Page 1

BP Pump 2
NIBP Simulator and Tester
Operators Manual
PN 2196592
June 2007
© 2007 Fluke Corporation, All rights reserved. Printed in USA
All product names are trademarks of their respective companies.
Page 2

Warranty and Product Support
Fluke Biomedical warrants this instrument against defects in materials and
workmanship for one year from the date of original purchase. During the warranty period, we will repair or at our option replace, at no charge, a product
that proves to be defective, provided you return the product, shipping prepaid,
to Fluke Biomedical. This warranty covers the original purchaser only and is
not transferable. The warranty does not apply if the product has been damaged
by accident or misuse or has been serviced or modified by anyone other than
an authorized Fluke Biomedical service facility. NO OTHER WARRANTIES,
SUCH AS FITNESS FOR A PARTICULAR PURPOSE, ARE EXPRESSED
OR IMPLIED. FLUKE SHALL NOT BE LIABLE FOR ANY SPECIAL,
INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES OR
LOSSES, INCLUDING LOSS OF DATA, ARISING FROM ANY CAUSE
OR THEORY.
This warranty covers only serialized products and their accessory items that
bear a distinct serial number tag. Recalibration of instruments is not covered
under the warranty
This warranty gives you specific legal rights and you may also have other
rights that vary in different jurisdictions. Since some jurisdictions do not allow
the exclusion or limitation of an implied warranty or of incidental or consequential damages, this limitation of liability may not apply to you. If any provision of this warranty is held invalid or unenforceable by a court or other decision-maker of competent jurisdiction, such holding will not affect the validity
or enforceability of any other provision.
07/07
Page 3
Notices
All Rights Reserved
© Copyright 2007, Fluke Biomedical. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into any language without the written
permission of Fluke Biomedical.
Copyright Release
Fluke Biomedical agrees to a limited copyright release that allows you to reproduce manuals and
other printed materials for use in service training programs and other technical publications. If
you would like other reproductions or distributions, submit a written request to Fluke Biomedical.
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for
damage. If damage is found, stop unpacking the instrument. Notify the carrier and ask for an
agent to be present while the instrument is unpacked. There are no special unpacking instructions,
but be careful not to damage the instrument when unpacking it. Inspect the instrument for physical damage such as bent or broken parts, dents, or scratches.
Technical Support
For application support or answers to technical questions, either email
techservices@flukebiomedical.com
1-425-446-6945.
Claims
Our routine method of shipment is via common carrier, FOB origin. Upon delivery, if physical
damage is found, retain all packing materials in their original condition and contact the carrier
immediately to file a claim. If the instrument is delivered in good physical condition but does not
operate within specifications, or if there are any other problems not caused by shipping damage,
please contact Fluke Biomedical or your local sales representative.
or call 1-800- 648-7952 or
Standard Terms and Conditions
Refunds and Credits
Please note that only serialized products and their accessory items (i.e., products and
items bearing a distinct serial number tag) are eligible for partial refund and/or credit.
Nonserialized parts and accessory items (e.g., cables, carrying cases, auxiliary modules,
etc.) are not eligible for return or refund. Only products returned within 90 days from the date
of original purchase are eligible for refund/credit. In order to receive a partial refund/credit of a
product purchase price on a serialized product, the product must not have been damaged by the
customer or by the carrier chosen by the customer to return the goods, and the product must be
returned complete (meaning with all manuals, cables, accessories, etc.) and in “as new” and resalable condition. Products not returned within 90 days of purchase, or products which are not in
“as new” and resalable condition, are not eligible for credit return and will be returned to the customer. The Return Procedure (see below) must be followed to assure prompt refund/credit.
Restocking Charges
Products returned within 30 days of original purchase are subject to a minimum restocking fee of
15 %. Products returned in excess of 30 days after purchase, but prior to 90 days, are subject to a
minimum restocking fee of 20 %. Additional charges for damage and/or missing parts and accessories will be applied to all returns.
Page 4
Return Procedure
All items being returned (including all warranty-claim shipments) must be sent freight-prepaid to
our factory location. When you return an instrument to Fluke Biomedical, we recommend using
United Parcel Service, Federal Express, or Air Parcel Post. We also recommend that you insure
your shipment for its actual replacement cost. Fluke Biomedical will not be responsible for lost
shipments or instruments that are received in damaged condition due to improper packaging or
handling.
Use the original carton and packaging material for shipment. If they are not available, we recommend the following guide for repackaging:
Use a double-walled carton of sufficient strength for the weight being shipped.
Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive
material around all projecting parts.
Use at least four inches of tightly packed, industry-approved, shock-absorbent
Returns for partial refund/credit:
Every product returned for refund/credit must be accompanied by a Return Material Authorization (RMA) number, obtained from our Order Entry Group at 1-800-648-7952 or 1-425-446-
6945.
Repair and calibration:
To find the nearest service center, go to www.flukebiomedical.com/service
In the U.S.A.:
Cleveland Calibration Lab
Tel: 1-800-850-4606
Email: globalcal@flukebiomedical.com
Everett Calibration Lab
Tel: 1-888-993-5853
Email: service.status@fluke.com
In Europe, Middle East, and Africa:
Eindhoven Calibration Lab
Tel: +31-402-675300
Email: ServiceDesk@fluke.com
In Asia:
Everett Calibration Lab
Tel: +425-446-6945
mail : se rvice.international@fluke.com
E
material around the instrument.
, or
Certification
This instrument was thoroughly tested and inspected. It was found to meet Fluke Biomedical’s
manufacturing specifications when it was shipped from the factory. Calibration measurements
are traceable to the National Institute of Standards and Technology (NIST). Devices for which
there are no NIST calibration standards are measured against in-house performance standards using accepted test procedures.
WARNING
Unauthorized user modifications or application beyond the published specifications may
result in electrical shock hazards or improper operation. Fluke Biomedical will not be responsible for any injuries sustained due to unauthorized equipment modifications.
Page 5

Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment
by Fluke Biomedical. Changes made to the information in this document will be incorporated in new editions of the publication. No responsibility is assumed by Fluke Biomedical for the use or reliability of software or equipment that is not supplied by Fluke Biomedical, or by its affiliated dealers.
Manufacturing Location
The BP Pump 2 Non-invasive Blood Pressure Simulator and Tester is manufactured in
Everett, Washington by Fluke Biomedical, 6920 Seaway Blvd., Everett, WA, U.S.A.
Page 6

Page 7

Table of Contents
Chapter Title Page
1 Introduction and Specifications.............................................. 1-1
Introduction .......................................................................................... 1-3
Key Features......................................................................................... 1-3
General Safety Considerations.............................................................. 1-4
Symbols ............................................................................................ 1-4
Warnings and Cautions..................................................................... 1-5
Instrument Familiarity .......................................................................... 1-7
Powering Up the Tester ........................................................................ 1-10
Specifications........................................................................................ 1-10
Accessories ........................................................................................... 1-12
2 Setup, Maintenance, and Support........................................... 2-1
Setting up the Tester ............................................................................. 2-3
Printer Output ................................................................................... 2-4
User-Defined Simulations................................................................. 2-5
Language .......................................................................................... 2-6
Units of Measure............................................................................... 2-6
Self Test............................................................................................ 2-8
Zero Pressure .................................................................................... 2-8
Enable ECG Signal ........................................................................... 2-9
Maintenance and Support ..................................................................... 2-10
Avoiding Damage............................................................................. 2-10
Cleaning............................................................................................ 2-10
Service and Calibration..................................................................... 2-11
Packing Instructions.......................................................................... 2-11
3 Operation .................................................................................. 3-1
Introduction .......................................................................................... 3-3
Configurations for Devices Under Test (DUT)................................. 3-4
Conversion Factors ........................................................................... 3-7
Initializing Tests and Simulations..................................................... 3-7
Error Messages ................................................................................. 3-7
i
Page 8

BP Pump 2
Operators Manual
Pressure Tests....................................................................................... 3-8
Pressure Leak Test............................................................................ 3-8
Pressure Relief Test.......................................................................... 3-10
Pressure Source Test ........................................................................ 3-11
Pressure Gauge Test ......................................................................... 3-12
Simulations........................................................................................... 3-13
Standard BP...................................................................................... 3-14
Patient Conditions ............................................................................ 3-15
Arrhythmias...................................................................................... 3-16
Respiratory Artifacts ........................................................................ 3-18
Neonate ............................................................................................ 3-19
Wrist................................................................................................. 3-20
User-Defined .................................................................................... 3-21
Auto Sequences.................................................................................... 3-21
Editing Auto Sequences ................................................................... 3-22
Printing Auto Sequences .................................................................. 3-23
Running Auto Sequences ................................................................. 3-24
Pressure Gauge............................................................................. 3-25
Leak Test...................................................................................... 3-25
Relief Valve Test.......................................................................... 3-26
Pressure Source ............................................................................ 3-26
Data Sheet Printout....................................................................... 3-27
BP Simulations............................................................................. 3-27
Remote Operation ................................................................................ 3-29
RS-232 Settings................................................................................ 3-29
Ansur Software Control.................................................................... 3-29
Appendices
A ECG Interface............................................................................... A-1
B Questions and Answers ................................................................ B-1
C Abbreviations ............................................................................... C-1
D Computer Control Commands...................................................... D-1
ii
Page 9

List of Tables
Table Title Page
1-1. Symbols ................................................................................................ 1-4
1-2. Top and Side Panel Components .......................................................... 1-8
1-3. Number Key Functions......................................................................... 1-9
1-4. Standard Accessories ............................................................................ 1-12
1-5. Optional Accessories ............................................................................ 1-12
3-1. Conversion Factors ............................................................................... 3-7
3-2. Standard Blood Pressure Simulations ................................................... 3-15
3-3. Patient Condition Simulations .............................................................. 3-16
3-4. Arrhythmia Simulations........................................................................ 3-17
3-5. Respiratory Artifact Simulations .......................................................... 3-18
3-6. Neonate Simulations............................................................................. 3-19
3-7. Wrist Simulations ................................................................................. 3-20
D-1. Computer Control Commands .............................................................. D-1
iii
Page 10

BP Pump 2
Operators Manual
iv
Page 11

List of Figures
Figure Title Page
1-1. Tester Top and Side Panel Components ............................................... 1-7
3-1. Tester Pneumatic Block Diagram ......................................................... 3-3
3-2. Connecting Tester to Single-hose NIBP Monitor (Int Cuff) ................. 3-4
3-3. Connecting Tester to Double-hose NIBP Monitor (Int Cuff)................ 3-5
3-4. Connecting Tester to Single-hose NIBP Monitor (Ext Cuff) ................ 3-5
3-5. Connecting Tester to Double-hose NIBP Monitor (Ext Cuff)............... 3-6
3-6. Connecting Tester to Single-hose NIBP Wrist Monitor (Ext Cuff) ...... 3-6
3-7. Sample Auto Sequence Test Printout.................................................... 3-24
3-8. Sample Data Sheet Printout .................................................................. 3-28
A-1. Optional ECG Interface Adapter........................................................... A-1
v
Page 12

BP Pump 2
Operators Manual
vi
Page 13

Chapter 1
Introduction and Specifications
Contents Page
Introduction .................................................................................. 1-3
Key Features................................................................................. 1-3
General Safety Considerations ..................................................... 1-4
Symbols .................................................................................... 1-4
Warnings and Cautions............................................................. 1-5
Instrument Familiarity.................................................................. 1-7
Powering Up the Tester................................................................ 1-10
Specifications ............................................................................... 1-10
Accessories................................................................................... 1-12
1-1
Page 14

BP Pump 2
Operators Manual
1-2
Page 15

Introduction and Specifications
Introduction
1
Introduction
The Fluke Biomedical BP Pump 2 Non-Invasive Blood Pressure Simulator and
Tester, hereafter known as the “Tester”, is a multi-purpose test instrument for
use with oscillometric Non-Invasive Blood Pressure Monitors (NIBPMs). The
Tester provides dynamic blood pressure simulations, static calibration,
automated leak testing, and pressure relief valve testing. The following
models are available:
• BP Pump 2L (Basic Model)
• BP Pump 2M (High-Accuracy Model)
The Tester allows you to verify the performance claims of different blood
pressure monitors. You can quickly recall the fixed onboard simulations or
define your own. With its internal pump, the Tester can generate pressures up
to 400 mmHg (53.3 kPa) for leak testing, pressure sourcing, and relief valve
testing.
In addition, you can define auto sequences that automate the sequencing of
tests and NIBP simulations and provide an optional printed report.
Key Features
Key features of the Tester include:
• Pressure leak testing on cuff, tubing, and connections
• Relief valve testing on the patient monitor
• Pressure gauge measurements
• Pressure source capability
• NIBP simulations including adult, neonate, arrhythmias, and respiratory
artifacts
• Auto sequences with optional reports
• Internal Adult and Neonatal Cuff simulation
Tester capabilities can be extended with optional accessories that allow:
• ECG synchronization with non-invasive output
• External wrist cuff simulations
1-3
Page 16
BP Pump 2
Operators Manual
Tester pressure accuracy can be improved by upgrading to a high-accuracy
pressure transducer. This is a factory service upgrade and is provided to
customers wanting to meet the DIN EN 1060 requirements for pressure
measurement accuracy. For more information, refer to “Setup, Maintenance,
and Support: Maintenance and Support.”
General Safety Considerations
This Tester complies with safety and technical requirements described in the
following directives:
• UL 3101-1, Electrical Equipment for Laboratory Use; Part 1: General
Requirements.
• CAN/CSA C22.2 No. 1010.1 (1992), Safety Requirements for Electrical
Equipment for Measurement, Control and Laboratory Use, Part 1: General
Requirements.
• EC 73/23/EEC (Amended 93/68/EEC) EN61010-1:2001, Safety
requirement for electrical equipment for measurement, control and
laboratory use, Part 1: General Requirements.
Symbols
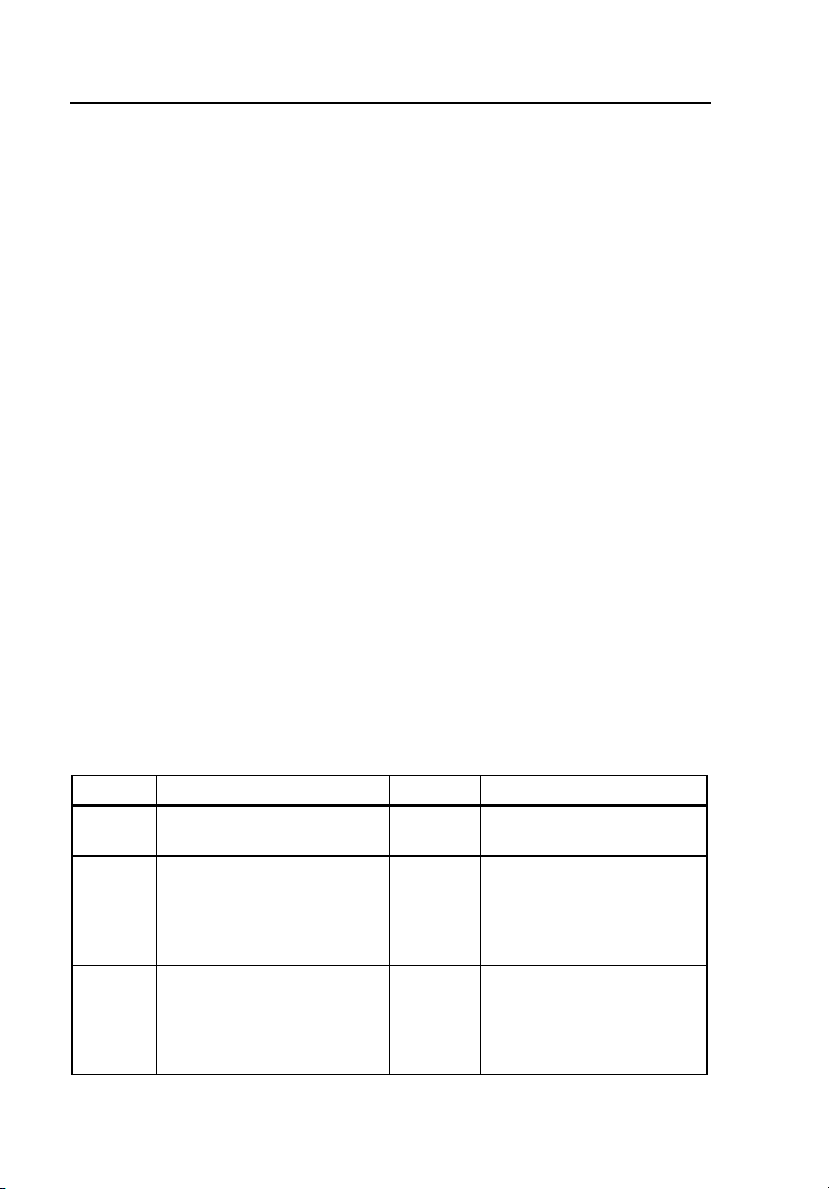
Table 1-1 describes symbols used in association with the Tester.
Table 1-1. Symbols
Symbol Description Symbol Description
W
Œ
~
1-4
Risk of danger. Important
information. See manual.
Intertek Electrical Test
Laboratory listed.
Conforms to relevant
Canadian and U.S.
standards.
Do not dispose of this
product as unsorted
municipal waste. Contact
Fluke or a qualified
recycler for disposal.
X
P
Hazardous voltage. Risk
of electrical shock.
Conforms to European
Union directives
Page 17

Introduction and Specifications
General Safety Considerations
Warnings and Cautions
Users are advised to read the manual carefully, observing all warnings and
cautions, before attempting to set up and operate the Tester.
A Warning identifies hazardous conditions and actions that could cause
bodily harm or death.
A Caution identifies conditions and actions that could damage the Tester or
the equipment under test, or cause permanent loss of data.
W X Warning
To avoid possible electric shock or personal injury, follow
these guidelines:
• Read the Users Manual before operating the Tester.
• Use this Tester only in the manner specified by the
manufacturer or the protection provided may be
impaired.
• Do not connect the Tester to a patient or equipment
connected to a patient. The Tester is intended for
equipment evaluation only and should never be used
in diagnostics, treatment or in any other capacity
where the Tester would come in contact with a patient.
1
• Do not use the product in wet locations, around
explosive gases or dust.
• Never open the Tester case, because dangerous
voltages are present. There are no user replaceable
parts in the Tester.
• The Tester must be properly earthed. Only use a
supply socket that has a protective earth contact. If
there is any doubt as to the effectiveness of the supply
socket earth, do not connect the Tester.
• Do not use a two-conductor adapter or extension cord;
this will break the protective ground connection.
• Ensure that the external power source is properly rated
for the system.
1-5
Page 18

BP Pump 2
Operators Manual
• Always connect the system power cord directly to a
three-prong receptacle with a functional ground. Never
use a two-prong plug adapter to connect primary
power to the Tester, thereby disconnecting the utility
ground.
• Disconnect the Tester from the power source before
changing the supply voltage. The Tester operates at a
range of 100 to 240 volts.
W Caution
To avoid damage to the Tester or adverse affects on its
performance, follow these guidelines:
• Allow only qualified technical personnel to service the
Tester.
• Do not expose the system to temperature extremes.
Ambient temperatures should remain between 15° C
and 40° C. System performance may be adversely
affected if temperatures fluctuate above or below this
range.
• Clean the Tester only by gently wiping down with a
clean, lint-free cloth dampened with a mild detergent
solution. Do not immerse the unit.
• Do not apply pressures greater than 400 mmHg (53
kPa) to the pressure port.
1-6
Page 19
Introduction and Specifications
Instrument Familiarity
1
Instrument Familiarity
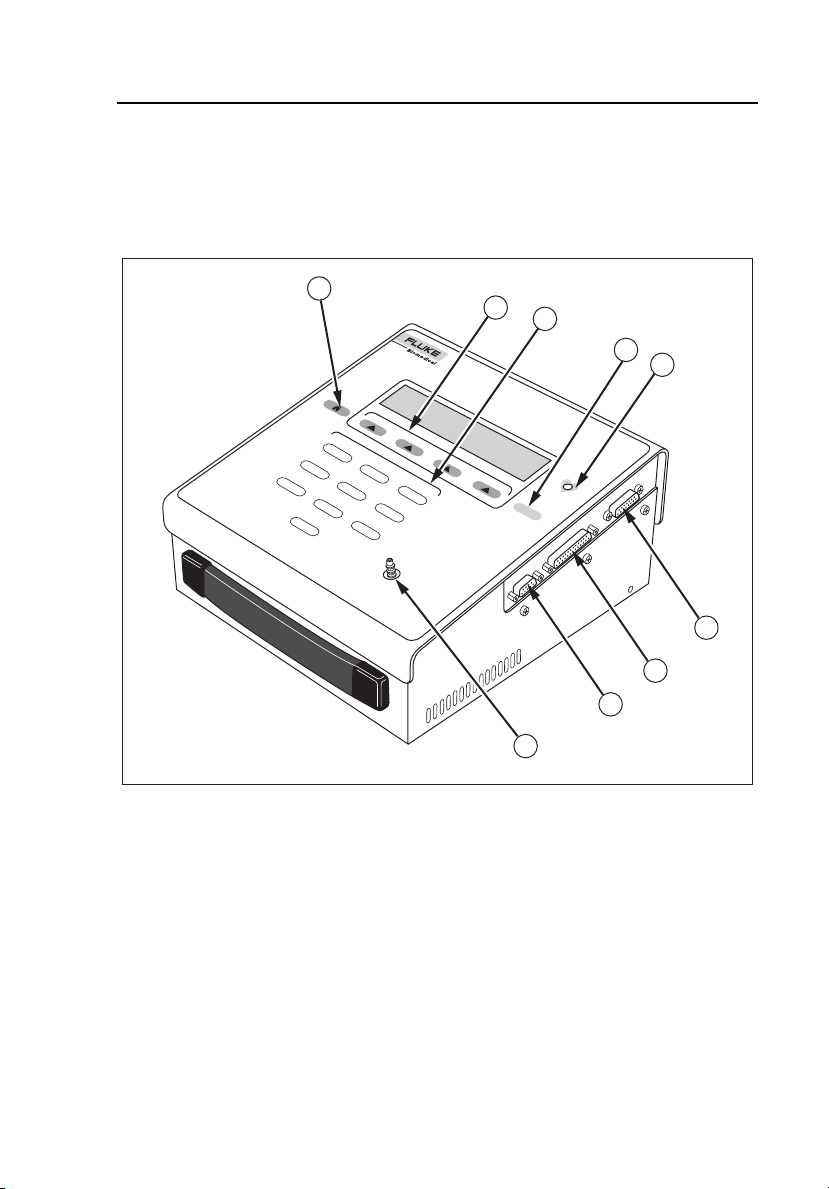
Figure 1-1 shows the Tester. The top and side panel components are described
in Table 1-2 and the number key functions are described in Table 1-3.
1
2
3
4
RESPIRI
ARTI
F
AC
7
USER DEFINED
0
STANDARD BP
TORY
T
4
NEON
8
PRESSURE
LEAK
1
A
T
E
CONDITIONS
TESTS AND SIMULA
PRESSURE
RELIEF
2
PA
TIEN
T
5
ARRHYTHMIAS
6
WRIST
9
PRESSURE PO
TION
S
PRESSURE
TA
3
RT
S
TI
C
BP Pump 2
NON-INV
ASIVE BLOOD PRESSURE MONITOR ANAL
YZE
R
ENT
8
9
Figure 1-1. Tester Top and Side Panel Components
5
6
7
fas10.eps
1-7
Page 20
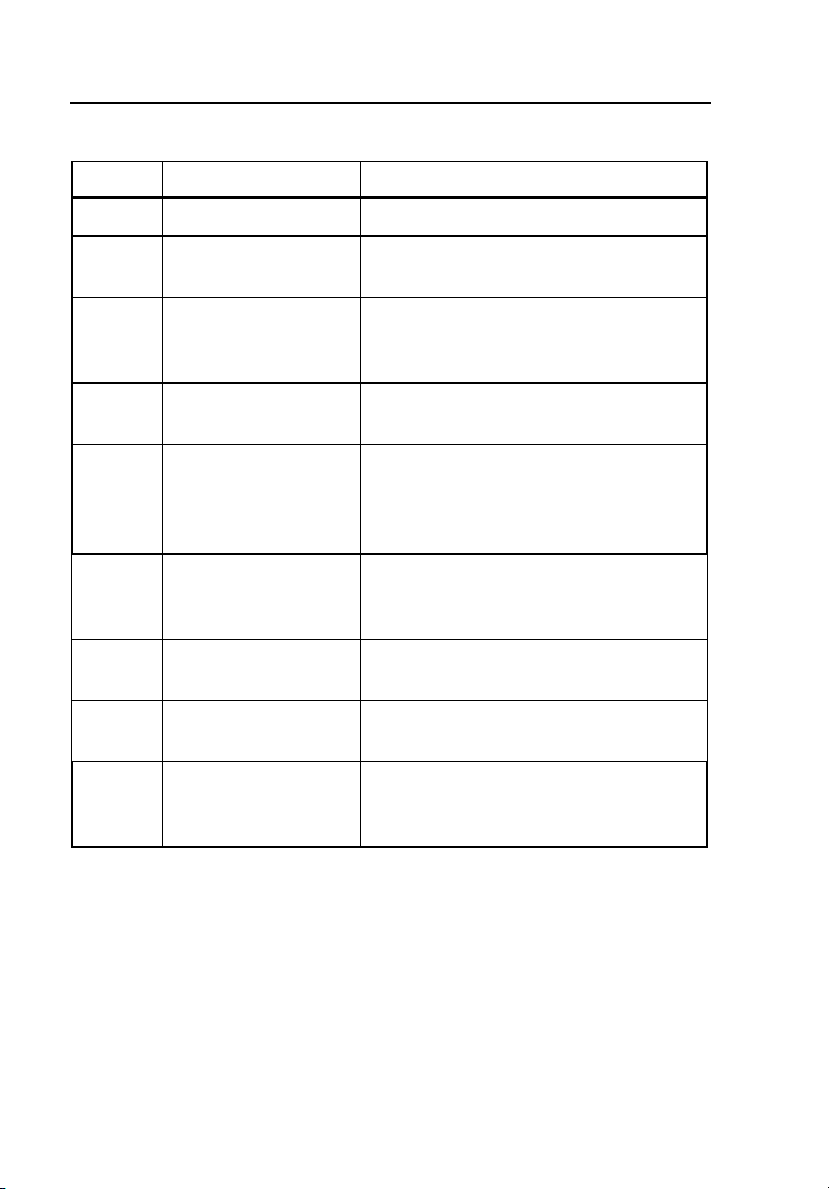
BP Pump 2
Operators Manual
Table 1-2. Top and Side Panel Components
Label Name Function
A Home Key Returns the operator to the Main Menu.
B Soft Keys 1 - 4
C
D Enter Key
E Pulse Indicator
F ECG Interface Port Allows connection of optional ECG
G Printer Port Provides D-25 female connector for
H RS-232 Serial Port Provides serial D-9 female connector for
I Pressure Port
Number (Test and
Simulations) Keys
Makes dynamic assignments based on
the current screen.
Allows the operator to perform auto
sequences and simulations using
numeric keys.
Advances to the next menu or
saves/selects options.
LED blinks in synchronization with
beeper, indicating that the pump is
generating a simulated blood pressure
pulse.
accessory (refer to Appendix, ECG
Option).
external parallel printer.
bi-directional computer control.
Connects to the Non-Invasive Blood
Pressure Monitor for all pressure
simulations and tests.
1-8
Page 21
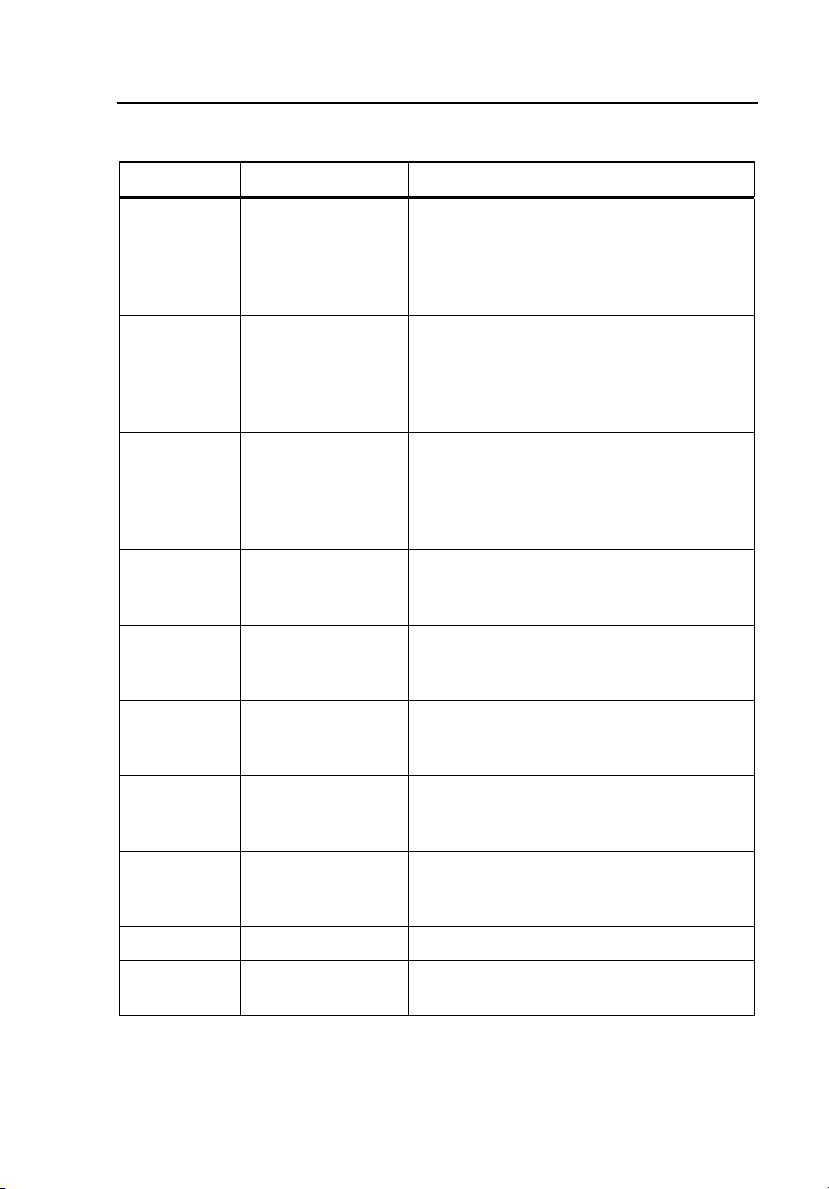
Introduction and Specifications
Instrument Familiarity
Table 1-3. Number Key Functions
Number Name Function
Pressurizes a pneumatic system to an
1
2
3
4 STANDARD BP
5
6 ARRHYTHMIAS
7
8 NEONATE
9 WRIST Tests wrist cuff NIBP monitors.
0 USER DEFINED
PRESSURE
LEAK
PRESSURE
RELIEF
STATIC
PRESSURE
PATIENT
CONDITIONS
RESPIRATORY
ARTIFACT
operator-defined target pressure up to
400 mmHg (53.3 kPa) and then
measures the loss of pressure over
time.
Increases the pressure in the
pneumatic system until the relief valve
on the NIBP monitor opens or until the
Setpoint is reached, whichever occurs
first.
Accessed via the Pressure Gauge
Test, which enables the Tester to
measure static pressure generated by
an external source in the range of 50
to 400 mmHg 6.7 to 53.3 kPa).
Provides seven variations of NIBP
simulations for both arm and wrist
cuffs.
Includes simulations for healthy,
geriatric, and obese patients, a well as
various levels of exercise.
Measures erratic heart rhythms,
including atrial fibrillation and
premature ventricular contraction.
Exhibits a beat-to-beat variation in the
blood pressure caused by intrathoracic
pressure.
Tests the ability of the NIBP monitors
to detect blood pressure on neonatal
patients.
Allows the operator to define blood
pressure simulations.
1
1-9
Page 22
BP Pump 2
Operators Manual
Powering Up the Tester
The Tester is very simple to power up. Follow these steps:
1. Plug in a three-pronged power cord to the back of the unit.
2. Plug the cord into an appropriate socket, ensuring that the external power
source is properly rated for the system.
3. Move the power switch above the plug to the on position. After two
momentary screens, the Tester displays the Main menu, from which all
Tester functions are selected.
Fluke Biomedical BP Bump 2
PERFORM
SIMULATION
PRESSURE
TESTS
AUTO
SEQUENCE
SETUP
fas31.eps
Specifications
The following are specifications for the Tester. Please contact your Fluke
Biomedical service representative for more information regarding the device
specifications.
Mains Voltage
Range ......................................................................100 - 240 V ac 50/60 Hz, 60 VA
Environmental Conditions
Operating Temperature ...........................................15 °C to 40 °C
Storage Temperature ..............................................-20 °C to +65 °C
Relative Humidity ....................................................90 % max
Pressure Measurement
Units ........................................................................kPa
mmHg
cmH
2
O
inH
2
psi
O
1-10
Page 23

Introduction and Specifications
Specifications
Range ..................................................................... 0 mmHg to +400 mmHg
Resolution............................................................... 0.1 kPa
Resolution (High Accuracy Version) ....................... 0.01 kPa
Accuracy (Standard Version)
0 to 300 mmHg ................................................... ±0.5 % of reading ±1 mmHg
301 to 400 mmHg .............................................. ±2 % of reading
High-Accuracy Version ........................................... <0.8 mmHg (0.1 kPa)
1 mmHg
O
1 cmH
2
O
1 inH
2
0.1 psi
0.1 mmHg
0.1 cmH
0.1 inH
0.01 psi
2
O
2
O
1
Pressure Generation
Pressure Generator, Static Pressure Range .......... 50 mmHg to +400 mmHg
Difference between target pressure
and actual pressure ................................................ ±10 mmHg from 100-400 mmHg with
Internal Leak Rate .................................................. <2 mmHg per minute, with a minimum
a minimum volume of 300 cc
volume of 300 cc
Electrical ECG
Signals .................................................................... RA, LA, RL, LL, V
Waveform ............................................................... Lead II
Amplitude................................................................ 1 mV peak (±10%)
Connections............................................................ Signals available via the optional ECG
adapter
Heart Rate for NIBP Simulations
Heart Rate Accuracy
With ECG disabled ............................................ ±1 BPM
With ECG enabled .............................................. ±1 BPM
Except for the following Patient Conditions:
Weak Pulse, Tachycardia, Obese, Geriatric....... ±1% ±1 BPM
Patient Condition Mild Exercise.......................... ±1.5% ±1 BPM
Patient Condition Strenuous Exercise ................ ±3% ±1 BPM
1-11
Page 24
BP Pump 2
Operators Manual
Accessories
The following are accessories for the Tester. To order, contact your Fluke
Biomedical equipment dealer and use the Fluke Biomedical part numbers
provided. Table 1-4 lists standard accessories shipped with the tester. Table
1-5 lists optional accessories that must be ordered separately.
Table 1-4. Standard Accessories
Description Quantity Shipped Part Number
Operator's Manual 1 2196592
Warranty Card 1 2241856
Tubing and Fittings 1 2196394
Country-specific Power Cord
USA
Schuko
UK
AU
1
1
1
1
284174
769422
769455
658641
Table 1-5. Optional Accessories
Description Part Number
Wrist Cuff Mandrel 2391875
ECG adapter 2391894
Carrying Case 2222822
RS-232 Serial Cable (9M-9F) 2238659
BP Pump 2 Ansur Plug-In 2755836
1-12
Page 25

Chapter 2
Setup, Maintenance, and Support
Contents Page
Setting up the Tester..................................................................... 2-3
Printer Output ........................................................................... 2-4
User-Defined Simulations ........................................................ 2-5
Language .................................................................................. 2-6
Units of Measure ...................................................................... 2-6
Self Test.................................................................................... 2-8
Zero Pressure............................................................................ 2-8
Enable ECG Signal................................................................... 2-9
Maintenance and Support............................................................. 2-10
Avoiding Damage..................................................................... 2-10
Cleaning.................................................................................... 2-10
Service and Calibration ............................................................ 2-11
Packing Instructions ................................................................. 2-11
2-1
Page 26

BP Pump 2
Operators Manual
2-2
Page 27

Setup, Maintenance, and Support
Setting up the Tester
2
Setting up the Tester
To set up the tester, carry out the following:
1. Insert the power cord into the Tester and plug the cord into an appropriate
ac power socket.
W X Warning
To avoid possible electric shock, burning of the skin, or
personal injury, ensure that the external power source is
properly rated for the system.
2. Turn the power switch on. When the Tester is powered up, the Main
menu appears.
Fluke Biomedical BP Bump 2
PERFORM
SIMULATION
PRESSURE
TESTS
AUTO
SEQUENCE
SETUP
3. The Tester has several configurable options available from the Setup
menu. You can reach the Setup menu by using the soft keys to follow
the menu path shown below:
MAIN MENU C SETUP
The Setup menu appears, showing the following configurable options:
BP Pump 2 Setup
PRINTER
OUTPUT
USER DEF
SIMULATION LANGUAGE
MORE*
4. Press the MORE soft key in this and each of the following screens to
display additional options:
fas31.eps
fas37.eps
2-3
Page 28

BP Pump 2
Operators Manual
BP Pump 2 Setup
UNITS OF
MEASURE
BP Pump 2 Setup
ZERO
PRESSURE
RS232
SETTINGS
ENABLE
ECG
SELF
TEST
MORE*
MORE*
fas38.eps
fas39.eps
Note
These parameters should be configured and the settings saved the
first time the Tester is used. They need to be configured only once.
Printer Output
Printouts are available for auto sequences. By default, the Printer Output is set
to NONE.
To control the production of when running auto sequences, take the following
actions:
1. Select the desired print format by using the soft keys to follow the menu
path shown below.
SETUP C PRINTER OUTPUT
The Printer Output screen appears, showing the available options:
Printer Output: None
A3CII HP PCL3 NONE
2. Select one option and press ENT to return to the SETUP menu.
3. If desired, press HOME to return to the Main menu.
2-4
fas22.eps
Page 29

Setup, Maintenance, and Support
Setting up the Tester
2
User-Defined Simulations
The Tester supports up to nine user-definable blood pressure simulations
configured through the Setup menu.
To access and modify a user-defined simulation definition, take the following
actions:
1. Use the soft keys to follow this menu path:
SETUP C USER DEF SIMULATION C Number Key (1-9) C
ENT
2. Alternatively, you can access the definition from the number keys:
0 USER DEFINED key C OPTIONS (to scroll through the
numbers) C EDIT
With either method, the User Defined Simulation screen appears:
User Defined Simulation 1
Sys: 120 Dia: 80 Pulse: 0.70cc HR: 75
<- FIELD FIELD ->
fas23.eps
This screen shows the number of the simulation, along with the following
parameters available for configuration. Their valid ranges are as follows:
• Sys: Systolic, 20 – 250 mmHg
• Dia: Diastolic, 10 – 200 mmHg
• Pulse: Pulse Volume, 0.1 cc – 2.4 cc in increments of 0.1 cc
• HR: Heart Rate, 30 – 250 bpm
The systolic and diastolic settings are interdependent. The diastolic must
always be below the systolic. The pulse volume and the heart rate are also
interdependent. The maximum pulse volume cannot be achieved at the
maximum heart rate.
3. Press the soft key <- FIELD or FIELD -> to scroll through options,
making changes to each field by entering numbers from the number keys.
4. Pres ENT to return to the SETUP menu.
5. If desired, press the HOME button to return to the Main menu.
2-5
Page 30

BP Pump 2
Operators Manual
Language
Besides the default of English, the Tester can support up to four additional
languages. Spanish is currently available; additional languages will be
released in the future.
To change the language, take the following actions:
1. Use the soft keys to follow the menu path shown below.
SETUP C LANGUAGE
The Display/Print Language screen appears, showing the available
options:
Display/Print Language: English
ENGLISH SPANISH
2. Select a language by pressing its soft key.
3. When finished, press ENT to return to the SETUP menu.
fas24.eps
4. If desired, press HOME to return to the Main menu.
Units of Measure
The Tester has separate definable measurement units for blood pressure
simulations and pressure tests. For the blood pressure simulations, units of
mmHg (default) and kPa are available. For the pressure tests, units of mmHg
(default), kPa, cmH
To define the pressure units, do the following:
1. Use the soft keys to follow the menu path shown below.
SETUP C *MORE C UNITS OF MEASURE
The Blood Pressure Units: screen appears, showing the available
options:
2-6
O, inH2O, and PSI are available.
2
Page 31

Setup, Maintenance, and Support
Setting up the Tester
Blood Pressure Units: mmHg
MMHG KPA
2. Select one option and press ENT.
The Pressure Measurement Units: screen appears, showing the
available options:
Pressure Measurement Units: mmHg
MMHG KPA CMH2O *MORE
3. Press *MORE to see additional options:
Pressure Measurement Units: mmHg
INH2O PSI *MORE
2
fas25.eps
fas26.eps
fas27.eps
4. Select one of the desired options and press ENT to return to the Setup
menu.
5. If desired, press HOME to return to the Main menu.
Note
Changing the UNITS OF MEASURE also changes the units used in
the auto sequences.
2-7
Page 32

BP Pump 2
Operators Manual
Self Test
The tester runs a self test, displaying the software version and checksum, the
serial number, and the model number. A motor check is also performed.
To run a self test, do the following:
1. Use the soft keys to follow the menu path shown below.
SETUP C *MORE C SELF TEST
The Self Test screen appears:
Fluke BP Pump 2
5180)
Heise Label: 002422 FW Version: 3.36
204634
mm Hg
fas28.eps
2. When finished, press ENT to return to the Setup menu.
3. If desired, press HOME to return to the Main menu.
Zero Pressure
The Zero pressure option allows the user to re-zero the pressure. The function
is similar to the tare of a scale. This re-zeroing lasts until the unit is zeroed
again or until the power is shut off.
To zero the pressure, do the following:
1. Use the soft keys to follow the menu path shown below.
SETUP C MORE C MORE C ZERO PRESSURE
The Zero Pressure (Remove connection …) screen appears:
Remove connection to pressure port and
press ZERO key
HA: 0.3 mmH 0g
ZERO
mm Hg
2. When finished, press ENT to return to the Setup menu.
2-8
fas29.eps
Page 33

Setup, Maintenance, and Support
Setting up the Tester
3. If desired, press HOME to return to the Main menu.
2
Enable ECG Signal
The ECG output is enabled or disabled by the operator. If the patient monitor
under test does not make use of ECG signals, we recommend that the ECG
output be disabled. (The factory default is for ECG to be disabled.) ECG
signals are present for all Standard BP, Patient Condition, and Arrhythmia
NIBP simulations. The ECG signals are present on the ECG Interface Port as
shown in Figure 1-2. An optional ECG Interface adapter, as described in
Appendix A, is available for purchase.
To enable or disable ECG signals, take the following actions:
1. Use the soft keys to follow the menu path shown below.
SETUP C *MORE C MORE C ENABLE ECG
The Enable ECG? screen appears, showing options YES and NO:
Enable ECG?
YES NO
2. Select one option and press ENT to return to the Setup menu.
3. If desired, press HOME to return to the Main menu.
2-9
Ye s
fas30.eps
Page 34

BP Pump 2
Operators Manual
Maintenance and Support
The Tester requires little maintenance or special care; however, it is a
calibrated measuring instrument and should be treated as such. The following
describes how to maintain the Tester and the method of company support.
Avoiding Damage
Do not drop the instrument or subject it to any mechanical abuse that could
cause a shift in the calibrated settings.
W Caution
To avoid damage to the Tester or adverse affects on its
performance, follow these guidelines:
• Do not expose the system to temperature extremes.
Ambient temperatures should remain between 15 and
40 °C. System performance may be adversely affected
if temperatures fluctuate above or below this range.
• Do not apply pressures greater than 400 mmHg (53
kPa) to the pressure port.
Cleaning
Clean the exterior of the Tester occasionally with a cloth dampened with a
mild detergent solution. Take care to keep liquids out of the pressure port.
W Caution
To avoid damage to the Tester or adverse affects on its
performance, clean it only by gently wiping down with a
clean, lint-free cloth dampened with a mild detergent
solution. Do not immerse the unit.
Carefully wipe down the hose adapters and inspect them for damage and
deterioration of the tubing and fittings.
2-10
Page 35

Setup, Maintenance, and Support
Maintenance and Support
2
Service and Calibration
If the Tester fails to operate successfully, please contact the Fluke Biomedical
Service Center immediately, as indicated under “Warranty and Product
Support.”
W Caution
To avoid damage to the Tester or adverse affects on its
performance, allow only qualified technical personnel to
service the Tester.
Annual calibration of the Tester by an authorized Fluke Biomedical Service
Center is recommended. Fluke Biomedical Service Centers have the
appropriate tools and procedures for performing calibrations as well as factoryauthorized updates.
International customers should contact their Fluke Biomedical dealers for
service/product support.
To obtain the name of a local dealer or service center, contact Fluke
Biomedical as indicated under “Return Procedures, Repair and Calibration.”
Packing Instructions
If repairs are required, return the Tester to the factory or the nearest service
center, packed in the original shipping container, using packing materials
supplied by Fluke Biomedical.
1. Before returning the Tester for factory service, contact the Fluke
Biomedical Service Center for a required Return Authorization Number.
2. Provide the following information:
• The Tester serial number
• The specific steps that reproduce the problem
• A daytime phone number
• Your name/company
• A fax number (if available)
3. Pack the instrument carefully, using the original packing materials. If the
original packing materials are not available, refer to “Return Procedures”
2-11
Page 36

BP Pump 2
Operators Manual
for a list of preferred materials or contact Fluke Biomedical for
replacement packing. Failure to pack the instrument properly could void
your warranty.
4. Place the Return Authorization Number in a prominent place on the
outside of the packing box, and refer to the number in any correspondence
with Fluke Biomedical Service.
5. Enclose your return address and Return Authorization Number.
6. Insure the unit for full retail value and ship to the nearest Fluke
Biomedical service center.
2-12
Page 37

Chapter 3
Operation
Contents Page
Introduction .................................................................................. 3-3
Configurations for Devices Under Test (DUT) ........................ 3-4
Conversion Factors ................................................................... 3-7
Initializing Tests and Simulations............................................. 3-7
Error Messages ......................................................................... 3-7
Pressure Tests............................................................................... 3-7
Pressure Leak Test.................................................................... 3-8
Pressure Relief Test .................................................................. 3-9
Pressure Source Test................................................................. 3-11
Pressure Gauge Test.................................................................. 3-12
Simulations................................................................................... 3-13
Standard BP .............................................................................. 3-14
Patient Conditions..................................................................... 3-15
Arrhythmias .............................................................................. 3-16
Respiratory Artifacts................................................................. 3-18
Neonate..................................................................................... 3-19
Wrist ......................................................................................... 3-20
User-Defined............................................................................. 3-21
Auto Sequences............................................................................ 3-21
Editing Auto Sequences............................................................ 3-22
Printing Auto Sequences........................................................... 3-23
Running Auto Sequences.......................................................... 3-24
Pressure Gauge...................................................................... 3-25
Leak Test............................................................................... 3-25
Relief Valve Test................................................................... 3-26
Pressure Source ..................................................................... 3-26
Data Sheet Printout ............................................................... 3-27
BP Simulations...................................................................... 3-27
Remote Operation......................................................................... 3-29
RS-232 Settings ........................................................................ 3-29
Ansur Software Control............................................................ 3-29
3-1
Page 38

BP Pump 2
Operators Manual
3-2
Page 39

Operation
Introduction
3
Introduction
The Tester contains a microprocessor that reads and controls the front panel
keyboard, the display, serial port, printer port, a diaphragm pump, two solenoid
valves, a step motor, a position sensor, and a pressure transducer (Figure 3-1).
Filter
DC
Diaphragm
Pump
Lead Screw
and Piston
Step
Motor
Home
Position
Sensor
Option
Hi Accuracy
Pressure
Sensor
Check Valve
Ring
12
Rolling
Seal
Standard
Pressure
Transducer
Figure 3-1. Tester Pneumatic Block Diagram
Ring Stud
12
N.C. Solenoid
Valve
Stud
Vent/Zero Port
Front Panel
Pressure Port
N.C. Solenoid
Valve
Internal Adult
Cuff Volume
290cc
fas14.eps
The diaphragm pump is used as a pressure source for the relief valve, leak, and
pressure source tests. The diaphragm pump pulls air through a filter and forces it
through a check valve into the main manifold of the instrument. This main
manifold has an internal volume of approximately 20 cc and is directly connected
to the pressure port on the front panel. Pressure in the manifold is measured by a
pressure transducer and can be released by a solenoid-operated valve. The volume
of the main manifold can be increased by approximately 290 cc, to simulate an
adult pressure cuff, by opening a second solenoid valve.
A stepper motor and lead screw move a piston into the manifold to decrease the
manifold volume, thereby creating pressure pulses to simulate a human subject. A
seal around the piston is maintained by a rolling diaphragm seal. The size and
3-3
Page 40

BP Pump 2
Operators Manual
shape of the pulses are controlled by the microprocessor driving the step motor.
The home position of the piston is detected by an optical interrupter. If the
optional high-accuracy pressure sensor is installed, it also measures the pressure in
the main manifold and is also controlled by the microprocessor.
Configurations for Devices Under Test (DUT)
Connect the Tester to the NIBPM unit with the tubing and fittings (part number
2391882) supplied with the Tester in the desired configurations, as shown in
Figures 3-2 through 3-6.
Basically, configurations involve connecting the NIBPM unit to the Tester directly
or connecting the unit, a cuff, and the Tester, using a “T” connector. When
connected properly, the tubing forms a closed system with the components.
BP Pump 2
NON-INVASIVE BLOOD PRESSURE MONITOR ANALYZER
Single Hose: Internal Cuff
NIBP Monitor
3-4
120
93
80
80
TESTS AND SIMULATIONS
PRESSURE
LEAK
1
STANDARD BP
4
RESPIRITORY
ARTIFACT
7
PRESSURE
RELIEF
2
PATIENT
CONDITIONS
5
NEONATE
8
USER DEFINED
0
S
TA
TIC
PRESSURE
3
ARRHYTHMIAS
6
WRIST
9
Figure 3-2.Connecting Tester to Single-hose NIBP Monitor
(Int Cuff)
PRESSURE PO
ENT
R
T
fas16.eps
Page 41

Operation
Introduction
BP Pump 2
Double Hose: Internal Cuff
NIBP Monitor
120
80
93
80
Figure 3-3. Connecting Tester to Double-hose NIBP Monitor
(Int Cuff)
Single Hose: External Cuff
NON-INVASIVE BLOOD PRESSURE MONITOR ANALYZER
TESTS AND SIMULATIONS
PRESSURE
LEAK
STANDARD BP
4
RESPIRITORY
ARTIFACT
1
7
S
T
A
T
PR
E
S
S
U
R
E
PR
E
S
S
R
E
L
IE
F
3
2
PATIENT
CONDITIONS
ARRHYTHMIAS
6
5
NEONATE
WRIST
8
9
USER DEFINED
0
BP Pump 2
NON-INVASIVE BLOOD PRESSURE MONITOR ANALYZER
IC
U
R
E
PRE
SS
URE
PORT
3
E
N
T
fas17.eps
NIBP Monitor
PRESSURE PO
ENT
R
T
120
93
80
80
Wrap Cuff
around Mandrel
TESTS AND SIMULATIONS
PRESSURE
LEAK
1
STANDARD BP
4
RESPIRITORY
ARTIFACT
7
PRESSURE
RELIEF
2
PATIENT
CONDITIONS
5
NEONATE
8
USER DEFINED
0
S
TA
PRESSURE
3
ARRHYTHMIAS
6
WRIST
9
TIC
Cuff
fas18.eps
Figure 3-4. Connecting Tester to Single-hose NIBP Monitor
(Ext Cuff)
3-5
Page 42

BP Pump 2
Operators Manual
Double Hose: External Cuff
BP Pump 2
NON-INVASIVE BLOOD PRESSURE MONITOR ANALYZER
NIBP Monitor
120
93
80
80
Wrap Cuff
around Mandrel
TESTS AND SIMULATIONS
PRESSURE
LEAK
1
STANDARD BP
4
RESPIRITORY
ARTIFACT
7
PR
E
S
S
R
E
L
IE
2
PATIENT
CONDITIONS
5
NEONATE
8
USER DEFINED
0
S
U
R
E
PR
F
ARRHYTHMIAS
WRIST
Cuff
Figure 3-5. Connecting Tester to Double-hose NIBP Monitor
(Ext Cuff)
BP Pump 2
Single Hose: External Wrist Cuff
NIBP Monitor
120
93
80
Wrist Cuff
80
NON-INVASIVE BLOOD PRESSURE MONITOR ANALYZER
TESTS AND SIMULATIONS
PRESSURE
PR
E
S
S
U
R
E
LEAK
R
E
L
IE
F
1
2
PATIENT
CONDITIONS
STANDARD BP
5
4
RESPIRITORY
NEONATE
ARTIFACT
7
8
USER DEFINED
0
T
A
TIC
E
S
S
U
R
E
3
6
9
S
PR
E
ARRHYTHMIAS
WRIST
E
N
T
PRESSURE
PORT
fas19.eps
E
N
T
T
A
T
IC
S
S
U
R
E
3
6
9
PRESSURE PORT
Bladder Wrist Mandrel
Be sure to attach the cuff so that the bladder is centered over the notch in the
mandrel insert.
Figure 3-6. Connecting Tester to Single-hose NIBP Wrist Monitor
(Ext Cuff)
3-6
fas20.eps
Page 43

Operation
Pressure Tests
3
Conversion Factors
Conversion factors for the Tester are shown in Table 3-1.
Table 3-1. Conversion Factors
Units mmHg
PSI_PER_MMHG 0.019337
CMH2O_PER_MMHG 1.3595
INH2O_PER_MMHG 0.53525
KPA_PER_MMHG 0.13332
Initializing Tests and Simulations
You can access functions of the Tester in two ways:
• Exclusive use of soft keys, the row of up arrow keys just below the display
• Combined use of the number keys and soft keys
For example, you may access a Pressure Source test as follows:
Soft keys only:
PRESSURE TESTS C STATIC PRESSURE C GAUGE C
SOURCE
Combined:
3 STATIC PRESSURE C GAUGE C SOURCE
Either method is acceptable, and it is assumed that you will develop your own,
most effective way of using the keys.
Error Messages
If you encounter any repeatable error messages, contact your Fluke Biomedical
Service Center.
Pressure Tests
The following tests assess the integrity and accuracy of the Tester, as well as the
instrument under test.
3-7
Page 44

BP Pump 2
Operators Manual
All tests begin from the Main menu:
Fluke Biomedical BP Bump 2
PERFORM
SIMULATION
PRESSURE
TESTS
AUTO
SEQUENCE
SETUP
fas31.eps
After pressing the PRESSURE TESTS soft key to display the Pressure Tests
menu, you can access the pressure tests described below:
PRESSURE
LEAK TEST
PRESSURE
RELIEF
STATIC
PRESSURE
fas35.eps
Pressure Leak Test
The Pressure Leak Test pressurizes a pneumatic system to an operator-defined
target pressure (labeled Setpoint) up to 400 mmHg (53.3 kPa) and then measures
the loss of pressure over time.
To assess leakage, take the following actions:
1. Define the Setpoint by using the soft keys to follow the menu path shown
below.
PRESSURE TESTS C PRESSURE LEAK TEST C SETUP
The Leak Test (Setpoint) screen appears.
Leak Test
Setpoint: 250
fas41.eps
2. Adjust the Setpoint by entering numbers with the number keys. The cursor
moves to the next position after a number is entered
3-8
Page 45

Operation
Pressure Tests
3
Note
Once defined, the Setpoint can be changed in increments of 1 least
significant digit (LSD).
3. Press ENT to display the Leak Test screen.
Leak Test
Cuff: External
CUFF SETUP VENT<START>
Leak Rate: _._ _
Setpoint: 250
Measured: 0
4. Press the VENT soft key to release any unwanted pressure in the system
before performing the test. This feature vents the system for approximately
five seconds and can be repeated as needed to return pressure to zero.
5. Press the <START> soft key to make the Tester deliver air to the system.
Once the system under test reaches the target pressure, the test begins. The
pressure leak rate of the system and the current system pressure are shown
during the test. The leak rate is expressed in mmHg/min by default or can
appear in kPa/min, cmH
O/min, inH2O/min, or psi/min, depending on the
2
pressure measurement unit selection. The leak rate of the Tester is < 2
mmHg/minute.
Note
When testing with an NIBP monitor in the system, it is necessary to put
the monitor in “Service” mode, because most monitors leave the system
open to the atmosphere.
6. Press HOME to return to the Main menu.
fas01.eps
Pressure Relief Test
The Pressure Relief Test increases the pressure in the pneumatic system until the
relief valve on the NIBP monitor opens, or until the Setpoint is reached, whichever
occurs first.
3-9
Page 46

BP Pump 2
Operators Manual
Note
Put the NIBP monitor in “Calibrate” or “Service” mode to close the vent
valve, allowing the Tester to inflate the pneumatic system. Refer to the
NIBP monitor’s service manual to find the method for entering “Service”
mode.
To assess the effectiveness of the relief valve, do the following:
1. Use the soft keys to follow the menu path shown below.
PRESSURE TESTS C PRESSURE RELIEF C SETUP
The Relief Valve Test (Setpoint) screen appears.
Relief Valve Test
Setpoint: 380 mm Hg
fas02.eps
2. If necessary, adjust the Setpoint, which defaults to 380 mmHg, by entering
numbers with the number keys. The cursor moves to the next position after a
number is entered.
3. Press ENT to display the Relief Valve Test screen.
Relief Valve Test
Peak : _._ _
Setpoint : 380
Measured : 0
SETUP VENT<START>
mm Hg
mm Hg
mm Hg
fas03.eps
4. Press the VENT soft key to release any unwanted pressure in the system
before performing the test. This feature vents the system for approximately
five seconds and can be repeated as needed to return pressure to zero.
5. Press the <START> soft key to make the Tester deliver air to the system.
While the Tester is delivering air to the system, the current pressure
(Measured) and peak pressure are being monitored.
If the Setpoint is reached and the monitor does not release the pressure, the
message No Relief Detected appears on the display.
3-10
Page 47

Operation
Pressure Tests
Note
It is recommended that three pressure relief measurements be taken to
check for a sticky relief valve.
Some NIPB monitors do not allow access to a “Service” mode, rendering
it impossible to close a vent valve so that the system can be pressurized
by an outside pump. As a last resort, it is possible to start a blood
pressure determination with the monitor (this closes the valve), then start
the Pressure Relief tests, so that two pumps inflate the system. The results
can vary, but the monitor generally opens a relief valve at some high
pressure.
6. Press HOME to return to the Main menu.
3
Pressure Source Test
The Pressure Source Test enables the Tester to simultaneously generate and
measure pressure.
The Pressure Source Test can be used for static calibration of Non-Invasive blood
pressure monitoring systems, checking sphygmomanometers, and evaluating any
medical device that measures pressure in the ranges of 50 to 400 mmHg (6.7 to
53.3 kPa). Pressures can be generated in 1-mmHg (0.1 kPa) increments.
To perform a Pressure Source test, do the following:
1. Use the soft keys to follow the menu path shown below.
PRESSURE TESTS C STATIC PRESSURE C SOURCE C SETUP
The Pressure Source (Setpoint) screen appears.
Pressure Source
2. If necessary, adjust the Setpoint, which defaults to 200 mmHg, by entering
numbers with the number keys. The cursor moves to the next position after a
number is entered.
3. Press ENT to display the Pressure Source screen.
3-11
Setpoint: 200 mm Hg
fas04.eps
Page 48

BP Pump 2
Operators Manual
Pressure Source
GAUGE SETUP VENT<START>
Measured : 0.4
Setpoint : 200
mm Hg
mm Hg
fas05.eps
4. Press the VENT soft key to release any unwanted pressure in the system
before performing the test. This feature vents the system for approximately
five seconds and can be repeated as needed to return pressure to zero.
5. Press the <START> soft key to make the Tester deliver air to the system. The
Tester pressurizes the system within 10 mmHg of the Setpoint value.
Note
Once the Setpoint has been reached, the Tester will not maintain the
pressure in the system. Therefore, it is recommended that the system be
checked for leaks prior to performing any static pressure tests.
6. Press HOME to return to the Main menu.
Pressure Gauge Test
The Pressure Gauge Test enables the Tester to measure static pressure generated
by an external source in the range of 0 to 400 mmHg (0 to 53.3 kPa).
To measure static pressure, take the following actions:
1. Use the soft keys to follow the menu path shown below.
PRESSURE TESTS C STATIC PRESSURE
The Pressure Gauge screen appears.
Pressure Guage
SOURCE VENT
Measured: 0 0.4 mm Hg
2. Press the VENT soft key to release any unwanted pressure in the system
before performing the test. This feature vents the system for approximately
five seconds and can be repeated as needed to return pressure to zero.
3-12
fas06.eps
Page 49

Operation
Simulations
3
Note
The <START> soft key is not applicable to the pressure gauge test and
does not display.
3. Apply pressure to the pressure port and read the displayed pressure in the
upper right corner of the screen.
Note
You can use a squeeze bulb or syringe to apply adequate pressure.
4. Press HOME to return to the Main menu.
Simulations
The following sections describe various simulations that the Tester accomplishes.
For all of the following tests, it is important to select the correct cuff.
To perform simulations, take the following actions:
1. Power on the NIBP unit.
Note
Either disable the alarm or be prepared to silence it when it sounds.
2. Use the soft keys, beginning from the Main menu:
Fluke Biomedical BP Bump 2
PERFORM
SIMULATION
PRESSURE
TESTS
AUTO
SEQUENCE
SETUP
fas31.eps
3. Press the PERFORM SIMULATION soft key to access the Select
Simulation Type menu. From this menu, you can access the simulations
described below:
3-13
Page 50

BP Pump 2
Operators Manual
Select Simulation Type
STANDARD
BP
PATIENT
CONDITION ARRHYTHMIA
MORE*
4. Press the MORE soft key on this and the following screens for additional
options:
Select Simulation Type
RESPIRATORY
ARTIFACTS
USER
DEFINED NEONAT E
MORE*
Select Simulation Type
WRIST
MORE*
Note
Alternatively, you can access all of the simulations from the number keys,
which is the method described in this section.
fas32.eps
fas33.eps
fas34.eps
Standard BP
The Tester provides many variations of NIBP simulations for both arm and wrist
cuffs.
To perform Standard BP tests, do the following:
1. Access these simulations by pressing the 4 STANDARD BP number key.
The Standard BP screen appears.
3-14
Page 51

Operation
Simulations
3
Standard BP
Preset # 1
Cuff: External
OPTIONS CUFF
ready ....
120/ 80 (93)
80 BPM 0.68 cc
2. Press the OPTIONS soft key to scroll through the simulation choices.
Parameters for Standard BP simulations are shown in Table 3-2.
Table 3-2. Standard Blood Pressure Simulations
Simulation
Number
1 120/80 (93) 80 0.68
2 150/100 (116) 80 0.65
3 200/150 (166) 80 0.60
4 255/195 (215) 80 0.55
5 60/30 (40) 80 0.75
6 80/50 (60) 80 0.71
7 100/65 (76) 80 0.69
Blood Pressure
(mmHg) (MAP)
Heart Rate (bpm)
Pulse volume
(cc)
fas07.eps
3. Press the CUFF soft key to select Internal Adult or External cuff.
4. Press Start on the NIBP unit.
5. Compare values on the NIBP unit with those on the Tester.
6. Press HOME to return to the Main menu.
Patient Conditions
The Patient Condition simulations are intended to provide some basic patient
variations.
To perform Patient Conditions simulations, do the following:
1. Access these simulations by pressing the 5 PATIENT CONDITIONS key.
The Patient Condition screen appears.
3-15
Page 52

BP Pump 2
Operators Manual
Patient Condition
Healthy
Cuff: External
OPTIONS CUFF
ready ....
120/ 80 (93)
75 BPM 0.68cc
fas08.eps
2. Press the OPTIONS soft key to scroll through the simulation choices.
Parameters for Patient Condition simulations are shown in Table 3-3.
Table 3-3. Patient Condition Simulations
Patient Condition
Healthy Heart 120/80 (93) 75 0.68
Weak Pulse 110/80 (90) 95 0.50
Mild Exercise #1 140/90 (106) 120 1.00
Strenuous Exercise #2 140/90 (106) 162 1.40
Obese Subject 120/80 (93) 90 0.50
Geriatric Subject 150/110 (123) 95 0.40
Tachycardia 120/105 (110) 130 0.40
Blood Pressure
(mmHg) (MAP)
Heart Rate
(bpm)
Pulse volume
(cc)
Bradycardia 120/60 (80) 45 1.10
3. Press the CUFF soft key to select Internal Adult or External cuff.
4. Press Start on the NIBP unit.
5. Compare values on the NIBP unit with those on the Tester.
6. Press HOME to return to the Main menu.
Arrhythmias
These waveforms cause erratic readings on some NIBPMs. The blood pressure
determination strongly depends on exactly what is happening with the subject's
blood pressure when the cuff pressure is at a particular level. Some NIBPMs
pause until they detect two or more equivalent beats. The pattern of step deflations
and the measured blood pressure depend on which beats occur during each step of
the cuff pressure.
3-16
Page 53

Operation
Simulations
3
To perform Arrhythmia Simulations, do the following:
1. Access these simulations by pressing the 6 ARRHYTHMIA key. The
Arrhythmia screen appears.
Arrhythmia Simulation
Pre Atrial Cont. 1
Cuff: External
OPTIONS CUFF
ready ....
138/ 53 (81)
80 BPM
2. Press the OPTIONS soft key to scroll through the simulation choices.
Parameters for Arrhythmia simulations are shown in Table 3-4.
Table 3-4. Arrhythmia Simulations
Arrhythmia Type
Premature Atrial Cont. #1 138/53 (81) 80
Premature Atrial Cont. #2 144/64 (90) 83
Premature Ventricular Cont. 118/61 (80) 83
Atrial Fib and PVCs 139/72 (94) 91
Blood Pressure (mmHg)
(MAP)
Heart Rate
(bpm)
3. Press the CUFF soft key to select Internal Adult or External cuff.
4. Press Start on the NIBP unit.
5. Compare values on the NIBP unit with those on the Tester.
fas09.eps
6. Press HOME to return to the Main menu.
3-17
Page 54

BP Pump 2
Operators Manual
Respiratory Artifacts
The Respiratory Artifact exhibits a beat-to-beat variation in the blood pressure
caused by intra-thoracic pressure. Changes in the intra-thoracic pressure affect
filling of the ventricles during diastole. This in turn affects the stroke volume of
the heart. A large stroke develops a higher systolic pressure than a small stroke.
To perform Respiratory Artifact simulations, do the following:
1. Access these simulations by pressing the 7 RESPIRATORY ARTIFACT
key. The Respiratory Artifact screen appears.
Respiratory Artifact
Spont Breath # 1
Cuff: External
OPTIONS CUFF
ready ....
138/ 65 (89)
104 BPM
2. Press the OPTIONS soft key to scroll through the simulation choices.
Parameters for Respiratory Artifact simulations are shown in Table 3-5.
Table 3-5. Respiratory Artifact Simulations
Blood
Artifact Type
Spontaneous Breathing #1 138/65 (89) 104 Varies
Spontaneous Breathing #2 149/65 (93) 105 Varies
Spontaneous Breathing #3 112/47 (68) 86 Varies
Controlled Ventilation 132/44 (73) 98 Varies
Pressure
(mmHg) (MAP)
Heart Rate
(bpm)
Pulse
volume (cc)
3. Press the CUFF soft key to select Internal Adult or External cuff.
4. Press Start on the NIBP unit.
5. Compare values on the NIBP unit with those on the Tester.
fas11.eps
6. Press HOME to return to the Main menu.
3-18
Page 55

Operation
Simulations
3
Neonate
The Neonate simulations are provided to test the ability of the NIBP monitors to
detect blood pressure on neonatal patients.
To perform Neonate simulations, do the following:
1. Access these simulations by pressing the 8 NEONATE key. The Neonate
Simulation screen appears.
Neonate Simulation
Neonate # 1
Cuff: Internal Neonate
OPTIONS CUFF
ready ....
35/ 15(22)
120 BPM 0.11 cc
2. Press the OPTIONS soft key to scroll through the simulation choices.
Parameters for Neonate simulations are shown in Table 3-6.
Table 3-6. Neonate Simulations
Simulation
Number
1 35/15 (22) 120 0.11
2 60/30 (40) 120 0.10
3 80/50 (60) 120 0.10
4 100/70 (80) 120 0.10
Blood Pressure
(mmHg) (MAP)
Heart Rate (bpm)
Pulse volume
(cc)
3. Press the CUFF soft key to select External or Internal Neonate.
4. Press Start on the NIBP unit.
5. Compare values on the NIBP unit with those on the Tester.
fas12.eps
6. Press HOME to return to the Main menu.
3-19
Page 56

BP Pump 2
Operators Manual
Wrist
The Wrist simulations are provided to test wrist cuff NIBP monitors.
To perform Wrist simulations, do the following:
1. Access these simulations by pressing the 9 WRIST key. The Wrist
Simulation screen appears.
Wrist Simulation
WristCuff # 1
Cuff: External
OPTIONS
ready ....
120/ 80 (93)
80 BPM 0.50 cc
fas13.eps
2. Press the OPTIONS soft key to scroll through the simulation choices. The
simulation is automatically set up to use the external cuff and cannot be
changed. Parameters for Wrist simulations are shown in Table 3-7.
Table 3-7. Wrist Simulations
Simulation
Number
Blood Pressure
(mmHg) (MAP)
Heart Rate (bpm)
Pulse volume
(cc)
1 120/80 (93) 80 0.50
2 160/100 (120) 80 0.50
3 80/55 (63) 80 0.50
7. Press Start on the NIBP unit.
8. Compare values on the NIBP unit with those on the Tester.
9. Press HOME to return to the Main menu.
3-20
Page 57

Operation
Auto Sequences
3
User-Defined
User-Defined simulations and how to create them are described in “Setting Up the
Tester.”
The following describes how to run such a simulation.
1. Select from any of the previously-defined simulations by pressing the 0
USER DEFINED key. The User Defined screen appears.
User Defined
Simulation 1:
Cuff: External
OPTIONS CUFF EDIT
ready ....
120/ 80
75 BPM 0.70 cc
fas15.eps
2. Press the OPTIONS soft key to scroll through the simulation choices.
3. Press the CUFF soft key to select External, Internal Adult, or Internal
Neonate cuff.
Note
It is important to select the correct cuff.
4. Press Start on the NIBP unit.
5. Compare values on the NIBP unit with those on the Tester.
6. Press HOME to return to the Main menu.
Auto Sequences
It is possible to create up to nine customized auto sequences. An auto sequence
contains all four pressure tests and five simulations. The operator can disable any
of these tests or simulations. A printout of the auto sequence result can also be
enabled. The operator must make a cuff selection; this determines what NIBP
simulations are displayed for that selection.
3-21
Page 58

BP Pump 2
Operators Manual
Editing Auto Sequences
Use the following steps to edit an auto sequence:
1. Press the AUTO SEQUENCE soft key to access the Auto Sequence
menu.
Auto Sequence
EDIT PRINTRUN
fas36.eps
2. Press the EDIT soft key.
3. At the Select Auto Sequence prompt, press a numeric key (1-9), followed
by the ENT key. You are prompted to select, enable, or disable each of the
components listed below. Press the ENT key at each of the prompts to
advance to the next selection.
• Print Auto Sequence Result
• Pressure Gauge Test
• Pressure Source Test
• Pressure Leak Test
• Pressure Relief Valve Test
• Cuff Selection
• NIBP Simulations (1-5)
Note
By default, each auto sequence is configured to perform the Leak Test,
Relief Test, and BP Simulation #1 (120/80) Changing the cuff selection
changes the available BP Simulation options.
4. When finished, either press the RUN soft key to run the sequence or press
HOME to return to the Main menu.
3-22
Page 59

Operation
Auto Sequences
3
Printing Auto Sequences
Use the following steps to print an auto sequence definition.
Note
Refer to ‘Setting up the Tester” for printer output setup instructions.
1. To determine which tests and simulations are defined in an auto sequence,
press the AUTO SEQUENCE soft key to access the Auto Sequence menu.
Auto Sequence
EDIT PRINTRUN
2. Press the PRINT soft key. The Select Auto Sequence (1-9) screen
appears.
Select Auto Sequence: 1
3. Press a numeric key (1-9), followed by the ENT key.
Figure 3-7 shows a typical generated auto sequence printout.
fas36.eps
fas42.eps
3-23
Page 60

BP Pump 2
Operators Manual
Auto Sequence #1 Definition
Gauge Enabled
Leak Setpoint: 200 mmHg
Relief Pressure:
Source Pressure: 200 mmHg
Cuff: External
Simulation 1: Preset #1 120/80
80BPM 0.7cc
Simulation 2: Preset #2 150/100
80BPM 0.7cc
Simulation 3: Preset #3 200/150
80BPM 0.7cc
Simulation 4: Preset #4 255/195
80BPM 0.7cc
Simulation 5: Preset #5 60/30
80BPM 0.7cc
Figure 3-7. Sample Auto Sequence Test Printout
Running Auto Sequences
Use the following steps to run an auto sequence.
380 mmHg
1. Press the AUTO SEQUENCE soft key to access the Auto Sequence
menu.
Auto Sequence
EDIT PRINTRUN
fas36.eps
2. Press the RUN soft key. The Select Auto Sequence (1-9) screen appears.
3-24
Page 61

Operation
Auto Sequences
Select Auto Sequence: 1
3
3. Press a numeric key (1-9), followed by the ENT key.
The auto sequence begins to execute, based on the options that were enabled
using the EDIT function.
Note
Tests and simulations not enabled in the executing auto sequence are
skipped.
Enabled auto sequences are executed in the following order:
• Pressure Gauge Test
• Pressure Leak Test
• Pressure Relief Valve Test
• Pressure Source Test
• Data Sheet Printout
• NIBP Simulations
fas42.eps
Pressure Gauge
This test monitors system pressure, which must be generated external to the Tester.
Press the NEXT> key to advance to the next test. Only the pressure displayed
when the NEXT> key is pressed appears on the printout.
Leak Test
This test performs a system leak test at the Setpoint pressure, which was defined
when the auto sequence was edited.
1. Press the START soft key to initiate this test. The system then pressurizes.
Once a stable pressure reading near the Setpoint is reached, a 60-second timer
begins to count down. Once the timer has elapsed, a Leak Rate appears on the
display.
3-25
Page 62

BP Pump 2
Operators Manual
2. If the Leak Rate is unsatisfactory, repair the possible cause of the leak and
press START again to repeat the test.
3. Press the NEXT> key to advance to the next test. Only the leak rate displayed
when the NEXT> key is pressed appears on the printout.
Note
The NEXT> key will not function until the Leak Test has been performed
at least once.
Relief Valve Test
This test increases the pressure in the pneumatic system until the relief valve on
the NIBP monitor opens or until the Setpoint is reached, whichever occurs first.
1. Press the START soft key to initiate this test. It is recommended that this test
be repeated multiple times.
2. Press the NEXT> key to advance to the next test.
Note
The NEXT> key will not function until the Relief Valve Test has been
performed at least once.
Pressure Source
This test causes the system to rise to the Setpoint pressure, which was defined
when the auto sequence was edited.
1. Press the START soft key to initiate this test. The system then begins to
pressurize until a stable pressure reading at or near the Setpoint is reached.
2. Record the monitor reading on the data sheet (if generated).
3. Press the NEXT> key to advance to the next test.
Note
The NEXT> key does not function until the Pressure Source test has been
performed.
3-26
Page 63

Operation
Auto Sequences
3
Data Sheet Printout
When this step is reached, a printout is generated, as shown in Figure 3-8, which
includes a standard header, the results of the previous tests, a place to record data
for the Pressure Source test (if enabled), and a list of BP Simulations that will be
executed.
BP Simulations
At the conclusion of the auto sequence, up to five different blood pressure
simulations are run in order of definition. Each simulation can be repeated as
many times as desired; however, each defined simulation must be run at least once
to advance to the next simulation.
Note
Some monitors cannot handle extreme changes in blood pressure. For
example, it may not be possible to perform a 255/195 simulation
following a 60/30 simulation on some monitors.
3-27
Page 64

BP Pump 2
Operators Manual
Fluke Biomedical
Tester Serial #:123456
Date:________ Time:_______
Serial #: ___________________
Control #: __________________
Mfg: ________________________
Model: ______________________
Location: ___________________
Technician: _________________
Work Order: _________________
Procedure ID: _______________
Gauge: 181 mmHg
Leak Test
Leak Rate: 5 mmHg/min
Start Pressure 203 mmHg
End Pressure: 198 mmHg
Relief Valve Test
Peak Pressure:
No Relief Detected
Pressure Source
Actual:
Source:
418 mmHg
_________
186 mmHg
Actual ___/___ ( ) ___BPM
Preset #1 120/80 80BPM 0.7cc
Actual ___/___ ( ) ___BPM
Preset #2 150/100 80BPM 0.7cc
Actual ___/___ ( ) ___BPM
Preset #3 200/150 80BPM 0.7cc
Actual ___/___ ( ) ___BPM
Preset #4 255/195 80BPM 0.7cc
Actual ___/___ ( ) ___BPM
Preset #5 60/30 80BPM 0.7cc
Figure 3-8. Sample Data Sheet Printout
3-28
Page 65

Operation
Remote Operation
3
Remote Operation
To prepare for remote operation, do the following:
1. Power down the Tester and connect the Tester to your PC, using a standard
bidirectional RS-232 cable.
2. Turn the Tester on: the operating system recognizes the Tester as new
hardware.
You are now ready to operate the Tester remotely.
RS-232 Settings
The Tester serial port parameters are fixed at the following settings:
• Baud Rate: 9600
• Parity: None
• Data Bits: 8
• Stop Bit: 1
Ansur Software Control
Ansur test automation systems allow a solutions-based approach to complete
testing of the medical device under test (DUT). Ansur helps you create standard
work using the test template/sequence (which is based on your written test
procedure) and integrates all test results into a single test report that can be printed
or archived. Ansur manages your test procedures by allowing both manual and
visual automated test sequences.
The software works hand-in-hand with Fluke Biomedical testers and simulators,
creating a seamless integration for:
• Visual inspections
• Preventive maintenance
• Work procedures
• Performance tests
• Safety tests
3-29
Page 66

BP Pump 2
Operators Manual
Ansur software utilizes plug-in modules to work with a wide array of Fluke
Biomedical instruments. The plug-in module is a software interface to the Ansur
program. Plug-ins provide test elements used by Ansur. This gives the benefit of
using the same user interface for all testers and simulators supported by an Ansur
plug-in. See the Fluke Biomedical Ansur BP Pump 2 Plug-in User Manual for
detailed information.
When you purchase a new Fluke Biomedical tester or simulator, you can update
your existing Ansur software by installing a new plug-in. Each plug-in module
lets you work only with the options and capabilities you need for the instrument
you are testing. The Fluke Part Number for the Ansur BP Pump 2 Plug-in is
2755836.
3-30
Page 67

Appendices
Appendix Title Page
A ECG Interface .............................................................................. A-1
B Questions and Answers................................................................ B-1
C Abbreviations............................................................................... C-1
D Computer Control Commands ..................................................... D-1
Page 68

BP Pump 2
Operators Manual
Page 69

Appendix A
ECG Interface
Optional ECG Interface Adapter
The optional ECG Interface Adapter (Fluke Biomedical Part Number
2780512), shown in Figure A-1, is provided to aid in testing NIBP monitors
that use ECG signals to assist in the detection of the pressure pulses. The ECG
attachment is labeled with the five leads, RA, RL, LA, LL, and V, and is
attached to the ECG Interface Port.
RL
LL
Figure A-1. Optional ECG Interface Adapter
Using the optional ECG Interface Adapter (Part Number 2780512), the
operator can observe simulated ECG signals that are in synchronization with
A-1
RA
LA
V
fas21.eps
Page 70

BP Pump 2
Operators Manual
the blood pressure simulations. These ECG signals are available for the
Standard BP, Patient Conditions and Arrhythmias simulations.
A-2
Page 71

Appendix B
Questions and Answers
Introduction
The following are questions submitted by customers and answered by
members of our technical staff.
Blood Pressure Issues
Q: Monitor's BP determinations vary.
“I connected the Tester to my Critikon DINAMAP monitor and used the preset
blood pressure of 120/80 (93) with a pulse rate of 80 beats per minute. I
performed three blood pressure determinations with the following results:
Trial # Systolic Mean Diastolic Pulse Rate
1 123 97 82 79
2 126 93 81 81
3 123 97 83 78
Why does the blood pressure determined by the DINAMAP vary?”
A: Some variance is normal and acceptable.
The Tester generates a very repeatable simulation. For this simulation, an ideal
NIBP monitor would show a variation of less than 2 mmHg from one
simulation to the next. Most of the variation seen here originates in the
DINAMAP. This is normal and acceptable.
Section 3.4.3 of the ANSI Standard for Electronic or Automated
Sphygmomanometers specifies the required efficacy of the blood pressure
determination:
B-1
Page 72

BP Pump 2
Operators Manual
“The mean difference of the paired measurements of the test system and the
comparison system shall be ± 5 mmHg or less with a standard deviation of
8 mmHg or less.”
This means that variations in individual readings of 5, 6, or even 10 mmHg are
quite normal and do not indicate that either the DINAMAP or the Tester are
malfunctioning. Some monitors are more repeatable than others, and
repeatability is one measure of the overall quality of the monitor.
Q: BP results vary using the same preset pressure.
“I checked another NIBP monitor using the same preset simulated blood
pressure of 120/80 (93) with a pulse rate of 80 bpm. This time I got the
following results:
Trial # Systolic Mean Diastolic Pulse Rate
1 120 89 71 80
2 120 87 73 80
3 121 91 72 80
Why does this monitor show such low Diastolic pressures?”
A: Monitors using different references – Ascultatory vs. Invasive data.
Neither the monitor nor the Tester is broken or giving incorrect readings.
Some monitors were designed to give readings close to those obtained by the
Ascultatory method of blood pressure determination. Other monitors have been
designed to agree with Invasive Blood Pressure readings. It is well known that
Invasive and Ascultatory BP readings on the same subject can be quite
different. Therefore it is not surprising that automated Oscillometric NIBP
monitors using Invasive readings as a reference would give different readings
than a monitor based on Ascultatory readings.
Cuff Issues
Q: Why is an Internal Cuff used?
“Why does the Tester use an Internal Simulated Cuff? Wouldn’t it be better to
include a real cuff in the measurement?”
A: Internal Cuff produces accurate and repeatable simulations.
The Tester uses an internal cuff to help ensure accurate and repeatable
simulations over time. The internal “cuff” is a 310-ml fixed volume that has
compliance very nearly equal to a normal adult cuff when used at typical adult
B-2
Page 73

Appendices
Questions and Answers B
mean pressures. Further, its compliance is constant over time and is
independent of cuff wrapping technique.
The compliance of a standard cuff depends on the amount of air it contains.
This, in turn, is dependent on what the cuff is wrapped around, and how tightly
it is wrapped.
Q: Why is compliance Important?
“Why is tightly controlled compliance so important?”
A: Air in the cuff affects oscillations.
Blood pulsing through the arm surrounded by a cuff actually causes
displacement of the air in the cuff. This must be converted into a pressure
oscillation before the NIBP monitor can sense what is happening.
For a given volume displacement, the size of the pressure oscillation is
inversely proportional to the volume of air in the cuff. Thus, a cuff full of air
gives a smaller pressure oscillation than one wrapped tightly around the arm
and containing little air.
The Tester works just like the subject’s arm. It creates a precisely controlled
volume displacement. The cuff is what converts this displacement into a
pressure oscillation.
By using an internal cuff of fixed volume, the Tester is assured of always
producing the same pressure oscillation for each test.
Q. Can an External Cuff be used with the Tester?
“Can the Tester be used with an External Cuff?”
A. Connectors provided in Accessory Kit
The Tester can easily be configured to work with an External Cuff.
The available cuff options are accessible via the CUFF soft key present during
the NIBP simulations.
Note
The exceptions are neonate, which allows only the internal neonate
cuff, and wrist cuff, which allows only the external cuff.
The external cuff is included in the pneumatic circuit using the “Tee” or “Y”
connectors in the accessory kit.
B-3
Page 74

BP Pump 2
Operators Manual
B-4
Page 75

Appendix C
Abbreviations
Abbreviations
The following list includes abbreviations used in this document.
A ampere
ANSI American National Standards Institute
AAMI
BLU blue (color)
BPM beats per minute
dB decibel
°C degrees Celsius (centigrade)
CQM Contact Quality Monitor
DMM digital multimeter
EEPROM electrically erasable PROM
ECG electrocardiograph or electrocardiogram
ESU Electrosurgery Unit
EUT equipment under test
°F degrees Fahrenheit
GRA gray (color)
GRN green (color)
Association for the Advancement of Medical
Instrumentation
C-1
Page 76

BP Pump 2
Operators Manual
Hz hertz
in inch
K kilo (103)
kg kilogram
kHz kilohertz
ke kilohm
lb pound
LED light-emitting diode
LCD liquid crystal display
M meg(a) (106)
MHz megahertz
Me megohm
m meter
m milli (10-3)
mA milliampere
mm millimeter
mV millivolt
p-p peak-to-peak
REM Return Electrode Monitor
s second
YEL yellow (color)
µ micro (10-6)
µA microampere
µV microvolt
Ω ohm
C-2
Page 77

Appendix D
Computer Control Commands
Introduction
The following commands are for use by software developers that have highlydeveloped technical knowledge. Operators should not try to control the Tester
using these commands; rather, they should use Ansur test automation software.
The Tester acknowledges each valid computer control command that it
receives. It responds to valid commands either with ACK (HEX 06), or ACK,
followed by the data, followed by CR-LF (HEX 0D0A). A NAK (HEX 15) is
returned for invalid commands.
The computer control commands are shown in Table D-1.
Table D-1. Computer Control Commands
Description Command
Internal Adult Cuff [CUFF_IA] ACK
Neonate Cuff [CUFF_IN] ACK
External Cuff [CUFF_EXT] ACK
Wrist Cuff Simulation [SIM_WC120_80] ACK
[SIM_WC160_100] ACK
[SIM_WC80_55] ACK
D-1
Returned
String
Page 78

BP Pump 2
Operators Manual
Table D-1. Computer Control Commands (cont.)
Description Command
Neonate Cuff Simulation [SIM_NEO35_15] NAK
[SIM_NEO60_30] NAK
[SIM_NEO80_50] NAK
[SIM_NEO100_70] NAK
Standard BP Simulation [SIM_STD120_80] ACK
[SIM_STD150_100] ACK
[SIM_STD200_150] ACK
[SIM_STD255_195] ACK
[SIM_STD60_30] ACK
[SIM_STD80_50] ACK
[SIM_STD100_65] ACK
Patient Conditions [SIM_HEALTHY] ACK
Returned
String
[SIM_WEAK_PULSE] ACK
[SIM_MILDEX] ACK
[SIM_STRENEX] ACK
[SIM_OBESE] ACK
[SIM_GERIATRIC] ACK
[SIM_TACHYCARDIA] ACK
[SIM_BRADYCARDIA] ACK
D-2
Page 79

Appendices
Table D-1. Computer Control Commands (cont.)
Computer Control Commands D
Description Command
Arrhythmias [SIM_PAC1] ACK
[SIM_PAC2] ACK
[SIM_PVC] ACK
[SIM_AFIBPVC] ACK
Respiratory Artifacts [SIM_SB1] ACK
[SIM_SB2] ACK
[SIM_SB3] ACK
[SIM_CV] ACK
Set Units of Blood Pressure [BP_UNITS_KPA] ACK
[BP_UNITS_MMHG] ACK
Set Units of Pressure [PRESS_UNITS_KPA] ACK
Returned
String
[PRESS_UNITS_MMHG] ACK
[PRESS_UNITS_CMH2O] ACK
[PRESS_UNITS_INH2O] ACK
[PRESS_UNITS_PSI] ACK
D-3
Page 80

BP Pump 2
Operators Manual
Table D-1. Computer Control Commands (cont.)
Description Command
Perform Leak Test at
XXXmmHg
Perform Pressure Source at
XXXmmHg
[BLEED_SYSTEM] ACK
Performs Relief Valve Test [RELIEF,XXX]
[LEAK,XXX]
50<=XXX<=400
[PSOURCE,XXX]
50<=XXX<=400
50<=XXX<=400
Returned
String
Returns ACK
then Leak Rate.
Returns Leak
Rate after 60
seconds,
followed by CRLF, or NAK if
XXX out of
range•
ACK or NAK if
XXX out of
range
Returns ACK
then Peak
Pressure,
followed by CRLF, or NAK if
XXX out of
range
Pressure Gauge [GAUGE] Returns ACK
then pressure
port value,
followed by CRLF
D-4
Page 81

Appendices
Table D-1. Computer Control Commands (cont.)
Computer Control Commands D
Description Command
[BP_SERIAL] Returns ACK
[BP_VERSION]• ACK then
Returned
String
then the serial
number,
followed by CRLF
2780201 Ver
X.XX, followed
by CR-LF•
D-5
Page 82

BP Pump 2
Operators Manual
D-6
 Loading...
Loading...