Page 1
Choosing a bath fluid
by Steve Iman
Hart Scientific
Application Note
Fluke Hart Scientific manufactures a
large number of constant tempera
ture baths that cover a temperature
range from –100 to 550°C. How
ever, the usable range of each bath
highly depends on the fluid chosen.
Hart’s definition of “usable range”,
is the temperature range over
which the fluid will provide the
best performance. The ideal bath
fluid would have a low viscosity,
high heat capacity, very low vapor
pressure, and a high flash point. It
would also need to cover a very
wide temperature range. Unfortu
nately, no single fluid has all of
these attributes, so care must be
taken when choosing a bath fluid.
From both an operational and
safety standpoint, some considerations are: safety precautions, flash
points, viscosity, heat capacity,
thermal conductivity, fluid expansion, specific gravity, vapor
pressure, gel time, usable life and
storage.
-
-
-
1.0 Fluid Safety
Always obtain data sheets and/or
material safety data sheets for the
fluids that will be used. It is impor
tant to read and understand all
safety requirements. When it comes
to safety, Hart strongly recommends
the following:
1. Have the appropriate fire
extinguishing equipment
nearby in case of a fire.
2. Never mix fluids or put any
chemicals into the fluid.
Doing so may cause
contamination or an adverse
chemical reaction.
3. Always exercise caution
when working around
extremely cold or hot bath
fluids. Wear protective
clothing to prevent accidental
injury.
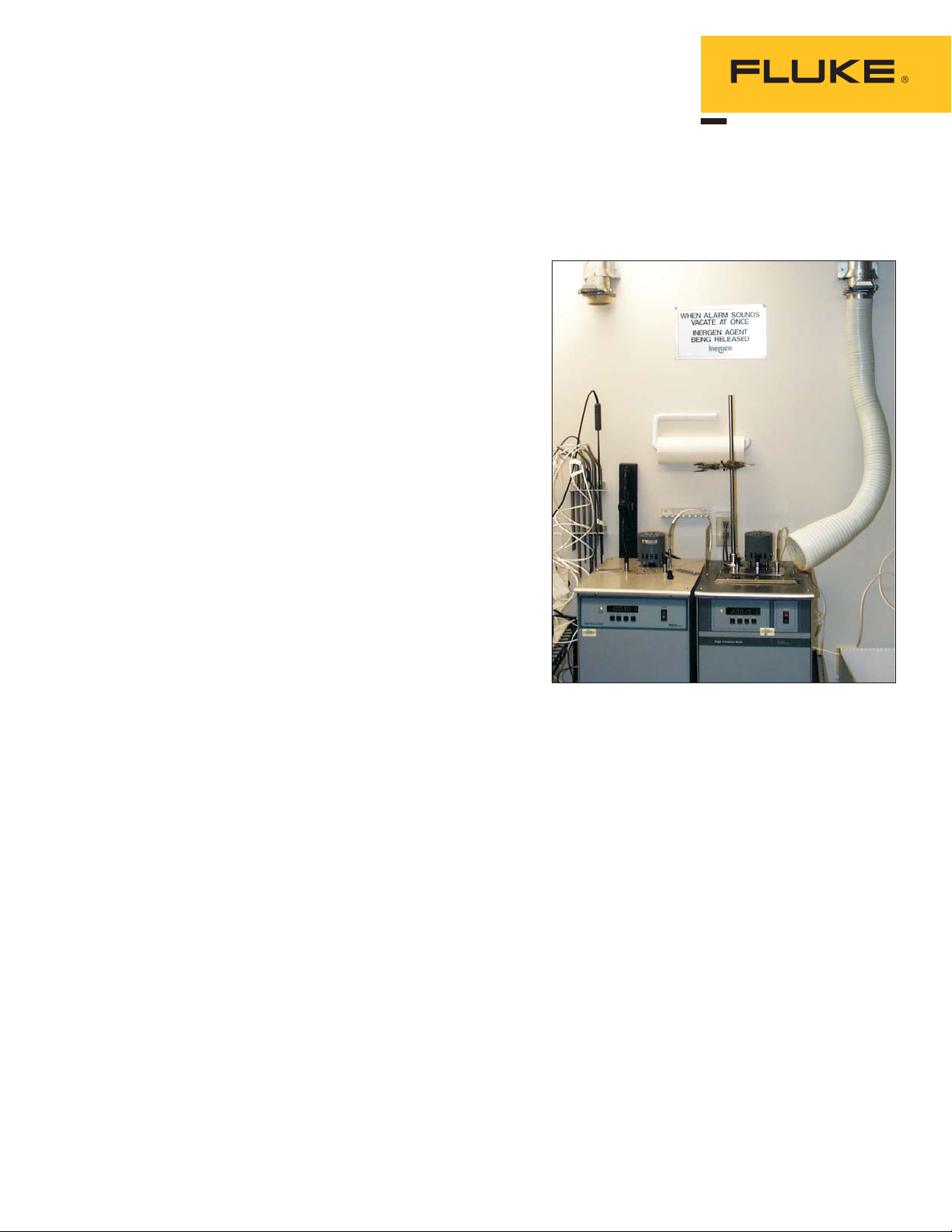
4. Use adequate ventilation for
fluids at elevated
temperatures. (See Figure 1).
5. Never operate a bath on or
around combustible
materials.
6. Provide safety training for all
personnel who will either
use the baths or be around
them.
7. Abide by federal and state
laws regarding storage and
disposal of any hazardous or
flammable liquid.
2.0 Terms and Definitions
Now that you have the data sheets,
what do all those terms mean?
2.1 Flash point
This is the temperature at which an
adequate mixture of fluid vapor and
air will ignite if in the presence of
an open flame or spark. It is important to note that if the fluid is nonflammable it’s only the vapor that
will burn and not the fluid. There
are two units of measure for flash
point.
1. Open Cup (oc). As the term
implies, the air and fluid
vapor are not enclosed. In an
open cup, there is a higher
ratio of air to fluid vapor.
2. Closed Cup (cc). The mixture
of air and fluid vapor are
-
contained in an enclosure. In
this instance, the ratio of
fluid vapor to air is higher.
When specifying the flash point,
fluid manufacturers only have a list
of options to pick from. They do not
have open text fields that they can
fill in. So when it says >101.1°C,
this is just stating that the flash
point is greater than 101.1°C. This
is just used to classify the material
(ie. flammable or combustible, etc.
for the hazard profile, not as an ac
tual value). It’s like saying how old
are you? Then giving you choices of
>15, >25, >35, etc. So when it co
mes to the flash point definitely go
with the product data sheet.
2.2 Viscosity
Viscosity is the unit of measure for
the thickness of a fluid at 25°C.
Generally it is a constant consis
tency under fixed pressure and
temperature. Ideal fluids offer no
resistance to shear and have zero
consistency. Viscosity dimensions
Figure 1 Ventilation system for removing oil vapors.
are force per area x time. The unit
of viscosity is the poise (P) =
1g/(cm) (sec) and is a measure of
mass flow of a liquid. One poise is
equal to 0.1 Pa.s in SI units. Where
Pa.s is the Pascal-second, the SI
unit for viscosity, equaling 1kg(m.s)
or 10 poise.
A common unit of measure with
bath fluids is kinematic viscosity.
-
This differs from viscosity in that it
is the measure of volume flow of a
liquid, defined as a stoke (st). A
-
stoke equals 1 cm
–4m2
10
/Sec. A centistoke, cst = 0.01
St = 1mm
can be converted to viscosity
(poise) by multiplying by the
density of the fluid.
-
used at more than one temperature,
the viscosity will change when it is
heated or cooled. Because the vis
cosity changes, each fluid will have
a “viscosity temperature coeffi
2
Since bath fluids generally are
2
/ Sec or
/sec. Kinematic viscosity
-
-
Page 2

Hart Scientific
cient.” Example: Over a range of
0°C to 100°C, VTC = 1–(viscos
ity@100°C/viscosity@0°C). Thus
the lower the VTC, the less change
there will be in viscosity over the
range.
Hart baths generally perform the
best with a viscosity that does not
exceed 10 cst. But in the real
world, the viscosity will change
with temperature. So viscosities
that do not exceed 50 cst will work
satisfactorily. Any higher than this,
and the stability and uniformity
may be very poor. In addition, if the
viscosity is too high, it will put too
much of a load on the stirring mo
tor. This may cause it to overheat or
to stop altogether.
2.3 Heat Capacity
The specific heat capacity of a solid
or liquid is defined as the heat re
quired to raise a unit of mass or
substance by one degree of
temperature.
ΔΔQmcT=
Where:
ΔQ = heat applied to fluid.
m = fluid mass.
c = specific heat capacity.
ΔT = rise in temperature.
2.4 Thermal Conductivity
Thermal conductivity is a fluid’s
ability to transfer heat from one
molecule to another. This can be
determined by:
λ() () () ()TaTcTpT
=× ×
p
Where:
λ = thermal conductivity
T = temperature
a = diffusivity of material
cp = specific heat
p = density
The better the heat transfer, the
quicker the fluid will heat or cool.
Better thermal conduction will help
with bath uniformity.
2.5 Coefficient of Volume
Expansion
All fluids have a thermal expansion
coefficient. This unit of measure
tells how much the fluid will either
expand or contract with changes in
temperature. Unless the bath is
equipped with an overflow device,
it must be considered, otherwise
the bath may overflow.
2.6 Specific Gravity
The specific gravity is a specifica
tion of the density or weight of a
fluid as compared
to that of water.
The specific grav
ity of water is 1. A
The specific gravity of water is
cubic foot of water
weighs 62.4
pounds.
The higher the
specific gravity,
the more the fluid
will weigh. If the
fluid is too heavy,
To calculate an unknown specific gravity:
Sp gr
=
Weight of an equal volume of water
Sp gr
Density of a fluid
=
Density of water
it may not work
well in a bath
equipped with a
Figure 2 Calculation of an unknown specific gravity.
pump mechanism
or circulator.
2.7 Vapor Pressure/Volatility
The temperature at which a liquid
is on the verge of vaporization is
called vapor pressure. At this tem
perature, the vapor pressure of the
liquid is equal to that of ambient
pressure. Another way of saying
this is that the vapor and ambient
pressures are at equilibrium. If the
temperature is below this point, the
vapor will condense into liquid.
Conversely, if the temperature is
above this point, the liquid will vaporize. A fluid that has a low vapor
pressure such as alcohol will evaporate quickly and require frequent
replenishment. Furthermore, rapid
evaporation at the fluid surface will
have a cooling effect, making tem
perature control more difficult.
These fluids generally are only
suitable for low temperature use.
With some liquids, the processes
of condensation and evaporation
can be delayed, which is referred
to as supersaturation and super
heating, respectively. A good exam
ple is adding ethylene glycol to
water. This raises the boiling point
of the water as well as the vapor
pressure.
2.8 Gel Time
Gel time is usually associated with
silicone oils when used at elevated
temperatures. This is the time that
it takes silicone oil to gel or poly
merize. Oxidation of the oil is the
root cause. When this occurs, it’s a
molecular chain reaction that hap
pens instantly and can cause the
fluid to nearly double in volume.
Polymerization is a metrologist’s
worst nightmare; the oil will either
turn to a jelly-like substance or
even worse, a “molasses in winter”
goop! It can be very difficult to re
62 4
.
==
62 4
1
.
Weight of the fluid
move from the bath and its parts.
Fortunately, Dow Corning makes a
solvent that can be used to remove
polymerized oil. The solvent is
called OS-2 and can be purchased
from an authorized distributor of
Dow Corning fluids. It will require
approximately 2 gallons of OS-2 for
every 7 gallons of polymerized oil.
Polymerization of silicone oil in
an open system may not be avoidable. However, there are steps that
can be taken to prolong the oil’s
life.
1. Keep the time that the bath is
at high temperatures to a
minimum.
2. If the bath isn’t being used,
either turn it off or set the
idling temperature below its
vapor point.
3. Avoid cross-contamination of
oils.
4. Keep oxidizers such as bath
salts out of the oil.
5. Change the oil if it becomes
-
too dark in color, to viscous,
or there is a notable
difference in bath stability.
3.0 Fluid Life and Storage
The life of a fluid depends on how
it is used, at what temperature it is
used, and the length of time at that
temperature. Generally, most bath
fluids will have a long life as long
as their limitations are not ex
ceeded. Unused liquids should be
left in their original unopened con
tainer. If storage life is a concern,
please check with the fluid manu
facturer for specifics about shelf life
and storage requirements.
-
-
-
-
2 Fluke Hart Scientific Choosing a bath fluid
Page 3

Hart Scientific
4.0 Choosing a Fluid
Having a bath for each temperature
or fluid type is the “ideal.” How
ever, this may not be within your
budget, so more than one fluid may
be needed. There are many bath
fluids on the market that may work
well for the temperature range of
interest. As stated earlier, a single
fluid may not be available to cover
the desired temperature range. So
try to choose fluids with the widest
range, lowest viscosity, and the
highest flash point. There may be
some overlap in the temperature
ranges of each fluid, but this is fine.
It is probably better to have some
overlap rather than being right on
the “fringe” of a fluid’s minimum or
maximum temperature.
To avoid cross-contamination of
fluids, thoroughly clean all wetted
parts before putting in the next
fluid. Another source for cross-con
tamination is moving thermometers
from a salt bath to an oil bath without cleaning off the salt.
4.1 Water
Water is one of the most commonly
used bath fluids. It is an ideal fluid
over its usable range. It’s inexpensive, has a low viscosity, and good
thermal characteristics. The drawbacks are limited temperature
range, hard water deposits, and the
formation of algae. Water generally
has a usable range from about 5 to
60°C but its upper limit depends on
atmospheric pressure. The eleva
tion at Hart Scientific is about 4500
ft above sea level, so here water
has a higher vapor pressure. This
limits the usable upper temperature
to about 40 to 45°C. Higher temper
ature settings will cause instability
due to the rapid cooling effect from
evaporation. The vapor pressure
can be reduced using a 50/50 mix
of Ethylene Glycol and water. In
Utah, this will raise the usable high
temperature to about 75°C.
To eliminate hard water depos
its, we recommend using either
distilled or deionized water. To re
duce the growth of algae, use a
good algaecide.
4.2 Silicone Oils
Silicone oils have unique properties
because they are not petroleum or
organic based. They were the first
and only polymer products made
from inorganic chemistry. Silicone
oils vary in viscosity and cover a
-
-
-
-
broad temperature range. (See Ta
ble 1.) They have good thermal
characteristics and low
flammability.
Even with their good qualities,
there are a few disadvantages. At
high temperatures, fuming occurs
and cleanup will require a solvent.
Because baths are “open” systems,
prolonged use at high temperatures
cause the thinner properties to boil
off. As the hot oil comes in contact
with air, the oil will oxidize. The
oxidation accumulates over time
and will eventually cause the oil to
gel or polymerize. When the color
changes from light honey to a
darker color, this is a sign that oxi
dation has taken place. The oxida
tion rate also depends on
contaminates. Bath salts are a
heavy oxidizer;
so avoid getting
them into the oil
To minimize
oxidation, turn
the bath off
when it’s not in
use or, keep it at
low idling temperature. To
avoid polymer-
Table 1 A few silicone oils and their characteristics.
Hart
Model # Description
5010 Silicone oil Type 200.05 –40 to 130°C 133°C
5012 Silicone oil Type 200.10 –30 to 209°C 211°C
5013 Silicone oil Type 200.20 10 to 230°C 232°C
5014 Silicone oil Type 200.50 30 to 278°C 280°C
ization, it’s recommended that the oil be changed
any time it becomes too dark or the
temperature becomes less stable.
Some silicone oils are designed
for low-temperature use. However,
at low temperatures condensation
will form. In locations where the
humidity is particularly high, more
moisture will condense. As with
many oils, water and oil remain
separated. At cold temperatures,
the water and oil will pass over the
cooling coils or cooled tank walls.
Generally, the water will freeze to
these parts of the bath. As the ice
thickens, it will reduce the heat
transfer between the cooling coil or
plate and the liquid. This will pre
vent the bath from reaching cold
temperatures.
4.3 Cooking Oils
Hydrogenated vegetable oils and
coconut oil can be used, but they
have a limited temperature range
and tend to fume more at high tem
peratures than do silicone oils.
Please note that vegetable and co
conut oils are subject to gelling, just
as silicone oils are.
4.4 White Mineral Oil
White mineral oil is a good choice
for resistor baths. It is inexpensive,
and has good thermal and electrical
resistive properties. Over time how
ever, the electrical resistivity may
decline. Generally this is due to
water contamination.
4.5 Perfluorocarbons
Perfluorocarbon fluids are excellent
for low temperature baths. They are
thermally and chemically stable,
nonflammable, and have low levels
of toxicity. They have a high dielec
tric strength and non-solvent char
acteristics, which make them ideal
for electronic control testing.
Cleanup is easy, as they leave
practically no residue. The disad
vantages are evaporation and cost.
4.6 Alcohols
Ethanol and methanol are excellent
cold bath fluids especially where
the ambient humidity is high. All
alcohols absorb moisture, which
can be an advantage. Unlike oils
where the water and oil remain
separated, ice will not form on
cooling coils or tank walls like it
will with oils. However, alcohols
can become overly saturated with
water. When saturation is reached,
the mixture forms a slurry of ice
and alcohol. At this stage, the stir
ring will be impeded resulting in
poor stability and uniformity. When
the alcohol becomes too saturated
with water, it must be changed.
Normally methanol freezes at
–98°C, but by adding water it will
lower the freezing point. This is
called the “freezing point depres
sion.” If water is not added for
–100°C operation, the methanol
will freeze to the bath cooling coils
as shown in Figure 3. When ice
forms on the cooling coil, the bath
will not reach temperature. By add
ing 5% water by volume, –100°C
may be achieved.
Methanol is very volatile, has a
low flash point, and has a high de
-
-
-
-
Usable
Range
-
-
-
-
Flash
Point
Fluke Hart Scientific Choosing a bath fluid 3
Page 4

Hart Scientific
gree of toxicity which can be ab
sorbed through the skin. Ethanol
also is very volatile, but unless in
gested it is less toxic. Isopropyl is
not as toxic or volatile as metha
nol, but it can become highly vis
-
cous at low temperatures because
it tends to be more hydroscopic.
Because of alcohol’s high volatil
ity and low flash points (see Ta
-
ble 2), all alcohols should only be
used for low temperature work.
When not in use, alcohols should
be kept in an appropriate con
tainer and stored in a cabinet de
-
-
signed for flammable liquids.
If alcohol is left in the bath,
the temperature should be main
tained at 0°C or lower to minimize
evaporation.
4.7 Bath Salts
Tempering A heat transfer salt is
very stable and experience has
shown that in the absence of
contaminants, it can give many
years of service if used at temperatures of 454°C and below. Salt is
usable from 200 to 550°C, but at
temperatures above 454°C, the
salt undergoes a slow thermal decomposition. This is accompanied
by a gradual rise in the freezing
point. Tempering salt is somewhat hygroscopic, so it is recommended that it be stored in a dry
place to prevent caking. Organic
chemicals and combustible materi
als should not be stored in the
same area.
Bath salt can be easily removed
from thermometers by rinsing it off
with warm water. NEVER allow
water to come in contact with mol
ten salt!
5.0 Conclusion
Bath fluids are an integral part of
constant temperature baths. It is
important to know their advantages
as well as their limitations, so that
you can get the maximum benefit
from their use. It is our hope that by
sharing our knowledge and experi
ence, that we can help you make
the right fluid choices. Should you
have any questions, please call or
email us. We are more than happy
to be of service.
Figure 3 Cooling coil coated with ice. The fluid is pure methanol (no water added).
Table 2 Usable temperature range for alcohols
Type Temperature Range (°C) Flash Point
Isopropyl –10 to 20 11.7°C
Ethanol –80 to 10 11.1°C
Methanol –100 to 10 (add 5% water by
volume for < –90°C)
12.8°C
Fluke Hart Scientific.
Total temperature solutions.
-
Fluke Hart Scientific
799 E Utah Valley Drive
American Fork, UT 84003
Tel: 801.763.1600
Fax: 801.763.1010
E-mail: info@hartscientific.com
For more information call:
Europe/Africa/Middle East:
Hart Scientific Europe
P.O. Box 1186, 5602 BD
-
Eindhoven, The Netherlands
Tel: (31 40) 2 675 200
Fax: (31 40) 2 675 222
Canada
Tel: 1-800-36-FLUKE or
905.890.7600
Fax: 905.890.6866
Other countries
Tel: 801.763.1600
Fax: 801.763.1010
Web access: www.hartscientific.com
©2005 Fluke Hart Scientific. All rights reserved. Printed
in U.S.A. 3/2005
4 Fluke Hart Scientific Choosing a bath fluid
 Loading...
Loading...