GC EUROPE Unifil Bond User Manual
Technical Manual
G
G
C
C
U
U
nii
n
Fiill
F
B
B
o
o
n
n
d
d
GC UniFil Bond Technical manual v ersion 1.10, November 2005, 1/23

Table of contents
1.0 Introduction ………………………………………………………………………. 3
2.0 Currently available adhesion concepts ……………………….. 3
3.0 Composition ………………………………………………………………………. 5
Initiators used ……………………………………………………………………… 5
Silica ……………………………………………………………………………………… 6
4.0 Application …………………………………………………………………………. 6
Self-Etching Primer …………………………………………………………….. 6
Bonding Agent .……………………………………………………………………. 6
5.0 Adhesion mechanism ………………………………………………………. 7
Self-etching primer / 4 – MET …………………………………………….. 7
Bonding agent ………………………………………………………………………. 8
6.0 Test results …………………………………………………………………………. 9
IR analysis ……………………………………………………………………………… 9
XPS analysis ………………………………………………………………………….. 9
Tensile Bond strength ……………………………………………………………. 10
Consequence of technique variables ……………………………………. 11
Consequence of variables in tooth structures ……………………… 12
7.0 Clinical investigations ………………………………………………………. 14
8.0 Literature …………………………………………………………………………….. 15
9.0 Packaging ……………………………………………………………………………. 21
10.0 Instructions for use …………………………………………………………… 22
GC UniFil Bond Technical manual v ersion 1.10, November 2005, 2/23

1.0 Introduction
The development of GC UniFil Bond is the result of GC’s commitment to
understanding and seeking solutions to the variable success parameters currently
restricting certain indications for composite resin restoratives.
While long-term success has been achieved with composite resin bonded to all
enamel surfaces, the same cannot be said for bonded composite where margins are
in dentine. Composite restoration failure often occurs at the interface between the
composite and dentine leading to staining, microbial leakage and progression of
caries.
Improvements therefore focused on solutions to the problems experienced at this
interface. The many causes of interface failure include application factors (variable
placement techniques, over etching, over drying, fluid contamination, surface
contaminants like excess acetone), site factors (variable dentin structure i.e. tubule
orientation, degree of mineralization) and stress factors (occlusal loading, tooth
flexure, polymerization shrinkage stress and variable coefficients of thermal
expansion).
GC UniFil Bond was developed seeking to provide solutions to many of these
problems and focused on three primary objectives:
• Provide strong adhesion via simple application procedures with minimal
opportunity for compromised results caused by technique variation
• Provide strong adhesion to a wide variety of dentine surfaces
Maintain an effective seal by utilizing both micromechan ical int erlocking and chemical
adhesion.
2.0 Currently available adhesion concepts
At present several adhesion concepts are considered for bonding to tooth surfaces.
The adhesion mechanisms are divided into three major categories
- micromechanical interlocking
- chemical (ion-exchange) adhesion
- combination of above two
• Micromechanical interlocking is achieved by etching both enamel and dentine
surfaces followed by application of resin bonding systems that contain functional
monomers with both hydrophilic and hydrophobic groups. These monomers are
able to penetrate and diffuse throughout the etched dentine surface layer to form
a hybrid zone that adheres to the dentin surface. Strong micromechanical
interlocking to enamel is also achieved where the resin penetrates the
irregularities in the etched enamel surface forming micro resin tags.
GC UniFil Bond Technical manual v ersion 1.10, November 2005, 3/23

Mechanical retention due to resin tag formation
• Chemical adhesion is the main mechanism by which glass ionomer cements are
bonded to dentine and enamel surfaces. The carboxyl group (COOH) of
polyalkenoic acids (the liquid component of glass ionomer cement ) is ionized by
reaction with powder into carboxylic acid ions (COO
strong ionic bonds with calcium ions (Ca
2+
) in the tooth apatite. The adhesion is
-
). These ions have extremely
so strong that laboratory testing often shows cohesive failure within the cement
rather than adhesive failure at the interface. Class V retention studies have
shown that long term stability of this adhesion is clinically relevant.
Chemical adhesion of glass ionomers to tooth structure
• A combined chemical adhesion / mechanical interlocking can be observed by
using resin reinforced glass ionomers in combination with the appropriate
conditioners. These resin reinforced glass ionomer cements have grown quickly in
popularity as highly successful adhesive materials for crown and bridge
cementation (GC Fuji PLUS), composite lining and bonding (GC Fuji Bond LC ) and
for orthodontic bonding of brackets and bands (GC Fuji ORTHO LC).
GC Fuji BOND LC GC Fuji PLUS GC Fuji BOND LC
Interface with dentine Interface with dentine Interface with dentine
GC UniFil Bond represents the result of GC’s application of glass ionomer adhesion
concepts into an advanced, user-friendly, resin bonding system.
GC UniFil Bond Technical manual v ersion 1.10, November 2005, 4/23

3.0 Composition
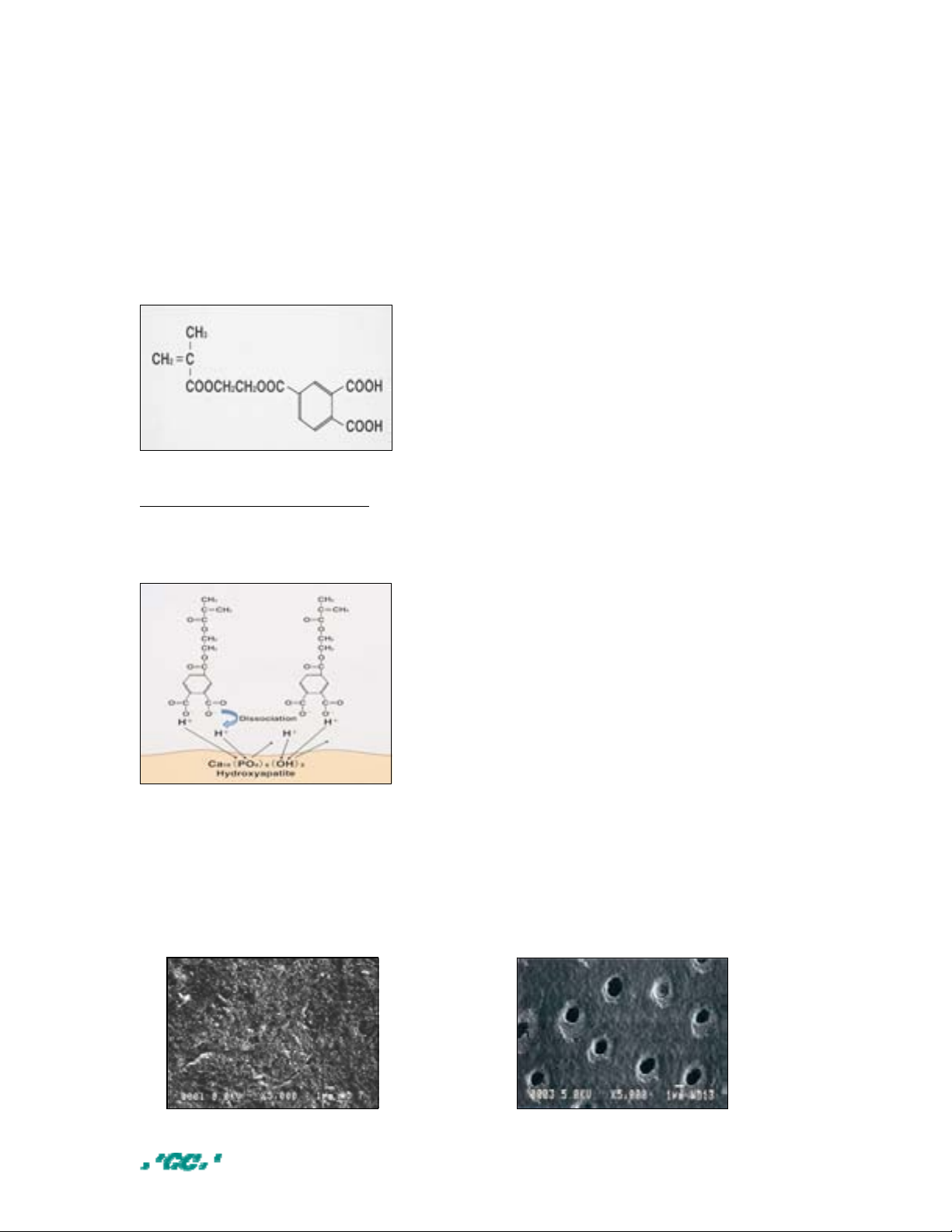
SELF-ETCHING PRIMER WT%
4-Methacryloxyethyl trimellitic acid
(4-MET)
Ethanol 48
Distilled water 40
HEMA 2
Initiator Trace
BONDING AGENT WT%
UDMA 50
TEGDMA 30
HEMA 16
Silica Filler 4
Initiator Trace
Initiators used
In GC UniFil Bond, a combination of camphorquinone and amine is used as the
catalyst. Light activation can be carried out with quartz halogen, plasma or LED
curing units.
To reinforce the outer enamel surface and the collagen network with a firm and
stable adhesive resin layer it is crucial that monomers from both the primer and
bonding agent are converted from a liquid into a solid state. When the initiators, as
in GC UniFil Bond, are contained in both the primer and bonding agent solut ions, the
co-polymerization of available monomers occurs more efficiently than when
contained only in either one of the solutions. Although in the latter case the initiator
might diffuse through both the primer and bonding agent immediately after coming
into contact with each other, it is preferable that both liquids include the initiator
separately.
10
GC UniFil Bond Technical manual v ersion 1.10, November 2005, 5/23
Silica
Colloidal silica is added to control the flow of the bonding agent. This will ensure that
the operator has a better control during the application.
In addition to this practical advantage, a slight increase in the modulus of elasticity
could be obtained – 2.8 (0.1) GPa with silica, versus 2.6 (0.1) GPa without.
4.0 Application
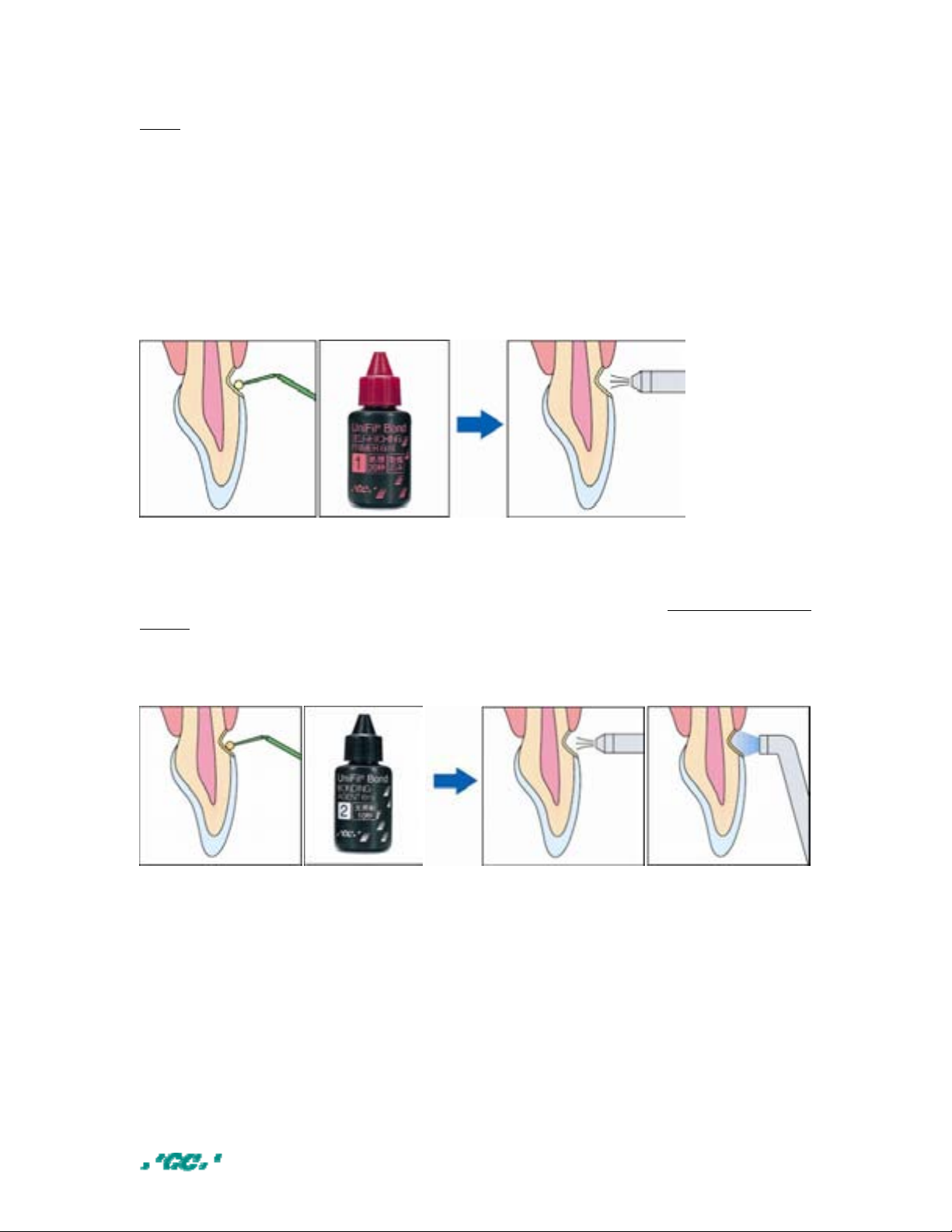
• Self – etching primer
Apply GC UniFil Bond self-etching primer to the dentine and enamel surfaces and
leave undisturbed for 20 seconds. Then gently dry with an air syringe for 5 seconds
and ensure that the primed surface has a glossy appearance. Do not rinse with
water.
• Bonding agent
GC UniFil Bond bonding agent is immediately applied to the primed enamel and
dentine surfaces. Then gently dry with an air syrin ge to form a thin film and light
cure for 10 seconds. GC GRADIA DIRECT composite can now be applied to the
treated surface.
GC UniFil Bond Technical manual v ersion 1.10, November 2005, 6/23
5.0 Adhesion mechanism
At the heart of GC UniFil Bond’s performance is the 4-MET molecule in the primer
solution.
4-MET is derived from 4-META by hydrolysis during the manufacturing process and
compared to the latter is more hydrophilic and acidic. The functional monomer 4MET is characterized by self etching and adhesive properties and inherently has very
good diffusion properties.
4-MET
Self-etching primer / 4 - MET
As can be seen from the figure below, the carboxyl group (COOH) of 4-MET can
dissociate into hydrogen (H
+
) and carboxylic (COO-) ions.
Dissociation of 4-MET when applied to tooth surfaces
This dissociation will result in both (self) etching of tooth surfaces and chemical
adhesion to calcium.
• Self-etching properties
Both enamel and dentine surfaces will be decalcified due to the freely availabl e
hydrogen ions and consequently dissolution of the outer surfaces (≤1µm) of the
hydroxyapatite will take place. The pH of GC UniFil Bond Primer is 2.0.
GC UniFil Bond Technical manual v ersion 1.10, November 2005, 7/23
 Loading...
Loading...