii / Predicate device (difference)
Page 68

68
Figure 3: FHR Bland Altman Novii / Predicate device (percent difference)
Figure 4: MHR Bland Altman Novii / Predicate device (difference)
Figure 5: MHR Bland Altman Novii / Predicate device (difference)
Page 69

69
Fault Finding
For further support visit www.monicahealthcare.com/support
Novii Interface Troubleshooting Table
ID
Symptom Description
Possible Causes
Actions and Solutions
S1
Power supply to Novii
interface is unplugged or
power cut occurs during
monitoring
Should the power supply to the Novii
interface be disconnected, switched off, or
fail due to a power cut, the monitoring will
not resume once the power is returned
To continue monitoring, once power is returned
remove the Novii POD from the patch and place in a
charging bay. The Interface will return to the start
screen and monitoring can be re-started
Should the power to the Maternal/Fetal
Monitor disconnect but the power to the
Novii Interface remains on
No action: The Novii system is still monitoring and
when the Maternal/Fetal Monitor is back on, the
recording will continue, but monitoring data during the
power loss will not be recoverable
S2
Novii Interface screen is
blank (no power).
The power lead is not plugged into the
Novii Interface and/or wall socket, or the
power is turned off
Ensure the power lead is plugged into the Novii
Interface and wall socket properly, and the power is
turned on. The green light on the Power Supply unit
should be illuminated. If no green light, replace Power
Supply
Power supply cable is damaged.
Visually inspect the cable for any signs of damage.
Replace the cable if necessary
Power supply is defective.
Confirm the green light on the power supply unit is on
and that the power supply is live. If not, replace the
Power Supply.
Novii Interface failure
Confirm that the Novii POD(s) are correctly positioned
in charging bays. Check if the POD blue light is
flashing or on continuously and make a note of the
status of the POD blue lights; if there is no display,
replace Novii Interface.
S3
Novii Interface screen
displays a frozen
image/no response
Power surge has crashed the display
Disconnect power, wait 30 seconds and reconnect
power. If the Novii Interface screen still frozen, with no
response, replace the Novii Interface.
Page 70
70
Novii Interface Troubleshooting Table continued
ID
Symptom Description
Possible Causes
Actions and Solutions
S4
Novii Interface does not
respond when a POD is
placed in charging bay
Monitoring session is in Progress
A POD placed in the empty charging bay, vacated by
the monitoring POD, will not be recognised by the
Interface. No action is needed. When the monitoring
session is ended by returning the POD to any empty
charging bay, the POD will be recognised
Faulty Pod
Confirm there is no ongoing monitoring session, the
POD is correctly positioned in the charging bay and the
Start Screen is being displayed on the Interface . The
blue light on the top of the POD should be flashing or
on continuously and the battery status should be
displayed on the Interface above the POD. If none of
this happens, replace the Novii POD.
Insufficient battery charge to allow POD to
switch on
Leave POD in charging bay for 20 mins. Blue POD
light should start to flash and Battery charge status
should appear on Interface display above POD, if not
replace the Novii POD
S5
Novii Interface does not
respond when a POD is
removed from the
charging bay
Monitoring session is in progress
If the Novii Interface is being used to monitor a Patient
then the Interface will not respond and the POD blue
lights will switch off when a POD is removed. This is
normal operation. The monitoring session must be
ended by removing the POD from the Patch and
returning it to the Interface charging bay.
Battery has insufficient charge
If the battery status display above the removed POD is
orange the POD will not switch on when removed.
Please replace POD in the charging bay and wait for
battery status to turn green.
Faulty POD
If none of the above apply, replace Novii POD
Interface is in Standby mode
Placing the Pod back on o the Interface will exit the
Standby mode.
Page 71
71
Novii POD Troubleshooting Table
ID
Symptom
Description
Possible Causes
Actions and Solutions
S6
Electrode check
repeatedly fails
during set-up
despite following
'Preparing Skin'
instructions on
Patch pouch.
User is not performing the skin preparation
properly.
Make sure to follow the skin preparation instruction
provided on patch pouch and in IFU.
Patch is out of date or electrodes have dried
out
Confirm the Patch is in date and the pack has not been
opened for a long time allowing the electrolyte on the
electrode central foam pad to dry out;
Wrong exfoliation finger pad or skin-prep tape
is used.
Confirm the skin-prep exfoliation finger pad (provided
with product) or 3M skin-prep tape are used.
POD has not seated correctly on Patch clip
Check that the POD is correctly seated in the Patch
clip by removing it and then clipping it back on and
pressing it firmly down. When the POD is removed,
check the patch connection gold pins on the bottom of
the POD for any evident damage. Replace POD if
necessary.
Dirt/grease/gel/water contaminating
POD/Patch connection
Look for dirt and grease/gel/water in the Patch plastic
clip/connector or on the POD pins. If necessary, clean
the inside of the Patch connector and wipe the pins on
the back of the POD using an alcohol wipe and dry
thoroughly.
Faulty POD
Remove POD and place in charging bay. Take other
POD and place in Patch. If electrode check passes
replace POD in charging bay.
Faulty Patch
Remove Patch, wash and dry abdomen and use a new
Patch placed over the same location – no further skinprep is required. If the electrode check fails, replace
Novii System
Page 72

72
Maternal/Fetal Monitoring Troubleshooting Table
ID
Symptom
Description
Possible Causes
Actions and Solutions
S8
FHR/UA or MHR
data not being
displayed by
Maternal/Fetal
Monitor
Maternal/Fetal Monitor is switched off
Confirm that the Maternal/Fetal Monitor is ON and
confirm that the Maternal/Fetal Monitor works using the
Ultrasound and TOCO transducers.
Cables are not correctly connected
Confirm that the Interface cables are securely plugged
into the correct port on the front of the Maternal/Fetal
Monitor and the back of the Novii Interface.
Novii FHR connects to the DECG port
Novii UA connects to the TOCO port
Novii MHR connects to the MECG port.
From the start screen select the ‘Test’ button and
follow the on-screen instructions. The Novii Interface
will send a FHR, MHR and UA signal to the monitor. If
none of the test FHR, MHR and UA values are
displayed, replace the Interface. If one is missing
check the cable(s) for damage. Replace cable(s) if
necessary
Cables are damaged
Confirm the cables are not damaged. Replace cables if
necessary. From the start screen select the ‘Test’
button and follow the on-screen instructions. The Novii
Interface will send a FHR, MHR and UA signal to the
monitor. If none of the test FHR, MHR and UA values
are displayed, replace the Interface. If one is missing,
check the cable(s) for damage. Replace cable(s) if
necessary
Page 73
73
Maternal/Fetal Monitoring Troubleshooting Table continued
ID
Symptom
Description
Possible Causes
Actions and Solutions
S8
FHR/UA or MHR
data not being
displayed by
Maternal/Fetal
Monitor
POD problems
Make sure the monitoring screen on the Interface is
being displayed and the signal quality, x3 green
squares, and the Battery Icon below the signal quality
is green. If not confirm the status of the two blue lights
on the POD connected to the Patch. If no blue lights
are visible, the POD has switched off. Remove from
Patch and place in a charging bay. Is it recognised by
the Interface (battery status above POD will appear)?
If not, wait 20minutes, if POD is still not recognised
replace POD. If the battery status was orange wait for
battery to charge. When battery status is green take
POD and place in Patch Clip. If electrode check is
good, but the POD switches off again, replace POD.
Was the ‘Bypass’ button on the Interface electrode
check screen used. If yes, remove POD from Patch
and place in charging bay. Wait a few moments for the
POD to be recognised (battery status above POD will
appear). If this does not happen replace the POD. If it
is recognised remove POD and place in Patch. Ensure
that all electrodes pass electrode check.
S9
FHR quality on the
Maternal/Fetal
Monitor trace is
poor in some
patients
Unfortunately this can happen in some
patients especially during stage 2.
Unless it is persistent and occurs on most/all patients it
is not a fault. The user should follow the Alert/Help
message on the Novii interface. If the FHR is
intermittent, the FHR can be confirmed with the
Doppler Transducer connected to US 2, but if the
problem persists then we would advise removing the
Novii POD/Patch and swapping to another monitoring
modality.
Page 74
74
FHR Gaps Troubleshooting Table
For further support visit www.monicahealthcare.com/support
ID
Possible Cause
Action and Solution1
1
During the ‘Electrode Check’ did you bypass a red X
or an orange O on the top or bottom midline
electrode? This could result in FHR gapping.
• Restart the monitoring session to identify which
electrode has O or X
• Peel the electrode back, remove excess gel from
skin. Wait until skin is dry then abrade skin and
reapply electrode.
2
In patients without a pannus the location of mid-line
lower electrode must be vertically above the point
2.4”/6cm horizontally above the symphysis pubis
• Confirm the placement of the midline lower
electrode and re-position
3
Electrode is fully or partially detached from
abdomen especially if the gap occurs after a
shower, clinical procedure or position change e.g.
sitting on chair
• Confirm that all electrodes are attached to the skin
and re-apply if necessary. The Interface should
alarm if an electrode comes detached, but partial
detachment or lifting e.g. skin folding when
bending forward do occur. If necessary use a strip
of micropore tape to prevent electrode lifting or
detachment.
4
Lost FHR, MHR and UA when patient has left room
• Check message on Interface and ask patient to
return to the room.
5
Lost FHR, MHR and UA when patient is in room
• Interface switched off or power supply to Interface
faulty. Bluetooth pairing with POD is lost. Re-start
Interface, remove POD from Patch, dock and start
new monitoring episode. Interface does not have
a battery back-up
6
Mid-line upper and lower electrodes have been
placed over a skin lesion, skin fold, stretch mark,
pronounced linea nigra
• Re-position Patch to avoid the skin problem
7
The mid-line electrode below Patch clip is over the
umbilicus, a skin fold or lesion and is not seated
correctly
• Re-position Patch to avoid the skin problem
8
It is important to wait 10-15 minutes after starting
Novii before commencing ambulation to allow the
electrode gel to penetrate the skin
• Return patient to bed and review causes 1 and 2
above. The patient should not be encouraged to
ambulate unless the FHR trace is good and the
signal indicator on the Novii Interface shows 3
green squares
9
The patient is ambulating
• Return patient to bed; review causes 1, 2 & 3
above
Page 75

75
ID
Possible Cause continued
Action and Solution1
10
Patient position/posture has changed
• If the patient is in bed, the simple use of a pillow
behind their back to make them more comfortable/
relaxed can improve FHR following
• If possible return patient to a position where Novii
worked well
• If possible turn/encourage patient to lie on left
• Review causes 1 and 2
• Has the patient a ‘mobile’ abdomen or pannus? If
yes has the Patch position changed with respect to
the uterus? If you suspect this has happened
consider supporting the abdomen with a pillow,
rolled blanket or support belt to try and re-position
the abdomen so the Patch is over the uterus
11
In a high BMI patient with a pannus that has
displaced the umbilicus and or covers the
symphysis pubis when supine or semi-supine the
lower midline electrode is not optimally placed2
• Remove the lower mid-line electrode and place it
lower on the abdomen positioned, if possible, to just
below the point where the surface curves back on
itself ensuring that the electrode is not folded
12
FHR/MHR/UA artefacts and gaps
• Check Y connector (replace it), or swap POD (look
for dirt or liquid ingress on the POD connector
and/or in Patch clip). Place Interface away from
potentially interfering devices like bar code reader
and Infusion pump.
13
Monitoring stops after a POD swap
• Current monitoring session must be ended by
removing POD from Patch and docking the POD.
Only then can the other POD be removed and
placed in Patch
14
None of the above
• Consider ‘filling’ FHR gaps using the US
transducer
• Remove Novii and swap back to conventional
monitoring modality
Notes:
This troubleshooting guide assumes that the patient is supine or semi-supine during Patch placement
and Novii set-up
1. Remember than any intervention will take 10 seconds before its impact will be seen on the trace
2. The user is familiar with the placement of Patch and lower mid-line electrode in high BMI patients
with a pannus
Page 76

76
Allergic Reaction to Patch
16.1 Overview
When an individual’s skin is exposed to ingredients to which they are allergic, any degree of
inflammation that occurs is clinically known as contact dermatitis. The severity of contact
dermatitis can vary from mild irritation and redness, to rash and even to blistering, depending on
the sensitivity of the skin.
This inflammatory response is the skin’s way of over protecting the rest of the body from the
allergen. An allergen is the substance that has caused the hypersensitive reaction. Almost any
substance can be an allergen for some individual, which is why we can never guarantee against
seeing allergic reactions.
It is worth remembering also, that sensitivity of the skin varies from individual to individual and
even may vary in the same individual from time to time.
Whilst allergic reactions are unpleasant, it is important to realize that they are an inevitable
occurrence as unfortunately, whilst Monica always takes steps to reduce the risk of allergy,
someone at some time will always be sensitive to certain ingredients in the skin contact parts.
16.2 Guidelines
The following are suggestions that have proven in the past to help reduce the occurrence of
contact dermatitis in relation to electrodes.
1. Ask the patient if they suffer from any allergies. It is proven that if individuals suffer from
any allergies, then their risk of developing contact dermatitis increases. But remember,
allergy can occur in any individual at any time.
2. If the answer is “yes” then the nurse needs to remain vigilant especially once an epidural
is given. If there is any concern, peel back electrode 4 (one just below the clip) and
check especially if the monitoring has extended over 12hrs.
3. If a severe allergic reaction has taken place: Review your department’s skin prep regime
and ensure that the skin preparation instructions are being followed.
i. If skin abrasion is too aggressive it can compromise the integrity of the skin, leaving
the individual at an increased risk of developing contact dermatitis.
ii. Monica recommends preparing skin using mild soap and paper towels. This
degreases and exfoliates the skin more gently and allows for a less aggressive
abrasion
4. Finally, inform patients that unfortunately, a few people do react to electrodes, but if they
experience any degree of itching or burning, then alert the nurse so that she can check
the skin condition by peeling back electrode 4, and if necessary remove the Patch at the
earliest opportunity. If individuals are already warned that a reaction may occur, then
Page 77

77
they are far more likely to accept this, and won’t be as upset if there appears to be
redness when the electrodes are removed.
16.3 Treatment
If contact dermatitis has occurred, initially the area should be thoroughly cleansed to remove
any allergen. In most cases, the best treatment is then to do nothing further to the affected area,
as contact dermatitis usually resolves spontaneously over time without complications once the
allergen has been removed.
Topical corticosteroids may reduce inflammation, but medical advice should be sought when
considering any treatment, as overuse of topical corticosteroids can itself bring about problems.
In the severest cases, systemic corticosteroids may need to be prescribed by medical personnel,
but this is extremely rare.
It is important to realize that allergies in general are on the increase, so if you have found a way
to reduce the occurrence in your department, pass on tips to your colleagues and this may this
may help to reduce the number of reactions in the future.
For further information, contact Monica Healthcare or contact your local GE representative.
Page 78

78
Servicing
Servicing of the Novii Interface must be carried out by Monica Healthcare authorized personnel.
Further information is available from your local GE representative or contact Monica Healthcare
below:
Company: Monica Healthcare Limited
Address: Unit 8, Interchange 25 Business Park
Bostocks Lane
Nottingham, NG10 5QG, UK
Phone: (+44) 115 949 6960
E-Mail: novii.info@ge.com
WARNING: No Modification of this equipment is allowed.
Page 79

79
Maintenance & Fault Reporting
18.1 Maintenance
There is no recommended maintenance schedule other than visual inspection for damage. Please
refer to the Troubleshooting Tables, Section 14 and 15 and in the event of device failure, please contact
your local GE service representative.
18.2 Calibration
No calibration is required. Users should use the TEST function to confirm calibration, function
and correct connection / setup of the Novii Interface, whenever the Novii Interface is moved and
connected to a new Maternal/Fetal Monitor.
18.3 Firmware version for Novii Interface and Pod
Periodically there will be a need to release new versions of the Firmware, please check the
Monica web site (www.monicahealthcare.com/support) or your local GE representative to see if
you have the latest version.
18.4 Disposal of Product Waste
As you use the Novii system, you will accumulate solid wastes that require proper disposal or
recycling. These include patient applied parts (Monica Novii Patch), packaging material and the
Monica Novii POD and Interface equipment.
Monica Novii Patch:
The Monica Novii Patch is a patient applied part intended for single use and should be disposed
of properly as medical waste in accordance with regional body controlled guideline.
Packaging material:
Retain original packaging materials for future use in storing or shipping the monitor and
accessories. This recommendation includes corrugated shippers and inserts. Whenever
possible recycle the packaging.
Monica Novii POD and Interface:
At the end of its service life, the Monica Novii Interface or Monica Novii POD, as well as its
accessories, must be disposed of in compliance with the guidelines regulating the disposal of
such products. If you have questions concerning disposal of the product, please contact Monica
Healthcare or its representatives.
CAUTION: The rechargeable battery in the Novii POD cannot be replaced and after 500+
charging cycles the ability to retain a charge will start to degrade. Eventually the
Page 80

80
retained battery charge will make the Novii POD unusable. It is essential that the
Novii POD and its battery are disposed of safely. Please contact Monica Healthcare
as listed in Section Section 17 -
The Disposal authority should contact Monica Healthcare for instructions to separate the battery
from the waste electronics prior to disposal.
Page 81

81
Monica Healthcare Limited
Unit 8, Interchange 25 Business Park, Bostocks Lane,
Nottingham, NG10 5QG, UK
T +44 115 949 6960 / E novii.info@ge.com
www.monicahealthcare.com
 Loading...
Loading...