Page 1
PN 3028662
Getting Started Manual
August 2007
© 2007 Fluke Corporation, All rights reserved. Printed in USA
All product names are trademarks of their respective companies.
Impulse 6000D
Defibrillator Analyzer
Impulse 7000DP
Defibrillator/Transcutaneous Pacer Analyzer
Page 2

Warranty and Product Support
Fluke Biomedical warrants this instrument against defects in materials and workmanship for one year from the date of original purchase OR two years if at the end of your first year you send the instrument to a Fluke Biomedical service center for
calibration. You will be charged our customary fee for such calibration. During the warranty period, we will repair or at our
option replace, at no charge, a product that proves to be defective, provided you return the product, shipping prepaid, to
Fluke Biomedical. This warranty covers the original purchaser only and is not transferable. The warranty does not apply if
the product has been damaged by accident or misuse or has been serviced or modified by anyone other than an authorized
Fluke Biomedical service facility. NO OTHER WARRANTIES, SUCH AS FITNESS FOR A PARTICULAR PURPOSE, ARE
EXPRESSED OR IMPLIED. FLUKE SHALL NOT BE LIABLE FOR ANY SPECIAL, INDIRECT, INCIDENTAL OR CONSEQUENTIAL DAMAGES OR LOSSES, INCLUDING LOSS OF DATA, ARISING FROM ANY CAUSE OR THEORY.
This warranty covers only serialized products and their accessory items that bear a distinct serial number tag. Recalibration
of instruments is not covered under the warranty.
This warranty gives you specific legal rights and you may also have other rights that vary in different jurisdictions. Since
some jurisdictions do not allow the exclusion or limitation of an implied warranty or of incidental or consequential damages,
this limitation of liability may not apply to you. If any provision of this warranty is held invalid or unenforceable by a court or
other decision-maker of competent jurisdiction, such holding will not affect the validity or enforceability of any other provision.
7/07
Page 3
Notices
All Rights Reserved
© Copyright 2007, Fluke Biomedical. No part of this publication may be reproduced, transmitted, transcribed, stored in a retrieval system, or translated into
any language without the written permission of Fluke Biomedical.
Copyright Release
Fluke Biomedical agrees to a limited copyright release that allows you to reproduce manuals and other printed materials for use in service training programs
and other technical publications. If you would like other reproductions or distributions, submit a written request to Fluke Biomedical.
Unpacking and Inspection
Follow standard receiving practices upon receipt of the instrument. Check the shipping carton for damage. If damage is found, stop unpacking the instrument.
Notify the carrier and ask for an agent to be present while the instrument is unpacked. There are no special unpacking instructions, but be careful not to damage the instrument when unpacking it. Inspect the instrument for physical damage such as bent or broken parts, dents, or scratches.
Technical Support
For application support or answers to technical questions, either email techservices@flukebiomedical.com or call 1-800- 648-7952 or 1-425-446-6945.
Claims
Our routine method of shipment is via common carrier, FOB origin. Upon delivery, if physical damage is found, retain all packing materials in their original
condition and contact the carrier immediately to file a claim. If the instrument is delivered in good physical condition but does not operate within specifications, or if there are any other problems not caused by shipping damage, please contact Fluke Biomedical or your local sales representative.
Page 4

Standard Terms and Conditions
Refunds and Credits
Please note that only serialized products and their accessory items (i.e., products and items bearing a distinct serial number tag) are eligible for
partial refund and/or credit. Nonserialized parts and accessory items (e.g., cables, carrying cases, auxiliary modules, etc.) are not eligible for return or refund. Only products returned within 90 days from the date of original purchase are eligible for refund/credit. In order to receive a partial refund/credit of a product purchase price on a serialized product, the product must not have been damaged by the customer or by the carrier chosen by the customer to return the goods, and the product must be returned complete (meaning with all manuals, cables, accessories, etc.) and in “as new” and resalable condition. Products not returned within 90 days of purchase, or products which are not in “as new” and resalable condition, are not eligible for credit return and
will be returned to the customer. The Return Procedure (see below) must be followed to assure prompt refund/credit.
Restocking Charges
Products returned within 30 days of original purchase are subject to a minimum restocking fee of 15 %. Products returned in excess of 30 days after purchase, but prior to 90 days, are subject to a minimum restocking fee of 20 %. Additional charges for damage and/or missing parts and accessories will be applied to all returns.
Return Procedure
All items being returned (including all warranty-claim shipments) must be sent freight-prepaid to our factory location. When you return an instrument to
Fluke Biomedical, we recommend using United Parcel Service, Federal Express, or Air Parcel Post. We also recommend that you insure your shipment for its
actual replacement cost. Fluke Biomedical will not be responsible for lost shipments or instruments that are received in damaged condition due to improper
packaging or handling.
Use the original carton and packaging material for shipment. If they are not available, we recommend the following guide for repackaging:
Use a double–walled carton of sufficient strength for the weight being shipped.
Use heavy paper or cardboard to protect all instrument surfaces. Use nonabrasive material around all projecting parts.
Use at least four inches of tightly packed, industry-approved, shock-absorbent material around the instrument.
Page 5
Returns for partial refund/credit:
Every product returned for refund/credit must be accompanied by a Return Material Authorization (RMA) number, obtained from our Order Entry Group at
1-800-648-7952 or 1-425-446-6945.
Repair and calibration:
To find the nearest service center, go to www.flukebiomedical.com/service
In the U.S.A.:
Cleveland Calibration Lab
Tel: 1-800-850-4606
Email: globalcal@flukebiomedical.com
Everett Calibration Lab
Tel: 1-888-99 FLUKE (1-888-993-5853)
Email: service.status@fluke.com
In Europe, Middle East, and Africa:
Eindhoven Calibration Lab
Tel: +31-402-675300
Email: ServiceDesk@fluke.com
In Asia:
Everett Calibration Lab
Tel: +425-446-6945
Email: service.international@fluke.com
or
Certification
This instrument was thoroughly tested and inspected. It was found to meet Fluke Biomedical’s manufacturing specifications when it was shipped from the
factory. Calibration measurements are traceable to the National Institute of Standards and Technology (NIST). Devices for which there are no NIST calibration standards are measured against in-house performance standards using accepted test procedures.
WARNING
Unauthorized user modifications or application beyond the published specifications may result in electrical shock hazards or improper operation. Fluke Biomedical will not be responsible for any injuries sustained due to unauthorized equipment modifications.
Page 6

Restrictions and Liabilities
Information in this document is subject to change and does not represent a commitment by Fluke Biomedical. Changes made to the information in
this document will be incorporated in new editions of the publication. No responsibility is assumed by Fluke Biomedical for the use or reliability
of software or equipment that is not supplied by Fluke Biomedical, or by its affiliated dealers.
Manufacturing Location
The Impulse 6000D and 7000DP Defibrillator/Transcutaneous Pacer Analyzers are manufactured at Fluke Biomedical, 6920 Seaway Blvd.,
Everett, WA, U.S.A.
Page 7

Table of Contents
Title Page
Introduction .................................................................................................................... 1
Intended Use.................................................................................................................. 1
Unpacking the Analyzer ................................................................................................. 1
Safety Information .......................................................................................................... 2
Instrument Familiarization .............................................................................................. 4
Turning the Analyzer On ................................................................................................ 7
Connecting a Defibrillator and Pacer to the Analyzer..................................................... 7
Accessing the Analyzer Tests ........................................................................................ 8
What to Do Next............................................................................................................. 12
Maintenance................................................................................................................... 12
Cleaning the Analyzer ............................................................................................... 12
Maintaining Peak Battery Condition........................................................................... 12
Accessories.................................................................................................................... 13
Specifications ................................................................................................................. 14
General Specifications............................................................................................... 14
Defibrillator Analyzer Specifications .......................................................................... 15
Energy Output Measurement................................................................................ 15
i
Page 8

Impulse 6000D, 7000DP
Getting Started Manual
ECG Waves ......................................................................................................... 17
Transcutaneous Pacemaker Analyzer Specifications (Impulse 7000DP only).......... 22
Test Load Selections............................................................................................ 22
Measurements...................................................................................................... 22
Demand and Asynchronous Mode Test ............................................................... 23
Sensitivity Test ..................................................................................................... 24
Refractory Period Tests........................................................................................ 24
ii
Page 9

List of Tables
Table Title Page
1. Symbols................................................................................................................................. 2
2. Top-Panel Controls and Connections.................................................................................... 5
3. Rear-Panel Connections ....................................................................................................... 7
4. Accessories ........................................................................................................................... 13
iii
Page 10

Impulse 6000D, 7000DP
Getting Started Manual
iv
Page 11

List of Figures
Figure Title Page
1. Top-Panel Controls and Connections.................................................................................... 4
2. Rear-Panel Connections ....................................................................................................... 6
3. Analyzer Ready Display ........................................................................................................ 7
4. Defib Function Menu ............................................................................................................. 8
5. Cursor Navigation Example................................................................................................... 8
6. Defibrillator Connections ....................................................................................................... 9
7. Pacer Connections ................................................................................................................ 11
8. ECG Connections.................................................................................................................. 11
v
Page 12

Impulse 6000D, 7000DP
Getting Started Manual
vi
Page 13

Defibrillator/Pacer Analyzer
Introduction
The Impulse 6000D and 7000DP (hereafter the Analyzer)
are portable, battery-powered precision instruments for
testing external defibrillators. The 7000DP has the added
capability of testing trancutaneous pacemakers. Where
the additional pacemaker testing capability is applicable,
this manual qualifies the description with “7000DP only.”
The model 7000DP appears in all product illustrations.
Intended Use
The Analyzer is intended for use by trained service
technicians to perform periodic inspections on a wide
range of cardiac resuscitation equipment. The testing
procedures are menu-driven, and simple to operate.
Unpacking the Analyzer
Carefully unpack all items from the box and check that
you have the following items:
• Impulse 6000D or 7000DP
• Battery charger
• Getting Started Manual
• Users Manual CD
• Defib paddle contact plates
• Impulse 6000D 7000DP Ansur Software CD (demo)
1
Page 14

Impulse 6000D, 7000DP
Getting Started Manual
Table 1. Symbols
Symbol Description
W Important information; refer to manual.
Do not dispose of this product as
~
;
)
X Hazardous voltage
P Conforms to European Union directives
CAT I
unsorted municipal waste. Go to Fluke’s
website for recycling information.
Conforms to relevant Australian EMC
requirements
Conforms to relevant Canadian and US
standards
IEC Measurement Category I – CAT I
equipment designed to protect against
transients in equipment on circuits not
directly connected to
circumstances should the terminals of
the Analyzer be connected to any
MAINS voltage.
MAINS. Under no
Safety Information
In this manual, a Warning identifies hazardous conditions
and actions that could cause bodily harm or death. A
Caution identifies conditions and actions that could
damage the Analyzer, the equipment under test, or cause
permanent loss of data.
XW Warning
To avoid possible electrical shock or personal
injury, follow these guidelines:
• Use this Analyzer only in the manner
specified by the manufacturer or the
protection provided may be impaired.
• Read the Users Manual before operating the
Analyzer.
• Do not use the product if it operates
abnormaly.
• Do not use the product in wet locations,
around explosive gases or dust.
• Use extreme caution when working with
voltages above 30 volts.
• Use the proper terminals, functions and
ranges for the test being performed.
2
Page 15

Defibrillator/Transcutaneous Pacer Analyzer
Safety Information
• Do not operate the Analyzer with the battery
eliminator connected, unless connected
directly to mains power. During battery
operation, completely remove the battery
eliminator/charger from both the Analyzer
and wall socket.
• Observe all precautions noted by the
Device Under Test (DUT) equipment
manufacturer when analyzing the DUT.
3
Page 16
Impulse 6000D, 7000DP
Getting Started Manual
Instrument Familiarization
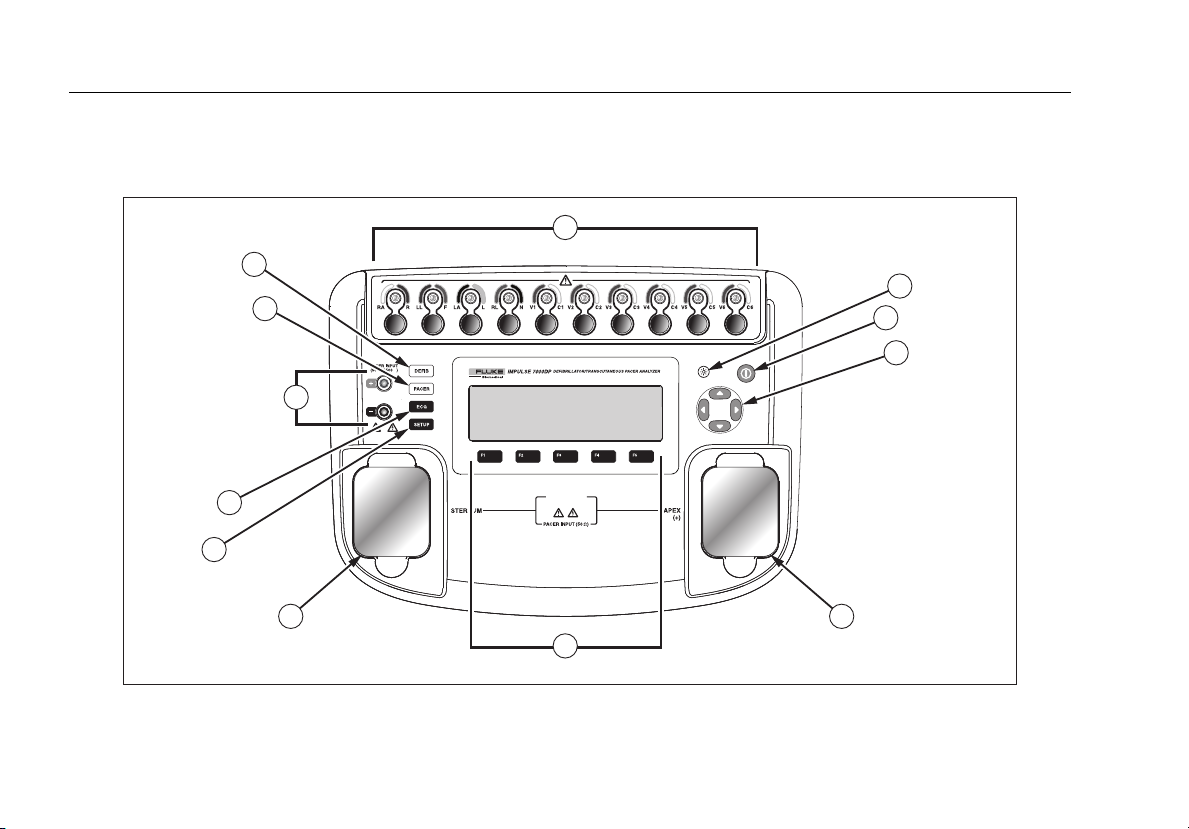
Figure 1 and Table 2 describes the top-panel controls and connections of the Analyzer .
1
11
10
2
3
4
4
9
8
275 V MAX
DEFIBRILLATOR
V
5000
p
MAX
7
5
5
6
Figure 1. Top-Panel Controls and Connections
fak07.eps
Page 17
Defibrillator/Transcutaneous Pacer Analyzer
Instrument Familiarization
Table 2. Top-Panel Controls and Connections
Item Name Description
1 ECG lead connectors
2 Backlight button Turns the LCD display backlight on and off.
3 Power button Turns the Analyzer on and off.
4 Navigation buttons Cursor control buttons for navigating menus and lists.
5 Defib connectors Defibrillator connections (Shown with removable defib paddle contact plates installed).
6 Function softkeys
7 Setup button Opens the setup menu.
8 ECG button Opens the main menu for ECG test functions.
9 Pacemaker inputs Input for low-level Pacer signal (7000DP only).
10 Pacer button Opens the main menu for pacer test functions (7000DP only).
11 Defibrillator button Opens the main menu for defibrillator test functions.
Outputs of low-level ECG signals (RA/R, LL/F, LA/L, RL/N, V1/C1, V2/C2, V3/C3, V4/C4,
V5/C5, and V6/C6).
Keys F1 through F5 are used to select from a number of selections that appear in the LCD
display above each function softkey.
5
Page 18

Impulse 6000D, 7000DP
Getting Started Manual
Figure 2 and Table 3 describes the rear-panel connections of the Analyzer.
6
CHARGE STATUS
21
SN
15VDC / 1.5 A
SERIAL NUMBER
FLUKE BIOMEDICAL
6920 SEAWAY BLVD
EVERETT, WA 98203
www.flukebiomedical.com
MADE IN USA
Figure 2. Rear-Panel Connections
HIGH LEVEL
SCOPE
ECG OUTPUT
OUTPUT
3 4 5
COMPUTER
PORT
fak08.eps
Page 19
Defibrillator/Transcutaneous Pacer Analyzer
Turning the Analyzer On
Table 3. Rear-Panel Connections
Item Name Description
1 Charge Status LED
Battery Charger
2
connector
3 Scope output Output signal jack for displaying the defib playback wave on an oscilloscope.
4 Hi-level ECG output High-level ECG signal output jack for oscilloscope viewing.
5 Computer Port Device Port (B-style USB) for controlling the Analyzer from a PC or instrument controller.
Indicates RED while battery is charging. Indicates GREEN when the battery is fully
charged and the charger is still connected.
Input connector for attaching the battery charger to the Analyzer.
Turning the Analyzer On
Note
When using the Analyzer for the first time, plug
the battery charger into the Analyzer and a power
outlet and charge the Analyzer for at least 4
hours. The Analyzer is still usable during this
period with the battery charger connected.
Press the power button (O) on the top panel to turn the
Analyzer on. After a short self-test period, the Analyzer will
display the screen shown in Figure 3 to indicate it is ready
for operation.
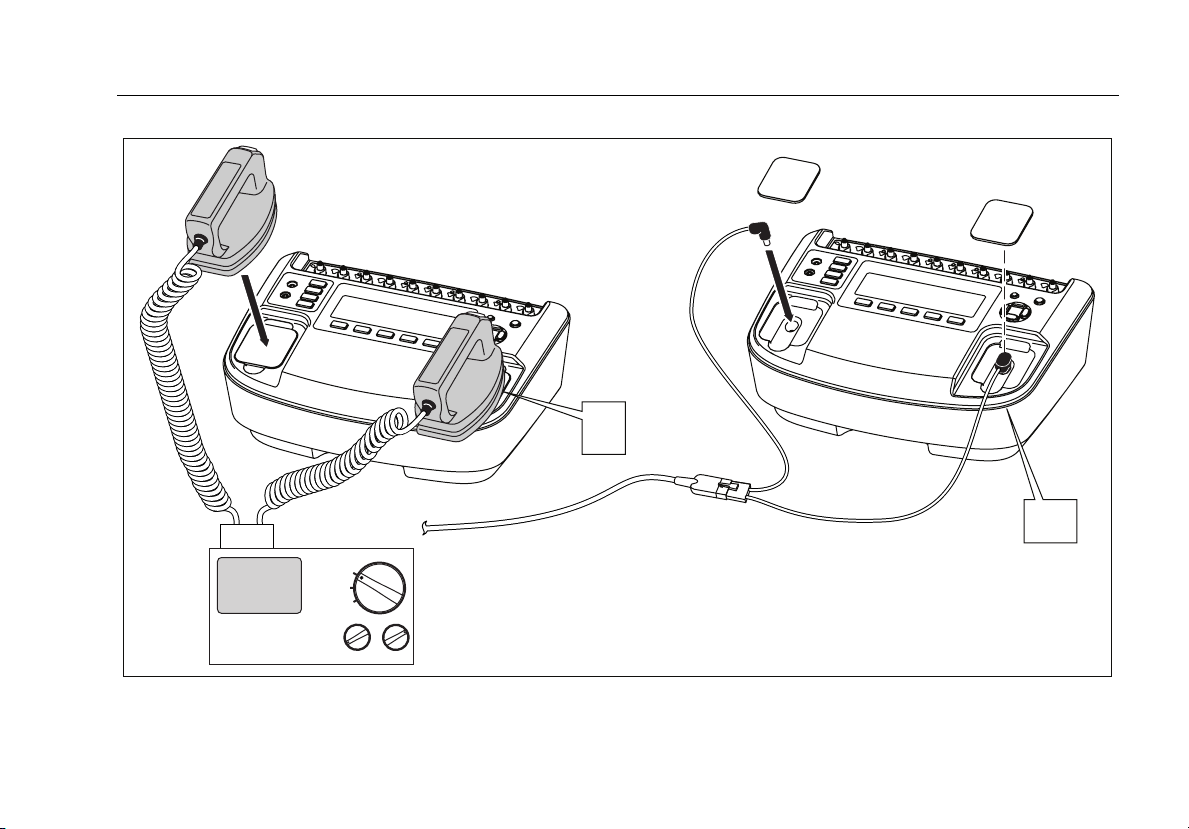
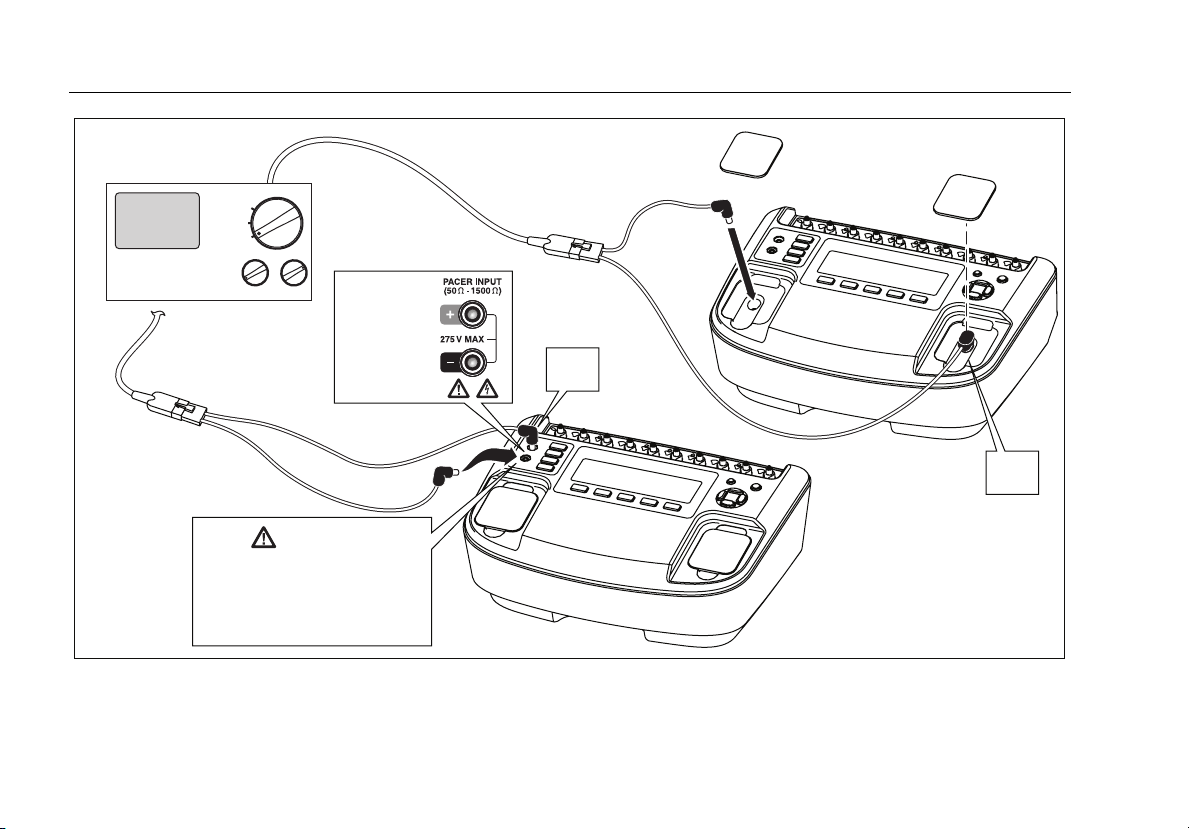
Connecting a Defibrillator and Pacer to the Analyzer
Figure 6 shows the two methods of connecting a
defibrillator to the Analyzer. The Defib Paddle Contact
Figure 3. Analyzer Ready Display
7
fak01.eps
Page 20
Impulse 6000D, 7000DP
Getting Started Manual
plates are inserted into the defibrillator jacks when
external defibrillator paddles are used on the defibrillator.
W Caution
To avoid damage to the Analyzer or
defibrillator, do not apply defibrillator pulses
to the pacer inputs.
Figure 7 shows the pacer connected to either the pacer
input jacks or the defibrillator jacks. While the pacer input
jacks have a selectable load from 50 to 1500 Ω, the
defibrillator input jacks have a fixed load of 50 Ω.
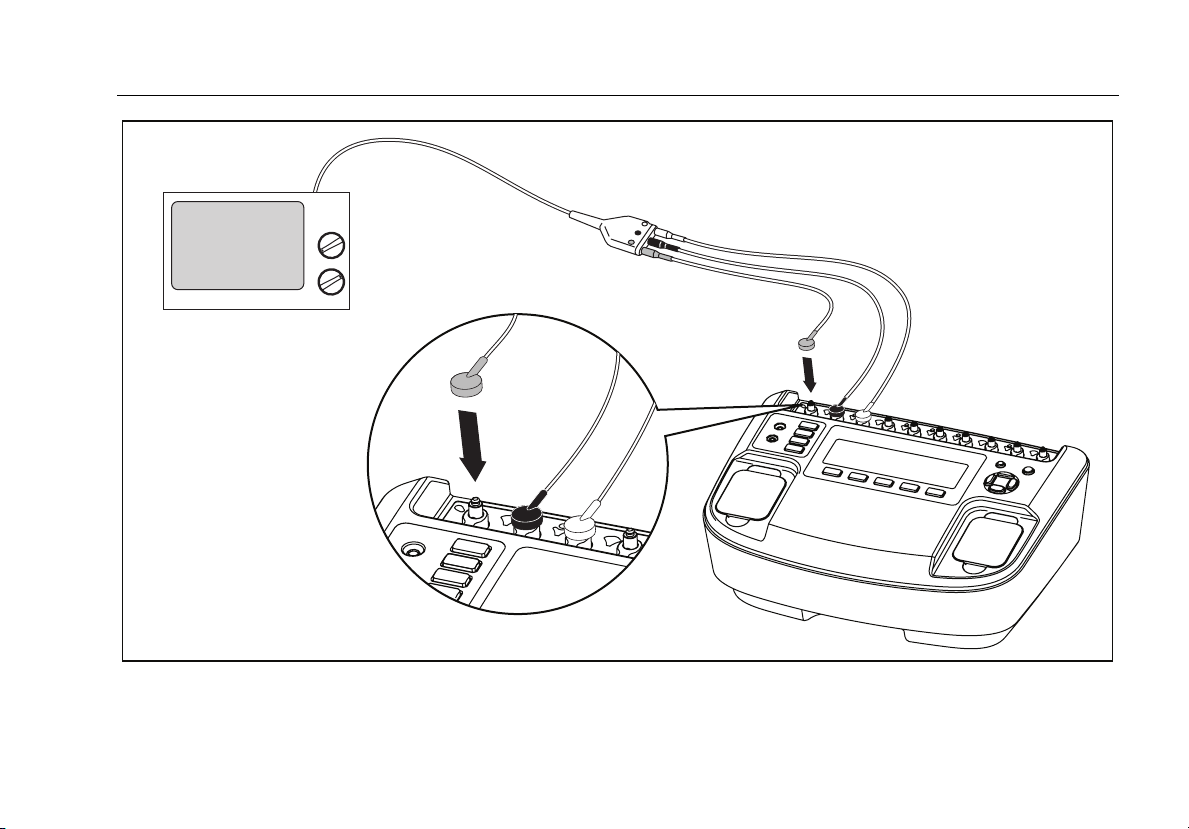
Figure 8 shows how to connect the ECG leads to the
Analyzer.
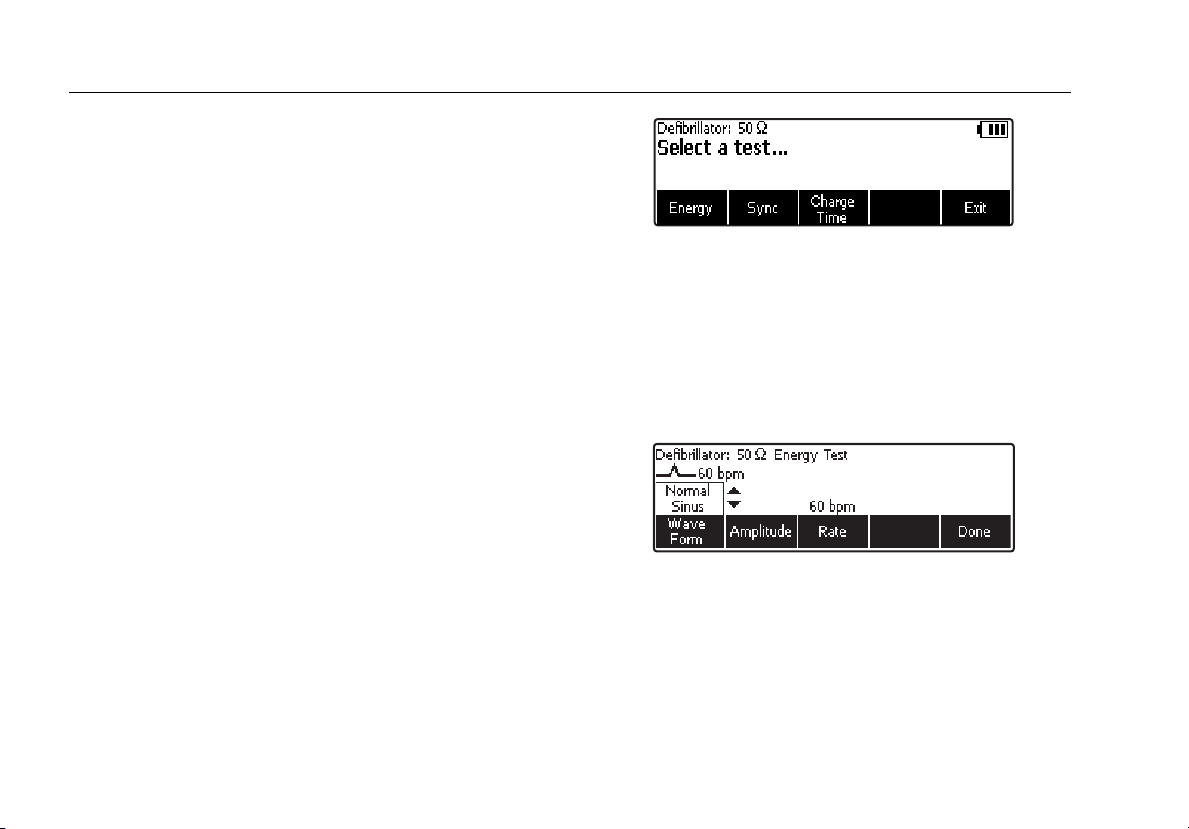
Accessing the Analyzer Tests
The Analyzer uses a series of menus to access various
Analyzer functions and setup variables. As shown in
Figure 4, the Analyzer indicates three different defibrillator
tests (Energy, Sync, and Charge Time) along the bottom
of the display. An Exit selection is also indicated as a way
of backing out of the defibrillator tests. Pressing a softkey
(F1 through F5) under a specific test will cause that test to
be selected.
fak02.eps
Figure 4. Defib Function Menu
Some menu selections reveal a list of selectable items by
displaying K to the right of the presently selected item.
See Figure 5. To change the selection, press either G or
H to scroll through the possible selections. Once the
desired selection appears, press the function softkey and
K disappears from the display.
fak03.eps
Figure 5. Cursor Navigation Example
8
Page 21
Defibrillator/Transcutaneous Pacer Analyzer
Accessing the Analyzer Tests
+
+
DEFIB
OFF
PACER
Defibrillator/Pacer
fak11.eps
Figure 6. Defibrillator Connections
9
Page 22
Impulse 6000D, 7000DP
Getting Started Manual
Defibrillator/Pacer
DEFIB
OFF
PACER
Caution
To avoid damage to the
Analyzer or defibrillator, do
not apply defibrillator pulses
to the pacer inputs.
50 - 1500 Ω
7000DP Only
+
Figure 7. Pacer Connections
50 Ω
Only
+
fak10.eps
10
Page 23
Defibrillator/Transcutaneous Pacer Analyzer
Accessing the Analyzer Tests
ECG Monitor
fak09.eps
Figure 8. ECG Connections
11
Page 24

Impulse 6000D, 7000DP
Getting Started Manual
What to Do Next
For more information on how to use the Analyzer, refer to
the Impulse 6000D, 7000DP Users Manual contained on
the accompanying CD.
Maintenance
The Analyzer needs little maintenance or special care.
However, treat it as a calibrated measuring instrument.
Avoid dropping or other mechanical abuse that could
cause a shift in the calibrated settings. The Analyzer has
no internal user serviceable parts.
Cleaning the Analyzer
W Caution
Do not pour fluid onto the Analyzer surface;
fluid seepage into the electrical circuitry may
cause the Analyzer to fail.
W Caution
Do not use spray cleaners on the Analyzer;
such action may force cleaning fluid into the
Analyzer and damage electronic components.
Clean the Analyzer occasionally utilizing a damp cloth and
mild detergent. Take care to prevent the entrance of
liquids.
Wipe down the adapter cables with the same care. Inspect
them for damage to and deterioration of the insulation.
Check the connections for integrity before each use.
Maintaining Peak Battery Condition
To maintain peak battery capacity, the Analyzer should be
charged completely at least once a month. If the Analyzer
is to be left idle for more than a month and it is
inconvenient to periodically connect to the battery charger,
keep it connected to the charger while idle.
Note
To obtain the specified performance, use the
battery charger specified in this manual.
12
Page 25
Defibrillator/Transcutaneous Pacer Analyzer
Accessories
Accessories
Table 4 lists the accessories for the Analyzer. Contact your local Fluke Biomedical sales representative or go to
www.flukebiomedical.com for an up-to-date accessories list.
Table 4. Accessories
Item
GE Medical RESPONDER1500/1700 4mm 3065423
Internal Defib Pdl Contacts 2/set 4mm 3065438
R2 Darox MRL/MDE/NK/Kimberly Clark 4mm 3065450
Med ERS /PhysioControl QUIK COMBO 4mm 3065461
Med ERS/PhysioControl QUIK PACE 4mm 3065477
Med ERS/PhysioControl FAST PATCH 4mm 3065489
Philips/HP/Agilent CODEMASTER 4mm 3065492
Philips/Agilent HEARTSTART FR2/MRX 4mm 3065509
ZOLL Medical PD-2200 MULTIFUNCTION 4mm 3065511
ZOLL Medical NTP/PD1400 4mm 3065527
Fluke Biomedical Model
Number
13
Page 26

Impulse 6000D, 7000DP
Getting Started Manual
Specifications
General Specifications
Temperature
Operating ............................................................10 °C to 40 °C (50
Storage................................................................ -20 °C to +60 °C (-4
Humidity................................................................. 10 % to 90 % non-condensing
Display ...................................................................LCD display
Communications................................................... USB device port for computer control
Modes of Operation ..............................................Manual and remote
Power .....................................................................Internal rechargeable NiMH battery pack for nine hours (typical) operation after full
charge, or the battery charger can operate the Analyzer and charge the battery
simultaneously.
Battery Charger ..................................................... 100 to 240 V input, 15 V/1.5 A output. For best performance, the battery charger should
be connected to a properly grounded ac receptacle.
Mechanical
Housing ............................................................... ABS Plastic
Size (H x W x L) ..................................................13 cm x 32 cm x 24 cm (5 in x 13 in x 9.5 in)
Weight ................................................................. 3.0 kg (6.6 lb)
Safety Standards
CE .......................................................................IEC/EN61010-1 2
CSA..................................................................... CAN/CSA- C22.2 No. 61010-1; UL61010-1
Electromagnetic Compatibility Standards (EMC)
European EMC.................................................... EN61326-1
°F to 104 °F)
°F to +140 °F)
nd
Edition; Pollution degree 2
14
Page 27

Defibrillator/Transcutaneous Pacer Analyzer
Specifications
Defibrillator Analyzer Specifications
Energy Output Measurement
Compatible Defibrillator Waveshapes.................Lown, Edmark, Trapezoidal, DC Bi-phasic, and AC Pulsed Bi-phasic
Autoranged Measurement ....................................0.1 to 600 J
Accuracy
0.1 to 360 J .....................................................±(1 % of reading + 0.1 J)
360 to 600 J ....................................................±(1 % of reading + 0.1 J), typical
Note
For Pulsed Bi-Phasic defibrillator, specified accuracy is ±(1.5 % of reading + 0.3 J) on both ranges.
Load resistance
Resistance...........................................................50 Ω
Accuracy..........................................................±1 %, non-inductive (<2 μH)
Variable external load resistance (optional) .......25, 75,100, 125, 150, or 175 Ω, All values ±1 %, non-inductive (<2 μH)
Pulse trigger level .................................................20 V
Pulse width
Range.................................................................. 1.0 to 50.0 ms
Accuracy..............................................................±0.1 ms
Voltage
Range.................................................................. 20 to 5000 V
Accuracy..............................................................±(1 % of reading + 2 V)
Current
Range.................................................................. 0.4 to 100.0 A
Accuracy..............................................................±(1 % of reading + 0.1 A)
15
Page 28

Impulse 6000D, 7000DP
Getting Started Manual
Sample rate............................................................250 kHz (4 μs sample)
Maximum Average Power.....................................12 W, equivalent to 10 defib pulses of 360 J every 5 minutes
Oscilloscope Output
Autorange............................................................ 2000:1, 400:1 and 80:1: dependant on the range
Waveform Playback
Output .............................................................BNC
Output impedance........................................... 50 Ω (nominal)
Delay............................................................... 50 ms (nominal)
Accuracy .........................................................±5 % of nominal
Charge Time Measurement
Range.................................................................. 0.1 to 100.0 s
Accuracy .............................................................±0.05 s, typical
Synchronization Test (Elective Cardioversion )
Delay Time Measurement
Timing window ................................................ECG R-wave peak to the defib pulse peak
Range..............................................................-120 to +380 ms; measures timing from 120 ms prior to the R-wave peak to up to 380 ms
following the R-wave peak.
Resolution ....................................................... 1 ms
Accuracy .........................................................±1 ms
ECG waves
Normal Sinus Rhythm (NSR) .......................... 30 to 180 (by 1) BPM
Atrial fibrillation................................................ Coarse and fine
Monomorphic Ventricular Tachycardia ...........120 to 240 (by 5) BPM
Asystole...........................................................Flat line
16
Page 29

Defibrillator/Transcutaneous Pacer Analyzer
Specifications
Automated Defibrillator Test ECG Waves
Normal Sinus.......................................................30 to 300 (by 1) BPM
Ventricular Fibrillation..........................................Coarse and fine
Monomorphic Ventricular Tachycardia................120 to 300 (by 5) BPM
Polymorphic Ventricular Tachycardia..................5 types
Asystole...............................................................Flat line
ECG Waves
ECG General
Lead configuration...............................................12-lead simulation. RA, LL, LA, RL, V1-6 with independent outputs
Lead to lead impedance ......................................1000 Ω (nominal)
Rate accuracy .....................................................±1 % of nominal
ECG Amplitudes
Reference lead ....................................................Lead 1
Settings ...............................................................0.05 to 0.45 (by 0.05) mV
Accuracy..............................................................±2 % of setting, lead I and 2 Hz square wave
For performance waves and R-wave detection, other leads are proportional to Lead I in percentage per:
Lead I ..............................................................100
Lead II .............................................................150
Lead III ............................................................50
Leads V1 through V6 ......................................100
For normal sinus waves, other leads are proportional to Lead I in percentage per:
Lead I ..............................................................100
Lead II .............................................................150
Lead III ............................................................50
0.5 to 5.0 (by 0.5) mV
17
Page 30

Impulse 6000D, 7000DP
Getting Started Manual
Lead V1........................................................... 24
Lead V2........................................................... 48
Lead V3........................................................... 100
Lead V4........................................................... 120
Lead V5........................................................... 112
Lead V6........................................................... 80
ECG Normal Sinus
Rates................................................................... 30 to 360 (by 1) BPM
ECG High Level Output (BNC Jack)
Amplitude ............................................................0.2 V/mV of Lead I amplitude
Accuracy .............................................................±5 %. 2 Hz Square Wave
Output Impedance............................................... 50 Ω output impedance
ECG on Defibrillator Input Load
Same as Lead II amplitude
ECG Performance Waves
Square wave .......................................................2.0 and 0.125 Hz
Triangular wave................................................... 2.0 and 2.5 Hz
Sine waves.......................................................... 0.05, 0.5, 5, 10, 40, 50, 60, 100, 150, and 200 Hz
Pulse ...................................................................30 and 60 BPM, 60 ms pulse width
R-Wave Detection
Waveform............................................................ Haver-triangle
Amplitude ............................................................0.05 to 0.45 (by 0.05) V
Rate..................................................................... 30, 60, 80, 120, 200, and 250 BPM
Widths .................................................................8, 10, 12 ms, and 20 to 200 (by 10) ms
Accuracy .............................................................±(1 % setting + 0.2 mV)
0.5 to 5.0 (by 0.5) V
18
Page 31

Defibrillator/Transcutaneous Pacer Analyzer
Specifications
Noise Immunity
Wave ................................................................... Sine
Line Frequency....................................................50 or 60 Hz (± 0.5 Hz)
Amplitude ............................................................0.0 to 10.0 (by 0.5) mV
Accuracy..............................................................± 5%
Transvenous Pacer Pulse Simulation
Widths
Range..............................................................0.1, 0.2, 0.5, 1.0, and 2.0 ms
Accuracy..........................................................±5 % of setting
Amplitudes...........................................................0 (off) and ±2, ±4, ±6, ±8, ±10, ±12, ±14, ±16, ±18, ±20, ±50, ±100, ±200, ±500, and
Accuracy..............................................................± (10 % of setting + 0.2 mV)
Arrhythmia Selections
Pacer Interactive (Transcutaneous pacer, Impulse 7000DP only)
Demand...........................................................30 to 360 (by 1) BPM
Asynchronous
Non-Capture
Non-Function
Threshold (Interactive pacing simulation only) 10 to 250 (by 10) mA
Supraventricular
Atrial Fibrillation Coarse
Atrial Fibrillation fine
Atrial Flutter
Sinus Arrhythmia
Missed Beat
Atrial Tachycardia
±700 mV
19
Page 32

Impulse 6000D, 7000DP
Getting Started Manual
Paroxysmal Atrial Tachycardia (PAT)
Nodal Rhythm
Supraventricular Tachycardia
Premature
Atrial PAC
Nodal PNC
PVC1 Left Ventricle
PVC1 LV Early
PVC1 LV R on T
PVC2 Right Ventricle
PVC2 RV Early
PVC2 RV R on T
Multifocal PVCs
Ventricular
PVCs 6/min
PVCs 12/min
PVCs 24/min
Freq Multifocal
Trigeminy
Bigeminy
Pair PVCs
Run 5 PVCs
Run 11 PVCs
Monomorphic Ventricular Tachycardia ...........120 to 300 (by 5) BPM
Polymorphic Ventricular Tachycardia .............1 to 5
Ventricular Fibrillation: Coarse and Fine
20
Page 33

Defibrillator/Transcutaneous Pacer Analyzer
Specifications
Asystole
Conduction
1° Block
2° Block Type I
2° Block Type II
3° Block
Right Bundle Branch Block RBBB
Left Bundle Branch Block LBBB
Transvenous Paced with selectable pacer spike amplitudes and widths
Atrial 80 BPM
Async 75 BPM
Demand with frequent sinus beats
Demand with occasional sinus beats
AV Sequential
Non-Capture
Non-Function
Selectable pacer pulse parameters for transvenous simulation. (Atrial and Ventricular channels are independently selectable):
Atrial Pacer Pulse
Width ....................................................0.1, 0.2, 0.5, 1.0, 2.0 ms
Polarity .................................................+ or -
Amplitude .............................................0 (off), 2 to 20 (by 2), 50, 100, 200, 500, 700 mV
Ventricular Pacer Pulse
Width ....................................................0.1, 0.2, 0.5, 1.0, 2.0 ms
Polarity .................................................+ or -
Amplitude .............................................0 (off), 2 to 20 (by 2), 50, 100, 200, 500, 700 mV
21
Page 34

Impulse 6000D, 7000DP
Getting Started Manual
Transcutaneous Pacemaker Analyzer Specifications (Impulse 7000DP only)
Test Load Selections
Defibrillator Input
Fixed Load ..........................................................50 Ω
Accuracy .............................................................±1 %, non-inductive (<2 μH)
Power Rating....................................................... 10 defib pulses of 360 J every 5 minutes
Pacemaker Input
Variable Load ...................................................... 50 to 1500 Ω in 50 Ω steps
Accuracy .............................................................±1 %, non-inductive (<2 μH)
Power Rating....................................................... 5 W (average), 40 W (peak) @ 1000 Ω
Measurements
Manufacturer Specific Algorithms
GE Responder (1500 & 1700)
MDE 300 (Medical Data Electronics)
Medtronic ERS/Physio Control LIFEPAK
MRL (Medical Research Laboratory/Welch Allyn)
Philips/Agilent/HP
Schiller Medical
ZOLL Medical
(plus a general purpose default algorithm selection)
Current
Range.................................................................. 4.00 to 250 mA
Accuracy .............................................................±(1% of reading + 0.02 mA)
22
Page 35

Defibrillator/Transcutaneous Pacer Analyzer
Specifications
Pulse Rate
Range.................................................................. 5.0 to 800 PPM
Accuracy..............................................................±(0.5% of reading + 0.1 PPM)
Pulse Width
Range.................................................................. 1.00 to 100.0 ms
Accuracy..............................................................±(0.5% of reading + 0.01 ms)
Energy
Range.................................................................. 1 µJ to 2.00 J
Accuracy..............................................................±(4% of reading + 10 µJ)
Demand and Asynchronous Mode Test
Input Pacer pulse rates.........................................30 to 200 PPM
ECG NSR wave
Rate..................................................................... 10 to 300 (by 1) BPM
Amplitude ............................................................1 mV
Underdrive rate....................................................10 BPM minimum
Overdrive rate......................................................300 BPM maximum
23
Page 36

Impulse 6000D, 7000DP
Getting Started Manual
Sensitivity Test
Automatic Interactive Threshold Detection
Compatible pacer rates ....................................... 30 to 120 PPM
ECG R wave:
Waveforms .......................................................... Square, Triangle, Sine
Width ................................................................... 1 to 19 (by 1) ms
Accuracy .............................................................± 5% of setting
Amplitude ............................................................0.05 to 0.95 (by 0.05) mV
Accuracy .............................................................± 5% of setting
20 to 95 (by 5) ms
100 to 300 (by 25) ms
1.0 to 5.0 (by 0.5) mV
Refractory Period Tests
Paced Refractory Period ......................................20 to 500 ms
Sensed Refractory Period ....................................15 to 500 ms
Accuracy................................................................±1 ms
Pacer pulse rate ....................................................20 to 200 PPM
ECG
Waveform............................................................ Triangle wave
Pulse width.......................................................... 40 ms
Amplitude ............................................................1.0 mV
24
 Loading...
Loading...