GE Healthcare 250cx Service Manual

GE Healthcare
Corometrics™ 250cx Series Monitor
Service Manual
Corometrics 250cx Series Monitor
English
2036947-001 Rev. C (paper)
© 2007 General Electric Company.
All Rights Reserved.


GE Healthcare
Corometrics™ 250cx Series Monitor
Service Manual
Corometrics 250cx Series Monitor
English
2036947-001 Rev. C (paper)
© 2007 General Electric Company.
All Rights Reserved.

GUARANTEE
All equipment sold by GE Medical Systems Information Technologies, is fully guaranteed as to
materials and workmanship for a period of 1 year. GE Medical Systems Information
Technologies reserves the right to perform guarantee service operations in its own factory, at
an authorized repair station, or in the customer’s installation.
Our obligation under this guarantee is limited to repairing, or, at our option, replacing any
defective parts of our equipment, except fuses or batteries, without charge, if such defects
occur in normal service.
Claims for damage in shipment should be filed promptly with the transportation company. All
correspondence covering the instrument should specify the model and serial numbers.
GE MEDICAL SYSTEMS Information Technologies
A GE Healthcare Company
GE Medical Systems Information Technologies will make available on request such circuit diagrams, component
diagrams, component parts lists, descriptions, calibration instructions, or other information which will assist the users
or appropriately qualified technical personnel to repair those parts of the equipment which are classified by GE
Medical Systems Information Technologies as repairable. Refer to the 250cx Series Service Manual for further
information.
NOTE: In addition to software version 4.50, the information in this manual also applies to previous software
revisions of Corometrics 250cx Series Monitor. There are no user-apparent differences among these software
versions. Due to continuing product innovation, specifications in this manual are subject to change without notice.
NOTE: For technical documentation purposes, the abbreviation GE is used for the legal entity name, GE Medical
Systems Information Technologies
Ohmeda Oximetry and other trademarks (OxyTip+®, PIr™, TruSat™, TruSignal™, TruTrak+®, SuperSTAT™) are the
property of GE Medical Systems Information Technologies, a division of General Electric Corporation. All other product
and company names are the property of their respective owners.
MASIMO SET
®
is a trademark of Masimo Corporation. Possession or purchase of this device does not convey any
express or implied license to use the device with replacement parts which would, alone, or in combination with this
device, fall within the scope of one or more of the patents relating to the device.
NELLCOR
TAT-5000™, Exergen
®
, OxiMax®, C-LOCK® and SatSeconds™ are trademarks of Nellcor Puritan Bennett.
®
, and TemporalScanner™ are trademarks of Exergen Corporation.
CAUTION: In the United States of America, Federal Law restricts this device to sale
by or on the order of a physician.
Corometrics and Marquette are registered trademarks of GE Medical Systems Information Technologies. GE is a
registered trademark of General Electric Company. All other product and brand names are trademarks or registered
trademarks of their respective companies. ©2005, 2006, 2007 GE Medical Systems Information Technologies. All rights
reserved. No part of this manual may be reproduced without the permission of GE Medical Systems Information
Technologies.
T-2 Corometrics 250cx Series Monitor Revision C
2036947-001 26-Sept-2007

CE
0086
Compliance
Components of the
Certified Systems
A GE brand Corometrics 250cx Series Monitor bears CE mark CE-0086
indicating its conformity with the provisions of the Council Directive
93/42/EEC concerning medical devices and fulfills the essential
requirements of Annex I of this directive.
The device is manufactured in India; the CE mark is applied under the
authority of Notified Body BSI (0086).
The country of manufacture and appropriate Notified Body can be
found on the equipment labeling.
The product complies with the requirements of standard EN 60601-12 “Electromagnetic Compatibility—Medical Electrical Equipment” and
standard EN 60601-1 “General Requirements for Safety.”
The IEC electromagnetic compatibility (EN) standards require
individual equipment (components and accessories) to be configured
as a system for evaluation. For systems that include a number of
different equipment that perform a number of functions, one of each
type of equipment shall be included in the evaluation.
The equipment listed below is representative of all possible
combinations. For individual equipment certification, refer to the
appropriate declarations of conformity.
Component Description
Exceptions
Monitor System EMC:
Immunity Performance
• 250cx Series Maternal/Fetal Monitor
• Model 146 Fetal Acoustic Stimulator
• Intrauterine Pressure Transducer
• FECG Cable/Legplate
• Ultrasound Transducers (x2)
• Blood Pressure Hose and Cuff
• MSpO
• MECG Cable
• FECG/MECG Adapter Cable
• Remote Event Marker
• RS-232C Interconnect Cables (x3)
• Central Nurses Station Interconnect Cable
• Model 2116B Keyboard and Interconnect Cable
• Model 1563AAO Telemetry Cable
•Exergen
None
Be aware that adding accessories or components, or modifying the
medical device or system may degrade the EMI performance. Consult
with qualified personnel regarding changes to the system
configuration.
Interconnect Cable and Sensor
2
®
TAT-5000™
CE- i

CE
0086
CE- ii

Contents
1 Introduction . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
Revision History . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-1
Safety Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-3
Responsibility of the Manufacturer . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
Responsibility of the User . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
References to Persons, Places, and Institutions . . . . . . . . . . . . . . . . . . . . . . . . . .1-3
Hazard Definitions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-4
Product Specific Hazards . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-5
Cautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-8
Electromagnetic Interference . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-9
Equipment Symbols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-10
Service Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 1-11
Equipment ID . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-11
Intended Audience . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-12
Intended Use . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-12
Related Manuals . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .1-13
2 Equipment Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-1
Equipment Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-3
Front Panel Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-4
Front Panel Displays . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-7
Display Example . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
Setup Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
Softkeys . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-8
Mode Title Softkeys . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-8
Waveform Softkeys . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-9
Dedicated Softkey Area . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-9
Rear Panel Description . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-12
Optional Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-14
Software Upgrades . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Adding Spectra Alerts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Adding Fetal Movement Detection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-14
Revision C 250cx Series Maternal/Fetal Monitor i
2036947-001

Peripheral Components . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-14
Nellcor® Puritan Bennett Model N-200 Maternal Pulse Oximeter . . . . . . . . . . . .2-14
Nellcor Puritan Bennett Model N-400 Fetal Pulse Oximeter . . . . . . . . . . . . . . . .2-15
DINAMAP® Models PRO Series 100-400 and ProCare . . . . . . . . . . . . . . . . . . . . . 2-16
ILC-1926 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-16
Centricity Perinatal (QS) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-16
Exergen® TAT-5000™ . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-16
. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-17
GE Healthcare Protocols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-17
250Plus Protocol . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-17
115 and 115X/R protocols . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-17
Theory of Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 2-18
Systems Overview . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .2-18
3 Installation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-1
Tools Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Connections . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-3
Fetal Acoustic Stimulator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-3
Remote Marks Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-3
ECG Out Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-3
J101 Connector (Model 340 Telemetry System Interface) . . . . . . . . . . . . . . . . . .3-4
J109, J110, and J111 Connectors (RS-232C) . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-5
Power . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-6
J112 (External Display Connector) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
Self-Test Routine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-7
Battery-Backed RAM Status . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-8
Setup . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-9
Loading Strip Chart Recorder Paper . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-9
Mounting a Strain Gauge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-12
Setup Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-12
Service Mode Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-12
Service Lock Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-13
Install Options Screens . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-14
Printing System Setup Information . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-20
ii 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

Communications Setup Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-21
Baudrate . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-21
Mode . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .3-21
Configuration Switches . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-22
Factory Defaults . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 3-23
4 Maintenance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-1
Maintenance Schedule . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-3
Visual Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-4
Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-5
Monitor Exterior . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-5
Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-6
Tocotransducer and Ultrasound Transducer . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-6
Leg Plates and MECG Cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-6
Maternal NIBP Cuffs and Hoses . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-7
SpO2 Sensors . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-8
Periodic Thermal Printhead Cleaning . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-8
Cleaning the UA Strain Gauge . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-8
Disposal of Product Waste . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-9
Patient Applied Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-9
Packaging Material . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
Electrical Safety Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-10
Initial Conditions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
AC Line . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
Ground Impedance . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-10
Chassis Leakage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-11
Patient-to-Ground Leakage for MECG/FECG . . . . . . . . . . . . . . . . . . . . . . . . . . .4-11
Patient-to-Line (ISO) Leakage for MECG/FECG . . . . . . . . . . . . . . . . . . . . . . . . .4-11
Patient-to-Ground Leakage for IUP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-12
Patient-to-Line (ISO) Leakage for IUP . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-12
Patient-to-Ground Leakage for US1/US2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-12
Patient-to-Line (ISO) Leakage for US1/US2 . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-13
Patient-to-Ground Leakage for SpO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4 -13
Patient-to-Line Leakage for SpO2 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-13
Dielectric (Hi-Pot) Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-14
Checkout . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-16
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-16
Equipment Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-17
Self-Test Routine . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-17
Revision C 250cx Series Maternal/Fetal Monitor iii
2036947-001

Front Panel Button Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-19
Connecting the Simulator . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-19
MECG Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-20
FECG Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-23
Ultrasound Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-26
Fetal Movement Detection Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-28
Ultrasound Transducer Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-29
Uterine Activity Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-30
Testing the Tocotransducers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-32
Strain Gauge Transducer Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-33
Pattern Memory Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-34
Dual Heart Rate Test (Non-Pattern) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-35
FECG/US Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-35
Dual Ultrasound Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-36
Alarm Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-37
MSpO
Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-39
2
NIBP Calibration and Testing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-40
Purpose . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-40
Required Hardware . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-40
General Calibration Sequence . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-41
Calibration Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-41
Calibrate Transducers . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-41
Overpressure Detection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-42
System Leakage . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-42
Display Check . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-43
Checking a Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-43
Verifying the DSP Board Operation . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-43
External Display . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-44
Maternal SpO
Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-44
2
Hardware Switches . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-44
Main Board SW1 Switch Settings . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-45
J102 Analog Output Connector DAC Static Test . . . . . . . . . . . . . . . . . . . . . . . . . 4-45
iv 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-46
RS-232C Connector Loopback Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-48
Making a Loopback Test Connector . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-48
Testing the Port(s) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-48
Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-50
Before You Begin Electronic Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-50
General . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-50
Handling Precautions . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-50
Power Supply Voltages—Verification . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-50
Main Board Power Supply Voltages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-50
Isolated Power Supply Board Voltages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-51
Isolated FECG/UA Board Voltages . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-51
Recorder Photosensor Calibration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-52
Adjusting the Paper-Low Photosensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-52
Adjusting the Paper-Out Photosensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-52
Adjusting the Paper-Loading Sensor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-53
Repair Log . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-54
Preventative Maintenance Inspection Report . . . . . . . . . . . . . . . . . . . . . . . . . . . . 4-55
Configuration . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-55
Tools Required . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-55
Visual Inspection . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-55
Comments: . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .4-59
5 Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-1
Diagnostic Tests . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Main Motherboard and DSP Board Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-3
Monitor Self-Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Error Log Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-3
Diagnostic Control Screen . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-5
Recorder Calibration Test . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
CPU Version . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
DSP Version . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-6
Run Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-7
Recorder Time . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-7
Recorder Servicing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-7
FAQs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-16
Revision C 250cx Series Maternal/Fetal Monitor v
2036947-001

System Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-37
General Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 5-46
Ultrasound Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-47
FECG Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-47
External Uterine Activity Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-48
Internal UA Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-49
MECG Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-49
Blood Pressure Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-50
Maternal Pulse Oximetry Troubleshooting . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .5-51
6 Parts List, Drawings, and Replacement . . . . . . . . . . . . . 6-1
Ordering Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Service Parts . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-3
Field-Replaceable Units (FRUs) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-4
FRU List . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-4
FRU Main Reference Guide Drawing . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-6
Assembly/Disassembly of FRUs . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 6-8
2025177-003 Speaker . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-8
2025177-037 Main Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-8
2025177-005 DSP Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-9
2025177-006 Main Power Supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-10
2025177-007 Dual Ultrasound Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-10
2025177-008 FECG/UA Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-11
2025177-009 Isolated Power Supply Board . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-12
2025177-010 SpO2 Carrier Board with Nellcor MSpO2 Module . . . . . . . . . . . . .6-12
2025177-011 SpO2 Carrier Board with Masimo MSpO2 Module . . . . . . . . . . . .6-13
2025177-012 SpO2 Carrier Board with TruSignal MSpO2 Module . . . . . . . . . . .6-13
2025177-013 Front-end Motherboard . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-14
2025177-036 Chassis . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-15
2025177-016 COMM Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-18
2025177-017 Recorder Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-18
2025177-018 Recorder Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-20
2025177-019 Cables . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-21
2025177-020 Pneumatics Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-22
2025177-021 Display Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-23
2025177-022 Front Bezel . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-24
2025177-023 Keypads . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-26
2025177-026 Trim Knob and Encoder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-27
2025177-027 Power Switch Assembly . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-28
2025177-028 Main Power Supply . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-29
2025177-029 MECG Board . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-30
2025177-031 Top Cover Gasket . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .6-31
vi 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

A Technical Specifications . . . . . . . . . . . . . . . . . . . . . . . . . .A-1
General Monitor . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-3
Operating Modes . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-4
Strip Chart Recorder . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . A-11
B Alarms Summary . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .B-1
C Electromagnetic Compatibility . . . . . . . . . . . . . . . . . . . . .C-1
Electromagnetic Compatibility (EMC) . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . C-3
Guidance and Manufacturer’s Declaration – Electromagnetic Emissions . . . . . . .C-3
Guidance and Manufacturer’s Declaration – Electromagnetic Immunity . . . . . . . .C-4
Recommended Separation Distances . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-6
Compliant Cables and Accessories . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .C-7
Revision C 250cx Series Maternal/Fetal Monitor vii
2036947-001

viii 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

1 Introduction
Revision History
Each page of this manual has a revision l etter located at the bottom of the page. This
letter identifies the revision level of the entire manual. This may be important if you
have different manuals and you do not know which is the most current.
For the initial release, all pages have the revision letter A. For the second update, all
pages receive the revision letter B. The latest letter of the alphabet added to the table
below corresponds to the most current revision.
Revision Date Comment
A 23 July, 2007 Initial release
B 26 July, 2007 Change selected FRU nos. Modify selected
wording.
C 16 September, 2007
Add Exergen
®
TAT-5000™
Revision C 250cx Series Maternal/Fetal Monitor 1-1
2036947-001

For your notes
1-2 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

Introduction: Safety Information
Safety Information
The information presented in this section is important for the safety of both the
patient and operator. This chapter describes how the terms Danger, Warning,
Caution, Important, and Note are used throughout the manual. In addition, standard
equipment symbols are defined.
Responsibility of the Manufacturer
GE is responsible for the effects on safety, reliability, and performance if:
assembly operations, extensions, readjustments, modifications, or
repairs are carried out by persons authorized by GE;
the electrical installation of the relevant room complies with the
requirements of appropriate regulations; and
the monitor is used in accordance with the instructions of use.
Responsibility of the User
This device is intended for use by clinical professionals who are expected to know
the medical procedures, practices, and terminology required to monitor obstetrical
patients. This manual documents all possible parameters available in the 250cx
Series of monitors. It is the responsibility of each hospital to ensure that the Labor
and Delivery staff is trained in all aspects of the selected model.
The 250cx Series Monitor is designed to assist the perinatal staff by providing
information regarding the clinical status of the mother and fetus during labor. Th e
monitor does not replace observation and evaluation of the mother and fetus at
regular intervals, by a qualified care provider, who will make diagnoses and decide
on treatments or interventions. Visual assessment of the monitor display and strip
chart must be combined with knowledge of patient history and risk factors to
properly care for the mother and fetus.
References to Persons, Places, and Institutions
References to persons, places, and institutions used within this manual are solely
intended to facilitate user comprehension of the 250cx Series Monitor’s use and
functions. Extreme care has been taken to use fictitious names and related
information in the examples and illustrations provided herein. Any similarity of this
data to persons either living or dead and to either current or previously existing
medical institutions should be regarded as coincidental.
Revision C 250cx Series Maternal/Fetal Monitor 1-3
2036947-001
Hazard Definitions
Introduction: Safety Information
Six types of special notices are used throughout this manual. They are: Danger,
Warning, Caution, Contraindication, Important, and Note. The warnings and
cautions in this Safety section relate to the equipment in general and apply to all
aspects of the monitor. Be sure to read the other chapters because there are
additional warnings and cautions which relate to specific features of the monitor.
When grouped, warnings and cautions are listed alphabetically and do not imply any
order of importance.
Definitions of Terminology
Danger A DANGER notice indicates an imminently
hazardous situation which, if not avoided, will result
in death or serious injury.
Warning A WARNING indicates a potentially hazardous
situation which, if not avoided, could result in death
or serious injury.
Caution A CAUTION indicates a potentially hazardous
situation which, if not avoided, may result in minor
or moderate injury. Cautions are also used to
avoid damage to equipment.
Contraindication A CONTRAINDICATION describes any special
symptom or circumstance that renders the use of a
remedy or the carrying out of a procedure
inadvisable, usually because of a risk.
Important An IMPORTANT notice indicates an emphasized
note. It is something you should be particularly
aware of; something not readily apparent.
Note A NOTE indicates a particular point of information;
something on which to focus your attention.
1-4 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

Product Specific Hazards
Introduction: Safety Information
WARNINGS
ACCIDENTAL SPILLS—In the event that fluids are accidentally
spilled on the monitor, take the monitor out of operation and
inspect for damage.
APPLICATION—This monitor is not designed for direct cardiac
connection.
CONDUCTIVE CONNECTIONS—Avoid making any
conductive connections to applied parts (patient connection)
which are likely to degrade safety.
CONDUCTIVE PARTS—Ensure that the conductive parts of the
lead electrodes and associated connectors do not contact other
conductive parts including earth.
CONNECTIONS—The correct way to connect a patient to the
monitor is to plug the electrode leads into the patient cable which
in turn connects to the monitor. The monitor is connected to the
wall socket by the power cord. Do not plug the electrode leads
into the power cord, a wall socket, or an extension cord.
DEFIBRILLATION—During defibrillation, all personnel must
avoid contact with the patient and monitor to avoid a dangerous
shock hazard. In addition, proper placement of the paddles in
relation to the electrodes is required to minimize harm to the
patient.
DEFIBRILLATION PROTECTION—When used with the GE
Medical Systems Information Technologies-recommended
accessories, the monitor is protected against the effects of
defibrillator discharge. If monitoring is disrupted by the
defibrillation, the monitor will recover.
ELECTRICAL SHOCK—To reduce the risk of electrical shock,
do not remove monitor cover. Refer servicing to qualified
personnel.
ELECTROMAGNETIC INTERFERENCE—Be aware that
strong electromagnetic fields may interfere with monitor
operation. Interference prevents the clear reception of signals by
the monitor. If the hospital is close to a strong transmitter such as
TV, AM or FM radio, police or fire stations, a HAM radio
operator, an airport, or cellular phone, their signals could be
picked up as monitor signals. If you feel interference is affecting
the monitor, contact your Service Representative to check the
monitor in your environment. Refer to Electromagnetic
Interference on p. 1-7 for additional information.
Revision C 250cx Series Maternal/Fetal Monitor 1-5
2036947-001

Introduction: Safety Information
WARNINGS
ELECTROSURGERY—The monitor is not designed for use with
high-frequency surgical devices. In addition, measurements may
be affected in the presence of strong electromagnetic sources such
as electrosurgery equipment.
EXPLOSION HAZARD—Do not use this equipment in the
presence of flammable anesthetics or inside an oxygen tent.
GROUNDING—Do not defeat the three-wire grounding feature
of the power cord by means of adaptors, plug modifications, or
other methods. A dangerous shock hazard to both patient and
operator may result.
INOPERABLE MECG—The MECG trace is not visible during a
LEADS OFF condition or an overload (saturation) of the frontend amplifier during differential input voltage of more than
±300mV.
INSTRUCTIONS—For continued and safe use of this equipment,
it is necessary to follow all listed instructions. However, the
instructions provided in this manual in no way supersede
established medical procedures concerning patient care. The
monitor does not replace observation and evaluation of the
patient, at regular intervals, by a qualified care provider who will
make diagnoses and decide on treatments and interventions.
INTERFACING OTHER EQUIPMENT—Monitoring equipment
must be interfaced with other types of medical equipment by
qualified biomedical engineering personnel. Be certain to consult
manufacturers’ specifications to maintain safe operation.
LEAKAGE CURRENT TEST—The interconnection of auxiliary
equipment with this device may increase the total leakage current.
When interfacing with other equipment, a test for leakage current
must be performed by qualified biomedical engineering personnel
before using with patients. Serious injury or death could result if
the leakage current exceeds applicable standards. The use of
accessory equipment not complying with the equivalent safety
requirements of this equipment may lead to a reduced level of
safety of the resulting system. Consideration relating to the choice
shall include: use of the accessory in the patient vicinity; and
evidence that the safety certification of the accessory has been
performed in accordance with the appropriate EN60601.1 and/or
EN60601.1.1 harmonized national standard.
1-6 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

Introduction: Safety Information
WARNINGS
LINE ISOLATION MONITOR TRANSIENTS—Line isolation
monitor transients may resemble actual cardiac waveforms, and
thus cause incorrect heart rate determinations and alarm activation
(or inhibition).
MRI USE—Do not use the electrodes during MRI scanning;
conducted current could potentially cause burns.
PATIENT CABLES AND LEADWIRES—Do not use patient
cables and electrode leads that permit direct connection to
electrical sources. Use only “safety” cables and leadwires. Use of
non-safety patient cables and leadwires creates risk of
inappropriate electrical connection which may cause patient shock
or death.
PACEMAKER PATIENTS—Rate meters may continue to count
the pacemaker rate during occurrences of cardiac arrest or some
arrhythmias. Do not rely entirely upon rate meter alarms. Keep
pacemaker patients under close surveillance. Refer to “Appendix
A, T echnical Specifications” for disclosure of the pacemaker pulse
rejection capability of the 250cx Series Monitor.
RF INTERF ACE—Known RF sources, such as cell phones, radio
or TV stations, and two-way radios, may cause unexpected or
adverse operation of this device.
SIMULTANEOUS DEVICES—Do not simultaneously connect
more than one device that uses electrodes to detect ECG and/or
respiration to the same patient. Use of more than one device in this
manner may cause improper operation of one or more of the
devices.
STRANGULATION—Make sure all patient cables, leadwires, and
tubing are positioned away from the patient’s head to minimize the
risk of accidental strangulation.
WATER BIRTHS—Do not use the monitor to directly monitor
patients during water births, in whirlpool or submersion water
baths, during showers, or in any other situation where the mother
is immersed in water. Doing so may result in electrical shock
hazard.
EXTERNAL VGA CONNECTIONS—Connect only to GE
recommended display. ONLY remove cover plate if external
display is used.
TELEMETRY CONNECTIONS—Connect only to GE
recommended telemetry system. Contact your GE service
representative for more information.
COLOR DISPLA Y—Certain colors may have limited visibility at
a distance. Color-blind individuals may experience this more often
Revision C 250cx Series Maternal/Fetal Monitor 1-7
2036947-001
.

Cautions
Introduction: Safety Information
EXERGEN® TAT-5000™ —Cable assembly 2036641-001 cannot
be field serviced. Do
This assembly must be returned to the factory for any repairs. This
assembly, as shipped, is important to patient safety.
DISPOSAL—This product consists of devices that may contain
mercury, which must be recycled or disposed of in accordance
with local, state, or country laws. (Within this system, the
backlight lamps in the monitor contain mercury.)
CAUTIONS
ANNUAL SERVICING—For continued safety and performance of the
monitor, verify the calibration, accuracy, and electrical safety of the
monitor annually. Contact your GE Medical Systems Information
Technologies Service Representative.
DAILY TESTING—It is essential that the monitor and accessories be
inspected every day. It is recommended practice to initiate the monitor’s selftest feature at the beginning of each monitoring session; follow the
instructions in “Chapter 5, Setup Procedures”.
NOT
attempt any repairs to this assembly.
ENVIRONMENT—The performance of the monitor has not been tested in
certain areas, such as x-ray and imaging suites. The monitor is not
recommended for use in these environments.
EQUIPMENT CONFIGURATION—The equipment or system
should not be used adjacent to, or stacked with, other equipment. If
adjacent or stacked use is necessary, the equipment or system
should be tested to verify normal op eratio n in the configuration in
which it is being used.
PERFORMANCE—Report all problems experienced with the monitor. If
the monitor is not working properly, contact your Service Representative
for service. The monitor should not be used if it is not working properly.
PINCHING—Keep fingers clear of the paper roller because the
roller could pinch your fingers.
STATIC ELECTRICITY—This assembly is extremely static
sensitive and should be handled using electrostatic discharge
precautions.
TRAPPING—Keep hands, hair, jewelry, and loose clothing away from the
paper roller because the roller could trap these items.
TRIPPING—Arrange monitoring equipment so that cords and
cables do not present a tripping hazard.
1-8 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

Introduction: Electromagnetic Interference
Electromagnetic Interference
This device has been tested and found to comply with the limits for medical devices to
the IEC 601-1-2:1993, EN60601-1-2:2001, Medical Device Directive 93/42/EEC.
These limits are designed to provide reasonable protection against harmful interference
in a typical medical installation.
However, because of the proliferation of radio-frequency transmitting equipment
and other sources of electrical noise in the health-care and home environments (for
example, cellular phones, mobile two-way radios, electrical appliances), it is
possible that high levels of such interference due to close proximity or strength of a
source, may result in disruption of performance of this device.
Refer to the Electromagnetic Immunity inf ormation in this product’s service manual
for EN 60601-1-2 (2001) Edition 2 compliance information and safety information
for this product.
This equipment generates, uses, and can radiate radio frequency energy and, if not
installed and used in accordance with these instructions, may cause harmful
interference with other devices in the vicinity. Disruption or interference may be
evidences by erratic readings, cessation of operation, or incorrect functioning. If this
occurs, the site of use should be surveyed to determine the source of this disruption,
and actions taken to eliminate the source.
The user is encouraged to try to correct the interference by one or more of the
following measures:
Turn equipment in the vicinity off and on to isolate the offending equipment.
Reorient or relocate the other receiving device.
Increase the separation between the interfering equipment and this equipment.
If assistance is required, contact your GE Medical Systems
Information Technologies Service Representative.
Revision C 250cx Series Maternal/Fetal Monitor 1-9
2036947-001
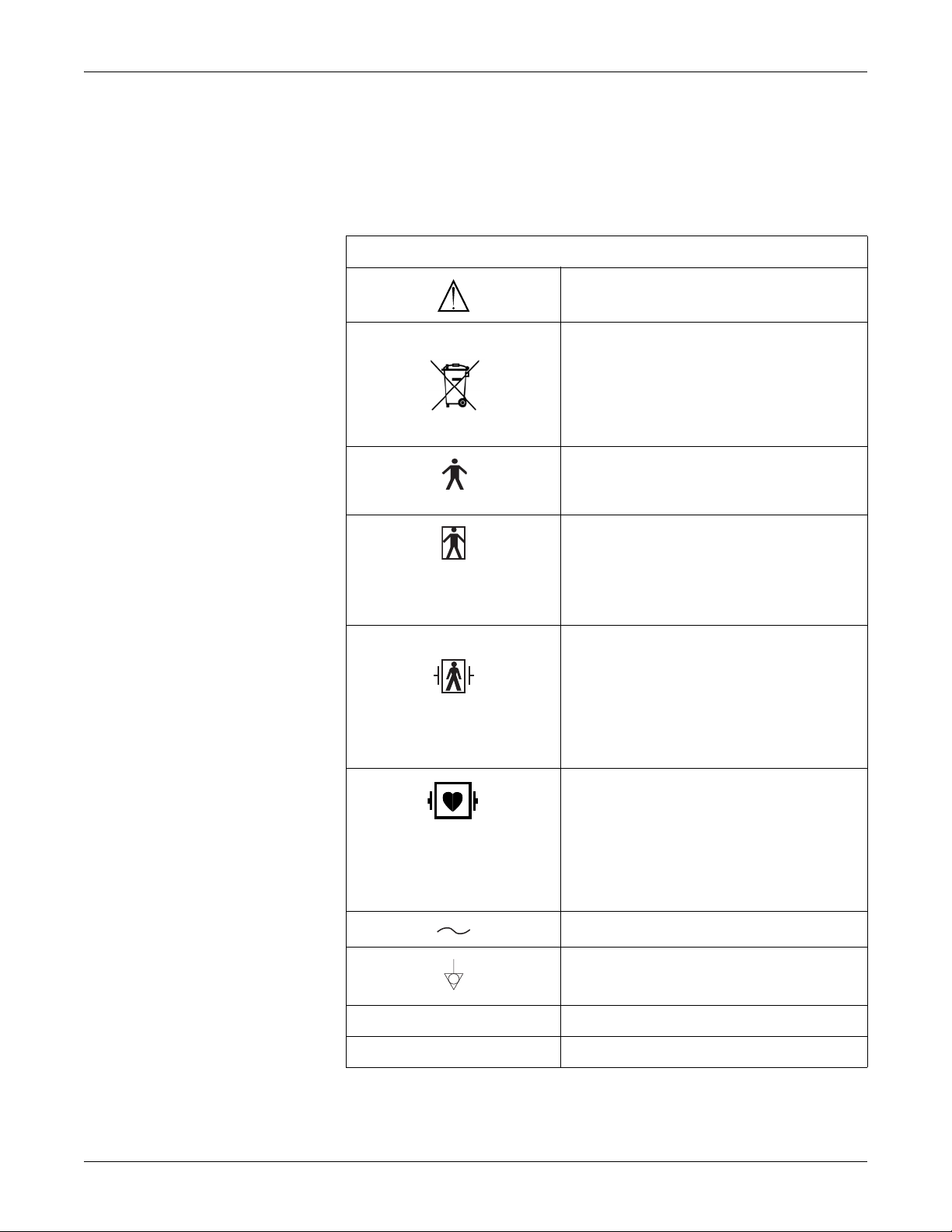
Equipment Symbols
The following is a list of symbols used on products manufactured by GE. Some
symbols may not appear on your unit.
Introduction: Equipment Symbols
Equipment Symbols
ATTENTION: Consult accompanying documents.
This symbol indicates that the waste of electrical
and electronic equipment must not be disposed as
unsorted municipal waste and must be collected
separately. Please contact the manufacturer or
other authorized disposal company to
decommission your equipment.
TYPE B EQUIPMENT. Type B equipment is suitable
for intentional external and internal application to the
patient, excluding direct cardiac application.
TYPE BF EQUIPMENT. Type BF equipment is
suitable for intentional external and internal
application to the patient, excluding direct cardiac
application. Type BF equipment has an F-type
applied part.
DEFIBRILLATOR-PROOF TYPE BF EQUIPMENT:
Type BF equipment is suitable for intentional
external and internal application to the patient,
excluding direct cardiac application. Type BF
equipment is type B equipment with an F-type
isolated (floating) part. The paddles indicate the
equipment is defibrillator proof.
TYPE CF EQUIPMENT. Type CF equipment is
suitable for intentional external and internal
application to the patient including direct cardiac
application. Type CF equipment is F-type applied
part that provides a higher degree of protection
against electric shock than that provided by Type
BF applied parts.
ALTERNATING CURRENT (AC).
EQUIPOTENTIALITY.
O POWER OFF: disconnection from the mains.
I POWER ON: connection to the mains.
1-10 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

Introduction: Service Requirements
Service Requirements
Follow the service requirements listed below.
Refer equipment servicing to GE Medical Systems Information
Technologies authorized service personnel only.
Any unauthorized attempt to repair equipment under warranty voids
that warranty.
It is the user’s responsibility to report the need for service to GE
Medical Systems Information Technologies or to one of GE’s
authorized agents.
Failure on the part of the responsible individual, hospital or
institution using this equipment to implement a satisfactory
maintenance schedule may cause undue equipment failure and
possible health hazards.
Regular maintenance, irrespective of usage, is essential to ensure
that the equipment will always be functional when required.
Equipment ID
The following graphic illustrates the components of the monitor’s serial number.
GEMS IT Global Serial Number Format
13- Digit
# # # # # # # # # # # # #
Manufacturing site
Sequential serial number (up to 9999)
Fiscal week
Year
Misc. : Prototype, refurbish, etc.
3-character product code
Global Serial Number Format
Revision C 250cx Series Maternal/Fetal Monitor 1-11
2036947-001

Intended Audience
Intended Use
General Use
Introduction: Service Requirements
This manual is intended for trained service professionals.
If the monitor is cold to the touch or below ambient temperature, allow it to reach
ambient, room temperature before use.
To ensure patient safety, use only parts and accessories manufactured or
recommended by GE Medical Systems Information Technologies. Parts and
accessories used shall meet the requirements of EN60601.1.1.
Disposable devices are intended for single use only. They should not be reused.
Periodically, and whenever the integrity of the monitor is in doubt, test all functions.
Refer to “Checkout” on page 4-16.
Fetal Monitoring
Maternal Monitoring
Blood Pressure
Refer to the “Maternal/Fetal Monitoring, Clinical Applications Manual” for
information concerning the limitations of internal and external feta l heart rate
monitoring techniques.
A Corometrics 250cx Series Monitor can be used for routine non-invasive and
invasive fetal monitoring throughout labor and delivery (i.e., fetal heart rate and
uterine activity monitoring). Fetal movement detection and Spectra Alerts are
options that may be purchased.
A Corometrics 250cx Series Maternal/Fetal Monitor is intended for monitoring
maternal vital signs to help assess maternal well-being. The vital signs which can be
measured with either of these monitors are summarized below.
This parameter is intended for use in the non-invasive monitoring of maternal blood
pressure (NIBP). This monitor is not intended for use in neonatal or pediatric blood
pressure monitoring.
Pulse Oximetry
This parameter is intended for use in the non-invasive monitoring of the functional
oxygen saturation of maternal arterial blood (MSpO
1-12 250cx Series Maternal/Fetal Monitor Revision C
2036947-001
).
2

Heart/Pulse Rate
Related Manuals
2020550-001 250cx Series Operator’s Manual
15457 Maternal/Fetal Monitoring, Clinical Application
2004435-001 Information For Prescribers
Introduction: Service Requirements
This parameter is intended for use in the non-invasive monitoring of the maternal
heart/pulse rate (MHR/P).
Manual Title
Revision C 250cx Series Maternal/Fetal Monitor 1-13
2036947-001

Introduction: Service Requirements
1-14 250cx Series Maternal/Fetal Monitor Revision C
2036947-001

2 Equipment Overview
Revision C 250cx Series Maternal/Fetal Monitor 2-1
2036947-001

For your notes
2-2 250cx Series Maternal/Fetal Monitor Revision C
2036947-001
 Loading...
Loading...